Abstract
Introduction:
Reconstruction of complex abdominal wall defects is both challenging and technically demanding for plastic surgeon. Objectives in abdominal wall reconstruction are consistent and include restoration of abdominal wall integrity, protection of intra abdominal viscera and prevention of herniation.
Materials:
We conducted a retrospective study on five patients in whom lateral thigh flaps such as anterolateral thigh (ALT) flaps and tensor fascia lata (TFL) myocutaneous flaps as pedicled or free flaps were used for complex abdominal wall Type II defects over a 5- years period between 2007 and 2012.
Results:
In two patients, free flaps were used for reconstruction of the upper abdomen and both were ALT. In three patients of lower abdominal defects, one patient had bilateral pedicled ALT flaps, one pedicled TFL myocutaneous and one free TFL myocutaneous in view of ipsilateral electric burn scars. There were no flap losses. Patients were followed up beyond 6 months and found to have a good abdominal contour and only one of five had clinical evidence of herniation.
Conclusion:
It can be concluded that flap from the Lateral thigh (ALT or TFL) is flap of choice for large Type II abdominal defects. Including vascularised fascia in the flap maintains abdominal wall integrity and use of synthetic mesh is not necessary. Upper abdominal defects need free flaps and in lower abdominal defects a pedicled flap suffices.
KEY WORDS: Complex anterior abdominal wall defects, pedicle and free anterolateral thigh flaps and Tensor Fascia Lata Flap, reconstruction, vascularised fascia
INTRODUCTION
Acquired defects of the abdominal wall can result from tumour excision, necrotizing infections and electrical injuries and need to be reconstructed with a view to provide integrity of the skin (where it is deficient or suboptimal) and also the deeper musculofascial layers to prevent herniation and its attendant symptoms such as pain and discomfort.[1,2,3] For ease of planning and reconstruction, the abdominal wall defects have been divided into two groups depending on defect components. The Type I defect has an intact or stable skin cover and the Type II defect has an unstable or absent skin cover as described by Mathes et al. classification.[4] Type II defects are further classified into zones [4] as shown in the Figure 1.
Figure 1.
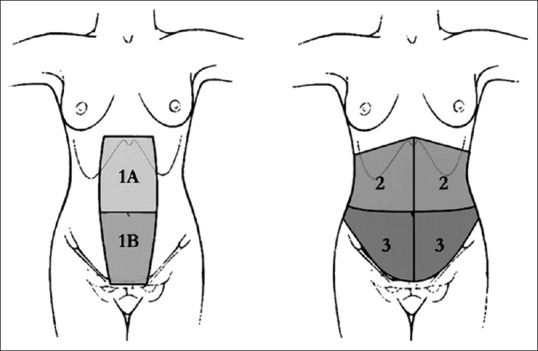
Classification of abdominal defects by Mathes ( Zone 1A: Upper midline defect, Zone 2: Upper quadrant defect of the abdomen, Zone IB: Lower midline defect, Zone 3: Lower quadrant defect of the abdomen (Extending across midline))
Though mesh repair and component separation methods can be used to manage Type I defects and small Type II defects, it is not applicable for moderate to large (>40 cm2) Type II defects as it creates excessive tension with risk of tissue necrosis or abdominal compartment syndrome; hence the import of vascularised tissues in the form of a skin flap is necessitated.[5]
Various local and regional flaps have been proposed utilizing nearby abdominal skin but are limited by size constraints.[6] Techniques that recruit the surrounding abdominal skin will necessitate the use of split skin grafts over the donor site. Restoration of integrity of the fascial layers has been conventionally done by the use of synthetic meshes.[7]
The use of free flaps prevents the violation of the residual abdominal wall and facilitates tension-free closure of the defect. Autogenous vascularised fascia has an advantage over synthetic meshes in that, they stand a better chance of incorporation into the body.[8] Flaps from the lateral thigh either anterolateral thigh (ALT) or tensor fascia lata (TFL) myocutaneous [9] (pedicled or free) are ideal for reconstruction of such defects, as the residual abdominal skin is not violated.
This article proposes to rationalize the choice of the flap to individual defects based on the anatomical location and suitability of available perforators arising from the lateral circumflex femoral vessels.
MATERIALS
A total of 5 patients underwent abdominal wall reconstruction during 2007-2012. All five were males with age ranging from 30 years to 52 years.
Three patients had sustained high-voltage electrical burns earlier and had underwent serial debridement with split skin grafting to achieve wound closure; they presented with abdominal ventral hernia [Figures 2a and 3a] Two patients had abdominal defects intra operatively following tumour excision [Figure 4] According to the classification of Mathes all the five patients had a large (>40 cm2) Type II defect (unstable or absent skin). The size of the defects varied from 15 to 20 cm long and 18 to 36 cm wide.
Figure 2a.
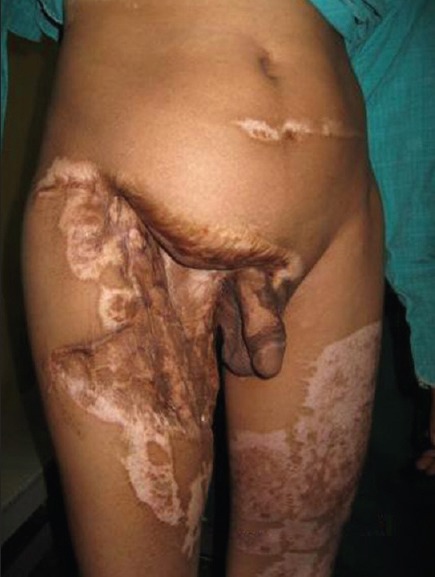
Pre-operative case of Type II electrical burns defects in the right lower abdomen and groin
Figure 3a.
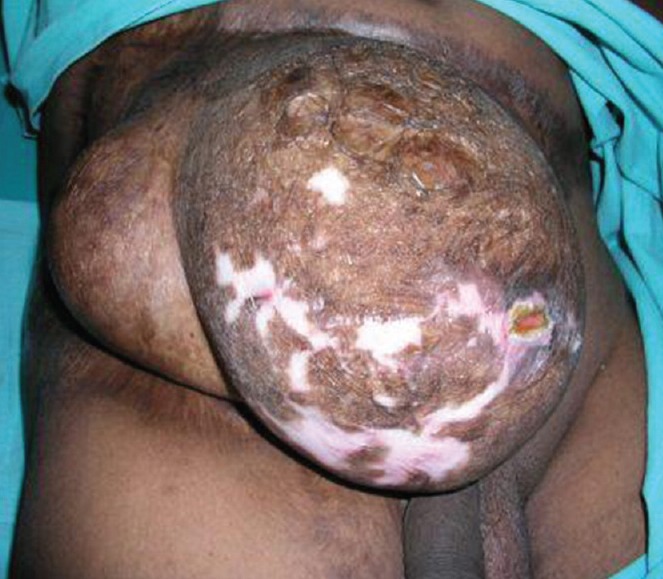
Pre-operative large lower midline and lower lateral quadrants of Type II electrical burns defect with unstable scar
Figure 4a.
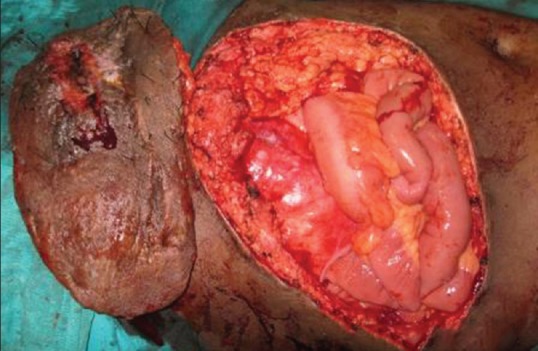
Intraoperative photograph showing after excision of the tumour with abdominal wall defect in left lower quadrant
Figure 2b.
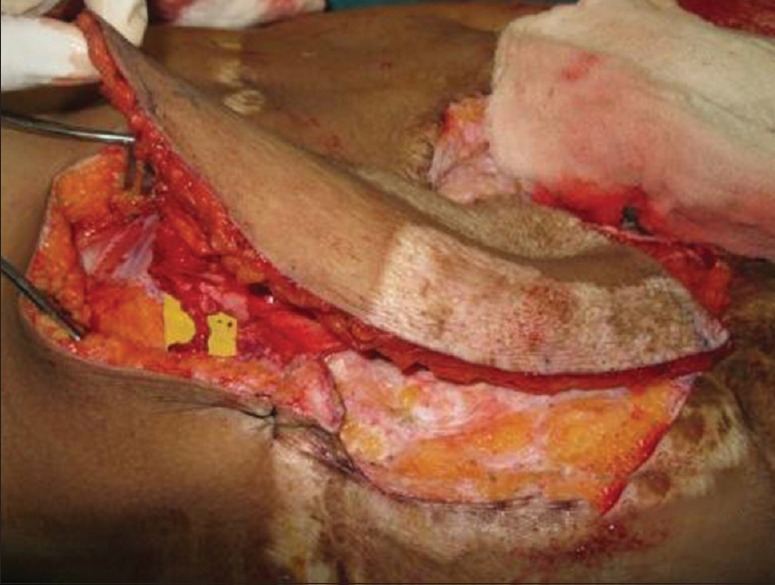
Intraoperative after complete harvesting of the tensor fascia lata free flap
Figure 3b.
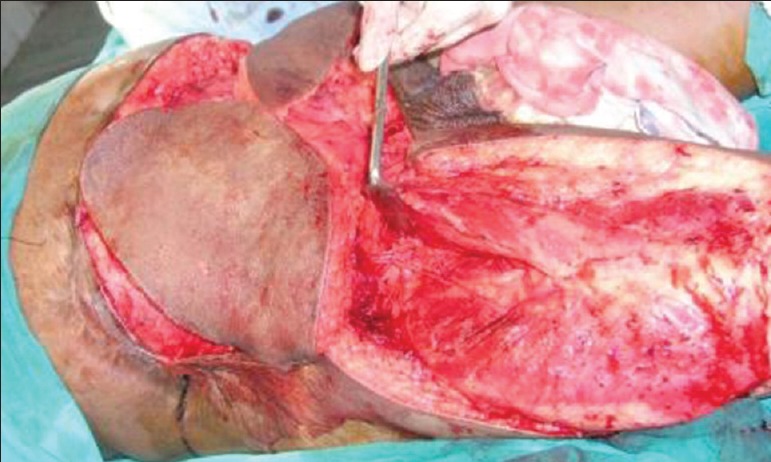
After transferring bilateral pedicled anterolateral thigh flaps over the defect
Figure 4c.
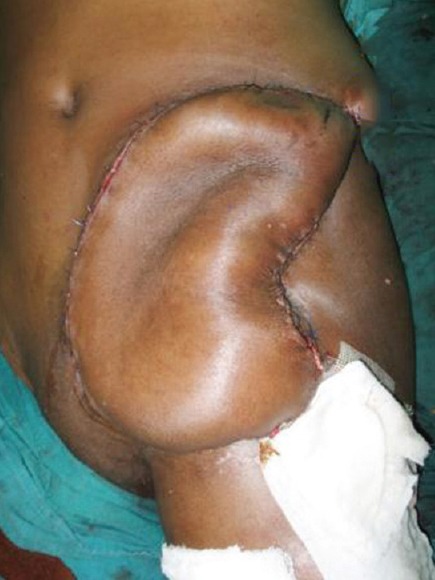
After complete inset of the flap
Figure 4d.
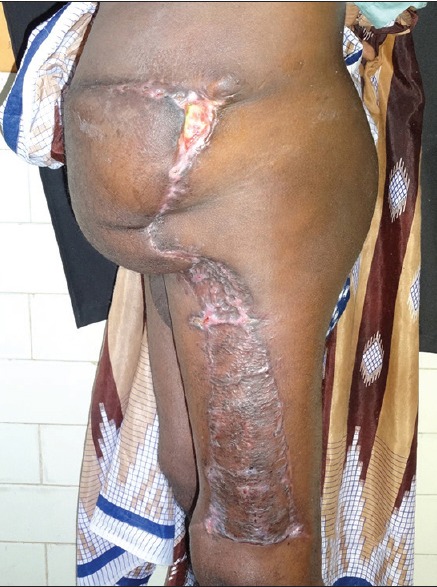
Post-operative follow-up
Amongst the two patients who presented intraoperatively with abdominal defect, one had a recurrent desmoid tumour for which he underwent only debulking as the abdominal wall tumour had an unresectable retroperitoneal extension [Figure 4a]. The other patient had a carcinoma of the splenic flexure of colon infiltrating to upper lateral and middle portions of the abdomen; he underwent wide local excision of tumour including a portion of the abdominal wall, left hemicolectomy and colo-colic anastomosis; both these patients had preoperative computed tomography scans of the abdomen.
The three patients who presented with ventral hernia had a routine ultrasound of the abdomen to exclude intra-abdominal pathology. Table 1 summarizes the pre-operative clinical findings of the five patients.
Table 1.
Preoperative clinical findings and special remarks

REVIEW OF LITERATURE
Type II defects need the use of local, regional or free flaps for providing skin cover and the use of autologous or prosthetic material in the form of a mesh to provide musculofascial integrity.[4]
Local flaps are the optimal choice for partial musculofascial defects of the lateral abdominal wall >3 cm in size. The thoracoepigastric flap has been described for repair of the upper third of the abdomen, but its use is limited by its arc of rotation.[10,11,12] The iliolumbar bipedicled flap is ideal for the middle third of the abdominal wall. Ohtsuka et al. described the iliolumbar bipedicled flap with a TFL graft for the repair of defects of the lateral abdominal wall.[13]
The lower third of the abdomen may be reconstructed with groin flap, which has a large arc of rotation allowing mobilization to the umbilicus.[12] Terashi et al. reported a case in which the groin flap was used in conjunction with a thigh adipofascial graft for the successful repair of large defect secondary to tumour resection.[14] Koshima described using the extended deep inferior epigastric artery (DIEP) flap to repair a defect secondary to resection of a malignant lymphoma.[15,16,17]
The rectus abdominis is the flap of choice for lateral defects.[11] The superiorly based rectus is for the upper two-thirds defects and inferiorly based rectus flap for lower one-third defects.[18,19,20] The external oblique flap for upper two-third defect,[21] and internal oblique flap for lower one-third and groin defects.[22] There is higher incidence of complications of skin necrosis and hernias with the use of local muscle flaps such as the rectus abdominis as studied by Defranzo et al. with 15 such cases.[20]
The criteria for special reconstruction techniques include a large defect – 40 square cm, absence of stable skin coverage, recurrence of the defect after previous closure attempts, infected or exposed mesh, systemic compromise (intercurrent malignancy), local tissue compromise (irradiation, corticosteroid dependence), and concomitant visceral complications (enterocutaneous fistula).[4]
Extensive upper midline abdominal wall and thoraco-abdominal defects usually require a free flap with or without the use of meshes.[6] A variety of free flaps is discussed in the literature, but the TFL is reported most commonly.[23,24,25] Free groin flap has been reported but has limitations with respect to myofascial support and pedicle consistency. Free innervated latissimus dorsi flap has also been reported to re-establish the contractile force and strength of the lost abdominal wall.[26]
The lateral thigh is an area of large skin availability and has the added advantage of vascularised fascia that can be incorporated in the repair of fascial tissues of the defect permitting avoidance of synthetic meshes. It has been used to replace loss of tendon substance, replace dura and patellar tendon also.[27,28] The free ALT flap alone or combination of vascularised fascia lata flap for reconstruction of soft tissue defect has been documented.[29,30,31] The choice of flap TFL myocutaneous or ALT as pedicled or free is likely to depend on the availability of recipient vessels, geometry of the defect and it's relation to the flap donor site.
OPERATIVE PLANNING
Three of the reconstructions of post electric burn sequelae had preoperative planning to determine the size of the defect and its relation to the proposed flap donor site. The planning for the two cases of skin defect following excision of tumours was made intra-operatively. Location of the perforators of the ALT flap on both thighs was mapped using a handheld Doppler probe to choose the most optimal donor site.
The decision to use a TFL myocutaneous as against an ALT flap was made based on availability of suitable perforator; whereas the decision to use a free as against a pedicled flap was made based on the location of the defect. In all the flaps harvested the fascia was included over the entire length of the skin flap and no thinning was done [Figure 4b].
Figure 4b.
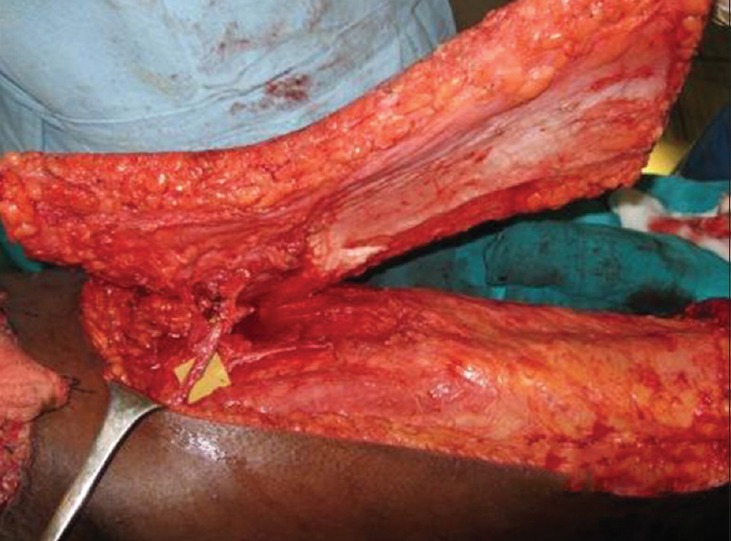
After complete islanding the flap on anterolateral thigh pedicle
In all the 3 free flap reconstructions the contralateral DIEP pedicle was used as the recipient vessels. Anastomosis was done under the operating microscope and in each case 1 artery and 2 veins using 9 '0' monofilament nylon interrupted sutures. The flap fascial edges were sutured to the fascial edges of the original defects carefully avoiding any obstruction or kinking of the flap vessels in the vicinity of the pedicle. One of the patients with the largest musculofascial defect had simultaneous polypropylene mesh reinforcement by suturing to the edges of the musculofascial defect using 2 '0' interrupted polypropylene sutures. Drains were placed subcutaneously and the subcutaneous tissues and skin were closed with interrupted suture of 3-0 polyglycaprone and skin staples.
All the patients needed split skin grafting at the donor site. All flaps were monitored postoperatively for vascular problems; intravenous fluids were continued till the return of bowel sounds usually on the 3rd day and Ryle's tube feeding was continued for 5 days thereafter. From the 7th day oral feeds were allowed and the diet was transitioned to a normal diet by the 10th day. Ambulation was started on the 5th post operative day and the patients were discharged between the 11th and 15th postoperative day with an advice to use an abdominal binder whilst ambulating for 6 weeks. All wounds (donor and recipient area) had healed by the end of 3 weeks. The patients were instructed not to lift heavy objects and climb stairs for 3 months.
All patients were followed up and clinically evaluated beyond 6 months. This was done to assess whether the patient had herniation on “straight leg raise test” and “eliciting a cough” in addition the patients were asked for relief, if any, from symptoms of pain or discomfort during exertional activities.
RESULTS
There were no re-explorations and all the flaps survived in entirety; skin graft at the donor site was required in each case. There were no infections but one patient had a graft loss at the donor area needing secondary skin grafting.
On follow-up evaluation with a “straight leg raise test” four of five patients had no evidence of herniation or bulge [Figures 2c and 3c]; the one patient with evidence of a bulge 7 months after follow-up was the one with the debulked desmoid tumour [Figure 11]. The other patient following reconstruction in the setting of colonic cancer was disease free at a follow-up of 18 months.
Figure 2c.
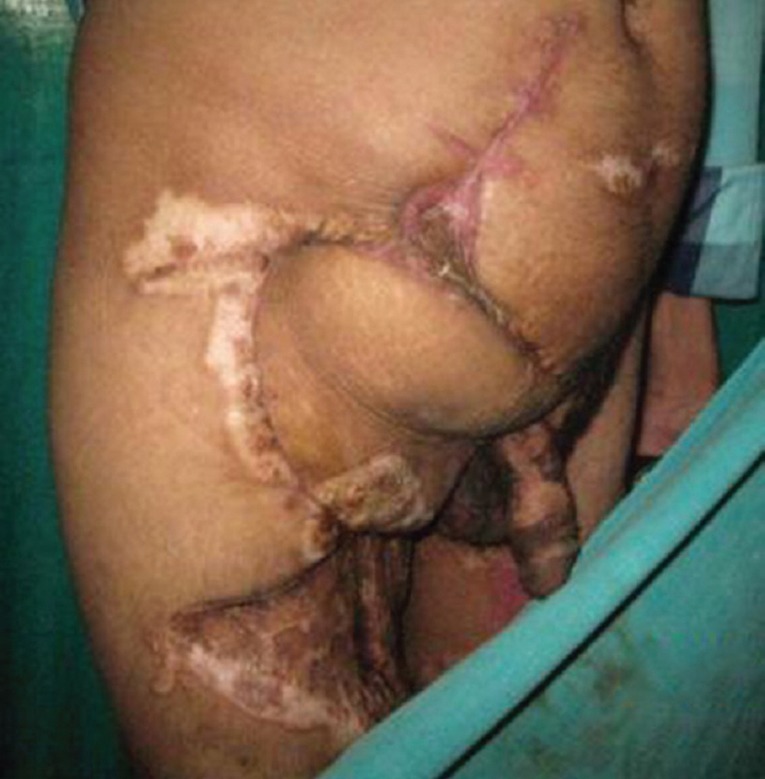
At 1-year post-operative follow-up
Figure 3c.
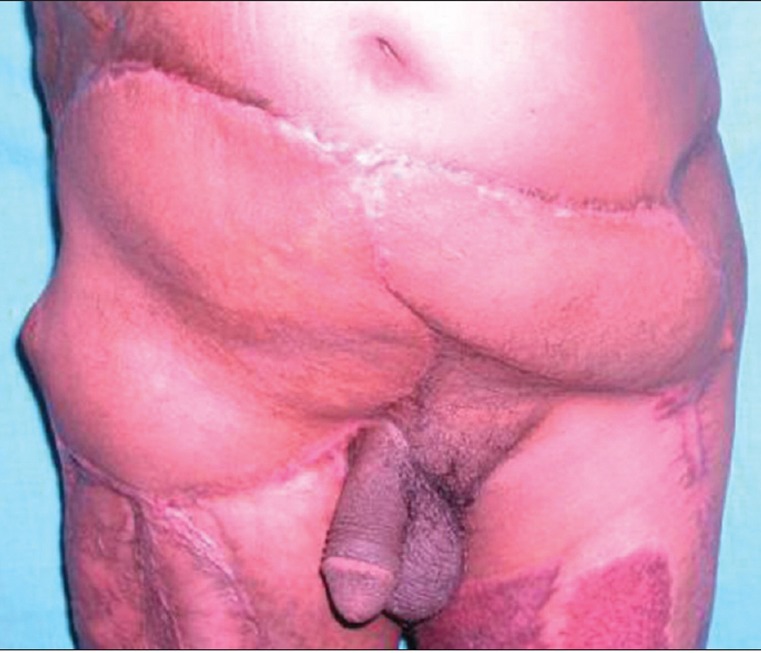
At 2-year post-operative follow-up
All three patients in whom reconstruction was done electively for ventral hernia had relief from symptoms of pain or discomfort during exertional activities.
DISCUSSION
The ideal reconstruction restores both the functional and aesthetic integrity of the abdominal wall.[32] The use of autologous tissue for definitive reconstruction of large abdominal wall defects using the skin and fascial component minimises the risk of hernia and avoids problems associated with synthetic materials. This series has demonstrated that the transfer of either pedicled or free lateral thigh flaps were reliable procedures for the reconstruction of complex defects of the abdominal wall. In this series 2 of 5 defects were in the upper abdomen and hence the free ALT flap was chosen; 3 of 5 defects were in the lower abdomen the largest of these needed a bilateral pedicled ALT flap; in one case a pedicled TFL flap was done and in the third a free TFL flap was done on account of a scarred ipsilateral thigh and groin (Table 2). Choosing between an ALT flap and TFL flap is based on finding a suitable perforator; when an ALT perforator was absent or situated at a site so as to prevent proper mobilisation of the flap to the defect a TLF musculocutaneous flap was chosen. Kayano et al. on a study of 20 patients had 8 free and 12 pedicled ALT flaps; although the defect size; when the free flap was used was larger than when a pedicled flap was used there were no other differences in outcome.[33] For the pedicled ALT flaps the skin of the flap was designed as an island distal to the vascular pedicle, allowing the flap to be tunnelled under intact groin skin to the abdominal defect.[32] If further mobilisation of the flap or lengthening of the pedicle was required, the primary muscle branch to the rectus femoris from the descending branch of the lateral circumflex femoral artery was divided and the flap was completely islanded to allow rotation of 180o.[31] Since all the other pedicles of the rectus femoris were retained vascularity to the muscle was confirmed before donor closure.
Table 2.
Choice of flap and transfer method
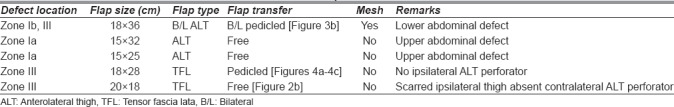
Amongst the four ALT flaps, there were two septocutaneous and two musculocutaneus perforators.
One patient with a defect in the lower abdomen needed a free TFL flap on account of severe scarring in the ipsilateral thigh; here a TFL instead of an ALT flap was chosen to take advantage of the location of the pedicle of the TFL flap in relation to the recipient vessels.
Only one patient with the largest defect 18 cm × 36 cm going across the midline needed the use of a polypropylene mesh to increase the safety factor in preventing recurrence.
CONCLUSION
Lateral thigh flaps are ideal choice for the reconstruction of large abdominal defects (size >40 cm2) as they can provide large surface area of skin with minimal impact on abdominal wall mechanics. Incorporating the vascularised deep fascia of the thigh flaps (ALT, TFL) into the abdominal musculofascial layers can restore the integrity of the abdominal wall and may make the use of synthetic meshes redundant. Lower abdominal defects are reconstructed using pedicled ALT or TFL myocutaneous flaps and upper abdominal defects with free ALT or TFL myocutaneous flaps. The decision on whether to use pedicled ALT or TFL depends on the availability and location of the ALT perforator.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgement
The authors would like to thank Dr. R. Srikanth, Professor and Head of the Department of Plastic Surgery, Nizam's Institute of Medical Sciences, Hyderabad, Telangana, India, for his invaluable input towards final draft preparation.
REFERENCES
- 1.Read RR. Ventral, epigastric, umbilical, spigelian and incisional hernias. In: Cameron JL, editor. Current Surgical Therapy. 5th ed. Philadelphia: Mosby; 1995. pp. 491–6. [Google Scholar]
- 2.Larson GM, Vandertoll DJ. Approaches to repair of ventral hernia and full-thickness losses of the abdominal wall. Surg Clin North Am. 1984;64:335–49. doi: 10.1016/s0039-6109(16)43289-5. [DOI] [PubMed] [Google Scholar]
- 3.Hurwotz DJ, Hollins RR. Reconstruction of the abdominal wall and groin. In: Cohen M, editor. Mastery of Plastic and Reconstructive Surgery. Boston: Little, Brown; 1994. pp. 1349–59. [Google Scholar]
- 4.Mathes SJ, Steinwald PM, Foster RD, Hoffman WY, Anthony JP. Complex abdominal wall reconstruction: A comparison of flap and mesh closure. Ann Surg. 2000;232:586–96. doi: 10.1097/00000658-200010000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tukiainen E, Leppäniemi A. Reconstruction of extensive abdominal wall defects with microvascular tensor fasciae latae flap. Br J Surg. 2011;98:880–4. doi: 10.1002/bjs.7489. [DOI] [PubMed] [Google Scholar]
- 6.Sharma RK, Verma GR, Biswas G. Reconstruction of a major abdominal and chest wall defect using latissimus dorsi and extended deep inferior epigastric artery flap. Ann Plast Surg. 1992;28:366–9. doi: 10.1097/00000637-199204000-00012. [DOI] [PubMed] [Google Scholar]
- 7.Bauer JJ, Harris MT, Gorfine SR, Kreel I. Rives-stoppa procedure for repair of large incisional hernias: Experience with 57 patients. Hernia. 2002;6:120–3. doi: 10.1007/s10029-002-0071-3. [DOI] [PubMed] [Google Scholar]
- 8.Nettelblad H, Tarpila E. Abdominal wall reconstruction with vascularised autologous tissue. Scand J Surg. 2003;92:297–300. doi: 10.1177/145749690309200410. [DOI] [PubMed] [Google Scholar]
- 9.Rifaat MA, Abdel Gawad WS. The use of tensor fascia lata pedicled flap in reconstructing full thickness abdominal wall defects and groin defects following tumor ablation. J Egypt Natl Canc Inst. 2005;17:139–48. [PubMed] [Google Scholar]
- 10.Hurwitz DJ, Hollins RR. Reconstruction of the abdominal wall and groin. In: Cohen M, editor. Mastery of Plastic and Reconstructive Surgery. 1st ed. Vol 1. Boston: Little, Brown; 1994. [Google Scholar]
- 11.Mathes SJ, Nahai F. Clinical Application of Muscle and Musculocutaneous Flaps. St. Louis: Mosby; 1982. pp. 364–85. [Google Scholar]
- 12.Mathes SJ, Nahai F. Reconstructive Surgery Principles Anatomy and Technique. New York: Churchill Livingstone; 1997. [Google Scholar]
- 13.Ohtsuka H, Ochi K, Seike H. Reconstruction of a large lateral abdominal wall defect with an ilio-lumbar bi-pedicled flap. Br J Plast Surg. 1984;37:327–9. doi: 10.1016/0007-1226(84)90075-4. [DOI] [PubMed] [Google Scholar]
- 14.Terashi H, Hashimoto H, Shibuya H, Ishii Y, Sato H, Takayasu S. Use of groin flap and anterolateral thigh adipofascial flap of tensor fascia lata for reconstruction of a wide lower abdominal wall defect. Ann Plast Surg. 1995;35:320–1. doi: 10.1097/00000637-199509000-00018. [DOI] [PubMed] [Google Scholar]
- 15.Koshima I, Nahai F. The versatile superficial inferior epigastric artery free flap. Br J Plast. 1992;45:270. doi: 10.1016/0007-1226(92)90050-8. [DOI] [PubMed] [Google Scholar]
- 16.Classen D. The extended deep inferior epigastruic artery skin flaps without rectus abdominis muscle. Br J Plast Surg. 1989;42:645. doi: 10.1016/0007-1226(89)90075-1. [DOI] [PubMed] [Google Scholar]
- 17.Gottlieb ME, Chandrasekhar B, Terz JJ, Sherman R. Clinical applications of the extended deep inferior epigastric flap. Plast Reconstr Surg. 1986;78:782–92. doi: 10.1097/00006534-198678060-00012. [DOI] [PubMed] [Google Scholar]
- 18.Mathes SJ, Bostwick J., 3rd A rectus abdominis myocutaneous flap to reconstruct abdominal wall defects. Br J Plast Surg. 1977;30:282–3. doi: 10.1016/0007-1226(77)90118-7. [DOI] [PubMed] [Google Scholar]
- 19.Parkash S, Palepu J. Rectus abdominis myocutaneous flap – Clinical experience with ipsilateral and contralateral flaps. Br J Surg. 1983;70:68–70. doi: 10.1002/bjs.1800700204. [DOI] [PubMed] [Google Scholar]
- 20.DeFranzo AJ, Kingman GJ, Sterchi JM, Marks MW, Thorne MT. Rectus turnover flaps for the reconstruction of large midline abdominal wall defects. Ann Plast Surg. 1996;37:18–23. doi: 10.1097/00000637-199607000-00003. [DOI] [PubMed] [Google Scholar]
- 21.Spear SL, Walker RK. The external oblique flap for reconstruction of the rectus sheath. Plast Reconstr Surg. 1992;90:608–13. doi: 10.1097/00006534-199210000-00009. [DOI] [PubMed] [Google Scholar]
- 22.Ramasastry SS, Futrell JW, Williams SL, Hurwitz DJ. Internal oblique muscle pedicle flap for coverage of a major soft tissue defect of the groin. Ann Plast Surg. 1985;15:57–60. doi: 10.1097/00000637-198507000-00007. [DOI] [PubMed] [Google Scholar]
- 23.Williams JK, Carlson GW, deChalain T, Howell R, Coleman JJ. Role of tensor fasciae latae in abdominal wall reconstruction. Plast Reconstr Surg. 1998;101:713–8. doi: 10.1097/00006534-199803000-00020. [DOI] [PubMed] [Google Scholar]
- 24.Caffee HH. Reconstruction of the abdominal wall by variations of the tensor fasciae latae flap. Plast Reconstr Surg. 1983;71:348–53. doi: 10.1097/00006534-198303000-00010. [DOI] [PubMed] [Google Scholar]
- 25.Hill HL, Nahai F, Vasconez LO. The tensor fascia lata myocutaneous free flap. Plast Reconstr Surg. 1978;61:517–22. doi: 10.1097/00006534-197804000-00004. [DOI] [PubMed] [Google Scholar]
- 26.Ninkovic M, Kronberger P, Harpf C, Rumer A, Anderl H. Free innervated latissimus dorsi muscle flap for reconstruction of full-thickness abdominal wall defects. Plast Reconstr Surg. 1998;101:971–8. doi: 10.1097/00006534-199804040-00013. [DOI] [PubMed] [Google Scholar]
- 27.Kuo YR, An PC, Kuo MH, Kueh NS, Yao SF, Jeng SF. Reconstruction of knee joint soft tissue and patellar tendon defects using a composite anterolateral thigh flap with vascularized fascia lata. J Plast Reconstr Aesthet Surg. 2008;61:195–9. doi: 10.1016/j.bjps.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 28.Houtmeyers P, Opsomer D, Van Landuyt K, Monstrey S. Reconstruction of the Achilles tendon and overlying soft tissue by free composite anterolateral thigh flap with vascularized fascia lata. J Reconstr Microsurg. 2012;28:205–9. doi: 10.1055/s-0032-1306367. [DOI] [PubMed] [Google Scholar]
- 29.Kuo YR, Kuo MH, Chou WC, Liu YT, Lutz BS, Jeng SF. One-stage reconstruction of soft tissue and Achilles tendon defects using a composite free anterolateral thigh flap with vascularized fascia lata: Clinical experience and functional assessment. Ann Plast Surg. 2003;50:149–55. doi: 10.1097/01.SAP.0000037270.95257.B9. [DOI] [PubMed] [Google Scholar]
- 30.Kuo YR, Seng-Feng J, Kuo FM, Liu YT, Lai PW. Versatility of the free anterolateral thigh flap for reconstruction of soft-tissue defects: Review of 140 cases. Ann Plast Surg. 2002;48:161–6. doi: 10.1097/00000637-200202000-00008. [DOI] [PubMed] [Google Scholar]
- 31.Kuo YR, Jeng SF, Kuo MH, Huang MN, Liu YT, Chiang YC, et al. Free anterolateral thigh flap for extremity reconstruction: Clinical experience and functional assessment of donor site. Plast Reconstr Surg. 2001;107:1766–71. doi: 10.1097/00006534-200106000-00019. [DOI] [PubMed] [Google Scholar]
- 32.Kuo YR, Kuo MH, Lutz BS, Huang YC, Liu YT, Wu SC, et al. One-stage reconstruction of large midline abdominal wall defects using a composite free anterolateral thigh flap with vascularized fascia lata. Ann Surg. 2004;239:352–8. doi: 10.1097/01.sla.0000114229.89940.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kayano S, Sakuraba M, Miyamoto S, Nagamatsu S, Taji M, Umezawa H, et al. Comparison of pedicled and free anterolateral thigh flaps for reconstruction of complex defects of the abdominal wall: Review of 20 consecutive cases. J Plast Reconstr Aesthet Surg. 2012;65:1525–9. doi: 10.1016/j.bjps.2012.05.003. [DOI] [PubMed] [Google Scholar]


