Abstract
In this article, we report data on vessel wall thickness parameters derived from different arterial segments of the circle of Willis and its primary branches in patients with and without cerebrovascular disease. Also data on inter-rater reliability and agreement of the derived vessel wall parameters are reported. For further interpretation and discussion please refer to the research article “ex vivo vessel wall thickness measurements of the human circle of Willis using 7T MRI” (Harteveld et al., in press) [1].
Specifications Table
| Subject area | Medicine |
| More specific subject area | Radiology, pathology |
| Type of data | Tables, figures |
| How data was acquired | MRI data acquisition: 7T whole-body system (Philips Healthcare, the Netherlands); MRI data analysis: CAAS MRA software program (Pie Medical Imaging, the Netherlands); Histological data acquisition: slide scanner with DotSlide software (Olympus, Japan); Histological data analysis: MeVisLab software program (MeVis Medical Solutions, Germany). |
| Data format | Analyzed |
| Experimental factors | Raw MRI data was processed offline using dedicated vascular analysis software. After MR imaging, samples were taken from different arterial segments and histologically processed to enable assessment of histological sections. |
| Experimental features | MRI measurements of vessel wall thickness parameters for different intracranial arteries; Inter-rater reliability and agreement of derived vessel wall parameters from MRI and histological data. |
| Data source location | Utrecht, Netherlands |
| Data accessibility | Data is within this article |
Value of the data
-
•
This data provides ex vivo vessel wall thickness measurement values of the main arteries of the circle of Willis and its primary branches of patients with and without cerebrovascular disease, using 7T MRI.
-
•
Inter-rater reliability and agreement of vessel wall thickness measurements using MRI compared to histology are provided.
-
•
The data presented would be useful as MRI-based reference values for vessel wall thickness parameters of intracranial arteries, and may stimulate future research investigating intracranial vessel wall thickness in a larger patient population with different risk factors and cerebrovascular disease burden.
1. Data
Segment averages of thickness measurements from all analyzed circle of Willis specimens are presented in Table 1. Mean vessel wall thickness MRI measurements over entire vessel segments are shown for each individual patient in Fig. 1. Inter-rater reliability and agreement of parameters derived from delineated vessel wall boundaries on MR images and histological sections are provided in Table 2.
Table 1.
MR imaging measurements of vessel wall thickness parameters for different arterial segments of the CoW in n = 10 patients with and without cerebrovascular disease.
| Segment | Lumen area (mm2) | Total vessel area (mm2) | Wall area (mm2) | Vessel wall thickness (mm) |
Normalized wall index | |||
|---|---|---|---|---|---|---|---|---|
| Mean | Minimum | Maximum | ||||||
| Anterior cerebral artery | A1 | 0.84 (0.47) | 2.92 (0.97) | 2.07 (0.56) | 0.45 (0.06) | 0.31 (0.04) | 0.52 (0.09) | 0.73 (0.09) |
| A2 | 0.75 (0.43) | 2.95 (0.88) | 2.20 (0.56) | 0.49 (0.08) | 0.32 (0.05) | 0.54 (0.13) | 0.75 (0.07) | |
| Internal carotid artery | 3.44 (1.86) | 8.90 (2.93) | 5.46 (1.46) | 0.66 (0.12) | 0.42 (0.09) | 0.86 (0.21) | 0.64 (0.12) | |
| Middle cerebral artery | 1.88 (1.01) | 5.62 (1.63) | 3.74 (0.89) | 0.58 (0.11) | 0.35 (0.07) | 0.78 (0.22) | 0.69 (0.12) | |
| Posterior cerebral artery | 1.07 (0.47) | 3.70 (1.05) | 2.63 (0.70) | 0.51 (0.08) | 0.34 (0.06) | 0.61 (0.12) | 0.72 (0.07) | |
| Basilar artery | proximal | 2.41 (1.01) | 6.79 (1.38) | 4.38 (1.12) | 0.61 (0.16) | 0.38 (0.11) | 0.73 (0.26) | 0.65 (0.11) |
| distal | 1.94 (1.00) | 5.37 (1.90) | 3.43 (1.14) | 0.53 (0.12) | 0.35 (0.08) | 0.61 (0.20) | 0.65 (0.10) | |
| Vertebral artery | 1.54 (0.97) | 4.74 (1.93) | 3.20 (1.15) | 0.54 (0.11) | 0.36 (0.07) | 0.59 (0.17) | 0.69 (0.09) | |
Values are given as mean (standard deviation).
For each vessel segment the measurements were averaged over the fixed length, and left and right were taken together (if applicable).
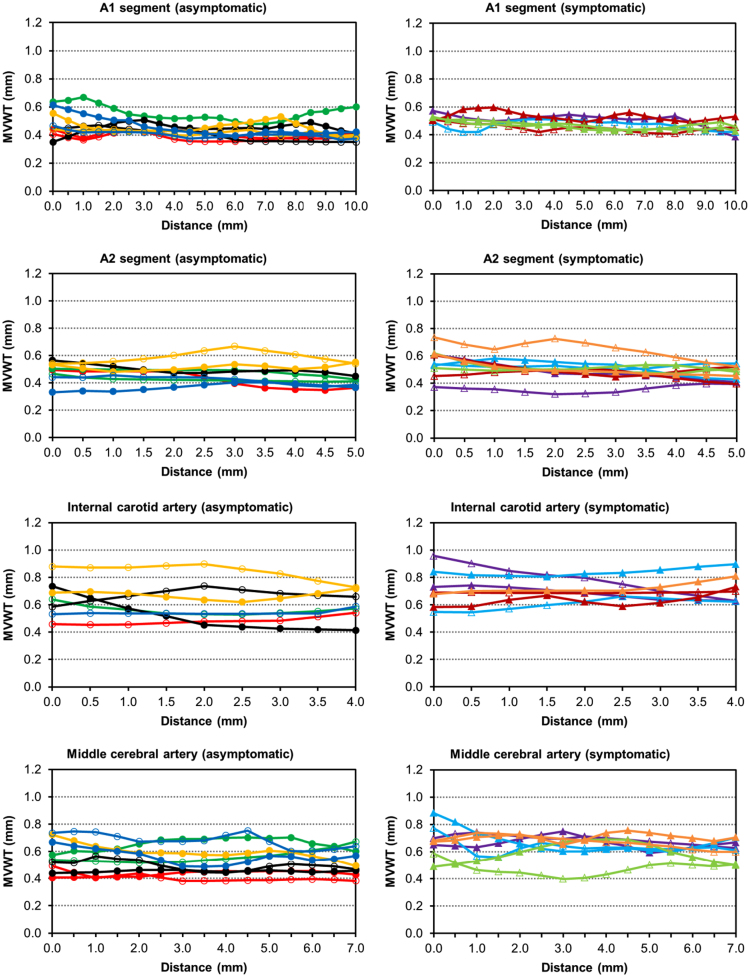
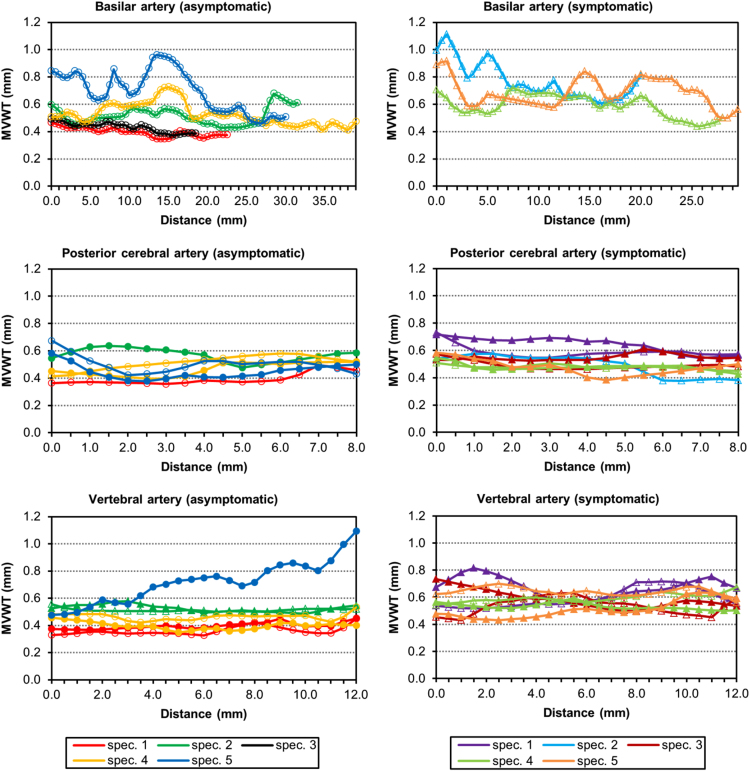
Fig. 1.
Mean vessel wall thickness (MVWT) MRI measurements over the fixed length of each analyzed vessel segment from the asymptomatic (graphs on the left) and symptomatic (graphs on the right) patients. For the basilar artery, the entire segment is shown. Each line color represents a different CoW specimen, where the markers indicate whether the vessel segment is located on the right (not filled) or left (filled). The distance represents the locations along the arterial segments from proximal to distal.
Table 2.
Inter-rater reliability and agreement of the derived vessel wall parameters.
| ICCa (95% CI) | Mean difference (95% LoA) | |||
|---|---|---|---|---|
| Histology | Total vessel area (mm2) | 1.000 (1.000–1.000) | 0.02 (− 0.01 to 0.06) | |
| Lumen area (mm2) | 1.000 (0.999–1.000) | 0.01 (− 0.09 to 0.11) | ||
| Wall area (mm2) | 1.000 (0.999–1.000) | 0.06 (− 0.06 to 0.17) | ||
| Vessel wall thickness (mm) | ||||
| mean | 0.999 (0.996–1.000) | 0.01 (− 0.01 to 0.04) | ||
| minimum | 0.996 (0.984–0.999) | 0.01 (− 0.01 to 0.03) | ||
| maximum | 0.996 (0.982–0.999) | 0.06 (− 0.06 to 0.18) | ||
| Normalized wall index | 1.000 (0.999–1.000) | 0.01 (− 0.01 to 0.02) | ||
| MRI | Total vessel area (mm2) | 0.993 (0.861–0.999) | − 0.46 (− 1.17 to 0.24) | |
| Lumen area (mm2) | 0.999 (0.992–1.000) | − 0.10 (− 0.32 to 0.12) | ||
| Wall area (mm2) | 0.985 (0.814–0.997) | − 0.36 (− 1.01 to 0.29) | ||
| Vessel wall thickness (mm) | ||||
| mean | 0.989 (0.929–0.998) | − 0.03 (− 0.11 to 0.04) | ||
| minimum | 0.849 (0.434–0.962) | − 0.02 (− 0.09 to 0.06) | ||
| maximum | 0.992 (0.970–0.998) | − 0.04 (− 0.20 to 0.13) | ||
| Normalized wall index | 0.995 (0.964–0.999) | − 0.02 (− 0.05 to 0.02) | ||
CI: confidence interval; ICC: intraclass correlation coefficient; LoA: limits of agreement.
Two-way mixed model with absolute agreement.
2. Experimental design, materials and methods
2.1. Specimens
In total 15 human circle of Willis specimens were used for data acquisition. Five specimens (from 5 patients) were used for evaluation of inter-rater reliability and agreement (3 male; mean age 66.2 years; range 42–92 years). The other 10 specimens from patients with (n = 5) and without (n = 5) cerebrovascular disease were used for vessel wall thickness measurements of the major arterial segments of the circle of Willis using MRI. Demographic and clinical data of these specimens are summarized in Table 3.
Table 3.
Baseline characteristics of donor patients used for vessel wall thickness measurements over the entire length of the major arterial segments of the CoW.
| Subject | Sex (m/f) | Age (years) | Clinical history cerebrovascular disease | Cause of death | Location cerebral ischemia |
|---|---|---|---|---|---|
| 1 | m | 77 | Ischemic stroke | Ischemic stroke | Left medial, right parietal cortex |
| 2 | f | 75 | Ischemic stroke | Intraparenchymal hemorrhage | Left middle cerebral artery territory |
| 3 | f | 75 | Ischemic stroke | Blood loss from hemorrhagic gastritis combined with heart failure | Right middle cerebral artery territory |
| 4 | m | 80 | TIA | Bilateral pneumonia | Left hemisphere |
| 5 | f | 80 | Ischemic stroke + TIAs | Intraparenchymal hemorrhage | Unknown |
| 6 | m | 84 | – | Subdural & intraparenchymal hemorrhage after trauma | N/A |
| 7 | f | 81 | – | Hypovolemic shock caused by intra-abdominal and retroperitoneal bleeding | N/A |
| 8 | f | 71 | – | Advanced metastatic thyroid cancer | N/A |
| 9 | f | 70 | – | Respiratory failure after (possible) postoperative (ileus) aspiration | N/A |
| 10 | m | 66 | – | Brain tumor (glioblastoma multiforme) | N/A |
2.2. MR imaging
Imaging was performed at room temperature on a 7T whole body system (Philips Healthcare, Best, the Netherlands), with an ultra-high resolution 3D T1-weighted sequence. Details regarding the applied scan parameters are described in Ref. [1].
2.3. Histological processing
Samples taken from different marked locations of each circle of Willis specimen that were used for evaluation of inter-rater reliability and agreement were histologically processed using an in-house developed protocol [2].
2.4. Vessel wall thickness analysis
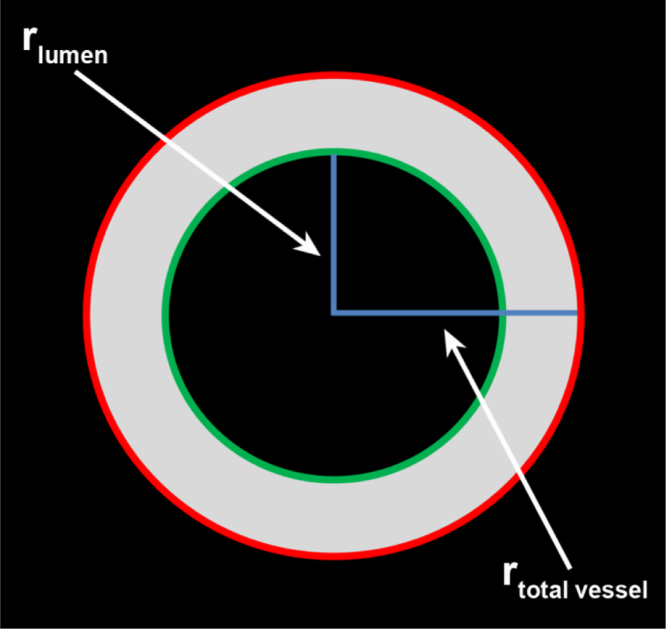
Vessel wall thickness parameters included: lumen area (LA), total vessel area (TVA), wall area (WA; TVA-LA), wall thickness (mean, minimum, and maximum), and normalized wall index (WA/TVA). Mean vessel wall thickness was calculated based on the vessel areas [3] (Fig. 2). Vessel wall thickness parameters were calculated from manually traced vessel boundary contours by dedicated software programs (MRI data: CAAS MRA, Pie Medical Imaging, the Netherlands; Histological data: MeVisLab, MeVis Medical Solutions, Germany).
Fig. 2.
Schematic clarification of the method used for calculation of mean vessel wall thickness based on the vessel areas [3]. The mean vessel wall thickness is calculated by taking the difference between the radius of a circle with area equal to that enclosed by the outer wall boundary (rtotal vessel) and the radius of a circle with area equal to that enclosed by the luminal boundary (rlumen). Outer wall and luminal boundary are indicated by the red and green line, respectively.
Inter-rater reliability and agreement of calculated vessel wall parameters were assessed using vessel wall boundaries drawn by two raters in the same set of histological sections and their matching MRI slices (n = 10 samples; randomly selected from all samples obtained from 5 specimens).
Footnotes
Transparency data associated with this article can be found in the online version at https://doi.org/10.1016/j.dib.2018.04.116.
Transparency document. Supplementary material
Supplementary material
References
- 1.A.A. Harteveld, N.P. Denswil, W. Van Hecke, et al., Ex vivo vessel wall thickness measurements of the human circle of Willis using 7T MRI, Atherosclerosis 273, 2018, 106-114. 10.1016/j.atherosclerosis.2018.04.023. [DOI] [PubMed]
- 2.van der Kolk A.G., Zwanenburg J.J., Denswil N.P. Imaging the intracranial atherosclerotic vessel wall using 7T MRI: initial comparison with histopathology. AJNR Am. J. Neuroradiol. 2015;36:694–701. doi: 10.3174/ajnr.A4178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rosero E.B., Peshock R.M., Khera A. Agreement between methods of measurement of mean aortic wall thickness by MRI. J. Magn. Reson. Imaging. 2009;29:576–582. doi: 10.1002/jmri.21697. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material