Abstract
Background
The aim of this study was to assess the effects of interleukin-1 (IL-1) receptor associated kinase (IRAK) inhibitors on intestinal injury induced by necrotizing enterocolitis (NEC) in neonatal rats and its regulation on the intestinal Toll-like receptor (TLR) inflammatory signaling pathway.
Material/Methods
The neonatal rat models of NEC were established though hypoxia-cold stimulation. All rats were divided into 3 groups: an NEC model group (NEC group), an IRAK inhibitor group (IRAKI group), and a normal control group (NC group). At 72 h after the models were established, intestinal tissues were collected for histopathological examination, enzyme-linked immunosorbent assay (ELISA), Western blotting, and immunohistochemistry.
Results
After IRAK inhibitor intervention, the symptoms of NEC in neonatal rats were alleviated, and the degree of weight loss was reduced. In the IRAK group, the intestinal pathology of neonatal rats was improved, pathological score was decreased, and the incidence rate of NEC was significantly reduced. The levels of tumor necrosis factor-alpha (TNF-α), IL-1β, and IL-6 in the IRAK group were significantly decreased compared with those in the NEC group. There were no significant differences in IRAK1 and IRAK4 protein expression levels between the IRAK group and the NEC group. The phosphorylated IRAK1 and IRAK4 in the IRAK group were significantly decreased. Nuclear factor-kappa B (NF-κB) level of intestinal tissues in the IRAK group was reduced compared with that in the NEC group.
Conclusions
IRAK inhibitors can inhibit the inflammatory response of the NEC model, reduce the release of pro-inflammatory cytokines, and alleviate the damage to intestinal tissues by inhibiting conduction of the TLR signaling pathway.
MeSH Keywords: Enterocolitis, Necrotizing; Interleukin-1 Receptor-Associated Kinases; Toll-Like Receptors
Background
Neonatal necrotizing enterocolitis (NEC) is a common and serious intestinal disease in the neonatal period, especially in premature infants [1]. The disease is more commonly seen in premature infants, especially those with extremely low or ultra-low birth weight. Since NEC has high morbidity and mortality rates, it is still an important cause of death in the intensive care unit for neonates. Although the cause and pathogenesis of NEC are not yet clear, it has been generally agreed in recent studies that the incidence of NEC is correlated with premature delivery, improper feeding, immature intestinal immune regulation, intestinal ischemia and hypoxia, abnormal bacterial colonization, and genetic factors [2]. The inflammatory cascade is caused by a variety of pathogenic factors important factor in the pathogenesis of NEC, and the final common pathway is the damage to intestinal tissues resulting from inflammatory mediators [3,4].
The intestinal mucosal innate immune signaling pathway plays a vital role in maintaining intestinal homeostasis and it can promote host anti-microbial defense and maintain mucosal balance [5]. Studies [6–8] have shown that the Toll-like receptor (TLR) signaling pathway plays important roles in maintaining the integrity of mucosal barriers and promoting host anti-microbial defense. TLR-mediated innate immunity greatly affects the pathogenesis of NEC. When the intestinal mucosa is stimulated by microbial ligands, the TLR inflammatory signaling pathway is activated, thus initiating the inflammatory cascade and inducing the inflammatory cascade effect. This process is closely related to the pathogenesis of NEC.
Studies [9,10] have shown that interleukin-1 (IL-1) receptor-associated kinase (IRAK) mainly mediates the conduction of the TLRs signaling pathway. IRAK1 and IRAK4, 2 important kinases in the TLR signaling pathway, exert positive regulatory effects on the signaling pathway and are believed to be key regulators in the processes of activating the TLR signaling pathway and causing inflammatory responses. When the body is stimulated by pathogenic microbial ligands, TLRs activate IRAK and further activate the downstream inflammatory pathways, resulting in the activation of transcription factors such as nuclear factor-kappa B(NF-κB), which causes a series of immune and inflammatory responses. Studies [11,12] on mice with IRAK gene deficiency or knockout have revealed that the secretions of inflammatory cytokines and chemokines mediated and produced by TLRs are impaired.
IRAKs play a pivotal role in the TLR signaling pathway. The TLR signaling pathway plays an important role in the pathogenesis of NEC. However, research on the relationship between IRAK and NEC is still limited. The effectiveness of IRAK inhibitors has been demonstrated in studies on systemic lupus erythematosus, chronic obstructive pulmonary disease, and hypoxic-ischemic brain damage to neurons [13–15]. In the present study, a neonatal rat model of NEC was established by artificial feeding and hypoxia-cold stimulation. IRAK inhibitors were used for intervention to study their effects on the intestinal injury of neonatal rats and the regulation of the intestinal TLR inflammatory signaling pathway.
Material and Methods
Experimental animals and model establishment
Neonatal rats within 2 h of birth and weighing 5–9 g were provided by the Tianjin Fourth Central Hospital Animal Center. The model of NEC was established by artificial feeding and hypoxia-cold stimulation: The formula substitute of rat milk [16] (4.60 g Liduojing stage I milk powder, 8.68 g albumen powder, 49.2 mL lipid emulsion (C14–24), addition of sterile double-distilled water to 100 mL, and total calorie: 581KJ) was injected into the neonatal rats by gavage. Rats were fed with the formula substitute of rat breast milk once every 4 h (q4h) at 0.2 mL/time within 24 h, at 0.3 mL/time within 24–48 h, and at 0.4 mL/time within 48–72 h. Rats were placed in a vacuum box, which was connected to nitrogen, and the flow rate was adjusted to 25 L/min. Count was started when the value on the oxygen meter was zero, the nitrogen was disconnected 90 s later, and the rats were quickly removed. Then, these rats were placed in a refrigerator chamber at the calibrated temperature of 4°C for 10 min of cold stimulation. Subsequently, the suckling rats were removed and returned to the incubator for rewarming and artificial feeding was continued. Hypoxia-cold stimulation was conducted once q12h for 3 consecutive days. This study was approved by the Animal Ethics Committee of Tianjin Fourth Central Hospital Animal Center.
Experimental grouping and processing methods
Rats in the experiment were divided into an NEC model group (NEC group), an IRAK inhibitor group (IRAKI group) in which IRAK inhibitors were intraperitoneally injected into neonatal rats after the first cold stimulation once every 24 h (q24h) for 3 consecutive times, and a normal control group (NC group) in which neonatal rats were in the same cage with mothers since birth and fed with rat breast milk.
Hematoxylin and eosin (H&E) staining and pathological scoring
At 72 h after the model was successfully established, neonatal rats were fasted for 12 h and then sacrificed to take intestinal tissues. The tissues were fixed with 10% neutral buffered formalin for 24 h, routinely embedded, and cut into 4-μm-thick paraffin sections. After these tissues were dewaxed using two-cylinder xylene, ethanol gradient dehydration was conducted, followed by H&E staining. The results were observed under a neutral gum capsule microscope. According to the pathological scoring standard (Table 1) proposed by Nadler et al. [17], the sections were scored by 2 double-blinded pathologists. The highest score given by the 2 pathologists was taken as the final result, and when the histological score was ≥2 points, NEC was confirmed.
Table 1.
The pathological scoring standard.
| Score | Pathological changes |
|---|---|
| 0 | The intestinal mucosa and villi were complete; the structure was completely normal |
| 1 | Slight submucosal and/or lamina propria separation |
| 2 | Moderate submucosal and/or lamina propria separation; submucosal and/or muscular layer edema |
| 3 | Severe submucosal and/or lamina propria separation; submucosal and/or muscular layer edema; local intestinal villi shedding |
| 4 | Intestinal villus disappearance with intestinal necrosis |
Enzyme-linked immunosorbent assay (ELISA)
Intestinal tissue specimens were weighed, cut into pieces, and added with 1 μL protease inhibitor and pre-cooled phosphate-buffered saline (PBS) in a certain proportion, followed by homogenization. These specimens were centrifuged at 5000×g for 5~10 min at 4°C using a refrigerated centrifuge, and the supernatant was removed for ELISA. The contents of tumor necrosis factor-alpha (TNF-α), IL-1β, and IL-6 were detected according to the instructions of the ELISA kit.
Western blotting
A total of 100 mg intestinal tissues was taken and placed in a culture dish, cut, and then homogenized on an ice bench. Tissues were placed in a centrifuge at the pre-cooled temperature of 4°C and centrifuged at 10 000 rpm for 5 min. The supernatant obtained from the centrifugation was the total protein extract, and the protein concentration was determined by bicinchoninic acid assay (BCA). An appropriate amount of proteins was taken for electrophoresis, and after membranes were transferred using polyvinylidene fluoride (PVDF), primary antibodies of the target protein were incubated. Electrochemiluminescence (ECL) was prepared and placed on a chemiluminescence apparatus for exposure after reaction.
Immunohistochemistry
After antigen retrieval of intestinal tissue sections, 3% hydrogen peroxide solution was added dropwise to block the endogenous peroxidase’s activity and an appropriate amount of serum was added dropwise for sealing. Primary antibodies of the target protein were added dropwise into the sections and placed in a humid chamber overnight at 4°C. After an appropriate amount of secondary antibodies was added dropwise, 3,3′-diaminobenzidine (DAB) development working solution was added dropwise for color development. Neutral resin glues were used for mounting, and observation was conducted under an optical microscope.
Statistical analysis
SPSS 19.0 software (Armonk, NY, USA) was used to process the experimental data. The obtained experimental results are expressed as mean ± standard deviation. The Kruskal-Wallis H test was used to analyze the pathological score of intestinal tissues. Analysis of variance was used for comparisons of intergroup differences. The Student-Newman-Keuls (SNK)-q test was used for multiple comparisons among samples. P<0.05 was defined as statistical significance.
Results
General state of neonatal rats
After modeling, abdominal distension and diarrhea gradually occurred in neonatal rats in the NEC group, and they excreted yellow-green mucous stool. In addition, their activities were reduced and response was poor, and they suffered from drowsiness. Group reaction was diminished or even disappeared, the disease degree became higher, and the subcutaneous fat gradually became thinner. In the IRAKI group, abdominal distension and diarrhea also gradually occurred in neonatal rats, and they excreted yellow-green mucous stool. However, the appearance time in the IRAKI group was later than that in the NEC group, and the degree in the former was lower than in the latter. In the NC group, neonatal rats had normal vitality with good response and no abdominal distension or diarrhea.
Body weight changes
The growth and development of neonates in the NC group were stable, the weight gain was obvious, the subcutaneous fat was full, the body weight increased gradually with age, and the growth trend was obvious. Neonatal rats in the NEC group and IRAKI group all had different degrees of growth and development retardation, gradually manifested as no weight gain and weight loss. Rats with weight loss in the IRAKI group were significantly decreased compared with those in the NEC group (Figure 1).
Figure 1.
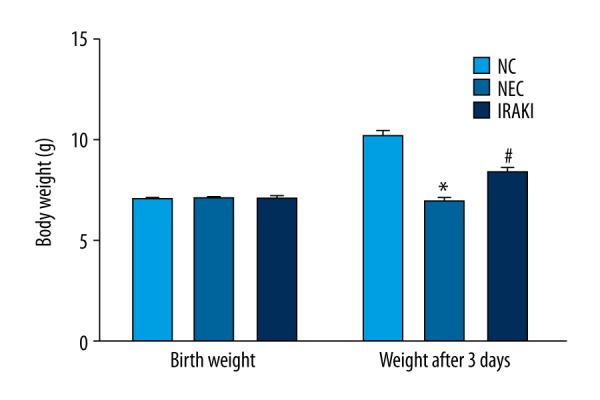
The effect of IRAK inhibitor on body weight in a neonatal rat model of necrotizing enterocolitis. * P<0.05 vs. NC group, # P<0.05 vs. NEC group.
Intestinal histopathological changes
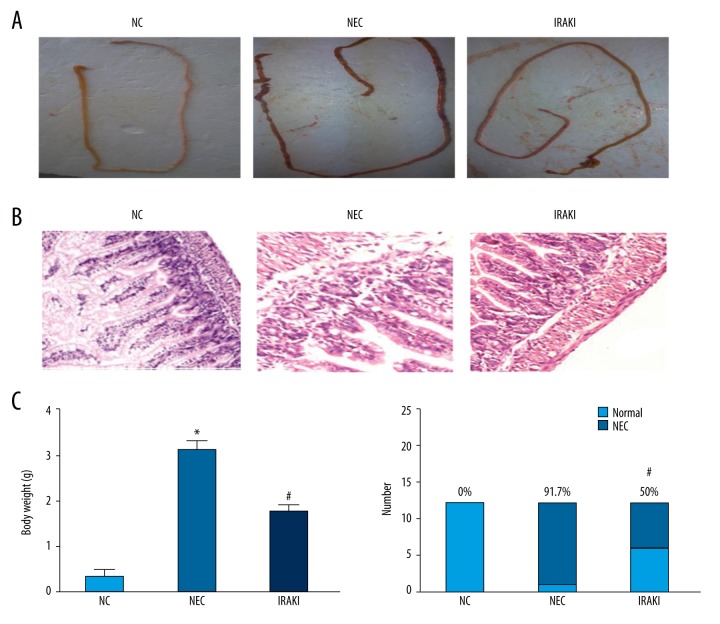
There were general changes in intestinal tissues. In the NC group, intestinal color was natural, manifested as pale yellow with no congestion, the elasticity of intestinal tissues was good, and no pneumatosis intestinalis or beaded changes were found. In the NEC group, intestinal congestion was obvious, intestinal color was dim, intestinal dilatation could be seen, and the elasticity of intestinal tissues was poor so that they were easily broken, pneumatosis intestinalis occurred, and severe cases were accompanied with beaded changes. In the IRAKI group, mild congestion and dilatation were also found in intestinal tissue appearance, the elasticity of intestinal tissues was not bad, and there was no significant pneumatosis intestinalis (Figure 2A).
Figure 2.
The effect of IRAK inhibitor on intestinal histopathology in a neonatal rat model of necrotizing enterocolitis. (A) The changes of gross morphology of intestinal tissues. (B) The pathologic changes of intestinal tissues under the microscope. (C) The analysis of pathological scores in different groups. (D) The analysis of necrotizing enterocolitis incidence in different groups. * P<0.05 vs. NC group, # P<0.05 vs. NEC group.
Observations were made using an optical microscope. In the NC group, the intestinal villus structure in sections appeared intact under the microscope, intestinal glands were arranged neatly, and there was no edema, swelling. or separation in the mucous layer, submucosa, or lamina propria. In the NEC group, intestinal villi were messy, part of the villi were shed or even missing, obvious edema appeared in the submucosa and muscular layer, and swelling and separation occurred in the lamina propria and muscular layer. In the IRAKI group, edema was found in epithelial cells of intestinal villi and the epithelium on the top of some villus tissues fell off (Figure 2B). The pathological score was 0.33±0.49 in the NC group, 3.08±0.99 in the NEC group, and 1.75±0.96 in the IRAKI group (Figure 2C). The pathological score in the IRAKI group was significantly decreased compared with that in the NEC group. The incidence rate of NEC was 0% (0/12) in the NC group, 91.7% (11/12) in the NEC group, and 50% (6/12) in the IRAKI group. The incidence rate of NEC in the IRAKI group was obviously lower compared with that in the NEC group (Figure 2D).
Levels of TNF-α, IL-1β, and IL-6 in intestinal tissues of neonatal rats
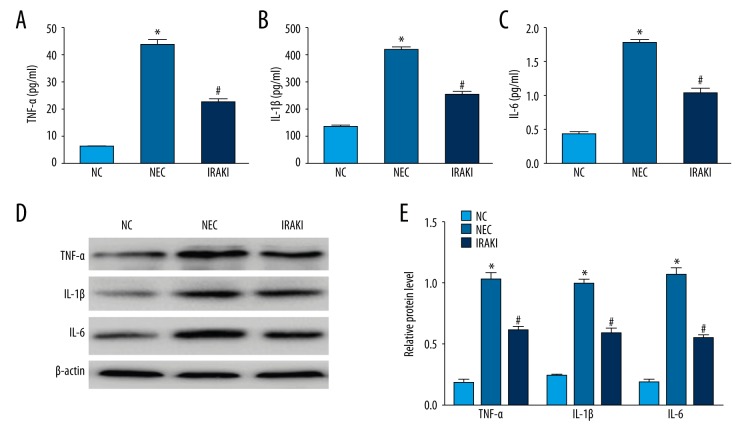
The inflammatory cascade is multifactorial and is important in the pathogenesis of NEC. ELISA results showed that the levels of IL-1β, IL-6, and TNF-α in intestinal tissue specimens in the NEC group were significantly elevated compared with those in the NC group, and the differences were statistically significant. The levels ofIL-1β, IL-6, and TNF-α in intestinal tissue specimens in the IRAKI group were significantly decreased compared with those in the NEC group, and the differences were statistically significant (Figure 3A–3C). Western blotting results were similar to ELISA results (Figure 3D, 3E).
Figure 3.
The effect of IRAK inhibitor on TNF-α, IL-1β, and IL-6 of intestinal tissues in a neonatal rat model of necrotizing enterocolitis. (A) The analysis of TNF-α by ELISA. (B) The analysis of IL-1β by ELISA. (C) The analysis of IL-6 by ELISA. (D) Western blotting showed the protein level of TNF-α, IL-1β, and IL-6. (E) Semi-quantitative analysis of the protein level of TNF-α, IL-1β, and IL-6. * P<0.05 vs. NC group, # P<0.05 vs. NEC group.
Protein level of IRAK1, IRAK4, and NF-κB in intestinal tissues of neonatal rats
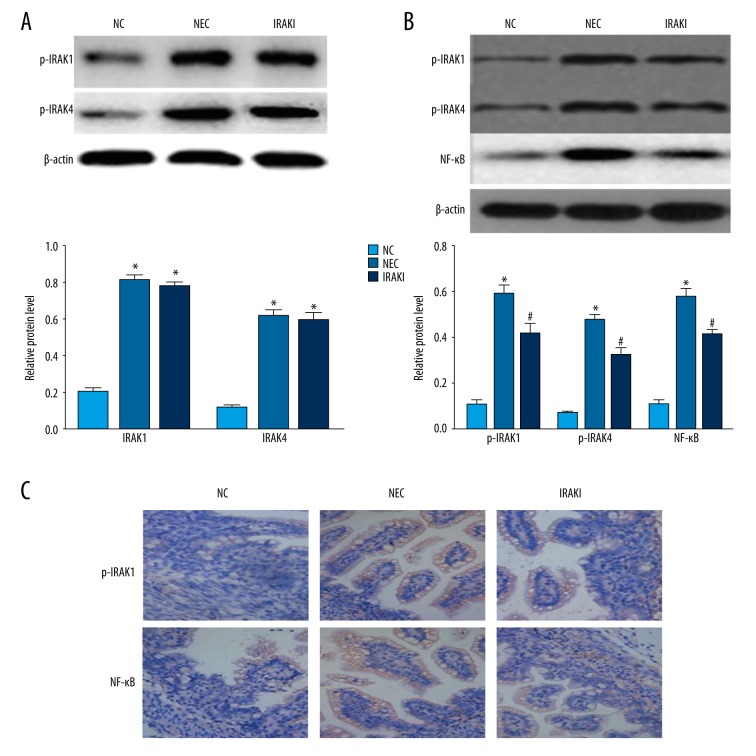
Western blotting results revealed that the total protein levels of IRAK1 and IRAK4 in the NEC group and IRAKI group were higher than those in the NC group. There were no significant differences in the total protein levels of IRAK1 and IRAK4 between the IRAKI group and NEC group (Figure 4A). The protein levels of phosphorylated IRAK1 and IRAK4 in the NEC group and IRAKI group were higher than those in the NC group. Compared with those in NEC group, the protein levels of phosphorylated IRAK1 and IRAK4 in the IRAKI group were significantly decreased. The levels of NF-κB in intestinal tissues in the NEC group and IRAKI group were higher than that in the normal control group, and levels in the IRAKI group were significantly lower than in the NEC group (Figure 4B). Immunohistochemistry results were similar to Western blotting results (Figure 4C).
Figure 4.
The effect of IRAK inhibitor on IRAK1, IRAK4, and NF-κB of intestinal tissues in a neonatal rat model of necrotizing enterocolitis. (A) The analysis of IRAK1 and IRAK4 by Western blotting. (B) The analysis of p-IRAK1, p-IRAK4, and NF-κB by Western blotting. (C) Representative images of immunohistochemistry of p-IRAK1 and NF-κB. * P<0.05 vs. NC group, # P<0.05 vs. NEC group.
Discussion
NEC is a common intestinal disease in the neonatal period, with intestinal inflammatory and necrotic changes as the main manifestations. It is characterized by high morbidity and mortality rates as well as difficulty in the early diagnosis, and it is currently a common critical illness in the intensive care unit for neonates [1]. NEC is a common disease that threatens the life and quality of life of neonates, and its specific pathogenesis and methods to effectively prevent and treat it are still not clear [18,19]. In this experiment, neonatal rats were used for modeling, and formula milk feeding and hypoxia-cold stimulation methods were used to simulate the various factors in the pathogenesis of NEC, better reflecting the pathogenetic process of NEC. In the process of modeling, neonatal rats manifested abdominal distension, diarrhea, poor response, and decreased activity. After the model was established, manifestations such as intestinal congestion, dilatation, poor elasticity of intestinal tissues so that they were easily broken, and pneumatosis intestinalis (even with accompanying beaded changes), and typical NEC-like changes also appeared in pathological sections. The method combined a variety of factors that might be pathogenic during the pathogenesis of NEC and reproduced the typical pathophysiological changes of NEC, making it an ideal NEC modeling method.
The TLR signaling pathway is an important part of the innate immune response, and the innate immune response involved in it is the most important basis for NEC. Studies have shown that IRAK1 and IRAK4 are very important kinases in the TLR signaling pathway and exert positive regulatory effects on the signaling pathway. They are considered as key regulatory factors in the processes of activating the TLR signaling pathway and causing the inflammatory response [20,21]. Therefore, IRAK was taken as a target in this experiment and its activity was inhibited using inhibitors, thus blocking the TLR signaling pathway so as to prevent the immune cascade reaction and reduce the inflammatory response. In this experiment, neonatal rats began to suffer from abdominal distension and diarrhea at about 24 h after modeling in the NEC group, and their activities were gradually decreased, responses were worsened, and sounds were weak. The above symptoms appeared in the neonatal rats in the IRAKI group at about 40 h after modeling, and the symptoms were milder than those in the NEC group. The intestinal pathological analysis after modeling revealed that the incidence rate of NEC in the IRAKI group was significantly lower than that in the NEC group. In addition, the average pathological score in the IRAKI group was obviously reduced compared with that in the NEC group. The above results indicate that IRAK inhibitor can reduce the systemic symptoms of NEC neonatal rats, alleviate damage to the intestinal tract, and reduce the incidence rate of NEC in the model.
Recent studies [22,23] have shown that the inflammatory cascade caused by a variety of pathogenic factors is important in the pathogenesis of NEC, and the final common pathway is the damage of resulting inflammatory mediators to intestinal tissues. In various NEC models, compared with that in the normal control group, the level of TNF-α in the NEC model group was significantly increased. TNF-α is also involved in the pathophysiological process of NEC, and further induces the apoptosis of intestinal epithelial cells [24]. A study used TNF-α specific antibody to intervene in the rat NEC model, and the results confirm that TNF-α-specific antibody protects the intestinal tract of NEC rats and also verify the role of TNF-α in the pathogenesis of NEC from the side. IL-1β and IL-6, secreted by mononuclear macrophages, are also the most common inflammatory mediators of several cell types, which can attract neutrophils to enter the diseased sites, thus causing tissue damage [25,26]. The contents of IL-1β in intestinal tissues and serum of NEC patients were significantly increased. Some scholars found that serum IL-6 level can be used as a marker of activation of the inflammatory cascade and can reflect the relationship between the inflammation response level and the severity of the disease [27]. Therefore, the levels of IL-1β, IL-6, and TNF-α were taken as indicators for evaluating the inflammatory response degree in animal models in the present study, revealing that the levels of IL-1β, IL-6, and TNF-α in intestinal specimens in the NEC group were significantly increased compared with those in the NC group, which is consistent with the above findings. The levels of IL-1β, IL-6, and TNF-α in intestinal tissues in the IRAKI group were significantly reduced compared with those in the NEC group, suggesting that IRAK inhibitor can to a certain extent reduce the inflammatory cascade of NEC.
IRAK inhibitors were used to intervene in neonatal rats during NEC modeling in this study. Intestinal tissue specimens were analyzed after modeling, which revealed that IRAK level in intestinal specimens in the IRAKI group were markedly increased compared with that in the normal control group, and there was no statistically significant difference in IRAK level between the IRAKI group and NEC group. Some scholars pointed out that IRAK inhibitors inhibit the activity of kinases and phosphorylation level of IRAK, but do not directly reduce its protein level [28]. However, the total IRAK level in tissue specimens includes phosphorylated and non-phosphorylated IRAKs. Therefore, in this experiment, the levels of IRAK1 and IRAK4 phosphorylated proteins were further examined, and the results revealed that IRAK inhibitors reduced the levels of IRAK1 and IRAK4 phosphorylated proteins in NEC neonatal rats, which is consistent with the results of previous studies. NF-κB is an important factor in the TLR signaling pathway and a key protein in the downstream of IRAK that leads to the inflammatory cascade. NF-κB is closely related to immune and inflammatory responses and is involved in the regulation of pro-inflammatory cytokine gene expression through the TLR signaling pathway [29]. The detection of NF-κB level showed that NF-κB level in the NEC group was significantly increased compared with that in the NC group, while that in the IRAKI group was significantly reduced compared with that in the NEC group. Experimental results, combined with the expression level of NF-κB, further confirmed that IRAK inhibitors can inhibit the conduction of the TLR signaling pathway, thus reducing the downstream inflammatory cascade.
Conclusions
In summary, we found that IRAK inhibitors inhibit the inflammatory response in the NEC model, reduce the release of pro-inflammatory cytokines, and alleviate the damage to intestinal tissues by inhibiting the conduction of the TLR signaling pathway, suggesting that use of IRAK inhibitors may be a potential method for the treatment or prevention of NEC.
Footnotes
Conflict of interest
None.
Source of support: Departmnetal sources
References
- 1.Eaton S, Rees CM, Hall NJ. Current research on the epidemiology, pathogenesis, and management of necrotizing enterocolitis. Neonatology. 2017;111:423–30. doi: 10.1159/000458462. [DOI] [PubMed] [Google Scholar]
- 2.Samuels N, van de Graaf RA, de Jonge R, et al. Risk factors for necrotizing enterocolitis in neonates: A systematic review of prognostic studies. BMC Pediatr. 2017;17:105. doi: 10.1186/s12887-017-0847-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tian F, Liu GR, Li N, Yuan G. Insulin-like growth factor I reduces the occurrence of necrotizing enterocolitis by reducing inflammatory response and protecting intestinal mucosal barrier in neonatal rats model. Eur Rev Med Pharmacol Sci. 2017;21:4711–19. [PubMed] [Google Scholar]
- 4.Shi Y, Liu T, Zhao X, et al. Vitamin D ameliorates neonatal necrotizing enterocolitis via suppressing TLR4 in a murine model. Pediatr Res. 2018 doi: 10.1038/pr.2017.329. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 5.Jenke AC, Zilbauer M, Postberg J, Wirth S. Human beta-defensin 2 expression in ELBW infants with severe necrotizing enterocolitis. Pediatr Res. 2012;72:513–20. doi: 10.1038/pr.2012.110. [DOI] [PubMed] [Google Scholar]
- 6.Meng D, Zhu W, Ganguli K, et al. Anti-inflammatory effects of Bifidobacterium longum subsp infantis secretions on fetal human enterocytes are mediated by TLR-4 receptors. Am J Physiol Gastrointest Liver Physiol. 2016;311:G744–53. doi: 10.1152/ajpgi.00090.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hui L, Dai Y, Guo Z, et al. Immunoregulation effects of different gamma delta T cells and toll-like receptor signaling pathways in neonatal necrotizing enterocolitis. Medicine (Baltimore) 2017;96:e6077. doi: 10.1097/MD.0000000000006077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meng D, Zhu W, Shi HN, et al. Toll-like receptor-4 in human and mouse colonic epithelium is developmentally regulated: A possible role in necrotizing enterocolitis. Pediatr Res. 2015;77:416–24. doi: 10.1038/pr.2014.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deguine J, Barton GM. MyD88: A central player in innate immune signaling. F1000Prime Rep. 2014;6:97. doi: 10.12703/P6-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ntoufa S, Vilia MG, Stamatopoulos K, et al. Toll-like receptors signaling: A complex network for NF-kappaB activation in B-cell lymphoid malignancies. Semin Cancer Biol. 2016;39:15–25. doi: 10.1016/j.semcancer.2016.07.001. [DOI] [PubMed] [Google Scholar]
- 11.Picard C, Puel A, Bonnet M, et al. Pyogenic bacterial infections in humans with IRAK-4 deficiency. Science. 2003;299:2076–79. doi: 10.1126/science.1081902. [DOI] [PubMed] [Google Scholar]
- 12.Kanakaraj P, Schafer PH, Cavender DE, et al. Interleukin (IL)-1 receptor-associated kinase (IRAK) requirement for optimal induction of multiple IL-1 signaling pathways and IL-6 production. J Exp Med. 1998;187:2073–79. doi: 10.1084/jem.187.12.2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li M, Yu D, Ni B, Hao F. Interleukin-1 receptor associated kinase 1 is a potential therapeutic target of anti-inflammatory therapy for systemic lupus erythematosus. Mol Immunol. 2017;87:94–101. doi: 10.1016/j.molimm.2017.03.018. [DOI] [PubMed] [Google Scholar]
- 14.Knobloch J, Chikosi SJ, Yanik S, et al. A systemic defect in Toll-like receptor 4 signaling increases lipopolysaccharide-induced suppression of IL-2-dependent T-cell proliferation in COPD. Am J Physiol Lung Cell Mol Physiol. 2016;310:L24–39. doi: 10.1152/ajplung.00367.2014. [DOI] [PubMed] [Google Scholar]
- 15.Yang YF, Chen Z, Hu SL, et al. Interleukin-1 receptor associated kinases-1/4 inhibition protects against acute hypoxia/ischemia-induced neuronal injury in vivo and in vitro. Neuroscience. 2011;196:25–34. doi: 10.1016/j.neuroscience.2011.08.059. [DOI] [PubMed] [Google Scholar]
- 16.Auestad N, Korsak RA, Bergstrom JD, Edmond J. Milk-substitutes comparable to rat’s milk; Their preparation, composition and impact on development and metabolism in the artificially reared rat. Br J Nutr. 1989;61:495–518. doi: 10.1079/bjn19890139. [DOI] [PubMed] [Google Scholar]
- 17.Nadler EP, Dickinson E, Knisely A, et al. Expression of inducible nitric oxide synthase and interleukin-12 in experimental necrotizing enterocolitis. J Surg Res. 2000;92:71–77. doi: 10.1006/jsre.2000.5877. [DOI] [PubMed] [Google Scholar]
- 18.Akotia DH, Durham JT, Arnell KM, et al. Relationship between near-infrared spectroscopy and transabdominal ultrasonography: Noninvasive monitoring of intestinal function in neonates. Med Sci Monit. 2016;22:61–68. doi: 10.12659/MSM.895730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barczuk-Falecka M, Bombinski P, Majkowska Z, et al. Hepatic portal venous gas in children younger than 2 years old – radiological and clinical characteristics in diseases other than necrotizing enterocolitis. Pol J Radiol. 2017;82:275–78. doi: 10.12659/PJR.899995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Finney CA, Lu Z, Hawkes M, et al. Divergent roles of IRAK4-mediated innate immune responses in two experimental models of severe malaria. Am J Trop Med Hyg. 2010;83:69–74. doi: 10.4269/ajtmh.2010.09-0753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fernandes-Alnemri T, Kang S, Anderson C, et al. Cutting edge: TLR signaling licenses IRAK1 for rapid activation of the NLRP3 inflammasome. J Immunol. 2013;191:3995–99. doi: 10.4049/jimmunol.1301681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xing T, Camacho SR, Chen YH. Animal models for studying epithelial barriers in neonatal necrotizing enterocolitis, inflammatory bowel disease and colorectal cancer. Tissue Barriers. 2017;5:e1356901. doi: 10.1080/21688370.2017.1356901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hodzic Z, Bolock AM, Good M. The role of mucosal immunity in the pathogenesis of necrotizing enterocolitis. Front Pediatr. 2017;5:40. doi: 10.3389/fped.2017.00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tayman C, Aydemir S, Yakut I, et al. TNF-alpha blockade efficiently reduced severe intestinal damage in necrotizing enterocolitis. J Invest Surg. 2016;29:209–17. doi: 10.3109/08941939.2015.1127449. [DOI] [PubMed] [Google Scholar]
- 25.Jiang F, Meng D, Weng M, et al. The symbiotic bacterial surface factor polysaccharide a on Bacteroides fragilis inhibits IL-1beta-induced inflammation in human fetal enterocytes via toll receptors 2 and 4. PLoS One. 2017;12:e172738. doi: 10.1371/journal.pone.0172738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meng D, Zhu W, Ganguli K, et al. Anti-inflammatory effects of Bifidobacterium longum subsp infantis secretions on fetal human enterocytes are mediated by TLR-4 receptors. Am J Physiol Gastrointest Liver Physiol. 2016;311:G744–53. doi: 10.1152/ajpgi.00090.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gregoric P, Sijacki A, Stankovic S, et al. SIRS score on admission and initial concentration of IL-6 as severe acute pancreatitis outcome predictors. Hepatogastroenterology. 2010;57:349–53. [PubMed] [Google Scholar]
- 28.Kim SJ, Cha JY, Kang HS, et al. Corosolic acid ameliorates acute inflammation through inhibition of IRAK-1 phosphorylation in macrophages. BMB Rep. 2016;49:276–81. doi: 10.5483/BMBRep.2016.49.5.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang P, Han X, Mo B, et al. LPS enhances TLR4 expression and IFNgamma production via the TLR4/IRAK/NFkappaB signaling pathway in rat pulmonary arterial smooth muscle cells. Mol Med Rep. 2017;16:3111–16. doi: 10.3892/mmr.2017.6983. [DOI] [PMC free article] [PubMed] [Google Scholar]