Abstract
Advances in understanding the mechanisms of tumour-induced immunosuppression have led to the development of immune-checkpoint inhibitors in cancer patients, including those with renal cell carcinoma (RCC). The optimal combination between immunotherapy and targeted agents (as well as the possible favourable sequential therapy of these two classes of drugs) remains an open question at this moment. Several trials are currently underway to assess the combination of anti-programmed-death 1 (PD-1) or anti-PD-ligand(L)1 agents with other immunotherapies or with anti-vascular endothelial growth factor receptor (VEGFR) tyrosine kinase inhibitors (TKIs). In this editorial, we described the results of the most recent clinical trials on the use of immunotherapies in RCC and the emerging data on the research for reliable biomarkers of tumour response in this setting. In addition, we have focused on the role of the gut microbiome and tumour microenvironment in the development of future therapeutic strategies for RCC patients.
Keywords: immunocheckpoint inhibitors, immunotherapy, PD-1, renal cell carcinoma, tyrosine kinase inhibitors
Introduction
Less than ten years ago, there were only a few drugs with demonstrated clinical efficacy in the management of metastatic renal cell carcinoma (mRCC) but without significant impact on clinical outcomes. However, in recent years, the approval of several agents has revolutionized treatment. Indeed, every new drug approved has provided a further step in the improvement of patient survival. Consequently, it is important to have a better understanding of tumour biological features as well as genetic assessment. Thus, we have discovered that renal cell carcinoma (RCC) involves a very large spectrum of tumours. Each of them is composed of a different spectrum of mutations and with different clinical behaviour. Clear cell RCC (ccRCC) accounts for approximately 75% of kidney cancer. In contrast, the other 25% of kidney cancer is classified as nonclear cell RCC (nccRCC). Of this last subgroup of tumours, the World Health Organization recognized a broad spectrum of over a dozen histopathological entities in 2016. Papillary renal cell carcinoma (pRCC) and chromophobe RCC (chRCC) are the most frequent subtypes of nccRCC; whereas, medullary, translocation and collecting duct RCC represent an infrequent diagnosis [1]. Each tumour subtype presents a specific and complex spectrum of gene and molecularly altered pathways resulting in a heterogeneous mixture of malignancies associated with different morphology, immunohistochemical features, clinical behaviour and prognosis.
Our growing understanding of the molecularly altered pathways related to cancer has led to the development of new classes of drugs that have rapidly replaced the first immunotherapies such as interleukin (IL)-2 and interferon-alfa (IFN-alfa) as the standard of care for RCC patients. Angiogenesis, the hallmark of RCC, is the first final target of several tyrosine kinase inhibitors (TKIs) (sunitinib, axitinib, sorafenib and pazopanib). After angiogenesis, the finding that deregulation of the PI3K–Akt–mTOR pathway, activated at different levels of the signalling cascade, drives RCC progression has led to the development of two mTOR inhibitors: everolimus and temsirolimus. Recently, the mesenchymal-epithelial transition (MET) and multityrosine kinases inhibitor cabozantinib has been included in clinical practice.
These drugs have led to an improvement in overall survival (OS) (sunitinib, pazopanib and cabozantinib) and in progression-free survival (PFS) (sunitinib, axitinib, cabozantinib, sorafenib, pazopanib, everolimus and temsirolimus), which was the endpoint of interest for FDA approval, until recently. All of these drugs showed a good safety profile combined with remarkable clinical activity in a disease that has always been difficult to treat [2–10].
Immune-checkpoint inhibitors
Although these drugs have significantly changed the course of this disease, the latest class of agents, the immune-checkpoint inhibitors, are predicted to provide further benefit. Programmed death receptor 1/programmed death receptor ligand 1 (PD-1/PD-L1) and cytotoxic T lymphocytes antigen 4 (CTLA-4) inhibitors target specific pathways related to the immune response, which are often hyperactivated by tumour cell interaction. By inhibiting these pathways, immune-checkpoint inhibitors could reactivate a specific immune response against tumour cells. The observation that RCC is related to a high mutation load and so maybe to high antigen expression has led to these drugs being tested at different stages of the disease. CheckMate 025 was the first large phase III clinical trial comparing the PD-1 inhibitor, nivolumab, to everolimus in patients with locally advanced or metastatic RCC who had progressed to at least one VEGF/VEGFR inhibitor. This study met its primary endpoint showing an OS benefit in patients receiving nivolumab. Furthermore, patients treated with immunotherapy showed a higher overall response rate (ORR) compared to everolimus with a considerable percentage of these achieving long-lasting response [11]. It is not surprising that the important results achieved in this trial led researchers to explore immunotherapy in other settings such as adjuvant/neoadjuvant stage and as first-line therapy [12–14]. Of note, Motzer and colleagues recently reported results of CheckMate 214, a phase III trial that tested the combination of ipilimumab and nivolumab over sunitinib in previously untreated patients with intermediate/poor risk (according to IMDC) metastatic or locally advanced ccRCC. The combination resulted in a significant OS benefit (median overall survival not reached versus 26.0 months with sunitinib, hazard ratio [HR] 0.63) in this population of patients. In addition, ORR was significantly better in the combination arm (42% with 9% of CR) compared to sunitinib (27% with 1% of CR).
Regarding this last point, two different strategies have been adopted.
Combination of an immune-checkpoint inhibitor and a VEGF inhibitor
The combination of an immune-checkpoint inhibitor and a VEGF inhibitor has been evaluated in phase II trials. In the IMmotion150 study, 305 patients with locally advanced/mRCC and untreated RCC were randomized to receive atezolizumab (an anti-PD-L1 inhibitor) plus bevacizumab, atezolizumab alone or sunitinib. The combination arm resulted in a longer PFS compared to atezolizumab (6.1 months) and sunitinib arms with a higher percentage ORR in the combination arm. Of note, patients with PD-L1 positive expression (≥1%) showed a longer PFS (14.7 months) and higher ORR (46%) in the atezolizumab monotherapy arm [15].
The combination of two immune-checkpoint inhibitors has been recently tested in a large phase III trial: CheckMate 214. In this study, patients were randomized to receive nivolumab (anti-PD-1) plus ipilimumab (anti-CTLA-4) or sunitinib as first-line therapy. At the 2017 ESMO Conference, Escudier presented primary results after 17.5 months of follow-up showing that the ipilimumab plus nivolumab combination resulted in higher ORR and CR in intermediate/poor-risk patients. Of note, patients with intermediate/poor-risk disease and PD-L1 expression ≥1% showed higher ORR and PFS compared to sunitinib; whereas, patients with favourable category of risk (which showed lower PD-L1 expression) showed a longer PFS and a higher ORR with sunitinib [16].
These encouraging results may only be the ‘tip of the iceberg’ and could suggest that we are entering a new era for the management of mRCC. Nonetheless, even if immunotherapy represents a new hope for patients with mRCC, the relatively older targeted therapy is far from being abandoned. In other words, although CheckMate 025 showed that nivolumab is better than everolimus, there are other agents that are extremely effective after VEGF/VEGFR inhibitors progression; thus, the decision for second-line treatment should be weighed on the basis of the clinical outcome pursued as well as patient preference and drug toxicity profile. Immunotherapy has shown very interesting result in first-line therapy. Looking to CheckMate 214 results, it is probable that this positive effect could be restricted in patients with specific clinical features such as intermediate/poor-risk disease; whereas, patients with a favourable profile could benefit more from a standard treatment [14]. This would suggest that the worst clinical profile of the disease could be related to a high mutation load of tumour cells resulting in a higher antigen expression. In addition, preliminary data seem to indicate that these patients present a higher percentage of tumours with positive PD-L1 expression. However, to date, no mature data about the role of immunotherapy in favourable risk patients have been released. Furthermore, in an exploratory analysis of CheckMate 214, immunotherapy also showed a not-to-be-ignored clinical activity in patients with favourable risk (CR 11 versus 6%) even if the same combination was associated with a worse ORR (29 versus 52%) compared to sunitinib. Future studies will help us to better understand the role of PD-L1 as a prognostic and predictive response factor because, to date, we have strongly diverging information. Indeed, a meta-analysis of six published studies revealed that a higher level of PD-L1 expression increased the risk of death by representing, therefore, a negative prognostic factor [17]. Different to what was expected, the improved OS with nivolumab was not correlated with PD-L1 expression in CheckMate 025; whereas, patients with positive PD-L1 expression seemed to show more clinical benefit from immune-checkpoint inhibitors in IMmotion150 and CheckMate 214 (Table 1).
Table 1.
Results obtained in trials exploring immune-checkpoint inhibitors in metastatic/locally advanced RCC.
| Study name Experimental arm Comparator arm |
Setting | N ITT |
N PD-L1+ |
OS ITT |
HR | OS PD-L1+ |
PFS ITT |
HR | PFS PD-L1+ |
HR | ORR ITT |
ORR PD-L1+ |
CR |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CHECKMATE 025 | |||||||||||||
| Nivolumab | Previously treated patients with locally advanced or mRCC | 410 | 94 | 25.0 | 0.73 | 21.8 | 4.6 | 0.88 | NR | NR | 25% | NR | 1% |
| Everolimus | 411 | 87 | 19.6 | 18.8 | 4.4 | NR | 5% | NR | <1% | ||||
| IMMOTION150 | |||||||||||||
| Atezolizumab + bevacizumab | Untreated patients with locally advanced or mRCC | 101 | 164 | NR | NR | NR | NR | NR | NR | NR | 32% | 46% | NR |
| Atezolizumab | 103 | NR | NR | NR | NR | 25% | 28% | NR | |||||
| Sunitinib | 101 | NR | NR | NR | NR | 29% | 27% | NR | |||||
| CHECKMATE 214 | |||||||||||||
| Ipilimumab + nivolumab | Untreated patients with locally advanced or mRCC | 550 | 204 | NR | NR | NR | 11.6 | 0.82 | 22.8* | 0.48 | NR | 58%* | 9.4%* |
| Sunitinib | 546 | 224 | NR | NR | NR | 8.4 | 5.9* | NR | 25%* | 1.2%* | |||
CR, complete response; NR, not reported; ORR, overall response rate; OS, overall survival; PFS, progression-free survival.
Intermediate/poor-risk patients with PD-L1 expression ≥1%.
Future challenges
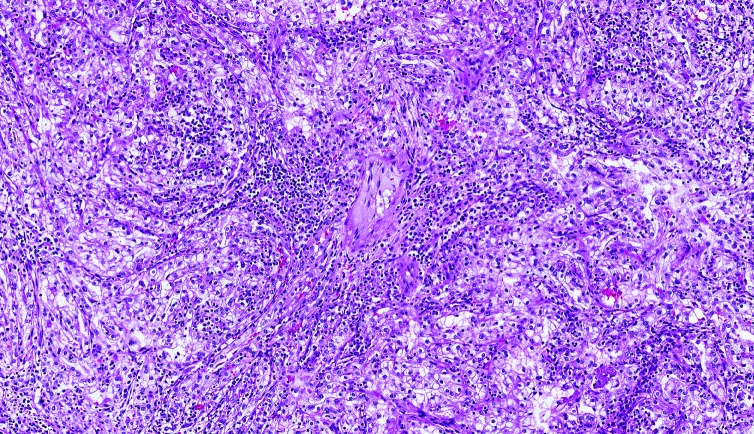
The complex interplay of inflammatory mediators and signalling pathways is absolutely crucial for RCC development and response to therapy [18–22] (Figure 1). Neutrophils, lymphocytes and macrophages have been implicated in promoting tumour angiogenesis and metastatic spread, as well as in the formation of pre-metastatic niches and in primary and acquired drug resistance [18–22]. In this scenario, the checkpoint molecules have gained wide interest since the introduction of anti-CTLA-4 and anti-PD-1/PD-L1 agents into daily clinical practice [23]. Beyond PD-1 and CTLA-4, a variety of molecules are emerging as potential therapeutic immunotargets in RCC [24]. This list includes the V-domain immunoglobulin containing suppressor of T-cell activation (VISTA), which has been recently shown to exert its inhibitory activity by acting as a ligand on antigen presenting cells (APCs) and as a receptor on T cells [25–27], chemokine receptors [28], the soluble lymphocyte-activation gene-3 (LAG-3), 4-1BB, B and T lymphocyte attenuator (BTLA) and OX40 (CD134) [29].
Figure 1.
Clear cell renal cell carcinoma (ccRCC) with chronic inflammation.
At present, we do have full knowledge of the underlying mechanisms of immune-checkpoint inhibitors-induced tumour response. To address this issue, Wei and colleagues investigated the effects of anti-PD-1 and anti-CTLA-4 inhibitors in human melanoma and murine tumour models [30]. They first revealed that these agents are able to target distinct tumour-infiltrating T-cell subpopulations. In particular, PD-1 blockade promotes the expansion of specific exhausted-like CD8 T-cell populations; whereas, CTLA-4 blockade induces both an ICOS+ Th1-like CD4 effector subset and exhausted-like CD8 T cells [30]. This evidence strongly supports the results of different trials exploring this combination therapy in different diseases and favours the combined use of current and future checkpoint inhibitors in cancer patients, which seems to be characterized by a good tolerable safety profile [14,31–33].
The results obtained in recent years have raised enormous enthusiasm in cancer researchers, aimed at identifying, isolating and validating biomarkers associated with the dynamics of the tumour environment and the therapeutic response [17,34,35]. Indeed, tumour responsiveness varies according to the mutation load and the expression of immunotargets in the tumour environment, which is variable in the different phases of RCC development and progression [36]. Based on this evidence, assessing the expression of PD-1/PD-L1 or other emerging immunotargets only at the diagnosis of metastatic disease may not reflect tumour dynamicity. To improve the feasibility and reduce the clinical impact of re-biopsy, assessing biomarkers on circulating tumour cells (CTCs) or exosomes [37] may represent a noninvasive strategy that can be performed several times during cancer therapy to reflect changes that occurred in the tumour environment. The early identification of validated biomarkers will be crucial to definitively carry immunotherapy into the era of precision medicine and to optimize the cost-effectiveness of these agents in cancer patients [38,39].
More recently, Routy and colleagues revealed that primary resistance to immune-checkpoint inhibitors can be correlated with abnormal gut microbiome composition [40]. In this study, the effectiveness of PD-1 blockade was enhanced by transplanting faecal microbiota from responder cancer patients into germ-free or antibiotic-treated mice [40], thus representing another step on the way to personalized and precision immunotherapy in cancer patients.
Another factor that is fundamental to improve the efficacy of immunotherapy in RCC patients is a better understanding of the immunological effects of TKIs and mTOR inhibitors [18,41]. Indeed, these agents can indirectly exert their anti-tumour activity by targeting immune cells in the RCC microenvironment [18], and this should be considered to combine or sequence them with currently available and future immunotherapies. In this regard, sunitinib has been shown to inhibit the colony forming units (CFUs) driven by GM-CSF and FLT3 ligand (FLT3L) [42] as well as dendritic cell antigen presentation [40] (by decreasing the secretion of cytokines and the expression of MHC and CD1a molecules), to suppress the myeloid-derived suppressor cells (MDSCs, involved in RCC progression and drug resistance), to enhance tumour cell sensitivity to natural killer (NK) cell killing [43] and to reduce the total count of CD3 and CD4 T cells and regulatory T cells [44,45]. On the other hand, pazopanib showed lower inhibitory potency and affinity against FLT3 and c-kit compared to sunitinib [46]. Interestingly, we previously showed that axitinib can increase the surface NKG2D ligand expression, thus promoting NK cell recognition and degranulation in A-498 RCC cells in a ROS-dependent manner [47]. At present, little evidence is available on the immunomodulatory effects of cabozantinib and lenvatinib, both of which were recently introduced into RCC clinical practice. With regard to the association between immunotherapy and TKIs, the association between atezolizumab and both bevacizumab [48] and cabozantinib [49] as well as pembrolizumab and axitinib [50] has shown promising results in terms of clinical activity and safety profile – thus justifying the planning of several different large trials exploring these associations in metastatic/locally advanced RCC (Table 2).
Table 2.
Ongoing phase III clinical trials testing the association between immunotherapy and TKIs.
| Trial | Treatment arms | n | Setting | Estimated primary completion date |
|---|---|---|---|---|
| NCT02811861 | Sunitinib versus lenvatinib + everolimus versus lenvatinib + pembrolizumab |
735 | First line | October 2019 |
| NCT02684006 | Avelumab + axitinib versus sunitinib |
830 | First line | December 2018 |
| NCT03141177 | Nivolumab + cabozantinib versus sunitinib |
630 | First line | September 2019 |
| NCT02420821 | Atezolizumab + bevacizumab versus sunitinib |
915 | First line | July 2020 |
| NCT02853331 | Pembrolizumab + axitinib versus sunitinib |
840 | First line | January 2020 |
Conclusions
Optimizing the combination of immunotherapy and target agents, as well as the possible favourable sequence of treatment between these two classes of drugs, remains as open questions at this moment. We have only few data provided from IMmotion150, which demonstrated that association between a PD-L1 inhibitor and bevacizumab is feasible with a satisfactory safety profile. However, we do not know if this association results in an effective clinical benefit from our patients. Finally, it is important to observe that all the cited studies explored immunotherapy in patients with ccRCC and the role of immunotherapy still remains unknown in nccRCC.
There are several questions that need to be answered. It is undeniable that immunotherapy represents a revolution for the management of RCC resulting in a dynamic and evolving scenario. Future studies will increase our knowledge about the role of immunotherapy in this no-more orphan disease.
Footnotes
Contributions: MS and FM conceived and designed the study and contributed equally to the preparation of the manuscript. VdN, ACo and ACi conducted the structured literature review. MSc, RM, LC, NB and ALB drafted the manuscript. All authors analysed and interpreted the data, critically revised successive drafts of the manuscript and have approved the final version.
Disclosure and potential conflicts of interest: The authors declare no conflicts of interest. The International Committee of Medical Journal Editors (ICMJE) Potential Conflicts of Interests form for the authors are available for download at http://www.drugsincontext.com/wp-content/uploads/2018/06/dic.212528-COI.pdf
Correct attribution: Copyright © 2018 Santoni M, Massari F, Di Nunno V, Conti A, Cimadamore A, Scarpelli M, Montironi R, Cheng L, Battelli N, Lopez-Beltran A. https://doi.org/10.7573/dic.212528. Published by Drugs in Context under Creative Commons License Deed CC BY NC ND 4.0.
Article URL: http://www.drugsincontext.com/immunotherapy-in-renal-cell-carcinoma-latest-evidence-and-clinical-implications
Provenance: invited; externally peer reviewed.
Drugs in Context is published by BioExcel Publishing Ltd. Registered office: Plaza Building, Lee High Road, London, England, SE13 5PT.
BioExcel Publishing Limited is registered in England Number 10038393. VAT GB 252772009.
For all manuscript and submissions enquiries, contact the Editorial office dic.editorial@bioexcelpublishing.com
For all permissions, rights and reprints, contact David Hughes david.hughes@bioexcelpublishing.com
Peer review comments to author: 27 March 2018
References
- 1.Moch H, Cubilla AL, Humphrey PA, Reuter VE, Ulbright TM. The 2016 WHO classification of tumours of the urinary system and male genital organs-part a: renal, penile, and testicular tumours. Eur Urol. 2016;70(1):93–105. doi: 10.1016/j.eururo.2016.02.029. https://doi.org/10.1016/j.eururo.2016.02.029. [DOI] [PubMed] [Google Scholar]
- 2.Motzer RJ, Hutson TE, Tomczak P, Michaelson MD, Bukowski RM, Rixe O, Oudard S, Negrier S, Szczylik C, Kim ST, Chen I, Bycott PW, Baum CM, Figlin RA. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. 2007;356:115–24. doi: 10.1056/NEJMoa065044. https://doi.org/10.1056/NEJMoa065044. [DOI] [PubMed] [Google Scholar]
- 3.Motzer RJ, Hutson TE, Cella D, Reeves J, Hawkins R, Guo J, Nathan P, Staehler M, de Souza P, Merchan JR, Boleti E, Fife K, Jin J, Jones R, Uemura H, De Giorgi U, Harmenberg U, Wang J, Sternberg CN, Deen K, McCann L, Hackshaw MD, Crescenzo R, Pandite LN, Choueiri TK. Pazopanib versus sunitinib in metastatic renal-cell carcinoma. N Engl J Med. 2013;369:722–31. doi: 10.1056/NEJMoa1303989. https://doi.org/10.1056/NEJMoa1303989. [DOI] [PubMed] [Google Scholar]
- 4.Sternberg CN, Hawkins RE, Wagstaff J, Salman P, Mardiak J, Barrios CH, Zarba JJ, Gladkov OA, Lee E, Szczylik C, McCann L, Rubin SD, Chen M, Davis ID. A randomised, double-blind phase III study of pazopanib in patients with advanced and/or metastatic renal cell carcinoma: final overall survival results and safety update. Eur J Cancer. 2013;49:1287–96. doi: 10.1016/j.ejca.2012.12.010. https://doi.org/10.1016/j.ejca.2012.12.010. [DOI] [PubMed] [Google Scholar]
- 5.Choueiri TK, Escudier B, Powles T, Tannir NM, Mainwaring PN, Rini BI, Hammers HJ, Donskov F, Roth BJ, Peltola K, Lee JL, Heng DYC, Schmidinger M, Agarwal N, Sternberg CN, McDermott DF, Aftab DT, Hessel C, Scheffold C, Schwab G, Hutson TE, Pal S, Motzer RJ METEOR investigators. Cabozantinib versus everolimus in advanced renal-cell carcinoma. N Engl J Med. 2015;373:1814–23. doi: 10.1056/NEJMoa1510016. https://doi.org/10.1056/NEJMoa1510016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Motzer RJ, Escudier B, Tomczak P, Hutson TE, Michaelson MD, Negrier S, Oudard S, Gore ME, Tarazi J, Hariharan S, Chen C, Rosbrook B, Kim S, Rini BI. Axitinib versus sorafenib as second-line treatment for advanced renal cell carcinoma: overall survival analysis and updated results from a randomised phase 3 trial. Lancet Oncol. 2013;14:552–62. doi: 10.1016/S1470-2045(13)70093-7. https://doi.org/10.1016/S1470-2045(13)70093-7. [DOI] [PubMed] [Google Scholar]
- 7.Motzer RJ, Escudier B, Oudard S, Hutson TE, Porta C, Bracarda S, Grünwald V, Thompson JA, Figlin RA, Hollaender N, Kay A, Ravaud A RECORD-1 Study Group. Phase 3 trial of everolimus for metastatic renal cell carcinoma: final results and analysis of prognostic factors. Cancer. 2010;116(18):4256–65. doi: 10.1002/cncr.25219. https://doi.org/10.1002/cncr.25219. [DOI] [PubMed] [Google Scholar]
- 8.Escudier B, Eisen T, Stadler WM, Szczylik C, Oudard S, Staehler M, Negrier S, Chevreau C, Desai AA, Rolland F, Demkow T, Hutson TE, Gore M, Anderson S, Hofilena G, Shan M, Pena C, Lathia C, Bukowski RM. Sorafenib for treatment of renal cell carcinoma: Final efficacy and safety results of the phase III treatment approaches in renal cancer global evaluation trial. J Clin Oncol. 2009;27:3312–8. doi: 10.1200/JCO.2008.19.5511. https://doi.org/10.1200/JCO.2008.19.5511. [DOI] [PubMed] [Google Scholar]
- 9.Hutson TE, Escudier B, Esteban E, Bjarnason GA, Lim HY, Pittman KB, Senico P, Niethammer A, Lu DR, Hariharan S, Motzer RJ. Randomized phase III trial of temsirolimus versus sorafenib as second-line therapy after sunitinib in patients with metastatic renal cell carcinoma. J Clin Oncol. 2014;32:760–7. doi: 10.1200/JCO.2013.50.3961. https://doi.org/10.1200/JCO.2013.50.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Motzer RJ, Hutson TE, Glen H, Michaelson MD, Molina A, Eisen T, Jassem J, Zolnierek J, Maroto JP, Mellado B, Melichar B, Tomasek J, Kremer A, Kim HJ, Wood K, Dutcus C, Larkin J. Lenvatinib, everolimus, and the combination in patients with metastatic renal cell carcinoma: a randomised, phase 2, open-label, multicentre trial. Lancet Oncol. 2015;16:1473–82. doi: 10.1016/S1470-2045(15)00290-9. https://doi.org/10.1016/S1470-2045(15)00290-9. [DOI] [PubMed] [Google Scholar]
- 11.Motzer RJ, Escudier B, McDermott DF, George S, Hammers HJ, Srinivas S, Tykodi SS, Sosman JA, Procopio G, Plimack ER, Castellano D, Choueiri TK, Gurney H, Donskov F, Bono P, Wagstaff J, Gauler TC, Ueda T, Tomita Y, Schutz FA, Kollmannsberger C, Larkin J, Ravaud A, Simon JS, Xu LA, Waxman IM, Sharma P CheckMate025 Investigators. Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med. 2015;373:1803–13. doi: 10.1056/NEJMoa1510665. https://doi.org/10.1056/NEJMoa1510665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ciccarese C, Di Nunno V, Iacovelli R, Massari F. Future perspectives for personalized immunotherapy in renal cell carcinoma. Expert Opin Biol Ther. 2017;17:1049–52. doi: 10.1080/14712598.2017.1339030. https://doi.org/10.1080/14712598.2017.1339030. [DOI] [PubMed] [Google Scholar]
- 13.Massari F, Di Nunno V, Ciccarese C, Graham J, Porta C, Comito F, Cubelli M, Iacovelli R, Heng DYC. Adjuvant therapy in renal cell carcinoma. Cancer Treat Rev. 2017;60:152–7. doi: 10.1016/j.ctrv.2017.09.004. https://doi.org/10.1016/j.ctrv.2017.09.004. [DOI] [PubMed] [Google Scholar]
- 14.Motzer RJ, Tannir NM, McDermott DF, Arén Frontera O, Melichar B, Choueiri TK, Plimack ER, Barthélémy P, Porta C, George S, Powles T, Donskov F, Neiman V, Kollmannsberger CK, Salman P, Gurney H, Hawkins R, Ravaud A, Grimm MO, Bracarda S, Barrios CH, Tomita Y, Castellano D, Rini BI, Chen AC, Mekan S, McHenry MB, Wind-Rotolo M, Doan J, Sharma P, Hammers HJ, Escudier B. CheckMate 214 Investigators Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N Engl J Med. 2018 doi: 10.1056/NEJMoa1712126. Epub ahead of print. https://doi.org/10.1056/NEJMoa1712126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Atkins MB, McDermott DF, Powles T, Motzer RJ, Rini BI, Fong L. IMmotion150: a phase II trial in untreated metastatic renal cell carcinoma (mRCC) patients (pts) of atezolizumab (atezo) and bevacizumab (bev) vs and following atezo or sunitinib (sun) J Clin Oncol. 2017;35:4505. [Google Scholar]
- 16.Motzer RJ, Tannir NM, McDermott DF, Arén Frontera O, Melichar B, Choueiri TK, Plimack ER, Barthélémy P, Porta C, George S, Powles T, Donskov F, Neiman V, Kollmannsberger CK, Salman P, Gurney H, Hawkins R, Ravaud A, Grimm MO, Bracarda S, Barrios CH, Tomita Y, Castellano D, Rini BI, Chen AC, Mekan S, McHenry MB, Wind-Rotolo M, Doan J, Sharma P, Hammers HJ, Escudier B CheckMate 214 Investigators. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N Engl J Med. 2018;378(14):1277–1290. doi: 10.1056/NEJMoa1712126. https://doi.org/10.1056/NEJMoa1712126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iacovelli R, Nolè F, Verri E, Paglino C, Santoni M, Cossu Rocca M, Giglione P, Aurilio G, Cullurà D, Cascinu S, Porta C. Prognostic role of PD-L1 expression in renal cell carcinoma. A systematic review and meta-analysis. Target Oncol. 2016;11:143–8. doi: 10.1007/s11523-015-0392-7. https://doi.org/10.1007/s11523-015-0392-7. [DOI] [PubMed] [Google Scholar]
- 18.Santoni M, Berardi R, Amantini C, Burattini L, Santini D, Santoni G, Cascinu S. Role of natural and adaptive immunity in renal cell carcinoma response to VEGFR-TKIs and mTOR inhibitor. Int J Cancer. 2014;134:2772–7. doi: 10.1002/ijc.28503. https://doi.org/10.1002/ijc.28503. [DOI] [PubMed] [Google Scholar]
- 19.Santoni M, Massari F, Amantini C, Nabissi M, Maines F, Burattini L, Berardi R, Santoni G, Montironi R, Tortora G, Cascinu S. Emerging role of tumor-associated macrophages as therapeutic targets in patients with metastatic renal cell carcinoma. Cancer Immunol Immunother. 2013;62:1757–68. doi: 10.1007/s00262-013-1487-6. https://doi.org/10.1007/s00262-013-1487-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lolli C, Basso U, Derosa L, Scarpi E, Sava T, Santoni M, Crabb SJ, Massari F, Aieta M, Conteduca V, Maruzzo M, La Russa F, Wheater M, Berardi R, Galli L, De Giorgi U. Systemic immune-inflammation index predicts the clinical outcome in patients with metastatic renal cell cancer treated with sunitinib. Oncotarget. 2016;7:54564–71. doi: 10.18632/oncotarget.10515. https://doi.org/10.18632/oncotarget.10515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Santoni M, De Giorgi U, Iacovelli R, Conti A, Burattini L, Rossi L, Burgio SL, Berardi R, Muzzonigro G, Cortesi E, Amadori D, Cascinu S. Pre-treatment neutrophil to lymphocyte ratio as an independent prognostic factor in patients with treated with everolimus for metastatic renal cell carcinoma. Br J Cancer. 2013;109:1755–9. doi: 10.1038/bjc.2013.522. https://doi.org/10.1038/bjc.2013.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Santoni M, Buti S, Conti A, Porta C, Procopio G, Sternberg CN, Bracarda S, Basso U, De Giorgi U, Rizzo M, Derosa L, Ortega C, Massari F, Milella M, Bersanelli M, Cerbone L, Muzzonigro G, Burattini L, Montironi R, Santini D, Cascinu S. Prognostic significance of host immune status in patients with late relapsing renal cell carcinoma treated with targeted therapy. Target Oncol. 2015;10:517–22. doi: 10.1007/s11523-014-0356-3. https://doi.org/10.1007/s11523-014-0356-3. [DOI] [PubMed] [Google Scholar]
- 23.Massari F, Santoni M, Ciccarese C, Santini D, Alfieri S, Brunelli M, Piva F, Berardi R, Montironi R, Porta C, Cascinu S, Tortora G. PD-1/PD-L1 blockade alone or in combination in renal cell carcinoma: current studies and future promises. Cancer Treat Rev. 2015;41:114–23. doi: 10.1016/j.ctrv.2014.12.013. https://doi.org/10.1016/j.ctrv.2014.12.013. [DOI] [PubMed] [Google Scholar]
- 24.Kucharczyk J, Matrana MR, Santoni M, Massari F, Scarpelli M, Cheng L, Lopez-Beltran A, Cascinu S, Montironi R, Moch H. Emerging immunotargets in metastatic renal cell carcinoma. Curr Drug Targets. 2016;17:771–6. doi: 10.2174/1389450117666151209115753. https://doi.org/10.2174/1389450117666151209115753. [DOI] [PubMed] [Google Scholar]
- 25.Montironi R, Santoni M, Massari F, Lopez-Beltran A, Cheng L, Berardi R, Scarpelli M. Emerging immunotargets in genitourinary tumors. Curr Drug Targets. 2016;17:748–9. doi: 10.2174/138945011707160412185542. https://doi.org/10.2174/138945011707160412185542. [DOI] [PubMed] [Google Scholar]
- 26.Montironi R, Santoni M, Cheng L, Lopez-Beltran A, Massari F, Matrana MR, Moch H, Scarpelli M. An overview of emerging immunotargets of genitourinary tumors. Curr Drug Targets. 2016;17:750–6. doi: 10.2174/1389450117666151209144649. https://doi.org/10.2174/1389450117666151209144649. [DOI] [PubMed] [Google Scholar]
- 27.Slovin SF. The need for immune biomarkers for treatment prognosis and response in genitourinary malignancies. Biomark Med. 2017;11:1149–59. doi: 10.2217/bmm-2017-0138. https://doi.org/10.2217/bmm-2017-0138. [DOI] [PubMed] [Google Scholar]
- 28.Santoni M, Bracarda S, Nabissi M, Massari F, Conti A, Bria E, Tortora G, Santoni G, Cascinu S. CXC and CC chemokines as angiogenic modulators in non-haematological tumors. Biomed Res Int. 2014;2014:768758. doi: 10.1155/2014/768758. http://dx.doi.org/10.1155/2014/768758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Massari F, Santoni M, Ciccarese C, Santini D. The immunocheckpoints in modern oncology: the next 15 years. Expert Opin Biol Ther. 2015;15:917–21. doi: 10.1517/14712598.2015.1035251. https://doi.org/10.1517/14712598.2015.1035251. [DOI] [PubMed] [Google Scholar]
- 30.Wei SC, Levine JH, Cogdil AP, Zhao Y, Anang NAS, Andrews MC, Sharma P, Wang J, Wargo JA, Pe’er D, Allison JP. Distinct cellular mechanisms underlie anti-Ctla-4 and anti-Pd-1 checkpoint blockade. Cell. 2017;170:1120–33. doi: 10.1016/j.cell.2017.07.024. https://doi.org/10.1016/j.cell.2017.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ciccarese C, Alfieri S, Santoni M, Santini D, Bergamini C, Licitra L, Montironi R, Tortora G, Massari F. New toxicity profile for novel immunotherapy agents: focus on checkpoint inhibitors. Expert Opin Drug Metab Toxicol. 2016;12:57–75. doi: 10.1517/17425255.2016.1120287. https://doi.org/10.1517/17425255.2016.1120287. [DOI] [PubMed] [Google Scholar]
- 32.Wolchok JD, Chiarion-Sileni V, Gonzalez R, Rutkowski P, Grob JJ, Cowey CL, Lao CD, Wagstaff J, Schadendorf D, Ferrucci PF, Smylie M, Dummer R, Hill A, Hogg D, Haanen J, Carlino MS, Bechter O, Maio M, Marquez-Rodas I, Guidoboni M, McArthur G, Lebbé C, Ascierto PA, Long GV, Cebon J, Sosman J, Postow MA, Callahan MK, Walker D, Rollin L, Bhore R, Hodi FS, Larkin J. Overall survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med. 2017;377(14):1345–56. doi: 10.1056/NEJMoa1709684. https://doi.org/10.1056/NEJMoa1709684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weber J, Mandala M, Del Vecchio M, Gogas HJ, Arance AM, Cowey CL, Dalle S, Schenker M, Chiarion-Sileni V, Marquez-Rodas I, Grob JJ, Butler MO, Middleton MR, Maio M, Atkinson V, Queirolo P, Gonzalez R, Kudchadkar RR, Smylie M, Meyer N, Mortier L, Atkins MB, Long GV, Bhatia S, Lebbé C, Rutkowski P, Yokota K, Yamazaki N, Kim TM, de Pril V, Sabater J, Qureshi A, Larkin J, Ascierto PA CheckMate 238 Collaborators. Adjuvant nivolumab versus ipilimumab in resected stage III or IV melanoma. N Engl J Med. 2017 Nov 9;377(19):1824–35. doi: 10.1056/NEJMoa1709030. https://doi.org/10.1056/NEJMoa1709030. [DOI] [PubMed] [Google Scholar]
- 34.Piva F, Santoni M, Scarpelli M, Briganti A, Lopez-Beltran A, Cheng L, Berardi R, Montorsi F, Montiron R. Re: Daniel M. Geynisman. Anti-programmed cell death protein 1 (PD-1) antibody nivolumab leads to a dramatic and rapid response in papillary renal cell carcinoma with sarcomatoid and rhabdoid features. Eur Urol. 2015;68:912–4. Eur Urol. 2016;70:e72–4. doi: 10.1016/j.eururo.2016.02.049. https://doi.org/10.1016/j.eururo.2016.02.049. [DOI] [PubMed] [Google Scholar]
- 35.Piva F, Giulietti M, Occhipinti G, Santoni M, Massari F, Sotte V, Iacovelli R, Burattini L, Santini D, Montironi R, Cascinu S, Principato G. Computational analysis of the mutations in BAP1, PBRM1 and SETD2 genes reveals the impaired molecular processes in renal cell carcinoma. Oncotarget. 2015;6:32161–8. doi: 10.18632/oncotarget.5147. https://doi.org/10.18632/oncotarget.5147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Santoni M, Santini D, Massari F, Conti A, Iacovelli R, Burattini L, Tortora G, Falconi M, Montironi R, Cascinu S. Heterogeneous drug target expression as possible basis for different clinical and radiological response to the treatment of primary and metastatic renal cell carcinoma: suggestions from bench to bedside. Cancer Metastasis Rev. 2014;33:321–31. doi: 10.1007/s10555-013-9453-5. https://doi.org/10.1007/s10555-013-9453-5. [DOI] [PubMed] [Google Scholar]
- 37.Ciccarese C, Santoni M, Massari F, Cheng L, Lopez-Beltran A, Scarpelli M, Conti A, Tortora G, Cascinu S, Montironi R. Present and future of personalized medicine in adult genitourinary tumors. Future Oncol. 2015;11:1381–8. doi: 10.2217/fon.15.30. https://doi.org/10.2217/fon.15.30. [DOI] [PubMed] [Google Scholar]
- 38.Montironi R, Santoni M, Tartari F, Lopez-Beltran A, Cheng L, Berardi R, Scarpelli M. Testing PD-1/PD-L1 expression in cancer therapy: pathological insights and economic sustainability. Arch Pathol Lab Med. 2016;140:501–2. doi: 10.5858/arpa.2015-0529-LE. https://doi.org/10.5858/arpa.2015-0529-LE. [DOI] [PubMed] [Google Scholar]
- 39.Tartari F, Santoni M, Burattini L, Mazzanti P, Onofri A, Berardi R. Economic sustainability of anti-PD-1 agents nivolumab and pembrolizumab in cancer patients: recent insights and future challenges. Cancer Treat Rev. 2016;48:20–4. doi: 10.1016/j.ctrv.2016.06.002. https://doi.org/10.1016/j.ctrv.2016.06.002. [DOI] [PubMed] [Google Scholar]
- 40.Routy B, Le Chatelier E, Derosa L, Duong CPM, Alou MT, Daillère R, Fluckiger A, Messaoudene M, Rauber C, Roberti MP, Fidelle M, Flament C, Poirier-Colame V, Opolon P, Klein C, Iribarren K, Mondragón L, Jacquelot N, Qu B, Ferrere G, Clémenson C, Mezquita L, Masip JR, Naltet C, Brosseau S, Kaderbhai C, Richard C, Rizvi H, Levenez F, Galleron N, Quinquis B, Pons N, Ryffel B, Minard-Colin V, Gonin P, Soria JC, Deutsch E, Loriot Y, Ghiringhelli F, Zalcman G, Goldwasser F, Escudier B, Hellmann MD, Eggermont A, Raoult D, Albiges L, Kroemer G, Zitvogel L. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science. 2018;359:91–7. doi: 10.1126/science.aan3706. https://doi.org/10.1126/science.aan3706. [DOI] [PubMed] [Google Scholar]
- 41.Santoni M, Rizzo M, Burattini L, Farfariello V, Berardi R, Santoni G, Cascinu S. Present and future of tyrosine kinase inhibitors in renal cell carcinoma: analysis of hematologic toxicity. Recent Pat Antinfect Drug Discov. 2012;7:104–10. doi: 10.2174/157489112801619719. https://doi.org/10.2174/157489112801619719. [DOI] [PubMed] [Google Scholar]
- 42.Pessina A, Albella B, Bueren J, Brantom P, Casati S, Gribaldo L, Croera C, Gagliardi G, Foti P, Parchment R, Parent-Massin D, Sibiril Y, Van Den Heuvel R. Prevalidation of a model for predicting acute neutropenia by colony forming unit granulocyte/macrophage (CFU-GM) assay. Toxicol In Vitro. 2001;6:729–40. doi: 10.1016/s0887-2333(01)00085-6. https://doi.org/10.1016/S0887-2333(01)00085-6. [DOI] [PubMed] [Google Scholar]
- 43.Hipp MM, Hilf N, Walter S, Werth D, Brauer KM, Radsak MP, Weinschenk T, Singh-Jasuja H, Brossart P. Sorafenib, but not sunitinib, affects function of dendritic cells and induction of primary immune responses. Blood. 2008;111:5610–20. doi: 10.1182/blood-2007-02-075945. https://doi.org/10.1182/blood-2007-02-075945. [DOI] [PubMed] [Google Scholar]
- 44.Powles T, Chowdhury S, Bower M, Saunders N, Lim L, Shamash J, Sarwar N, Sadev A, Peters J, Green J, Boleti K, Augwal S. The effect of sunitinib on immune subsets in metastatic clear cell renal cancer. Urol Int. 2011;86:53–9. doi: 10.1159/000319498. https://doi.org/10.1159/000319498. [DOI] [PubMed] [Google Scholar]
- 45.Adotevi O, Pere H, Ravel P, Haicheur N, Badoual C, Merillon N, Medioni J, Peyrard S, Roncelin S, Verkarre V, Mejean A, Fridman WH, Oudard S, Tartour E. A decrease of regulatory T cells correlates with overall survival after sunitinib-based antiangiogenic therapy in metastatic renal cancer patients. J Immunother. 2010;33:991–8. doi: 10.1097/CJI.0b013e3181f4c208. https://doi.org/10.1097/CJI.0b013e3181f4c208. [DOI] [PubMed] [Google Scholar]
- 46.Kumar R, Crouthamel M-C, Rominger DH, Gontarek RR, Tummino PJ, Levin RA, King AG. Myelosuppression and kinase selectivity of multikinase angiogenesis inhibitors. Br J Cancer. 2009;101:1717–23. doi: 10.1038/sj.bjc.6605366. https://doi.org/10.1038/sj.bjc.6605366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Morelli MB, Amantini C, Santoni M, Soriani A, Nabissi M, Cardinali C, Santoni A, Santoni G. Axitinib induces DNA damage response leading to senescence, mitotic catastrophe, and increased NK cell recognition in human renal carcinoma cells. Oncotarget. 2015;6:36245–59. doi: 10.18632/oncotarget.5768. https://doi.org/10.18632/oncotarget.5768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McDermott DF, Atkins MB, Motzer RJ, Rini BI, Escudier BJ, Fong L, Joseph RW, Pal SK, Sznol M, Hainsworth JD, Stadler WM, Hutson TE, Ravaud A, Bracarda S, Suarez C, Choueiri TK, Choi YJ, Huseni MA, Fine GD, Powles T. A phase II study of atezolizumab (atezo) with or without bevacizumab (bev) versus sunitinib (sun) in untreated metastatic renal cell carcinoma (mRCC) patients. J Clin Oncol. 2017;35(6_suppl):431. https://doi.org/10.1200/JCO.2017.35.6_suppl.431. [Google Scholar]
- 49.Maia MC, Agarwal N, McGregor BA, Vaishampayan UN, Choueiri TK, Green MC, Hessel C, Scheffold C, Schwab G, Powles T, Pal SK. Phase 1b trial of cabozantinib in combination with atezolizumab in patients with locally advanced or metastatic urothelial carcinoma (UC) or renal cell carcinoma (RCC) J Clin Oncol. 2018;36(6_suppl) Published online before print. https://doi.org/10.1200/JCO.2018.36.5_suppl.TPS42. [Google Scholar]
- 50.Atkins MB, Plimack ER, Puzanov I, Fishman MN, McDermott DF, Cho DC, Vaishampayan U, George S, Olencki TE, Tarazi JC, Rosbrook B, Fernandez KC, Lechuga M, Choueiri TK. Axitinib in combination with pembrolizumab in patients with advanced renal cell cancer: a non-randomised, open-label, dose-finding, and dose-expansion phase 1b trial. Lancet Oncol. 2018;19(3):405–15. doi: 10.1016/S1470-2045(18)30081-0. https://doi.org/10.1016/S1470-2045(18)30081-0. [DOI] [PMC free article] [PubMed] [Google Scholar]