Summary
CNS cortical histogenesis depends on polarity signaling pathways that regulate cell adhesion and motility. Here we report that conditional deletion of the Rho GTPase Cdc42 in cerebellar granule cell precursors (GCPs) results in abnormalities in cerebellar foliation revealed by iDISCO clearing methodology, a loss of columnar organization of proliferating GCPs in the external germinal layer (EGL), disordered parallel fiber organization in the molecular layer (ML), and a failure to extend a leading process and form a neuron-glial junction during migration along Bergmann glia (BG). Notably, GCPs lacking Cdc42 had a multi-polar morphology and slowed migration rate. In addition, secondary defects occurred in BG development and organization, especially in the lateral cerebellar hemispheres. By phosphoproteomic analysis, affected Cdc42 targets included regulators of the cytoskeleton, cell adhesion and polarity. Thus, Cdc42 signaling pathways are critical regulators of GCP polarity and the formation of neuron-glial junctions during cerebellar development.
Subject Areas: Optical Imaging, Developmental Neuroscience, Techniques in Neuroscience
Graphical Abstract

Highlights
-
•
Conditional deletion of Cdc42 in GCPs perturbs cerebellar cortical histogenesis
-
•
Loss of Cdc42 in GCPs disrupts GCP neuron-glial junctions
-
•
Cdc42 deficiency causes a loss of GCP polarity and slows their migration
-
•
Phosphoproteomics reveals changes in cytoskeletal, adhesion, and polarity proteins
Optical Imaging; Developmental Neuroscience; Techniques in Neuroscience
Introduction
The histogenesis of the laminar architecture of cortical regions of the mammalian brain depends on a finely orchestrated series of developmental processes that includes the migration of post-mitotic neuronal precursors from pseudo-stratified germinal zones to specific layers, where they mature and form synaptic connections with afferent axons. Each step in neuronal development and layer formation depends on characteristic, stage-specific, polarized neuronal morphologies, through coordinated reorganization of the actin and microtubule cytoskeletons. The Rho GTPases control key signaling pathways that regulate cell polarization by interacting with a variety of effector proteins that regulate cytoskeletal dynamics. They have been implicated in every stage of neuronal development, including axon guidance, neuronal migration, dendrite and spine formation, and synaptic plasticity (Azzarelli et al., 2014, Govek et al., 2011). Of the small Rho GTPases, Cdc42 has emerged as a critical regulator of cell polarity and directed cell migrations. As first shown in non-neuronal cells, Cdc42 functions as a critical regulator of cell polarity, centrosome orientation, cell-cell junction formation, and actin dynamics in motile cells (Heasman and Ridley, 2008). During CNS development, Cdc42 deficiency in telencephalic progenitor cells causes defects in forebrain development (Cappello et al., 2006, Chen et al., 2006). Cdc42 also regulates axon formation (Hall and Lalli, 2010) and dendrite (Scott et al., 2003) and dendritic spine morphogenesis (Kreis et al., 2007). Although electroporation of Cdc42 mutants in utero has been shown to retard radial migration in the developing neocortex (Konno et al., 2005), the function of Cdc42 in the regulation of neuronal polarity in glial-guided CNS migration has not been defined.
The cerebellar cortex has long provided a model for critical steps in cortical histogenesis, including neurogenesis, glial-guided migration, and the formation of neuronal layers. During postnatal cerebellar development, precursors of the granule cell, one of two principal cerebellar neurons, proliferate in the superficial zone of the external germinal layer (EGL) before exiting the cell cycle in the deeper zone of the EGL, where they extend bipolar parallel fiber axons that form synapses with Purkinje cells (PCs), the sole output neuron of the cerebellum. Subsequently, granule cell precursors (GCPs) extend a descending, leading process along radially aligned Bergmann glia (BG), which guides their migration through the molecular layer (ML) (Edmondson and Hatten, 1987, Solecki et al., 2004, Solecki et al., 2009). The highly stereotyped sequence of changes in GCP polarity during axon extension, glial-guided migration, and dendrite formation provides a classic paradigm for developmental-stage-specific changes in CNS neuronal polarity (see diagram in Figure S1).
To examine the function of Cdc42 in axon patterning and glial-guided migration during cerebellar histogenesis, we conditionally deleted Cdc42 in cerebellar GCPs. Surprisingly, a conditional loss of Cdc42 in GCPs caused striking changes in GCP polarity and neuron-glial interactions, as well as thinner, more elongated, and undulated cerebellar folia, the severity of which was revealed by iDISCO methodology. These changes involved deficits in polarity, cell interactions, and motility at each stage of development, including failure to align into pseudo-columns among BG fibers in the proliferative zone, defects in parallel fiber axon fasciculation in the ML, and failure to extend a single, descending leading process along or form a migration junction with BG as they traversed the ML. By phosphoproteomic analyses, changes occurred in the phosphorylation of cytoskeletal, adhesion, and polarity proteins in Cdc42-deficient GCPs. Thus, Cdc42 signaling pathways are critical regulators of GCP polarity, neuron-glial interactions, and migration during cerebellar histogenesis.
Results
Expression of Dominant Negative Form of Cdc42 Perturbs GCP Polarity
To examine the role of Cdc42 signaling in cerebellar GCP development, we first over-expressed Venus fluorophore-tagged dominant negative (DN) Cdc42 (Venus-Cdc42N17) to block Cdc42 activity and Venus fluorophore-tagged constitutively active (CA) Cdc42 (Venus-Cdc42V12). We electroporated Venus-Cdc42N17, Venus-Cdc42V12, or Venus alone as a control into postnatal day (P) 8 mouse cerebella and generated organotypic slices of cerebellar cortex. Expression of DN Cdc42 resulted in a dramatic change in GCP morphology. DN Cdc42-expressing cells in the ML, where GCPs undergo glial-guided migration, expressed multiple branched processes (Figure 1B) rather than the classic bipolar morphology of migrating GCPs (Figure 1A). In contrast, after CA Cdc42 expression, some GCPs had small changes in the amount of ruffling of the leading process, but overall, they had a classic bipolar shape, typical of migrating neurons (Figure 1C). These experiments suggested that Cdc42 activity was an important determinant of GCP morphology associated with normal migration along BG fibers.
Figure 1.
Polarity and Neuroanatomical Changes Caused by Inhibition and Loss of Cdc42
(A–C) Inhibition of Cdc42 activity, by electroporating DN Cdc42 (Venus-Cdc42N17, B) into GCPs of P8 wild-type cerebellum and culturing organotypic slices, caused extreme, multiple branching and loss of polarity of GCPs migrating through the molecular layer (ML). Venus control (A) and CA Cdc42 (Venus-Cdc42V12, C) electroporated GCPs exhibited the typical bipolar phenotype of migrating GCPs. Scale bar represents 10 μm.
(D–G) iDISCO clearing, volumetric imaging, and computer rendering of TAG1-immunostained P7 cerebella revealed defects in foliation patterning in the Cdc42 cKO mutant mouse (E and G) compared with control (D and F). Dorsal images of the cerebellum (D and E). Ventral images of the cerebellum (F and G). Lobes in the vermis are numbered. Lateral hemisphere lobes are labeled S, simplex; CI and II, Crus I and II; Pm, paramedian; PF, paraflocculus; F, flocculus. Scale bar represents 1 mm.
(H–K) Loss of Cdc42 caused a decrease in the area and width of the cerebellar vermis in the sagittal plane of mutant cerebella (I–K) compared with control cerebella (H, J, and K). Immunostaining for NeuroD1 (green) and β-tubulin (red) was performed to highlight the morphology of the cerebellum. Scale bar represents 500 μm. Data are represented as mean ± SD (***p < 0.001).
See also Figures S1–S3 and File S4.
Conditional Loss of Cdc42 Function Causes Defects in Cerebellar Development
To provide a genetic model for the role of Cdc42 in GCP polarity and cerebellar development, we conditionally deleted Cdc42 in mouse GCPs by crossing a floxed Cdc42 line with a Tg(Atoh1-Cre) Cre-deleter line (Figures S2A–S2F). P7 Atoh1-Cre+/-; Cdc42 loxP/loxP mutant mice lacking Cdc42 in GCPs had a striking cerebellar phenotype compared with control Cdc42 loxP/loxP mice with normal Cdc42 levels in GCPs. Overall, the cerebellar shape differed significantly in mutant compared with control animals (stereoscopic images in Figures S2G and S2H). To obtain 3D images of mutant cerebella, we used iDISCO clearing (Liebmann et al., 2016, Renier et al., 2014), immunostained the tissue for the GCP axonal marker TAG1 and the PC marker Calbindin (CALB1), and performed light sheet flourescence microscopy (LSFM). Computer rendering of these images for surface structure revealed the striking undulations of the lateral portions of mutant cerebella compared with the smooth lobes of control cerebella (Figures 1D–1G and S3; File S4). In addition, we observed a fusion of lobes VI–VII in the Cdc42 cKO. Overall, the mutant cerebellum was longer and thinner than the wild-type cerebellum.
The severity of the lateral undulations in mutant cerebella was also evident in serial, sagittal sections immunostained for GCP and PC markers (Figure S2I). Notably, the lobes in lateral sections were greatly disorganized compared with those of the control. In addition, the presence of more lateral sections in the mutant compared with the control confirmed elongation of the lobes. Sagittal sections of the medial portion of the cerebellum, the vermis, from mutant mice also revealed that the cerebellum was 33% smaller in total area and 23.5% smaller in width in the sagittal plane than those of control mice (Figures 1H–1K).
To study developmental processes, we examined cerebella from embryonic age (E) 16.5 through P15. Sagittal cryostat sections from embryonic and postnatal mice were immunostained with the neuronal marker NeuN and the PC marker CALB1. Although there was no difference in size at E16.5, at later stages, the size of mutant cerebella decreased: 16%, 26%, and 55% at P0, P7, and P15, respectively, compared with control cerebella (Figures S2J–S2Q). As with P7 mice, lateral sagittal sections of P15 mice also revealed an undulating pattern of the principal layers in the lateral aspects of lobes in mutant mice (Figure S2R).
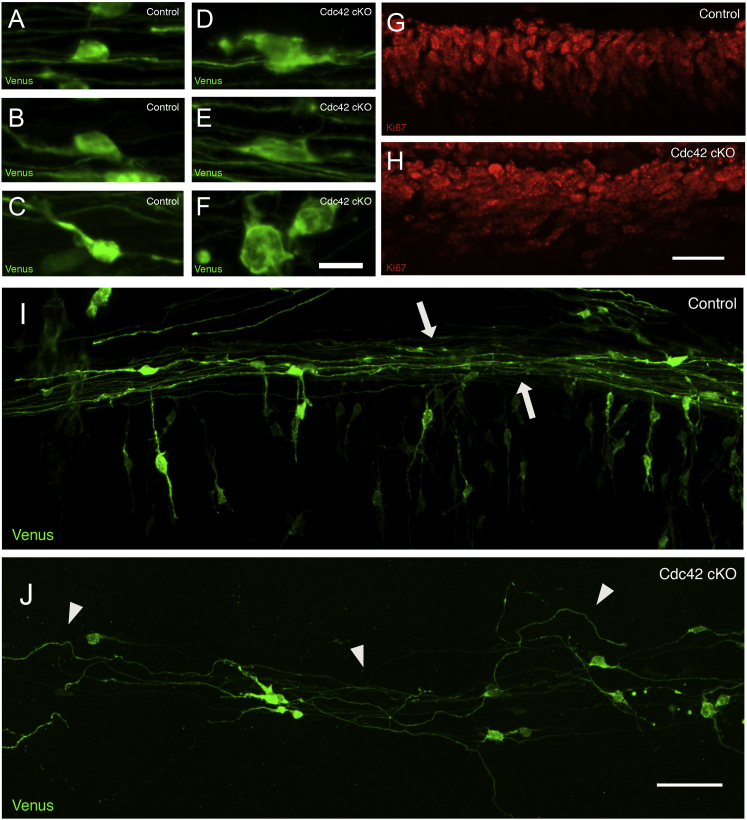
To determine whether changes in cerebellar patterning related to the rate of proliferation of GCPs, we assayed EdU incorporation. Proliferation assays at P7 did not reveal significant changes in the proliferation rate of early postnatal GCPs lacking Cdc42 (Figures S4A–S4C). Ki67 immunostaining also failed to show differences in the localization of proliferating GCPs in the outer aspect of the EGL (Figures 2G, 2H, and 4A–4D).
Figure 2.
Loss of Cdc42 Causes Polarity Defects in GCPs in the External Granule Layer of the Cerebellum
(A–F) Venus-expressing GCPs in the EGL of organotypic cerebellar slices of P8 Cdc42 cKO animals have larger, misshapen cell somas (D–F) compared with those of control animals (A–C). Scale bar represents 10 μm.
(G and H) Immunostaining of P8 mouse sections for Ki67 to label cells in the cell cycle revealed disorganization of the pseudo-columnar arrangement of GCPs in the outer EGL of the cerebellar cortex of Cdc42 cKO animals (H) compared with control animals (G). Scale bar represents 12.5 μm.
(I and J) Parallel fibers of Cdc42 cKO GCPs expressing Venus fluorophore (J) are disorganized and defasciculated compared with those of control GCPs (I). Arrows in the control panel indicate the relatively straight and parallel packing of the parallel fibers of control GCPs. Arrowheads in the mutant panel indicate disorganization and lack of fasciculation of the parallel fibers of mutant GCPs. Scale bar represents 50 μm.
See also Figure S1.
Figure 4.
Loss of Cdc42 Causes Slowed Glial-Guided Migration In Vivo
(A–D) Cerebella of P5 control (A and C) and Cdc42 cKO (B and D) mice were injected with BrdU, and the mice were sacrificed 72 hr later. Immunostaining cerebellar sections for BrdU showed an increased percentage of GCPs in the EGL and ML layers of Cdc42 cKO animals (B and D) compared with control animals (A and C) and a decreased percentage in the IGL. Immunostaining for Ki67 labeled cells in the cell cycle at the time that the animals were sacrificed. Scale bar for A and B represents 200 μm. Scale bar for C and D represents 50 μm.
(E) Quantitation of BrdU+ cells in the EGL, ML, and IGL of the cerebellar cortex of control and Cdc42 cKO animals.
(F) Quantitation of the thickness of the different regions of the cerebellar cortex in control and Cdc42 cKO animals.
Data are represented as mean ± SEM (*p < 0.05, **p < 0.01).
See also Figure S1.
To assess whether the aberrant shape of the cerebellum related to changes in the pattern or rate of cell death during development, we assayed cell death by activated Caspase 3 immunostaining. Again, no significant changes were observed (Figures S4D–S4H). Thus, an autonomous loss of Cdc42 in GCPs did not affect proliferation during the peak of postnatal GCP proliferation or cell death in the developing cerebellum.
Visualization of GCP Developmental Defects by Imaging Venus-Labeled Neurons
To visualize the morphology and cell interactions of developing GCPs in more detail, we used electroporation to express the fluorophore Venus in GCPs in P8 organotypic slices of cerebellar cortex and imaged labeled cells by spinning disc confocal microscopy. After expression of Venus in organotypic slice cultures for 60 hr, a time that would label both proliferating and migrating GCPs, dramatic changes were observed in the polarity of Cdc42-deficient GCPs at each developmental stage. Proliferating Cdc42-deficient GCPs in the outer aspect of the EGL had irregular, enlarged morphologies (Figures 2D–2F) compared with the classic rounded and/or elongated shapes seen in controls (Figures 2A–2C). Ki67 staining of tissue sections of P8 cerebellum also showed that the nuclei of GCPs lacking Cdc42 were more rounded (Figures 2G and 2H). Importantly, the organization of proliferating Cdc42-deficient GCPs differed dramatically from the controls, where progenitors were arranged in pseudo-columns (Figure 2G). In the Cdc42 mutant cerebellum, this pseudo-columnar organization was not apparent. Instead, proliferating GCPs were scattered randomly (Figure 2H).
By Venus labeling, although postmitotic Cdc42-deficient GCPs extended parallel fiber axons in the deeper aspects of the EGL, they were not fasciculated. Instead GCP parallel fibers coursed in and out of the parallel plane in a highly disorganized manner compared with control GCPs (Figures 2I and 2J). Thus, by confocal imaging, loss of Cdc42 perturbed GCP axon patterning at early stages of development.
To assay the polarity of migrating GCPs, we imaged labeled cells crossing the ML. The majority of GCPs in the ML of control animals had a bipolar shape with a single leading process in the direction of migration (Figures 3A, 3C, and 3E), whereas many of the Venus-labeled GCPs in the ML of mutant animals were multipolar (Figures 3B, 3D, and 3E). Thus, the morphology of Cdc42-deficient GCPs closely resembled that of GCPs that expressed DN Cdc42 (Figures 3B, 3D, and 1B). The loss of classic GCP polarity, especially the failure to extend a single, leading process along BG fibers, which is characteristic of migrating GCPs (Edmondson and Hatten, 1987, Gregory et al., 1988), suggested defects in glial-guided migration.
Figure 3.
Loss of Cdc42 Causes Defects in the Polarity of the Leading Process of Migrating GCPs in the Molecular Layer (ML)
(A and B) Venus-expressing control GCPs (A) and Cdc42 cKO GCPs (B) in organotypic slices of P8 cerebellar cortex. Arrows denote GCPs with a bipolar phenotype. Arrowheads denote GCPs with a multipolar phenotype. Scale bar represents 50 μm.
(C and D) Mutant GCPs tend to be multipolar (D) instead of possessing a single leading process extended in the direction of migration like control GCPs (C). Scale bar represents 10 μm. Arrow denotes GCP with a bipolar phenotype.
(E) Quantitation of the percentage of bipolar and multipolar GCPs in the ML for control and Cdc42 cKO cerebella. Data are represented as mean ± SEM (**p < 0.01).
See also Figure S1.
In Vivo Migration Assay of GCPs Lacking Cdc42
To determine the rate of migration of Cdc42-deficient GCPs along BG, we used a BrdU incorporation assay. In these experiments, we injected the cerebella of P5 mutant and control mice with BrdU and assayed the distance migrated after 72 hr. Measurements of the distribution of BrdU+ GCPs in the EGL, where proliferation occurs, the ML, where glial-guided migration occurs, and the internal granular layer (IGL), where post-migratory GCPs are located, showed a pronounced decrease in the percentage of post-migratory cells in the IGL (Figures 4A–4E). Thus, BrdU labeling experiments revealed a major delay in GCP migration from the EGL to the IGL.
Secondary Defects in Bergmann Glial Development
Since prior studies showed that defects in GCP axon extension and migration have secondary effects on the development and alignment of BG fibers (Hatten et al., 1984) and GC target neurons, the PCs (Adams et al., 2002), we examined the development of these cells in mutant mice. Loss of Cdc42 in GCPs had a profound effect on BG fiber morphology in postnatal cerebellum. Control BG aligned their cell bodies in a layer just below the PCs and extended a radial array of processes to the pial surface (Figures 5A, 5C, and S5A–S5C), whereas the cell bodies of BG in mutant cerebella were mis-localized above and below the PC layer, the processes were often out of the radial plane, and glial end feet were thicker and aberrantly fenestrated compared with those in control mice (Figures 5B, 5D, and S5D–S5F). Defects in BG development and organization were especially prominent in the elongated lateral hemispheres (Figure 5D). Defects in PC morphology and/or alignment were also noted in Cdc42 cKO animals, with PC dendritic arbors appearing smaller or tilted out of the sagittal plane and their cell bodies more disorganized compared with those of control cells in lateral cerebellar sections (Figures 5E–5J).
Figure 5.
Development of Bergmann Glia and Purkinje Cells in Cdc42 cKO Mice
(A–D) P8 mid-sagittal (A and B) and lateral (C and D) cerebellar sections from control (A and C) and Cdc42 cKO (B and D) mice immunostained for GFAP (red) to label glial cells and BrdU (A and B, green) or NeuN (C and D, green) to label GCPs show that GFAP fibers that span the cerebellar cortex in control mice are disorganized in mutant mice. In addition, BG cell bodies, denoted by arrowheads, are mis-localized in Cdc42 cKO animals and do not align as in control animals. Arrows point to the ends of the glial fibers, which appeared to be thickened and aberrantly fenestrated in Cdc42 cKO animals compared with control animals. Scale bar for A and B represents 30 μm. Scale bar for C and D represents 25 μm.
(E–J) P8 cerebellar sections were immunostained for calbindin (red) to label PCs in control (E, G, and I) and mutant (F, H, and J) cerebella. PCs in mid-sagittal cerebellar sections were similar in morphology in control (E) and mutant (F) animals, whereas PC dendrites in lateral sections were less elaborate in mutant (H and J) compared with control (G and I) animals. Scale bar represents 25 μm.
See also Figure S5.
Ultrastructural Features of GCPs Lacking Cdc42: Defects in Neuron-Glial Interactions
To analyze ultrastructural features of neuron-glial interactions of developing GCPs lacking Cdc42, we carried out electron microscopy (EM) of P7 cerebella. Strikingly, the BG fibers coursed between the colonnades of GCPs in the EGL, with GCPs apparently aligned among the radial glial fibers (Figure 6A), whereas BG fibers were not apparent in the EGL of the Cdc42 cKO cerebellum and GCPs were not arranged in a pseudo-columnar pattern (Figure 6B). Instead, irregularly shaped and randomly oriented GCPs were scattered across the EGL among fewer BG fibers that were not strictly radial. Thus, our EM analysis revealed a previously unreported pseudo-columnar pattern of organization in the EGL of control cerebella and an overall loss of this columnar organization in mutant cerebella.
Figure 6.
Ultrastructural Features of GCPs Lacking Cdc42 Revealed Defects in Neuron-Glial Interactions
(A and B) GCPs in control cerebellar cortex (A) were aligned among the radially extended glial fibers, whereas those in mutant cerebellar cortex (B) were scattered randomly throughout the EGL. BG fibers were difficult to detect in the mutant cerebella. Arrows indicate glial fibers. Scale bar represents 10 μm.
(C and D) GCPs lacking Cdc42 (D) migrating through the molecular layer (ML) had more irregularly shaped nuclei that were not strictly apposed to glial fibers as in the control condition (C). Arrows indicate glial fibers. Arrowhead indicates GCP nucleus apposed to a glial fiber. Scale bar represents 10 μm.
(C′ and D′) Slight zoom of areas boxed in C and D. Note the close apposition of the control cells' nuclei to the glial fiber, whereas the nuclei of most mutant cells near a glial fiber are not aligned with the fiber. Scale bar represents 5 μm. Arrows indicate glial fibers. Arrowheads indicate GCP nuclei apposed to a glial fiber.
(E and F) Zoom of control (E) and mutant (F) GCP migrating through the ML. Note the close apposition of the control cell's nucleus to the glial fiber, whereas the nuclei of mutant cells near a glial fiber are not aligned with the fiber. Scale bar represents 5 μm. Arrows indicate glial fibers. Arrowheads indicate GCP nuclei apposed to a glial fiber.
See also Figure S1.
EM analysis of GCPs migrating across the ML also revealed defects in neuron-glial interactions of migrating GCPs, with mutant GCPs failing to form the classic migration junction with BG fibers (Gregory et al., 1988). As reported earlier, BG fibers coursed in and out of the radial plane (Figures 6D, 5B, and 5D). Importantly, GCPs lacking Cdc42 did not have the characteristic, elongated cell soma closely apposed to BG fibers (Gregory et al., 1988, Rakic, 1971). Instead, GCPs lacking Cdc42 had irregularly shaped cell somas that were not strictly apposed to glial fibers (Figures 6D, 6D′, and 6F) like control cells (Figures 6C, 6C′, and 6E). Thus, loss of Cdc42 caused changes in the polarity of GCPs and failure to form a migration junction with BG fibers.
Changes in Gene Expression and Protein Phosphorylation in GCPs Lacking Cdc42
To determine whether changes in GCP polarity, axon patterning, and glial-guided migration related to mPar6 signaling, a key effector of Cdc42, which we previously showed is critical to radial neuronal migration (Solecki et al., 2004), we used Western blotting to probe for changes in mPar6 protein levels or the phosphorylation and thus activation of aPKC, the principle downstream effector of mPar6 signaling, and the aPKC target GSK3β (Figure S6). Surprisingly, no changes in mPar6 levels, aPKC phosphorylation, or GSK3β phosphorylation were detected.
We next took a more global approach and assayed whether loss of Cdc42 caused general changes in gene transcription. RNA-seq analysis of Cdc42-deficient GCPs did not reveal changes in RNA levels for genes involved in cell polarity pathways or neuron-glial interactions (File S1).
Since we did not observe general transcription changes or changes in Par6 levels or activity (Figure S6), we used global phosphoproteomics of P7 GCPs purified from mutant and control cerebella to identify affected phospho targets in Cdc42-deficient GCPs. Using a 2-fold cut-off, we identified a decrease in the phosphorylation of 1204 sites corresponding to 740 proteins and an increase in the phosphorylation of 170 sites corresponding to 143 proteins (Table 1 and File S2). Among the proteins identified with changes in phosphorylation were Rho GTPase and Cdc42 regulators and effectors, including Trio, Itsn1, Srgap1/3, and the actin regulatory proteins Pak1/2/4, which are main effector proteins of Cdc42 (Figure S7). A large number of cytoskeletal proteins with changes in phosphorylation were also identified, including Pxn, Fmn2, Dbn1, and Map2. Polarity regulators, such as Numbl and Scrib, also exhibited changes in phosphorylation, as well as a large number of cell adhesion proteins, including α-catenin, σ-catenin, ZO-1, and ZO-2. Biological pathway analysis using the Database for Annotation, Visualization and Integrated Discovery (DAVID) revealed enrichment of phosphorylation sites for “regulation of actin cytoskeleton,” “focal adhesion,” “tight junction,” and "adherens junction,” among others (File S3), suggesting that structural changes in the cytoskeleton underlie the changes in polarity and that changes in cell-cell adhesion contribute to the defects in GCP organization, axon fasciculation, and migration in developing GCPs lacking Cdc42.
Table 1.
Phosphoproteomics of Cdc42-Deficient GCPs
| Protein | aa | Position | Down or Up | Localization Prob | Peptide Sequence |
|---|---|---|---|---|---|
| Alpha Catenin | S | 654 | Down | 0.97 | SRTSVQTEDDQLIAGQSAR |
| Alpha Catenin | T | 657 | Down | 1.00 | SRTSVQTEDDQLIAGQSAR |
| APC | S | 1006 | Down | 1.00 | YSDEQLNSGRQSPSQNER |
| APC | S | 2296 | Down | 0.98 | NSISPGRNGISPPNK |
| APC | S | 2800 | Down | 0.94 | RHSGSYLVTSV |
| Cortactin | S | 370 | Down | 1.00 | KQTPPASPSPQPIEDRPPSSPIYEDAAPFK |
| Cortactin | S | 381 | Down | 0.80 | KQTPPASPSPQPIEDRPPSSPIYEDAAPF |
| Dcx | S | 297 | Down | 1.00 | SPGPMRR |
| Dcx | S | 304 | Down | 1.00 | SKSPADSANGTSSSQLSTPK |
| Drebrin | T | 383 | Up | 0.84 | ALDEVTSSQPPPPPPPPPPTQEAQETTPSLDEELSK |
| Enah | S | 336 | Down | 0.97 | NSRPSSPVNTPSSQPPAAK |
| Filamin-A | T | 1742 | Down | 0.98 | FGGEHVPNSPFQVTALAGDQPTVQTPLR |
| Formin-2 | S | 493 | Down | 0.98 | GATADDSGGGSPVLAAK |
| Ncam1 | T | 757 | Down | 0.97 | EPIVEVRTEEERTPNHDGGK |
| Numbl | S | 263 | Down | 1.00 | KAEAAAAPAVAPGPAQPGHVSPTPATTSPGEK |
| Numbl | S | 305 | Down | 1.00 | QGSFRGFPALSQK |
| Pak1 | S | 222 | Down | 0.98 | SVIEPLPVTPTRDVATSPISPTENNTTPPDALTR |
| Pak2 | S | 141 | Down | 1.00 | YLSFTPPEK |
| Pak2 | S | 197 | Down | 1.00 | SVIDPIPAPVGDSNVDSGAK |
| Pak4 | S | 104 | Down | 1.00 | RESPPPPAR |
| Pak4 | S | 181 | Down | 1.00 | RPLSGPDVSTPQPGSLTSGTK |
| Pak4 | S | 293 | Down | 1.00 | ALAAPAVPPAPGPPGPRSPQREPQR |
| Paxillin | S | 244 | Down | 0.89 | GLEDVRPSVESLLDELESSVPSPVPAITVNQGEMSSPQR |
| Srgap1 | S | 906 | Down | 1.00 | GLNNDSPERR |
| Wipf2 | S | 235 | Down | 1.00 | LHPGREGHPAPPPVKPPPSPVNIR |
Table of phosphorylation sites that changed 2-fold or more for selected Cdc42, cytoskeletal, and adhesion-related proteins. The protein name is provided, and the identity of the phosphorylated amino acid (aa) is given as serine (S) or threonine (T). The position of the phosphorylated aa in the protein sequence is given, as well as whether the phosphorylation is upregulated (Up) or downregulated (Down). The localization probability is the probability that the identified residue is the correct phosphorylation site, and the matched peptide sequence is provided. For proteins of interest, all phosphorylation sites are shown if multiples were detected.See also Figure S7 and Files S2 and S3.
Discussion
The present findings demonstrate a novel, critical role for Cdc42 signaling pathways in key steps in cerebellar histogenesis, including axon patterning, glial-guided migration, and foliation of the cerebellar cortex. At each stage of development, loss of Cdc42 resulted in defects in neuronal polarity and neuron-glial interactions, including loss of GCP organization in the EGL, disorganized parallel fiber patterning, failure of migrating GCPs to form a leading process along BG fibers, and failure to form a classic migration junction with BG during migration. Unexpectedly, our study also revealed secondary defects in glial fiber organization, which perturbed the apparent organization of proliferating GCPs in the outer EGL and affected cerebellar folia formation. Molecular analyses of downstream signaling pathways in GCPs lacking Cdc42 revealed changes in the phosphorylation of prominent Cdc42 signaling proteins, including the actin regulatory proteins Pak1/2/4.
The changes we observed in the cerebellar cortex patterning of Cdc42 cKO animals, a decrease in width and elongation in length with severe undulation of the lateral lobes, have not been reported previously. These changes were especially apparent in iDISCO clearing and LSFM imaging of whole cerebellum, which revealed the extent of the undulation of the lateral lobes and the fusion of lobes VI–VII. The increase in overall length was also supported by an increased number of serial sections of mutant cerebella compared with controls. Although the thinner folia and smaller area and width of mid-sagittal sections of mutant cerebella gave the appearance of a smaller cerebellum, the increased length of folia suggested that the overall size of the cerebellum did not change, which is consistent with our finding that proliferation rates at the peak of GCP proliferation did not change. The surface rendering provided by the iDISCO/LSFM methodology used in this study is a critical advance for analyzing foliation patterning during cortical histogenesis. Previously, this level of detail was only possible with MRI imaging.
The changes we observed in foliation patterning likely relate to changes in the timing of neuronal migration and subsequent secondary defects in BG organization, which possibly affected neuron-glial interactions involved in migration and PC development. This interpretation is consistent with the fact that defects in BG fiber orientation in mutant cerebellum were most pronounced in the lateral hemispheres where foliation defects were also observed. Notably, defects in PC dendritic arborization and alignment were also seen in the lateral hemispheres of the cerebellum. The defects in PC dendritic arborization could relate to slowed GC migration (Adams et al., 2002), which would alter the dynamics of parallel fiber-PC synapse formation, or to secondary defects in BG, as PC dendritic orientation is thought to relate, at least in part, to contacts with BG. As discussed later, these secondary defects likely emanate from loss of Cdc42 function in GC polarity and adhesions required to maintain BG development.
The present study revealed a previously unreported organization of proliferating GCPs into pseudo-columns among radially aligned BG fibers in the external EGL. This organization was lost in Cdc42-deficient GCPs, apparently because of both a change in GCP polarity from small, rounded cells to irregularly shaped cells, and more importantly because of secondary, non-autonomous effects on BG fiber organization, which skewed the glial fibers out of the radial plane in mutant cerebellum. Defects in both glial cell body localization and radial orientation of BG fibers were apparent in light microscopy and EM of cerebella with Cdc42-deficient GCPs. The conclusion that defects in BG development were secondary to loss of neuron-glial interactions is consistent with prior studies showing secondary defects in BG development in the cerebellum of mice with autonomous neuronal defects in neuron-glial interactions, such as the weaver mouse (Hatten et al., 1984). As we did not observe changes in the number of EdU or Ki67-labeled cells in Cdc42-deficient GCPs, the defects in the columnar arrangement of proliferative GCPs did not correlate with significant changes in the proliferation of GCPs in the EGL, the zone where neurogenesis occurs. This result is in contrast to studies on the role of Cdc42 in cortical neurogenesis, where loss of Cdc42 resulted in defects in the position of mitoses, cell fate, and localization of cortical progenitors (Cappello et al., 2006, Chen et al., 2006).
Although Cdc42-deficient GCPs formed parallel fibers, striking defects in parallel fiber fasciculation, which organizes them into the classic parallel array, occurred in the absence of Cdc42 activity. Some insights into the defects in parallel fiber fasciculation observed can perhaps be gained from the results of our RNA-Seq and phosphoproteomic assays. Known regulators of axon fasciculation include cadherins and Ncam proteins. Interestingly, we observed an increase in mRNA expression of Fat2, an atypical cadherin capable of homophilic interactions that localizes to GCP parallel fibers (Nakayama et al., 2002) in our RNA-Seq analysis. We also found a decrease in the phosphorylation of the neural cell adhesion molecule Ncam1, which has been implicated in cell-cell adhesion, neurite outgrowth, synaptic plasticity, and learning and memory and interacts with the neuronal cytoskeleton. It is also possible that loss of Cdc42 activity affected the regulation of actin dynamics that control growth cone motility along parallel fiber tracts. These defects in parallel fiber packing could have affected GCP contacts with PCs, causing the secondary defects in PC development and alignment in the lateral hemispheres of the cerebellum of mutant mice, and they are consistent with prior studies showing secondary defects in PC development and alignment in the cerebellum of Astn1 null mice (Adams et al., 2002). Our findings on cerebellar GCP axon extension differ from results of studies on hippocampal neurons, where loss of Cdc42 or perturbation of Cdc42 activity and signaling using CA and DN Cdc42 mutants impaired axon formation (Garvalov et al., 2007, Schwamborn and Puschel, 2004).
The most striking change in GCP polarity and motility was that Cdc42-deficient GCPs had multipolar, branched processes rather than the classic bipolar descending process seen in GCPs migrating along BG fibers (Edmondson and Hatten, 1987, Gregory et al., 1988). This prominent change in polarity occurred in spite of the fact that GCPs lacking Cdc42 formed a T-shaped axon similar to control GCPs in cerebellar slices. The finding that expression of DN Cdc42 in vivo resulted in defects in the polarity of GCPs quite similar to those seen in GCPs with a conditional deletion of Cdc42 supports our interpretation that Cdc42 activity is required to express the classic morphology of migrating cerebellar granule cells. Although perturbation of Cdc42 activity in cortical precursors impairs radial migration (Konno et al., 2005), the present study is the first indication that Cdc42 activity regulates both the extension of a leading process and the formation of a migration junction with the glial fiber during CNS neuronal migration.
The results of our BrdU assays indicate, that Cdc42-deficient GCPs had a slowed rate of migration through the ML. Since prior studies showed that the mPar6 signaling complex regulates coordinated movement of the centrosome and cell soma during migration (Solecki et al., 2004), we anticipated that Cdc42 activity would regulate Par6 and PKCζ activity in GCPs. This was apparently not the case, as loss of Cdc42 activity did not alter Par6 expression or aPKC phosphorylation in GCPs. However, our phosphoproteomic analysis of Cdc42-deficient GCPs suggested that cytoskeletal and adhesion pathways were the molecular pathways underlying the changes in GCP organization, polarization, and migration that we observed.
Global phosphoproteomics is a powerful means to reveal signaling pathway networks, as well as identify specific targets and effectors, including those of small Rho GTPases (Gnad et al., 2013, Nishioka et al., 2012). In the present study, pathway analysis of the global phosphorylation changes we observed after deletion of Cdc42 in GCPs identified principal pathways likely to function in GCP polarity, axon fasciculation, and migration, including “regulation of actin cytoskeleton,” “focal adhesion,” “tight junction,” and “adherens junction” (File S3). We identified changes in a number of Rho GTPase and Cdc42 regulators, including Trio, Itsn1, and Srgap1/3. Of the main effector proteins of Cdc42 (Figure S7), we identified changes in the phosphorylation of Pak1/2/4, as well as a number of cytoskeletal proteins that are Pak targets, including Pxn, Cttn, Flna, and Stmn1. We also identified a number of cell adhesion molecules, including classical adhesion proteins, such as α-catenin, σ-catenin, ZO-1, and ZO-2. Although we identified Numbl and Scrib polarity proteins among those with decreases in phosphorylation, other regulators of polarity, such as the Pak proteins, Trio and Pxn, were also identified. Taken together, the large number of cytoskeletal and adhesion proteins affected in our phosphoproteomic analysis underscores the importance of Cdc42 activity in cytoskeletal dynamics during the polarization and extension of the leading process during glial-guided migration and cell adhesion required for cell organization and migration.
The current study underscores the importance of Cdc42 signaling to neuronal polarity and cell-cell interactions required for parallel fiber axon patterning and glial-guided migration during cerebellar development. The loss of Cdc42 signaling had a dramatic effect on GCP development with secondary effects on BG development, resulting in unique changes in the overall length and width of the cerebellum. Importantly, our studies revealed a previously unappreciated role for BG fibers in the organization of GCPs into pseudo-columns within the EGL. Although it will be important to further analyze the roles of Cdc42 targets identified in our phosphoproteomic analysis, it is clear that Cdc42 affects many signaling pathways that converge to organize cerebellar histogenesis. Understanding the role of Rho GTPase signaling in cortical development will likely provide important insights on the assembly of CNS circuits and behavior.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Data Software and Availability
All raw LC-MS/MS data and peptide identifications have been uploaded to the PRIDE data repository: Project Name: Cdc42 phosphorylation study, Project accession: PXD008148, Username: reviewer79582@ebi.ac.uk, Password: WILJxtep All. RAW files and matched peptides were deposited at PRIDE.
Acknowledgments
We are especially grateful to Dr. Linda Van Aelst at Cold Spring Harbor Laboratory for critical discussions on these experiments and for providing CA and DN Cdc42 cDNAs, Dr. Carol A. Mason at Columbia for critical comments on the electron microscopy, and Dr. Alexandra Joyner at Memorial Sloan Kettering Cancer Center (MSKCC) for advice on foliation patterning and defects. We thank Dr. Yi Zheng at Cincinnati Children's Hospital for the floxed Cdc42 mice and Dr. David Rowitch at UCSF and Dr. Alex Joyner at MSKCC for providing the Atoh1-Cre mice. Drs. Linda Van Aelst, David J. Solecki, Hourinaz Behesti, and Zachi Horn provided critical comments on the manuscript and reviewed the phosphoproteomic data. We thank Drs. Brian Chait at The Rockefeller University and Darryl Pappin of Cold Spring Harbor Laboratory for advice on our phosphoproteomic analysis. Dr. Kunihiro Uryu of The Rockefeller University Electron Microscopy Resource Center provided help with the electron microscopy. We also thank Dr. Connie Zhao of The Rockefeller Genomics Resource Center for performing the RNA sequencing and Dr. Nicolas Robine of the New York Genome Center (NYGC) and Dr. Yupu Liang of the Rockefeller CCTS Bioinformatics Program for performing bioinformatic analysis of the RNA-seq data. We thank Weslie Janeway for making and preparing DNA constructs and Raquel Hernandez-Solis and Rebecca de Frates for analyzing cerebellar morphology and size at different developmental stages. This work was supported by NIH grant R01 5R01NS051778-09 (M.E.H.) and a grant from the Eugene W. Chinery 2012 Trust (M.E.H.).
Author Contributions
M.E.H. and E.-E.G. designed all experiments and wrote the manuscript. E.-E.G. carried out the anatomical analyses, light microscopy, and biochemical assays. M.T.-L. and Z.W. designed and carried out the iDISCO clearing and LSFM. D.A. carried out the electron microscopy, H.M. carried out the global phosphoproteomics, and K.R. did the bioinformatics analysis of the phosphoproteomic datasets. X.Z. assisted with phosphoproteomic and Western blotting analyses, and Y.F. assisted with BrdU assays, immunohistochemistry, and mouse husbandry.
Declaration of Interests
The authors declare no competing interests.
Published: March 8, 2018
Footnotes
Supplemental Information includes Transparent Methods, seven figures, and four data files and can be found with this article online at https://doi.org/10.1016/j.isci.2018.01.004.
Supplemental Information
Excel spreadsheet of protein coding RNAs that changed 1.7-fold or greater in Cdc42 cKO GCPs compared with control GCPs. The gene name, log2-fold change, and adjusted p-value are provided.
Excel spreadsheet of phosphorylation sites that changed 2-fold or greater in Cdc42 cKO GCPs compared with control GCPs. The gene name, protein name, and accession number according to Uniprot are provided. The position of the phosphorylated amino acid in the protein sequence and its identity (S, serine; T, threonine) are also indicated, as well as whether the phosphorylation is upregulated or downregulated. The localization probability (the probability that the identified residue is the correct phosphorylation site) and the matched peptide sequence are provided. All raw LC-MS/MS data and data analysis files have been uploaded to the PRIDE data repository: Project Name: Cdc42 phosphorylation study, Project accession:PXD008148, Username:reviewer79582@ebi.ac.uk, Password: WILJxtep.
The Database for Annotation, Visualization and Integrated Discovery (DAVID) program was used for pathway analysis on proteins with phosphorylation sites that changed 2-fold or greater. The category, term, count (the number of proteins from the 2-fold or greater list of phosphorylation sites in that pathway), percentage (%, the number of proteins from the 2-fold or greater list of phosphorylation sites/total number of proteins in the pathway), p-value, and Benjamini significance are provided.
References
- Adams N.C., Tomoda T., Cooper M., Dietz G., Hatten M.E. Mice that lack astrotactin have slowed neuronal migration. Development. 2002;129:965–972. doi: 10.1242/dev.129.4.965. [DOI] [PubMed] [Google Scholar]
- Azzarelli R., Kerloch T., Pacary E. Regulation of cerebral cortex development by Rho GTPases: insights from in vivo studies. Front. Cell. Neurosci. 2014;8:445. doi: 10.3389/fncel.2014.00445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cappello S., Attardo A., Wu X., Iwasato T., Itohara S., Wilsch-Brauninger M., Eilken H.M., Rieger M.A., Schroeder T.T., Huttner W.B. The Rho-GTPase cdc42 regulates neural progenitor fate at the apical surface. Nat. Neurosci. 2006;9:1099–1107. doi: 10.1038/nn1744. [DOI] [PubMed] [Google Scholar]
- Chen L., Liao G., Yang L., Campbell K., Nakafuku M., Kuan C.Y., Zheng Y. Cdc42 deficiency causes Sonic hedgehog-independent holoprosencephaly. Proc. Natl. Acad. Sci. USA. 2006;103:16520–16525. doi: 10.1073/pnas.0603533103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmondson J.C., Hatten M.E. Glial-guided granule neuron migration in vitro: a high-resolution time-lapse video microscopic study. J. Neurosci. 1987;7:1928–1934. doi: 10.1523/JNEUROSCI.07-06-01928.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garvalov B.K., Flynn K.C., Neukirchen D., Meyn L., Teusch N., Wu X., Brakebusch C., Bamburg J.R., Bradke F. Cdc42 regulates cofilin during the establishment of neuronal polarity. J. Neurosci. 2007;27:13117–13129. doi: 10.1523/JNEUROSCI.3322-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gnad F., Young A., Zhou W., Lyle K., Ong C.C., Stokes M.P., Silva J.C., Belvin M., Friedman L.S., Koeppen H. Systems-wide analysis of K-Ras, Cdc42, and PAK4 signaling by quantitative phosphoproteomics. Mol. Cell. Proteomics. 2013;12:2070–2080. doi: 10.1074/mcp.M112.027052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govek E.E., Hatten M.E., Van Aelst L. The role of Rho GTPase proteins in CNS neuronal migration. Dev. Neurobiol. 2011;71:528–553. doi: 10.1002/dneu.20850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory W.A., Edmondson J.C., Hatten M.E., Mason C.A. Cytology and neuron-glial apposition of migrating cerebellar granule cells in vitro. J. Neurosci. 1988;8:1728–1738. doi: 10.1523/JNEUROSCI.08-05-01728.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall A., Lalli G. Rho and Ras GTPases in axon growth, guidance, and branching. Cold Spring Harb. Perspect. Biol. 2010;2:a001818. doi: 10.1101/cshperspect.a001818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatten M.E., Liem R.K., Mason C.A. Defects in specific associations between astroglia and neurons occur in microcultures of weaver mouse cerebellar cells. J. Neurosci. 1984;4:1163–1172. doi: 10.1523/JNEUROSCI.04-04-01163.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heasman S.J., Ridley A.J. Mammalian Rho GTPases: new insights into their functions from in vivo studies. Nat. Rev. Mol. Cell Biol. 2008;9:690–701. doi: 10.1038/nrm2476. [DOI] [PubMed] [Google Scholar]
- Konno D., Yoshimura S., Hori K., Maruoka H., Sobue K. Involvement of the phosphatidylinositol 3-kinase/rac1 and cdc42 pathways in radial migration of cortical neurons. J. Biol. Chem. 2005;280:5082–5088. doi: 10.1074/jbc.M408251200. [DOI] [PubMed] [Google Scholar]
- Kreis P., Thevenot E., Rousseau V., Boda B., Muller D., Barnier J.V. The p21-activated kinase 3 implicated in mental retardation regulates spine morphogenesis through a Cdc42-dependent pathway. J. Biol. Chem. 2007;282:21497–21506. doi: 10.1074/jbc.M703298200. [DOI] [PubMed] [Google Scholar]
- Liebmann T., Renier N., Bettayeb K., Greengard P., Tessier-Lavigne M., Flajolet M. Three-dimensional study of Alzheimer's disease hallmarks using the iDISCO clearing method. Cell Rep. 2016;16:1138–1152. doi: 10.1016/j.celrep.2016.06.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama M., Nakajima D., Yoshimura R., Endo Y., Ohara O. MEGF1/fat2 proteins containing extraordinarily large extracellular domains are localized to thin parallel fibers of cerebellar granule cells. Mol. Cell. Neurosci. 2002;20:563–578. doi: 10.1006/mcne.2002.1146. [DOI] [PubMed] [Google Scholar]
- Nishioka T., Nakayama M., Amano M., Kaibuchi K. Proteomic screening for Rho-kinase substrates by combining kinase and phosphatase inhibitors with 14-3-3zeta affinity chromatography. Cell Struct. Funct. 2012;37:39–48. doi: 10.1247/csf.11044. [DOI] [PubMed] [Google Scholar]
- Rakic P. Neuron-glia relationship during granule cell migration in developing cerebellar cortex. A Golgi and electron microscopic study in Macacus Rhesus. J. Comp. Neurol. 1971;141:283–312. doi: 10.1002/cne.901410303. [DOI] [PubMed] [Google Scholar]
- Renier N., Wu Z., Simon D.J., Yang J., Ariel P., Tessier-Lavigne M. iDISCO: a simple, rapid method to immunolabel large tissue samples for volume imaging. Cell. 2014;159:896–910. doi: 10.1016/j.cell.2014.10.010. [DOI] [PubMed] [Google Scholar]
- Schwamborn J.C., Puschel A.W. The sequential activity of the GTPases Rap1B and Cdc42 determines neuronal polarity. Nat. Neurosci. 2004;7:923–929. doi: 10.1038/nn1295. [DOI] [PubMed] [Google Scholar]
- Scott E.K., Reuter J.E., Luo L. Small GTPase Cdc42 is required for multiple aspects of dendritic morphogenesis. J. Neurosci. 2003;23:3118–3123. doi: 10.1523/JNEUROSCI.23-08-03118.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solecki D.J., Model L., Gaetz J., Kapoor T.M., Hatten M.E. Par6alpha signaling controls glial-guided neuronal migration. Nat. Neurosci. 2004;7:1195–1203. doi: 10.1038/nn1332. [DOI] [PubMed] [Google Scholar]
- Solecki D.J., Trivedi N., Govek E.E., Kerekes R.A., Gleason S.S., Hatten M.E. Myosin II motors and F-actin dynamics drive the coordinated movement of the centrosome and soma during CNS glial-guided neuronal migration. Neuron. 2009;63:63–80. doi: 10.1016/j.neuron.2009.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Excel spreadsheet of protein coding RNAs that changed 1.7-fold or greater in Cdc42 cKO GCPs compared with control GCPs. The gene name, log2-fold change, and adjusted p-value are provided.
Excel spreadsheet of phosphorylation sites that changed 2-fold or greater in Cdc42 cKO GCPs compared with control GCPs. The gene name, protein name, and accession number according to Uniprot are provided. The position of the phosphorylated amino acid in the protein sequence and its identity (S, serine; T, threonine) are also indicated, as well as whether the phosphorylation is upregulated or downregulated. The localization probability (the probability that the identified residue is the correct phosphorylation site) and the matched peptide sequence are provided. All raw LC-MS/MS data and data analysis files have been uploaded to the PRIDE data repository: Project Name: Cdc42 phosphorylation study, Project accession:PXD008148, Username:reviewer79582@ebi.ac.uk, Password: WILJxtep.
The Database for Annotation, Visualization and Integrated Discovery (DAVID) program was used for pathway analysis on proteins with phosphorylation sites that changed 2-fold or greater. The category, term, count (the number of proteins from the 2-fold or greater list of phosphorylation sites in that pathway), percentage (%, the number of proteins from the 2-fold or greater list of phosphorylation sites/total number of proteins in the pathway), p-value, and Benjamini significance are provided.