Summary
We report a case of a 63-year-old man who developed diabetic ketoacidosis (DKA) associated with canagliflozin, a sodium glucose co-transporter 2 (SGLT-2) inhibitor. He presented acutely unwell with a silent myocardial infarction, diverticulitis and DKA with a minimally raised blood glucose level. Standard therapy for DKA was initiated. Despite this, ketonaemia persisted for a total of 12 days after discontinuation of canagliflozin. Glucosuria lasting for several days despite discontinuation of the medications is a recognised phenomenon. However, this is the longest duration of ketonaemia to be reported. The cause of prolonged SGLT-2 inhibition remains uncertain. Deviation from the normal DKA treatment protocol and use of personalised regimens may be required in order to prevent relapse into ketoacidosis while avoiding hypoglycaemia in those that develop this condition.
Learning points:
Diabetic ketoacidosis (DKA) may develop in the presence of lower-than-expected blood glucose levels in patients treated with a sodium glucose co-transporter 2 (SGLT-2) inhibitor.
Certain individuals prescribed with SGLT-2 inhibitors may be more at risk of DKA, for example, those with a low beta cell function reserve, excessive alcohol consumption and a low carbohydrate diet.
In order to reduce the risk of SGLT-2 inhibitor-associated DKA, all patients must be carefully selected before prescription of the medication and appropriately educated.
Increased serum ketone levels and glucosuria have been reported to persist for several days despite discontinuation of their SGLT-2 inhibitor.
Physicians should consider individualised treatment regimens for subjects with prolonged DKA in the presence of SGLT-2 inhibition.
Background
Sodium glucose co-transporter 2 (SGLT-2) inhibitors are the latest guideline-approved therapy for the treatment of type 2 diabetes mellitus (NICE). Their attributes go beyond HbA1c reduction (1). Improvement in weight, blood pressure and cardiovascular outcomes have been reported with the drug class (2). Concerns were raised regarding the occurrence of diabetic ketoacidosis (DKA) in patients taking SGLT-2 inhibitors (3). The precise risk of DKA associated with prescription of the drug still remains uncertain. A meta-analysis of randomised controlled clinical trials reported a negligible effect of the medications on the presence of DKA (4, 5). These patients would be carefully selected and monitored throughout the study and the risk may be higher outside of this environment. In the United States, a claims database of insured patients was analysed to assess the risk of DKA in comparison to prescription of a dipeptidyl peptidase-4 inhibitor (6). Over 50 000 patients were prescribed an SGLT-2 inhibitor. The risk of DKA was doubled for those taking SGLT-2 inhibitors. The presentation of DKA may be atypical with SGLT-2 inhibition. Cases are often hyperglycaemic but may have lower-than-expected glucose levels and patients can even be euglycaemic (7). Most reported cases, even those with euglycaemia, resolve rapidly with standard DKA therapy. However, urinary glucose loss has been reported to persist for several days (8).
Case presentation
We report a case of a 63-year-old white British man who was admitted to a district general hospital in the United Kingdom. The patient had a 23-year duration of type 2 diabetes with micro- and micro-vascular complications including retinopathy, maculopathy and intermittent claudication. He also had moderate chronic obstructive pulmonary disease (COPD), but the patient was independent with his activities of daily living, was an ex-smoker and drank minimal amounts of alcohol. His medications on the day of his admission included metformin, aspirin, simvastatin and twice daily pre-mixed insulin. He was prescribed canagliflozin 7 months prior to hospital admission. His most recent body mass index was 27.2 kg/m2 and HbA1c was 60 mmol/mol.
The patient presented to the Accident and Emergency Department of our district general hospital with a 2-day history of vomiting, diarrhoea, anorexia and right upper quadrant abdominal pain. He had discontinued all medications, including canagliflozin, at the onset of vomiting. Physical observations were respiratory rate: 22 breaths/min, blood pressure: 117/69 mmHg, heart rate: 105 bpm, oxygen saturation: 95% on room air and temperature: 37.1°C. The patient was alert and orientated. Physical examination revealed mild right upper quadrant tenderness with no peritonism.
Investigation
An electrocardiogram revealed antereo-lateral ST segment depression. Finger prick blood glucose was 13.3 mmol/L and 3-hydroxybutyrate 5.2 mmol/L. Venous blood glucose was determined; pH: 7.15 and bicarbonate: 8 mmol/L. Creatinine was mildly raised 79 µmol/L (baseline: 56 µmol/L), troponin I was initially normal but increased to 10 148 (0–40 ng/L) and CRP was significantly elevated at 452 mg/L (0–10 mg/L). A CT scan of the abdomen revealed hepatic flexure diverticulitis. The patient was diagnosed with DKA, silent myocardial infarction and diverticulitis.
Treatment
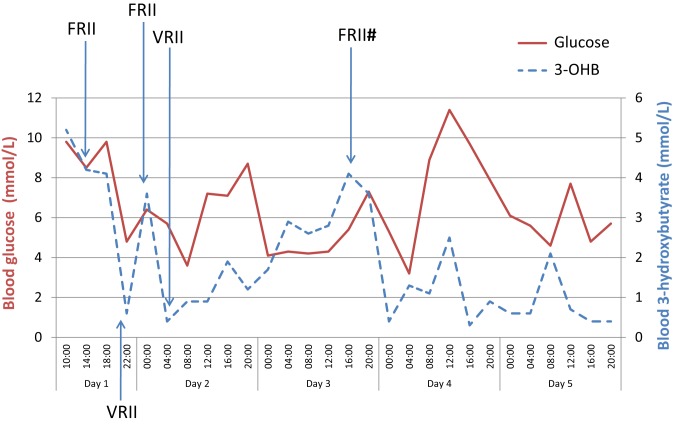
After initial resuscitation, the patient remained haemodynamically stable throughout his admission. He was transferred to a general medical ward and all oral diabetes medications were discontinued. The patient was commenced on standard DKA therapy consistent with the British National Guidelines (9). Therefore, fixed-rate insulin infusion (9 units/h), intravenous fluid therapy and subcutaneous basal insulin were delivered. He was treated with intravenous antibiotics and dual crystalloid oral anti-platelet therapy. Upon improvement of standard DKA parameters (e.g. glucose, pH, bicarbonate and 3-hydroxybutyrate), fixed-rate high-dose insulin was switched to a variable-rate insulin infusion. In view of his euglycaemia, the insulin dose was substantially lower on a variable-rate infusion at approximately 1–2 units/h. As demonstrated in Fig. 1, ketonaemia worsened several times upon reduction of insulin doses. On day 3, he relapsed into DKA despite receiving intravenous insulin on a variable rate infusion. Again, this resolved rapidly on high-dose fixed-rate insulin infusion. He required intravenous insulin until day 5 of his admission before commencing a multiple daily injection regime. Ketonaemia persisted despite euglycaemia and improved clinical status of the patient. Intravenous fluids therapy was delivered and oral diet was maintained throughout. 3-hydroxybutyrate finally fell below 1.0 mmol/L on day 10 of admission, 12 days after discontinuing canagliflozin.
Figure 1.
Blood glucose and blood ketone (3-hydroxybutyrate) levels of the case during the first five days of admission. #The patient relapsed into diabetic ketoacidosis. FRII, fixed-rate insulin infusion; VRII, variable-rate insulin infusion.
Outcome and follow-up
The patient steadily improved during his admission with a reduction in inflammatory markers and no further cardiovascular complications. He was transferred to a tertiary cardiology centre for an angiogram, which revealed triple-vessel disease. Therefore, he was discharged for routine coronary artery bypass grafting surgery. Subsequent investigations revealed normal anti-glutamic acid decarboxylase antibody levels but a low C-peptide level, 69.5 ρmol/L (298–2350 ρmol/L).
Discussion
We report a case of DKA related to SGLT-2 inhibition with significantly prolonged ketonaemia, including a relapse of DKA. The mechanism of typical DKA is well described and occurs as a consequence of an imbalance between insulin and counter-regulatory hormones such as glucagon. In typical hyperglycaemic DKA, ketone bodies are produced in response to a lowered insulin:glucagon ratio and resultant lipolysis (7, 10). Ketone production overwhelms the normal buffering capacity of the body leading to raised anion gap metabolic acidosis. Our understanding of SGLT-2 inhibitor-associated DKA remains incomplete. SGLT-2 inhibition results in elevated ketone levels through a variety of means (11, 12). SGLT-2 inhibition causes glucosuria, lowering blood glucose levels, which leads to a lowering in the insulin:glucagon ratio. SGLT-2 inhibitors may act directly on pancreatic islet cells disturbing this imbalance further. Additionally, renal clearance of ketones may be reduced. DKA may occur in the presence of euglycaemia as glucosuria lowers serum glucose levels (7). Factors that may further disrupt the already altered insulin:glucagon hormone ratio may precipitate DKA (Table 1). Our case was at risk of DKA in view of his long duration of diabetes and beta-cell failure. His severe acute illness and reduced carbohydrate intake then precipitated the presentation. DKA has been described to occur most often in insulin-deficient patients as a result of type 1 diabetes, latent autoimmune diabetes of adulthood and, as in our case, long-standing type 2 diabetes (8). Subjects with beta-cell depletion as well as other at risk individuals may not be suitable for SGLT-2 inhibitors and commencement of treatment must be carefully considered in all patients. Once prescribed, patients should be appropriately educated on DKA prevention and detection. Our case developed DKA despite following ‘sick-day rules’. However, discontinuation of SGLT-2 inhibitors at the onset of illness, before surgery or in the presence of reduced carbohydrate intake may help in reducing the incidence of DKA.
Table 1.
Possible risk factors for the development of SGLT-2 inhibitor-associated DKA (24).
| Surgical procedures |
| Vigorous exercise |
| Acute illness e.g. myocardial infarction, infection |
| Prolonged fasting |
| Beta cell failure i.e. LADA, type 1 diabetes and prolonged type 2 diabetes |
| Dehydration |
| Reduction in dosing or discontinuation of insulin |
| Weight loss |
| Alcohol |
DKA, diabetic ketoacidosis; LADA, latent-onset autoimmune diabetes in adults; SGLT-2, sodium glucose co-transporter.
Case reporting of SGLT-2 inhibitor-associated DKA is often of variable quality (8). Cases have been reported with ketosis but not frank acidosis and the inpatient course of the patient is infrequently discussed. Reports define illness resolution with inconsistency making it difficult to precisely compare our case with others in the literature. However, to the knowledge of the authors, we present the longest duration of ketonaemia associated with SGLT-2 inhibitors. It is recognised that urinary loss of glucose may persist beyond the 10- to 13-h half-life of SGLT-2 inhibitors (13). Although many cases of DKA rapidly resolve with standard treatment (3, 14), there are several which are much more resistant. Euglycaemic DKA has been reported to last 36 h (15, 16), 48 h (17, 18, 19) and 72 h (20, 21). Further cases describe an even more prolonged disease course. ‘Metabolic recovery’ has been reported to take as long as 6 days since SGLT-2 inhibitor discontinuation, although the specific blood results to define resolution were not provided (3). Glucosuria has been described to persist 11 days after discontinuation of SGLT-2 inhibitor treatment despite normal serum glucose levels (22). Ketonaemia has been reported to take as long as 10 days to resolve, since discontinuation of SGLT-2 inhibitor treatment (23). However, in this case, insulin was not commenced until several days into the admission, which will have contributed to prolonged ketonaemia.
Based on the small number of cases, it is premature to conclude that prolonged ketonaemia is directly attributable to SGLT-2 inhibitors. It seems biologically plausible due to the fact that all cases had euglycaemia and significant glucosuria. SGLT-2 inhibitors are recognised to cause glucosuria for several days after discontinuation (8, 24). Therefore, the blood glucose lowering, insulin:glucagon imbalance and ketogenic effect of SGLT-2 inhibitors may also be extended. The reason why SGLT-2 inhibition is prolonged in certain cases is unclear but warrants further study. In the reported cases of prolonged glucosuria or ketonaemia, there was no clear factor linking all the cases. Age, HbA1c, duration of diabetes, presence of beta-cell failure, precipitating event and medications all varied in these cases. All of the three most commonly used medications were implicated: dapagliflozin (23), empaglaflozin (16, 19) and canagliflozin (3, 15, 17, 21, 22). Unfortunately, we did not monitor urinary glucose levels but would expect them to be elevated as a result of prolonged SGLT-2 inhibition.
Reduction in insulin dosing is a known precipitant of SGLT-2 inhibitor-associated DKA. Therefore, as described in our case, a rise in ketonaemia is perhaps expected on reduction in intravenous insulin dose if SGLT-2 inhibition is still present. In the event of similar cases of prolonged SGLT-2 inhibitor action, personalised DKA therapy may be required. In DKA associated with SGLT-2 inhibition, fixed-rate insulin combined with 10% dextrose infusions may be necessary to suppress ketone levels while preventing hypoglycaemia. Careful blood glucose and ketone monitoring is required. Reduction in insulin dosing may need to be performed in a stepwise fashion rather than the significant reduction that will occur when changing from fixed-rate to variable-rate infusions as per DKA guidelines. In the future, detailed reporting of the hospital course of atypical DKA cases may inform on changes in guidelines in SGLT-2 inhibition.
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Funding
This research did not receive any specific grant from any funding agency in the public, commercial or not-for-profit sector.
Patient consent
Written consent has been obtained.
Author contribution statement
Gordon Sloan was the primary author. Tania Kakoudaki was the co-author and assisted with the writing of the manuscript, editing and creation of the figure. Nishant Ranjan was the physician responsible for the care of the patient and had oversight of the manuscript creation.
References
- 1.Ferrannini E, Solini A. SGLT2 inhibition in diabetes mellitus: rationale and clinical prospects. Nature Reviews Endocrinology 2012. 8 495–502. ( 10.1038/nrendo.2011.243) [DOI] [PubMed] [Google Scholar]
- 2.Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, et al Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. New England Journal of Medicine 2015. 373 2117–2128. ( 10.1056/NEJMoa1504720) [DOI] [PubMed] [Google Scholar]
- 3.Peters AL, Buschur EO, Buse JB, Cohan P, Diner JC, Hirsch IB. Euglycemic diabetic ketoacidosis: a potential complication of treatment with sodium-glucose cotransporter 2 inhibition. Diabetes Care 2015. 38 1687–1693. ( 10.2337/dc15-0843) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Monami M, Nreu B, Zannoni S, Lualdi C, Mannucci E. Effects of SGLT-2 inhibitors on diabetic ketoacidosis: a meta-analysis of randomised controlled trials. Diabetes Research and Clinical Practice 2017. 130 53–60. ( 10.1016/j.diabres.2017.04.017) [DOI] [PubMed] [Google Scholar]
- 5.Neal B, Perkovic V, Matthews DR. Canagliflozin and cardiovascular and renal events in type 2 diabetes. New England Journal of Medicine 2017. 377 2099. [DOI] [PubMed] [Google Scholar]
- 6.Fralick M, Schneeweiss S, Patorno E. Risk of diabetic ketoacidosis after initiation of an SGLT2 inhibitor. New England Journal of Medicine 2017. 376 2300–2302. ( 10.1056/NEJMc1701990) [DOI] [PubMed] [Google Scholar]
- 7.Rosenstock J, Ferrannini E. Euglycemic diabetic ketoacidosis: a predictable, detectable, and preventable safety concern with SGLT2 inhibitors. Diabetes Care 2015. 38 1638–1642. ( 10.2337/dc15-1380) [DOI] [PubMed] [Google Scholar]
- 8.Handelsman Y, Henry RR, Bloomgarden ZT, Dagogo-Jack S, DeFronzo RA, Einhorn D, Ferrannini E, Fonseca VA, Garber AJ, Grunberger G, et al American Association of Clinical Endocrinologists and American College of Endocrinology position statement on the association of SGLT-2 inhibitors and diabetic ketoacidosis. Endocrine Practices 2016. 22 753–762. ( 10.4158/EP161292.PS) [DOI] [PubMed] [Google Scholar]
- 9.Savage MW, Dhatariya KK, Kilvert A, Rayman G, Rees JA, Courtney CH, Hilton L, Dyer PH, Hamersley MS. & Joint British Diabetes Societies. Joint British Diabetes Societies guideline for the management of diabetic ketoacidosis. Diabetic Medicine 2011. 28 508–515. ( 10.1111/j.1464-5491.2011.03246.x) [DOI] [PubMed] [Google Scholar]
- 10.Laffel L. Ketone bodies: a review of physiology, pathophysiology and application of monitoring to diabetes. Diabetes/Metabolism Research and Reviews 1999. 15 412–426. () [DOI] [PubMed] [Google Scholar]
- 11.Ferrannini E, Baldi S, Frascerra S, Astiarraga B, Heise T, Bizzotto R, Mari A, Pieber TR, Muscelli E. Shift to fatty substrate utilization in response to sodium-glucose cotransporter 2 inhibition in subjects without diabetes and patients with type 2 diabetes. Diabetes 2016. 65 1190–1195. ( 10.2337/db15-1356) [DOI] [PubMed] [Google Scholar]
- 12.Qiu H, Novikov A, Vallon V. Ketosis and diabetic ketoacidosis in response to SGLT2 inhibitors: basic mechanisms and therapeutic perspectives. Diabetes/Metabolism Research and Reviews 2017. 33 Epub. ( 10.1002/dmrr.2886) [DOI] [PubMed] [Google Scholar]
- 13.Scheen AJ. Pharmacokinetics, pharmacodynamics and clinical use of SGLT2 inhibitors in patients with type 2 diabetes mellitus and chronic kidney disease. Clinical Pharmacokinetics 2015. 54 691–708. ( 10.1007/s40262-015-0264-4) [DOI] [PubMed] [Google Scholar]
- 14.Farjo PD, Kidd KM, Reece JL. A case of euglycemic diabetic ketoacidosis following long-term empagliflozin therapy. Diabetes Care. 2016. 39 e165–e166. ( 10.2337/dc16-0728) [DOI] [PubMed] [Google Scholar]
- 15.Andrews TJ, Cox RD, Parker C, Kolb J. Euglycemic diabetic ketoacidosis with elevated acetone in a patient taking a sodium-glucose cotransporter-2 (SGLT2) inhibitor. Journal of Emergency Medicine 2017. 52 223–226. ( 10.1016/j.jemermed.2016.07.082) [DOI] [PubMed] [Google Scholar]
- 16.Roach P, Skierczynski P. Euglycemic diabetic ketoacidosis in a patient with type 2 diabetes after treatment with empagliflozin. Diabetes Care 2016. 39 e3 ( 10.2337/dc15-1797) [DOI] [PubMed] [Google Scholar]
- 17.González Sanchidrián S, Gómez-Martino Arroyo JR, Labrador Gómez PJ. Diabetic ketoacidosis associated to canagliflozin in type 2 diabetes. Medicina Clínica 2017. 148 e19–e20. ( 10.1016/j.medcli.2016.11.016) [DOI] [PubMed] [Google Scholar]
- 18.Hayami T, Kato Y, Kamiya H, Kondo M, Naito E, Sugiura Y, Kojima C, Sato S, Yamada Y, Kasagi R, et al Case of ketoacidosis by a sodium-glucose cotransporter 2 inhibitor in a diabetic patient with a low-carbohydrate diet. Journal of Diabetes Investigation 2015. 6 587–590. ( 10.1111/jdi.12330) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Candelario N, Wykretowicz J. The DKA that wasn’t: a case of euglycemic diabetic ketoacidosis due to empagliflozin. Oxford Medical Case Reports 2016. 2016 144–146. ( 10.1093/omcr/omw061) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Adachi J, Inaba Y, Maki C. Euglycemic diabetic ketoacidosis with persistent diuresis treated with canagliflozin. Internal Medicine 2017. 56 187–190. ( 10.2169/internalmedicine.56.7501) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gelaye A, Haidar A, Kassab C, Kazmi S, Sinha P. Severe ketoacidosis associated with canagliflozin (invokana): a safety concern. Case Reports in Critical Care 2016. 2016 1656182 ( 10.1155/2016/1656182) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kelmenson DA, Burr K, Azhar Y, Reynolds P, Baker CA, Rasouli N. Euglycemic diabetic ketoacidosis with prolonged glucosuria associated with the sodium-glucose cotransporter-2 canagliflozin. Journal of Investigative Medicine High Impact Case Reports 2017. 5 2324709617712736 ( 10.1177/2324709617712736) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pujara S, Ioachimescu A. Prolonged ketosis in a patient with euglycemic diabetic ketoacidosis secondary to dapagliflozin. Journal of Investigative Medicine High Impact Case Reports 2017. 5 2324709617710040 ( 10.1177/2324709617710040) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goldenberg RM, Berard LD, Cheng AY, Gilbert JD, Verma S, Woo VC, Yale JF. SGLT2 inhibitor-associated diabetic ketoacidosis: clinical review and recommendations for prevention and diagnosis. Clinical Therapeutics 2016. 38 2654.e1–2664.e1. ( 10.1016/j.clinthera.2016.11.002) [DOI] [PubMed] [Google Scholar]



 This work is licensed under a
This work is licensed under a