Short abstract
Therapies treating psoriasis can be categorized into five classes according to their mechanism: anti-metabolites (AM), anti-interleukin-12/23 agents (anti-IL12/23), anti-interleukin-17 agents (anti-IL17), anti-T-cell agent (ANT), and anti-tumor necrosis factor-α agent (anti-TNF-α). This network meta-analysis (NMA) aimed to give a quantitative and systemic evaluation of safety and efficacy for the five kinds of therapies mentioned above. Odds ratios and mean differences were calculated to evaluate binary and continuous outcomes, respectively. Forest plots were conducted to show the performance of pair-wise comparison of above therapies in each outcome, and surface under the cumulative ranking curves was given to evaluate the relative ranking of above therapies in each outcome. Node splitting was conducted to evaluate the consistency between direct and indirect evidence. Direct comparisons from 65 studies (32,352 patients) were included in this NMA. Our results showed an excellent efficacy of anti-IL12/23 and anti-IL17. However, these two therapies and anti-TNF-α were revealed to have a high possibility to cause adverse effects (AEs) such as infections. Additionally, node splitting showed that no inconsistency appeared between the direct and indirect comparisons. Anti-IL12/23 was the most recommended therapy according to this NMA. Anti-IL17 had similar efficacy to anti-IL12/23 but should be applied with caution since it has poor performance in safety outcomes.
Keywords: Psoriasis, network meta-analysis, efficacy, safety
Introduction
Psoriasis, characterized by quick and excessive growth of the skin’s epidermal layer,1 is a common, chronic, and systemic disease, affecting 1–3% of the world population. It is widely considered as a genetic disease and could be affected by some environmental factors.2 Up to now, various therapies are available for psoriasis, including phototherapy, topical treatment, systemic therapies, and biologic drugs.3
The biologic drugs can be classified into five classes based on their mechanism: anti-metabolites (AM), anti-tumor necrosis factor-α agent (anti-TNF-α), anti-T-cell agent (ANT), anti-interleukin-12/23 agents (anti-IL12/23), and anti-interleukin-17 agents (anti-IL17). It is reported that methotrexate, an AM, has been applied as a valid systemic treatment for psoriasis patients over 48 years.4 However, it is relevant to hepatotoxicity and myelosuppression.5 Besides, the TNF is widely regarded as an important cytokine involved in the pathophysiology of psoriasis. Therefore, monoclonal antibodies, such as adalimumab and infliximab, which antagonized TNF, were applied in the treatment of psoriasis.6,7
Relevant studies showed that psoriasis was possibly an autoimmune disease where the activation of skin-directed T-cells performed an important role.8 Alefacept, a recombinant protein, has the ability to block T-cell’s proliferation and activation by combining with CD2 on the surface of T-cells. Alefacept can also induce selective CD45RO+ T-cell apoptosis by interacting with the immunoglobulin receptors FcγRIII on the accessory cells.9 Other drugs, such as efalizumab and itolizumab, are humanized monoclonal antibodies which could directly deal with the pathogenic T-cells by binding to CD11a or CD6 and inhibit T-cell functions, such as activation, trafficking, and migration.10–12
Meanwhile, it has been discovered that TNF is produced by the immune pathways stimulated by two interleukins, interleukin-12 (IL-12) and interleukin-23 (IL-23).13 This discovery indicates that both IL12 and IL23 play a pivotal role in the psoriasis development. In addition, IL-17A and IL-17RA were also found related to the disease severity because of the elevated levels of IL-17A in the diseased skin and blood of patients with psoriasis.14 Etanercept, a human fusion TNF soluble receptor, is used to prevent the TNF-mediated inflammatory response and applied for the therapy of psoriatic arthritis and chronic plaque.15
To make an effective and safe decision in treatment of psoriasis, it is necessary to conduct reliable evidences of comparison among these drugs. A number of traditional meta-analysis studies had been done to make comparison between two therapies, which indicated that all the therapies are more effective than placebo (PBO). But they cannot compare several therapies simultaneously. Therefore, the network meta-analysis (NMA) is required to synthesize all valuable evidences from randomized control trials (RCTs), combining both direct and indirect evidences, to convincingly draw the conclusions about competitive efficacy and safety information.
Woolacott et al. made the comparisons among three biological therapies, efalizumab, infliximab, and etanercept, and two nonbiological therapies.16 Adalimumab was taken into consideration in the work of Bansback et al.17 After then, with the development of biological treatment, more NMA were conducted, such as the works of Reich et al.18 and Lin et al.,19 who added the ustekinumab, which antagonizes IL-12/23p40. Besides, Nast et al.20 assessed the efficacy and safety of treatments of systemic long-term treatments. Recently, Gomez-Garcia et al.21 used the new 2015 PRISMA statement for the NMA and evaluated the comparative short-term efficacy and tolerance of the agents. Jabbar-Lopez et al. established the relative efficacy and tolerability of six monoclonal antibodies. None of them compared the biotics from the level of large classes.
The primary objective of our study was to give an extension to the existing NMAs to evaluate the efficacy and safety of different treatment agents. More agents were taken in account to provide more reliable conclusion. Moreover, the ranking possibility in specific efficacy and safety were also presented to help making optimal decision in clinical drug using. Besides, no NMA similar to this study, with sufficient samples and consideration of all therapies, had been done yet.
Materials and methods
Search strategy
To get the relevant studies, the following three electronic databases were taken into our retrieval: Chinese National Knowledge Infrastructure, PubMed, and Embase. Regardless of the limitation of language, key terms “psoriasis,” “antimetabolites,” “macrolides,” “antibodies, monoclonal,” “etanercept” as well as their acronyms were searched in this work. Besides, the reference lists were examined to identify the potentially available studies.
Inclusion and exclusion criteria
All included trials must satisfy the following criteria: (i) the studies must be RCTs; (ii) the patients involved in the studies must be diagnosed as psoriasis; and (iii) relevant outcomes should be contained. Besides, duplicate RCTs or the studies with isolated comparison were excluded.
Outcome measure and data extraction
Data extraction was conducted by two reviewers independently, and following characteristics of each study were extracted from the original documents: (i) the basic information, including the first author, country, published year, and blinding; (ii) the patients characteristics, including ages, gender ratio, and disease duration; (iii) efficacy outcomes, including the Psoriasis Area and Severity Index (PASI), Dermatology Life Quality Index (DLQI), and Physician’s Global Assessment (PGA); (iv) safety outcomes, including the incidence of all AEs, infection, nasopharyngitis, headache, and upper respiratory tract infection (URTI).
Statistical analysis
Odds ratio (OR) with corresponding 95% credible interval (CrI) were used to evaluate the binary outcomes. Mean difference with corresponding 95% CrI were applied to assess the continuous outcomes. Meanwhile, forest plots were drawn to visually present the relative efficacy and safety of different comparisons for each outcome. Consistency between direct and indirect comparison was analyzed by the node-splitting method. p<0.05 indicated a significant inconsistency for a specific comparison. Moreover, surface under the cumulative ranking curves (SUCRAs) were calculated to present the ranking probability of each treatment to find the relatively optimal treatment to improve the efficacy and decrease the incidence of adverse events. Software R (version 3.2.3) and STATA (version 13.0) were used to implement the NMA.
Results
Included studies
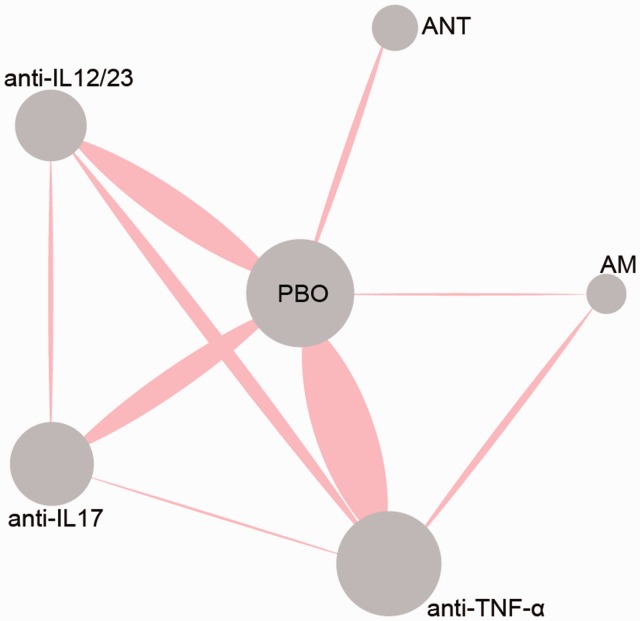
In the retrieval, 1562 records were identified at the beginning, and 17 records were added manually. Among them, 573 duplicates were removed, and 989 records were left. After 242 records excluded during screening, 432 records were full-text assessed. Finally, 75 studies and 25,108 patients were included in our NMA.4–6,9–14,22–87 The flow chart was shown in Figure 1. The details of specific treatment, the characteristics of patients, and outcomes of each trail were provided in Table 1. The study sample sizes ranged from 33 to 1831. The follow-up period ranged from six weeks to 120 weeks. Among 58 trails, five trails failed to provide age range of the patients,27,29,49,59,62,70 while six trails failed to provide gender ratios of the patients.14,27,49,59,70,86 The mean disease duration was 17.5 years (range 5.6–22.8). Besides, disease severity was assessed containing all the trails with a baseline PASI score of 19.9 (range 5.5–33.1) and a body surface area (BSA) of 28.8% (range 5.1–49.8). Jadad score of included RCTs was shown in Table S1. Meanwhile, the network diagram was shown in Figure 2. The area of dots represents the number of patients in the therapy, and the width of lines stands for the number of references including the comparison.
Figure 1.
PRISMA flow chart.
RCTs: randomized control trials.
Table 1.
Baseline population characteristics of included studies.
| Author | Year | Country | Follow-up | Type | Intervention | N | Age | Male (%) | Disease | HPA | Affected BSA (%) | PASI |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Duration (years) | ||||||||||||
| Papp | 2008 | Canada | 12 weeks | anti-IL12/23 | Ustekinumab | 820 | 45.1 ± 12.1 | 69.2 | 19.3 ± 11.7 | 26.2 | 25.9 ± 15.5 | 19.4 ± 6.8 |
| PBO | Placebo | 410 | 47.0 ± 12.5 | 69 | 20.8 ± 12.2 | 25.6 | 26.1 ± 17.4 | 19.4 ± 7.5 | ||||
| Revicki | 2008 | USA | 16 weeks | anti-TNF-α | Adalimumab | 103 | 42.8 ± 12.3 | 64.1 | 17.6 ± 10.0 | 22.3 | 33.7 ± 20.0 | 20.1 ± 7.4 |
| AM | Methotrexate | 108 | 41.9 ± 11.9 | 66.7 | 19.0 ± 10.3 | 17.6 | 32.6 ± 20.7 | 19.5 ± 7.4 | ||||
| PBO | Placebo | 53 | 40.7 ± 11.4 | 67 | 18.9 ± 8.71 | 20.8 | 28.4 ± 16.1 | 19.2 ± 6.9 | ||||
| Blauvelt | 2015 | USA | 12 weeks | anti-IL17 | Secukinumab | 118 | 45.1 ± 12.6 | 64.4 | 18.0 ± 11.9 | – | 33.3 ± 17.9 | 20.7 ± 7.9 |
| PBO | Placebo | 59 | 46.5 ± 14.1 | 66.1 | 20.2 ± 14.2 | – | 32.2 ± 17.4 | 21.1 ± 8.5 | ||||
| Dubertret | 2006 | Germany | 12 weeks | ANT | Efalizumab | 529 | 44.0 ± 12.0 | 67.3 | 19.3 ± 11.5 | – | 37.1 ± 20.2 | 23.6 ± 6.7 |
| PBO | Placebo | 264 | 45.3 ± 12.1 | 67.4 | 21.0 ± 10.2 | – | 36.2 ± 20.7 | 23.0 ± 9.6 | ||||
| Barker | 2011 | UK | 16 weeks | anti-TNF-α | Infliximab | 653 | 44.1 ± 33.9 | 67 | 18.8 ± 11.6 | – | 31.9 ± 16.5 | 21.4 ± 8.0 |
| AM | Methotrexate | 215 | 41.9 ± 27.1 | 69 | 17.0 ± 10.3 | – | 31.0 ± 15.0 | 21.1 ± 7.6 | ||||
| Asahina | 2010 | Japan | 16 weeks | anti-TNF-α | Adalimumab | 123 | 47.7 ± 12.8 | 84.2 | 14.2 ± 9.29 | – | 43.3 ± 19.4 | 25.4 ± 8.9 |
| PBO | Placebo | 46 | 43.9 ± 10.7 | 89.1 | 15.5 ± 8.83 | – | 46.7 ± 19.9 | 29.1 ± 11.8 | ||||
| Chaudhari | 2001 | USA | 10 weeks | anti-TNF-α | Infliximab | 22 | 35.1 ± 11.2 | 72.7 | – | – | – | 26.6 ± 10.3 |
| PBO | Placebo | 11 | 45.0 ± 12.0 | 72.7 | – | – | – | 20.3 ± 5.5 | ||||
| Gordon | 2003 | USA | 12 weeks | ANT | Efalizumab | 369 | 45.2 ± 14.3 | 68 | 19.1 ± 15.2 | – | 28.0 ± 21.2 | 19 ± 6.9 |
| PBO | Placebo | 187 | 45.7 ± 13.7 | 71 | 19.0 ± 13.0 | – | 27.0 ± 20.0 | 19 ± 6.7 | ||||
| Gottlieb | 2004 | USA | 10 weeks | anti-TNF-α | Infliximab | 198 | 44.1 ± 14.1 | 73.7 | 16.0 ± 11.1 | 29.3 | – | – |
| PBO | Placebo | 51 | 45.0 ± 16.3 | 60.8 | 16.0 ± 11.8 | 33.3 | – | – | ||||
| Gottlieb | 2011 | USA | 12 weeks | anti-IL12/23 | Briakinumab | 138 | 43.6 ± 14.3 | 64.5 | 16.1 ± 12.5 | 19.6 | 23.6 ± 16.6 | 18.4 ± 7.2 |
| anti-TNF-α | Etanercept | 141 | 43.1 ± 12.5 | 69.5 | 17.0 ± 12.7 | 22.7 | 24.1 ± 15.0 | 19.4 ± 8.0 | ||||
| PBO | Placebo | 68 | 44.0 ± 13.6 | 69.1 | 19.1 ± 13.2 | 20.6 | 23.8 ± 15.5 | 18.5 ± 6.9 | ||||
| Gottlieb | 2003 | USA | 24 weeks | anti-TNF-α | Etanercept | 57 | 48.2 ± 11.7 | 58 | – | 28 | – | – |
| PBO | Placebo | 55 | 46.5 ± 14.7 | 67 | – | 35 | – | – | ||||
| Menter | 2008 | USA | 12 weeks | anti-TNF-α | Adalimumab | 814 | 44.1 ± 13.2 | 67.1 | 18.1 ± 11.9 | 27.5 | 25.8 ± 15.5 | 19.0 ± 7.1 |
| PBO | Placebo | 398 | 45.4 ± 13.4 | 64.6 | 18.4 ± 11.9 | 28.4 | 25.6 ± 14.8 | 18.8 ± 7.1 | ||||
| Ohtsuki | 2014 | Japan | 12 weeks | anti-IL17 | Secukinumab | 58 | 51.9 ± 11.8 | 89.7 | 15.6 ± 10.3 | 13.8 | 42.0 ± 23.4 | 26.7 ± 10.5 |
| PBO | Placebo | 29 | 50.2 ± 13.6 | 79.3 | 14.1 ± 10.9 | 13.8 | 32.7 ± 16.9 | 21.4 ± 10.3 | ||||
| Ortonne | 2003 | France | 24 weeks | ANT | Alefacept | 339 | – | – | 19.0 ± 17.0 | – | 20.0 ± 19.7 | 13.0 ± 12.2 |
| PBO | Placebo | 168 | – | – | 20.0 ± 18.5 | – | 23.5 ± 20.7 | 14.0 ± 9.7 | ||||
| Leonardi | 2003 | USA | 12 weeks | anti-TNF-α | Etanercept | 486 | 44.8 ± 0.8 | 65 | 18.6 ± 10.9 | – | 29.9 ± 10.6 | 18.4 ± 6.7 |
| PBO | Placebo | 166 | 45.6 ± 1.0 | 63 | 18.4 ± 10.9 | – | 28.8 ± 10.4 | 18.3 ± 6.6 | ||||
| Paul | 2015 | France | 12 weeks | anti-IL17 | Secukinumab | 121 | 46.6 ± 14.2 | 76.7 | 21.0 ± 13.5 | 23.3 | 26.4 ± 12.8 | 18.9 ± 6.4 |
| PBO | Placebo | 61 | 43.7 ± 12.7 | 62.3 | 19.9 ± 12.2 | 19.7 | 25.7 ± 14.7 | 19.4 ± 6.7 | ||||
| Krueger | 2002 | USA | 12 weeks | ANT | Alefacept | 367 | 45.4 ± 15.8 | 71 | – | – | – | – |
| PBO | Placebo | 186 | 45 ± 14.5 | 68 | – | – | – | – | ||||
| Mease | 2000 | USA | 12 weeks | anti-TNF-α | Etanercept | 30 | 46.0 ± 10.0 | 53 | 19.0 ± 7.5 | 100 | – | – |
| PBO | Placebo | 30 | 43.5 ± 9.7 | 60 | 17.5 ± 7.2 | 100 | – | – | ||||
| Feldman | 2005 | USA | 10 weeks | anti-TNF-α | Infliximab | 198 | – | – | – | – | – | – |
| PBO | Placebo | 51 | – | – | – | – | – | – | ||||
| Menter | 2005 | USA | 12 weeks | ANT | Efalizumab | 369 | 45.3 ± 14.2 | 68 | 19.3 ± 15.2 | – | – | – |
| PBO | Placebo | 187 | 44.9 ± 11.4 | 70.6 | 19.3 ± 13.0 | – | – | – | ||||
| Leonardi | 2005 | USA | 12 weeks | ANT | Efalizumab | 328 | 45.5 ± 13.5 | 71.1 | 16.7 ± 14.7 | – | 29.9 ± 18.2 | 18.9 ± 11.4 |
| PBO | Placebo | 170 | 41.7 ± 12.5 | 72.9 | 18.5 ± 13.7 | – | 29.4 ± 18.7 | 19.0 ± 12.0 | ||||
| Papp | 2006 | Canada | 12 weeks | ANT | Efalizumab | 450 | 45.6 ± 12.5 | 67.3 | 18.4 ± 12.1 | – | 27.7 ± 15.8 | 19.14 ± 7.5 |
| PBO | Placebo | 236 | 46.3 ± 12.1 | 59.3 | 17.5 ± 11.1 | – | 26.8 ± 15.2 | 18.69 ± 7.0 | ||||
| Kimball | 2008 | USA | 12 weeks | anti-IL12/23 | Briakinumab | 150 | 46.0 ± 15.0 | 77 | 18.0 ± 10.9 | 30 | 23.0 ± 12.6 | 19.0 ± 6.3 |
| PBO | Placebo | 30 | 49.0 ± 14.4 | 73 | 21.0 ± 12.4 | 30 | 21.0 ± 9.21 | 16.0 ± 2.9 | ||||
| Reich | 2005 | Germany | 10 weeks | anti-TNF-α | Infliximab | 301 | 42.6 ± 11.7 | 69 | 19.1 ± 11.0 | 31 | 34.1 ± 19.0 | 22.9 ± 9.3 |
| PBO | Placebo | 77 | 43.8 ± 12.6 | 79 | 17.3 ± 11.1 | 29 | 33.5 ± 18.0 | 22.8 ± 8.7 | ||||
| Tyring | 2006 | USA | 12 weeks | anti-TNF-α | Etanercept | 311 | 45.8 ± 12.8 | 65 | 20.1 ± 12.3 | 35 | 27.2 ± 18.2 | 18.3 ± 7.6 |
| PBO | Placebo | 307 | 45.6 ± 12.1 | 70 | 19.7 ± 11.4 | 33 | 27.2 ± 17.2 | 18.1 ± 7.4 | ||||
| Lebwohl | 2003 | USA | ANT | Alefacept | 339 | 45.3 ± 14.7 | 62 | 19.0 ± 17.0 | – | 20.0 ± 19.7 | 13.2 ± 12.3 | |
| PBO | Placebo | 168 | 46.5 ± 15.0 | 65 | 20.0 ± 18.5 | – | 23.5 ± 20.7 | 14.3 ± 9.9 | ||||
| Papp | 2005 | Canada | 12 weeks | anti-TNF-α | Etanercept | 390 | 44.5 ± 14.7 | 67 | 18.1 ± 14.9 | 26 | 25.0 ± 17.5 | 16.1 ± 12.6 |
| PBO | Placebo | 193 | 44.0 ± 15.5 | 64 | 17.5 ± 12.4 | 26 | 20.0 ± 21.2 | 16.0 ± 13.8 | ||||
| Torii | 2010 | Japan | 10 weeks | anti-TNF-α | Infliximab | 35 | 46.9 ± 13.0 | 62.9 | 14.2 ± 8.91 | 28.6 | – | 31.9 ± 12.8 |
| PBO | Placebo | 19 | 43.3 ± 12.3 | 73.7 | 11.1 ± 6.51 | 36.8 | – | 33.1 ± 15.6 | ||||
| Menter | 2007 | USA | anti-TNF-α | Infliximab | 627 | 44.5 ± 13.0 | 65 | 19.1 ± 11.7 | 28.3 | 28.7 ± 16.4 | 20.4 ± 7.5 | |
| PBO | Placebo | 208 | 44.4 ± 12.5 | 69.2 | 17.8 ± 10.8 | 26 | 28.4 ± 17.6 | 19.8 ± 7.7 | ||||
| Igarashi | 2012 | Japan | 12 weeks | anti-IL12/23 | Ustekinumab | 126 | – | 75.8 | 17.3 ± 10.7 | 11.3 | 46.6 ± 19.7 | 28.7 ± 11.2 |
| PBO | Placebo | 32 | – | 83.9 | 16.0 ± 11.2 | 3.1 | 49.8 ± 22.5 | 30.3 ± 11.8 | ||||
| Gordon | 2006 | USA | 12 weeks | anti-TNF-α | Adalimumab | 95 | 44.0 ± 15.5 | 66 | 18.0 ± 11.5 | 24 | 25.0 ± 19.5 | 14.5 ± 10.0 |
| PBO | Placebo | 52 | 43.0 ± 12.5 | 65 | 19.0 ± 9.72 | 31 | 28.0 ± 17.0 | 16.0 ± 8.725 | ||||
| Krueger | 2007 | USA | 12 weeks | anti-IL12/23 | – | 256 | 44.0 ± 13.0 | 81 | 17.3 ± 13.5 | 20 | 27.4 ± 18.1 | 19.0 ± 7.9 |
| PBO | Placebo | 64 | 44.0 ± 14.0 | 72 | 16.9 ± 11.0 | 19 | 26.6 ± 18.4 | 19.9 ± 8.3 | ||||
| Saurat | 2008 | Switzerland | 16 weeks | anti-TNF-α | Adalimumab | 108 | 42.9 ± 12.6 | 64.8 | 17.9 ± 10.1 | 21.3 | – | 20.2 ± 7.5 |
| AM | Methotrexate | 110 | 41.6 ± 12.0 | 66.4 | 18.9 ± 10.2 | 17.3 | – | 19.4 ± 7.4 | ||||
| PBO | Placebo | 53 | 40.7 ± 11.4 | 66 | 18.8 ± 8.70 | 20.8 | – | 19.2 ± 6.9 | ||||
| van de Kerkhof | 2008 | Netherlands | 12 weeks | anti-TNF-α | Etanercept | 96 | 45.9 ± 12.8 | 61.5 | 19.3 ± 11.3 | 15.6 | 26.5 ± 15.0 | 21.4 ± 9.3 |
| PBO | Placebo | 46 | 43.6 ± 12.6 | 54.4 | 17.3 ± 8.20 | 10.9 | 30.3 ± 17.8 | 21.0 ± 8.7 | ||||
| Landells | 2015 | Multi | 12 weeks | anti-IL12/23 | Ustekinumab | 73 | 14.8 ± 1.7 | 44.4 | 5.60 ± 3.80 | – | 31.9 ± 23.2 | 21.7 ± 10.4 |
| PBO | Placebo | 37 | 15.6 ± 1.5 | 54.1 | 6.20 ± 5.00 | – | 27.4 ± 16.4 | 20.8 ± 8.0 | ||||
| Poulin | 2014 | Canada | 16 weeks | anti-TNF-α | Adalimumab | 49 | 49.0 ± 11.4 | 42.9 | 14.9 ± 16.3 | 14.3 | 8.90 ± 11.9 | 8.8 ± 8.2 |
| PBO | Placebo | 23 | 54.8 ± 11.4 | 34.8 | 11.5 ± 9.94 | 4.3 | 5.10 ± 6.96 | 5.7 ± 4.5 | ||||
| Langley | 2014 | Canada | 12 weeks | anti-IL17 | Secukinumab | 490 | 44.9 ± 13.5 | 69 | 17.4 ± 11.1 | 23.3 | 32.8 ± 19.3 | 22.5 ± 9.2 |
| PBO | Placebo | 248 | 45.4 ± 12.6 | 69.4 | 17.3 ± 12.4 | 27.4 | 29.7 ± 15.9 | 21.4 ± 9.1 | ||||
| Ellis | 2001 | USA | 12 weeks | ANT | Alefacept | 170 | 44.0 ± 11.5 | 72.4 | 18.0 ± 15.0 | – | 25.0 ± 18.75 | 20.0 ± 11.5 |
| PBO | Placebo | 59 | 42.0 ± 12.2 | 59.3 | 18.0 ± 9.75 | – | 20.0 ± 17.5 | 15.0 ± 17.2 | ||||
| Papp | 2001 | Canada | 8 weeks | ANT | hu1124 | 97 | 44.5 ± 12.9 | 63 | 22.8 ± 12.6 | – | 29.4 ± 13.9 | 19.1 ± 7.3 |
| PBO | Placebo | 48 | 42.3 ± 12.3 | 67 | 17.8 ± 10.0 | – | 21.5 ± 10.4 | 16.2 ± 4.4 | ||||
| Nakagawa | 2016 | Multi | 12 weeks | anti-IL17 | Brodalumab | 113 | 46.4 ± 11.8 | 78.4 | 14.9 ± 10.9 | 13.5 | 43.7 ± 25.9 | 27.9 ± 14.3 |
| PBO | Placebo | 38 | 46.6 ± 10.8 | 71.1 | 16.8 ± 11.4 | 18.4 | 37.8 ± 21.4 | 23.9 ± 8.9 | ||||
| Gordon | 2014 | USA | 16 weeks | anti-TNF-α | Adalimumab | 43 | – | 70 | 19.3 ± 12.8 | 26 | 26.8 ± 16.8 | 20.2 ± 7.6 |
| anti-IL12/23 | Guselkumab | 208 | – | 72 | 18.5 ± 12.2 | 25 | 24.6 ± 14.5 | 20.9 ± 8.0 | ||||
| PBO | Placebo | 42 | – | 67 | 18.0 ± 13.3 | 29 | 27.5 ± 19.3 | 21.8 ± 9.9 | ||||
| Griffiths | 2015 | UK | 12 weeks | anti-IL17 | Ixekizumab | 771 | 46.0 ± 13.0 | 66 | 18.0 ± 12.0 | – | 28.0 ± 17.0 | 21.0 ± 8.0 |
| anti-TNF-α | Etanercept | 382 | 46.0 ± 14.0 | 70 | 18.0 ± 12.0 | – | 28.0 ± 17.0 | 21.0 ± 8.0 | ||||
| PBO | Placebo | 193 | 46.0 ± 12.0 | 71 | 18.0 ± 13.0 | – | 29.0 ± 17.0 | 21.0 ± 8.0 | ||||
| Lebwohl | 2015 | USA | 12 weeks | anti-IL17 | Brodalumab | 1222 | 45.0 ± 13.0 | 69 | 19.0 ± 12.0 | 19 | 27.0 ± 17.0 | 20.3 ± 8.2 |
| anti-IL12/23 | Ustekinumab | 300 | – | – | – | – | – | – | ||||
| PBO | Placebo | 309 | – | – | – | – | – | – | ||||
| Thaçi | 2015 | Germany | 16 weeks | anti-IL17 | Secukinumab | 337 | 45.2 ± 13.9 | 68 | 19.6 ± 12.9 | 20.5 | 32.6 ± 17.8 | 21.7 ± 8.5 |
| anti-IL12/23 | Ustekinumab | 339 | 44.6 ± 13.6 | 74.3 | 16.1 ± 11.2 | 15.9 | 32.0 ± 16.8 | 21.5 ± 8.1 | ||||
| Youn | 2010 | South Korea | 12 weeks | anti-IL12/23 | Ustekinumab | 61 | 40.9 ± 12.7 | 82 | 11.9 ± 7.50 | 16.4 | 41.8 ± 24.4 | 25.2 ± 11.9 |
| PBO | Placebo | 60 | 40.4 ± 10.1 | 88.3 | 13.9 ± 7.30 | 11.7 | 35.8 ± 21.4 | 22.9 ± 8.6 | ||||
| Zhu | 2013 | China | 12 weeks | anti-IL12/23 | Ustekinumab | 160 | 40.1 ± 12.4 | 78.1 | 14.6 ± 8.90 | 8.8 | 35.1 ± 18.5 | 23.2 ± 9.5 |
| PBO | Placebo | 162 | 39.2 ± 12.2 | 75.9 | 14.2 ± 8.60 | 8.6 | 35.1 ± 19.6 | 22.7 ± 9.5 | ||||
| Gordon | 2010 | USA | 12 weeks | anti-IL12/23 | Briakinumab | 139 | 44.9 ± 12.9 | 66.9 | 16.3 ± 12.0 | 23.7 | 24.9 ± 17.8 | 19.4 ± 7.9 |
| anti-TNF-α | Etanercept | 139 | 45.2 ± 14.8 | 61.2 | 15.2 ± 2.10 | 33.1 | 24.7 ± 13.9 | 18.5 ± 6.0 | ||||
| PBO | Placebo | 72 | 45.0 ± 13.9 | 63.9 | 15.5 ± 11.7 | 20.8 | 22.1 ± 13.4 | 18.3 ± 6.4 | ||||
| Papp | 2014 | Canada | 12 weeks | anti-IL12/23 | Briakinumab | 981 | 45.7 ± 13.2 | 67.9 | 18.9 ± 12.3 | 29.6 | 24.8 ± 16.3 | 19.1 ± 7.5 |
| PBO | Placebo | 484 | 45.1 ± 13.5 | 70.9 | 19.2 ± 11.9 | 31 | 25.7 ± 16.9 | 19.3 ± 7.3 | ||||
| Papp | 2015 | Canada | 16 weeks | anti-IL12/23 | Tildrakizumab | 309 | 43.2 ± 12.9 | 74 | – | – | – | – |
| PBO | Placebo | 46 | 45.9 ± 11.7 | 83 | – | – | – | – | ||||
| Papp | 2014 | Multi | 120 weeks | anti-IL17 | Brodalumab | 148 | – | – | – | – | – | – |
| PBO | Placebo | 33 | – | – | – | – | – | – | ||||
| Krupashankar | 2014 | Multi | 12 weeks | ANT | Itolizumab | 180 | 40.7 ± 11.0 | 76.7 | – | – | – | 21.3 ± 8.5 |
| PBO | Placebo | 43 | 43.3 ± 13.0 | 74.4 | – | – | – | 21.9 ± 8.9 | ||||
| Bachelez | 2015 | France | 12 weeks | anti-TNF-α | Etanercept | 335 | 42.0 ± 14.0 | 70 | 18.0 ± 15.2 | 21 | 25.0 ± 20.9 | 19.4 ± 12.9 |
| PBO | Placebo | 107 | 46.0 ± 15.0 | 66 | 17.0 ± 14.0 | 24 | 26.0 ± 17.0 | 19.5 ± 10.5 | ||||
| Micali | 2015 | Italy | 6 weeks | anti-TNF-α | Etanercept | 58 | 41.8 ± 13.0 | 65.5 | – | – | – | 20.2 ± 13.7 |
| PBO | Placebo | 62 | 41.5 ± 16.7 | 72.6 | – | – | – | 19.4 ± 12.6 | ||||
| Papp | 2013 | Canada | 12 weeks | anti-IL17 | Secukinumab | 103 | 46.1 ± 12.6 | 69 | 19.8 ± 12.6 | 31 | 26.0 ± 19.3 | 21.6 ± 11.5 |
| PBO | Placebo | 22 | 45.9 ± 10.8 | 63.6 | 21.4 ± 14.8 | 27.3 | 26.0 ± 18.8 | 21.7 ± 8.5 | ||||
| Reich | 2013 | Germany | 10 weeks | anti-TNF-α | Infliximab | 222 | 45.7 ± 13.5 | 68 | 20.5 ± 12.0 | – | – | 21.5 ± 8.7 |
| PBO | Placebo | 219 | 43.3 ± 13.0 | 71 | 17.5 ± 11.0 | – | – | 21.2 ± 7.7 | ||||
| Mease | 2016 | USA | 24 weeks | PBO | Placebo | 106 | 50.6 ± 12.3 | 45.3 | 16.0 ± 13.8 | – | – | 6.2 ± 7.5 |
| anti-TNF-α | Adalimumab | 101 | 48.6 ± 12.4 | 50.5 | 15.7 ± 12.7 | – | – | 5.5 ± 6.5 | ||||
| Kavanaugh | 2016 | USA | 24 weeks | PBO | Placebo | 92 | 47.4 ± 12.8 | 48.9 | 16.0 ± 12.6 | – | 28.4 ± 26.1 | 13.9 ± 12.5 |
| anti-IL12/23 | Ustekinumab | 164 | 45.7 ± 11.7 | 57.9 | 15.9 ± 11.5 | – | 30.1 ± 25.6 | 14.8 ± 12.4 | ||||
| Blauvelt | 2016 | Portland | 52 weeks | anti-IL17 | Secukinumab | 337 | 45.2 ± 13.9 | 68 | 19.7 ± 12.8 | 20.5 | – | 21.7 ± 8.5 |
| anti-IL12/23 | Ustekinumab | 339 | 44.6 ± 13.7 | 74.3 | 16.1 ± 11.2 | 15.9 | – | 21.5 ± 8.1 | ||||
| Blauvelt | 2017 | Multi | 16 weeks | anti-IL12/23 | Guselkumab | 329 | 43.9 ± 12.74 | 72.9 | 17.9 ± 6.22 | – | 28.3 ± 17.1 | 22.1 ± 9.49 |
| anti-TNF-α | Adalimumab | 334 | 42.9 ± 12.58 | 82.9 | 29.8 ± 6.48 | – | 28.6 ± 16.66 | 22.4 ± 8.97 | ||||
| PBO | Placebo | 174 | 44.9 ± 12.9 | 83.3 | 28.9 ± 6.89 | – | 25.8 ± 15.93 | 20.4 ± 8.74 | ||||
| Kavanaugh | 2017 | USA | 24 weeks | anti-TNF-α | Golimumab | 241 | 45.7 ± 11.3 | 53.1 | 6.2 ± 6 | – | 196 ± 81.3 | 11 ± 9.9 |
| PBO | Placebo | 239 | 46.7 ± 12.5 | 50.6 | 5.3 ± 5.9 | – | 198 ± 82.8 | 8.9 ± 9 | ||||
| Lacour | 2017 | Multi | 12 weeks | anti-IL17 | Secukinumab | 121 | 43.9 ± 14.41 | 71 | 20.6 ± 14.54 | – | 30.1 ± 16.66 | 22 ± 8.85 |
| PBO | Placebo | 60 | 43.7 ± 12.74 | 62.3 | 19.9 ± 12.2 | – | 25.7 ± 19.7 | 19.4 ± 6.7 | ||||
| Nash | 2017 | Multi | 24 weeks | anti-IL17 | Ixekizumab | 245 | 52.6 ± 13.6 | 52 | 15.7 ± 12.3 | – | 12.5 | 6.4 ± 7.9 |
| PBO | Placebo | 118 | 51.5 ± 10.4 | 47 | 15.3 ± 12.6 | – | 9 | 5.2 ± 6.3 | ||||
| Papp | 2017 | Multi | 16 weeks | anti-TNF-α | Adalimumab | 77 | 13 ± 3.3 | 45 | 5 ± 3.8 | – | 17.7 ± 20.4 | 18.9 ± 10 |
| AM | MTX | 37 | 13.4 ± 3.5 | 30 | 5.1 ± 3.8 | – | 30.3 ± 21.2 | 19.2 ± 10 | ||||
| Papp | 2016 | Multi | 12 weeks | anti-IL17 | Brodalumab | 351 | 46 ± 12 | 73 | 20 ± 13 | – | 25.1 ± 15.3 | 19.4 ± 6.6 |
| PBO | Placebo | 220 | 47 ± 13 | 73 | 21 ± 12 | – | 26.9 ± 17.1 | 19.7 ± 7.7 | ||||
| Reich | 2017 | USA | 16 weeks | anti-IL12/23 | Guselkumab | 496 | 43.7 ± 12.2 | 70.4 | 17.9 ± 12 | – | 28.5 ± 16.4 | 21.9 ± 8.8 |
| anti-TNF-α | Adalimumab | 284 | 43.2 ± 11.9 | 68.5 | 17.6 ± 11.7 | – | 19.1 ± 16.5 | 21.7 ± 9 | ||||
| PBO | Placebo | 284 | 43.3 ± 12.4 | 69.8 | 17.9 ± 11.9 | –– | 28 ± 16.5 | 21.5 ± 8 | ||||
| Reich | 2017 | Germany | 16 weeks | anti-TNF-α | Etanercept | 83 | 40 ± 14.1 | 59 | 18.1 ± 1.7 | – | 29.9 ± 6.8 | 18.1 ± 11.7 |
| PBO | Placebo | 84 | 43.4 ± 14.9 | 70.2 | 16.6 ± 12.1 | – | 29.5 ± 6.6 | 16.6 ± 12.1 | ||||
| Reich | 2017 | Germany | 16 weeks | anti-IL12/23 | Tildrakizumab | 617 | 46.4 ± 13.1 | 67 | – | – | 29.7 ± 17.44 | 20 ± 7.85 |
| PBO | Placebo | 155 | 47.9 ± 13.5 | 65 | – | – | 29.6 ± 17.28 | 19.3 ± 7.07 | ||||
| 16 weeks | anti-IL12/23 | Tildrakizumab | 621 | 44.6 ± 13.6 | 72 | – | – | 34.2 ± 18.44 | 20.5 ± 7.63 | |||
| anti-TNF-α | Etanercept | 313 | 46.4 ± 12.2 | 72 | – | – | 31.3 ± 14.75 | 20 ± 7.57 | ||||
| PBO | Placebo | 156 | 45.8 ± 14 | 71 | – | – | 31.6 ± 16.58 | 20.2 ± 7.36 | ||||
| Reich | 2017 | Germany | 12 weeks | anti-IL12/23 | Ustekinumab | 166 | 44 ± 13.33 | 67.5 | 18.2 ± 12 | – | 27.5 ± 16.7 | 39.4 ± 30.8 |
| anti-IL17 | Ixekizumab | 136 | 42.7 ± 12.7 | 66.2 | 18 ± 11.1 | – | 26.7 ± 16.5 | 42.9 ± 33.3 | ||||
| Paller | 2008 | Multi | 12 weeks | anti-TNF-α | Etanercept | 106 | 14 ± 3.25 | 52 | – | – | – | 16.7 ± 9.9 |
| PBO | Placebo | 105 | 13 ± 3.25 | 50 | – | – | – | 16.4 ± 11.175 | ||||
| Bachelez | 2015 | Multi | 12 weeks | anti-TNF-α | Etanercept | 335 | 42 ± 14 | 70 | 18 ± 15.25 | 21 | 25 ± 20.875 | 19.4 ± 12.9 |
| PBO | Placebo | 107 | 46 ± 15 | 66 | 17 ± 14 | 24 | 26 ± 17 | 19.5 ± 10.55 | ||||
| Cai | 2016 | China | 12 weeks | anti-TNF-α | Adalimumab | 338 | 43.1 ± 11.91 | 75.1 | 14.8 ± 10.15 | 12.7 | 24.4 ± 3.48 | 28.2 ± 12 |
| PBO | Placebo | 87 | 43.8 ± 12.45 | 66.7 | 15.8 ± 10.31 | 12.5 | 23.6 ± 2.86 | 25.6 ± 10.98 | ||||
| Gordon | 2016 | Multi | 12 weeks | anti-IL17 | Ixekizumab | 875 | 46 ± 13 | 66.9 | 19 ± 12 | – | – | 20 ± 7 |
| PBO | Placebo | 431 | 46 ± 13 | 70.3 | 20 ± 12 | – | – | 20 ± 9 | ||||
| Gordon | 2015 | Multi | 16 weeks | anti-TNF-α | Adalimumab | 43 | 50 | 70 | 91.6 ± 19.88 | – | – | 20.2 ± 7.58 |
| PBO | Placebo | 42 | 46.5 | 67 | 93.6 ± 22.62 | – | – | 21.8 ± 9.98 | ||||
| anti-IL12/23 | Guselkumab | 208 | – | 70 | – | – | – | – | ||||
| Gottlieb | 2016 | Multi | 16 weeks | anti-IL17 | Secukinumab | 137 | 52.4 ± 12.6 | 58.8 | 7.5 ± 8.8 | – | 28.8 ± 5.7 | 8.7 ± 10.4 |
| PBO | Placebo | 68 | 50.9 ± 13 | 50 | 11.8 ± 10.4 | – | 28.8 ± 5.7 | 7.7 ± 7.3 | ||||
| Leonardi | 2012 | Multi | 12 weeks | anti-IL17 | Ixekizumab | 58 | 48 ± 11 | 57 | 21 ± 12 | – | 22 ± 18 | 19.2 ± 8 |
| PBO | Placebo | 27 | 45 ± 13 | 52 | 15 ± 11 | – | 19 ± 12 | 16.5 ± 5.3 |
HPA: history of psoriatic arthritis (%); BSA: biologic systemic agents; PASI: psoriasis area and severity index; AM: anti-metabolites; anti-TNF-α: anti-tumor necrosis factor-α agents; ANT: anti-T-cell agents; anti-IL12/23: anti-interleukin-12/23 agents; anti-IL17: anti-interleukin-17agents; PBO: placebo; MTX: methotrexate.
Figure 2.
Network diagram of all included studies. Each node represents a medicine type; the diameters of circles represent the number of people involved, and the widths of lines between two nodes represent the number of study involved in the head-to-head comparison.
AM: anti-metabolites; anti-IL12/23: anti-interleukin-12/23 agents; anti-IL17: anti-interleukin-17 agents; ANT: anti-T-cell agent; anti-TNF-α: anti-tumor necrosis factor-α agent.
NMA results for PASI reduction
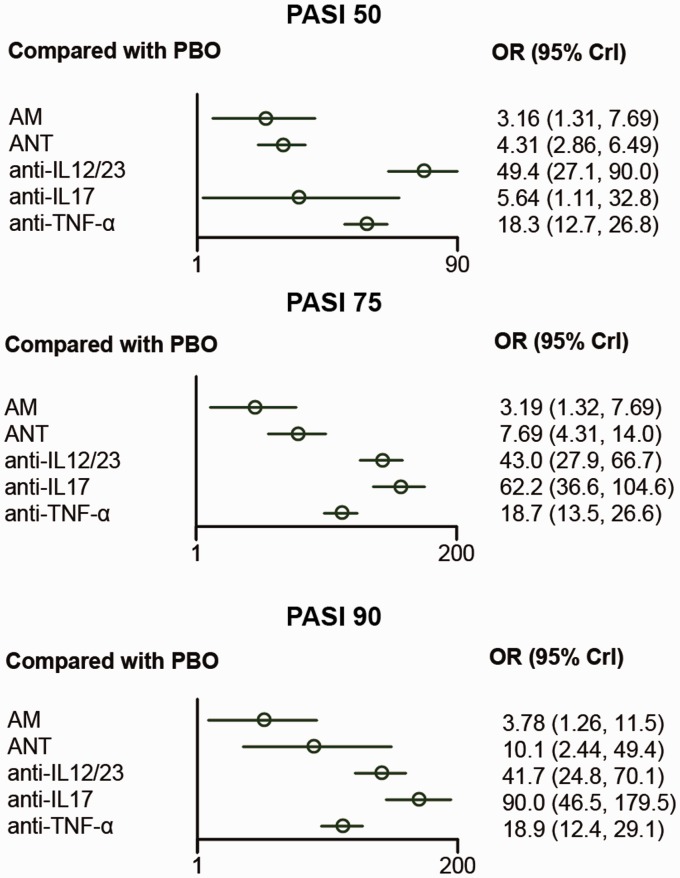
Table 2 showed the network comparison of different treatments for PASI reduction. In terms of PASI 75, it shows that anti-IL12/23 and anti-IL17 are significantly superior compared with PBO (OR=43.0, 95% CrI: 27.9–66.7; OR=62.2, 95% CrI: 36.6–104.6, respectively). (OR=5.9, 95% CrI: 2.5–13.7) and ANT (OR=2.44, 95% CrI: 1.3–4.8).
Table 2.
Network comparison of different treatments for PASI reduction in psoriasis patients.
| PBO | AM | ANT | Anti-IL12/23 | Anti-IL17 | Anti-TNF-α | |
|---|---|---|---|---|---|---|
| PASI 50 | ||||||
| PBO | 1 | 3.16 (1.31, 7.69) | 4.31 (2.86, 6.49) | 49.4 (27.11, 90.02) | 5.64 (1.11, 32.79) | 18.36 (12.68, 26.84) |
| AM | 0.32 (0.13, 0.76) | 1 | 1.36 (0.51, 3.63) | 15.64 (5.37, 45.60) | 1.79 (0.28, 12.81) | 5.81 (2.51, 13.74) |
| ANT | 0.23 (0.15, 0.35) | 0.73 (0.28, 1.97) | 1 | 11.47 (5.58, 23.81) | 1.31 (0.24, 7.92) | 4.26 (2.46, 7.46) |
| Anti-IL12/23 | 0.02 (0.01, 0.04) | 0.06 (0.02, 0.19) | 0.09 (0.04, 0.18) | 1 | 0.12 (0.02, 0.73) | 0.37 (0.18, 0.75) |
| Anti-IL17 | 0.18 (0.03, 0.90) | 0.56 (0.08, 3.60) | 0.76 (0.13, 4.14) | 8.67 (1.36, 49.40) | 1 | 3.25 (0.53, 17.64) |
| Anti-TNF-α | 0.05 (0.04, 0.08) | 0.17 (0.07, 0.40) | 0.23 (0.13, 0.41) | 2.69 (1.34, 5.42) | 0.31 (0.06, 1.88) | 1 |
| PASI 75 | ||||||
| PBO | 1 | 3.19 (1.32, 7.69) | 7.69 (4.31, 14.01) | 42.95 (27.94, 66.69) | 62.18 (36.6, 104.58) | 18.73 (13.46, 26.58) |
| AM | 0.31 (0.13, 0.76) | 1 | 2.41 (0.85, 7.03) | 13.60 (5.21, 35.16) | 19.49 (7.03, 52.98) | 5.93 (2.53, 13.74) |
| ANT | 0.13 (0.07, 0.23) | 0.41 (0.14, 1.17) | 1 | 5.58 (2.69, 11.59) | 8.00 (3.63, 17.46) | 2.44 (1.25, 4.81) |
| Anti-IL12/23 | 0.02 (0.01, 0.04) | 0.07 (0.03, 0.19) | 0.18 (0.09, 0.37) | 1 | 1.43 (0.77, 2.64) | 0.44 (0.27, 0.71) |
| Anti-IL17 | 0.02 (0.01, 0.03) | 0.05 (0.02, 0.14) | 0.12 (0.06, 0.28) | 0.70 (0.38, 1.30) | 1 | 0.30 (0.17, 0.56) |
| Anti-TNF-α | 0.05 (0.04, 0.07) | 0.17 (0.07, 0.39) | 0.41 (0.21, 0.80) | 2.29 (1.40, 3.71) | 3.29 (1.79, 5.99) | 1 |
| PASI 90 | ||||||
| PBO | 1 | 3.78 (1.26, 11.47) | 10.07 (2.44, 49.40) | 41.68 (24.78, 70.11) | 90.02 (46.53, 179.47) | 18.92 (12.43, 29.08) |
| AM | 0.26 (0.09, 0.79) | 1 | 2.66 (0.43, 18.17) | 11.02 (3.35, 35.52) | 24.05 (6.69, 84.77) | 5.00 (1.75, 14.30) |
| ANT | 0.10 (0.02, 0.41) | 0.38 (0.06, 2.32) | 1 | 4.14 (0.77, 18.73) | 9.03 (1.62, 42.52) | 1.88 (0.36, 8.25) |
| Anti-IL12/23 | 0.02 (0.01, 0.04) | 0.09 (0.03, 0.30) | 0.24 (0.05, 1.30) | 1 | 2.18 (1.03, 4.62) | 0.45 (0.26, 0.80) |
| Anti-IL17 | 0.01 (0.01, 0.02) | 0.04 (0.01, 0.15) | 0.11 (0.02, 0.62) | 0.46 (0.22, 0.97) | 1 | 0.21 (0.10, 0.44) |
| Anti-TNF-α | 0.05 (0.03, 0.08) | 0.20 (0.07, 0.57) | 0.53 (0.12, 2.75) | 2.20 (1.25, 3.90) | 4.81 (2.27, 10.07) | 1 |
Bold: data with statistically significant difference, which is highlighted in the upper region of each outcome. PASI 50: ≥50% reduction in psoriasis area and severity index; PASI 75: ≥75% reduction in psoriasis area and severity index; PASI 90: ≥90% reduction in psoriasis area and severity index; AM: anti-metabolites; anti-TNF-α: anti-tumor necrosis factor-α agents; ANT: anti-T-cell agents; anti-IL12/23: anti-interleukin-12/23 agents; anti-IL17: anti-interleukin-17 agents; PBO: placebo.
According to PASI 75, all the therapies had significantly higher ORs compared with PBO. Furthermore, anti-IL12/23, anti-IL17, and anti-TNF-α were estimated to be more effective than AM (OR=13.6, 95% CrI: 5.2–35.2; OR=19.5, 95% CrI: 7.0–53.0; OR=5.9, 95% CrI: 2.5–13.7, respectively) and ANT considering PASI 75 (OR=5.6, 95% CrI: 2.7–11.6; OR=8.0, 95% CrI: 3.6–17.5; OR=2.44, 95% CrI: 1.3–4.8, respectively).
For the comparison of treatments under PASI 90 reduction, all treatments were statistically more effective than PBO. Moreover, anti-IL17 was significantly better than other treatments. Meanwhile, it was revealed that anti-IL12/23 had significantly higher ORs than AM (OR=11.0, 95% CrI: 3.3–35.5) and anti-TNF-α (OR=2.39, 95% CrI: 1.19–4.62). Besides, anti-TNF-α had a better performance than AM (OR=2.2, 95% CrI: 1.2–3.9). The visualized result was also provided in Figure 3.
Figure 3.
Forest plots for different treatment effects in psoriasis area and severity index reduction in psoriasis patients.
AM: anti-metabolites; anti-IL12/23: anti-interleukin-12/23agents; anti-IL17: anti-interleukin-17 agents; ANT: anti-T-cell agent; anti-TNF-α: anti-tumor necrosis factor-α agent; PBO: placebo; PASI 50: ≥50% reduction in psoriasis area and severity index; PASI 75: ≥75% reduction in psoriasis area and severity index; PASI 90: ≥90% reduction in psoriasis area and severity index.
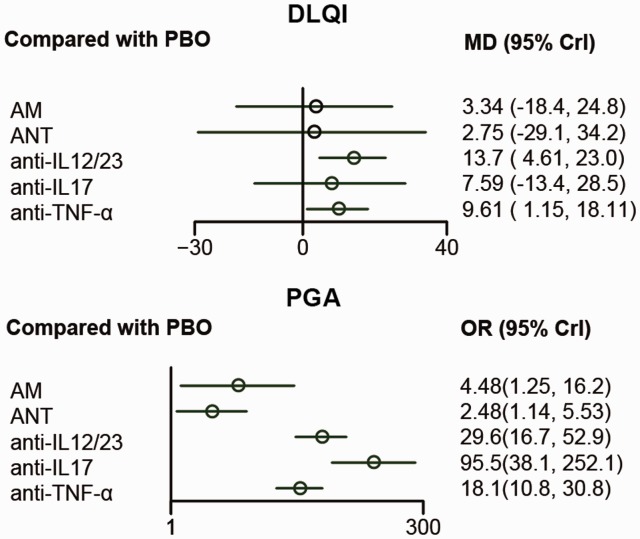
NMA result for DLQI and PGA
Table 3 showed the network comparison of different treatments for DLQI and PGA. In view of DQLI, only two drugs, anti-IL12/23 and anti-TNF-α were superior compared with PBO (OR=13.8, CrI: 4.6–23.3; OR=9.6, CrI: 1.2–18.1, respectively). Considering PGA, all drugs were superior compared with PBO, and anti-IL12/23, anti-IL17, and anti-TNF-α had better PGA compared with AM (OR=6.62, 95% CrI: 1.68–25.79; OR=21.54, 95% CrI: 4.57–101.49; OR=4.06, 95% CrI: 1.20–13.60, respectively) and ANT (OR=11.94, 95% CrI: 4.48–31.82; OR=38.36, 95% CrI: 11.47–134.29; OR=7.32, 95% CrI: 2.8–18.73, respectively). In addition, anti-IL17 was estimated to be superior to anti-TNF-α (OR=5.31, 95% CrI: 1.93–14.88). The forest plots were presented in Figure 4.
Table 3.
Network comparison of different treatments for Dermatology Life Quality Index and Physician’s Global Assessment in psoriasis patients.
| PBO | AM | ANT | Anti-IL12/23 | Anti-IL17 | Anti-TNF-α | |
|---|---|---|---|---|---|---|
| Dermatology Life Quality Index | ||||||
| PBO | 1 | 3.34 (−18.48, 24.82) | 2.75 (−29.15, 34.17) | 13.75 (4.61, 23.03) | 7.59 (−13.44, 28.50) | 9.61 (1.15, 18.11) |
| AM | −3.34 (−24.82, 18.48) | 1 | −0.55 (−39.1, 37.58) | 10.44 (−12.65, 33.71) | 4.29 (−25.36, 33.83) | 6.25 (−14.6, 27.26) |
| ANT | −2.75 (−34.17, 29.15) | 0.55 (−37.58, 39.10) | 1 | 10.98 (−21.69, 44.20) | 4.80 (−33.03, 42.39) | 6.83 (−25.58, 39.77) |
| Anti-IL12/23 | −13.75 (−23.03, −4.61) | −10.44 (−33.71, 12.65) | −10.98 (−44.2, 21.69) | 1 | −6.15 (−29.1, 16.45) | −4.13 (−15.66, 7.49) |
| Anti-IL17 | −7.59 (−28.5, 13.44) | −4.29 (−33.83, 25.36) | −4.80 (−42.39, 33.03) | 6.15 (−16.45, 29.10) | 1 | 2.02 (−19.49, 23.74) |
| Anti-TNF-α | −9.61 (−18.11, −1.15) | −6.25 (−27.26, 14.60) | −6.83 (−39.77, 25.58) | 4.13 (−7.49, 15.66) | −2.02 (−23.74, 19.49) | 1 |
| Physician’s Global Assessment | ||||||
| PBO | 1 | 4.48 (1.25, 16.28) | 2.48 (1.14, 5.53) | 29.67 (16.78, 52.98) | 95.58 (38.09, 252.14) | 18.17 (10.80, 30.88) |
| AM | 0.22 (0.06, 0.80) | 1 | 0.55 (0.12, 2.51) | 6.62 (1.68, 25.79) | 21.54 (4.57, 101.49) | 4.06 (1.20, 13.60) |
| ANT | 0.40 (0.18, 0.88) | 1.80 (0.40, 8.17) | 1 | 11.94 (4.48, 31.82) | 38.86 (11.47, 134.29) | 7.32 (2.80, 18.73) |
| Anti-IL12/23 | 0.03 (0.02, 0.06) | 0.15 (0.04, 0.59) | 0.08 (0.03, 0.22) | 1 | 3.25 (1.21, 8.94) | 0.61 (0.32, 1.20) |
| Anti-IL17 | 0.01 (0.00, 0.03) | 0.05 (0.01, 0.22) | 0.03 (0.01, 0.09) | 0.31 (0.11, 0.83) | 1 | 0.19 (0.07, 0.52) |
| Anti-TNF-α | 0.06 (0.03, 0.09) | 0.25 (0.07, 0.84) | 0.14 (0.05, 0.36) | 1.63 (0.84, 3.16) | 5.31 (1.93, 14.88) | 1 |
Bold: data with statically significant difference, which is highlighted in the upper region of each outcome. AM: anti-metabolites; anti-TNF-α: anti-tumor necrosis factor-α agents; ANT: anti-T-cell agents; anti-IL12/23: anti-interleukin-12/23 agents; anti-IL17: anti-interleukin-17 agents; PBO: placebo.
Figure 4.
Forest plots for different treatment effects of Dermatology Life Quality Index and Physician’s Global Assessment in psoriasis patients.
AM: anti-metabolites; anti-IL12/23: anti-interleukin-12/23 agents; anti-IL17: anti-interleukin-17 agents; ANT: anti-T-cell agent; anti-TNF-α: anti-tumor necrosis factor-α agent; PBO: placebo; DLQI: Dermatology Life Quality Index; PGA: Physician’s Global Assessment – minimal or cleared.
NMA result for AEs
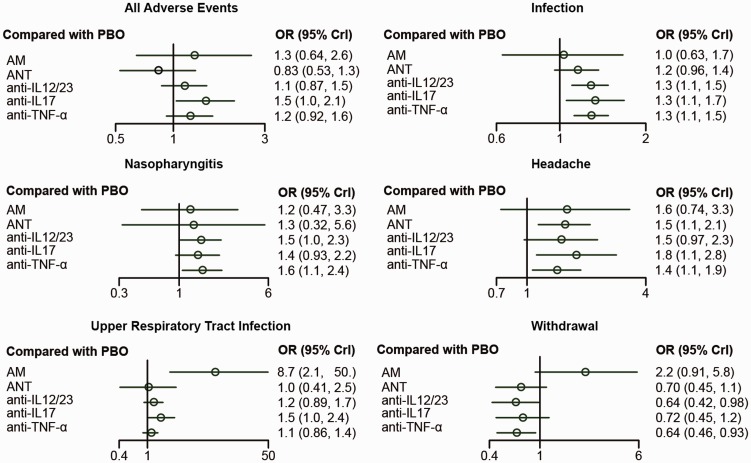
Table 4 showed the network comparison of different treatments for adverse events. According to all adverse events (AAE), only anti-IL17 showed a significant result that it had more AAE than PBO (OR=0.68, 95% CrI: 0.48–0.97). In view of incidence of infection, anti-IL12/23, anti-IL17, and anti-TNF-α showed more infection cases than PBO (OR=1.28, 95% CrI: 1.11–1.48; OR=1.32, 95% CrI: 1.05–1.68; OR=1.28, 95% CrI: 1.12–1.48, respectively). Besides, anti-TNF-α was associated with statistically significant increased odds of nasopharyngitis compared with PBO (OR=0.64, 95% CrI: 0.42–0.94). In view of headache, few of them demonstrated a significant difference. ANT, anti-IL17, and anti-TNF-α was assessed to be worse compared with PBO (OR=1.54, 95% CrI: 1.14–2.10; OR=1.77, 95% CrI: 1.12–2.83; OR=1.40, 95% CrI: 1.06–1.88, respectively). As for URTI, only AM was associated with statistically stronger URTI compared with PBO and all the other inventions. Meanwhile, the network comparisons for incidence of withdrawal due to the AE showed that compared with patients using PBO, patients using IL12/23 or TNF-α had statistically higher possibility to keep on (OR=0.64, 95% CrI: 0.42–0.98; OR=0.64, 95% CrI: 0.46–0.92, respectively). In addition, AM was associated with higher withdrawal probability than other inventions. The forest plots of the random-effects model were shown in Figure 5.
Table 4.
Network comparison of different treatments for adverse events in psoriasis patients.
| PBO | AM | ANT | Anti-IL12/23 | Anti-IL17 | Anti-TNF-α | ||
|---|---|---|---|---|---|---|---|
| All adverse events | |||||||
| PBO | 1 | 1.02(0.63, 1.67) | 1.15 (0.96, 1.36) | 1.28 (1.11, 1.48) | 1.32 (1.05, 1.68) | 1.28 (1.12, 1.48) | Infection |
| AM | 0.79 (0.39, 1.55) | 1 | 1.12 (0.66, 1.90) | 1.25 (0.76, 2.05) | 1.30 (0.75, 2.20) | 1.25 (0.78, 2.03) | |
| ANT | 1.21 (0.76, 1.90) | 1.54 (0.67, 3.53) | 1 | 1.11 (0.89, 1.40) | 1.15 (0.86, 1.57) | 1.12 (0.89, 1.40) | |
| Anti-IL12/23 | 0.88 (0.67, 1.15) | 1.12 (0.55, 2.29) | 0.73 (0.43, 1.23) | 1 | 1.04 (0.84, 1.27) | 1.01 (0.84, 1.19) | |
| Anti-IL17 | 0.68 (0.48, 0.97) | 0.87 (0.40, 1.86) | 0.57 (0.32, 1.00) | 0.77 (0.53, 1.13) | 1 | 0.97 (0.75, 1.26) | |
| Anti-TNF-α | 0.82 (0.63, 1.08) | 1.05 (0.55, 2.01) | 0.68 (0.40, 1.16) | 0.93 (0.68, 1.30) | 1.21 (0.79, 1.86) | 1 | |
| Nasopharyngitis | |||||||
| PBO | 1 | 1.58 (0.73, 3.32) | 1.54 (1.14, 2.10) | 1.48 (0.97, 2.27) | 1.77 (1.12, 2.83) | 1.40 (1.06, 1.88) | Headache |
| AM | 0.81 (0.31, 2.12) | 1 | 0.98 (0.44, 2.23) | 0.94 (0.41, 2.23) | 1.12 (0.47, 2.75) | 0.90 (0.44, 1.84) | |
| ANT | 0.76 (0.18, 3.13) | 0.93 (0.17, 5.26) | 1 | 0.96 (0.57, 1.63) | 1.14 (0.66, 2.01) | 0.91 (0.61, 1.38) | |
| Anti-IL12/23 | 0.66 (0.43, 0.98) | 0.81 (0.29, 2.25) | 0.86 (0.2, 3.82) | 1 | 1.19 (0.72, 2.01) | 0.95 (0.58, 1.54) | |
| Anti-IL17 | 0.70 (0.44, 1.07) | 0.85 (0.30, 2.44) | 0.91 (0.21, 4.10) | 1.06 (0.64, 1.77) | 1 | 0.8 (0.46, 1.35) | |
| Anti-TNF-α | 0.64 (0.42, 0.94) | 0.79 (0.31, 1.95) | 0.84 (0.19, 3.71) | 0.97 (0.59, 1.60) | 0.91 (0.52, 1.62) | 1 | |
| Upper respiratory tract infection | |||||||
| PBO | 1 | 2.25 (0.91, 5.81) | 0.70 (0.44, 1.13) | 0.64 (0.42, 0.98) | 0.73 (0.45, 1.19) | 0.64 (0.46, 0.92) | Withdrawal |
| AM | 0.11 (0.02, 0.48) | 1 | 0.31 (0.11, 0.86) | 0.28 (0.10, 0.75) | 0.32 (0.11, 0.89) | 0.29 (0.12, 0.67) | |
| ANT | 1.01 (0.39, 2.51) | 9.12 (1.63, 62.18) | 1 | 0.90 (0.49, 1.70) | 1.03 (0.53, 1.99) | 0.91 (0.52, 1.65) | |
| Anti-IL12/23 | 0.83 (0.61, 1.12) | 7.46 (1.70, 41.26) | 0.82 (0.31, 2.18) | 1 | 1.14 (0.66, 1.97) | 1.01 (0.62, 1.67) | |
| Anti-IL17 | 0.66 (0.41, 1.00) | 5.93 (1.31, 39.25) | 0.66 (0.23, 1.82) | 0.80 (0.49, 1.26) | 1 | 0.89 (0.51, 1.58) | |
| Anti-TNF-α | 0.90 (0.71, 1.15) | 8.17 (1.95, 45.60) | 0.90 (0.34, 2.39) | 1.09 (0.77, 1.57) | 1.38 (0.86, 2.29) | 1 | |
Bold: data with statically significant difference; in the upper regions, columns are compared with rows, while lower regions are opposite. AM: anti-metabolites; anti-TNF-α: anti-tumor necrosis factor-α agents; ANT: anti-T-cell agents; anti-IL12/23: anti-interleukin-12/23 agents; anti-IL17: anti-interleukin-17 agents; PBO: placebo.
Figure 5.
Forest plots for different treatment effects of adverse events in psoriasis patients.
AM: anti-metabolites; anti-IL12/23: anti-interleukin-12/23 agents; anti-IL17: anti-interleukin-17 agents; ANT: anti-T-cell agent; anti-TNF-α: anti-tumor necrosis factor-α agent; PBO: placebo.
Ranking of treatments
The SUCRA values for different treatments for all outcomes was calculated in order to determine the best method for curing psoriasis, and the calculated numbers were listed in Table 5. The result showed that anti-IL12/23 had better efficacy in ≥50% reduction in PASI (0.997) and led to better Dermatology Life Quality (0.842), but for better efficacy in PASI, it is indicated that anti-IL17 had the most possibility to rank the first among all six drugs (PASI 75: 0.980, PASI 90: 0.995, PGA: 0.998). Meanwhile, anti-TNF-α had good performance in PASI 50, 75, and 90. However, AM and ANT showed less efficacy. As for ranking of incidence of AE, it showed that none of the interventions were better than PBO except for ANT in AAE outcome. And anti-IL17 showed worse effect in both AAE and infection (AAE: 0.281, infection: 0.352).
Table 5.
Surface under the cumulative ranking curve (SUCRA) values for different treatments for all outcomes in psoriasis patients.
| Outcomes | PBO | AM | ANT | Anti-IL12/23 | Anti-IL17 | Anti-TNF-α |
|---|---|---|---|---|---|---|
| PASI 50 | 0.171 | 0.421 | 0.520 | 0.997 | 0.571 | 0.819 |
| PASI 75 | 0.167 | 0.341 | 0.493 | 0.854 | 0.980 | 0.666 |
| PASI 90 | 0.168 | 0.356 | 0.518 | 0.829 | 0.995 | 0.632 |
| DLQI | 0.342 | 0.495 | 0.506 | 0.842 | 0.617 | 0.699 |
| PGA | 0.171 | 0.465 | 0.368 | 0.823 | 0.998 | 0.676 |
| AAE | 0.782 | 0.474 | 0.904 | 0.580 | 0.281 | 0.480 |
| Infection | 0.910 | 0.761 | 0.643 | 0.427 | 0.352 | 0.407 |
| Nasopharyngitis | 0.874 | 0.643 | 0.588 | 0.454 | 0.517 | 0.423 |
| Headache | 0.972 | 0.501 | 0.495 | 0.554 | 0.363 | 0.615 |
| URTI | 0.861 | 0.171 | 0.745 | 0.602 | 0.415 | 0.706 |
| Withdrawal | 0.359 | 0.179 | 0.702 | 0.800 | 0.669 | 0.791 |
PASI 50: ≥50% reduction in psoriasis area and severity index; PASI 75: ≥75% reduction in psoriasis area and severity index; PASI 90: ≥90% reduction in psoriasis area and severity index; DLQI: Dermatology Life Quality Index; PGA: Physician’s Global Assessment – minimal or cleared; AAE: all adverse events; URTI: upper respiratory tract infection; AM: anti-metabolites; anti-TNF-α: anti-tumor necrosis factor-α agents; ANT: anti-T-cell agents; anti-IL12/23: anti-interleukin-12/23 agents; anti-IL17: anti-interleukin-17 agents; PBO: placebo.
Inconsistency analysis
The direct and indirect evidences for each comparison under all outcomes, as well as network results, were presented in Table 6. p<0.05 suggested a significant inconsistency between direct and indirect evidence. Overall, no inconsistency was found for each comparison under all outcomes (all p>0.05), which indicated reliable results of the current NMA.
Table 6.
Comparison of direct and indirect evidences of treatments for psoriasis.
| Study | PASI 75 |
PGA |
AAE |
Nasopharyngitis |
Headache |
URTI |
Withdrawal |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| p value | OR | p value | OR | p value | OR | p value | OR | p value | OR | p value | OR | p value | OR | |
| AM vs. PBO | ||||||||||||||
| Direct | 2.50 (0.70, 9.80) | 3.70 (0.35, 36.0) | 1.10 (0.33, 4.00) | 1.20 (0.28, 5.90) | 1.20 (0.32, 5.00) | – | 4.30 (0.45200) | |||||||
| Indirect | 0.544 | 4.50 (1.20, 17.0) | 0.756 | 5.50 (1.00, 30.0) | 0.988 | 1.10 (0.48, 2.70) | 0.989 | 1.20 (0.27, 5.20) | 0.555 | 2.10 (0.72, 5.60) | – | – | 0.538 | 2.00 (0.73, 5.90) |
| Network | 3.10 (1.30, 7.40) | 4.40 (1.02, 17.0) | 1.30 (0.65, 2.60) | 1.20 (0.47, 3.20) | 1.60 (0.69, 3.30) | – | 2.30 (0.89, 5.90) | |||||||
| Anti-IL12/23 vs. PBO | ||||||||||||||
| Direct | 51.0 (30.0, 86.0) | – | 1.10 (0.76, 1.50) | 1.30 (0.82, 2.50) | 1.10 (0.69, 2.00) | 1.10 (0.81, 1.60) | 0.65 (0.40, 1.10) | |||||||
| Indirect | 0.146 | 16.0 (3.70, 71.0) | – | – | 0.403 | 1.60 (0.68, 3.80) | 0.604 | 1.40 (0.66, 5.20) | 0.246 | 2.10 (0.86, 5.10) | 0.433 | 1.60 (0.69, 4.00) | 0.642 | 0.89 (0.26, 2.90) |
| Network | 43.0 (27.0, 67.0) | – | 1.10 (0.87, 1.50) | 1.50 (1.00, 2.30) | 1.50 (0.99, 2.20) | 1.20 (0.89, 1.70) | 0.65 (0.42, 0.99) | |||||||
| Anti-IL17 vs. PBO | ||||||||||||||
| Direct | 50.0 (28.0, 86.0) | – | 1.60 (1.10, 2.50) | 1.60 (0.96, 2.50) | 2.00 (1.20, 3.50) | 1.70 (1.00, 3.00) | 0.83 (0.47, 1.50) | |||||||
| Indirect | 0.101 | 140 (42.0, 960) | – | – | 0.356 | 1.10 (0.55, 2.30) | 0.558 | 1.20 (0.49, 2.70) | 0.396 | 1.30 (0.52, 3.10) | 0.494 | 1.20 (0.54, 2.70) | 0.394 | 0.49 (0.17, 1.50) |
| Network | 62.0 (37.0, 130) | – | 1.50 (1.00, 2.10) | 1.40 (0.94, 2.20) | 1.80 (1.10, 2.80) | 1.50 (1.00, 2.50) | 0.73 (0.45, 1.20) | |||||||
| Anti-IL17 vs. Anti-IL12/23 | ||||||||||||||
| Direct | 2.30 (0.85, 6.50) | 2.10 (0.43, 11.0) | 1.10 (0.62, 1.80) | 0.82 (0.39, 1.80) | 0.96 (0.46, 2.00) | 1.00 (0.49, 2.30) | 0.82 (0.36, 1.90) | |||||||
| Indirect | 0.224 | 1.10 (0.51, 2.30) | 0.463 | 4.60 (1.20, 20.0) | 0.266 | 1.60 (0.94, 2.80) | 0.568 | 0.87 (0.54, 2.20) | 0.399 | 1.50 (0.72, 3.30) | 0.500 | 1.50 (0.80, 2.80) | 0.387 | 1.30 (0.65, 2.60) |
| Network | 1.40 (0.76, 2.60) | 3.30 (1.20, 9.10) | 1.30 (0.88, 1.90) | 0.93 (0.57, 1.60) | 1.20 (0.60, 1.50) | 1.30 (0.79, 2.10) | 1.10 (0.64, 2.00) | |||||||
| Anti-TNF-α vs. Anti-IL12/23 | ||||||||||||||
| Direct | 0.40 (0.21, 0.79) | 0.51 (0.22, 1.10) | 1.10 (0.69, 1.80) | 1.00 (0.47, 2.20) | – | 0.77 (0.45, 1.30) | 1.10 (0.50, 2.30) | |||||||
| Indirect | 0.588 | 0.53 (0.25, 1.10) | 0.511 | 0.73 (0.29, 1.80) | 0.840 | 1.00 (0.54, 1.90) | 0.916 | 1.00 (0.48, 2.10) | – | – | 0.470 | 1.00 (0.63, 1.60) | 0.871 | 1.00 (0.54, 2.10) |
| Network | 0.44 (0.27, 0.73) | 0.62 (0.31, 1.20) | 1.10 (0.77, 1.50) | 1.00 (0.62, 1.70) | – | 0.91 (0.62, 1.30) | 1.00 (0.63, 1.60) | |||||||
| Anti-TNF-α vs. Anti-IL17 | ||||||||||||||
| Direct | 0.19 (0.03, 1.00) | 0.19 (0.02, 1.60) | – | – | – | – | 0.51 (0.13, 1.90) | |||||||
| Indirect | 0.568 | 0.33 (0.18, 0.65) | 0.931 | 0.17 (0.05, 0.54) | – | – | – | – | – | – | – | – | 0.368 | 1.00 (0.56, 1.90) |
| Network | 0.30 (0.17, 0.55) | 0.18 (0.06, 0.54) | – | – | – | – | 0.89 (0.52, 1.60) | |||||||
URTI: upper respiratory tract infection; PASI 75: ≥75% reduction in psoriasis area and severity index; PGA: Physician’s Global Assessment – minimal or cleared; AAE: all adverse events; AM: anti-metabolites; anti-TNF-α: anti-tumor necrosis factor-α agents; ANT: anti-T-cell agents; anti-IL12/23: anti-interleukin-12/23 agents; anti-IL17: anti-interleukin-17 agents; PBO: placebo.
Discussion
Undoubtedly, as the NMA results revealed, all included therapies showed significant efficacy when compared with PBO in terms of all the efficacy outcomes except for DLQI, which in general corresponded to the results of previous RCTs. Meanwhile, the efficacy and safety of these therapies were certainly different from each other.
First of all, as was shown in the NMA results, anti-IL12/23 was proved to be the most ideal therapy among the included therapies. Its excellent efficacy as well as mild AEs was revealed. Additionally, its extraordinary efficacy and safety were also proved by previous RCTs, which corresponded with the results of previous RCT studies.25,29 Ustekinumab, an antibody agent binding to the shared p40 subunit of IL 12/23, was the most widely researched agent among the therapies mentioned above. It bound to the interleukins specifically and prevented their binding with respective receptors, thus blocked the downstream signaling cascades.25 Meanwhile, briakinumab, another research focus with analogous structure and function with ustekinumab, also showed an excellent performance clinically. Tildrakizumab and guselkumab are also experimental monoclonal antibodies (Statement on a Nonproprietary Name Adopted by the USAN Council—Tildrakizumab; Statement on A Nonproprietary Name Adopted by the USAN Council—Guselkumab) designed to block IL-23. However, such agents still required more research to promote its clinical appliance.
Second, anti-IL17 showed a satisfactory efficacy performance in this NMA. It was revealed that this therapy had a similar efficacy with anti-IL12/23. These anti-IL17 monoclonal antibody agents including ixekizumab, brodalumab, and secukinumab selectively bind to the IL 17 and neutralize the bioactivity of this cytokine.22 Though its efficacy was excellent, the safety of this therapy was not very good.
Third, ANT showed a weaker efficacy; however, its total AE ranked the first among the relevant therapy, and as a result, it can be regarded as a milder treatment in the clinical appliance.
Fourth, anti-TNF-α showed weaker efficacy than anti-IL-17 or anti-IL12/23 accompanied with a stronger AE; as a result, it was not recommended in this NMA research. As revealed in the introduction part, interleukins stimulate T-cells to produce TNF causing psoriasis. These biological agents work on the stimulation interleukins, the producer T-cells, and the final production TNF, respectively. The selectivity of these therapies gave them totally different mechanism and excellent efficacy. However, infection, the major AE of the above-mentioned biological agents according to the NMA results and previous studies,88,89 was still a severe problem to be solved. Additionally, a number of relevant biological agents were still at the stage of laboratory research, requiring more clinical studies and appliances.
Finally, AM did not work well in both efficacy and AE outcomes, which made is the least satisfactory therapy.
In this NMA research, there also existed some limitations. First of all, most of included studies reported the latest biological agents comparing with PBO or traditional therapy AM. However, direct RCT studies between these different treatments were still required for the unchallengeable authority of clinical experimental data. Besides, this NMA did not evaluate the treatment of mild psoriasis and topical therapies indicated for patients whose affected area is less than 10% of the BSA.90,91 The majority of this research and relevant works focused on severe psoriasis, and little attention was paid to the topical therapies like vitamin D and emollient. Finally, in this NMA, we divided the drugs treating psoriasis into five classes and regarded each whole class as a therapy; the efficacy and safety performance of interclass drug was not revealed in this NMA.
In conclusion, the efficacy and safety of some therapies of psoriasis were evaluated comprehensively and quantitatively in this NMA; monoclonal antibody agents of IL 12/23 and IL 17 were two recommended agents according to the results, while anti-IL17 should be used in caution since it has severe side effects.
Supplemental Material
Supplemental material, Table S1 for Quantitative evaluation to efficacy and safety of therapies for psoriasis: A network meta-analysis by Jingjing Lv, Dongmei Zhou, Yan Wang, Jingxia Zhao, Zhaoxia Chen, Jinchao Zhang, Tingting Di, Jing Hu, Bo Li, Ping Li and Feng Huang in Molecular Pain
Author Contributions
Research conception and design: DZ, YW, and JZ. Data analysis and interpretation: ZC, JZ, and BL. Statistical analysis: TD and JH. Drafting of the manuscript: PL. Critical revision of the manuscript: JL. All authors approved the final manuscript.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by Beijing Financial Research Institute Project: Clinical and Biological Studies of Eczema and Psoriasis (No.PXM2017_026273_000001).
References
- 1.Wang J Zhan Q andZhang L.. A systematic review on the efficacy and safety of infliximab in patients with psoriasis. Hum Vaccin Immunother 2016; 12: 431–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xiong HZ Gu JY He ZG Chen WJ Zhang X Wang JY andShi YL.. Efficacy and safety of secukinumab in the treatment of moderate to severe plaque psoriasis: a meta-analysis of randomized controlled trials. Int J Clin Exp Med 2015; 8: 3156–3172. [PMC free article] [PubMed] [Google Scholar]
- 3.Jabbar-Lopez ZK Yiu ZZN Ward V Exton LS Mohd Mustapa MF Samarasekera E Burden AD Murphy R Owen CM Parslew R Venning V Warren RB andSmith CH.. Quantitative evaluation of biologic therapy options for psoriasis: a systematic review and network meta-analysis. J Investig Dermatol 2017; 137: 1646–1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saurat JH, Stingl G, Dubertret L, Papp K, Langley RG, Ortonne JP, Unnebrink K, Kaul M, Camez A. and CHAMPION Study investigators. Efficacy and safety results from the randomized controlled comparative study of adalimumab vs. methotrexate vs. placebo in patients with psoriasis (CHAMPION). Br J Dermatol 2008; 158: 558–566. [DOI] [PubMed] [Google Scholar]
- 5.Bachelez H Pc VDK Strohal R Kubanov A Valenzuela F Lee JH Yakusevich V Chimenti S Papacharalambous J andProulx J.. Tofacitinib versus etanercept or placebo in moderate-to-severe chronic plaque psoriasis: a phase 3 randomised non-inferiority trial. Lancet 2015; 386: 552. [DOI] [PubMed] [Google Scholar]
- 6.Poulin Y Crowley JJ Langley RG Unnebrink K Goldblum OM andValdecantos WC.. Efficacy of adalimumab across subgroups of patients with moderate-to-severe chronic plaque psoriasis of the hands and/or feet: post hoc analysis of REACH. J Eur Acad Dermatol Venereol 2014; 28: 882–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reich K Wozel G Zheng H van Hoogstraten HJ Flint L andBarker J.. Efficacy and safety of infliximab as continuous or intermittent therapy in patients with moderate-to-severe plaque psoriasis: results of a randomized, long-term extension trial (RESTORE2). Br J Dermatol 2013; 168: 1325–1334. [DOI] [PubMed] [Google Scholar]
- 8.Feldman SR Gottlieb AB Bala M Wu Y Eisenberg D Guzzo C Li S Dooley LT andMenter A.. Infliximab improves health-related quality of life in the presence of comorbidities among patients with moderate-to-severe psoriasis. Br J Dermatol 2008; 159: 704–710. [DOI] [PubMed] [Google Scholar]
- 9.Gordon KB Duffin KC Bissonnette R Prinz JC Wasfi Y Li S Shen YK Szapary P Randazzo B andReich K.. A phase 2 trial of guselkumab versus adalimumab for plaque psoriasis. N Engl J Med 2015; 373: 136–144. [DOI] [PubMed] [Google Scholar]
- 10.Gordon KB Blauvelt A Papp KA Langley RG Luger T Ohtsuki M Reich K Amato D Ball SG andBraun DK.. Phase 3 trials of ixekizumab in moderate-to-severe plaque psoriasis. N Engl J Med 2016; 375: 345–356. [DOI] [PubMed] [Google Scholar]
- 11.Gottlieb A Sullivan J Van DM Kubanov A You R Parneix A Hugot S andMilutinovic M.. Secukinumab shows significant efficacy in palmoplantar psoriasis: results from GESTURE, a randomized controlled trial. J Am Acad Dermatol 2017; 76: 70. [DOI] [PubMed] [Google Scholar]
- 12.Krupashankar DS Dogra S Kura M Saraswat A Budamakuntla L Sumathy TK Shah R Gopal MG Narayana Rao T Srinivas CR Bhat R Shetty N Manmohan G Sai Krishna K Padmaja D Pratap DV Garg V Gupta S Pandey N Khopkar U Montero E Ramakrishnan MS Nair P andGanapathi PC.. Efficacy and safety of itolizumab, a novel anti-CD6 monoclonal antibody, in patients with moderate to severe chronic plaque psoriasis: results of a double-blind, randomized, placebo-controlled, phase-III study. J Am Acad Dermatol 2014; 71: 484–492. [DOI] [PubMed] [Google Scholar]
- 13.Leonardi C Matheson R Zachariae C Cameron G Li L Edsonheredia E Braun D andBanerjee S. . Anti-interleukin-17 monoclonal antibody ixekizumab in chronic plaque psoriasis. N Engl J Med 2012; 367: 274. [DOI] [PubMed] [Google Scholar]
- 14.Paller AS Siegfried EC Langley RG Gottlieb AB Pariser D Landells I Hebert AA Eichenfield LF Patel V Creamer K andJahreis A.. Etanercept treatment for children and adolescents with plaque psoriasis. N Engl J Med 2008; 358: 241–251. [DOI] [PubMed] [Google Scholar]
- 15.Reich K Nestle FO Papp K Ortonne JP Wu Y Bala M Evans R Guzzo C Li S andDooley LT.. Improvement in quality of life with infliximab induction and maintenance therapy in patients with moderate-to-severe psoriasis: a randomized controlled trial. Br J Dermatol 2006; 154: 1161. [DOI] [PubMed] [Google Scholar]
- 16.Woolacott N, Hawkins N, Mason A, Kainth A, Khadjesari Z, Vergel YB, Misso K, Light K, Chalmers R, Sculpher M, Riemsma R. Etanercept and efalizumab for the treatment of psoriasis: a systematic review. Health Technol Assess 2006; 10: 1–233. [DOI] [PubMed]
- 17.Bansback N, Sizto S, Sun H, Feldman S, Willian MK, Anis A. Efficacy of systemic treatments for moderate to severe plaque psoriasis: systematic review and meta-analysis. Dermatology 2009; 219: 209–218. [DOI] [PubMed] [Google Scholar]
- 18.Reich K, Burden AD, Eaton JN, Hawkins NS. Efficacy of biologics in the treatment of moderate to severe psoriasis: a network meta-analysis of randomized controlled trials. Br J Dermatol 2012; 166: 179–188. [DOI] [PubMed] [Google Scholar]
- 19.Lin VW Ringold S andDevine EB.. Comparison of ustekinumab with other biological agents for the treatment of moderate to severe plaque psoriasis: a Bayesian network meta-analysis. Arch Dermatol 2012; 148: 1403–1410. [DOI] [PubMed] [Google Scholar]
- 20.Nast A Jacobs A Rosumeck S andWerner RN.. Efficacy and safety of systemic long-term treatments for moderate-to-severe psoriasis: a systematic review and meta-analysis. J Investig Dermatol 2015; 135: 2641–2648. [DOI] [PubMed] [Google Scholar]
- 21.Gomez-Garcia F Epstein D Isla-Tejera B Lorente A Velez Garcia-Nieto A andRuano J.. Short-term efficacy and safety of new biological agents targeting the interleukin-23-T helper 17 pathway for moderate-to-severe plaque psoriasis: a systematic review and network meta-analysis. Br J Dermatol 2017; 176: 594–603. [DOI] [PubMed] [Google Scholar]
- 22.Blauvelt A Prinz JC Gottlieb AB Kingo K Sofen H Ruer-Mulard M Singh V Pathan R Papavassilis C andCooper S.. Secukinumab administration by pre-filled syringe: efficacy, safety and usability results from a randomized controlled trial in psoriasis (FEATURE). Br J Dermatol 2015; 172: 484–493. [DOI] [PubMed] [Google Scholar]
- 23.Menter A Feldman SR Weinstein GD Papp K Evans R Guzzo C Li S Dooley LT Arnold C andGottlieb AB.. A randomized comparison of continuous vs. intermittent infliximab maintenance regimens over 1 year in the treatment of moderate-to-severe plaque psoriasis. J Am Acad Dermatol 2007; 56: 31.e31–e15. [DOI] [PubMed] [Google Scholar]
- 24.Papp K Thaçi D Reich K Riedl E Langley RG Krueger JG Gottlieb AB Nakagawa H Bowman EP Mehta A Li Q Zhou Y andShames R.. Tildrakizumab (MK-3222), an anti-interleukin-23p19 monoclonal antibody, improves psoriasis in a phase IIb randomized placebo-controlled trial. Br J Dermatol 2015; 173: 930–939. [DOI] [PubMed] [Google Scholar]
- 25.Papp KA Langley RG Lebwohl M Krueger GG Szapary P Yeilding N Guzzo C Hsu MC Wang Y Li S Dooley LT andReich K.. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 52-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 2). Lancet 2008; 371: 1675–1684. [DOI] [PubMed] [Google Scholar]
- 26.Reich K Wozel G Zheng H Van Hoogstraten HJF Flint L andBarker J.. Efficacy and safety of infliximab as continuous or intermittent therapy in patients with moderate-to-severe plaque psoriasis: results of a randomized, long-term extension trial (RESTORE2). Br J Dermatol 2013; 168: 1325–1334. [DOI] [PubMed] [Google Scholar]
- 27.Feldman SR Gordon KB Bala M Evans R Li S Dooley LT Guzzo C Patel K Menter A andGottlieb AB.. Infliximab treatment results in significant improvement in the quality of life of patients with severe psoriasis: a double-blind placebo-controlled trial. Br J Dermatol 2005; 152: 954–960. [DOI] [PubMed] [Google Scholar]
- 28.Krueger GG Langley RG Leonardi C Yeilding N Guzzo C Wang Y Dooley LT andLebwohl M.. A human interleukin-12/23 monoclonal antibody for the treatment of psoriasis. N Engl J Med 2007; 356: 580–592. [DOI] [PubMed] [Google Scholar]
- 29.Igarashi A Kato T Kato M Song M andNakagawa H.. Efficacy and safety of ustekinumab in Japanese patients with moderate-to-severe plaque-type psoriasis: long-term results from a phase 2/3 clinical trial. J Dermatol 2012; 39: 242–252. [DOI] [PubMed] [Google Scholar]
- 30.Kimball AB Gordon KB Langley RG Menter A Chartash EK andValdes J.. Safety and efficacy of ABT-874, a fully human interleukin 12/23 monoclonal antibody, in the treatment of moderate to severe chronic plaque psoriasis: results of a randomized, placebo-controlled, phase 2 trial. Arch Dermatol 2008; 144: 200–207. [DOI] [PubMed] [Google Scholar]
- 31.Krueger GG Papp KA Stough DB Loven KH Gulliver WP andEllis CN.. A randomized, double-blind, placebo-controlled phase III study evaluating efficacy and tolerability of 2 courses of alefacept in patients with chronic plaque psoriasis. J Am Acad Dermatol 2002; 47: 821–833. [DOI] [PubMed] [Google Scholar]
- 32.Akcali C Guven EH Kirtak N Inaloz HS Ozgoztasi O andGuvenc U.. Serum concentrations of interleukin-2 and tumour necrosis factor-alpha under cyclosporine versus acitretin treatment in plaque-type psoriasis. J Int Med Res 2014; 42: 1118–1122. [DOI] [PubMed] [Google Scholar]
- 33.Ohtsuki M Morita A Abe M Takahashi H Seko N Karpov A Shima T Papavassilis C andNakagawa H.. Secukinumab efficacy and safety in Japanese patients with moderate-to-severe plaque psoriasis: subanalysis from ERASURE, a randomized, placebo-controlled, phase 3 study. J Dermatol 2014; 41: 1039–1046. [DOI] [PubMed] [Google Scholar]
- 34.Gottlieb AB Evans R Li S Dooley LT Guzzo CA Baker D Bala M Marano CW andMenter A.. Infliximab induction therapy for patients with severe plaque-type psoriasis: a randomized, double-blind, placebo-controlled trial. J Am Acad Dermatol 2004; 51: 534–542. [DOI] [PubMed] [Google Scholar]
- 35.Leonardi CL Powers JL Matheson RT Goffe BS Zitnik R Wang A andGottlieb AB.. Etanercept as monotherapy in patients with psoriasis. N Engl J Med 2003; 349: 2014–2022. [DOI] [PubMed] [Google Scholar]
- 36.Gottlieb AB Leonardi C Kerdel F Mehlis S Olds M andWilliams DA.. Efficacy and safety of briakinumab vs. etanercept and placebo in patients with moderate to severe chronic plaque psoriasis. Br J Dermatol 2011; 165: 652–660. [DOI] [PubMed] [Google Scholar]
- 37.Gordon KB Langley RG Leonardi C Toth D Menter MA Kang S Heffernan M Miller B Hamlin R Lim L Zhong J Hoffman R andOkun MM.. Clinical response to adalimumab treatment in patients with moderate to severe psoriasis: double-blind, randomized controlled trial and open-label extension study. J Am Acad Dermatol 2006; 55: 598–606. [DOI] [PubMed] [Google Scholar]
- 38.Nakagawa H Niiro H andOotaki K.. Brodalumab, a human anti-interleukin-17-receptor antibody in the treatment of Japanese patients with moderate-to-severe plaque psoriasis: efficacy and safety results from a phase II randomized controlled study. J Dermatol Sci 2016; 81: 44–52. [DOI] [PubMed] [Google Scholar]
- 39.Menter A Gordon K Carey W Hamilton T Glazer S Caro I Li N andGulliver W.. Efficacy and safety observed during 24 weeks of efalizumab therapy in patients with moderate to severe plaque psoriasis. Arch Dermatol 2005; 141: 31–38. [DOI] [PubMed] [Google Scholar]
- 40.Papp KA Tyring S Lahfa M Prinz J Griffiths CEM Nakanishi AM Zitnik R andVan De Kerkhof PCM.. A global phase III randomized controlled trial of etanercept in psoriasis: safety, efficacy, and effect of dose reduction. Br J Dermatol 2005; 152: 1304–1312. [DOI] [PubMed] [Google Scholar]
- 41.Chaudhari U Romano P Mulcahy LD Dooley LT Baker DG andGottlieb AB.. Efficacy and safety of infliximab monotherapy for plaque-type psoriasis: a randomised trial. Lancet 2001; 357: 1842–1847. [DOI] [PubMed] [Google Scholar]
- 42.Dubertret L Sterry W Bos JD Chimenti S Shumack S Larsen CG Shear NH andPapp KA.. Clinical experience acquired with the efalizumab (Raptiva) (CLEAR) trial in patients with moderate-to-severe plaque psoriasis: results from a phase III international randomized, placebo-controlled trial. Br J Dermatol 2006; 155: 170–181. [DOI] [PubMed] [Google Scholar]
- 43.Asahina A Nakagawa H Etoh T andOhtsuki M.. Adalimumab in Japanese patients with moderate to severe chronic plaque psoriasis: efficacy and safety results from a phase II/III randomized controlled study. J Dermatol 2010; 37: 299–310. [DOI] [PubMed] [Google Scholar]
- 44.Leonardi CL Papp KA Gordon KB Menter A Feldman SR Caro I Walicke PA Compton PG andGottlieb AB.. Extended efalizumab therapy improves chronic plaque psoriasis: results from a randomized phase III trial. J Am Acad Dermatol 2005; 52: 425–433. [DOI] [PubMed] [Google Scholar]
- 45.Lebwohl M Christophers E Langley R Ortonne JP Roberts J andGriffiths CE.. An international, randomized, double-blind, placebo-controlled phase 3 trial of intramuscular alefacept in patients with chronic plaque psoriasis. Arch Dermatol 2003; 139: 719–727. [DOI] [PubMed] [Google Scholar]
- 46.Griffiths CEM Reich K Lebwohl M Van De Kerkhof P Paul C Menter A Cameron GS Erickson J Zhang L Secrest RJ Ball S Braun DK Osuntokun OO Heffernan MP Nickoloff BJ andPapp K.. Comparison of ixekizumab with etanercept or placebo in moderate-to-severe psoriasis (UNCOVER-2 and UNCOVER-3): results from two phase 3 randomised trials. Lancet 2015; 386: 541–551. [DOI] [PubMed] [Google Scholar]
- 47.Tyring S Gottlieb A Papp K Gordon K Leonardi C Wang A Lalla D Woolley M Jahreis A Zitnik R Cella D andKrishnan R.. Etanercept and clinical outcomes, fatigue, and depression in psoriasis: double-blind placebo-controlled randomised phase III trial. Lancet 2006; 367: 29–35. [DOI] [PubMed] [Google Scholar]
- 48.Paul C Lacour JP Tedremets L Kreutzer K Jazayeri S Adams S Guindon C You R andPapavassilis C.. Efficacy, safety and usability of secukinumab administration by autoinjector/pen in psoriasis: a randomized, controlled trial (JUNCTURE). J Eur Acad Dermatol Venereol 2015; 29: 1082–1090. [DOI] [PubMed] [Google Scholar]
- 49.Ortonne JP. Clinical response to alefacept: results of a phase 3 study of intramuscular administration of alefacept in patients with chronic plaque psoriasis. J Eur Acad Dermatol Venerol 2003; 17: 12–16. [DOI] [PubMed] [Google Scholar]
- 50.Youn JI Tsai TF Song M Shen YK Li S Choi JH Kim KJ andHo JC.. Efficacy and safety of ustekinumab for the treatment of moderate-to-severe psoriasis: results of a phase 3 trial in Taiwanese and Korean patients. J Dermatol 2010; 37: 121–122. [Google Scholar]
- 51.Reich K Nestle FO Papp K Ortonne JP Evans R Guzzo C Li S Dooley LT andGriffiths CE.. Infliximab induction and maintenance therapy for moderate-to-severe psoriasis: a phase III, multicentre, double-blind trial. Lancet 2005; 366: 1367–1374. [DOI] [PubMed] [Google Scholar]
- 52.Micali G Wilsmann-Theis D Mallbris L Gallo G Marino V Brault Y andGermain JM.. Etanercept reduces symptoms and severity of psoriasis after cessation of cyclosporine therapy: results of the SCORE study. Acta Derm Venerol 2015; 95: 57–61. [DOI] [PubMed] [Google Scholar]
- 53.Barker J Hoffmann M Wozel G Ortonne JP Zheng H van Hoogstraten H andReich K.. Efficacy and safety of infliximab vs. methotrexate in patients with moderate-to-severe plaque psoriasis: results of an open-label, active-controlled, randomized trial (RESTORE1). Br J Dermatol 2011; 165: 1109–1117. [DOI] [PubMed] [Google Scholar]
- 54.Papp KA Bressinck R Fretzin S Goffe B Kempers S Gordon KB Caro I Walicke PA Wang X andMenter A.. Safety of efalizumab in adults with chronic moderate to severe plaque psoriasis: a phase IIIb, randomized, controlled trial. Int J Dermatol 2006; 45: 605–614. [DOI] [PubMed] [Google Scholar]
- 55.Mease PJ Goffe BS Metz J VanderStoep A Finck B andBurge DJ.. Etanercept in the treatment of psoriatic arthritis and psoriasis: a randomised trial. Lancet 2000; 356: 385–390. [DOI] [PubMed] [Google Scholar]
- 56.van de Kerkhof PC Segaert S Lahfa M Luger TA Karolyi Z Kaszuba A Leigheb G Camacho FM Forsea D Zang C Boussuge MP Paolozzi L andWajdula J.. Once weekly administration of etanercept 50 mg is efficacious and well tolerated in patients with moderate-to-severe plaque psoriasis: a randomized controlled trial with open-label extension. Br J Dermatol 2008; 159: 1177–1185. [DOI] [PubMed] [Google Scholar]
- 57.Gordon K Langley R Gottlieb A Papp K Menter A Krueger G Strober B Gu Y andValdes J.. Efficacy and safety results from a phase III, randomized controlled trial comparing two dosing regimens of ABT-874 to placebo in patients with moderate to severe psoriasis. J Eur Acad Dermatol Venereol 2010; 24: 30–31. [Google Scholar]
- 58.Gordon KB Papp KA Hamilton TK Walicke PA Dummer W Li N Bresnahan BW andMenter A.. Efalizumab for patients with moderate to severe plaque psoriasis: a randomized controlled trial. JAMA 2003; 290: 3073–3080. [DOI] [PubMed] [Google Scholar]
- 59.Lebwohl M Strober B Menter A Gordon K Weglowska J Puig L Papp K Spelman L Toth D Kerdel F Armstrong AW Stingl G Kimball AB Bachelez H Wu JJ Crowley J Langley RG Blicharski T Paul C Lacour JP Tyring S Kircik L Chimenti S Callis Duffin K Bagel J Koo J Aras G Li J Song W Milmont CE Shi Y Erondu N Klekotka P Kotzin B andNirula A.. Phase 3 studies comparing brodalumab with ustekinumab in psoriasis. N Engl J Med 2015; 373: 1318–1328. [DOI] [PubMed] [Google Scholar]
- 60.Gottlieb AB Matheson RT Lowe N Krueger GG Kang S Goffe BS Gaspari AA Ling M Weinstein GD Nayak A Gordon KB andZitnik R.. A randomized trial of etanercept as monotherapy for psoriasis. Arch Dermatol 2003; 139: 1627–1632; discussion 1632. [DOI] [PubMed] [Google Scholar]
- 61.Torii H andNakagawa H.. Infliximab monotherapy in Japanese patients with moderate-to-severe plaque psoriasis and psoriatic arthritis. A randomized, double-blind, placebo-controlled multicenter trial. J Dermatol Sci 2010; 59: 40–49. [DOI] [PubMed] [Google Scholar]
- 62.Gordon KB Kimball AB Chau D Viswanathan HN Li J Revicki DA Kricorian G andOrtmeier BG.. Impact of brodalumab treatment on psoriasis symptoms and health-related quality of life: use of a novel patient-reported outcome measure, the Psoriasis Symptom Inventory. Br J Dermatol 2014; 170: 705–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Langley RG Elewski BE Lebwohl M Reich K Griffiths CEM Papp K Puig L Nakagawa H Spelman L Sigurgeirsson B Rivas E Tsai TF Wasel N Tyring S Salko T Hampele I Notter M Karpov A Helou S andPapavassilis C.. Secukinumab in plaque psoriasis – results of two phase 3 trials. N Engl J Med 2014; 371: 326–338. [DOI] [PubMed] [Google Scholar]
- 64.Papp KA Sundaram M Bao Y Williams DA Gu Y Signorovitch JE Wang Y Valdes JM andMulani PM.. Effects of briakinumab treatment for moderate to severe psoriasis on health-related quality of life and work productivity and activity impairment: results from a randomized phase III study. J Eur Acad Dermatol Venereol 2014; 28: 790–798. [DOI] [PubMed] [Google Scholar]
- 65.Zhu X Zheng M Song M Shen YK Chan D Szapary PO andWang B.. Efficacy and safety of ustekinumab in Chinese patients with moderate to severe plaque-type psoriasis: results from a phase 3 clinical trial (LOTUS). J Drugs Dermatol 2013; 12: 166–174. [PubMed] [Google Scholar]
- 66.Ellis CN andKrueger GG.. Treatment of chronic plaque psoriasis by selective targeting of memory effector T lymphocytes. N Engl J Med 2001; 345: 248–255. [DOI] [PubMed] [Google Scholar]
- 67.Papp K Bissonnette R Krueger JG Carey W Gratton D Gulliver WP Lui H Lynde CW Magee A Minier D Ouellet JP Patel P Shapiro J Shear NH Kramer S Walicke P Bauer R Dedrick RL Kim SS White M andGarovoy MR.. The treatment of moderate to severe psoriasis with a new anti-CD11a monoclonal antibody. J Am Acad Dermatol 2001; 45: 665–674. [DOI] [PubMed] [Google Scholar]
- 68.Thaçi D Blauvelt A Reich K Tsai TF Vanaclocha F Kingo K Ziv M Pinter A Hugot S You R andMilutinovic M.. Secukinumab is superior to ustekinumab in clearing skin of subjects with moderate to severe plaque psoriasis: CLEAR, a randomized controlled trial. J Am Acad Dermatol 2015; 73: 400–409. [DOI] [PubMed] [Google Scholar]
- 69.Menter A Tyring SK Gordon K Kimball AB Leonardi CL Langley RG Strober BE Kaul M Gu Y Okun M andPapp K.. Adalimumab therapy for moderate to severe psoriasis: a randomized, controlled phase III trial. J Am Acad Dermatol 2008; 58: 106–115. [DOI] [PubMed] [Google Scholar]
- 70.Papp K Leonardi C Menter A Thompson EH Milmont CE Kricorian G Nirula A andKlekotka P.. Safety and efficacy of brodalumab for psoriasis after 120 weeks of treatment. J Am Acad Dermatol 2014; 71: 1183–1190. [DOI] [PubMed] [Google Scholar]
- 71.Papp KA Langley RG Sigurgeirsson B Abe M Baker DR Konno P Haemmerle S Thurston HJ Papavassilis C andRichards HB.. Efficacy and safety of secukinumab in the treatment of moderate-to-severe plaque psoriasis: a randomized, double-blind, placebo-controlled phase II dose-ranging study. Br J Dermatol 2013; 168: 412–421. [DOI] [PubMed] [Google Scholar]
- 72.Revicki D Willian MK Saurat JH Papp KA Ortonne JP Sexton C andCamez A.. Impact of adalimumab treatment on health-related quality of life and other patient-reported outcomes: results from a 16-week randomized controlled trial in patients with moderate to severe plaque psoriasis. Br J Dermatol 2008; 158: 549–557. [DOI] [PubMed] [Google Scholar]
- 73.Blauvelt A Reich K Tsai TF Tyring S Vanaclocha F Kingo K Ziv M Pinter A Vender R Hugot S You R Milutinovic M andThaci D.. Secukinumab is superior to ustekinumab in clearing skin of subjects with moderate-to-severe plaque psoriasis up to 1 year: results from the CLEAR study. J Am Acad Dermatol 2017; 76: 60–69.e9. [DOI] [PubMed] [Google Scholar]
- 74.Kavanaugh A Puig L Gottlieb AB Ritchlin C You Y Li S Song M Randazzo B Rahman P andMcInnes IB.. Efficacy and safety of ustekinumab in psoriatic arthritis patients with peripheral arthritis and physician-reported spondylitis: post-hoc analyses from two phase III, multicentre, double-blind, placebo-controlled studies (PSUMMIT-1/PSUMMIT-2). Ann Rheum Dis 2016; 75: 1984–1988. [DOI] [PubMed] [Google Scholar]
- 75.Landells I Marano C Hsu MC Li S Zhu Y Eichenfield LF Hoeger PH Menter A Paller AS Taieb A Philipp S Szapary P andRandazzo B.. Ustekinumab in adolescent patients age 12 to 17 years with moderate-to-severe plaque psoriasis: results of the randomized phase 3 CADMUS study. J Am Acad Dermatol 2015; 73: 594–603. [DOI] [PubMed] [Google Scholar]
- 76.Mease PJ van der Heijde D Ritchlin CT Okada M Cuchacovich RS Shuler CL Lin CY Braun DK Lee CH andGladman DD.. Ixekizumab, an interleukin-17A specific monoclonal antibody, for the treatment of biologic-naive patients with active psoriatic arthritis: results from the 24-week randomised, double-blind, placebo-controlled and active (adalimumab)-controlled period of the phase III trial SPIRIT-P1. Ann Rheum Dis 2017; 76: 79–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Blauvelt A Papp KA Griffiths CE Randazzo B Wasfi Y Shen YK Li S andKimball AB.. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the continuous treatment of patients with moderate to severe psoriasis: results from the phase III, double-blinded, placebo- and active comparator-controlled VOYAGE 1 trial. J Am Acad Dermatol 2017; 76: 405–417. [DOI] [PubMed] [Google Scholar]
- 78.Cai L Gu J Zheng J Zheng M Wang G Xi LY Hao F Liu XM Sun QN Wang Y Lai W Fang H Tu YT Sun Q Chen J Gao XH Gu Y Teixeira HD Zhang JZ andOkun MM.. Efficacy and safety of adalimumab in Chinese patients with moderate-to-severe plaque psoriasis: results from a phase 3, randomized, placebo-controlled, double-blind study. J Eur Acad Dermatol Venereol 2017; 31: 89–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kavanaugh A Husni ME Harrison DD Kim L Lo KH Leu JH andHsia EC.. Safety and efficacy of intravenous golimumab in patients with active psoriatic arthritis: results through week 24 of the GO-VIBRANT study. Arthritis Rheumatol 2017; 69: 2151–2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lacour JP Paul C Jazayeri S Papanastasiou P Xu C Nyirady J Fox T andPapavassilis C.. Secukinumab administration by autoinjector maintains reduction of plaque psoriasis severity over 52 weeks: results of the randomized controlled JUNCTURE trial. J Eur Acad Dermatol Venereol 2017; 31: 847–856. [DOI] [PubMed] [Google Scholar]
- 81.Nash P Kirkham B Okada M Rahman P Combe B Burmester GR Adams DH Kerr L Lee C Shuler CL andGenovese M.. Ixekizumab for the treatment of patients with active psoriatic arthritis and an inadequate response to tumour necrosis factor inhibitors: results from the 24-week randomised, double-blind, placebo-controlled period of the SPIRIT-P2 phase 3 trial. Lancet 2017; 389: 2317–2327. [DOI] [PubMed] [Google Scholar]
- 82.Papp K Thaci D Marcoux D Weibel L Philipp S Ghislain PD Landells I Hoeger P Kotkin C Unnebrink K Seyger M andWilliams D.. Efficacy and safety of adalimumab every other week versus methotrexate once weekly in children and adolescents with severe chronic plaque psoriasis: a randomised, double-blind, phase 3 trial. Lancet 2017; 390: 40–49. [DOI] [PubMed] [Google Scholar]
- 83.Papp KA Reich K Paul C Blauvelt A Baran W Bolduc C Toth D Langley RG Cather J Gottlieb AB Thaci D Krueger JG Russell CB Milmont CE Li J Klekotka PA Kricorian G andNirula A.. A prospective phase III, randomized, double-blind, placebo-controlled study of brodalumab in patients with moderate-to-severe plaque psoriasis. Br J Dermatol 2016; 175: 273–286. [DOI] [PubMed] [Google Scholar]
- 84.Reich K Armstrong AW Foley P Song M Wasfi Y Randazzo B Li S Shen YK andGordon KB.. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the treatment of patients with moderate to severe psoriasis with randomized withdrawal and retreatment: results from the phase III, double-blind, placebo- and active comparator-controlled VOYAGE 2 trial. J Am Acad Dermatol 2017; 76: 418–431. [DOI] [PubMed] [Google Scholar]
- 85.Reich K Gooderham M Green L Bewley A Zhang Z Khanskaya I Day RM Goncalves J Shah K Piguet V andSoung J.. The efficacy and safety of apremilast, etanercept and placebo in patients with moderate-to-severe plaque psoriasis: 52-week results from a phase IIIb, randomized, placebo-controlled trial (LIBERATE). J Eur Acad Dermatol Venereol 2017; 31: 507–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Reich K Papp KA Blauvelt A Tyring SK Sinclair R Thaci D Nograles K Mehta A Cichanowitz N Li Q Liu K La Rosa C Green S andKimball AB.. Tildrakizumab versus placebo or etanercept for chronic plaque psoriasis (reSURFACE 1 and reSURFACE 2): results from two randomised controlled, phase 3 trials. Lancet 2017; 390: 276–288. [DOI] [PubMed] [Google Scholar]
- 87.Reich K Pinter A Lacour JP Ferrandiz C Micali G French LE Lomaga M Dutronc Y Henneges C Wilhelm S Hartz S andPaul C.. Comparison of ixekizumab with ustekinumab in moderate-to-severe psoriasis: 24-week results from IXORA-S, a phase III study. Br J Dermatol 2017; 177: 1014–1023. [DOI] [PubMed] [Google Scholar]
- 88.Dommasch ED Abuabara K Shin DB Nguyen J Troxel AB andGelfand JM.. The risk of infection and malignancy with tumor necrosis factor antagonists in adults with psoriatic disease: a systematic review and meta-analysis of randomized controlled trials. J Am Acad Dermatol 2011; 64: 1035–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.de Vries AC Thio HB de Kort WJ Opmeer BC van der Stok HM de Jong EM Horvath B Busschbach JJ Nijsten TE andSpuls PI.. A prospective randomized controlled trial comparing infliximab and etanercept in patients with moderate-to-severe chronic plaque-type psoriasis: the Psoriasis Infliximab vs. Etanercept Comparison Evaluation (PIECE) study. Br J Dermatol 2017; 176: 624–633. [DOI] [PubMed] [Google Scholar]
- 90.Hong CH Papp KA Lophaven KW Skallerup P andPhilipp S.. Patients with psoriasis have different preferences for topical therapy, highlighting the importance of individualized treatment approaches: randomized phase IIIb PSO-INSIGHTFUL study. J Eur Acad Dermatol Venereol 2017; 31: 1876–1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bagel J Duffin KC Moore A Ferris LK Siu K Steadman J Kianifard F Nyirady J andLebwohl M.. The effect of secukinumab on moderate-to-severe scalp psoriasis: results of a 24-week, randomized, double-blind, placebo-controlled phase 3b study. J Am Acad Dermatol 2017; 77: 667–674. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Table S1 for Quantitative evaluation to efficacy and safety of therapies for psoriasis: A network meta-analysis by Jingjing Lv, Dongmei Zhou, Yan Wang, Jingxia Zhao, Zhaoxia Chen, Jinchao Zhang, Tingting Di, Jing Hu, Bo Li, Ping Li and Feng Huang in Molecular Pain