Abstract
Maternal malnutrition during pregnancy impacts fetal growth, with developmental consequences that extend to later life outcomes. In underdeveloped countries, this malnutrition typically takes the form of poor dietary protein content and quality, even if adequate calories are consumed. Here, we report the establishment of a nonhuman primate model of gestational protein restriction (PR) in order to understand how placental function and pregnancy outcomes are affected by protein deficiency. Rhesus macaques were assigned to either a control diet containing 26% protein or switched to a 13% PR diet prior to conception and maintained on this PR diet throughout pregnancy. Standard fetal biometry, Doppler ultrasound of uteroplacental blood flow, ultrasound-guided amniocentesis, and contrast-enhanced ultrasound (CE-US) to assess placental perfusion were performed mid-gestation (gestational day 85 [G85] where term is G168) and in the early third trimester (G135). Our data demonstrate that a 50% reduction in dietary protein throughout gestation results in reduced placental perfusion, fetal growth restriction, and a 50% rate of pregnancy loss. In addition, we demonstrate reduced total protein content and evidence of fetal hypoxia in the amniotic fluid. This report highlights the use of CE-US for in vivo assessment of placental vascular function. The ability to detect placental dysfunction, and thus a compromised pregnancy, early in gestation, may facilitate the development of interventional strategies to optimize clinical care and improve long-term offspring outcomes, which are future areas of study in this new model.
Keywords: contrast-enhanced ultrasound, protein restriction, placental perfusion, in vivo imaging, placental dysfunction, nonhuman primate
Introduction
Numerous studies have demonstrated that maternal well-being and diet impact not only fetal development but also the long-term health outcomes of children. Our group has previously utilized a nonhuman primate model of diet-induced obesity to study the impact of maternal overnutrition on placental function, fetal development, and offspring outcomes.1-3 However, the effects of undernutrition, in particular protein deficiency, a global health concern that impacts predominantly low- and middle-income countries, on maternal–placental–fetal health are not well understood.
Maternal malnutrition is known to be detrimental to fetal development, with fetal growth restriction (FGR) commonly reported in both human epidemiological studies and in animal models of nutrient restriction.4-6 In addition, poor maternal diet is associated with impaired neurodevelopmental and behavioral outcomes in children,7 as well as an increased risk of other health complications such as metabolic and cardiovascular dysfunction.8-10 The timing and severity of any insult during pregnancy is a key determinant of outcome. Evidence from rodent models has demonstrated differential effects of nutrient deficiency on placental growth and fetal development, which are dependent on early versus mid-gestational pregnancy exposure.11-14 However, these models often employ short-term intrapartum malnutrition, which does not mimic the typical human situation in which dietary deficiency is usually a chronic issue that is not limited to pregnancy.
As the mediator between mother and fetus, the placenta has a pivotal role in conveying any dietary impact. The placenta is a multifunctional organ that integrates maternal and fetal nutritional and growth cues to optimize fetal growth potential. This dynamic organ has a substantial reserve capacity, enabling some compensatory adaptations to an adverse in utero environment or maternal perturbations.15 However, the effects of maternal protein restriction (PR) preconception and throughout pregnancy on in vivo placental function are poorly understood. Indeed, placental studies are largely restricted to postdelivery in vitro assessments conducted when the placenta has performed its function and exceeded its life span. In vivo studies are limited by current imaging capabilities and the reliability of biomarkers assessed in maternal blood, or in the amniotic fluid, which may reflect placental function and allow detection of placental insufficiency. Understanding placental function in real time, and thus facilitating the identification of at-risk pregnancies for potential intervention, is an important area of focus at present.16 As placental blood flow is a critical determinant of nutrient and oxygen transfer, and hence fetal growth, application and development of advanced noninvasive imaging modalities are rapidly expanding in placental functional studies. Doppler ultrasound is routinely used for clinical management of pregnancy, but although this semi-quantitative method is suitable for blood flow measurements in major vessels, its application to the direct study of placental perfusion is not possible. Measurement of the umbilical artery pulsatility index (PI) reflects hemodynamics of the placental villi with an increase in PI reflecting an increase in resistance in the microcirculation.17 However, a significant increase in PI only occurs when ∼60% of terminal vascular branches are destroyed,18 and thus, this is not a sensitive measure of placental dysfunction. We have recently implemented contrast-enhanced ultrasound (CE-US) to visualize and quantify microvascular perfusion of the placental intervillous space (IVS).19 This is a technique that relies on the acoustic detection of lipid-encapsulated, gas-filled microbubble contrast agents for the visualization of vascular perfusion20 and is widely used in cardiac diagnostic imaging.21,22 By applying custom algorithms to the data, replenishment kinetic curves can be generated to allow quantitative measurement of microvascular flux rate.23 The use of CE-US has advanced our placental imaging capabilities.19 Earlier identification of at-risk pregnancies may allow for better clinical management and intervention to improve maternal and fetal health.
Given the numerous and far-reaching consequences of maternal health and diet on the long-term health of the offspring, we have established a new nonhuman primate model of gestational PR for the study of maternal, placental, fetal, and neonatal outcomes. This clinically relevant translational animal model supports a multidisciplinary approach to understanding the mechanisms underlying impaired neonatal growth and development following gestational PR. Our long-term goal is the development of safe and effective interventional strategies to address the issue of maternal malnutrition and improve global health. In this initial report from this new animal model, we present our findings of the impact of PR on maternal body composition and metabolic regulation, pregnancy outcomes, and fetal growth parameters. Our specific focus was the assessment of in vivo placental function using advanced ultrasound imaging modalities. We hypothesized that reduced maternal dietary protein content would reduce perfusion of the placental IVS and compromise fetal growth.
Methods
Animals
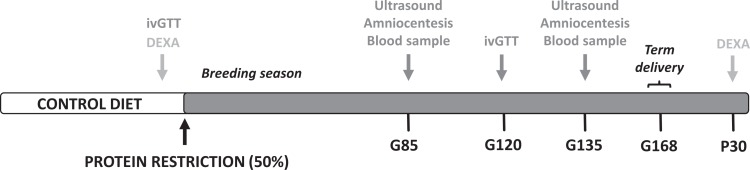
All protocols were approved by the Institutional Animal Care and Utilization Committee of the Oregon National Primate Research Center (ONPRC), and guidelines for humane animal care were followed. Adult rhesus macaques (Macaca mulatta) were maintained on either a control chow diet consisting of 26% protein (CON) or a protein-modified diet containing 13% protein (PR). Animals in the PR cohort were switched from the CON to the PR diet 1 month prior to being housed with a male. Both diets were matched for vitamin and micronutrient content, with the calorie content deficit made up with additional carbohydrates in the PR diet (TestDiet, St Louis, Missouri). Animals were socially housed in indoor/outdoor pens with 10 females and 1 male in each dietary cohort with ad libitum access to food and water. Animals were allowed to breed naturally, and pregnancies were identified by routine early first-trimester 2-dimensional ultrasound (GE Voluson 730 Expert, Kretztechnik, Austria) with fetal biometry for gestational dating. Fetal biometry measurements are well established in our rhesus colony and are routinely used for gestational dating. The breeding season lasted approximately 3 months for both diet cohorts. Total body fat for each animal was determined by conducting dual-energy X-ray absorptiometry (DEXA) scans (Hologic QDR Discovery A; Hologic, Inc, Bedford, Massachusetts). Animals were sedated with 10 mg/kg ketamine, intramuscular (IM) (Henry Schein Animal Health, Dublin, Ohio) and positioned supine on the bed of the scanner. Total body scans (core; collar bones through to hip bones and periphery; limbs) were performed on each animal. QDR software (Hologic) was used to calculate body composition. Intravenous glucose tolerance tests (ivGTTs) were performed on sedated dams (10 mg/kg ketamine, IM) after overnight food withdrawal to establish baseline glucose, normal glucose clearance, baseline insulin, and C-peptide levels. The ivGTTs were performed as described previously24 at 2 time points: prior to the diet switch and in the early third trimester at gestational day 120 (G120; Figure 1).
Figure 1.
Study design overview. Timeline of the experimental design indicating the dietary switch prior to the natural breeding season and the study time points. Maternal metabolic measurements (DEXA and ivGTT) and imaging studies consisting of ultrasound, amniocentesis, and blood sampling are indicated. Animals were allowed to deliver naturally at term and offspring were followed postnatally until 7 months of age. DEXA indicates dual-energy X-ray absorptiometry; G, gestational day; ivGTT, intravenous glucose tolerance tests; P, postnatal day.
Imaging Studies
Term Gestation in the rhesus macaque is ∼168 days. At gestational days 85 and 135, following an overnight fast, animals were sedated by IM injection with 10 mg/kg of ketamine. Animals were then intubated and maintained under anesthesia with 1% to 2% inhaled isoflurane gas for the duration of each imaging study. All studies were conducted in the ONPRC surgical unit. For ultrasound, participants were positioned in dorsal recumbency, and physiological vital signs were monitored throughout the procedure. Image-directed pulsed and color Doppler equipment (GE Voluson 730 Expert; Kretztechnik, Zipf, Austria) with a 5 to 9 MHz sector probe was used for ultrasonographic data collection by using an ultrasonographer (J.O.L. or A.E.F.). The lowest high-pass filter level was used (100 Hz), and an angle of 15° or less between the vessel and Doppler beam was deemed acceptable. Standard fetal biometry measurements consisting of biparietal diameter (BPD), head circumference (HC), abdominal circumference, and femur length (FL) were obtained. Blood flow velocity waveforms were obtained from the proximal portion of the uterine artery as described previously.25 Measurements of the waveforms were facilitated by the software supplied on the ultrasound machine, and the PI, velocity time integral, and heart rate were obtained. The diameter of the uterine artery was measured using power angiography as previously described25 and utilized in our prior nonhuman primate studies.1,15,26,27 The cross-sectional area (CSA) of the vessel was calculated as CSA = π (d/2)2 where d is diameter. The calculated uterine artery volume blood flow (cQuta) was calculated as velocity time integral × CSA × heart rate. For the quantitative estimation of blood flow on the fetal side of the placenta, the umbilical venous volume blood flow (cQuv) was calculated as mean velocity × CSA × 60, where the mean velocity was calculated as 0.5 of the maximum velocity, as described previously.1
Ultrasound-guided amniocentesis was accomplished by sterilizing the maternal abdomen with ChloraPrep (a chlorhexidine/alcohol solution) followed by ultrasound localization of a suitable sample site. A 22-gauge spinal needle was inserted transabdominally into the amniotic cavity with care taken to avoid the placenta and fetus. A 5 mL amniotic fluid sample was removed using a 10 mL syringe. Samples were centrifuged at 1,800 xg for 15 minutes, aliquoted, and stored at −80°C for later analysis.
Contrast-enhanced ultrasound was performed using a multiphase amplitude modulation and phase-inversion algorithm on a Sequoia system (Siemens Medical Systems, Mountain View, California) equipped with a 15L8 transducer at a transmit frequency of 7 MHz with a 0.18 mechanical index (MI) and a 55 dB dynamic range. Lipid-shelled octafluoropropane microbubble contrast reagent (Definity; Lantheus Medical Imaging, Billerica, Massachusetts) was prepared in 0.9% saline at a final concentration of 5%. Intravenous infusion via a cephalic vein catheter was initially performed at a rate of 60 mL/h for visualization of uteroplacental blood flow. The acoustic beam was centered over individually identified maternal spiral artery sources, and the microbubbles within the path of the beam were destroyed by a brief (2-second) increase in MI to 1.9. Microbubble reentry in the spiral artery and the IVS was recorded at 1 frame/75 milliseconds (rapidly refilling vessels) or 1 frame/125 milliseconds (slower refilling vessels) until the area of interest reached signal saturation. Upon completion of placental imaging, the intravenous infusion rate was reduced to 10 mL/h to obtain baseline measurements of the maternal blood pool at 2 sites: the brachial artery of the noncatheterized arm and within the uterine artery by the uterocervical junction. Digital video clips were recorded for 10-second durations to measure video intensity of the blood pool. Three replicates of all recordings were obtained during each study for data acquisition validation. Digital imaging data were analyzed using a custom-designed CE-US analysis program as previously described in detail.19 In brief, regions of interest were drawn over the IVS perfused by 1 maternal spiral artery input source. Replenishment kinetic curves were generated in a custom software program, and microvascular flux rate (β) was calculated as the rate of refilling of the vascular space until signal saturation was reached.
Amniotic Fluid Analysis
Individual amniotic fluid samples (G85 CON, n = 8; G85 PR, n = 8; G135 CON, n = 7; G135 PR, n = 7) were assayed for protein content (DC Protein Assay; BioRad, Richmond, California, Cat# 500-0116) and for erythropoietin levels by enzyme-linked immunosorbent assay according to the manufacturers’ instructions (MyBiosource, San Diego, CA, Cat# MBS2509449).
Statistical Analysis
Maternal body weight, glucose tolerance measurements, ultrasound parameters, and expression levels of erythropoietin were compared between the 2 diet groups at both G85 and G135 using a 2-way analysis of variance (ANOVA) with either a Tukey or a Sidak multiple comparison post hoc test performed using statistical analysis software (GraphPad Prism, La Jolla, California). In addition, linear regression analysis was performed for CE-US data replicates. A P < .05 was considered to be significant.
Results
Maternal Phenotype
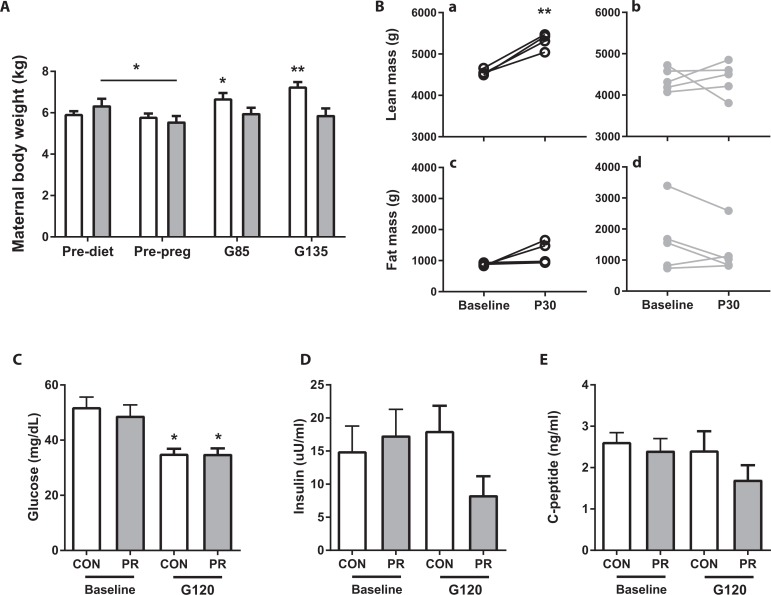
Animals switched from a CON diet to a 50% PR diet prior to the breeding season (Figure 1) demonstrated initial weight loss (pre-diet to pre-pregnancy time point; Figure 2A). In CON animals, there was appropriate weight gain during pregnancy. By comparison, PR animals maintained but did not increase their body weight (Figure 2A). Maternal body composition was assessed by DEXA scan prior to the diet switch and 30 days postpartum (P30, Figure 1). There was an increase in lean mass in the CON animals but no change in fat mass in either animal cohort (Figure 2B). There was no change in bone mineral content or density in either diet cohort (data not shown).
Figure 2.
Maternal phenotype. A, Maternal body weight at 4 time points: prior to diet switch (pre-diet), post-diet switch, preconception (pre-preg), and at G85 and G135; (B) DEXA data demonstrating lean mass in (a) CON and (b) PR and fat mass in (c) CON and (d) PR animals prior to dietary switch (baseline) and 30 days postinfant delivery (P30). Fasting glucose (C), fasting insulin (D), and C-peptide levels (E) in CON and PR animals prepregnancy (baseline) and in the early third trimester (G120). Data are mean + SEM. *P < .05 third trimester versus baseline, **P < .01 P30 versus baseline, by 2-way ANOVA with Sidak post hoc test. ANOVA indicates analysis of variance; DEXA dual-energy X-ray absorptiometry; PR, protein restriction; SEM, standard error of the mean.
Maternal fasting glucose concentrations were significantly reduced from baseline (pre-pregnancy) to third trimester (G120) but were not affected by a chronic PR diet (Figure 2C). Fasting insulin and C-peptide levels were not significantly affected either by pregnancy or consumption of the PR diet (Figure2D and E), although levels appeared to be slightly reduced in the PR cohort compared to CON in the early third trimester.
Maternal blood samples were taken at both G85 and G135 in conjunction with the ultrasound imaging time points. By blood gas analysis, maternal hemoglobin (Hgb) levels were within the normal range28 but were lower in PR compared to CON pregnant animals (Hgb:G85: 12.43 ± 0.89 vs. 14.45 ± 0.35, G135: 11.21 ± 0.38 vs. 13.37 ± 0.46*, mean ± standard error of the mean [SEM], *P < .05 2-way ANOVA with Sidak post hoc test), indicating maternal anemia in the PR group.
Gestational PR, Pregnancy Outcome, and Fetal Growth
Conception rates were not adversely affected in this study, with the pregnancy rate in the CON cohort being 9 of 10, and in the gestational PR cohort, all 10 animals became pregnant. However, there was a 50% pregnancy loss rate in the PR cohort, with 5 of 10 pregnancies resulting in miscarriage (at gestational ages 46, 48, 104, 146, and 153 days) compared to 2 losses in the CON group (at gestational ages 67 and 133 days).
Of the pregnancies that continued to full term, the mean gestational age at delivery was 165.4 days in the CON cohort (range: 159-170 days) and 170.6 days in the PR cohort (range: 165-188). The ratio of male:female offspring was 3:4 in CON and 2:3 in PR animals, respectively. Standard fetal biometry measurements obtained by ultrasound are reported in Table 1. Abdominal circumference was significantly reduced at both G85 and G135 in PR compared to CON fetuses (Table 1). Similarly, there was evidence of reduced BPD, HC, and FL at both gestational age time points in the PR fetuses (Table 1).
Table 1.
Fetal Biometry Measures.a
| G85 | G135 | |||
|---|---|---|---|---|
| CON (n = 8) | PR (n = 8) | CON (n = 7) | PR (n = 7) | |
| Biparietal diameter, mm | 29.16 ± 0.52 | 27.52 ± 0.86 | 44.34 ± 0.54 | 42.61 ± 1.10 |
| Head circumference, mm | 10.67 ± 0.26 | 10.26 ± 0.28 | 16.78 ± 0.16 | 16.08 ± 0.38 |
| Abdominal circumference, mm | 9.201 ± 0.13 | 8.347 ± 0.24b | 13.6 ± 0.34 | 12.2 ± 0.46c |
| Femur length, mm | 18.31 ± 0.43 | 16.89 ± 0.51d | 36.61 ± 0.31 | 33.92 ± 1.53 |
Abbreviations: PR, protein restriction; SEM, standard error of the mean.
aData are mean ± SEM.
b P < .01.
c P < .05.
d P = .052.
Uteroplacental Blood Flow
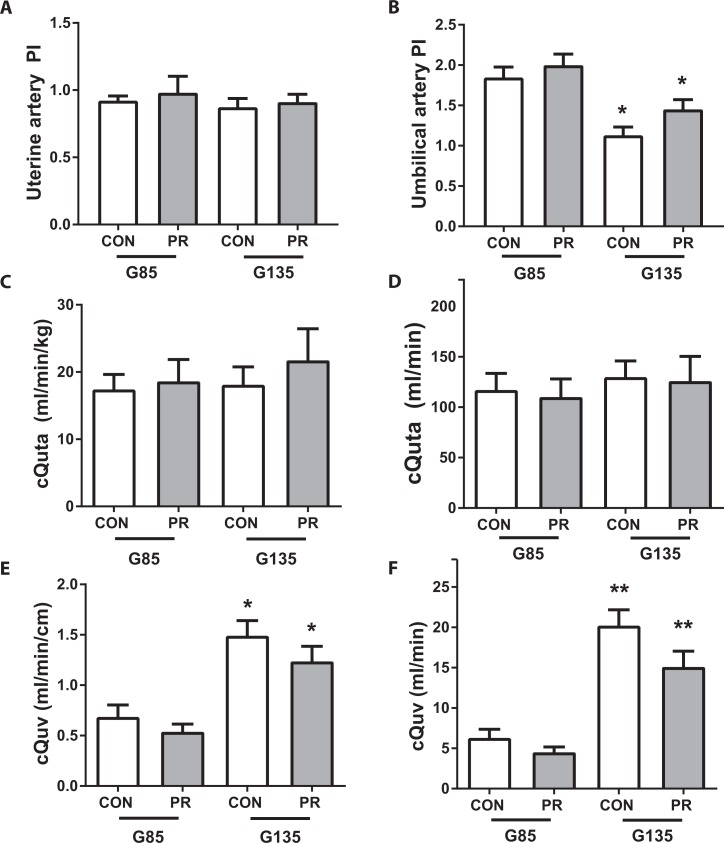
The uterine artery PI was not different either by diet group or gestational age (Figure 3A). The umbilical artery PI was not affected by diet group but was significantly reduced with advancing gestation (Figure 3B, P < .01). Maternal uterine artery blood flow (cQuta) and placental blood flow (cQuv) were calculated from Doppler ultrasound measurements of vessel diameter and velocity time integral as previously described.1,26,27 There was no difference in cQuta either across gestation (G85 to G135) or between the 2 diet cohorts (Figure 3C and D). We demonstrate a gestational age-dependent increase in cQuv but no significant difference between the CON and PR cohorts (Figure 3E and F).
Figure 3.
Uteroplacental blood flow assessed by Doppler ultrasound. A, Uterine artery pulsatility index and (B) umbilical artery pulsatility index; (C) cQuta normalized to maternal body weight and (D) unadjusted cQuta; (E) cQuv expressed as blood flow in mL/min normalized to fetal abdominal circumference and (F) unadjusted cQuv. All measurements taken by Doppler ultrasound in CON and PR animals at G85 (n = 8/group) and G135 (n = 7/group). Data are mean + SEM. *P < .05, **P < .01, G135 versus G85, by 2-way ANOVA with Tukey post hoc test. ANOVA indicates analysis of variance; PR, protein restriction; SEM, standard error of the mean.
Placental Perfusion
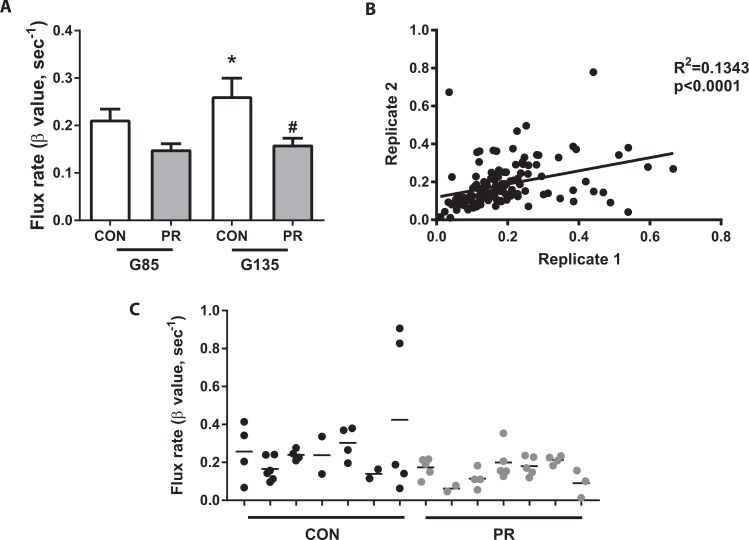
Contrast-enhanced ultrasound with Definity microbubble contrast agent was used to locate and visualize maternal spiral arteries supplying individual placental cotyledons at G85 and G135. Our CE-US data analysis does not allow for absolute quantification of blood flow volumes but instead reflects the change in vascular resistance. Thus, an increase in flux rate indicates an increase in perfusion of the placental IVS. In CON animals, we demonstrate a gestational age–dependent increase in placental perfusion from G85 to G135 (Figure 4A). By comparison, the flux rate was numerically lower at G85 in PR animals versus CON and significantly diminished at G135, with no increase in perfusion with gestational age in this cohort (Figure 4A). Video link 1 shows an example from a CON animal where the microbubbles within the acoustic window are destroyed and then rapid refilling of the maternal spiral artery and placental IVS can be seen. By contrast, in video link 2, we demonstrate delayed and slow refilling in a PR-fed animal that subsequently had a late pregnancy loss at G146. For validation of data reproducibility, we analyzed the variability within data acquisition replicates (Figure 4B). Importantly, these data were highly correlated (P < .0001). Additionally, data variability within individual spiral arteries of each animal demonstrates regional perfusion differences across the placenta (Figure 4C).
Figure 4.
Placental perfusion by contrast-enhanced ultrasound. A, Flux rate (β) calculated from the rate of vascular refilling at G85 and G135. Data are mean + SEM from n = 29 (CON) and n = 39 (PR) spiral artery sources from 8 animals/cohort at G85, and n = 27 (CON) and n = 28 (PR) spiral artery sources from 7 animals/cohort at G135. *P < .05 G135 CON versus G85 CON, #P < .05 G135 PR versus G135 CON, by 2-way ANOVA with Tukey post hoc test. B, Linear regression plot of 2 replicate measures of microvascular flux taken in repeated data acquisition sets of 3. R 2 = .1343, P < .0001. C, Flux values from individual spiral artery sources in CON and PR (n = 7/group) animals at G135, demonstrating variance in microvascular flux rates within each placenta. ANOVA indicates analysis of variance; PR, protein restriction; SEM, standard error of the mean.
Amniotic Fluid Analysis
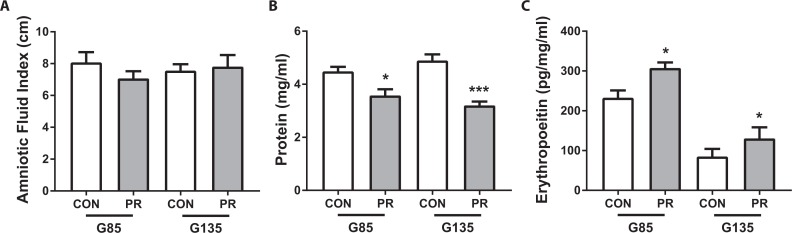
The amniotic fluid index (AFI) was calculated in standard fashion as the sum of the deepest amniotic fluid pocket in 4 quadrants. In addition, amniotic fluid samples were obtained by ultrasound-guided amniocentesis at G85 and G135. There was no difference in AFI either with gestational age or diet (Figure 5A). However, total protein content within the amniotic fluid was significantly lower in PR animals compared to CON at both time points (Figure 5B). In both diet cohorts, erythropoietin expression in the amniotic fluid was significantly higher at G85 than G135 (Figure 5C). In addition, erythropoietin levels were significantly elevated in the PR animals compared to controls at both time points, suggesting some evidence of fetal hypoxia in this model of gestational PR.
Figure 5.
Amniotic fluid analysis. A, Amniotic fluid index measured by ultrasound, (B) total protein content of the amniotic fluid, and (C) erythropoietin levels in amniotic fluid corrected for total protein content in CON and PR animals (n = 8/cohort at G85 and n = 7/cohort at G135). *P < .05, ***P < .001 PR vs CON, by 2-way ANOVA with Tukey post hoc test. ANOVA indicates analysis of variance; PR, protein restriction.
Discussion
Here we report observations and data from a newly established nonhuman primate model of gestational PR for the study of maternal, placental, fetal, and offspring outcomes. Animal models can provide valuable insights into human disease conditions as they allow for experimental manipulation and can be conducted in controlled environments to eliminate variables that often confound human studies. The nonhuman primate, like humans, has a long gestation, a hemochorial placenta, and similar pre- and postnatal developmental milestones, making this species well suited for translational studies. This initial report has focused on the effects of gestational PR on placental function and pregnancy outcomes. Importantly, we demonstrate data to show that a 50% reduction in dietary protein generates a maternal phenotype in the nonhuman primate that reflects the human condition of maternal malnourishment, where poor diet and nutritional reserves detrimentally impact fetal growth and development.29 Specifically, the cohort of PR-fed females lost weight initially (pre-pregnancy) and, despite being able to maintain body weight across gestation, failed to gain an appropriate amount of weight typically required to match the metabolic demands needed for maintenance of a healthy pregnancy. Interestingly, we demonstrate no change in fat mass in either diet cohort but an increase in lean mass in the control animals. This can likely be explained by the change in housing environment; prior to the study, these animals were indoor cage housed, and following the diet switch, they were moved to group housing in larger indoor–outdoor pens. Increased physical activity accompanied this change in housing setting. Our data show that 50% PR does not significantly alter glucose sensitivity in the nonhuman primate. However, the PR cohort was associated with decreased maternal Hgb levels reported at G85 and G135, which is consistent with prior studies in canines30 and swine31 that reported anemia secondary to protein deficiency; when protein supply is limited, tissues compete for available resources and consequently Hgb production is diminished.32
Interestingly, there was no negative impact of protein deficiency on conception rates, but we observed a high incidence of fetal loss in the PR cohort, with 50% of the pregnancies resulting in miscarriage. This finding corresponds to the higher stillbirth rates reported in human epidemiological studies33 and fits with poor maternal health and the inability to support the physiological demands of pregnancy. The rate of pregnancy loss determined over a 10-year period in rhesus macaques at the California primate center was 17%.34 An estimate of spontaneous pregnancy loss in the general ONPRC rhesus macaque colony is lower, at 5% to 7%, and we have previously reported a 7% loss rate in Japanese macaques.1 Thus, it is important to acknowledge the higher rate of pregnancy loss in our CON cohort (2 of 9). This is likely explained by instability within the newly established animal group induced by male aggression that occurred after pregnancies were identified. Unfortunately, these groups were newly formed, so the dominance hierarchy was not well established and this resulted in an unstable group environment.
One challenge of this new model was the lack of direct access to placental tissue as the pregnancies were continued to full term for natural delivery in group housing where viable tissue cannot be collected postdelivery. This issue was addressed using 2 approaches: (1) by implementing in vivo ultrasound modalities to monitor placental vascular function, and (2) by sampling amniotic fluid for in vitro analysis as a proxy for placental function/health. In vivo tools to assess placental function are crucial to advance our ability to detect placental insufficiency at a time in pregnancy when effective interventions could be implemented. Doppler ultrasound is an established antenatal surveillance method in the clinical management of high-risk pregnancies35-37; however, current Doppler ultrasound measurements lack the sensitivity and specificity to identify pregnancies at risk of placental dysfunction when applied as an early screening test.38 Advances in ultrasound technology permit semi-quantitative estimation of total uterine artery volume blood flow (reported as cQuta in our data set) to assess blood flow on the maternal side of the placenta, and these measurements correlate well with directly measured blood flow volume in the uterine artery in sheep studies25,39 and have been extensively used by our group in a number of nonhuman primate models of placental dysfunction.1,15,26,27 However, we estimate that vascular insufficiency greater than 50% is needed before detection by Doppler is feasible. Unsurprisingly, using these Doppler calculations, we were unable to detect any apparent differences in uteroplacental blood flow in the 2 diet cohorts. However, the use of CE-US allowed us to detect significantly reduced maternal perfusion of the IVS in the placenta of the PR animals, highlighting its potential to identify placental dysfunction beyond current clinical capabilities. This reduced placental perfusion will undoubtedly impact nutrient and oxygen transfer as maternal blood supply to the placenta is a key determinant of maternal–fetal exchange function. Although the placenta has the ability to make adaptive changes in response to perturbations, or a suboptimal in utero environment, inadequate nutrient availability can result in FGR.40 Indeed, we find FGR following PR diet consumption in this cohort. Future studies with this model will include scheduled cesarean section deliveries to allow for placental tissue collection and analysis. Such studies will allow us to examine the causal link between the PR diet and FGR. We might speculate, based on the slower and delayed rate of refilling of the IVS seen by CE-US, that FGR is a consequence of poor perfusion and thus decreased capacity for maternal–fetal exchange rather than the converse situation where a small fetus has a decreased demand for placental blood flow. In vivo imaging data will be correlated with in vitro assessments of transport function and tissue morphology in the planned studies.
The mechanisms underlying reduced placental perfusion with PR are not currently understood. We could speculate that it may be due to structural differences in spiral artery remodeling in early pregnancy. Data from a swine model of protein deficiency in which growth restriction and reduced litter size were reported demonstrate decreased availability of arginine accompanied by reduced nitric oxide synthase (NOS) activity in the placenta and endometrium of low protein-fed animals.41 The authors suggest that decreased nitric oxide synthesis impairs angiogenesis leading to reduced placental–fetal blood flow and impaired amino acid transport by the placenta. In support of this, evidence from an endothelial NOS knockout mice model shows impaired blood flow and intrauterine growth restriction.42 In addition, reduced spiral artery remodeling is reported with both a reduction in the amount of coiling and in the length of the spiral arteries noted.43 Structural alterations in the placenta have also been reported in a guinea pig model of maternal food restriction.44 In that study, a 30% reduction in chow consumption prior to pregnancy and up to mid-gestation with 10% reduction for the remainder of pregnancy reduced the placental exchange surface area and increased barrier thickness, resulting in impaired function and FGR.44 The potential role of nitric oxide–mediated mechanisms and structural changes in placental tissue will be examined in our future studies.
An alternative cause of reduced placental perfusion is tissue inflammation. In our ongoing investigations, we are using molecular imaging with phosphatidylserine (PS)-containing microbubbles to address this. The PS microbubbles are charged, but net neutral, and can be used in combination with ultrasound to identify sites of leukocyte activation,45 allowing inflammation to be visualized in real time in the IVS of the placenta.
The amniotic fluid is a protein-rich environment. Here, we report no difference in the volume of amniotic fluid between the 2 diet cohorts but a significant decrease in total protein content of the amniotic fluid in PR animals. The reason for this decrease is not known, but the unaltered AFI suggests that it is not largely due to a reduction in fetal renal filtration. It may be secondary to reduced maternal protein availability and allocation of resources, but further studies are needed to understand this finding. Fetal hypoxia was assessed by measuring erythropoietin levels in the amniotic fluid. Erythropoietin does not cross the placenta; therefore, levels in the amniotic fluid reflect fetal synthesis of erythropoietin in response to reduced oxygen availability.46 We found an increase in erythropoietin levels with PR in mid-gestation and in the early third trimester, indicating some relative fetal hypoxia resulting from maternal protein deficiency.
In summary, we report multiple adverse effects of gestational PR on maternal health, placental vascular function, and fetal growth in this new nonhuman primate model. Future work will focus on understanding the mechanisms underlying abnormal placental perfusion due to maternal PR and how transport function is impacted. We recently demonstrated encouraging safety results in both nonhuman primates and early human pregnancies, suggesting that CE-US does not affect placental tissue integrity.19 Thus, in conclusion, we suggest that CE-US may provide a practical clinical tool for in vivo assessment of placental perfusion and may facilitate the early identification of pregnancies at risk of placental dysfunction. Our ultimate goal is to utilize this nonhuman primate model for the development of novel interventional strategies to alter the trajectory of at-risk pregnancies and improve offspring outcomes from protein-deficient mothers.
Supplementary Material
Footnotes
Authors’ Note: The work presented in this manuscript was performed at the Oregon National Primate Research Center (ONPRC). The ONPRC abides by the Animal Welfare Act and regulations enforced by the USDA. Part of the work presented in this manuscript was published as an abstract in Reproductive Sciences following an oral presentation at the Society for Reproductive Investigation Annual Meeting in 2016.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Funding for this project was provided by The Bill & Melinda Gates Foundation (OPP1110865). Additional support provided by NIH grants R21 HD076265, R24 DK090964, and P51 OD011092.
Supplemental material: Supplementary material for this article is available online.
References
- 1. Frias AE, Morgan TK, Evans AE, et al. Maternal high-fat diet disturbs uteroplacental hemodynamics and increases the frequency of stillbirth in a nonhuman primate model of excess nutrition. Endocrinology. 2011;152(6):2456–2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. McCurdy CE, Bishop JM, Williams SM, et al. Maternal high-fat diet triggers lipotoxicity in the fetal livers of nonhuman primates. J Clin Invest. 2009;119(2):323–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sullivan EL, Grayson B, Takahashi D, et al. Chronic consumption of a high-fat diet during pregnancy causes perturbations in the serotonergic system and increased anxiety-like behavior in nonhuman primate offspring. J Neurosci. 2010;30(10):3826–3830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lumey LH, Stein AD. Offspring birth weights after maternal intrauterine undernutrition: a comparison within sibships. Am J Epidemiol. 1997;146(10):810–819. [DOI] [PubMed] [Google Scholar]
- 5. Elias AA, Ghaly A, Matushewski B, Regnault TR, Richardson BS. Maternal nutrient restriction in guinea pigs as an animal model for inducing fetal growth restriction. Reprod Sci. 2016;23(2):219–227. [DOI] [PubMed] [Google Scholar]
- 6. Gonzalez PN, Gasperowicz M, Barbeito-Andres J, Klenin N, Cross JC, Hallgrimsson B. Chronic protein restriction in mice impacts placental function and maternal body weight before fetal growth. PLoS One. 2016;11(3):e0152227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Morgane PJ, Austin-LaFrance R, Bronzino J, et al. Prenatal malnutrition and development of the brain. Neurosci Biobehav Rev. 1993;17(1):91–128. [DOI] [PubMed] [Google Scholar]
- 8. Barker DJ, Gluckman PD, Godfrey KM, Harding JE, Owens JA, Robinson JS. Fetal nutrition and cardiovascular disease in adult life. Lancet. 1993;341(8850):938–941. [DOI] [PubMed] [Google Scholar]
- 9. Singhal A, Lucas A. Early origins of cardiovascular disease: is there a unifying hypothesis? Lancet. 2004;363(9421):1642–1645. [DOI] [PubMed] [Google Scholar]
- 10. Painter RC, de Rooij SR, Bossuyt PM, et al. Early onset of coronary artery disease after prenatal exposure to the Dutch famine. Am J Clin Nutr. 2006;84(2):322–327; quiz 466-327. [DOI] [PubMed] [Google Scholar]
- 11. Fernandez-Twinn DS, Ozanne SE, Ekizoglou S, et al. The maternal endocrine environment in the low-protein model of intra-uterine growth restriction. Br J Nutr. 2003;90(4):815–822. [DOI] [PubMed] [Google Scholar]
- 12. Rutland CS, Latunde-Dada AO, Thorpe A, Plant R, Langley-Evans S, Leach L. Effect of gestational nutrition on vascular integrity in the murine placenta. Placenta. 2007;28(7):734–742. [DOI] [PubMed] [Google Scholar]
- 13. Coan PM, Vaughan OR, Sekita Y, et al. Adaptations in placental phenotype support fetal growth during undernutrition of pregnant mice. J Physiol. 2010;588(pt 3):527–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Vaughan OR, Sferruzzi-Perri AN, Coan PM, Fowden AL. Environmental regulation of placental phenotype: implications for fetal growth. Reprod Fertil Dev. 2011;24(1):80–96. [DOI] [PubMed] [Google Scholar]
- 15. Roberts VH, Rasanen JP, Novy MJ, et al. Restriction of placental vasculature in a non-human primate: a unique model to study placental plasticity. Placenta. 2012;33(1):73–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Guttmacher AE, Maddox YT, Spong CY. The Human Placenta Project: placental structure, development, and function in real time. Placenta. 2014;35(5):303–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Acharya G, Erkinaro T, Makikallio K, Lappalainen T, Rasanen J. Relationships among Doppler-derived umbilical artery absolute velocities, cardiac function, and placental volume blood flow and resistance in fetal sheep. Am J Physiol Heart Circ Physiol. 2004;286(4):H1266–H1272. [DOI] [PubMed] [Google Scholar]
- 18. Thompson RS, Trudinger BJ. Doppler waveform pulsatility index and resistance, pressure and flow in the umbilical placental circulation: an investigation using a mathematical model. Ultrasound Med Biol. 1990;16(5):449–458. [DOI] [PubMed] [Google Scholar]
- 19. Roberts VH, Lo JO, Salati JA, et al. Quantitative assessment of placental perfusion by contrast-enhanced ultrasound in macaques and human subjects. Am J Obstet Gynecol. 2016;214(3):369.e361–e368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kaufmann BA, Wei K, Lindner JR. Contrast echocardiography. Curr Probl Cardiol. 2007;32(2):51–96. [DOI] [PubMed] [Google Scholar]
- 21. Heppner P, Lindner JR. Contrast ultrasound assessment of angiogenesis by perfusion and molecular imaging. Expert Rev Mol Diagn. 2005;5(3):447–455. [DOI] [PubMed] [Google Scholar]
- 22. Lindner JR. Contrast ultrasound molecular imaging of inflammation in cardiovascular disease. Cardiovasc Res. 2009;84(2):182–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wei K, Jayaweera AR, Firoozan S, Linka A, Skyba DM, Kaul S. Quantification of myocardial blood flow with ultrasound-induced destruction of microbubbles administered as a constant venous infusion. Circulation. 1998;97(5):473–483. [DOI] [PubMed] [Google Scholar]
- 24. Comstock SM, Pound LD, Bishop JM, et al. High-fat diet consumption during pregnancy and the early post-natal period leads to decreased alpha cell plasticity in the nonhuman primate. Mol Metab. 2012;2(1):10–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Acharya G, Sitras V, Erkinaro T, et al. Experimental validation of uterine artery volume blood flow measurement by Doppler ultrasonography in pregnant sheep. Ultrasound Obstet Gynecol. 2007;29(4):401–406. [DOI] [PubMed] [Google Scholar]
- 26. Lo JO, Schabel MC, Roberts VH, et al. Vitamin C supplementation ameliorates the adverse effects of nicotine on placental hemodynamics and histology in nonhuman primates. Am J Obstet Gynecol. 2015;212(3):370.e1–e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Roberts VH, Pound LD, Thorn SR, et al. Beneficial and cautionary outcomes of resveratrol supplementation in pregnant nonhuman primates. FASEB J. 2014;28(6):2466–2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Golub MS, Hogrefe CE, Tarantal AF, et al. Diet-induced iron deficiency anemia and pregnancy outcome in rhesus monkeys. Am J Clin Nutr. 2006;83(3):647–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Harding JE. The nutritional basis of the fetal origins of adult disease. Int J Epidemiol. 2001;30(1):15–23. [DOI] [PubMed] [Google Scholar]
- 30. Weech AA, Wollstein M, Goettsch E. Nutritional Edema in the Dog. V. Development of deficits in erythrocytes and hemoglobin on a diet deficient in protein. J Clin Invest. 1937;16(5):719–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cartwright GE, Wintrobe MM. Studies on free erythrocyte protoporphyrin, plasma copper, and plasmairon in protein-deficient and iron-deficient swine. J Biol Chem. 1948;176(2):571–583. [PubMed] [Google Scholar]
- 32. Shahidi NT, Diamond LK, Shwachman H. Anemia associated with protein deficiency. A study of 2 cases with cystic fibrosis. J Pediatr. 1961;59:533–542. [DOI] [PubMed] [Google Scholar]
- 33. Lumey LH. Reproductive outcomes in women prenatally exposed to undernutrition: a review of findings from the Dutch famine birth cohort. Proc Nutr Soc. 1998;57(1):129–135. [DOI] [PubMed] [Google Scholar]
- 34. Hendrie TA, Peterson PE, Short JJ, et al. Frequency of prenatal loss in a macaque breeding colony. Am J Primatol. 1996;40(1):41–53. [DOI] [PubMed] [Google Scholar]
- 35. Trudinger BJ, Cook CM, Giles WB, Connelly A, Thompson RS. Umbilical artery flow velocity waveforms in high-risk pregnancy. Randomised controlled trial. Lancet. 1987;1(8526):188–190. [DOI] [PubMed] [Google Scholar]
- 36. Baschat AA, Gembruch U, Reiss I, Gortner L, Weiner CP, Harman CR. Relationship between arterial and venous Doppler and perinatal outcome in fetal growth restriction. Ultrasound Obstet Gynecol. 2000;16(5):407–413. [DOI] [PubMed] [Google Scholar]
- 37. Alfirevic Z, Stampalija T, Gyte GM. Fetal and umbilical Doppler ultrasound in high-risk pregnancies. Cochrane Database Syst Rev. 2010(1):CD007529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Stampalija T, Gyte GM, Alfirevic Z. Utero-placental Doppler ultrasound for improving pregnancy outcome. Cochrane Database Syst Rev. 2010(9):CD008363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Konje JC, Kaufmann P, Bell SC, Taylor DJ. A longitudinal study of quantitative uterine blood flow with the use of color power angiography in appropriate for gestational age pregnancies. Am J Obstet Gynecol. 2001;185(3):608–613. [DOI] [PubMed] [Google Scholar]
- 40. Romo A, Carceller R, Tobajas J. Intrauterine growth retardation (IUGR): epidemiology and etiology. Pediatr Endocrinol Rev. 2009;6(suppl 3):332–336. [PubMed] [Google Scholar]
- 41. Wu G, Pond WG, Flynn SP, Ott TL, Bazer FW. Maternal dietary protein deficiency decreases nitric oxide synthase and ornithine decarboxylase activities in placenta and endometrium of pigs during early gestation. J Nutr. 1998;128(12):2395–2402. [DOI] [PubMed] [Google Scholar]
- 42. Kulandavelu S, Whiteley KJ, Qu D, Mu J, Bainbridge SA, Adamson SL. Endothelial nitric oxide synthase deficiency reduces uterine blood flow, spiral artery elongation, and placental oxygenation in pregnant mice. Hypertension. 2012;60(1):231–238. [DOI] [PubMed] [Google Scholar]
- 43. Kulandavelu S, Whiteley KJ, Bainbridge SA, Qu D, Adamson SL. Endothelial NO synthase augments fetoplacental blood flow, placental vascularization, and fetal growth in mice. Hypertension. 2013;61(1):259–266. [DOI] [PubMed] [Google Scholar]
- 44. Roberts CT, Sohlstrom A, Kind KL, et al. Maternal food restriction reduces the exchange surface area and increases the barrier thickness of the placenta in the guinea-pig. Placenta. 2001;22(2-3):177–185. [DOI] [PubMed] [Google Scholar]
- 45. Lindner JR, Song J, Xu F, et al. Noninvasive ultrasound imaging of inflammation using microbubbles targeted to activated leukocytes. Circulation. 2000;102(22):2745–2750. [DOI] [PubMed] [Google Scholar]
- 46. Teramo KA, Widness JA. Increased fetal plasma and amniotic fluid erythropoietin concentrations: markers of intrauterine hypoxia. Neonatology. 2009;95(2):105–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.