Abstract
The human milk oligosaccharide 3′-sialyllactose (3′SL) has previously been shown to activate murine dendritic cells (DC) in a Toll-like receptor (TLR) 4-mediated manner ex vivo. In this study we aimed to investigate whether 3′SL has similar immunomodulatory properties on human DC. 3′SL was shown to induce NF-κB activation via human TLR4. However, LPS was detected in the commercially obtained 3′SL from different suppliers. After the removal of LPS from 3′SL, we studied its ability to modify DC differentiation in vitro. In contrast to LPS and 3′SL, LPS-free 3′SL did not induce functional and phenotypical changes on immature DC (iDC). iDC that were differentiated in the presence of LPS or 3′SL showed a semi-mature phenotype (i.e., fewer CD83+CD86+ DC), produced IL-10 and abrogated IL-12p70 and tumor necrosis factor-alpha levels upon stimulation with several TLR ligands. Differentiation into these tolerogenic DC was completely abrogated by LPS removal from 3′SL. In contrast to previous reports in mice, we found that LPS-free 3′SL does not activate NF-κB via human TLR4. In conclusion, removing LPS from (oligo)saccharide preparations is necessary to study their potential immunomodulatory function.
Keywords: 3′-sialyllactose, DC differentiation, human tolerogenic DC, LPS contamination, TLR4 signaling
Introduction
The organization and function of the neonatal mucosal immune system is largely shaped during the first months of life under the influence of microbial colonization and breast milk (Brugman et al. 2015). Breast milk is an unique exogenous source of oligosaccharides which promotes growth and immune function (Bode 2012; Brugman et al. 2015; Charbonneau et al. 2016). Human milk oligosaccharides (HMOs) consist of a disaccharide lactose backbone at the reducing end that can be elongated into a trisaccharide containing a neutral (fucose) or acidic (sialic acid) fraction or more complex oligosaccharides. The binding of the sialic acid N-acetylglucosamine to the backbone via either a α-2,3- or α-2,6 linkage results in 3′-sialyllactose (3′SL) or 6′-sialyllactose (6′SL), respectively. 3′SL and 6′SL are abundant acidic oligosaccharides present in human and bovine milk (ten Bruggencate et al. 2014).
HMOs escape enzymatic hydrolysis and are fermented in the colon, facilitating the outgrowth of beneficial gut bacteria. Low levels of HMOs can be absorbed in the small intestine and reach systemic circulation where they may interact with immune cells (Rudloff et al. 2012; Ruhaak et al. 2014). HMOs are also postulated to regulate mucosal immune function, although their mechanism is poorly understood (Bode 2012; Kulinich and Liu 2016). The architecture of HMOs is considered essential for their effect on the immune system (Bode and Jantscher-Krenn 2012). Human milk components, including HMOs, have been postulated to modulate Toll-like receptor (TLR) signaling on immune cells and induce regulatory responses (He et al. 2016; Kulinich). Moreover, oligosaccharide mixtures used for infant nutrition were shown to activate human epithelial cell lines (Ortega-González et al. 2014), human monocytes (Lehmann et al. 2015) and murine monocytes (Capitán-Cañadas et al. 2014) in a TLR4-mediated manner in vitro. In mice, 3′SL was shown to induce colitis and isolated mesenteric lymph node dendritic cells (DC) were shown to be activated ex vivo by 3′SL in a TLR4-dependent manner as the cause of inflammation (Kurakevich et al. 2013). On the other hand, TLR4 activation was shown to be essential for proper immune education, which protected mice from autoimmune diabetes (Vatanen et al. 2016). Activation of the immune system by such HMOs via TLR4 therefore may be essential for immune development in early life. The aim of the current study was to investigate the immunomodulatory properties of 3′SL on human DC development.
Results
In line with a previous mouse study, 3′SL showed NF-κB activation via human TLR4 (Figures 1A) and not via TLR2 or TLR2-1 (Figure S1). Since LPS is a major bacterial contaminant that signals via TLR4, we wanted to exclude contamination with LPS in 3′SL. Unexpectedly, two batches from different suppliers of commercial 3′SL were tested positive in a recombinant factor C LAL assay, which resulted in human TLR4-mediated NF-κB activation (Figure S2). The 3′SL used in study contained 10 ± 0.17 EU/mg 3′SL (Figure 1B). Therefore, 3′SL was treated with an optimized Triton X-114 method (Teodorowicz et al. 2017) to remove any trace of LPS (Supplementary Materials and Methods). HPLC analysis confirmed that the concentration of 3′SL was unaltered after applying the Triton X-114 method (Figure S3). This methodology decreased the LPS contamination drastically to 0.11 ± 0.08 EU/mg (Figure 1B). Next, we investigated whether 3′SL, LPS-free 3′SL, or ultrapure LPS were able to activate NF-κB via human TLR4. The TLR4 signaling activity in 3′SL was completely abrogated by LPS removal (Figure 1A).
Fig. 1.
LPS in 3′SL induces TLR4-mediated NF-κB activation. (A) 3′SL (1 mg/ml), LPS-free 3′SL (1 mg/ml) and LPS (10 EU/ml) were tested for their NF-κB activation via TLR4 (n = 3). (B) 3′SL was tested for LPS contamination using a recombinant factor C LAL assay. LPS-free 3′SL was included with or without a 5 EU spiked control (n = 2). Data are represented as mean ± SEM.
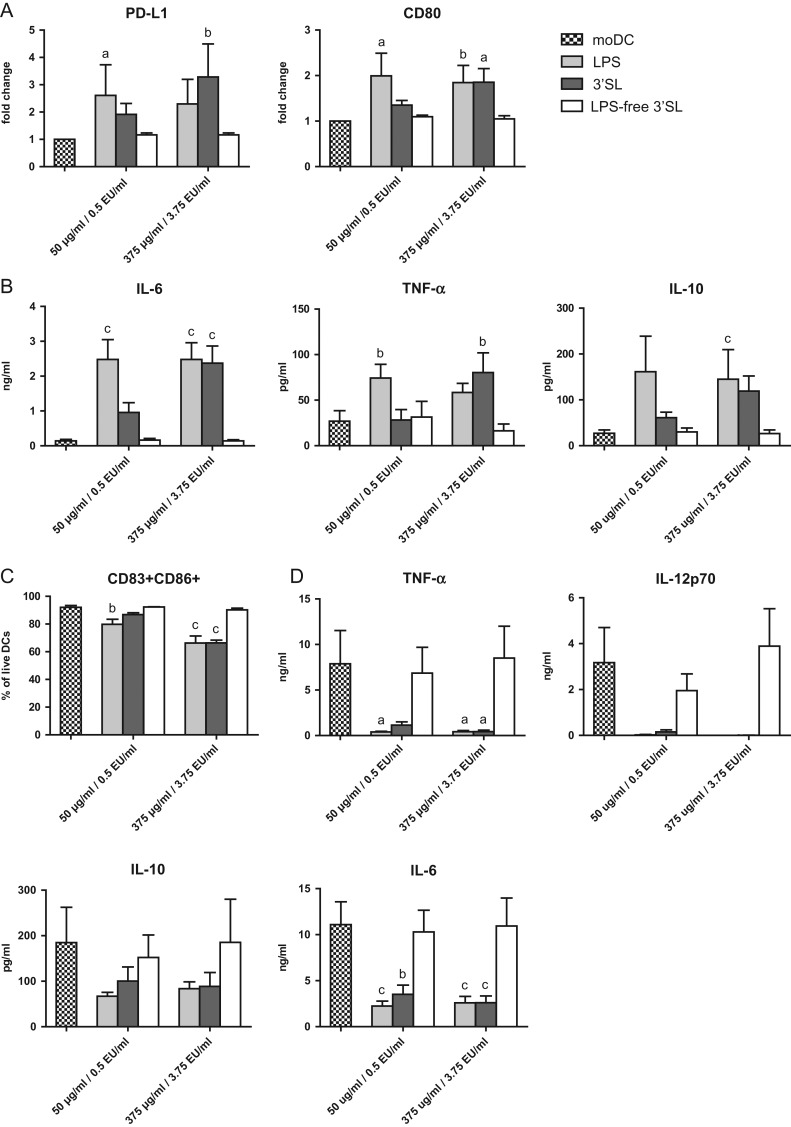
To study the effect of LPS contamination of 3′SL on DC differentiation, we cultured freshly isolated monocytes for 6 days in the presence of IL-4 and GM-CSF with or without 3′SL, LPS-free 3′SL or ultrapure LPS to generate immature DC (iDC). The concentration of ultrapure LPS was matched to the concentration of LPS contamination measured in the 3′SL sample (10 EU/mg; Figure 1B). The concentrations of 3′SL used in this study were based on breast milk concentrations, which range between 76 and 300 μg/ml (ten Bruggencate et al. 2014). Surface marker expression on DC was measured by flow cytometry (Supplementary Materials and Methods). DC differentiated in the presence of LPS or 3′SL showed a dose-dependent increase in the expression of the regulatory DC markers CD80 and PD-L1 and the production of IL-6, tumor necrosis factor-alpha (TNF-α) and IL-10 (Figures 2A,B and S4). However, the surface marker expression and cytokine production was not elevated compared to monocyte-derived DC (moDC) when monocytes were cultured in the presence of LPS-free 3′SL (Figure 2A,B). Thus phenotypical changes on iDC induced by 3′SL were shown to be fully driven by LPS contamination. Next, we investigated the response of the different DC types to subsequent stimulations with LPS or Poly I:C + R848. iDC differentiated in the presence of LPS or 3′SL resulted in tolerogenic DC; showing a semi-mature phenotype that produced significantly less TNF-α and abrogated IL-12p70 levels (Figure 2C,D and S5). In contrast, DC differentiated in the presence of LPS-free 3′SL showed a fully mature phenotype (CD86+CD83+) capable of producing IL-6, IL-12p70 and TNF-α in similar levels compared to moDC upon stimulation with Poly I:C + R848 (Figure 2C,D) or LPS (Figure S6).
Fig. 2.
LPS induced tolerogenic DC. Monocytes were cultured with IL-4 and GM-CSF with or without LPS-free 3′SL, 3′SL or LPS. (A) The relative CD80 and PD-L1 expression is shown on iDC. The MFI (median fluorescence intensity) was normalized to the expression on moDC of the respective donor. (B) The production of IL-6, TNF-α and IL-10 was measured in the supernatants (n = 6). (C) Next, these iDC were stimulated with Poly I:C and R848. The percentage of CD86+CD83+ DC was assessed after 48 hours. (D) The cytokines TNF-α, IL-10, IL-6 and IL-12p70 were measured by CBA (n = 3). Significance differences compared to moDC are indicated with; a = P < 0.001, b = P < 0.01 and c = P < 0.05. Data are represented as mean ± SEM.
Discussion and conclusions
In literature, HMOs, plant-derived oligosaccharides, and other polysaccharides are shown to be immunogenic via interacting with TLR4 on human and murine myeloid (Kurakevich et al. 2013; Capitán-Cañadas et al. 2014; Lehmann et al. 2015) and epithelial cells (Ortega-González et al. 2014). Kurakevich and colleagues demonstrated that mouse pups receiving milk devoid of 3′SL were less prone to develop colitis. Interestingly, the authors showed that 3′SL, which was tested negative for LPS contamination, activated mesenteric lymph node DC in a TLR4-dependent manner in vitro (Kurakevich et al. 2013). However, here we show that 3′SL is not capable of activating NF-κB via human TLR4 after the removal of LPS. This suggests that the specificity of mouse TLR4 is different from human TLR4. Interestingly, TLR4-mediated activation of moDC by a mixture of the prebiotic oligosaccharides scGOS/lcFOS was shown to induce IL-10 production and increased the number of FoxP3+ T cells (Lehmann et al. 2015). The authors show that these moDC released a distinct array of cytokines upon LPS stimulation compared to scGOS/lcFOS stimulation, which contained endotoxin levels of <3 ng/ml. It has been widely acknowledged that human and murine myeloid cells are particularly sensitive to LPS contamination (Schwarz et al. 2014). LPS induces a negative feedback loop in monocytes involving IL-6- and IL-10-mediated SOCS3 and STAT3 activation that dampens TLR signaling (Yoshimura et al. 2007; Frick et al. 2010). STAT3 was also shown to bind the PD-L1 promotor directly and to regulate its expression in TLR activated monocytes (Wölfle et al. 2011), explaining upregulation of membranous PD-L1 by LPS. We showed that DC exposed to LPS during the differentiation period produced abrogated IL-12p70 levels to multiple TLR ligands. Moreover, these DC showed a semi-mature phenotype, which is known to be involved in the polarization of naive T cells into regulatory T cells (Hubo et al. 2013; Nikolic and Roep 2013). Insufficient removal of LPS results in activation of human monocytes and DC, which makes them hyporesponsive towards secondary stimulation with TLR ligands. Thus, false conclusions about the immunomodulatory capacity of the molecule of interest may be drawn due to LPS contamination. The data shown here demonstrate that the presence of >0.5 EU/ml LPS as measured in commercially available 3′SL preparations during DC differentiation results in tolerogenic DC. Hence, it is an absolute necessity to remove LPS from glycan samples before studying their immunomodulatory capacity in vitro.
Supplementary data
Funding
This work was supported by the Netherlands Organisation of Scientific Research (NWO) as part of the technology foundation STW (project number 13017).
Conflict of interest statement
RJJvN is an employee of FrieslandCampina.
Abbreviations
CD, cluster of differentiation; DC, dendritic cells; EU, endotoxin unit; iDC, immature dendritic cell; IFNγ, interferon gamma; IL, interleukin; HMO, human milk oligosaccharide; moDC, monocyte-derived dendritic cell; PBMC, peripheral blood mononuclear cells; PD-L1, programmed death ligand 1; Poly I:C, polyinosinic:polycytidylic acid; R848, resiquimod; TLR, toll-like receptor; TNF-α, tumor necrosis factor-alpha; 3′SL, 3′-sialyllactose.
Supplementary Material
Acknowledgements
We thank Barry Schoemaker for conducting HPAEC-PAD analysis on Triton X-114 treated 3′SL and Prof. Wells of the Host-Microbe Interactomics group, Wageningen University & Research, for providing us with the HEK TLR cell lines.
References
- Bode L. 2012. Human milk oligosaccharides: Every baby needs a sugar mama. Glycobiology. 22(9):1147–1162, doi:10.1093/glycob/cws074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bode L, Jantscher-Krenn E. 2012. Structure-function relationships of human milk oligosaccharides. Adv Nutr. 3(3):383S–391S, doi:10.3945/an.111.001404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brugman S, Perdijk O, van Neerven RJ, Savelkoul HF. 2015. Mucosal immune development in early life: Setting the stage. Arch Immunol Ther Exp (Warsz). 63(4):251–268, doi:10.1007/s00005-015-0329-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capitán-Cañadas F, Ortega-González M, Guadix E, Zarzuelo A, Suárez MD, de Medina FS, Martínez-Augustin O. 2014. Prebiotic oligosaccharides directly modulate proinflammatory cytokine production in monocytes via activation of TLR4. Mol Nutr Food Res. 58(5):1098–1110, doi:10.1002/mnfr.201300497. [DOI] [PubMed] [Google Scholar]
- Charbonneau MR, O’Donnell D, Blanton LV, Totten SM, Davis JCC, Barratt MJ, Cheng J, Guruge J, Talcott M, Bain JR et al. 2016. Sialylated milk oligosaccharides promote microbiota-dependent growth in models of infant undernutrition. Cell. 164(5):859–871, doi:10.1016/j.cell.2016.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frick J-S, Grünebach F, Autenrieth IB. 2010. Immunomodulation by semi-mature dendritic cells: A novel role of toll-like receptors and interleukin-6. Int J Med Microbiol. 300(1):19–24, doi:10.1016/j.ijmm.2009.08.010. [DOI] [PubMed] [Google Scholar]
- He Y, Nathan TL, Newburg DS. 2016. Human milk components modulate toll-like receptor-mediated inflammation. Adv Nutr. 7(1):102–111, doi:10.3945/an.115.010090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubo M, Trinschek B, Kryczanowsky F, Tuettenberg A, Steinbrink K, Jonuleit H. 2013. Costimulatory molecules on immunogenic versus tolerogenic human dendritic cells. Front Immunol. 4, doi:10.3389/fimmu.2013.00082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulinich A, Liu L. 2016. Human milk oligosaccharides: The role in the fine-tuning of innate immune responses. Carbohydr Res. 432:62–70, doi:10.1016/j.carres.2016.07.009. [DOI] [PubMed] [Google Scholar]
- Kurakevich E, Hennet T, Hausmann M, Rogler G, Borsig L. 2013. Milk oligosaccharide sialyl(α2,3)lactose activates intestinal CD11c+ cells through TLR4. Proc Natl Acad Sci USA. 110(43):17444–17449, doi:10.1073/pnas.1306322110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann S, Hiller J, van Bergenhenegouwen J, Knippels LMJ, Garssen J, Traidl-Hoffmann C. 2015. In vitro evidence for immune-modulatory properties of non-digestible oligosaccharides: Direct effect on human monocyte derived dendritic cells. PLoS One. 10(7):e0132304, doi:10.1371/journal.pone.0132304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolic T, Roep BO. 2013. Regulatory multitasking of tolerogenic dendritic cells—Lessons taken from vitamin D3-treated tolerogenic dendritic cells. Front Immunol. 4, doi:10.3389/fimmu.2013.00113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortega-González M, Ocón B, Romero-Calvo I, Anzola A, Guadix E, Zarzuelo A, Suárez MD, de Medina FS, Martínez-Augustin O. 2014. Nondigestible oligosaccharides exert nonprebiotic effects on intestinal epithelial cells enhancing the immune response via activation of TLR4-NFκB. Mol Nutr Food Res. 58(2):384–393, doi:10.1002/mnfr.201300296. [DOI] [PubMed] [Google Scholar]
- Rudloff S, Pohlentz G, Borsch C, Lentze MJ, Kunz C. 2012. Urinary excretion of in vivo 13C-labelled milk oligosaccharides in breastfed infants. Br J Nutr. 107(7):957–963, doi:10.1017/S0007114511004016. [DOI] [PubMed] [Google Scholar]
- Ruhaak LR, Stroble C, Underwood MA, Lebrilla CB. 2014. Detection of milk oligosaccharides in plasma of infants. Anal Bioanal Chem. 406(24):5775–5784, doi:10.1007/s00216-014-8025-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz H, Schmittner M, Duschl A, Horejs-Hoeck J. 2014. Residual endotoxin contaminations in recombinant proteins are sufficient to activate human CD1c+ dendritic cells. PLoS One. 9(12):e113840, doi:10.1371/journal.pone.0113840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ten Bruggencate SJ, Bovee-Oudenhoven IM, Feitsma AL, van Hoffen E, Schoterman MH. 2014. Functional role and mechanisms of sialyllactose and other sialylated milk oligosaccharides. Nutr Rev. 72(6):377–389, doi:10.1111/nure.12106. [DOI] [PubMed] [Google Scholar]
- Teodorowicz M, Perdijk O, Verhoek I, Govers C, Savelkoul HFJ, Tang Y, Wichers H, Broersen K. 2017. Optimized triton X-114 assisted lipopolysaccharide (LPS) removal method reveals the immunomodulatory effect of food proteins. PloS One. 12(3):e0173778, doi:10.1371/journal.pone.0173778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vatanen T, Aleksandar DK, d’Hennezel E, Siljander H, Franzosa EA, Yassour M, Kolde R, Vlamakis H, Arthur TD, Hämäläinen AM et al. 2016. Variation in microbiome LPS immunogenicity contributes to autoimmunity in humans. Cell. 165(4):842–853, doi:10.1016/j.cell.2016.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wölfle SJ, Strebovsky J, Bartz H, Sähr A, Arnold C, Kaiser C, Dalpke AH, Heeg K. 2011. PD-L1 Expression on tolerogenic APCs is controlled by STAT-3. Eur J Immunol. 41(2):413–424, doi:10.1002/eji.201040979. [DOI] [PubMed] [Google Scholar]
- Yoshimura A, Naka T, Kubo M. 2007. SOCS proteins, cytokine signalling and immune regulation. Nat Rev Immunol. 7(6):454–465, doi:10.1038/nri2093. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.