Abstract
Collagen is one of the most important components of the extracellular matrix that is involved in the strength of tissues, cell adhesion and cell proliferation. Mutations in several collagen and post-translational modification enzyme genes cause Ehlers–Danlos syndrome (EDS) characterized by joint and skin hyperextensibility as well as fragility of various organs. Carbohydrate sulfotransferase 14/dermatan 4-O-sulfotransferase-1 (CHST14/D4ST1) is a critical enzyme for biosynthesis of dermatan sulfate, a side chain of various proteoglycans including biglycan that regulates collagen fibrils through their interaction. Mutations in CHST14 were found to cause a new form of EDS, named musculocontractural type EDS (mcEDS-CHST14). Large subcutaneous hematomas are one of the most serious complications accompanied by decreased quality of life and potential lethality. In this study, Chst14 gene-deleted mice were expected to be an animal model of the vascular abnormalities of mcEDS-CHST14. However, only limited numbers of adult mice were generated because of perinatal lethality in most Chst14 gene-deleted homozygote (Chst14−/−) mice. Therefore, we investigated the placentas of these fetuses. The placentas of Chst14−/− fetuses showed a reduced weight, alterations in the vascular structure, and ischemic and/or necrotic-like changes. Electron microscopy demonstrated an abnormal structure of the basement membrane of capillaries in the placental villus. These findings suggest that Chst14 is essential for placental vascular development and perinatal survival of fetuses. Furthermore, placentas of Chst14−/− fetuses could be a useful model for vascular manifestations in mcEDS-CHST14, such as the large subcutaneous hematomas.
Keywords: carbohydrate sulfotransferase 14 (Chst14), dermatan 4-O-sulfotransferase-1 (D4ST1), Ehlers–Danlos syndrome, placenta, vascular abnormality
Introduction
Extracellular matrix (ECM), which contains high amounts of proteoglycans (PGs) and glycoproteins, is observed in basement membranes and intercellular spaces. The ECM is involved in the strength of tissues, cell adhesion and cell proliferation (Kim et al. 2011). Collagen, a critical ECM component, undergoes post-translational modification by several enzymes to form mature collagen fibers. Collagen monomers are wrapped around each other to form a triple helix structure (Okuyama 2008). Collagen fibers consist of many collagen fibrils (trimer assembly) in the ECM. Mutations in several collagen genes and post-translational modification enzyme genes cause Ehlers–Danlos syndromes (EDSs), a heterogeneous group of heritable connective tissue disorders characterized by joint and skin hyperextensibility as well as fragility of various organs (Colige et al. 1999, 2004; Schwarze et al. 2001; Zweers et al. 2003; Cabral et al. 2005; Malfait et al. 2005; Makareeva et al. 2006).
Chondroitin sulfate (CS) and dermatan sulfate (DS) are glycosaminoglycans (GAGs) which are linear polysaccharide chains consisting of repeating disaccharide units. Biosynthesis of DS and CS is initiated by synthesis of a tetrasaccharide linker region, glucuronic acidβ1-3galactoseβ1-3galactoseβ1-4xyloseβ1-O-(GlcA-Gal-Gal-Xyl-), on serine residues of specific core proteins of PGs (Kitagawa et al. 1998; Okajima et al. 1999; Gotting et al. 2000; Bai et al. 2001). CS contains disaccharide units consisting of glucuronic acid (GlcA) and N-acetylgalactosamine (GalNAc), which are commonly sulfated at C-4 and/or C-6 of GalNAc residues (Kitagawa et al. 2001, 2003; Uyama et al. 2002). DS differs from CS by containing iduronic acid (IdoUA) in place of GlcUA (Maccarana et al. 2006; Pacheco et al. 2009). Both chains frequently exist as CS/DS hybrid chains in mammalian cells and tissues, and covalently attach to a core protein to form a PG. Decorin (DCN) and biglycan (BGN) are core proteins of these GAG chains (Schonherr et al. 1995). Furthermore, these PGs connect collagen fibrils via GAG chains and influence the structure and functions of the ECM (Pogany et al. 1994; Schonherr et al. 1995).
The carbohydrate sulfotransferase 14 (CHST14) gene encodes dermatan 4-O-sulfotransferase-1 (CHST14/D4ST1) that catalyzes transference of a sulfate group from the sulfate donor 3′-phosphoadenosine 5′-phosphosulfate to the C-4 position of the GalNAc residue in DS chains in vitro (Evers et al. 2001; Mikami et al. 2003). CHST14/D4ST1 is a critical enzyme for biosynthesis of DS in vivo (Miyake et al. 2014). Dermatan chains are matured by sulfation reactions catalyzed by CHST14/D4ST1 and uronosyl 2-O-sulfotransferase (UST) encoded by CHST14 and UST, respectively. CHST14/D4ST1 and UST transfer the sulfate group from the sulfate donor 3′-phosphoadenosine 5′-phosphosulfate to the C-4 position of GalNAc and C-2 position of IdoUA residues in dermatan, respectively (Kobayashi et al. 1999; Evers et al. 2001; Mikami et al. 2003).
Biallelic loss-of-function mutations in CHST14 cause a recently delineated type of EDS designated as musculocontractural EDS caused by CHST14 mutations (mcEDS-CHST14) in the 2017 International Nosology of EDS (Kosho 2016; Malfait et al. 2017). This disorder is clinically characterized by multiple congenital malformations (craniofacial features and multiple congenital contractures) and progressive multisystem fragility-related complications (skin hyperextensibility and fragility, recurrent dislocations, progressive talipes or spinal deformities and large subcutaneous hematomas). The large subcutaneous hematomas, which are speculated to be caused by ruptures of small arteries, are one of the most serious complications accompanied by decreased quality of life and potential lethality. Multisystem fragility is supposed to be caused by impaired assembly of collagen fibrils, resulting from loss of the DS side chain of DCN, which promotes electrostatic binding between collagen fibrils (Miyake et al. 2010; Kosho 2016).
Chst14 gene-deleted homozygote (Chst14−/−) mice have been generated by homologous recombination and targeting of only the coding exon (exon 1) of the Chst14 gene (Tang et al. 2010; Bian et al. 2011). Previous studies have reported that, upon injury of nerves or spinal cord, CHST14/D4ST1 is related to recovery of these tissues in mice (Akyuz et al. 2013; Rost et al. 2016). However, the vascular phenotypes, which supposedly underlie the large subcutaneous hematomas, have not been investigated in the Chst14−/− mouse.
The Chst14−/− mouse is potentially a critical model for investigation of the pathophysiology of mcEDS-CHST14. However, the perinatal lethality of Chst14−/− fetuses makes investigations difficult (Akyuz et al. 2013). This is the first study of the placenta of the Chst14−/− mouse, which implicates the etiology of vascular abnormalities in patients with mcEDS-CHST14 as well as perinatal loss of Chst14−/− mice.
Results
Characteristics of Chst14−/− fetuses
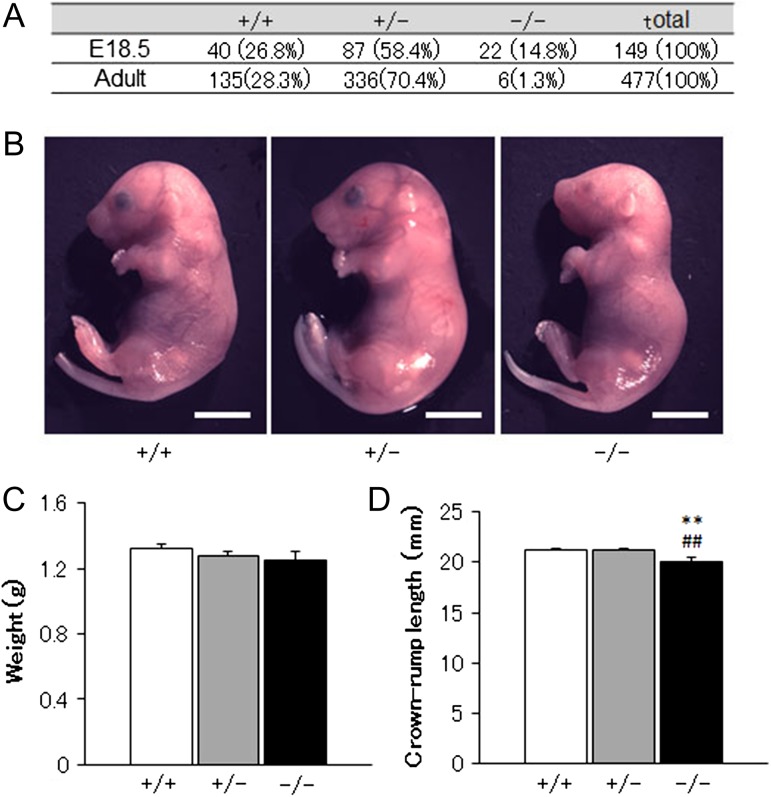
Chst14 −/− mice were observed in 1.3 and 14.8% of all adult mice and fetuses at the perinatal period (E18.5), respectively (Figure 1A). Body weights of Chst14−/− fetuses were not different from those of Chst14+/− or Chst14+/+ fetuses (Figure 1C). Crown-rump lengths of Chst14−/− fetuses were significantly shorter than those of Chst14+/− or Chst14+/+ fetuses, whereas the appearance of Chst14−/− fetuses did not appear to be different from that of Chst14+/− or Chst14+/+ fetuses (Figure 1B and C).
Fig. 1.
Observations of Chst14 gene-delated fetuses. (A) Absolute numbers and percentages of wild-type (+/+), heterozygous (+/−) and homozygous (−/−) mice among offspring of heterozygous breeding pairs analyzed at embryonic and postnatal ages. (B) Appearances of the fetuses. Bar: 5 mm. (C) Body weights of the fetuses (means + SEM). Wild-type: n = 16; heterozygous: n = 37; homozygous: n = 8. (D) Crown-rump lengths of the fetuses (means + SEM). Wild-type: n = 26; heterozygous: n = 56; homozygous: n = 12. **P < 0.01, compared with wild-type (+/+); ##P < 0.01, compared with heterozygous (+/−); one-way ANOVA followed by the Tukey–Kramer post hoc test.
Glycobiological findings of the placentas
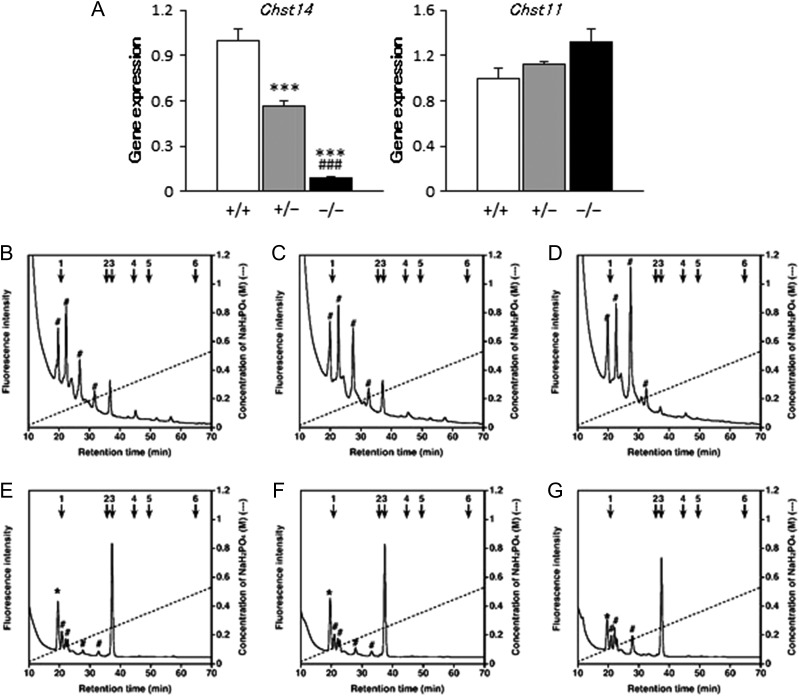
Gene expression of Chst14 was significantly decreased in the placentas of Chst14−/− fetuses compared with those of Chst14+/− or Chst14+/+ fetuses, whereas expression of Chst11, which encodes chondroitin 4-O-sulfotransferase-1, was not significantly different among each group (Figure 2A). DS or CS moiety in CS/DS chains obtained from samples was digested with chondroitinase B (Figure 2B–D) or AC (Figure 2E–G), respectively, into disaccharides for their analyses. HPLC profiles were obtained from the digested DS (Figure 2B–D) and CS (Figure 2E–G) moieties in CS/DS chains prepared from placentas of wild-type (Figure 2B and E), heterozygous (Figure 2C and F) and homozygous (Figure 2D and G) fetuses. The amount of DS disaccharides was significantly decreased in the placentas of Chst14−/− fetuses compared with those of Chst14+/− and Chst14+/+ fetuses, whereas the amount of CS disaccharides did not change (Table I).
Fig. 2.
Decreased gene expression of Chst14 and HPLC profiles of DS and CS moieties in CS/DS chains of Chst14−/− mice. (A) Relative mRNA levels of Chst14 and Chst11 in placentas of wild-type (+/+), heterozygous (+/−) and homozygous (−/−) fetuses (means + SEM, n = 6/group). ***P < 0.001, compared with wild-type; ###P < 0.001, compared with heterozygous; one-way ANOVA followed by the Tukey–Kramer post hoc test. (B–G) HPLC profiles of the digests of DS (B–D) and CS (E–G) moieties in CS/DS chains prepared from placentas of wild-type (B, E), heterozygous (C, F) and homozygous (D, G) fetuses following digestion with chondroitinase B (B–D) or AC (E–G) into disaccharides for analysis of DS or CS moieties, respectively. Each digest was labeled with 2AB, and 2AB-labeled CS/DS disaccharides were separated by anion-exchange HPLC on an amine-bound silica PA-G column using a linear gradient of NaH2PO4, as indicated by the dashed line. The amount of resultant disaccharides in each sample was calculated based on the peak area in reference to that of standard unsaturated disaccharides. The elution positions of authentic 2AB-labeled CS/DS disaccharides are indicated by the numbered arrows: 1, ΔHexUA-GalNAc; 2, ΔHexUA-GalNAc(6S); 3, ΔHexUA-GalNAc(4S); 4, ΔHexUA(2S)-GalNAc(6S); 5, ΔHexUA-GalNAc(4S,6S); 6, ΔHexUA(2S)-GalNAc(4S,6S). *Peak of ΔHexUA-GlcNAc (degradation product of hyaluronic acid). Peaks detected between standards 1 and 2 (indicated by #) are impurities that were also detected in chromatograms of the negative controls without enzymatic digestion (data not shown).
Table I.
Disaccharide composition of DS and CS moieties in CS/DS chains isolated from placentas
| Disaccharide contents in DS moieties of CS/DS chains | pmol/mg Protein | ||
|---|---|---|---|
| Chst14+/+ | Chst14+/− | Chst14−/− | |
| ∆HexUA-GalNA | N.D. | N.D. | N.D. |
| ∆HexUA-GalNAc(6S) | N.D. | N.D. | N.D. |
| ∆HexUA-GalNAc(4S) | 29.0 ± 7.0 | 34.2 ± 4.1 | 4.25 ± 2.5*,## |
| ∆HexUA(2S)-GalNAc(6S) | N.D. | N.D. | N.D. |
| ∆HexUA-GalNAc(4S,6S) | N.D. | N.D. | N.D. |
| ∆HexUA(2S)-GalNAc(4S,6S) | N.D. | N.D. | N.D. |
| Total disaccharides | 29.0 ± 7.0 | 34.2 ± 4.1 | 4.25 ± 2.5*,## |
| Disaccharide contents in CS moieties of CS/DS chains | pmol/mg Protein | ||
|---|---|---|---|
| Chst14+/+ | Chst14+/− | Chst14−/− | |
| ∆HexUA-GalNA | 154.3 ± 16.9 | 139.0 ± 5.2 | 113.4 ± 40.7 |
| ∆HexUA-GalNAc(6S) | N.D. | N.D. | N.D. |
| ∆HexUA-GalNAc(4S) | 1182.0 ± 147.0 | 1186.6 ± 137.6 | 768.8 ± 303.5 |
| ∆HexUA(2S)-GalNAc(6S) | N.D. | N.D. | N.D. |
| ∆HexUA-GalNAc(4S,6S) | N.D. | N.D. | N.D. |
| ∆HexUA(2S)-GalNAc(4S,6S) | N.D. | N.D. | N.D. |
| Total disaccharides | 1336.0 ± 157.1 | 1325.6 ± 141.4 | 882.65 ± 342.1 |
Placental samples were individually digested with either chondroitinase B or AC for analysis of DS or CS moieties in CS/DS chains, respectively. Each digest was treated with 2AB to label the yielded CS/DS-derived disaccharides, and was analyzed by anion-exchange HPLC. Disaccharide contents calculated from the peak area of the HPLC profile are summarized as the means ± SEM. Wild-type: n = 4; heterozygous: n = 5; homozygous: n = 4. *P < 0.05, compared with wild-type (Chst14+/+); ##P < 0.01, compared with heterozygous (Chst14+/−); one-way ANOVA followed by the Tukey–Kramer post hoc test. Each string: ∆HexUA: 4,5-unsaturated hexuronic acid; 2S, 4S, 6S: 2-O-sulfate, 4-O-sulfate, 6-O-sulfate; N.D.: not detected (<0.1 pmol/mg).
General appearances of the placenta of Chst14−/− fetuses
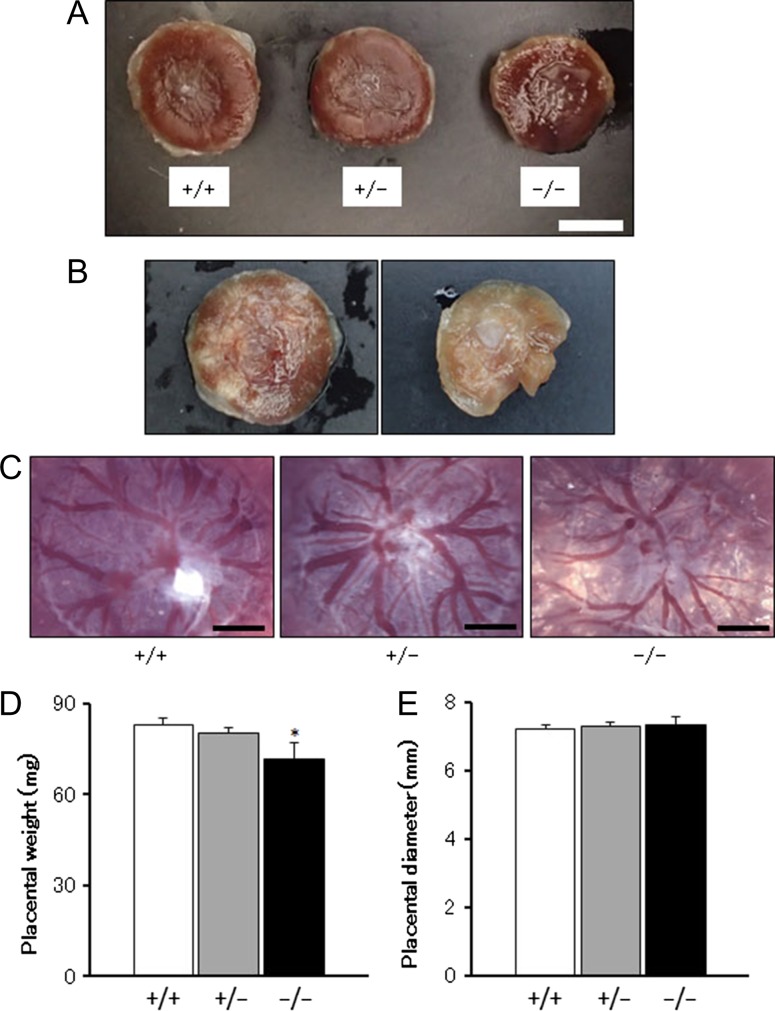
Most placentas of Chst14+/+, Chst14+/− and Chst14−/− fetuses showed similar gross appearances (Figure 3A), but some placentas of Chst14−/− fetuses showed hypoxia-like and/or necrosis-like abnormalities (Figure 3B). Vascular diameters of chorionic plates in the placentas of Chst14−/− fetuses were smaller than those in the placentas of Chst14+/− or Chst14−/− fetuses (Figure 3C). Placental weights of Chst14−/− fetuses were decreased significantly compared with those of Chst14+/+ or Chst14+/− fetuses (Figure 3D and E), although the diameters were not different among these groups.
Fig. 3.
Vascular development of the placenta is abnormal in Chst14 gene-deleted fetuses. (A) Appearance of the placentas of wild-type (+/+), heterozygous (+/−) and homozygous (−/−) fetuses (bar: 5 mm). (B) Hypoxia-like (left) and necrosis-like (right) appearances of the placentas of homozygous fetuses. (C) Microphotographs of the chorionic plate side of placentas (bar: 1 mm). (D) Placental weight (means + SEM). Wild-type: n = 16; heterozygous: n = 38; homozygous: n = 8. *P < 0.05, compared with wild-type; one-way ANOVA followed by the Tukey–Kramer post hoc test. (E) Placental diameter (means + SEM). Wild-type: n = 12; heterozygous: n = 27; homozygous: n = 6.
Structure of the placental labyrinth zone
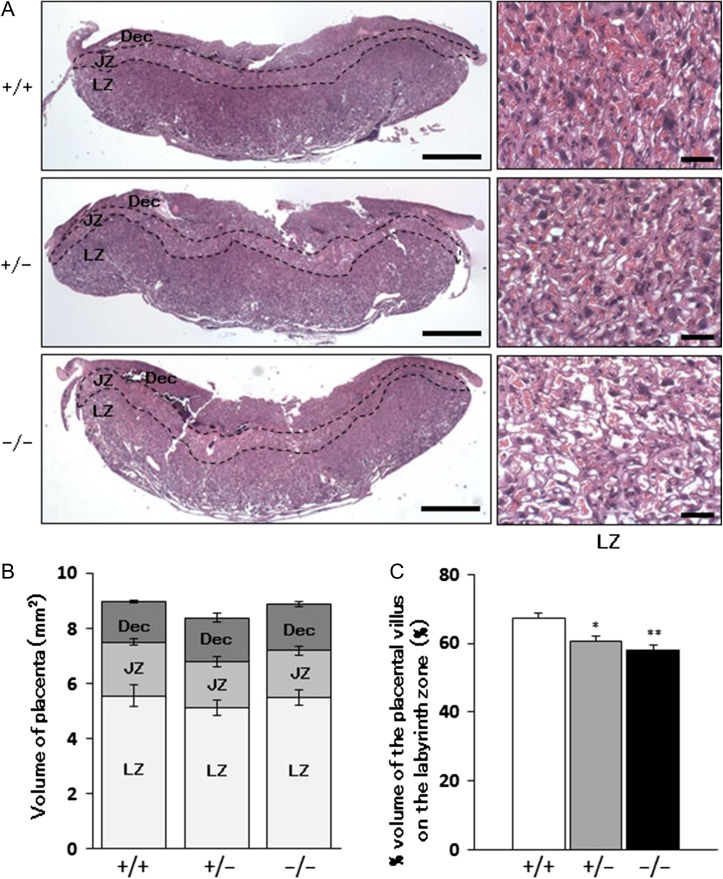
Three placental zones, the decidua basalis, junctional zone and labyrinth zone, were distinguished by observation of hematoxylin and eosin (H&E)-stained sections of placentas from Chst14+/+, Chst14+/− and Chst14−/− fetuses (Figure 4A). The volume of each placental zone was found to be similar (Figure 4B), but the percentage volume of the placental villus on the labyrinth zone was decreased significantly in the placentas of Chst14−/− fetuses compared with those of Chst14+/+ fetuses (Figure 4C).
Fig. 4.
Reduced volume of the placental villus of Chst14 gene-deleted fetuses. (A) H&E-stained sections of placentas from wild-type (+/+), heterozygous (+/−) and homozygous (−/−) fetuses. Left column shows low power photomicrographs of transversal sections of whole placentas (bar: 1 mm). Dec, decidua basalis; JZ, junctional zone; LZ, labyrinth zone. Right column shows higher magnifications micrographs of the labyrinth zone (bar: 50 μm). (B) Areas of the placental zones in Chst14 gene-deleted placentas (means ± SEM, n = 5 or 6). (C) Comparison of the percentage volume of the placental villus on the labyrinth zone (means + SEM, n = 6/group). *P < 0.05, **P < 0.01, compared with wild-type; One-way ANOVA followed by the Tukey–Kramer post hoc test.
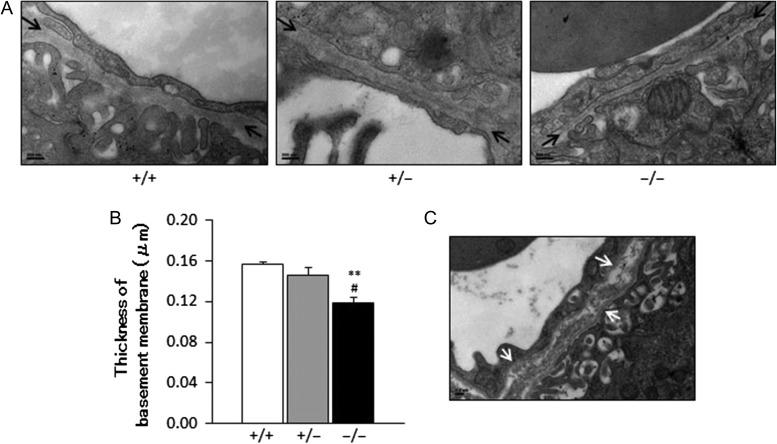
Ultrastructure of placental vessels
In the labyrinth zone, transmission electron microscopy (TEM) images revealed a significant decrease in the thickness of basement membranes in capillaries of the villus in placentas of Chst14−/− fetuses compared with those of Chst14+/+ or Chst14+/− fetuses (Figure 5A and B). Fragmentation of the basement membrane was also observed in some capillaries of the placentas of Chst14−/− fetuses (Figure 5C).
Fig. 5.
Ultrastructure of the capillary basement membrane in the labyrinth zone decreases in the Chst14−/− placenta. (A) TEM of capillaries in the labyrinth zone of wild-type (+/+), heterozygous (+/−) and homozygous (−/−) fetuses (bar: 200 nm). The capillary basement membrane is indicated by arrows. (B) Thickness of the basement membrane of capillaries in the labyrinth zone (mean + SEM, n = 3/group). **P < 0.01, compared with wild-type; #P < 0.05, compared with heterozygous; one-way ANOVA followed by the Tukey–Kramer post hoc test. (C) Structural abnormality (arrows) of the capillary basement membrane in the labyrinth zone of a homozygous fetus revealed by TEM (bar: 0.2 μm).
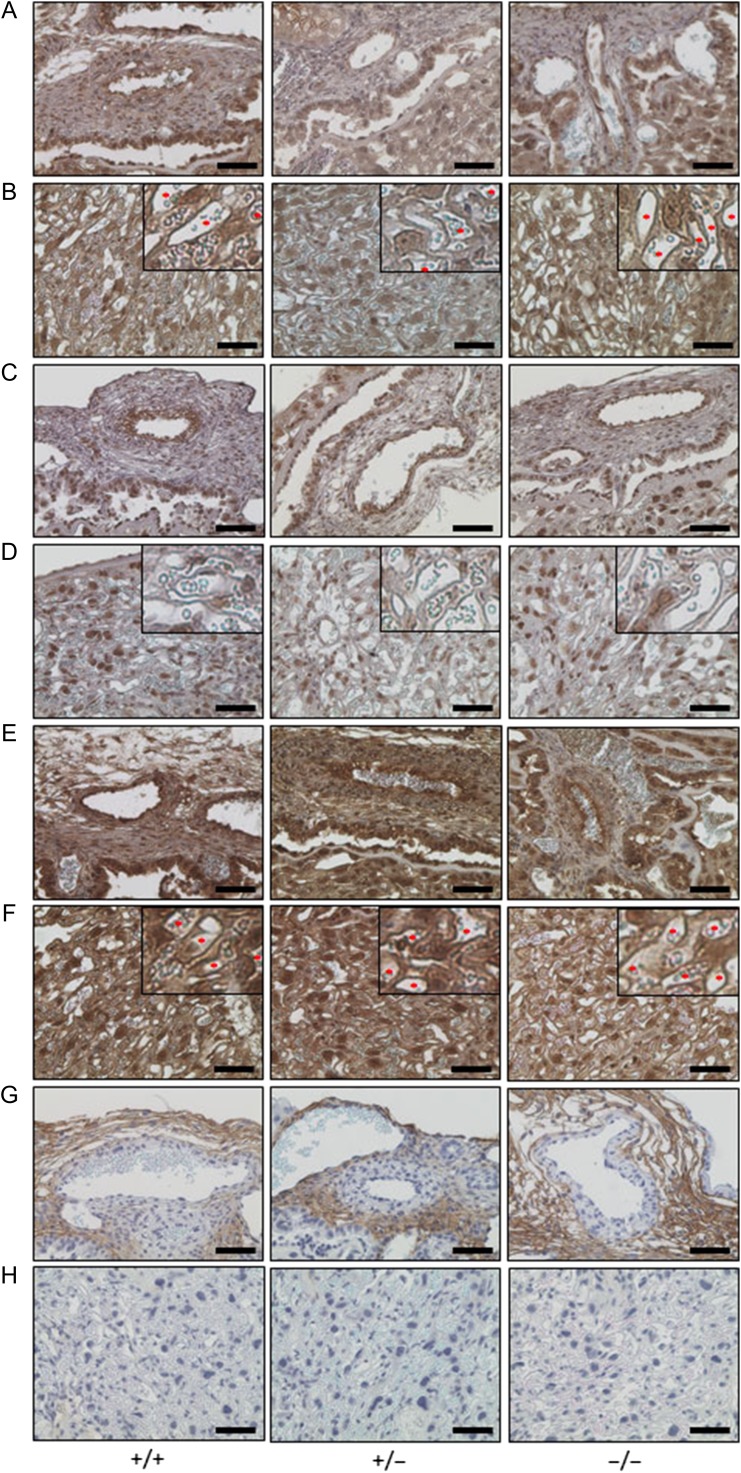
Localizations of vasculature-related collagens and PGs on the fetal placenta
Immunostaining of the fetal placenta: Chorionic plate (Figure 6A, C, E and G) and placental villus (Figure 6B, D, F and H) showed localizations of vasculature-related collagens and PGs. Collagen IV (COL4) was distributed on vascular endothelial cells in the chorionic plate and capillaries of the placental villus in each group (Figure 6A and B). Collagen XV (COL15) was also detected on vascular endothelial cells in the chorionic plate (Figure 6C), but was hardly expressed in capillaries of the placental villus (Figure 6D). BGN was extensively expressed in the fetal placenta. In particular, BGN was highly expressed on vascular endothelial cells in the chorionic plate and capillaries of the placental villus (Figure 6E and F). DCN was detected in the interstitial tissues of the chorionic plate, but not in blood vessels (Figure 6G and H). There were no differences in each staining among placentas from wild-type, heterozygous and homozygous mice.
Fig. 6.
Immunostaining of vasculature-related collagens and PGs on the fetal placenta. Immunostaining of collagen IV (A and B), collagen XV (C and D), biglycan (E and F) and decorin (G and H) on the chorionic plate (A, C, E and G) and placental villus (B, D, F and H) (bar: 50 μm). The upper left image is a ×2.5 enlargement of each photograph (B, D and F). *Capillary of the placental villus that highly expressed these immunogens (B and F).
Expression of ECM-related genes
Expression of ECM-related genes, including collagen Iα (Col1a), collagen IIα (Col2a), collagen IIIα (Col3a), collagen IVα (Col4a), collagen XV (Col15), biglycan (Bgn) and decorin (Dcn), showed no differences among placentas of Chst14+/+, Chst14+/− or Chst14−/− fetuses (Supplemental Figure S1).
Discussion
Body lengths of Chst14−/− fetuses were shorter than those of Chst14+/− or Chst14+/+ fetuses, whereas body weights and general appearances were similar. In a study by Akyüz et al. (2013), the body weight of Chst14−/− mice was lower at 8 weeks of age. However, fetal growth was not mentioned in the study. Patients with mcEDS-CHST14 have been described to show mild growth impairment prenatally and postnatally with slenderness and relative macrocephaly (Shimizu et al. 2011). Because the percentage of homozygotes (Chst14−/−) was decreased markedly from 14.8% at E18.5 to 1.3% at adulthood, our current study supports that major fatal events occurred between E18.5 and birth.
We have presented detailed and comprehensive findings of the placentas of Chst14−/− fetuses for the first time. Decreases in the placental weight and percentage volume of the placental villus on the labyrinth zone were observed in placentas of Chst14−/− fetuses. Smaller vascular diameters in chorionic plates as well as decreased thickness and fragmentation of basement membranes were observed in capillaries of the villus. Changes in vascularity (e.g., changes in the blood vessel number, distribution point and/or blood vessel divergence) were not found in the placentas among wild-type, heterozygous and homozygous fetuses. Hypoxia-like and/or necrosis-like changes were found in 6.1% of placentas of Chst14−/− fetuses, which were not observed in Chst14+/− or Chst14+/+ fetuses (data not shown). Thus, hypoxia and/or necrosis-related events might be causal for perinatal loss in some fetuses. Variation between the different knockout placentas might be dependent on the difference in severity of hypoxia and/or necrosis-related events or other kinds of events in the fetuses. These results suggest that loss of CHST14/D4ST1 induces a change in the blood vessels of fetal placentas and can induce an abnormality of placental functions.
The placenta, a critical organ for growth and survival of fetuses, is a fetomaternal organ with two components: the fetal placenta (e.g., chorionic plate and placental villus), which develops from the same blastocyst that forms the fetus, and the maternal placenta (decidua) that develops from maternal uterine tissue. The genotypes of fetal and maternal placentas correspond with those of the fetus (Chst14+/+, Chst14+/− or Chst14−/−) and mother (Chst14+/−), respectively. Because it was difficult to completely separate maternal and fetal placentas, whole placentas were used for the analyses in the study. Chondroitin 4-O-sulfotransferase-2 (C4ST2) encoded by CHST12 has both a chondroitin 4-O-sulfotransferase activity and dermatan 4-O-sulfotransferase activity in vitro (Mikami et al. 2003). However, DS is not detected in the skin of mcEDS-CHST14 patients (Miyake et al. 2010), suggesting that C4ST2 might not be primarily involved in the sulfation of DS moieties in vivo. Hence, some detections of Chst14 expression and disaccharides from DS moieties in the whole placenta of Chst14−/− fetuses might be derived from the maternal placenta. These observations indicate loss of CHST14/D4ST1 in the fetal placenta of Chst14−/− fetuses. Placental villi, which are part of the fetal placenta, are rich in blood capillaries and could potentially be a disease model of the vascular abnormality of mcEDS-CHST14.
We found no altered expression of ECM-related genes including Col1a, Col2a, Col3a, Col4a, Col15, Dcn and Bgn. In immunostaining, COL4, COL15, DCN and BGN were found on fetal placentas. In particular, COL4 and BGN were strongly stained in vascular endothelial cells of the chorionic plate and capillaries of the placental villus by corresponding antibodies. DCN was distributed in the interstitial tissue of the chorionic plate, but not in placental blood vessels. COL4 is a major and crucial component of all basement membranes and essential for maintenance of the ECM (Poschl et al. 2004). BGN is a leucine-rich repeat PG with a protein core and two GAG chains consisting of either CS or DS (Roughley and White 1989). BGN interacts with collagen via both the core protein and GAG chains (Pogany et al. 1994; Schonherr et al. 1995). Based on these reports and our findings, the decreased thickness and fragmentation of basement membranes in placental villus capillaries might be the primary placental abnormalities, which may be related to aberrant formation of collagen fibrils (a change in quality of the ECM not quantity) resulting from loss of DS in the GAG chains of BGN.
In conclusion, this is the first investigation of vascular abnormalities in the placentas of Chst14−/− fetuses, which demonstrated vascular-related manifestations including hypoxia-like and/or necrosis-like abnormalities, smaller vascular diameters of chorionic plates, a decreased thickness and fragmentation of basement membranes in capillaries of the villus, decreased placental weight, and decreased percentage volume of the placental villus on the labyrinth zone. These findings might explain the underlying pathophysiology of not only the perinatal lethality of Chst14−/− fetuses but also the vascular abnormalities (large subcutaneous hematomas) in patients with mcEDS-CHST14.
Materials and methods
Animals
All experimental procedures were carried out in accordance with the Regulations for Animal Experimentation of Shinshu University. The animal protocol was approved by the Committee for Animal Experiments of Shinshu University (Approval number 270030). Based on the national regulations and guidelines, all experimental procedures were reviewed by the Committee for Animal Experiments and finally approved by the president of Shinshu University.
Chst14 gene-deleted mice were obtained from the Mutant Mouse Regional Resource Center (MMRRC; https://www.mmrrc.org) (Tang et al. 2010) and inbreeded for more than 12 generations. Mice were housed in a micro isolator (Shin Toyo Seisakusho, Saitama, Japan) at 23 ± 2°C with constant humidity and a 12-h light/dark cycle. Animals had free access to tap water and standard mouse chow (Funabashi Farm, Chiba, Japan).
Sample collection
Proestrus female Chst14+/− mice were bred with male Chst14+/− mice overnight. Female mice were then checked for vaginal plugs. Days when plugs were found were designated as E0.5. Placentas and fetuses were obtained on E18.5 after sacrificing female mice. After washing in phosphate-buffered saline (PBS), the placentas were fixed with 10% formaldehyde or were stored at −80°C. The fetuses were used for genotyping after obtaining photographs under a stereoscopic microscope (M165C; Leica Microsystems, Wetzlar, Germany) and measuring the body weight.
Genotyping
To extract DNA, tails of the fetuses were treated with Mighty Prep reagent for DNA (TAKARA BIO INC., Shiga, Japan). Primer sequences for wild-type genotyping, which were designed for exon 1 of Chst14 gene, were 5′-GGACCACCGCAGTGACTTG-3′ and 5′-ACAGGCATCCAATGCTCATTC-3′. Primer sequences for the neomycin resistance gene in knockout polymerase chain reaction (PCR) were 5′-TGGCTCTCCTCAAGCGTATT-3′ and 5′-GTTTTCCCAGTCACGACGTT-3′. The extracted DNA solutions were used for PCR with HS Perfect Mix (TAKARA BIO INC.). The PCR conditions were 94°C for 1 min and then 35 cycles of 94°C for 5 s and 65°C for 15 s. PCR products were detected by agarose gel electrophoresis. The electrophoretic patterns are shown in Supplementary Figure S2.
Pathological analysis
After taking photographs of their appearance, fixed placentas were embedded in paraffin and cut into 5 μm-thick sections for histological examination. The paraffin-embedded sections were immersed in Hemo-De (Falma, Tokyo, Japan) for deparaffinization and then subjected to hydrophilic treatment by sequential immersion in a series of 100, 95, 80 and 70% ethanol solutions followed by deionized water. For immunostaining, sections were treated with Antigen Retrieval Reagent (Nichirei, Tokyo, Japan) and then 5% hydrogen peroxide for inactivation of endogenous peroxidases. After blocking with 2% bovine serum albumin, an anti-collagen IV (Bioss Antibodies, Woburn, MA), anti-collagen XV (Gene Tex, Irvine, CA), anti-biglycan (Gene Tex), or anti-decorin (R&D Systems, Minneapolis, MN) antibody solution was reacted with the sections at 4°C overnight. Then, the sections were treated with a peroxidase-conjugated secondary antibody (Nichirei, Tokyo, Japan). Antigen-positive sites were stained brown with a 3,3′-diaminobenzidine tetrahydrochloride hydrate solution (Nichirei, Tokyo, Japan). The stained sections were observed under a microscope (CKX41; Olympus, Tokyo, Japan). The areas of placental zones and the percentage volume of the placental villus (three fields/sample) were calculated with Photoshop CS5 software (Adobe, San Jose, CA).
Transmission electron microscopy
Placental tissues were collected and cut into pieces with a scalpel on ice. Then, the samples were fixed with 2.5% glutaraldehyde and 4% osmium tetroxide, embedded in epoxy resin, and cut into ultrathin sections. These sections were electron-stained with uranyl acetate and lead citrate, subjected to carbon shadowing, and observed by TEM (JEM1400; JEOL, Tokyo, Japan). The basement membrane thickness was calculated with the Measure Tool of Photoshop CS5 software.
Gene expression analyses
Frozen placentas were homogenized in TRI Reagent (Molecular Research Center, Cincinnati, OH) using a Bio-Gen PRO200 homogenizer (PRO Scientific, Oxford, CT) to extract total RNA. The homogenate was treated with DNase (TURBO-DNA-free Kit; Life Technologies, Carlsbad, CA) to remove contaminating DNA. The RNA was then subjected to reverse transcription using a High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA). Quantitative reverse transcription-polymerase chain reaction (RT-PCR) was carried out using a Step One Plus Realtime PCR system (Applied Biosystems) with SYBR Green Realtime PCR Master Mix Plus (Toyobo, Osaka, Japan). Values were normalized to 18S ribosomal RNA levels. The primer sequences used are listed in Supplementary Table SI.
Quantification of DS and CS
Placentas were washed, homogenized, and sonicated. Extraction and purification of GAG fractions were carried out as described previously (Mizumoto and Sugahara 2012). The samples were treated individually with chondroitinase B (EC 4.2.2.19) (IBEX Technologies, Montreal, Canada) or a mixture of chondroitinase AC-I (EC 4.2.2.5) and AC-II (EC 4.2.2.5) (Seikagaku Corp., Tokyo, Japan) to analyze the disaccharide composition of DS or CS moieties in CS/DS chains, respectively. Chondroitinase B cleaves N-acetylgalactosaminidic linkages in consecutive GalNAc(4S)-IdoUA sequences into disaccharides, whereas chondroitinase AC degrades N-acetylgalactosaminidic linkages in consecutive GalNAc(±4S, ±6S)-GlcUA sequences. However, it should be noted that N-acetylgalactosaminidic linkages in the alternating sequence of GalNAc(4S)-IdoUA and GalNAc(±4S, ±6S)-GlcUA are resistant to both enzymes. Therefore, the amounts of CS and DS moieties might be underestimated, although such alternating sequences are rare in CS/DS chains. The digests were labeled with the fluorophore 2-aminobenzamide (2AB), and samples were analyzed for each disaccharide by anion-exchange high performance liquid chromatography (HPLC) on a PA-G column (YMC Co., Kyoto, Japan) as described previously (Mizumoto and Sugahara 2012). Disaccharide standards were also labeled with 2AB and were analyzed by HPLC. The standards used were as follows: ΔHexUA-GalNAc, ΔHexUA-GalNAc(6S), ΔHexUA-GalNAc(4S), ΔHexUA(2S)-GalNAc(6S), ΔHexUA-GalNAc(4S,6S) and ΔHexUA(2S)-GalNAc(4S,6S). Unsaturated DS and CS disaccharides observed in the digests were identified by comparison with the elution positions of the authentic 2AB-labeled disaccharide standards, and quantitated based on their peak area in reference to that of standard unsaturated disaccharides.
Statistical analysis
Data are reported as the mean ± standard error of the mean (SEM). Statistical comparisons between groups were carried out using Stat Mate statistical software (ATMS, Tokyo, Japan). The data were analyzed by one-way analysis of variance (ANOVA) followed by the Tukey–Kramer post hoc test (for multiple comparisons). In all analyses, P < 0.05 was considered to indicate statistical significance.
Supplementary Material
Acknowledgements
We are grateful to Ms. Nana Tsumita and Mr. Shota Saka (Scleroprotein and Leather Research Institute, Tokyo University of Agriculture and Technology, Faculty of Agriculture, Tokyo, Japan) for breeding the mice. The mouse strain used in this study, B6;129S5-Chst14tm1Lex/Mmcd (identification number 031,629-UCD), was obtained from the MMRRC, a National Center for Research Resources-National Institutes of Health-funded strain repository, which was donated to the MMRRC by Lexicon Genetics Incorporated. We are grateful to all staff of the Research Center for Supports to Advanced Science, Shinshu University, for assisting our experiments. We thank Mitchell Arico from Edanz Group (www.edanzediting.com/ac) for editing a draft of this manuscript.
Supplementary data
Funding
Practical Research Project for Rare/Intractable Diseases from the Japan Agency for Medical Research and Development (AMED) (to T.Y., S.M., J.N., T.O., K.M., S.Y. and T.K.). Grant-in Aid for Young Scientists (B) 16K19396 (to T.Y.) and for Scientific Research (C) 16K08251 (to S.M.) from the Japan Society for the Promotion of Science.
Conflict of interest statement
None declared.
Abbreviations
ANOVA, analysis of variance; CS, chondroitin sulfate; DS, dermatan sulfate; EDS, Ehlers–Danlos syndrome; ECM, extracellular matrix; GAGs, glycosaminoglycans; H&E, hematoxylin and eosin; HPLC, high performance liquid chromatography; PBS, phosphate-buffered saline; PGs, proteoglycans; SEM, standard error of the mean; TEM, transmission electron microscopy.
References
- Akyuz N, Rost S, Mehanna A, Bian S, Loers G, Oezen I, Mishra B, Hoffmann K, Guseva D, Laczynska E et al. 2013. Dermatan 4-O-sulfotransferase1 ablation accelerates peripheral nerve regeneration. Exp Neurol. 247:517–530. [DOI] [PubMed] [Google Scholar]
- Bai X, Zhou D, Brown JR, Crawford BE, Hennet T, Esko JD. 2001. Biosynthesis of the linkage region of glycosaminoglycans: cloning and activity of galactosyltransferase II, the sixth member of the beta 1,3-galactosyltransferase family (beta 3GalT6). J Biol Chem. 276:48189–48195. [DOI] [PubMed] [Google Scholar]
- Bian S, Akyuz N, Bernreuther C, Loers G, Laczynska E, Jakovcevski I, Schachner M. 2011. Dermatan sulfotransferase Chst14/D4st1, but not chondroitin sulfotransferase Chst11/C4st1, regulates proliferation and neurogenesis of neural progenitor cells. J Cell Sci. 124:4051–4063. [DOI] [PubMed] [Google Scholar]
- Cabral WA, Makareeva E, Colige A, Letocha AD, Ty JM, Yeowell HN, Pals G, Leikin S, Marini JC. 2005. Mutations near amino end of alpha1(I) collagen cause combined osteogenesis imperfecta/Ehlers–Danlos syndrome by interference with N-propeptide processing. J Biol Chem. 280:19259–19269. [DOI] [PubMed] [Google Scholar]
- Colige A, Nuytinck L, Hausser I, van Essen AJ, Thiry M, Herens C, Ades LC, Malfait F, Paepe AD, Franck P et al. 2004. Novel types of mutation responsible for the dermatosparactic type of Ehlers–Danlos syndrome (Type VIIC) and common polymorphisms in the ADAMTS2 gene. J Invest Dermatol. 123:656–663. [DOI] [PubMed] [Google Scholar]
- Colige A, Sieron AL, Li SW, Schwarze U, Petty E, Wertelecki W, Wilcox W, Krakow D, Cohn DH, Reardon W et al. 1999. Human Ehlers–Danlos syndrome type VII C and bovine dermatosparaxis are caused by mutations in the procollagen I N-proteinase gene. Am J Hum Genet. 65:308–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evers MR, Xia G, Kang HG, Schachner M, Baenziger JU. 2001. Molecular cloning and characterization of a dermatan-specific N-acetylgalactosamine 4-O-sulfotransferase. J Biol Chem. 276:36344–36353. [DOI] [PubMed] [Google Scholar]
- Gotting C, Kuhn J, Zahn R, Brinkmann T, Kleesiek K. 2000. Molecular cloning and expression of human UDP-d-Xylose:proteoglycan core protein beta-d-xylosyltransferase and its first isoform XT-II. J Mol Biol. 304:517–528. [DOI] [PubMed] [Google Scholar]
- Kim SH, Turnbull J, Guimond S. 2011. Extracellular matrix and cell signalling: The dynamic cooperation of integrin, proteoglycan and growth factor receptor. J Endocrinol. 209:139–151. [DOI] [PubMed] [Google Scholar]
- Kitagawa H, Izumikawa T, Uyama T, Sugahara K. 2003. Molecular cloning of a chondroitin polymerizing factor that cooperates with chondroitin synthase for chondroitin polymerization. J Biol Chem. 278:23666–23671. [DOI] [PubMed] [Google Scholar]
- Kitagawa H, Tone Y, Tamura J, Neumann KW, Ogawa T, Oka S, Kawasaki T, Sugahara K. 1998. Molecular cloning and expression of glucuronyltransferase I involved in the biosynthesis of the glycosaminoglycan-protein linkage region of proteoglycans. J Biol Chem. 273:6615–6618. [DOI] [PubMed] [Google Scholar]
- Kitagawa H, Uyama T, Sugahara K. 2001. Molecular cloning and expression of a human chondroitin synthase. J Biol Chem. 276:38721–38726. [DOI] [PubMed] [Google Scholar]
- Kobayashi M, Sugumaran G, Liu J, Shworak NW, Silbert JE, Rosenberg RD. 1999. Molecular cloning and characterization of a human uronyl 2-sulfotransferase that sulfates iduronyl and glucuronyl residues in dermatan/chondroitin sulfate. J Biol Chem. 274:10474–10480. [DOI] [PubMed] [Google Scholar]
- Kosho T. 2016. CHST14/D4ST1 deficiency: new form of Ehlers–Danlos syndrome. Pediatr Int. 58:88–99. [DOI] [PubMed] [Google Scholar]
- Maccarana M, Olander B, Malmstrom J, Tiedemann K, Aebersold R, Lindahl U, Li JP, Malmstrom A. 2006. Biosynthesis of dermatan sulfate: chondroitin-glucuronate C5-epimerase is identical to SART2. J Biol Chem. 281:11560–11568. [DOI] [PubMed] [Google Scholar]
- Makareeva E, Cabral WA, Marini JC, Leikin S. 2006. Molecular mechanism of alpha 1(I)-osteogenesis imperfecta/Ehlers–Danlos syndrome: unfolding of an N-anchor domain at the N-terminal end of the type I collagen triple helix. J Biol Chem. 281:6463–6470. [DOI] [PubMed] [Google Scholar]
- Malfait F, Coucke P, Symoens S, Loeys B, Nuytinck L, De Paepe A. 2005. The molecular basis of classic Ehlers–Danlos syndrome: a comprehensive study of biochemical and molecular findings in 48 unrelated patients. Hum Mutat. 25:28–37. [DOI] [PubMed] [Google Scholar]
- Malfait F, Francomano C, Byers P, Belmont J, Berglund B, Black J, Bloom L, Bowen JM, Brady AF, Burrows NP et al. 2017. The 2017 international classification of the Ehlers–Danlos syndromes. Am J Med Genet C Semin Med Genet. 175:8–26. [DOI] [PubMed] [Google Scholar]
- Mikami T, Mizumoto S, Kago N, Kitagawa H, Sugahara K. 2003. Specificities of three distinct human chondroitin/dermatan N-acetylgalactosamine 4-O-sulfotransferases demonstrated using partially desulfated dermatan sulfate as an acceptor: implication of differential roles in dermatan sulfate biosynthesis. J Biol Chem. 278:36115–36127. [DOI] [PubMed] [Google Scholar]
- Miyake N, Kosho T, Matsumoto N. 2014. Ehlers–Danlos syndrome associated with glycosaminoglycan abnormalities. Adv Exp Med Biol. 802:145–159. [DOI] [PubMed] [Google Scholar]
- Miyake N, Kosho T, Mizumoto S, Furuichi T, Hatamochi A, Nagashima Y, Arai E, Takahashi K, Kawamura R, Wakui K et al. 2010. Loss-of-function mutations of CHST14 in a new type of Ehlers–Danlos syndrome. Hum Mutat. 31:966–974. [DOI] [PubMed] [Google Scholar]
- Mizumoto S, Sugahara K. 2012. Glycosaminoglycan chain analysis and characterization (glycosylation/epimerization). Methods Mol Biol. 836:99–115. [DOI] [PubMed] [Google Scholar]
- Okajima T, Yoshida K, Kondo T, Furukawa K. 1999. Human homolog of Caenorhabditis elegans sqv-3 gene is galactosyltransferase I involved in the biosynthesis of the glycosaminoglycan-protein linkage region of proteoglycans. J Biol Chem. 274:22915–22918. [DOI] [PubMed] [Google Scholar]
- Okuyama K. 2008. Revisiting the molecular structure of collagen. Connect Tissue Res. 49:299–310. [DOI] [PubMed] [Google Scholar]
- Pacheco B, Malmstrom A, Maccarana M. 2009. Two dermatan sulfate epimerases form iduronic acid domains in dermatan sulfate. J Biol Chem. 284:9788–9795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogany G, Hernandez DJ, Vogel KG. 1994. The in vitro interaction of proteoglycans with type I collagen is modulated by phosphate. Arch Biochem Biophys. 313:102–111. [DOI] [PubMed] [Google Scholar]
- Poschl E, Schlotzer-Schrehardt U, Brachvogel B, Saito K, Ninomiya Y, Mayer U. 2004. Collagen IV is essential for basement membrane stability but dispensable for initiation of its assembly during early development. Development. 131:1619–1628. [DOI] [PubMed] [Google Scholar]
- Rost S, Akyuz N, Martinovic T, Huckhagel T, Jakovcevski I, Schachner M. 2016. Germline ablation of dermatan-4O-sulfotransferase1 reduces regeneration after mouse spinal cord injury. Neuroscience. 312:74–85. [DOI] [PubMed] [Google Scholar]
- Roughley PJ, White RJ. 1989. Dermatan sulphate proteoglycans of human articular cartilage. The properties of dermatan sulphate proteoglycans I and II. Biochem J. 262:823–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schonherr E, Witsch-Prehm P, Harrach B, Robenek H, Rauterberg J, Kresse H. 1995. Interaction of biglycan with type I collagen. J Biol Chem. 270:2776–2783. [DOI] [PubMed] [Google Scholar]
- Schwarze U, Schievink WI, Petty E, Jaff MR, Babovic-Vuksanovic D, Cherry KJ, Pepin M, Byers PH. 2001. Haploinsufficiency for one COL3A1 allele of type III procollagen results in a phenotype similar to the vascular form of Ehlers–Danlos syndrome, Ehlers–Danlos syndrome type IV. Am J Hum Genet. 69:989–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu K, Okamoto N, Miyake N, Taira K, Sato Y, Matsuda K, Akimaru N, Ohashi H, Wakui K, Fukushima Y et al. 2011. Delineation of dermatan 4-O-sulfotransferase 1 deficient Ehlers–Danlos syndrome: observation of two additional patients and comprehensive review of 20 reported patients. Am J Med Genet A. 155A:1949–1958. [DOI] [PubMed] [Google Scholar]
- Tang T, Li L, Tang J, Li Y, Lin WY, Martin F, Grant D, Solloway M, Parker L, Ye W et al. 2010. A mouse knockout library for secreted and transmembrane proteins. Nat Biotechnol. 28:749–755. [DOI] [PubMed] [Google Scholar]
- Uyama T, Kitagawa H, Tamura Ji J, Sugahara K. 2002. Molecular cloning and expression of human chondroitin N-acetylgalactosaminyltransferase: the key enzyme for chain initiation and elongation of chondroitin/dermatan sulfate on the protein linkage region tetrasaccharide shared by heparin/heparan sulfate. J Biol Chem. 277:8841–8846. [DOI] [PubMed] [Google Scholar]
- Zweers MC, Bristow J, Steijlen PM, Dean WB, Hamel BC, Otero M, Kucharekova M, Boezeman JB, Schalkwijk J. 2003. Haploinsufficiency of TNXB is associated with hypermobility type of Ehlers–Danlos syndrome. Am J Hum Genet. 73:214–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.