Abstract
From an evolutionary perspective keratan sulfate (KS) is the newest glycosaminoglycan (GAG) but the least understood. KS is a sophisticated molecule with a diverse structure, and unique functional roles continue to be uncovered for this GAG. The cornea is the richest tissue source of KS in the human body but the central and peripheral nervous systems also contain significant levels of KS and a diverse range of KS-proteoglycans with essential functional roles. KS also displays important cell regulatory properties in epithelial and mesenchymal tissues and in bone and in tumor development of diagnostic and prognostic utility. Corneal KS-I displays variable degrees of sulfation along the KS chain ranging from non-sulfated polylactosamine, mono-sulfated and disulfated disaccharide regions. Skeletal KS-II is almost completely sulfated consisting of disulfated disaccharides interrupted by occasional mono-sulfated N-acetyllactosamine residues. KS-III also contains highly sulfated KS disaccharides but differs from KS-I and KS-II through 2-O-mannose linkage to serine or threonine core protein residues on proteoglycans such as phosphacan and abakan in brain tissue. Historically, the major emphasis on the biology of KS has focused on its sulfated regions for good reason. The sulfation motifs on KS convey important molecular recognition information and direct cell behavior through a number of interactive proteins. Emerging evidence also suggest functional roles for the poly-N-acetyllactosamine regions of KS requiring further investigation. Thus further research is warranted to better understand the complexities of KS.
Keywords: keratan sulfate, KS-I, KS-II, KS-III, glycosaminoglycans, KS-proteoglycans
Introduction
A historical perspective on keratan sulfate
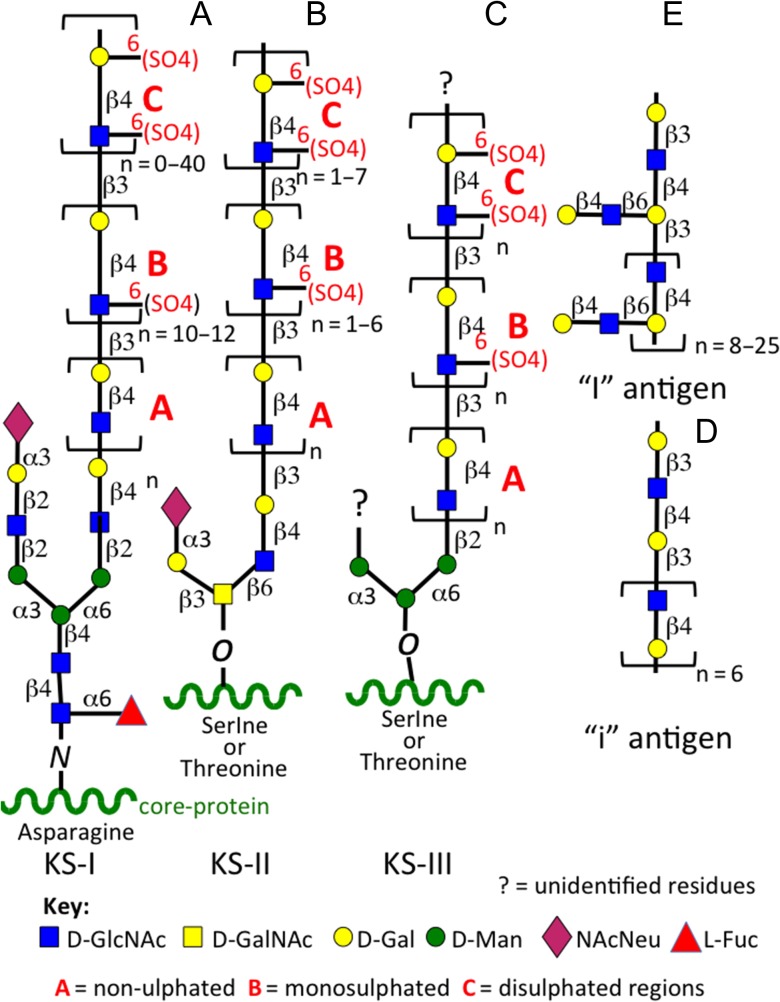
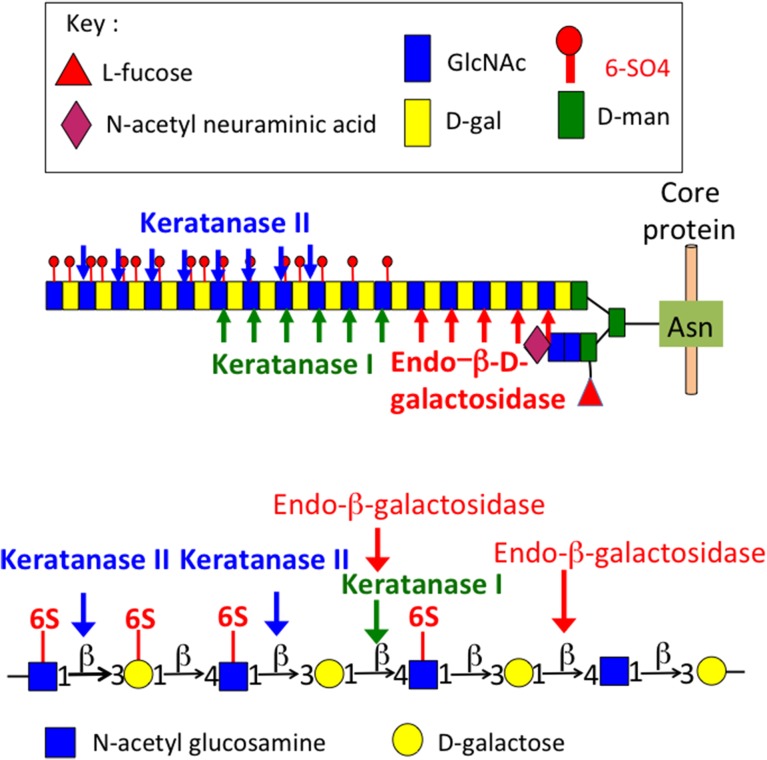
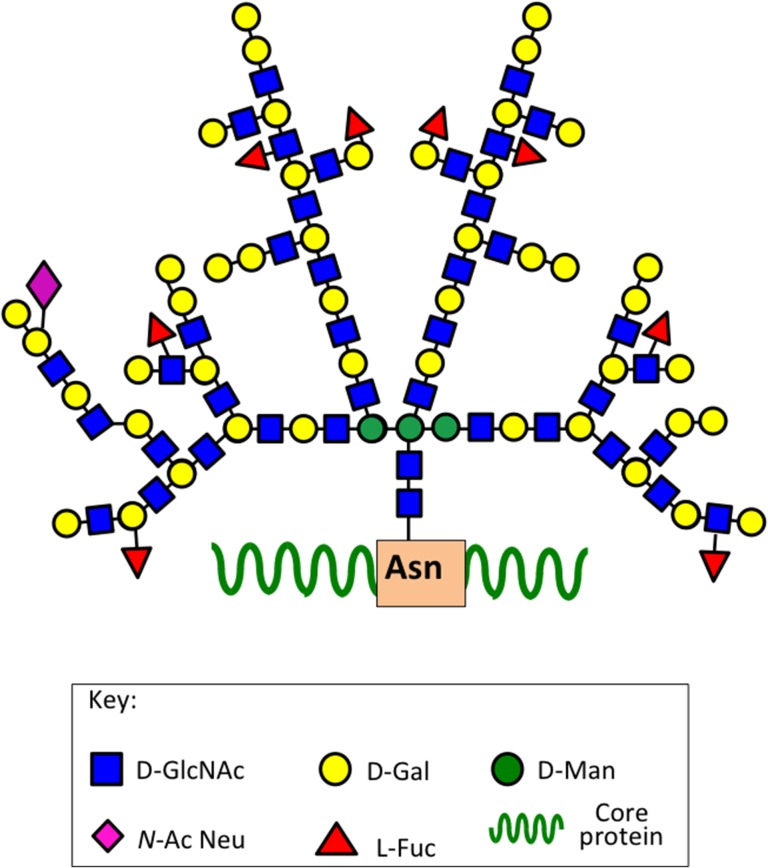
Keratan sulfate (KS) was first identified in the cornea by Suzuki and colleagues in 1939 (Suzuki 1939), it was identified as a mucoid type material containing galactose and glucose in equimolar amounts, and also acetyl and sulfate groups. Karl Meyer and colleagues subsequently characterized this mucinous mucopolysaccharide in a series of studies renaming it kerato-sulfate (Meyer et al. 1953, 1967; Davidson et al. 1956; Hoffman et al. 1958, 1967; Seno et al. 1965; Bhavanandan and Meyer 1966, 1968; Bray et al. 1967; Shulman and Meyer 1968; Choi and Meyer 1975; Kikuchi et al. 1987; Meyer-Puttlitz et al. 1995). Ongoing studies on the characterization of KS and KS-proteoglycans established the current name of this mucopolysaccharide as keratan sulfate. Investigations on the kerato-sulfates were initially compositional studies, and structural analyses on the linkage region of kerato-sulfate to protein (Bray et al. 1967; Bhavanandan and Meyer 1968; Choi and Meyer 1975; Brown et al. 1996). Most work was initially conducted on corneal KS and this was subsequently classified as KS-I but despite this naming KS-I is also found in tissues other than cornea as a side chain components of members of the SLRP family members keratocan, fibromodulin and lumican (Plaas et al. 1990, 2001; Lauder et al. 1996). Comparative studies on corneal KS and the KS of cartilaginous tissues in a number of species subsequently identified a different O-linkage group to protein through serine or threonine residues and variable levels of l-fucose and N-acetylneuraminic acid capping residues (Choi and Meyer 1975). Thus skeletal KS was subsequently classified as KS-II to distinguish it from corneal KS-I. Ongoing studies on KS in brain tissues, the next richest source of KS in the human body after the cornea, identified a further O-linkage region through D-mannose residues in KS and this was classified as KS-III (Krusius et al. 1986) (Figure 1). Although variations in chain length and sulfation patterns and differences in the types of linkage groups were noted all three forms of KS were found to share common structural elements including stretches of non-sulfated poly-N-acetyllactosamine, and variable regions of mono- and disulfated regions of 6-sulfated d-galactose and N-acetylglucosamine, the two glycosaminoglycans of the characteristic repeat disaccharide unit of KS. The variable presence of minor l-fucose and N-acetylneuraminic acid further distinguished KS-I, II, III. While these residues conferred resistance to the KS chain to degradation by keratanase-I, II and endo-β-d-galactosidase (Figure 2) the full significance of these glycan components on the interactive properties of KS are only now beginning to be uncovered. Up till now, most GAG interactive studies have been conducted using the CS-A, CS-B, CS-D, CS-E isomers and HS and there is a massive literature published on these GAGs. However, a few studies have now shown that KS also has interactive properties with a number of regulatory proteins. Surface plasmon resonance studies have shown that corneal KS interacts with SHH, FGF1 and FGF2 (Weyers et al. 2013). The core proteins of the KS-SLRPs are also highly interactive proteins due to their LRR motifs.
Fig. 1.
Structural complexity of KS. Corneal KS-I, skeletal KS-II, i antigen, I antigen.
Fig. 2.
The cleavage sites of keratanase-I, keratanase II and endo-β-d-galactosidase on a typical KS-I chain. For information on the KS-oligosaccharides released, see Brown et al. (1994a, 1994b) and Tai et al. (1996, 1997).
A recent study with corneal KS using a proteomics screen with a microarray of 8268 proteins and custom array of 85 extracellular nerve growth factor protein epitopes has uncovered a wealth of data pointing to potential roles for KS in cell-signaling processes in neural tissues (Conrad et al. 2010). Highly sulfated KS interacted with 217 of the microarray proteins examined including 75 kinases, several membrane and secreted proteins, cytoskeletal proteins and a number of nerve regulatory proteins. These interactions were confirmed using plasmon resonance and binding constants determined. Of the 85 ECM nerve-related epitopes examined, KS bound to almost half of these, including Slit, two Robo’s, nine ephrin receptors, eight ephrins, eight semaphorins and two nerve growth factor receptors. The SLIT-ROBO cell-signaling pathway has important roles to play in axonal guidance and neural angiogenic processes (Tessier-Lavigne and Goodman 1996; Bashaw et al. 2000; Nguyen-Ba-Charvet and Chedotal 2002). Slit is a secreted protein which provides a repulsive cue for the directional guidance of axons during neuronal development and repair processes (Rothberg et al. 1990), Robo is its transmembrane protein cellular receptor. Vertebrates contain four Robo receptors and three Slits (Yuan et al. 1999). Slits are modular proteins containing many LRRs, 7–9 EGF repeat modules and an Agrin, Laminin, Perlecan, Slit (ALPS) interactive domain (Howitt et al. 2004; Hussain et al. 2006). These LRRs and EGFs have well-known functional interactive properties with proteins. Robos are transmembrane receptors which contain five IgG-like domains, three fibronectin type III repeats and an intracellular cytoplasmic domain containing up to four conserved CCO, CC1, CC2 and CC3 motifs containing potential sites of tyrosine phosphorylation, netrin-1 binding activity and binding sites for Ena/Vasp proteins (Stein and Tessier-Lavigne 2001). Ena/Vasp homology proteins are a family of closely related proteins involved in cellular motility through regulatory properties exerted on the spatial polymerization of actin structures required to promote chemotaxis in response to attractive and repulsive cues (Wong et al. 2002). Interactions of KS with Robo-Slit results in downstream activation of Rho GTPases which mediate actin depolymerization, cytoskeletal re-organization and cell signaling. Ephrins are a family of protein ligands for the Ephrin receptors. This is the largest subfamily of receptor protein–tyrosine kinases. These are membrane-bound proteins, which regulate intracellular signaling pathways arising from direct cell–cell interaction. Semaphorins are secreted and membrane-bound axonal growth cone guidance proteins which act as short-range inhibitory signals through multimeric plexin and neuropilin receptors to regulate Rho family GTPases and are critical to axonal guidance in neural development and repair processes. KS research is therefore entering an exciting era. Functional studies on KS in neural tissues are eagerly anticipated and may yield information of application in neural repair biology.
Tissue distribution of KS, KS–proteoglycans and KS–glycoproteins in tissues
KS (Figures 1 and 2) is a widely distributed glycosaminoglycan (GAG) in tensional and weight-bearing connective tissues (cornea, bone, cartilage, intervertebral disc, tendon), epithelial tissues and the central and peripheral nervous system (CNS/PNS) (Funderburgh 2000, 2002). KS is attached to a number of proteoglycan and glycoprotein core proteins (Table I). KS–proteoglycans (KSPGs) display adaptable and variable functional interactive properties in situ and are localized within tissues in a specific spatio-temporal manner with important roles in tissue morphogenesis. Like all GAGs the variable but specific sulfation status of KS is an important functional determinant encoding a significant level of information which cells can interpret to influence cellular metabolism and behavior to effect tissue homeostasis, modulation of tissue structure and the assembly of key extracellular matrix (ECM) assemblies in tissue morphogenesis critical in the determination of tissue form and function. The ability to perceive ion-fluxes by KS is a conduit to the sensory capability of cells which allows them to perceive and respond dynamically to biomechanical changes in their microenvironment equipping them with the ability to modulate the synthesis and assembly of ECM components to form a matrix which better protects them from extrinsic forces. In some cells, the ability to sense and control ion-fluxes is highly advanced such as in neurons which generate action potentials (Camire and Topolnik 2014), the basis of synaptic function that has even been proposed as a mechanism whereby cognitive memory is generated (Eccles 1983). Synaptic vesicle protein-2 (SV2) is a transport proteoglycan for neurotransmitters (Bajjalieh et al. 1992; Feany et al. 1992; Scranton et al. 1993; Carlson 1996; Nowack et al. 2010; Wan et al. 2010). Glial cells (oligodendrocytes, astrocytes, Schwann cells) produce KSPGs and KS-substituted glycoproteins (Fryer et al. 1992; Geisert et al. 1992; Geisert and Bidanset 1993; Burg and Cole 1994; Robson and Geisert 1994; Junghans et al. 1995; Meyer-Puttlitz et al. 1995; Jones and Tuszynski 2002; Papageorgakopoulou et al. 2002; Dobbertin et al. 2003; Vitureira et al. 2005; Sinouris et al. 2009; Vitureira et al. 2010), with roles in the demarcation of functional areas of the PNS/CNS and assembly of the myelin sheath encompassing neurons (Table I). KS has associated Ca2+ counterions which may act as a calcium reserve for egg shell production (Ha et al. 2007; Du et al. 2015) and in the mineralization of bone in laying birds and in the generation of action potentials in neurons. KS-substituted small leucine repeat proteoglycans (SLRPs) also have roles in bone formation (Kinne and Fisher 1987; Sommarin et al. 1998; Wendel et al. 1998; Gori et al. 2001; Nakamura et al. 2001; Igwe et al. 2011; Nikdin et al. 2012).
Table I.
The biodiverse structural forms and functions of KS proteoglycans
| Protein | Distribution | Functions | Reference |
|---|---|---|---|
| KS–proteoglycans of tensional and weight-bearing connective tissues | |||
| Aggrecan lectican KS/CSPG | Large ECM PG of cartilage, CNS, tendon, IVD | Tissue hydration, weight bearing. Inhibits neurite outgrowth, repulsive cue on axonal guidance | Fryer et al. (1992), Kiani et al. (2002) |
| Fibromodulin | Widespread ECM distribution in cornea, cartilage, tendon, IVD, meniscus | Regulate collagen fibrillogenesis and inflammatory cytokines/growth factors, cell proliferation and cell signaling. Lumican is a tumor marker | Chen and Birk (2013), Merline et al. (2009), Schaefer and Iozzo (2008) |
| Keratocan | Blochberger et al. (1992), Corpuz et al. (1996), Oldberg et al. (1989), Sommarin et al. (1998) | ||
| Lumican | |||
| Osteoadherin (osteomodulin) | Cartilage/bone growth plate interface | Cell binding bone KSPG, may regulate mineralization | Sommarin et al. (1998) |
| Mimecan (osteoglycin) | Broad distribution in connective tissues | Corneal mimecan is sulfated but not sulfated in other tissues. Has roles in bone induction | Corpuz et al. (2000), Funderburgh et al. (1997) |
| CD44 | Epidermal/CNS KS-CD44 isoform | Ubiquitous HA receptor occurring as alternatively spliced forms substituted with KS, CS or HS | Takahashi et al. (1996) |
| Bone sialoprotein-II (BSP-II) | 80 kDa core protein, substituted with sialic acid and N- and O-linked KS chains | BSP-II, KSPG in compact rabbit bone, BSP-II from other species does not contain KS. Related KSPG identified in rat calvaria BSP-II in medullary bone in laying birds is a KSPG | Ganss et al. (1999), Hadley et al. (2016), Kinne and Fisher (1987), Masubuchi et al. (1975), Nakamura et al. (2001) |
| KS–proteoglycans of mucinous tissues | |||
| MUC1 | epithelial distribution | transmembrane epithelial KSPG, heavily O-glycosylated, sialylated forms 200–500 nm layer on cell surface | Aplin et al. (1998), Brayman et al. (2004) |
| Endometrial ECM | |||
| Mucous KSPG | 220 kDa 5D4+ve KSPG | KSPG of cervical mucous secretions | Fischer et al. (2001) |
| Podocalyxcin | 240 kDa 3–10 G +ve | Mucin-like, sialomucin cell surface KS–PG related to CD34. Anti-adhesive. Widespread epithelial distribution | Nielsen and McNagny (2009), Vitureira et al. (2010) |
| Zona pellucida protein-3 (PZP-3) | zona pellucida | An N-linked polylactosamine sulfated KS protein with oocyte–sperm receptor interactive activity | Nakano et al. (1996), Noguchi and Nakano (1992) |
| Oocyte membrane glycoprotein | |||
| Keratinocyte perlecan | Epidermis | Hybrid KS-HS-CS basement membrane proteoglycan with roles in ECM stabilization and growth factor binding | Knox et al. (2005) |
| Embryoglycan | Cell surface PG of Pluripotent and early embryonic stem cells | Highly branched polylactosamine non-sulfated KS chains contain 3-G10, EMCA-2, 3, TRA-1-60 and TRA-1-81, GCTM-2, SSEA carbohydrate motifs | Dvorak et al. (1998), Muramatsu (2017), Ozawa et al. (1985a) |
| KS–proteoglycans of the PNS/CNS | |||
| Abakan | Astrocyte KSPG, co-distributed with glial fibrillary acidic protein | Provides repulsive axonal guidance cues which regulate neuritogenesis in CNS development | Geisert and Bidanset (1993) |
| Claustrin/MAP1B | Claustrin, ECM PG synthesized by CNS astrocytes. MAP1B is a microtubule associated protein | Claustrin, anti-adhesive neural proteoglycan inhibits neurite outgrowth, N-terminal truncated MAP-1B, a 225 kDa microtubule and dendritic PG of neurons and glial cells | Burg and Cole (1994), Edelmann et al. (1996) |
| Synapse vesicle protein 2 (SV2) | 12 span membrane KSPG, 100/250 kDa forms and 3 isoforms SV2A, B, C | Storage/neurotransmitter transport in synaptic vesicles/neuroendocrine cells. KS of SV2 interactive component of a smart gel delivery system | Bajjalieh et al. (1993), Feany et al. (1992), Scranton et al. (1993) |
| PG1000 | KS/CS proteoglycan Electric organ ECM | Forms 2–6 monomer complexes concentrated in the reticular laminae of electric organ basement membranes surrounding nerve fibers/terminals | Carlson et al. (1996), Iwata and Carlson (1991) |
| Phosphacan, RPTP-β/PTPζ, (DSD-1 in mice) | PNS, CNS KSPG also has CS and HNK-1 substitution. Phosphacan is the ecto-domain of PTPζ | PTPζ is a type I transmembrane glycoprotein, carbonic anhydrase motif interacts with pleiotrophin and midkine to promote neurite outgrowth activity | Faissner et al. (2006), Garwood et al. (1999), Morise et al. (2014) |
| Miscellaneous KS-substituted proteins | |||
| Transferrin, thyroglobulin | Associated with papillary thyroid carcinoma | KS epitope is capped with α2-3 N-acetylneuraminic acid | Magro et al. (2003) |
| Cytokeratin | epidermal protein | Human keratinocytes contain keratin filaments containing KS chains | Schafer and Sorrell (1993) |
| Prostaglandin-D synthase | 28 kDa KS-glycoprotein produced by bovine corneal keratocytes | Corneal retinoid transporter, also found in seminal plasma, rat brain and spinal cord, rat cochlea, human prostate, human and rat epididymis and testes | Berryhill et al. (2001) |
| Mammallin | Avian KS proteoglycan | Role in egg shell production, binds Ca2+ and maintains a Calcium reserve. Mammallin awaits full characterization. The KS content of egg shells correlates directly with their strength | Du et al. (2015), Ha et al. (2007) |
CNS, central nervous system; SV2, synaptic vesicle protein 2; RPTPβ, receptor protein tyrosine phosphatase β; PTP ζ, protein tyrosine phosphatase ζ.
The biodiversity of KSPGs
KSPGs are widely distributed and display a diverse range of functional properties (Table I). In tissues, KS chains can be either N- or O-linked to the proteoglycan core protein.
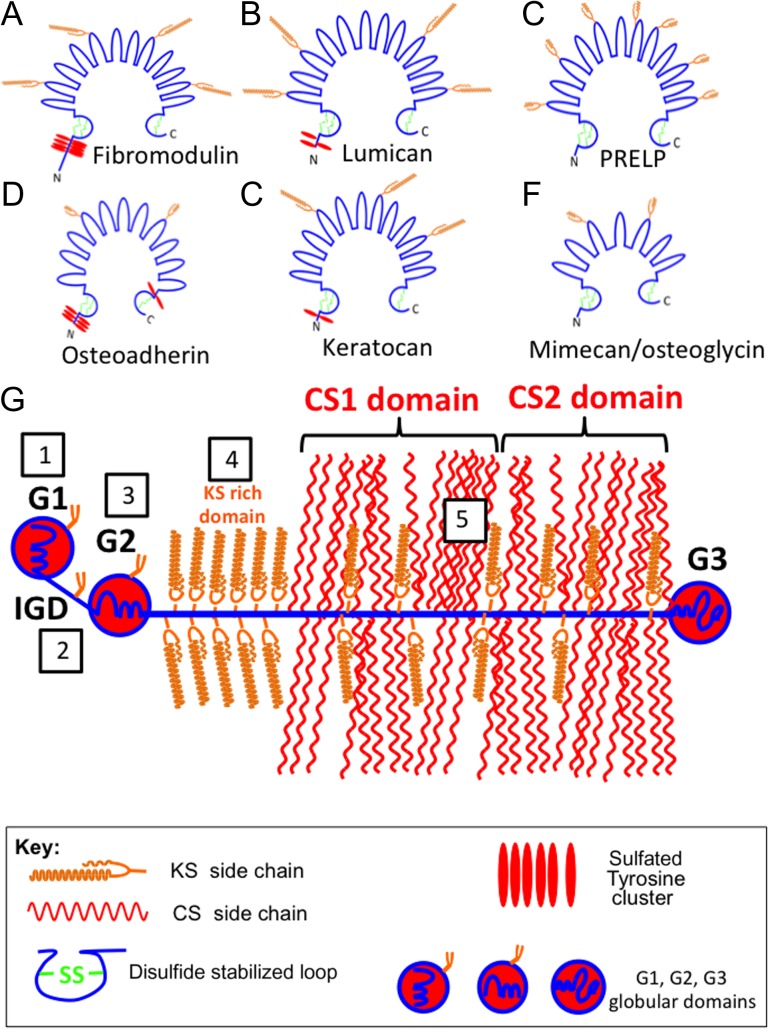
Several members of the small leucine repeat proteoglycan (SLRP) family (fibromodulin, lumican, keratocan, mimecan, osteoadherin) contain small N-linked KS chains (Figure 3A–F). The SLRPs contain leucine rich repeat regions (LRRs) that provide interactive properties with ECM proteins (Figure 3A–F). The SLRPs regulate collagen fibrillogenesis, and interact with cytokines and growth factors which regulate cell proliferation, cell signaling and matrix assembly (Hocking et al. 1998; Merline et al. 2009; Dellett et al. 2012; Chen and Birk 2013; Chen et al. 2014). Some SLRP members (PRELP, mimecan, osteoadherin) have small minimally sulfated KS chains and roles in the laying down of bone, cellular adhesion and anchorage of the basement membrane to adjacent connective tissue (Bengtsson et al. 1995, 2002; Sommarin et al. 1998; Johnson et al. 2006).
Fig. 3.
Structural diagrams of some selected extracellular matrix KS-proteoglycans. Fibromodulin (A), lumican (B), PRELP (C), Osteoadherin (D), keratocan (E) and mimecan/osteoglycin (F). These are horseshoe-shaped members of the small leucine rich repeat proteoglycan (SLRP) family. Aggrecan (G) is a member of the lectican proteoglycan family. KS chains in aggrecan occur in five regions indicated in the boxed areas in (G). These are (1) the G1 hyaluronan binding region (HABR), (2) interglobular domain (IGD), (3) G2 globular domain, (4) KS-rich region. (5) KS chains are also interspersed throughout the CS1 and CS2 domains of the CS-rich region.
Aggrecan is a large KS and CS substituted proteoglycan of the lectican family which has important space filling and water imbibing properties in cartilaginous tissues (Kiani et al. 2002) (Figure 3G). Aggrecan in cartilage forms massive macro-aggregate link protein-stabilized ternary structures with hyaluronan which have impressive water trapping properties within tissues providing the tissue with a resistance to compression (Roughley and Mort 2014). Aggrecan is widely distributed in articular, hyaline, elastic and fibrocartilages in diarthrodial joints, rib, nasal and tracheal cartilages as well as the larynx, outer ear and epiglottis (Roughley et al. 2003, 2006). Aggrecan equips these tissues with the ability to withstand compression and provides mechanical support to the elastic or collagenous fibers that convey and control reversible tissue deformability or the ability to withstand tensional forces as well as providing strong interconnections between muscle and bone. In adult cartilage, aggrecan contains ~100 CS and ~25–50 KS chains which collectively represent ~90% of the mass of this molecule (Kiani et al. 2002). KS is localized in a region adjacent to the CS rich region in all species examined except rodent aggrecan which has a truncated core protein devoid of a KS-rich region (Barry et al. 1994). Small KS chains are also found in the G1 and G2 globular domains of aggrecan and in the interglobular domain (IGD) between G1 and G2 regions (Barry et al. 1992, 1995; Fosang et al. 2009). The role of the KS chains within the KS-rich region is not known; however, the other KS chains located towards the N-terminus of aggrecan have roles in the suppression of a T cell-mediated response to free G1 when used as an arthritogen (Leroux et al. 1996; Glant et al. 1998; Guerassimov et al. 1998) and also regulate aggrecanolysis by aggrecanases (Poon et al. 2005). Although not formally considered a KSPG, versican G1 domains also contains KS chains whose roles await determination (Sztrolovics et al. 2002).
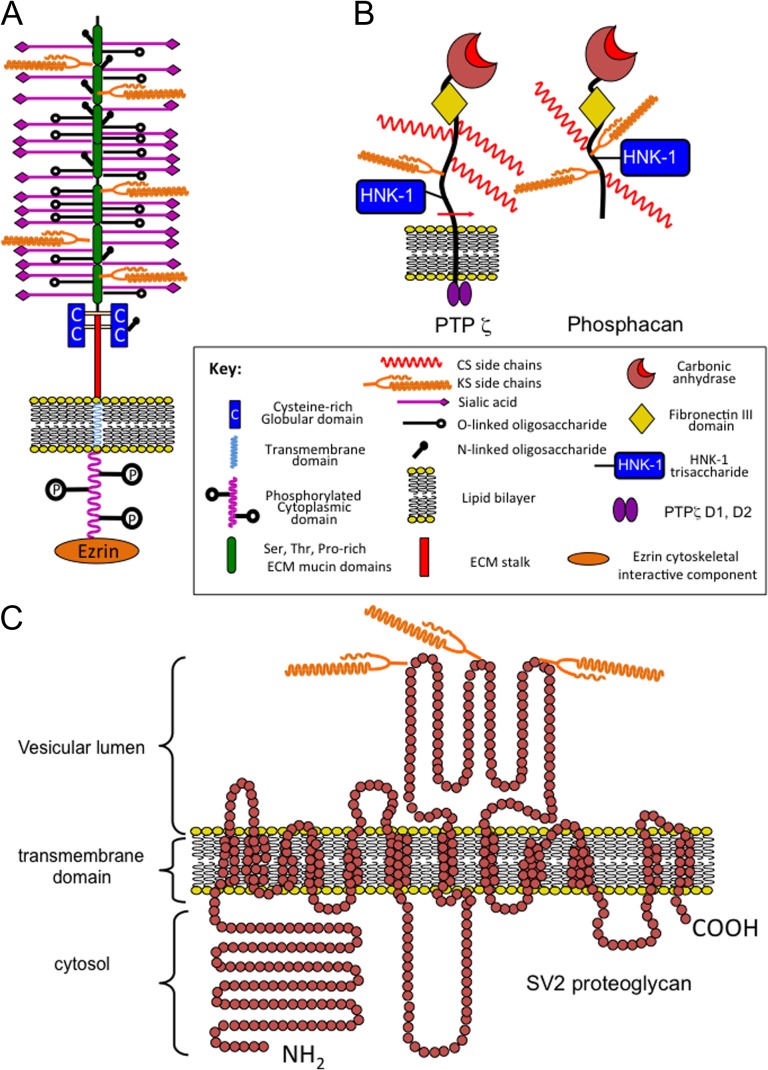
KSPGs and KS-substituted glycoproteins have been identified in epithelial tissues. MUC1 is a widely distributed mucin glycoprotein that contains small minimally sulfated KS chains. Podocalyxcin is a 240 kDa sialylated mucin-like cell membrane proteoglycan, which contains small low sulfation KS chains in embryonic and highly sulfated KS chains in adult tissues (Figure 4A). Endometrial KSPG and PZP-3 have roles in fertilization and implantation. Variants of CD44 and perlecan have also been described bearing KS chains, the role of these KS chains remains to be established; however, it is likely that they may modulate growth factor binding and matrix organization.
Fig. 4.
Schematic depictions of cell-associated KS-proteoglycans. Podocalyxcin (A). Protein Tyrosine Phosphatase Receptor-β/ζ/Phosphacan (B) and synapse vesicle proteoglycan-2 (SV-2) (C). The podocalyxcin core protein is heavily substituted with N- and O-linked oligosaccharides and these are potential linkage sites for KS. Phosphacan is the ecto-domain of the transmembrane Protein Tyrosine Phosphatase Receptor-β/ζ which contains KS, CS and HNK-1 trisaccharide GAG substitution. SV-2 is a 12 span transmembrane KS–proteoglycan with transport functions for neurotransmitters in synaptic vesicles and occurs as low (100 kDa) and high (250 kDa) molecular weight forms containing KS substitution on three N-linked glycosylation sites at amino acids 498, 548 and 573. The free core protein of SV-2 is 80 kDa. SV2 occurs as three alternatively spliced isoforms SV2A, B, C of variable tissue distribution in the CNS/PNS.
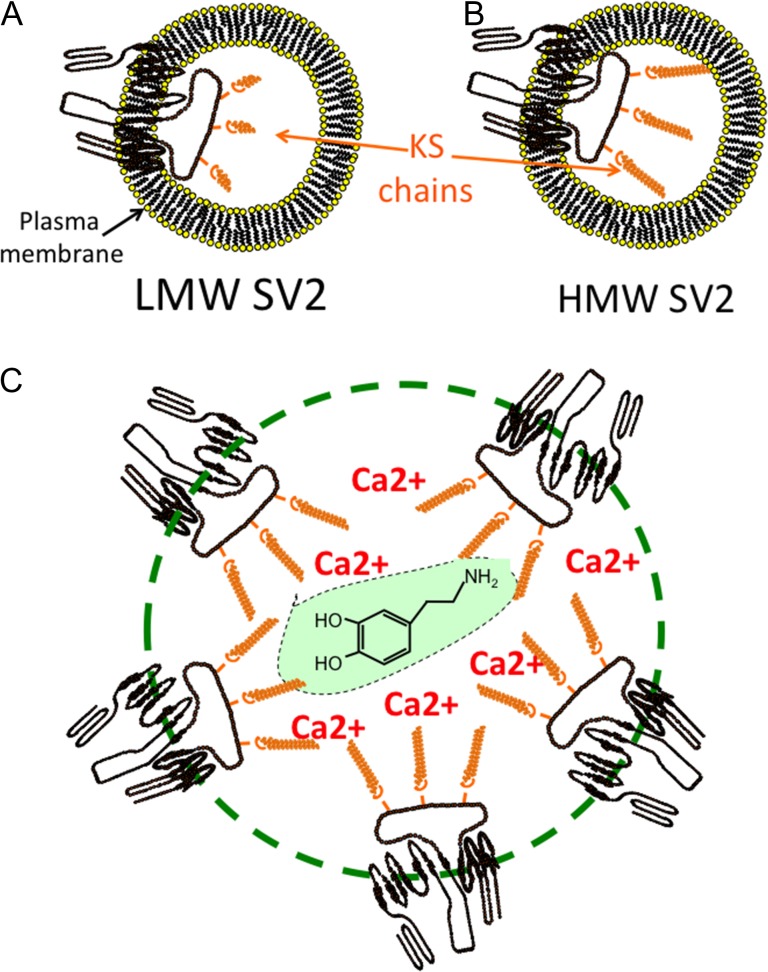
After the cornea, the brain is the next richest source of KS in the human body and contains a number of large KSPGs, which contain highly sulfated KS-III chains. Abakan is synthesized by astrocytes, directs neuritogenesis and defines the functional boundaries of areas of the brain. Claustrin has anti-adhesive properties and also directs axonal growth and repair. Phosphacan is one of the most abundant KSPGs in brain tissue and has roles in the regulation of neuronal development and repair processes (Figure 4B). PG1000 is a large KSPG detected in the electric organ and may have roles in synaptic assembly processes and neurotransmission. Synaptic protein-2 (SV2) is a 12 span proteoglycan with novel transport properties (Figure 4C). SV-2 contains three large highly sulfated KS chains that interact with neurotransmitters in a smart gel complex within the synaptic vesicle which functions in neurotransmitter storage and transmission.
Embryoglycan is a highly branched mucin-like sialylated cell surface glycoprotein of embryonic pluripotent stem cells that contains a number of developmentally regulated carbohydrate motifs and small non-sulfated poly-N-acetyllactosamine residues similar to in corneal KS (Figure 5).
Fig. 5.
Schematic representation of the structural organization of embryoglycan, a cell membrane associated KS–proteoglycan of pluripotent embryonic stem cells.
Besides its well-documented roles in cartilaginous tissues, aggrecan also has roles in the development of heart tissue and in the valve leaflets (Zanin et al. 1999; Lincoln et al. 2006) and in perineural net formation in the cortex, hippocampus, thalamus, brain stem and spinal cord (Virgintino et al. 2009). Perineural nets are macroaggregates assembled from HA, link protein and tenascin-R which surround and protect neuron dendritic synaptic contact areas (Bandtlow and Zimmermann 2000; Peal et al. 2009; Virgintino et al. 2009). Another KSPG, SV2 has roles in the development and function of electro-conductive tissue in the heart and PNS/CNS. SV2 occurs as a low and a high molecular weight form which reflects the relative size of the KS chain on each form (Figure 6A and B). SV2A expression is down-regulated and SV2C up-regulated in epileptic foci in humans with intractable temporal lobe epilepsy (Crevecoeur et al. 2014). SV2 knockout mice lacking SV2A display abnormal neurotransmission and epileptic seizures (Crowder et al. 1999; Tokudome et al. 2016). Preclinical and clinical targeting of SV2A with the anti-convulsant drug leveritacetam in humans and in laboratory studies in SV2A deficient mice demonstrate the important roles SV2 plays in normal brain function (Loscher et al. 1998; Niespodziany et al. 2001; Margineanu et al. 2008). SV2 acts as a calcium reservoir; Ca2+ has roles in the generation of synaptic action potentials. SV2 also has transport properties for neurotransmitters into synaptic vesicles and acts as a smart gel delivery system important for neuron functional properties (Figure 6C). Mammallin is another KS–proteoglycan which act as a Ca2+ reservoir in egg shell assembly. A KS–glycoprotein in medullary bone, bone sialoprotein-II (BSP-II) is synthesized in synchrony with the egg laying cycle in egg laying birds. (Hadley et al. 2016). Bone sialoprotein-II (BSP-II) from compact rabbit bone is a KSPG; however, some studies have shown that BSP-II from other species may not contain KS (Masubuchi et al. 1975; Kinne and Fisher 1987; Ganss et al. 1999; Hadley et al. 2016). KS chains have also been detected on transferrin and thyroglobulin in papillary thyroid carcinoma where the KS chains may be of diagnostic value and on keratin filaments produced by keratinocytes but the roles of these KS chains are unknown.
Fig. 6.
Schematic representation of the functional organization of SV-2 proteoglycan of synaptic vesicles, low (100 kDa) and high (250 kDa) molecular weight forms are depicted in (A) and (B). The KS chains (orange) of SV2 distributed around a synaptic vesicle (green dotted line) interact with Ca2+ and neurotransmitters such as dopamine as illustrated in the central green highlighted region in a smart gel proteoglycan delivery complex (C).
Functional properties of KSPGs
KS occurs on proteoglycans in connective tissues that contain chondroitin sulfate (CS) and both of these GAGs influence tissue hydration and organization; however, some KS-specific functional properties have also been demonstrated. Mouse macrophages express a high-affinity cell surface receptor for the KSPG lumican; these receptors are modified with mono-sulfated oligolactosamine. These cells do not bind lumican that carries sulfated KS chains, nor will they attach and spread on plastic surfaces coated with KS-substituted lumican. Removal of KS with endo-β-galactosidase restores attachment and spreading of these cells in vitro indicating that low sulfation KS is involved in this process (Funderburgh et al. 1997). Human melanoma A375 cells grown on lumican coated culture surfaces undergo morphological changes apparently due to rearrangement in their actin cytoskeletons which may underly the inhibitory effect lumican displays on the migration of melanoma cells (Radwanska et al. 2008). A 17 amino acid peptide derived from LRR-9 of lumican has been isolated (lumcorin), and shown to decrease melanoma progression (Zeltz et al. 2009; Pietraszek et al. 2013). This anti-tumor activity resides in the ability of lumcorin to inhibit cell motility by inhibition of focal adhesion kinase phosphorylation and by blocking melanoma cell interactions with α2β1 integrin preventing the development of focal adhesion complexes which are required for cell migration. Lumican also exerts angiostatic properties on endothelial cells which provides tumor inhibitory activity in melanoma and a number of other tumors (Brezillon et al. 2013). Furthermore, lumican inhibits the expression and activation of MMP-9 and 14 (Pietraszek et al. 2013) which also contributes to its anti-angiogenic/anti-tumor activity.
Anti-adhesive properties for KS have been observed in a number of studies. KSPGs constitute a barrier to neurite outgrowth in vitro and directs axon growth patterns during development and regeneration in vivo (Burg and Cole 1994; Olsson et al. 1996). This “barrier” function of KS is also evident in the KS chains of aggrecan G1 domains which block development of a T cell-mediated immune response in vivo and in vitro to the G1 domain when it is used as an arthritogen, suppressing development of antigen-induced osteoarthritis (Guerassimov et al. 1998) (Leroux et al. 1996). The KS content of endometrial uterine lining tissues varies markedly during the menstrual cycle, reaching a peak at the time of embryo implantation (Graham et al. 1994). KS substitution on MUC1 and other PGs in the endometrial lining suggests a potential regulatory role for KS in this implantation process (Aplin and Hey 1995; Aplin et al. 1998; DeLoia et al. 1998; Cipollone et al. 2012). A number of KSPGs have been identified in endometrial tissue (DeLoia et al. 1998) with apparent roles in tissue organization and implantation. Zona pellucida glycoprotein-3 (PZP-3) contains low sulfation N-linked poly-N-acetyllactosamine residues which promote oocyte–sperm interactions and regulate fertilization (Noguchi and Nakano 1992; Yonezawa et al. 1995; Gupta et al. 1996; Nakano et al. 1996). The KS chains within the IGD of aggrecan are smaller and of lower charge density than the O-linked KS chains in the KS-rich region and have critical roles to play in the aggrecanolysis process by aggrecanases (Poon et al. 2005) (Figure 3G). The basement membrane anchoring molecule PRELP also contains small low sulfation KS chains; however, their functional roles have yet to be determined (Bengtsson et al. 1995).
Mimecan/osteoglycin, osteoadherin and keratocan are KSPGs with proposed roles in the laying down of bone (Funderburgh et al. 1997; Sommarin et al. 1998; Wendel et al. 1998; Tasheva et al. 2002; Igwe et al. 2011; Nikdin et al. 2012). Keratocan is expressed by osteoblasts and can modulate osteoblast differentiation (Igwe et al. 2011). Specific KSPGs (e.g. phosphacan) have also been demonstrated in the CNS/PNS which contain highly charged KS chains and which depending on cellular context can display anti-adhesive properties on neural cells for tenascin-C and laminin which promote neuronal/axonal repair processes (Garwood et al. 2001, 2003; Dobbertin et al. 2003; Butler et al. 2004; Faissner et al. 2006). Other brain KSPGs (Abakan, PG1000, SV2) contain highly charged KS chains which confer interactive properties in neurotransmission, brain ECM and synaptic organization and function.
KS is interactive with a number of cell stimulatory molecules which have important roles to play in skeletogenesis and in the regulation of tissue homeostasis (Osawa et al. 2006). KS chains bind the cardiotoxins CTX A3 and T (Vyas et al. 1998) and insulin-like growth factor binding protein-2 (IGFBP2) (Russo et al. 1997). Surface plasmon resonance has shown that corneal KS interacts with SHH, FGF1 and FGF2 (Weyers et al. 2013). The core proteins of the KS-SLRPs are also highly interactive due to their LRRs. Fibromodulin and lumican bind to collagen and regulate fibrillogenesis, with fibromodulin promoting the formation of thick fibers while lumican promotes the formation of thin collagen fibers. Fibromodulin also binds to TGF-β and controls its bioavailability (Hildebrand et al. 1994). The core proteins of keratocan and lumican interact with inflammatory cytokines such as CXCL1 (Carlson et al. 2007). Lumican interacts with CD14 and activates the TLR-4 pattern recognition receptor as part of the innate immune response promoting phagocytosis of invading bacteria (Wu et al. 2007; Shao et al. 2013).
Mimecan/osteoglycin interacts with TGF-βs and BMPs (Iozzo and Schaefer 2010), binding of mimecan to BMP2 and BMP3 provides osteoinductive activity (Bentz et al. 1989). Mimecan acts as a carrier molecule releasing TGFβ and BMPs into the ECM during tissue development (Iozzo and Schaefer 2010). TGF-βs enhance chondrocyte proliferation in early differentiation, but inhibit chondrocytes in their terminal differentiation stage prior to bone formation (Zhang et al. 2004). Co-ordination of BMP2 and BMP3 with TGF-β2 activity potentiates cartilage and bone formation (Serra and Chang 2003; Wan and Cao 2005).
Interactive Properties of KS
GAGs interact with proteins in many different ways. Clusters of basic amino acids have been proposed as consensus GAG binding sites (Cardin and Weintraub 1989) while in other cases, interactions between GAGs and proteins appear to be purely charge-mediated (Ruoslahti and Engvall 1980) influenced by charge density due to the proximity of multiple GAG chains on PGs (Oldberg and Ruoslahti 1982). A microarray analysis has shown that corneal KS interacts with a number of kinases, membrane and secreted proteins, cytoskeletal components and a number of nerve receptors and effector molecules (Conrad et al. 2010). Plasmon resonance studies confirmed these interactions and binding constants were determined. KS interacts with a number of proteins of the Slit-Robo cell-signaling pathway which have roles in axonal guidance. Slits contain variable numbers of LRRs and 7–9 epidermal growth factor (EGF) repeats which are highly interactive modules with KS resulting in downstream activation of Rho GTPases which mediate actin depolymerization, cytoskeletal re-organization and cell signaling. KS is interactive with Ephrins and Ephrin receptors, the largest subfamily of receptor protein–tyrosine kinases. These are membrane-bound proteins, which regulate intracellular signaling pathways arising from direct cell–cell interaction resulting in the activation of Rho family GTPases critical to axonal guidance in neural development and repair processes.
KS structure
KS is composed of the basic repeating disaccharide d-galactose β1–4 linked to GlcNAc-6-sulfate. The poly-N-acetyllactosamine structure of KS is also found in glycoproteins of the N- and O-linkage families of mucin type glycoproteins and these can also bear KS chains in specific tissue contexts (Cooper et al. 2002; Brayman et al. 2004; Karlsson and McGuckin 2012). Some cell surface KSPGs also have mucin type and other developmental carbohydrate structures (Orlando et al. 2001; Schopperle et al. 2003; Riccioni et al. 2006; Schopperle and DeWolf 2007; Graves et al. 2016). Three forms of KS have been identified on the basis of linkage type to PG core proteins, and the structural organization of their constituent saccharides. KS type I is attached to PG core proteins via an N-glycan linkage to asparagine via a high mannose type oligosaccharide, KS type II is an O-linked-glycan attached to threonine or serine residues via a mucin type structure on the core protein. A further KS type (type III) has been identified in the PNS/CNS attached via an O-linked 2-O mannose residue to serine residues on proteoglycan core proteins but is antigenically distinct from KS-I and KS-II (Funderburgh 2002) (Figure 1). A related mucin-like polylactosamine molecule, embryoglycan, has also been described which bears some similarities to minimally sulfated embryonic KS chains but is a highly branched molecule whereas KS-I is a linear molecule (Muramatsu 2017).
In mature tissues, the poly-N-acetyllactosamine region of KS-I can contain ~50 disaccharides and reach 20–25 kDa in size and consist of a mixture of non-sulfated, mono-sulfated (Gal-GlcNAc6S), and disulfated (Gal6S-GlcNAc6S) disaccharide units (Figure 1). Skeletal KS-II chains also contain capping l-fucose and N-acetylneuraminic acid residues to variable degree however this appears to vary within different tissues and these confer KS with resistant properties to digestion by keratanases and endo-β-d-galactosidase (Figure 2). Several members of the SLRP family contain small N-linked KS chains distributed in their central LRR regions. PRELP contains several small low sulfation KS chains however their functional roles have yet to be defined (Johnson et al. 2006) (Figure 3A–F). The KS chains of aggrecan from weight-bearing tissues such as articular cartilage and IVD contain 1–3 fucose and 2–6 N-acetyl-neuraminic acid residues (Funderburgh et al. 1990, 1991; Kiani et al. 2002); however, these are absent in aggrecan from non-weight-bearing nasal and tracheal cartilages (Nieduszynski et al. 1990). Aggrecan also contains a number of small N- and O-linked KS chains in the G1 and G2 domains and within the IGD of lower sulfation to the KS chains in the KS-rich region of the core protein (Figure 3G). Occasional proteins such as the cytokeratins and transferrin/thyroglobulin can also be decorated with KS chains in specific developmental contexts and these may be of value as biomarkers of the tissue pathology status (Table I).
Biosynthesis of KS
KS biosynthesis involves the sequential action of the β-1,3-N-acetylglucosaminyltransferase (β3GnT), N-acetylglucosaminyl-6-sulfotransferase (GlcNAc6ST), β1,4-galactosyl transferase (β4GalT-1) and KS galactosyl sulphotransferase (KSGalST) biosynthetic enzymes. KS-I and II differ from other GAGs in that their biosynthesis is initiated through an N-linkage between GlcNAc and asparagine (Seno et al. 1965; Choi and Meyer 1975; Plaas et al. 1990), or O- linkage between GalNAc and serine/threonine, in KS-II (Bray et al. 1967; Choi and Meyer 1975; Kikuchi et al. 1987). Biosynthesis of KS-I begins in the ER where dolichol phosphate on the ER membrane acts as a glycosyl receptor for the formation of high mannose N-linked oligosaccharide. The complex is then transferred to the Golgi apparatus where an GlcNAc residue is added and this is sulfated by GlcNAc6ST. The substrate donor molecule 3′-phosphoadenosine 5′-phosphosulphate (PAPS) is also required for sulfation to proceed (Dunlevy et al. 1998; Akama et al. 2001; Kusche-Gullberg and Kjellen 2003). If addition of GlcNAc does not occur, the N-linked mannose may be converted to a complex N-linked oligosaccharide (Hassell et al. 1986; Dunlevy et al. 1998). In contrast, the biosynthesis of the KS-II linkage region and attachment of GlcNAc does not begin until the proteoglycan protein precursor reaches the Golgi apparatus (Hassell et al. 1986). As with KS-I, chain elongation depends on the expression of glycosyltransferase and sulphotransferases and the availability of UDP-sugar precursors to alternately add GlcNAc then Gal residues to the KS poly-N-acetyllactosamine backbone then some of these residues are selected for sulfation at C6.
Galactosyltransferases
Glycosyltransferases are responsible for the sequential addition of Gal and GlcNAc to the growing KS chain (Funderburgh 2000). Several families of galactosyltransferases (Gal-T) have been identified (Amado et al. 1999). β4GalT-1 catalyzes the addition of UDP-Gal to a non-reducing terminal GlcNAc acceptor, via a β1–4 glycosidic linkage generating the non-sulfated poly-N-acetyllactosamine domains which comprise the basic unit of the KS molecule (Brew et al. 1968; Schanbacher and Ebner 1970). Another galactosyl transferase, β4 GalT-4, is the only galactosyl transferase which catalyzes transfer of Gal to a non-reducing terminal GlcNAc-6-sulfate acceptor residue (Seko et al. 2003) and is essential for the production of mono- and disulfated disaccharides in the KS chain. β4 GalT-4 is also the only GalT enzyme which generates the initial branch points found in the 2 O-linked poly-N-acetyllactosamine regions of the KS core structure.
N-Acetylglucosaminyltransferases
Seven β3 N-acetyl glucoaminyltransferases (β3GnT-1, 2, 3, 4, 5, 6, 7) can catalyze the addition of GlcNAc via β3 linkage to non-reducing terminal Gal or GalNAc (Seko and Yamashita 2004). One of these enzymes acts most efficiently in the elongation of the linear poly-N-acetyllactosamine region known as the “i” antigen and is designated iGnT and has a broad tissue distribution.
Sulphotransferases
Sulphotransferase enzymes transfer sulfate groups from the PAPS donor to C6 of Gal or GlcNAc in KS. Gal-6-sulphotransferase (KSGal6ST) transfers sulfate groups to C6 of Gal and GlcNAc-6-sulphotransferase (GlcNAc6ST) transfers sulfate to the C6 of GlcNAc. Sulfation of Gal is normally incomplete in corneal KS but occurs at a higher level in skeletal KS whereas sulfation of GlcNAc residues is nearly complete. Chondroitin sulfate sulphotransferase (C6ST) catalyzes the addition of a sulfate group to GalNAc in CS, but also to Gal in cartilage and corneal KS (Habuchi et al. 1996). Five GlcNAc-6-sulphotransferase genes have been identified (Seko et al. 2003). GlcNAc6ST-5 sulfates the non-reducing terminal GlcNAc in both human (Akama et al. 2001) and mouse tissues (Uchimura et al. 1998). KSGal6ST catalyzes the addition of sulfate to an internal Gal residue within the KS chain (Fukuta et al. 1997). Sulfation of Gal in unsulfated KS disaccharides is much lower than in disaccharides where GlcNAc is already sulfated, indicating that GlcNAc sulfation precedes and may be a prerequisite for Gal sulfation during the biosynthesis of KS. This also explains why it is only the GlcNAc of the KS disaccharide unit which is sulfated in regions of monosulfation along the KS chain.
KS Synthesis and Corneal Development
The cornea is the richest source of KS in the human body. Corneal KS is N-linked to one of three proteoglycan core proteins, lumican, keratocan or mimecan (Table I). The cornea is a tissue which is composed of ~90% stroma and is covered anteriorly with epithelium and posteriorly with endothelium. The cornea is a curved transparent tissue that focuses light into the eye. The remarkable transparency and optical clarity of the cornea results from the strict regularity of the orthogonal distribution of collagen fibers of uniform diameter and interfibrillar spacing in this tissue. Four SLRPs in the cornea, decorin, lumican, mimecan and keratocan critically regulate collagen fibril size and interfibrillar spacing essential for the correct functional properties of this tissue. KS synthesis in the cornea starts at early stages of fetal development, and progressive developmental changes occur in KS antigenic determinants in specific regions of the cornea and conjunctiva over time. SundarRaj et al. (1985) raised 25 monoclonal antibodies to KS-I, and one clone to KS-II [J14] to examine the distribution of KS epitopes in lapine corneal development. The specific epitopes identified by each antibody clone were not reported however differences were evident in the spatio-temporal distribution of KS epitopes in the developmental rabbit cornea and conjunctival stroma over time and an apparent lack of KS in early stages of rabbit corneal development. However, this was a methodological deficiency since KS antibodies such as 5-D-4 detect highly sulfated KS epitopes only. Low sulfation KS would not have been detected. Since MAb 1-B-4 (Mehmet et al. 1986) and MAb R-10G (Kawabe et al. 2013; Nakao et al. 2017) which detect these were not used in this study.
The Sulfation Status of KS Chains as Regulatory Motifs in Tissue Morphogenesis
Chick corneal KS is initially synthesized in E5–E7 as a non-sulfated poly-N-acetyllactosamine form, sulfation occurs over E12–E18 to form KS ensuring correct spacing of collagen fibrils essential for optical clarity, this also fine-tunes corneal hydration as the stroma undergoes compaction during development (Borcherding et al. 1975; Liles et al. 2010). Many antibodies have been raised to KS, the majority of these detect highly sulfated determinants on KS (Table II). A few antibodies have also been raised which specifically detect low sulfation and non-sulfated epitopes in poly-N-acetyllactosamine located on the embryonic cell surface (Andrews et al. 1984; Pera et al. 1988; Adewumi et al. 2007; Kawabe et al. 2013). These antibodies were originally developed to identify pluripotent embryonic stem cells and human induced pluripotent cells (Adewumi et al. 2007) to differentiate these from embryonic carcinoma stem cell populations which contain highly sulfated KS (Ozawa and Muramatsu 1985; Ozawa et al. 1985a; Muramatsu and Muramatsu 2004). KS is synthesized by the chick cornea between E5 and E7, but only becomes highly sulfated by E14 (Hart 1976) with a switch in KS biosynthesis from an unsulfated form to sulfated KS between E12 and E15 (Cornuet et al. 1994). The low sulfation form of KS in early corneal development displays antigenic determinants in the poly-N-acetyllactosamine chain identified by EMCA-2, EMCA-3, TRA-1-60/TRA-1-81 and R-10G (Kawabe et al. 2013; Nakao et al. 2017). Human auto-antibodies to the blood group substances i- and I-antigen also identify determinants in embryonic KS chains (Liles et al. 2010). These epitopes are masked by the sulfated KS epitopes in mature tissues (Tang et al. 1986). The glycosylation status of cell surface KS is an important regulatory determinant in tissue development (Ohtsubo and Marth 2006). Antibodies which detect highly sulfated KS on the cell surface of tumor cell proteoglycans such as podocalyxcin have found application as diagnostic biomarkers.
Table II.
KS antibodies and the epitopes they identify illustrate KS structural complexity
| Antibody | Epitope identified | Reference |
|---|---|---|
| EMCA-2, 3¶ | Mucin core antigens, mucin-like polylactosamine | Aplin et al. (1998), Dixon et al. (1993) |
| TRA-1-60 | Epitope sensitive to neuraminidase, keratanase-I, II and endo-β-d-galactosidase. terminal type 1 lactosamine: Galβ1–3GlcNAcβ1–3Galβ1–4GlcNAc and Galβ1–3GlcNAcβ1–3Galβ1–4GlcNAcβ1–6 (Galβ1–3GlcNAcβ1–3)Galβ1–4Glc oligosaccharide, expressed on podocalyxcin | Adewumi et al. (2007), Andrews et al. (1984), Badcock et al. (1999), Natunen et al. (2011), Schopperle and DeWolf (2007) |
| TRA-1-81 | Epitope is resistant to neuraminidase but sensitive to endo-β-d-galactosidase, keratanase-I, II. Epitope is a terminal type 1 lactosamine: Galβ1–3GlcNAcβ1–3Galβ1–4GlcNAc and Galβ1–3GlcNAcβ1–3Galβ1–4GlcNAcβ1–6(Galβ1–3GlcNAcβ1–3)Galβ1–4Glc oligosaccharide, expressed on cell surface podocalyxcin | Adewumi et al. (2007), Andrews et al. (1984), Badcock et al. (1999), Natunen et al. (2011), Schopperle and DeWolf (2007) |
| R-10G | Low sulfation KS expressed on cell surface podocalyxcin on pluripotent embryonic stem cells | Kawabe et al. (2013), Makanga et al. (2015), Nakao et al. (2017) |
| SSEA-1¶ | Cell surface glycan of murine embryonic pluripotent stem cells, epitope expressed on proteoglycan and glycoprotein core proteins and bioactive lipids | Ozawa et al. (1985b) |
| “i” antigen¶ | Human autoantibody to non-branched epitope in non-sulfated poly-N-acetyllactosamine (see Figure 1) | Feizi (1989), Feizi et al. (1979), Feizi et al. (1971), Young et al. (2007a, 2007b) |
| “I” antigen¶ | Human autoantibody to branched epitope in non-sulfated poly-N-acetyllactosamine (see Figure 1) | Feizi (1989), Feizi et al. (1979), Feizi et al. (1971), Young et al. (2007a, 2007b) |
| 4C4 | Highly sulfated KS on podocalyxcin in embryonic tumor cells | Fukuma et al. (2003) |
| 5D4 | Hexa sulfated KS octa-saccharide and a linear dodecasaccharide containing N-sulfated glucosamine | Caterson et al. (1983), Mehmet et al. (1986) |
| MZ15 | Hepta and octa-saccharide KS oligosaccharides | Mehmet et al. (1986) |
| 1B4 | Tetrasulfated hexasaccharide in linear KS | Mehmet et al. (1986) |
| 4D1 | Sulfated linear poly-N-acetyllactosamine epitope | B. Kerr PhD Thesis, University of Cardiff (2005). |
| 2D3 | Highly sulfated linear poly-N-acetyllactosamine epitope | |
| 3D12/H7 | Trisulfated fucosylated poly-N-acetyllactosamine linkage region KS chains interspersed in the CS1 and 2 region of aggrecan (see Figure 1) | Fischer et al. (1996) |
| D9B1 | A sialo-KS epitope on endometrial KSPGs | Aplin et al. (1998), Hoadley et al. (1990), Smith et al. (1989) |
| 6D2/B5 | Fucosyl-KS epitope also detects fucoidan | Baker et al. (1989) |
| SV1, SV2, SV4 | High sulfation KS chains in SV2 proteoglycan | Scranton et al. (1993), Sinouris et al. (2009) |
| EFG-11 | Tri-KS disaccharides | Papageorgakopoulou et al. (2002) |
| 122 | Highly sulfated KS | Papageorgakopoulou et al. (2002) |
| 1/14/16H9 | Specific equine KS antibody | Okumura and Fujinaga (1998), Okumura et al. (2000) |
| LC8.13 | Lesser reactivity following keratanase pretreatment | Keiser and Diamond (1987) |
| F1.2 | Conformation dependent KS epitope on aggrecan core protein, un-reactive with KS-peptides released into media | Keiser and Diamond (1987) |
| BKS-1(+) | Keratanase-generated KS stub neoepitope | Akhtar et al. (2008) |
EMCA, epithelial mucin core antigen; TRA, Trafalgar antigen, some authors also refer to this as tumor rejection antigen; SSEA. Stage specific embryonic antigen; ¶ these antibodies identify non-sulfated epitopes thus by definition these are not KS epitopes but poly-N-acetyllactosamine stretches occurring in KS.
KS and human disease
KS in Macular Degeneration
Macular dystrophy is a relatively rare eye condition linked to inherited genetic mutations. Macular dystrophy causes deterioration of the most sensitive part of the central retina (macula), which has the highest concentration of light-sensitive cells (photoreceptors). Alterations in the degree of sulfation of KS through mutations in the CHST6 gene result in corneal opacity in macular corneal dystrophy in humans (Edward et al. 1990; Funderburgh et al. 1990; El-Ashry et al. 2002, 2005; Liskova et al. 2008; Dang et al. 2009; Sultana et al. 2009; Patel et al. 2011).
KS in Cornea Plana Type 2 and Keratoconus
Mutations in the KERA gene cause the disorder cornea plana type 2 (CNA2) (Liskova et al. 2007; Roos et al. 2015; Kumari et al. 2016). In patients with CNA2 the cornea lacks the normal convex profile which prevents the correct refraction of light through the lens. Defective KS chain elongation occurs in keratoconus (Funderburgh et al. 1989; Akhtar et al. 2011; Garcia et al. 2016). Keratoconus is a disorder of the eye which results in progressive thinning of the cornea leading to blurry vision, double-vision, near-sightedness, astigmatism and light sensitivity (Funderburgh et al. 1989; Edrington et al. 1995; Espandar and Meyer 2010; Romero-Jimenez et al. 2010). To ascertain the importance of KS sulfation on KS functionality, mice with a targeted gene deletion in Chst5 have been developed (Hayashida et al. 2006). Chst5 encodes an N-acetylglucosamine-6-O-sulfotransferase that is integral to the sulfation of corneal KS chains. Corneas of homozygous mutants were significantly thinner than those of WT or heterozygous mice and lacked high-sulfated KS, but contained the core protein of the major corneal KSPG, lumican. The corneal stroma of the Chst5-null mouse exhibited widespread structural alterations in collagen fibrillar architecture, decreased interfibrillar spacing and spatially disorganized collagen arrays indicating that the KS sulfation had important roles to play in collagen matrix organization (Hayashida et al. 2006).
Aberrant KS Biosynthesis in Amyotrophic Lateral Sclerosis
ALS (Lou Gehrig’s disease), or amyotrophic lateral sclerosis, is a progressive neurodegenerative disease that affects nerve cells in the brain and the spinal cord.
KS chains have essential roles to play in ALS, a motor neuron disease which rapidly targets motor neurons and is a progressive, fatal neurological condition affecting voluntary muscle control (Hirano et al. 2013; Foyez et al. 2015). Motor neurons are located in the brain, brain stem, and spinal cord and are vital lines of communication between the nervous system and voluntary muscle groups. Progressive motor neuronal death results in muscle weakening, atrophy and eventually a complete inability to control voluntary muscular movement. Respiratory failure is a common cause of death in ALS patients (Hirano et al. 2013). Nonsense and missense mutations in the CHST6 gene for Carbohydrate sulphotransferase 6 cause ALS. The CHST6 gene product, corneal N-acetylglucosamine-6-sulfotransferase (C-GlcNac6ST), is important for the production of sulfated KS. Lack of activity of this enzyme results in the production of unsulfated KS, leading to a loss of transparency in the corneas of affected patients (Edward et al. 1990; Akama et al. 2000; El-Ashry et al. 2002, 2005; Iida-Hasegawa et al. 2003; Aldave et al. 2004; Patel et al. 2011).
The Role of KS in Mouse Models of COPD
A KS disaccharide ([SO3−-6]Galβ1–4[SO3−-6]GlcNAc) has been examined as a therapeutic agent in a murine model of elastase-induced-emphysema and in an LPS-induced exacerbation model of cigarette smoke-induced emphysema (Gao et al. 2017). The KS–disaccharide treatment attenuated alveolar destruction, resulted in a reduced neutrophil influx and a reduced levels of inflammatory cytokines and MMPs in lung tissues resulting in reduced inflammation and lung tissue destruction. KS disaccharide also blocked the chemotactic migration of neutrophils in vitro and was as effective as dexamethasone in preventing the accumulation of inflammatory neutrophils in lung tissues in vivo. Thus KS-disaccharide displayed potential as an agent for the treatment of chronic obstructive pulmonary disease (COPD) deserving further evaluation.
Alzheimers Disease
Aberrant sulfation levels of KS are found in the brains of Alzheimers patients (Zhang et al. 2017).
The interactivity of KS with neuroregulatory effector proteins, nerve growth factor and receptor proteins, synaptic proteins, neurotransmitters and cytoskeletal components (Conrad et al. 2010) suggests likely effects which may explain the impact on cognitive learning and memory associated with Alzheimers disease (Lindahl et al. 1996; Snow et al. 1996).
KS antibodies and their use in the elucidation of KS structural complexity
The first antibody identified that specifically recognized a native “GAG structure-specific epitope” was 5-D-4 (Caterson et al. 1983). Interestingly, of all four classes of GAGs, KS has the largest number of antibodies which have been developed to various KS antigenic epitopes lending tacit support to the importance of emerging roles for KS in the pathobiology of connective tissues (Table II). The range of antigenic determinants detected using these antibodies testifies to the structural complexity and biodiversity of KS. GAGs are information dense molecules which provide molecular recognition and directive information to cells influencing cellular behavior in tissue development, tissue morphogenesis and the maintenance of tissue homeostasis (Melrose 2016).
EMCA is detected in early embryonic stem cells and pluripotent stem cells particularly in the intestinal epithelium which is covered by a continuous layer of mucus (Buisine et al. 1998). MUC-1 in the glandular endometrial endothelium carries small sulfated KS chains identified by MAb 5D4 and the anti-sialo KS antibody D9B1 (Aplin et al. 1998). These KS chains are believed to influence the adhesive/anti-adhesive properties of MUC1 and may have roles in the spatio-temporal regulation of embryo implantation (Smith et al. 1989; Hoadley et al. 1990; Aplin 1991; Graham et al. 1994; Aplin et al. 1998). Endometrial MUC1 also bears sialyl Lewis X epitope which has roles in selectin-mediated cell adhesion and tissue morphogenesis.
GCTM-2 is an epitope on the core protein of a mucin-like KS/CS pericellular matrix PG, podocalyxcin expressed by human pluripotent stem cells (Pebay et al. 2005). MAb TRA-1-60 and TRA-1-81 also recognize epitopes on carbohydrate side chains on the same molecule (Nakao et al. 2017). A related diagnostic MAb to EMCA detects serum epitheliamucin epitopes arising from breast cancer tumors (Dixon et al. 1993). EMCA is also a carbohydrate component of embryoglycan, a branched poly-d-N-acetyllactosamine mucin-like cell surface glycan expressed by embryonic cells. TRA-1-60 and TRA-1-81 antigens are commonly used as markers of undifferentiated pluripotent human stem cells (Schopperle and DeWolf 2007). Glycan array analysis of more than 500 oligosaccharides has identified the TRA-1-60 and TRA-1-81 epitopes as terminal type 1 lactosamine: Galβ1–3GlcNAcβ1–3Galβ1–4GlcNAc and Galβ1–3GlcNAcβ1–3Galβ1–4GlcNAcβ1–6(Galβ1–3GlcNAcβ1–3)Galβ1–4Glc. Effective antibody binding requires an extended tetrasaccharide structure where the type 1 disaccharide is β1,3-linked to type 2 lactosamine (Natunen et al. 2011). The TRA-1-81 epitope is resistant to neuraminidase digestion, unlike the TRA-1-60 epitope. The “TRA” antigen is named after “The battle of Trafalgar”; however, some authors also refer to this as tumor rejection antigen (Schopperle and DeWolf 2007).
The TRA-1-60 and TRA-1-81 and GCTM-2 epitopes on KS are unique to primate pluripotent stem cells (Pebay et al. 2005; Adewumi et al. 2007). These epitopes are sensitive to endo-β-d-galactosidase, keratanase-I, keratanase II and N-glycanase digestion and are completely lost upon differentiation of the stem cells. Podocalyxcin, a cell surface mucin-like stem cell KSPG bears the TRA-1-60, TRA-1-81 KS epitopes (Andrews et al. 1984; Nakao et al. 2017). Antibody R-10G also identifies low sulfation KS chains on podocalyxcin (Cooper et al. 2002) in induced pluripotent stem cells and embryonic stem cells distinguishing these from embryonal carcinoma cells expressing podocalyxcin containing oversulfated KS chains detected using MAb 5-D-4. Another antibody to oversulfated KS (MAb 4-C-4) also detects cell surface KS on embryonic carcinoma cells (Fukuma et al. 2003). Thus podocalyxcin apparently follows a similar developmental pathway to corneal KSPGs. Corneal KS is initially synthesized in the chick embryo as a non-sulfated chain in which the polylactosamine i-antigen can be detected (Liles et al. 2010), with development the i-antigen becomes obscured through branching to form the I antigenic structure. Sulfation of the polylactosamine chain with tissue maturation produces oversulfated KS obscuring detection of the “i” epitope. The “i” (Galβ1–4GlcNAcβ1–3Galβ1–4GlcNAcβ1–3Gal-) and branched “I” antigens (Figure 2) are recognized by human cold reactive monoclonal IgM auto-antibodies (Feizi 1977).
A related bi-, tri- or higher antennary branched 120–440 kDa proteoglycan composed of a complex core polylactosamine assembled from β1–3-linked d-GlcNAc and d-Gal units has also been observed on embryonic cells (Dvorak et al. 1998; Muramatsu 2017). Embryoglycan carries a number of developmentally regulated glycan marker molecules such as the trisaccharide Gal(β1→4)-[Fuc(α1→3)] GlcNAc Lewis X epitope, also known as CD15/SSEA-1 (stage-specific embryonic antigen) (Son et al. 2009), a cell surface marker of embryonic stem cells, embryonal carcinoma cells and multipotential cells of early embryos (Muramatsu and Muramatsu 2004). These glycans mediate cell adhesion in pre-implantation embryos, aggregation of endothelial cells (Kojima et al. 1994; Boubelik et al. 1996) and cell–cell interaction of galactosyl transferase and N-acetylglucosamine in polylactosaminoglycans. Lewis X acts as a recognition molecule for FGF-2 and plays an active role in the formation of ligand–receptor complexes (Dvorak et al. 1998). Lectin studies employing Helix pomatia agglutinin, soybean agglutinin, Sophora japonica agglutinin, Ricinus communis agglutinin-1, Griffonia simplicifolia agglutinin-I, Dolichos biflorus agglutinin and peanut agglutinin identify N-acetylgalactosamine, N-acetylglucosamine and/or galactose as components of the side chains of embryoglycan (Ozawa and Muramatsu 1985). Embryoglycan contains variable levels of sialylation and fucosylation (Figure 5). The ectodomains of embryoglycan regulate FGF-2 activity (Dvorak et al. 1998). Normal embryonic stem cells also synthesize low sulfation KS detected by MAb R-10G (Kawabe et al. 2013; Nakao et al. 2017).
Antibody SSEA-1 detects stage-specific embryonic antigen-1 (CD15/Lewis X/3-fucosyl-N-acetyllactosamine) on cell surface glycoproteins, glycolipids and PGs on murine pluripotent stem cells and embryos at the pre-implantation stage. SSEA-1 is also expressed as a developmental marker in Xenopus laevis in early stage embryos but appears as a 200 kDa glycoprotein in later stage embryos facilitating cell–cell adhesion and interactions which promote tissue morphogenesis through the selectin cell adhesion glycoprotein family (Yoshida-Noro et al. 1999). Multipotent haematopoietic stem cells expressing the SSEA-1 epitope populate skin wounds and actively promote skin healing (Muramatsu and Muramatsu 2004; Li et al. 2016).
MAbs 5-D-4, 1-B-4 and MZ-15 are highly specific for sulfated poly-N-acetyllactosamine sequences found in KS but not non-sulfated poly-N-acetyllactosamine sequences of I- and i-antigen (Mehmet et al. 1986). The minimum size of KS oligosaccharide detected by KS antibodies 5-D-4, 1-B-4 and MZ-15 is a linear heptasulfated hexasaccharide, although 1-B-4 also reacts with a tetrasulfated analog (Mehmet et al. 1986). MZ-15 reacts with hepta and octa-saccharide KS oligosaccharides. Antibody 5-D-4 differs from the 1-B-4 and MZ-15 antibodies in that it reacts strongly with a hexa sulfated octa-saccharide, and most strongly with a linear dodecasaccharide which may contain N-sulfated glucosamine residues (Mehmet et al. 1986). A sialo-KS epitope in sulfated KS is also recognized by Mab D9B1 (Aplin et al. 1998). MAb 4-C-4 detects a sialyl fucosyl-KS proteoglycan containing highly sulfated KS on embryonic carcinoma cells (Fukuma et al. 2003). Monoclonal antibody, 6D2/B5, recognizes a fucosyl-KS epitope in cartilage proteoglycans and also detects fucoidan (Baker et al. 1989). A novel anti-KS antibody 3D12/H7 identifies KS chains interspersed within the CS rich region of the aggrecan core protein in articular cartilage and to a significantly lesser extent in tracheal cartilage aggrecan and corneal extracts (Fischer et al. 1996). MAb 3D12/H7 does not identify the KS chains from the KS-rich region on aggrecan. The 3D12/H7 KS antibody does not recognize fragmented KS species released by keratanase I/keratanase II digestion of the intact KS chains. It does, however, identify intact KS chains from the CS-rich region of aggrecan isolated by chondroitinase ABC and papain digestion. The specific KS epitope identified by 3D12/H7 is within the oligosaccharide linkage region of the KS chain which contains three sulfate groups and two fucose residues on the GlcNAc residues in this region of the KS chain. These KS chains are preferentially expressed within the CS-rich region on the aggrecan core protein (Fischer et al. 1996).
Further KS antibodies have been developed to a moderately sulfated (MAb 4-D-1) and a highly sulfated epitope in linear poly-N-acetyllactosamine (MAb 2-D-3); however, these still require detailed characterization (B. Kerr PhD Thesis, University of Cardiff, University of Cardiff, 2005). A number of KS antibodies identify tri-KS disaccharides (BVD-4, EFG-11) or ill-defined highly sulfated KS epitopes (122, LC8.13, F1.2) (Table II). MAb F1.2 is claimed to identify a conformation dependant KS epitope on the aggrecan core protein but not in small KS-peptides.
Variation in GAG chains on cell-associated and tissue KSPGs
In common with other PGs, KSPGs are important ECM and cell-associated components which provide hydration, stabilization and organization, and facilitate cell adhesion, cell migration and proliferation, neurotransmitter processing, synaptic function and neurotransmission. KSPGs and their GAG side chains act as a cell recognition and information biosensor system which regulates cellular behavior (Melrose 2016). The information contained within the sulfated sugars and poly-N-acetyllactosamine residues of KS regulate growth factor and morphogen binding and through interactions with FGF, IGFBP2, Wnt, Shh and BMPs they regulate essential physiological and developmental processes (Russo et al. 1997; Weyers et al. 2013). The interaction of KS with IGFBP2 may be significant. The Cdk4 pathway is operative in neuritogenesis and is activated by insulin. This induces neural cell proliferation and terminal differentiation (Chirivella et al. 2017). Microarray studies with corneal KS demonstrate that it also interacts with the Ephrin 4 receptor and may promote neuronal cell proliferation and differentiation through Ephrin tyrosine kinase activity (Liu et al. 2017).
Podocalyxcin
Podocalyxcin is a cell surface mucin-like 250 kDa sialo KSPG expressed by stem cells which is also associated with pluripotency and bears the TRA-1-60 and TRA-1-81 KS epitopes (Andrews et al. 1984). GCTM-2 Ab also identifies 240 and 415 kDa KSPGs on the surface of embryonal carcinoma cells (Pera et al. 1988). A novel antibody to KS (R-10G) does not identify oversulfated KS (Kawabe et al. 2013) but identifies low sulfation KS chains on podocalyxcin (Cooper et al. 2002). Thus podocalyxcin follows a similar developmental pathway to corneal KSPGs. Corneal KS is initially synthesized in the chick embryo as a non-sulfated chain in which the polylactosamine i-antigen can be detected (Liles et al. 2010). As embryonic development ensues the i-antigen becomes obscured due to branching to in the I-antigenic structure and progressive sulfation of components of the poly-N-acetyllactosamine epitopes occurs with tissue maturation leading to an accumulation of highly sulfated KS. Normal embryonic stem cells synthesize low sulfation KS chains detected by MAb R-10G (Kawabe et al. 2013; Nakao et al. 2017). This is carried by the cell surface mucin-like KS–proteoglycan podocalyxcin. Embryonal carcinoma cells or mature human tissues also express podocalyxcin but it contains an oversulfated form of KS, the sulfation status of podocalyxcin correlates with the invasiveness, metastatic and growth potential of tumor cells (Lin et al. 2014a, 2014b). The sulfation motifs on KS represent an important molecular switch which regulates cellular behavior. When this occurs in an appropriate ordered fashion the tissue progresses from an embryonic phenotype containing low sulfation KS to a normal functional adult tissue containing highly sulfated KS. When this process is dysregulated with the occurrence of early oversulfated KS in embryonic tissues tumor development occurs. The form of lumican expressed by tumor cells also bears oversulfated KS and correlates with the metastatic potential of these cells (Lu et al. 2002; Naito et al. 2002; Holland et al. 2004; Seya et al. 2006; Ishiwata et al. 2007; Matsuda et al. 2008; Brezillon et al. 2013; Coulson-Thomas et al. 2013; Nikitovic et al. 2014; Cappellesso et al. 2015).
Organization of KS Saccharides Effect the Functional Properties of Intact KS Chains
As noted earlier, three types of KS chains have been categorized on the basis of linkage type to PG core proteins, internal saccharide organization and the presence or absence of internal or non-reducing terminal α1-3 fucose or α2,3 neuraminic acid capping saccharides. A number of monoclonal antibodies have been developed which identify the sulfation status of the KS chains. MAbs such as 5-D-4, MZ-15, EFG-4, EFG-11, 122, SV-1, SV-2, SV-4, 3D12/H7 and 4-C-4 detect highly sulfated KS sulfation epitopes (Table II). MAb 1-B-4, 2-D-1, 2-D-3, LC8.13 and R-10G detect lower sulfation KS sulfation epitopes. MAbs to non-sulfated epitopes within the poly-N-acetyllactosamine regions of KS chains including anti “i” (Tho), anti-I (Den) antigens have also been identified. A KS neoepitope antibody to KS has also been developed, BKS-1(+) (Akhtar et al. 2008). This antibody detects a keratanase-generated “stub epitope” on the KS chains. The susceptibility of the KS chains to degradation by depolymerizing enzymes such as keratanase-I, keratanase-II and endo-β-d-glycosidase (Figure 2) have been used to confirm the sulfation status of the KS chains and undertake their structural characterization, GAG sequencing and estimations of GAG chain size.
KSPGs Associated with Oocyte Implantation and Fertilization
The KS content of endometrial uterine lining tissues varies markedly during the menstrual cycle, reaching a peak at the time of embryo implantation (Graham et al. 1994). KS substitution on MUC1 and other PGs in the endometrial lining suggests a potential role for KS in this implantation process. A number of large KSPGs have been identified in endometrial tissue (DeLoia et al. 1998) with roles in tissue organization and in uterine mucous secretions however the fine structure of their KS chains have not been determined. PZP-3 is one such KS-substituted protein containing low sulfation N-linked lactosamine residues with roles in the promotion of oocyte–sperm interactions which regulate fertilization (Noguchi and Nakano 1992; Yonezawa et al. 1995; Gupta et al. 1996; Nakano et al. 1996).
Variation in the Sulfation of KS Chains of Other Proteoglycans in Normal Tissues
Fibromodulin from young articular cartilage, contains KS chains (Lauder et al. 1997) devoid of non-reducing terminal (α2–6)-linked N-acetylneuraminic acid or fucose (α1–3)-linked to sulfated N-acetylglucosamine residues (Lauder et al. 1998). An age-related increase in the abundance of both (α2–6)-linked N-acetylneuraminic acid and (α1–3)-linked fucose occurs on the KS chains of fibromodulin in articular cartilage. KS isolated from non-articular tissues does not contain these capping structures irrespective of tissue age.
PRELP is a further “non-GAG” member of the SLRP family containing low sulfation KS chains. PRELP contains four potential N-linked glycosylation core protein sites as are present in the related KSPGs lumican and fibromodulin. Shorter low sulfation KS chains may however be present in PRELP. A study in 1995 by Bengtsson and colleagues showed that keratanase digestion of PRELP produced a small size shift on SDS-PAGE, suggesting the presence of either small KS chains or non-sulfated poly-N-acetylactosamine. Attempts to identify KS chains on the intact protein and on peptide fragments using several monoclonal KS antibodies, however, were inconclusive but it was not reported whether antibodies capable of detecting low sulfation KS epitopes were employed (Bengtsson et al. 1995). PRELP has also been identified as a component of the cornea and sclera where it was present as a 60–116 and 55–60 kDa protein, respectively. Digestion with endo-β-d-galactosidase converted the corneal PRELP to 45–50 kDa in size and the scleral PRELP to a protein with a molecular weight of 50 kDa (Johnson et al. 2006). Digestion with N-glycanase resulted in a further reduction in size to 42–45 and 45KDa in the corneal and scleral PRELP samples demonstrating N-linked KS chains of low sulfation. Keratanase-I and II were ineffective in depolymerizing the PRELP KS chains confirming that they were not highly sulfated. Thus PRELP can occur as a KS-substituted glycoprotein in these tissues and contains small low sulfated KS chains (Johnson et al. 2006).
Variation in Highly Sulfated KS Levels in Connective Tissues with Ageing
Although there is a generalized decline in connective tissue GAG levels with ageing, the relative KS content of these tissues increases with tissue maturation particularly in tissues subjected to high compressive load such as the IVD (Olczyk 1993, 1994; Scott et al. 1994). Highly sulfated KS is absent in the fetal IVD, detectable in the newborn and its levels progressively increase with the maturation of the IVD (Melrose et al. 1998, 2000). Fibromodulin was more abundant in the AF than in NP at all ages, its levels were elevated in the adult NP (Sztrolovics et al. 1999). With increasing age, the glycoprotein form of fibromodulin lacking KS was the predominant molecular form present in IVD tissues. The abundance of fibromodulin increases with disc degeneration (Sztrolovics et al. 1999). Lumican is more abundant in the NP than AF juvenile IVD; however, in the adult it was present at comparable levels in both tissues.
KS Structure and Function in Aggrecan
Aggrecan is a major KS–proteoglycan which equips tissues with the ability to resist compression. KS substitution in aggrecan occurs at several locations (i) individual small N- and O-linked KS chains are attached to the N-terminal G1 and G2 domains, (ii) small N- and O-linked KS chains are also located in the IGD between G1 and G2, (iii) a KS-rich region is located between the G2 and CS1 domains which contains O-linked KS chains and (iv) occasional isolated single or doublet KS chains also occur in the CS1 and CS2 domains of aggrecan (Figure 3G).
KS Chains in the G1 Domain Inhibit a T Cell-Mediated Response When Aggrecan G1 is Used as an Arthritogen
A KS side chain in adult aggrecan G1 domain obscures the recognition of arthritogenic T cell epitopes (Leroux et al. 1996). A cross-reactive and arthritogenic T cell epitope in the G1 domain of human and murine aggrecan (Glant et al. 1998) elicits an immune response in RA when the KS chains in the G1 domain are absent (Leroux et al. 1996, Guerassimov et al. 1998). In aggrecan isolated from mature articular cartilage the G1 hyaluronan binding region contains a small KS chain attached to Threonine 42 within loop A. This is not present in the immature G1 domain. A small N-linked KS chain is attached to Asn 220 in the B loop of the G1 domain in immature and mature aggrecan, but in the immature G1 the KS chain is shorter. Asn 314 of the B’ loop of mature G1 domain contains KS, however immature G1 does not. The versican G1 domain also contains KS chains; however, these have not been characterized and it is not generally referred to as a KSPG (Sztrolovics et al. 2002). The aggrecan G2 domain contains a KS chain located close to the MAb 1-C-6 HABR epitope. This KS chain obscures antibody binding to the 1-C-6 epitope (Fosang and Hardingham 1991). The biological significance of this KS chain is un-determined.
KS Substitution in the Aggrecan IGD Effects MMP and ADAMTS Cleavage
The aggrecan interglobular domain (IGD) can be cleaved by a number of proteinases including aggrecanase-1 and 2 and MMPs (Fosang et al. 1996; Lark et al. 1997; van Meurs et al. 1998; 1999; Chambers et al. 2001; Struglics et al. 2006a, 2006b). The KS attachment sites in the IGD of porcine and bovine aggrecan are located in a 32 amino acid sequence between the MMP and ADAMTS cleavage sites (Barry et al. 1992, 1994, 1995). This amino acid sequence is highly conserved and has functional roles in the modulation of aggrecanase-1 and 2 activity (Fosang et al. 2008). KS chains are also located in close proximity to the IGD aggrecanase cleavage site (Fosang et al. 2009). In cartilage, proteolysis in the IGD releases the entire CS-rich region of aggrecan. Cleavage at E373↓374A in the IGD is a signature feature of the aggrecanases but is not actually the preferred cleavage site which is in the CS-2 domain (Tortorella et al. 2000, 2002). ADAMTS-1, ADAMTS-4 and cathepsin B can also cleave at the MMP IGD cleavage site N341↓342F (Mort et al. 1998; Rodriguez-Manzaneque et al. 2002; Westling et al. 2002).
Differences in the KS Chains in the KS-Rich Region, IGD and G1, G2 Domains
The GAG chains in the KS-rich region have been extensively studied in human and bovine aggrecan (Tai et al. 1991, 1993, 1994, Dickenson et al. 1992, Brown et al. 1998). However, there is only one study published on the KS microstructure in the IGD (Fosang et al. 2009) and a handful of studies on the G1 and G2 KS chains (Barry et al. 1995). The KS chains in the IGD of pig aggrecan are significantly less sulfated than the KS chains from the KS-rich region (Fosang et al. 2009). KS in the KS-rich region has a high proportion of disulfated disaccharides (55%) and a low proportion (11%) of unsulfated disaccharides, whereas KS in the IGD has less disulfated disaccharides (33%) and a significantly higher proportion of unsulfated disaccharides (33%) (Fosang et al. 2009).
Species Differences in KS Substitution in Aggrecan
While Swarm rat chondrosarcoma aggrecan contains KS oligosaccharides, these do not undergo elongation and sulfation into mature KS chains. The KS-rich region in rodent aggrecan is spliced out (Oegema et al. 1975; Venn and Mason 1985) but KS is present in the IGD (Fosang et al. 2008) and of low sulfation as seen in pig and rat chondrosarcoma (Oegema et al. 1975), and not detected by antibodies to highly sulfated KS epitopes such as 5D4 (Caterson et al. 1983, Mehmet et al. 1986) and MZ-15 (Zanetti et al. 1985).
Variation in KS Substitution in Aggrecan with Ageing
In humans, the KS content of aggrecan increases from a minimal level at birth to a value of ~25% of the total GAG in adulthood (Brown et al. 1998). The microstructure of KS in the KS-rich region of aggrecan undergoes age-dependant changes (Brown et al. 1998). KS from young articular cartilage (0–9 years) has low sulfation levels compared to KS from adult articular cartilage (18–85 years). Skeletal KS-II in aggrecan isolated from adult articular cartilage is also more highly modified by fucosylation and sialylation than KS from immature tissue. The KS chains of aggrecan from weight-bearing tissues such as articular cartilage and IVD contain 1–3 fucose and 2–6 N-acetylneuraminic acid residues (Kiani et al. 2002), these are absent in aggrecan isolated from non-weight-bearing nasal and tracheal cartilages (Nieduszynski et al. 1990). Increased fucosylation and sialylation of KS confers an increased resistance to depolymerization by keratanase-I and II and endo-β-d-galactosidase (Melrose and Ghosh 1988). KS substitution on aggrecan increases with ageing (Barry et al. 1995, Pratta et al. 2000) and in the IGD of aggrecan (Barry et al. 1995). The IGD of aggrecan in aged tissues may be more susceptible to proteolysis by aggrecanases (Pratta et al. 2000).
Functional roles for KS–proteoglycans in brain tissue
Role of the ECM in Brain Tissue Development
The ECM of the brain, a supportive scaffolding network and platform for cellular attachment, is a source of intuitive cues which direct cell behavior. KSPGs also have key functional roles to play in brain tissue (Table I). The ECM provides cues for the assembly and repair of functional network structures where secreted molecules produced by glial cells and neurons are used to assemble transmitter and effector receptors and ion channels which effect brain function and neuronal control over tissues throughout the human body. Significant inroads have been made on specific molecules which modulate the incorporation of effector molecules into network assemblies in the brain or the shedding of transmembrane activity molecules through proteolytic release of signaling messengers. The ECM is plastic and responsive to such network activity. Dysregulation of the brain ECM is linked to major psychiatric and neurodegenerative diseases.
Proteoglycans play important regulatory roles in the development and function of the brain. Perineural nets are prominent structures formed by interaction of the lectican family of CS–proteoglycans, link protein, hyaluronan and tenascin-R. The perineural nets surround and protect neurons; however, the charge localization provided by the CS-A and CS-C side chains in these assembled structures provides repulsive cues which inhibit neuronal outgrowth in cases of axonal damage. The charge properties of the GAG chains of other brain proteoglycans, however, can promote repair processes and its functional organization. The brain contains several KSPGs (Table I) including Abakan, PG1000, claustrin, phosphacan and SV-2 with important functional roles to play in the CNS/PNS. In contrast to the CS-A and CS-C chains of the lectican proteoglycan family which inhibit neural repair, the KS chains of phosphacan provide anti-adhesive cues to neuronal cells preventing their interaction with tenascin-C fostering neurite outgrowth following injury and promote axonal repair (Dobbertin et al. 2003).
Roles for the SLRPs in Neuro-regulation
The SLRP family contains a number of KSPGs which regulate signaling pathways in neural development and in neural maintenance (Dellett et al. 2012). A distinguishing feature of the SLRPs is their central LRRs which adopt a horseshoe-like configuration which facilitates protein–protein interactions (Kobe and Deisenhofer 1994; Scott 1996; Iozzo 1997) and molecular recognition processes, including cell adhesion, signal transduction and DNA repair (Hocking et al. 1998; Schonherr and Hausser 2000; Wei et al. 2008; Winther and Walmod 2014) (Figure 3). LRRs are present in more than 6000 proteins (Wei et al. 2008) and approximately 140 genes encode extracellular LRR proteins (Winther and Walmod 2014). The GAG chains of SLRPs function in the maintenance of the spacing of collagen fibers, normal tissue hydration, and interact with growth factors and their receptors (Kobe and Deisenhofer 1994; Scott 1996; Iozzo 1997; Schonherr and Hausser 2000). Many members of the SLRP family exist without GAG chains and are considered as “part time” proteoglycans but may still have anchoring roles in the ECM due to their LRR containing core proteins (Iozzo 1997; Hocking et al. 1998). The SLRPs also act as matricellular proteins co-ordinating a number of signaling pathways to regulate the development of the neural system through secreted signaling molecules such as Nodal, FGFs, Wnts and Shh (Dellett et al. 2012). A number of LRR proteins of the SLRP family have been identified in brain tissues including Tsukushi, decorin, biglycan and fibromodulin (Snow et al. 1992; Stichel et al. 1995; Kappler et al. 1998; Ito et al. 2010). Cultured meningeal cells and astrocytes synthesize biglycan (Koops et al. 1996, Kikuchi et al. 2000). Biglycan stimulates growth of microglial cells (Kikuchi et al. 2000), has neurotrophic activity (Junghans et al. 1995) and supports the survival of neocortical neurons (Koops et al. 1996). Decorin is developmentally regulated in postnatal rat brains (Kappler et al. 1998). Decorin and biglycan are differentially expressed after injury in the adult rat brain (Stichel et al. 1995), decorin has also been immunolocalized in plaques and neurofibrillary tangles in Alzheimers disease (Snow et al. 1992). Fibromodulin has been detected in the hippocampus of a mouse model of Alzheimers disease (Alvarez-Lopez et al. 2013). Biglycan and Fibromodulin have both been detected in the hypothalamus and hippocampus in a transcriptomic epigenomic study demonstrating roles for these proteoglycans in gene networks regulating cell metabolism, cell communication, inflammation and neuronal signaling of relevance as genetic causal risks in metabolic, neurological and psychiatric disorders in humans (Meng et al. 2016).
SV2 Proteoglycan
SV2 proteoglycan has critical neuroregulatory and synaptic regulatory roles via its ability to regulate presynaptic Ca2+ and neurotransmitter levels, malfunctioning of SV2 is implicated in epilepsy (Wan et al. 2010). SV2 is phosphorylated on serine and threonine thus is a substrate for serine/threonine kinases (Pyle et al. 2000) which have downline effects on cytoskeletal organization, cell signaling and interactions with neurotransmitters. KS in SV-2 proteoglycan occurs as a large GAG side chain up to 50 Da in size (Figure 4C). SV-2 occurs as 3 isoforms (SV2A, SV2B, SV2C) of variable distribution in the CNS/PNS and as low (100 kDa) and high molecular weight forms (250 kDa). SV-2 has a 80 kDa core protein and contains three N-linked KS substitution sites at amino acids 498, 548 and 573 (Bajjalieh et al. 1992, 1993, 1994; Feany et al. 1992). The KS chains of SV2 interact with neurotransmitters forming a smart gel storage matrix within synaptic vesicles.
Phosphacan
Phosphacan is the enzymatically released proteoglycan ecto-domain of receptor-type protein tyrosine phosphatase beta (RPTP-β) and is one of the principal ECM proteoglycans of the CNS promoting neuron–glial interactions, neuronal differentiation, myelination and axonal repair (Figure 3B). RPTP-β is expressed in the developing nervous system and contains an extracellular carbonic anhydrase and fibronectin type III repeat domain which foster protein–protein interactions. RPTP is expressed in 3 alternatively spliced forms RPTP-γ, RPTP-β/ζ and a truncated form. The transient nature of cell signaling by phosphorylation requires specific phosphatases in order to control and regulate this process. The complexity of this regulatory system is well illustrated by the many protein tyrosine phosphatases spatially and temporally expressed in specific regions of the developing brain. The carbonic anhydrase (CAH) domain of RPTP-β promotes protein–protein recognition inducing cell adhesion and neurite outgrowth of primary neurons, and differentiation of neuroblastoma cells. Contactin is a neuronal receptor for RPTP-β and its interaction with phosphacan generates unidirectional/bidirectional neural signaling and promotes axonal repair.
Podocalyxcin
Podocalyxcin is an anti-adhesive transmembrane neural KS-polysialylated-proteoglycan/glycoprotein with essential roles to play in neural development (Vitureira et al. 2005, 2010) and is also a marker of human embryonic and induced pluripotent stem cells (Toyoda et al. 2017). Podocalyxcin is up-regulated in glioblastomas and astrocytomas (Hayatsu et al. 2008b; Toyoda et al. 2017), leading to its use diagnostically in the detection of various cancers (Nielsen and McNagny 2009; Wang et al. 2017) including esophageal and gastric adenocarcinoma (Laitinen et al. 2015; Borg et al. 2016), colorectal (Larsson et al. 2013, 2016), breast (Sizemore et al. 2007; Snyder et al. 2015; Graves et al. 2016), hepatocellular (Flores-Tellez et al. 2015), pancreatic ductal (Heby et al. 2015; Saukkonen et al. 2015), oral squamous cell (Lin et al. 2014b), urothelial bladder (Boman et al. 2013), ovarian (Ye et al. 2012), renal (Hsu et al. 2010), thyroid carcinoma (Yasuoka et al. 2008) and lymphoblastic and myeloid leukemia (Kelley et al. 2005; Riccioni et al. 2006; Nielsen and McNagny 2009). KS chains on podocalyxcin in normal embryonic cells are of low sulfation detected by MAb R-10G (Kawabe et al. 2013; Nakao et al. 2017; Toyoda et al. 2017) while tumor cells produce a high sulfation KS chain detected by antibodies such as 5-D-4 or MZ-14 (Caterson et al. 1983; Saphos et al. 1993; Yoon et al. 2002). Two cytosolic adapter proteins, Na+/H+-Exchanger Regulatory Factor 2 (NHERF2) and Ezrin, interact with the cytoplasmic tail of podocalyxcin exerting regulatory effects on cell signaling and downline effects on neural behavior during the development and repair of the CNS/PNS. Neural migration and axonal guidance are governed by cues from many ECM molecules (Netrins, Semaphorins) which exert either attractive or repulsive cues. Podocalyxcin is not essential for neural migration to occur but can modulate this process. Cell–cell contact and adhesion to the ECM contribute to neural assembly processes. Adhesion molecules such as NCAM and L1 have important roles to play in axonal growth, neural migration and synapse formation. Podocalyxcin has essential roles to play in neuritogenesis and synaptogenesis (Kiss and Rougon 1997; Eckhardt et al. 2000; Angata et al. 2007). Podocalyxcin co-localizes with synapsin and synaptophysin in synapse vesicle formations (Vitureira et al. 2010). Synaptophysin is a major synaptic vesicle protein which co-ordinates the endocytosis of synaptic vesicles during neural stimulation (Kwon and Chapman 2011), synapsin tethers synaptic vesicles to cytoskeletal components preventing premature vesicle release into the synaptic gap co-ordinating neurotransmitter release from the synaptic vesicles to communicating neurons (Cesca et al. 2010; Fornasiero et al. 2010; Valtorta et al. 2011; Song and Augustine 2015). Podocalyxcin is related to the sialomucins CD34 and endoglycan (Horvat et al. 1986; Delia et al. 1993; Kershaw et al. 1997; Sassetti et al. 1998; Hara et al. 1999; Miettinen et al. 1999; Sassetti et al. 2000; Doyonnas et al. 2005; Nielsen and McNagny 2008, 2009).
Abakan
Abakan is a large brain KSPG with at least one CS side chain and one additional carbohydrate chain containing a terminal 3-sulpho-glucuronic acid (HNK-1) (Geisert et al. 1992). Abakan is up-regulated during corticol injury in neonatal rats and is associated with astrocytes in the optic nerve and developing brain. At birth, Abakan levels are low in the sensory cortex but its levels steadily increase with tissue maturation reaching maximal levels in the adult brain (Seo and Geisert 1995). Abakan blocks neural attachment and neurite outgrowth in culture and marks the boundaries of structural regions of the developing brain (Geisert and Bidanset 1993; Robson and Geisert 1994).
Conclusions
KSPGs are a biodiverse multifunctional group of molecules. KS has a widespread distribution in the cornea, cartilage and brain, however, compared to other GAGs relatively little is known of its functional properties (Table I). KSPGs of widely differing modular design such as aggrecan and fibromodulin occur in hyaline cartilage and fibrocartilage, lumican and keratocan in cornea, SV2 proteoglycan in synaptic vesicles and claustrin, abakan, phosphacan and aggrecan in brain (Iozzo and Schaefer 2015). One isoform of CD44 (epican) contains KS and the perlecan synthesized by HEK 293 kidney keratinocytes also contains KS. A number of molecules have been demonstrated to contain small low sulfation KS chains (PRELP, transferrin, thyroglobulin, early embryonic KSPGs, podocalyxcin); however, the functional significance of these KS chains is not known. The uniform spacing between collagen fibrils provided by lumican and keratocan in cornea points to their well-established roles in collagen fibrillogenesis, fibril spacing and ECM organization. Roles for brain KSPGs in neurotransmission, synapse organization and nerve regeneration have also been elucidated. Endometrial and uterine KSPGs have roles in implantation and fertilization. Highly sulfated KS chains on a number of cell surface and secreted KSPGs have found diagnostic utility and prognostic capability for the assessment of a number of tumors. KSPGs act as Ca2+ reservoirs in egg shell assembly, bone mineralization and in the generation of action potentials in neurons. SV2 is a transport proteoglycan and forms a smart gel complexes with neurotransmitters within synaptic vesicles. KS is interactive with a large number of protein kinases and nerve associated proteins and new evidence shows KS regulates a number of growth factors, morphogens and inflammatory cytokines in tissue morphogenesis, remodeling and development. It is remarkable that these properties are achieved by a relatively simple GAG. The KS structure is based on the N-acetyl-polylactosamine backbone -Galββ(1–4) GlcNAcβ(1–3)-, GlcNAc is normally 6-O-sulfated and the Gal disaccharide component can also be 6-O sulfated but this varies with tissue location, age and species. α1–3 linked l-fucose on GlcNAc in the main lactosamine repeat region occurs in articular cartilage but rarely in non-articular tissues (e.g. nasal septum, trachea). Skeletal KSII has two major capping sequences α2–3 and α2–6 linked N-acetylneuraminic acid whereas corneal KS has over seven different sugar/linkage combinations involving N-acetyl, N-glycolyl-neuraminic acids, GalNAc and α-Gal (Whitham et al. 1999; Prydz 2015). The significance of these capping structures in terms of molecular recognition is not known but they do confer resistance to depolymerization to KS by keratanase-I/II and endo-β-d-galactosidase.
The identification of a range of molecules with low sulfation small KS chains points to an area of the KS molecule which is poorly investigated. While the charge density of the highly sulfated KS chains is a driving force for many KS-mediated interactions, a high charge density in glycans is not essential for these to impart important recognition and directional cues to cells and the regulation of a number of physiological processes through interactions with lectins and pattern recognition receptors in the human body (Melrose 2017). It may well be that such interactions with low sulfation KS chains afford a more subtle level of cellular control than the high charge density mediated interactions provided by oversulfated KS chains such as those detected by MAb 5-D-4. There is still a lot to understand about KS and this area of glycobiology is entering an interesting era.
Acknowledgements
This study received no funding other than infrastructure support from The Institute of Bone and Joint Research, University of Sydney.
Abbreviations
ADAMTS, a disintegrin and metalloproteinase with thrombospondin motifs; ALS, amyotrophic lateral sclerosis; BMP, bone morphogenetic protein; BSP-II, bone sialoprotein-II; β3GnT, β-1,3-N-acetylglucosaminyltransferase; GlcNAc6ST-1, N-acetylglucosaminyl-6-sulphotransferase, Gn6ST1 (encoded by gene CHST2); β4GalT1, β1,4-galactosyl transferase1; β4GalT4, β1,4-galactosyl transferase4; KSGalST, KS galactosyl sulphotransferase; CNS, central nervous system; CXCL1, chemokine (C-X-C motif) ligand 1; ECM, extracellular matrix; EGF, epidermal growth factor; EMCA, embryonic mucin core antigen; FACE, fluorescence assisted carbohydrate electrophoresis; FGF, fibroblast growth factor; Fmod, fibromodulin; GAG, glycosaminoglycan; GCTM-2, human embryonal carcinoma tumor antigen-2; HNK-1, human natural killer antigen-1; IGD, interglobular domain; IGFBP2, insulin-like growth factor binding protein-2; KS, keratan sulfate; KSPGs, keratan sulfate proteoglycans; LRRs, leucine-rich repeats; Lum, lumican; MAb, monoclonal antibody; MMP, matrix metalloprotease; MUC1, cell surface-associated mucin; PRELP, Proline and arginine end leucine-rich repeat protein, Prolargin; PNS, peripheral nervous system; PZP-3, Zona pellucida glycoprotein-3; PGs, proteoglycans; RA, rheumatoid arthritis; RPTP-β, receptor-type protein tyrosine phosphatase beta; SSEA-1, stage-specific embryonic antigen; SV2, synaptic vesicle proteoglycan-2; SLRPs, small leucine-rich repeat proteoglycans; TLR4, Toll-like receptor-4; TGF-β, transforming growth factor-β; TRA 1–60, human embryonal carcinoma marker antigen “TRA”—battle of Trafalgar or tumor rejection antigen; TSP-1, thrombospondin-1
Conflict of interest statement
None declared.
Disclosures
The authors have no financial disclosures or conflicts of interest to make.
Authors’ contributions
B.C. and J.M. contributed equally to the writing and review of this manuscript.
References
- Adewumi O, Aflatoonian B, Ahrlund-Richter L, Amit M, Andrews PW, Beighton G, Bello PA, Benvenisty N, Berry LS, Bevan S et al. . 2007. Characterization of human embryonic stem cell lines by the International Stem Cell Initiative. Nat Biotechnol. 25:803–816. [DOI] [PubMed] [Google Scholar]
- Akama TO, Nakayama J, Nishida K, Hiraoka N, Suzuki M, McAuliffe J, Hindsgaul O, Fukuda M, Fukuda MN.. 2001. Human corneal GlcNac 6-O-sulfotransferase and mouse intestinal GlcNac 6-O-sulfotransferase both produce keratan sulfate. J Biol Chem. 276:16271–16278. [DOI] [PubMed] [Google Scholar]
- Akama TO, Nishida K, Nakayama J, Watanabe H, Ozaki K, Nakamura T, Dota A, Kawasaki S, Inoue Y, Maeda N et al. . 2000. Macular corneal dystrophy type I and type II are caused by distinct mutations in a new sulphotransferase gene. Nat Genet. 26:237–241. [DOI] [PubMed] [Google Scholar]
- Akhtar S, Bron AJ, Hayes AJ, Meek KM, Caterson B.. 2011. Role of keratan sulphate (sulphated poly-N-acetyllactosamine repeats) in keratoconic cornea, histochemical, and ultrastructural analysis. Graefes Arch Clin Exp Ophthalmol. 249:413–420. [DOI] [PubMed] [Google Scholar]
- Akhtar S, Kerr BC, Hayes AJ, Hughes CE, Meek KM, Caterson B.. 2008. Immunochemical localization of keratan sulfate proteoglycans in cornea, sclera, and limbus using a keratanase-generated neoepitope monoclonal antibody. Invest Ophthalmol Vis Sci. 49:2424–2431. [DOI] [PubMed] [Google Scholar]
- Aldave AJ, Yellore VS, Thonar EJ, Udar N, Warren JF, Yoon MK, Cohen EJ, Rapuano CJ, Laibson PR, Margolis TP et al. . 2004. Novel mutations in the carbohydrate sulfotransferase gene (CHST6) in American patients with macular corneal dystrophy. Am J Ophthalmol. 137:465–473. [DOI] [PubMed] [Google Scholar]
- Alvarez-Lopez MJ, Castro-Freire M, Cosin-Tomas M, Sanchez-Roige S, Lalanza JF, Del Valle J, Parrizas M, Camins A, Pallas M, Escorihuela RM et al. . 2013. Long-term exercise modulates hippocampal gene expression in senescent female mice. J Alzheimers Dis. 33:1177–1190. [DOI] [PubMed] [Google Scholar]
- Amado M, Almeida R, Schwientek T, Clausen H.. 1999. Identification and characterization of large galactosyltransferase gene families: Galactosyltransferases for all functions. Biochim Biophys Acta. 1473:35–53. [DOI] [PubMed] [Google Scholar]
- Andrews PW, Banting G, Damjanov I, Arnaud D, Avner P.. 1984. Three monoclonal antibodies defining distinct differentiation antigens associated with different high molecular weight polypeptides on the surface of human embryonal carcinoma cells. Hybridoma. 3:347–361. [DOI] [PubMed] [Google Scholar]
- Angata K, Huckaby V, Ranscht B, Terskikh A, Marth JD, Fukuda M.. 2007. Polysialic acid-directed migration and differentiation of neural precursors are essential for mouse brain development. Mol Cell Biol. 27:6659–6668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aplin JD. 1991. Glycans as biochemical markers of human endometrial secretory differentiation. J Reprod Fertil. 92:525–541. [DOI] [PubMed] [Google Scholar]
- Aplin JD, Hey NA.. 1995. MUC1, endometrium and embryo implantation. Biochem Soc Trans. 23:826–831. [DOI] [PubMed] [Google Scholar]
- Aplin JD, Hey NA, Graham RA.. 1998. Human endometrial MUC1 carries keratan sulfate: Characteristic glycoforms in the luminal epithelium at receptivity. Glycobiology. 8:269–276. [DOI] [PubMed] [Google Scholar]
- Badcock G, Pigott C, Goepel J, Andrews PW.. 1999. The human embryonal carcinoma marker antigen TRA-1-60 is a sialylated keratan sulfate proteoglycan. Cancer Res. 59:4715–4719. [PubMed] [Google Scholar]
- Bajjalieh SM, Frantz GD, Weimann JM, McConnell SK, Scheller RH.. 1994. Differential expression of synaptic vesicle protein 2 (SV2) isoforms. J Neurosci. 14:5223–5235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajjalieh SM, Peterson K, Linial M, Scheller RH.. 1993. Brain contains two forms of synaptic vesicle protein 2. Proc Natl Acad Sci USA. 90:2150–2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajjalieh SM, Peterson K, Shinghal R, Scheller RH.. 1992. SV2, a brain synaptic vesicle protein homologous to bacterial transporters. Science. 257:1271–1273. [DOI] [PubMed] [Google Scholar]
- Baker J, Walker T, Morrison K, Neame P, Christner J.. 1989. The specificity of a mouse monoclonal antibody to human aorta proteoglycans. Matrix. 9:92–98. [DOI] [PubMed] [Google Scholar]
- Bandtlow CE, Zimmermann DR.. 2000. Proteoglycans in the developing brain: New conceptual insights for old proteins. Physiol Rev. 80:1267–1290.11015614 [Google Scholar]
- Barry FP, Gaw JU, Young CN, Neame PJ.. 1992. Hyaluronan-binding region of aggrecan from pig laryngeal cartilage. Amino acid sequence, analysis of N-linked oligosaccharides and location of the keratan sulphate. Biochem J. 286(Pt 3):761–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry FP, Neame PJ, Sasse J, Pearson D.. 1994. Length variation in the keratan sulfate domain of mammalian aggrecan. Matrix Biol. 14:323–328. [DOI] [PubMed] [Google Scholar]
- Barry FP, Rosenberg LC, Gaw JU, Koob TJ, Neame PJ.. 1995. N- and O-linked keratan sulfate on the hyaluronan binding region of aggrecan from mature and immature bovine cartilage. J Biol Chem. 270:20516–20524. [DOI] [PubMed] [Google Scholar]
- Bashaw GJ, Kidd T, Murray D, Pawson T, Goodman CS.. 2000. Repulsive axon guidance: Abelson and Enabled play opposing roles downstream of the roundabout receptor. Cell. 101:703–715. [DOI] [PubMed] [Google Scholar]
- Bengtsson E, Morgelin M, Sasaki T, Timpl R, Heinegard D, Aspberg A.. 2002. The leucine-rich repeat protein PRELP binds perlecan and collagens and may function as a basement membrane anchor. J Biol Chem. 277:15061–15068. [DOI] [PubMed] [Google Scholar]
- Bengtsson E, Neame PJ, Heinegard D, Sommarin Y.. 1995. The primary structure of a basic leucine-rich repeat protein, PRELP, found in connective tissues. J Biol Chem. 270:25639–25644. [DOI] [PubMed] [Google Scholar]
- Bentz H, Nathan RM, Rosen DM, Armstrong RM, Thompson AY, Segarini PR, Mathews MC, Dasch JR, Piez KA, Seyedin SM.. 1989. Purification and characterization of a unique osteoinductive factor from bovine bone. J Biol Chem. 264:20805–20810. [PubMed] [Google Scholar]
- Berryhill BL, Beales MP, Hassell JR.. 2001. Production of prostaglandin D synthase as a keratan sulfate proteoglycan by cultured bovine keratocytes. Invest Ophthalmol Vis Sci. 42:1201–1207. [PubMed] [Google Scholar]
- Bhavanandan VP, Meyer K.. 1966. Mucopolysaccharides: N-acetylglucosamine- and galactose-6-sulfates from keratosulfate. Science. 151:1404–1405. [DOI] [PubMed] [Google Scholar]
- Bhavanandan VP, Meyer K.. 1968. Studies on keratosulfates. Methylation, desulfation, and acid hydrolysis studies on old human rib cartilage keratosulfate. J Biol Chem. 243:1052–1059. [PubMed] [Google Scholar]
- Blochberger TC, Vergnes JP, Hempel J, Hassell JR.. 1992. cDNA to chick lumican (corneal keratan sulfate proteoglycan) reveals homology to the small interstitial proteoglycan gene family and expression in muscle and intestine. J Biol Chem. 267:347–352. [PubMed] [Google Scholar]
- Boman K, Larsson AH, Segersten U, Kuteeva E, Johannesson H, Nodin B, Eberhard J, Uhlen M, Malmstrom PU, Jirstrom K.. 2013. Membranous expression of podocalyxin-like protein is an independent factor of poor prognosis in urothelial bladder cancer. Br J Cancer. 108:2321–2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borcherding MS, Blacik LJ, Sittig RA, Bizzell JW, Breen M, Weinstein HG.. 1975. Proteoglycans and collagen fibre organization in human corneoscleral tissue. Exp Eye Res. 21:59–70. [DOI] [PubMed] [Google Scholar]
- Borg D, Hedner C, Nodin B, Larsson A, Johnsson A, Eberhard J, Jirstrom K.. 2016. Expression of podocalyxin-like protein is an independent prognostic biomarker in resected esophageal and gastric adenocarcinoma. BMC Clin Pathol. 16:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boubelik M, Draberova L, Draber P.. 1996. Carbohydrate-mediated sorting in aggregating embryonal carcinoma cells. Biochem Biophys Res Commun. 224:283–288. [DOI] [PubMed] [Google Scholar]
- Bray BA, Lieberman R, Meyer K.. 1967. Structure of human skeletal keratosulfate. The linkage region. J Biol Chem. 242:3373–3380. [PubMed] [Google Scholar]
- Brayman M, Thathiah A, Carson DD.. 2004. MUC1: A multifunctional cell surface component of reproductive tissue epithelia. Reprod Biol Endocrinol. 2:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brew K, Vanaman TC, Hill RL.. 1968. The role of alpha-lactalbumin and the A protein in lactose synthetase: A unique mechanism for the control of a biological reaction. Proc Natl Acad Sci USA. 59:491–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brezillon S, Pietraszek K, Maquart FX, Wegrowski Y.. 2013. Lumican effects in the control of tumour progression and their links with metalloproteinases and integrins. FEBS J. 280:2369–2381. [DOI] [PubMed] [Google Scholar]
- Brown GM, Huckerby TN, Abram BL, Nieduszynski IA.. 1996. Characterization of a non-reducing terminal fragment from bovine articular cartilage keratan sulphates containing alpha(2-3)-linked sialic acid and alpha(1-3)-linked fucose. A sulphated variant of the VIM-2 epitope. Biochem J. 319(Pt 1):137–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown GM, Huckerby TN, Bayliss MT, Nieduszynski IA.. 1998. Human aggrecan keratan sulfate undergoes structural changes during adolescent development. J Biol Chem. 273:26408–26414. [DOI] [PubMed] [Google Scholar]
- Brown GM, Huckerby TN, Morris HG, Abram BL, Nieduszynski IA.. 1994. a. Oligosaccharides derived from bovine articular cartilage keratan sulfates after keratanase II digestion: Implications for keratan sulfate structural fingerprinting. Biochemistry. 33:4836–4846. [DOI] [PubMed] [Google Scholar]
- Brown GM, Huckerby TN, Nieduszynski IA.. 1994. b. Oligosaccharides derived by keratanase II digestion of bovine articular cartilage keratan sulphates. Eur J Biochem. 224:281–308. [DOI] [PubMed] [Google Scholar]
- Buisine MP, Devisme L, Savidge TC, Gespach C, Gosselin B, Porchet N, Aubert JP.. 1998. Mucin gene expression in human embryonic and fetal intestine. Gut. 43:519–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burg MA, Cole GJ.. 1994. Claustrin, an antiadhesive neural keratan sulfate proteoglycan, is structurally related to MAP1B. J Neurobiol. 25:1–22. [DOI] [PubMed] [Google Scholar]
- Butler CD, Schnetz SA, Yu EY, Davis JB, Temple K, Silver J, Malouf AT.. 2004. Keratan sulfate proteoglycan phosphacan regulates mossy fiber outgrowth and regeneration. J Neurosci. 24:462–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camire O, Topolnik L.. 2014. Dendritic calcium nonlinearities switch the direction of synaptic plasticity in fast-spiking interneurons. J Neurosci. 34:3864–3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cappellesso R, Millioni R, Arrigoni G, Simonato F, Caroccia B, Iori E, Guzzardo V, Ventura L, Tessari P, Fassina A.. 2015. Lumican is overexpressed in lung adenocarcinoma pleural effusions. PLoS One. 10:e0126458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardin AD, Weintraub HJ.. 1989. Molecular modeling of protein–glycosaminoglycan interactions. Arteriosclerosis. 9:21–32. [DOI] [PubMed] [Google Scholar]
- Carlson EC, Lin M, Liu CY, Kao WW, Perez VL, Pearlman E.. 2007. Keratocan and lumican regulate neutrophil infiltration and corneal clarity in lipopolysaccharide-induced keratitis by direct interaction with CXCL1. J Biol Chem. 282:35502–35509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson SS. 1996. SV2proteoglycan: A potential synaptic vesicle transporter and nerve terminal extracellular matrix receptor. Perspect Dev Neurobiol. 3:373–386. [PubMed] [Google Scholar]
- Carlson SS, Iwata M, Wight TN.. 1996. A chondroitin sulfate/keratan sulfate proteoglycan, PG-1000, forms complexes which are concentrated in the reticular laminae of electric organ basement membranes. Matrix Biol. 15:281–292. [DOI] [PubMed] [Google Scholar]
- Caterson B, Christner JE, Baker JR.. 1983. Identification of a monoclonal antibody that specifically recognizes corneal and skeletal keratan sulfate. Monoclonal antibodies to cartilage proteoglycan. J Biol Chem. 258:8848–8854. [PubMed] [Google Scholar]
- Cesca F, Baldelli P, Valtorta F, Benfenati F.. 2010. The synapsins: Key actors of synapse function and plasticity. Prog Neurobiol. 91:313–348. [DOI] [PubMed] [Google Scholar]
- Chambers MG, Cox L, Chong L, Suri N, Cover P, Bayliss MT, Mason RM.. 2001. Matrix metalloproteinases and aggrecanases cleave aggrecan in different zones of normal cartilage but colocalize in the development of osteoarthritic lesions in STR/ort mice. Arthritis Rheum. 44:1455–1465. [DOI] [PubMed] [Google Scholar]
- Chen S, Birk DE.. 2013. The regulatory roles of small leucine-rich proteoglycans in extracellular matrix assembly. FEBS J. 280:2120–2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Young MF, Chakravarti S, Birk DE.. 2014. Interclass small leucine-rich repeat proteoglycan interactions regulate collagen fibrillogenesis and corneal stromal assembly. Matrix Biol. 35:103–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirivella L, Kirstein M, Ferron SR, Domingo-Muelas A, Durupt FC, Acosta-Umanzor C, Cano-Jaimez M, Perez-Sanchez F, Barbacid M, Ortega S et al. . 2017. Cyclin-dependent kinase 4 regulates adult neural stem cell proliferation and differentiation in response to insulin. Stem Cells. 35:2403–2416. [DOI] [PubMed] [Google Scholar]
- Choi HU, Meyer K.. 1975. The structure of keratan sulphates from various sources. Biochem J. 151:543–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cipollone JA, Graves ML, Kobel M, Kalloger SE, Poon T, Gilks CB, McNagny KM, Roskelley CD.. 2012. The anti-adhesive mucin podocalyxin may help initiate the transperitoneal metastasis of high grade serous ovarian carcinoma. Clin Exp Metastasis. 29:239–252. [DOI] [PubMed] [Google Scholar]
- Conrad AH, Zhang Y, Tasheva ES, Conrad GW.. 2010. Proteomic analysis of potential keratan sulfate, chondroitin sulfate A, and hyaluronic acid molecular interactions. Invest Ophthalmol Vis Sci. 51:4500–4515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper S, Bennett W, Andrade J, Reubinoff BE, Thomson J, Pera MF.. 2002. Biochemical properties of a keratan sulphate/chondroitin sulphate proteoglycan expressed in primate pluripotent stem cells. J Anat. 200:259–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornuet PK, Blochberger TC, Hassell JR.. 1994. Molecular polymorphism of lumican during corneal development. Invest Ophthalmol Vis Sci. 35:870–877. [PubMed] [Google Scholar]
- Corpuz LM, Dunlevy JR, Hassell JR, Conrad AH, Conrad GW.. 2000. Molecular cloning and relative tissue expression of keratocan and mimecan in embryonic quail cornea. Matrix Biol. 19:693–698. [DOI] [PubMed] [Google Scholar]
- Corpuz LM, Funderburgh JL, Funderburgh ML, Bottomley GS, Prakash S, Conrad GW.. 1996. Molecular cloning and tissue distribution of keratocan. Bovine corneal keratan sulfate proteoglycan 37A. J Biol Chem. 271:9759–9763. [DOI] [PubMed] [Google Scholar]
- Coulson-Thomas VJ, Coulson-Thomas YM, Gesteira TF, Andrade de Paula CA, Carneiro CR, Ortiz V, Toma L, Kao WW, Nader HB.. 2013. Lumican expression, localization and antitumor activity in prostate cancer. Exp Cell Res. 319:967–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crevecoeur J, Kaminski RM, Rogister B, Foerch P, Vandenplas C, Neveux M, Mazzuferi M, Kroonen J, Poulet C, Martin D et al. . 2014. Expression pattern of synaptic vesicle protein 2 (SV2) isoforms in patients with temporal lobe epilepsy and hippocampal sclerosis. Neuropathol Appl Neurobiol. 40:191–204. [DOI] [PubMed] [Google Scholar]
- Crowder KM, Gunther JM, Jones TA, Hale BD, Zhang HZ, Peterson MR, Scheller RH, Chavkin C, Bajjalieh SM.. 1999. Abnormal neurotransmission in mice lacking synaptic vesicle protein 2A (SV2A). Proc Natl Acad Sci USA. 96:15268–15273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang X, Zhu Q, Wang L, Su H, Lin H, Zhou N, Liang T, Wang Z, Huang S, Ren Q et al. . 2009. Macular corneal dystrophy in a Chinese family related with novel mutations of CHST6. Mol Vis. 15:700–705. [PMC free article] [PubMed] [Google Scholar]
- Davidson E, Hoffman P, Linker A, Meyer K.. 1956. The acid mucopolysaccharides of connective tissue. Biochim Biophys Acta. 21:506–518. [DOI] [PubMed] [Google Scholar]
- Delia D, Lampugnani MG, Resnati M, Dejana E, Aiello A, Fontanella E, Soligo D, Pierotti MA, Greaves MF.. 1993. CD34 expression is regulated reciprocally with adhesion molecules in vascular endothelial cells in vitro. Blood. 81:1001–1008. [PubMed] [Google Scholar]
- Dellett M, Hu W, Papadaki V, Ohnuma S.. 2012. Small leucine rich proteoglycan family regulates multiple signalling pathways in neural development and maintenance. Dev Growth Differ. 54:327–340. [DOI] [PubMed] [Google Scholar]
- DeLoia JA, Krasnow JS, Brekosky J, Babaknia A, Julian J, Carson DD.. 1998. Regional specialization of the cell membrane-associated, polymorphic mucin (MUC1) in human uterine epithelia. Hum Reprod. 13:2902–2909. [DOI] [PubMed] [Google Scholar]
- Dickenson JM, Huckerby TN, Nieduszynski IA.. 1992. Skeletal keratan sulphate chains isolated from bovine intervertebral disc may terminate in alpha(2—6)-linked N-acetylneuraminic acid. Biochem J. 282(Pt 1):267–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon AR, Price MR, Hand CW, Sibley PE, Selby C, Blamey RW.. 1993. Epithelial mucin core antigen (EMCA) in assessing therapeutic response in advanced breast cancer—A comparison with CA15.3. Br J Cancer. 68:947–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobbertin A, Rhodes KE, Garwood J, Properzi F, Heck N, Rogers JH, Fawcett JW, Faissner A.. 2003. Regulation of RPTPbeta/phosphacan expression and glycosaminoglycan epitopes in injured brain and cytokine-treated glia. Mol Cell Neurosci. 24:951–971. [DOI] [PubMed] [Google Scholar]
- Doyonnas R, Nielsen JS, Chelliah S, Drew E, Hara T, Miyajima A, McNagny KM.. 2005. Podocalyxin is a CD34-related marker of murine hematopoietic stem cells and embryonic erythroid cells. Blood. 105:4170–4178. [DOI] [PubMed] [Google Scholar]
- Du J, Hincke MT, Rose-Martel M, Hennequet-Antier C, Brionne A, Cogburn LA, Nys Y, Gautron J.. 2015. Identifying specific proteins involved in eggshell membrane formation using gene expression analysis and bioinformatics. BMC Genomics. 16:792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlevy JR, Neame PJ, Vergnes JP, Hassell JR.. 1998. Identification of the N-linked oligosaccharide sites in chick corneal lumican and keratocan that receive keratan sulfate. J Biol Chem. 273:9615–9621. [DOI] [PubMed] [Google Scholar]
- Dvorak P, Hampl A, Jirmanova L, Pacholikova J, Kusakabe M.. 1998. Embryoglycan ectodomains regulate biological activity of FGF-2 to embryonic stem cells. J Cell Sci. 111(Pt 19):2945–2952. [DOI] [PubMed] [Google Scholar]
- Eccles JC. 1983. Calcium in long-term potentiation as a model for memory. Neuroscience. 10:1071–1081. [DOI] [PubMed] [Google Scholar]
- Eckhardt M, Bukalo O, Chazal G, Wang L, Goridis C, Schachner M, Gerardy-Schahn R, Cremer H, Dityatev A.. 2000. Mice deficient in the polysialyltransferase ST8SiaIV/PST-1 allow discrimination of the roles of neural cell adhesion molecule protein and polysialic acid in neural development and synaptic plasticity. J Neurosci. 20:5234–5244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelmann W, Zervas M, Costello P, Roback L, Fischer I, Hammarback JA, Cowan N, Davies P, Wainer B, Kucherlapati R.. 1996. Neuronal abnormalities in microtubule-associated protein 1B mutant mice. Proc Natl Acad Sci USA. 93:1270–1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edrington TB, Zadnik K, Barr JT.. 1995. Keratoconus. Optom Clin. 4:65–73. [PubMed] [Google Scholar]
- Edward DP, Thonar EJ, Srinivasan M, Yue BJ, Tso MO.. 1990. Macular dystrophy of the cornea. A systemic disorder of keratan sulfate metabolism. Ophthalmology. 97:1194–1200. [DOI] [PubMed] [Google Scholar]
- El-Ashry MF, Abd El-Aziz MM, Shalaby O, Wilkins S, Poopalasundaram S, Cheetham M, Tuft SJ, Hardcastle AJ, Bhattacharya SS, Ebenezer ND.. 2005. Novel CHST6 nonsense and missense mutations responsible for macular corneal dystrophy. Am J Ophthalmol. 139:192–193. [DOI] [PubMed] [Google Scholar]
- El-Ashry MF, Abd El-Aziz MM, Wilkins S, Cheetham ME, Wilkie SE, Hardcastle AJ, Halford S, Bayoumi AY, Ficker LA, Tuft S et al. . 2002. Identification of novel mutations in the carbohydrate sulfotransferase gene (CHST6) causing macular corneal dystrophy. Invest Ophthalmol Vis Sci. 43:377–382. [PubMed] [Google Scholar]
- Espandar L, Meyer J.. 2010. Keratoconus: Overview and update on treatment. Middle East Afr J Ophthalmol. 17:15–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faissner A, Heck N, Dobbertin A, Garwood J.. 2006. DSD-1-Proteoglycan/Phosphacan and receptor protein tyrosine phosphatase-beta isoforms during development and regeneration of neural tissues. Adv Exp Med Biol. 557:25–53. [DOI] [PubMed] [Google Scholar]
- Feany MB, Lee S, Edwards RH, Buckley KM.. 1992. The synaptic vesicle protein SV2 is a novel type of transmembrane transporter. Cell. 70:861–867. [DOI] [PubMed] [Google Scholar]
- Feizi T. 1977. Human Blood Groups. Basel: Karger. [Google Scholar]
- Feizi T. 1989. KS Oligosaccharides: Members of a Family of Antigens of the Poly-N-Acetyl-lactosamine series. London: The Biochemical Society. [Google Scholar]
- Feizi T, Childs RA, Watanabe K, Hakomori SI.. 1979. Three types of blood group I specificity among monoclonal anti-I autoantibodies revealed by analogues of a branched erythrocyte glycolipid. J Exp Med. 149:975–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feizi T, Kabat EA, Vicari G, Anderson B, Marsh WL.. 1971. Immunochemical studies on blood groups.XLIX. The I antigen complex: Specificity differences among anti-I sera revealed by quantitative precipitin studies; partial structure of the I determinant specific for one anti-I serum. J Immunol. 106:1578–1592. [PubMed] [Google Scholar]
- Fischer DC, Haubeck HD, Eich K, Kolbe-Busch S, Stocker G, Stuhlsatz HW, Greiling H.. 1996. A novel keratan sulphate domain preferentially expressed on the large aggregating proteoglycan from human articular cartilage is recognized by the monoclonal antibody 3D12/H7. Biochem J. 318(Pt 3):1051–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer DC, Kuth A, Winkler M, Handt S, Hauptmann S, Rath W, Haubeck HD.. 2001. A large keratan sulfate proteoglycan present in human cervical mucous appears to be involved in the reorganization of the cervical extracellular matrix at term. J Soc Gynecol Investig. 8:277–284. [DOI] [PubMed] [Google Scholar]
- Flores-Tellez TN, Lopez TV, Vasquez Garzon VR, Villa-Trevino S.. 2015. Co-expression of Ezrin-CLIC5-podocalyxin is associated with migration and invasiveness in hepatocellular carcinoma. PLoS One. 10:e0131605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornasiero EF, Bonanomi D, Benfenati F, Valtorta F.. 2010. The role of synapsins in neuronal development. Cell Mol Life Sci. 67:1383–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fosang AJ, Hardingham TE.. 1991. 1-C-6 epitope in cartilage proteoglycan G2 domain is masked by keratan sulphate. Biochem J. 273(Pt 2):369–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fosang AJ, Last K, Maciewicz RA.. 1996. Aggrecan is degraded by matrix metalloproteinases in human arthritis. Evidence that matrix metalloproteinase and aggrecanase activities can be independent. J Clin Invest. 98:2292–2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fosang AJ, Last K, Poon CJ, Plaas AH.. 2009. Keratan sulphate in the interglobular domain has a microstructure that is distinct from keratan sulphate elsewhere on pig aggrecan. Matrix Biol. 28:53–61. [DOI] [PubMed] [Google Scholar]
- Fosang AJ, Rogerson FM, East CJ, Stanton H.. 2008. ADAMTS-5: The story so far. Eur Cell Mater. 15:11–26. [DOI] [PubMed] [Google Scholar]
- Foyez T, Takeda-Uchimura Y, Ishigaki S, Narentuya, Zhang Z, Sobue G, Kadomatsu K, Uchimura K.. 2015. Microglial keratan sulfate epitope elicits in central nervous tissues of transgenic model mice and patients with amyotrophic lateral sclerosis. Am J Pathol. 185:3053–3065. [DOI] [PubMed] [Google Scholar]
- Fryer HJ, Kelly GM, Molinaro L, Hockfield S.. 1992. The high molecular weight Cat-301 chondroitin sulfate proteoglycan from brain is related to the large aggregating proteoglycan from cartilage, aggrecan. J Biol Chem. 267:9874–9883. [PubMed] [Google Scholar]
- Fukuma M, Abe H, Okita H, Yamada T, Hata J.. 2003. Monoclonal antibody 4C4-mAb specifically recognizes keratan sulphate proteoglycan on human embryonal carcinoma cells. J Pathol. 201:90–98. [DOI] [PubMed] [Google Scholar]
- Fukuta M, Inazawa J, Torii T, Tsuzuki K, Shimada E, Habuchi O.. 1997. Molecular cloning and characterization of human keratan sulfate Gal-6-sulfotransferase. J Biol Chem. 272:32321–32328. [DOI] [PubMed] [Google Scholar]
- Funderburgh JL. 2000. Keratan sulfate: Structure, biosynthesis, and function. Glycobiology. 10:951–958. [DOI] [PubMed] [Google Scholar]
- Funderburgh JL. 2002. Keratan sulfate biosynthesis. IUBMB Life. 54:187–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funderburgh JL, Corpuz LM, Roth MR, Funderburgh ML, Tasheva ES, Conrad GW.. 1997. Mimecan, the 25-kDa corneal keratan sulfate proteoglycan, is a product of the gene producing osteoglycin. J Biol Chem. 272:28089–28095. [DOI] [PubMed] [Google Scholar]
- Funderburgh JL, Funderburgh ML, Mann MM, Conrad GW.. 1991. Unique glycosylation of three keratan sulfate proteoglycan isoforms. J Biol Chem. 266:14226–14231. [PubMed] [Google Scholar]
- Funderburgh JL, Funderburgh ML, Rodrigues MM, Krachmer JH, Conrad GW.. 1990. Altered antigenicity of keratan sulfate proteoglycan in selected corneal diseases. Invest Ophthalmol Vis Sci. 31:419–428. [PubMed] [Google Scholar]
- Funderburgh JL, Mitschler RR, Funderburgh ML, Roth MR, Chapes SK, Conrad GW.. 1997. Macrophage receptors for lumican. A corneal keratan sulfate proteoglycan. Invest Ophthalmol Vis Sci. 38:1159–1167. [PubMed] [Google Scholar]
- Funderburgh JL, Panjwani N, Conrad GW, Baum J.. 1989. Altered keratan sulfate epitopes in keratoconus. Invest Ophthalmol Vis Sci. 30:2278–2281. [PubMed] [Google Scholar]
- Ganss B, Kim RH, Sodek J.. 1999. Bone sialoprotein. Crit Rev Oral Biol Med. 10:79–98. [DOI] [PubMed] [Google Scholar]
- Gao C, Fujinawa R, Yoshida T, Ueno M, Ota F, Kizuka Y, Hirayama T, Korekane H, Kitazume S, Maeno T et al. . 2017. A keratan sulfate disaccharide prevents inflammation and the progression of emphysema in murine models. Am J Physiol Lung Cell Mol Physiol. 312:L268–L276. [DOI] [PubMed] [Google Scholar]
- Garcia B, Garcia-Suarez O, Merayo-Lloves J, Alcalde I, Alfonso JF, Fernandez-Vega Cueto L, Meana A, Vazquez F, Quiros LM.. 2016. Differential expression of proteoglycans by corneal stromal cells in keratoconus. Invest Ophthalmol Vis Sci. 57:2618–2628. [DOI] [PubMed] [Google Scholar]
- Garwood J, Heck N, Reichardt F, Faissner A.. 2003. Phosphacan short isoform, a novel non-proteoglycan variant of phosphacan/receptor protein tyrosine phosphatase-beta, interacts with neuronal receptors and promotes neurite outgrowth. J Biol Chem. 278:24164–24173. [DOI] [PubMed] [Google Scholar]
- Garwood J, Rigato F, Heck N, Faissner A.. 2001. Tenascin glycoproteins and the complementary ligand DSD-1-PG/ phosphacan—Structuring the neural extracellular matrix during development and repair. Restor Neurol Neurosci. 19:51–64. [PubMed] [Google Scholar]
- Garwood J, Schnadelbach O, Clement A, Schutte K, Bach A, Faissner A.. 1999. DSD-1-proteoglycan is the mouse homolog of phosphacan and displays opposing effects on neurite outgrowth dependent on neuronal lineage. J Neurosci. 19:3888–3899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisert EE Jr., Bidanset DJ.. 1993. A central nervous system keratan sulfate proteoglycan: Localization to boundaries in the neonatal rat brain. Brain Res Dev Brain Res. 75:163–173. [DOI] [PubMed] [Google Scholar]
- Geisert EE Jr., Williams RC, Bidanset DJ.. 1992. A CNS specific proteoglycan associated with astrocytes in rat optic nerve. Brain Res. 571:165–168. [DOI] [PubMed] [Google Scholar]
- Glant TT, Buzas EI, Finnegan A, Negroiu G, Cs-Szabo G, Mikecz K.. 1998. Critical roles of glycosaminoglycan side chains of cartilage proteoglycan (aggrecan) in antigen recognition and presentation. J Immunol. 160:3812–3819. [PubMed] [Google Scholar]
- Gori F, Schipani E, Demay MB.. 2001. Fibromodulin is expressed by both chondrocytes and osteoblasts during fetal bone development. J Cell Biochem. 82:46–57. [DOI] [PubMed] [Google Scholar]
- Graham RA, Li TC, Cooke ID, Aplin JD.. 1994. Keratan sulphate as a secretory product of human endometrium: Cyclic expression in normal women. Hum Reprod. 9:926–930. [DOI] [PubMed] [Google Scholar]
- Graves ML, Cipollone JA, Austin P, Bell EM, Nielsen JS, Gilks CB, McNagny KM, Roskelley CD.. 2016. The cell surface mucin podocalyxin regulates collective breast tumor budding. Breast Cancer Res. 18:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerassimov A, Zhang Y, Banerjee S, Cartman A, Leroux JY, Rosenberg LC, Esdaile J, Fitzcharles MA, Poole AR.. 1998. Cellular immunity to the G1 domain of cartilage proteoglycan aggrecan is enhanced in patients with rheumatoid arthritis but only after removal of keratan sulfate. Arthritis Rheum. 41:1019–1025. [DOI] [PubMed] [Google Scholar]
- Gupta SK, Chadha K, Harris JD, Yurewicz EC, Sacco AG, Kolluri SK, Afzalpurka A.. 1996. Mapping of epitopes on porcine zona pellucida-3 alpha by monoclonal antibodies inhibiting oocyte–sperm interaction. Biol Reprod. 55:410–415. [DOI] [PubMed] [Google Scholar]
- Ha YW, Son MJ, Yun KS, Kim YS.. 2007. Relationship between eggshell strength and keratan sulfate of eggshell membranes. Comp Biochem Physiol A Mol Integr Physiol. 147:1109–1115. [DOI] [PubMed] [Google Scholar]
- Habuchi O, Hirahara Y, Uchimura K, Fukuta M.. 1996. Enzymatic sulfation of galactose residue of keratan sulfate by chondroitin 6-sulfotransferase. Glycobiology. 6:51–57. [DOI] [PubMed] [Google Scholar]
- Hadley JA, Horvat-Gordon M, Kim WK, Praul CA, Burns D, Leach RM Jr.. 2016. Bone sialoprotein keratan sulfate proteoglycan (BSP-KSPG) and FGF-23 are important physiological components of medullary bone. Comp Biochem Physiol A Mol Integr Physiol. 194:1–7. [DOI] [PubMed] [Google Scholar]
- Hara T, Nakano Y, Tanaka M, Tamura K, Sekiguchi T, Minehata K, Copeland NG, Jenkins NA, Okabe M, Kogo H et al. . 1999. Identification of podocalyxin-like protein 1 as a novel cell surface marker for hemangioblasts in the murine aorta-gonad-mesonephros region. Immunity. 11:567–578. [DOI] [PubMed] [Google Scholar]
- Hart GW. 1976. Biosynthesis of glycosaminolgycans during corneal development. J Biol Chem. 251:6513–6521. [PubMed] [Google Scholar]
- Hassell JR, Kimura JH, Hascall VC.. 1986. Proteoglycan core protein families. Annu Rev Biochem. 55:539–567. [DOI] [PubMed] [Google Scholar]
- Hayashida Y, Akama TO, Beecher N, Lewis P, Young RD, Meek KM, Kerr B, Hughes CE, Caterson B, Tanigami A et al. . 2006. Matrix morphogenesis in cornea is mediated by the modification of keratan sulfate by GlcNAc 6-O-sulfotransferase. Proc Natl Acad Sci USA. 103:13333–13338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayatsu N, Kaneko MK, Mishima K, Nishikawa R, Matsutani M, Price JE, Kato Y.. 2008. a. Podocalyxin expression in malignant astrocytic tumors. Biochem Biophys Res Commun. 374:394–398. [DOI] [PubMed] [Google Scholar]
- Hayatsu N, Ogasawara S, Kaneko MK, Kato Y, Narimatsu H.. 2008. b. Expression of highly sulfated keratan sulfate synthesized in human glioblastoma cells. Biochem Biophys Res Commun. 368:217–222. [DOI] [PubMed] [Google Scholar]
- Heby M, Elebro J, Nodin B, Jirstrom K, Eberhard J.. 2015. Prognostic and predictive significance of podocalyxin-like protein expression in pancreatic and periampullary adenocarcinoma. BMC Clin Pathol. 15:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrand A, Romaris M, Rasmussen LM, Heinegard D, Twardzik DR, Border WA, Ruoslahti E.. 1994. Interaction of the small interstitial proteoglycans biglycan, decorin and fibromodulin with transforming growth factor beta. Biochem J. 302(Pt 2):527–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano K, Ohgomori T, Kobayashi K, Tanaka F, Matsumoto T, Natori T, Matsuyama Y, Uchimura K, Sakamoto K, Takeuchi H et al. . 2013. Ablation of keratan sulfate accelerates early phase pathogenesis of ALS. PLoS One. 8:e66969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoadley ME, Seif MW, Aplin JD.. 1990. Menstrual-cycle-dependent expression of keratan sulphate in human endometrium. Biochem J. 266:757–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hocking AM, Shinomura T, McQuillan DJ.. 1998. Leucine-rich repeat glycoproteins of the extracellular matrix. Matrix Biol. 17:1–19. [DOI] [PubMed] [Google Scholar]
- Hoffman P, Linker A, Meyer K.. 1958. The acid mucopolysaccharides of connective tissue. III. The sulfate linkage. Biochim Biophys Acta. 30:184–185. [DOI] [PubMed] [Google Scholar]
- Hoffman P, Mashburn TA Jr., Meyer K.. 1967. Proteinpolysaccharide of bovine cartilage. II. The relation of keratan sulfate and chondroitin sulfate. J Biol Chem. 242:3805–3809. [PubMed] [Google Scholar]
- Holland JW, Meehan KL, Redmond SL, Dawkins HJ.. 2004. Purification of the keratan sulfate proteoglycan expressed in prostatic secretory cells and its identification as lumican. Prostate. 59:252–259. [DOI] [PubMed] [Google Scholar]
- Horvat R, Hovorka A, Dekan G, Poczewski H, Kerjaschki D.. 1986. Endothelial cell membranes contain podocalyxin—The major sialoprotein of visceral glomerular epithelial cells. J Cell Biol. 102:484–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howitt JA, Clout NJ, Hohenester E.. 2004. Binding site for Robo receptors revealed by dissection of the leucine-rich repeat region of Slit. EMBO J. 23:4406–4412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu YH, Lin WL, Hou YT, Pu YS, Shun CT, Chen CL, Wu YY, Chen JY, Chen TH, Jou TS.. 2010. Podocalyxin EBP50 ezrin molecular complex enhances the metastatic potential of renal cell carcinoma through recruiting Rac1 guanine nucleotide exchange factor ARHGEF7. Am J Pathol. 176:3050–3061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain SA, Piper M, Fukuhara N, Strochlic L, Cho G, Howitt JA, Ahmed Y, Powell AK, Turnbull JE, Holt CE et al. . 2006. A molecular mechanism for the heparan sulfate dependence of slit-robo signaling. J Biol Chem. 281:39693–39698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igwe JC, Gao Q, Kizivat T, Kao WW, Kalajzic I.. 2011. Keratocan is expressed by osteoblasts and can modulate osteogenic differentiation. Connect Tissue Res. 52:401–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iida-Hasegawa N, Furuhata A, Hayatsu H, Murakami A, Fujiki K, Nakayasu K, Kanai A.. 2003. Mutations in the CHST6 gene in patients with macular corneal dystrophy: Immunohistochemical evidence of heterogeneity. Invest Ophthalmol Vis Sci. 44:3272–3277. [DOI] [PubMed] [Google Scholar]
- Iozzo RV. 1997. The family of the small leucine-rich proteoglycans: Key regulators of matrix assembly and cellular growth. Crit Rev Biochem Mol Biol. 32:141–174. [DOI] [PubMed] [Google Scholar]
- Iozzo RV, Schaefer L.. 2010. Proteoglycans in health and disease: Novel regulatory signaling mechanisms evoked by the small leucine-rich proteoglycans. FEBS J. 277:3864–3875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iozzo RV, Schaefer L.. 2015. Proteoglycan form and function: A comprehensive nomenclature of proteoglycans. Matrix Biol. 42:11–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishiwata T, Cho K, Kawahara K, Yamamoto T, Fujiwara Y, Uchida E, Tajiri T, Naito Z.. 2007. Role of lumican in cancer cells and adjacent stromal tissues in human pancreatic cancer. Oncol Rep. 18:537–543. [PubMed] [Google Scholar]
- Ito A, Shinmyo Y, Abe T, Oshima N, Tanaka H, Ohta K.. 2010. Tsukushi is required for anterior commissure formation in mouse brain. Biochem Biophys Res Commun. 402:813–818. [DOI] [PubMed] [Google Scholar]
- Iwata M, Carlson SS.. 1991. A large chondroitin sulfate basement membrane-associated proteoglycan exists as a disulfide-stabilized complex of several proteins. J Biol Chem. 266:323–333. [PubMed] [Google Scholar]
- Johnson JM, Young TL, Rada JA.. 2006. Small leucine rich repeat proteoglycans (SLRPs) in the human sclera: Identification of abundant levels of PRELP. Mol Vis. 12:1057–1066. [PubMed] [Google Scholar]
- Jones LL, Tuszynski MH.. 2002. Spinal cord injury elicits expression of keratan sulfate proteoglycans by macrophages, reactive microglia, and oligodendrocyte progenitors. J Neurosci. 22:4611–4624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junghans U, Koops A, Westmeyer A, Kappler J, Meyer HE, Muller HW.. 1995. Purification of a meningeal cell-derived chondroitin sulphate proteoglycan with neurotrophic activity for brain neurons and its identification as biglycan. Eur J Neurosci. 7:2341–2350. [DOI] [PubMed] [Google Scholar]
- Kappler J, Stichel CC, Gleichmann M, Gillen C, Junghans U, Kresse H, Muller HW.. 1998. Developmental regulation of decorin expression in postnatal rat brain. Brain Res. 793:328–332. [DOI] [PubMed] [Google Scholar]
- Karlsson NG, McGuckin MA.. 2012. O-Linked glycome and proteome of high-molecular-mass proteins in human ovarian cancer ascites: Identification of sulfation, disialic acid and O-linked fucose. Glycobiology. 22:918–929. [DOI] [PubMed] [Google Scholar]
- Kawabe K, Tateyama D, Toyoda H, Kawasaki N, Hashii N, Nakao H, Matsumoto S, Nonaka M, Matsumura H, Hirose Y et al. . 2013. A novel antibody for human induced pluripotent stem cells and embryonic stem cells recognizes a type of keratan sulfate lacking oversulfated structures. Glycobiology. 23:322–336. [DOI] [PubMed] [Google Scholar]
- Keiser HD, Diamond BA.. 1987. Monoclonal antibodies reactive with keratan sulfate-bearing tryptic fragments of bovine nasal cartilage proteoglycan. Connect Tissue Res. 16:131–141. [DOI] [PubMed] [Google Scholar]
- Kelley TW, Huntsman D, McNagny KM, Roskelley CD, Hsi ED.. 2005. Podocalyxin: a marker of blasts in acute leukemia. Am J Clin Pathol. 124:134–142. [DOI] [PubMed] [Google Scholar]
- Kershaw DB, Beck SG, Wharram BL, Wiggins JE, Goyal M, Thomas PE, Wiggins RC.. 1997. Molecular cloning and characterization of human podocalyxin-like protein. Orthologous relationship to rabbit PCLP1 and rat podocalyxin. J Biol Chem. 272:15708–15714. [DOI] [PubMed] [Google Scholar]
- Kiani C, Chen L, Wu YJ, Yee AJ, Yang BB.. 2002. Structure and function of aggrecan. Cell Res. 12:19–32. [DOI] [PubMed] [Google Scholar]
- Kikuchi A, Tomoyasu H, Kido I, Takahashi K, Tanaka A, Nonaka I, Iwakami N, Kamo I.. 2000. Haemopoietic biglycan produced by brain cells stimulates growth of microglial cells. J Neuroimmunol. 106:78–86. [DOI] [PubMed] [Google Scholar]
- Kikuchi Y, Yasumoto K, Meyer K.. 1987. Amino acid sequence of a peptide from keratan sulfate II-core protein linkage regions. J Biochem. 102:1519–1524. [DOI] [PubMed] [Google Scholar]
- Kinne RW, Fisher LW.. 1987. Keratan sulfate proteoglycan in rabbit compact bone is bone sialoprotein II. J Biol Chem. 262:10206–10211. [PubMed] [Google Scholar]
- Kiss JZ, Rougon G.. 1997. Cell biology of polysialic acid. Curr Opin Neurobiol. 7:640–646. [DOI] [PubMed] [Google Scholar]
- Knox S, Fosang AJ, Last K, Melrose J, Whitelock J.. 2005. Perlecan from human epithelial cells is a hybrid heparan/chondroitin/keratan sulfate proteoglycan. FEBS Lett. 579:5019–5023. [DOI] [PubMed] [Google Scholar]
- Kobe B, Deisenhofer J.. 1994. The leucine-rich repeat: A versatile binding motif. Trends Biochem Sci. 19:415–421. [DOI] [PubMed] [Google Scholar]
- Kojima N, Fenderson BA, Stroud MR, Goldberg RI, Habermann R, Toyokuni T, Hakomori S.. 1994. Further studies on cell adhesion based on Le(x)–Le(x) interaction, with new approaches: Embryoglycan aggregation of F9 teratocarcinoma cells, and adhesion of various tumour cells based on Le(x) expression. Glycoconj J. 11:238–248. [DOI] [PubMed] [Google Scholar]
- Koops A, Kappler J, Junghans U, Kuhn G, Kresse H, Muller HW.. 1996. Cultured astrocytes express biglycan, a chondroitin/dermatan sulfate proteoglycan supporting the survival of neocortical neurons. Brain Res Mol Brain Res. 41:65–73. [DOI] [PubMed] [Google Scholar]
- Krusius T, Finne J, Margolis RK, Margolis RU.. 1986. Identification of an O-glycosidic mannose-linked sialylated tetrasaccharide and keratan sulfate oligosaccharides in the chondroitin sulfate proteoglycan of brain. J Biol Chem. 261:8237–8242. [PubMed] [Google Scholar]
- Kumari D, Tiwari A, Choudhury M, Kumar A, Rao A, Dixit M.. 2016. A novel KERA mutation in a case of autosomal recessive cornea plana with primary angle-closure glaucoma. J Glaucoma. 25:e106–109. [DOI] [PubMed] [Google Scholar]
- Kusche-Gullberg M, Kjellen L.. 2003. Sulfotransferases in glycosaminoglycan biosynthesis. Curr Opin Struct Biol. 13:605–611. [DOI] [PubMed] [Google Scholar]
- Kwon SE, Chapman ER.. 2011. Synaptophysin regulates the kinetics of synaptic vesicle endocytosis in central neurons. Neuron. 70:847–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laitinen A, Bockelman C, Hagstrom J, Kokkola A, Fermer C, Nilsson O, Haglund C.. 2015. Podocalyxin as a prognostic marker in gastric cancer. PLoS One. 10:e0145079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lark MW, Bayne EK, Flanagan J, Harper CF, Hoerrner LA, Hutchinson NI, Singer II, Donatelli SA, Weidner JR, Williams HR et al. . 1997. Aggrecan degradation in human cartilage. Evidence for both matrix metalloproteinase and aggrecanase activity in normal, osteoarthritic, and rheumatoid joints. J Clin Invest. 100:93–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson AH, Lehn S, Wangefjord S, Karnevi E, Kuteeva E, Sundstrom M, Nodin B, Uhlen M, Eberhard J, Birgisson H et al. . 2016. Significant association and synergistic adverse prognostic effect of podocalyxin-like protein and epidermal growth factor receptor expression in colorectal cancer. J Transl Med. 14:128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson AH, Nodin B, Syk I, Palmquist I, Uhlen M, Eberhard J, Jirstrom K.. 2013. Podocalyxin-like protein expression in primary colorectal cancer and synchronous lymph node metastases. Diagn Pathol. 8:109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauder RM, Huckerby TN, Nieduszynski IA.. 1996. The structure of the keratan sulphate chains attached to fibromodulin isolated from articular cartilage. Eur J Biochem. 242:402–409. [DOI] [PubMed] [Google Scholar]
- Lauder RM, Huckerby TN, Nieduszynski IA.. 1997. The structure of the keratan sulphate chains attached to fibromodulin from human articular cartilage. Glycoconj J. 14:651–660. [DOI] [PubMed] [Google Scholar]
- Lauder RM, Huckerby TN, Nieduszynski IA, Plaas AH.. 1998. Age-related changes in the structure of the keratan sulphate chains attached to fibromodulin isolated from articular cartilage. Biochem J. 330(Pt 2):753–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leroux JY, Guerassimov A, Cartman A, Delaunay N, Webber C, Rosenberg LC, Banerjee S, Poole AR.. 1996. Immunity to the G1 globular domain of the cartilage proteoglycan aggrecan can induce inflammatory erosive polyarthritis and spondylitis in BALB/c mice but immunity to G1 is inhibited by covalently bound keratan sulfate in vitro and in vivo. J Clin Invest. 97:621–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Jalili RB, Ghahary A.. 2016. Accelerating skin wound healing by M-CSF through generating SSEA-1 and -3 stem cells in the injured sites. Sci Rep. 6:28979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liles M, Palka BP, Harris A, Kerr B, Hughes C, Young RD, Meek KM, Caterson B, Quantock AJ.. 2010. Differential relative sulfation of Keratan sulfate glycosaminoglycan in the chick cornea during embryonic development. Invest Ophthalmol Vis Sci. 51:1365–1372. [DOI] [PubMed] [Google Scholar]
- Lin CW, Sun MS, Liao MY, Chung CH, Chi YH, Chiou LT, Yu J, Lou KL, Wu HC.. 2014. a. Podocalyxin-like 1 promotes invadopodia formation and metastasis through activation of Rac1/Cdc42/cortactin signaling in breast cancer cells. Carcinogenesis. 35:2425–2435. [DOI] [PubMed] [Google Scholar]
- Lin CW, Sun MS, Wu HC.. 2014. b. Podocalyxin-like 1 is associated with tumor aggressiveness and metastatic gene expression in human oral squamous cell carcinoma. Int J Oncol. 45:710–718. [DOI] [PubMed] [Google Scholar]
- Lincoln J, Lange AW, Yutzey KE.. 2006. Hearts and bones: Shared regulatory mechanisms in heart valve, cartilage, tendon, and bone development. Dev Biol. 294:292–302. [DOI] [PubMed] [Google Scholar]
- Lindahl B, Eriksson L, Spillmann D, Caterson B, Lindahl U.. 1996. Selective loss of cerebral keratan sulfate in Alzheimer’s disease. J Biol Chem. 271:16991–16994. [DOI] [PubMed] [Google Scholar]
- Liskova P, Hysi PG, Williams D, Ainsworth JR, Shah S, de la Chapelle A, Tuft SJ, Bhattacharya SS.. 2007. Study of p.N247S KERA mutation in a British family with cornea plana. Mol Vis. 13:1339–1347. [PubMed] [Google Scholar]
- Liskova P, Veraitch B, Jirsova K, Filipec M, Neuwirth A, Ebenezer ND, Hysi PG, Hardcastle AJ, Tuft SJ, Bhattacharya SS.. 2008. Sequencing of the CHST6 gene in Czech macular corneal dystrophy patients supports the evidence of a founder mutation. Br J Ophthalmol. 92:265–267. [DOI] [PubMed] [Google Scholar]
- Liu T, Zeng X, Sun F, Hou H, Guan Y, Guo D, Ai H, Wang W, Zhang G.. 2017. EphB4 regulates self-renewal, proliferation and neuronal differentiation of human embryonic neural stem cells in vitro. Cell Physiol Biochem. 41:819–834. [DOI] [PubMed] [Google Scholar]
- Loscher W, Honack D, Rundfeldt C.. 1998. Antiepileptogenic effects of the novel anticonvulsant levetiracetam (ucb L059) in the kindling model of temporal lobe epilepsy. J Pharmacol Exp Ther. 284:474–479. [PubMed] [Google Scholar]
- Lu YP, Ishiwata T, Kawahara K, Watanabe M, Naito Z, Moriyama Y, Sugisaki Y, Asano G.. 2002. Expression of lumican in human colorectal cancer cells. Pathol Int. 52:519–526. [DOI] [PubMed] [Google Scholar]
- Magro G, Perissinotto D, Schiappacassi M, Goletz S, Otto A, Muller EC, Bisceglia M, Brown G, Ellis T, Grasso S et al. . 2003. Proteomic and postproteomic characterization of keratan sulfate-glycanated isoforms of thyroglobulin and transferrin uniquely elaborated by papillary thyroid carcinomas. Am J Pathol. 163:183–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makanga JO, Kobayashi M, Ikeda H, Christianto A, Toyoda H, Yamada M, Kawasaki T, Inazu T.. 2015. Generation of rat induced pluripotent stem cells using a plasmid vector and possible application of a keratan sulfate glycan recognizing antibody in discriminating teratoma formation phenotypes. Biol Pharm Bull. 38:127–133. [DOI] [PubMed] [Google Scholar]
- Margineanu DG, Matagne A, Kaminski RM, Klitgaard H.. 2008. Effects of chronic treatment with levetiracetam on hippocampal field responses after pilocarpine-induced status epilepticus in rats. Brain Res Bull. 77:282–285. [DOI] [PubMed] [Google Scholar]
- Masubuchi M, Miura S, Yosizawa Z.. 1975. Glycosaminoglycans and glycoproteins isolated from rabbit femur. J Biochem. 77:617–626. [DOI] [PubMed] [Google Scholar]
- Matsuda Y, Yamamoto T, Kudo M, Kawahara K, Kawamoto M, Nakajima Y, Koizumi K, Nakazawa N, Ishiwata T, Naito Z.. 2008. Expression and roles of lumican in lung adenocarcinoma and squamous cell carcinoma. Int J Oncol. 33:1177–1185. [PubMed] [Google Scholar]
- Mehmet H, Scudder P, Tang PW, Hounsell EF, Caterson B, Feizi T.. 1986. The antigenic determinants recognized by three monoclonal antibodies to keratan sulphate involve sulphated hepta- or larger oligosaccharides of the poly(N-acetyllactosamine) series. Eur J Biochem. 157:385–391. [DOI] [PubMed] [Google Scholar]
- Melrose J. 2016. The Glycosaminoglycan/glycan Interactome: A Bioinformatics Platform. An Evolutionary Conserved Biosensor Platform Controlling Cell Behaviour, Tissue Morphogenesis and Tissue Assembly. Saarbrucken: Scholars Press, Omniscriptum GmbH and Co KG. [Google Scholar]
- Melrose J. 2017. Glycans Provide Molecular Recognition Motifs which Regulate Endoplasmic Protein Folding, Transport, Lysosomal Targeting, and are used by Pattern Recognition Receptors in Pathogen Surveyance and Innate Immunity. Glycosaminoglycans (GAGs): Biosynthesis, Functions and Clinical Significance. New York: NOVA Pubs. [Google Scholar]
- Melrose J, Ghosh P.. 1988. The quantitative discrimination of corneal type I, but not skeletal type II, keratan sulfate in glycosaminoglycan mixtures by using a combination of dimethylmethylene blue and endo-beta-d-galactosidase digestion. Anal Biochem. 170:293–300. [DOI] [PubMed] [Google Scholar]
- Melrose J, Little CB, Ghosh P.. 1998. Detection of aggregatable proteoglycan populations by affinity blotting using biotinylated hyaluronan. Anal Biochem. 256:149–157. [DOI] [PubMed] [Google Scholar]
- Melrose J, Smith S, Ghosh P.. 2000. Differential expression of proteoglycan epitopes by ovine intervertebral disc cells. J Anat. 197(Pt 2):189–198. [DOI] [PubMed] [Google Scholar]
- Meng Q, Ying Z, Noble E, Zhao Y, Agrawal R, Mikhail A, Zhuang Y, Tyagi E, Zhang Q, Lee JH et al. . 2016. Systems nutrigenomics reveals brain gene networks linking metabolic and brain disorders. EBioMedicine. 7:157–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merline R, Schaefer RM, Schaefer L.. 2009. The matricellular functions of small leucine-rich proteoglycans (SLRPs). J Cell Commun Signal. 3:323–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer K, Bhavanandan VP, Yung D, Lee LT, Howe C.. 1967. The keratosulfate-like mucopolysaccharide of chick allantoic fluid. Proc Natl Acad Sci USA. 58:1655–1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer K, Linker A, Davidson EA, Weissmann B.. 1953. The mucopolysaccharides of bovine cornea. J Biol Chem. 205:611–616. [PubMed] [Google Scholar]
- Meyer-Puttlitz B, Milev P, Junker E, Zimmer I, Margolis RU, Margolis RK.. 1995. Chondroitin sulfate and chondroitin/keratan sulfate proteoglycans of nervous tissue: Developmental changes of neurocan and phosphacan. J Neurochem. 65:2327–2337. [DOI] [PubMed] [Google Scholar]
- Miettinen A, Solin ML, Reivinen J, Juvonen E, Vaisanen R, Holthofer H.. 1999. Podocalyxin in rat platelets and megakaryocytes. Am J Pathol. 154:813–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morise J, Kizuka Y, Yabuno K, Tonoyama Y, Hashii N, Kawasaki N, Manya H, Miyagoe-Suzuki Y, Takeda S, Endo T et al. . 2014. Structural and biochemical characterization of O-mannose-linked human natural killer-1 glycan expressed on phosphacan in developing mouse brains. Glycobiology. 24:314–324. [DOI] [PubMed] [Google Scholar]
- Mort JS, Magny MC, Lee ER.. 1998. Cathepsin B: An alternative protease for the generation of an aggrecan ‘metalloproteinase’ cleavage neoepitope. Biochem J. 335(Pt 3):491–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muramatsu T. 2017. Embryoglycan: A highly branched poly-N-acetyllactosamine in pluripotent stem cells and early embryonic cells. Glycoconj J. 34:701–712. [DOI] [PubMed] [Google Scholar]
- Muramatsu T, Muramatsu H.. 2004. Carbohydrate antigens expressed on stem cells and early embryonic cells. Glycoconj J. 21:41–45. [DOI] [PubMed] [Google Scholar]
- Naito Z, Ishiwata T, Kurban G, Teduka K, Kawamoto Y, Kawahara K, Sugisaki Y.. 2002. Expression and accumulation of lumican protein in uterine cervical cancer cells at the periphery of cancer nests. Int J Oncol. 20:943–948. [PubMed] [Google Scholar]
- Nakamura H, Hirata A, Tsuji T, Yamamoto T.. 2001. Immunolocalization of keratan sulfate proteoglycan in rat calvaria. Arch Histol Cytol. 64:109–118. [DOI] [PubMed] [Google Scholar]
- Nakano M, Yonezawa N, Hatanaka Y, Noguchi S.. 1996. Structure and function of the N-linked carbohydrate chains of pig zona pellucida glycoproteins. J Reprod Fertil Suppl. 50:25–34. [PubMed] [Google Scholar]
- Nakao H, Nagai Y, Kojima A, Toyoda H, Kawasaki N, Kawasaki T.. 2017. Binding specificity of R-10G and TRA-1-60/81, and substrate specificity of keratanase II studied with chemically synthesized oligosaccharides. Glycoconj J. 34:789–795. [DOI] [PubMed] [Google Scholar]
- Natunen S, Satomaa T, Pitkanen V, Salo H, Mikkola M, Natunen J, Otonkoski T, Valmu L.. 2011. The binding specificity of the marker antibodies Tra-1-60 and Tra-1-81 reveals a novel pluripotency-associated type 1 lactosamine epitope. Glycobiology. 21:1125–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen-Ba-Charvet KT, Chedotal A.. 2002. Role of slit proteins in the vertebrate brain. J Physiol Paris. 96:91–98. [DOI] [PubMed] [Google Scholar]
- Nieduszynski IA, Huckerby TN, Dickenson JM, Brown GM, Tai GH, Morris HG, Eady S.. 1990. There are two major types of skeletal keratan sulphates. Biochem J. 271:243–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen JS, McNagny KM.. 2008. Novel functions of the CD34 family. J Cell Sci. 121:3683–3692. [DOI] [PubMed] [Google Scholar]
- Nielsen JS, McNagny KM.. 2009. The role of podocalyxin in health and disease. J Am Soc Nephrol. 20:1669–1676. [DOI] [PubMed] [Google Scholar]
- Niespodziany I, Klitgaard H, Margineanu DG.. 2001. Levetiracetam inhibits the high-voltage-activated Ca(2+) current in pyramidal neurones of rat hippocampal slices. Neurosci Lett. 306:5–8. [DOI] [PubMed] [Google Scholar]
- Nikdin H, Olsson ML, Hultenby K, Sugars RV.. 2012. Osteoadherin accumulates in the predentin towards the mineralization front in the developing tooth. PLoS One. 7:e31525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikitovic D, Papoutsidakis A, Karamanos NK, Tzanakakis GN.. 2014. Lumican affects tumor cell functions, tumor–ECM interactions, angiogenesis and inflammatory response. Matrix Biol. 35:206–214. [DOI] [PubMed] [Google Scholar]
- Noguchi S, Nakano M.. 1992. Structure of the acidic N-linked carbohydrate chains of the 55-kDa glycoprotein family (PZP3) from porcine zona pellucida. Eur J Biochem. 209:883–894. [DOI] [PubMed] [Google Scholar]
- Nowack A, Yao J, Custer KL, Bajjalieh SM.. 2010. SV2 regulates neurotransmitter release via multiple mechanisms. Am J Physiol Cell Physiol. 299:C960–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oegema TR Jr., Hascall VC, Dziewiatkowski DD.. 1975. Isolation and characterization of proteoglycans from the swarm rat chondrosarcoma. J Biol Chem. 250:6151–6159. [PubMed] [Google Scholar]
- Ohtsubo K, Marth JD.. 2006. Glycosylation in cellular mechanisms of health and disease. Cell. 126:855–867. [DOI] [PubMed] [Google Scholar]
- Okumura M, Fujinaga T.. 1998. Establishment of a monoclonal antibody (1/14/16H9) for detection of equine keratan sulfate. Am J Vet Res. 59:1203–1208. [PubMed] [Google Scholar]
- Okumura M, Tagami M, Fujinaga T.. 2000. Consideration of the role of antigenic keratan sulphate reacting to a 1/14/16H9 antibody as a molecular marker to monitor cartilage metabolism in horses. J Vet Med Sci. 62:281–285. [DOI] [PubMed] [Google Scholar]
- Olczyk K. 1993. Age-related changes in glycosaminoglycans of human intervertebral discs. Folia Histochem Cytobiol. 31:215–220. [PubMed] [Google Scholar]
- Olczyk K. 1994. Age-related changes in proteoglycans of human intervertebral discs. Z Rheumatol. 53:19–25. [PubMed] [Google Scholar]
- Oldberg A, Antonsson P, Lindblom K, Heinegard D.. 1989. A collagen-binding 59-kDa protein (fibromodulin) is structurally related to the small interstitial proteoglycans PG-S1 and PG-S2 (decorin). EMBO J. 8:2601–2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldberg A, Ruoslahti E.. 1982. Interactions between chondroitin sulfate proteoglycan, fibronectin, and collagen. J Biol Chem. 257:4859–4863. [PubMed] [Google Scholar]
- Olsson L, Stigson M, Perris R, Sorrell JM, Lofberg J.. 1996. Distribution of keratan sulphate and chondroitin sulphate in wild type and white mutant axolotl embryos during neural crest cell migration. Pigment Cell Res. 9:5–17. [DOI] [PubMed] [Google Scholar]
- Orlando RA, Takeda T, Zak B, Schmieder S, Benoit VM, McQuistan T, Furthmayr H, Farquhar MG.. 2001. The glomerular epithelial cell anti-adhesin podocalyxin associates with the actin cytoskeleton through interactions with ezrin. J Am Soc Nephrol. 12:1589–1598. [DOI] [PubMed] [Google Scholar]
- Osawa A, Kato M, Matsumoto E, Iwase K, Sugimoto T, Matsui T, Ishikura H, Sugano S, Kurosawa H, Takiguchi M et al. . 2006. Activation of genes for growth factor and cytokine pathways late in chondrogenic differentiation of ATDC5 cells. Genomics. 88:52–64. [DOI] [PubMed] [Google Scholar]
- Ozawa M, Muramatsu T.. 1985. The glycoprotein-bound large carbohydrates from embryonal carcinoma cells carry receptors for several lectins recognizing N-acetylgalactosamine and galactose. J Biochem. 97:317–324. [DOI] [PubMed] [Google Scholar]
- Ozawa M, Muramatsu T, Kemler R.. 1985. a. Molecular properties of ECMA 2 and ECMA 3 antigens defined by monoclonal antibodies against embryonal carcinoma cells. J Biochem. 97:307–315. [DOI] [PubMed] [Google Scholar]
- Ozawa M, Muramatsu T, Solter D.. 1985. b. SSEA-1, a stage-specific embryonic antigen of the mouse, is carried by the glycoprotein-bound large carbohydrate in embryonal carcinoma cells. Cell Differ. 16:169–173. [DOI] [PubMed] [Google Scholar]
- Papageorgakopoulou N, Theocharis AD, Skandalis SS, Vynios DH, Theocharis DA, Tsiganos CP.. 2002. Immunological studies of sheep brain keratan sulphate proteoglycans. Biochimie. 84:1225–1228. [DOI] [PubMed] [Google Scholar]
- Patel DA, Harocopos GJ, Chang SH, Vora SC, Lubniewski AJ, Huang AJ.. 2011. Novel CHST6 gene mutations in 2 unrelated cases of macular corneal dystrophy. Cornea. 30:664–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peal DS, Burns CG, Macrae CA, Milan D.. 2009. Chondroitin sulfate expression is required for cardiac atrioventricular canal formation. Dev Dyn. 238:3103–3110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pebay A, Wong RC, Pitson SM, Wolvetang EJ, Peh GS, Filipczyk A, Koh KL, Tellis I, Nguyen LT, Pera MF.. 2005. Essential roles of sphingosine-1-phosphate and platelet-derived growth factor in the maintenance of human embryonic stem cells. Stem Cells. 23:1541–1548. [DOI] [PubMed] [Google Scholar]
- Pera MF, Blasco-Lafita MJ, Cooper S, Mason M, Mills J, Monaghan P.. 1988. Analysis of cell-differentiation lineage in human teratomas using new monoclonal antibodies to cytostructural antigens of embryonal carcinoma cells. Differentiation. 39:139–149. [DOI] [PubMed] [Google Scholar]
- Pietraszek K, Brezillon S, Perreau C, Malicka-Blaszkiewicz M, Maquart FX, Wegrowski Y.. 2013. Lumican-derived peptides inhibit melanoma cell growth and migration. PLoS One. 8:e76232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plaas AH, Neame PJ, Nivens CM, Reiss L.. 1990. Identification of the keratan sulfate attachment sites on bovine fibromodulin. J Biol Chem. 265:20634–20640. [PubMed] [Google Scholar]
- Plaas AH, West LA, Midura RJ.. 2001. Keratan sulfate disaccharide composition determined by FACE analysis of keratanase II and endo-beta-galactosidase digestion products. Glycobiology. 11:779–790. [DOI] [PubMed] [Google Scholar]
- Poon CJ, Plaas AH, Keene DR, McQuillan DJ, Last K, Fosang AJ.. 2005. N-Linked keratan sulfate in the aggrecan interglobular domain potentiates aggrecanase activity. J Biol Chem. 280:23615–23621. [DOI] [PubMed] [Google Scholar]
- Pratta MA, Tortorella MD, Arner EC.. 2000. Age-related changes in aggrecan glycosylation affect cleavage by aggrecanase. J Biol Chem. 275:39096–39102. [DOI] [PubMed] [Google Scholar]
- Prydz K. 2015. Determinants of glycosaminoglycan (GAG) structure. Biomolecules. 5:2003–2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyle RA, Schivell AE, Hidaka H, Bajjalieh SM.. 2000. Phosphorylation of synaptic vesicle protein 2 modulates binding to synaptotagmin. J Biol Chem. 275:17195–17200. [DOI] [PubMed] [Google Scholar]
- Radwanska A, Baczynska D, Nowak D, Brezillon S, Popow A, Maquart FX, Wegrowski Y, Malicka-Blaszkiewicz M.. 2008. Lumican affects actin cytoskeletal organization in human melanoma A375 cells. Life Sci. 83:651–660. [DOI] [PubMed] [Google Scholar]
- Riccioni R, Calzolari A, Biffoni M, Senese M, Riti V, Petrucci E, Pasquini L, Cedrone M, Lo-Coco F, Diverio D et al. . 2006. Podocalyxin is expressed in normal and leukemic monocytes. Blood Cells Mol Dis. 37:218–225. [DOI] [PubMed] [Google Scholar]
- Robson JA, Geisert EE Jr.. 1994. Expression of a keratin sulfate proteoglycan during development of the dorsal lateral geniculate nucleus in the ferret. J Comp Neurol. 340:349–360. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Manzaneque JC, Westling J, Thai SN, Luque A, Knauper V, Murphy G, Sandy JD, Iruela-Arispe ML.. 2002. ADAMTS1 cleaves aggrecan at multiple sites and is differentially inhibited by metalloproteinase inhibitors. Biochem Biophys Res Commun. 293:501–508. [DOI] [PubMed] [Google Scholar]
- Romero-Jimenez M, Santodomingo-Rubido J, Wolffsohn JS.. 2010. Keratoconus: A review. Cont Lens Anterior Eye. 33:157–166; quiz 205. [DOI] [PubMed] [Google Scholar]
- Roos L, Bertelsen B, Harris P, Bygum A, Jensen H, Gronskov K, Tumer Z.. 2015. Case report: A novel KERA mutation associated with cornea plana and its predicted effect on protein function. BMC Med Genet. 16:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothberg JM, Jacobs JR, Goodman CS, Artavanis-Tsakonas S.. 1990. slit: An extracellular protein necessary for development of midline glia and commissural axon pathways contains both EGF and LRR domains. Genes Dev. 4:2169–2187. [DOI] [PubMed] [Google Scholar]
- Roughley PJ, Barnett J, Zuo F, Mort JS.. 2003. Variations in aggrecan structure modulate its susceptibility to aggrecanases. Biochem J. 375:183–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roughley PJ, Melching LI, Heathfield TF, Pearce RH, Mort JS.. 2006. The structure and degradation of aggrecan in human intervertebral disc. Eur Spine J. 15(Suppl 3):S326–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roughley PJ, Mort JS.. 2014. The role of aggrecan in normal and osteoarthritic cartilage. J Exp Orthop. 1:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruoslahti E, Engvall E.. 1980. Complexing of fibronectin glycosaminoglycans and collagen. Biochim Biophys Acta. 631:350–358. [DOI] [PubMed] [Google Scholar]
- Russo VC, Bach LA, Fosang AJ, Baker NL, Werther GA.. 1997. Insulin-like growth factor binding protein-2 binds to cell surface proteoglycans in the rat brain olfactory bulb. Endocrinology. 138:4858–4867. [DOI] [PubMed] [Google Scholar]
- Saphos CA, Dey P, Lark MW, Moore VL.. 1993. Sensitivity of monoclonal antibody, 5-D-4, for the detection of aggrecan, aggrecan fragments, and keratan sulfate. Agents Actions. 39(Spec No):C154–156. [DOI] [PubMed] [Google Scholar]
- Sassetti C, Tangemann K, Singer MS, Kershaw DB, Rosen SD.. 1998. Identification of podocalyxin-like protein as a high endothelial venule ligand for L-selectin: Parallels to CD34. J Exp Med. 187:1965–1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sassetti C, Van Zante A, Rosen SD.. 2000. Identification of endoglycan, a member of the CD34/podocalyxin family of sialomucins. J Biol Chem. 275:9001–9010. [DOI] [PubMed] [Google Scholar]
- Saukkonen K, Hagstrom J, Mustonen H, Juuti A, Nordling S, Fermer C, Nilsson O, Seppanen H, Haglund C.. 2015. Podocalyxin is a marker of poor prognosis in pancreatic ductal adenocarcinoma. PLoS One. 10:e0129012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer L, Iozzo RV.. 2008. Biological functions of the small leucine-rich proteoglycans: From genetics to signal transduction. J Biol Chem. 283:21305–21309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer IA, Sorrell JM.. 1993. Human keratinocytes contain keratin filaments that are glycosylated with keratan sulfate. Exp Cell Res. 207:213–219. [DOI] [PubMed] [Google Scholar]
- Schanbacher FL, Ebner KE.. 1970. Galactosyltransferase acceptor specificity of the lactose synthetase A protein. J Biol Chem. 245:5057–5061. [PubMed] [Google Scholar]
- Schonherr E, Hausser HJ.. 2000. Extracellular matrix and cytokines: A functional unit. Dev Immunol. 7:89–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schopperle WM, DeWolf WC.. 2007. The TRA-1-60 and TRA-1-81 human pluripotent stem cell markers are expressed on podocalyxin in embryonal carcinoma. Stem Cells. 25:723–730. [DOI] [PubMed] [Google Scholar]
- Schopperle WM, Kershaw DB, DeWolf WC.. 2003. Human embryonal carcinoma tumor antigen, Gp200/GCTM-2, is podocalyxin. Biochem Biophys Res Commun. 300:285–290. [DOI] [PubMed] [Google Scholar]
- Scott JE. 1996. Proteodermatan and proteokeratan sulfate (decorin, lumican/fibromodulin) proteins are horseshoe shaped. Implications for their interactions with collagen. Biochemistry. 35:8795–8799. [DOI] [PubMed] [Google Scholar]
- Scott JE, Bosworth TR, Cribb AM, Taylor JR.. 1994. The chemical morphology of age-related changes in human intervertebral disc glycosaminoglycans from cervical, thoracic and lumbar nucleus pulposus and annulus fibrosus. J Anat. 184(Pt 1):73–82. [PMC free article] [PubMed] [Google Scholar]
- Scranton TW, Iwata M, Carlson SS.. 1993. The SV2 protein of synaptic vesicles is a keratan sulfate proteoglycan. J Neurochem. 61:29–44. [DOI] [PubMed] [Google Scholar]
- Seko A, Dohmae N, Takio K, Yamashita K.. 2003. Beta 1,4-galactosyltransferase (beta 4GalT)-IV is specific for GlcNAc 6-O-sulfate. Beta 4GalT-IV acts on keratan sulfate-related glycans and a precursor glycan of 6-sulfosialyl-Lewis X. J Biol Chem. 278:9150–9158. [DOI] [PubMed] [Google Scholar]
- Seko A, Yamashita K.. 2004. beta1,3-N-Acetylglucosaminyltransferase-7 (beta3Gn-T7) acts efficiently on keratan sulfate-related glycans. FEBS Lett. 556:216–220. [DOI] [PubMed] [Google Scholar]
- Seno N, Meyer K, Anderson B, Hoffman P.. 1965. Variations in keratosulfates. J Biol Chem. 240:1005–1010. [PubMed] [Google Scholar]
- Seo H, Geisert EE Jr.. 1995. A keratan sulfate proteoglycan marks the boundaries in the cortical barrel fields of the adult rat. Neurosci Lett. 197:13–16. [DOI] [PubMed] [Google Scholar]
- Serra R, Chang C.. 2003. TGF-beta signaling in human skeletal and patterning disorders. Birth Defects Res C Embryo Today. 69:333–351. [DOI] [PubMed] [Google Scholar]
- Seya T, Tanaka N, Shinji S, Yokoi K, Koizumi M, Teranishi N, Yamashita K, Tajiri T, Ishiwata T, Naito Z.. 2006. Lumican expression in advanced colorectal cancer with nodal metastasis correlates with poor prognosis. Oncol Rep. 16:1225–1230. [PubMed] [Google Scholar]
- Shao H, Scott SG, Nakata C, Hamad AR, Chakravarti S.. 2013. Extracellular matrix protein lumican promotes clearance and resolution of Pseudomonas aeruginosa keratitis in a mouse model. PLoS One. 8:e54765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman HJ, Meyer K.. 1968. Cellular differentiation and the aging process in cartilaginous tissues. Mucopolysaccharide synthesis in cell cultures of chondrocytes. J Exp Med. 128:1353–1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinouris EA, Skandalis SS, Kilia V, Theocharis AD, Theocharis DA, Ravazoula P, Vynios DH, Papageorgakopoulou N.. 2009. Keratan sulfate-containing proteoglycans in sheep brain with particular reference to phosphacan and synaptic vesicle proteoglycan isoforms. Biomed Chromatogr. 23:455–463. [DOI] [PubMed] [Google Scholar]
- Sizemore S, Cicek M, Sizemore N, Ng KP, Casey G.. 2007. Podocalyxin increases the aggressive phenotype of breast and prostate cancer cells in vitro through its interaction with ezrin. Cancer Res. 67:6183–6191. [DOI] [PubMed] [Google Scholar]
- Smith RA, Seif MW, Rogers AW, Li TC, Dockery P, Cooke ID, Aplin JD.. 1989. The endometrial cycle: The expression of a secretory component correlated with the luteinizing hormone peak. Hum Reprod. 4:236–242. [DOI] [PubMed] [Google Scholar]
- Snow AD, Mar H, Nochlin D, Kresse H, Wight TN.. 1992. Peripheral distribution of dermatan sulfate proteoglycans (decorin) in amyloid-containing plaques and their presence in neurofibrillary tangles of Alzheimer’s disease. J Histochem Cytochem. 40:105–113. [DOI] [PubMed] [Google Scholar]
- Snow AD, Nochlin D, Sekiguichi R, Carlson SS.. 1996. Identification in immunolocalization of a new class of proteoglycan (keratan sulfate) to the neuritic plaques of Alzheimer’s disease. Exp Neurol. 138:305–317. [DOI] [PubMed] [Google Scholar]
- Snyder KA, Hughes MR, Hedberg B, Brandon J, Hernaez DC, Bergqvist P, Cruz F, Po K, Graves ML, Turvey ME et al. . 2015. Podocalyxin enhances breast tumor growth and metastasis and is a target for monoclonal antibody therapy. Breast Cancer Res. 17:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommarin Y, Wendel M, Shen Z, Hellman U, Heinegard D.. 1998. Osteoadherin, a cell-binding keratan sulfate proteoglycan in bone, belongs to the family of leucine-rich repeat proteins of the extracellular matrix. J Biol Chem. 273:16723–16729. [DOI] [PubMed] [Google Scholar]
- Son MJ, Woolard K, Nam DH, Lee J, Fine HA.. 2009. SSEA-1 is an enrichment marker for tumor-initiating cells in human glioblastoma. Cell Stem Cell. 4:440–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song SH, Augustine GJ.. 2015. Synapsin isoforms and synaptic vesicle trafficking. Mol Cells. 38:936–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein E, Tessier-Lavigne M.. 2001. Hierarchical organization of guidance receptors: Silencing of netrin attraction by slit through a Robo/DCC receptor complex. Science. 291:1928–1938. [DOI] [PubMed] [Google Scholar]
- Stichel CC, Kappler J, Junghans U, Koops A, Kresse H, Muller HW.. 1995. Differential expression of the small chondroitin/dermatan sulfate proteoglycans decorin and biglycan after injury of the adult rat brain. Brain Res. 704:263–274. [DOI] [PubMed] [Google Scholar]
- Struglics A, Larsson S, Lohmander LS.. 2006. a. Estimation of the identity of proteolytic aggrecan fragments using PAGE migration and Western immunoblot. Osteoarthritis Cartilage. 14:898–905. [DOI] [PubMed] [Google Scholar]
- Struglics A, Larsson S, Pratta MA, Kumar S, Lark MW, Lohmander LS.. 2006. b. Human osteoarthritis synovial fluid and joint cartilage contain both aggrecanase- and matrix metalloproteinase-generated aggrecan fragments. Osteoarthritis Cartilage. 14:101–113. [DOI] [PubMed] [Google Scholar]
- Sultana A, Klintworth GK, Thonar EJ, Vemuganti GK, Kannabiran C.. 2009. Immunophenotypes of macular corneal dystrophy in India and correlation with mutations in CHST6. Mol Vis. 15:319–325. [PMC free article] [PubMed] [Google Scholar]
- SundarRaj N, Willson J, Gregory JD, Damle SP.. 1985. Monoclonal antibodies to proteokeratan sulfate of rabbit corneal stroma. Curr Eye Res. 4:49–54. [DOI] [PubMed] [Google Scholar]
- Suzuki M. 1939. Biochemical studies on carbohydrates. I prosthetic group of cornea mucoid. J Biochemistry. 30:185–191. [Google Scholar]
- Sztrolovics R, Alini M, Mort JS, Roughley PJ.. 1999. Age-related changes in fibromodulin and lumican in human intervertebral discs. Spine (Phila Pa 1976). 24:1765–1771. [DOI] [PubMed] [Google Scholar]
- Sztrolovics R, Grover J, Cs-Szabo G, Shi SL, Zhang Y, Mort JS, Roughley PJ.. 2002. The characterization of versican and its message in human articular cartilage and intervertebral disc. J Orthop Res. 20:257–266. [DOI] [PubMed] [Google Scholar]
- Tai GH, Brown GM, Morris HG, Huckerby TN, Nieduszynski IA.. 1991. Fucose content of keratan sulphates from bovine articular cartilage. Biochem J. 273(Pt 2):307–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai GH, Huckerby TN, Nieduszynski IA.. 1993. N.m.r. spectroscopic studies of fucose-containing oligosaccharides derived from keratanase digestion of articular cartilage keratan sulphatesInfluence of fucose residues on keratanase cleavage. Biochem J. 291(Pt 3):889–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai GH, Huckerby TN, Nieduszynski IA.. 1994. 600 MHz 1H NMR study of a fucose-containing heptasaccharide derived from a keratanase digestion of bovine articular cartilage keratan sulphate. Carbohydr Res. 255:303–309. [DOI] [PubMed] [Google Scholar]
- Tai GH, Huckerby TN, Nieduszynski IA.. 1996. Multiple non-reducing chain termini isolated from bovine corneal keratan sulfates. J Biol Chem. 271:23535–23546. [DOI] [PubMed] [Google Scholar]
- Tai GH, Nieduszynski IA, Fullwood NJ, Huckerby TN.. 1997. Human corneal keratan sulfates. J Biol Chem. 272:28227–28231. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Stamenkovic I, Cutler M, Dasgupta A, Tanabe KK.. 1996. Keratan sulfate modification of CD44 modulates adhesion to hyaluronate. J Biol Chem. 271:9490–9496. [DOI] [PubMed] [Google Scholar]
- Tang PW, Scudder P, Mehmet H, Hounsell EF, Feizi T.. 1986. Sulphate groups are involved in the antigenicity of keratan sulphate and mask i antigen expression on their poly-N-acetyllactosamine backbones. An immunochemical and chromatographic study of keratan sulphate oligosaccharides after desulfation or nitrosation. Eur J Biochem. 160:537–545. [DOI] [PubMed] [Google Scholar]
- Tasheva ES, Koester A, Paulsen AQ, Garrett AS, Boyle DL, Davidson HJ, Song M, Fox N, Conrad GW.. 2002. Mimecan/osteoglycin-deficient mice have collagen fibril abnormalities. Mol Vis. 8:407–415. [PubMed] [Google Scholar]
- Tessier-Lavigne M, Goodman CS.. 1996. The molecular biology of axon guidance. Science. 274:1123–1133. [DOI] [PubMed] [Google Scholar]
- Tokudome K, Okumura T, Shimizu S, Mashimo T, Takizawa A, Serikawa T, Terada R, Ishihara S, Kunisawa N, Sasa M et al. . 2016. Synaptic vesicle glycoprotein 2A (SV2A) regulates kindling epileptogenesis via GABAergic neurotransmission. Sci Rep. 6:27420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tortorella M, Pratta M, Liu RQ, Abbaszade I, Ross H, Burn T, Arner E.. 2000. The thrombospondin motif of aggrecanase-1 (ADAMTS-4) is critical for aggrecan substrate recognition and cleavage. J Biol Chem. 275:25791–25797. [DOI] [PubMed] [Google Scholar]
- Tortorella MD, Liu RQ, Burn T, Newton RC, Arner E.. 2002. Characterization of human aggrecanase 2 (ADAM-TS5): substrate specificity studies and comparison with aggrecanase 1 (ADAM-TS4). Matrix Biol. 21:499–511. [DOI] [PubMed] [Google Scholar]
- Toyoda H, Nagai Y, Kojima A, Kinoshita-Toyoda A.. 2017. Podocalyxin as a major pluripotent marker and novel keratan sulfate proteoglycan in human embryonic and induced pluripotent stem cells. Glycoconj J. 34:817–823. [DOI] [PubMed] [Google Scholar]
- Uchimura K, Muramatsu H, Kadomatsu K, Fan QW, Kurosawa N, Mitsuoka C, Kannagi R, Habuchi O, Muramatsu T.. 1998. Molecular cloning and characterization of an N-acetylglucosamine-6-O-sulfotransferase. J Biol Chem. 273:22577–22583. [DOI] [PubMed] [Google Scholar]
- Valtorta F, Pozzi D, Benfenati F, Fornasiero EF.. 2011. The synapsins: Multitask modulators of neuronal development. Semin Cell Dev Biol. 22:378–386. [DOI] [PubMed] [Google Scholar]
- van Meurs JB, van Lent PL, Singer II, Bayne EK, van de Loo FA, van den Berg WB.. 1998. Interleukin-1 receptor antagonist prevents expression of the metalloproteinase-generated neoepitope VDIPEN in antigen-induced arthritis. Arthritis Rheum. 41:647–656. [DOI] [PubMed] [Google Scholar]
- van Meurs JB, van Lent PL, van de Loo AA, Holthuysen AE, Bayne EK, Singer II, van den Berg WB. 1999. Increased vulnerability of postarthritic cartilage to a second arthritic insult: Accelerated MMP activity in a flare up of arthritis. Ann Rheum Dis. 58:350–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venn G, Mason RM.. 1985. Absence of keratan sulphate from skeletal tissues of mouse and rat. Biochem J. 228:443–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virgintino D, Perissinotto D, Girolamo F, Mucignat MT, Montanini L, Errede M, Kaneiwa T, Yamada S, Sugahara K, Roncali L et al. . 2009. Differential distribution of aggrecan isoforms in perineuronal nets of the human cerebral cortex. J Cell Mol Med. 13:3151–3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitureira N, Andres R, Perez-Martinez E, Martinez A, Bribian A, Blasi J, Chelliah S, Lopez-Domenech G, De Castro F, Burgaya F et al. . 2010. Podocalyxin is a novel polysialylated neural adhesion protein with multiple roles in neural development and synapse formation. PLoS One. 5:e12003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitureira N, McNagny K, Soriano E, Burgaya F.. 2005. Pattern of expression of the podocalyxin gene in the mouse brain during development. Gene Expr Patterns. 5:349–354. [DOI] [PubMed] [Google Scholar]
- Vyas KA, Patel HV, Vyas AA, Wu W.. 1998. Glycosaminoglycans bind to homologous cardiotoxins with different specificity. Biochemistry. 37:4527–4534. [DOI] [PubMed] [Google Scholar]
- Wan M, Cao X.. 2005. BMP signaling in skeletal development. Biochem Biophys Res Commun. 328:651–657. [DOI] [PubMed] [Google Scholar]
- Wan QF, Zhou ZY, Thakur P, Vila A, Sherry DM, Janz R, Heidelberger R.. 2010. SV2 acts via presynaptic calcium to regulate neurotransmitter release. Neuron. 66:884–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Zhao Y, Qi R, Zhu X, Huang C, Cheng S, Wang S, Qi X.. 2017. Prognostic role of podocalyxin-like protein expression in various cancers: A systematic review and meta-analysis. Oncotarget. 8:52457–52464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei T, Gong J, Jamitzky F, Heckl WM, Stark RW, Rossle SC.. 2008. LRRML: A conformational database and an XML description of leucine-rich repeats (LRRs). BMC Struct Biol. 8:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendel M, Sommarin Y, Heinegard D.. 1998. Bone matrix proteins: Isolation and characterization of a novel cell-binding keratan sulfate proteoglycan (osteoadherin) from bovine bone. J Cell Biol. 141:839–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westling J, Fosang AJ, Last K, Thompson VP, Tomkinson KN, Hebert T, McDonagh T, Collins-Racie LA, LaVallie ER, Morris EA et al. . 2002. ADAMTS4 cleaves at the aggrecanase site (Glu373-Ala374) and secondarily at the matrix metalloproteinase site (Asn341-Phe342) in the aggrecan interglobular domain. J Biol Chem. 277:16059–16066. [DOI] [PubMed] [Google Scholar]
- Weyers A, Yang B, Solakyildirim K, Yee V, Li L, Zhang F, Linhardt RJ.. 2013. Isolation of bovine corneal keratan sulfate and its growth factor and morphogen binding. FEBS J. 280:2285–2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitham KM, Hadley JL, Morris HG, Andrew SM, Nieduszynski IA, Brown GM.. 1999. An improved method for the structural profiling of keratan sulfates: Analysis of keratan sulfates from brain and ovarian tumors. Glycobiology. 9:285–291. [DOI] [PubMed] [Google Scholar]
- Winther M, Walmod PS.. 2014. Neural cell adhesion molecules belonging to the family of leucine-rich repeat proteins. Adv Neurobiol. 8:315–395. [DOI] [PubMed] [Google Scholar]
- Wong K, Park HT, Wu JY, Rao Y.. 2002. Slit proteins: Molecular guidance cues for cells ranging from neurons to leukocytes. Curr Opin Genet Dev. 12:583–591. [DOI] [PubMed] [Google Scholar]
- Wu F, Vij N, Roberts L, Lopez-Briones S, Joyce S, Chakravarti S.. 2007. A novel role of the lumican core protein in bacterial lipopolysaccharide-induced innate immune response. J Biol Chem. 282:26409–26417. [DOI] [PubMed] [Google Scholar]
- Yasuoka H, Tsujimoto M, Hirokawa M, Tori M, Nakahara M, Miyauchi A, Kodama R, Sanke T, Nakamura Y.. 2008. Podocalyxin expression in undifferentiated thyroid carcinomas. J Clin Pathol. 61:1228–1229. [DOI] [PubMed] [Google Scholar]
- Ye F, Hu Y, Zhou C, Chen H.. 2012. Podocalyxin-like protein 1 expression and correlation with clinical characteristics in epithelial serous and mucinous ovarian carcinoma and tumor-like lesions. Pathobiology. 79:307–313. [DOI] [PubMed] [Google Scholar]
- Yonezawa N, Aoki H, Hatanaka Y, Nakano M.. 1995. Involvement of N-linked carbohydrate chains of pig zona pellucida in sperm–egg binding. Eur J Biochem. 233:35–41. [DOI] [PubMed] [Google Scholar]
- Yoon JH, Brooks R, Halper J.. 2002. Immunoblotting assays for keratan sulfate. Anal Biochem. 306:298–304. [DOI] [PubMed] [Google Scholar]
- Yoshida-Noro C, Heasman J, Goldstone K, Vickers L, Wylie C.. 1999. Expression of the Lewis group carbohydrate antigens during Xenopus development. Glycobiology. 9:1323–1330. [DOI] [PubMed] [Google Scholar]
- Young RD, Akama TO, Liskova P, Ebenezer ND, Allan B, Kerr B, Caterson B, Fukuda MN, Quantock AJ.. 2007. a. Differential immunogold localisation of sulphated and unsulphated keratan sulphate proteoglycans in normal and macular dystrophy cornea using sulfation motif-specific antibodies. Histochem Cell Biol. 127:115–120. [DOI] [PubMed] [Google Scholar]
- Young RD, Gealy EC, Liles M, Caterson B, Ralphs JR, Quantock AJ.. 2007. b. Keratan sulfate glycosaminoglycan and the association with collagen fibrils in rudimentary lamellae in the developing avian cornea. Invest Ophthalmol Vis Sci. 48:3083–3088. [DOI] [PubMed] [Google Scholar]
- Yuan W, Zhou L, Chen JH, Wu JY, Rao Y, Ornitz DM.. 1999. The mouse SLIT family: Secreted ligands for ROBO expressed in patterns that suggest a role in morphogenesis and axon guidance. Dev Biol. 212:290–306. [DOI] [PubMed] [Google Scholar]
- Zanetti M, Ratcliffe A, Watt FM.. 1985. Two subpopulations of differentiated chondrocytes identified with a monoclonal antibody to keratan sulfate. J Cell Biol. 101:53–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanin MK, Bundy J, Ernst H, Wessels A, Conway SJ, Hoffman S.. 1999. Distinct spatial and temporal distributions of aggrecan and versican in the embryonic chick heart. Anat Rec. 256:366–380. [DOI] [PubMed] [Google Scholar]
- Zeltz C, Brezillon S, Perreau C, Ramont L, Maquart FX, Wegrowski Y.. 2009. Lumcorin: A leucine-rich repeat 9-derived peptide from human lumican inhibiting melanoma cell migration. FEBS Lett. 583:3027–3032. [DOI] [PubMed] [Google Scholar]
- Zhang X, Ziran N, Goater JJ, Schwarz EM, Puzas JE, Rosier RN, Zuscik M, Drissi H, O’Keefe RJ.. 2004. Primary murine limb bud mesenchymal cells in long-term culture complete chondrocyte differentiation: TGF-beta delays hypertrophy and PGE2 inhibits terminal differentiation. Bone. 34:809–817. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Takeda-Uchimura Y, Foyez T, Ohtake-Niimi S, Narentuya, Akatsu H, Nishitsuji K, Michikawa M, Wyss-Coray T, Kadomatsu K et al. . 2017. Deficiency of a sulfotransferase for sialic acid-modified glycans mitigates Alzheimer’s pathology. Proc Natl Acad Sci USA. 114:E2947–E2954. [DOI] [PMC free article] [PubMed] [Google Scholar]