Abstract
The myxoma virus (a microparasite) reduced wild rabbit numbers worldwide when introduced in the 1950s, and is known to interact with co-infecting helminths (macroparasites) causing both increases and decreases in macroparasite population size. In the 1990s Rabbit Haemorrhagic Disease Virus (RHDV) infected rabbits and also significantly reduced rabbit numbers in several countries. However, not much is known about RHDV interactions with macroparasites. In this study, we compare prevalence and intensity of infection for three gastrointestinal nematode species (Trichostrongylus retortaeformis, Graphidium strigosum and Passalurus ambiguus) before and after RHDV spread across host populations in Scotland and New Zealand. During one common season, autumn, prevalence of T. retortaeformis was higher after RHDV spread in both locations, whereas it was lower for G. strigosum and P. ambiguus after RHDV arrived in New Zealand, but higher in Scotland. Meanwhile, intensity of infection for all species decreased after RHDV arrived in New Zealand, but increased in Scotland. The impact of RHDV on worm infections was generally similar across seasons in Scotland, and also similarities in seasonality between locations suggested effects on infection patterns in one season are likely similar year-round. The variable response by macroparasites to the arrival of a microparasite into Scottish and New Zealand rabbits may be due to differences in the environment they inhabit, in existing parasite community structure, and to some extent, in the relative magnitude of indirect effects. Specifically, our data suggest that bottom-up processes after the introduction of a more virulent strain of RHDV to New Zealand may affect macroparasite co-infections by reducing the availability of their shared common resource, the rabbits. Clearly, interactions between co-infecting micro- and macroparasites vary in host populations with different ecologies, and significantly impact parasite community structure in wildlife.
Keywords: Co-infection, Community ecology, European rabbit, Helminth, Macroparasite, Microparasite, RHDV, Virus, Within-host ecology
Graphical abstract
Highlights
-
•
Nematode communities in Scotland and New Zealand were compared pre and post Rabbit Haemorrhagic Disease Virus introduction.
-
•
Similar species occur in both rabbit populations, but prevalence and intensity changed in opposing directions after RHDV.
-
•
RHDV had a major impact on rabbit populations, and our data show differing impacts on macroparasites in the two countries.
-
•
Variability in rabbit environment, parasite community structure, and indirect interaction processes may explain differences.
-
•
Results can help understand interactions between co-infecting parasites and their epidemiology in wild and domestic animals.
1. Introduction
It is becoming increasingly clear that ecological rules and processes important to the structure and dynamics of species populations in communities of aquatic and terrestrial environments apply to parasite community structure, and the dynamics of co-infecting parasite populations living within earth's third environment, i.e. the living, interacting tissues within hosts (Sukhdeo, 1997; Pederson and Fenton, 2007; Graham, 2008; Griffiths et al., 2014). Co-infecting parasite species can interact directly while competing for physical space needed to mature and reproduce (Schaad, 1963), and indirectly through, either bottom-up processes that control species' abundance when competing for shared food resources, or top-down processes that control population size via host immune modulation or stimulation (Schaad, 1966; Lello et al., 2004; Pederson and Fenton, 2007; Graham, 2008). Although insight into interactions among co-infecting parasites of wildlife is growing, interactions among all types of parasites are not clear. For example, interactions between co-infecting micro- and macroparasites are not completely understood. In this study, we examine patterns of micro- and macroparasite infection in wild European rabbits (Oryctolagus cuniculus) to determine how macroparasites respond to the introduction of a similarly novel microparasite into host populations with distinct ecologies in Scotland and New Zealand.
Evidence for parasite interactions within hosts primarily comes from controlled laboratory experiments, experimental removal of species from natural within-host communities, and observational field studies. Laboratory co-infection experiments have shed light on the relative strength of parasite-parasite interactions, although hosts in nature are frequently infected with more than two parasite species (Cox, 2001; Lello et al., 2004; Pederson and Fenton, 2007; Graham, 2008). Manipulative field studies on wild host populations have measured how multispecies parasite communities respond to perturbations, such as the removal of a species, but are more difficult and rare (e.g. Ferrari et al., 2009; Knowles et al., 2013; Pedersen and Antonovics, 2013). More commonly, our understanding of within-host parasite community dynamics in wild populations comes from observational field studies (Lello et al., 2004; Telfer et al., 2008), although apparent within-host parasite interactions are thought to potentially be a consequence of statistical associations rather than true interspecific interactions (Fenton et al., 2010; Johnson and Buller, 2011). Nevertheless, observations from wild hosts are critical because they ultimately help guide a priori predictions testable through controlled experimentation in the lab or field (Graham, 2008). Thus far, a general pattern emerging from field and laboratory experiments, as well as observations of wild host populations, is that species-specific ecological differences can influence mechanisms, direction and interaction strength among co-infecting parasites (Knowles et al., 2013; Griffiths et al., 2014).
The European rabbit (Oryctolagus cuniculus) is considered a pest species in Australia, New Zealand and the United Kingdom, and has been a good model system to study within-host parasite community dynamics. This is partly because it can be easily harvested throughout the year in some countries, i.e. there is no closed season as it is a widely distributed and numerous small mammal (Flux and Fullagar, 1983). European rabbits can be infected by a wide variety of micro- and macroparasites, including helminths, protozoa and viruses (Taylor et al., 2015), and success in controlling rabbit populations by introducing microparasites (e.g. viruses) as biological control agents has had some impact on hosts and co-infecting macroparasites. For example, the introduction of the flea-borne myxoma virus (myxomatosis) causing cutaneous fibromas into Australia and the United Kingdom (Findlay, 1929; Fenner and Ratcliffe, 1965; Fenner and Ross, 1994), and of the faecal-oral transmitted Rabbit Haemorrhagic Disease Virus (RHDV) causing liver, spleen and lung necrosis into New Zealand, Australia and the United Kingdom (Cooke and Fenner, 2002; Abrantes et al., 2012; Cooke, 2014; Rouco et al., 2014), reduced rabbit populations by significant numbers. At the same time, introduction of myxomatosis led to larger populations of co-infecting intestinal macroparasites (Mykytowycz, 1959; Boag, 1988). This was initially suggested to be due to myxomatosis compromising host immunity (Boag, 1988), and evidence of it was subsequently confirmed experimentally (Cattadori et al., 2007; Berto-Moran et al., 2013). Some strains of the myxoma virus became attenuated over time (Kerr et al., 2012), and the hosts more resistant (Marshall and Fenner, 1958), resulting in rabbit numbers increasing significantly in Britain until the mid 1990s when RHDV became established in wild populations (Aebischer et al., 2011).
Unlike myxomatosis, there are few reports of possible interactions between RHDV and other micro- or macroparasites. The RHDV may have initially existed as a non-pathogenic strain in Scotland before the pathogenic strain was introduced in 1997 (Forrester et al., 2009). A single European strain was introduced to Australia in 1991, and phylogenetic evidence suggests a single importation event into New Zealand from Australia gave rise to all descendant viruses in New Zealand without any mixing with Australian strains (Eden et al., 2015). The presence of two strains has been detected in New Zealand (Forrester et al., 2003), and since the introduction of the pathogenic strain in 1997 reduced rabbit populations significantly (Thompson and Clark, 1997; Norburry et al., 2002), the virus is becoming less pathogenic allowing rabbit populations to gradually increase there (Parkes et al., 2008). RHDV can induce host acquired immunity (Parkes et al., 2008; Abrantes et al., 2012), and evidence from Australia indicates RHDV can influence the dynamics of co-infecting myxomatosis (Mutze et al., 2002), but whether macroparasites co-infecting rabbit populations responded similarly to RHDV as they did to myxomatosis, is not known. As such, the present epidemiological study draws on long-term field observations of wild rabbit populations from Scotland and New Zealand to investigate the potential impact that introducing RHDV has had on the population dynamics of co-infecting macroparasites.
The mechanism, direction and strength of interaction among species in parasite communities is expected to vary between Scottish and New Zealand rabbit populations due to ecological differences between regions, such as differences in community structure, in host density, or in environment possibly influencing infection variabilities. For example, the founder populations of European rabbits in New Zealand arrived with a depauperate macroparasite community structure (Bull, 1953; Rhodes, 1985). There is no myxomatosis in New Zealand rabbits, and the only parasites they have in common with rabbits from Scotland are three directly transmitted intestinal nematode macroparasites, Trichostrongylus retortaeformis in the small intestine, Graphidium strigosum in the stomach and Passalurus ambiguus in the large intestine (Mykytowycz, 1959; Boag, 1988). Rabbits in Scotland can be additionally co-infected with the trophically transmitted intestinal cestodes Cittotaenia denticulata, Mosgovoyia pectinata, Coenurus pisiformis and Cysiticercus serialis, as well as the liver fluke Fasciola hepatica, ectoparasites such as the flea Spilopyllus cuniculi, and the vector-borne blood protozoan Trypanosoma nabiasi (Boag, 1985; Hamilton et al., 2005). Not only have parasite communities in New Zealand experienced a bottleneck, but also rabbit numbers have previously crashed after a sustained poisoning campaign in the 1940s, which resulted in congruent decreases in nematode infection levels (Bull, 1957). Environment also differs between regions and this could have an impact on both hosts and parasites. For example, annual rainfall is lower in the inland south island of New Zealand studied (Robertson, 1959), than it is in the central east coast of Scotland (Barnett et al., 2006). Rainfall is positively correlated with the incidence of non-pathogenic RHDV (Liu et al., 2014), as well as biomass of grass for rabbits (Silvertown et al., 1994), and thus can potentially affect both parasite and host density. Analysis of summary networks for all possible human co-infections reported in a single year suggest that bottom-up processes, i.e. resource availability, are more involved in structuring parasite interactions than top-down processes, i.e. immune control (Griffiths et al., 2014). However, the relative importance of bottom-up or top-down processes in parasite communities is generally poorly understood (Graham, 2008), and it is not known whether they played an important role in macroparasite community structure after RHDV was introduced to wild rabbit populations in Scotland and New Zealand.
To assess what effects introducing a novel microparasite (RHDV) into rabbit populations in Scotland and New Zealand had on the three gastrointestinal nematode macroparasite species that they share in common (i.e. T. retortaeformis, G. strigosum, and P. ambiguus), we compared the (a) prevalence of infection with each nematode species, and (b) intensity of infection before and after the introduction of RHDV. We first asked whether seasonal prevalence and intensity of infection for each macroparasite species in New Zealand rabbits varied from that of rabbits sampled in congruent seasons in Scotland in order to discern whether regional environmental variability influenced parasite ecology before the arrival of RHDV. Seasonal differences in average macroparasite infection size in rabbits have long been known to exist (Dunsmore and Dudzinski, 1968), and seasonal shifts in response to RHDV have been observed in co-infecting myxomatosis epidemiology (Mutze et al., 2002). Thus, we next asked whether patterns in seasonal infection prevalence and intensity of each macroparasite species from Scottish rabbits varied before and after RHDV spread. The introduction of another microparasite, myxomatosis, has been shown to mostly increase worm burdens for several macroparasite species in various regions (Dunsmore and Dudzinski, 1968; Boag, 1988). Consequently, we also asked whether infection prevalence and intensity of all three macroparasite species in rabbits sampled in a common season before and after the introduction of RHDV changed congruously in Scotland and New Zealand. Finally, we examined the relationship between serum antibody levels to RHDV, and the abundance of each macroparasite species infecting a sub-sample of rabbits collected over one year from Scotland, to further assess the relative importance of indirect (top-down) interactions between micro- and macroparasites.
2. Materials and methods
The data from Scotland is from a monthly long-term study on rabbits undertaken at sites in the Sidlaw Hills (approximately 56.5000° N, 3.1667° W) in eastern Scotland beginning in 1977 (Boag, 1985; Boag et al., 2001, 2013; Lello et al., 2004). The 1984–1985 data from New Zealand (pre-RHDV) was obtained from an MSc thesis by Rhodes (1985) who collected rabbits from farms around Alexandra, Central Otago, New Zealand (approximately 45.2328° S, 169.3724° E) in the months of November 1984 (Austral Spring), and February (Austral Summer), May (Austral Autumn) and August 1985 (Austral Winter). One of the authors (BB) collected the data for 2002 (post-RHDV) from Alexandra on 30th March 2002 (Austral Autumn) where rabbits were supplied from local farms. To make the data from Scotland more comparable with the data from New Zealand we only selected rabbits sampled in Scotland during the months of February (Palearctic Winter), May (Palearctic Spring), August (Palearctic Summer) and November (Palearctic Autumn) between 1977 and 1996 (pre-RHDV) and 2005–2010 (post-RHDV).
The protocol used to sample rabbits and parasites in all surveys was the same. All rabbits were shot using a 0.22 calibre rifle equipped with a silencer. In the laboratory the animals were sexed, weighed, measured and their alimentary tracts separated into three regions, i.e. stomach, small intestine and large intestine, each section being opened, washed by hand and the contents passed through a 100 mesh (150sieve. The residue was then collected and either examined fresh (within 24 h) or stored in 10% formalin for parasite identification and enumeration using a dissecting microscope with at least a 40× magnification (Boag, 1984). In the samples collected by BB, if numbers of nematodes were high (>2000) then a dilution of up to 1:25 was counted, and if counts were low then at least half of the contents were counted.
Host age and the aggregated nature of parasite populations are thought to be important factors influencing patterns indicative of parasite interactions based on observational field studies (Fenton et al., 2010). Body mass can be a proxy for age (Myers and Gilbert, 1968; Cowan, 1983), and therefore individual rabbits were classified into age-mass classes as defined by Cattadori et al. (2005) who used rabbits sampled in all twelve months from the Sidlaw Hills between 1977 and 2002 (Boag et al., 2001). Eight age-mass classes corresponded to three major age categories: kittens (class 1: 100–200 g, class 2: 201–480 g, class 3: 481–750 g); juveniles (class 4: 751–1030 g, class 5:1031–1300 g); and adults (class 6: 1301–1580 g, class 7:1581–1860 g, class 8: ≥1861 g).
Blood sera were obtained monthly from 252 rabbits in Scotland sampled between January and December 2005 (range: 10–38 rabbits per month) and analysed for the presence of antibodies against RHDV using ELISA tests following the protocol by Forrester et al. (2009). Briefly, an optimized concentration of recombinant RHDV protein (Marin et al., 1995) was coated onto ELISA plates overnight at 4 °C in coating buffer. After washing the plates in PBS-Tween (0.1%), twofold dilutions of serum were added for 1 h at 37 °C. Plates were washed, and a 1:1000 dilution of polyvalent goat anti-rabbit serum conjugated with horseradish peroxidase (Sigma-Aldrich) added for 1 h at 37 °C. Plates were washed and substrate (OPD, Sigma-Aldrich) was added. After 30 min, absorption for each reaction was estimated at 492 nm. Absorption readings of at least twice that at the equivalent dilution of negative control serum (serum from a commercially supplied rabbit certified as negative) were considered positive for RHDV. Titres of 1:10 were considered negative.
2.1. Statistical analysis
All analyses were conducted using R statistical software v.3.4.1 (R Core Team, 2017). We studied macroparasite infection prevalence, or proportion of rabbits infected with a particular parasite species (see Bush et al., 1997), by classifying each individual rabbit sampled as either uninfected or infected. We treated these data as a series of Bernoulli trials with a binary response variable, which allowed us to examine the likelihood of observing nematode infections in rabbits across seasons, locations, and periods before and after the introduction of RHDV. We constructed generalized linear mixed models (glmer function, package lme4, Bates et al., 2015) of the binomial family with a logit link function for each nematode species to first test if there were differences in infection prevalence for rabbits sampled every season (winter, spring, summer, autumn) in Scotland vs. New Zealand before RHDV spread. Next, we tested for differences in prevalence of infection in rabbits sampled every season from Scotland before and after the arrival of RHDV. Finally, we tested if prevalence of infection in rabbits sampled in autumn season from Scotland and New Zealand differed before and after the arrival of RHDV. To control for possible effects of an individual's sex, age and mass, which are known to influence worm infection (Boag et al., 2001; Cattadori et al., 2005), rabbit sex (female or male) and age-mass class (class 1–8) were included as random effects in all models, with sex nested into age-mass class.
General linear mixed effect models (lme function, package NLME, Pinheiro and Bates, 2000) were used to examine differences in the mean intensity of infection for each nematode species, or the mean number of worms per rabbit infected with a particular macroparasite (see Bush et al., 1997). The number of worms per rabbit was log (x) transformed for normality since zeros were excluded, and used as the response variable in relation to the following possible fixed explanatory variables: occurrence of RHDV (pre-RHDV or post-RHDV), location (Scotland or New Zealand), and season. To control for possible effects of individual's sex, age and mass (Boag et al., 2001; Cattadori et al., 2005), rabbit sex and age-mass class were included as random effects, with sex nested into age-mass class. We first tested if there were differences in the mean intensity of infection between rabbits sampled every season in Scotland vs. New Zealand before RHDV spread. Next, we tested if there were differences in mean intensity in rabbits sampled every season from Scotland before and after the arrival of RHDV. Finally, we tested if there were differences in mean intensity between rabbits sampled in autumn season from Scotland and New Zealand before and after the arrival of RHDV.
Simple linear regression models (lm function, package stats, R Core Team et al., 2017) were used to examine whether RHDV antibody levels were related to worm counts for each nematode species in all rabbits sampled from Scotland during 2005. The number of worms per rabbits sampled (i.e. abundance of infection (see Bush et al., 1997), was log (x+1) transformed and regressed against the log (x+1) antibody levels from ELISA titres, which were measured as the inverse of the dilution at which sera were considered positive (Forrester et al., 2009).
3. Results
3.1. Did parasite prevalence and intensity of infection vary similarly across seasons in Scotland and New Zealand before the spread of RHDV?
A total of 885 rabbits were collected from Scotland between 1977 and 1996 (female: 406, male: 479) and 400 from New Zealand (in comparable seasons) in 1985 (female: 203, male: 197). The prevalence and mean intensity of infection with each macroparasite is consistent with what has been reported from other regions of Britain, continental Europe and New Zealand in that large variabilities are typical between populations in some species (SM Tables 1–3). In our data, prevalence of infection with T. retortaeformis did not vary significantly between Scotland and New Zealand, but was significantly higher for G. strigosum in Scotland and for P. ambiguus in New Zealand, regardless of season (Table 1). The prevalence of all three nematode species varied significantly between seasons, regardless of location (Table 1). The interaction between season and location had no significant effect on prevalence of T. retortaeformis or G. strigosum, but did on P. ambiguus (Table 1). In New Zealand, prevalence of P. ambiguus was higher every season relative to spring, whereas in Scotland it was lower in summer relative to spring, but higher in autumn and winter (SM Table 2).
Table 1.
Results of the generalized linear mixed-effects models (GLMM) and general linear mixed-effects models (LME) of best fit for nematode infection prevalence and mean intensity, respectively, from rabbits sampled seasonally in New Zealand (NZ) and Scotland before the arrival of RHDV. All comparisons are made against the intercept of the first level of each factor, i.e. NZ for location and Spring for season. Test statistic (z values) are marked as: ****P < 0.001, ***P < 0.01, **P < 0.05.
| Parasite species | Fixed effects |
Prevalence Models |
Mean Intensity Models |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Factor | Level | Estim. | SE | z value | Pr (>|z|) | Estim. | SE | t value | P value | |
|
Trichostrongylus retortaeformis |
Location | NZ vs. Scotland | – | – | – | – | −0.58 | 0.09 | −6.18 | <0.0001 |
| Season | Spring vs. Summer | 0.66 | 0.2 | 3.22 | 0.0012*** | 0.26 | 0.12 | 2.12 | 0.03 | |
| Spring vs. Autumn | 0.67 | 0.23 | 2.86 | 0.0042*** | 0.21 | 0.14 | 1.56 | 0.12 | ||
| Spring vs. Winter | 1.3 | 0.27 | 4.87 | 1.1 × 10−6**** | 0.5 | 0.14 | 3.59 | 0.0003 | ||
|
Graphidium strigosum |
Location | NZ vs. Scotland | 1.2 | 0.15 | 7.75 | 8.9 × 10−15**** | −1.11 | 0.15 | −7.34 | <0.0001 |
| Season | Spring vs. Summer | −0.92 | 0.19 | −4.74 | 2.1 × 10−6**** | −0.73 | 0.21 | −3.54 | 0.0004 | |
| Spring vs. Autumn | 0.45 | 0.21 | 2.16 | 0.031** | 0.03 | 0.19 | 0.14 | 0.88 | ||
| Spring vs. Winter | 0.85 | 0.22 | 3.84 | 0.0001**** | 0.91 | 0.19 | 4.83 | <0.0001 | ||
|
Passalurus ambiguus |
Location | NZ vs. Scotland | −2.08 | 0.29 | −7.01 | 2.32 × 10−12**** | −2.33 | 0.26 | −8.93 | <0.0001 |
| Season | Spring vs. Summer | −0.08 | 0.32 | −0.24 | 0.81 | 0.73 | 0.36 | 2.0 | 0.0455 | |
| Spring vs. Autumn | 0.7 | 0.33 | 2.11 | 0.035** | 0.98 | 0.32 | 3.11 | 0.0021 | ||
| Spring vs. Winter | 1.33 | 0.38 | 3.46 | 0.0005 **** | 1.33 | 0.31 | 4.22 | <0.0001 | ||
| Location:Season | Scotland:Summer | −1.56 | 0.48 | −3.26 | 0.001 *** | – | – | – | – | |
| Scotland:Autumn | −0.48 | 0.44 | −1.09 | 0.27 | – | – | – | – | ||
| Scotland:Winter | −1.23 | 0.48 | −2.56 | 0.01** | – | – | – | – | ||
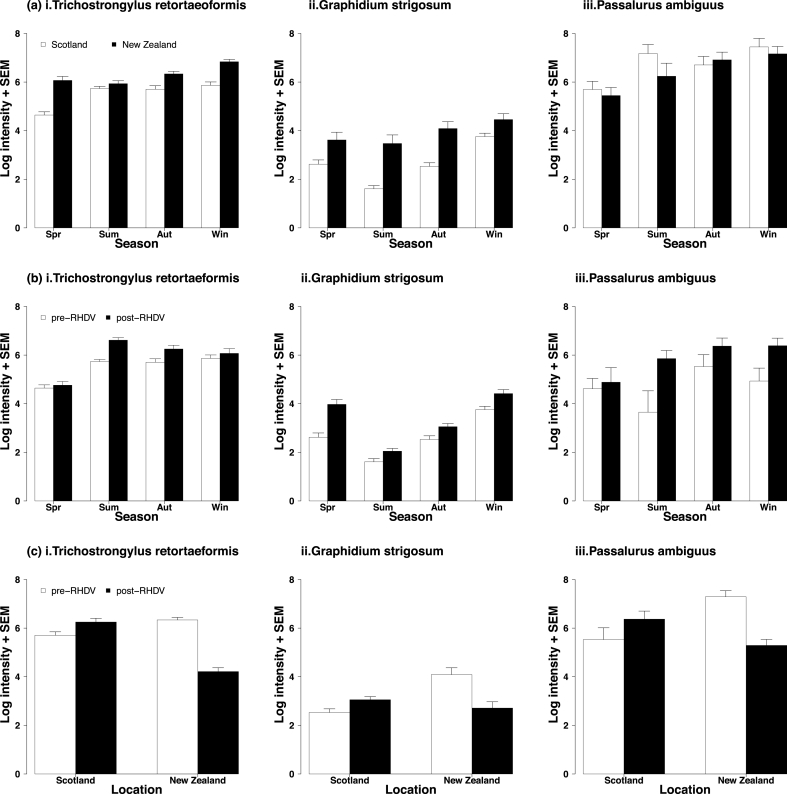
Mean intensity of infection for all three nematode species was significantly higher in New Zealand than Scotland, regardless of season (Table 1). Season had a significant effect on mean intensity of all three nematode species, regardless of location (Table 1). The mean number of worms per rabbit was generally lowest in spring and summer, and highest by autumn and winter, for all three nematodes (Fig. 1a). There was no significant interaction between season and location affecting the mean intensity of any macroparasite.
Fig. 1.
Differences in mean intensity of nematode parasite infection in rabbits sampled seasonally from New Zealand and Scotland before the spread of RHDV (a), rabbits sampled seasonally from Sotland before and after RHDV (b), and rabbits sampled in autumn season from New Zealand and Scotland before and after RHDV (c). Nematode parasites included T. retortaeformis (i) G. strigosum (ii) and P. ambiguus (iii) found in rabbits sampled in spring (Spr), summer (Sum), autumn (Aut) and winter (Win).
3.2. Did seasonal prevalence and intensity of infection in Scotland vary similarly before and after the spread of RHDV?
In addition to the rabbits collected in Scotland between 1977 and 1996 (pre-RHDV), 589 rabbits (267 female, 322 male) were collected between 2005 and 2010 (post-RHDV). The prevalence of T. retortaeformis and G. strigosum was significantly higher after the spread of RDHV, regardless of season (Table 2). There were significant differences between most seasons in prevalence of all three nematode species, regardless of RHDV presence (Table 2). The interaction between season and presence of RHDV had a significant effect on prevalence of T. retortaeformis (Table 2), with a bigger increase in the proportion of individuals infected between spring and summer before the arrival of RHDV relative to the rise between spring and summer post-RHDV (SM Table 2). Similarly, there were significant interactions between season and RHDV presence on the prevalence of P. ambiguus, with a decrease in prevalence between spring and summer pre-RHDV while it increased post-RHDV, and the increase between spring and winter was higher post-RHDV than pre-RHDV (Table 2, SM Table 2).
Table 2.
Results of the generalized linear mixed-effects models (GLMM) and general linear mixed-effects models (LME) of best fit for nematode infection prevalence and mean intensity, respectively, from rabbits sampled seasonally in Scotland before and after the arrival of RHDV. All comparisons are made against the intercept of the first level of each factor, i.e. Absent for RHDV and Spring for season. Test statistic (z values) are marked as: ****P < 0.001, ***P < 0.01, **P < 0.05.
| Parasite species | Fixed effects |
Prevalence Models |
Mean Intensity Models |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Factor | Level | Estim. | SE | z value | Pr (>|z|) | Estim. | SE | t value | P value | |
|
Trichostrongylus retortaeformis |
RHDV | Absent vs. Present | 0.44 | 0.2 | 2.16 | 0.031** | 0.44 | 0.09 | 5.1 | <0.0001 |
| Season | Spring vs. Summer | 0.64 | 0.23 | 2.76 | 0.006*** | 0.72 | 0.12 | 5.89 | <0.0001 | |
| Spring vs. Autumn | 1.02 | 0.32 | 3.23 | 0.0012*** | 0.38 | 0.14 | 2.69 | 0.007 | ||
| Spring vs.Winter | 1.44 | 0.34 | 4.21 | 2.6 × 10−5**** | 0.37 | 0.15 | 2.45 | 0.014 | ||
| RHDV:Season | Present:Summer | −0.79 | 0.37 | −2.14 | 0.032 ** | – | – | – | – | |
| Present:Autumn | −0.23 | 0.47 | −0.5 | 0.618 | – | – | – | – | ||
| Present:Winter | −0.89 | 0.47 | −1.88 | 0.06 | – | – | – | – | ||
|
Graphidium strigosum |
RHDV | Absent vs. Present | 1.18 | 0.15 | 7.7 | 1.35 × 10−14**** | 1.37 | 0.2 | 6.82 | <0.0001 |
| Season | Spring vs. Summer | −0.52 | 0.18 | −2.89 | 0.0038*** | −0.82 | 0.2 | −4.06 | 0.0001 | |
| Spring vs. Autumn | 1.03 | 0.25 | 4.04 | 5.33 × 10−5**** | −0.22 | 0.2 | −1.1 | 0.27 | ||
| Spring vs. Winter | 1.32 | 0.3 | 4.49 | 7.15 × 10−6**** | 0.91 | 0.2 | 4.5 | <0.0001 | ||
| RHDV:Season | Present:Summer | – | – | – | – | −0.76 | 0.27 | −2.75 | 0.006 | |
| Present:Autumn | – | – | – | – | −0.8 | 0.28 | −2.79 | 0.005 | ||
| Present:Winter | – | – | – | – | −0.7 | 0.29 | −2.41 | 0.016 | ||
|
Passalurus ambiguus |
RHDV | Absent vs. Present | −0.33 | 0.32 | −1.03 | 0.303 | 1.09 | 0.32 | 3.35 | 0.001 |
| Season | Spring vs. Summer | −1.71 | 0.38 | −4.53 | 5.91 × 10−6**** | 0.09 | 0.51 | 0.17 | 0.86 | |
| Spring vs. Autumn | 0.19 | 0.3 | 0.64 | 0.52 | 1.1 | 0.44 | 2.48 | 0.014 | ||
| Spring vs. Winter | 0.08 | 0.31 | 0.26 | 0.79 | 0.84 | 0.44 | 1.91 | 0.058 | ||
| RHDV:Season | Present:Summer | 1.95 | 0.49 | 4.01 | 6.14 × 10−5**** | – | – | – | – | |
| Present:Autumn | 0.61 | 0.44 | 1.38 | 0.17 | – | – | – | – | ||
| Present:Winter | 1.46 | 0.45 | 3.21 | 0.001*** | – | – | – | – | ||
The mean intensity of infection for all three nematode species was significantly higher after the spread of RHDV, regardless of season (Table 2). Season had a significant effect on mean intensity of all three nematode species, regardless of RHDV presence (Table 2). There was also a significant negative interaction between season and presence of RHDV on G. strigosum mean intensity, but not on the other two nematode species (Table 2). For G. strigosum, the mean number of worms was consistently higher after RHDV, but overall, it was lower in summer and autumn relative to spring (Fig. 1b).
3.3. Did parasite prevalence and intensity of infection in autumn season vary between rabbits collected in Scotland and New Zealand before and after the circulation of RHDV?
The total number of rabbits collected in autumn from Scotland pre-RHDV was 123 (55 female, 68 male), and 98 were collected post-RHDV (42 female, 56 male). A total of 100 rabbits (49 female, 51 male) were collected in autumn from New Zealand (in May 1985) pre-RHDV, and 100 rabbits (42 female, 58 male) were collected (on 30th March 2002) post-RHDV. The presence of RHDV, regardless of location, significantly increased the prevalence of infection with T. retortaeformis, and decreased it for G. strigosum and P. ambiguus infections, albeit not significantly (Table 3). The prevalence of G. strigosum was significantly higher in Scotland than New Zealand, regardless of the RHDV presence, and for P. ambiguus it was lower in Scotland (Table 3). There was no significant effect of location on the prevalence of T. retortaeformis. There was a significant interaction between RHDV presence and location on prevalence of G. strigosum (Table 3), but not for the other nematode species. In New Zealand, prevalence of G. strigosum when RHDV was present was lower, whereas in Scotland it was higher (SM Table 3).
Table 3.
Results of the generalized linear mixed-effects models (GLMM) and general linear mixed-effects models (LME) of best fit for nematode infection prevalence and mean intensity, respectively, from rabbits sampled in autumn from New Zealand (NZ) and Scotland before and after the arrival of RHDV. All comparisons are made against the intercept of the first level of each factor, i.e. Absent for RHDV and NZ for location. Test statistic (z values) are marked as: ****P < 0.001, **P < 0.05.
| Parasite species | Fixed effects |
Prevalence Models |
Mean Intensity Models |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Factor | Level | Estim. | SE | z value | Pr (>|z|) | Estim. | SE | t value | P value | |
|
Trichostrongylus retortaeformis |
RHDV | Absent vs. Present | 0.58 | 0.29 | 1.99 | 0.046** | −2.29 | 0.2 | −11.29 | <0.0001 |
| Location | NZ vs. Scotland | – | – | – | – | −0.88 | 0.2 | −4.38 | <0.0001 | |
| RHDV:Location | Present:Scotland | – | – | – | – | 2.84 | 0.28 | 10.29 | <0.0001 | |
|
Graphidium strigosum |
RHDV | Absent vs. Present | −0.54 | 0.29 | −1.87 | 0.06 | −1.39 | 0.34 | −4.13 | <0.0001 |
| Location | NZ vs. Scotland | 1.54 | 0.3 | 5.07 | 3.97 × 10−7**** | −1.55 | 0.26 | −5.88 | <0.0001 | |
| RHDV:Location | Present:Scotland | 1.86 | 0.56 | 3.31 | 0.0009**** | 1.92 | 0.4 | 4.8 | <0.0001 | |
|
Passalurus ambiguus |
RHDV | Absent vs. Present | −0.5 | 0.33 | −1.51 | 0.13 | −2.0 | 0.36 | −5.63 | <0.0001 |
| Location | NZ vs. Scotland | −2.56 | 0.34 | −7.54 | 4.51 × 10-14**** | −1.76 | 0.47 | −3.74 | 0.0002 | |
| RHDV:Location | Present:Scotland | 0.85 | 0.45 | 1.89 | 0.058 | 2.84 | 0.67 | 4.26 | <0.0001 | |
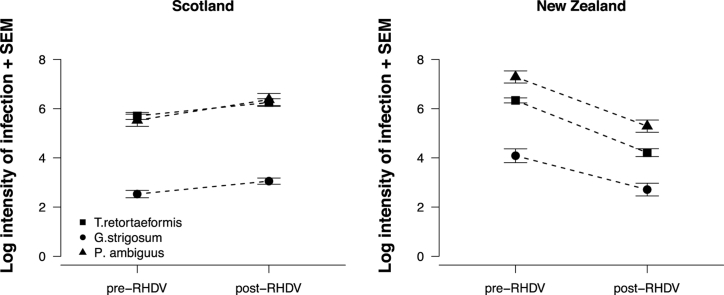
The presence of RHDV had a significant negative effect on mean intensity for all three nematode species, regardless of location (Table 3). The mean intensity of all three nematode species was significantly higher in New Zealand than Scotland, regardless of RHDV presence (Table 3). There was a significant interaction between location and presence of RHDV on mean intensity for all three nematode species (Table 3), with number of worms decreasing after RHDV spread in New Zealand, and increasing after RHDV spread in Scotland (Fig. 1c).
3.4. Were RHDV antibody levels related to worm burden for nematode species infecting rabbits from Scotland in 2005?
RHDV antibody levels in rabbits from Scotland collected in 2005 were not significantly related to worm counts of T. retortaeformis (F1,250 = 1.31, p = 0.25, R2 = 0.001, y = 3.89 + 0.11x) or P. ambiguus (F1,250 = 2.11, p = 0.15, R2 = 0.004, y = 0.35 + 0.1x). Only rabbits with higher numbers of G. strigosum had significantly higher RHDV titres, although the linear regression model had a low, albeit significant, R2 value (F1,250 = 11.99, p = 0.0006, R2 = 0.042, y = 0.86 + 0.22x).
4. Discussion
The introduction of RHDV appears to relate differently with macroparasite in Scotland and New Zealand. Evidence from one common season (autumn) suggests that prevalence of T. retortaeformis was generally higher after the virus spread in both locations, whereas it was lower for G. strigosum and P. ambiguus after RHDV arrived in New Zealand, but higher in Scotland. Meanwhile, mean intensity of all three nematode species decreased after RHDV arrived in New Zealand, but it increased in Scotland. The impact of RHDV on worm infections was consistent across seasons in Scotland, and the similarities in seasonality between locations suggest that the effects on infection patterns observed in one season are likely to be similar year-round.
The seasonal patterns of macroparasite infection did not vary very much between Scotland and New Zealand before RHDV arrived possibly because both locations experience similar environmental changes across seasons typical of temperate climates, which may be important to the transmission and general life history of the nematode species. For example, the prevalence and mean intensity of infection with T. retortaeformis was lowest in spring in both locations relative to other seasons, and this may coincide with the beginning of the rabbits' breeding season when there are fewer breeding pairs and higher numbers of young-of-the-year that have not had exposure to parasite infective stages in the environment for very long (Cattadori et al., 2005). Rabbits develop an immune response that can reduce or remove T. retortaeformis, although not fully protect against reinfections (Cattadori et al., 2005, 2008; Murphy et al., 2011). Thus, the general rise in prevalence and intensity of T. retortaeformis infection at both locations as the rabbit breeding-season progressed may be because of similarly increasing host densities. In contrast, prevalence and intensity of infection with G. strigosum peaks in autumn and winter in both locations because this species of parasite accumulates with host age with no evidence of worm mortality even though a robust species-specific immune response occurs (Cattadori et al., 2005, 2008; Murphy et al., 2011). Less is known about the seasonal biology of P. ambiguus, but our data suggest that the rise in prevalence and intensity of infection starting in the spring may also be related to increasing host densities associated with rabbit breeding.
Seasonal patterns in prevalence and intensity of infection in Scotland did not vary greatly before and after the spread of RHDV. However, overall prevalence and intensity of infection of all three nematodes did increase post-RHDV, and in every season. A rise in G. strigosum numbers in Scottish rabbits over decades of parallel increases in temperature suggests that climate may be driving changes to the population dynamics of this parasite, while T. retortaeformis populations are unchanged because host immunity plays a bigger role in regulating this species than it does G. strigosum (Hudson et al., 2006). Indeed, laboratory and field studies measuring the effects of elevated temperatures on egg hatching and survival of T. retortaeformis and G. strigosum indicate higher temperatures accelerate hatching and reduce survival in both species, but G. strigosum free living stages are more sensitive to both high and low temperatures (Hernandez et al., 2013). Thus, the higher prevalence and intensity of infection with G. strigosum in Scottish rabbits in the periods when RHDV was introduced might coincide with higher temperatures increasing worm transmission, but a similar increase in T. retortaeformis infection is not as clearly explained by environmental change.
The variabilities in how macroparasites responded to the introduction of RHDV into Scotland versus New Zealand could be due to inherent ecological differences, such as in parasite community structure, host population densities, or possibly environment-driven differences in host feeding behaviour. The bottleneck after the introduction of rabbits into New Zealand resulted in a depauperate community of macroparasites relative to the more diverse communities that can infect Scottish rabbits, which is common after animals are introduced into a new environment (Torchin et al., 2003). Direct and indirect interactions between co-infecting species can influence population dynamics of parasites in Scottish rabbits. For example, Lello et al. (2004) found that the presence of C. denticulata was associated with a 51% increase in T. retortaeformis, while M. pectinata was associated with a decrease in G. strigosum intensity of 19%. Thus, it may be possible that the elevated T. retortaeformis, G. strigosum and P. ambiguus intensities measured in Scotland rabbits post-RHDV in our study may have also been affected, in part, by their interactions with cestodes and other types of parasites that do not occur in New Zealand.
The introduction of RHDV may have also significantly decreased the mean intensity of all three nematodes in New Zealand, but not Scotland, because RHDV killed a higher percentage of rabbits in New Zealand, whereas it had no detectable detrimental impact on the density of rabbits in Scotland (Patterson and Howie, 1995). Parasite populations are known to relate to the density of their hosts (Arneberg et al., 1998; Hudson et al., 1998). Rabbit numbers have been significantly reduced by RHDV in the Otago area by 67% (Parkes et al., 2002), and Norbury et al. (2002) reported decreases of up to 90%. At the same time, rabbit numbers in the Sidlaw Hills of Scotland remained high even while a second strain of the RHDV was reported to be present (Forrester et al., 2006, 2009). Thus, macroparasite densities may have decreased in New Zealand because RHDV killed more hosts there.
Another possible reason the impact of RHDV in Scotland is less than in New Zealand may be that regional climate variabilities can differentially impact not only parasites, but also hosts. For example, annual rainfall in the Alexandra area is ca. 30–50 cm (Robertson, 1959; Radcliffe, 1974) compared with ca. 64–86 cm in areas near the Sidlaw Hills (Barnett et al., 2006), and there is relatively more grass growth in Scotland than in New Zealand (BB, pers. obs.). Differences in rainfall may impact the feeding behaviour of rabbits by influencing how far they travel to find suitable food, which may explain why home ranges in Scotland are relatively small (∼3.9ha, Hulbert et al., 1996), while those in New Zealand can be larger (Gibb and Morgan-Williams, 1994). Rabbits do favour short swards, probably as an anti-predator strategy (Iason et al., 2002), but there are no predators in New Zealand. As the size of the patch probably depends upon the numbers of rabbits in a warren, the resulting faecal contaminations will remain relatively constant. However, in the more arid area around Alexandra, the short vegetation is widespread and hence rabbit distribution is not restricted to areas immediately surrounding warrens to the same extend as in Scotland. Consequently, contamination of the pasture with faeces, and hence parasites, is more likely to be host-density dependent. This further supports the idea that the marked decrease in rabbit numbers after RHDV spread can help explain the significant decrease in mean macroparasite intensity measured in New Zealand.
A comparison of the parasites used in the present investigation with published data from previous parasitological studies on rabbits shows large variations in both prevalence and intensity of infection can be expected for G. strigosum and P. ambiguus (SM Table 1), which do not have close host affinity (Mykytowycz, 1956). Whereas, published data for T. retortaeformis shows that this species has a closer relationship with its host and is generally found at a high level of prevalence (>72%) wherever surveys have occurred (SM Table 1). The lack of G. strigosum in records by Hulbert and Boag (2001) may be because of the infective stages' sensitivity to high and low temperatures (Hernandez et al., 2013), and the fact rabbits collected in their survey were taken at 750 m asl where temperatures are often below freezing. In contrast, those collected by Boag and Kolb (1989) were just above sea level where frosts are fewer. The absence of G. strigosum in the survey by Boag (1972) was possibly due to this population of parasites having lost its association with its host when the number of rabbits was reduced by myxomatosis by up to 99% (Thompson and Worden, 1956). The reason P. ambiguus was absent from 2 published surveys (SM Table 1) was either due to the animals being very young (Boag and Garson, 1993), or the low temperatures encountered overwinter at 550 m asl (Hulbert and Boag, 2001). The low T. retortaeoformis prevalence recorded by Boag and Garson (1993) was again due to the rabbits being very young, while that recorded by Allan et al. (1999) was due to a poorly followed accepted protocol (i.e. no microscopic examination of small intestine contents). Data collected by Boag (2007, unpublished data) at the same time of year, and from the same site, subsequently found higher T. retortaeformis prevalence and intensity compared with Allan et al. (1999; SM Table 1). Collectively, data from all of these surveys support the suggestion by Knowles et al. (2013) that there can be stability in infection prevalence within parasite communities of wild mammals, but that intensity of infection can be variable across host populations.
There was a significant positive relationship between serum antibody levels to RHDV and the abundance of G. strigosum worms in Scotland, but that relationship was very weak with only 4% of the variability explained by the model. Nonetheless, this result is suggestive of possible within-host interactions whereby higher RHDV titres indirectly facilitate higher numbers of G. strigosum, potentially via immunomodulation by depleting both B and T lymphocytes in virulent infections (Abrantes et al., 2012). However, it's more likely that given the relatively weak relation between RHDV and G. strigosum worm burdens, and the lack thereof with the other two nematode species, that any possible impact RHDV might have on mean intensity of macroparasites is independent of the size of RHDV infection.
5. Conclusion
There was no evidence to suggest that RHDV had an impact on macroparasites similar to that of myxomatosis, and this was probably due to the two viruses acting differently. Myxomatosis takes longer to kill its host, allowing it time to compromise its immune status, while RHDV kills its host within a short period of time and its survival can depend upon the environment (Henning et al., 2005). Bottom-up processes may have played an important role in macroparasite community structure after RHDV was introduced to Scotland and New Zealand. The higher frequency of infected rabbits and higher worm burdens after the arrival of RHDV to Scotland might suggest that, like myxomatosis, RHDV could interact indirectly with co-infecting macroparasites via a top-down processes such as immune modulation. However, the direction of change in infection prevalence and mean intensity differed between macroparasite species after the arrival of RHDV into New Zealand, and may instead be an indirect result of a more virulent virus reducing host density and their period alive during which worms could successfully mature and reproduce. This is in essence a bottom-up process whereby RHDV is competing for a shared resource, i.e. the host organism, by expending it to a level low enough in the ecosystem that it becomes less abundant for other micro- and macroparasites to (co-) infect or exploit. One reason for the variability in direction and magnitude of change in infection prevalence and mean intensity between locations may be that the more diverse parasite community in rabbits from Scotland may mitigate potential detrimental effects of more virulent microparasites that often emerge in some wildlife populations such as RHDV. Clearly, interactions between co-infecting micro- and macroparasites can vary in host populations with different ecologies, but nevertheless significantly impact parasite community structure in wildlife.
Acknowledgements
We should like to thank the landowners who allowed the rabbits to be collected on their land. We're grateful to AJJ MacIntosh and two anonymous reviewers for comments and suggestions on earlier drafts of this paper. ADH was supported from funds from the Department of Biology and College of Liberal Arts & Sciences at Kutztown University.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.ijppaw.2018.05.002.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- Abrantes J., van der Loo W., Le Pendu J., Esteves P.J. Rabbit haemorrhagic disease (RHD) and rabbit haemorrhagic disease virus (RHDV): a review. Vet. Res. 2012;43:12. doi: 10.1186/1297-9716-43-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aebischer N.J., Davey P.D., Kingdon N.G. Game Conservancy Trust; Fordingbridge: 2011. National Gamebag Census: Mammal Trends to 2009. [Google Scholar]
- Allan J.C., Craig P.S., Sherington J., Rogan M.T., Storey D.M., Iball K. Helminth parasites of the wild rabbit Oryctolagus cuniculus near Malham Tarn, Yorkshire, UK. J. Helminthol. 1999;73:289–294. doi: 10.1017/s0022149x99000487. [DOI] [PubMed] [Google Scholar]
- Arneberg P., Skorping A., Grengell B., Read A.F. Host densities as determinants of abundance in parasite communities. P. Roy. Soc. B-Biol Sci. 1998;265:1283–1289. [Google Scholar]
- Barnett C., Hossell J., Perry M., Procter C., Hughes G. SNIFFER Project CC03, Scotland and Northern Ireland Forum for Environmental Research. 2006. A handbook of climate trends across Scotland. [Google Scholar]
- Bates D., Maechler M., Bolker B., Walker S. Fitting linear mixed-effects models using lme4. J. Stat. Software. 2015;67:1–48. [Google Scholar]
- Berto-Moran A., Pacios I., Serrano E., Moreno S., Rouco C. Coccidian and nematode infections influence prevalence of antibody to myxoma and rabbit haemorrhagic disease viruses in European rabbits. J. Wildl. Dis. 2013;49:10–17. doi: 10.7589/2011-12-343. [DOI] [PubMed] [Google Scholar]
- Boag B. Helminth parasites of the wild rabbit Oryctolagus cuniculus (L.) in North East England. J. Helminthol. 1972;46:73–79. doi: 10.1017/s0022149x00022136. [DOI] [PubMed] [Google Scholar]
- Boag B. Effect of different concentrations of formalin on the preservation of Trichostrongylus retortaeformis (Zeder, 1800) (Nematoda) Proc. Helm. Soc. Wash. 1984;51:331. [Google Scholar]
- Boag B. The incidence of helminth parasites from the wild rabbit Oryctolagus cuniculus (L.) in Eastern Scotland. J. Helminthol. 1985;59:61–69. doi: 10.1017/s0022149x00034507. [DOI] [PubMed] [Google Scholar]
- Boag B. Observations on the seasonal incidence of myxomatosis and its interactions with helminth parasites in the European rabbit (Oryctolagus cunuiculus) J. Wildl. Dis. 1988;24:450–455. doi: 10.7589/0090-3558-24.3.450. [DOI] [PubMed] [Google Scholar]
- Boag B. 2007. Unpublished Data from Malham Tarn Yorkshire England. [Google Scholar]
- Boag B., Garson P.J. Helminth infections of weaning rabbits from Holy Island. Northumberland. J. Zool. 1993;230:323–327. [Google Scholar]
- Boag B., Kolb H.H. Influence of host age and sex on nematode populations in the wild rabbit (Oryctolagus cuniculus L.) Proc. Helm. Soc. Wash. 1989;56:116–119. [Google Scholar]
- Boag B., Hernandez A.D., Cattadori I.M. Observations on the epidemiology and interactions between myxomatosis, coccidiosis and helminth parasites in a wild rabbit population in Scotland. Eur. J. Wildl. Res. 2013;59:557–562. [Google Scholar]
- Boag B., Lello J., Fenton A., Tompkins D.M., Hudson P.J. Patterns of parasite aggregation in the wild European rabbit (Oryctolagus cuniculus) Int. J. Parasitol. 2001;31:1421–1428. doi: 10.1016/s0020-7519(01)00270-3. [DOI] [PubMed] [Google Scholar]
- Bull P.C. Parasites of the wild rabbit, Oryctolagus cuniculus (L.) in New Zealand. NZ J. Sci. Tech. 1953;34:341–372. [Google Scholar]
- Bull P.C. Changing incidence of parasitism in a declining rabbit population. Proc. N. Z. Ecol. Soc. 1957;5:11–12. [Google Scholar]
- Bush A., Lafferty K.D., Lotz J.M., Shostak A.W. Parasitology meets ecology on its own terms: Margolis et al. revisited. J. Parasitol. 1997;83:575–583. [PubMed] [Google Scholar]
- Cattadori I.M., Albert R., Boag B. Variation in host susceptibility and infectiousness generated by co-infection: the myxoma–Trichostrongylus retortaeformis case in wild rabbits. J. Roy. Soc. Interface. 2007;4:831–840. doi: 10.1098/rsif.2007.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattadori I.M., Boag B., Bjornstad O.N., Cornell S.J., Hudson P.J. Peak shift and epidemiology in a seasonal host-nematode system. Proc. Roy. Soc. B-Biol Sci. 2005;272:1163–1169. doi: 10.1098/rspb.2004.3050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattadori I.M., Boag B., Hudson P.J. Parasite co-infection and interaction as drivers of host heterogeneity. Int. J. Parasitol. 2008;38:371–380. doi: 10.1016/j.ijpara.2007.08.004. [DOI] [PubMed] [Google Scholar]
- Cooke B.D. CSIRO Publishing; Clayton, Australia: 2014. Australia's War against Rabbits: the Story of Rabbit Haemorrhagic Disease. [Google Scholar]
- Cooke B.D., Fenner F. Rabbit haemorrhagic disease and the biological control of wild rabbits, Oryctolagus cuniculus, in Australia and New Zealand. Wildl. Res. 2002;29:689–706. [Google Scholar]
- Cowan D.P. Royal Holloway College; London: 1983. Aspects of the Behavioural Ecology of a Free-living Population of the European Wild Rabbit, Oryctolagus cuniculus L in Southern England. Ph.D. thesis. [Google Scholar]
- Cox F.E.G. Concomitant infections, parasites and immune responses. Parasitology. 2001;122:S23–S38. doi: 10.1017/s003118200001698x. [DOI] [PubMed] [Google Scholar]
- Dunsmore J.D., Dudzinski M.L. Relationship of numbers of parasites in wild rabbits Orycytolagus cuniculus (L.), to host sex, age, and season. J. Parasitol. 1968;54:462–474. [Google Scholar]
- Eden J.-S., Kovaliski J., Duckworth J.A., Swain G., Mahar J.E., Strive T. Comparative phylodynamics of rabbit haemorrhagic disease virus in Australia and New Zealand. J. Virol. 2015;89:9548–9558. doi: 10.1128/JVI.01100-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenner F., Ratcliffe F.N. Cambridge University Press, Cambridge; Cambridge: 1965. Myxomatosis. [Google Scholar]
- Fenner F., Ross J. Myxomatosis. In: Thompson H.V., King C.M., editors. The European Rabbit: the History and Biology of a Successful Colonizer. Oxford University Press; Oxford: 1994. pp. 205–239. [Google Scholar]
- Fenton A., Viney M.E., Lello J. Detecting interspecific macroparasite interactions from ecological data: patterns and processes. Ecol. Lett. 2010;13:606–615. doi: 10.1111/j.1461-0248.2010.01458.x. [DOI] [PubMed] [Google Scholar]
- Ferrari N., Cattadori I.M., Rizzoli A., Hudson P.J. Heligmosomoides polygyrus reduces infection of Ixodes ricinus in free-living yellow-necked mice Apodemus flavicollis. Parasitology. 2009;136:305–316. doi: 10.1017/S0031182008005404. [DOI] [PubMed] [Google Scholar]
- Findlay G.M. Notes on infectious myxomatosis of rabbits. Br. J. Exp. Pathol. 1929;10:214–219. [Google Scholar]
- Flux J.E.C., Fullagar P.J. World distribution of the rabbit Oryctolagus cuniculus. Acta Zool. Fennica. 1983;174:75–77. [Google Scholar]
- Forrester N.L., Boag B., Buckley A., Moureau G., Gould E.A. Co-circulation of widely disparate strains of rabbit haemorrhagic disease virus could explain localised epidemiology in the United Kingdom. Virology. 2009;393:42–48. doi: 10.1016/j.virol.2009.07.008. [DOI] [PubMed] [Google Scholar]
- Forrester N.L., Trout R.C., Turner S.L., Kelly D., Boag B., Moss S., Gould E.A. Unravelling the paradox of rabbit haemorrhagic disease virus emergence, using phylogenetic analysis; possible implications for rabbit conservation strategies. Biol. Conserv. 2006;131:296–306. [Google Scholar]
- Forrester N.L., Boag B., Moss S.R., Turner S.L., Trout R.C., White P.J., Hudson P.J., Gould E.A. Long-term survival of New Zealand rabbit haemorrhagic disease virus RNA in wild rabbits, revealed by RT-PCR and phylogenetic analysis. J. Gen. Virol. 2003;84:3079–3086. doi: 10.1099/vir.0.19213-0. [DOI] [PubMed] [Google Scholar]
- Gibb J.A., Morgan-Williams J. The rabbit in New Zealand. In: Thompson H.V., King C.M., editors. The European Rabbit: the History and Biology of a Successful Colonizer. Oxford University Press; Oxford: 1994. pp. 158–204. [Google Scholar]
- Graham A.L. Ecological rules governing helminth-microparasite coinfection. P. Natl. Acad. Sci. USA. 2008;105:566–570. doi: 10.1073/pnas.0707221105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths E.C., Pedersen A.B., Fenton A., Petchey O.L. Analysis of a symmary network of co-infection in humans reveals that parasites interact most via shared resources. Proc. Roy. Soc. B-Biol Sci. 2014;281:20132286. doi: 10.1098/rspb.2013.2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton P.B., Stevens J.R., Holz P., Boag B., Cooke B., Gibson W.C. The inadvertent introduction into Australia of Trypanosoma nabiasi, the trypanosome of the European rabbit (Oryctolagus cuniculus ), and its potential for biocontrol. Mol. Ecol. 2005;14:3167–3175. doi: 10.1111/j.1365-294X.2005.02602.x. [DOI] [PubMed] [Google Scholar]
- Henning J., Meers J., Davies R., Morris R.S. Survival of rabbit haemorrhagic disease virus (RHDV) in the environment. Epidemiol. Infect. 2005;133:719–730. doi: 10.1017/s0950268805003766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez A.D., Poole A., Cattadori I.M. Climate changes influence free-living stages of soil-transmitted parasites of European rabbits. Global Change Biol. 2013;19:1028–1042. doi: 10.1111/gcb.12106. [DOI] [PubMed] [Google Scholar]
- Hudson P.J., Cattadori I.M., Boag B., Dobson A.P. Climate disruption and parasitehost dynamics: patterns and processes associated with warming and the frequency of extreme climatic events. J. Helminthol. 2006;80:175–182. doi: 10.1079/joh2006357. [DOI] [PubMed] [Google Scholar]
- Hudson P.J., Dobson A.P., Newborn D. Prevention of population cycles by parasite removal. Science. 1998;282:2256–2258. doi: 10.1126/science.282.5397.2256. [DOI] [PubMed] [Google Scholar]
- Hulbert I.A.R., Boag B. The potential role of habitat on intestinal helminths of mountain hares, Lepus timidus. J. Helminthol. 2001;75:345–349. [PubMed] [Google Scholar]
- Hulbert I.A.R., Iason G.R., Elston D.A., Racey P.A. Home range size in a stratified landscape of two lagomorphs with different feeding strategies. J. Appl. Ecol. 1996;33:1479–1488. [Google Scholar]
- Iason G.R., Manso T., Sim D.A., Hartley F.G. The functional response does not predict the local distribution of European Rabbits (Oryctolagus cuniculus) on grass swards: experimental evidence. Funct. Ecol. 2002;16:394–402. [Google Scholar]
- Johnson P.T.J., Buller I.D. Parasite competition hidden by correlated coinfection: using surveys and experiments to understand parasite interactions. Ecology. 2011;92:535–554. doi: 10.1890/10-0570.1. [DOI] [PubMed] [Google Scholar]
- Kerr P.J., Ghedin E., Depasse J.V., Fitch A., Cattadori I.M., Hudson P.J., Tscharke D.C., Read A.F., Holmes E.C. Evolutionary history and attenuation of myxoma virus on two continents. PLoS Pathog. 2012;8:1–9. doi: 10.1371/journal.ppat.1002950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles S.C.L., Fenton A., Petchey O.L., Jones T.R., Barber R., Pedersen A.B. Stability of within-host-parasite communities in a wild mammal system. P. Roy. Soc. B-Biol Sci. 2013;280:20130598. doi: 10.1098/rspb.2013.0598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lello J., Boag B., Fenton A., Stevenson I.R., Hudson P.J. Competition and mutualism amoung the gut helminths of a mammalian host. Nature. 2004;428:840–844. doi: 10.1038/nature02490. [DOI] [PubMed] [Google Scholar]
- Liu J., Fordham D.A., Cooke B.D., Cox T., Mutze G., Strive T. Distribution and prevalence of the Australian non-pathogenic rabbit calicicvirus is correlated with rainfall and temperature. PLoS One. 2014;9 doi: 10.1371/journal.pone.0113976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin M.S., Martin Alonso J.M., Perez Ordoyo Garcia L.I., Boga J.A., Arguello-Villares J.L., Casais R., Venugopal K., Jiang W., Gould E.A., Parra F. Immunogenic properties of rabbit haemorrhagic disease virus structural protein VP60 expressed by a recombinant baculovirus: an efficient vaccine. Virus Res. 1995;39:119–128. doi: 10.1016/0168-1702(95)00074-7. [DOI] [PubMed] [Google Scholar]
- Marshall I.D., Fenner F. Studies in the epidemiology of infectious myxomatosis of rabbits. VI Changes in the innate resistance of Australian wild rabbits exposed to myxomatosis. J. Hyg.-Cambridge. 1958;56:288–302. doi: 10.1017/s0022172400037773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy L., Nalpas N., Stear M., Cattadori I.M. Explaining patterns of infection in free living populations using laboratory immune experiments. Parasite Immunol. 2011;33:287–302. doi: 10.1111/j.1365-3024.2011.01281.x. [DOI] [PubMed] [Google Scholar]
- Mutze G., Bird P., Kovaliski J., Peacock D., Jennings S., Cook B. Emerging epidemiological patterns in rabbit haemorrhagic disease, its interaction with myxomatosis, and their effects on rabbit populations in Southern Australia. Wildl. Res. 2002;29:577–590. [Google Scholar]
- Myers K., Gilbert N. Determination of age of wild rabbits in Australia. J. Wildl. Manag. 1968;32:841–849. [Google Scholar]
- Mykytowycz R. A survey of endoparasites of the wild rabbit, Oryctolagus cuniculus (L.) in Australia. CSIRO Wildlife Res. 1956;1:19–25. [Google Scholar]
- Mykytowycz R. Effect of infection with myxomatosis virus on endoparasites of rabbits. Nature. 1959;183:555–556. doi: 10.1038/183555b0. [DOI] [PubMed] [Google Scholar]
- Norbury G., Heyward R., Parke J. Short-term ecological effects of rabbit haemorrhagic disease in the short-tussock grassland of the South Island, New Zealand. Wildl. Res. 2002;29:599–604. [Google Scholar]
- Parkes J.P., Glentworth B., Sullivan G. Changes in immunity to rabbit haemorrhagic disease virus, and abundance and rates of increase of wild rabbits in Mackenzie Basin, New Zealand. Wildl. Res. 2008;35:775–779. [Google Scholar]
- Parkes J.P., Norbury G., Sullivan G., Hayward R.P. Epidemiology of rabbit haemorrhagic disease (RHD) in South Island, New Zealand. 1997-2001. Wildl. Res. 2002;29:543–555. [Google Scholar]
- Patterson I.A., Howie F.E. Rabbit haemorrhagic disease in Scotland. Vet. Rec. 1995;137:523. doi: 10.1136/vr.137.20.523-a. [DOI] [PubMed] [Google Scholar]
- Pederson A.B., Antonovics J. Anthelmintic treatment alters the parasite community in a wild mouse host. Biol. Lett. 2013;9:20130205. doi: 10.1098/rsbl.2013.0205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pederson A.B., Fenton A. Emphasizing the ecology in parasite community ecology. Trends Ecol. Evol. 2007;22:133–139. doi: 10.1016/j.tree.2006.11.005. [DOI] [PubMed] [Google Scholar]
- Pinheiro J., Bates D. Springer-Verlag; New York: 2000. Mixed-effects Models in S and S-plus. [Google Scholar]
- Radcliffe J.E. Seasonal distribution of pasture production in New Zealand I: Methods of measurement. New Zeal. J. Exp. Agr. 1974;2:337–340. [Google Scholar]
- R Core Team . R Foundation for Statistical Computing; Vienna, Austria: 2017. R: a Language and Environment for Statistical Computing.http://www.R-project.org/ URL. [Google Scholar]
- Rhodes D.S. University of Otago; Dunedin, New Zealand: 1985. Parasites from the Wild Rabbit from Central Otago; pp. 1–160. PhD thesis. [Google Scholar]
- Robertson N.G. The climate of New Zealand. In: McLintock A.H., editor. Descriptive Atlas of New Zealand. Government Printer; Wellington, New Zealand: 1959. pp. 19–23. [Google Scholar]
- Rouco C., Norbury G., Ramsay D. Kill rates by hunters before and 16 years after the introduction of rabbit haemorrhagic disease in southern South Island, New Zealand. Wildl. Res. 2014;41:136–140. [Google Scholar]
- Schaad G.A. Niche diversification in a parasitic species flock. Nature. 1963;198:404–406. [Google Scholar]
- Schaad G.A. Immunity, competition, and natural regulation of helminth populations. Am. Nat. 1966;100:359–364. [Google Scholar]
- Silvertown J., Dodd M.E., McConway K., Potts J., Crawley M. Rainfall, biomass variation, and community composition in the Park Grass Experiment. Ecology. 1994;75:2430–2437. [Google Scholar]
- Sukhdeo M.V.K. Earth's third environment: The worm's eye view. Bioscience. 1997;47:141–149. [Google Scholar]
- Taylor M.A., Coop R.L., Wall R.L. Wiley Blackwell; Hoboken: 2015. Veterinary Parasitology. [Google Scholar]
- Telfer S., Birtles R., Bennet M., Lambin X., Paterson S., Begon M. Parasite interactions in natural populations: insight from longitudinal data. Parasitology. 2008;135:767–781. doi: 10.1017/S0031182008000395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson H.V., Worden A.N. Collins New Naturalist Series; London: 1956. The Rabbit. [Google Scholar]
- Thompson J., Clark G. Rabbit calicivirus disease is now established in New Zealand. Surveillance. 1997;24:5–6. [Google Scholar]
- Torchin M.E., Lafferty K.D., Dobson A.P., McKenzie V.J., Kuris A.M. Introduced species and their missing parasites. Nature. 2003;421:628–630. doi: 10.1038/nature01346. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.