Abstract
To assess the clinicopathological significance of survivin in ovarian carcinoma through this meta-analysis. PubMed, EMBASE, Web of Science, and The Cochrane Library databases were searched for relevant studies published through September, 2017. Included studies reported the case-control study of surviving expression with ovarian cancer and its clinicopathological characteristics. The quality assessment was performed according to the Newcastle–Ottawa Scale (NOS) for quality assessment of case–control studies. Statistical analysis was performed with the software Stata 12.0. Twelve eligible studies with a total of 1097 patients were included in this meta-analysis. Survivin overexpression was closely related to FIGO stage (I-II vs. III-IV) of ovarian carcinoma (odds ratio [OR] = 0.26,95% confidence interval [CI]:0.16,0.42),P<0.00001),tumor grade (G1-G2 vs. G3) (OR = 0.29,95%CI(0.17, 0.51),P <0.0001), but was not significantly associated with lymphatic metastasis (OR = 1.53, 95%CI(0.77, 3.03, P = 0.23),ascites (OR = 0.89,95%CI(0.39,2.05),P = 0.79). Our meta-analysis shows that survivin is strongly associated with FIGO stage and tumor grade of ovarian carcinoma. Maybe survivin is a novel clinicopathological marker of ovarian carcinoma.
Introduction
Presently, ovarian cancer still remains the most fatal cancer of the female reproductive tract [1] due to vague symptomatology and the absence of reliable screening tests in the early stages[2]. The majority of these patients are diagnosed when treatment options are limited, and the overall 5-year survival rates do not exceed 30% [3]. Survivin is a member of the inhibitors of the apoptosis protein (IAP) family, and undetectable in normal adult tissues but highly expressed in several types of cancer, with a potential involvement in malignant transformation and tumor growth [4, 5]. High expression levels of this antiapoptotic protein have been previously found and shown to predict poorer prognosis and shorter survival in a wide range of human cancers, including the gastrocolic carcinoma [6, 7], breast cancer [8], lung [9], and ovary cancer [10, 11]. Thus, survivin is considered a novel clinicopathological marker for numerous human malignant tumors and may be an important prognostic marker in cancer. However, the clinicopathological features associated with survivin expression in ovarian carcinoma remain controversial. To more precisely evaluate the relationship between surviving expression and clinicopathological outcome in ovarian carcinoma, we conducted a meta-analysis of 12 published studies.
Methods
Search strategy
We systematically searched PubMed, EMBASE, Web of Science, and The Cochrane Library databases for studies in humans on survivin expression with ovarian cancer and its clinicopathological characteristics, which were published from inception to September, 2017. Computer searches used combinations of subject headings or other key words by the following search strategy: (Ovarian tumor OR Ovarian cancer OR Ovarian carcinoma OR Ovarian malignancy OR Ovarian Neoplasms) AND (Survivin). PubMed database was searched as following: #1 Ovarian tumor[Title/Abstract] OR Ovarian cancer[Title/Abstract] OR Ovarian carcinoma[Title/Abstract] OR Ovarian malignancy[Title/Abstract] OR Ovarian Neoplasms[Title/Abstract] #2 "Ovarian Neoplasms"[Mesh] #3 #1 OR #2 #4 Survivin [Title/Abstract] #5 “Survivin”[Mesh] #6 #4 OR #5 #7 #3 AND #6.
Study selection
Included studies must meet the following criteria: (1) case-control study focus on the association between the survivin expression and ovarian cancer and its clinicopathological variables;(2)immunohistochemistry(IHC) or real-time PCR (RT-PCR) analysis to evaluate survivin expression in ovarian carcinoma; (3) None of the patients received radiotherapy, chemotherapy or tumor drug treatment before surgery; (4) All cases were not limited with race, nationality or age. The following studies were excluded: (1) conference abstracts, letters, reviews, case reports, commentaries, or expert opinions; (2) studies with insufficient information on clinicopathological characteristics.
Quality assessment
Study quality was independently assessed by two investigators according to the Newcastle-Ottawa Scale (NOS) for quality assessment of case–control studies [12]. The criteria were quality of selection, comparability, exposure and outcome; the scales allocate stars, with a maximum of nine. The study was evaluated as low quality when the score ≤5 and excluded from our study, by contrast, the study was evaluated as high quality when the score was ≥ 6 and included in our meta-analysis.
Data extraction
Two investigators independently extracted data that met our inclusion and exclusion criteria. Any discrepancies were resolved by consensus. We extracted the following information: name of the first author, year of publication, country, specific outcomes, total number of individuals, number of cases and controls, clinicopathological characteristics.
Statistical analysis
Pooled estimates of ORs with 95% CIs were used to evaluate the associations between survivin expression and clinicopathological characteristics of ovarian cancer. Heterogeneity among studies was evaluated with the Cochran Q test and I2 statistic [13], inter-study heterogeneity was assumed in cases in which I2 >50%, and ORs were pooled according to random-effects models. Alternatively, fixed-effects models were used. Sensitivity analyses evaluated whether the results could have been affected markedly by a single study [14]. In addition, Begg’s funnel plots and Egger’s test were used to statistically assess publication bias, there existed publication bias when p-value less than 0.05 [15]. We used STATA version 12.0 for all statistical analyses and to produce the forest plot. A p-value less than .05 was considered statistically significant.
Results
The initial search in the electronic databases identified 1195 articles. After excluding duplicate citations and studies that did not meet the inclusion criteria, twelve publications [11, 16–26] were finally included in the meta-analysis (Fig 1). A description of the baseline characteristics are summarized in Table 1. The analysis included 566 ovarian carcinoma cases. Study quality scores (range, 0–9) averaged 7.0, with a proportion of high-quality studies (quality score C8) of 33.3%.
Fig 1. Flow diagram of the study selection process.
Table 1. Characteristics and results of the included studies.
| Study | Country | No.of P.(1097) | Method | FIGOStage (Ⅰ-Ⅱ/Ⅲ-Ⅳ) | tumor grade(G1/G2/G3) | lymph nodly metastasis(yes /no) | Survivin(+) | NOS | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ovarian carcinoma | Borderline ovarian tumor | Ovarian benign tumor | Normal ovarian tissues | ||||||||
| Sui[11]2002 | Japan | 103 | IHC | 19/28 | 21/13/13 | 24/19 | 24 | 11 | 7 | 7 | |
| Ju LL[16]2016 | China | 60 | IHC | 19/20 | 8/31* | 8 | 3 | 1 | 8 | ||
| Kanter M[17]2016 | Turkey | 98 | IHC | 37 | 4 | 3 | 8 | ||||
| Plewka D[18]2015 | Europe | 157 | IHC | 41 | 22 | 13 | 0 | 6 | |||
| Turan G[19]2014 | Turkey | 62 | IHC | 21 | 10 | 6 | 8 | ||||
| Qian X[20]2011 | China | 91 | IHC | 55 | 4 | 0 | 7 | ||||
| Huang Y[21]2011 | China | 65 | IHC | 7/18 | 2/23* | 26 | 6 | 5 | 8 | ||
| Liguang Z[22]2007 | China | 114 | RT-PCR | 28/35 | 30Δ/33 | 34/29 | 46 | 9 | 4 | 0 | 6 |
| Gao Q[23]2007 | China | 70 | IHC | 10/36 | 22Δ/24 | 28 | 5 | 0 | 7 | ||
| Yin RT[24]2006 | China | 69 | IHC | 10/28 | 13Δ/25 | 18/13 | 29 | 9 | 0 | 7 | |
| Ma XY[25]2006 | China | 143 | IHC | 41/43 | 32/34/18 | 53 | 12 | 0 | 7 | ||
| Zhang SL[26]2003 | China | 65 | RT-PCR | 14/21 | 24Δ/11 | 13/22 | 29 | 8 | 2 | 0 | 6 |
*, G2-G3
Δ, G1-G2
No. of P, number of patients; NOS, Newcastle-Ottawa quality assessment scale; RT-PCR, reverse transcription polymerase chain reaction; IHC, immunohistochemistry
Ovarian carcinoma vs normal ovarian tissues
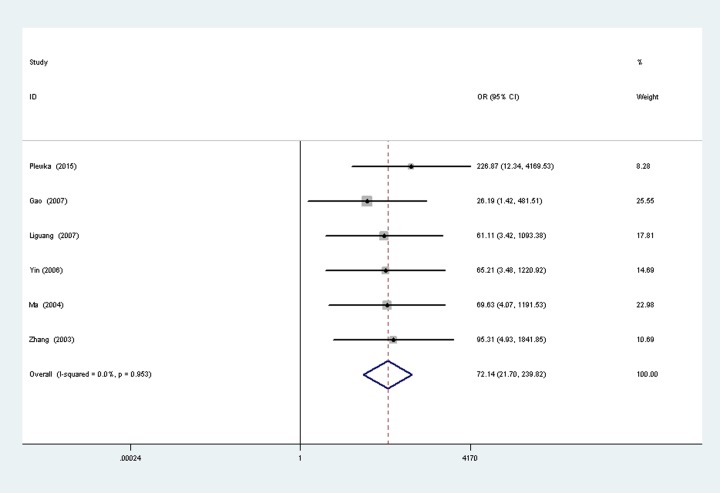
A total of six studies [18, 22–26] reported the survivin expression in ovarian cancer vs normal ovarian tissues, with 314 cases of ovarian cancer patients and 79 normal women. There was no significant heterogeneity between the two groups (P = 0.95, I2 = 0.0%), so fixed-effects models was used to analysis (Fig 2). Results show that the survivin expression in ovarian cancer is higher than normal group, the difference was statistically significant (OR = 72.14, 95% CI (21.70, 21.70), P < 0.00001).
Fig 2. Forest plot depiction of survivin expression and odds ratio (OR) for ovarian carcinoma vs normal ovarian tissues.
Ovarian carcinoma vs ovarian benign tumor
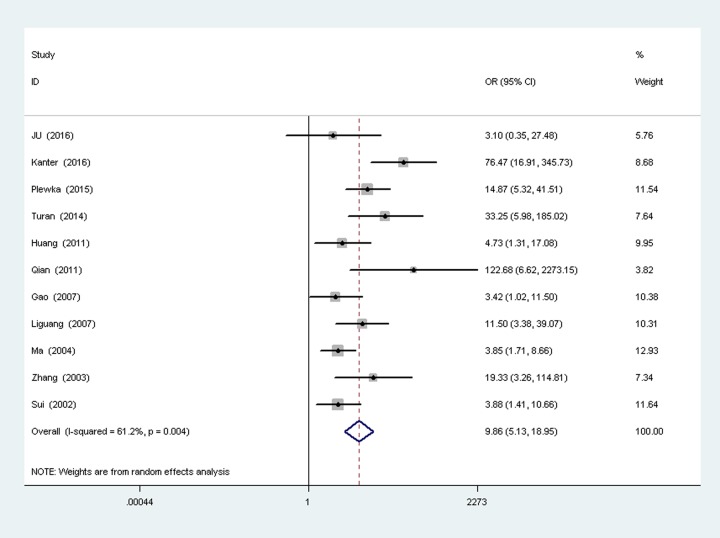
A total of eleven studies [11, 16–23, 25, 26] reported the survivin expression in ovarian cancer vs ovarian benign tumor, with 528 cases of ovarian cancer patients and 262 ovarian benign tumor patients. There was heterogeneity between the two groups (P = 0.004,I 2 = 61.2%), so random-effects models was used to analysis (Fig 3). Results show that the surviving expression in ovarian cancer is higher than ovarian benign tumor, the difference was statistically significant (OR = 9.86, 95%CI(5.13,18.95), P<0.00001).
Fig 3. Forest plot depiction of survivin expression and odds ratio (OR) for ovarian carcinoma vs ovarian benign tumor.
Ovarian carcinoma vs borderline ovarian tumor
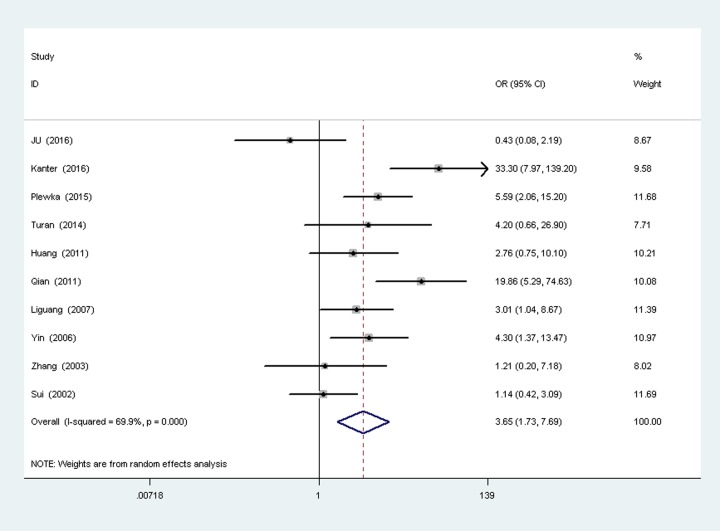
A total of ten studies [11, 16–22, 24, 26] reported the survivin expression in ovarian cancer vs borderline ovarian tumor, with 436 cases of ovarian cancer patients and 190 ovarian borderline tumor patients. There was heterogeneity between the two groups (P = 0.0000,I2 = 69.9%), so random-effects models was used to analysis (Fig 4). Results show that the surviving expression in ovarian cancer is higher than borderline ovarian tumor, the difference was statistically significant (OR = 3.65,9%CI(1.73,7.69), P = 0.000).
Fig 4. Forest plot depiction of survivin expression and odds ratio (OR) for ovarian carcinoma vs borderline ovarian tumor.
Survivin expression with clinicopathological characteristics
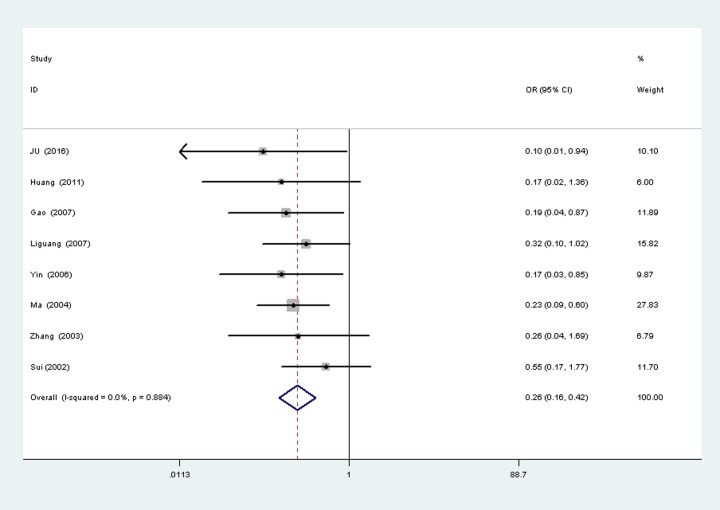
Survivin expression with FIGO stage A total of eight studies [11, 16, 21–26] reported the surviving expression in different FIGO stage of ovarian cancer, with 148 cases of Ⅰ-Ⅱ and cases of Ⅲ- Ⅳ. There was no significant heterogeneity between the two groups (P = 0.884,I2 = 0%), so fixed-effects models was used to analysis (Fig 5). Results show that the surviving Expression in Ⅰ-Ⅱ is higher than Ⅲ- Ⅳ, the difference was statistically significant (OR = 0.26, 95%CI(0.16,0.42), P<0.00001).
Fig 5. Tumor FIGO stage.
Survivin expression with tumor grade
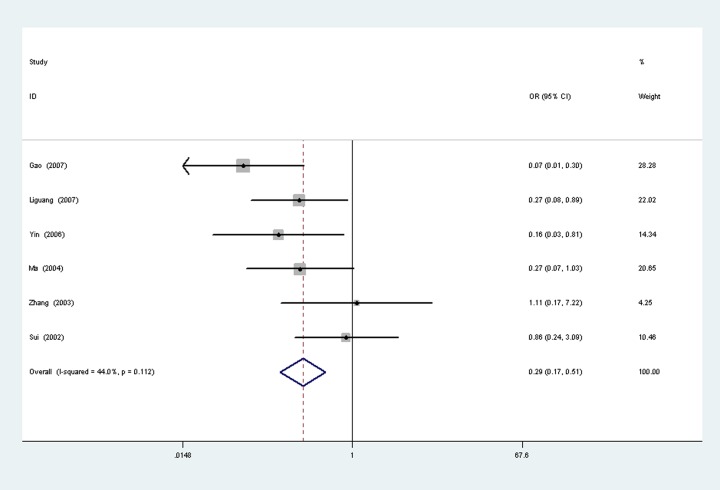
A total of six studies [11, 22–26] reported the survivin expression in different tumor grade of ovarian cancer, with 189 cases of G1-G2 and 124 cases of G3. There was no significant heterogeneity between the two groups (P = 0.112,I 2 = 44%), so fixed-effects models was used to analysis (Fig 6). Results show that the survivin expression in G1-G2 is lower than G3, the difference was statistically significant (OR = 0.29,95%CI(0.17,0.51), P<0.0001).
Fig 6. Tumor grade.
Survivin expression with lymphatic metastasis
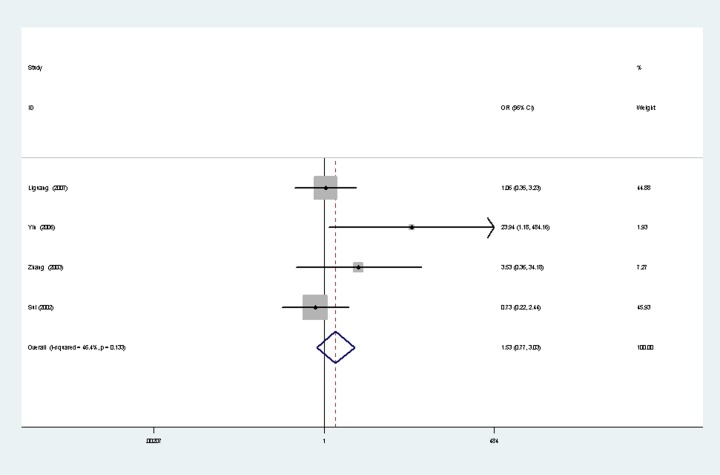
A total of four studies [11, 22, 24, 26] reported the survivin expression in lymphatic metastasis of ovarian cancer, with 89 cases of metastasis and 83 cases of non-metastasis. There was no significant heterogeneity between the two groups (P = 0.133, I2 = 46.4%), so fixed-effects models was used to analysis (Fig 7). Results show that survivin expression was not significantly associated with lymphatic metastasis (OR = 1.53,95%CI(0.77, 3.03, P = 0.23).
Fig 7. Lymphatic metastasis.
Sensitivity analysis
A sensitivity analysis omitting one study at a time and calculating the pooled ORs for the remainder of the studies in ovarian cancer vs ovarian benign tumor showed that the studies by Kanter et al.[17], Qian et al. [20] and Ma et al.[25] substantially influenced the pooled risk estimates. By excluding these three studies, heterogeneity decreased dramatically (I2 = 33.4%; Table 2). In ovarian carcinoma vs borderline ovarian tumor, the studies by JU et al.[16], Kanter etal. [17], Qian et al.[20] and Sui et al. [11] substantially influenced the pooled risk estimates. By excluding these four studies, heterogeneity decreased dramatically (I2 = 0%; Table 2).
Table 2. Sensitivity analyses.
| Groups | Studies(n) | OR (95%CI) | heterogeneity test | |
|---|---|---|---|---|
| P | I2(%) | |||
| Ovarian cancer vs Ovarian benign tumor | ||||
| All studies | 11 | 9.86(5.13–18.95) | 0.004 | 61.2 |
| Omitting Kanter M[17] | 10 | 7.25(4.95–10.61) | 0.057 | 45.5 |
| Omitting Qian X[20] | 10 | 8.85(4.68–16.72) | 0.008 | 59.5 |
| Omitting Ma XY[25] | 10 | 11.36(5.60–23.07) | 0.010 | 58.5 |
| Omitting Kanter M[17],Qian X[20],Ma XY[25] | 8 | 7.66(4.88–12.02) | 0.161 | 33.4 |
| Ovarian cancer vs Borderline ovarian tumor | ||||
| All studies | 10 | 3.65(1.73–7.69) | 0.000 | 69.9 |
| Omitting Ju LL[16] | 9 | 4.46(2.18–9.10) | 0.004 | 65.1 |
| Omitting Kanter M[17] | 9 | 2.92(1.49–5.73) | 0.011 | 59.8 |
| Omitting Qian X[20] | 9 | 3.03(1.46–6.28) | 0.003 | 65.2 |
| Omitting Sui[11] | 9 | 4.27(1.98–9.21) | 0.003 | 66.3 |
| Omitting Ju LL[16], Kanter M[17],QianX[20], and Sui[11] | 6 | 3.58(2.18–5.90) | 0.765 | 0.0 |
Publication bias
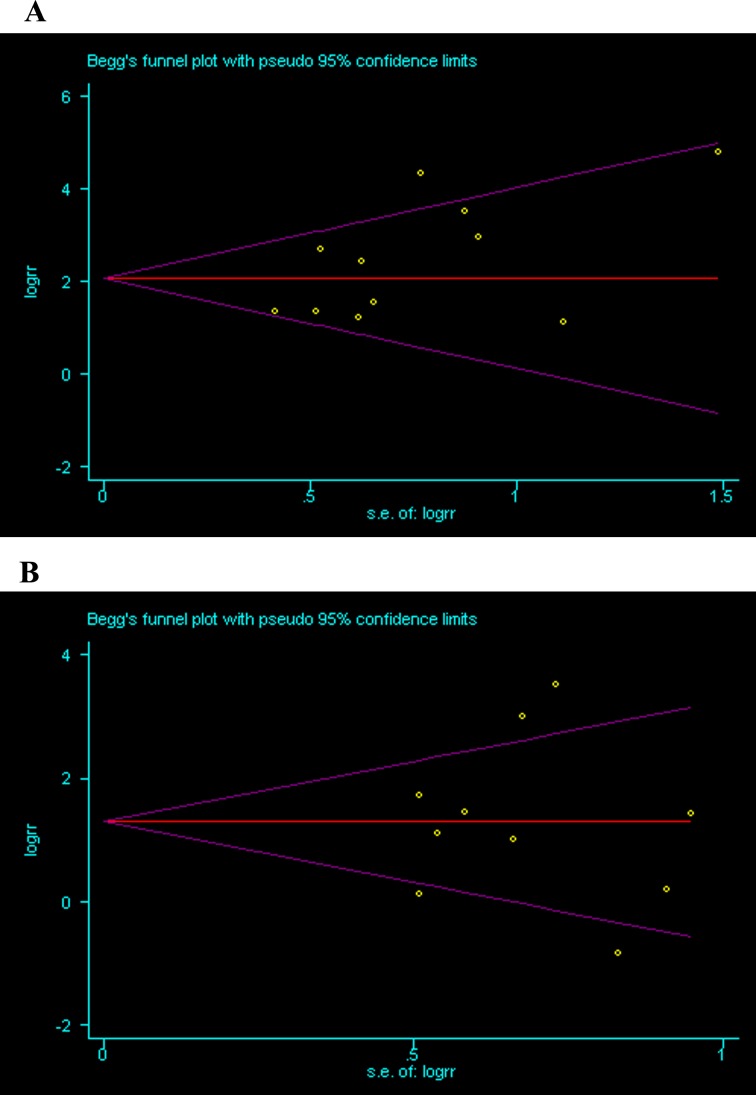
Begg’s funnelplots and Egger’s test were used to assess publication bias in the meta-analysis. There was no indication for publication bias in the Egger’s test of ovarian carcinoma vs ovarian benign tumor (P = 0.073) and ovarian carcinoma vs borderline ovarian tumor (P = 1.000) (Fig 8A and 8B).
Fig 8.
A Ovarian carcinoma vs ovarian benign tumor, B ovarian carcinoma vs borderline ovarian tumor.
Discussion
Ovarian cancer is one of the most common female cancers and the most frequent cause of gynecologic cancer-related deaths in the word. Hence, presence of biomarkers for the diagnosis, treatment and prediction of prognosis of ovarian neoplasms might guide the management of these patients. Survivin overexpression was found to be related with the aggressiveness of the tumor[27]. The use of survivin expression as a clinicopathological marker of malignancy has received increasing attention. Over expression of survivin has been observed in esophageal, gastric, lung and cervical cancer tissues and represents a poor prognostic factor in these cancer patients [28–32]. Inhibition of apoptosis by survivin is associated with the mitotic spindle assembly checkpoint [33, 34]. Thus, survivin may be a potential target in the treatment of epithelial ovarian cancers. However, despite presence of many studies, definitive role of survivin in the progress of ovarian cancer remains unknown [10, 11, 20, 35] In this study, we investigated the expression and clinical significance of survivin in ovarian cancer in order to find the relationship between them. We found that survivin expression was positively correlated with the FIGO stage and tumor grade. These findings may implicate that survivin has a possible role in malignant potential of ovarian neoplasms. The results of quality evaluation of included studies in our meta-analysis showed that the average of NOS score was 7, and no study was evaluated less than 5, which illustrated that the quality of included studies in our meta-analysis were high. Our meta-analysis results indicate that, survivin expression in the ovarian cancer group is significantly higher than in the normal tissues and benign ovarian tumors (the OR value are as high as 72.14 and 9.86 respectively), with significant difference (P < 0.00001). In normal ovarian tissues and benign ovarian tumor could hardly detect survivin, indicate that survivin has not been activated, survivin was not involved in apoptosis regulation of normal ovarian cells and benign ovarian tissues. However, survivin expressed in ovarian cancer stablely, abnormal expression of survivin may result in the occurrence of ovarian cancer by inhibiting apoptosis. Survivin expression in ovarian cancer is closely related to FIGO stage, tumor grade. The differences of I—II compared with III—IV, G1-G2 compared with G3 were statistically significant (OR = 0.26, OR = 0.29 respectively, P < 0.00001). This suggest the later clinical stage, lower histological differentiation degree, the higher survivin positive expression. Survivin is correlated with the malignant degree of ovarian cancer. Altieri [36] found that survivin over expression was related to tumor metastasis. Zhou [37] found that survivin could promote the transfer of corpus cancer, but our study shows that survivin positive rate between lymph node metastasis and non-lymph metastasis was no statistically different (OR = 1.53, 95%CI(0.77,3.03, P = 0.23,P>0.05). This may be relevant to the different tumor types, or insufficiency of the number of research, or the various different experiment conditions, or the judgment of positive deviation. However, there were limitations to this meta-analysis. First, the techniques used to detect survivin may have differed between the included studies. We determined that IHC and RT-PCR are equally important for the detection of survivin. Second, the survivin antibodies used in the included studies do not discriminate survivin isoforms, so the results may reflect increases in total survivin levels. We suggest that these preliminary findings warrant further analyses in the future. Third, there is the possibility of publication bias, because small studies with null results tend not to be published, as well as outcome reporting biases. In conclusion, despite the above limitations, our meta-analysis supports that survivin overexpression is associated with FIGO stage and tumor grade of ovarian cancer. However, larger clinical studies must be performed to more thoroughly investigate the precise clinicopathological features associated with survivin.
Supporting information
(ZIP)
Acknowledgments
KHY contributed to study concept and design; LY and HFW contributed to literature search, review of the studies, and data extractions; HLW and XHC contributed to data analysis and the study quality evaluation; XYH, SYM contributed to verify the statistical analysis and scrutinized data and supervision throughout the whole study. All the authors contributed writing the manuscript. All authors approved the final version of the manuscript.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The fund that supported the study was paid by the first authors.
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA: a cancer journal for clinicians. 2009;59(4):225–49. Epub 2009/05/29. doi: 10.3322/caac.20006 . [DOI] [PubMed] [Google Scholar]
- 2.Goff BA, Mandel L, Muntz HG, Melancon CH. Ovarian carcinoma diagnosis. Cancer. 2000;89(10):2068–75. Epub 2000/11/07. . [DOI] [PubMed] [Google Scholar]
- 3.FIGO (International Federation of Gynecology and Obstetrics) annual report on the results of treatment in gynecological cancer. International journal of gynaecology and obstetrics: the official organ of the International Federation of Gynaecology and Obstetrics. 2003;83 Suppl 1:ix–xxii, 1–229. Epub 2004/02/07. . [PubMed] [Google Scholar]
- 4.Yamashita S, Masuda Y, Kurizaki T, Haga Y, Murayama T, Ikei S, et al. Survivin expression predicts early recurrence in early-stage breast cancer. Anticancer research. 2007;27(4c):2803–8. Epub 2007/08/19. . [PubMed] [Google Scholar]
- 5.Gordon GJ, Mani M, Mukhopadhyay L, Dong L, Yeap BY, Sugarbaker DJ, et al. Inhibitor of apoptosis proteins are regulated by tumour necrosis factor-alpha in malignant pleural mesothelioma. The Journal of pathology. 2007;211(4):439–46. Epub 2007/01/27. doi: 10.1002/path.2120 . [DOI] [PubMed] [Google Scholar]
- 6.Kawasaki H, Altieri DC, Lu CD, Toyoda M, Tenjo T, Tanigawa N. Inhibition of apoptosis by survivin predicts shorter survival rates in colorectal cancer. Cancer research. 1998;58(22):5071–4. Epub 1998/11/21. . [PubMed] [Google Scholar]
- 7.Sarela AI, Macadam RC, Farmery SM, Markham AF, Guillou PJ. Expression of the antiapoptosis gene, survivin, predicts death from recurrent colorectal carcinoma. Gut. 2000;46(5):645–50. Epub 2000/04/15. doi: 10.1136/gut.46.5.645 ; PubMed Central PMCID: PMCPMC1727921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tanaka K, Iwamoto S, Gon G, Nohara T, Iwamoto M, Tanigawa N. Expression of survivin and its relationship to loss of apoptosis in breast carcinomas. Clinical cancer research: an official journal of the American Association for Cancer Research. 2000;6(1):127–34. Epub 2000/02/03. . [PubMed] [Google Scholar]
- 9.Monzo M, Rosell R, Felip E, Astudillo J, Sanchez JJ, Maestre J, et al. A novel anti-apoptosis gene: Re-expression of survivin messenger RNA as a prognosis marker in non-small-cell lung cancers. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 1999;17(7):2100–4. Epub 1999/11/24. doi: 10.1200/jco.1999.17.7.2100 . [DOI] [PubMed] [Google Scholar]
- 10.Takai N, Miyazaki T, Nishida M, Nasu K, Miyakawa I. Expression of survivin is associated with malignant potential in epithelial ovarian carcinoma. International journal of molecular medicine. 2002;10(2):211–6. Epub 2002/07/18. . [PubMed] [Google Scholar]
- 11.Sui L, Dong Y, Ohno M, Watanabe Y, Sugimoto K, Tokuda M. Survivin expression and its correlation with cell proliferation and prognosis in epithelial ovarian tumors. International journal of oncology. 2002;21(2):315–20. Epub 2002/07/16. . [PubMed] [Google Scholar]
- 12.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. European journal of epidemiology. 2010;25(9):603–5. Epub 2010/07/24. doi: 10.1007/s10654-010-9491-z . [DOI] [PubMed] [Google Scholar]
- 13.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Statistics in medicine. 2002;21(11):1539–58. Epub 2002/07/12. doi: 10.1002/sim.1186 . [DOI] [PubMed] [Google Scholar]
- 14.Tobias A. Assessing the influence of a single study in the meta-analysis estimate. Stata Technical Bulletin. 1999;8(41):7526–9. [Google Scholar]
- 15.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ (Clinical research ed). 1997;315(7109):629–34. Epub 1997/10/06. ; PubMed Central PMCID: PMCPMC2127453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ju LL, Zhao CY, Ye KF, Yang H, Zhang J. Expression and clinical implication of Beclin1, HMGB1, p62, survivin, BRCA1 and ERCC1 in epithelial ovarian tumor tissues. European review for medical and pharmacological sciences. 2016;20(10):1993–2003. Epub 2016/06/02. . [PubMed] [Google Scholar]
- 17.Kanter M, Turan G, Usta C, Usta A, Esen HH, Tavli L, et al. Survivin and cycline D1 expressions are associated with malignant potential in mucinous ovarian neoplasms. Journal of molecular histology. 2016;47(2):145–52. Epub 2016/01/28. doi: 10.1007/s10735-016-9661-8 . [DOI] [PubMed] [Google Scholar]
- 18.Plewka D, Jakubiec-Bartnik B, Morek M, Bogunia E, Bienioszek M, Wolski H, et al. Survivin in ovary tumors. Ginekologia polska. 2015;86(7):525–30. Epub 2015/09/18. . [DOI] [PubMed] [Google Scholar]
- 19.Turan G, Usta CS, Usta A, Kanter M, Tavli L, Karacan M, et al. The expression of HER-2/neu (c-erbB2), survivin and cycline D1 in serous ovarian neoplasms: their correlation with clinicopathological variables. Journal of molecular histology. 2014;45(6):679–87. Epub 2014/08/12. doi: 10.1007/s10735-014-9591-2 . [DOI] [PubMed] [Google Scholar]
- 20.Qian X, Xi X, Li L. Nuclear survivin is associated with malignant potential in epithelial ovarian carcinoma. Applied immunohistochemistry & molecular morphology: AIMM. 2011;19(2):126–32. Epub 2010/08/21. doi: 10.1097/PAI.0b013e3181e30dcd . [DOI] [PubMed] [Google Scholar]
- 21.Huang Y, Jin H, Liu Y, Zhou J, Ding J, Cheng KW, et al. FSH inhibits ovarian cancer cell apoptosis by up-regulating survivin and down-regulating PDCD6 and DR5. Endocrine-related cancer. 2011;18(1):13–26. Epub 2010/10/15. doi: 10.1677/ERC-09-0308 . [DOI] [PubMed] [Google Scholar]
- 22.Liguang Z, Peishu L, Hongluan M, Hong J, Rong W, Wachtel MS, et al. Survivin expression in ovarian cancer. Experimental oncology. 2007;29(2):121–5. Epub 2007/08/21. . [PubMed] [Google Scholar]
- 23.Gao Q, Xing L, Wang Y. The expression of survivin and P-gp proteins in epithelial ovarian carcinoma and its relationship with multidrug resistance in ovarian carcinoma. Journal of Xian Jiaotong University. 2007. [Google Scholar]
- 24.Yin RT, Peng ZL, Yang KX, Kang DY. [Survivin expression in ovarian carcinoma]. Sichuan da xue xue bao Yi xue ban = Journal of Sichuan University Medical science edition. 2006;37(2):215–7, 33. Epub 2006/04/13. . [PubMed] [Google Scholar]
- 25.Ma XY, He FX, Wu SF, Lu YP, Ma D. [Expression of survivin in ovarian epithelial carcinoma and its correlation with expression of Fas and FasL]. Ai zheng = Aizheng = Chinese journal of cancer. 2004;23(2):173–6. Epub 2004/02/13. . [PubMed] [Google Scholar]
- 26.Zhang SL, Zhao CQ, Lin B, Li Y, Gao H. [Expression of survivin gene and its relation with the expression of bcl-2 and bax protein in epithelial ovarian cancer]. Zhonghua fu chan ke za zhi. 2003;38(4):203–6. Epub 2003/07/30. . [PubMed] [Google Scholar]
- 27.Zaffaroni N, Pennati M, Colella G, Perego P, Supino R, Gatti L, et al. Expression of the anti-apoptotic gene survivin correlates with taxol resistance in human ovarian cancer. Cellular and molecular life sciences: CMLS. 2002;59(8):1406–12. Epub 2002/10/05. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lu CD, Altieri DC, Tanigawa N. Expression of a novel antiapoptosis gene, survivin, correlated with tumor cell apoptosis and p53 accumulation in gastric carcinomas. Cancer research. 1998;58(9):1808–12. Epub 1998/05/15. . [PubMed] [Google Scholar]
- 29.Zhang LQ, Wang J, Jiang F, Xu L, Liu FY, Yin R. Prognostic value of survivin in patients with non-small cell lung carcinoma: a systematic review with meta-analysis. PloS one. 2012;7(3):e34100 Epub 2012/03/30. doi: 10.1371/journal.pone.0034100 ; PubMed Central PMCID: PMCPMC3311582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Okada E, Murai Y, Matsui K, Isizawa S, Cheng C, Masuda M, et al. Survivin expression in tumor cell nuclei is predictive of a favorable prognosis in gastric cancer patients. Cancer letters. 2001;163(1):109–16. Epub 2001/02/13. . [DOI] [PubMed] [Google Scholar]
- 31.Li C, Li Z, Zhu M, Zhao T, Chen L, Ji W, et al. Clinicopathological and prognostic significance of survivin over-expression in patients with esophageal squamous cell carcinoma: a meta-analysis. PloS one. 2012;7(9):e44764 Epub 2012/10/03. doi: 10.1371/journal.pone.0044764 ; PubMed Central PMCID: PMCPMC3459962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu HQ, Wang YH, Wang LL, Hao M. P16INK4A and survivin: Diagnostic and prognostic markers in cervical intraepithelial neoplasia and cervical squamous cell carcinoma. Experimental and molecular pathology. 2015;99(1):44–9. Epub 2015/04/26. doi: 10.1016/j.yexmp.2015.04.004 . [DOI] [PubMed] [Google Scholar]
- 33.Altieri DC. The case for survivin as a regulator of microtubule dynamics and cell-death decisions. Current opinion in cell biology. 2006;18(6):609–15. Epub 2006/08/29. doi: 10.1016/j.ceb.2006.08.015 . [DOI] [PubMed] [Google Scholar]
- 34.Wheatley SP, McNeish IA. Survivin: a protein with dual roles in mitosis and apoptosis. International review of cytology. 2005;247:35–88. Epub 2005/12/14. doi: 10.1016/S0074-7696(05)47002-3 . [DOI] [PubMed] [Google Scholar]
- 35.Ferrandina G, Legge F, Martinelli E, Ranelletti FO, Zannoni GF, Lauriola L, et al. Survivin expression in ovarian cancer and its correlation with clinico-pathological, surgical and apoptosis-related parameters. British journal of cancer. 2005;92(2):271–7. Epub 2005/01/19. doi: 10.1038/sj.bjc.6602332 ; PubMed Central PMCID: PMCPMC2361852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Altieri DC. Validating survivin as a cancer therapeutic target. Nature reviews Cancer. 2003;3(1):46–54. Epub 2003/01/02. doi: 10.1038/nrc968 . [DOI] [PubMed] [Google Scholar]
- 37.Zhou XL, Wang M. Expression levels of survivin, Bcl-2, and KAI1 proteins in cervical cancer and their correlation with metastasis. Genetics and molecular research: GMR. 2015;14(4):17059–67. Epub 2015/12/19. doi: 10.4238/2015.December.16.6 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(ZIP)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.