Abstract
The ‘SCOOP’ study was a two-arm randomised controlled trial conducted in the UK in 12 483 eligible women aged 70 to 85 years. It compared a screening programme using the FRAX® risk assessment tool in addition to BMD measures versus usual management. The SCOOP study found a reduction in the incidence of hip fractures in the screening arm, but there was no evidence of a reduction in the incidence of all osteoporosis related fractures. In order to make decisions about whether to implement any screening programme we should also consider whether the programme is likely to be a good use of health care resources, i.e., is it cost-effective? The cost per gained quality adjusted life year (QALY) of screening for fracture risk has not previously been demonstrated in an economic evaluation alongside a clinical trial. We conducted a ‘within trial’ economic analysis alongside the ‘SCOOP’ study from the perspective of a National Health payer, the UK National Health Service (NHS). The main outcome measure in the economic analysis was the cost per quality adjusted life year (QALY) gained over a 5-year time period. We also estimated cost per osteoporosis-related fracture prevented and the cost per hip fracture prevented. The screening arm had an average incremental QALY gain of 0.0237 (-0.0034, 0.0508) for the five year follow-up. The incremental cost per QALY gained was £2,772 compared to the control arm. Cost-effectiveness acceptability curves indicated a 93% probability of the intervention being cost-effective at values of a QALY greater than £20,000. The intervention arm prevented fractures at a cost of £4,478 and £7,694 per fracture for osteoporosis-related and hip fractures respectively. The current study demonstrates that a systematic, community-based screening programme of fracture risk in older women in the UK represents a highly cost-effective intervention.
Keywords: Health Economics, DXA, screening, osteoporosis, fracture prevention
Introduction
There are approximately 9 million osteoporotic or fragility (low trauma) fractures worldwide per year.(1) In developed nations, around one in three women and one in five men aged 50 years or more will suffer a fragility fracture during their remaining lifetime, most commonly at sites such as the hip, distal forearm, vertebrae and humerus. In the UK, around 536,000 people suffer fragility fractures each year, including 79,000 hip fractures, with a cost in 2010 estimated at £3.5 billion expected to rise to £5.5 billion per year by 2025.(2) For the individual, a hip fracture can be devastating with loss of independence and less than one third of patients make a full recovery; mortality at one year post-fracture is approximately 20%.(3)
Advances in osteoporosis management over the last two decades have included the development of bone-strengthening treatments and also fracture risk assessment tools, such as FRAX®, improving the ability to target treatment to those most likely to fracture. These elements provide the potential for a community-based screening programme to reduce fracture rates. The aim of the SCOOP (‘screening for prevention of fractures in older women’) trial was to assess the effectiveness and cost-effectiveness of a FRAX-based screening programme for older UK women. This screening programme used a baseline questionnaire to assess 10-year risk using the FRAX risk algorithm. (4) Individuals judged to have sufficiently high risk were invited to undergo DXA based bone mineral density (BMD) measurement and this information was used to recalculate the 10-year hip fracture probability. This information was communicated to the participant and their family doctor. We have recently reported the effectiveness results,(5) which concluded that there was a potential to reduce hip fracture rates substantially over five years (hazard ratio: 0.72, p=0.002), though not fractures at other sites (hazard ratio: 0.94, p=0.178).
There is an extensive literature evaluating the cost-effectiveness of interventions for osteoporosis. However, this has almost exclusively used economic modelling. The breadth of this literature is illustrated by two recent systematic reviews of models to estimate the cost-effectiveness of preventing osteoporotic fractures. Si et al examined the evolution of health economic models aimed at strategies for preventing osteoporotic fractures and identified 104 studies relating to 74 different models, published between 1980 and 2013.(6) They found that models have evolved in terms of complexity and emphasis. Hiligsmann et al looked at cost-effectiveness analyses of drugs for postmenopausal osteoporosis and identified 39 studies, between 2008 and 2013.(7) These authors concluded that active osteoporotic drugs were generally cost-effective in postmenopausal women over the age of 60, particularly if they had other risk factors. Many of these studies have estimated outcomes in terms of cost per quality adjusted life year (QALY). However, we are aware of no published study that has estimated cost per QALY using an economic evaluation conducted alongside a randomised controlled trial of screening to prevent fractures. The aim of the current study was to use resource-use and outcome data collected as part of the SCOOP study to estimate the cost-effectiveness of the SCOOP screening intervention over a five-year time horizon.
Materials and Methods
The Clinical Trial
The SCOOP trial has been described elsewhere.(5, 8) In brief, SCOOP is an evaluation of screening aimed at identifying older women at increased risk of fragility fractures. The study was conducted across seven UK geographical regions as a pragmatic, randomised controlled trial. A total of 12,483 women, aged 70 to 85 years were consented into the trial by post via primary care. Women already on prescriptions for anti-osteoporosis medicines (apart from vitamin D or calcium) were excluded. Participants randomised to the screening arm had 10-year hip fracture probabilities computed from clinical risk factors using the FRAX tool. Those above an age-dependent threshold were invited to have a bone mineral density (BMD) assessment using dual-energy X-ray absorptiometry (DXA). Individuals subsequently above a second age-dependent threshold, with the inclusion of the BMD measure, were recommended for treatment via their General Practitioner (GP). Participants in the control arm received standard management: this included referral for DXA scans and anti-osteoporosis treatments if deemed clinically appropriate by their GP. Data collection followed at six months post-randomisation and annually thereafter for five years. The primary outcome measure was the proportion of individuals sustaining at least one osteoporosis-related fracture, the assessment of which is summarised below. A number of secondary outcomes measures were also collected in the trial: including; hip fractures, all clinical fractures; mortality; health related quality of life; and health care resource use data.
Measurement of outcomes
Data were requested on an annual basis from 2009 to 2014 from NHS Digital, formerly the Health and Social Care Information Centre (HSCIC). (9) This comprised admitted patient care (inpatient), outpatient, and accident and emergency (A&E) datasets. Data were interrogated to identify fractures in study participants from randomisation to the end of follow-up. Primary care records were also screened for fractures based on their GP Read codes. Participants could also self-report fractures at each follow-up. In the case of self-report and A&E reported fractures, or for other sources where there was missing information on dates or anatomical site, further verification was sought. This included requests to primary care practices or searches of radiological records at local hospitals. Only verified fractures were included as outcomes.(5)
The main outcome measure used in the economic evaluation was the quality adjusted life year (QALY). This was assessed using the 3-level EQ-5D(10) by means of a postal questionnaire and scored using the published tariff.(11) The EQ-5D was assessed at baseline, 6-months, 12-months and then annually thereafter up to five years’ follow-up. We estimated QALY by ‘area-under-the-curve’ at these time points assuming a linear relationship between each EQ-5D value. Any participant who died was assumed to have an EQ-5D score of zero. A secondary analysis was performed using cost per fracture avoided for both any probable osteoporotic-related fracture (defined as fractures of hip, wrist and spine), and hip fractures only.
Costs
Cost of the Screening Intervention
Primary care practices identified eligible women from their lists who were then invited to participate. Those individuals who agreed constituted the SCOOP cohort and were randomised to the intervention or control arms. Those in the intervention arm had their fracture risk assessed, and participants and GPs were notified of fracture risk. The resources required to undertake the relevant processes (BMD measurement via DXA scans, calculation and clinical review of final fracture risk, written notification of initial and final fracture risk, and a GP consultation for identified high fracture risk individuals) were recorded as part of the SCOOP study. All costs were either costed using data collected as part of the study, or were costed using appropriate unit cost data.(12, 13) A breakdown of these costs is given in Table 1.
Table 1.
The costs of the screening intervention
| Category of resource use | Number of items | Cost per item | Total cost | SCOOP denominator | Cost per person |
|---|---|---|---|---|---|
| Identification of eligible patients | 52,033 | £10.70 | £556,753 | 12483 | £44.60 |
| Resource to administer screening questionnaire | 6,515 | £3.65 | £23,780 | 6515 | £3.65 |
| Calculation of initial WHO risk algorithm | 6,233 | £0.49 | £3,054 | 6,233 | £0.49 |
| Notification of initial fracture risk, letters to participants and GPs | 6,233 | £1.30 | £8,103 | 6,233 | £1.30 |
| BMD assessment using DXA scans | 3,064 | £69.00 | £211,416 | 6,233 | £33.92 |
| Calculation of final fracture risk | 3,064 | £0.21 | £643 | 6,233 | £0.10 |
| Clinical review of final fracture risk | 3,064 | £0.00 | £0 | 6,233 | £0.00 |
| Notification of final fracture risk result (questionnaire + DXA in selected cases) | 3,064 | £1.30 | £3,983 | 6,233 | £0.64 |
| Oversight of screening process | 6,233 | £0.00 | £0 | 6,233 | £0.00 |
| GP consultations | 898 | £134.00 | £120,332 | 6,233 | £19.31 |
| Total | £104 | ||||
Costs associated with fracture related health care contacts
Health Resource Group codes (HRGs) were not available from NHS Digital, therefore, inpatient, outpatient and A&E datasets were each run through the HRG 4+ grouper to derive the HRG codes. (14) Costs of resource use were drawn from HRGs linked to National Health Service (NHS) reference costs via data from HSCIC. (13) Inpatient stay costs were derived from HRG codes corresponding to each Finished Consultant Episode (FCE). Allowances were made for: type of admission (elective or non-elective); length of stay; short stays; and excess bed days. A short stay was defined as less than two days in hospital. A long stay was costed in the same manner as elective admission costs, but to reflect non-elective NHS reference cost data. Outpatient attendances were costed according to speciality and type of attendance (for example first or follow-up appointments). Procedure costs, where recorded, were included. The completeness of A&E data was much lower than inpatient and outpatient data, leading to the generation of missing HRG codes. A weighted average cost of £129 per A&E attendance was used in these cases.(13) Medication data were available for anti-osteoporosis medicines for the full period of follow-up for all study participants, these were costed using prices from the British National Formulary No.66.(15) All costs are for the year 2013/14 in pounds sterling.
Analysis
A ‘within trial’ economic analysis was undertaken on an ‘Intention-to-Treat’ basis from the perspective of a national health payer, the United Kingdom (UK) NHS. The main cost-effectiveness analysis used QALYs as the outcome measure (cost-utility study). Two additional economic evaluations were performed using osteoporotic fractures and hip fractures as outcomes (cost-effectiveness studies). The study had a 5 year horizon so discounting was used to allow for differential timing of costs and benefits. Discounting was not used for the first year of follow-up. In subsequent years costs, QALYs, and fractures were discounted using a rate of 3.5%, as recommended by the National Institute for Health and Care Excellence (NICE).(16)
EQ-5D values were completed by postal questionnaires, with some telephone questionnaire completion for non-responders. Response rates were high in the SCOOP study but complete data required up to 7 EQ-5D returns over 5 years so increasing the potential for missing data. Missing data is a common problem in economic analysis and can lead to both bias and a lack of precision.(17) To estimate QALYs we needed EQ-5D values for all points of follow-up when the participant was alive. Each EQ-5D questionnaire has five questions, all of which need to be completed to obtain an EQ-5D score. Where there was a single missing EQ-5D question but the participant had completed the other four questions, we imputed the missing question using a ‘hot-decking’ approach.(18) Using this method the four completed responses were compared with individuals with complete data who had the same pattern of responses to those four items. The missing value was replaced by the modal response for that item taken from those with complete data. Individuals with complete EQ-5D data, including those imputed using ‘hot-decking’, were defined as the complete case analysis (CCA) set. Where participants had missed more than one EQ-5D question, or where the questionnaire had not been returned, that EQ-5D score was deemed missing and a QALY could not be calculated. For these cases, multiple imputation (using five imputed data sets) was used. Discounted QALY scores were imputed using the following variables: baseline EQ-5D, age at randomisation, days alive, time without osteoporotic fracture, and time without hip fracture. Imputation was conducted separately for each study group.(17) This analysis was conducted in SPSS version 23.
Analysis of the costs and outcomes data were undertaken using seemingly unrelated regression, which allows for correlation between costs and outcomes and is generally considered robust for skewed data.(19) This was conducted using the sureg command in STATA. Both costs and effects used baseline EQ-5D, age, and study group, as explanatory variables. Means and standard errors from imputed data were calculated using Rubin’s rules.(20) For the cost per QALY analysis using imputed QALY data, cost-effectiveness acceptability curves (CEACs) were estimated using 1,000 samples for each of the five imputed data sets, i.e., 5000 samples in total. For analyses where data were not imputed, CEACs were estimated using boot-strapping with 2,000 samples. CEACs show the probability that each of the study groups is the most cost-effective option at different valuations of the outcome variable.(21) Analyses were performed in SPSS 23 and STATA 11.
Sensitivity analysis
To evaluate the effect of only using cases where we were able to estimate QALY without multiple imputation, we estimated cost per QALY for the CCA set. We felt that this would likely provide a biased estimator, as cases were unlikely to be missing at random. Since we had data on cost per fracture avoided for all study participants we therefore repeated this analysis, but restricted to only those cases in the CCA set. This allowed assessment of whether the results for cost per fracture when restricted to the CCA set were similar to results when all data were used.
Ethical approval was obtained from the North Western - Haydock Research Ethics Committee of England in September 2007 (REC 07/H1010/70). The trial was registered on the International Standard Randomised Controlled Trial Register in June 2007 (ISRCTN 55814835). The Arthritis Research United Kingdom (ARUK), formerly the Arthritis Research Campaign (ARC), and the Medical Research Council (MRC) of the UK, jointly funded this trial.
Role of the funding bodies
The funders of the study played no role in the study design, data collection, data analysis, data interpretation or writing of this report. The corresponding author had full access to all data used in this study and had final responsibility for the decision to submit for publication.
Results
There were 12,483 participants in the SCOOP study, 6,250 in the control and 6,233 in the intervention group. Comparisons of baseline characteristics between study groups have been published elsewhere; the two study groups were found to be very similar.(5) The number of cases for whom we were able to estimate QALY was 6,881 (55%), this comprised 3,404 (54%) from the control group and 3,477 (56%) from the intervention group. When the ‘hot-decking’ (18) method was used to impute responses when a single EQ-5D question was missing this rose to 7,975 cases (64%); this comprised the CCA set. A comparison of the CCA set with cases missing one or more EQ-5D values indicated that missing cases had statistically significantly lower baseline EQ-5D, more incident fractures and higher fracture related healthcare costs (Table 2).
Table 2.
Characteristics of complete case analysis and those missing full QALY data
| CCA | Missing any EQ-5D | Difference | p | |
|---|---|---|---|---|
| Age | 75.2 | 76.3 | 1.08 | <0.001 |
| Number of Incident Osteoporotic Fractures | 0.140 | 0.194 | 0.05 | <0.001 |
| Number of Incident Hip Fractures | 0.023 | 0.045 | 0.02 | <0.001 |
| Baseline EQ-5D | 0.769 | 0.690 | -0.08 | <0.001 |
| Total Health care costs | £781 | £1,206 | £426 | <0.001 |
The average costs for the intervention were £104 per person (Table 1). The main components of this cost were case finding, DXA scans, and GP consultations for identified cases. The total average discounted costs of both intervention and fracture related health care for the five year follow-up are given in Table 3; estimates are provided for both the full data set and the CCA analysis. For the whole sample, it can be seen that estimated costs are £968 and £900 for the intervention and control groups respectively. The major component of costs was inpatient stay with other secondary care costs also being important. When costs are examined for the CCA set only, it can be seen that estimated total costs are lower and the difference between the intervention and control is higher at £104, reflecting a lower proportion of fractures in the CCA than the whole sample. Table 4 provides EQ-5D for all available time points for the CCA, as well as QALY estimates without adjustment for baseline EQ-5D. Also provided are baseline EQ-5D and unadjusted QALY for the imputed analysis. Estimates of the discounted QALY difference for the intervention group compared to the control are -0.005 and 0.008 for the CCA and imputed analysis respectively, neither statistically significantly different. However, in both cases baseline EQ-5D values are lower in the intervention group, which would tend to bias QALY estimates in favour of the control group.
Table 3.
Total costs in both arms of the study by cost category.
| Whole Sample | CCA | |||||
|---|---|---|---|---|---|---|
| Cost type | Control | Intervention | Difference (95% CI) | Control | Intervention | Difference (95% CI) |
| Inpatient | £531 | £482 | -49.6 (-133 , 34) | £393 | £378 | -14.5 (-105 , 76) |
| A&E | £162 | £160 | -2 ( -10.7, 6.7) | £138 | £134 | -3.9 (-13 , 6) |
| Outpatient | £191 | £201 | 9.8 (-4, 24) | £181 | £194 | 12.6 (-5, 30) |
| Medicines | £8 | £13 | 5.6 (3.5, 7.8) | £8 | £14 | 5.7 ( 3, 8) |
| Non SCOOP DXA | £9 | £9 | -0.1 ( -0.6 , 0.4) | £9 | £9 | 0.4 ( -0.2 , 1) |
| Cost of intervention | - | £104 | - | - | £104 | - |
| Total | £900 | £968 | 68 (-21, 157) | £728 | £833 | 104 ( 8 , 201) |
Table 4.
Unadjusted EQ-5D and QALY estimates for both complete case analysis (CCA) and imputed analysis
| Control | Intervention | Difference | Lower 95% CI | Upper 95% CI | |
|---|---|---|---|---|---|
| CCA EQ-5D - Baseline | 0.773 | 0.764 | -0.009 | -0.018 | 0.001 |
| CCA EQ-5D - 6 months | 0.760 | 0.759 | -0.001 | -0.011 | 0.009 |
| CCA EQ-5D - 12 months | 0.750 | 0.754 | 0.004 | -0.007 | 0.014 |
| CCA EQ-5D -24 months | 0.736 | 0.734 | -0.002 | -0.013 | 0.009 |
| CCA EQ-5D -36 months | 0.712 | 0.710 | -0.002 | -0.014 | 0.010 |
| CCA EQ-5D - 48 months | 0.690 | 0.693 | 0.003 | -0.010 | 0.015 |
| CCA EQ-5D - 60 months | 0.671 | 0.666 | -0.005 | -0.018 | 0.009 |
| CCA Discounted QALY | 3.373 | 3.368 | -0.005 | -0.051 | 0.040 |
| Imputed analysis Baseline EQ-5D | 0.743 | 0.738 | -0.006 | -0.014 | 0.002 |
| Imputed analysis Discounted QALY | 3.266 | 3.274 | 0.008 | -0.028 | 0.044 |
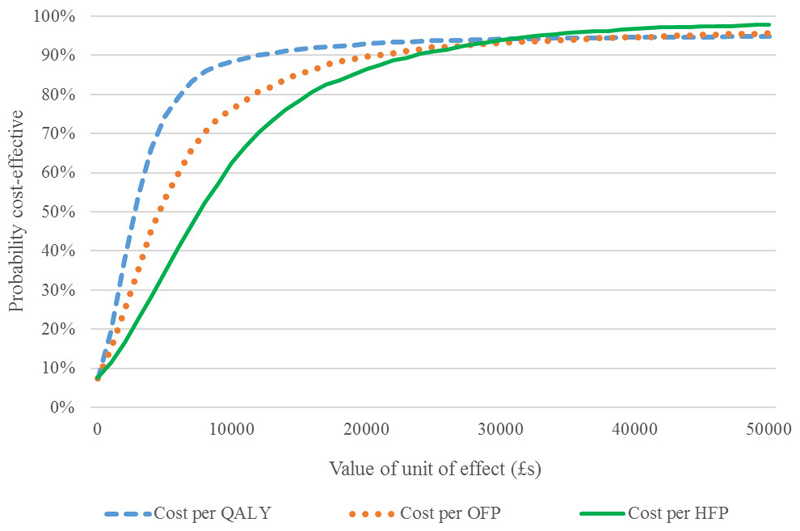
The results of the economic evaluations are shown in Table 5. These results were obtained using seemingly unrelated regression and adjust for differences in baseline age and EQ-5D. The estimate of incremental QALYs was 0.0237 per person (95% CI: -0.003 to 0.051). The confidence interval crosses zero; thus, the difference in QALY is not statistically significantly at the 5% level. The estimate of the incremental cost-effectiveness ratio (ICER) was £2,772. Also shown in Table 5 are the two analyses of cost per fracture prevented. The incremental estimate of fractures prevented was 0.0146 (95% CI: 0.0002 to 0.029) and 0.0085 (95% CI: 0.0026 to 0.0144) for osteoporotic-related and hip fractures respectively. These results are for the five years of follow-up. The intervention group had an incremental cost per fracture prevented of £4,478 per osteoporotic-related fracture and £7,694 per hip fracture. The uncertainty surrounding these estimates are shown in cost-effectiveness acceptability curves (CEACs) provided in Figure 1 for all three analyses. In terms of cost per QALY there is a 93% probability the intervention is cost-effective at the NICE threshold of £20,000 per QALY. For fractures the CEACs are slightly lower; for example, there would be an 87% probability the intervention would be considered cost-effective if preventing a hip fracture was valued at £20,000.
Table 5.
Cost effectiveness results for the base case analysis (imputed and full data) and sensitivity analysis
| Incremental Cost | 95% CI | Incremental effect | 95% CI | ICER | |
|---|---|---|---|---|---|
| Base Case Analysis | |||||
| Cost per QALY - Imputed | £66 | (-21.7 , 153) | 0.0237 | (-0.0034 , 0.0508) | £2,772 |
| Osteoporotic Fracture prevented | £65 | (-23.7 , 154.5) | 0.0146 | (0.00015 , 0.029) | £4,478 |
| Hip Fracture prevented | £65 | (-23.4 , 154.1) | 0.0085 | (0.0026 , 0.0144) | £7,694 |
| Sensitivity Analysis | |||||
| Cost per QALY - CCA | £99 | (3 , 196) | 0.0214 | (-0.0113 , 0.054) | £4 646 |
| Osteoporotic Fracture prevented (CCA set) | £99 | (3.2 , 195.5) | 0.0094 | (-0.0073 , 0.0262) | £10 564 |
| Hip Fracture prevented (CCA set) | £99 | (3.4 , 195.2) | 0.0045 | (-0.0018 , 0.0108) | £22 067 |
Figure 1.
Cost-Effectiveness acceptability curves (CEACs) for cost per QALY, osteoporotic (OFP) and hip fracture prevented (HFP) for the base case analyses (imputed and full data)
Sensitivity analysis
The above analysis was repeated for the CCA set, again shown in Table 5. The incremental effect in terms of QALYs was 0.0214 (95% CI: -0.011 to 0.054). The ICER for this analysis was £4,646. For the CCA the probability that the intervention would be cost-effective at the NICE threshold of £20,000 per QALY was approximately 83%. When analysis of osteoporosis-related and hip fractures was restricted to only those cases that were also in the CCA data set we found a marked difference in results (Table 5). For osteoporotic fractures the mean estimate of incremental effect was 0.0094 (95% CI: -0.0073 to 0.026). For hip fractures the mean estimate of incremental effect was 0.0049 (95% CI: -0.0018 to 0.0108). These were both considerably lower than the values for the whole sample given above. For both types of fractures the estimates of effect were no longer statistically significant between groups and estimated ICERs were more than double those estimated from the full data sets.
Discussion
Participants in the intervention arm accrued, on average, an additional 0.0237 QALYs though this difference was not statistically significant. The additional cost per QALY was £2,772 compared to the control group in our base case analysis. Although these gains in QALY appear modest given the five-year follow-up, it should be borne in mind that these are mean incremental values for the whole of the intervention cohort compared to the control. As this is a screening intervention the majority of participants in the intervention arm received no change in their health care, and hence would not be expected to generate a QALY gain. The CEAC presented in Figure 1, which allows for the uncertainty inherent in the data, indicated that at the NICE threshold value of £20,000 for a QALY, the intervention had a 93% probability of being cost-effective. The intervention also generated reductions in fractures with a cost per fracture prevented of £4,478 for all osteoporotic fractures and £7,694 for hip fractures. Together, these results provide strong evidence that screening in the community to reduce fractures in older women represents an efficient use of healthcare resources.
This economic evaluation was based on the SCOOP study. An important aim of resource data collection was to minimise burden on participants and achieve high completion rates of those resources felt likely to be most important in relation to fractures, e.g., fracture-related inpatient care. Data on fracture related outcomes and on the resource implications of fracture related care were obtained from routine data sources e.g. from NHS Data. Considerable effort was invested in ensuring these were as complete as possible. This also meant that completeness of these data were independent of factors that might normally be expected to affect response, such as poor health. There were also pragmatic issues related to the research burden of collecting resource use data from a large number of practices. These considerations meant that some items of resource use were not recorded. Examples included: routine primary care contacts and admissions to nursing homes. The former may understate some of the costs of providing anti-osteoporosis medicines should prescription of these drugs lead to an increase in primary care consultations. The latter might understate any potential resource savings associated with preventing fractures.
The SCOOP cohort generally had extremely high rates for the return of questionnaires: at the first follow-up (6-months), 11,967 out of 12,483 participants responded (96%), and at the 60 month follow-up, of the 11 408 participants still living, 10,661 (93%) responded. However, the nature of QALY estimation using a repeated series of EQ-5D questionnaires makes estimation of QALYs vulnerable to problems of missing data. Additionally, if participants would be less likely to return EQ-5D in the period immediately after a fracture there may be fewer observations in a time period that would be expected to have the largest effect on EQ-5D scores. Furthermore, since the EQ-5D were completed at set times, it is possible that acute changes in quality-of-life secondary to a fracture that had occurred six months prior, for example, might not be captured. As the comparison of the CCA with missing data (Table 2), and results for cost per fracture prevented when restricted to the CCA data (sensitivity analysis, Table 5); both indicated that results were likely to be biased for the CCA data we used imputed data as our base case QALY analysis.
A number of modelling studies have evaluated the cost-effectiveness of osteoporotic fracture prevention for the UK,(22–25) or for a number of different countries including the UK.(26–30) UK based models have generally found treatment for osteoporosis to be cost-effective but have found variations in estimates of cost-effectiveness. Factors likely to be important were reported as prevalence of osteoporosis, costs of treating fractures, and costs of treatment. One UK study also evaluated the cost-effectiveness in relation to risk of fracture.(25) Treatment in cases with higher risk of fractures was associated with increased probability of being cost-effective, suggestion strategies to identify higher risk individuals could be beneficial. The disadvantages of a modelling approach include the requirement for data from a variety of sources and the necessity for a number of assumptions to be made. All primary data used in the current study came from the same source, i.e., the SCOOP study. We are aware of no other study that has conducted an economic evaluation looking at cost per QALY alongside a randomised study for the prevention of osteoporotic fractures.
The SCOOP study also differs from a number of the published models in its length of follow-up. The effect of variable follow-up time has been investigated using an economic model. (31) In this study Kanis et al investigated the effect on ICERs of a ten-year follow-up compared to lifetime follow-up for 70 year old women. Increasing the length of follow-up led to a decrease in estimated ICERs (i.e. improved cost-effectiveness). Furthermore, there is often a selection bias in randomised trials with those consenting involvement likely to be different in characteristics from decliners. The clinical study reported that mortality in SCOOP was less than 50% of that expected based on age distribution at entry and that generally participants tended to be better educated and of higher socio economic status than those who declined. (5) Conversely, the SCOOP study also appeared to have higher than expected numbers of fractures. (5) The fact that the SCOOP study was an RCT also may have affected the costs associated with the screening programme. Costs of identification of £44 were paid to practices to reflect the fact that this was a task only carried out because of the trial. If this screening programme was rolled out in practice these costs may be lower. For these reasons, the estimates of cost-effectiveness from SCOOP may represent conservative ones.
Conclusions
The SCOOP clinical trial demonstrated that community screening, based upon the FRAX probability of hip fracture, leads to a significant reduction in hip fractures in older women.(5) The current study provides strong evidence that community screening, based upon the FRAX probability of hip fracture in older women, would likely be cost-effective and represent an efficient use of health care resources.
Acknowledgments
The Arthritis Research United Kingdom (ARUK), formerly the Arthritis Research Campaign (ARC), and the Medical Research Council (MRC) of the UK, jointly funded this trial. The SCOOP study was designed and conducted with substantial input from the Norwich Clinical Trials Unit, UK, particularly the construction of the study database and provision of on-line randomisation (completed by Mr Tony Dyer). Invaluable advice and support were provided by Mrs Margaret McWilliams and Mrs Ann Pulford, the study’s public and patient involvement (PPI) representatives. We would like to acknowledge and thank our Trial Steering Committee and Data Monitoring Committee.
Footnotes
The ’SCOOP Study Team’ consists of the authors plus the following individuals who contributed to or worked directly on the SCOOP study
Birmingham : Nicola Crabtree, Helen Duffy, Jim Parle, Farzana Rashid, Katie Stant,
Bristol : Shane Clarke, Alison Heawood, Niamh Redmond, Tim Peters, Kate Taylor, Clare Thomas (nee Emmett)
Manchester : Emma Knox, Cherry Tenneson, Helen Williams,
Norwich: David Adams, Veronica Bion, Jeanette Blacklock, Tony Dyer, Ian Harvey, Tarnya Marshall
Sheffield : Selina Bratherton (nee Simpson), Matt Fidler, Katharine Knight, Carol McGurk, Katie Smith, Stacey Young
Southampton : Karen Collins, Janet Cushnaghan
York : Catherine Arundel, Kerry Bell, Laura Clark, Sue Collins, Sarah Gardner, Natasha Mitchell
Declarations of Interest
Professor N Harvey has received consultancy, lecture fees and honoraria from Alliance for Better Bone Health, AMGEN, MSD, Eli Lilly, Servier, Shire, UCB, Consilient Healthcare and Internis Pharma
Professor McCloskey has been, or currently is, an advisor or speaker for ActiveSignal, Amgen, AstraZeneca, Consilient Healthcare, GSK, Hologic, Internis, Lilly, Medtronic, Merck, Novartis, Pfizer, Roche, Sanofi-Aventis, Servier, Synexus, Tethys, UCB, Warner Chilcott. He has also received research support from these plus I3 Innovus, the IOF and Unilever.
Professor Cooper has received consultancy fees and honoraria from Amgen, Danone, Eli Lilly, GSK, Medtronic, Merck, Nestle, Novartis, Pfizer, Roche, Servier, Shire, Takeda and UCB.
Professor Gittoes has been, or currently is, an advisor or speaker for Alexion, Shire, PARADIGHM, Amgen, Kyowa Kirin.
No other declarations of interest are reported.
Contributions
LS was the Chief Investigator and EL was responsible for the organisation and co-ordination of for SCOOP trial. RF was the health economics investigator for SCOOP. The cost analysis was conducted by RFSK and EL for the intervention and by RFSK for the health service costs. DAT conducted the economic evaluation, analysed and interpreted data, with advice from LS & RF, and drafted the manuscript. All authors contributed to the writing of the final trial manuscript. DAT takes responsibility for the integrity of the data analysis.
No supplementary data is included with the submission.
References
- 1.Johnell O, Kanis JA. An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos Int. 2006;17(12):1726–33. doi: 10.1007/s00198-006-0172-4. [DOI] [PubMed] [Google Scholar]
- 2.Hernlund E, Svedbom A, Ivergård M, Compston J, Cooper C, Stenmark J, et al. Osteoporosis in the European Union : medical management, epidemiology and economic burden. A report prepared in collaboration with the International Osteoporosis Foundation and the European Federation of Pharmaceutical Industry Associations (EFPIA) Arch Osteoporos. 2013;8(1–2):136. doi: 10.1007/s11657-013-0136-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sernbo I, Johnell O. Consequences of a hip fracture: a prospective study over 1 year. Osteoporosis International. 1993;3(3):148–53. doi: 10.1007/BF01623276. [DOI] [PubMed] [Google Scholar]
- 4.Kanis JA, Johnell O, Oden A, Johansson H, McCloskey E. FRAX and the assessment of fracture probability in men and women from the UK. Osteoporosis International. 2008;19(4):385–97. doi: 10.1007/s00198-007-0543-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shepstone L, Lenaghan E, Cooper C, Clarke S, Fong-Soe-Khioe R, Fordham R, et al. Screening in the community to reduce fractures in older women (SCOOP): a randomised controlled trial. Lancet. 2017 doi: 10.1016/S0140-6736(17)32640-5. Epub ahed of print. [DOI] [PubMed] [Google Scholar]
- 6.Si L, Winzenberg TM, Palmer AJ. A systematic review of models used in cost-effectiveness analyses of preventing osteoporotic fractures. Osteoporos Int. 2014;25(1):51–60. doi: 10.1007/s00198-013-2551-y. [DOI] [PubMed] [Google Scholar]
- 7.Hiligsmann M, Evers SM, Ben Sedrine W, Kanis JA, Ramaekers B, Reginster JY, et al. A Systematic Review of Cost-Effectiveness Analyses of Drugs for Postmenopausal Osteoporosis. PharmacoEconomics. 2015;33(3):205–24. doi: 10.1007/s40273-014-0231-1. [DOI] [PubMed] [Google Scholar]
- 8.Shepstone L, Fordham R, Lenaghan E, Harvey I, Cooper C, Gittoes N, et al. A pragmatic randomised controlled trial of the effectiveness and cost-effectiveness of screening older women for the prevention of fractures: Rationale, design and methods for the SCOOP study. Osteoporosis International. 2012;23(10):2507–15. doi: 10.1007/s00198-011-1876-7. [DOI] [PubMed] [Google Scholar]
- 9.NHS Digital. London: Goverment Statistical Office; Dec, 2017. Accessed at: https://digital.nhs.uk/ [Google Scholar]
- 10.Brooks R. EuroQol: the current state of play. Health Policy. 1996;37(1):53–72. doi: 10.1016/0168-8510(96)00822-6. [DOI] [PubMed] [Google Scholar]
- 11.MVH. The measurement and valuation of health. Final report on the modelling of valuation tariffs. York: Centre for Health Economics; 1995. [Google Scholar]
- 12.Curtis L. Unit costs of health and social care. Canterbury, Kent: Personal Social Services Research Unit; 2014. [Google Scholar]
- 13.NHS Reference costs 2013 to 2014. London: Department of Health; 2014. [Google Scholar]
- 14.The National Casemix Office. HRG4+ Grouper Reference Manual Reference Costs 13/14. HSCIC Health & Social Care Information Centre; 2014. Accessed at: http://content.digital.nhs.uk/media/13823/HRG4-201314-RC-Grouper-Reference-Manual/pdf/HRG4__201314_Reference_Costs_Grouper_Reference_Manual_v1.0.pdf. March 2016. [Google Scholar]
- 15.Joint Formulary Committee. British National Formulary. 66 ed. BMJ Group and Pharmaceutical Press; 2014. [Google Scholar]
- 16.NICE. Guide to the methods of technology apprisal. London: National Institute for Health and Care Excellence (NICE); 2013. [PubMed] [Google Scholar]
- 17.Faria R, Gomes M, Epstein D, White IR. A guide to handling missing data in cost-effectiveness analysis conducted within randomised controlled trials. PharmacoEconomics. 2014;32(12):1157–70. doi: 10.1007/s40273-014-0193-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Little R, Rubin D. Statistical analysis with missing data. New York: Wiley; 1987. [Google Scholar]
- 19.Willan AR, Briggs A, Hoch JS. Regression methods for covariate adjustment and subgroup analysis for non-censored cost-effectiveness data. Health Economics. 2004;13(5):461–75. doi: 10.1002/hec.843. [DOI] [PubMed] [Google Scholar]
- 20.Marshall A, Altman DG, Holder RL, Royston P. Combining estimates of interest in prognostic modelling studies after multiple imputation: current practcie and guidelines. BMC Medical Research Methodology. 2009;9(1) doi: 10.1186/1471-2288-9-57. Article number 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fenwick E, O'Brien BJ, Briggs A. Cost-effectiveness acceptability curves - facts, fallacies and frequently asked questions. Health Economics. 2004;13(5):405–15. doi: 10.1002/hec.903. [DOI] [PubMed] [Google Scholar]
- 22.Borgström F, Ström O, Coelho J, Johansson H, Oden A, McCloskey EV, et al. The cost-effectiveness of risedronate in the UK for the management of osteoporosis using the FRAX®. Osteoporosis International. 2010;21(3):495–505. doi: 10.1007/s00198-009-0989-8. [DOI] [PubMed] [Google Scholar]
- 23.Borgström F, Ström O, Coelho J, Johansson H, Oden A, McCloskey E, et al. The cost-effectiveness of strontium ranelate in the UK for the management of osteoporosis. Osteoporos Int. 2010;21(2):339–49. doi: 10.1007/s00198-009-0971-5. [DOI] [PubMed] [Google Scholar]
- 24.Stevenson MD, Brazier JE, Calvert NW, Lloyd-Jones M, Oakley JE, Kanis J. Description of an individual patient methodology for calculating the cost-effectiveness of treatments for osteoporosis in women. Journal of the Operational Research Society. 2005;56(2):214–21. [Google Scholar]
- 25.van Staa TP, Kanis JA, Geusens P, Boonen A, Leufkens HGM, Cooper C. The cost-effectiveness of bisphosphonates in postmenopausal women based on individual long-term fracture risks. Value in Health. 2007;10(5):348–57. doi: 10.1111/j.1524-4733.2007.00188.x. [DOI] [PubMed] [Google Scholar]
- 26.Borgström F, Carlsson Å, Sintonen H, Boonen S, Haentjens P, Burge R, et al. The cost-effectiveness of risedronate in the treatment of osteoporosis: An international perspective. Osteoporos Int. 2006;17(7):996–1007. doi: 10.1007/s00198-006-0094-1. [DOI] [PubMed] [Google Scholar]
- 27.Borgström F, Johnell O, Kanis JA, Jönsson B, Rehnberg C. At what hip fracture risk is it cost-effective to treat?: International intervention thresholds for the treatment of osteoporosis. Osteoporos Int. 2006;17(10):1459–71. doi: 10.1007/s00198-006-0107-0. [DOI] [PubMed] [Google Scholar]
- 28.Ström O, Borgström F, Sen SS, Boonen S, Haentjens P, Johnell O, et al. Cost-effectiveness of alendronate in the treatment of postmenopausal women in 9 European countries - an economic evaluation based on the fracture intervention trial. Osteoporos Int. 2007;18(8):1047–61. doi: 10.1007/s00198-007-0349-5. [DOI] [PubMed] [Google Scholar]
- 29.Borgström F, Ström O, Kleman M, McCloskey E, Johansson H, Odén A, et al. Cost-effectiveness of bazedoxifene incorporating the FRAX® algorithm in a European perspective. Osteoporos Int. 2011;22(3):955–65. doi: 10.1007/s00198-010-1291-5. [DOI] [PubMed] [Google Scholar]
- 30.Kim K, Svedbom A, Luo X, Sutradhar S, Kanis JA. Comparative cost-effectiveness of bazedoxifene and raloxifene in the treatment of postmenopausal osteoporosis in Europe, using the FRAX algorithm. Osteoporosis International. 2014;25(1):325–37. doi: 10.1007/s00198-013-2521-4. [DOI] [PubMed] [Google Scholar]
- 31.Kanis JA, Adams J, Borgström F, Cooper C, Jönsson B, Preedy D, et al. The cost-effectiveness of alendronate in the management of osteoporosis. Bone. 2008;42(1):4–15. doi: 10.1016/j.bone.2007.10.019. [DOI] [PubMed] [Google Scholar]