Abstract
Purpose of review
To provide an overview of shared dysregulation of the hypothalamic-pituitary-adrenal (HPA) and brain-gut-microbiome (BGM) axes associated with depression and type 2 diabetes (T2D). Clinical implications and future research are also discussed.
Recent findings
Both depression and T2D are associated with dysregulation of the HPA and BGM axes. These pathways regulate immune function, glucose metabolism, and sleep, and are altered in both illnesses. Dysregulation of homeostatic brain-body pathways may be positively influenced though different therapeutic actions, including psychotherapy, healthy eating, physical activity, sleep promotion, and certain anti-inflammatory or antidepressant medications.
Summary
While the causal nature of the relationship between depression and T2D remains unclear, these conditions share dysregulation of homeostatic brain-body pathways that are central to mental and physical health. Better understanding of this dysregulation may provide opportunities for interventions that could benefit both conditions. Future research should examine the additive burden of depression and T2D on HPA and BGM dysregulation, and differentiate depression from emotional distress.
Keywords: Type 2 diabetes, depression, biological pathways, hypothalamic-pituitary-adrenal axis, brain-gut-microbiome axis
Introduction
Depression is among the most common mental disorders in the United States affecting around 16 million (6.7%) US adults in 2015 (1). Likewise, type 2 diabetes (T2D) is one of the most common chronic illnesses in the United States, with experts predicting that 1 in 3 Americans may have diabetes by 2050 if current trends continue (2). There is also abundant evidence that depression and T2D are related. Meta-analyses suggest that depression is 60-100% more common among individuals diagnosed with T2D compared to those without this diagnosis (3,4). However, the causal or temporal relationship between these two conditions remains unclear. Depression is often considered to be a comorbid condition resulting from diabetes burden with data from the prospective population-based ZARADEMP study showing that having T2D increases the risk of prevalent and incident depression by 41% and 26%, respectively (5). However, depression appears to be a stronger predictor of T2D than it is a consequence, as evidenced by data from the same project showing that depression increases the chance of developing T2D by 60% (6).
Several explanations have been proposed for this comorbidity and the bidirectional influence between depression and T2D (5,6). One explanation is the excessive emotional burden of chronic diabetes, especially considering the demanding nature of the treatment regimen and lifestyle changes that are required for illness self-management. In support of this, reported symptoms of depression are more common among diabetes patients on a more burdensome treatment regimen (i.e., those on insulin treatment compared to oral medications only) (7), and are more common among those with diagnosed compared to undiagnosed diabetes (8). A second explanation is that depression is associated with low self-esteem and neglect of one’s needs, which may contribute to unhealthy lifestyle behaviors such as physical inactivity, smoking and an unhealthy diet (9). Indeed, research supports a link of moderate effect size between depression symptoms and problems with diabetes self-management (10).
Finally, a third interpretation is that depression and T2D co-occur due to shared biological changes (9). Specific environmental and lifestyle factors could contribute to dysregulation of homeostatic brain-body pathways in genetically vulnerable individuals that are associated with an increased mental and physical morbidity, including depression and T2D. Recent research has revealed the presence of inter-related homeostatic brain-body pathways that play central roles in maintaining mental and physical health and may represent shared pathways linking depression and diabetes. In this review, we will focus on the hypothalamic-pituitary-adrenal (HPA) axis and the brain-gut-microbiome (BGM) axis. The microbiome refers to the community of microorganisms that reside within our GI tracts, which will be described in further detail in the next section.
The HPA system has received a significant amount of research attention as it appears to be a primary pathway through which emotional symptoms and physical health relate to one another (11). The BGM system has received less research attention in humans, but holds significant promise for future discoveries in relation to depression and T2D and their co-occurrence. Here we will introduce each axis, describe its dysregulation, and discuss how dysregulation impacts immune function, food and glucose metabolism, and sleep. We then relate these immune and metabolic shifts to depression and T2D. Given the complexity of the bi-directional regulation between brain-body axes and physiological systems, it can be difficult to establish causal relationships. Thus, our focus is on describing the most prominent inter-relationships between these systems as they relate to depression and T2D.
1. Overview of Homeostatic Brain-Body Pathways
a. Introduction
It is evolutionarily beneficial for complex organisms like humans to adapt to a range of physical and psychosocial stressors in order to preserve overall homeostasis, defined as the stability of a complex biological system. Such adaptations allow for flexibility in varying environments and promote better use of changing environmental resources (12). However, flexible adaptation to a variety of stressors also requires brain-body coordination. Indeed, a substantial body of research supports a shared chemical language between brain and body consisting of various signaling molecules including cytokines, hormones, neurotransmitters and their receptors, and these signaling molecules appear to play a central role in coordinating homeostasis, including complex integration and communication between the HPA and BMG axes (12–15).
Increasing evidence suggests that the HPA and BGM axes are central to overall mental and physical health, and their dysregulation appears to underlie the increased occurrence (and co-occurrence) of various mental and physical illnesses (16,17). Contemporary culture in industrialized environments encourages lifestyles with a considerable negative impact on these homeostatic brain-body pathways by introducing several disruptors that contribute to dysregulation when prolonged (Figure 1). These disruptors emerge from dramatic changes in diet, increased use of medications such as antibiotics, a sedentary lifestyle, and sleep changes, and importantly, our increasingly complex society has led to a growing number of psychosocial stressors that have become more abstract, chronic, and separated from physical stress (18–21). Our biological systems respond to these psychosocial stressors as being indicative of potential physical harm, and the attempt to maintain homeostasis in the face of continual challenge, also referred to as allostatic load (21), can contribute to dysregulation of immune, metabolic and sleep systems. This dysregulation in turn contributes to the increased occurrence of mental and physical illnesses associated with immune and metabolic functions.
Figure 1.
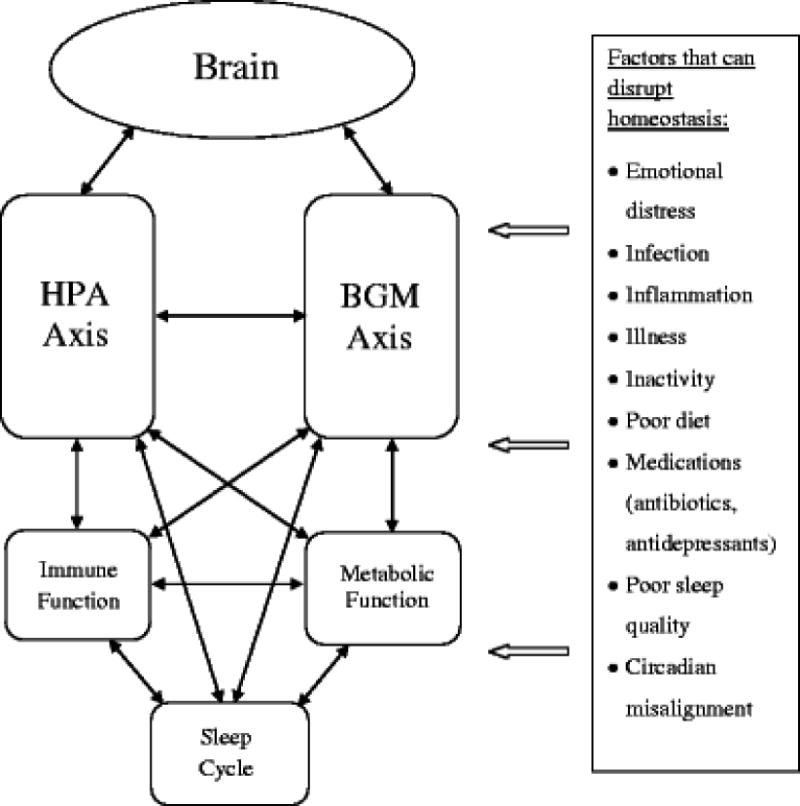
Homeostatic Dysregulation of Brain-Body Pathways. Figure shows inter-regulation between physiological systems, which allows for maximum flexibility in maintaining homeostasis. The introduction of chronic disruptors to this homeostatic system at every level can contribute to dysregulation. Dysregulation of core homeostatic brain-body pathways, namely the hypothalamic-pituitary-adrenal (HPA) and brain-gut-microbiome (BGM) axes, are associated with mental and physical illness, including depression and type 2 diabetes.
b. Hypothalamic Pituitary Adrenal (HPA) Axis
The HPA axis is a homeostatic brain-body system that is fundamentally involved in immune, metabolic and reproductive regulation, as well as psychological well-being (22), illustrated in Figure 2. Activation of the HPA axis is initiated by the paraventricular nucleus of the hypothalamus in the brain, and this nucleus can be activated by perceptions of threat as well as internal physiological processes (12). This leads to the release of corticotropin-releasing factor/hormone (CRF/CRH), which stimulates the release of adrenocorticotrophic hormone (ACTH) into the vascular system from the pituitary gland. This in turn activates the adrenal glands situated atop the kidneys, which secrete the glucocorticoid hormone cortisol. Cortisol secretion follows a diurnal pattern that regulates daily physiological processes such as waking, glucose intake, and immune functioning. In times of stress, the system increases its cortisol output above typical levels to coordinate temporary fight-or-flight responses (e.g., release of adrenaline, increased heart rate, etc.) and compensate for potential overshooting of immune responses (22). Several stressors have shown to activate the HPA axis in an acute fashion, including social stress and separation (23), strong emotional responses (24), and carbon dioxide inhalation (25).
Figure 2.
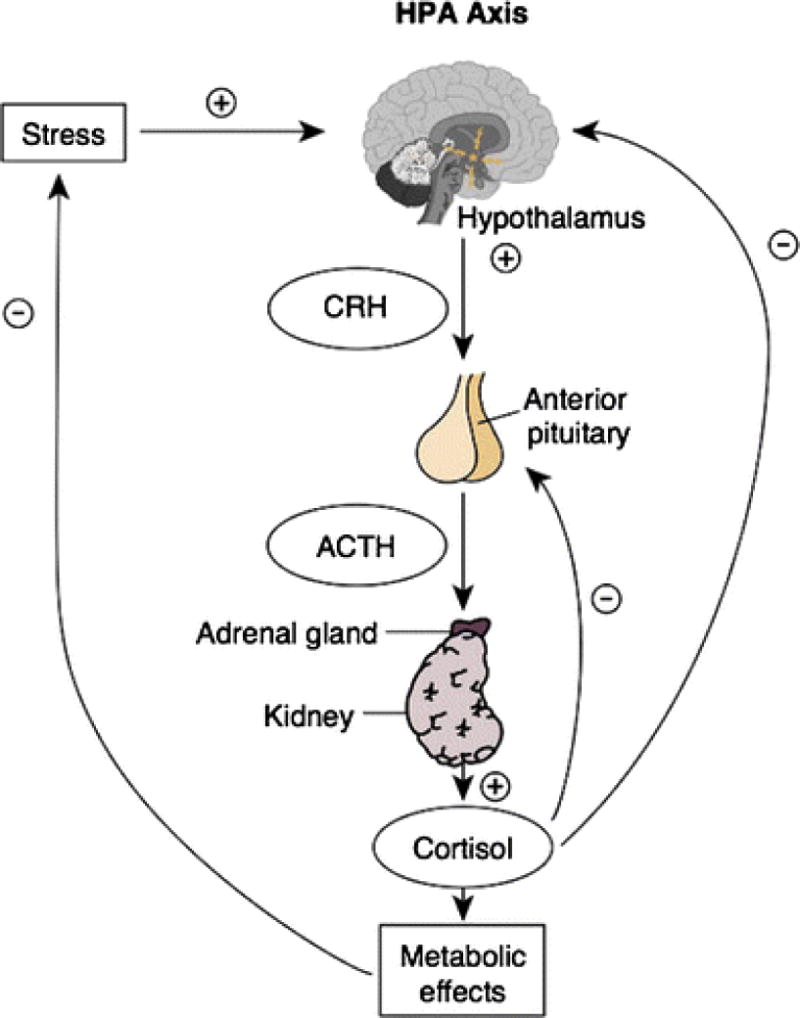
Overview of the HPA axis. Activation of the HPA axis is initiated by the paraventricular nucleus of the hypothalamus in the brain, and this nucleus can be activated by perceptions of threat as well as internal physiological processes. This leads to the release of corticotropin-releasing factor/hormone (CRF/CRH), which stimulates the release of adrenocorticotrophic hormone (ACTH) into the vascular system from the pituitary gland. This in turn activates the adrenal glands situated atop the kidneys, which secrete the glucocorticoid hormone cortisol. (Figure reproduced from: Hiller-Sturmhöfel and Bartke. Alcohol Research and Health 1998;22(3):153) [101].
Long-term HPA axis activation above normal daily levels can lead to eventual cortisol dysregulation and negative physical consequences (22,26). When cortisol is chronically elevated, it alters feedback controls, which changes the daily pattern of cortisol over time. Specifically, cortisol becomes elevated and the diurnal curve begins to flatten, with relatively lower cortisol levels observed in the morning and higher levels observed at night compared to individuals with a normally-functioning HPA axis (22); and these changes to HPA axis function have been linked to the presence of depression and T2D. For example, a meta-analysis of 361 studies conducted over four decades of research suggests that clinical depression is associated with HPA axis dysregulation, though the degree of dysregulation varies significantly among patient groups (26). This is consistent with a second meta-analysis showing particularly high HPA dysregulation among elderly individuals with depression, which was related in part to the presence of comorbid illness (27); however, aging is also associated with HPA dysregulation in physically healthy individuals (28). HPA axis dysregulation has also been associated with obesity (29), cardiovascular disease (30) and T2D (31), with longer diabetes duration associated with higher dysregulation (32) . Under acute conditions, cortisol indirectly increases and maintains glucose levels in the blood by acting on insulin, though dysregulation contributes to insulin resistance as well as increased hunger and obesity (33).
c. Brain-Gut-Microbiome (BGM) Axis
The BGM pathway coordinates hunger, gut motility, inflammatory responses, and signals related to mood between the brain and enteric nervous system of the GI tract (17,34), illustrated in Figure 3. The enteric nervous system, recently referred to as the ‘second brain,’ is a neural network of approximately 100 million neurons embedded in the lining of the gastrointestinal tract that have bidirectional connections to the CNS via motor and sensory fibers, and particularly the vagus nerve (17). Moreover, there is an immense ecological community of bacteria, fungi, viruses and archaea that reside within our GI tracts, collectively called the gut microbiome, that exerts control over the brain and physiological processes of the host (17,35). While the gut microbiome differs between individuals, several core strains are shared (36,37). We have developed a symbiotic (helpful) dependence on these microorganisms, as our intestines provide nutrition and a competitive environment for a variety of symbiotic, commensal (neutral), and pathogenic (harmful) microorganisms. At the same time, these microorganisms contribute to nutrient processing and are essential to many biological processes (36,37), and a stable and diverse microbiota is central to BGM axis homeostasis and host health (17,20).
Figure 3.
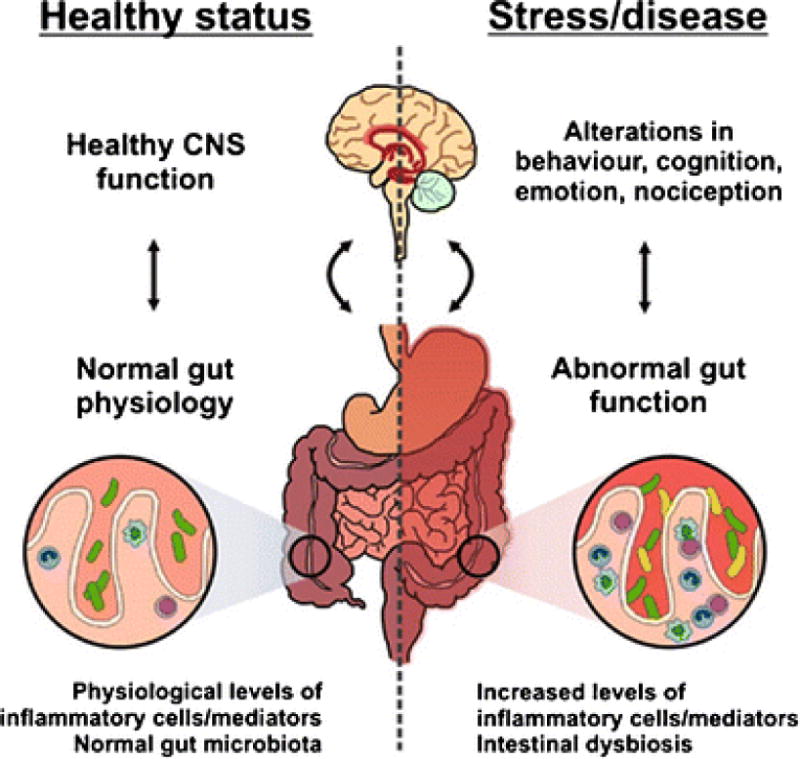
Brain–gut–microbe communication in health and disease. A stable gut microbiota is essential for normal gut physiology and contributes to appropriate signaling along the brain–gut axis and to the healthy status of the individual as shown on the left hand side of the diagram. Conversely, as shown on the right hand side of the diagram, intestinal dysbiosis can adversely influence gut physiology leading to inappropriate brain–gut axis signaling and associated consequences for CNS functions and disease states. Stress at the level of the CNS can also impact on gut function and lead to perturbations of the microbiota. (Legend and figure reproduced from: Grenham S, et al. Frontiers in Physiology 2011;2:94) [17].
Dysbiosis results when there is an imbalance between symbiotic, commensal and pathogenic microorganisms, involving decreased diversity and an increased presence of pathogenic bacteria (20). Emerging evidence suggests that factors such as genetic predisposition, an unhealthy diet, the use of antibiotics, and psychological and physical stress can disrupt the gut microbiome and contribute to dysbiosis (20). For example, exam stress was found to increase the presence of harmful bacteria in the stool of college students (38), and stress from confinement training promoted the growth of harmful fecal bacteria in astronauts (39). In animal models, the stress-related chemical messenger, norepinephrine, has been shown to bind directly to pathogenic bacteria and increase their virulence (34), while changes in depression-related serotonin and CRF/CRH signaling can contribute to altered motility, fluid secretion, and heightened intestinal permeability, indirectly influencing dysbiosis (34,40).
Gut microbiota have been associated with depressive behaviors in animals (41), and depression in humans (42). While research involving humans is limited, it is important to note that serotonin is a key signaling molecule at both ends of the BGM pathway, is synthesized and regulated by microbiota (43), and is central to BGM homeostasis (15). Additionally, there is growing support for a link between gut microbiota and various chronic conditions, including obesity, metabolic syndrome, (44) and T2D (45). Preliminary work in humans supports that those with T2D have a reduced abundance of Bacteroidetes and a higher abundance of Firmicutes (45), and one experimental study showed that transfer of gut microbiota from individuals who were lean to those with metabolic syndrome, improved gut microbiome diversity and insulin sensitivity among the recipients (46). Further, a comprehensive systematic review and meta-analysis recently documented that gastric bypass surgery, which contributes to weight loss and a reduced risk of T2D, also leads to changes in gut microbiota composition, particularly enrichment of Bacteroidetes (47).
3. Homeostatic Dysregulation: Inflammation, Metabolism and Sleep
The ability to fight infections and store energy is essential for survival, and immune and metabolic processes deeply depend on one another. Immune cells rely on energy (i.e., glucose) from the metabolic system to fight infections, while food digestion requires help from the immune system and helpful microbiota to differentiate between nutrients and pathogens within the GI tract (48). As previously mentioned, the HPA and BGM axes, and fundamental physiological processes (immune and metabolic function, diurnal sleep rhythm) use the same chemical language that involves various hormones, cytokines, and other signaling proteins that allow for flexible communication and regulation (13) (See Figure 1).
a. Immune function
Chronic inflammation appears to be a central mechanism through which homeostatic dysregulation negatively influences physical health. When the HPA axis is functioning normally, acute surges of cortisol initially activate immune responses, and later contribute to restraining neuroendocrine and inflammatory responses (22,49). However, when chronic stressors alter diurnal cortisol patterns, targeted cells (e.g., immune cells) begin to show a reduced response, termed glucocorticoid resistance (50). Thus, HPA axis dysregulation ultimately prolongs activation of the inflammatory response (22,50). Several studies highlight that glucocorticoid resistance is central to the effects of chronic stress and HPA axis dysregulation on prolonged inflammation (50). Glucocorticoid resistance has also been tied to depression (51) and physical illness (30,52). Emerging evidence further suggests that dysregulation of the BGM axis contributes to systemic inflammation (53). This is in large part because dysbiosis contributes to epithelial permeability, or leakiness of the gut lining, which allows pathogenic bacteria to enter the body that then produces an inflammatory response (20,53).
It is known that about a third of patients treated with interferon-alpha in several diseases (e.g., cancer, hepatitis C) develop ‘sickness behavior’, which shares similarities with depression such as depressed mood, reduced appetite, sleep changes, fatigue and psychomotor slowing, and termination of the medication alleviates these symptoms (14,54). Since this discovery, many studies have examined the relationship between inflammation and depression with mixed findings (e.g., (55)). A recent meta-analysis of 24 studies examining cytokines associated with depression showed consistent findings of elevated pro-inflammatory markers Tumor Necrosis Factor-Alpha (TNF-α) and interleukin-6 (IL-6) and interleukin-1 beta (IL-1β) among individuals meeting the Diagnostic and Statistical Manual of Mental Disorders (DSM) criteria for depression compared to a control group (56), strengthening the notion that depression is associated with a chronic inflammatory state.
The increased presence of TNF-α, IL-6, and IL-1β in depression is noteworthy, as they are primary messengers when it comes to immune regulation of the HPA axis (e.g., (57), and are the same pro-inflammatory markers implicated in T2D (58). Cumulative research suggests that inflammation is related to T2D pathogenesis, with chemical messengers IL-6, IL-1β and TNF- α playing noteworthy roles in the development of obesity and insulin resistance, or cellular insensitivity to insulin (58). Excessive nutrient intake activates the IL-system in the pancreas, while the presence of excess adipose tissue increases circulating IL-6 and TNF- α (58). Reducing TNF-α secretion from adipose tissue in animal models improved glycemic control and insulin sensitivity (59).
b. Food and Glucose Metabolism
The HPA axis regulates metabolism while metabolic factors (e.g., insulin) also regulate HPA axis function, which is believed to increase homeostatic flexibility in times of feast or famine (60). Hypercortisolemia increases the release of glucose from the liver, while at the same time interfering with insulin action, including inhibiting insulin release from pancreatic beta cells (61), and weakening the insulin-dependent transport of glucose into cells (62), which together contribute to hyperglycemia. Cortisol also influences fat storage and distribution, and hypercortisolemia contributes to central adiposity (63).
Gut bacteria appear to play a key role in the digestive process, and have also been shown to be related to glucose metabolism. In fact, humans would not be able to metabolize a large portion of their food without the help of gut microbiota (17). Gut microbiota also control the amount of energy that is taken from food and thus play an important role in weight gain, showing increased energy harvest from food in obesity (63). Gut microbiota are associated with weight gain, fat distribution, and adipose tissue inflammation(44,63), which contributes to insulin resistance (63) and thus glycemic control.
Depression and the presence of emotional distress are both associated with changes in glucose metabolism, and are particularly associated with insulin resistance (64,65). A recent systematic review and meta-analysis of 18 studies examining depression and insulin resistance, showed a small but significant association (64). Another meta-analysis of 24 studies also showed a small to moderate association between depression and hyperglycemia in diabetes patients (66). While these relationships have been documented, further experimental and longitudinal studies are needed to better understand causal or temporal associations between depression and insulin resistance (64) and/or glycemic control (67,68).
In summary, the HPA and BGM axes play significant roles in regulating various metabolic functions related to T2D, an illness characterized by insulin resistance in the presence of beta cell dysfunction that together contribute to a hyperglycemic state (69). While there is a considerable genetic component to developing T2D (70) insulin resistance and beta cell dysfunction are also strongly influenced by behavioral and environmental factors associated with our industrialized society (69), including psychological and emotional factors (62).
c. Diurnal Sleep Rhythm
The HPA axis is known to contribute to sleep regulation, while relationships are less clear when it comes to sleep and the BGM axis. Peaks and troughs of the healthy diurnal cortisol pattern align with times of activity and rest (71), and the development of the diurnal cortisol secretion pattern is closely related to the initiation of the circadian sleep cycle in newborns (72). The potential influence of the BGM on the diurnal sleep rhythm has been recently assessed in mice, showing circadian fluctuations in microbiome composition and gene expression that relate to host feeding schedules (73), and serotonin changes associated with the brain-gut axis are associated with sleep regulation (15). Further, animal models of sleep apnea also show that intermittent hypoxia contributes to a higher abundance of phylum Firmicutes and a decrease in Bacteroidetes and Proteobacteria phyla compared to controls (74).
Fatigue and sleep disturbances are common among individuals with depression, as well as individuals with T2D. In fact, insomnia and hypersomnia are symptoms that contribute to a diagnosis of MDD (75). Depression is also genetically linked to clock gene polymorphisms, suggesting a common biological predisposition with sleep dysregulation (76). However, the specific relationship between depression and changes in circadian sleep rhythm are not completely clear, with several hypotheses related to disturbance in circadian, social and sleep rhythms alignment (e.g., the phase-shift, phase advance, and social rhythms hypotheses) (77). Fatigue and sleep disturbances are also commonly reported by individuals with diabetes, with a recent study showing that 50% of diabetes patients reported insomnia compared to 27% of patients without diabetes (78). Large prospective studies show that reduced self-reported sleep duration (<6 hrs) is related to an increased risk of T2D, and this increased risk appears to be due to heightened inflammation, insulin resistance, appetite and weight gain associated with sleep loss (79,80). Obstructive sleep apnea is also common, affecting 58%-86% of individuals with T2D, and is associated with increased insulin resistance and poor glycemic control (79,81). Few studies have examined the causal effect of sleep deprivation on glycemic control in diabetes samples, and these studies suggest that sleep deprivation reduces glycemic control, which is consistent with studies in healthy adults (79).
4. Clinical Implications
Given the preliminary nature of much of this research, it is premature to propose concrete recommendations for health practitioners. Our review of initial evidence suggests psychological, behavioral and pharmacological therapies that target dysregulated homeostatic brain-body pathways may have positive effects on depression and T2D control. In particular, perceived stress is directly related to activation of the HPA and BGM axes, and adaptive coping strategies likely have powerful influences on health (21). For example, interpersonal therapy, which is focused on finding solutions to current interpersonal problems, has been shown to be more effective in reducing depressive symptoms among individuals with depressive disorders than placebo or CBT (82). . However, it is important to note that interventions alleviating depression and distress in T2D have generally failed to improve glycemic control (83).
Health behaviors such as a healthy diet, physical activity and adequate sleep can also reduce homeostatic dysregulation. For instance, a diet high in fiber and low in fat promotes the growth of helpful bacteria and thus can reduce gut dysbiosis (63). In addition, initial meta-analyses of RCTs testing probiotics (which increase the presence of helpful bacteria) as a potential treatment, are showing promising results for reducing depressive symptoms among physically healthy individuals (84), and improving glycemic control and insulin resistance in T2D patients (85). However, future studies would benefit from having larger sample sizes, analyzing individual probiotic strains, and considering probiotic survivability in the GI tract (84). Furthermore, physical activity is known to regulate HPA axis function and contribute to increased insulin sensitivity (86), and has been found to be as effective as selective serotonin reuptake inhibitors (SSRIs) in alleviating depression (87). Treatment of sleep issues may also improve depression and glycemic control; initial evidence suggests that successful CBT for insomnia also improves depressive symptoms not related to sleep (e.g., hopefulness, energy levels, suicidality), though these effects require further replication (91). Meta-analyses assessing treatment of sleep apnea among individuals with T2D have shown inconclusive results (79). It is possible that individuals with comorbid depression and T2D require interventions that target multiple aspects of mental and physical health concurrently. For example, an integrative treatment that paired physical activity with cognitive behavioral therapy showed promise for improving depressive symptoms and glycemic control among individuals with T2D and comorbid depression (88), though specific potential mechanisms were not assessed in this study. Measurement of homeostatic dysregulation in future studies, which we discuss in more detail in the next section, would allow researchers to test potential explanatory mechanism for differences in health outcomes.
5. Research Implications and Future Directions
a. Clinical Depression and Emotional Distress
Since chronic psychological stress as well as depression is associated with dysregulation of the HPA and BGM axes, it is unclear how a clinical diagnosis of depression is related to homeostatic dysregulation specifically. Some researchers have proposed that chronic emotional distress is a contributor to dysregulation, while depression may be a consequence (e.g., (89), though this remains to be clarified. This is particularly relevant, given that previous literature supports that diabetes distress is more closely related to glycemic control in T2D compared to clinical depression per se (68). Future research should continue to differentiate between clinical depression and chronic emotional distress when considering the co-occurrence of mental and physical diseases, and dysregulation of the HPA and BGM axes.
Related to this, there is large diversity of symptoms within the construct of MDD, as well as heterogeneity in the assessment of depression (symptom report/screening scales vs. diagnostic interview). The gold standard for diagnosing depression is a diagnostic interview that assesses whether a particular individual meets the criteria presented in the DSM (75), though different minimally-overlapping symptom constellations can contribute to the same diagnosis (67). In research, the presence or absence of depression among those with diabetes is often assessed utilizing cutoff scores for self-report measures. However, the use of self-report questionnaire cutoff scores leads to a substantial overdiagnosis of depression, and depressive symptoms greatly overlap with general emotional distress, diabetes-specific distress, and physical symptoms of T2D (67), making it difficult to tease apart whether studies are assessing depression, emotional distress more generally, or symptoms of T2D. This heterogeneity in MDD symptoms and assessment may mask relations to dysregulation of homeostatic pathways, and it is possible that particular depression symptoms such as fatigue (90) are more closely related to homeostatic dysregulation than other (i.e., cognitive) symptoms of depression.
b. Measurement of Homeostatic Dysregulation
It is of primary importance that dysregulation of brain-body pathways are more clearly characterized in patients with depression, T2D, and in those with both conditions. Routine assessment of one or more of the following measures would help quantify and illuminate the role of homeostatic pathways in depression and diabetes: diurnal cortisol patterns, the dexamethasone-CRH (DEX/CRH) test assessing HPA axis dysregulation and glucocorticoid resistance, homeostasis model assessment of insulin resistance (HOMA-R), the diversity and prevalence of harmful (e.g., phylum Firmicutes) and helpful (e.g., phyla Proteobacteria and Bacteroidetes) gut microbiota, degree of endothelial permeability in the gut, degree of chronic systemic low grade inflammation (e.g., CRP), and sleep quality/duration over time. C-Reactive protein is a sensitive non-specific measure of low-grade inflammation and thus a good measure to assess homeostatic dysregulation, while measurements of IL-6, the IL-1 family, and TNF-α would allow for more specific characterization of inflammation specific to the biological overlap between depression and T2D. Further, somatic symptoms such as fatigue have been shown to be related to homeostatic dysregulation (90), and thus have the potential to act as an indirect patient-reported measure to accompany biological measures. The theoretical model that we have introduced provides researchers with specific biological measures to assess, and may help strengthen future research design.
Future studies should examine whether depressed and non-depressed T2D patients differ in measures of homeostatic dysregulation mentioned above; as this could help to establish whether comorbidity is associated with greater disruption of homeostasis, or whether co-occurrence is due to other factors. An initial study by Laake and colleagues (91) showed a relationship between increased inflammatory markers and depressive symptoms in 1,790 participants recently diagnosed with T2D, providing evidence of increased homeostatic dysregulation among individuals with both disorders. However, these relationships require replication and further exploration.
c. Medication Treatments
The theoretical model we have presented here may also strengthen our understanding of how antidepressant medications relate to depression and T2D. It is well-known that CRF/CFH release from the hypothalamus is under serotonergic control, and serotonin also appears to have direct action on the pituitary and adrenal cortex, thus influencing ACTH and cortisol release independent of CRF/CRH (89). Serotonin also plays a critical role in immune cell signaling and inflammatory processes (92), and successful antidepressant treatment appears to work in part by influencing glucocorticoid receptor function and restoring communication between the HPA axis and TNF-α system (93). Treatment of T2D with antidepressant medications with anti-inflammatory properties could theoretically address symptoms of depression and disease simultaneously (94), though further research is needed.
One issue is that serotonin is one of the most abundant and heterogeneous chemical signaling molecules in the body, and SSRIs, serotonin-norepineprine reuptake inhibitors (SNRIs), and tricyclic (TCAs) and atypical antidepressants each have unique mechanisms of action that are often not fully understood (95,96). Currently, literature shows mixed findings related to the positive and negative effects of various antidepressant medications on health. A meta-analysis showed that mirtazapine (an atypical), amitriptyline (a TCA), and paroxetine (an SSRI) increased the risk for weight gain, while bupropion (an atypical) and fluoxetine (an SSRI) contributed to weight loss (97). This is consistent with findings showing that fluoxetine improves glucose control and nortripyline (a TCA) can worsen glucose control (98).
For these reasons, potential effects of antidepressants in relation to depression, T2D, and homeostatic regulation should be examined by class, and sometimes even by specific antidepressant, as there can be variations in serotonin signaling and receptor binding between and within classes (95,96). For example, recent epidemiological studies linking antidepressant use to future T2D diabetes risk combined antidepressant classes when testing a potential causal biological relationship (e.g. (99,100), though Kivimaki and colleagues (100) did look at SSRIs and TCAs individually in follow-up analyses and Rubin and colleagues (99) removed antidepressants associated with weight loss (e.g., bupropion). Bench work elucidating the full range of serotonin signaling in the body, alongside scientific clarification of specific mechanisms of action for various antidepressants, would contribute to stronger clinical and epidemiological study designs when it comes to biological pathways linking depression, antidepressants, and risk for T2D.
6. Conclusion
While the complexity of the described inter-related physiological systems increases flexibility and adaptability for survival, the complexity also allows for multiple opportunities of dysregulation. This becomes more problematic in contemporary society, which introduces several sources of continual disruption including psychological stress, antibiotic use, poor diet, physical inactivity, and shifts in diurnal patterns. It is important for future research to differentiate chronic emotional distress from depression, as emotional distress appears to be a powerful driver of homeostatic dysregulation. This is consistent with epidemiological and population-based data supporting that depression may be a stronger predictor for T2D than vice versa (5,6). The evidence that depression and T2D involve shared dysregulation of homeostatic brain-body pathways, suggests that this dysregulation may be an important treatment target for depression, T2D, and their comorbidity. However, research examining the BGM axis is in its infancy, and researchers as well as clinicians should be cautiously optimistic about the future connections with mental and physical health that may be made. Future studies should attempt to establish the various causal relationships underlying HPA and BGM axis dysregulation in relation to depression, emotional distress, and the development of T2D.
Acknowledgments
Support was provided by The Drs. David and Jane Willner Bloomgarden Family Fellowship Fund.
Footnotes
SpringerLink Header: Psychosocial Aspects (S Jaser, Section Editor)
Compliance with Ethical Standards
Conflict of Interest
Claire J. Hoogendoorn, Juan F. Roy, and Jeffrey S. Gonzalez declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.National Institutes of Mental Health. Major depression among adults. 2017 Available at: http://www.nimh.nih.gov/health/statistics/prevalence/major-depression-among-adults.shtml. Accessed 3/17.
- 2.Boyle JP, Thompson TJ, Gregg EW, Barker LE, Williamson DF. Projection of the year 2050 burden of diabetes in the US adult population: dynamic modeling of incidence, mortality, and prediabetes prevalence. Population health metrics. 2010;8(1):29. doi: 10.1186/1478-7954-8-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ali S, Stone M, Peters J, Davies M, Khunti K. The prevalence of co-morbid depression in adults with Type 2 diabetes: a systematic review and meta-analysis. Diabetic Med. 2006;23(11):1165–1173. doi: 10.1111/j.1464-5491.2006.01943.x. [DOI] [PubMed] [Google Scholar]
- 4.Anderson RJ, Freedland KE, Clouse RE, Lustman PJ. The prevalence of comorbid depression in adults with diabetes: a meta-analysis. Diabetes Care. 2001 Jun;24(6):1069–1078. doi: 10.2337/diacare.24.6.1069. [DOI] [PubMed] [Google Scholar]
- 5.De Jonge P, Roy J, Saz P, Marcos G, Lobo A. Prevalent and incident depression in community-dwelling elderly persons with diabetes mellitus: results from the ZARADEMP project. Diabetologia. 2006;49(11):2627–2633. doi: 10.1007/s00125-006-0442-x. [DOI] [PubMed] [Google Scholar]
- 6.Campayo A, de Jonge P, Roy JF, Saz P, de la Cámara C, Quintanilla MA, et al. Depressive disorder and incident diabetes mellitus: the effect of characteristics of depression. Am J Psychiatry. 2010;167(5):580–588. doi: 10.1176/appi.ajp.2009.09010038. [DOI] [PubMed] [Google Scholar]
- 7.Gonzalez JS, Safren SA, Cagliero E, Wexler DJ, Delahanty L, Wittenberg E, et al. Depression, self-care, and medication adherence in type 2 diabetes: relationships across the full range of symptom severity. Diabetes Care. 2007 Sep;30(9):2222–2227. doi: 10.2337/dc07-0158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nouwen A, Nefs G, Caramlau I, Connock M, Winkley K, Lloyd CE, et al. Prevalence of depression in individuals with impaired glucose metabolism or undiagnosed diabetes: a systematic review and meta-analysis of the European Depression in Diabetes (EDID) Research Consortium Diabetes Care. 2011 Mar;34(3):752–762. doi: 10.2337/dc10-1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moulton CD, Pickup JC, Ismail K. The link between depression and diabetes: the search for shared mechanisms. The Lancet Diabetes & Endocrinology. 2015;3(6):461–471. doi: 10.1016/S2213-8587(15)00134-5. [DOI] [PubMed] [Google Scholar]
- 10.Gonzalez JS, Peyrot M, McCarl LA, Collins EM, Serpa L, Mimiaga MJ, et al. Depression and diabetes treatment nonadherence: a meta-analysis. Diabetes Care. 2008 Dec;31(12):2398–2403. doi: 10.2337/dc08-1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miller GE, Chen E, Zhou ES. If it goes up, must it come down? Chronic stress and the hypothalamic-pituitary-adrenocortical axis in humans. Psychol Bull. 2007;133(1):25. doi: 10.1037/0033-2909.133.1.25. [DOI] [PubMed] [Google Scholar]
- 12.Vedder H. Physiology of the hypothalamic–pituitary–adrenocortical axis. NeuroImmune Biology. 2007;7:17–31. [Google Scholar]
- 13.Blalock J. The immune system as the sixth sense. J Intern Med. 2005;257(2):126–138. doi: 10.1111/j.1365-2796.2004.01441.x. [DOI] [PubMed] [Google Scholar]
- 14.Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nature reviews neuroscience. 2008;9(1):46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O’Mahony S, Clarke G, Borre Y, Dinan T, Cryan J. Serotonin, tryptophan metabolism and the brain-gut-microbiome axis. Behav Brain Res. 2015;277:32–48. doi: 10.1016/j.bbr.2014.07.027. [DOI] [PubMed] [Google Scholar]
- 16.Eskandari F, Sternberg EM. Neural-immune interactions in health and disease. Ann N Y Acad Sci. 2002;966(1):20–27. doi: 10.1111/j.1749-6632.2002.tb04198.x. [DOI] [PubMed] [Google Scholar]
- 17.Grenham S, Clarke G, Cryan JF, Dinan TG. Brain–gut–microbe communication in health and disease. Frontiers in Physiology. 2011;2:94. doi: 10.3389/fphys.2011.00094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dhabhar FS. Enhancing versus suppressive effects of stress on immune function: implications for immunoprotection and immunopathology. Neuroimmunomodulation. 2009;16(5):300–317. doi: 10.1159/000216188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chakravarthy MV, Booth FW. Eating, exercise, and “thrifty” genotypes: connecting the dots toward an evolutionary understanding of modern chronic diseases. J Appl Physiol 1985. 2004 Jan;96(1):3–10. doi: 10.1152/japplphysiol.00757.2003. [DOI] [PubMed] [Google Scholar]
- 20.Myers SP. The causes of intestinal dysbiosis: a review. Altern Med Rev. 2004;9(2):180–197. [PubMed] [Google Scholar]
- 21.McEwen BS. Stress, adaptation, and disease: Allostasis and allostatic load. Ann N Y Acad Sci. 1998;840(1):33–44. doi: 10.1111/j.1749-6632.1998.tb09546.x. [DOI] [PubMed] [Google Scholar]
- 22.Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions 1. Endocr Rev. 2000;21(1):55–89. doi: 10.1210/edrv.21.1.0389. [DOI] [PubMed] [Google Scholar]
- 23.Kudielka BM, Schommer NC, Hellhammer DH, Kirschbaum C. Acute HPA axis responses, heart rate, and mood changes to psychosocial stress (TSST) in humans at different times of day. Psychoneuroendocrinology. 2004;29(8):983–992. doi: 10.1016/j.psyneuen.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 24.Miller DB, O’Callaghan JP. Neuroendocrine aspects of the response to stress. Metab Clin Exp. 2002;51(6):5–10. doi: 10.1053/meta.2002.33184. [DOI] [PubMed] [Google Scholar]
- 25.Kaye J, Buchanan F, Kendrick A, Johnson P, Lowry C, Bailey J, et al. Acute carbon dioxide exposure in healthy adults: evaluation of a novel means of investigating the stress response. J Neuroendocrinol. 2004;16(3):256–264. doi: 10.1111/j.0953-8194.2004.01158.x. [DOI] [PubMed] [Google Scholar]
- 26.Stetler C, Miller GE. Depression and hypothalamic-pituitary-adrenal activation: a quantitative summary of four decades of research. Psychosom Med. 2011 Feb-Mar;73(2):114–126. doi: 10.1097/PSY.0b013e31820ad12b. [DOI] [PubMed] [Google Scholar]
- 27.Murri MB, Pariante C, Mondelli V, Masotti M, Atti AR, Mellacqua Z, et al. HPA axis and aging in depression: systematic review and meta-analysis. Psychoneuroendocrinology. 2014;41:46–62. doi: 10.1016/j.psyneuen.2013.12.004. [DOI] [PubMed] [Google Scholar]
- 28.Ferrari E, Casarotti D, Muzzoni B, Albertelli N, Cravello L, Fioravanti M, et al. Age-related changes of the adrenal secretory pattern: possible role in pathological brain aging. Brain Res Rev. 2001;37(1):294–300. doi: 10.1016/s0165-0173(01)00133-3. [DOI] [PubMed] [Google Scholar]
- 29.Rosmond R, Björntorp P. Occupational Status, Cortisol Secretory Pattern, and Visceral Obesity in Middle-aged Men. Obes Res. 2000;8(6):445–450. doi: 10.1038/oby.2000.55. [DOI] [PubMed] [Google Scholar]
- 30.Walker BR. Glucocorticoids and cardiovascular disease. Eur J Endocrinol. 2007 Nov;157(5):545–559. doi: 10.1530/EJE-07-0455. [DOI] [PubMed] [Google Scholar]
- 31.Chan O, Inouye K, Riddell MC, Vranic M, Matthews SG. Diabetes and the hypothalamo-pituitary-adrenal (HPA) axis. Minerva Endocrinol. 2003 Jun;28(2):87–102. [PubMed] [Google Scholar]
- 32.Roy M, Collier B, Roy A. Dysregulation of the hypothalamo-pituitary-adrenal axis and duration of diabetes. J Diabet Complications. 1991;5(4):218–220. doi: 10.1016/0891-6632(91)90079-5. [DOI] [PubMed] [Google Scholar]
- 33.McEwen BS. Allostasis and allostatic load: implications for neuropsychopharmacology. Neuropsychopharmacology. 2000;22(2):108–124. doi: 10.1016/S0893-133X(99)00129-3. [DOI] [PubMed] [Google Scholar]
- 34.Rhee SH, Pothoulakis C, Mayer EA. Principles and clinical implications of the brain–gut–enteric microbiota axis. Nature Reviews Gastroenterology and Hepatology. 2009;6(5):306–314. doi: 10.1038/nrgastro.2009.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cryan JF, Dinan TG. Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nature reviews neuroscience. 2012;13(10):701–712. doi: 10.1038/nrn3346. [DOI] [PubMed] [Google Scholar]
- 36.Turnbaugh PJ, Hamady M, Yatsunenko T, Cantarel BL, Duncan A, Ley RE, et al. A core gut microbiome in obese and lean twins. Nature. 2009;457(7228):480–484. doi: 10.1038/nature07540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Qin J, Li Y, Cai Z, Li S, Zhu J, Zhang F, et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature. 2012;490(7418):55–60. doi: 10.1038/nature11450. [DOI] [PubMed] [Google Scholar]
- 38.Knowles SR, Nelson EA, Palombo EA. Investigating the role of perceived stress on bacterial flora activity and salivary cortisol secretion: a possible mechanism underlying susceptibility to illness. Biol Psychol. 2008;77(2):132–137. doi: 10.1016/j.biopsycho.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 39.Holdeman LV, Good IJ, Moore WE. Human fecal flora: variation in bacterial composition within individuals and a possible effect of emotional stress. Appl Environ Microbiol. 1976 Mar;31(3):359–375. doi: 10.1128/aem.31.3.359-375.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bailey MT, Dowd SE, Parry NM, Galley JD, Schauer DB, Lyte M. Stressor exposure disrupts commensal microbial populations in the intestines and leads to increased colonization by Citrobacter rodentium. Infect Immun. 2010 Apr;78(4):1509–1519. doi: 10.1128/IAI.00862-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dinan TG, Cryan JF. Melancholic microbes: a link between gut microbiota and depression? Neurogastroenterology & Motility. 2013;25(9):713–719. doi: 10.1111/nmo.12198. [DOI] [PubMed] [Google Scholar]
- 42.Naseribafrouei A, Hestad K, Avershina E, Sekelja M, Linløkken A, Wilson R, et al. Correlation between the human fecal microbiota and depression. Neurogastroenterology & Motility. 2014;26(8):1155–1162. doi: 10.1111/nmo.12378. [DOI] [PubMed] [Google Scholar]
- 43.Yano JM, Yu K, Donaldson GP, Shastri GG, Ann P, Ma L, et al. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell. 2015;161(2):264–276. doi: 10.1016/j.cell.2015.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44•.Boulangé CL, Neves AL, Chilloux J, Nicholson JK, Dumas M. Impact of the gut microbiota on inflammation, obesity, and metabolic disease. Genome medicine. 2016;8(1):42. doi: 10.1186/s13073-016-0303-2. This review provides a thorough overview of current knowledge regarding the mechanistic interactions between the gut microbiota, and host energy metabolism and immune system, as it relates to obesity and metabolic disease. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Forslund K, Hildebrand F, Nielsen T, Falony G, Le Chatelier E, Sunagawa S, et al. Disentangling type 2 diabetes and metformin treatment signatures in the human gut microbiota. Nature. 2015;528(7581):262–266. doi: 10.1038/nature15766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vrieze A, Van Nood E, Holleman F, Salojärvi J, Kootte RS, Bartelsman JF, et al. Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology. 2012;143(4):913–916 e7. doi: 10.1053/j.gastro.2012.06.031. [DOI] [PubMed] [Google Scholar]
- 47.Magouliotis DE, Tasiopoulou VS, Sioka E, Chatedaki C, Zacharoulis D. Impact of Bariatric Surgery on Metabolic and Gut Microbiota Profile: a Systematic Review and Meta-analysis. Obesity Surg. 2017:1–13. doi: 10.1007/s11695-017-2595-8. [DOI] [PubMed] [Google Scholar]
- 48.Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444(7121):860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- 49.Charmandari E, Tsigos C, Chrousos G. Endocrinology of the stress response 1. Annu Rev Physiol. 2005;67:259–284. doi: 10.1146/annurev.physiol.67.040403.120816. [DOI] [PubMed] [Google Scholar]
- 50.Cohen S, Janicki-Deverts D, Doyle WJ, Miller GE, Frank E, Rabin BS, et al. Chronic stress, glucocorticoid receptor resistance, inflammation, and disease risk. Proc Natl Acad Sci U S A. 2012 Apr 17;109(16):5995–5999. doi: 10.1073/pnas.1118355109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pariante CM, Miller AH. Glucocorticoid receptors in major depression: relevance to pathophysiology and treatment. Biol Psychiatry. 2001;49(5):391–404. doi: 10.1016/s0006-3223(00)01088-x. [DOI] [PubMed] [Google Scholar]
- 52.Franchimont DP, Chrousos GP. Glucocorticoid Resistance in Inflammatory Diseases. NeuroImmune Biology. 2007;7:349–358. [Google Scholar]
- 53.Macia L, Thorburn AN, Binge LC, Marino E, Rogers KE, Maslowski KM, et al. Microbial influences on epithelial integrity and immune function as a basis for inflammatory diseases. Immunol Rev. 2012;245(1):164–176. doi: 10.1111/j.1600-065X.2011.01080.x. [DOI] [PubMed] [Google Scholar]
- 54.Raison CL, Capuron L, Miller AH. Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends Immunol. 2006;27(1):24–31. doi: 10.1016/j.it.2005.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Steptoe A, Kunz-Ebrecht S, Owen N. Lack of association between depressive symptoms and markers of immune and vascular inflammation in middle-aged men and women. Psychol Med. 2003;33(04):667–674. doi: 10.1017/s0033291702007250. [DOI] [PubMed] [Google Scholar]
- 56.Dowlati Y, Herrmann N, Swardfager W, Liu H, Sham L, Reim EK, et al. A meta-analysis of cytokines in major depression. Biol Psychiatry. 2010;67(5):446–457. doi: 10.1016/j.biopsych.2009.09.033. [DOI] [PubMed] [Google Scholar]
- 57.Beishuizen A, Thijs LG. Review: endotoxin and the hypothalamo-pituitary-adrenal (HPA) axis. J Endotoxin Res. 2003;9(1):3–24. doi: 10.1179/096805103125001298. [DOI] [PubMed] [Google Scholar]
- 58.Donath MY, Shoelson SE. Type 2 diabetes as an inflammatory disease. Nature Reviews Immunology. 2011;11(2):98–107. doi: 10.1038/nri2925. [DOI] [PubMed] [Google Scholar]
- 59.Ilan Y, Maron R, Tukpah AM, Maioli TU, Murugaiyan G, Yang K, et al. Induction of regulatory T cells decreases adipose inflammation and alleviates insulin resistance in ob/ob mice. Proc Natl Acad Sci U S A. 2010 May 25;107(21):9765–9770. doi: 10.1073/pnas.0908771107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dallman MF, Strack AM, Akana SF, Bradbury MJ, Hanson ES, Scribner KA, et al. Feast and famine: critical role of glucocorticoids with insulin in daily energy flow. Front Neuroendocrinol. 1993;14(4):303–347. doi: 10.1006/frne.1993.1010. [DOI] [PubMed] [Google Scholar]
- 61.Lambillotte C, Gilon P, Henquin JC. Direct glucocorticoid inhibition of insulin secretion. An in vitro study of dexamethasone effects in mouse islets. J Clin Invest. 1997 Feb 1;99(3):414–423. doi: 10.1172/JCI119175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rosmond R. Stress induced disturbances of the HPA axis: a pathway to Type 2 diabetes? Med Sci Monit. 2003 Feb;9(2):RA35–9. [PubMed] [Google Scholar]
- 63.Ley RE. Obesity and the human microbiome. Curr Opin Gastroenterol. 2010 Jan;26(1):5–11. doi: 10.1097/MOG.0b013e328333d751. [DOI] [PubMed] [Google Scholar]
- 64.Kan C, Silva N, Golden SH, Rajala U, Timonen M, Stahl D, et al. A systematic review and meta-analysis of the association between depression and insulin resistance. Diabetes Care. 2013 Feb;36(2):480–489. doi: 10.2337/dc12-1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang J, Niaura R, Dyer JR, Shen BJ, Todaro JF, McCaffery JM, et al. Hostility and urine norepinephrine interact to predict insulin resistance: the VA Normative Aging Study. Psychosom Med. 2006 Sep-Oct;68(5):718–726. doi: 10.1097/01.psy.0000228343.89466.11. [DOI] [PubMed] [Google Scholar]
- 66.Lustman PJ, Anderson RJ, Freedland KE, de Groot M, Carney RM, Clouse RE. Depression and poor glycemic control: a meta-analytic review of the literature. Diabetes Care. 2000 Jul;23(7):934–942. doi: 10.2337/diacare.23.7.934. [DOI] [PubMed] [Google Scholar]
- 67•.Snoek FJ, Bremmer MA, Hermanns N. Constructs of depression and distress in diabetes: time for an appraisal. The Lancet Diabetes & Endocrinology. 2015;3(6):450–460. doi: 10.1016/S2213-8587(15)00135-7. This review describes evidence that diabetes distress and depression are distinct overlapping constructs, and reviews evidence that diabetes distress may mediate the relationship between depression and glycemic control. Further, the review outlines three distinct data-driven depression symptom profiles that appear to show differential relationships with metabolic outcomes. [DOI] [PubMed] [Google Scholar]
- 68.Fisher L, Skaff M, Mullan J, Arean P, Glasgow R, Masharani U. A longitudinal study of affective and anxiety disorders, depressive affect and diabetes distress in adults with type 2 diabetes. Diabetic Med. 2008;25(9):1096–1101. doi: 10.1111/j.1464-5491.2008.02533.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kahn SE, Cooper ME, Del Prato S. Pathophysiology and treatment of type 2 diabetes: perspectives on the past, present, and future. The Lancet. 2014;383(9922):1068–1083. doi: 10.1016/S0140-6736(13)62154-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dupuis J, Langenberg C, Prokopenko I, Saxena R, Soranzo N, Jackson AU, et al. New genetic loci implicated in fasting glucose homeostasis and their impact on type 2 diabetes risk. Nat Genet. 2010;42(2):105–116. doi: 10.1038/ng.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dallman MF, Strack AM, Akana SF, Bradbury MJ, Hanson ES, Scribner KA, et al. Feast and famine: critical role of glucocorticoids with insulin in daily energy flow. Front Neuroendocrinol. 1993;14(4):303–347. doi: 10.1006/frne.1993.1010. [DOI] [PubMed] [Google Scholar]
- 72.de Weerth C, Zijl RH, Buitelaar JK. Development of cortisol circadian rhythm in infancy. Early Hum Dev. 2003;73(1):39–52. doi: 10.1016/s0378-3782(03)00074-4. [DOI] [PubMed] [Google Scholar]
- 73.Trinder M, Bisanz JE, Burton JP, Reid G. Bacteria Need “Sleep” Too?: Microbiome Circadian Rhythmicity, Metabolic Disease, and Beyond. University of Toronto Medical Journal. 2015;92(3) [Google Scholar]
- 74.Moreno-Indias I, Torres M, Montserrat JM, Sanchez-Alcoholado L, Cardona F, Tinahones FJ, et al. Intermittent hypoxia alters gut microbiota diversity in a mouse model of sleep apnoea. Eur Respir J. 2015 Apr;45(4):1055–1065. doi: 10.1183/09031936.00184314. [DOI] [PubMed] [Google Scholar]
- 75.American Psychiatric Association. Diagnostic and statistic0al manual of mental disorders (DSM-5®) American Psychiatric Pub; 2013. [Google Scholar]
- 76.Desan PH, Oren DA, Malison R, Price LH, Rosenbaum J, Smoller J, et al. Genetic polymorphism at the CLOCK gene locus and major depression. American Journal of Medical Genetics Part A. 2000;96(3):418–421. doi: 10.1002/1096-8628(20000612)96:3<418::aid-ajmg34>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 77.Germain A, Kupfer DJ. Circadian rhythm disturbances in depression. Hum Psychopharmacol Clin Exp. 2008;23(7):571–585. doi: 10.1002/hup.964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bhaskar S, Hemavathy D, Prasad S. Prevalence of chronic insomnia in adult patients and its correlation with medical comorbidities. Journal of family medicine and primary care. 2016;5(4):780. doi: 10.4103/2249-4863.201153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79•.Reutrakul S, Van Cauter E. Interactions between sleep, circadian function, and glucose metabolism: implications for risk and severity of diabetes. Ann N Y Acad Sci. 2014;1311(1):151–173. doi: 10.1111/nyas.12355. This review provides an update on current evidence from experimental, prospective and interventional studies linking sleep disturbance, circadian dysregulation, and glucose metabolism. [DOI] [PubMed] [Google Scholar]
- 80.Knutson KL, Spiegel K, Penev P, Van Cauter E. The metabolic consequences of sleep deprivation. Sleep medicine reviews. 2007;11(3):163–178. doi: 10.1016/j.smrv.2007.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Aronsohn RS, Whitmore H, Van Cauter E, Tasali E. Impact of untreated obstructive sleep apnea on glucose control in type 2 diabetes. American journal of respiratory and critical care medicine. 2010;181(5):507–513. doi: 10.1164/rccm.200909-1423OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.de Mello MF, de Jesus Mari J, Bacaltchuk J, Verdeli H, Neugebauer R. A systematic review of research findings on the efficacy of interpersonal therapy for depressive disorders. Eur Arch Psychiatry Clin Neurosci. 2005;255(2):75–82. doi: 10.1007/s00406-004-0542-x. [DOI] [PubMed] [Google Scholar]
- 83.Ismail K, Winkley K, Rabe-Hesketh S. Systematic review and meta-analysis of randomised controlled trials of psychological interventions to improve glycaemic control in patients with type 2 diabetes. The Lancet. 2004;363(9421):1589–1597. doi: 10.1016/S0140-6736(04)16202-8. [DOI] [PubMed] [Google Scholar]
- 84.Pirbaglou M, Katz J, de Souza RJ, Stearns JC, Motamed M, Ritvo P. Probiotic supplementation can positively affect anxiety and depressive symptoms: a systematic review of randomized controlled trials. Nutr Res. 2016;36(9):889–898. doi: 10.1016/j.nutres.2016.06.009. [DOI] [PubMed] [Google Scholar]
- 85.Kasińska MA, Drzewoski J. Effectiveness of probiotics in type 2 diabetes: a meta-analysis. Pol Arch Med Wewn. 2015;125(11):803–813. doi: 10.20452/pamw.3156. [DOI] [PubMed] [Google Scholar]
- 86.Tsatsoulis A, Fountoulakis S. The protective role of exercise on stress system dysregulation and comorbidities. Ann N Y Acad Sci. 2006;1083(1):196–213. doi: 10.1196/annals.1367.020. [DOI] [PubMed] [Google Scholar]
- 87.Blumenthal JA, Babyak MA, Doraiswamy PM, Watkins L, Hoffman BM, Barbour KA, et al. Exercise and pharmacotherapy in the treatment of major depressive disorder. Psychosom Med. 2007 Sep-Oct;69(7):587–596. doi: 10.1097/PSY.0b013e318148c19a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.De Groot M, Doyle T, Kushnick M, Shubrook J, Merrill J, Rabideau E, et al. Can lifestyle interventions do more than reduce diabetes risk? Treating depression in adults with type 2 diabetes with exercise and cognitive behavioral therapy. Current diabetes reports. 2012;12(2):157–166. doi: 10.1007/s11892-012-0261-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Van Praag HM, de Kloet ER, van Os J. Stress, the brain and depression. Cambridge University Press; 2004. [Google Scholar]
- 90.Swain MG. Fatigue in chronic disease. Clin Sci (Lond) 2000 Jul;99(1):1–8. [PubMed] [Google Scholar]
- 91.Laake JP, Stahl D, Amiel SA, Petrak F, Sherwood RA, Pickup JC, et al. The association between depressive symptoms and systemic inflammation in people with type 2 diabetes: findings from the South London Diabetes Study. Diabetes Care. 2014 Aug;37(8):2186–2192. doi: 10.2337/dc13-2522. [DOI] [PubMed] [Google Scholar]
- 92.Baganz NL, Blakely RD. A dialogue between the immune system and brain, spoken in the language of serotonin. ACS chemical neuroscience. 2012;4(1):48–63. doi: 10.1021/cn300186b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Himmerich H, Binder EB, Künzel HE, Schuld A, Lucae S, Uhr M, et al. Successful antidepressant therapy restores the disturbed interplay between TNF-α system and HPA axis. Biol Psychiatry. 2006;60(8):882–888. doi: 10.1016/j.biopsych.2006.03.075. [DOI] [PubMed] [Google Scholar]
- 94.Guseva D, Holst K, Kaune B, Meier M, Keubler L, Glage S, et al. Serotonin 5-HT7 receptor is critically involved in acute and chronic inflammation of the gastrointestinal tract. Inflamm Bowel Dis. 2014 Sep;20(9):1516–1529. doi: 10.1097/MIB.0000000000000150. [DOI] [PubMed] [Google Scholar]
- 95.Naughton M, Mulrooney JB, Leonard BE. A review of the role of serotonin receptors in psychiatric disorders. Hum Psychopharmacol Clin Exp. 2000;15(6):397–415. doi: 10.1002/1099-1077(200008)15:6<397::AID-HUP212>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 96.Nemeroff CB, Owens MJ. Pharmacologic differences among the SSRIs: focus on monoamine transporters and the HPA axis. CNS spectrums. 2004;9(S4):23–31. doi: 10.1017/s1092852900025475. [DOI] [PubMed] [Google Scholar]
- 97.Serretti A, Mandelli L. Antidepressants and body weight: a comprehensive review and meta-analysis. J Clin Psychiatry. 2010 Oct;71(10):1259–1272. doi: 10.4088/JCP.09r05346blu. [DOI] [PubMed] [Google Scholar]
- 98.Deuschle M. Effects of antidepressants on glucose metabolism and diabetes mellitus type 2 in adults. Curr Opin Psychiatry. 2013 Jan;26(1):60–65. doi: 10.1097/YCO.0b013e32835a4206. [DOI] [PubMed] [Google Scholar]
- 99.Rubin RR, Ma Y, Marrero DG, Peyrot M, Barrett-Connor EL, Kahn SE, et al. Elevated depression symptoms, antidepressant medicine use, and risk of developing diabetes during the diabetes prevention program. Diabetes Care. 2008 Mar;31(3):420–426. doi: 10.2337/dc07-1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kivimaki M, Hamer M, Batty GD, Geddes JR, Tabak AG, Pentti J, et al. Antidepressant medication use, weight gain, and risk of type 2 diabetes: a population-based study. Diabetes Care. 2010 Dec;33(12):2611–2616. doi: 10.2337/dc10-1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hiller-Sturmhöfel S, Bartke A. The endocrine system: an overview. Alcohol Research and Health. 1998;22(3):153. [PMC free article] [PubMed] [Google Scholar]


