Abstract
MicroRNA miR-126 has been shown to be required for proper angiogenesis in several models. However, its expression, regulation and function in the mouse choroid remain unclear. Our previous data has shown that miR-126 expression is enriched in the endothelial cells (ECs) of the mouse choroid. Here we report that a 5.5 kb Egfl7/miR-126 promoter drives the expression of miR-126 in the choroid ECs during choroidal vascular development. The expression of miR-126 in the ECs is regulated by flow stress likely through Krüppel-like transcriptional factors. miR-126−/− mice show mildly delayed choroidal vascular development, but adult knockout mice develop periphery choroidal vascular lesions. This study suggests that miR-126 is largely dispensable for mouse choroidal development but required for maintaining choroidal vasculature integrity.
Keywords: miR-126, Choroid, Vascular development, Endothelial cells
1. Introduction
The choroid is a highly vascularized tissue positioned between the retinal pigment epithelium (RPE)/Bruch's membrane and the sclera in the eye (Lutty et al., 2010). It contains three layers, including the inner capillary layer, Sattler's layer of intermediate arteries in the middle, and the outer Haller's layer with large veins. Choroidal circulation is responsible for the supply and removal of metabolites to and from the outer retina. Not surprisingly, a defective choroidal vasculature is associated with ocular diseases, including age-related macular degeneration (AMD) (Grunwald et al., 1998; McLeod et al., 2009). Choroidal neovascularization (CNV) is the major cause of vision loss in patients with wet AMD. The development of CNV begins with new vessel formation and sprouting from the choroid, through Bruch's membrane and into the RPE and subretinal space. Choroidal capillary degeneration was also observed in both wet AMD and late-stage dry AMD subjects (McLeod et al., 2009). Human choroidal vascular development originates from periocular mesenchyme through haemovasculogenesis (Lutty et al., 2010; Saint-Geniez and D'Amore, 2004). Subsequently, angiogenesis accounts for the new vessel formation from the existing vessels. The mouse eye has been an excellent model system to study vascular development and disease. Vascular endothelial growth factor (VEGF) has been shown expressed in the RPE cells throughout mouse choroidal vascular development and in the adults. VEGF receptor VEGFR2 is expressed in the choroicapillaries underlying the RPE (Saint-Geniez et al., 2006). RPE-derived VEGF is critical for choroidal vascular development and maintenance (Le et al., 2010; Saint-Geniez et al., 2009). Besides, by studying the phenotype of transgenic mice expressing dominant-negative FGF receptor 1 (FGFR-1) in the RPE, RPE-derived FGF signaling is also shown to be important for choroid vascular development (Rousseau et al., 2000). Despite the research progress, the genetic, cellular and molecular mechanisms underlying mouse choroidal vascular development and maintenance remain unclear.
microRNAs (miRNA or miRs) are ~22-nucleotide noncoding RNAs that function by repressing the expression of multiple target genes through inducing mRNA cleavage or repressing protein translation (Bartel, 2004). Recent studies have shown that a list of miRNAs are enriched in the vasculature, and play important regulatory functions in angiogenesis and vascular diseases (Bonauer et al., 2010; Qin and Zhang, 2011; Wang and Olson, 2009). The term “angiomiR” has been used to name miRNAs that regulate angiogenesis either cell autonomously or non-cell autonomously (Wang and Olson, 2009; Wurdinger et al., 2008). miR-126 is one of the best characterized angiomiRs. It has been shown to be required for proper angiogenesis and maintaining vascular integrity (Fish et al., 2008; Kuhnert et al., 2008; van Solingen et al., 2009; Wang et al., 2008; Zhou et al., 2016). miR-126 expression has been shown to be enriched in vascular endothelium in the choroid (Zhou et al., 2016). However, the regulation of miR-126 in vascular EC and its function in choroidal vascular development and maintenance are still unclear. Here we provide evidence that miR-126 expression is regulated by flow in ECs, and miR-126 promoter activity is enriched in the choroidal ECs during choroidal vascular development. miR-126 is largely dispensable for mouse choroidal vascular development but required for the maintenance of choroidal vascular integrity in the adult.
2. Materials and methods
2.1. Animals
Animal studies were conducted in accordance with the ARVO statement for the Use of Animals in Ophthalmic and Vision Research and were approved by the Institutional Animal Care and Use Committees at Tulane University. miR-126−/− mice and their WT littermate controls were generated from miR-126+/− (backcrossed to C57BL6J background for 10 generations) breeding as described (Wang et al., 2008). Egfl7/miR-126 promoter-LacZ transgenic mice were generated as described (Zhou et al., 2016).
2.2. Fluorescein angiography
Fluorescein angiography was performed using a Micron III Fundus Camera connected to the Image system (Phoenix Research Labs, CA) as described (Zhou et al., 2017). 3 months WT and miR-126−/− mice were anesthetized with an intraperitoneal injection of Ketamine (100 mg/kg) plus Xylazine (5 mg/kg). Eyes were dilated with one drop of each of 2.5% Phenylephrine HCL and 1% Cyclopentolate HCL. Topical analgesic was applied (1 drop of proparacaine 0.5%). Fundus pictures were taken before or after fluorescein injection. Fluorescein dye (0.1 mL/kg of 10% fluorescein sodium) was injected intraperitoneally, and multiple pictures were taken at 30–300 s after injection of the dye. The same mice were reexamined by fluorescent angiography at 6, 12 and 18 months old.
2.3. Hematoxylin & Eosin (H&E), LacZ antibody staining, and immunostaining
Hematoxylin & Eosin staining was performed as described (Wang et al., 2008). For flat mount staining of the choroidal vasculature, pigment bleaching in RPE cells and choroidal melanocytes was performed as described (Kim and Assawachananont, 2016). Briefly, pigmented eyes were fixed in 4% paraformaldehyde PBS for 30 min to 2 h. After washing with PBS three times, the cornea and lens were removed before 3% hydrogen peroxide bleaching at 55 °C for 30 min to 2 h depending on the stage, or at 4 °C for several days. Immunofluorescence staining was performed using standard procedures. Primary antibodies used include: Beta-galactosidase (LacZ) from chicken (1:300, Abcam), Endomucin from rat (1:200, Santa Cruz Biotechnology), PECAM-1 from rat (1:50, BD Pharmingen), ICAM-2 from rat (1: 200, BD Pharmingen). These three EC markers, Endomucin, PECAM-1 and ICAM-2, were used interchangeably, but some antibodies worked better than others due to different sample processing. Secondary antibodies include: goat antichicken (or rat) AlexaFluor 488 (1:500, Invitrogen) and goat anti-rat AlexaFluor 594 (1:500, Invitrogen).
2.4. Cell culture, flow treatment and RT-PCR
HUVEC (ATCC) cells were grown in EC growth medium EGM-2 (Lonza). Steady laminar flow experiments was performed as described (Kwon et al., 2014). Briefly, confluent HUVECs were cultured in the absence of serum for 24 h and exposed to fluid shear stress (12 dynes/cm2) for 4 and 24 h before processing for miRNA gene expression analysis. Total RNA was isolated using TRIzol reagent (Invitrogen). miRNA real-time RT-PCR was performed using miRCURY LNA Universal microRNA RT-PCR system (Exiqon). miR-126-3p, miR-92 primers from Exiqon, with U6 as control, were used as primers for PCR.
2.5. Plasmid construction and reporter assay
The 0.9 kb region enhancer of mouse Egfl7/miR-126 was amplified by PCR, and cloned into pGL3 vector upstream of an engineered ANF basal promoter. Of note, the 0.9kb region is also the region-2 shown in our previous paper (Wang et al., 2008). Primer sequences include: 5′-ACAGAGGTCTGGGCATGTTC-3′ and 5′- TGCACAGAGGCTCCTCCCG GGT-3’. COS-7 cells in 24-well plates were transfected with 50 ng of reporter plasmid in the presence or absence of ETS1 and/or KLF2 expression plasmids (Meadows et al., 2009; Wang et al., 2008). Reporter assays were performed as described (Zhou et al., 2016).
2.6. Statistics
Number of animals was indicated in the text when necessary. Student's t-Tests were used to determine statistical significance between groups. P-values of less than 0.05 were considered to be statistically significant.
3. Results
3.1. Egfl7/miR-126 promoter activity during mouse choroidal vascular development
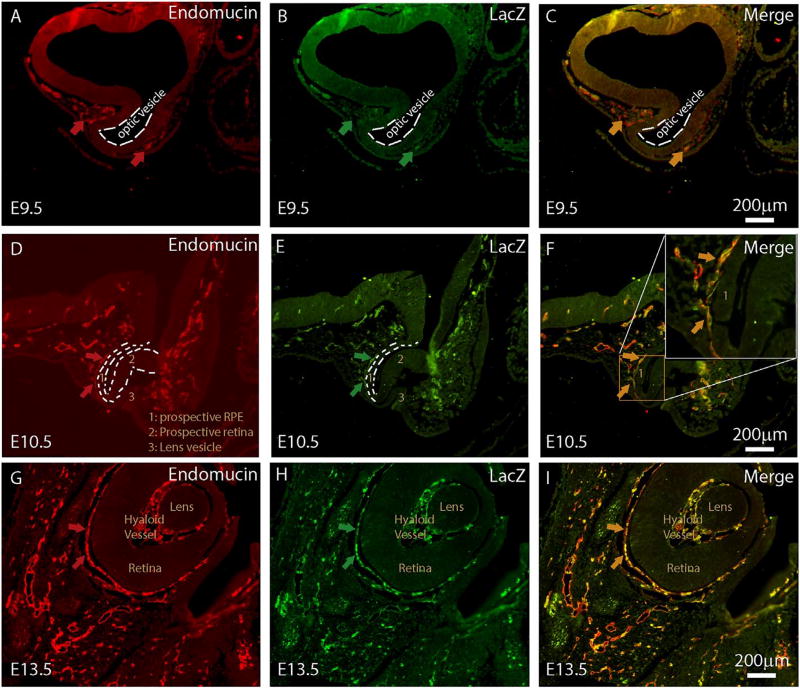
miR-126 is an EC-enriched miRNA located in the intron of Egfl7 gene. A 5.5kb promoter has been shown to drive EC-enriched Egfl7/miR-126 expression (Wang et al., 2008). Our published data has shown that miR-126 is expressed in the adult mouse choroidal vasculature (Zhou et al., 2016). To define a role for miR-126 in choroidal vascular development, Egfl7/miR-126 promoter activity was visualized by LacZ antibody staining and EC marker co-staining in a stable transgenic mouse line that expresses LacZ reporter under the control of the 5.4 Egfl7/miR-126 promoter. The development of the mouse choroidal vascular system has not been systematically defined in literature. In humans, the ocular vascular system emerges from the mesoderm surrounding the newly formed optic cup (Saint-Geniez and D'Amore, 2004). In the E9.5 mouse embryo, isolated Endomucin-positive EC cells were observed at the presumptive choroidal region that surrounds the optic vesicle laterally (arrows in Fig. 1A). A few of the Endomucin-positive EC cells also showed Egfl7/miR-126 promoter activity as shown by LacZ co-staining (Fig. 1B–C). At E10.5, when the prospective retina and retinal pigment epithelium (RPE) start to form, co-localization of Endomucin and LacZ staining was observed in the cells behind the RPE that surround the optic cup. At E13.5, Endomucin and LacZ co-staining persists in the choroid plexus behind the retina/RPE layer. Of note, double-positive staining was also seen in the hyaloid vasculature at this stage. Based on these, we conclude that Egfl7/miR-126 promoter activity is enriched in the choroidal ECs during early choroidal vascular development.
Fig. 1. Egfl7/miR-126 promoter activity during choroidal vascular development.
(A) Cross section of 5.5 kb pEgfl7/miR-126-LacZ transgenic E9.5 embryo showing Endomucin staining surrounding optical vesicle; (B) Sample in (A) co-stained with LacZ antibody; (C) Merge of (A) and (B); (D) Cross section of 5.5 kb pEgfl7/miR-126-LacZ transgenic E10.5 embryo showing Endomucin staining behind the prospective RPE. Several labeled regions are: 1: prospective RPE, 2: prospective retina, 3: lens vesicle; (E) Sample in (D) co-stained with LacZ antibody; (F) Merge of (D) and (E) with boxed region highlighted in the insert in the right upper corner; (G) Cross section of 5.5 kb pEgfl7/miR-126-LacZ transgenic E13.5 embryo showing Endomucin staining behind the RPE. Lens, hyaloid vessel and retina structures are labeled; (H) Sample in (G) co-stained with LacZ antibody; (I) Merge of (G) and (H). scale bar equals 200µm.
3.2. Regulation of miR-126 by flow in ECs
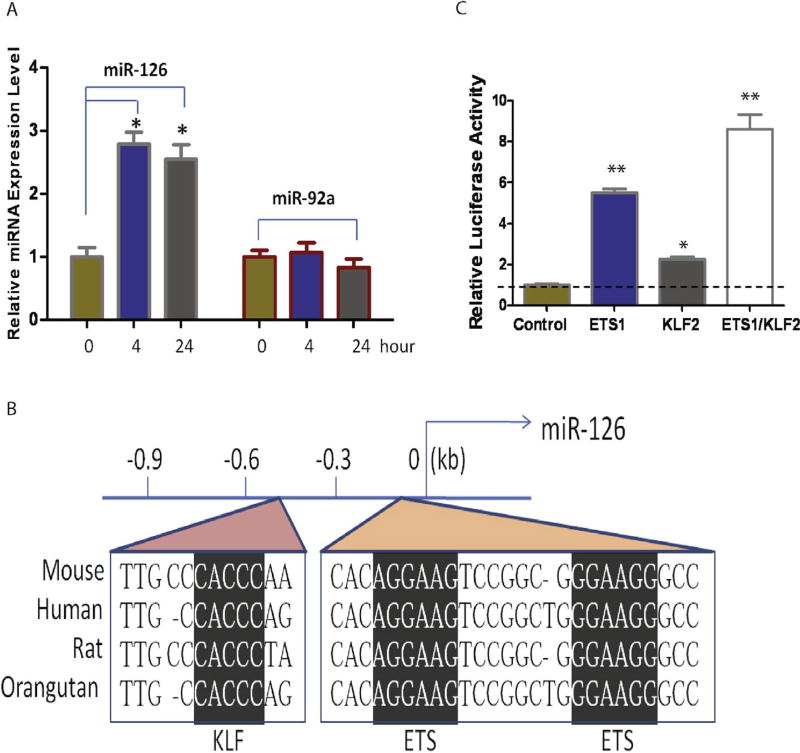
The choroid blood flow has multiple functions, including vascular remodeling and supplying nutrients and oxygen for the outer retina (Nickla and Wallman, 2010). Impairment of the choroid blood flow may cause Age-related Macular Degeneration (AMD) (Grunwald et al., 1998). miR-126 has been shown to integrate mechanosensory stimulus and growth factor signaling to guide angiogenesis in zebrafish (Nicoli et al., 2010). To study the regulation of miR-126, we hypothesized that miR-126 is regulated by flow stress in ECs. To test it, miR-126 expression was examined at 4–24 h of laminar shear stress at 12 dyn/cm2 in human umbilical vein endothelial cells (HUVECs). miR-126 expression was upregulated at 4 (~2.8 fold) and 24 h (~2.6 fold) after flow stress by qRT-PCR using LNA modified sybgreen microRNA probe (Fig. 2A). As a control, the expression of miR-92a, a microRNA shown to suppress angiogenesis (Bonauer et al., 2009), was not affected by flow stress. Krüppel-like factor-2 (KLF2) is an essential regulator of vascular hemodynamic forces and vessel formation (Dekker et al., 2002; Kuo et al., 1997; Lee et al., 2006; Parmar et al., 2006). ETS transcription factors are the major regulators of endothelial transcription program (De Val and Black, 2009). KLFs bind a consensus recognition sequence of CACCC (Bieker, 2001; Dang et al., 2000). A conserved KLF binding site was found in a 0.9 kb promoter region of EGFL7/miR-126, which is located in the proximal region of the 5.5kb promoter and contains two conserved ETS sites (Fig. 2B). To test whether KLF2 is sufficient to regulate the 0.9 kb Egfl7/miR-126 promoter, the 0.9 kb promoter was cloned in front of a promoter-less luciferase vector, and co-transfected with ETS1 and/or KLF2 expression plasmid in COS-7 cells. ETS1 co-transfection led to ~5.5-fold increase of the luciferase activity, while KLF2 co-transfection led to ~2-fold increase of the reporter activity. ETS1 and KLF2 co-transfection led to ~8.5-fold increase in the reporter activity (Fig. 2C), suggesting both ETS and KLF transcriptional factors are regulators of miR-126 expression induced by flow in ECs.
Fig. 2. Regulation of miR-126 by flow stress.
(A). miR-126 and miR-92a expression detected in HUVEC cells cultured under flow by qRT-PCR using LNA modified sybgreen microRNA probe, U6 was used as a control for normalization. (B). Conserved KLF and ETS binding sites in the promoter of pEgfl7/miR-126; (C). Synergistic activation of miR-126 promoter by ETS1 and KLF2 as revealed by luciferase assays. *, p < 0.05; **, p < 0.01.
3.3. Choroid vascular development is mildly delayed in miR-126−/− mice
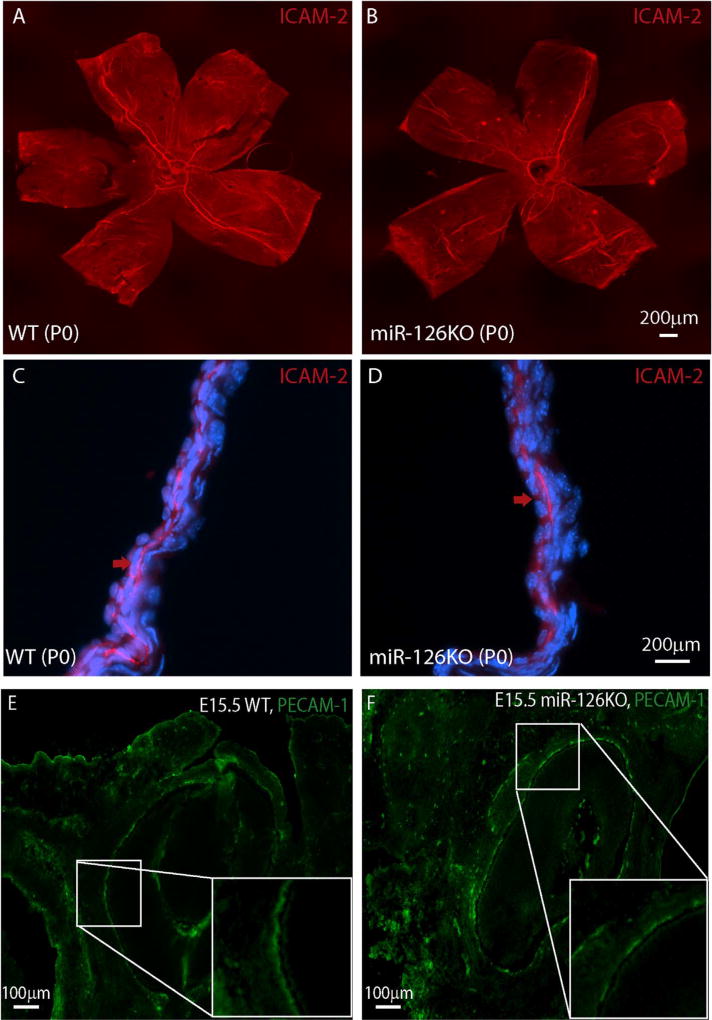
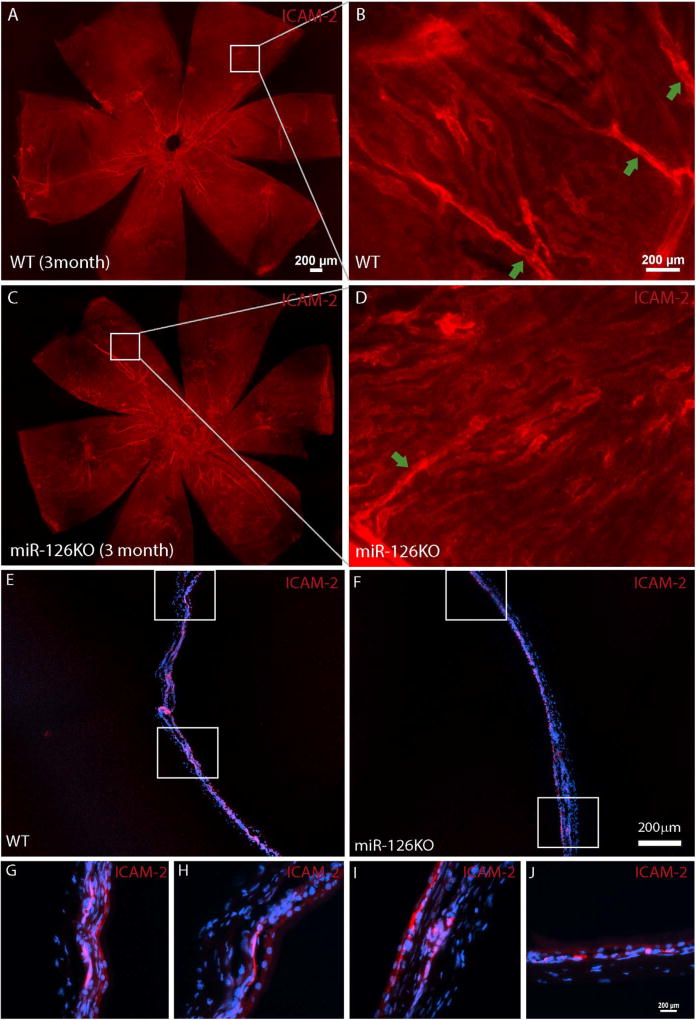
To determine whether miR-126 is required for choroid vascular development, the choroid vasculature was examined by ICAM-2 staining in newborn miR-126−/− mice. By ICAM-2 flatmount staining, the choroid vasculature coverage appeared to be normal in the miR-126−/− mice (Fig. 3A–B). By section, the choroidal vasculature in the miR-126−/− mice appeared less continuous compared to that in the wild-type (WT) control mice (Fig. 3C–D). A similar phenomenon was observed at E15.5 (Fig. 3E–F). PECAM-1 staining in E15.5 miR-126−/− embryos appeared to be patchy, suggesting a delayed choroidal vascular development in miR-126−/− mice. In the adult mice, the choroidal vascular bed was populated with choroid capillaries as stained by ICAM-2 in both WT and miR-126−/− mice. However, there was an insignificantly less major vascular branches in the KO mice compared to the WT controls (vessel area: 2.8 ± 0.2% in WT, 1.9 ± 0.3% in KO, p=0.11) (Fig. 4A–D). By section, the connections of choroidal vessels appeared to be normal in the miR-126−/− mice (Fig. 4E–J). Based on the mild choroidal vascular phenotype in miR-126−/− mice, miR-126 is largely dispensable for mouse choroid vascular development.
Fig. 3. Mildly delayed choroidal vascular development in miR-126−/− mice.
(A) Flatmount and ICAM-2 staining of a P0 WT choroid; (B) Flatmount ICAM-2 staining of a P0 miR-126−/− choroid; (C) Frozen section of the sample in (A). Arrow indicates the continuous ICAM-2 staining; (D) Frozen section of the sample in (B). Arrow indicates the patchy ICAM-2 staining; (E) PECAM-1 staining of E15.5 WT cross section sample showing choroidal vessels behind the RPE/retina. Some regions of the choroidal vessels show continuous PECAM-1 staining at this stage, as shown by the magnified picture of the boxed region; (F) PECAM-1 staining of E15.5 miR-126−/− cross section sample showing choroidal vessels. Most choroidal vessels show discontinuous PECAM-1 staining at this stage, as shown by the magnified picture of the boxed region.
Fig. 4. Less choroid branches but largely normal choroidal vasculature in adult miR-126−/− mice.
(A) Flatmount ICAM-2 staining of a 3 month WT choroid; (B) Magnified picture of the boxed region in (A) showing several vascular branches connecting to the choroidal vascular bed; (C) Flatmount ICAM-2 staining of a 3 month miR-126−/− choroid; (D) Magnified picture of the boxed region in (C) showing one vascular branch connecting to the choroidal vascular bed; (E) Frozen section of the sample in (A); (F) Frozen section of the sample in (B); (G–H) Boxed regions in (A); (I–J) Boxed regions in (B). Scale bar equals 200µm.
3.4. miR-126 is required for maintaining choroidal vascular integrity
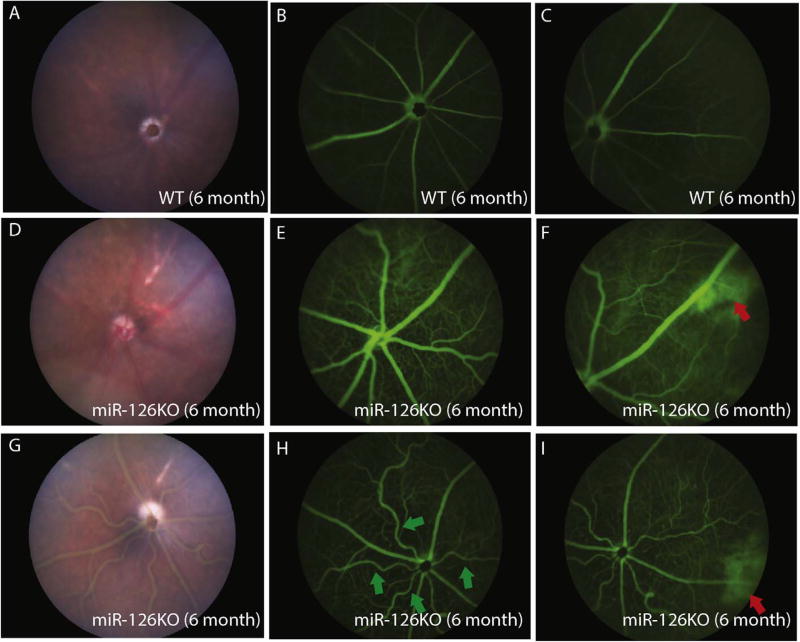
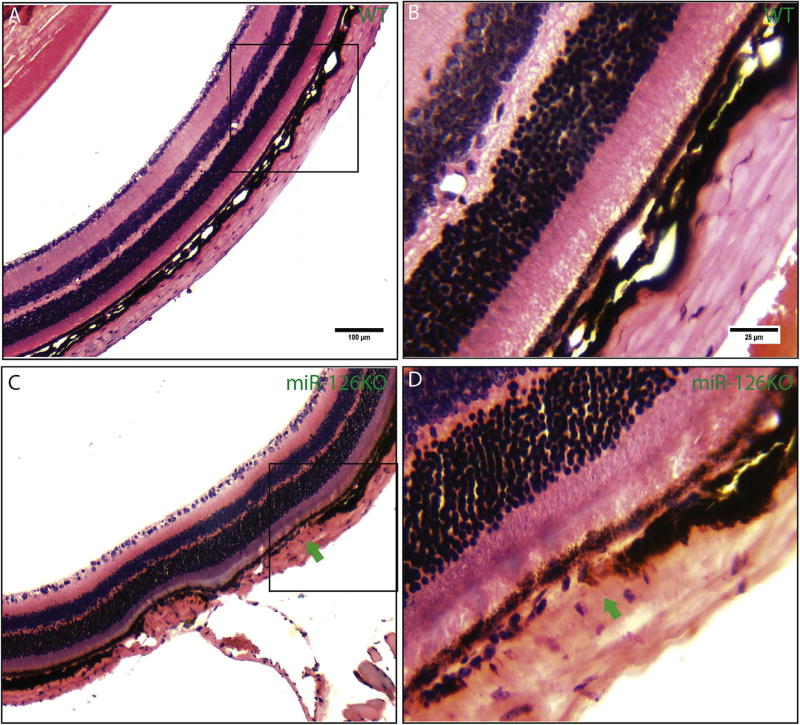
Fundus scope imaging did not reveal obvious defects in the retinal structure in 6 months-old miR-126−/− mice (Fig. 5A, D and F). Fluorescein angiography was used to monitor the ocular vascular phenotype in adult miR-126−/− mice. Compared to the normal retinal/choroid vessel structure, areas of hyper-fluorescence (red arrows in Fig. 4F and I) were observed in the periphery region only at late phase (after 5 min) of angiogram in miR-126−/− mice, suggesting choroid/RPE abnormality in the knockout mice (n=10 in WT with no lesion, 10 of 11 of the KO mice have lesions, Fig. 5B–C, E–F and H–I). Retinal tortuosity was observed in one of the eleven 6-month miR-126−/− mice examined (green arrows in Fig. 5H). These phenotypes did not progress significantly with age when the same mice were re-examined at 12 and 18 months old. Histological section and H&E staining of the hyperfluorescent region revealed that focal choroidal vascular atrophy was present in the miR-126−/− mice, whereas the RPE layer appeared to be pigmented and morphologically normal (Fig. 6A–D, arrows in Fig. 6C–D shows choroid lesion). These suggest that miR-126 is required to maintain choroidal vascular integrity in mice.
Fig. 5. Peripheral choroidal vascular lesion in miR-126−/− mice as shown by fluorescein angiography.
(A) Fundus picture of a 6 months-old WT mouse; (B) Central view of fluorescein angiogram in the mouse in (A) at 10 min after fluorescein injection; (C) Peripheral view of fluorescein angiogram in the mouse in (A) at 10 min after fluorescein injection; (D) Fundus picture of a 6 months-old miR-126−/− mouse; (E) Central view of fluorescein angiogram in the mouse in (D) at 10 min after fluorescein injection; (F) Peripheral view of fluorescein angiogram in the mouse in (D) at 10 min after fluorescein injection showing focal peripheral hyperfluorescence (red arrow); (G) Fundus picture of a 6 months-old miR-126−/− mouse; (H) Central view of fluorescein angiogram in the mouse in (G) at 10 min after fluorescein injection showing tortuous retinal vessels (green arrow); (I) Peripheral view of fluorescein angiogram in the mouse in (G) at 10 min after fluorescein injection showing focal peripheral hyperfluorescence (red arrow). (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Fig. 6. Focal choroidal vascular atrophy in adult miR-126−/− mice.
(A) Representative H&E staining in an 18-months-old WT mice showing the retina/choroid region; (B) Magnified picture of the boxed region in (A); (C) H&E staining in an 18-months-old miR-126−/− mice showing focal choroidal vascular atrophy (green arrow). (D) Magnified picture of the boxed region in (C). (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
4. Discussion
The role of miRNAs in choroidal vascular development and maintenance is currently unknown. Using in vitro models, as well as the miR-126 promoter reporter mice and miR-126−/− mice established in the lab, the following findings were observed in this study: (1) miR-126 promoter activity is enriched in the choroid ECs as early as stage E9.5 during mouse choroid development; (2) miR-126 expression in ECs is regulated by flow stress; (3) miR-126 is largely dispensable for choroidal vascular development; (4) miR-126−/− mice develop focal choroidal vascular atrophy. Our study revealed an important regulatory mechanism of miR-126 in ECs and a critical function for miR-126 in maintaining choroidal vascular integrity.
4.1. Expression and regulation of miR-126 during choroidal vascular development
Our previous study has established the expression of miR-126 in mouse adult choroidal vasculature by miRNA in situ hybridization (Zhou et al., 2016). To define a role of miR-126 during choroidal vascular development, we utilized a 5.5-kb Egfl7/miR-126 promoter reporter mouse line, which has been shown to visualize miR-126 expression in other vascular systems (Wang et al., 2008). miR-126 promoter activity, as shown by LacZ antibody staining, was detected as early as E9.5 in a subset of ECs at the presumptive choroid region (Fig. 1). At later stages, the promoter activity co-localizes with EC marker Endomucin in the choroid. These suggest that miR-126 can label at least a subset of choroidal ECs during choroid development before the onset of choroidal blood flow. Of note, miR-126 is also expressed in the hyaloid vessels and retinal vessels in the eye (Fig. 1, and Zhou et al., 2016). Although the initial human choroidal vascular development is believed to occur via haemovasculogenesis, whether mouse choroidal vasculature is initialized through similar mechanisms, or whether miR-126 is expressed in haemangioblast cells in the presumptive choroid region, still awaits future studies (Lutty et al., 2010).
Given the importance of choroid blood flow in maintaining retinal homeostasis, as well as the important role of miR-126 in flow-regulated angiogenesis, we asked whether miR-126 expression is regulated by flow (Grunwald et al., 1998; Nicoli et al., 2010). We found that miR-126 is upregulated by laminal shear stress in ECs, which is consistent with a recent report (Mondadori dos Santos et al., 2015). Conserved binding sites for KLF2, a master regulator of flow-induced angiogenesis, was further found in the Egfl7/miR-126 promoter. KLF2, together with key EC gene expression regulator ETS1, regulates Egfl7/miR-126 promoter activity. These suggest flow stress regulates miR-126 expression likely through KLF transcription factors. Further study is needed to examine whether miR-126 is regulated by flow in vivo, and whether miR-126 expression is downregulated in AMD subjects who show reduced choroidal blood flow (Grunwald et al., 1998).
4.2. Role of miR-126 in choroidal vascular development and maintenance
We found that, while miR-126 is largely dispensable for mouse choroidal vascular development, there is some mild delay in choroidal vascular development in miR-126−/− mice. At E15.5 and P0, the ECs appeared not well-connected in miR-126−/− mice compared to the WT control mice. In adult miR-126−/− mice, the choroidcapillaris in the vascular bed is well populated with choroid vessels. There appeared to be insignificantly less vascular branches connecting the choroid vascular bed. This is in line with the decreased angiogenesis observed in other organs of miR-126−/− mice. In our previous report, the retinal vascular development phenotype in miR-126−/− mice appears to be more severe than the choroidal vascular phenotype (Zhou et al., 2016). That could be attributed to specific features of choroidal EC cells, which are fenestrated, or the specific expression pattern of miR-126 target genes in choroidal ECs. EC heterogeneity has been well-recognized in the vascular field (Ribatti et al., 2002).
Despite normal choroidal vascularity in miR-126−/− mice, fluorescent angiography revealed peripheral hyperfluorescent lesions in majority of the miR-126−/− mice. These lesions were confirmed as regions of choroidal vascular atrophy. Interestingly, this phenotype did not progress significantly with age. We speculate that the lesion may result from the mild choroidal vascular developmental phenotype. Future work is needed to further characterize the phenotype and identify the underlying mechanisms. Nevertheless, these results suggest that miR-126 is required for maintaining choroidal vascular integrity. Given the findings that choroidal circulation is reduced and choroidal capillary degeneration occurs in AMD subjects, further work is need to define whether miR-126 has a role in AMD (Grunwald et al., 1998; McLeod et al., 2009).
Acknowledgments
We thank Dr. Pual Krieg from the University of Arizona for providing the KLF2 expression plasmid. S.W. was supported by a startup fund from Tulane University, President's Research Council New Investigator Award from UT Southwestern Medical Center, NIH Grants EY021862 and EY026069, a career development award from the Research to Prevent Blindness foundation, and a Bright Focus Foundation Award in Age-related Macular Degeneration.
References
- Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- Bieker JJ. Kruppel-like factors: three fingers in many pies. J. Biol. Chem. 2001;276:34355–34358. doi: 10.1074/jbc.R100043200. [DOI] [PubMed] [Google Scholar]
- Bonauer A, Boon RA, Dimmeler S. Vascular microRNAs. Curr. Drug Targets. 2010;11:943–949. doi: 10.2174/138945010791591313. [DOI] [PubMed] [Google Scholar]
- Bonauer A, Carmona G, Iwasaki M, Mione M, Koyanagi M, Fischer A, Burchfield J, Fox H, Doebele C, Ohtani K, et al. MicroRNA-92a controls angiogenesis and functional recovery of ischemic tissues in mice. Science. 2009;324:1710–1713. doi: 10.1126/science.1174381. [DOI] [PubMed] [Google Scholar]
- Dang DT, Pevsner J, Yang VW. The biology of the mammalian Kruppel-like family of transcription factors. Int. J. Biochem. Cell Biol. 2000;32:1103–1121. doi: 10.1016/s1357-2725(00)00059-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Val S, Black BL. Transcriptional control of endothelial cell development. Dev. Cell. 2009;16:180–195. doi: 10.1016/j.devcel.2009.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekker RJ, van Soest S, Fontijn RD, Salamanca S, de Groot PG, VanBavel E, Pannekoek H, Horrevoets AJ. Prolonged fluid shear stress induces a distinct set of endothelial cell genes, most specifically lung Kruppel-like factor (KLF2) Blood. 2002;100:1689–1698. doi: 10.1182/blood-2002-01-0046. [DOI] [PubMed] [Google Scholar]
- Fish JE, Santoro MM, Morton SU, Yu S, Yeh RF, Wythe JD, Ivey KN, Bruneau BG, Stainier DY, Srivastava D. miR-126 regulates angiogenic signaling and vascular integrity. Dev. Cell. 2008;15:272–284. doi: 10.1016/j.devcel.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunwald JE, Hariprasad SM, DuPont J, Maguire MG, Fine SL, Brucker AJ, Maguire AM, Ho AC. Foveolar choroidal blood flow in age-related macular degeneration. Invest. Ophthalmol. Vis. Sci. 1998;39:385–390. [PubMed] [Google Scholar]
- Kim SY, Assawachananont J. A new method to visualize the intact subretina from retinal pigment epithelium to retinal tissue in whole mount of pigmented mouse eyes. Transl Vis Sci Technol. 2016;5:6. doi: 10.1167/tvst.5.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhnert F, Mancuso MR, Hampton J, Stankunas K, Asano T, Chen CZ, Kuo CJ. Attribution of vascular phenotypes of the murine Egfl7 locus to the microRNA miR-126. Development. 2008;135:3989–3993. doi: 10.1242/dev.029736. [DOI] [PubMed] [Google Scholar]
- Kuo CT, Veselits ML, Barton KP, Lu MM, Clendenin C, Leiden JM. The LKLF transcription factor is required for normal tunica media formation and blood vessel stabilization during murine embryogenesis. Genes Dev. 1997;11:2996–3006. doi: 10.1101/gad.11.22.2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon IS, Wang W, Xu S, Jin ZG. Histone deacetylase 5 interacts with Kruppel-like factor 2 and inhibits its transcriptional activity in endothelium. Cardiovasc. Res. 2014;104:127–137. doi: 10.1093/cvr/cvu183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le YZ, Bai Y, Zhu M, Zheng L. Temporal requirement of RPE-derived VEGF in the development of choroidal vasculature. J. Neurochem. 2010;112:1584–1592. doi: 10.1111/j.1471-4159.2010.06573.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JS, Yu Q, Shin JT, Sebzda E, Bertozzi C, Chen M, Mericko P, Stadtfeld M, Zhou D, Cheng L, et al. Klf2 is an essential regulator of vascular hemodynamic forces in vivo. Dev. Cell. 2006;11:845–857. doi: 10.1016/j.devcel.2006.09.006. [DOI] [PubMed] [Google Scholar]
- Lutty GA, Hasegawa T, Baba T, Grebe R, Bhutto I, McLeod DS. Development of the human choriocapillaris. Eye. 2010;24:408–415. doi: 10.1038/eye.2009.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLeod DS, Grebe R, Bhutto I, Merges C, Baba T, Lutty GA. Relationship between RPE and choriocapillaris in age-related macular degeneration. Invest Ophth Vis Sci. 2009;50:4982–4991. doi: 10.1167/iovs.09-3639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meadows SM, Salanga MC, Krieg PA. Kruppel-like factor 2 cooperates with the ETS family protein ERG to activate Flk1 expression during vascular development. Development. 2009;136:1115–1125. doi: 10.1242/dev.029538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondadori dos Santos A, Metzinger L, Haddad O, M'Baya-Moutoula E, Taibi F, Charnaux N, Massy ZA, Hlawaty H, Metzinger-Le Meuth V. miR-126 is involved in vascular remodeling under laminar shear stress. BioMed Res. Int. 2015;2015:497280. doi: 10.1155/2015/497280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickla DL, Wallman J. The multifunctional choroid. Prog. Retin. Eye Res. 2010;29:144–168. doi: 10.1016/j.preteyeres.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoli S, Standley C, Walker P, Hurlstone A, Fogarty KE, Lawson ND. MicroRNA-mediated integration of haemodynamics and Vegf signalling during angiogenesis. Nature. 2010;464:1196–1200. doi: 10.1038/nature08889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parmar KM, Larman HB, Dai G, Zhang Y, Wang ET, Moorthy SN, Kratz JR, Lin Z, Jain MK, Gimbrone MA., Jr Integration of flow-dependent endothelial phenotypes by Kruppel-like factor 2. J. Clin. Invest. 2006;116:49–58. doi: 10.1172/JCI24787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin S, Zhang C. MicroRNAs in vascular disease. J. Cardiovasc. Pharmacol. 2011;57:8–12. doi: 10.1097/FJC.0b013e318203759b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribatti D, Nico B, Vacca A, Roncali L, Dammacco F. Endothelial cell heterogeneity and organ specificity. J. Hematother. Stem Cell Res. 2002;11:81–90. doi: 10.1089/152581602753448559. [DOI] [PubMed] [Google Scholar]
- Rousseau B, Dubayle D, Sennlaub F, Jeanny JC, Costet P, Bikfalvi A, Javerzat S. Neural and angiogenic defects in eyes of transgenic mice expressing a dominant-negative FGF receptor in the pigmented cells. Exp. Eye Res. 2000;71:395–404. doi: 10.1006/exer.2000.0892. [DOI] [PubMed] [Google Scholar]
- Saint-Geniez M, D'Amore PA. Development and pathology of the hyaloid, choroidal and retinal vasculature. Int. J. Dev. Biol. 2004;48:1045–1058. doi: 10.1387/ijdb.041895ms. [DOI] [PubMed] [Google Scholar]
- Saint-Geniez M, Kurihara T, Sekiyama E, Maldonado AE, D'Amore PA. An essential role for RPE-derived soluble VEGF in the maintenance of the choriocapillaris. Proc. Natl. Acad. Sci. U. S. A. 2009;106:18751–18756. doi: 10.1073/pnas.0905010106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saint-Geniez M, Maldonado AE, D'Amore PA. VEGF expression and receptor activation in the choroid during development and in the adult. Invest. Ophthalmol. Vis. Sci. 2006;47:3135–3142. doi: 10.1167/iovs.05-1229. [DOI] [PubMed] [Google Scholar]
- van Solingen C, Seghers L, Bijkerk R, Duijs JM, Roeten MK, van Oeveren-Rietdijk AM, Baelde HJ, Monge M, Vos JB, de Boer HC, et al. Antagomir-mediated silencing of endothelial cell specific microRNA-126 impairs ischemia-induced angiogenesis. J. Cell Mol. Med. 2009;13:1577–1585. doi: 10.1111/j.1582-4934.2008.00613.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Aurora AB, Johnson BA, Qi X, McAnally J, Hill JA, Richardson JA, Bassel-Duby R, Olson EN. The endothelial-specific microRNA miR-126 governs vascular integrity and angiogenesis. Dev. Cell. 2008;15:261–271. doi: 10.1016/j.devcel.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Olson EN. AngiomiRs–key regulators of angiogenesis. Curr. Opin. Genet. Dev. 2009;19:205–211. doi: 10.1016/j.gde.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurdinger T, Tannous BA, Saydam O, Skog J, Grau S, Soutschek J, Weissleder R, Breakefield XO, Krichevsky AM. miR-296 regulates growth factor receptor overexpression in angiogenic endothelial cells. Canc. Cell. 2008;14:382–393. doi: 10.1016/j.ccr.2008.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q, Anderson C, Hanus J, Zhao F, Ma J, Yoshimura A, Wang S. Strand and cell type-specific function of microRNA-126 in angiogenesis. Mol. Ther. 2016;24:1823–1835. doi: 10.1038/mt.2016.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q, Frost RJA, Anderson C, Zhao F, Ma J, Yu B, Wang S. Let-7 contributes to diabetic retinopathy but represses pathological ocular angiogenesis. Mol. Cell Biol. 2017;37(16):1–17. doi: 10.1128/MCB.00001-17. [DOI] [PMC free article] [PubMed] [Google Scholar]