Abstract
Background
Chemoradiotherapy (CRT) has improved organ preservation or overall survival (OS) of locoregionally advanced head and neck squamous cell cancer (LAHNSCC), but in clinical trials of conventional CRT, increasing CRT intensity has not been shown to improve OS. In the Adjuvant ChemoTherapy with S-1 after curative treatment in patients with Head and Neck Cancer (ACTS-HNC) phase III study, OS of curative locoregional treatments improved more with adjuvant chemotherapy with S-1 (tegafur gimeracil oteracil potassium) than with tegafur/uracil (UFT). ACTS HNC study showed the significant efficacy of S-1 after curative radiotherapy in sub-analysis. We explored the efficacy of S-1 after curative CRT in a subset of patients from the ACTS-HNC study.
Methods
Patients with stage III, IVA, or IVB LAHNSCC were enrolled in this study to evaluate the efficacy of S-1 compared with UFT as adjuvant chemotherapy after curative CRT in the ACTS-HNC study. Patients received S-1 at 80–120 mg/day in two divided doses for 2 weeks, followed by a 1-week rest, or UFT 300 or 400 mg/day in two or three divided doses daily, for 1 year. The endpoints were OS, disease-free survival, locoregional relapse-free survival, distant metastasis-free survival (DMFS), and post-locoregional relapse survival.
Results
One hundred eighty patients (S-1, n = 87; UFT, n = 93) were included in this study. Clinical characteristics of the S-1 and UFT arms were similar. S-1 after CRT significantly improved OS (hazard ratio [HR], 0.46; 95% confidence interval [CI], 0.22–0.93) and DMFS (HR, 0.50; 95% CI, 0.26–0.97) compared with UFT.
Conclusion
As adjuvant chemotherapy, S-1 demonstrated better efficacy for OS and DMFS than UFT in patients with LAHNSCC after curative CRT and may be considered a treatment option following curative CRT. For this study was not preplanned in the ACTS-HNC study, the results is hypothesis generating but not definitive.
Introduction
Chemoradiotherapy (CRT) administered as curative or postoperative treatment for locoregionally advanced head and neck squamous-cell carcinoma (LAHNSCC) has been demonstrated to improve locoregional control, overall survival (OS), and organ preservation [1–10]. However, recent phase III studies that explored whether the combination of induction chemotherapy or molecularly targeted drug therapy and concurrent CRT enhances the treatment effectiveness of concurrent CRT failed to demonstrate improvement in OS [11–14]. Concurrent CRT with nonstandard fractionation schedules also failed to prolong OS [15, 16]. New therapeutic strategies to prolong the survival time of CRT have been investigated.
Although adjuvant chemotherapy after curative treatment for HNSCC has not been shown to improve OS [17–21], it has been reported to reduce the incidence of distant metastasis [17, 19, 21]. A previous report showed that tegafur/uracil (UFT) as adjuvant chemotherapy was preferable, in terms of rate of distant metastasis, to non-treatment after curative surgery in cases of HNSCC, but that there was no difference in disease-free survival (DFS) [21].
Like UFT, S-1 is an oral fluoropyrimidine preparation, but because it contains gimeracil, a dihydropyrimidine dehydrogenase inhibitor, S-1 has greater antitumor activity than UFT [22]. Indeed, S-1 as adjuvant chemotherapy after curative surgery of rectal cancers significantly increased the proportion of patients achieving relapse-free survival compared with UFT [23]. In the Adjuvant Chemotherapy with S-1 after definitive Treatment in Patients with Head and Neck Cancer (ACTS-HNC) study, OS was significantly longer with S-1 than with UFT [24]. If adjuvant therapy with S-1 after CRT can reduce the rate of distant metastasis and prolong OS in patients with LAHNSCC, S-1 may be of benefit.
Therefore, we examined the efficacy of S-1 as adjuvant chemotherapy after curative CRT. The present analysis compared OS, DFS, distant metastasis-free survival (DMFS), locoregional relapse-free survival (LRRFS), and post-LRR survival (post-LRRS) between S-1 and UFT among ACTS-HNC study (ClinicalTrial. gov: NCT00336947) patients who had undergone curative CRT.
Methods
Compliance with ethical standards
Because the current study was an explanatory analysis using the dataset of a previously reported clinical trial (ACTS-HNC study), no ethical approval was required.
The ACTS-HNC study
Patients, study design, and methods of the ACTS-HNC study have been reported [24]. Briefly, patients with stage III, IVA, or IVB squamous-cell carcinoma of the maxillary sinus, oral cavity, oropharynx, hypopharynx, or larynx that disappeared, as confirmed by diagnostic imaging or biopsy, after a combination of curative treatments (radiotherapy or surgery) with or without chemotherapy were eligible for this trial. If residual tumor was suspected after curative therapies, additional treatment was followed. Additional treatment defined as either the addition of surgery to the curative radiotherapy ± chemotherapy, or the addition of radiotherapy ± chemotherapy to the curative surgery before random allocation. After completion of definitive treatment confirmed no residual tumor within 2 months, patients were randomly assigned (1:1) to receive either UFT or S-1. In the UFT group, patients received 300 mg/day [body surface area(BSA)<1.5 m2] or 400 mg/day (BSA≥1.5 m2) of UFT in two or three divided doses daily. In the S-1 group, patients received 80 mg/day (BSA<1.25 m2), 100 mg/day (BSA≥1.25 and <1.5 m2), or 120 mg/day (BSA≥1.5 m2) of S-1 in two divided doses for 2 weeks, followed by one week off treatment. Treatment duration for both arms was 1 year. Stratification factors for dynamic allocation included subset, stage (III, IVA, or IVB), type of curative therapy (surgery, radiotherapy, or both), and institution. Criteria for dose reduction were developed based on adverse events.
Patient groups for analysis
Based on the type of curative treatments (surgery, radiotherapy), patients were divided into either the CRT group (concurrent or consecutive administration of chemotherapy with radiotherapy) or the Other Therapy group (surgery alone, surgery with chemotherapy, or radiotherapy alone) (Fig 1).
Fig 1. Subsets by curative treatment before random allocation.
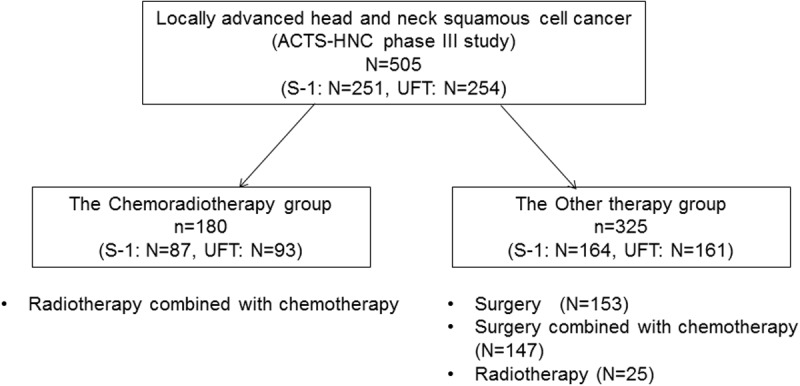
Abbreviations: ACTS-HNC, Adjuvant chemotherapy with S-1 after curative treatment in patients with Head and Neck Cancer. CRT, chemoradiotherapy.
Outcomes evaluated
OS, DFS, LRRFS, DMFS, and post-LRRS were evaluated in both groups. Of these, OS, DFS, post-LRRS were assessed in the phase III trial [24]. For the present study, additional outcomes were defined as follows: LRRFS was the time from the date of randomization to the first date of local recurrence, cervical lymph node recurrence, or disease-specific death. DMFS was the time to the first date of distant recurrence or distant metastasis in areas other than local or cervical lymph nodes, or death from any cause. The censoring time for DMFS was the date of the last observation that showed no recurrence. Post-LRRS was the time from the date of randomization to the date of disease-specific death after confirmed locoregional relapse (LRR). This censoring time was the date of the last observation indicating survival.
Statistical analyses
This reanalysis was performed by using analysis dataset of ACTS-HNC study [24]. Clinical information was collected before randomization in the phase III trial. Clinical characteristics were compared using Fisher’s exact test for categorical variables such as sex (male or female), age (20–59, 60–69, or 70–75), performance status (0 or 1), subsite (maxillary sinus, oral cavity, oropharynx, hypopharynx, or larynx), stage (III, IVA, or IVB), and additional treatments. The Kaplan–Meier method was used to estimate OS, DFS, LRRFS, DMFS, and post-LRRS rates. Hazard ratios (HRs) and 95% confidence intervals (CIs) were estimated using Cox proportional-hazard models to evaluate effect size. The robustness of results for OS, DMFS, and post-LRRS was assessed by adjusting the effect of baseline characteristics in the log-rank test. Sex, age, performance status, subsite, stage, and additional treatment were added to the stratified log-rank test as stratification factors in the sensitivity analysis. Differences with a P value of <.05 were considered statistically significant. All statistical analyses were performed using SAS/STAT® software, Version 9.3 (SAS Institute Inc., Cary, NC, USA) and R, Version 3.3.2 [25].
Results
Patient characteristics by curative therapy
Of the 505 ACTS-HNC study patients analyzed, 180 were in the CRT group (S-1 arm, n = 87; UFT, n = 93) and 325 were in the Other Therapy group (S-1 arm, n = 164; UFT, n = 161) (Table 1 and Fig 1).
Table 1. Comparison of clinical characteristics between the chemoradiotherapy group and the other therapy group.
| Variable | Category | Chemoradiation (%) | Other therapy (%) | P value | ||||
|---|---|---|---|---|---|---|---|---|
| Total | S-1 | UFT | Total | S-1 | UFT | CRT vs. Others | ||
| No. of patients | 180 | 87 | 93 | 325 | 164 | 161 | ||
| Sex | Male | 155 (86) | 73 (84) | 82 (88) | 269 (83) | 136 (83) | 133 (83) | 0.3761 |
| Female | 25 (14) | 14 (16) | 11 (12) | 56 (17) | 28 (17) | 28 (27) | ||
| Age | Median | 62 | 61 | 63 | 61 | 62 | 61 | 0.1888 |
| Range | 36–75 | 40–75 | 36–75 | 26–75 | 26–75 | 29–75 | ||
| PS | 0 | 162 (90) | 77 (89) | 85 (91) | 303 (93) | 154 (94) | 149 (93) | 0.2289 |
| 1 | 18 (10) | 10 (11) | 8 (9) | 22 (7) | 10 (6) | 12 (7) | ||
| Stage | III | 55 (31) | 27 (31) | 28 (30) | 78 (24) | 40 (24) | 38 (24) | 0.0622 |
| IVA | 115 (64) | 55 (63) | 60 (65) | 238 (73) | 121 (74) | 117 (73) | ||
| IVB | 10 (6) | 5 (6) | 5 (5) | 9 (3) | 3 (2) | 6 (4) | ||
| Primary Site | Maxillary Sinus | 16 (9) | 9 (10) | 7 (8) | 23 (7) | 12 (7) | 11 (7) | <0.001 |
| Oral Cavity | 15 (8) | 6 (7) | 9 (10) | 105 (32) | 53 (32) | 52 (32) | ||
| Oropharynx | 55 (31) | 22 (25) | 33 (36) | 53 (16) | 31 (19) | 22 (14) | ||
| Hypopharynx | 71 (39) | 37 (43) | 34 (37) | 71 (22) | 34 (21) | 37 (23) | ||
| Larynx | 23 (13) | 13 (15) | 10 (11) | 73 (22) | 34 (21) | 39 (24) | ||
| Additional Treatment | Yes | 53 (29) | 30 (35) | 23 (25) | 199 (61) | 102 (62) | 97 (60) | <0.001 |
| None | 127 (71) | 57 (65) | 70 (75) | 126 (49) | 62 (38) | 64 (40) | ||
Data presented as Median [Range] or n (%).
Abbreviations: PS performance status.
Comparisons between the CRT and Other Therapy groups and between S-1 and UFT in the CRT group
Comparisons of clinical characteristics between the CRT group and the other therapy group revealed significant differences in subsite (P <.001) and additional treatment (P <.001) (Table 1). However, baseline characteristics were generally similar between the S-1 and UFT arms within each group.
Outcomes in the present study
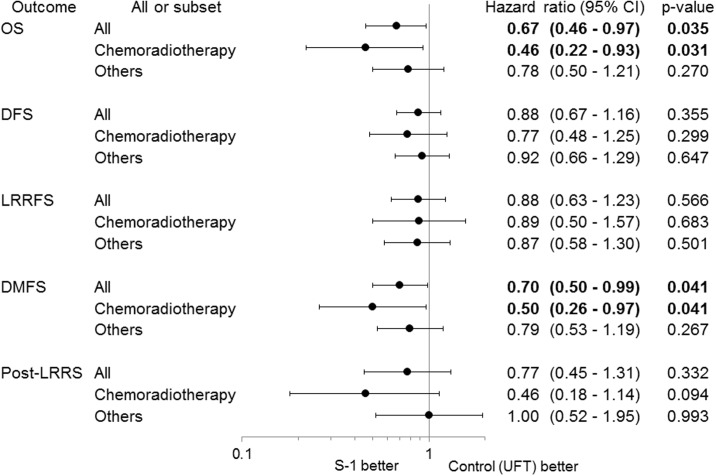
Median follow-up for all patients was 1356 days (range, 35–2116 days). The results of the analysis for OS, DFS, RFS, LRRFS, DMFS, and post-LRRS between the S-1 and UFT arms of the CRT group are presented in Fig 2.
Fig 2. Forest plot for subset analysis.
Abbreviations: CI, confidence interval. DFS, disease-free survival. DMFS, distant metastasis-free survival. LRRFS, locoregional relapse-free survival. OS, overall survival. post-LRRS, post-locoregional relapse-free survival. UFT, tegafur/uracil.
Overall survival
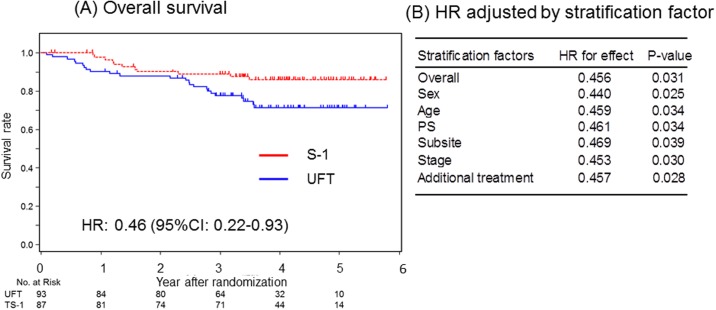
In the CRT group, S-1 significantly improved OS (HR, 0.46; 95% CI, 0.22–0.93; P = .031) (Fig 2A). Kaplan-Meier curves comparing the effect of S-1 and UFT on OS are shown in Fig 3A. In sensitivity analysis, the treatment effect of S-1 on OS remained significantly better after adjustment for all stratification factors (sex, age, performance status, subsite, stage, and additional treatment) (Fig 3B).
Fig 3. Overall survival in the CRT group.
(A) OS derived from Kaplan–Meier curves. (B) HR and corresponding CI were calculated using Cox proportional hazard model. P values were calculated based on stratified log-rank test. Abbreviations: CI, confidence interval. HR, hazard ratio. OS, overall survival.
Distant metastasis-free survival
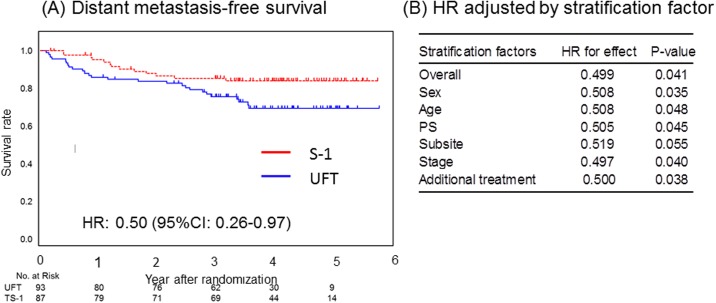
In the CRT group, S-1 significantly improved DMFS (HR, 0.50; 95% CI, 0.26–0.97; P = .041) (Fig 2A). Kaplan-Meier curves comparing the effect of S-1 and UFT on DMFS are shown in Fig 4A. In sensitivity analysis, when adjusting for stratification factors, the treatment effect of S-1 on DMFS remained significantly better excluding only after adjustment for subsite (Fig 4B).
Fig 4. Distant metastasis-free survival in the CRT group.
(A) DMFS derived from Kaplan–Meier curves. (B) HR and corresponding CI were calculated using Cox proportional hazard model. P values were calculated based on stratified log-rank test. Abbreviations: CI, confidence interval; HR, hazard ratio; DMFS, distant metastasis-free survival.
Post- locoregional relapse survival
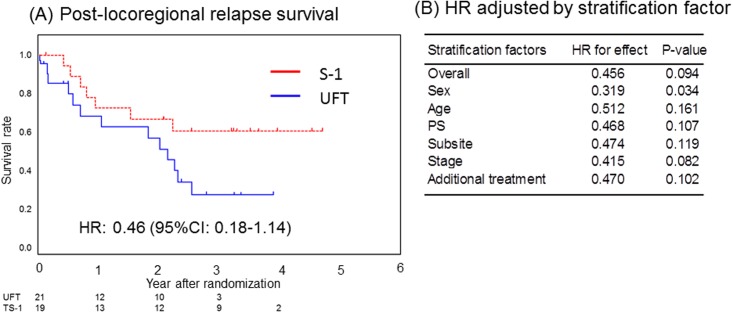
In the CRT group, A trend was observed in improvement of post-LRRS by S-1, but the difference was not statistically significant (HR, 0.46, 95% CI, 0.18–1.14; P = .094) (Fig 2). Kaplan-Meier curves comparing the effect of S-1 and UFT on post-LRRS are shown in Fig 5A. In sensitivity analysis, when adjusting for stratification factors, the treatment effect of S-1 on post-LRRS remained significantly better only after adjustment for sex (Fig 5B).
Fig 5. Post- locoregional relapse survival in the CRT group.
(A) Post-LRRS derived from Kaplan–Meier curves. (B) HR and corresponding CI were calculated using Cox proportional hazard model. P values were calculated based on stratified log-rank test. Abbreviations: CI, confidence interval; HR, hazard ratio; post-LRRS, post-locoregional relapse-free survival.
Disease-free survival and Locoregional relapse-free survival
DFS and LRRFS did not differ significantly between S-1 and UFT (Fig 2).
Discussion
The present study has shown a survival effect of adjuvant chemotherapy with S-1 in a subset of patients who underwent CRT as initial curative treatment (CRT group) in a phase III trial. In the CRT group, adjuvant chemotherapy with S-1 was associated with significant improvement in OS and DMFS, and with a trend toward better post-LRRS, compared with UFT. These outcomes of OS and DMFS were also verified in all patients in the ACTS-HNC phase III study, and these significant improvements with S-1 in the CRT group remained after sensitivity analysis with adjustment of HR for stratification factors.
In the CRT group, 127 patients (71%) had no residual tumor without additional surgery. These patients might be consisted with responder of IC plus radiotherapy or concurrent CRT, since non-responders to IC or concurrent CRT would be recommended for surgical resection. In previous phase III studies, IC plus radiotherapy or concurrent CRT significantly reduced the number of occurrences of distant metastasis [1–3, 5, 10]. If reduced rate of distant metastasis was a suitable factor for evaluating the effectiveness of adjuvant chemotherapy, those patients who responded to IC or concurrent CRT would have further reduction of distant metastasis with adjuvant chemotherapy following CRT. Indeed, the rate of distant recurrence in the CRT group was significantly lower than that of the Other Therapy group (P = .044) (data not shown) and, in addition to decreasing the rate of distant recurrence, S -1 displayed a survival effect more clearly than did UFT.
Although locoregional control was similar between the S-1 and UFT arms, S-1 showed a greater trend toward better post-LRRS in the CRT group, which may further explain the efficacy of S-1. There have been no phase III studies confirming an OS benefit from adjuvant chemotherapy [17–21]. Three studies [17, 19, 21] indicated that adjuvant chemotherapy reduced distant metastasis in patients undergoing curative surgery, and in two of the studies [17, 19], surgery plus radiotherapy was considered definitive treatment. Definitive treatment after LRR is surgery or radiotherapy; after surgery, postoperative radiotherapy with or without chemotherapy is recommended, so that salvage treatment of LRR after curative surgery is limited and will not improve OS. In contrast, because many patients in the CRT group had no residual tumor without additional surgery, salvage surgery after LRR might be an option. In addition, the curability of salvage surgery might have been increased by S-1 more than by UFT because it limited the extent of recurrence. Future clinical studies of adjuvant chemotherapy must incorporate not only salvage therapy following LRR, but also patient follow-up, in their designs.
It has been reported that the prognosis of oropharyngeal cancer is improving because oropharyngeal cancer related to human papillomavirus (HPV) responds well to chemotherapy and radiotherapy, but that HPV-related oropharyngeal cancer has been increasing over the years [26, 27]. It has also been reported that the prognosis in patients with HPV-related oropharyngeal cancer is well preserved in nonsmokers and that smoking is a poor prognostic factor. In the present study, data on HPV expression and smoking status were not available for patients with oropharyngeal cancer. However, it is unlikely that the lack of information on HPV status contributed to better results with S-1 because fewer patients with oropharyngeal cancer were treated with S-1 than with UFT.
The present study may be limited by the possibility of statistical error from ad hoc analysis or subset analysis from trial data because what we studied here was not preplanned in the ACTS-HNC study. Caution should therefore be exercised, and readers should be aware that the study is only an exploratory subset analysis. We were unable to determine the proportions of patients who received induction versus concurrent CRT. However, no previous study of adjuvant chemotherapy shown improvement in the OS of curative locoregional treatment or curative CRT; therefore, the outcomes of this study, that S-1 as adjuvant chemotherapy after curative CRT may improve OS and DMFS and possibly ameliorate poor prognosis of LAHNSCC, are still important. Clinical trials that clarify the efficacy of S-1 as adjuvant chemotherapy after curative CRT should be encouraged.
Conclusion
The results of the present study suggest that adjuvant chemotherapy with S-1 may improve OS and DMFS compared with UFT in patients who have received CRT as curative treatment that resulted in no residual tumor. Adjuvant chemotherapy with S-1 may therefore be considered a treatment option following CRT.
Acknowledgments
We greatly appreciate the late Professor Mamoru Tsukuda, who played an important role in the ACTS HNC study. We are grateful to all of the patients, the co-investigators, and the site staff members for their cooperation in the ACTS- HNC study.
Members of "the Adjuvant Chemotherapy with S-1 after Curative Treatment in Patients with Squamous-cell Carcinoma of the Head and Neck" (ACTS-HNC) study group were as follows:
Steering Committee: M. Fujii, A. Kubota, K. Kawabata, N. Kohno, K. Tomita, K. Yoshino, and M. Fukushima; Independent Data and SafetyMonitoring Committee: A. Kida, S. Kamata, T. Yoshihara, M. Sugita,M. Asai; Study Statistician: S. Teramukai.
The following institutions participated in this study:
Tokyo Medical University Hachioji Medical Center (Hachioji), Yokohama Rosai Hospital (Yokohama), Aichi Cancer Center Hospital (Nagoya), Hyogo College of Medicine (Nishinomiya), Yokohama City University School of Medicine (Yokohama), Kusatsu General Hospital (Kusatsu), Tochigi Cancer Center (Utsunomiya), Osaka Medical Center for Cancer and Cardiovascular Diseases (Osaka), National Hospital Organization Kyushu Cancer Center (Fukuoka), Hyogo Cancer Center (Akashi), Saitama Cancer Center (Saitama), Kagoshima City Hospital (Kagoshima), Yamagata University School of Medicine (Yamagata), Kagoshima University Medical And Dental Hospital (Kagoshima), Hirosaki University School of Medicine and Hospital (Hirosaki), Keio University (Tokyo), Yokohama City University Medical Center (Yokohama), University of the Ryukyus (Nakagami), Akita University Graduate School of Medicine and Faculty of Medicine (Akita), Hamamatsu University School of Medicine (Hamamatsu), Fujita Health University Banbuntane Houtokukai Hospital (Nagoya), Kansai Medical University Hirakata Hospital (Hirakata), Kanazawa University School of Medicine (Kanazawa), National Hospital Organization Tokyo Medical Center (Tokyo), Osaka Saiseikai
Nakatsu Hospital (Osaka), Oita Prefectural Hospital (Oita), Gunma University Hospital (Maebashi), Tokyo Dental College Ichikawa General Hospital (Ichikawa), Gifu University Hospital (Gifu), Kobe University (Kobe), Kobe City Medical Center General Hospital (Kobe), Ehime University (Matsuyama), Oita University (Yufu), National Hospital Organization Kumamoto Medical Center (Kumamoto), Kyushu University Hospital (Fukuoka), Fukushima Medical University (Fukushima), Niigata Prefectural Central Hospital (Jouetsu), Juntendo University Hospital (Tokyo), Shinshu University (Matsumoto), Kinki University Faculty of Medicine (Osaka-sayama), Osaka General Medical Center (Osaka), Nara Medical University (Kashihara), Yamaguchi University School of Medicine (Ube), Kagawa University Faculty of Medicine (Kagawa), Hokkaido University Graduate School of Medicine (Sapporo), Graduate School of Medicine and School of Medicine, Chiba University (Chiba), Tokyo Medical University (Tokyo), Kyorin University (Mitaka), Kanagawa Cancer Center (Yokohama), Tokai University (Isehara), Seirei Hamamatsu General Hospital (Hamamatsu), Tenri Hospital (Tenri), Hiroshima University Hospital (Hiroshima), Shimane University Faculty of Medicine (Izumo), Tottori University Faculty of Medicine (Yonago), Kumamoto University (Kumamoto), Jichi Medical University (Shimotsuke), Mie University Hospital (Tsu), Osaka City University Graduate School of Medicine (Osaka), National Kyushu Medical Center (Fukuoka), Saga Medical School Faculty of Medicine, Saga University (Saga), Tohoku University School of Medicine (Sendai), University of Yamanashi Graduate School of Medical Science (Kofu), Dokkyo Medical University Hospital (Tochigi), Dokkyo Medical University Koshigaya Hospital (Koshigaya), Niigata Cancer Center Hospital (Niigata), Tokyo Medical and Dental University (Tokyo), Kitasato University (Sagamihara), Fujita Health University (Toyoake), University of Fukui (Fukui), Fukuoka University (Fukuoka), Kurume University School of Medicine (Kurume), Asahikawa Medical University (Asahikawa), Sapporo Medical University (Sapporo), Iwate Medical University Hospital (Morioka), Saiseikai Utsunomiya Hospital (Utsunomiya), Saitama Medical
University International Medical Center (Hidaka), Nippon Medical School (Tokyo), Cancer Institute Hospital (Tokyo), Ome Municipal General Hospital (Ome), Showa University Fujigaoka Hospital (Yokohama), Shizuoka Cancer Center (Nagaizumi), Japanese Red Cross Nagoya Daiichi Hospital (Nagoya), Yokkaichi Municipal Hospital (Yokkaichi), Kyoto Prefectural University of Medicine (Kyoto), Osaka Medical College Hospital (Takatsuki), Wakayama Medical University (Wakayama), Kanazawa Medical University (Kanazawa), National Hospital Organization Himeji Medical Center (Himeji), Kawasaki Medical School (Kurashiki), University of Miyazaki (Miyazaki) Lead author: Akira Kubota E-mail: kubota-a@kcch.jp
The authors appreciate the careful review of this manuscript by Hideaki Kaneda, MD, PhD; Shinsuke Kojima, MD, PhD; Mikio Yoshitomi, PhD; and Atsuhiko Kawamoto, MD, PhD, at the Translational Research Center for Medical Innovation (TRI), Foundation for Biomedical Research and Innovation at Kobe (FBRI). The authors also thank Emiko Uno for data management and Masanori Fukushima, MD, PhD, director of the TRI Center. Finally, we thank Denise Di Salvo, MS, from Edanz Group (http://www.edanzediting.com/ac) for editing a draft of this manuscript.
Abbreviations
- ACTS-HNC
Adjuvant Chemotherapy with S-1 after curative treatment in patients with Head and Neck Cancer
- BSA
body surface area
- CI
confidence interval
- CRT
chemoradiotherapy
- DFS
disease-free survival
- DMFS
distant metastasis-free survival
- HPV
human papillomavirus
- HR
hazard ratio
- LAHNSCC
locoregionally advanced head and neck squamous cell cancer
- LRR
locoregional relapse
- LRRFS
locoregional relapse-free survival
- OS
overall survival
- post-LRRS
post-LRR survival
- UFT
tegafur/uracil
Data Availability
When the ACTS-HNC study began, the authors did not obtain informed consent regarding the disclosure of raw data from the patients. Thus, raw data cannot be made freely available in the manuscript, Supporting Information files, or a public repository. The ACTS-HNC dataset is available on request to Translational Research Center for Medical Innovation, Foundation for Biomedical Research and Innovation at Kobe, 1-5-4 Minatojima-minamimachi, Chuo-ku, Kobe, Japan 650-0047 Email: tri.data-sharing@tri-kobe.org. All requests must be approved by the Steering Committee and must include an analysis plan.
Funding Statement
This study was sponsored by the FBRI, with funding from Taiho Pharmaceutical Co., Ltd., Japan (http://www.taiho.co.jp/english/) under the study contract. The funding source of the study participated in study design but had no role in data collection, data management, data analysis, or data interpretation.
References
- 1.Wolf GT, Fisher SG, Hong WK, Hillman R, Spaulding M, Laramore GE, et al. ; Department of Veterans Affairs Laryngeal Cancer Study Group. Induction chemotherapy plus radiation compared with surgery plus radiation in patients with advanced laryngeal cancer. N Engl J Med. 1991;324:1685–90. doi: 10.1056/NEJM199106133242402 [DOI] [PubMed] [Google Scholar]
- 2.Lefèbvre JL, Chevalier D, Luboinski B, Kirkpatrick A, Collette L, Sahmoud T. Larynx preservation in pyriform sinus cancer: preliminary results of a European Organization for Research and Treatment of Cancer phase III trial. EORTC Head and Neck Cancer Cooperative Group. J Natl Cancer Inst. 1996;88:890–9. [DOI] [PubMed] [Google Scholar]
- 3.Al-Sarraf M, LeBlanc M, Giri PG, Fu KK, Cooper J, Vuong T, et al. Chemoradiation versus radiotherapy in patients with advanced nasopharyngeal cancer: phase III randomized Intergroup study 0099. J Clin Oncol. 1998;16:1310–7. doi: 10.1200/JCO.1998.16.4.1310 [DOI] [PubMed] [Google Scholar]
- 4.Calais G, Alfonsi M, Bardet E, Sire C, Germain T, Bergerot P, et al. Randomized trial of radiation therapy versus concomitant chemotherapy and radiation therapy for advanced-stage oropharynx carcinoma. J Natl Cancer Inst. 1999;91:2081–6. [DOI] [PubMed] [Google Scholar]
- 5.Forastiere AA, Goepfert H, Maor M, Pajak TF, Weber R, Morrison W, et al. Concurrent chemotherapy and radiotherapy for organ preservation in advanced laryngeal cancer. N Engl J Med. 2003;349:2091–8. doi: 10.1056/NEJMoa031317 [DOI] [PubMed] [Google Scholar]
- 6.Adelstein DJ, Li Y, Adams GL, Wagner H Jr, Kish JA, Ensley JF, et al. An intergroup phase III comparison of standard radiation therapy and two schedules of concurrent chemoradiotherapy in patients with unresectable squamous cell head and neck cancer. J Clin Oncol. 2003;21:92–8. doi: 10.1200/JCO.2003.01.008 [DOI] [PubMed] [Google Scholar]
- 7.Bernier J, Domenge C, Ozsahin M, Matuszewska K, Lefèbvre JL, Greiner RH, et al. Postoperative irradiation with or without concomitant chemotherapy for locally advanced head and neck cancer. N Engl J Med. 2004;350:1945–52. doi: 10.1056/NEJMoa032641 [DOI] [PubMed] [Google Scholar]
- 8.Cooper JS, Pajak TF, Forastiere AA, Jacobs J, Campbell BH, Saxman SB, et al. (2004) Postoperative concurrent radiotherapy and chemotherapy for high-risk squamous-cell carcinoma of the head and neck. N Engl J Med. 2004;350:1937–44. doi: 10.1056/NEJMoa032646 [DOI] [PubMed] [Google Scholar]
- 9.Bernier J, Cooper JS, Pajak TF, van Glabbeke M, Bourhis J, Forastiere A, et al. Defining risk levels in locally advanced head and neck cancers: a comparative analysis of concurrent postoperative radiation plus chemotherapy trials of the EORTC (#22931) and RTOG (#9501). Head Neck. 2005;27:843–50. doi: 10.1002/hed.20279 [DOI] [PubMed] [Google Scholar]
- 10.Pignon JP, le Maître A, Maillard E, Bourhis J; MACH-NC Collaborative Group. Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): an update on 93 randomised trials and 17,346 patients. Radiother Oncol. 2009;92:4–14. doi: 10.1016/j.radonc.2009.04.014 [DOI] [PubMed] [Google Scholar]
- 11.Haddad R, O’Neill A, Rabinowits G, Tishler R, Khuri F, Adkins D, et al. Induction chemotherapy followed by concurrent chemoradiotherapy (sequential chemoradiotherapy) versus concurrent chemoradiotherapy alone in locally advanced head and neck cancer (PARADIGM): a randomised phase 3 trial. Lancet Oncol. 2013;14:257–64. doi: 10.1016/S1470-2045(13)70011-1 [DOI] [PubMed] [Google Scholar]
- 12.Hitt R, Grau JJ, López-Pousa A, Berrocal A, García-Girón C, Irigoyen A, et al. ; Spanish Head and Neck Cancer Cooperative Group (TTCC). A randomized phase III trial comparing induction chemotherapy followed by chemoradiotherapy versus chemoradiotherapy alone as treatment of unresectable head and neck cancer. Ann Oncol. 2014;25:216–25. doi: 10.1093/annonc/mdt461 [DOI] [PubMed] [Google Scholar]
- 13.Cohen EE, Karrison TG, Kocherginsky M, Mueller J, Egan R, Huang CH, et al. Phase III randomized trial of induction chemotherapy in patients with N2 or N3 locally advanced head and neck cancer. J Clin Oncol. 2014;32:2735–43. doi: 10.1200/JCO.2013.54.6309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ang KK, Zhang Q, Rosenthal DI, Nguyen-Tan PF, Sherman EJ, Weber RS, et al. Randomized phase III trial of concurrent accelerated radiation plus cisplatin with or without cetuximab for stage III or IV head and neck carcinoma: RTOG 0522. J Clin Oncol. 2014;32:2940–50. doi: 10.1200/JCO.2013.53.5633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bourhis J, Sire C, Graff P, Grégoire V, Maingon P, Calais G, et al. Concomitant chemoradiotherapy versus acceleration of radiotherapy with or without concomitant chemotherapy in locally advanced head and neck carcinoma (GORTEC 99–02): an open-label phase 3 randomised trial. Lancet Oncol. 2012;13:145–53. doi: 10.1016/S1470-2045(11)70346-1 [DOI] [PubMed] [Google Scholar]
- 16.Nguyen-Tan PF, Zhang Q, Ang KK, Weber RS, Rosenthal DI, Soulieres D, et al. Randomized phase III trial to test accelerated versus standard fractionation in combination with concurrent cisplatin for head and neck carcinomas in the Radiation Therapy Oncology Group 0129 trial: long-term report of efficacy and toxicity. J Clin Oncol. 2014;32:3858–66. doi: 10.1200/JCO.2014.55.3925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Head and Neck Contracts Program. Adjuvant chemotherapy for advanced head and neck squamous carcinoma. Final report of the Head and Neck Contracts Program. Cancer. 1987;60:301–11. [DOI] [PubMed] [Google Scholar]
- 18.Rossi A, Molinari R, Boracchi P, Del Vecchio M, Marubini E, Nava M, et al. Adjuvant chemotherapy with vincristine, cyclophosphamide, and doxorubicin after radiotherapy in local-regional nasopharyngeal cancer: results of a 4-year multicenter randomized study. J Clin Oncol. 1988;6:1401–10. doi: 10.1200/JCO.1988.6.9.1401 [DOI] [PubMed] [Google Scholar]
- 19.Laramore GE, Scott CB, al-Sarraf M, Haselow RE, Ervin TJ, Wheeler R, et al. Adjuvant chemotherapy for resectable squamous cell carcinomas of the head and neck: report on Intergroup Study 0034. Int J Radiat Oncol Biol Phys. 1992;23:705–13. [DOI] [PubMed] [Google Scholar]
- 20.Kwong DL, Sham JS, Au GK, Chua DT, Kwong PW, Cheng AC, et al. Concurrent and adjuvant chemotherapy for nasopharyngeal carcinoma: a factorial study. J Clin Oncol. 2004;22:2643–53. doi: 10.1200/JCO.2004.05.173 [DOI] [PubMed] [Google Scholar]
- 21.Tsukuda M, Ogasawara H, Kaneko S, Komiyama S, Horiuchi M, Inuyama Y, et al. A prospective randomized trial of adjuvant chemotherapy with UFT for head and neck carcinoma. Head and Neck UFT study Group [in Japanese]. Gan To Kagaku Ryoho. 1994;21:1169–77. [PubMed] [Google Scholar]
- 22.Tatsumi K, Fukushima M, Shirasaka T, Fujii S. Inhibitory effects of pyrimidine, barbituric acid and pyridine derivatives on 5-fluorouracil degradation in rat liver extracts. Jpn J Cancer Res. 1987;78:748–55. [PubMed] [Google Scholar]
- 23.Oki E, Murata A Yoshida K, Maeda K, Ikejiri K, Munemoto Y, et al. A randomized phase 3 trial comparing S-1 versus UFT as adjuvant chemotherapy for stage II/III rectal cancer (JFMC35-C1: ACTS-RC). Ann Oncol. 2016;27:1266–72. doi: 10.1093/annonc/mdw162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tsukahara K, Kubota A, Hasegawa Y, Takemura H, Terada T, Taguchi T, et al. ; ACTS-HNC Group. Randomized phase III trial of adjuvant chemotherapy with S-1 after curative treatment in patients with squamous-cell carcinoma of the head and neck (ACTS-HNC). PLoS One. 2015;10(2):e0116965 doi: 10.1371/journal.pone.0116965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.R Core Team. R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing; 2015. http://www.R-project.org/. Accessed November 2016. [Google Scholar]
- 26.Ang KK, Harris J, Wheeler R, Weber R, Rosenthal DI, Nguyen-Tân PF, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363:24–35. doi: 10.1056/NEJMoa0912217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dahlstrom KR, Calzada G, Hanby JD, Garden AS, Glisson BS, Li G, et al. An evolution in demographics, treatment, and outcomes of oropharyngeal cancer at a major cancer center: a staging system in need of repair. Cancer. 2013;119:81–9. doi: 10.1002/cncr.27727 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
When the ACTS-HNC study began, the authors did not obtain informed consent regarding the disclosure of raw data from the patients. Thus, raw data cannot be made freely available in the manuscript, Supporting Information files, or a public repository. The ACTS-HNC dataset is available on request to Translational Research Center for Medical Innovation, Foundation for Biomedical Research and Innovation at Kobe, 1-5-4 Minatojima-minamimachi, Chuo-ku, Kobe, Japan 650-0047 Email: tri.data-sharing@tri-kobe.org. All requests must be approved by the Steering Committee and must include an analysis plan.