Abstract
The emergence and dissemination of carbapenemases, bacterial enzymes able to inactivate most β-lactam antibiotics, in Enterobacteriaceae is of increasing concern. The concurrent spread of resistance against colistin, an antibiotic of last resort, further compounds this challenge further. Whole-genome sequencing (WGS) can play a significant role in the rapid and accurate detection/characterization of existing and emergent resistance determinants, an essential aspect of public health surveillance and response activities to combat the spread of antimicrobial resistant bacteria. In the current study, WGS data was used to characterize the genomic content of antimicrobial resistance genes, including those encoding carbapenemases, in 10 multidrug-resistant Klebsiella pneumoniae isolates from Pakistan. These clinical isolates represented five sequence types: ST11 (n = 3 isolates), ST14 (n = 3), ST15 (n = 1), ST101 (n = 2), and ST307 (n = 1). Resistance profiles against 25 clinically-relevant antimicrobials were determined by broth microdilution; resistant phenotypes were observed for at least 15 of the 25 antibiotics tested in all isolates except one. Specifically, 8/10 isolates were carbapenem-resistant and 7/10 isolates were colistin-resistant. The blaNDM-1 and blaOXA-48 carbapenemase genes were present in 7/10 and 5/10 isolates, respectively; including 2 isolates carrying both genes. No plasmid-mediated determinants for colistin resistance (e.g. mcr) were detected, but disruptions and mutations in chromosomal loci (i.e. mgrB and pmrB) previously reported to confer colistin resistance were observed. A blaOXA-48-carrying IncL/M-type plasmid was found in all blaOXA-48-positive isolates. The application of WGS to molecular epidemiology and surveillance studies, as exemplified here, will provide both a more complete understanding of the global distribution of MDR isolates and a robust surveillance tool useful for detecting emerging threats to public health.
Introduction
The Gram-negative bacterium Klebsiella pneumoniae is a clinically relevant pathogen that has a propensity to acquire multidrug resistance (MDR), thus limiting therapeutic options for treating community-acquired and nosocomial infections such as pneumonia, septicemia, wound, and urinary tract infections (UTIs) [1]. MDR and extensively drug-resistant (XDR) strains are defined as non-susceptible to at least one agent in three or more antimicrobial classes or non-susceptible to at least one agent in all but two or fewer antimicrobial classes, respectively [2]. The rapid worldwide spread of MDR K. pneumoniae, as well as other Enterobacteriaceae, poses a serious threat to global health [3]. K. pneumoniae, the most common Klebsiella species causing human infections, is one of the top three pathogens of international concern documented in the 2014 World Health Organization (WHO) Global Report on Surveillance of Antimicrobial Resistance [4].
Extended-spectrum β-lactamases (ESBLs) are bacterial enzymes that hydrolyze and inactivate most β-lactam antibiotics such as penicillins, broad-spectrum cephalosporins, and monobactams, but not cephamycins or carbapenems. Their production by bacterial pathogens confers resistance against a number of commonly used classes of β-lactam antibiotics and primarily restricts the choice of antimicrobial therapy to carbapenem antibiotics [5]. Thus, carbapenems are often employed as last resort antibiotics for the treatment of severe infections caused by MDR bacterial pathogens [1]. The acquisition of carbapenemases by Enterobacteriaceae has thus been especially worrisome as it threatens the clinical utility of these important therapeutic agents [1]. Indeed, the United States Centers for Disease Control and Prevention (CDC) has identified carbapenem-resistant Enterobacteriaceae (CRE) as one of the most urgent MDR threats [6].
The most common carbapenemases identified in K. pneumoniae to date are: i) class A β-lactamases (e.g., K. pneumoniae carbapenemase; KPC); ii) class B β-lactamases/metallo- β-lactamases (e.g., New Delhi metallo-β-lactamase-1 [NDM-1]), and iii) class D β-lactamases (e.g., oxacillinase-48; OXA-48-like carbapenemases) [1]. While these plasmid-encoded carbapenemases have been increasingly reported worldwide, their prevalence varies geographically [1,3].
Therapeutic options to treat infections caused by MDR carbapenemase-producing K. pneumoniae strains are limited to drugs that are less effective, more toxic, and/or not widely available, such as colistin (polymyxin E), polymyxin B, fosfomycin, tigecycline, and select aminoglycosides [1]. In the last few years, resistance to colistin has emerged due, in part, to its extensive use in livestock feed [7]. Endogenous resistance to colistin in K. pneumoniae can result from any of several genetic changes, including alteration of the mgrB gene and several non-synonymous point mutations in the genes encoding the two-component regulatory systems PhoPQ and PmrAB [8–10].
The continued emergence of antibiotic resistance in K. pneumoniae, and more broadly Enterobacteriaceae, presents a considerable clinical challenge. The steep decline in the discovery of effective antibiotics by pharmaceutical companies further exacerbates the threat posed by MDR pathogens. Rapid, accurate detection and characterization of antimicrobial resistance determinants and genomic mutations conferring resistance are crucial to countering the mounting burden of infections caused by MDR bacteria. Such information could help direct hospital resources to prevent nosocomial spread of MDR organisms and guide best-choice antimicrobial therapy to improve patient outcomes [11]. Unfortunately, some phenotypic detection methods can be unreliable for the detection of carbapenemase-producing bacteria, depending on the test used and the carbapenemase produced. For instance, the Modified Hodge Test (MHT) is a simple and easy laboratory test based on the inactivation of a carbapenem by a carbapenemase-producing isolate. While this culture-based test, originally the only Clinical Laboratory Standards Institute (CLSI)-recommended carbapenemase screening method [12], performs well for the detection of KPC and OXA-48 producers, it often fails to detect NDM-producing organisms [13]. Since 2017, CLSI has recommended two additional phenotypic tests (Carba NP, and mCIM) and removed MHT early 2018 [14].
Hospital laboratories are increasingly using whole genome sequencing (WGS) for the unambiguous identification of previously identified and characterized genes encoding antimicrobial resistance determinants [11]. This method can be used to identify resistance genes located on both the bacterial chromosome and on mobile genetic elements, as well as to track the emergence and persistence of resistance in previously susceptible bacterial pathogens [11]. Additionally, WGS provides a wealth of data that can be used for multiple analyses (e.g., concurrently determining sequence types or the presence of both virulence and resistance genes), thus helping to optimize resources and support appropriate clinical intervention. It is imperative to track the spread of existing determinants of antimicrobial resistance to previously susceptible organisms and recognize the emergence of new or novel combinations of determinants [15].
In the current study, we have characterized the resistome of carbapenem- and colistin-resistant clinical isolates of K. pneumoniae that were isolated in Pakistan between 2010 and 2013. Our findings provide important insight into the genetic diversity of these challenging MDR bacterial pathogens in a high prevalence area of the world. The characterization of these historical isolates will help facilitate an understanding of the emergence and spread of antimicrobial resistance in K. pneumoniae. Further, WGS-based context will help inform hospital infection control measures and aid the elucidation of contributing factors that promote the development of antimicrobial resistance in the non-hospital environment. Such information provides an essential foundation to support the development of novel diagnostic and therapeutic strategies for detecting and treating infections caused by MDR bacteria.
Material and methods
Samples
Ten MDR clinical isolates of K. pneumoniae that were cultured from blood, urine, or other sites (e.g. wound) between 2010 and 2013 (Fig 1) were obtained from the Department of Pathology and Laboratory Medicine at the Aga Khan University Hospital (AKUH) in Karachi, Pakistan. Limited clinical information was available for all isolates. Initial species identification and antimicrobial susceptibility testing (AST) were performed at AKUH using Vitek 2 (bioMérieux, France). To get a snapshot of the resistome of MDR K. pneumoniae in Pakistan, isolates had been randomly selected among those resistant to at least one carbapenem. Of the 10 selected isolates resistant to at least one carbapenem, 7 were resistant to colistin while 3 were susceptible (Table 1). Three of the isolates (CFSAN044564, CFSAN044572, CFSAN044573) were from the same patient who had been hospitalized multiple times.
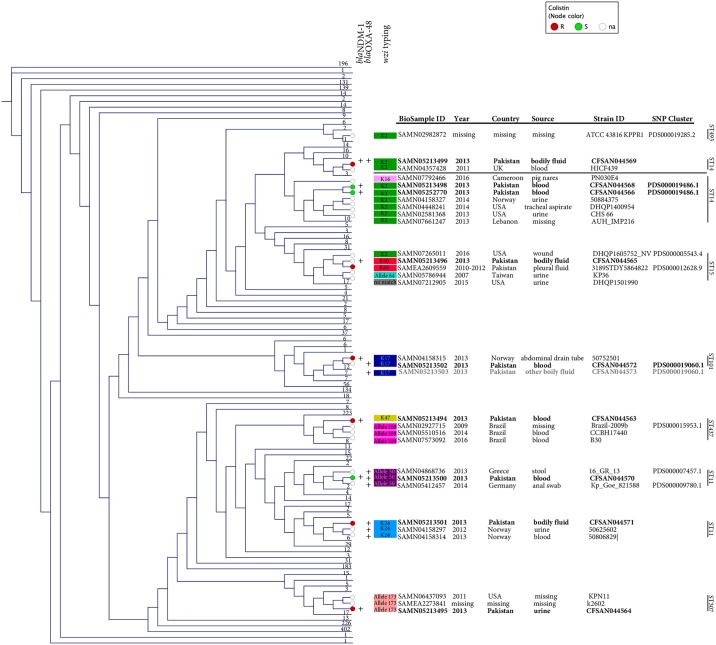
Fig 1. Cladogram of the Kmer distance tree derived from the NCBI Pathogen Detection database comprising 5169 Klebsiella pneumoniae isolates at time of writing.
The tree also shows MLST, wzi typing, and the presence of blaOXA-48 and blaNDM-1 genes for the 10 clinical isolates and sequence control strain (ATCC 43816), as well as their closest relatives. For space optimization, all other isolates were collapsed into straight lines comprising different numbers (aka leaves). Colistin susceptibility is indicated with different colored dots: green (susceptible); red (resistant); and white (no information). The identification for the BioSample and SNP cluster (when available) is also provided. The complete tree file from NCBI Pathogen detection (PDG000000012.284.reference_target.tree.asn—as of March 24th, 2018), can be downloaded at https://doi.org/10.6084/m9.figshare.5708347.v2 and can be opened with NCBI software Genome Workbench (https://www.ncbi.nlm.nih.gov/tools/gbench/). Given that the kmer tree only includes a reference isolate from each SNP clusters plus singleton isolates, CFSAN044573 (marked in gray) is not included in the original tree but it is listed here for completeness of information.
Table 1. Antibiotic susceptibility profiles of MDR K. pneumoniae isolates subjected to resistome analysis.
| ID CFSAN0 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| List of antibiotics (n = 25 agents) | 44563 | 44564 | 44565 | 44566 | 44568 | 44569 | 44570 | 44571 | 44572 | 44573 | |
| Penicillins | Ampicillin | R | R | R | R | R | R | R | R | R | R |
| β-lactam/ β-lactamase inhibitor combinations | Amoxicillin-clavulanic acid | R | R | R | R | R | R | R | R | R | R |
| Ampicillin-sulbactam | R | R | R | R | R | R | R | R | R | R | |
| Piperacillin-tazobactam | R | R | R | R | R | R | R | R | R | R | |
| Cephalosporins | Cefazolin—C1Ga | R | R | R | R | R | R | R | R | R | R |
| Cefoxitin—C2Gb | R | R | R | R | R | R | R | R | I | I | |
| Cefotaxime -C3Gc | R | R | R | R | R | R | R | R | R | R | |
| Ceftazidime—C3G | R | R | R | R | R | R | R | S | R | R | |
| Ceftriaxone—C3G | R | R | R | R | R | R | R | R | R | R | |
| Cefepime—C4Gd | R | R | R | R | R | R | R | I | R | R | |
| Monobactams | Aztreonam | R | R | R | R | R | R | R | S | R | R |
| Carbapenems | Imipenem | R | R | R | R | R | R | R | R | I | I |
| Doripenem | R | R | R | R | R | R | R | R | S | S | |
| Meropenem | R | R | R | R | R | R | R | R | S | S | |
| Ertapenem | R | R | R | R | R | R | R | R | R | R | |
| Aminoglycosides | Amikacin | R | R | R | R | R | R | R | S | R | R |
| Gentamicin | R | R | R | R | R | R | R | I | R | R | |
| Tobramycin | R | R | R | R | R | R | R | I | R | R | |
| Fluoroquinolones | Ciprofloxacin | R | R | R | R | R | R | R | R | R | R |
| Levofloxacin | R | I | R | S | S | S | R | R | R | R | |
| Folate Pathways inhibitors | Trimethoprim-sulfamethoxazole | R | R | S | R | R | R | R | S | S | S |
| Polymyxins | Colistin | R | R | R | S | S | R | S | R | R | R |
| Chloramphenicol | Chloramphenicol | R | S | S | S | S | S | R | I | S | S |
| Tetracyclines | Tetracycline | R | S | S | R | R | S | S | S | S | S |
| Tigecycline | S | S | S | S | S | S | S | S | S | S | |
β-lactam antibiotic class are shaded grey. Red/R indicates resistance, green/S susceptibility, and yellow/I intermediate. Strains from the same patient are underlined.
aC1G: first generation cephalosporin
bC2G: second generation cephalosporin
cC3G: third generation cephalosporin
dC4G: fourth generation cephalosporin
The 10 selected isolates were sent to University of Virginia (Charlottesville, VA, USA [UVA]) for further studies [16]. DNA was extracted using the Qiagen DNeasy Blood & Tissue Kit (Germantown, MD, USA). DNA samples prepared at UVA were sent to the Center for Food Safety and Applied Nutrition of the US Food and Drug Administration (College Park, MD, USA) where WGS was carried out. K. pneumoniae ATCC 43816 (CFSAN044574) was included as a sequencing control. All 10 isolates were sent to the Centers for Disease Control and Prevention (Atlanta, GA, USA) for confirmation of AST testing using the reference BMD method.
Antimicrobial susceptibility testing (AST)
The MIC values for 25 antimicrobial agents were determined by BMD, according to CLSI guidelines [12,14,17]. Escherichia coli ATCC 25922 and Pseudomonas aeruginosa ATCC 27853 were used as control strains for antimicrobial susceptibility testing. Susceptibilities were interpreted using clinical breakpoints established by the CLSI for 23/25 drugs. The European Committee for Antimicrobial Susceptibility Testing (EUCAST) breakpoints [18] were used for colistin and tigecycline, since CLSI breakpoints are not currently available. Table 1 indicates the antimicrobials agents tested, as well as isolates susceptibilities.
Sequencing and assembly of genomes
DNA libraries and genomic assemblies were prepared as previously described [19]. Briefly, DNA was prepared using the Nextera XT DNA Library Preparation Kit (Illumina, San Diego, CA, USA), and WGS was carried out on a MiSeq platform using the 2 × 250 bp paired-end MiSeq Reagent Kit v2 (Illumina, San Diego, CA, USA). SPAdes Genome Assembler (version 3.9) was used to obtain de novo assemblies, which are available under accession numbers MAG(C/E/F/G/H/I/J/K/L/M)00000000 [19]. To support global distribution and provide epidemiological linkages, all genomes in this study were uploaded to the publicly available NCBI Pathogen Detection website (https://www.ncbi.nlm.nih.gov/pathogens/).
DNA sequences were analyzed using the IS finder web resource (https://www-is.biotoul.fr/blast.php), to identify insertion sequences (ISs) and IS fragments [20]. Potential integrons were further annotated using INTEGRALL (http://integrall.bio.ua.pt) [21]]. Identified amino acid substitutions were checked in the Protein Variation Effect Analyzer tool (PROVEAN—http://provean.jcvi.org/index.php) to predict their effect on the biological function of the protein (i.e. neutral or deleterious) [22]. Average nucleotide identities (ANIs) between genomes of interest were calculated using JSpeciesWS (http://jspecies.ribohost.com) [23]. Progressive Mauve was used to align genomes to identify conserved or disparate regions and/or SNPs (http://darlinglab.org/mauve/mauve.html) [24]. PHASTER (PHAge Search Tool Enhanced Release) was used for the identification and annotation of prophage sequences within obtained genomes (http://phaster.ca) [25].
Molecular typing of isolates and plasmid profiling
The Bacterial Isolate Genome Sequence Database (BIGSdb) (http://bigsdb.web.pasteur.fr/perl/bigsdb/bigsdb.pl?db=pubmlst_klebsiella_seqdef_public) was used to characterize each of the K. pneumoniae isolates by determining the ST (multi-locus sequence type) and capsular serotype. In particular, to predict the capsular (K) types of the examined isolates (among the currently identified 79 K types), we used the sequences of the wzi gene, one of the six conserved genes in the cps locus [26]. PlasmidFinder (https://cge.cbs.dtu.dk/services/PlasmidFinder/) was used to type plasmids via the identification of major incompatibility (Inc) groups in Enterobacteriaceae species [27]. The minimum percentage of sequence identity was set at 100%, with an alignment length of >98%.
To increase the size of our analysis, we have included comparisons of the 10 isolates sequenced herein with more than 5100 other K. pneumoniae isolates obtained worldwide, using the NCBI Pathogen Detection tool (https://www.ncbi.nlm.nih.gov/pathogens/). This pipeline uses WGS data to: i) produce a phylogenetic tree based on Kmer distance; ii) perform single-linkage clustering (with a SNP distance of 50 SNPs) to find closely related isolates, and iii) determine the antibiotic resistance (AMR) profiles of isolates. A Kmer distance tree was obtained from NCBI Pathogen Detection on March 24th, 2018. This Kmer tree includes a reference isolate from each SNP clusters plus singleton isolates, which are not currently included in SNP clusters.
Identification of resistance determinants
Three different approaches were used to identify antimicrobial resistance genes and select efflux pumps: ResFinder, the Comprehensive Antibiotic Research Database (CARD), and NCBI Pathogen Detection. Assembled genomes were uploaded to the web resource ResFinder v2.1 (http://cge.cbs.dtu.dk/services/ResFinder/) for the identification of acquired antimicrobial resistance genes [28]. The minimum percentage of sequence identity threshold was set at 98%, with an alignment length of at least 80%. Assemblies were also analyzed with the tools available at CARD for strict and perfect hits, with identity >96% (http://arpcard.mcmaster.ca) [29], and using the NCBI Pathogen Detection tool. When conflicting nomenclatures were encountered, the NCBI nomenclature was used. The presence of acquired colistin resistance genes (i.e. mcr variants) was determined using the database resources described above. Disruptions or alterations of chromosomal loci conferring colistin-resistance (e.g. mgrB, pmrAB, phoPQ) were determined in silico using CLC Genomics Workbench v.9 (CLC Bio, Aarhus, Denmark) [8–10]. Chromosomal point mutations in the Quinolone Resistance Determining Region (QRDR) of gyrA, gyrB and parC, parE genes were investigated for characterization of quinolone resistance using PointFinder [30].
Results
Antimicrobial susceptibility profiles
Isolate-specific antimicrobial susceptibilities are shown in Table 1. One of the blaNDM-producing isolates, CFSAN044563, was resistant to 24/25 antibiotics tested, and susceptible to only tigecycline. Conversely, one of the blaOXA-48-producing strains, CFSAN44571, exhibited the highest number of intermediate and susceptible results (n = 10 antibiotics). Interestingly, CFSAN044572 and CFSAN044573 were susceptible to meropenem and doripenem but intermediate to imipenem.
All isolates were resistant to the same 9 antibiotics (8 β-lactams and ciprofloxacin). Non-susceptibility to 3 other β-lactam antibiotics was observed in 70% of the isolates, including two isolates (CFSAN044572 and CFSAN044573) which were intermediate to cefoxitin and imipenem and one strain (CFSAN044571) intermediate to cefepime (Table 1). For carbapenems, 80% of the strains were resistant to all four carbapenems tested. Of the remaining antibiotics, 90% of the strains were resistant to all aminoglycosides; 70% to colistin; 60% to trimethoprim-sulfamethoxazole; 30% to tetracycline and 40% to chloramphenicol (Table 1). All isolates were susceptible to the tetracycline derivative tigecycline.
Molecular typing and plasmid profiling
The 10 K. pneumoniae clinical isolates analyzed here were determined to belong to 5 different sequence types (STs): ST11 (n = 3 isolates), ST14 (n = 3), ST15 (n = 1), ST101 (n = 2), and ST307 (n = 1) (Fig 1 and Table 2). CFSAN044574 (ATCC 43816) belonged to ST493, as previously reported in the Bacterial Isolate Genome Sequence Database (BIGSdb). Examination of the wzi sequences identified 7 alleles, which were associated with specific capsular types (Fig 1). The most common capsular type was K2 (n = 3 isolates); followed by K17 (n = 2); K24, K47, and K60 (n = 1 each). The K2 capsular type was present in multiple STs (ST14, and ST15). Two isolates possessed wzi alleles that, based on BIGSdb, were either associated with multiple capsular serotypes (allele 50, CFSAN044570) or not yet assigned to a specific capsular serotype (allele 173, CFSAN044564) (Fig 1).
Table 2. List of antimicrobial resistance genes and plasmid replicon-types.
| ID CFSAN0 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| ST11 | ST307 | ST14 | ST15 | ST101 | |||||||
| Class | Gene | 44570 | 44563 | 44571 | 44564 | 44569 | 44566 | 44568 | 44565 | 44572 | 44573 |
| A | armA | x | x | x | x | x | |||||
| rmt | F1 | C | F1 | F1 | |||||||
| aadA | 2 | 1 | 1 | 1 | |||||||
| aac(3)- | IIa | IIa | II | IIa | IIa | IIa | IIa | IIa | |||
| aph(3')- | Ia | VI | |||||||||
| aph(3'')-Ib | x | x | x | x | x | x | x | ||||
| aph(6)-Id | x | x | x | x | x | ||||||
| aac(6')-Ib | x | xa | x | ||||||||
| aac(6')-Ib-cr | x | x | x | x | x | x | x | ||||
| B | blaCTX-M-15 | x | x | x | x | x | x | x | x | x | |
| blaSHV- | 11 | 11 | 11 | 28 | 28 | 28 | 28 | 28 | 1 | 1 | |
| blaOXA-1 | x | x | x | x | x | x | x | x | |||
| blaOXA-10 | x | x | x | ||||||||
| blaOXA-48 | x | x | x | x | x | ||||||
| blaNDM-1 | x | x | x | x | x | x | x | ||||
| blaTEM-1 | x | x | x | x | x | x | |||||
| blaCMY- | 16 | 16 | 6 | ||||||||
| C | cmlA5 | x | x | x | |||||||
| catA1 | x | ||||||||||
| F | qnr | S1 | B1 | B1 | B1 | ||||||
| G | ble | x | x | x | x | x | x | x | |||
| M | mph | A | E | E | E | E | |||||
| Mrx | x | ||||||||||
| R | arr | x | x | x | x | x | x | ||||
| S | sul | 1 | 1 | 1 | 1,2 | 1 | 1,2 | 1,2 | 1 | 2 | 2 |
| Te | tet(A) | x | x | x | |||||||
| Tr | dfrA | 12 | 1 | 14 | 14 | 14 | 14 | ||||
| Efflux pumps | qacEdelta1 | x | x | x | x | x | x | x | x | ||
| msr(E) | x | x | x | x | x | ||||||
| Plasmid replicon types | IncL/M | x | x | x | x | x | |||||
| IncFIB(pQiL) | x | x | x | x | |||||||
| IncFIB(Mar) | x | ||||||||||
| IncFIB(K) | x | ||||||||||
| IncFII(pKPX1) | x | x | x | ||||||||
| IncFII(K) | x | ||||||||||
| IncHI1B | x | ||||||||||
| IncA/C2 | x | x | x | x | |||||||
Classes of antimicrobial resistance genes are indicated as follows: A (Aminoglycosides); B (β-lactams); C (Chloramphenicol); F (Fluoroquinolones); G (Glycopeptides); M (Macrolide); R (Rifampicin); S (Sulfonamides); Te (Tetracycline); Tr (Trimethoprim). An x or an allele designation indicates the detection of the gene using NCBI Pathogen Detection, ResFinder (98%ID threshold, 80% minimum length), and/or CARD (perfect and strict hits, with identity >96%). A bolded x or allele designation indicates 100% identity. Possible gene duplications are underlined. For the plasmid replicon analysis, an x indicates the presence of a replicon type as determined using by PlasmidFinder (%ID threshold: 100% and a query vs. HSP length ratio of >98%). The oqxAB genes, which were present in all isolates, are not listed in the Table. In CFSAN044572, aac(6’)-lb is annotated as a partial sequence.
None of the 10 strains was grouped in a SNP cluster with any other K. pneumoniae strains in the NCBI Pathogen Detection database, containing 5169 strains at time of writing. However, 2 pairs of strains (CFSAN044572/CFSAN044573 and CFSAN044566/CFSAN044568) were found to be closely related to each other and grouped in a SNP cluster. Specifically, CFSAN044572 and CFSAN044573 were grouped in SNP cluster PDS000019060.1, with a calculated distance between them of an average of 800 SNPs. CFSAN044566 and CFSAN044568 were grouped in SNP cluster PDS000019486.1, with a calculated distance of 1 SNP. Additionally, CFSAN044574 (ATCC 43816) was grouped in SNP cluster PDS000019285.1 with KPPR1, a spontaneous rifampin-resistant isolate derived from ATCC 43816 [31], confirming the accuracy of sequencing and assembly (Fig 1). The list of isolates, their AMR genotypes, overall results, and Kmer tree are available at the K. pneumoniae species webpage at NCBI Pathogen Detection (https://www.ncbi.nlm.nih.gov/pathogens/). The isolates reported here represent a diverse population, based on the spectrum of observed STs and differences in resistomes. In all cases, K types were clearly defined based on either the ST or cluster, except for ST15 and a subset of ST11 in which four and two different capsular types were observed, respectively (Fig 1). Among the ST11 isolates, CFSAN044570 (ST11, 2010, positive for both blaNDM-1 and blaOXA-48) clustered with a ST11 strains isolated in 2013 from stool in Greece (16_GR_13) and two other ST11 isolates linked to a blaOXA-48-positive K. pneumoniae outbreak in Germany in 2013–2014 (SNP cluster PDS000009780.1, n = 2). These latter two isolates were obtained from skin and rectal swabs and each was associated with colonization in refugees from North Africa (Fig 1). CFSAN044571 clustered with two ST11 strains isolated in Norway: 50625602 from urine (2012) and 50806829 from blood (2013) (Fig 1). CFSAN044563 grouped with blaKPC-2 positive isolates from Brazil belonging to ST437 (SLV—single locus variant of ST11): Brazil-2009b (belonging to SNP cluster PDS000015953.1, n = 4); CCBH17440 (2014, sepsis/blood); and B30 (2016, blood). Of the three ST14 isolates, the genomes of CFSAN044566 and CFSAN044568 were most closely related as they were grouped in a SNP cluster, and differed by 1 SNP. CFSAN044569 grouped with HICF439, a ST14 strain from the UK (2011, blood/bacteremia). The ST15 isolate CFSAN044565 grouped with other ST15 strains: DHQP1501990 (2015, urine, USA); DHQP1605752_NV (2016, wound, USA, SNP cluster PDS000005543.4, n = 5); KP36 (2007, urine, Taiwan, 2007) [32]; and 3189STDY5864822. DHQP1605752_NV has been recently described as one of the first K. pneumoniae isolates in the US to be non-susceptible to all 26 drugs tested, including all ß -lactams, colistin, and tigecycline. The isolate carried two plasmids (IncA/C2 and IncFIB), and four ß-lactamase genes (plasmid-mediated blaNDM-1 and blaCMY-6, and chromosomal blaCTX-M-15 and blaSHV-28) [33]. 3189STDY5864822 belongs to SNP cluster PDS000012628.9, comprising 40 clinical isolates from Pakistan, collected between 2010 and 2012, and of which 4 were blaNDM-1 positive. The two ST101 isolates were highly related, sharing an average ANI of 99.9% and belonged to the same SNP cluster, as described above. This observation is consistent with their isolation from different sites of the same patient, blood for CFSAN044572 and catheter tip for CFSAN044573. The same patient was also infected with another strain (CFSAN044564) that belonged to a different ST (ST307), which is located on a separate branch on the phylogenetic tree (Fig 1). The closest relatives for this isolate were two ST307 clinical isolates: KPN11 from the US (2011), and k2602 from the BSAC Resistance Surveillance program (Fig 1).
Eight plasmid replicon-types were observed with a percent ID of 100%: IncL/M(pOXA-48) (n = 5 isolates), IncA/C2 and IncFIB(pQil) (n = 4); IncFII(pKPX1) (n = 3); and IncFIB(Mar), IncHI1B, IncFII(K) and IncFIB(K) (n = 1 each) (Table 2). The IncL/M(pOXA-48) replicon was present in the five K. pneumoniae strains in which the blaOXA-48 gene was detected (Table 2). No clear relationship between replicon type and ST was observed, except for the two ST101 isolates that shared the same plasmid profile (Table 2). No replicon-types were detected in the control strain K. pneumoniae ATCC 43816 (CFSAN044574), since that strain does not contain any plasmid [30]. Overall, the highest number of different replicon-types was observed in ST11 strains (n = 8 types), followed by ST14 (n = 3), ST101 (n = 2), and ST307 and ST15 (n = 1 each) (Table 2).
Antimicrobial-resistance determinants
In most cases, ResFinder, CARD and NCBI Pathogen Detection were in agreement in identifying the predominant antibiotic resistance genes; however, some differences in nomenclature and reference sequences were noted. Table 2 shows a selection of the antimicrobial resistance determinants that were identified using the three platforms (n = 41 genes). Overall, ResFinder and the NCBI pipeline detected, on average, a smaller number of genes (19 per strain) than CARD (78 per strain). This discrepancy is due to the former two primarily identifying plasmid-associated resistance determinants and not chromosomal loci associated with antibiotic resistance.
According to all three platforms, the highest diversity of resistance genes was observed for aminoglycosides (n = 13 genes) and β-lactams (n = 11), with at least two resistance determinants present for each antibiotic class present in every isolate (Table 2). Consistent with BMD results, CFSAN044563 had the highest number of resistance determinants (n = 23 genes), while CFSAN044571 had the fewest (n = 6); both were identified as ST11 isolates. Among the genes present in multiple isolates, 4 were associated with a specific ST: blaSHV-1 in ST101; blaSHV-11 in ST11; qnrB1 and blaCMY-16 in ST14. The remaining genes were found in isolates belonging to different STs (Table 2).
Two ESBL genes were identified (blaCTX-M-15 and blaSHV-28). blaCTX-M-15 was the most frequent ESBL gene detected (n = 9 isolates) and was present in combination with blaSHV-28 (n = 5) or the following non-ESBL genes: blaTEM-1 (n = 6) and other blaSHV genes (n = 5). No blaKPC genes were found. blaNDM-1 was the most frequently detected carbapenemase gene (n = 7 isolates), followed by blaOXA-48 (n = 5). In two isolates (CFSAN044570 and CFSAN044569), both blaNDM-1 and blaOXA-48 were present (Table 2). CFSAN044571 possessed the fewest β-lactam and carbapenem resistance genes, and was susceptible to ceftazidime (C3G) and aztreonam (monobactam), but had intermediate susceptibility to cefepime (C4G).
None of the ten strains analyzed possessed any of mcr genes, the plasmid-borne determinants of colistin resistance [34]. In all cases, chromosomal mutations associated with colistin resistance were identified. Specifically, while no amino acid substitutions were observed in mgrB; disruption of mgrB and point mutations within prmB were observed in colistin-resistant strains (Table 3). The mgrB of strains CFSAN044564, CFSAN044565, and CFSAN044569 was disrupted by three different classes of IS families (ISKpn25, IS5, and IS1) inserted at different nucleotide positions (mgrB23, mgrB38, and mgrB45, respectively) (Table 3). In one isolate (CFSAN044563), no mgrB gene sequence could be identified, indicating the possible loss of the entire mgrB locus. No substitutions were observed in phoP, while only changes encoding neutral amino acid substitutions were found in pmrA and phoQ (S1 Table). In contrast, multiple non-synonymous substitutions were observed in pmrB (n = 14 substitutions), with most of them (n = 10) being considered deleterious to protein structure/function. A mutation resulting in a Thr157Pro amino acid substitution, previously confirmed to be responsible for colistin resistance [9], was found in all (n = 3) of the colistin-resistant strains with an intact mgrB gene (Table 3).
Table 3. Disruptions/insertion in the mgrB gene and point mutations causing neutral and deleterious amino acid substitutions (in pink) in the pmrB gene.
| mgrB | pmrB | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ID CFSAN0 | T93P | N110T | T112P | T127P | T128P | L130P | L141P | V151G | T157P | L159P | L164P | L213M | A246T | R256G | |
| 44563 | deletion | x | x | x | x | ||||||||||
| 44564 | ISKpn25 at mgrB23 | x | |||||||||||||
| 44565 | IS5-like at mgrB38 | x | x | x | x | x | x | ||||||||
| 44569 | IS1-like at mgrB45 | x | x | ||||||||||||
| 44571 | intact | x | |||||||||||||
| 44572 | intact | x | x | ||||||||||||
| 44573 | intact | x | x | x | x | x | x | x | |||||||
| 44570 | intact | ||||||||||||||
| 44566 | intact | x | x | x | |||||||||||
| 44568 | intact | x | x | ||||||||||||
Colistin-resistant strains are underlined, and amino acid substitutions known to be associated with colistin resistance are in bold. The genome sequence for K. pneumoniae HS11286 was used as a reference (GenBank assembly accession GCA_000240185.2).
For quinolone-resistant isolates, three different genotype profiles were observed: i) a gyrA mutation (S83I) and a parC mutation (S80I) in 3 isolates (CFSAN044563, CFSAN044564 and CFSAN044570); ii) 2 gyrA mutations (S83F/Y) and a parC mutation (S80I) in 4 isolates (CFSAN044565, CFSAN044571, CFSAN044572, and CFSAN044573); and no mutations at all in 3 isolates (CFSAN044566, CFSAN044568, and CFSAN044569). The following plasmid-mediated quinolone resistance genes were identified: aac(6’)-lb-cr (6 isolates), qnrB1 (all ST14 isolates), and qnrS1 (1 ST11 isolate), along with the oqxAB efflux pump (all isolates). The 3 isolates (CFSAN044566, CFSAN044568, and CFSAN044569) with no mutations in the topoisomerase type II enzymes, both carried qnrB1 and aac6’-Ib-cr. Additional genes detected included the tet(A) gene in the three tetracycline-resistant strains; and dfrA/sul1 in the 6 trimethoprim-sulfamethoxazole resistant-strains (Table 2).
blaNDM-1 common region and gene duplication
In the seven blaNDM-1-positive isolates, the regions immediately flanking the blaNDM-1 locus were conserved and included the bleomycin resistance protein (bleMBL) and the N-(5’-phosphoribosyl)anthranilate isomerase (trpF) genes (Fig 2). In four isolates, the interrupted sequence of the left end of ISAba125 was located upstream of the blaNDM-1 gene, while the complete sequence of ISAba125 was present only in one isolate (CFSAN044563). In two of the isolates (CFSAN044566 and CFSAN044568), the original ISAba125-bracketed blaNDM-1 region seems to have been further mobilized by an ISEC28-mediated event downstream of a class 1 integron, thereby generating an extended multidrug-resistance scaffold and a duplication event (Fig 2).
Fig 2. Schematic representation of the genetic environment of the blaNDM-1 common region and gene duplication organization in ST14 isolates CFSAN044566 and CFSAN044568.
blaNDM-1 is in red, other genes in blue, duplicated copies in green, and insertion sequences are in light blue. The blaNDM-1 common region includes a truncated ISaba125, the blaNDM-1 gene, the bleomycin resistance protein (bleMBL) and phosphoribosylanthranilate isomerase (trpF) genes. Duplicate copies of four genes (blaOXA-10, aadA1, qacEdelta1, and sul1) were found. The tat gene is present in two copies split in the middle in one instance. A straight line indicates gaps between the ORFs.
Two copies of a gene cassette comprising blaOXA-10, aadA1, qacEdelta1, and sul1 were present in CFSAN044566 and CFSAN044568 (Fig 2). The latter three genes were identical in both repeats and in both isolates. In the assembled genome of CFSAN044568, the first repeat of blaOXA-10 shows a non-synonymous mutation (G663A) that results in a premature stop codon. However, when reads were mapped back to the assembly, this mutation was not observed, possibly due to an assembly error caused by repetitive nucleotide sequences in this region. In both isolates, the insertion of a partial ISAba125 element truncates the first 33 nucleotides of the second copy of blaOXA-10.
To evaluate the number of reads specific for the genes seen in duplicate copies and therefore estimate the sequence coverage in CFSAN044566 and CFSAN044568, CLC Genomics Workbench v.9 was used to map raw sequence reads to reference sequences for the blaNDM-1, blaOXA-10, aadA1, qacEdelta1 and sul1 genes (NG_049326.1, NG_050979.1, NG_052030.1, NG_048042.1, NG_048100.1, respectively). CFSAN044563 was used as a control as all 5 genes were present without duplication. We observed a higher average coverage for blaOXA-10, aadA1, qacEdelta1, and sul1 in CFSAN044566 (80X, 80X, 85X, 81X, respectively) and CFSAN044568 (127X, 114X, 117X, 94X) compared to blaNDM-1 (40X and 58X). In CFSAN044563, the coverages were the following: blaOXA-10 (39X), aadA1 (43X), qacEdelta1 (58X) and sul1 (46X), and blaNDM-1 (37X).
Discussion
WGS data for 10 MDR K. pneumoniae strains isolated between 2010 and 2013 in Pakistan were analyzed to provide a comparative genetic context for carbapenem and colistin resistance that will help inform infectious diseases epidemiology and the identification of antimicrobial resistance determinants. Knowledge of the genomic content of these historical isolates will also be useful for elucidating the spread of antimicrobial resistance in K. pneumoniae. The analyzed isolates represent a diverse population as indicated by the Kmer distance tree, assigned ST and capsular types, and the resistomes.
WGS findings reflect the considerable antimicrobial resistance displayed by these isolates to multiple antibiotics, with resistance to β-lactams (including carbapenems) and aminoglycosides, fluoroquinolones, and colistin ranging from 60% to 100%. Only 30–40% of the isolates exhibited resistance to tetracycline and non-susceptibility to chloramphenicol, which may reflect the reduced clinical use of these agents in Pakistan [35]. Indeed, a previous study reported that cephalosporins were the first option for empirical treatment of bacterial infections caused by MDR-pathogens in Pakistan and that there was limited use of carbapenems due to their high cost [36]. However, sales of carbapenems almost tripled between 2005 and 2010 [35], possibly in response to a rise in infections caused by ESBL-producing pathogens.
The isolates examined in this study belong to either established (ST11, ST15, and ST14) or emerging (ST101 and ST307) antibiotic resistant high-risk clones of K. pneumoniae [5]. K. pneumoniae ST258 emerged in the middle 2000s in the US and has become a worldwide-propagated clone, along with its related variants belonging to clonal group 258 (CG258) [5]. ST11, a SLV of ST258, can capture multiple plasmids and is typically associated with MDR pathogens [5]. This ability is reflected by the different antimicrobial resistance and plasmid profiles of the 3 ST11 isolates we examined. Specifically, the three ST11 strains were highly diverse as they were represented by a NDM-1-producer (CFSAN044563), an OXA-48-producer (CFSAN044571), and one carrying both the blaNDM-1/blaOXA-48 genes (CFSAN044570). These 3 ST11 strains were either colistin-susceptible (CFSAN044570) or colistin-resistant through different chromosomal mechanisms of resistance (mgrB deletion or T157P mutation in pmrB for CFSAN044563 and CFSAN044571, respectively). The plasmid replicon-type and AMR profiles also differed within ST11, comprising between 3 and 4 different plasmid replicon-types (IncFIB, IncFII, IncL/M and IncA/C2) and sharing only 3 common AMR markers: aac(3)-IIa, SHV-11, and qacEdelta1. Non-susceptibility to chloramphenicol was observed in only two ST11 strains; one strain (CFSAN044563) which harbored both cmlA5 and catA1; and one (CFSAN044570) which did not carry any genes associated with phenicol-resistance, thus suggesting the involvement of another mechanism (e.g.: overexpression of efflux systems). After alignment with MAUVE, a large region (contig MAGJ05, 110951 bp) was observed to be present in CFSAN044563 but absent in both CFSAN044570 and CFSAN044571. This contig contains a replicon with 95.73% identity to IncFIB from pKPHS1, but carries no resistance genes and is recognized by PHASTER as an intact phage, with a length of 110821 bp, attL and attR attachment sites, 117 proteins and a GC content of 48.92%. The most common related phage is listed as SSU5 from Salmonella [37], which has been described as a temperate phage with a circular plasmid prophage, as it is homologous to circular plasmids in several Enterobacteriaceae genomes [38]. Blasting of the MAGJ05 sequence showed different percentages of identity and query coverage vs.: SSU5 (83% identity with 48% coverage); and plasmids pKPHS1 (98% identity with 88% coverage), and pPMK1-B and p1605752FIB_2 (both 99% identity and 91% coverage). Additionally, the DEAD/DEAH box helicase gene (BAY54_20090 and BAY54_20675) in the MAGJ05 contig overlaps for 127 bp. Future research with long read sequence will be needed to confirm the genomic location and characteristics of this region.
Both ST14 and its SLV ST15 frequently carry and disseminate resistance determinants, such as multiple β-lactamases [1,5]. ST14 strains have been associated with pediatric and neonatal infections, sometimes carrying blaNDM-1 or blaOXA-48 [39–42], and ST15 strains have been reported in intensive care facilities [43]. Of the three ST14 strains, two blaNDM-1- possessing strains (CFSAN044566 and CFSAN044568), were susceptible to colistin and are closely related as they belong to the same SNP cluster and share a similar plasmid profile (IncA/C2), comprising 24 different AMR markers for 6 different antibiotic families: β-lactams (blaCTX-M-15, blaSHV-28, blaOXA-1, blaOXA-10, blaTEM-1, blaCMY-16); aminoglycosides (armA, aadA1, aac(3)-IIa, aph3”-Ib, aph6-Id, aac6’-Ib); chloramphenicol (cmlA5); cotrimoxazole (sul1&sul2), trimethoprim (dfrA14); macrolides (msrE); along with class 1 integron markers (qacEdelta1, sul1). The third ST14 strain (CFSAN044569), co-possessing blaNDM-1 and blaOXA-48, was colistin-resistant due to an IS1-like insertion at mgrB45, with a different plasmid profile and shared 14 out of the 24 detected AMR genes. The absence of the tet(A) gene in CFSAN044569 and the presence of alterations in either mgrB or pmrB in CFSAN044566/68 likely explains differences in susceptibility to tetracycline and colistin, respectively.
Beginning in 2014, ST307 has emerged in Italy replacing the predominant hyper-epidemic ST258 clone of K. pneumoniae [44]. A 2017 US study showed that, between 2011 and 2015, strains of CG307 were more prevalent than those belonging to CG258 [45]. ST307 has also been reported to be a major blaCTX-M producing clone in Pakistan (2009–2010) [46], the US (2010) [47], Morocco (2012) [48], Serbia (2013–2016) [49], South Korea (2015) [50], and Colombia (2012–2014) [42], where it has been associated with infections with a mortality rate >50%. ST307 strains have been associated with capsular type wzi-173 [44], as also observed here.
ST101 has been described as an emerging pandemic clone found in several countries e.g. Romania, 2012 [51]; Japan, 2012 [52]; Spain, 2012–2014 [53]; Algeria, 2014–2015 [54]; and others [1]. The two ST101 strains from the same patient (blood and catheter) were closely related and showed a similar plasmid profile, except for the aac6’-lb gene which was not identified in the assembly for CFSAN044572. However, the aac6’-lb gene was annotated as incomplete and when raw reads from CFSAN044572 were mapped to the aac6’-Ib sequence from CFSAN044573, the full gene was observed. The Mauve alignment showed 1194 SNPs in 543 regions (annotated genes, hypothetical proteins and unannotated/intergenic regions), with n = 505 showing between 1–5 SNPs; 32 between 6–10 SNPs, and 6 with >10 SNPs. In particular, the highest numbers of SNPs were observed in a glutamate dehydrogenase (n = 17, BAY53_19965); followed by 2 unannotated regions (n = 13 and 12, respectively); and 3 genes with 11 SNPs each (a multidrug RND transporter, BAY53_01340; the competence/damage-inducible protein A, BAY53_20960; and the exodeoxyribonuclease V subunit alpha A, BAY53_6365). Additionally, their phage profile appears to be identical, with one incomplete, two questionable, and two complete phages identified by PHASTER (S2 Table). The two ST101 strains were likely resistant to colistin due to a T157P mutation in pmrB; however, there were 7 additional SNPs in pmrB between CFSAN044572 and CFSAN044573, 6 of which are non-synonymous: L130P, L141P, V151G, T157P, L159P, L164P. Three neutral substitutions (L213M, A246T, and R256G) have been previously observed, but they do not appear to be associated with colistin resistance. In fact, complementation with the wild-type pmrB gene in isolates with these substitutions did not restore colistin susceptibility. Additional reports also suggest that these pmrB mutations are not related to colistin resistance [55–57]. In particular, R256G has been observed in both colistin-resistant (13 out of 17) and colistin-susceptible (10 out of 20) isolates [58]. Overall, the 7 colistin resistant isolates had different chromosomal mutations associated with colistin resistance and belonged to 5 different sequence types, confirming that colistin resistance is multifactorial and chromosomal determinants are independent of the sequence types [59].
The isolates analyzed herein grouped largely based on their ST in the Kmer distance tree. Given the presence of different mobile elements, the Kmer distance tree could potentially be confounding for the tips of the tree; however, Kmer analysis is able to capture differences across genomes (e.g. mutations, insertions/deletions, recombination, and differences in gene content) [60]. WGS results are consistent with the average short-term evolutionary rate for the two ST101 isolates from the same patient, suggesting that diversity within individual patients is low. While CFSAN044572 and CFSAN044573 are indeed closely related, given the observed number of SNPs and considering the potential artifacts introduced by short reads technology and assembly, it is difficult to determine if some evolution events occurred within the patient or that the patient was concurrently infected by two closely related strains. Future research with long-read sequencing will be necessary to better elucidate the relationship between these two isolates. However, the isolation of a different strain (ST307) from the same patient clearly indicates a non-clonal relationship that may have resulted from a subsequent infection with a different strain or the presence of diverse pathogen populations within the same individual, each of which complicates empirical treatment.
Capsular polysaccharide (CPS) is one of the main virulence factors of K. pneumoniae and capsular types are related to the clinical severity of the infections [61]. Wzi encodes for an outer membrane protein involved in capsule attachment to the surface of the cell [26]. The identification of distinct ST15 lineages, as suggested by the different capsular types associated with this ST, has been linked to the circulation of distinct lineages with differences in relative occurrence, geographical, niche distribution, and/or host susceptibility [62]. A similar finding was observed herein for the capsular type related to wzi-109 of ST437 (Fig 1), a ST belonging to the same clonal complex of ST11 and frequently observed between 2007 and 2009 in Brazil [63].
The variety of AMR genes was higher among the blaNDM-1-positive isolates (up to 23 genes), than among the blaOXA-48-positive isolates (up to 11 genes). All 7 blaNDM-1-positive isolates identified herein are carrying both blaCTX-M-15, with either blaSHV-28 (n = 5) or blaSHV-11 (n = 2), along with additional β-lactamases (blaOXA-1, blaOXA-10 or blaOXA-48). The main AMR genes identified in most blaNDM-positive isolates of that study were sul1 (n = 6), armA, aph3”-Ib, aph(6)-Id (n = 5), mphE (n = 4), dfrA14 (n = 4), qacEdelta1 (n = 7), and msrE (n = 5). All the blaOXA-48 positive isolates were resistant to colistin except one strain. Of the 5 blaOXA-48-positive isolates, 4 carried both blaCTX-M-15 and one isolate carried both blaCTX-M-15 and blaSHV-28. The only additional β-lactamase identified was blaOXA-1 (n = 4) for blaOXA-48-positive isolates. Other AMR determinants associated with blaOXA-48 were rmtF1 (n = 3), aac(3)-IIa (n = 4), aph3”-Ib, sul1 (n = 5), qacEdelta1 (n = 3). The plasmid replicon-type IncL/M was identified in all blaOXA-48-positive isolates along with additional types: IncFIb (n = 1), IncFII (n = 2), IncFIB + IncFII (n = 1), IncFIB + IncA/C2 (n = 1).
Overall, clonal diversity was observed in blaNDM-1- and blaOXA-48-positive K. pneumoniae isolates, as they belonged to four and three different STs, respectively, and the bla genes were harbored on different plasmids, except for CFSAN044566 and CFSAN044568 which likely carry the same plasmid. This finding is in contrast to blaKPC-positive K. pneumoniae, which historically have been linked to ST258 and its related variant in CG258 [5]. Differences in geographical distribution likely explain the absence of blaKPC genes; KPC K. pneumoniae isolates maybe be rare in Pakistan as no KPC-associated isolates/cases were reported in a 2015 literature review [64], and only 2 strains carrying blaKPC-3 were detected in a study analyzing Pakistani isolates obtained between 2012–2013 [65].
The concurrent presence of blaNDM-1 and blaOXA-48 has been described in isolates from Morocco, 2011 [66]; Tunisia, 2012 [67]; United Arab Emirates, 2011–2013 [68,69]; Turkey, Switzerland and Australia, 2013 [70–72]. Since then, these isolates have been commonly reported and include those with different combinations of blaOXA-48 variants with blaNDM- genes [73–81]. In the NCBI Pathogen Detection database, the combination of any of the variants of blaOXA-48 [82] with any blaNDM- gene [83], was observed in 34 out of 5278 isolates (0.64%) (as of April 18th, 2018). Two isolates were from the present study and the remaining were isolated between 2011–2017 in Vietnam, Europe, South Korea, Thailand and the US. The identification of both genes in two isolates of different STs, one from 2010 and one from 2013, appears to be the first description of blaNDM-1 and blaOXA-48 co-producing K. pneumoniae clones originating from Pakistan thus predating what is currently described in the literature.
The gene encoding OXA-48 β-lactamase was first identified in 2000; since then, this class D β-lactamase and its variants have become clinically significant worldwide [1]. As CRE-producing OXA-48-like enzymes may be difficult to recognize since they only weakly hydrolyze both cephalosporins and carbapenems, their incidence is possibly underestimated [3]. A highly-transferable pOXA-48 plasmid (IncL group), generally containing no other antibiotic resistance genes, was reported to be primarily responsible for spreading the blaOXA-48 gene in K. pneumoniae [1]. Confirming the ability of high-risk clones to accumulate resistance determinants, the blaOXA-48 gene was detected in strains belonging to different STs (ST101, ST11, and ST14). In particular, ST101 does not appear to have been previously observed in Pakistan. To the best of our knowledge, this is the first description of a blaOXA-48 producing K. pneumoniae strain of ST101 isolated in Pakistan. In fact, the only report of a blaOXA-48-producing K. pneumoniae linked to Pakistan appears to be from a December 2012 case of osteomyelitis in an infant that had sustained a burn injury in Karachi, Pakistan before returning to Canada, where the blaOXA-48 producing strain was isolated [84]. This highlights the complex issues and challenges presented by MDR organisms, as clinicians around the world need to be aware of global trends in antimicrobial resistance as focusing only on local patterns might not be sufficient to make prudent clinical decisions.
Seventy percent of the K. pneumoniae isolates examined were found to carry blaNDM-1, consistent with it being endemic in South Asia (i.e., Bangladesh, India, and Pakistan) [1]. Specifically, blaNDM-1 has been identified in the majority (75%) of carbapenem-resistant K. pneumoniae in 2011 in Pakistan [36]; since 2015, the country has been associated with single hospital outbreaks of NDM-producing isolates [64]. As observed for blaOXA-48, blaNDM-1 was associated with ST11, ST14, ST15, and ST307, which is troubling as these STs frequently contain blaCTX-M and blaKPC, and represent both well-established and emerging clones. Additionally, our blaNDM-1-positive ST14 isolates belonged to serotype K2, which is considered to be one of the predominant virulent serotypes [61]. The blaNDM genes have been found on a wide variety of different broad-host-range plasmids, thus facilitating the spread of such genes by horizontal gene transfer to various Enterobacteriaceae species [85]. Among the seven blaNDM-1 positive isolates, several different replicon-types associated with blaNDM-1 were identified, confirming the high variability of the blaNDM-1 genomic context.
The immediate genetic environment of the blaNDM-1 gene is conserved [85]. A blaNDM-1 common region usually comprises a truncated ISAba125 element immediately upstream of blaNDM-1, followed by the bleMBL gene and the trpF gene (either complete or truncated) [85,86]. ISAba125 is thought to be responsible for the initial mobilization of blaNDM-1 from its progenitor [85,86]. In CFSAN044566 and CFSAN044568, the arrangement of the blaNDM-1 region suggests there were multiple insertion events, as two copies of an ISCR1 element are present. It may be that this region was first mobilized onto a plasmid by an ISAba125-mediated event, followed by transfer onto an IncA/C2-like plasmid by an ISCR1-mediated transpositional event. The presence of different IS elements has been suggested to aid the efficiency by which resistance genes spread and both ISCR1 and IS26 have been observed to facilitate the transposition and/or expression of resistance genes located near them [87]. The flanking genetic elements may act as hot spots for recombination, responsible for the mobilization of the conserved blaNDM-1 region and the high dissemination rate of blaNDM-1 worldwide, even without an apparent epidemiological linkage between NDM-1-positive isolates [85,88].
Intriguingly, the blaNDM-1 common region may have resulted in the duplication of blaOXA-10, aadA1, qacEdelta1, and sul1. In 2011, duplication had been observed for the non-ESBL blaSHV-11 gene in different strains of K. pneumoniae, which was linked to a 16-fold higher level of resistance to amoxicillin [89]. More recently, multiple copies of resistance genes (blaSHV-12, blaOXA-9, and blaTEM-1) were reported in a K. pneumoniae ST11 strain isolated in 2013 in South Korea [90]. When mapping reads to a specific genomic region, the number of aligned reads should be proportional to the number of times the region is present in the isolate [91]. In our simple estimation of relative coverage in CFSAN044566 and CFSAN044568, the four genes (blaOXA-10, aadA1, qacEdelta1, and sul1) showed a higher coverage when compared to that of blaNDM-1. Future research with long-read sequencing will be necessary to confirm gene duplication, in addition to closing putative plasmids and determining if they match a previously described plasmid or represent a new variant.
Considering the identification of high-risk clones with extended multidrug resistance, gene duplication, and high prevalence of blaNDM-1 and blaOXA-48 genes, sometimes concurrently, our findings highlight the serious challenges posed by MDR K. pneumoniae and underscores the importance of implementing worldwide surveillance for antimicrobial resistance. Additionally, the numerous potential transmission routes at the human-animal-environment interface stresses the importance of a One Health approach [14] for effective surveillance, control and prevention. The presence of about 5,200 isolates of K. pneumoniae in a shared database such as NCBI Pathogen Detection should help track the global spread of these deadly pathogens, but it is only as good as the data deposited—a shared responsibility of the clinical research community [92]. The application of WGS to molecular epidemiology studies could provide a better understanding of the worldwide dissemination of MDR isolates and offer a robust surveillance tool that will be useful in detecting and characterizing both existing and emerging threats.
Disclaimer
The findings and conclusions in this report are those of the author(s) and do not necessarily represent the official position of the U.S. Food & Drug Administration and U.S. Centers for Disease Control and Prevention. The mention of company names or products does not constitute endorsement by the FDA or CDC.
Supporting information
Susceptibility or resistance to colistin is indicated with S or R, respectively. The genome sequence for K. pneumoniae HS11286 was used as a reference (GenBank assembly accession GCA_000240185.2).
(XLSX)
The tool identified 1 incomplete, 2 questionable, and 2 complete phages.
(XLSX)
Data Availability
The genome assemblies described in this manuscript are available in DDBJ/ENBL/GenBank under the accession numbers MAGC00000000 (CFSAN044573), MAGE00000000 (CFSAN044569), MAGF00000000 (CFSAN044570), MAGG00000000 (CFSAN044571), MAGH00000000 (CFSAN044572), MAGI00000000 (CFSAN044568), MAGJ00000000 (CFSAN044563), MAGK00000000 (CFSAN044564), MAGL00000000 (CFSAN044565), and MAGM00000000 (CFSAN044566). The complete tree file from NCBI Pathogen detection (PDG000000012.284.reference_target.tree.asn—as of March 24th, 2018), can be downloaded at https://doi.org/10.6084/m9.figshare.5708347.v2 and can be opened with NCBI software Genome Workbench (https://www.ncbi.nlm.nih.gov/tools/gbench/).
Funding Statement
SL received support by appointment to the Research Participation Program at the Center for Food Safety and Applied Nutrition, U.S. Food & Drug Administration, administered by the Oak Ridge Institute for Science and Education (https://orise.orau.gov) through an interagency agreement between the U.S. Department of Energy and the U.S. Food & Drug Administration. MAC was supported by U.S. National Institutes of Health (https://www.nih.gov/) grant F32 AI108249. CL received support from the U.S. Department of Homeland Security, Science and Technology Directorate (https://www.dhs.gov/science-and-technology) Agreement no. HSHQPM-16-X-00066. MAH was supported by the U.S. National Institutes of Health (https://www.nih.gov/) grant R01 AI099097 and a Henry Rose Carter Research Award (https://med.virginia.edu/office-for-research/funding-opportunities/). The funders listed above provided support in the form of salaries for authors [SL, MAC, CL, MAH], but did not have any role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. IHRC, Inc., a commercial entity that provides scientific personnel for the US government, had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The specific roles of all authors are articulated in the ‘author contributions’ section.
References
- 1.Lee C-R, Lee JH, Park KS, Kim YB, Jeong BC, Lee SH. Global dissemination of carbapenemase-producing Klebsiella pneumoniae: epidemiology, genetic context, treatment options, and detection methods. Front Microbiol. 2016;7: 895 doi: 10.3389/fmicb.2016.00895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Magiorakos A-P, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect 2012;18: 268–281. doi: 10.1111/j.1469-0691.2011.03570.x [DOI] [PubMed] [Google Scholar]
- 3.van Duin D, Doi Y. The global epidemiology of carbapenemase-producing Enterobacteriaceae. Virulence. 2017;8: 460–469. doi: 10.1080/21505594.2016.1222343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Organization WH. Antimicrobial Resistance: Global Report on Surveillance. Geneva: World Health Organization; 2014. http://www.who.int/drugresistance/documents/surveillancereport/en/ Cited 18 April 2018 [Google Scholar]
- 5.Navon-Venezia S, Kondratyeva K, Carattoli A. Klebsiella pneumoniae: a major worldwide source and shuttle for antibiotic resistance. FEMS Microbiol Rev. 2017;41: 252–275. doi: 10.1093/femsre/fux013 [DOI] [PubMed] [Google Scholar]
- 6.CDC. Antibiotic resistance threats in the United States, 2013 U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; 2013. https://www.cdc.gov/drugresistance/pdf/ar-threats-2013-508.pdf Cited 18 April 2018
- 7.Rhouma M, Beaudry F, Thériault W, Letellier A. Colistin in pig production: chemistry, mechanism of antibacterial action, microbial resistance emergence, and One Health perspectives. Front Microbiol. 2016;7 doi: 10.3389/fmicb.2016.01789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cannatelli A, Giani T, D’Andrea MM, Di Pilato V, Arena F, Conte V, et al. MgrB inactivation is a common mechanism of colistin resistance in KPC-producing Klebsiella pneumoniae of clinical origin. Antimicrob Agents Chemother. 2014;58: 5696–5703. doi: 10.1128/AAC.03110-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nordmann P, Jayol A, Poirel L. Rapid detection of polymyxin resistance in Enterobacteriaceae. Emerg Infect Dis. 2016;22: 1038–1043. doi: 10.3201/eid2206.151840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jayol A, Poirel L, Brink A, Villegas M-V, Yilmaz M, Nordmann P. Resistance to colistin associated with a single amino acid change in protein PmrB among Klebsiella pneumoniae isolates of worldwide origin. Antimicrob Agents Chemother. 2014;58: 4762–4766. doi: 10.1128/AAC.00084-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pecora ND, Li N, Allard M, Li C, Albano E, Delaney M, et al. Genomically informed surveillance for carbapenem-resistant Enterobacteriaceae in a health care system. mBio. 2015;6: e01030 doi: 10.1128/mBio.01030-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.CLSI. Performance Standards for Antimicrobial Susceptibility Testing; 26th informational supplement. CLSI document M100-S. Wayne, PA.; 2016.
- 13.Nordmann P, Gniadkowski M, Giske CG, Poirel L, Woodford N, Miriagou V, et al. Identification and screening of carbapenemase-producing Enterobacteriaceae. Clin Microbiol Infect 2012;18: 432–438. doi: 10.1111/j.1469-0691.2012.03815.x [DOI] [PubMed] [Google Scholar]
- 14.CLSI. Performance standards for antimicrobial susceptibility testing; 27th informational supplement. CLSI document M100-S. Wayne, PA.; 2017.
- 15.Robinson TP, Bu DP, Carrique-Mas J, Fèvre EM, Gilbert M, Grace D, et al. Antibiotic resistance is the quintessential One Health issue. Trans R Soc Trop Med Hyg. 2016;110: 377–380. doi: 10.1093/trstmh/trw048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Crawford MA, Fisher DJ, Leung LM, Lomonaco S, Lascols C, Cannatelli A, et al. CXC chemokines exhibit bactericidal activity against multidrug-resistant Gram-negative pathogens. Bush K, editor. mBio. 2017;8: e01549–17. doi: 10.1128/mBio.01549-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.CLSI. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 10th Edition. CLSI document M07. Wayne, PA.; 2015.
- 18.EUCAST. Breakpoint tables for interpretation of MICs and zone diameters, version 6.0. 2016. http://www.eucast.org/clinical_breakpoints/ Cited 18 April 2018
- 19.Crawford MA, Timme R, Lomonaco S, Lascols C, Fisher DJ, Sharma SK, et al. Genome sequences of multidrug-resistant, colistin-susceptible and -resistant Klebsiella pneumoniae clinical isolates from Pakistan. Genome Announc. 2016;4 doi: 10.1128/genomeA.01419-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Siguier P, Perochon J, Lestrade L, Mahillon J, Chandler M. ISfinder: the reference centre for bacterial insertion sequences. Nucleic Acids Res. 2006;34: D32–36. doi: 10.1093/nar/gkj014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moura A, Soares M, Pereira C, Leitão N, Henriques I, Correia A. INTEGRALL: a database and search engine for integrons, integrases and gene cassettes. Bioinforma Oxf Engl. 2009;25: 1096–1098. doi: 10.1093/bioinformatics/btp105 [DOI] [PubMed] [Google Scholar]
- 22.Choi Y, Chan AP. PROVEAN web server: a tool to predict the functional effect of amino acid substitutions and indels. Bioinformatics. 2015;31: 2745–2747. doi: 10.1093/bioinformatics/btv195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Richter M, Rosselló-Móra R, Oliver Glöckner F, Peplies J. JSpeciesWS: a web server for prokaryotic species circumscription based on pairwise genome comparison. Bioinforma Oxf Engl. 2016;32: 929–931. doi: 10.1093/bioinformatics/btv681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Darling AE, Mau B, Perna NT. progressiveMauve: multiple genome alignment with gene gain, loss and rearrangement. PLoS ONE. 2010;5: e11147 doi: 10.1371/journal.pone.0011147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arndt D, Grant JR, Marcu A, Sajed T, Pon A, Liang Y, et al. PHASTER: a better, faster version of the PHAST phage search tool. Nucleic Acids Res. 2016;44: W16–21. doi: 10.1093/nar/gkw387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brisse S, Passet V, Haugaard AB, Babosan A, Kassis-Chikhani N, Struve C, et al. wzi gene sequencing, a rapid method for determination of capsular type for Klebsiella strains. J Clin Microbiol. 2013;51: 4073–4078. doi: 10.1128/JCM.01924-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carattoli A, Zankari E, García-Fernández A, Voldby Larsen M, Lund O, Villa L, et al. In silico detection and typing of plasmids using PlasmidFinder and plasmid multilocus sequence typing. Antimicrob Agents Chemother. 2014;58: 3895–3903. doi: 10.1128/AAC.02412-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zankari E, Hasman H, Cosentino S, Vestergaard M, Rasmussen S, Lund O, et al. Identification of acquired antimicrobial resistance genes. J Antimicrob Chemother. 2012;67: 2640–2644. doi: 10.1093/jac/dks261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jia B, Raphenya AR, Alcock B, Waglechner N, Guo P, Tsang KK, et al. CARD 2017: expansion and model-centric curation of the comprehensive antibiotic resistance database. Nucleic Acids Res. 2017;45: D566–D573. doi: 10.1093/nar/gkw1004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zankari E, Allesøe R, Joensen KG, Cavaco LM, Lund O, Aarestrup FM. PointFinder: a novel web tool for WGS-based detection of antimicrobial resistance associated with chromosomal point mutations in bacterial pathogens. J Antimicrob Chemother. 2017;72: 2764–2768. doi: 10.1093/jac/dkx217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Broberg CA, Wu W, Cavalcoli JD, Miller VL, Bachman MA. Complete genome sequence of Klebsiella pneumoniae strain ATCC 43816 KPPR1, a rifampin-resistant mutant commonly used in animal, genetic, and molecular biology studies. Genome Announc. 2014;2 doi: 10.1128/genomeA.00924-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lin W-H, Zheng P-X, Liu T, Tseng C-C, Chen W-C, Wang M-C, et al. Complete genome sequence of community-acquired Klebsiella pneumoniae KP36, a strain isolated from a patient with an upper urinary tract infection. Genome Announc. 2016;4 doi: 10.1128/genomeA.01403-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.de Man TJB, Lutgring JD, Lonsway DR, Anderson KF, Kiehlbauch JA, Chen L, et al. Genomic analysis of a pan-resistant isolate of Klebsiella pneumoniae, United States 2016. Jacoby GA, editor. mBio. 2018;9: e00440–18. doi: 10.1128/mBio.00440-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu Y-Y, Wang Y, Walsh TR, Yi L-X, Zhang R, Spencer J, et al. Emergence of plasmid-mediated colistin resistance mechanism mcr-1 in animals and human beings in China: a microbiological and molecular biological study. Lancet Infect Dis. 2016;16: 161–168. doi: 10.1016/S1473-3099(15)00424-7 [DOI] [PubMed] [Google Scholar]
- 35.Center for Disease Dynamics, Economics & Policy. State of the World’s Antibiotics, 2015. CDDEP: Washington, D.C.; 2015. https://cddep.org/publications/state_worlds_antibiotics_2015/ Cited 18 April 2018
- 36.Fakhuruddin null, Durrani MA, Ahmed R, Kumar M, Bakar I. Frequency of class B carbapenemases (MbetaL) in Enterobacteriacae. JPMA J Pak Med Assoc. 2014;64: 519–523. [PubMed] [Google Scholar]
- 37.Kim M, Kim S, Ryu S. Complete genome sequence of bacteriophage SSU5 specific for Salmonella enterica serovar Typhimurium rough strains. J Virol. 2012;86: 10894–10894. doi: 10.1128/JVI.01796-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grose JH, Casjens SR. Understanding the enormous diversity of bacteriophages: The tailed phages that infect the bacterial family Enterobacteriaceae. Virology. 2014;468–470: 421–443. doi: 10.1016/j.virol.2014.08.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Arena F, Giani T, Becucci E, Conte V, Zanelli G, D’Andrea MM, et al. Large oligoclonal outbreak due to Klebsiella pneumoniae ST14 and ST26 producing the FOX-7 AmpC β-lactamase in a neonatal intensive care unit. J Clin Microbiol. 2013;51: 4067–4072. doi: 10.1128/JCM.01982-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mshana SE, Hain T, Domann E, Lyamuya EF, Chakraborty T, Imirzalioglu C. Predominance of Klebsiella pneumoniae ST14 carrying CTX-M-15 causing neonatal sepsis in Tanzania. BMC Infect Dis. 2013;13: 466 doi: 10.1186/1471-2334-13-466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Garcia-Fulgueiras V, Araujo L, Bado I, Cordeiro NF, Mota MI, Laguna G, et al. Allodemic distribution of plasmids co-harbouring blaCTX-M-15/aac(6′)-Ib -cr/qnrB in Klebsiella pneumoniae is the main source of extended-spectrum β-lactamases in Uruguay’s paediatric hospital. J Glob Antimicrob Resist. 2017;9: 68–73. doi: 10.1016/j.jgar.2017.01.008 [DOI] [PubMed] [Google Scholar]
- 42.Ocampo AM, Chen L, Cienfuegos AV, Roncancio G, Chavda KD, Kreiswirth BN, et al. A two-year surveillance in five colombian tertiary care hospitals reveals high frequency of non-CG258 clones of carbapenem-resistant Klebsiella pneumoniae with distinct clinical characteristics. Antimicrob Agents Chemother. 2016;60: 332–342. doi: 10.1128/AAC.01775-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Markovska R, Stoeva T, Schneider I, Boyanova L, Popova V, Dacheva D, et al. Clonal dissemination of multilocus sequence type ST15 KPC-2-producing Klebsiella pneumoniae in Bulgaria. APMIS Acta Pathol Microbiol Immunol Scand. 2015;123: 887–894. doi: 10.1111/apm.12433 [DOI] [PubMed] [Google Scholar]
- 44.Villa L, Feudi C, Fortini D, Iacono M, Bonura C, Endimiani A, et al. Complete genome sequence of KPC-3- and CTX-M-15-Producing Klebsiella pneumoniae Sequence Type 307. Genome Announc. 2016;4: e00213–16. doi: 10.1128/genomeA.00213-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Long SW, Olsen RJ, Eagar TN, Beres SB, Zhao P, Davis JJ, et al. Population genomic analysis of 1,777 extended-spectrum beta-lactamase-producing Klebsiella pneumoniae isolates, Houston, Texas: unexpected abundance of Clonal Group 307. mBio. 2017;8 doi: 10.1128/mBio.00489-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Habeeb MA, Haque A, Nematzadeh S, Iversen A, Giske CG. High prevalence of 16S rRNA methylase RmtB among CTX-M extended-spectrum β-lactamase-producing Klebsiella pneumoniae from Islamabad, Pakistan. Int J Antimicrob Agents. 2013;41: 524–526. doi: 10.1016/j.ijantimicag.2013.02.017 [DOI] [PubMed] [Google Scholar]
- 47.Castanheira M, Farrell SE, Wanger A, Rolston KV, Jones RN, Mendes RE. Rapid expansion of KPC-2-producing Klebsiella pneumoniae isolates in two Texas hospitals due to clonal spread of ST258 and ST307 lineages. Microb Drug Resist Larchmt N. 2013;19: 295–297. doi: 10.1089/mdr.2012.0238 [DOI] [PubMed] [Google Scholar]
- 48.Girlich D, Bouihat N, Poirel L, Benouda A, Nordmann P. High rate of faecal carriage of extended-spectrum β-lactamase and OXA-48 carbapenemase-producing Enterobacteriaceae at a University hospital in Morocco. Clin Microbiol Infect. 2014;20: 350–354. doi: 10.1111/1469-0691.12325 [DOI] [PubMed] [Google Scholar]
- 49.Novović K, Trudić A, Brkić S, Vasiljević Z, Kojić M, Medić D, et al. Molecular epidemiology of colistin-resistant, carbapenemase-producing Klebsiella pneumoniae, Serbia, 2013–2016. Antimicrob Agents Chemother. 2017; doi: 10.1128/AAC.02550-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim JO, Song SA, Yoon E-J, Shin JH, Lee H, Jeong SH, et al. Outbreak of KPC-2-producing Enterobacteriaceae caused by clonal dissemination of Klebsiella pneumoniae ST307 carrying an IncX3-type plasmid harboring a truncated Tn4401a. Diagn Microbiol Infect Dis. 2017;87: 343–348. doi: 10.1016/j.diagmicrobio.2016.12.012 [DOI] [PubMed] [Google Scholar]
- 51.Czobor I, Novais Â, Rodrigues C, Chifiriuc MC, Mihăescu G, Lazăr V, et al. Efficient transmission of IncFIIY and IncL plasmids and Klebsiella pneumoniae ST101 clone producing OXA-48, NDM-1 or OXA-181 in Bucharest hospitals. Int J Antimicrob Agents. 2016;48: 223–224. doi: 10.1016/j.ijantimicag.2016.05.004 [DOI] [PubMed] [Google Scholar]
- 52.Hashimoto A, Nagamatsu M, Ohmagari N, Hayakawa K, Kato Y, Kirikae T. Isolation of OXA-48 carbapenemase-producing Klebsiella pneumoniae ST101 from an overseas traveler returning to Japan. Jpn J Infect Dis. 2014;67: 120–121. [DOI] [PubMed] [Google Scholar]
- 53.Oteo J, Pérez-Vázquez M, Bautista V, Ortega A, Zamarrón P, Saez D, et al. The spread of KPC-producing Enterobacteriaceae in Spain: WGS analysis of the emerging high-risk clones of Klebsiella pneumoniae ST11/KPC-2, ST101/KPC-2 and ST512/KPC-3. J Antimicrob Chemother. 2016;71: 3392–3399. doi: 10.1093/jac/dkw321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Loucif L, Kassah-Laouar A, Saidi M, Messala A, Chelaghma W, Rolain J-M. Outbreak of OXA-48-producing Klebsiella pneumoniae involving a sequence type 101 clone in Batna University Hospital, Algeria. Antimicrob Agents Chemother. 2016;60: 7494–7497. doi: 10.1128/AAC.00525-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cheng Y-H, Lin T-L, Pan Y-J, Wang Y-P, Lin Y-T, Wang J-T. Colistin resistance mechanisms in Klebsiella pneumoniae strains from Taiwan. Antimicrob Agents Chemother. 2015;59: 2909–2913. doi: 10.1128/AAC.04763-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Aires CAM, Pereira PS, Asensi MD, Carvalho-Assef APD. mgrB mutations mediating polymyxin B resistance in Klebsiella pneumoniae isolates from rectal surveillance swabs in Brazil. Antimicrob Agents Chemother. 2016;60: 6969–6972. doi: 10.1128/AAC.01456-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Haeili M, Javani A, Moradi J, Jafari Z, Feizabadi MM, Babaei E. MgrB alterations Mediate colistin resistance in Klebsiella pneumoniae isolates from Iran. Front Microbiol. 2017;8: 2470 doi: 10.3389/fmicb.2017.02470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang X, Liu Y, Qi X, Wang R, Jin L, Zhao M, et al. Molecular epidemiology of colistin-resistant Enterobacteriaceae in inpatient and avian isolates from China: high prevalence of mcr-negative Klebsiella pneumoniae. Int J Antimicrob Agents. 2017;50: 536–541. doi: 10.1016/j.ijantimicag.2017.05.009 [DOI] [PubMed] [Google Scholar]
- 59.Pragasam AK, Shankar C, Veeraraghavan B, Biswas I, Nabarro LEB, Inbanathan FY, et al. Molecular mechanisms of colistin resistance in Klebsiella pneumoniae causing bacteremia from India—a first report. Front Microbiol. 2016;7: 2135 doi: 10.3389/fmicb.2016.02135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lees JA, Vehkala M, Välimäki N, Harris SR, Chewapreecha C, Croucher NJ, et al. Sequence element enrichment analysis to determine the genetic basis of bacterial phenotypes. Nat Commun. 2016;7: 12797 doi: 10.1038/ncomms12797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Liu Y, Liu P, Wang L, Wei D, Wan L-G, Zhang W. Capsular polysaccharide types and virulence-related traits of epidemic KPC-producing Klebsiella pneumoniae isolates in a Chinese University Hospital. Microb Drug Resist. 2017; doi: 10.1089/mdr.2016.0222 [DOI] [PubMed] [Google Scholar]
- 62.Zhou K, Lokate M, Deurenberg RH, Tepper M, Arends JP, Raangs EGC, et al. Use of whole-genome sequencing to trace, control and characterize the regional expansion of extended-spectrum β-lactamase producing ST15 Klebsiella pneumoniae. Sci Rep. 2016;6 doi: 10.1038/srep20840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Andrade LN, Curiao T, Ferreira JC, Longo JM, Climaco EC, Martinez R, et al. Dissemination of blaKPC-2 by the spread of Klebsiella pneumoniae clonal complex 258 clones (ST258, ST11, ST437) and plasmids (IncFII, IncN, IncL/M) among Enterobacteriaceae species in Brazil. Antimicrob Agents Chemother. 2011;55: 3579–3583. doi: 10.1128/AAC.01783-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zahedi Bialvaei A, Samadi Kafil H, Ebrahimzadeh Leylabadlo H, Asgharzadeh M, Aghazadeh M. Dissemination of carbapenemases producing Gram negative bacteria in the Middle East. Iran J Microbiol. 2015;7: 226–246. [PMC free article] [PubMed] [Google Scholar]
- 65.Pesesky MW, Hussain T, Wallace M, Wang B, Andleeb S, Burnham C-AD, et al. KPC and NDM-1 genes in related Enterobacteriaceae strains and plasmids from Pakistan and the United States. Emerg Infect Dis. 2015;21: 1034–1037. doi: 10.3201/eid2106.141504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Barguigua A, El otmani F, Lakbakbi el yaagoubi F, Talmi M, Zerouali K, Timinouni M. First report of a Klebsiella pneumoniae strain coproducing NDM-1, VIM-1 and OXA-48 carbapenemases isolated in Morocco. APMIS. 2013;121: 675–677. doi: 10.1111/apm.12034 [DOI] [PubMed] [Google Scholar]
- 67.Ben Nasr A, Decré D, Compain F, Genel N, Barguellil F, Arlet G. Emergence of NDM-1 in association with OXA-48 in Klebsiella pneumoniae from Tunisia. Antimicrob Agents Chemother. 2013;57: 4089–4090. doi: 10.1128/AAC.00536-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sonnevend Á, Ghazawi A, Hashmey R, Haidermota A, Girgis S, Alfaresi M, et al. Multihospital occurrence of pan-resistant Klebsiella pneumoniae sequence type 147 with an IS Ecp1 -directed blaOXA-181 insertion in the mgrB gene in the United Arab Emirates. Antimicrob Agents Chemother. 2017;61: e00418–17. doi: 10.1128/AAC.00418-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dash N, Panigrahi D, Zarouni MA, Darwish D, Ghazawi A, Sonnevend A, et al. High incidence of New Delhi metallo-beta-lactamase-producing Klebsiella pneumoniae isolates in Sharjah, United Arab Emirates. Microb Drug Resist. 2014;20: 52–56. doi: 10.1089/mdr.2013.0040 [DOI] [PubMed] [Google Scholar]
- 70.Seiffert SN, Marschall J, Perreten V, Carattoli A, Furrer H, Endimiani A. Emergence of Klebsiella pneumoniae co-producing NDM-1, OXA-48, CTX-M-15, CMY-16, QnrA and ArmA in Switzerland. Int J Antimicrob Agents. 2014;44: 260–262. doi: 10.1016/j.ijantimicag.2014.05.008 [DOI] [PubMed] [Google Scholar]
- 71.Sidjabat HE, Townell N, Nimmo GR, George NM, Robson J, Vohra R, et al. Dominance of IMP-4-producing Enterobacter cloacae among carbapenemase-producing Enterobacteriaceae in Australia. Antimicrob Agents Chemother. 2015;59: 4059–4066. doi: 10.1128/AAC.04378-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kilic A, Baysallar M. The first Klebsiella pneumoniae isolate co-producing OXA-48 and NDM-1 in Turkey. Ann Lab Med. 2015;35: 382 doi: 10.3343/alm.2015.35.3.382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Solgi H, Badmasti F, Aminzadeh Z, Giske CG, Pourahmad M, Vaziri F, et al. Molecular characterization of intestinal carriage of carbapenem-resistant Enterobacteriaceae among inpatients at two Iranian university hospitals: first report of co-production of blaNDM-7 and blaOXA-48. Eur J Clin Microbiol Infect Dis. 2017;36: 2127–2135. doi: 10.1007/s10096-017-3035-3 [DOI] [PubMed] [Google Scholar]
- 74.Cizmeci Z, Aktas E, Otlu B, Acikgoz O, Ordekci S. Molecular characterization of carbapenem- resistant Enterobacteriaceae yields increasing rates of NDM-1 carbapenemases and colistin resistance in an OXA-48- endemic area. J Chemother. 2017;29: 344–350. doi: 10.1080/1120009X.2017.1323149 [DOI] [PubMed] [Google Scholar]
- 75.Samuelsen Ø, Overballe-Petersen S, Bjørnholt JV, Brisse S, Doumith M, Woodford N, et al. Molecular and epidemiological characterization of carbapenemase-producing Enterobacteriaceae in Norway, 2007 to 2014. Butaye P, editor. PLOS ONE. 2017;12: e0187832 doi: 10.1371/journal.pone.0187832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shaw E, Rombauts A, Tubau F, Padullés A, Càmara J, Lozano T, et al. Clinical outcomes after combination treatment with ceftazidime/avibactam and aztreonam for NDM-1/OXA-48/CTX-M-15-producing Klebsiella pneumoniae infection. J Antimicrob Chemother. 2017; doi: 10.1093/jac/dkx496 [DOI] [PubMed] [Google Scholar]
- 77.Guducuoglu H, Gursoy NC, Yakupogullari Y, Parlak M, Karasin G, Sunnetcioglu M, et al. Hospital outbreak of a colistin-resistant, NDM-1- and OXA-48-producing Klebsiella pneumoniae: high mortality from pandrug resistance. Microb Drug Resist. 2017; doi: 10.1089/mdr.2017.0173 [DOI] [PubMed] [Google Scholar]
- 78.Uwaezuoke NS, Kieffer N, Iregbu KC, Nordmann P. First report of OXA-181 and NDM-1 from a clinical Klebsiella pneumoniae isolate from Nigeria. Int J Infect Dis. 2017;61: 1–2. doi: 10.1016/j.ijid.2017.05.004 [DOI] [PubMed] [Google Scholar]
- 79.Székely E, Damjanova I, Jánvári L, Vas KE, Molnár S, Bilca DV, et al. First description of blaNDM-1, blaOXA-48, blaOXA-181 producing Enterobacteriaceae strains in Romania. Int J Med Microbiol. 2013;303: 697–700. doi: 10.1016/j.ijmm.2013.10.001 [DOI] [PubMed] [Google Scholar]
- 80.Xie L, Dou Y, Zhou K, Chen Y, Han L, Guo X, et al. Coexistence of blaOXA-48 and truncated blaNDM-1 on different plasmids in a Klebsiella pneumoniae isolate in China. Front Microbiol. 2017;8 doi: 10.3389/fmicb.2017.00133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Avolio M, Vignaroli C, Crapis M, Camporese A. Co-production of NDM-1 and OXA-232 by ST16 Klebsiella pneumoniae, Italy, 2016. Future Microbiol. 2017;12: 1119–1122. doi: 10.2217/fmb-2017-0041 [DOI] [PubMed] [Google Scholar]
- 82.Mairi A, Pantel A, Sotto A, Lavigne J-P, Touati A. OXA-48-like carbapenemases producing Enterobacteriaceae in different niches. Eur J Clin Microbiol Infect Dis 2017; doi: 10.1007/s10096-017-3112-7 [DOI] [PubMed] [Google Scholar]
- 83.Khan AU, Maryam L, Zarrilli R. Structure, Genetics and worldwide spread of New Delhi metallo-β-lactamase (NDM): a threat to public health. BMC Microbiol. 2017;17: 101 doi: 10.1186/s12866-017-1012-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sepehri S, Poliquin G, Alfattoh N, Boyd D, Mulvey M, Denisuik A, et al. Osteomyelitis due to multiple carbapenemase-producing Gram-negative bacteria: The first case report of a GES-13-producing Pseudomonas aeruginosa isolate in Canada. Can J Infect Dis Med Microbiol. 2014;25: 229–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Dortet L, Poirel L, Nordmann P. Worldwide dissemination of the NDM-type carbapenemases in Gram-negative bacteria. BioMed Res Int. 2014;2014: 249856 doi: 10.1155/2014/249856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chen Y-T, Siu LK, Tsai Y-K, Lin F-M, Koh TH, Chen J-H. A common flanking region in promiscuous plasmids encoding blaNDM-1 in Klebsiella pneumoniae isolated in Singapore. Microb Drug Resist. 2016;22: 109–114. doi: 10.1089/mdr.2015.0132 [DOI] [PubMed] [Google Scholar]
- 87.He S, Hickman AB, Varani AM, Siguier P, Chandler M, Dekker JP, et al. Insertion sequence IS 26 reorganizes plasmids in clinically isolated multidrug-resistant bacteria by replicative transposition. mBio. 2015;6: e00762–15. doi: 10.1128/mBio.00762-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chen Y-T, Lin A-C, Siu LK, Koh TH. Sequence of closely related plasmids encoding blaNDM-1 in two unrelated Klebsiella pneumoniae isolates in Singapore. Chang Y-F, editor. PLoS ONE. 2012;7: e48737 doi: 10.1371/journal.pone.0048737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Duvernay C, Coulange L, Dutilh B, Dubois V, Quentin C, Arpin C. Duplication of the chromosomal blaSHV-11 gene in a clinical hypermutable strain of Klebsiella pneumoniae. Microbiol Read Engl. 2011;157: 496–503. doi: 10.1099/mic.0.043885-0 [DOI] [PubMed] [Google Scholar]
- 90.DSouza R, Pinto NA, Hwang I, Younjee H, Cho Y, Kim H, et al. Molecular epidemiology and resistome analysis of multidrug-resistant ST11 Klebsiella pneumoniae strain containing multiple copies of extended-spectrum β-lactamase genes using whole-genome sequencing. New Microbiol. 2017;40: 38–44. [PubMed] [Google Scholar]
- 91.Magi A, Tattini L, Pippucci T, Torricelli F, Benelli M. Read count approach for DNA copy number variants detection. Bioinformatics. 2012;28: 470–478. doi: 10.1093/bioinformatics/btr707 [DOI] [PubMed] [Google Scholar]
- 92.Allard MW, Strain E, Melka D, Bunning K, Musser SM, Brown EW, et al. Practical value of food pathogen traceability through building a whole-genome sequencing network and database. J Clin Microbiol. 2016;54: 1975–1983. doi: 10.1128/JCM.00081-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Susceptibility or resistance to colistin is indicated with S or R, respectively. The genome sequence for K. pneumoniae HS11286 was used as a reference (GenBank assembly accession GCA_000240185.2).
(XLSX)
The tool identified 1 incomplete, 2 questionable, and 2 complete phages.
(XLSX)
Data Availability Statement
The genome assemblies described in this manuscript are available in DDBJ/ENBL/GenBank under the accession numbers MAGC00000000 (CFSAN044573), MAGE00000000 (CFSAN044569), MAGF00000000 (CFSAN044570), MAGG00000000 (CFSAN044571), MAGH00000000 (CFSAN044572), MAGI00000000 (CFSAN044568), MAGJ00000000 (CFSAN044563), MAGK00000000 (CFSAN044564), MAGL00000000 (CFSAN044565), and MAGM00000000 (CFSAN044566). The complete tree file from NCBI Pathogen detection (PDG000000012.284.reference_target.tree.asn—as of March 24th, 2018), can be downloaded at https://doi.org/10.6084/m9.figshare.5708347.v2 and can be opened with NCBI software Genome Workbench (https://www.ncbi.nlm.nih.gov/tools/gbench/).