Abstract
In order to predict which species can successfully cope with global warming and how other environmental stressors modulate their vulnerability to climate‐related environmental factors, an understanding of the ecophysiology underpinning thermal limits is essential for both conservation biology and invasion biology.
Heat tolerance and the extent to which heat tolerance differed with oxygen availability were examined for four native and four alien freshwater peracarid crustacean species, with differences in habitat use across species. Three hypotheses were tested: (1) Heat and lack of oxygen synergistically reduce survival of species; (2) patterns in heat tolerance and the modulation thereof by oxygen differ between alien and native species and between species with different habitat use; (3) small animals can better tolerate heat than large animals, and this difference is more pronounced under hypoxia.
To assess heat tolerances under different oxygen levels, animal survival was monitored in experimental chambers in which the water temperature was ramped up (0.25°C min−1). Heat tolerance (CTmax) was scored as the cessation of all pleopod movement, and heating trials were performed under hypoxia (5 kPa oxygen), normoxia (20 kPa) and hyperoxia (60 kPa).
Heat tolerance differed across species as did the extent by which heat tolerance was affected by oxygen conditions. Heat‐tolerant species, for example, Asellus aquaticus and Crangonyx pseudogracilis, showed little response to oxygen conditions in their CTmax, whereas the CTmax of heat‐sensitive species, for example, Dikerogammarus villosus and Gammarus fossarum, was more plastic, being increased by hyperoxia and reduced by hypoxia.
In contrast to other studies on crustaceans, alien species were not more heat‐tolerant than native species. Instead, differences in heat tolerance were best explained by habitat use, with species from standing waters being heat tolerant and species from running waters being heat sensitive. In addition, larger animals displayed lower critical maximum temperature, but only under hypoxia. An analysis of data available in the literature on metabolic responses of the study species to temperature and oxygen conditions suggests that oxygen conformers and species whose oxygen demand rapidly increases with temperature (low activation energy) may be more heat sensitive.
The alien species D. villosus appeared most susceptible to hypoxia and heat stress. This may explain why this species is very successful in colonizing new areas in littoral zones with rocky substrate which are well aerated due to continuous wave action generated by passing ships or prevailing winds. This species is less capable of spreading to other waters which are poorly oxygenated and where C. pseudogracilis is the more likely dominant alien species.
A http://onlinelibrary.wiley.com/doi/10.1111/1365-2435.13050/suppinfo is available for this article.
Keywords: amphipods, global warming, hypoxia, invasive species, isopods, pollution
1. INTRODUCTION
Freshwater animals face new thermal challenges owing to climate change, local discharge of cooling water and to globalization resulting in redistribution and invasions of species into habitats with a different thermal regime. Patterns in the geographical distribution of aquatic ectotherms have been linked to their thermal tolerance and hypoxia tolerance (e.g. Calosi, Bilton, Spicer, Votier, & Atfield, 2010; Deutsch, Ferrel, Seibel, Pörtner, & Huey, 2015). Increasing our understanding of the physiological mechanisms underlying thermal responses is essential for both conservation biology and invasion biology as it enables us to better predict which species can successfully cope with global warming and how other environmental stressors modulate their responses to changing thermal regimes of their habitat (Chown, 2012; Huey et al., 2012; Verberk & Bilton, 2013). With increasing temperature, the demand for oxygen increases more than the rate at which oxygen can be supplied, due to capacity limitations (Fry & Hart, 1948; Pörtner, 2010; Verberk, Bilton, Calosi, & Spicer, 2011; Winterstein, 1905). Consequently, oxygen could become limiting, constraining aerobic energy metabolism necessary for reproduction, growth, and physical activities, including predator avoidance, feeding, and locomotion (Pörtner, 2010). However, there is an ongoing debate on the universality of this oxygen‐ and capacity‐limited thermal tolerance (OCLTT) hypothesis and the validity of its assumptions (e.g. Ern et al., 2015; Jutfelt et al., 2014; Klok, Sinclair, & Chown, 2004; Pörtner, 2014; Verberk, Overgaard, et al., 2016).
The problem of insufficient oxygen under warm conditions may be more immediate in water than in air as aquatic gas exchange is challenging due to the lower rate of oxygen diffusion in water and the larger effort required for ventilation as water has a higher density and viscosity (Verberk & Atkinson, 2013). Similarly, this challenge of breathing underwater is thought to explain the recurrent evolution of air breathing in crustaceans from tropical waters that may be more prone to severe hypoxia (Fusi et al., 2016; Giomi et al., 2014). Moreover, oxygen availability is more variable in an aquatic setting, declining at night and increasing during the day by primary producers, and options for thermoregulation to adaptively modulate body temperature are limited. Consequently, interactions between warming, dissolved oxygen concentrations and organic pollution may be relevant especially in aquatic systems (Diaz & Rosenberg, 2008; Meire, Soetaert, & Meysman, 2013; Moran & Woods, 2010; Posch, Köster, Salcher, & Pernthaler, 2012; Verberk, Durance, Vaughan, & Ormerod, 2016).
In freshwater ecosystems, amphipod and isopod crustaceans can be highly abundant, are crucial for the decomposition of organic matter, show an omnivorous feeding pattern and are themselves important food sources for fish and invertebrate predators, thus being important actors in aquatic food webs (Väinölä et al., 2008; Wallace & Webster, 1996). Freshwater amphipods and isopods inhabit a wide range of standing and running water bodies, characterized by various thermal regimes and oxygen conditions. Running waters such as rivers and streams exhibit on average lower temperatures and higher oxygen contents than standing waters such as ponds, reservoirs or stratified lakes (Wetzel, 2001). Hence, species using different habitats are hypothesized to differ in their susceptibility to hypoxia and warming. Similarly, the origin of a species, that is, native or alien, may influence the thermal tolerances under different oxygen conditions. Alien species may have experienced low or changing oxygen conditions during the invasion process, for example, during ballast water transport or crossing different water bodies, explaining why species that successfully spread outside their native range could better cope with hypoxia and heat (Bates et al., 2013; Jewett, Hines, & Ruiz, 2005; Lenz et al., 2011). Finally, it has been suggested that large‐bodied animals may be more prone to oxygen limitation (Chapelle & Peck, 1999; Verberk & Bilton, 2011), explaining why warming may benefit smaller animals (e.g. Daufresne, Lengfellner, & Sommer, 2009). However, size dependency of oxygen limitation has been difficult to demonstrate in arthropods, for example, in aquatic pycnogonids (Woods, Moran, Arango, Mullen, & Shields, 2009) and terrestrial insects (Harrison, Klok, & Waters, 2014).
Understanding if and how oxygen contents affect upper thermal limits will improve our ability to predict the susceptibility of ectotherm species to global warming (Chown, 2012) and improve our predictions of invasion risk associated with alien species (Bates et al., 2013), many of which are crustaceans (Leuven et al., 2009; Van der Velde, Rajagopal, Kelleher, Muskó, & Bij de Vaate, 2000). However, few studies have focussed on patterns of heat tolerance in relation to oxygen limitation in aquatic crustaceans (Verberk, Overgaard, et al., 2016), and available studies have yielded mixed outcomes (Ern et al., 2015; Frederich & Pörtner, 2000). Here, we investigate heat tolerance and the extent to which heat tolerance is modulated by dissolved oxygen content in eight species of freshwater peracarid crustaceans, two preferring running waters, two preferring standing waters and four species occurring in both habitats. Half of the species of each group were native and the other half alien. This experimental setup allows us to test whether differences across species in habitat use, origin or both are associated to differences in heat tolerance and whether patterns in heat tolerance vary with oxygen content. We hypothesize that (1) heat and lack of oxygen synergistically reduce survival of species; (2) oxygen conditions have a stronger influence on the thermal tolerance of native species than alien species and on the thermal tolerance of those that prefer running rather than standing waters; and (3) small animals better tolerate heat compared with large animals and that this difference is more pronounced when oxygen is most limiting (i.e. under hypoxic conditions). We also tested whether differences in thermal tolerance between our study species were related to their oxygen demand (e.g. Verberk & Bilton, 2011), by collating published data on oxygen consumption rates in response to temperature and ambient oxygen conditions.
2. MATERIALS AND METHODS
2.1. Animal collection and maintenance
Heat tolerance was assessed for four native and four alien species of peracarid Crustacea, which were collected in The Netherlands and Germany. Individuals of the native Asellus aquaticus (Linnaeus, 1758) were sampled from a pond in the city of Nijmegen (N51°49′13″, E5°52′28″), Gammarus fossarum Koch, in Panzer, 1835 from a brooklet (N51°49′24″, E5°56′32″), Gammarus pulex (Linnaeus, 1758) from a ditch (N51°55′18″, E6°40′47″) and Gammarus roeselii Gervais, 1835 from a lowland brook (N51°44′17″, E5°57′19″). The alien Crangonyx pseudogracilis Bousfield, 1958 was collected from a ditch in the vicinity of Lienden (N51°55′46″, E5°31′41″), individuals of Dikerogammarus villosus (Sowinsky, 1894) were collected from the River Waal (N51°51′22″, E5°52′55″), individuals of Echinogammarus berilloni (Catta, 1878) from a smaller river near the city of Hamm (North Western Germany (N51°37′55″, E7°55′57), and Gammarus tigrinus Sexton, 1939 from the Zijkanaal C of the Noordzeekanaal near Spaarndam (N52°25′13″, E4°41′42″) in March and August 2014. In addition to their origin (native vs. alien), species also differed in their preferred habitat. A. aquaticus and C. pseudogracilis are more often found in standing waters, whereas D. villosus and G. fossarum prefer running waters. E. berilloni, G. pulex, G. roeselii, and G. tigrinus are found in both types of habitat (Eggers & Martens, 2001; Verberk, Verdonschot, Van Haaren, & Van Maanen, 2012; Wijnhoven, Van Riel, & Van der Velde, 2003). Individuals were maintained in the laboratory at 10°C in Dutch standard water (demineralized water with 0.20 g/L CaCl2·2H2O, 0.18 g/L MgSO4·7H2O, 0.10 g/L NaHCO3, and 0.02 g/L KHCO3; pH = 7.9 ± 0.1 and conductivity = 576 ± 44 μS/cm; NEN6503, 1980) at a 12 L:12 D light regime and fed with living chironomid larvae ad libitum. Before recording critical temperatures, all animals were acclimated for at least 7 days to reduce variability in thermal history (Terblanche, Deere, Clusella‐Trullas, Janion, & Chown, 2007). Animals were also gradually acclimated to Dutch Standard Water.
2.2. Heat tolerance trials
To assess the heat tolerances of the various species under various oxygen levels, animals were placed in five parallel flow‐through chambers (70 × 70 × 30 mm) using the experimental setup described in detail elsewhere (Verberk & Bilton, 2011). In these flow‐through chambers, we could manipulate water temperature by means of a Grant R5 water bath with a GP200 pump unit (Grant Instrument Ltd, Cambridge, UK) connected to the heat exchanger and oxygen conditions by bubbling nitrogen‐oxygen gas mixtures obtained with a WITT gas‐mixer KM 100‐3 MEM (1) (WITT‐Gasetechnik GmbH & Co KG, Salinger Feld, Germany). Flow rate per chamber was 0.016 L/sec, resulting in a refresh time of 9–10 s. The flow‐through chambers were enriched with artificial stones (made of white modelling clay) providing substrate for individuals to cling on to. After 1 h of acclimatization in the flow‐through chambers with Dutch Standard Water, the water temperature of 10°C was increased at 0.25°C min−1 by a water bath with a pump unit (Grant R5, GP200 pump, Grant Instrument Ltd, Cambridge, UK). Temperatures were logged using a HH806AU digital thermometer (Omega Engineering Inc., Stamford, CT, USA). Three different endpoints were noted. During heating, animals increased pleopod movement, until pleopod movement became irregular (first endpoint). Further heating resulted in pleopod movement faltering and the temperature when pleopod movement ceased for more than 2 s was taken as the second endpoint which was on average 1.7°C higher. Finally, all pleopod movement stopped, and the corresponding temperature was defined as the critical maximum temperature (CTmax). This temperature, on average 2.3°C higher, could be most consistently scored and exhibited the lowest variability. Therefore, we focused our analysis on this last endpoint, but note that all three endpoints where strongly related (GLMM using species as a random factor: F 1,229 > 67.71; p < .0001; conditional R 2 > .73). Temperatures were increased for a further 8 min (2°C) after the last individual stopped moving its pleopods to make sure that ceasing of pleopod movement was not transient. The pleopod movements of the exposed individuals were assessed visually. As the pleopods of A. aquaticus are ventrally situated, tilted mirrors were inserted in each flow‐through chamber to enable the observation of pleopod movements of this species.
Trials were performed under hypoxia (5 kPa Oxygen), normoxia (20 kPa) and hyperoxia (60 kPa). Temperature and oxygen contents were checked during the experiments via a HH806 AU digital Thermometer (Omega Engineering In., Stamford, CT, USA) and an optical Fibox 3 LCD‐trace oxygen meter (PreSens, Precision Sensing GmbH, Regensburg, Germany), respectively.
At the end of each warming trial, each individual was sexed and its biomass (wet weight after blotting the animals dry to remove adhering water) was determined. For the heat tolerance trials, only non‐gravid individuals were used as the occurrence of eggs may alter the pleopod movement frequency and oxygen requirements. Individuals differed in body mass and individual variation was larger across species than within species: A. aquaticus (32.8 ± 2.4 mg wet weight, M ± SE), C. pseudogracilis (8.7 ± 0.2 mg), D. villosus (62.9 ± 4.3 mg), E. berilloni (22.6 ± 1.6 mg), G. fossarum (22.1 ± 1.1 mg), G. pulex (50.2 ± 2.3 mg), G. roeselii (51.9 ± 2.8 mg) and G. tigrinus (11.7 ± 0.7 mg).
2.3. Published data on oxygen consumption rates
We collated data on oxygen consumption rates published in the scientific literature for our study species. Studies were included that reported oxygen consumption rates at different temperatures or at different levels of oxygen. We found 25 studies that reported such data for 6 out of 8 species (no oxygen consumption data were retrieved for C. pseudogracilis and E. berilloni).
To assess whether thermal responses in oxygen consumption rates differed across species, we calculated activation energy (Ea values), which express the thermal sensitivity of oxygen consumption. In most cases, one Ea value was calculated for each species in a given study, but some studies separately reported data for males and females or for different populations and allowed gender‐ or location‐specific calculations of Ea values. In total, 33 Ea values for six species were calculated. Whether animals were acclimated to the test temperature for at least 24 hr prior to measurements of their metabolic rate (acclimated response) or not (acute response) was also noted, as this has been shown to affect the thermal sensitivity (Seebacher, White, & Franklin, 2015).
To assess whether species differed in their respiratory responses when subject to hypoxia, we extracted the critical oxygen level (Pc) from figures and tables, yielding 23 values for four species (A. aquaticus, G. fossarum, G. pulex and G. roeselii) and expressed these as a percentage of oxygen levels at normoxia. The critical oxygen level marks the shift from oxygen regulation (whereby metabolic rate is independent of ambient oxygen levels) to oxygen conformation (whereby metabolic rate is largely dependent of ambient oxygen levels). Thus, oxygen regulators can maintain a constant oxygen consumption until the critical oxygen level, whereas oxygen conformers cannot. In practice, this distinction is rarely absolute, and even in oxygen regulators, oxygen consumption rates at the critical oxygen level may have dropped relative to the initial oxygen consumption rates at normoxia (e.g. Brodersen, Pedersen, Walker, & Jensen, 2008; Mueller & Seymour, 2011). Therefore, in addition to the critical oxygen level (Pc), we also noted the oxygen consumption rates at Pc (expressed as a % of those at normoxia). Temperatures employed during these measurements were also noted as respiratory responses when subject to hypoxia are temperature dependent (e.g. Ern, Norin, Gamperl, & Esbaugh, 2016).
2.4. Data analysis
To investigate the effects of oxygen content across all species, we constructed a linear mixed‐effects model with species as a random factor (model 1) and a similar model that additionally included body size and the interaction between body mass and oxygen conditions (testing whether potential effects of body mass were dependent on the oxygen conditions) (model 2). Additional models were used to test for an effect of species origin (native or alien) (model 3) and species habitat preference (running waters, indifferent, standing waters) (model 4). The statistical significance of the fixed effects was tested using likelihood ratio tests on models fitted with the function {lme} which were fitted using maximum likelihood. Marginal and conditional pseudo‐R 2 values were calculated using the function {r.squaredGLMM}, respectively, indicating the variance explained by fixed effects, and by both fixed and random effects, respectively. Contrasts were tested after a Bonferroni correction, using the function {testInteractions}.
To test whether effects of oxygen conditions varied between heat‐tolerant and heat‐sensitive species, we calculated the difference between the mean CTmax under hyperoxia and under hypoxia for each species. Next, we used linear regressions to relate this difference to their mean CTmax observed under normoxia across all eight species. The above calculations were performed in R (version 3.1.1) (R Core Team, 2013), using packages nlme (Pinheiro, Bates, DebRoy, & Sarkar, 2013), mumin (Barton, 2011) and phia (Martinez, 2015).
For each species, differences in CTmax among oxygen treatments were tested using ANOVA with Scheffé post hoc tests when normal distribution and homogeneity of variances were given. In G. fossarum, requirements were not met, and Mann–Whitney U‐tests with Bonferroni corrections were used. These calculations were performed with PASW (IBM SPSS Statistics, v 22, Chicago, USA).
To test for differences in thermal sensitivity of oxygen consumption (expressed as activation energy, Ea value) between species of different habitat preference or origin, we ran mixed effect models with literature source as a random factor. Preliminary analyses showed Ea values were not significantly influenced by either the thermal range (p = .631) over which Ea values were calculated, nor the type of response (acclimated or acute; p = .726), so we excluded these factors from the model. Therefore, the model included either habitat preference or origin to test for associated differences in thermal sensitivity of oxygen consumption.
We ran a linear model to test for differences in critical oxygen level (Pc) between species of different habitat preference. We also included the oxygen consumption rate at Pc, expressed as a percentage of the initial oxygen consumption rates at normoxia in our model as preliminary analysis showed that these were related to critical oxygen levels. To prevent overfitting, given the low number of data points (n = 23), simpler models were favoured over complex ones. For this reason, we excluded test temperature (F = 0.46; p = .517) and we did not include literature source as a random factor. Mixed effect models with species as a random factor were not deemed suitable, given that we had three categories of habitat preference and only had data on four species (i.e. 1–2 species for each category). Also, all four species were native of origin, preventing us from testing whether species origin had an effect on the respiratory responses when subject to hypoxia.
3. RESULTS
Critical thermal maxima varied considerably among treatments and among species, ranging from 30.0 ± 0.9°C (M ± SD) for D. villosus under hypoxic conditions to 37.3 ± 0.4°C for C. pseudogracilis under hyperoxic conditions (Table 1; See Figure S1 in Supporting Information). Across all species, oxygen conditions during heating trials significantly affected CTmax (Figure 1; LR test: p < .0001), with hypoxia reducing CTmax on average by 0.78°C (p < .0001), while hyperoxia significantly improved CTmax by on average 0.66°C (p < .0001). When considering species individually, hypoxia significantly reduced CTmax in four species relative to normoxia, while hyperoxia significantly increased CTmax in four species relative to normoxia. When directly comparing hypoxia and hyperoxia, seven species showed an increase in CTmax under hyperoxia (Table 1).
Table 1.
Lethal temperatures (°C; M ± SD) of the investigated species for the hypoxia, normoxia and hyperoxia treatment. Different letters indicate significant differences among oxygen treatments (small letters: ANOVA with Scheffé post hoc tests; capital letters: Mann–Whitney U‐test with Bonferroni correction)
| Species | Hypoxia | Normoxia | Hyperoxia |
|---|---|---|---|
| Asellus aquaticus | 35.1 ± 0.7 a | 35.6 ± 0.5 ab | 36.1 ± 0.2 b |
| Crangonyx pseudogracilis | 37.3 ± 0.2 a | 36.8 ± 0.3 b | 37.3 ± 0.4 a |
| Dikerogammarus villosus | 30.0 ± 0.9 a | 32.3 ± 0.7 b | 32.9 ± 0.9 b |
| Echinogammarus berilloni | 33.3 ± 0.4 a | 34.0 ± 0.6 b | 35.0 ± 0.4 c |
| Gammarus fossarum | 30.7 ± 0.7 A | 32.9 ± 0.4 B | 33.4 ± 0.2 C |
| Gammarus pulex | 33.8 ± 0.4 a | 34.9 ± 0.2 b | 35.1 ± 0.4 b |
| Gammarus roeselii | 33.1 ± 1.0 a | 33.6 ± 0.4 ab | 34.2 ± 0.3 b |
| Gammarus tigrinus | 34.5 ± 0.5 a | 34.7 ± 0.3 a | 36.3 ± 0.6 b |
Figure 1.
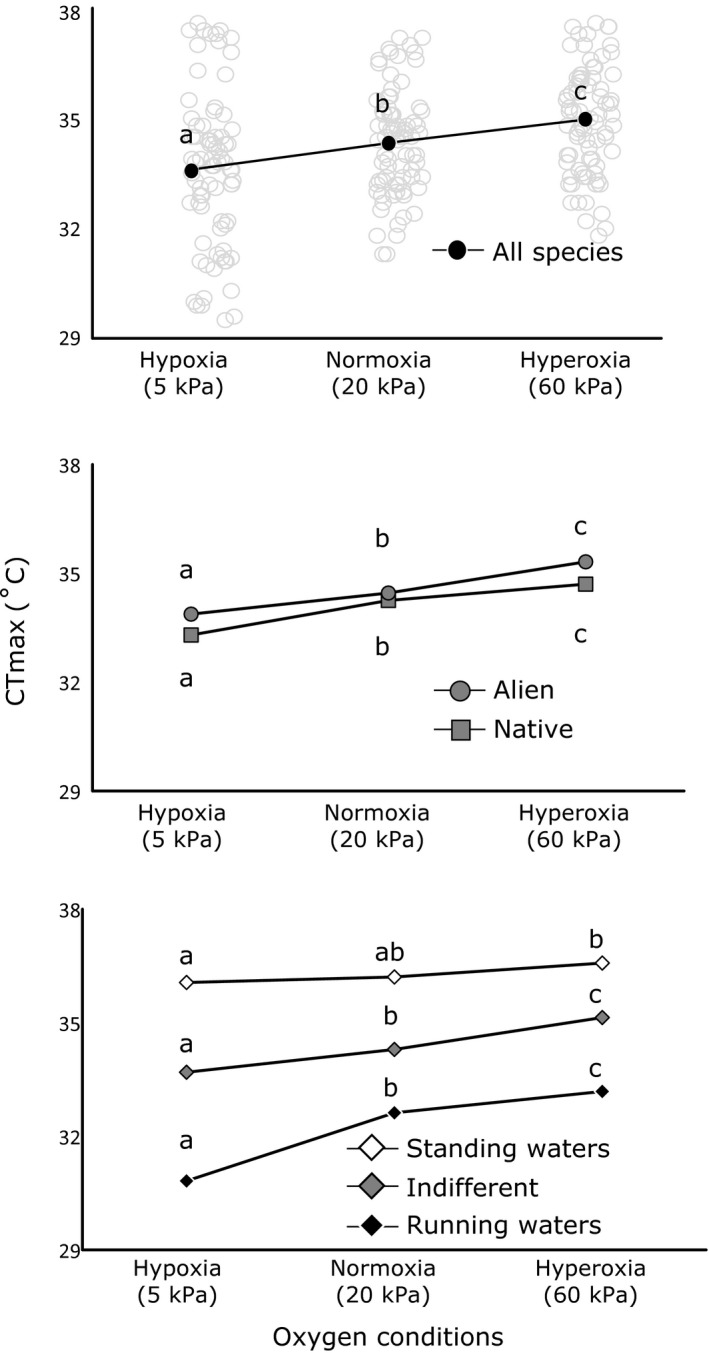
Lethal temperatures (°C) under different oxygen conditions for all species (a), native vs. alien species (b) and species with different habitat use (c). Different letters indicate significant differences between oxygen conditions during heating trials (p < .05; as revealed by testing the contrasts of the linear mixed‐effects models with Chi‐square tests and a Bonferroni correction)
Native and alien species did not differ in their CTmax (LR test: p = .215) nor in how their CTmax was influenced by oxygen conditions during heating trials (LR test: p = .118) (Figure 1b; Table 2). This was also reflected in a higher AIC value for model 3 which included species origin compared to model 2 that did not (502.2 vs. 500.6; Table 2). In contrast, CTmax varied greatly between species from different habitats (LR test: p < .0001), with species from standing waters being most heat resistant while those from running waters being most susceptible (Figure 1c; Table 2). Furthermore, the effects of oxygen conditions during heating trials on CTmax were different across species from different habitats (LR test: p < .0001). Hypoxia caused the strongest reductions in CTmax in species from running waters (on average 1.8°C), while having negligible effects on species from standing waters (average difference of 0.1°C). Species that were indifferent with respect to water type occupied an intermediate position (average difference of 0.6°C) (Figure 1c). The importance of habitat use as an explanatory factor is also illustrated by the low AIC (438.4) of model 4 and the high marginal R 2 of .83 (Table 2). Adding body mass and the interaction between body mass and oxygen conditions also improved model fit significantly (p < .047 and p < .027 for body mass and the interaction, respectively; Table 2). On average, larger animals displayed lower CTmax, and the lower CTmax in larger bodied animals was most prominent under hypoxia (Figure S2).
Table 2.
Results of the linear mixed‐effects model analyses of the effects of oxygen treatment, species origin (native vs. exotic species) and species habitat use (running water, standing water or indifferent) on the heat tolerance (critical maximum temperature; response variable) of the eight investigated species. Significance of fixed factors was tested by likelihood ratio (LR) tests and the difference in AIC of the model without the fixed factor as compared to the full model is given (negative values indicate that including the fixed factor reduces AIC value [i.e. a better model fit]). Significant fixed factors are indicated boldfaced. For each model, we also provide marginal and conditional pseudo‐R 2 values (indicating the variance explained by fixed effects, and by both fixed and random effects, respectively) as well as AIC values
| Fixed factors | Models | |||
|---|---|---|---|---|
| 1. O2 | 2. O2 and body mass | 3. As model 2 + origin | 4. As model 2 + habitat use | |
| Oxygen conditions (O 2 ) | −139.9139 (p < .0001) | −151.715 (p < .0001) | −152.0196 (p < .0001) | −198.4787 (p < .0001) |
| Body mass | – | −11.9126 (p = .0005) | −4.7363 (p = .0132) | −1.9751 (p = .0465) |
| O 2 × body mass | – | −13.067 (p = .0002) | −5.2567 (p = .0098) | −3.24672 (p = .0267) |
| Origin (native or alien) | – | – | 1.5302 (p = .215) | – |
| O2 × Origin | – | – | −0.2761 (p = .1179) | – |
| Habitat use | – | – | – | −62.2585 (p < .0001) |
| O 2 × habitat | – | – | – | −47.61827 (p < .0001) |
| Model fit | ||||
| Marginal R 2 | .10641 | .12696 | .15559 | .83228 |
| Conditional R 2 | .87406 | .87518 | .87851 | .90554 |
| AIC value | 512.535 | 500.623 | 502.153 | 438.364 |
The extent to which oxygen conditions affected the outcome of the heating trials, calculated as the difference between CTmax assessed under hyperoxia minus the CTmax when assessed under hypoxia, was clearly different across species. We found a strong negative relationship (adj. R 2 = .78; F 1,6 = 25.26; p = .0024) between the extent to which oxygen conditions modulated CTmax and the average heat resistance when assessed under normoxia (Figure 2). Heat‐tolerant taxa, such as A. aquaticus and C. pseudogracilis, showed little response to oxygen conditions, whereas the CTmax of heat sensitive species, for example, D. villosus and G. fossarum, was more plastic, being increased by hyperoxia and strongly reduced by hypoxia.
Figure 2.
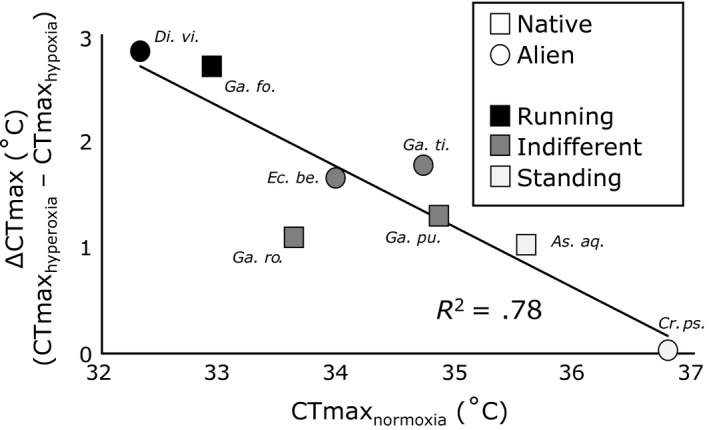
Regression between the difference in lethal temperatures of hyperoxia and hypoxia and the lethal temperatures at normoxia for the investigated species (As. aq. = Asellus aquaticus, Cr. ps. = Crangonyx pseudogracilis, Di. vi. = Dikerogammarus villosus, Ec. be. = Echinogammarus berilloni, Ga. fo. = Gammarus fossarum, Ga. pu. = G. pulex, Ga. ro. = G. roeselii, Ga. ti. = G. tigrinus)
Thermal sensitivity of metabolic rate, expressed as activation energy (Ea values) calculated from literature data, tended to be slightly higher in animals from standing waters (t = 2.81; p = .016; Table 3). Ea values did not vary with origin of species (t = −0.350; p = .732). Reported critical oxygen levels were positively correlated with the ability to maintain oxygen consumption rates at the critical oxygen level such that low critical oxygen levels were frequently accompanied by marked reductions in oxygen consumption rates at these levels (Figure S4). When no critical oxygen levels could be detected (i.e. oxygen consumption rates declined monotonically with falling oxygen levels), critical oxygen levels were taken to equal 100% normoxia where animals by definition fully maintained oxygen consumption rates, which may have strengthened the relationship mentioned above. Differences in respiratory responses when subject to hypoxia across the four species could also be related to habitat preference (Table 3), with the species preferring standing waters (A. aquaticus), having relatively low critical oxygen levels (t = −4.62; p < .001), and in this species, low reported values for critical oxygen levels were not necessarily coupled to marked reductions in oxygen consumption rate at Pc, expressed as a percentage of the initial oxygen consumption rates at normoxia (t = 3.633; p = .002). No differences in respiratory responses when subject to hypoxia could be detected between the species preferring running waters (G. fossarum) and the two species that were indifferent with respect to water type (G. pulex and G. roeselii).
Table 3.
Metrics for metabolic rate reported in the literature. Activation Energy (Ea) values (in eV) indicate the thermal sensitivity of oxygen consumption rates. Critical oxygen levels (Pc) are listed alongside with the oxygen consumption rate at Pc, expressed as a percentage of the initial oxygen consumption rates at normoxia (see text for further explanation). Numbers refer to literature sources: 1: Adcock, 1982; 2: Becker, Ortmann, Wetzel, & Koop, 2016; 3: Bruijs, Kelleher, Van der Velde, & Bij de Vaate, 2001; 4: Dorgelo, 1973; 5: Foucreau, Cottin, Piscart, & Hervant, 2014; 6: Franke, 1977; 7: Hervant, Mathieu, & Messana, 1998; 8: Issartel, Hervant, Voituron, Renault, & Vernon, 2005; 9: Lukacsovics, 1958; 10: Maazouzi et al., 2011; 11: Micherdzinski, 1958; 12: Mösslacher & Creuzé des Châtelliers, 1996; 13: Nilsson, 1974; 14: Pieper, 1978; 15: Prus, 1976; 16: Rotvit & Jacobsen, 2013; 17: Roux & Roux, 1967; 18: Roux, 1975; 19: Roux, Roux, & Opdam, 1980; 20: Rumpus & Kennedy, 1974; 21: Suomalainen, 1958; 22: Toman & Dall, 1998; 23: Walshe‐Maetz, 1956; 24: Wautier & Troiani, 1960; 25: Woynárvich, 1961
| Species | Ea value ± SE | Literature sources | Critical oxygen level (Pc) (% of normoxia) | Metabolic rate at Pc (% of normoxia) | Literature sources |
|---|---|---|---|---|---|
| Asellus aquaticus | 0.87 ± 0.14 | 1, 15, 16 | 15.41 ± 6.12 | 63.22 ± 16.31 | 7, 12, 16 |
| Dikerogammarus villosus | 0.54 ± 0.084 | 2, 3, 9, 10 | |||
| Gammarus fossarum | 0.41 ± 0.11 | 2, 4, 8, 14, 17, 18 | 30.00 ± 4.59 | 63.73 ± 4.59 | 6, 7, 11, 22 |
| Gammarus pulex | 0.49 ± 0.062 | 5, 10, 13, 16, 17, 18, 20, 21, 24 | 50.40 ± 12.64 | 74.82 ± 8.95 | 16, 21, 22, 23, 24 |
| Gammarus roeselii | 0.38 ± 0.027 | 2, 9, 19, 25 | 26.55 ± 26.55 | 58.73 ± 41.27 | 22 |
| Gammarus tigrinus | 0.52 | 4 |
4. DISCUSSION
It is important to understand interactive effects of warming and water oxygenation as it is becoming increasingly clear that the effects of each stressor are not merely additive for many aquatic taxa, including molluscs, insects and fish (Koopman, Collas, Van der Velde, & Verberk, 2016; McBryan, Anttila, Healy, & Schulte, 2013; Verberk, Overgaard, et al., 2016). Studies investigating heat tolerance in aquatic crustaceans have mainly focused on heat tolerance under normoxic conditions (Wijnhoven, Van Riel, & Van der Velde, 2003; but see Ern et al., 2015 who also manipulated oxygen conditions). Here, we report patterns in heat tolerance across eight crustacean species in relation to water oxygen content. When averaged across all species, small (<1.0°C) but statistically significant effects were found on heat tolerance, not only for hypoxia but also for hyperoxia, the latter being considered a stronger test of oxygen‐limited thermal tolerance (Verberk, Overgaard, et al., 2016). While these effects were generally small, there is evidence for mayflies that small effects of oxygen on lethal limits become more pronounced for sublethal limits manifested at lower temperatures under field conditions (Verberk, Durance, et al., 2016). Previous work on A. aquaticus, one of the most heat‐ and hypoxia‐resistant species in our study, also showed strong interactive effects of temperature and oxygen on growth (Hoefnagel & Verberk, 2015), while employing temperatures (14–24°C) and oxygen levels (10–40 kPa) more moderate than those used in the present study. Still in half of the species we studied, thermal tolerance was not affected by hypoxia or by hyperoxia, reflecting the mixed results reported earlier (Ern et al., 2015; Frederich & Pörtner, 2000). Furthermore, the lowest and highest CTmax recorded did differ by more than 7°C (Table 1) and an explicit aim of this study was, therefore, to investigate if these differences in heat tolerance and their sensitivity to oxygenation can be explained from differences in origin or habitat use of species.
Our hypothesis that alien species would be consistently more heat tolerant was not confirmed. In fact, the alien D. villosus was one of the most heat‐sensitive species, confirming the results by Maazouzi, Piscart, Legier, and Hervant (2011) who also observed greater heat tolerance in the native G. pulex than in D. villosus. However, this pattern contrasts those of other studies on crustaceans (Kenna, Fincham, Dunn, Brown, & Hassall, 2017; Sareyka et al., 2011) and ectotherms more generally (Bates et al., 2013; Lenz et al., 2011; Verbrugge, Schipper, Huijbregts, Van der Velde, & Leuven, 2012). If we investigate more species, this result could change, but at the very least, it suggests that classifying species based on their origin (native or alien) to derive the taxa vulnerability to global warming or to predict invasion success should be done with caution.
Our hypothesis that large‐bodied animals would be more susceptible to heat stress was partly supported by the data. Larger animals displayed lower CTmax under hypoxia, where oxygen limitation is most likely to occur. Size dependency of thermal tolerance implies size dependency of the mechanism setting thermal tolerance. This mechanism has been suggested to be oxygen limitation (Chapelle & Peck, 1999; Pörtner, 2010; Verberk et al., 2011), and limits to oxygen provisioning are implicated in setting upper body size limits (e.g. Kaiser et al., 2007; Lane et al., 2017). However, other studies did not find evidence for size dependency of oxygen limitation (Harrison et al., 2014; Woods et al., 2009). Only when comparing stonefly nymphs spanning several orders of magnitude in size, did Verberk, Sommer, Davidson, and Viant (2013) observe a weak negative relationship between body size and CTmax. In the present study, body mass also explained only a small amount of the variation in heat tolerance under hypoxia (c.1°C difference across a 10‐fold difference in body mass).
Most of the variation in heat tolerance across species was linked to differences in their habitat use (Figure 1c), with model four showing the highest marginal R 2 of .83 (Table 2). Moreover, the extent to which oxygen‐limited heat tolerance differed across species differing in habitat use. In a recent review on arthropods, Verberk, Overgaard, et al. (2016) suggested that whether taxa show oxygen limitation at thermal extremes may be contingent on their capacity to regulate oxygen uptake, which in turn, could help explain differences in support for oxygen limitation between aquatic and terrestrial ectotherms. Indeed, studies on insects and freshwater snails indicate that the extent to which taxa show oxygen‐limited thermal tolerance depends on the ability of animals to regulate oxygen uptake (Boardman & Terblanche, 2015; Koopman et al., 2016; Verberk & Bilton, 2013, 2015). All the species investigated in our study have similar gas exchange mechanisms (i.e. aquatic gas exchange across respiratory surfaces such as the gills and coxal plates). However, species in standing waters are more frequently confronted with periods of low oxygen while conditions for oxygen uptake should be consistently better in running waters where permanent water flow greatly reduces the ventilation effort needed, while the resultant mixing of air and water ensures high water oxygenation. The adaptations that species possess to cope with such environmental conditions in their preferred habitat likely also help to explain differences in heat tolerance and their sensitivity to oxygenation. Adaptations to ensure adequate oxygen provisioning under heat or hypoxia may explain why oxygen had little effect on the heat tolerance of the two most tolerant species (A. aquaticus and C. pseudogracilis), and these may involve low oxygen demand or high capacity for oxygen uptake (via enhanced ventilation or circulation). A. aquaticus was shown to be an oxygen conformer (Table 3; Rotvit & Jacobsen, 2013), which may explain its five times higher tolerance to hypoxia compared to G. pulex (Maltby, 1995). While no data on respiratory responses when subject to hypoxia could be retrieved for C. pseudogracilis, it was shown to be five times more tolerant to hypoxia compared with G. pulex (Macneil & Dick, 2014). Sareyka et al. (2011) compared two amphipod species and reported greater hypoxia tolerance in the most heat‐tolerant species. In addition, to respiratory responses when subject to hypoxia, differences between species in their thermal sensitivity of oxygen demand, expressed as Ea values, may explain their thermal tolerance. Stonefly nymphs with high Q10 values have been reported to have a lower CTmax (Verberk & Bilton, 2011), which seems straightforward when high Q10 values indicate that the oxygen demand of an individual is very sensitive to increasing temperatures, making it more susceptible to oxygen‐limited heat tolerance. Yet, our analysis on Ea values extracted from the literature showed higher Ea values in animals preferring standing waters if anything (Table 3). The problem here is that a high Ea value may be taken as a proxy for increases in oxygen demand with temperature, but in order to satisfy such increases in demand, an animal needs sufficient capacity to extract and transport oxygen. Consequently, without data on scope for oxygen consumption across temperatures, high Ea values could also be interpreted as a high capacity for oxygen delivery under warmer temperatures. Both viewpoints may be correct but in different contexts, such that oxygen consumption transitions from being demand driven to being supply driven when metabolic rates approach maxima (see also Verberk, Bartolini, et al., 2016).
Intriguingly, our results show that the inherent heat tolerance (CTmax under normoxia) is a strong predictor for the extent to which oxygen content affects heat tolerance (the difference between CTmax when assessed under hypoxia and hyperoxia) (Figure 2). This suggests that oxygen limitation could be involved in setting thermal limits in heat‐susceptible taxa, but that in heat‐resistant taxa, the role of oxygen is smaller (A. aquaticus) or even absent (C. pseudogracilis) and mechanisms other than insufficient oxygen become more influential. The relatively low number of species in our study precluded us from taking phylogeny into account. Other studies did not found a clear association between thermal tolerance and phylogeny across amphipods and isopods (Best & Stachowicz, 2013). In our case, the two species preferring standing water are both distantly related to the remaining species, and they are also distantly related to each other (Figure S5). The most divergent species A. aquaticus, a descendent of the Asellota already occurring in the Triassic (Wilson, 2009), did not exhibit the most divergent physiology, which was exhibited by C. pseudogracilis. Similarly, G. pulex and G. fossarum which are very closely related (Hou & Sket, 2016; Figure S5) showed quite divergent physiology, and differences between these species were consistent with the pattern of oxygen having smaller effects in heat‐resistant taxa. Thus, the relationship reported in Figure 2 does not seem to be confounded by phylogenetic relationships, but studies encompassing a greater number of species allowing phylogenetic relatedness to be taken into account are needed to verify this intriguing observation.
Our study has implications for both predicting susceptibility to global warming and invasion success. Species from running waters (e.g. G. fossarum and D. villosus) are likely to be more susceptible to interactive effects of warming and hypoxia than those of standing waters (e.g. A. aquaticus and C. pseudogracilis), but at the same time, running water taxa may profit most from improvements in water quality (e.g. reduction in nutrient load), and such improvements may even off‐set the effects of warming (Durance & Ormerod, 2009; Verberk, Durance, et al., 2016). It is striking that D. villosus appears most susceptible to hypoxia and heat stress. This may explain why this species is very successful in colonizing new areas, but mainly in the rocky substrate with continuous wave action, flow and water displacements by passing ships or prevailing winds, resulting in continuous aeration (Gabel, 2012; Gabel et al., 2008; Platvoet, Dick, MacNeil, Van Riel, & Van der Velde, 2009). This likely also explains why this species is much less adept in spreading to other waters which are less well oxygenated and where C. pseudogracilis is the more likely alien species.
In conclusion, oxygen availability had stronger effects in crustaceans that prefer running waters, and these taxa were also found to be more heat susceptible. Conversely, taxa that prefer standing waters were more heat resistant and had better oxyregulatory capacity, and heat tolerance was much less affected by oxygen availability. Such context‐dependence effects of oxygen on thermal limits could potentially reconcile the mixed support for the oxygen and capacity limitation of thermal tolerance hypothesis (Ern et al., 2015; Frederich & Pörtner, 2000).
DATA ACCESSIBILITY
Data available from the Dryad Digital Repository https://doi.org/10.5061/dryad.tf641 (Verberk, Leuven, van der Velde, & Gabel, 2018).
Supporting information
ACKNOWLEDGEMENTS
We thank M. Orbons, K.R. Koopman and K.N. Hoefnagel for help in the laboratory and E. Jongejans for statistical advice. We thank Johannes Overgaard, Folco Giomi and an anonymous reviewer for their useful and constructive comments on earlier versions. W.C.E.P.V. gratefully acknowledges funding by the European Research Council (Marie‐Curie Fellowship FP7‐PEOPLE‐2012‐CIG).
Verberk WCEP, Leuven RSEW, van der Velde G, Gabel F. Thermal limits in native and alien freshwater peracarid Crustacea: The role of habitat use and oxygen limitation. Funct Ecol. 2018;32:926–936. https://doi.org/10.1111/1365-2435.13050
REFERENCES
- Adcock, J. A. (1982). Energetics of a population of Asellus aquaticus (Crustacea, Isopoda): Respiration and energy budgets. Freshwater Biology, 12, 257–269. https://doi.org/10.1111/j.1365-2427.1982.tb00620.x [Google Scholar]
- Barton, K. (2011). MuMIn: multi‐model inference. R package version 1.0.0. Retrieved from: http://CRAN.R-project.org/package=MuMIn
- Bates, A. E. , McKelvie, C. M. , Sorte, C. J. B. , Morley, S. A. , Jones, N. A. R. , Mondon, J. A. , … Quinn, G. (2013). Geographical range, heat tolerance and invasion success in aquatic species. Proceedings of the Royal Society B‐Biological Sciences, 280, 20131958 https://doi.org/10.1098/rspb.2013.1958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker, J. , Ortmann, C. , Wetzel, M. A. , & Koop, J. H. E. (2016). Metabolic activity and behavior of the invasive amphipod Dikerogammarus villosus and two common Central European gammarid species (Gammarus fossarum, Gammarus roeselii): Low metabolic rates may favor the invader. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology, 191, 119–126. https://doi.org/10.1016/j.cbpa.2015.10.015 [DOI] [PubMed] [Google Scholar]
- Best, R. J. , & Stachowicz, J. J. (2013). Phylogeny as a proxy for ecology in seagrass amphipods: Which traits are most conserved? PLoS ONE, 8, e57550 https://doi.org/10.1371/journal.pone.0057550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boardman, L. , & Terblanche, J. S. (2015). Oxygen safety margins set thermal limits in an insect model‐system. Journal of Experimental Biology, 218, 1677–1685. https://doi.org/10.1242/jeb.120261 [DOI] [PubMed] [Google Scholar]
- Brodersen, K. P. , Pedersen, O. , Walker, I. R. , & Jensen, M. T. (2008). Respiration of midges (Diptera; Chironomidae) in British Columbian lakes: Oxy‐regulation, temperature and their role as palaeo‐indicators. Freshwater Biology, 53, 593–602. https://doi.org/10.1111/j.1365-2427.2007.01922.x [Google Scholar]
- Bruijs, M. C. M. , Kelleher, B. , Van der Velde, G. , & Bij de Vaate, A. (2001). Oxygen consumption, temperature and salinity tolerance of the invasive amphipod Dikerogammarus villosus: Indicators of further dispersal via ballast water transport. Archiv für Hydrobiologie, 152, 633–646. https://doi.org/10.1127/archiv-hydrobiol/152/2001/633 [Google Scholar]
- Calosi, P. , Bilton, D. T. , Spicer, J. I. , Votier, S. C. & Atfield, A. (2010). What determines a species’ geographical range? Thermal biology and latitudinal range size relationships in European diving beetles (Coleoptera: Dytiscidae). Journal of Animal Ecology, 79, 194–204. https://doi.org/10.1111/j.1365-2656.2009.01611.x [DOI] [PubMed] [Google Scholar]
- Chapelle, G. , & Peck, L. S. (1999). Polar gigantism dictated by oxygen availability. Nature, 399, 114–115. https://doi.org/10.1038/20099 [Google Scholar]
- Chown, S. L. (2012). Trait‐based approaches to conservation physiology: Forecasting environmental change risks from the bottom up. Philosophical Transactions of the Royal Society B . Biological Sciences, 367, 1615–1627. https://doi.org/10.1098/rstb.2011.0422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daufresne, M. , Lengfellner, K. , & Sommer, U. (2009). Global warming benefits the small in aquatic ecosystems. Proceedings of the National Academy of Sciences of the United States of America, 106, 12788–12793. https://doi.org/10.1073/pnas.0902080106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutsch, C. , Ferrel, A. , Seibel, B. , Pörtner, H.‐O. , & Huey, R. B. (2015). Climate change tightens a 686 metabolic constraint on marine habitats. Science, 348, 1132–1135. https://doi.org/10.1126/science.aaa1605 [DOI] [PubMed] [Google Scholar]
- Diaz, R. J. , & Rosenberg, R. (2008). Spreading dead zones and consequences for marine ecosystems. Science, 321, 926–929. https://doi.org/10.1126/science.1156401 [DOI] [PubMed] [Google Scholar]
- Dorgelo, J. (1973). Comparative ecophysiology of gammarids (Crustacea: Amphipoda) from marine, brackish and fresh‐water habitats exposed to the influence of salinity‐temperature combinations. III. Oxygen uptake. Netherlands Journal of Sea Research, 7, 253–266. https://doi.org/10.1016/0077-7579(73)90049-5 [Google Scholar]
- Durance, I. , & Ormerod, S. J. (2009). Trends in water quality and discharge confound long‐term warming effects on river macro‐invertebrates. Freshwater Biology, 54, 388–405. https://doi.org/10.1111/j.1365-2427.2008.02112.x [Google Scholar]
- Eggers, T. O. , & Martens, A. (2001). A key to the freshwater Amphipoda (Crustacea) of Germany. Lauterbornia, 42, 1–70. [Google Scholar]
- Ern, R. , Huong, D. T. T. , Phuong, N. T. , Madsen, P. T. , Wang, T. , & Bayley, M. (2015). Some like it hot: Thermal tolerance and oxygen supply capacity in two eurythermal crustaceans. Scientific Reports, 5, 10743 https://doi.org/10.1038/srep10743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ern, R. , Norin, T. , Gamperl, A. K. , & Esbaugh, A. J. (2016). Oxygen dependence of upper thermal limits in fishes. Journal of Experimental Biology, 219, 3376–3383. https://doi.org/10.1242/jeb.143495 [DOI] [PubMed] [Google Scholar]
- Foucreau, N. , Cottin, D. , Piscart, C. , & Hervant, F. (2014). Physiological and metabolic responses to rising temperature in Gammarus pulex (Crustacea) populations living under continental or Mediterranean climates. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology, 168, 69–75. https://doi.org/10.1016/j.cbpa.2013.11.006 [DOI] [PubMed] [Google Scholar]
- Franke, U. (1977). Experimentelle Untersuchungen zur Respiration von Gammarus fossarum Koch 1835 (Crustacea: Amphipoda) in Abhängigkeit von Temperatur, Sauerstoffkonzentration und Wasserbewegung. Archiv für Hydrobiologie, 48 (Suppl), 369–411. [Google Scholar]
- Frederich, M. , & Pörtner, H.‐O. (2000). Oxygen limitation of thermal tolerance defined by cardiac and ventalitory performance in spider crab, Maja squinado. American Journal of Physiology – Regulatory . Integrative and Comparative Physiology, 279, R1531–R1538. https://doi.org/10.1152/ajpregu.2000.279.5.R1531 [DOI] [PubMed] [Google Scholar]
- Fry, F. , & Hart, J. (1948). The relation of temperature to oxygen consumption in the goldfish. Biological Bulletin, 94, 66–77. https://doi.org/10.2307/1538211 [PubMed] [Google Scholar]
- Fusi, M. , Cannicci, S. , Daffonchio, D. , Mostert, B. , Pörtner, H.‐O. , & Giomi, F. (2016). The trade‐off between heat tolerance and metabolic cost drives the bimodal life strategy at the air‐water interface. Scientific Reports, 6, 19158 https://doi.org/10.1038/srep19158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabel, F . (2012). Impacts of ship‐induced waves on benthic macroinvertebrates (dissertation). Humboldt‐Universität zu Berlin
- Gabel, F. , Garcia, X.‐F. , Brauns, M. , Sukhodolov, A. , Leszinski, M. , & Pusch, M. T. (2008). Resistance to ship‐induced waves of benthic invertebrates in various littoral habitats. Freshwater Biology, 53, 1567–1578. https://doi.org/10.1111/j.1365-2427.2008.01991.x [Google Scholar]
- Giomi, F. , Fusi, M. , Barausse, A. , Mostert, B. , Pörtner, H.‐O. , & Cannicci, S. (2014). Improved heat tolerance in air drives the recurrent evolution of air‐breathing. Proceedings of the Royal Society B, Biological Sciences, 281, 20132927 https://doi.org/10.1098/rspb.2013.2927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison, J. F. , Klok, C. J. , & Waters, J. S. (2014). Critical PO2 is size‐independent in insects: Implications for the metabolic theory of ecology. Current Opinion in Insect Science, 4, 54–59. https://doi.org/10.1016/j.cois.2014.08.012 [DOI] [PubMed] [Google Scholar]
- Hervant, F. , Mathieu, J. , & Messana, G. (1998). Oxygen consumption and ventilation in declining oxygen tension and posthypoxic recovery in epigean and hypogean crustaceans. Journal of Crustacean Biology, 18, 717–727. https://doi.org/10.2307/1549148 [Google Scholar]
- Hoefnagel, K. N. , & Verberk, W. C. E. P. (2015). Is the temperature‐size rule mediated by oxygen in aquatic ectotherms? Journal of Thermal Biology, 54, 56–65. https://doi.org/10.1016/j.jtherbio.2014.12.003 [DOI] [PubMed] [Google Scholar]
- Hou, Z. , & Sket, B. (2016). A review of Gammaridae (Crustacea: Amphipoda): The family extent, its evolutionary history, and taxonomic redefinition of genera. Zoological Journal of the Linnean Society, 176, 323–348. https://doi.org/10.1111/zoj.12318 [Google Scholar]
- Huey, R. B. , Kearney, M. R. , Krockenberger, A. , Holtum, J. A. M. , Jess, M. , & Williams, S. E. (2012). Predicting organismal vulnerability to climate warming: Roles of behavior, physiology and adaptation. Philosophical Transactions of the Royal Society B, Biological Sciences, 367, 1665–1679. https://doi.org/10.1098/rstb.2012.0005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Issartel, J. , Hervant, F. , Voituron, Y. , Renault, D. , & Vernon, P. (2005). Behavioural, ventilatory and respiratory responses of epigean and hypogean crustaceans to different temperatures. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology, 141, 1–7. https://doi.org/10.1016/j.cbpb.2005.02.013 [DOI] [PubMed] [Google Scholar]
- Jewett, E. B. , Hines, A. H. , & Ruiz, G. M. (2005). Epifaunal disturbance by periodic low levels of dissolved oxygen: Native vs. invasive species response. Marine Ecology‐Progress Series, 304, 31–44. https://doi.org/10.3354/meps304031 [Google Scholar]
- Jutfelt, F. , Gräns, A. , Jönsson, E. , Wiklander, K. , Seth, H. , Olsson, C. , … Sandblom, E. (2014) Response to ‘How and how not to investigate the oxygen and capacity limitation of thermal tolerance (OCLTT) and aerobic scope ‐ remarks on the article by Gräns et al’ . Journal of Experimental Biology, 217, 4433–4435 https://doi.org/10.1242/jeb.115410 [DOI] [PubMed] [Google Scholar]
- Kaiser, A. , Klok, C. J. , Socha, J. J. , Lee, W.‐K. , Quinlan, M. C. , & Harrison, J. F. (2007). Increase in tracheal investment with beetle size supports hypothesis of oxygen limitation on insect gigantism. Proceedings of the National Academy of Sciences of the United States of America, 104, 13198–13203. https://doi.org/10.1073/pnas.0611544104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenna, D. , Fincham, W. N. W. , Dunn, A. M. , Brown, L. E. , & Hassall, C. (2017). Antagonistic effects of biological invasion and environmental warming on detritus processing in freshwater ecosystems. Oecologia, 183, 875–886. https://doi.org/10.1007/s00442-016-3796-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klok, C. J. , Sinclair, B. J. , & Chown, S. L. (2004). Upper thermal tolerance and oxygen limitation in terrestrial arthropods. Journal of Experimental Biology, 207, 2361–2370. https://doi.org/10.1242/jeb.01023 [DOI] [PubMed] [Google Scholar]
- Koopman, K. R. , Collas, F. P. L. , Van der Velde, G. , & Verberk, W. C. E. P. (2016). Oxygen can limit heat tolerance in freshwater gastropods: Difference between gill and lung breathers. Hydrobiologia, 763, 301–312. https://doi.org/10.1007/s10750-015-2386-y [Google Scholar]
- Lane, S. J. , Shishido, C. M. , Moran, A. L. , Tobalske, B. W. , Arango, C. P. , & Woods, H. A. (2017). Upper limits to body size imposed by respiratory–structural trade‐offs in Antarctic pycnogonids. Proceedings of the Royal Society B, Biological Sciences, 284, 20171779 https://doi.org/10.1098/rspb.2017.1779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenz, M. , da Gama, B. A. P. , Gerner, N. V. , Gobin, J. , Gröner, F. , Harry, A. , … Wahl, M. (2011). Non‐native marine invertebrates are more tolerant towards environmental stress than taxonomically related native species: Results from a globally replicated study. Environmental Research, 111, 943–952. https://doi.org/10.1016/j.envres.2011.05.001 [DOI] [PubMed] [Google Scholar]
- Leuven, R. S. E. W. , Van der Velde, G. , Baijens, I. , Snijders, J. , Van der Zwart, C. , Lenders, H. J. R. , & Bij de Vaate, A. (2009). The river Rhine: A global highway for dispersal of aquatic invasive species. Biological Invasions, 11, 1989–2008. https://doi.org/10.1007/s10530-009-9491-7 [Google Scholar]
- Lukacsovics, F. (1958). Összehasonlító oxigénfogyasztási vizsgálatok álló‐és folyóvízi Amphipodákon (Dicerogammarus villosus bispinosus, Gammarus roeseli). (Vergleichende Untersuchungen über den Sauerstoffverbrauch von Amphipoden aus stehenden und fliessenden Gewässern). Annales Insiituti Hiologici. Tihany, 25, 57–67. [Google Scholar]
- Maazouzi, C. , Piscart, C. , Legier, F. , & Hervant, F. (2011). Ecophysiological responses to temperature of the ‘killer shrimp’ Dikerogammarus villosus: Is the invader really stronger than the native Gammarus pulex? Comparative Biochemistry and Physiology, Part A, 159, 268–274. https://doi.org/10.1016/j.cbpa.2011.03.019 [DOI] [PubMed] [Google Scholar]
- Macneil, C. , & Dick, J. T. A. (2014). Physicochemical tolerance, habitat use and predation are drivers of patterns of coexistence and exclusion among invasive and resident amphipods. Freshwater Biology, 59, 1956–1969. https://doi.org/10.1111/fwb.12399 [Google Scholar]
- Maltby, L. (1995). Sensitivity of the crustaceans Gammarus pulex (L.) and Asellus aquaticus (L.) to short‐term exposure to hypoxia and unionized ammonia: Observations and possible mechanisms. Water Research, 29, 781–787. https://doi.org/10.1016/0043-1354(94)00231-U [Google Scholar]
- Martinez, H. D. R. (2015) Analysing interactions of fitted models. Retrieved from https://cran.r-project.org/web/packages/phia/vignettes/phia.pdf
- McBryan, T. L. , Anttila, K. , Healy, T. M. , & Schulte, P. M. (2013). Responses to temperature and hypoxia as interacting stressors in fish: Implications for adaptation to environmental change. Integrative and Comparative Biology, 53, 648–659. https://doi.org/10.1093/icb/ict066 [DOI] [PubMed] [Google Scholar]
- Meire, L. , Soetaert, K. E. R. , & Meysman, F. J. R. (2013). Impact of global change on coastal oxygen dynamics and risk of hypoxia. Biogeosciences, 10, 2633–2653. https://doi.org/10.5194/bg-10-2633-2013 [Google Scholar]
- Micherdzinski, W. (1958). O2‐verbrauch einiger Süsswasser‐Amphipoden. Folia Biologica, 6, 145–162. [Google Scholar]
- Moran, A. L. , & Woods, H. A. (2010). Limits to diffusive O2 transport: Flow, form, and function in nudibranch egg masses from temperate and polar regions. PLoS ONE, 5(8), e12113 https://doi.org/10.1371/journal.pone.0012113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mösslacher, F. , & Creuzé des Châtelliers, M. (1996) Physiological and behavioural adaptations of an epigean and a hypogean dwelling population of Asellus aquaticus (L.) (Crustacea, Isopoda). Archiv für Hydrobiologie, 138, 187–198 [Google Scholar]
- Mueller, C. A. , & Seymour, R. S. (2011). The regulation index: A new method for assessing the relationship between oxygen consumption and environmental oxygen. Physiological and Biochemical Zoology, 84, 522–532. https://doi.org/10.1086/661953 [DOI] [PubMed] [Google Scholar]
- NEN6503 . (1980) Water‐benodigdheden, werkwijze en medium voor het kweken van Daphnia magna en van de hiervoor als voedsel benodigde algen. Delft, the Netherlands: Nederlands Normalisatie Instituut; [Google Scholar]
- Nilsson, L. M. (1974). Energy budget of a laboratory population of Gammarus pulex (Amphipoda). Oikos, 25, 35–42. https://doi.org/10.2307/3543543 [Google Scholar]
- Pieper, H.‐G. (1978) Ökophysiologische und produktionsbiologische Untersuchungen an Jugendstadien von Gammarus fossarum KOCH 1835. Schlitzer produktionsbiologische Studien (31). Archiv für Hydrobiologie/Supplement (Monographische Beiträge), 54(3), 257–327. [Google Scholar]
- Pinheiro, J. , Bates, D. , DebRoy, S. , Sarkar, D. & R Core Team . (2013). nlme: Linear and nonlinear mixed effects models: R package version 3.1‐109. Retrieved from https://cran.r-project.org/web/packages/nlme/nlme.pdf. [Google Scholar]
- Platvoet, D. , Dick, J. T. A. , MacNeil, C. , Van Riel, M. C. , & Van der Velde, G. (2009). Invader‐invader interactions in relation to environmental heterogeneity leads to zonation of two invasive amphipods, Dikerogammarus villosus (Sowinsky) and Gammarus tigrinus Sexton: Amphipod pilot species project (AMPIS) report 6. Biological Invasions, 11, 2085–2093. https://doi.org/10.1007/s10530-009-9488-2 [Google Scholar]
- Pörtner, H.‐O. (2010). Oxygen‐ and capacity‐limitation of thermal tolerance: A matrix for integrating climate‐related stressor effects in marine ecosystems. Journal of Experimental Biology, 213, 881–893. https://doi.org/10.1242/jeb.037523 [DOI] [PubMed] [Google Scholar]
- Pörtner, H.‐O. (2014). How to and how not to investigate the oxygen and capacity limitation of thermal tolerance (OCLTT) and aerobic scope. Journal of Experimental Biology, 217, 4432–4433. https://doi.org/10.1242/jeb.114181 [DOI] [PubMed] [Google Scholar]
- Posch, T. , Köster, O. , Salcher, M. M. , & Pernthaler, J. (2012). Harmful filamentous cyanobacteria favoured by reduced water turnover with lake warming. Nature Climate Change, 2, 809–813. https://doi.org/10.1038/nclimate1581 [Google Scholar]
- Prus, T. (1976). Experimental and field studies on ecological energetics of Asellus aquaticus L. (Isopoda) II. Respiration at various temperatures as an element of energy budget. Ekologia Polska, 24, 607–621. [Google Scholar]
- R Core Team (2013). R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; [Google Scholar]
- Rotvit, L. , & Jacobsen, D. (2013) Temperature increase and respiratory performance of macroinvertebrates with different tolerances to organic pollution. Limnologica – Ecology and Management of Inland Waters, 43, 510–515 https://doi.org/10.1016/j.limno.2013.04.003 [Google Scholar]
- Roux, C. (1975). Les variations de la courbe métabolisme/temperature de Gammarus pulex (L,) et Gammarus fossarum Koch (Amphipodes) sous influence de divers facteurs écologiques. Crustaceana, 29, 33–48. https://doi.org/10.1163/156854075X00036 [Google Scholar]
- Roux, C. , & Roux, A. L. (1967). Temperature et métabolisme réspiratoire d'espèces sympatriques de Gammares du groupe Pulex (Crustacés, Amphipodes). Annales de Limnotogie, 3, 3–16. https://doi.org/10.1051/limn/1967020 [Google Scholar]
- Roux, C. , Roux, A. L. , & Opdam, Y. (1980). Répartition écologique et métabolisme respiratoire de Gammarus roeselii Gervais, 1835. Crustaceana, 6 (Suppl), 148–159. [Google Scholar]
- Rumpus, A. E. , & Kennedy, C. R. (1974). The effect of the acanthocephalan Pomphorhynchus laevis upon the respiration of its intermediate host, Gammarus pulex . Parasitology, 68, 271–284. https://doi.org/10.1017/S0031182000045789 [DOI] [PubMed] [Google Scholar]
- Sareyka, J. , Kraufvelin, P. , Lenz, M. , Lindström, M. , Tollrian, R. , & Wahl, M. (2011). Differences in stress tolerance and brood size between a non‐indigenous and an indigenous gammarid in the northern Baltic Sea. Marine Biology, 158, 2001–2008. https://doi.org/10.1007/s00227-011-1708-5 [Google Scholar]
- Seebacher, F. , White, C. R. , & Franklin, C. E. (2015). Physiological plasticity increases resilience of ectothermic animals to climate change. Nature Climate Change, 5, 61–66. https://doi.org/10.1038/nclimate2457 [Google Scholar]
- Suomalainen, P. (1958). Der Sauerstoffverbrauch einiger finnischer Gammarus‐arten. Verhandlungen der internationalen Vereinigung für theoretische und angewandte Limnologie, 13, 873–878. [Google Scholar]
- Terblanche, J. S. , Deere, J. A. , Clusella‐Trullas, S. , Janion, C. , & Chown, S. L. (2007). Critical thermal limits depend on methodological context. Proceedings of the Royal Society B, Biological Sciences, 274, 2935–2942. https://doi.org/10.1098/rspb.2007.0985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toman, M. J. , & Dall, P. C. (1998). Respiratory levels and adaptations in four freshwater species of Gammarus (Crustacea: Amphipoda). International Review of Hydrobiology, 83, 251–263. https://doi.org/10.1002/(ISSN)1522-2632 [Google Scholar]
- Väinölä, R. , Witt, J. D. S. , Grabowski, M. , Bradbury, J. H. , Jazdzewski, K. , & Sket, B. (2008). Global diversity of amphipods (Amphipoda; Crustacea) in freshwater. Hydrobiologia, 595, 241–255. [Google Scholar]
- Van der Velde, G. , Rajagopal, S. , Kelleher, B. , Muskó, I. B. , & Bij de Vaate, A. (2000) Ecological impact of crustacean invaders: General considerations and examples from the Rhine River In: Von Vaupel Klein J. C. & Schram F. R. (Eds.), The biodiversity crisis and Crustacea (Vol. 12, pp. 3–33). Crustacean Issues. [Google Scholar]
- Verberk, W. C. E. P. , & Atkinson, D. (2013). Why polar gigantism and Palaeozoic gigantism are not equivalent: Effect of oxygen and temperature on the body size of ectotherms. Functional Ecology, 27, 1275–1285. https://doi.org/10.1111/1365-2435.12152 [Google Scholar]
- Verberk, W. C. E. P. , Bartolini, F. , Marshall, D. J. , Pörtner, H.‐O. , Terblanche, J. S. , White, C. R. , & Giomi, F. (2016). Can respiratory physiology predict thermal niches? Annals of the New York Academy of Sciences, 1365, 73–88. https://doi.org/10.1111/nyas.12876 [DOI] [PubMed] [Google Scholar]
- Verberk, W. C. E. P. , & Bilton, D. T. (2011). Can oxygen set thermal limits in an insect and drive gigantism? PLoS ONE, 6, e22610 https://doi.org/10.1371/journal.pone.0022610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verberk, W. C. E. P. , & Bilton, D. T. (2013). Respiratory control in aquatic insects dictates their vulnerability to global warming. Biology Letters, 9, 20130473 https://doi.org/10.1098/rsbl.2013.0473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verberk, W. C. E. P. , & Bilton, D. T. (2015). Oxygen limited thermal tolerance is seen in a plastron breathing insect, and can be induced in a bimodal gas exchange. Journal of Experimental Biology, 218, 2083–2088. https://doi.org/10.1242/jeb.119560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verberk, W. C. E. P. , Bilton, D. T. , Calosi, P. , & Spicer, J. I. (2011). Oxygen supply in aquatic ectotherms: Partial pressure and solubility together explain biodiversity and size patterns. Ecology, 92, 1565–1572. https://doi.org/10.1890/10-2369.1 [DOI] [PubMed] [Google Scholar]
- Verberk, W. C. E. P. , Durance, I. , Vaughan, I. P. , & Ormerod, S. J. (2016). Field and laboratory studies reveal interacting effects of stream oxygenation and warming on aquatic ectotherms. Global Change Biology, 22, 1769–1778. https://doi.org/10.1111/gcb.13240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verberk, W. C. E. P. , Leuven, R. S. E. W. , van der Velde, G. , & Gabel, F. (2018). Data from: Thermal limits in native and alien freshwater peracarid Crustacea: The role of habitat use and oxygen limitation. Dryad Digital Repository, https://doi.org/10.5061/dryad.tf641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verberk, W. C. E. P. , Overgaard, J. , Ern, R. , Bayley, M. , Wang, T. , Boardman, L. , & Terblanche, J. S. (2016). Does oxygen limit thermal tolerance in arthropods? A critical review of current evidence. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology, 192, 64–78. https://doi.org/10.1016/j.cbpa.2015.10.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verberk, W. C. E. P. , Sommer, U. , Davidson, R. L. , & Viant, M. R. (2013). Anaerobic metabolism at thermal extremes: A metabolomic test of the oxygen limitation hypothesis in an aquatic insect. Integrative and Comparative Biology, 53, 609–619. https://doi.org/10.1093/icb/ict015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verberk, W. C. E. P. , Verdonschot, P. F. M. , Van Haaren, T. , & Van Maanen, B. (2012) Milieu‐ en habitatpreferentie van Nederlandse zoetwater‐macrofauna. WEW Themanummer 23, Van de Garde‐Jemé, Eindhoven. 32 pp
- Verbrugge, L. N. H. , Schipper, A. M. , Huijbregts, M. A. J. , Van der Velde, G. , & Leuven, R. S. E. W. (2012). Sensitivity of native and non‐native mollusc species to changing river water temperature and salinity. Biological Invasions, 14, 1187–1199. https://doi.org/10.1007/s10530-011-0148-y [Google Scholar]
- Wallace, J. B. , & Webster, J. R. (1996). The role of macroinvertebrates in stream ecosystem function. Annual Review of Entomology, 41, 115–139. https://doi.org/10.1146/annurev.en.41.010196.000555 [DOI] [PubMed] [Google Scholar]
- Walshe‐Maetz, B. M. (1956). Controle respiratoire et métabolisme chez les Crustacés. Vie Milieu, 7, 523–543. [Google Scholar]
- Wautier, J. , & Troiani, D. (1960). Contribution á l’étude du métabolisme respiratoire de quelques Gammaridae. Annales de la Station Centrale d'Hydrobiologie Appliqée, 8, 7–50. [Google Scholar]
- Wetzel, R. G. (2001). Limnology. Lake and river ecosystems. London, UK: Elsevier Academic Press. [Google Scholar]
- Wijnhoven, S. , Van Riel, M. C. , & Van der Velde, G. (2003). Exotic and indigenous freshwater gammarid species: Physiological tolerance to water temperature in relation to ionic content of the water. Aquatic Ecology, 37, 151–158. https://doi.org/10.1023/A:1023982200529 [Google Scholar]
- Wilson, G. D. F. (2009). The phylogenetic position of the Isopoda in the Peracarida (Crustacea: Malacostraca). Arthropod Systematics & Phylogeny, 67, 159–198. [Google Scholar]
- Winterstein, H. (1905). Wärmelähmung und Narkose. Zeitschrift für allgemeine Physiologie, 5, 323–350. [Google Scholar]
- Woods, H. A. , Moran, A. L. , Arango, C. P. , Mullen, L. , & Shields, C. (2009). Oxygen hypothesis of polar gigantism not supported by performance of Antarctic pycnogonids in hypoxia. Proceedings of the Royal Society of London B, 276, 1069–1075. https://doi.org/10.1098/rspb.2008.1489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woynárvich, E. (1961). Sauerstoffverbrauch einiger Wassertiere bei verschiedenen Temperaturen. Verhandlungen der internationalen Vereinigung für theoretische und angewandte Limnologie, 14, 1014–1018. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data available from the Dryad Digital Repository https://doi.org/10.5061/dryad.tf641 (Verberk, Leuven, van der Velde, & Gabel, 2018).


