Abstract
Borrelia burgdorferi sensu lato (Bbsl), the causative agent of Lyme disease, establishes an initial infection in the host’s skin following a tick bite, and then disseminates to distant organs, leading to multisystem manifestations. Tick-to-vertebrate host transmission requires that Bbsl survives during blood feeding. Complement is an important innate host defense in blood and interstitial fluid. Bbsl produces a polymorphic surface protein, CspA, that binds to a complement regulator, Factor H (FH) to block complement activation in vitro. However, the role that CspA plays in the Bbsl enzootic cycle remains unclear. In this study, we demonstrated that different CspA variants promote spirochete binding to FH to inactivate complement and promote serum resistance in a host-specific manner. Utilizing a tick-to-mouse transmission model, we observed that a cspA-knockout B. burgdorferi is eliminated from nymphal ticks in the first 24 hours of feeding and is unable to be transmitted to naïve mice. Conversely, ectopically producing CspA derived from B. burgdorferi or B. afzelii, but not B. garinii in a cspA-knockout strain restored spirochete survival in fed nymphs and tick-to-mouse transmission. Furthermore, a CspA point mutant, CspA-L246D that was defective in FH-binding, failed to survive in fed nymphs and at the inoculation site or bloodstream in mice. We also allowed those spirochete-infected nymphs to feed on C3-/- mice that lacked functional complement. The cspA-knockout B. burgdorferi or this mutant strain complemented with cspA variants or cspA-L246D was found at similar levels as wild type B. burgdorferi in the fed nymphs and mouse tissues. These novel findings suggest that the FH-binding activity of CspA protects spirochetes from complement-mediated killing in fed nymphal ticks, which ultimately allows Bbsl transmission to mammalian hosts.
Author summary
Lyme disease, the most common vector-borne disease in the United States, is caused by the bacterium, Borrelia burgdorferi. This bacterium is transmitted to humans via the bite of a tick, and then spreads from the bite site to multiple tissues. Tick-to-human transmission of this bacterium requires bacterial survival in fed ticks because the blood and body fluid that ticks consume contain complement, an important mechanism that kills bacteria. To prevent host cell damage by this powerful mechanism, vertebrate animals produce factor H to inhibit complement. Lyme disease bacteria produce a surface protein CspA that binds to factor H to inhibit complement, but the role that CspA-mediated factor H-binding activity plays in tick-to-human transmission remains unexplained. To investigate this, we infected mice with Lyme disease strains that were identical except for the CspA variant or mutant with no factor H-binding ability they produced. We found that the factor H-binding activity of CspA appears to prevent the bacteria from being killed by complement in fed nymphal ticks. Such ability further facilitates bacterial transmission to mice. These results will promote the development of strategies against CspA to intervene in Lyme disease bacterial transmission from ticks to humans.
Introduction
Lyme disease is caused by spirochetes of Bbsl and is transmitted to humans by the bites of infected Ixodes ticks. It is the most common vector-borne disease in North America and Europe [1, 2]. Upon blood feeding, spirochetes migrate from the ticks’ midguts to salivary glands, where they are transmitted to the host’s skin at the tick bite sites [2, 3]. In humans, Lyme borreliae initiate local skin infection often leading to erythema migrans, commonly known as a “bull’s-eye” rash [1, 2]. If left untreated, spirochetes are capable of entering the bloodstream and spreading to multiple tissues and organs, leading to arthritis, carditis, neuroborreliosis, and acrodermatitis chronica atrophicans [4]. The three main Lyme disease causing species, B. afzelii, B. garinii, and B. burgdorferi sensu stricto (hereafter B. burgdorferi), survive not only in humans, but also in other vertebrate animals [5]. These spirochete species tend to be associated with different vertebrate hosts: B. afzelii is typically isolated from small mammals, B. garinii from birds, and B. burgdorferi from both hosts [6, 7]. Specific spirochete-host associations are thought to be caused by the selective ability of these spirochetes to evade innate immune responses of different hosts. One such immune response, complement, is the first-line defense mechanism in humans and other vertebrates [7, 8].
The fluid-phase of complement is comprised of serum proteins, which are sequentially activated in response to invading pathogens [9, 10]. Complement can be initiated by three different pathways (the classical, alternative, and lectin pathways) which result in the formation of two distinct C3 convertases, C4b2a and C3bBb [11]. These C3 convertases recruit other complement components to generate C5 convertases, resulting in the release of pro-inflammatory peptides (C3a and C5a), the deposition of opsonins (C3b and iC3b) on microbial surfaces, and in the case of gram-negative organisms, lysis via insertion of the pore-forming membrane attack complex (C5b-9 also known as MAC) [11]. Humans and other vertebrates produce complement regulators to down-regulate the excessive complement activity to prevent host cell damage from non-specific attack by complement [9]. For example, factor H (FH) and factor H-like protein-1 (FHL-1, the spliced form of FH [12]) bind to C3b to promote its cleavage to iC3b, which is hemolytically inactive [9]. Interestingly, FH sequences from different vertebrate animals are diverse (e.g. 60 to 70% identity between mice and humans), suggesting a selective adaptation of FH to efficiently regulate the host-specific complement [13, 14].
Invading pathogens produce a variety of surface components such as capsules, lipopolysaccharides (e.g., O-antigens), and complement regulator-binding proteins to combat complement-mediated killing [15–18]. These complement regulator-binding proteins recruit these complement regulators to promote pathogen survival in the blood or serum (also known as serum resistance) [19, 20]. Like other pathogens, Lyme borreliae produce surface proteins that bind complement regulators to block the formation of lethal pores generated by the MAC. The resulting localization (and hence locally high concentration) of complement regulators on the spirochete surface permits survival of Lyme borreliae despite high concentrations of complement in the blood [21, 22]. B. burgdorferi produces five complement regulator acquiring surface proteins (CRASPs) including CspA (CRASP-1), CspZ (CRASP-2), ErpP (CRASP-3), ErpC (CRASP-4), and ErpA (CRASP-5) [23]. These CRASP proteins share activities in binding to FH (for all 5 CRASPs) and FHL-1 (for CspA and CspZ) [23] and degrading C3 or C5 convertases by binding to plasminogen (for all 5 CRASPs) [24, 25]. Additionally, CspA also inhibits complement by binding to C7 and C9 to block the formation of MAC [24, 25]. Unlike other CRASP genes, cspA is expressed when Lyme borreliae are in both fed and unfed nymphal ticks or at the inoculation site immediately after infection, suggesting that CspA may play a unique role in tick-to-mammal transmission [26, 27]. Ectopic production of CspA confers resistance to human serum in an in vitro gain-of-function study [24, 25], while deleting cspA from an infectious and serum-resistant B. burgdorferi strain makes this strain susceptible in human serum in vitro [28, 29]. These results indicate that CspA functions as a key factor to promote spirochete survival in serum.
CspA is highly conserved within B. burgdorferi (greater than 90% identity) but displays less than 50% sequence identity across different spirochete species [25]. Consistent with this variation, CspA variants from B. burgdorferi, B. spielmannii, or B. afzelii exhibit varying capacities to bind human FH and differ in their abilities to resist human serum [25, 30, 31]. These findings led us to hypothesize that the CspA-mediated FH-binding activity promotes spirochete serum survival in a vertebrate animal-specific manner. In addition, while the role that CspA plays in vitro has been extensively characterized, the function of this protein for Lyme borreliae in the enzootic cycle is still unclear. In this study, we elucidate the role of CspA-FH interactions in promoting complement evasion in vitro and tick-to-host transmission of Lyme borreliae.
Results
Polymorphic CspA variants have differential binding affinities to factor H from different vertebrate animals
The sequences of CspA variants among B. burgdorferi sensu lato are extremely polymorphic (S1 Fig) [25]. We thus sought to examine whether CspA polymorphisms account for variant-to-variant differences in their abilities to bind to FH from different vertebrate species. Thus, we tested the ability of recombinant CspA proteins derived from cspA sequences of B. burgdorferi strain B31 (CspAB31), B. afzelii strain PKo (CspAPKo), or B. garinii strain ZQ1 (CspAZQ1) to bind to FH from different vertebrate animals. These animals include mouse (Mus musculus), Coturnix quail (rodent or avian model of Lyme disease [32, 33]), human (incidental host), and horse (dead-end host). We used ELISA and surface plasmon resonance (SPR) to evaluate the FH-binding affinity of CspA variants. A CspA mutant, CspAB31L246D, in which the leucine at position 246 was replaced by aspartic acid rendering it incapable of binding to human FH [34, 35], was included as negative control. As expected, while the irrelevant negative control protein B. burgdorferi DbpA did not bind to FH from these animals, both methods for assessing binding affinity indicated three distinct binding patterns of CspA variants in a host-specific manner (S2 and S3 Figs, and Table 1): (1) CspAB31 exhibited a versatile binding pattern to FH from all tested species (ELISA: KD = 0.23–0.92 μM, SPR: KD = 0.07–0.55 μM); (2) CspAPKo possessed less flexible binding, with preference for human and mouse FH (ELISA: KD = 0.16–0.76 μM, SPR: KD = 0.11–0.46 μM), but not horse or quail FH; (3) CspAZQ1 bound only to FH from quail (ELISA: KD = 0.36 μM). As expected, the recombinant CspAB31L246D, which retained a secondary structure similar to wild type CspAB31 by far-UV CD analysis (S1 and S4 Figs and S1 Table), was unable to bind to FH from any of the tested species (S2 and S3 Figs and Table 1). These results indicate that CspA variants from different Lyme borreliae species bind variably to FH from different animals, and that leucine-246 of CspAB31 is essential for its ability to bind to FH from all tested animals.
Table 1. CspA variants differ in binding to factor H from different animals.
| CspA variant | Factor H source | ELISA KD (μM)b | -----Surface Plasmon Resonance---- | ||
|---|---|---|---|---|---|
| KD (μM)a | kon (105s-1M-1) | koff (s-1) | |||
| B. burgdorferi | |||||
| CspAB31 | Human | 0.41±0.09 | 0.20±0.07 | 1.14±0.42 | 0.017±0.005 |
| Mouse | 0.23±0.08 | 0.07±0.01 | 1.88±0.36 | 0.012±0.005 | |
| Horse | 0.88±0.33 | 0.55±0.18 | 0.30±0.01 | 0.014±0.003 | |
| Quail | 0.92±0.28 | n.d.a | n.d. | n.d. | |
| CspAB31L246D | Human | n.s.b | n.b. | n.b. | n.b. |
| Mouse | n.s. | n.b. | n.b. | n.b. | |
| Horse | n.b.c | n.b. | n.b. | n.b. | |
| Quail | n.b. | n.d. | n.d. | n.d. | |
| DbpAB31d | Human | n.b. | n.d. | n.d. | n.d. |
| Mouse | n.b. | n.d. | n.d. | n.d. | |
| Horse | n.b. | n.d. | n.d. | n.d. | |
| Quail | n.b. | n.d. | n.d. | n.d. | |
| B. afzelii | |||||
| CspAPko | Human | 0.16±0.05 | 0.11±0.02 | 1.52±0.62 | 0.014±0.004 |
| Mouse | 0.76±0.64 | 0.46±0.14 | 1.15±0.31 | 0.048±0.011 | |
| Horse | n.b. | n.b. | n.b. | n.b. | |
| Quail | n.b. | n.d. | n.d. | n.d. | |
| B. garinii | |||||
| CspAZQ1 | Human | n.b. | n.b. | n.b. | n.b. |
| Mouse | n.b. | n.b. | n.b. | n.b. | |
| Horse | n.b. | n.b. | n.b. | n.b. | |
| Quail | 0.36±0.13 | n.d. | n.d. | n.d. | |
All values represent the mean ± SEM of three experiments.
aNot determined.
bNot saturated
cNo binding activity was detected.
dDbpAB31 was included as a negative control.
Allelic variable CspA promotes spirochete binding to FH in a host-specific manner
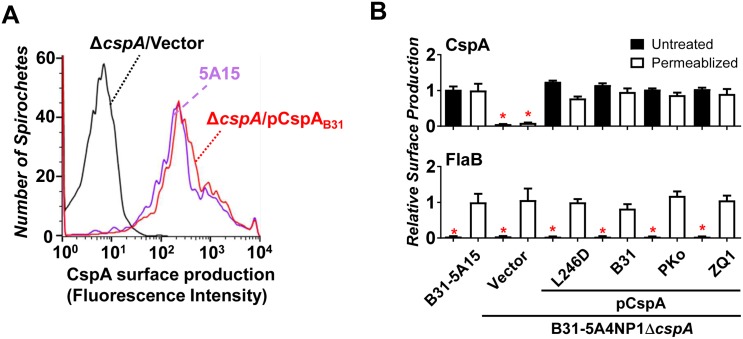
To examine whether the FH-binding characteristics of CspA proteins described above are maintained when these variants are produced on the surface of the spirochetes, we transformed shuttle plasmids encoding cspAB31, cspAPKo, cspAZQ1, or cspAB31L246D under the control of the cspA promoter from B. burgdorferi strain B31 into B. burgdorferi strain B31-5A4NP1ΔcspA (5A4NP1ΔcspA), a cspA deficient strain previously generated from an infectious background B. burgdorferi strain B31-5A4NP1 (S2 Table) [28]. Because strain 5A4NP1ΔcspA was identified to have lost the plasmid lp21, B. burgdorferi strain B31-5A15 (B31-5A15), which has the same plasmid profile as strain 5A4NP1ΔcspA and is fully virulent, was used as a wild type (WT) positive control [28, 36]. We first used flow cytometry analysis to verify that the CspA variants or mutants produced in strain 5A4NP1ΔcspA are localized on the surface of spirochetes and at levels similar to the WT strain B31-5A15 (Fig 1).
Fig 1. Localization of CspA on the surface of B. burgdorferi.
Flow cytometry analysis of CspA localized on the surface of B. burgdorferi strains B31-5A15 (“B31-5A15”), B31-5A4NP1ΔcspA harboring the vector pBSV2G (“ΔcspA/Vector”), or this cspA mutant strain producing CspAB31 (“ΔcspA/pCspAB31”), CspAPKo (“ΔcspA/pCspAPKo”), CspAZQ1 (“ΔcspA/pCspAZQ1”), or CspAB31L246D (“ΔcspA/pCspAB31L246D”). (A) Representative histograms of flow cytometry analysis showing the levels of CspA surface production to indicated B. burgdorferi strains. (B) The production of CspA and FlaB (negative control) on the surface of indicated B. burgdorferi strains was detected by flow cytometry (see Materials and methods). Values are shown relative to the production levels of CspA or FlaB on the surface of permeabilized B. burgdorferi strain B31-5A15. Each bar represents the mean of four independent determinations ± the standard deviation. (*): indicates that relative surface production of the indicated proteins was significantly lower (P < 0.05 by one-way ANOVA with post hoc Bonferroni correction) than that of CspA or FlaB by B. burgdorferi strain B31-5A15.
We then incubated each of the CspA-producing 5A4NP1ΔcspA-derived strains, strain 5A4NP1ΔcspA harboring the vector alone (hereafter termed 5A4NP1ΔcspA-V), or the WT strain B31-5A15, with FH purified from human, mouse, horse, or quail. We then determined their FH-binding activity using flow cytometry. A high passage and non-infectious B. burgdorferi strain B313 (B313) harboring the shuttle vector alone was also included as a negative control as this strain does not encode cspA and does not bind human FH [37]. Consistent with previous findings [37], strain B313 (vector alone) did not bind human FH (Fig 2A right panel), or to FH from mouse, horse, and quail (Fig 2B to 2D right panel). The WT strain B31-5A15 bound FH from all these animals (Fig 2A to 2D right panel), in agreement with findings in previous studies [21, 28]. Strain 5A4NP1ΔcspA-V displayed undetectable levels of human FH-binding activity (Fig 2A), similar to previous observations [28]. Note that the infectious B. burgdorferi strain B31, the background strain of 5A4NP1ΔcspA-V, produces other human FH-binding proteins when cultivated in vitro [23, 26, 38]. However, these FH-binding proteins are either produced in extremely low levels [26] or display lower levels of FH-binding ability, compared to CspA [28, 39, 40]. These previous studies support our finding that nearly no human FH-binding activity was observed in this strain. Additionally, strain 5A4NP1ΔcspA-V did not show binding ability to mouse, horse, or quail FH (Fig 2B to 2D). These data indicate that CspA is essential for this infectious B. burgdorferi strain to bind to FH from all tested animals.
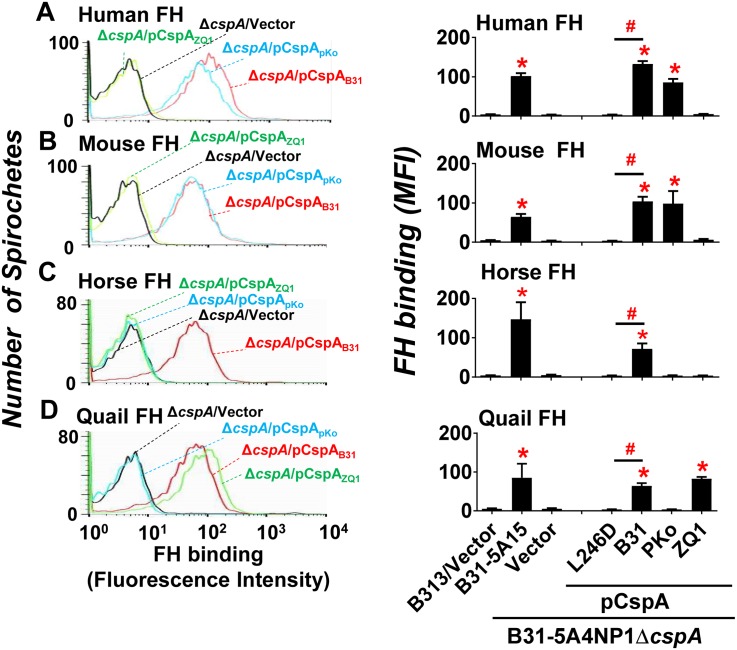
Fig 2. CspA variants differ in their ability in promoting spirochetes binding to FH from different vertebrate animals.
B. burgdorferi strain B31-5A15 (“B31-5A15”), B31-5A4NP1ΔcspA harboring the vector pBSV2G (“ΔcspA/Vector”), or this cspA mutant strain producing CspAB31 (“ΔcspA/pCspAB31”), CspAPKo (“ΔcspA/pCspAPKo”), CspAZQ1 (“ΔcspA/pCspAZQ1”), or CspAB31L246D (“ΔcspA/pCspAB31L246D”), or B313 carrying the vector pBSV2G (“B313/Vector”, negative control) was incubated with FH from human, mouse, horse, or quail. The bacteria were stained with a sheep anti-FH polyclonal IgG (for the spirochetes incubated with human, mouse, or horse FH) or a mouse anti-FH monoclonal antibody VIG8 (for the spirochetes incubated with quail FH) followed by an Alexa 647-conjugated donkey anti-sheep IgG or goat anti-mouse IgG prior to being applied to flow cytometry analysis. (Left panel) Representative histograms of flow cytometry analysis showing the levels of FH from (A) human, (B) mouse, (C) horse, or (D) quail binding to indicated B. burgdorferi strains. (Right panel) The levels of B. burgdorferi binding to FH from (A) human, (B) mouse, (C) horse, or (D) quail were measured by flow cytometry and presented as mean fluorescence index (MFI). Each bar represents the mean of three independent determinations ± SEM. Significant differences (P < 0.05 by one-way ANOVA with post hoc Bonferroni correction) in the levels of FH binding relative to the B313/Vector (“*”) or between two strains relative to each other (“#”).
Further, expression of CspA variants in strain 5A4NP1ΔcspA restores the levels of binding to FH in a host-specific manner, reflecting the results obtained with the recombinant proteins (Fig 2): (1) The production of CspAB31 restored binding to FH from human, mouse, horse, and quail; (2) CspAPKo increased binding to human and mouse FH but not to horse or quail FH; (3) CspAZQ1 promoted binding only to quail FH but not to FH from other animals. Additionally, the cspAB31L246D-complemented strain showed no detectable binding to FH from all four animals tested (Fig 2A to 2D right panel). We also incubated the above-mentioned spirochete strains with C3-depleted human or mouse serum (C3-depleted horse or quail serum is not available) to verify the FH-binding activity in the context of serum components. C3-depleted serum was used because the strains that show reduced FH-binding would have greater levels of C3b deposited on their surfaces [28], which in turn would bind FH thereby confounding interpretation of results. We observed binding of human and mouse FH in C3 depleted sera as with purified FH (S5 Fig). These results indicate that the leucine-246 of this protein plays an essential role in facilitating spirochete binding to FH from these animals.
Species to species variation of CspA-mediated FH binding contributes to different levels of complement deposition on the spirochete surface
We next aimed to determine the role of host-specific FH-binding of CspA variants in inhibiting complement deposition on the spirochete surface. We first incubated human, mouse, or horse serum with strain 5A4NP1ΔcspA-V or this strain producing CspAB31, CspAPKo, CspAZQ1, or CspAB31L246D as well as the WT strain B31-5A15 or the negative control strain B313. The levels of C3 fragments (C3b and iC3b) and the MAC bound by spirochetes were quantified using flow cytometry. Quail serum was not included as antibodies against quail C3b or MAC were not available. FH-depleted serum was not used as the lack of FH in serum causes an uncontrollable complement activation, which consumes C3 resulting in no C3b/iC3b deposition on spirochete surface [41]. Consistent with previous observations [42], a significant amount of C3b and MAC was detected on the surface of the strain B313 carrying vector alone upon incubation with human (Fig 3B and 3D top panel), mouse, or horse serum (Fig 3B and 3D middle and bottom panels). Conversely, incubation of the WT strain B31-5A15 with serum from these animals resulted in at least 2-fold reduction of C3b deposition compared to strain B313, and virtually undetectable MAC deposition (Fig 3B and 3D, p < 0.05). Strain 5A4NP1ΔcspA-V had similar levels of C3b and MAC deposition compared to strain B313 (Fig 3B and 3D p > 0.05)[28]. These results indicate that CspA is required to inhibit human and non-human complement bound to the spirochete surface.
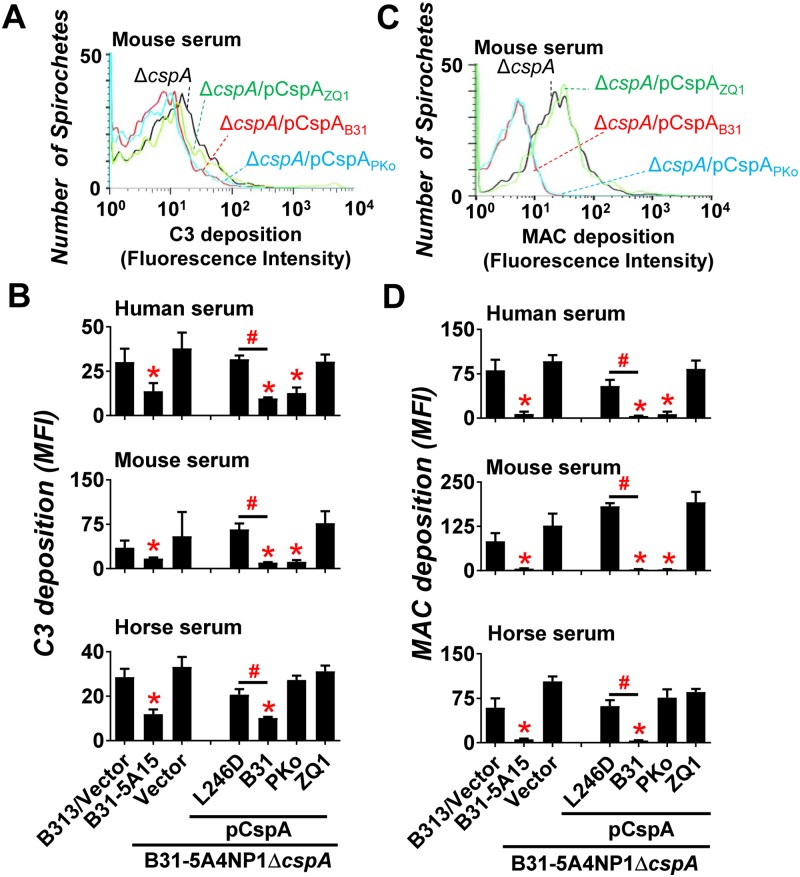
Fig 3. CspA variants differ in their ability to reduce the deposition of C3b or MAC from different vertebrate animals on the spirochete surface.
B. burgdorferi strain B31-5A15 (“B31-5A15”), B31-5A4NP1ΔcspA harboring the vector pBSV2G (“ΔcspA/Vector”), or this cspA mutant strain producing CspAB31 (“ΔcspA/pCspAB31”), CspAPKo (“ΔcspA/pCspAPKo”), CspAZQ1 (“ΔcspA/pCspAZQ1”), or CspAB31L246D (“ΔcspA/pCspAB31L246D”), or B313 carrying the vector pBSV2G (“B313/Vector”, negative control) was incubated with serum from human, mouse, or horse with a final concentration of 20%. The bacteria were stained with a guinea pig anti-C3 polyclonal IgG, a mouse anti-C5b-9 monoclonal antibody aE11 (for spirochetes incubated with human or horse serum), or a rabbit anti-C5b-9 polyclonal IgG (for spirochetes incubated with mouse serum) followed by an Alexa 647-conjugated goat anti-guinea pig IgG or goat anti-mouse IgG, or goat anti-rabbit IgG prior to being applied to flow cytometry analysis. Representative histograms of flow cytometry analysis showing the deposition levels of mouse (A) C3b or (C) MAC on the surface of indicated B. burgdorferi strains. The deposition levels of (B) C3b or (D) MAC of indicated animals on the surface of B. burgdorferi were measured by flow cytometry and presented as mean fluorescence index (MFI). Each bar represents the mean of three independent determinations ± SEM. Significant differences (P < 0.05 by one-way ANOVA with post hoc Bonferroni correction) in the deposition levels of C3b or MAC relative to the B313/Vector (“*”) or between two strains relative to each other (“#”).
We also observed a correlation between the origin of the serum and the ability of CspA variants to inhibit deposition of C3 fragment or MAC: (1) Compared to strain 5A4NP1ΔcspA-V, expression of cspAB31 significantly decreased levels of C3b and MAC deposition on the spirochete surface in human serum (Fig 3B and 3D top panels, consistent with a previous finding [25]) and in mouse or horse sera (Fig 3A and 3C, the middle and bottom panels of Fig 3B and 3D). (2) Expression of cspAPKo in the strain 5A4NP1ΔcspA significantly reduced human and mouse C3b and MAC deposition compared to strain 5A4NP1ΔcspA-V (Fig 3A and 3C, top and middle panels of Fig 3B and 3D), in agreement with a previous observation [25], but not in horse serum (bottom panels of Fig 3B and 3D). (3) Expression of cspAZQ1 resulted in similar C3b and MAC deposition as the strain 5A4NP1ΔcspA-V in all three sera (Fig 3A to 3D). We also compared the complement activating abilities of isogenic 5A4NP1ΔcspA producing either CspAB31 or the FH-binding deficient point mutant CspAB31L246D. Although CspAB31 has also been shown to bind complement C7 and C9, and plasminogen [24, 25, 43], CspAB31L246D was selectively defective in FH-binding, but still bound to C7, C9, or plasminogen at levels similar to CspAB31 (S1 Table and S6 Fig). Thus, the CspAB31L246D producing strain showed significantly greater levels of C3 and MAC deposition than the strain producing CspAB31 (Fig 3B and 3D), suggesting that the FH-binding activity of CspA mediates inhibition of complement deposition on the surface of B. burgdorferi.
CspA-mediated FH-binding activity facilitates bacterial serum survival in a host-specific fashion
The ability of pathogens to inhibit the host complement in the bloodstream correlates with their ability to survive in the serum [7]. Thus, we sought to investigate how species-to-species variation of CspA promotes bacterial survival in serum from different vertebrate hosts. We incubated WT strain B31-5A15, as well as strain 5A4NP1ΔcspA-V or this plasmid encoding cspAB31, cspAPKo, cspAZQ1, or cspAB31L246D with serum from human, horse, or quail and negative control serum (C3-depleted or heat treated human serum) for four hours. Mouse serum was not used, as its complement is highly labile ex vivo [44], and the ability to kill spirochetes in vitro has not been consistently observed [45, 46]. More than 75% of the strain B31-5A15 survived in human serum or serum without active complement (C3-depleted serum) (Fig 4A and 4B). In addition, this strain survived in horse and quail serum, with average spirochete serum survival above 75% (Fig 4C and 4D). Less than 20% of the strain 5A4NP1ΔcspA-V remained motile after incubation with human serum (less than the survival percentage of WT strain B31-5A15, p < 0.05), but more than 85% of the strain 5A4NP1ΔcspA-V survived in the C3-depleted or heat inactivated human serum (Fig 4A and 4B). Similarly, 5A4NP1ΔcspA-V was killed by horse and quail serum, indicating that CspA plays an essential role in promoting spirochete survival in not only human serum as shown previously [28], but also in horse and quail serum (Fig 4C and 4D).
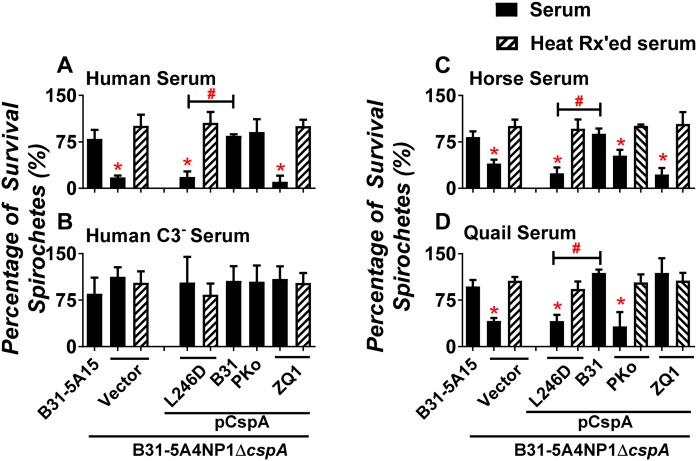
Fig 4. CspA variants mediate distinct levels of spirochete survival in serum from different vertebrate animals.
B. burgdorferi strain B31-5A15 (“B31-5A15”), B31-5A4NP1ΔcspA harboring the vector pBSV2G (“ΔcspA/Vector”), or this cspA mutant strain producing CspAB31 (“ΔcspA/pCspAB31”), CspAPKo (“ΔcspA/pCspAPKo”), CspAZQ1 (“ΔcspA/pCspAZQ1”), or CspAB31L246D (“ΔcspA/pCspAB31L246D”) was incubated for four hours with untreated (filled bars) or heat-inactivated (HI, hatched bars) serum with a final concentration of 40%. These sera include (A) human serum or (B) C3-depleted human serum (Human C3- serum) or the serum from (C) horse or (D) quail. The number of motile spirochete was assessed microscopically. The percentage of survival for those B. burgdorferi strains was calculated using the number of mobile spirochetes at four hours post incubation normalized to that prior to the incubation with serum. Each bar represents the mean of three independent determinations ± SEM. Significant differences (P < 0.05 by one-way ANOVA with post hoc Bonferroni correction) in the percentage survival of spirochetes relative to the ΔcspA/Vector (“*”) or between two strains relative to each other (“#”).
We also found that CspA variants produced on the strain 5A4NP1ΔcspA confer serum survival in a host-specific manner: (1) More than 75% of motile cspAB31-complemented B. burgdorferi were detected when incubated with sera from all these animals (Fig 4A, 4C and 4D). (2) A cspAPKo-complemented strain was able to survive in human serum (Fig 4A), but less than 50% of the spirochetes survived when incubated with horse or quail sera (Fig 4C and 4D). (3) Less than 20% of a cspAZQ1-complemented strain survived following the treatment with human or horse serum (Fig 4A and 4C), but over 75% of this strain survived in quail serum (Fig 4D). Additionally, less than 25% of cspAB31L246D-complemented strain remained motile after treatments with human, horse, or quail serum, which was about three-fold lower than the cspAB31-complemented strain (Fig 4A, 4C and 4D). Conversely, more than 75% of cspAB31L246D-complemented strain survived in either C3-depleted or heat treated-serum, suggesting that the FH-binding ability of CspA promotes spirochete survival in serum (Fig 4A to 4D).
B. burgdorferi CspA is up-regulated in post-molting but down-regulated in post-feeding nymphal ticks
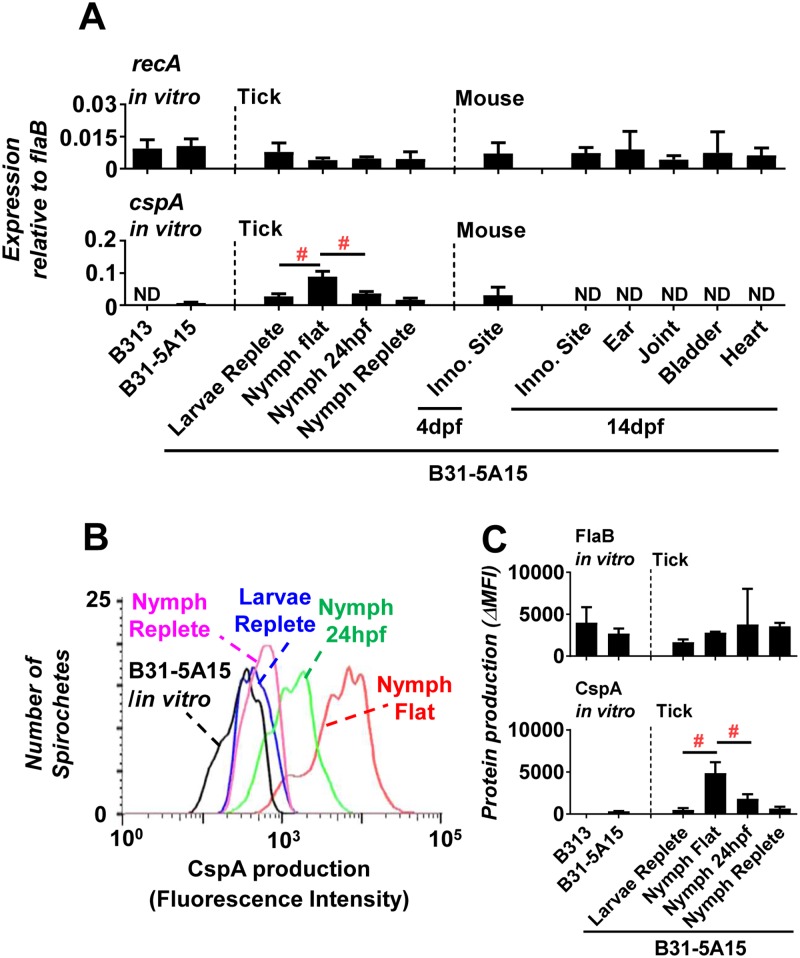
We and others have demonstrated the binding of FH to CspA in vitro and the role of CspA in inactivating complement on spirochete surface and serum survival [25, 28, 30, 31] (Figs 2 to 4). How CspA promotes Lyme infection in vivo is still unclear. Thus, we examined regulation of CspA throughout the enzootic cycle (S7B Fig). The naïve larval Ixodes scapularis ticks were first allowed to feed on mice previously infected with the infectious B. burgdorferi strain B31-5A15. After the engorged and infected larval ticks molted into nymphal ticks, they were placed on naïve C3H/HeN mice for blood feeding. We then determined the expression levels of spirochete genes cspA, recA, and flaB in ticks and mouse tissues using quantitative RT-PCR and calculated the expression levels of cspA and recA relative to that of flaB. recA expression levels relative to flaB levels remained unchanged throughout the enzootic cycle (Fig 5A top panel). As reported previously [26], cspA expression was detected in both larval and nymphal ticks, as well as at tick bite sites on the mouse skin at 72 hours post feeding, but not in mouse tissues after spirochetes disseminate (Fig 5A bottom panel). B. burgdorferi in flat nymphs expressed more than 2-fold increased levels of cspA compared to levels prior to molting (replete larvae, p < 0.05), but the spirochetes’ cspA expression was reduced approximately 2-fold in nymphs following 24 hours of feeding, compared to expression levels in flat nymphs (Fig 5A bottom panel, p < 0.05).
Fig 5. B. burgdorferi expresses distinct levels of CspA at different stages of enzootic cycle.
C3H/HeN mice were infected with 105 B. burgdorferi strain B31-5A15. At 14 days post infection, the uninfected I. scapularis larval ticks were allowed to feed on these mice to repletion. After the replete larvae molted into nymphs, these B. burgdorferi-infected nymphs were allowed to feed on naïve C3H/HeN mice for different period of time or to repletion. The mice were euthanized at 7 or 14 days post feeding (“7dpf” or “14dpf”) to collect the inoculation site of skin (for mice at 7 and 14 days post feeding), ears, tibiotarsus joints, bladder, and heart (for mice at 14 days post feeding). RNA was extracted from replete larvae (“larvae replete”), flat nymphs (“nymph flat”), fed nymphs at 24 hours post feeding (“24hpf”) or replete nymphs (“nymph replete”) as well as in vitro cultured B. burgdorferi strain B313 (“B313”, negative control) or B31-5A15 (“B31-5A15”, positive control) in BSKII medium. (A) RNA was also extracted from mouse tissues including tick biting site (“inoc. site”), ears, tibiotarsus joints, bladder, and heart at 7 and/or 14 days post feeding. The extracted RNA was then used to determine the expression levels of cspA and constitutive expressed genes flaB and recA using qRT-PCR (see Materials and methods). The expression levels of (Top panel; negative control) recA and (Bottom panel) cspA are presented by normalizing the expression levels of flaB (negative control) (see Materials and methods). Each bar represents the mean of five independent determinations ± SEM (“#”). Significant differences (P < 0.05 by one-way ANOVA with post hoc Bonferroni correction) in the normalized expression levels of cspA between two conditions relative to each other. (B and C) The bacteria isolated from ticks or in vitro cultured B. burgdorferi strains were applied to flow cytometry. (B) Representative histograms of flow cytometry analysis showing the production levels of CspA in B. burgdorferi in the indicated environment. (C) The production of FlaB (Top panel; negative control) and CspA (Bottom panel) are presented as “ΔMFI”, the mean fluorescence index obtained from each of these strains subtracting that obtained from the strains stained only by the secondary antibody. Each bar represents the mean of three independent determinations ± SEM. Significant differences (P < 0.05 by one-way ANOVA with post hoc Bonferroni correction) in the production of CspA relative to the B313 cultured in vitro (“*”) or between two conditions relative to each other (“#”).
We next sought to investigate CspA production in different stages of ticks. A previous study reported that the production of CspA could not be visually detected in spirochetes using fluorescence microscopy when spirochetes are present in nymphs [47]. One possibility for this discrepancy is that visual detection using microscopy may be subjective and not be sensitive enough to detect small differences in protein production [26]. We therefore gently lysed different stages of ticks, sorted B. burgdorferi from the tick lysates by their granularity and size, and quantitated the levels of CspA (and a constitutively produced protein FlaB) in the spirochetes using flow cytometry (S8 Fig). The strain B313, which does not carry the plasmid encoding cspA, was included as a negative control. There was no significant difference of FlaB production by strain B31-5A15 and the negative control strain B313 cultured in vitro (Fig 5C top panel). Additionally, we did not observe a significant difference of FlaB production by strain B31-5A15 in different stages of ticks (Fig 5C top panel). In spirochetes cultured in vitro, CspA production was detected in B31-5A15, but not in the strain B313 (Fig 5C bottom panel). Interestingly, we were able to detect B. burgdorferi CspA production in all examined stages of the ticks (significantly greater MFI values than strain B313; p < 0.05) (Fig 5C bottom panel). Further, the CspA production more than quadrupled in flat nymphs compared to fed larvae (p < 0.05), but thereafter halved after nymphs had fed for 24 hours (Fig 5B and 5C bottom panel). Our findings on the spirochetes’ CspA protein production in ticks are consistent with the dynamic changes of cspA mRNA levels (Fig 5A)[26], suggesting that CspA is up-regulated after larval ticks molt but is down-regulated after nymphal ticks feed.
The in vitro FH-binding activity of CspA is correlated with spirochete survival in nymphs and transmission to mice
We then examined the role of CspA to facilitate spirochete survival in the enzootic cycle. As expected, when we inoculated C3H/HeN mice by subcutaneous needle infection with strain 5A4NP1ΔcspA-V or the WT strain B31-5A15 (S7A Fig), these strains colonized tissues to similar degrees (S9 Fig, 14 days post infection). Similarly, at early stages of infection, strain 5A4NP1ΔcspA-V colonized the skin of the mouse inoculation site and triggered bacteremia with a burden similar to the WT strain B31-5A15 (S10 Fig, 4 days post infection). These results indicate that CspA is not essential during early and disseminated stages of mouse infection through needle inoculation.
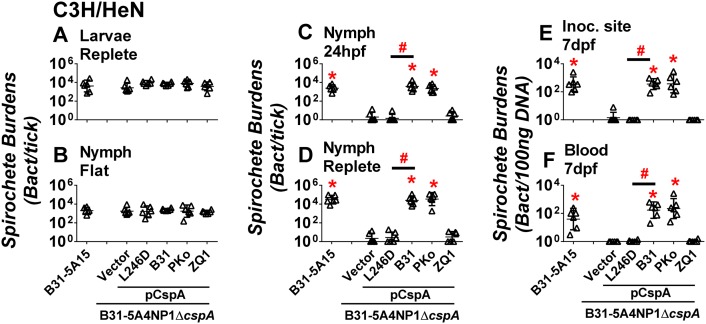
WT strain B31-5A15 and the strain 5A4NP1ΔcspA-V display similar levels of infectivity at 14 days post needle infection in mice (S9 Fig). Thus, to examine whether CspA plays a role in vivo during tick infection, we allowed the larval ticks to feed until replete on mice previously infected for 14 days with either WT strain B31-5A15 or strain 5A4NP1ΔcspA-V (S7B Fig). Nymphal ticks that developed after replete larval ticks molted were fed on naïve C3H/HeN mice until removed or replete, and the bacterial burdens in the nymphs and nymph-infected mouse blood and tissues were determined (S7B Fig). The bacterial loads of the strain 5A4NP1ΔcspA-V did not differ from the WT strain B31-5A15 in fed larvae or flat nymphs (Fig 6A and 6B). However, strain 5A4NP1ΔcspA-V did not survive in nymphs fed for 24, 48, or 72 hours or in replete nymphs (approximately 1000-fold less than the WT strain, Fig 6C and 6D and S11 Fig). Further, this strain was incapable of surviving in the mouse blood and colonizing tissues at 7 and 14 days post nymph feeding (at least 40-fold less than the WT strain, Fig 6E and 6F and S12 Fig). These results indicate that CspA enables the spirochete to survive in fed nymphs, which subsequently permits spirochete transmission to mice.
Fig 6. FH-binding ability of CspA promotes spirochete survival in nymphal ticks upon feeding.
C3H/HeN mice were infected with 105 B. burgdorferi strain B31-5A15 (“B31-5A15”), B31-5A4NP1ΔcspA harboring the vector pBSV2G (“ΔcspA/Vector”), or this cspA mutant strain producing CspAB31 (“ΔcspA/pCspAB31”), CspAPKo (“ΔcspA/pCspAPKo”), CspAZQ1 (“ΔcspA/pCspAZQ1”), or CspAB31L246D (“ΔcspA/pCspAB31L246D”). At 14 days post infection, the uninfected I. scapularis larval ticks were allowed to feed on each of these mice until they are replete. After the replete larvae molt into nymphs, those B. burgdorferi-infected nymphs were allowed to feed on naïve C3H/HeN mice to repletion. The bacterial loads in the (A) replete larvae (“larvae replete”), (B) flat nymphs (“nymph flat”), fed nymphs at (C) 24 hours post feeding (“nymph 24hpf”) or (D) replete nymphs (“nymph replete”), or (E) the site where nymphal ticks fed (“inoc. site”) or (F) blood at 7 days post nymph feeding (“blood 7dpf”) were determined by qPCR. The bacterial loads in mouse tissues or blood were normalized to 100 ng total DNA. Shown are the geometric mean of bacterial loads ± 95% confidence interval of six ticks or mice per group. Significant differences (P < 0.05 by one-way ANOVA with post hoc Bonferroni correction) in the spirochete burdens relative to the ΔcspA/Vector (“*”) or between two strains relative to each other (“#”).
We then investigated the ability of CspA variants to promote spirochete survival in infected nymphs and in mice infected by tick infection. Mice were initially infected by needle injection with the strain 5A4NP1ΔcspA producing CspAB31, CspAZQ1, or CspAPKo. All strains exhibited similar levels of tissue colonization (S9 Fig). After the larval ticks fed on these mice and molted into nymphs, the infected nymphs were placed on naïve mice (S7B Fig). All strains displayed similar levels of survival in fed larvae and flat nymphs (Fig 6A and 6B). The isogenic strain producing CspAB31 or CspAPko was able to survive in fed nymphs, colonize the mouse inoculation site, and survive in blood at the levels at least 150-fold more than the strain 5A4NP1ΔcspA-V (P < 0.05) (Fig 6C to 6F). Conversely (and similar to 5A4NP1ΔcspA-V), the isogenic strain producing CspAZQ1 did not survive in nymphs fed for 24 hours or in replete nymphs and was incapable of surviving in the mouse blood and colonizing tissues (p > 0.05, Fig 6C to 6F). We then compared the burdens of the isogenic B. burgdorferi strain producing CspAB31 or the point mutant CspAB31L246D at different stages of tick and mouse infection. Similar levels of both strains were seen in fed larvae as well as in flat nymphs (Fig 6A and 6B). Interestingly, no CspAB31L246D-producing isogenic spirochetes were detectable in fed nymphs or at mouse inoculation site and in the bloodstream (similar strain 5A4NP1ΔcspA-V, p >0.05) (Fig 6C to 6F). These findings suggest that the in vitro FH-binding ability of CspA is correlated with spirochete survival in fed nymphs and with subsequent transmission to the mammalian host.
FH binding to CspA enables complement evasion to permit spirochete survival in fed nymphal ticks and transmission to mammalian hosts
We have demonstrated that CspA is critical to promote spirochete survival in nymphal ticks in the first 24 hours of feeding (Fig 6). At this time point, the small amount of blood and the interstitial fluid that contain complement components enter nymphs’ midguts [48, 49]. The fact that the majority of B. burgdorferi have been found in the tick midgut at this time point (and is thus in contact with the host blood and interstitial fluid) [48, 50] led us to hypothesize that CspA-mediated complement inactivation facilitates host complement evasion by spirochetes in fed nymphs. Thus, we fed the nymphs infected with the WT strain B31-5A15 or the strain 5A4NP1ΔcspA-V on a mouse strain deficient in C3, the central molecule of complement required for opsonophagocytosis and formation of MAC (S7C Fig) [51]. WT BALB/c mice, which the C3-deficient mice were back-crossed into, served as controls. The spirochete burdens in the nymphs prior to and post feeding on mice were determined using qPCR. Consistent with infection in C3H/HeN mice, strain 5A4NP1ΔcspA-V was undetectable in the nymphs fed on WT BALB/c mice (Fig 7A and 7B, left panel) and at the bite site of the mouse skin, or in the blood (Fig 7C and 7D, left panel). However, this strain survived in nymphs fed on C3-deficient mice for 24 hours or to repletion at levels indistinguishable from the WT strain B31-5A15 (p > 0.05, Fig 7A and 7B, right panel). Strain 5A4NP1ΔcspA-V was also observed at bite sites and in the blood of C3-deficient mice at 7 days post nymph feeding (Fig 7C and 7D, right panel). These results suggest that CspA plays an essential role in evading the complement present in fed nymphs and for tick-to-mammal transmission.
Fig 7. CspA-mediated FH-binding activity facilitates spirochete evading complement present in fed nymphs resulting in nymph-to-mouse transmission.
C3H/HeN mice were infected with 105 B. burgdorferi strain B31-5A15 (“B31-5A15”), B31-5A4NP1ΔcspA harboring the vector pBSV2G (“ΔcspA/Vector”), or this cspA mutant strain producing CspAB31 (“ΔcspA/pCspAB31”), CspAPKo (“ΔcspA/pCspAPKo”), CspAZQ1 (“ΔcspA/pCspAZQ1”), or CspAB31L246D (“ΔcspA/pCspAB31L246D”). At 14 days post infection, the uninfected I. scapularis larval ticks were allowed to feed on each of these mice until they are replete. After the replete larvae molt into nymphs, those B. burgdorferi-infected nymphs were allowed to feed on naïve BALB/c or BALB/c C3-/- mice to repletion. The bacterial loads in the nymphs fed on BALB/c (Left panel) or BALB/c C3-/- (Right panel) for (A) 24 hours post feeding (“24hpf”) or (B) replete nymphs (“replete”), or at (C) the site where nymphs fed (“inoc. site”) on mice or (D) mouse blood at 7 days post nymph feeding (“7dpf”) were determined by qPCR. The bacterial loads in mouse tissues or blood were normalized to 100 ng total DNA. Shown are the geometric mean of bacterial loads ± 95% confidence interval of six ticks or mice per group. Significant differences (P < 0.05 by one-way ANOVA with post hoc Bonferroni correction) in the spirochete burdens relative to the ΔcspA/Vector (“*”) or between two strains relative to each other (“#”).
We then examined the ability of B. burgdorferi strain B31-5A4NP1ΔcspA complemented with cspAB31, cspAZQ1, or cspAPKo to survive in nymphs fed on WT BALB/c or C3-deficient mice. In agreement with findings using WT C3H/HeN mice, the cspAB31- or cspAPKo- but not cspAZQ1-complemented B. burgdorferi was detectable in the nymphs fed on WT BALB/c mice (Fig 7A and 7B, left panel) and at the bite sites of the skin and in blood of these mice (Fig 7C and 7D, left panel). Interestingly, the strain 5A4NP1ΔcspA-complemented with each of these three cspA alleles exhibited nearly identical bacterial levels in nymphs fed on C3-deficient mice for 24 hours or to repletion (Fig 7A and 7B, right panel). These cspA-complemented strains also colonized the tick bite sites and bloodstream at 7 days after the onset of nymph feeding at levels similar to WT bacteria (Fig 7C and 7D, right panel). These results suggest that the CspA variants differentially promote spirochete complement evasion in fed nymphs, which is required for spirochete transmission from ticks to mammals.
Nymphs infected with the cspAB31- or cspAB31-L246D-complemented strain were also allowed to feed on WT or C3-deficient BALB/c mice to test how FH binding by CspA promotes complement evasion in fed nymphs and facilitates tick-to-mammal transmission. We observed that the cspAB31L246D-complemented strain displayed approximately 5000-fold lower burdens than the cspAB31-complemented strain in nymphs fed on BALB/c mice for 24 hours or to repletion (Fig 7A and 7B, left panel). Similar to C3H/HeN mice, the cspAB31L246D strain was not detectable at the bite site or blood of BALB/c mice (Fig 7C and 7D, left panel). However, this mutant strain was detected in nymphs fed on C3-deficient mice for 24 hours, replete nymphs (Fig 7A and 7B, right panel), and at the bite sites and blood of C3 deficient mice at 7 days post feeding at levels similar to the cspAB31-complemented strain (Fig 7C and 7D, right panel). Our findings thus strongly suggest that the in vitro FH-binding activity of CspA is correlated with the ability of B. burgdorferi to evade mouse complement in fed nymphs, which would promote spirochete survival in those ticks and facilitate transmission from ticks to mammalian hosts.
Discussion
Each of the Lyme borreliae species has been associated with specific vertebrate host(s) [7, 52, 53]. This spirochete-host association has been correlated with the ability of B. burgdorferi to survive in the blood (or serum) from the corresponding hosts [7, 54–56], but the mechanism that drives this association is still unclear. An attractive hypothesis is that the spirochetes exhibit host-specific immune evasion, which leads to the observed spirochete-host association. This could be due to variable spirochete outer surface proteins that interact with components of host complement in a host-specific manner. One such protein is CspA, which displays variant-to-variant differences in binding to human FH [25]. However, the recombinant CspA protein from B. burgdorferi strain B31 is incapable of binding to any other vertebrate animals’ FH, when the FH has been subjected to SDS-PAGE followed by a far-western blot [57]. In contrast, FH from the serum of multiple animals including human and mouse recognizes CspA variants run on a similar blot [58, 59]. This discrepancy may be due to structural alternations of animals’ FH on SDS-PAGE and the following far-western blot [8, 22, 23]. Therefore, we further verified the binding of CspA to FH from mouse, horse, and quail by demonstrating that the CspA of three main Lyme borreliae (B. burgdorferi, B. afzelii, or B. garinii) bind to purified FH in a host-specific manner. Additionally, we observed that the ability of different CspA variants to bind to FH correlates with their ability to inhibit complement activation on the spirochete surface and facilitate spirochete survival in serum in a host-specific manner. We found that these correlations not only apply to human complement (consistent with a previous study [28]) but also to complement of other animals. Further, the high-resolution structure of CspA suggests the recombinant version of this protein forms a dimer [35]. Two FH-binding regions have been localized on the central cleft of the CspA dimer and on the C-termini of this protein including leucine-246 [34, 60]. By ectopically producing a CspA point mutant (CspA-L246D) selectively lacking FH-binding ability on spirochetes, we further demonstrated that the CspA-mediated FH-binding activity contributes to the inactivation of host complement and spirochete survival in sera. Note that similar non-polar features of this position (leucine-246) of CspAB31 and the equivalent location of other CspA variants (phenylalanine-237 of CspAPKo and leucine-252 of CspAZQ1) suggests a possibility that this amino acid is critical for the FH-binding activity of these variants (S1 Fig).
FH is polymorphic between humans and mice (61% amino acid identity) [13, 14]. However, we have demonstrated a similar ability of CspA to bind to both human and mouse FH as well as inhibit complement deposition of both human and mouse sera on the spirochete surface. These findings imply that CspA plays a similar role in mouse and human during infections. Thus, the murine model was selected to test the role of the CspA-mediated FH binding activity. In addition, we have demonstrated that spirochetes require CspA to survive in fed nymphs during tick-to-mouse transmission. This result is consistent with the fact that cspA is uniquely expressed when spirochetes are in fed nymphs [26]. In contrast, CspA was not essential for spirochetes to be transferred from mouse to larvae or establish infection in mouse skin at early stages of infection, even though cspA expression was detectable at these stages. This may be due to the previous observation that other genes encoding functionally redundant FH-binding proteins (e.g. cspZ, erpP, and erpA) are co-expressed with cspA in spirochetes at these stages [26]. Unexpectedly, CspA was detected in spirochetes when spirochetes are in flat nymphs even though this protein was not required for B. burgdorferi to survive in these ticks. One possibility is that spirochetes may need to maintain certain levels of CspA in preparation to survive in the first 24 hours of feeding when the small amount of blood and interstitial fluid containing complement components enter the ticks’ midgut. This possibility is supported by the observations that a cspA deficient B. burgdorferi is eliminated from human serum within 1 hour [28], but a significant shift in gene expression is not detectable until the spirochetes are treated with blood for 48 hours [61].
In addition to CspA, B. burgdorferi requires other proteins to promote persistent survival in fed nymphs and to be transmitted to vertebrate animals [62–66]. Some of these proteins have been thought to be important for nutrient acquisition and metabolism in ticks [62, 67–72], while the functions of the other proteins are still unclear. Blood and interstitial fluid from vertebrate hosts contain diverse innate immune defense mechanisms, including complement [9]. In fact, B. burgdorferi displays increased infectivity in C3-deficient mice, which lack the ability to deposit opsonic C3 fragments or to generate the pore-forming MAC that can lyse spirochetes [73, 74]. This finding suggests that spirochetes need to evade hosts’ complement to survive in vertebrate animals. Moreover, Rathinavelu et al. reported that the blood meals from either WT or C3-deficient mice do not eliminate the WT B. burgdorferi in fed ticks [75], raising the possibility that spirochetes produce factors to facilitate the complement evasion in fed ticks. In this study, we observed a clear correlation of CspA variants or mutants in their FH-binding activity with their ability to promote spirochete survival in nymphs fed on WT mice and tick-to-mouse transmission. Conversely, this correlation was not detectable when these ticks fed on C3-deficient mice. These findings identify that CspA-mediated FH-binding activity is necessary for the spirochetes’ evasion of complement in fed nymphs and eventually to be transmitted to mammalian hosts. It is noteworthy that Woodman et al. reported that FH-deficient mice were susceptible to WT B. burgdorferi infection, leading to the conclusion that spirochetes did not require FH-binding activity to evade mouse complement [74]. However, the lack of the FH leads to the spontaneous complement activation and subsequent complement consumption in these mice, rendering them functionally complement deficient [41]. Thus, FH-deficient mice cannot be used to study the role of FH-binding microbial proteins in complement evasion.
It is noteworthy that B. garinii ZQ1 was originally isolated from ticks [76]. Therefore, the infectivity of this strain in vertebrate animals is still unclear. We found that variant to variant differences of CspA-mediated FH-binding activity are correlated with these variants’ ability to confer spirochete transmission from nymphs to mice. Particularly, the cspA-ZQ1-complemented strain infected C3-deficient mice but not wild type mice via ticks. Thus, our observation of CspA-ZQ1 selectively binding to quail FH implies that this variant may promote spirochete infectivity in quail by evading this animal’s complement. (Note that the complement among different avian hosts may vary. Thus, that one CspA variant promotes infectivity in quail may not necessarily indicate that the same variant contributes to the infectivity in other birds). More in-depth studies on avian hosts promoted by CspA-ZQ1 are warranted. In this study, we demonstrated the molecular mechanisms by which CspA of B. burgdorferi facilitates complement evasion of spirochetes in fed nymphal ticks (Fig 8). The host-specific differences in FH-binding capabilities conferred by CspA variants illuminate the possibility of a complement-driven host-specificity and selective transmission of Lyme disease spirochetes.
Fig 8. A proposed model showing CspA-mediated FH-binding activity promotes spirochete evasion of complement in feeding nymphs to facilitate tick-to-host transmission.
CspA of spirochetes binds to FH present in the host’s blood and interstitial fluid in feeding nymphs to facilitate spirochete escape from the complement-mediated killing. Such CspA-mediated immune evasion ultimately facilitates spirochete transmission from nymphs to hosts.
Materials and methods
Ethics statement
All mouse experiments were performed in strict accordance with all provisions of the Animal Welfare Act, the Guide for the Care and Use of Laboratory Animals, and the PHS Policy on Humane Care and Use of Laboratory Animals. The protocol was approved by the Institutional Animal Care and Use Committee (IACUC) of Wadsworth Center, New York State Department of Health (Protocol docket number 16–451), and University of Massachusetts Medical School (Protocol docket number 1930). All efforts were made to minimize animal suffering.
Mouse, tick, and bacterial strains
C3H/HeN, BALB/c and Swiss-Webster mice were purchased from Charles River (Boston, MA) and Taconic (Hudson, NY), respectively. C3-/- mice (C57BL/6) purchased from Jackson Laboratory (Bar Harbor, ME) were backcrossed for 11 generations into BALB/c background. Mice were genotyped for the C3 allele by PCR analysis of mouse tail DNA. Ixodes scapularis tick larvae were purchased from National Tick Research and Education Center, Oklahoma State University (Stillwater, OK) or obtained from BEI Resources (Manassas, VA). B. burgdorferi-infected nymphs were generated as described in the section “Mouse infection experiments by ticks.” The Borrelia and Escherichia coli strains used in this study are described in S2 Table. E. coli strains DH5α, M15, and derivatives were grown in Luria-Bertani (BD Bioscience, Franklin lakes, NJ) broth or agar, supplemented with kanamycin (50 μg/mL), ampicillin (100 μg/mL), or no antibiotics where appropriate. All B. burgdorferi, B. afzelii, and B. garinii strains were grown in BSK-II completed medium supplemented with kanamycin (200 μg/mL), streptomycin (50 μg/mL), gentamicin (50 μg/mL), or no antibiotics (see S2 Table).
Generation of recombinant CspA proteins and antisera
The open reading frames lacking the putative signal sequences of bba68 (cspAB31) from B. burgdorferi strains B31 or zqa68 (cspAZQ1) from B. garinii strain ZQ1 were amplified using primers listed in S3 Table to generate recombinant histidine-tagged CspA proteins. In addition, an altered open reading frame encoding CspAB31L246D (residue 26 to 252 of CspAB31 with leucine-246 replaced by aspartate) was amplified using the primers described in S3 Table. Amplified fragments were engineered to encode a BamHI site at the 5’ end and a stop codon followed by a SalI site at the 3’ end. PCR products were sequentially digested with BamHI and SalI and then inserted into the BamHI and SalI sites of pQE30Xa (Qiagen, Valencia, CA). The plasmids were transformed into E. coli strain M15, and the plasmid inserts were sequenced (Wadsworth ATGC core facility). The resulting M15 derived strains and the M15 strain carrying the plasmid encoding the open reading frames lacking the putative signal sequences of bafPKo_A0067 (cspAPKo) from B. afzelii strains PKo [25] were used to produce respective recombinant CspA variants or mutants (S2 Table). The histidine-tagged CspA variants or mutants were produced and purified by nickel affinity chromatography according to the manufacturer’s instructions (Qiagen, Valencia, CA). Antisera against CspAB31, CspAPKo, or CspAZQ1 were generated by immunizing four-week-old Swiss Webster mice with each of the CspA proteins as described previously [77]. The ability of each of these antisera to recognize CspAB31, CspAPKo, CspAZQ1, or CspAB31L246D was verified using ELISA. Basically, one microgram of the above-mentioned CspA variants or mutant proteins, or BSA (negative control) was immobilized on microtiter plates (MaxiSorp, ThermoFisher). The antisera raised from the mice immunized with each of these CspA variants (1: 1, 000x) were added to the wells. The pre-immune mouse serum was also included as a negative control. HRP-conjugated goat anti-mouse IgG (1: 1,000x) (ThermoFisher) was then added as antibody to detect the binding of the mouse anti-sera to CspA variants. The plates were washed three times with PBST (0.05% Tween 20 in PBS), and 100 μL of ortho-phenylenediamide dihydrochloride solution (Sigma-Aldrich) were added to each well and incubated for five minutes. The reaction was stopped by adding 50 μL of 2.6M hydrosulfuric acid to each well. Plates were read at 405nm using a Tecan Sunrise Microplate reader (Tecan, Morrisville NC). The anti-sera of CspAB31, CspAPKo, CspAZQ1, or CspAB31L246D exhibited similar levels of binding to each of these CspA variants or mutant proteins (S13 Fig).
Circular Dichroism (CD) spectroscopy
CD analysis was performed on a Jasco 810 spectropolarimeter (Jasco Analytical Instrument, Easton, MD) under nitrogen. CD spectra were measured at room temperature (RT, 25°C) in a 1 mm path length quartz cell. Spectra of CspAB31 (10 μM) or CspAB31L246D (10 μM) were recorded in phosphate based saline buffer (PBS) at RT, and three far-UV CD spectra were recorded from 190 to 250 nm for far-UV CD in 1 nm increments. The background spectrum of PBS without proteins was subtracted from the protein spectra. CD spectra were initially analyzed by the software Spectra Manager Program (Jasco). Analysis of spectra to extrapolate secondary structures and the prediction of the spectrum using the amino acid sequences of CspAB31 were performed by Dichroweb (http://dichroweb.cryst.bbk.ac.uk/html/process.shtml) using the K2D and Selcon 3 analysis programs [78].
Purification of horse and quail FH
The procedure to purify FH from serum of various vertebrate animals has been described previously [79]. Basically, the serum collected from horses originally from New Zealand, (ThermoFisher, Waltham, MA) or Coturnix coturnix quail (Canola Live Poultry Market, Brooklyn, NY) was centrifuged to remove aggregates prior to being diluted with two volumes deionized water. Then, 6g of cyanogen bromide (CNBr)-activated Sepharose 4B resin (GE Healthcare, Piscataway, NJ) was mixed with 100mg of Trinitrophenyl-Bovine Serum Albumin (TNP-BSA) (LGC Biosearch Technology, Petaluma, CA) for 2 hours followed by incubation with the blocking buffer (PBS with 100mM ethanolamine-HCl, 150mM NaCl at pH8.5) at room temperature for 2 hours. The TNP-BSA CNBr resin was then equilibrated with PBS and packed into a column. After the diluted serum was applied into the TNP-BSA CNBr column, the column was washed by PBS until the OD280 values of the effluent below 0.04 Arbitrary Unit. Bound proteins were then eluted by the elution buffer (PBS with 0.5mM EDTA and 1M sodium chloride at pH7.4). The eluent was subsequently applied to a NAb Protein G Spin Column (ThermoFisher) according to the manufacturer’s instruction to remove the immunoglobulin in the serum. The purified factor H was confirmed by ELISA [77]. A sheep anti-FH polyclonal IgG (ThermoFisher) (1:200x), which has been shown to recognize horse FH [58] or a mouse anti-FH monoclonal antibody VIG8 (1: 200x), which has been observed to recognize avian FH [80] was used as a primary antibody. A horse radish peroxidase (HRP) conjugated donkey anti-sheep (1:2,000x) (ThermoFisher) or goat anti-mouse (1: 1,000x) was used as a secondary antibody.
FH binding assay by quantitative ELISA
Quantitative ELISA for FH, C7, C9, or plasminogen binding by CspA proteins was performed similarly to that previously described [78]. For FH binding, one microgram of BSA (negative control; Sigma-Aldrich) or FH from human (ComTech, Tyler, Texas), mouse (MyBiosource, San Diego, CA)[81], horse, or quail was coated onto microtiter plate wells. For FH binding, one hundred microliters of increasing concentrations (0.03125, 0.0625, 0.125, 0.25, 0.5, 1, 2 μM) of histidine-tagged DbpA from B. burgdorferi strain B31 (negative control) or a CspA variant or mutant, including CspAB31, CspAPKo, CspAZQ1, or CspAB31L246D was then added to the wells. Mouse anti-histidine tag (Sigma-Aldrich, St. Louis, MO; 1:200) and HRP-conjugated goat anti-mouse IgG (1: 1,000x) were used as primary and secondary antibodies, respectively, to detect the binding of histidine-tagged proteins. The plates were washed three times with PBST (0.05% Tween 20 in PBS), and 100 μL of tetramethyl benzidine (TMB) solution (ThermoFisher) were added to each well and incubated for five minutes. The reaction was stopped by adding 100 μL of 0.5% hydrosulfuric acid to each well. Plates were read at 405 nm using a Tecan Sunrise Microplate reader. To determine the dissociation constant (KD), the data were fitted by the following equation using GraphPad Prism software (Version 7, La Jolla, CA).
| (1) |
Surface plasmon resonance (SPR)
Interactions of CspA with FH were analyzed by a SPR technique using a Biacore 3000 (GE Healthcare). Ten micrograms of FH from human, mouse, or horse were conjugated to a CM5 chip (GE Healthcare) as described previously [78]. A control flow cell was injected with PBS without FH. For quantitative SPR experiments to determine FH-binding, ten microliters of increasing concentrations of CspA variants or mutants, including CspAB31, CspAPKo, CspAZQ1, or CspAB31L246D, were injected into the control cell and flow cell immobilized with different animals’ FH, human plasminogen (Sigma-Aldrich), C7 (ComTech), or C9 (ComTech) at 10 μL/min, 25°C. To obtain the kinetic parameters of the interaction, sensogram data were fitted by means of BIAevaluation software version 3.0 (GE Healthcare), using the one step biomolecular association reaction model (1:1 Langmuir model), resulting in optimum mathematical fit with the lowest Chi-square values.
Shuttle plasmid construction
cspAB31, cspAPKo, cspAZQ1, or cspAB31L246D was first PCR amplified with the addition of a SalI site and a BamHI site at the 5’and 3’ ends, respectively, using Taq polymerase (Qiagen) and the primers (see S3 Table) to generate the plasmids encoding cspA alleles. The unpaired nucleotides at 5’ and 3’ end of the amplified DNA fragments were removed by exonuclease from CloneJet PCR cloning kit (ThermoFisher) and then inserted into the vector pJET1.2/blunt (ThermoFisher). The plasmids were then digested with SalI and BamHI to release the cspA alleles, which were then inserted into the SalI and BamHI sites of pBSV2G (S2 Table) [82]. The promoter region of cspA from B. burgdorferi B31, 400bp upstream from the start codon of cspA, was also PCR amplified. SphI and SalI sites were added at the 5’and 3’ ends of amplified DNA, respectively, using primers pcspAfp and pcspArp (S3 Table). Promoter fragments were then inserted into the SphI and SalI sites of pBSV2G to drive the expression of cspAB31, cspAPKo, cspAZQ1, and cspAB31L246D.
Plasmid transformation into B. burgdorferi
Electrocompetent B. burgdorferi B31-5A4NP1ΔcspA prepared as described [77, 83] was transformed separately with 80 μg of each of the shuttle plasmids encoding cspAB31, cspAPKo, cspAZQ1, or cspAB31L246D (S2 Table) and cultured in BSK II medium at 33°C for 24 hours. Liquid plating transformations were performed in the presence of antibiotic selection (50 μg/mL gentamicin, 200μg/mL kanamycin, 50μg/mL streptomycin, as required), as described previously [84, 85]. After incubation at 33°C in 5% CO2 for two weeks, kanamycin-, gentamicin-, and streptomycin-resistant colonies of cspA-complemented B. burgdorferi were obtained and expanded at 33°C in liquid BSK II medium containing these antibiotics, followed by genomic DNA preparation as previously described [86]. PCR was performed with primers (S3 Table) specific for aphI (encoding the kanamycin resistance gene), aacC1 (encoding the gentamicin resistance gene), and aadA (encoding the streptomycin resistance gene) to verify their presence in the transformants. The plasmid profiles of the cspA deficient mutant complemented with cspA alleles were examined as described previously [36]. The plasmid profiles of these strains were found to be identical to those of the parental strain 5A4NP1ΔcspA and the strain 5A4NP1ΔcspA-V.
Surface localization of CspA detected by flow cytometry
The determination of surface localization of CspA by Flow cytometry has been described previously [77, 87, 88]. Basically, 1 x 108 B. burgdorferi cells producing CspAB31, CspAPKo, CspAZQ1, or CspAB31L246D were washed three times with HBSC buffer containing glucose and BSA (25 mM Hepes acid, 150 mM sodium chloride, 1 mM MnCl2, 1 mM MgCl2, 0.25 mM CaCl2, 0.1% glucose, and 0.2% BSA, final concentration) and then resuspended into 500 μL of the same buffer. To prepare permeabilized spirochetes, 1 × 108 B. burgdorferi was incubated with 100% methanol for an hour, followed by washing three times with HBSC buffer containing glucose and BSA. A mixture of mouse antisera raised against CspAB31, CspAPKo, or CspAZQ1 or monoclonal mouse antibody against B. burgdorferi FlaB (negative control) was used as a primary antibody (1:250x). An Alexa 647-conjugated goat anti-mouse IgG (ThermoFisher) (1:250x) was used as a secondary antibody. Three hundred microliters of formalin (0.1%) were then added for fixing. Surface production of CspA was measured by flow cytometry using a Becton-Dickinson FACSCalibur (BD Bioscience). All flow cytometry experiments were performed within two days of collection of B. burgdorferi samples. Spirochetes in the suspension were distinguished on the basis of their distinct light scattering properties in the flow cytometer equipped with a 15 mW, 488 nm air-cooled argon laser, a standard three-color filter arrangement, and CELLQuest Software (BD Bioscience). The mean fluorescence index (MFI) of each sample was obtained from FlowJo software (Three-star Inc, Ashland, OR) representing the surface production of the indicated proteins. Mean Fluorescence Index (MFI) normalized to that of CspA from permeabilized B. burgdorferi obtained from S5 Fig was used to compare the surface production of CspA in different strains. These results represent the mean of three independent determinations ± the standard deviation of mean. Each standard deviation of mean value was no more than 7% of its mean value.
FH-binding assay of B. burgdorferi detected by flow cytometry
To quantitatively determine the ability of B. burgdorferi strains producing CspAB31, CspAPKo, CspAZQ1, or CspAB31L246D in binding to FH, 1 x 107 B. burgdorferi strains were washed twice by PBS, resuspended into 100μL of the same buffer, and then incubated with FH from human, mouse, horse, or quail (1 μg per reaction) or C3-depleted human serum (ComTech) or serum from BALB/c C3-/- mice (Final concentration: 20%) at 25°C for 1 hour. Following incubation, the spirochetes were washed three times with PBS and resuspended in 100μL of HBSC buffer containing DB. A sheep anti-FH polyclonal IgG (ThermoFisher) (1:250x), which has been shown to recognize FH from human, mouse and horse [58] or a mouse anti-FH monoclonal antibody VIG8 (1:250x), which has been observed to recognize avian FH [80] were used as primary antibodies. An Alexa 647-conjugated donkey anti-sheep IgG (ThermoFisher) (1: 250x) or goat anti-mouse IgG (1:250x) was used as secondary antibodies. Three hundred microliters of formalin (0.1%) was then added for fixing. The mean fluorescence index (MFI) of each B. burgdorferi strain was measured to determine the FH-binding capability promoted by CspA variants or mutants using a Becton-Dickinson FACSCalibur and analyzed by FlowJo software as described above.
C3b and MAC deposition assay on B. burgdorferi detected by flow cytometry
The deposition of C3b and MAC on the surface of B. burgdorferi producing CspAB31, CspAPKo, CspAZQ1, or CspAB31L246D was quantitatively determined by flow cytometry as revised from previous reported methodologies [89]. B. burgdorferi strains were washed twice, resuspended in PBS, and then incubated with serum from human (MP Biomedical, Santa Ana, CA), mouse (Southern Biotech, Birmingham, AL), horse, or quail (Final concentration: 20%) at 25°C for 1 hour. Twenty percent serum was used in this study because more than 80% of B. burgdorferi strains were capable of surviving in this concentration of serum [28], but C3b and MAC have been constantly detected on spirochete surface when these strains are incubated with 20% serum [28]. Prior to being mixed with B. burgdorferi, those sera were screened with the C6 Lyme ELISA kit (Diamedix, Miami Lakes, FL) to determine whether the individual from which it was collected had prior exposure to B. burgdorferi by detecting the antibody against the C6 peptide of a B. burgdorferi protein VlsE [90]. Then, the spirochetes were washed three times with PBS and resuspended in HBSC buffer containing glucose and BSA. A guinea pig anti-C3 polyclonal IgG (ThermoFisher) (1:250x), which has been shown to recognize C3 from human, mouse and horse, was used as a primary antibody to detect C3b. A mouse anti-C5b-9 monoclonal antibody aE11 (ThermoFisher) (1:250x), which has been observed to recognize MAC from human and horse, and a rabbit anti-C5b-9 polyclonal IgG (Abcam, Cambridge, MA) (1:250x), which has been verified to bind to MAC from human and mouse, were used as primary antibodies. An Alexa 647-conjugated goat anti-guinea pig IgG (ThermoFisher) (1:250x), goat anti-mouse IgG (ThermoFisher) (1:250x), or goat anti-rabbit IgG (ThermoFisher) (1:250x) were used as secondary antibodies. Three hundred microliters of formalin (0.1%) were then added for fixing. The mean fluorescence index (MFI) of each B. burgdorferi strain was measured to determine the levels of C3b or MAC deposition on the surface of B. burgdorferi strains using a Becton-Dickinson FACSCalibur and analyzed by FlowJo software as described above.
Serum sensitivity assay
The serum sensitivity of B. burgdorferi strain B31-5A15, B31-5A4NP1ΔcspA-V and this cspA mutant strain producing CspAB31, CspAPKo, CspAZQ1, or CspAB31L246D was measured using a published procedure [91]. Briefly, triplicate samples of each strain were grown to mid-log phase and diluted to a final concentration of 5×106 bacteria per milliliter into BSKII medium without rabbit serum with a final concentration of 40% normal serum from human, mouse, horse, or quail or C3-depleted human serum. We also included the spirochetes mixed with 40% heat-inactivated serum from these vertebrate hosts, which was incubated at 55 °C for 2 hours prior to being mixed with spirochetes. At 0 and 4 hours after the addition of serum, an aliquot was taken from each condition and counted by Petroff-Hausser counting chamber (Hausser Scientific, Horsham, PA) using a Nikon Eclipse E600 darkfield microscope (Nikon, Melville, NY). Though the strain B31-5A4NP1ΔcspA has been shown to be eliminated by incubating human serum (final concentration 40%) in one hour [28], the survival of the B31-5A4NP1ΔcspA-derived strains was evaluated at 4 hours post incubation to delineate the potential partial serum survival of these strains. The percentage of survival for those B. burgdorferi strains was calculated using the number of mobile spirochetes at 4 hours post incubation normalized to that prior to the incubation with serum.
Mouse infection experiments using needle inoculation
Four-week-old female C3H/HeN mice were used for experiments involved in needle infection of B. burgdorferi. Mice were infected by intradermal injection as previously described [77] with 105 of different strains of B. burgdorferi strain B31-5A15, B31-5A4NP1ΔcspA-V or this cspA mutant strain producing CspAB31, CspAPKo, CspAZQ1, or CspAB31L246D. The plasmid profiles and the presence of the shuttle vector of each of these B. burgdorferi strains were verified prior to infection as described to ensure the stability of the vector and no loss of plasmids (S7A and S7B Fig) [36]. Mice were sacrificed at 14 days post-infection, the inoculation site of the skin, the tibiotarsus joints, ears, bladder, and heart were collected to quantitatively evaluate levels of colonization during infection.
Mouse infection experiments by ticks
The procedure of the tick infection has been shown in S7B and S7C Fig and described previously [92]. Basically, four-week-old male and female C3H/HeN mice were infected with 105 of B. burgdorferi strain B31-5A15, the cspA knockout mutant strain B31-5A4NP1ΔcspA-V or this cspA mutant strain producing CspAB31, CspAPKo, CspAZQ1, or CspAB31L246D by intradermal injection as described above. The ear punches from those mice were collected and placed into BSKII medium at 7 days post infection, and the spirochete growth in the medium was evaluated to confirm the infection of these mice. At 14 days post infection, the uninfected larvae were allowed to feed to repletion on those B. burgdorferi-infected C3H/HeN mice as described previously [92]. Approximately 100 to 200 larvae were allowed to feed on each mouse. The engorged larvae were collected and allowed to molt into nymphs in 4 to 6 weeks in a desiccator at room temperature and 95% relative humidity in a room with light dark control (light to dark, 12: 12 hours). DNA was extracted from engorged larvae and post molting flat nymphs to examine the plasmid profiles and the presence of the shuttle vector the B. burgdorferi strains carried by these ticks as described to ensure no loss of plasmids during acquisition and molting (S7B Fig) [36]. The flat nymphs molted from larvae were placed in a chamber on four to six-week old male and female C3H/HeN, BALB/c, or C3-/- mice in BALB/c background as described [93]. Ten nymphs were allowed to feed on each mouse. After the nymphs were forcibly removed by forceps at 24, 48, or 72 hours post feeding, the rest of the ticks were allowed to feed to repletion. The mice were then euthanized at 7 or 14 days after tick feeding, and the blood, the feeding site of the skin, the tibiotarsus joints, bladder, ears, and heart were collected.
Quantification of cspA expression levels using quantitative RT-PCR
The B. burgdorferi strain B31-5A15, ticks, or mouse tissues were mixed with glass beads and then homogenized by a Precellys 24 High-Powered Bead Mill Homogenizer (Bertin, Rockville, MD). RNA was extracted from these homogenized bacteria, ticks or tissue samples using Direct-Zol RNA MiniPrep Plus Kit (Zymo Research, Irvine, CA) according to the manufacturer’s instructions. Contaminating DNA was removed using RQ1 RNase-Free DNase (Promega, Madison, WI) following vendor’s instruction. cDNA was synthesized from 1 μg of RNA measured by spectrophotometer using a qScript cDNA SuperMix (Quanta Bioscience, Beverly, MA) according to the manufacturer’s instructions. Then, the quantification of cspA, flaB, or recA expression from cDNA was performed using an Applied Biosystems 7500 Real-Time PCR system (ThermoFisher) in conjunction with PowerUp SYBR Green Master Mix (ThermoFisher), based on amplification of the B. burgdorferi cspA, flaB, or recA gene using primers BBCspAfp and BBCspArp (for cspA), BBFlaBfp and BBFlaBrp (for flaB), or BBRecAfp and BBRecArp (for recA) as described previously [94] (S3 Table), respectively. Cycling parameters for SYBR green-based reactions were 50°C for 2 minutes, 95°C for 10 minutes, 45 cycles of 95°C for 15 seconds, and 60°C for 1 minute. Melting curve analysis for purity was performed on each sample by performing 80 cycles of increasing temperature for 10 seconds, each beginning at 55°C. Three biological replicates were included. Each biological replicate was run in duplicates and checked for intra-run variation. The gene expression of cspA or recA was normalized to that of flaB using the ΔCT method, where the relative expression of target (cspA or recA), normalized to the expression of flaB, is given by 2−ΔCT, where Ct is the cycle number of the detection threshold (see Eq 2). All analyses and calculations were performed using the Applied Biosystem sequence detection software version 7.5.1 (ThermoFisher).
| (2) |
Determination of CspA production levels of B. burgdorferi in ticks using flow cytometry
Ticks mixed with Enzyme Free cell dissociation buffer (ThermoFisher) were gently disrupted by pipette tips and then incubated at 37°C for 30 minutes to release the spirochetes into the buffer. The ticks-spirochetes mixtures were subsequently spun down, washed by PBS, and permeabilized by incubating the mixture with 100% methanol for one hour. After these mixtures were washed three times with HBSC buffer containing glucose and BSA, they were incubated with mouse antisera raised against CspAB31 (1:250x) or monoclonal mouse antibody against FlaB (1:250x) as primary antibody followed by an Alexa 647-conjugated goat anti-mouse IgG (1:250x) as a secondary antibody. Three hundred microliters of formalin (0.1%) were then added for fixing. The spirochetes were first sorted using a FACSAria cell sorter II equipped with FACSDiva software (BD Bioscience). The purity of sorted populations was greater than 70% in all experiments (S8 Fig). Then, the production of CspA and FlaB (negative control) in these spirochetes was measured by this equipment and software. The mean fluorescence index (MFI) of each sample was obtained from FlowJo software representing the production of the indicated proteins. The “ΔMFI” values are the mean fluorescence index obtained from each of these strains subtracting that obtained from the strains stained only by the secondary antibody. The production levels of CspA and FlaB in different stages of the enzootic cycle and in vitro cultured B. burgdorferi were presented as “ΔMFI” (Fig 5C). These results represent the mean of three independent determinations ± the standard deviation of mean.
Quantification of B. burgdorferi in infected ticks, tissues and blood samples
The ticks collected from the chambers on the mice were mixed with glass beads and then homogenized by a Precellys 24 High‑Powered Bead Mill Homogenizer (Bertin, Rockville, MD). The DNA from mouse tissues or blood or homogenized ticks was extracted using the EZ-10 Genomic DNA kit (for mouse tissues and blood, Biobasic, Amherst, New York) or the insect DNA kit (for ticks, OMEGA Biotek, Norcross, GA). The quantity and quality of DNA for each tissue sample have been assessed by measuring the concentration of DNA and the ratio of the UV absorption at 280 to 260 using a nanodrop 1000 UV/Vis spectrophotometer (ThermoFisher). The amount of DNA used in this study was 100 ng for each sample, and the 280:260 ratio was between 1.75 to 1.85, indicating the lack of contaminating RNA or proteins. Quantitative PCR (qPCR) was then performed to quantitate bacterial loads, using 100 ng of DNA per reaction. B. burgdorferi genomic equivalents were calculated using an Applied Biosystems 7500 Real-Time PCR system (ThermoFisher) in conjunction with PowerUp SYBR Green Master Mix (ThermoFisher), based on amplification of the B. burgdorferi recA gene using primers BBRecAfp and BBRecArp (S3 Table), as described previously [77]. Cycling parameters for SYBR green-based reactions were 50°C for 2 minutes, 95°C for 10 minutes, 45 cycles of 95°C for 15 seconds, and 60°C for 1 minute. The number of recA copies was calculated by establishing a threshold cycle (Ct) standard curve of a known number of recA gene extracted from B. burgdorferi strain B31, then comparing the Ct values of the experimental samples. To determine that the shuttle vectors expressing CspA variants is not missing during tick-mouse studies, the colE1 gene on the shuttle vector was amplified using primers BBColE1fp and BBColE1rp (S3 Table) and the same cycling parameters used to amplify recA described above. The bacterial burdens determined using colE1 primers were compared with the burdens obtained using primers to amplify the recA gene, the gene on the chromosome of spirochetes. The shuttle vectors were not missing as the bacterial loads determined using colE1 primers is close to 100% of the bacterial loads determined using recA primers. To assure the low signals were not simply a function of the presence of PCR inhibitors in the DNA preparation, we subjected 5 samples from blood, tibiotarsus joints, and bladder of the mice infected by B. burgdorferi strain B31-5A15, B31-5A4NP1ΔcspA-V or this cspA mutant strain producing CspAB31, CspAPKo, CspAZQ1, or CspAB31L246D to qPCR using mouse nidogen primers mNidfp and mNidrp (S3 Table) as an internal standard [77]. As predicted, we detected 107 copies of the nidogen gene from 100ng of each DNA sample, ruling out the presence of PCR inhibitors in these samples.
Statistical analysis
Significant differences between samples were determined using Student’s T test or the one-way ANOVA with post hoc Bonferroni correction. P-values were determined for each sample. A P-value < 0.05 (*) or (#) was considered to be significant.
Supporting information
The amino acid sequences of CspA variants from B. burgdorferi strains B31 (Bburg_B31_BBA68) and ZS7 (Bburg_ZS7_A59), B. afzelii strains MMS (Bafz_MMS_MMSA71) and PKo (Bafz_PKo_A0067), B. garinii strain ZQ1 (Bgar_ZQ1_ZQA68), B. bavariensis strain PBi (Bbav_PBi_BGA66), and B. spielmanii strain A14S (Bspiel_A14S_A0067) were aligned using M-Coffee with default parameters. Black shaded residues are identical among all of these variants. The red asterisk indicates the leucine-246 of CspAB31, which is required for human, mouse, horse, and quail FH-binding activity of this protein.
(TIF)
The indicated concentrations of various recombinant histidine-tagged CspAB31 (“B31”), CspAPKo (“PKo”), CspAZQ1 (“ZQ1”), CspAB31L246D (“L246D”), or DbpA (“DbpA”, negative control) were added to triplicate wells coated with FH from (A) human, (B) mouse, (C) horse, or (D) quail, and protein binding was quantitated by ELISA. The experiments were performed on three independent occasions; within each occasion, samples were run in duplicate. All experiments were performed with a single preparation of recombinant proteins. Shown is a representative experiment from the average of two replicates. The KD values (Table 1) representing the FH-binding affinity of each protein were determined from the average of three experiments.
(TIF)
Ten micrograms of FH from (A to C) human, (D to F) mouse, or (G and H) horse were conjugated on a SPR chip, resulting the response unit (RU) as 563, 511, and 433 for human, mouse, and horse FH, respectively. Different concentrations (0.125 to 2 or 4μM) of histidine tagged CspAB31 (“B31”), CspAPKo (“PKo”), CspAZQ1 (“ZQ1”), or CspAB31L246D (“L246D”) were flowed over a surface of the chip. Binding was measured in response units (RU) by SPR (see Materials and methods). The experiments were performed on three independent occasions; within each occasion, samples were run in duplicate. All experiments were performed with a single preparation of recombinant proteins. The kon, koff, and KD values (Table 1) were determined from the average of these three experiments. Panel A, D, and F are representative experiments applying 1 μM of indicated CspA proteins to the chip coated with FH from indicated species.
(TIF)
Far-UV CD analysis of CspAB31 and CspAB31L246D. The molar ellipticity, Φ, was measured from 190 to 250 nm for 10μM of each protein in PBS. The predicted spectrum of CspAB31 was generated applying the full-length amino acid sequences of this protein to DichroWeb (http://dichroweb.cryst.bbk.ac.uk/html/links.shtml).
(TIF)
B. burgdorferi strain B31-5A15 (“B31-5A15”), B31-5A4NP1ΔcspA harboring the vector pBSV2G (“ΔcspA/Vector”), or this cspA mutant strain producing CspAB31 (“ΔcspA/pCspAB31”), CspAPKo (“ΔcspA/pCspAPKo”), CspAZQ1 (“ΔcspA/pCspAZQ1”), or CspAB31L246D (“ΔcspA/pCspAB31L246D”), or B313 carrying the vector pBSV2G (“B313/Vector”, negative control) was incubated with C3-depleted human or mouse serum. The bacteria were stained with a sheep anti-FH polyclonal IgG followed by an Alexa 647-conjugated donkey anti-sheep IgG prior to being applied to flow cytometry analysis. (Left panel) Representative histograms of flow cytometry analysis showing the levels of FH from (A) human or (B) mouse binding to indicated B. burgdorferi strains. (Right panel) The levels of B. burgdorferi binding to FH from (A) human or (B) mouse were measured by flow cytometry and presented as mean fluorescence index (MFI). Each bar represents the mean of three independent determinations ± SEM. Significant differences (P < 0.05 by one-way ANOVA with post hoc Bonferroni correction) in the levels of FH binding relative to the B313/Vector (“*”) or between two strains relative to each other (“#”).
(TIF)
Ten micrograms of human (A and B) C7, (C and D) C9, or (E and F) plasminogen (PLG) were conjugated on a SPR chip, resulting the response unit (RU) as 2823, 2034, and 2106 for C7, C9, and PLG, respectively. Different concentrations (0.0625 to 1 μM) of histidine tagged (A, C, and E) CspAB31 (“B31”) or (B, D and F) CspAB31L246D (“L246D”) were flowed over the chip. Binding was measured in RU by SPR (see Materials and methods). The experiments were performed on three independent occasions; within each occasion, samples were run in duplicate. All experiments were performed with a single preparation of recombinant proteins. The kon, koff, and KD values (S1 Table) were determined from the average of these three experiments.
(TIF)
Experimental infection of (A) C3H/HeN mice using needle infection (B) C3H/HeN mice or (C) BALB/c or BALB/c C3-/- mice by larvae acquisition and nymph transmission.
(TIF)
I. scapularis ticks carrying B. burgdorferi strain B31-5A15 were disrupted by pipette tips followed by incubation with enzyme free cell dissociation buffer. The resulting suspensions were subjected to FACS to isolate B. burgdorferi. To enhance the sorting efficiency, the spirochetes were permeabilized by methanol and then stained by a mouse monoclonal antibody against B. burgdorferi flagellin (FlaB) followed by Alexa 647 conjugated antibody against mouse IgG. Shown is the FACS data from gating for B. burgdorferi in the suspensions of disrupted nymphal ticks at 24 hours post fed on naïve C3H/HeN mice. (A) To discriminate between B. burgdorferi and debris of ticks or aggregations, the B. burgdorferi-tick suspensions were introduced into FACS and plotted by forward scattering (FSC) or side scattering (SSC). Two populations with distinct FSC and SSC (R1 and R2) were sorted. (B) The populations of R1 and R2 from panel A were examined for their fluorescence intensity of Alexa 647. The majority of the cells in R1 was Alexa 647 positive whereas the population in R2 was Alexa 647 negative, suggesting B. burgdorferi was sorted at R1. (C) The same disrupted ticks-B. burgdorferi suspensions were also permeabilized and then stained by Alexa 647 conjugated antibody against mouse IgG but not antibody against B. burgdorferi flagellin (FlaB) to detect the background staining. The same populations of R1 and R2 were also gated. (D) Both R1 and R2 from panel C were Alexa 647 negative, indicating low levels of background staining for each of these two populations. The (E) pre- and (F) post-sorted cells were also imaged under a dark-field microscope (40x, bar = 5μm). The arrows indicate B. burgdorferi spirochetes.
(TIF)
C3H/HeN mice were infected by needles with 105 B. burgdorferi strains B31-5A15 (“B31-5A15”), B31-5A4NP1ΔcspA harboring the vector pBSV2G (“ΔcspA/Vector”), or this cspA mutant strain producing CspAB31 (“ΔcspA/pCspAB31”), CspAPKo (“ΔcspA/pCspAPKo”), CspAZQ1 (“ΔcspA/pCspAZQ1”), or CspAB31L246D (“ΔcspA/pCspAB31L246D”). At 14 days post infection, the bacterial loads in (A) the inoculation site of skin (“inoc. Site”), (B) tibiotarsus joints, (C) heart, (D) bladder, and (E) ears were determined by qPCR and normalized to 100 ng total DNA. Shown are the geometric mean of bacterial loads ± 95% confidence interval of 5 mice per group. No Significant differences (P > 0.05 by one-way ANOVA with post hoc Bonferroni correction) of bacterial burdens was observed in these tissues from the mice infected by each of those B. burgdorferi strains.
(TIF)
C3H/HeN mice were infected via needles with 105 B. burgdorferi strains B31-5A15 (“5A15”) or B31-5A4NP1ΔcspA harboring the vector pBSV2G (“5A4NP1ΔcspA/Vector”). At 4 days post infection (“4dpi”), the bacterial loads in the inoculation site of skin (“Inoc. Site”) and blood were determined by qPCR and normalized to 100 ng total DNA. Shown are the geometric mean of bacterial loads ± 95% confidence interval of 5 mice per group. No Significant differences (P > 0.05 by Student’s t test) of bacterial burdens were observed from the mice infected between these B. burgdorferi strains.
(TIF)
The nymphs infected with B. burgdorferi strain B31-5A15 (“5A15”) or a cspA mutant strain B31-5A4NP1ΔcspA harboring the vector pBSV2G (“5A4NP1ΔcspA/Vector”) were allowed to feed on C3H/HeN mice for 48 and 72 hours (“48hpf” and “72hpf”). Bacterial loads in those fed nymphs were determined by qPCR. Shown are the geometric mean of bacterial loads ± 95% confidence interval of four nymphs (for nymphs carrying strain B31-5A15) or five nymphs (for nymphs carrying the strain B31-5A4NP1ΔcspA harboring the vector) per group. “*” indicates statistically (P < 0.05 by Student’s t test) different between two different strains-infected nymphs.
(TIF)
C3H/HeN mice were infected by needles with 105 B. burgdorferi strain B31-5A15 (“5A15”) or B31-5A4NP1ΔcspA harboring the vector pBSV2G (“5A4NP1ΔcspA/Vector”). At 14 days post infection, the uninfected I. scapularis larval ticks were allowed to feed on each of these mice to repletion. After the replete larvae molt into nymphs, those B. burgdorferi-infected nymphs were allowed to feed on naïve C3H/HeN mice to repletion. At 14 days after nymphal tick feeding (“14dpf”), the bacterial loads in the inoculation site of skin (“inno. site”), tibiotarsus and knee joints, bladder, and heart were determined by qPCR and normalized to 100 ng total DNA. Shown are the geometric mean of bacterial loads ± 95% confidence interval of four mice per group. Significant differences (P < 0.05, Student’s t test) in the spirochete burdens between two strains relative to each other (“*”).
(TIF)
One microgram of CspAB31, CspAPKo, CspAZQ1, or CspAB31L246D, or BSA (as a negative control) was coated on triplicate wells in a microtiter plate. The anti-sera raised against (A) CspAB31 (“αCspAB31”), (B) CspAPKo (“αCspAPKo”), (C) CspAZQ1 (“αCspAZQ1”) or (D) pre-immune serum (“Preimmune”, negative control) were added to these wells. Bound antibody was measured by ELISA (see Materials and methods), and the mean OD405 values ± standard deviations were determined. Asterisk (“*”) indicates significant different levels of recognition by particular anti-sera to CspA proteins (P < 0.05) compared that to BSA determined by one-way ANOVA with post hoc Bonferroni correction.
(TIF)
(PDF)
(PDF)
(PDF)
Acknowledgments
We thank Jennifer Yates, Aurelie Kern, Linden Hu, and John Leong for valuable technical advice, Linda Gebhardt, Ing-Nang Wang, Melissa Caimano and Xiuli Yang for critical reading of the manuscript, Brian Sa for technical support of serum resistance assay, Patti Rosa for providing B. burgdorferi strain B313 and the vector pBSV2G, Darrin Akins for providing B. burgdorferi strain B31-5A15 and B31-5A4NP1ΔcspA, Jorge Benach for monoclonal mouse antibody against B. burgdorferi FlaB (CB1), Sudha Chaturvedi allowing us to use her Bead Homogenizer. We thank Leslie Eisele and Renjie Song of Wadsworth Biochemistry and Immunology Core for assistance with Circular Dichroism and flow cytometry and bacteria sorting, Karen Chave of the Wadsworth Protein Expression Core for purifying factor H proteins.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by NIH R01AI121401 (to PK and YL), NIH R01HL094463 and NIH R21HL136271 (to RJL), and NIH R01HL125371 (to RJL and FZ), and New York State Department of Health Wadsworth Center Start-Up Grant (to YL and TH). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Steere AC, Strle F, Wormser GP, Hu LT, Branda JA, Hovius JW, et al. Lyme borreliosis. Nat Rev Dis Primers. 2016;2:16090 doi: 10.1038/nrdp.2016.90 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Radolf JD, Caimano MJ, Stevenson B, Hu LT. Of ticks, mice and men: understanding the dual-host lifestyle of Lyme disease spirochaetes. Nat Rev Microbiol. 2012;10(2):87–99. doi: 10.1038/nrmicro2714 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rosa PA, Tilly K, Stewart PE. The burgeoning molecular genetics of the Lyme disease spirochaete. Nat Rev Microbiol. 2005;3(2):129–43. doi: 10.1038/nrmicro1086 . [DOI] [PubMed] [Google Scholar]
- 4.Steere AC, Coburn J, Glickstein L. The emergence of Lyme disease. J Clin Invest. 2004;113(8):1093–101. doi: 10.1172/JCI21681 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brisson D, Drecktrah D, Eggers CH, Samuels DS. Genetics of Borrelia burgdorferi. Annu Rev Genet. 2012;46:515–36. doi: 10.1146/annurev-genet-011112-112140 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Dam AP. Diversity of Ixodes-borne Borrelia species—clinical, pathogenetic, and diagnostic implications and impact on vaccine development. Vector Borne Zoonotic Dis. 2002;2(4):249–54. doi: 10.1089/153036602321653833 . [DOI] [PubMed] [Google Scholar]
- 7.Kurtenbach K, De Michelis S, Etti S, Schafer SM, Sewell HS, Brade V, et al. Host association of Borrelia burgdorferi sensu lato—the key role of host complement. Trends Microbiol. 2002;10(2):74–9. . [DOI] [PubMed] [Google Scholar]
- 8.Kraiczy P. Travelling between Two Worlds: Complement as a Gatekeeper for an Expanded Host Range of Lyme Disease Spirochetes. Vet Sci 2016;3(2):12–26. doi: 10.3390/vetsci3020012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zipfel PF, Skerka C. Complement regulators and inhibitory proteins. Nat Rev Immunol. 2009;9(10):729–40. doi: 10.1038/nri2620 . [DOI] [PubMed] [Google Scholar]
- 10.Ricklin D, Hajishengallis G, Yang K, Lambris JD. Complement: a key system for immune surveillance and homeostasis. Nat Immunol. 2010;11(9):785–97. doi: 10.1038/ni.1923 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Merle NS, Church SE, Fremeaux-Bacchi V, Roumenina LT. Complement System Part I—Molecular Mechanisms of Activation and Regulation. Front Immunol. 2015;6:262 doi: 10.3389/fimmu.2015.00262 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zipfel PF, Skerka C, Hellwage J, Jokiranta ST, Meri S, Brade V, et al. Factor H family proteins: on complement, microbes and human diseases. Biochem Soc Trans. 2002;30(Pt 6):971–8. . [DOI] [PubMed] [Google Scholar]
- 13.Ripoche J, Day AJ, Harris TJ, Sim RB. The complete amino acid sequence of human complement factor H. Biochem J. 1988;249(2):593–602. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ripoche J, Erdei A, Gilbert D, Al Salihi A, Sim RB, Fontaine M. Two populations of complement factor H differ in their ability to bind to cell surfaces. Biochem J. 1988;253(2):475–80. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miajlovic H, Smith SG. Bacterial self-defence: how Escherichia coli evades serum killing. FEMS Microbiol Lett. 2014;354(1):1–9. doi: 10.1111/1574-6968.12419 . [DOI] [PubMed] [Google Scholar]
- 16.Guerry P, Poly F, Riddle M, Maue AC, Chen YH, Monteiro MA. Campylobacter polysaccharide capsules: virulence and vaccines. Front Cell Infect Microbiol. 2012;2:7 doi: 10.3389/fcimb.2012.00007 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kenedy MR, Lenhart TR, Akins DR. The role of Borrelia burgdorferi outer surface proteins. FEMS Immunol Med Microbiol. 2012;66(1):1–19. doi: 10.1111/j.1574-695X.2012.00980.x . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Taeye SW, Kreuk L, van Dam AP, Hovius JW, Schuijt TJ. Complement evasion by Borrelia burgdorferi: it takes three to tango. Trends Parasitol. 2013;29(3):119–28. doi: 10.1016/j.pt.2012.12.001 . [DOI] [PubMed] [Google Scholar]
- 19.Zipfel PF, Hallstrom T, Riesbeck K. Human complement control and complement evasion by pathogenic microbes—tipping the balance. Mol Immunol. 2013;56(3):152–60. doi: 10.1016/j.molimm.2013.05.222 . [DOI] [PubMed] [Google Scholar]
- 20.Lambris JD, Ricklin D, Geisbrecht BV. Complement evasion by human pathogens. Nat Rev Microbiol. 2008;6(2):132–42. doi: 10.1038/nrmicro1824 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kraiczy P. Hide and Seek: How Lyme Disease Spirochetes Overcome Complement Attack. Front Immunol. 2016;7:385 doi: 10.3389/fimmu.2016.00385 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marcinkiewicz A, Kraiczy P, Lin Y-P. There is a method to the madness: Strategies to study host complement evasion by Lyme disease and relapsing fever spirochetes. Frontiers in Microbiology. 2017;8(328). doi: 10.3389/fmicb.2017.00328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kraiczy P, Stevenson B. Complement regulator-acquiring surface proteins of Borrelia burgdorferi: Structure, function and regulation of gene expression. Ticks Tick Borne Dis. 2013;4(1–2):26–34. doi: 10.1016/j.ttbdis.2012.10.039 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hallstrom T, Siegel C, Morgelin M, Kraiczy P, Skerka C, Zipfel PF. CspA from Borrelia burgdorferi inhibits the terminal complement pathway. MBio. 2013;4(4). doi: 10.1128/mBio.00481-13 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hammerschmidt C, Koenigs A, Siegel C, Hallstrom T, Skerka C, Wallich R, et al. Versatile roles of CspA orthologs in complement inactivation of serum-resistant Lyme disease spirochetes. Infect Immun. 2014;82(1):380–92. doi: 10.1128/IAI.01094-13 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bykowski T, Woodman ME, Cooley AE, Brissette CA, Brade V, Wallich R, et al. Coordinated expression of Borrelia burgdorferi complement regulator-acquiring surface proteins during the Lyme disease spirochete’s mammal-tick infection cycle. Infect Immun. 2007;75(9):4227–36. doi: 10.1128/IAI.00604-07 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miller JC, von Lackum K, Babb K, McAlister JD, Stevenson B. Temporal analysis of Borrelia burgdorferi Erp protein expression throughout the mammal-tick infectious cycle. Infect Immun. 2003;71(12):6943–52. doi: 10.1128/IAI.71.12.6943-6952.2003 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kenedy MR, Vuppala SR, Siegel C, Kraiczy P, Akins DR. CspA-mediated binding of human factor H inhibits complement deposition and confers serum resistance in Borrelia burgdorferi. Infect Immun. 2009;77(7):2773–82. doi: 10.1128/IAI.00318-09 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brooks CS, Vuppala SR, Jett AM, Alitalo A, Meri S, Akins DR. Complement regulator-acquiring surface protein 1 imparts resistance to human serum in Borrelia burgdorferi. J Immunol. 2005;175(5):3299–308. . [DOI] [PubMed] [Google Scholar]
- 30.Wallich R, Pattathu J, Kitiratschky V, Brenner C, Zipfel PF, Brade V, et al. Identification and functional characterization of complement regulator-acquiring surface protein 1 of the Lyme disease spirochetes Borrelia afzelii and Borrelia garinii. Infect Immun. 2005;73(4):2351–9. doi: 10.1128/IAI.73.4.2351-2359.2005 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kraiczy P, Skerka C, Brade V, Zipfel PF. Further characterization of complement regulator-acquiring surface proteins of Borrelia burgdorferi. Infect Immun. 2001;69(12):7800–9. doi: 10.1128/IAI.69.12.7800-7809.2001 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ma Y, Seiler KP, Eichwald EJ, Weis JH, Teuscher C, Weis JJ. Distinct characteristics of resistance to Borrelia burgdorferi-induced arthritis in C57BL/6N mice. Infect Immun. 1998;66(1):161–8. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Isogai E, Tanaka S, Braga IS 3rd, Itakura C, Isogai H, Kimura K, et al. Experimental Borrelia garinii infection of Japanese quail. Infect Immun. 1994;62(8):3580–2. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kraiczy P, Hanssen-Hubner C, Kitiratschky V, Brenner C, Besier S, Brade V, et al. Mutational analyses of the BbCRASP-1 protein of Borrelia burgdorferi identify residues relevant for the architecture and binding of host complement regulators FHL-1 and factor H. Int J Med Microbiol. 2009;299(4):255–68. doi: 10.1016/j.ijmm.2008.09.002 . [DOI] [PubMed] [Google Scholar]
- 35.Cordes FS, Roversi P, Kraiczy P, Simon MM, Brade V, Jahraus O, et al. A novel fold for the factor H-binding protein BbCRASP-1 of Borrelia burgdorferi. Nat Struct Mol Biol. 2005;12(3):276–7. doi: 10.1038/nsmb902 . [DOI] [PubMed] [Google Scholar]
- 36.Purser JE, Norris SJ. Correlation between plasmid content and infectivity in Borrelia burgdorferi. Proc Natl Acad Sci U S A. 2000;97(25):13865–70. doi: 10.1073/pnas.97.25.13865 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hartmann K, Corvey C, Skerka C, Kirschfink M, Karas M, Brade V, et al. Functional characterization of BbCRASP-2, a distinct outer membrane protein of Borrelia burgdorferi that binds host complement regulators factor H and FHL-1. Mol Microbiol. 2006;61(5):1220–36. doi: 10.1111/j.1365-2958.2006.05318.x . [DOI] [PubMed] [Google Scholar]
- 38.Hefty PS, Jolliff SE, Caimano MJ, Wikel SK, Radolf JD, Akins DR. Regulation of OspE-related, OspF-related, and Elp lipoproteins of Borrelia burgdorferi strain 297 by mammalian host-specific signals. Infect Immun. 2001;69(6):3618–27. doi: 10.1128/IAI.69.6.3618-3627.2001 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Siegel C, Hallstrom T, Skerka C, Eberhardt H, Uzonyi B, Beckhaus T, et al. Complement factor H-related proteins CFHR2 and CFHR5 represent novel ligands for the infection-associated CRASP proteins of Borrelia burgdorferi. PLoS One. 2010;5(10):e13519 doi: 10.1371/journal.pone.0013519 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hammerschmidt C, Hallstrom T, Skerka C, Wallich R, Stevenson B, Zipfel PF, et al. Contribution of the infection-associated complement regulator-acquiring surface protein 4 (ErpC) to complement resistance of Borrelia burgdorferi. Clin Dev Immunol. 2012;2012:349657 doi: 10.1155/2012/349657 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pickering MC, Cook HT, Warren J, Bygrave AE, Moss J, Walport MJ, et al. Uncontrolled C3 activation causes membranoproliferative glomerulonephritis in mice deficient in complement factor H. Nat Genet. 2002;31(4):424–8. doi: 10.1038/ng912 . [DOI] [PubMed] [Google Scholar]
- 42.Grosskinsky S, Schott M, Brenner C, Cutler SJ, Simon MM, Wallich R. Human complement regulators C4b-binding protein and C1 esterase inhibitor interact with a novel outer surface protein of Borrelia recurrentis. PLoS Negl Trop Dis. 2010;4(6):e698 doi: 10.1371/journal.pntd.0000698 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hallstrom T, Haupt K, Kraiczy P, Hortschansky P, Wallich R, Skerka C, et al. Complement regulator-acquiring surface protein 1 of Borrelia burgdorferi binds to human bone morphogenic protein 2, several extracellular matrix proteins, and plasminogen. J Infect Dis. 2010;202(3):490–8. doi: 10.1086/653825 . [DOI] [PubMed] [Google Scholar]
- 44.Lachmann PJ. Preparing serum for functional complement assays. J Immunol Methods. 2010;352(1–2):195–7. doi: 10.1016/j.jim.2009.11.003 . [DOI] [PubMed] [Google Scholar]
- 45.Ristow LC, Miller HE, Padmore LJ, Chettri R, Salzman N, Caimano MJ, et al. The beta(3)-integrin ligand of Borrelia burgdorferi is critical for infection of mice but not ticks. Mol Microbiol. 2012;85(6):1105–18. doi: 10.1111/j.1365-2958.2012.08160.x . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Caine JA, Coburn J. A short-term Borrelia burgdorferi infection model identifies tissue tropisms and bloodstream survival conferred by adhesion proteins. Infect Immun. 2015;83(8):3184–94. doi: 10.1128/IAI.00349-15 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.von Lackum K, Miller JC, Bykowski T, Riley SP, Woodman ME, Brade V, et al. Borrelia burgdorferi regulates expression of complement regulator-acquiring surface protein 1 during the mammal-tick infection cycle. Infect Immun. 2005;73(11):7398–405. doi: 10.1128/IAI.73.11.7398-7405.2005 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dunham-Ems SM, Caimano MJ, Pal U, Wolgemuth CW, Eggers CH, Balic A, et al. Live imaging reveals a biphasic mode of dissemination of Borrelia burgdorferi within ticks. J Clin Invest. 2009;119(12):3652–65. doi: 10.1172/JCI39401 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schwan TG, Piesman J. Temporal changes in outer surface proteins A and C of the lyme disease-associated spirochete, Borrelia burgdorferi, during the chain of infection in ticks and mice. J Clin Microbiol. 2000;38(1):382–8. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ohnishi J, Piesman J, de Silva AM. Antigenic and genetic heterogeneity of Borrelia burgdorferi populations transmitted by ticks. Proc Natl Acad Sci U S A. 2001;98(2):670–5. doi: 10.1073/pnas.98.2.670 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wessels MR, Butko P, Ma M, Warren HB, Lage AL, Carroll MC. Studies of group B streptococcal infection in mice deficient in complement component C3 or C4 demonstrate an essential role for complement in both innate and acquired immunity. Proc Natl Acad Sci U S A. 1995;92(25):11490–4. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kurtenbach K, Schafer SM, Sewell HS, Peacey M, Hoodless A, Nuttall PA, et al. Differential survival of Lyme borreliosis spirochetes in ticks that feed on birds. Infect Immun. 2002;70(10):5893–5. doi: 10.1128/IAI.70.10.5893-5895.2002 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Matuschka FR, Fischer P, Heiler M, Richter D, Spielman A. Capacity of European animals as reservoir hosts for the Lyme disease spirochete. J Infect Dis. 1992;165(3):479–83. . [DOI] [PubMed] [Google Scholar]
- 54.Kurtenbach K, Sewell HS, Ogden NH, Randolph SE, Nuttall PA. Serum complement sensitivity as a key factor in Lyme disease ecology. Infect Immun. 1998;66(3):1248–51. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bhide MR, Travnicek M, Levkutova M, Curlik J, Revajova V, Levkut M. Sensitivity of Borrelia genospecies to serum complement from different animals and human: a host-pathogen relationship. FEMS Immunol Med Microbiol. 2005;43(2):165–72. doi: 10.1016/j.femsim.2004.07.012 . [DOI] [PubMed] [Google Scholar]
- 56.Kraiczy P. Travelling between Two Worlds: Complement as a Gatekeeper for an Expanded Host Range of Lyme Disease Spirochetes. Vet Sci. 2016;3(2). doi: 10.3390/vetsci3020012 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.McDowell JV, Hovis KM, Zhang H, Tran E, Lankford J, Marconi RT. Evidence that the BBA68 protein (BbCRASP-1) of the Lyme disease spirochetes does not contribute to factor H-mediated immune evasion in humans and other animals. Infect Immun. 2006;74(5):3030–4. doi: 10.1128/IAI.74.5.3030-3034.2006 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bhide MR, Escudero R, Camafeita E, Gil H, Jado I, Anda P. Complement factor H binding by different Lyme disease and relapsing fever Borrelia in animals and human. BMC Res Notes. 2009;2:134 doi: 10.1186/1756-0500-2-134 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bhide M, Bhide K, Pulzova L, Madar M, Mlynarcik P, Bencurova E, et al. Variable regions in the sushi domains 6–7 and 19–20 of factor H in animals and human lead to change in the affinity to factor H binding protein of Borrelia. J Proteomics. 2012;75(14):4520–8. doi: 10.1016/j.jprot.2012.04.013 . [DOI] [PubMed] [Google Scholar]
- 60.McDowell JV, Harlin ME, Rogers EA, Marconi RT. Putative coiled-coil structural elements of the BBA68 protein of Lyme disease spirochetes are required for formation of its factor H binding site. J Bacteriol. 2005;187(4):1317–23. doi: 10.1128/JB.187.4.1317-1323.2005 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tokarz R, Anderton JM, Katona LI, Benach JL. Combined effects of blood and temperature shift on Borrelia burgdorferi gene expression as determined by whole genome DNA array. Infect Immun. 2004;72(9):5419–32. doi: 10.1128/IAI.72.9.5419-5432.2004 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Caimano MJ, Kenedy MR, Kairu T, Desrosiers DC, Harman M, Dunham-Ems S, et al. The hybrid histidine kinase Hk1 is part of a two-component system that is essential for survival of Borrelia burgdorferi in feeding Ixodes scapularis ticks. Infect Immun. 2011;79(8):3117–30. doi: 10.1128/IAI.05136-11 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kumar M, Kaur S, Kariu T, Yang X, Bossis I, Anderson JF, et al. Borrelia burgdorferi BBA52 is a potential target for transmission blocking Lyme disease vaccine. Vaccine. 2011;29(48):9012–9. doi: 10.1016/j.vaccine.2011.09.035 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mulay VB, Caimano MJ, Iyer R, Dunham-Ems S, Liveris D, Petzke MM, et al. Borrelia burgdorferi bba74 is expressed exclusively during tick feeding and is regulated by both arthropod- and mammalian host-specific signals. J Bacteriol. 2009;191(8):2783–94. doi: 10.1128/JB.01802-08 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Promnares K, Kumar M, Shroder DY, Zhang X, Anderson JF, Pal U. Borrelia burgdorferi small lipoprotein Lp6.6 is a member of multiple protein complexes in the outer membrane and facilitates pathogen transmission from ticks to mice. Mol Microbiol. 2009;74(1):112–25. doi: 10.1111/j.1365-2958.2009.06853.x . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rogers EA, Terekhova D, Zhang HM, Hovis KM, Schwartz I, Marconi RT. Rrp1, a cyclic-di-GMP-producing response regulator, is an important regulator of Borrelia burgdorferi core cellular functions. Mol Microbiol. 2009;71(6):1551–73. doi: 10.1111/j.1365-2958.2009.06621.x . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nogueira SV, Smith AA, Qin JH, Pal U. A surface enolase participates in Borrelia burgdorferi-plasminogen interaction and contributes to pathogen survival within feeding ticks. Infect Immun. 2012;80(1):82–90. doi: 10.1128/IAI.05671-11 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jewett MW, Lawrence KA, Bestor A, Byram R, Gherardini F, Rosa PA. GuaA and GuaB are essential for Borrelia burgdorferi survival in the tick-mouse infection cycle. J Bacteriol. 2009;191(20):6231–41. doi: 10.1128/JB.00450-09 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Eggers CH, Caimano MJ, Malizia RA, Kariu T, Cusack B, Desrosiers DC, et al. The coenzyme A disulphide reductase of Borrelia burgdorferi is important for rapid growth throughout the enzootic cycle and essential for infection of the mammalian host. Mol Microbiol. 2011;82(3):679–97. doi: 10.1111/j.1365-2958.2011.07845.x . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Strother KO, de Silva A. Role of Borrelia burgdorferi linear plasmid 25 in infection of Ixodes scapularis ticks. J Bacteriol. 2005;187(16):5776–81. doi: 10.1128/JB.187.16.5776-5781.2005 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ouyang Z, Kumar M, Kariu T, Haq S, Goldberg M, Pal U, et al. BosR (BB0647) governs virulence expression in Borrelia burgdorferi. Mol Microbiol. 2009;74(6):1331–43. doi: 10.1111/j.1365-2958.2009.06945.x . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Purser JE, Lawrenz MB, Caimano MJ, Howell JK, Radolf JD, Norris SJ. A plasmid-encoded nicotinamidase (PncA) is essential for infectivity of Borrelia burgdorferi in a mammalian host. Mol Microbiol. 2003;48(3):753–64. . [DOI] [PubMed] [Google Scholar]
- 73.Lawrenz MB, Wooten RM, Zachary JF, Drouin SM, Weis JJ, Wetsel RA, et al. Effect of complement component C3 deficiency on experimental Lyme borreliosis in mice. Infect Immun. 2003;71(8):4432–40. doi: 10.1128/IAI.71.8.4432-4440.2003 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Woodman ME, Cooley AE, Miller JC, Lazarus JJ, Tucker K, Bykowski T, et al. Borrelia burgdorferi binding of host complement regulator factor H is not required for efficient mammalian infection. Infect Immun. 2007;75(6):3131–9. doi: 10.1128/IAI.01923-06 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rathinavelu S, Broadwater A, de Silva AM. Does host complement kill Borrelia burgdorferi within ticks? Infect Immun. 2003;71(2):822–9. doi: 10.1128/IAI.71.2.822-829.2003 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kraiczy P, Hartmann K, Hellwage J, Skerka C, Kirschfink M, Brade V, et al. Immunological characterization of the complement regulator factor H-binding CRASP and Erp proteins of Borrelia burgdorferi. Int J Med Microbiol. 2004;293 Suppl 37:152–7. . [DOI] [PubMed] [Google Scholar]
- 77.Lin YP, Benoit V, Yang X, Martinez-Herranz R, Pal U, Leong JM. Strain-specific variation of the decorin-binding adhesin DbpA influences the tissue tropism of the lyme disease spirochete. PLoS Pathog. 2014;10(7):e1004238 doi: 10.1371/journal.ppat.1004238 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lin YP, Greenwood A, Nicholson LK, Sharma Y, McDonough SP, Chang YF. Fibronectin binds to and induces conformational change in a disordered region of leptospiral immunoglobulin-like protein B. J Biol Chem. 2009;284(35):23547–57. doi: 10.1074/jbc.M109.031369 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yu BB, Moffatt BE, Fedorova M, Villiers CG, Arnold JN, Du E, et al. Purification, quantification, and functional analysis of Complement Factor H. Methods Mol Biol. 2014;1100:207–23. doi: 10.1007/978-1-62703-724-2_17 . [DOI] [PubMed] [Google Scholar]
- 80.van Burgel ND, Kraiczy P, Schuijt TJ, Zipfel PF, van Dam AP. Identification and functional characterisation of Complement Regulator Acquiring Surface Protein-1 of serum resistant Borrelia garinii OspA serotype 4. BMC Microbiol. 2010;10:43 doi: 10.1186/1471-2180-10-43 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Marcinkiewicz AL, Lieknina I, Kotelovica S, Yang X, Kraiczy P, Pal U, et al. Eliminating Factor H-Binding Activity of Borrelia burgdorferi CspZ Combined with Virus-Like Particle Conjugation Enhances Its Efficacy as a Lyme Disease Vaccine. Front Immunol. 2018;9:181 doi: 10.3389/fimmu.2018.00181 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tilly K, Krum JG, Bestor A, Jewett MW, Grimm D, Bueschel D, et al. Borrelia burgdorferi OspC protein required exclusively in a crucial early stage of mammalian infection. Infect Immun. 2006;74(6):3554–64. doi: 10.1128/IAI.01950-05 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Samuels DS. Electrotransformation of the spirochete Borrelia burgdorferi. Methods Mol Biol. 1995;47:253–9. doi: 10.1385/0-89603-310-4:253 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yang XF, Pal U, Alani SM, Fikrig E, Norgard MV. Essential role for OspA/B in the life cycle of the Lyme disease spirochete. J Exp Med. 2004;199(5):641–8. doi: 10.1084/jem.20031960 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Moriarty TJ, Shi M, Lin YP, Ebady R, Zhou H, Odisho T, et al. Vascular binding of a pathogen under shear force through mechanistically distinct sequential interactions with host macromolecules. Mol Microbiol. 2012;86(5):1116–31. doi: 10.1111/mmi.12045 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Parveen N, Leong JM. Identification of a candidate glycosaminoglycan-binding adhesin of the Lyme disease spirochete Borrelia burgdorferi. Mol Microbiol. 2000;35(5):1220–34. . [DOI] [PubMed] [Google Scholar]
- 87.Lin YP, Chen Q, Ritchie JA, Dufour NP, Fischer JR, Coburn J, et al. Glycosaminoglycan binding by Borrelia burgdorferi adhesin BBK32 specifically and uniquely promotes joint colonization. Cell Microbiol. 2015;17(6):860–75. doi: 10.1111/cmi.12407 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lin YP, Bhowmick R, Coburn J, Leong JM. Host cell heparan sulfate glycosaminoglycans are ligands for OspF-related proteins of the Lyme disease spirochete. Cell Microbiol. 2015;17(10):1464–76. doi: 10.1111/cmi.12448 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Karnchanaphanurach P, Mirchev R, Ghiran I, Asara JM, Papahadjopoulos-Sternberg B, Nicholson-Weller A, et al. C3b deposition on human erythrocytes induces the formation of a membrane skeleton-linked protein complex. J Clin Invest. 2009;119(4):788–801. doi: 10.1172/JCI36088 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lawrenz MB, Hardham JM, Owens RT, Nowakowski J, Steere AC, Wormser GP, et al. Human antibody responses to VlsE antigenic variation protein of Borrelia burgdorferi. J Clin Microbiol. 1999;37(12):3997–4004. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Alitalo A, Meri T, Ramo L, Jokiranta TS, Heikkila T, Seppala IJ, et al. Complement evasion by Borrelia burgdorferi: serum-resistant strains promote C3b inactivation. Infect Immun. 2001;69(6):3685–91. doi: 10.1128/IAI.69.6.3685-3691.2001 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kern A, Zhou CW, Jia F, Xu Q, Hu LT. Live-vaccinia virus encapsulation in pH-sensitive polymer increases safety of a reservoir-targeted Lyme disease vaccine by targeting gastrointestinal release. Vaccine. 2016;34(38):4507–13. doi: 10.1016/j.vaccine.2016.07.059 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Coleman AS, Yang X, Kumar M, Zhang X, Promnares K, Shroder D, et al. Borrelia burgdorferi complement regulator-acquiring surface protein 2 does not contribute to complement resistance or host infectivity. PLoS One. 2008;3(8):3010e doi: 10.1371/journal.pone.0003010 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hodzic E, Feng S, Freet KJ, Barthold SW. Borrelia burgdorferi population dynamics and prototype gene expression during infection of immunocompetent and immunodeficient mice. Infect Immun. 2003;71(9):5042–55. doi: 10.1128/IAI.71.9.5042-5055.2003 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The amino acid sequences of CspA variants from B. burgdorferi strains B31 (Bburg_B31_BBA68) and ZS7 (Bburg_ZS7_A59), B. afzelii strains MMS (Bafz_MMS_MMSA71) and PKo (Bafz_PKo_A0067), B. garinii strain ZQ1 (Bgar_ZQ1_ZQA68), B. bavariensis strain PBi (Bbav_PBi_BGA66), and B. spielmanii strain A14S (Bspiel_A14S_A0067) were aligned using M-Coffee with default parameters. Black shaded residues are identical among all of these variants. The red asterisk indicates the leucine-246 of CspAB31, which is required for human, mouse, horse, and quail FH-binding activity of this protein.
(TIF)
The indicated concentrations of various recombinant histidine-tagged CspAB31 (“B31”), CspAPKo (“PKo”), CspAZQ1 (“ZQ1”), CspAB31L246D (“L246D”), or DbpA (“DbpA”, negative control) were added to triplicate wells coated with FH from (A) human, (B) mouse, (C) horse, or (D) quail, and protein binding was quantitated by ELISA. The experiments were performed on three independent occasions; within each occasion, samples were run in duplicate. All experiments were performed with a single preparation of recombinant proteins. Shown is a representative experiment from the average of two replicates. The KD values (Table 1) representing the FH-binding affinity of each protein were determined from the average of three experiments.
(TIF)
Ten micrograms of FH from (A to C) human, (D to F) mouse, or (G and H) horse were conjugated on a SPR chip, resulting the response unit (RU) as 563, 511, and 433 for human, mouse, and horse FH, respectively. Different concentrations (0.125 to 2 or 4μM) of histidine tagged CspAB31 (“B31”), CspAPKo (“PKo”), CspAZQ1 (“ZQ1”), or CspAB31L246D (“L246D”) were flowed over a surface of the chip. Binding was measured in response units (RU) by SPR (see Materials and methods). The experiments were performed on three independent occasions; within each occasion, samples were run in duplicate. All experiments were performed with a single preparation of recombinant proteins. The kon, koff, and KD values (Table 1) were determined from the average of these three experiments. Panel A, D, and F are representative experiments applying 1 μM of indicated CspA proteins to the chip coated with FH from indicated species.
(TIF)
Far-UV CD analysis of CspAB31 and CspAB31L246D. The molar ellipticity, Φ, was measured from 190 to 250 nm for 10μM of each protein in PBS. The predicted spectrum of CspAB31 was generated applying the full-length amino acid sequences of this protein to DichroWeb (http://dichroweb.cryst.bbk.ac.uk/html/links.shtml).
(TIF)
B. burgdorferi strain B31-5A15 (“B31-5A15”), B31-5A4NP1ΔcspA harboring the vector pBSV2G (“ΔcspA/Vector”), or this cspA mutant strain producing CspAB31 (“ΔcspA/pCspAB31”), CspAPKo (“ΔcspA/pCspAPKo”), CspAZQ1 (“ΔcspA/pCspAZQ1”), or CspAB31L246D (“ΔcspA/pCspAB31L246D”), or B313 carrying the vector pBSV2G (“B313/Vector”, negative control) was incubated with C3-depleted human or mouse serum. The bacteria were stained with a sheep anti-FH polyclonal IgG followed by an Alexa 647-conjugated donkey anti-sheep IgG prior to being applied to flow cytometry analysis. (Left panel) Representative histograms of flow cytometry analysis showing the levels of FH from (A) human or (B) mouse binding to indicated B. burgdorferi strains. (Right panel) The levels of B. burgdorferi binding to FH from (A) human or (B) mouse were measured by flow cytometry and presented as mean fluorescence index (MFI). Each bar represents the mean of three independent determinations ± SEM. Significant differences (P < 0.05 by one-way ANOVA with post hoc Bonferroni correction) in the levels of FH binding relative to the B313/Vector (“*”) or between two strains relative to each other (“#”).
(TIF)
Ten micrograms of human (A and B) C7, (C and D) C9, or (E and F) plasminogen (PLG) were conjugated on a SPR chip, resulting the response unit (RU) as 2823, 2034, and 2106 for C7, C9, and PLG, respectively. Different concentrations (0.0625 to 1 μM) of histidine tagged (A, C, and E) CspAB31 (“B31”) or (B, D and F) CspAB31L246D (“L246D”) were flowed over the chip. Binding was measured in RU by SPR (see Materials and methods). The experiments were performed on three independent occasions; within each occasion, samples were run in duplicate. All experiments were performed with a single preparation of recombinant proteins. The kon, koff, and KD values (S1 Table) were determined from the average of these three experiments.
(TIF)
Experimental infection of (A) C3H/HeN mice using needle infection (B) C3H/HeN mice or (C) BALB/c or BALB/c C3-/- mice by larvae acquisition and nymph transmission.
(TIF)
I. scapularis ticks carrying B. burgdorferi strain B31-5A15 were disrupted by pipette tips followed by incubation with enzyme free cell dissociation buffer. The resulting suspensions were subjected to FACS to isolate B. burgdorferi. To enhance the sorting efficiency, the spirochetes were permeabilized by methanol and then stained by a mouse monoclonal antibody against B. burgdorferi flagellin (FlaB) followed by Alexa 647 conjugated antibody against mouse IgG. Shown is the FACS data from gating for B. burgdorferi in the suspensions of disrupted nymphal ticks at 24 hours post fed on naïve C3H/HeN mice. (A) To discriminate between B. burgdorferi and debris of ticks or aggregations, the B. burgdorferi-tick suspensions were introduced into FACS and plotted by forward scattering (FSC) or side scattering (SSC). Two populations with distinct FSC and SSC (R1 and R2) were sorted. (B) The populations of R1 and R2 from panel A were examined for their fluorescence intensity of Alexa 647. The majority of the cells in R1 was Alexa 647 positive whereas the population in R2 was Alexa 647 negative, suggesting B. burgdorferi was sorted at R1. (C) The same disrupted ticks-B. burgdorferi suspensions were also permeabilized and then stained by Alexa 647 conjugated antibody against mouse IgG but not antibody against B. burgdorferi flagellin (FlaB) to detect the background staining. The same populations of R1 and R2 were also gated. (D) Both R1 and R2 from panel C were Alexa 647 negative, indicating low levels of background staining for each of these two populations. The (E) pre- and (F) post-sorted cells were also imaged under a dark-field microscope (40x, bar = 5μm). The arrows indicate B. burgdorferi spirochetes.
(TIF)
C3H/HeN mice were infected by needles with 105 B. burgdorferi strains B31-5A15 (“B31-5A15”), B31-5A4NP1ΔcspA harboring the vector pBSV2G (“ΔcspA/Vector”), or this cspA mutant strain producing CspAB31 (“ΔcspA/pCspAB31”), CspAPKo (“ΔcspA/pCspAPKo”), CspAZQ1 (“ΔcspA/pCspAZQ1”), or CspAB31L246D (“ΔcspA/pCspAB31L246D”). At 14 days post infection, the bacterial loads in (A) the inoculation site of skin (“inoc. Site”), (B) tibiotarsus joints, (C) heart, (D) bladder, and (E) ears were determined by qPCR and normalized to 100 ng total DNA. Shown are the geometric mean of bacterial loads ± 95% confidence interval of 5 mice per group. No Significant differences (P > 0.05 by one-way ANOVA with post hoc Bonferroni correction) of bacterial burdens was observed in these tissues from the mice infected by each of those B. burgdorferi strains.
(TIF)
C3H/HeN mice were infected via needles with 105 B. burgdorferi strains B31-5A15 (“5A15”) or B31-5A4NP1ΔcspA harboring the vector pBSV2G (“5A4NP1ΔcspA/Vector”). At 4 days post infection (“4dpi”), the bacterial loads in the inoculation site of skin (“Inoc. Site”) and blood were determined by qPCR and normalized to 100 ng total DNA. Shown are the geometric mean of bacterial loads ± 95% confidence interval of 5 mice per group. No Significant differences (P > 0.05 by Student’s t test) of bacterial burdens were observed from the mice infected between these B. burgdorferi strains.
(TIF)
The nymphs infected with B. burgdorferi strain B31-5A15 (“5A15”) or a cspA mutant strain B31-5A4NP1ΔcspA harboring the vector pBSV2G (“5A4NP1ΔcspA/Vector”) were allowed to feed on C3H/HeN mice for 48 and 72 hours (“48hpf” and “72hpf”). Bacterial loads in those fed nymphs were determined by qPCR. Shown are the geometric mean of bacterial loads ± 95% confidence interval of four nymphs (for nymphs carrying strain B31-5A15) or five nymphs (for nymphs carrying the strain B31-5A4NP1ΔcspA harboring the vector) per group. “*” indicates statistically (P < 0.05 by Student’s t test) different between two different strains-infected nymphs.
(TIF)
C3H/HeN mice were infected by needles with 105 B. burgdorferi strain B31-5A15 (“5A15”) or B31-5A4NP1ΔcspA harboring the vector pBSV2G (“5A4NP1ΔcspA/Vector”). At 14 days post infection, the uninfected I. scapularis larval ticks were allowed to feed on each of these mice to repletion. After the replete larvae molt into nymphs, those B. burgdorferi-infected nymphs were allowed to feed on naïve C3H/HeN mice to repletion. At 14 days after nymphal tick feeding (“14dpf”), the bacterial loads in the inoculation site of skin (“inno. site”), tibiotarsus and knee joints, bladder, and heart were determined by qPCR and normalized to 100 ng total DNA. Shown are the geometric mean of bacterial loads ± 95% confidence interval of four mice per group. Significant differences (P < 0.05, Student’s t test) in the spirochete burdens between two strains relative to each other (“*”).
(TIF)
One microgram of CspAB31, CspAPKo, CspAZQ1, or CspAB31L246D, or BSA (as a negative control) was coated on triplicate wells in a microtiter plate. The anti-sera raised against (A) CspAB31 (“αCspAB31”), (B) CspAPKo (“αCspAPKo”), (C) CspAZQ1 (“αCspAZQ1”) or (D) pre-immune serum (“Preimmune”, negative control) were added to these wells. Bound antibody was measured by ELISA (see Materials and methods), and the mean OD405 values ± standard deviations were determined. Asterisk (“*”) indicates significant different levels of recognition by particular anti-sera to CspA proteins (P < 0.05) compared that to BSA determined by one-way ANOVA with post hoc Bonferroni correction.
(TIF)
(PDF)
(PDF)
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.