Abstract
Inherited bone marrow failure syndrome (IBMFS) including Shwachman Diamond Syndrome (SDS) can present initially to the hematologist with myelodysplastic syndrome (MDS). Accurate diagnosis affects choice of chemotherapy, donor selection, and transplant conditioning. We report a case of delayed diagnosis of SDS in a family with another child with aplastic anemia, and review reported cases of SDS in Asia. This highlights the gap in identifying inherited bone marrow failure syndromes in adults with hematologic malignancies.
Keywords: Shwachman diamond syndrome, Inherited bone marrow failure syndrome, Telomere
1. Introduction
SDS is an autosomal recessive disease characterised classically by exocrine pancreatic insufficiency and chronic neutropenia, but considerable phenotypic variation has been described even among siblings. In 90% of cases, SDS is caused by mutations in the Shwachman-Bodian-Diamond syndrome (SBDS) gene that produces a protein with critical roles in ribosome biogenesis, mitotic spindle stabilization, and normal chromosomal segregation. While many pathogenic variants in the SBDS gene have been described, no correlation has been found between genotype and phenotype [1]. Three other causal SDS genes, DNAJC21, EFL1, and SRP54 have been reported. These genes have roles in the assembly of ribosomal subunits, the ribosomal subunit maturation, and ribosomal biogenesis respectively [2]. The epidemiology of SDS in Asia is not well studied and we performed a comprehensive review of reported cases to understand the clinical features and genetic variants here. A total of 65 cases have been described, most of which are from Japan. Those with clinical data are summarized in Table 1 [3], [4], [5], [6], [7], [8], [9], [10], [11], [12], [13]. Similar to previous reports, clinical manifestations including hematological abnormalities, pancreatic dysfunction, and short stature were common. The reported genetic mutations affecting patients in Asia were similar to their Western counterparts.
Table 1.
Cases of Shwachman-diamond syndrome reported in Asia.
| Country | Sex | Age at diagnosis | Pancreatic insufficiency | Hematological abnormalities | Skeletal abnormalities | Height (SD) | Body weight (SD) | SBDSmutations | Status at reporting |
|---|---|---|---|---|---|---|---|---|---|
| Japan | M | 3mo | + | Pancytopenia | - | − 2.1 | −1 | 183–184TA>CT/258+2T>C | NA |
| Japan | F | 8y | + | Pancytopenia | + | −2 | −1.5 | 183–184TA>CT/258+2T>C | NA |
| Japan | F | 5mo | + | Pancytopenia | – | −3 | −2 | 428C>G/258+2T>C | NA |
| Japan | M | 1mo | + | Pancytopenia | – | −3 | −1.8 | 183–184TA>CT/258+2T>C | NA |
| Japan | M | 12y | + | Thrombocytopenia | – | −3 | −1 | 183–184TA>CT/258+2T>C | NA |
| Japan | F | 5y | + | Pancytopenia | + | −2.9 | −1.4 | 183–184TA>CT/258+2T>C | NA |
| Japan | M | 2mo | + | Pancytopenia | + | −2 | NA | 183–184TA>CT/258+2T>C | NA |
| Japan | M | 8y | + | Pancytopenia | – | −2 | −1.5 | 258+2T>C/259–1G>A | NA |
| Japan | M | 6mo | + | Neutropenia | – | −3.1 | −2.1 | 183–184TA>CT/unknown | NA |
| Japan | M | 9mo | + | Pancytopenia | – | −3.6 | −3.4 | 183–184TA>CT/258+2T>C | NA |
| Japan | M | 6mo | + | AML | + | −4.8 | −1.8 | 258+2T>C/292–295delAAAG | Alive |
| Japan | M | 2y | + | MDS-->AML | + | −2.8 | −1.8 | 258+2T>C/unknown | Dead |
| Japan | M | 1mo | + | MDS-->AML | + | −2.3 | −1.4 | 183–184TA>CT/258+2T>C | Dead |
| Japan | M | 4y | + | Pancytopenia | + | −1.3 | −1.1 | 183–184TA>CT/258+2T>C | Alive |
| Japan | M | 2y | + | – | – | −3.5 | −1.7 | 183–184TA>CT/258+2T>C | Alive |
| Japan | F | 8y | + | Pancytopenia | + | −3.5 | −1.3 | 183–184TA>CT/258+2T>C | Alive |
| Japan | M | 5mo | + | Pancytopenia | – | −3 | −3.5 | 183–184TA>CT/258+2T>C | Alive |
| Japan | F | 15mo | + | Neutropenia | – | −5.2 | −6.6 | 183–184TA>CT/258+2T>C | Dead |
| Japan | F | 2y7mo | + | MDS | – | −1.2 | −2.3 | 183–184TA>CT/258+2T>C | Alive |
| Japan | F | 4mo | – | Neutropenia | + | −7.5 | NA | 79T>C / 183TA> CT | Alive |
| Korea | F | 3y6mo | + | Neutropenia | + | <3% | <3% | 183–184TA>CT/258+2T>C | Alive |
| Korea | M | 13y9mo | + | Bicytopenia (WBC/PLT) | + | <3% | <3% | 183–184TA>CT/258+2T>C | Alive |
| Korea | M | 15y6mo | + | bicytopenia (WBC/PLT) | + | 3% | 10% | 258+2T>C homozygous | Alive |
| India | M | 4y | + | Pancytopenia | – | NA | NA | 258+2T>C homozygous | Dead |
| China | M | 8y | + | Bicytopenia (WBC/RBC) | + | <3% | 3% | 183–184TA>CT/258+2T>C | Alive |
| China | M | 1y3mo | + | Neutropenia | + | NA | <3% | 183–184TA>CT/258+2T>C | Alive |
| China | F | 6mo | + | Pancytopenia | + | NA | NA | 183–184TA>CT/258+2T>C | Alive |
Abbreviations: M, male; F, female; y, year; mo, month; WBC, white blood cell; RBC, red blood cell; +, present; -, absent; NA, not available; SD, standard deviation; SBDS, Shwachman-Biodan-Diamond syndrome; MDS, myelodysplastic syndrome; AML, acute myeloid leukemia.
Individuals with SDS are at increased risk for developing myelodysplastic syndrome (MDS) and acute myeloid leukemia (AML). Our review identified case reports of three patients who presented with MDS or AML without a prior diagnosis of SDS in Asia [9], [14], [15]. Similarly, SDS continues to be underdiagnosed internationally. Lindsley et al. found that 7 of 241 young adults with MDS enrolled in the Center for International Blood and Marrow Transplant Research (CIMBTR) repository had SBDS mutations, and 5 of these patients did not have a diagnosis of SDS before transplant [16]. Other studies utilizing gene panel testing to screen for genetic predisposition to AML/MDS in young patients presenting with MDS identified SBDS gene mutations in 1 out of 110 patients and 2 out of 197 patients [17], [18]. While underlying SDS is rare in young adults presenting with MDS/AML, the growing use of next-generation sequencing technologies may facilitate identification.
2. Case report
Our patient was a 19 year old male patient who presented with a one-month history of fever, dyspnea, and skin abscesses. His full blood count showed pancytopenia and 14% blasts. Bone marrow aspirate was markedly hypocellular (10% cellularity) with features of dyserythropoiesis and dysmegakaryopoiesis and 3% blasts (Fig. 1). Flow cytometry detected 2.8% blasts that are CD34+ partial CD117+ and CD33+. Trephine was markedly hypocellular with rare hypolobated megakaryocytes and 8% CD117+ CD34+ myeloblasts. Cytogenetics with bone marrow karyotype showed a complex monosomal karyotype as follows, 42–45,XY,add(5)(q15),add(5)(q31),add(6)(q13), der(8)ins(8;?)(q13;?),del(9)(q21q22), dic(14;22)(p11.2;p12),tas(21;16)(q22.3;p13.3), add(17)(q21),-18,-21,+der(?)t(?;8)(?;q13),+r, +1–2mar[cp21]. These findings were compatible with primary hypocellular MDS or IBMFS evolving into MDS.
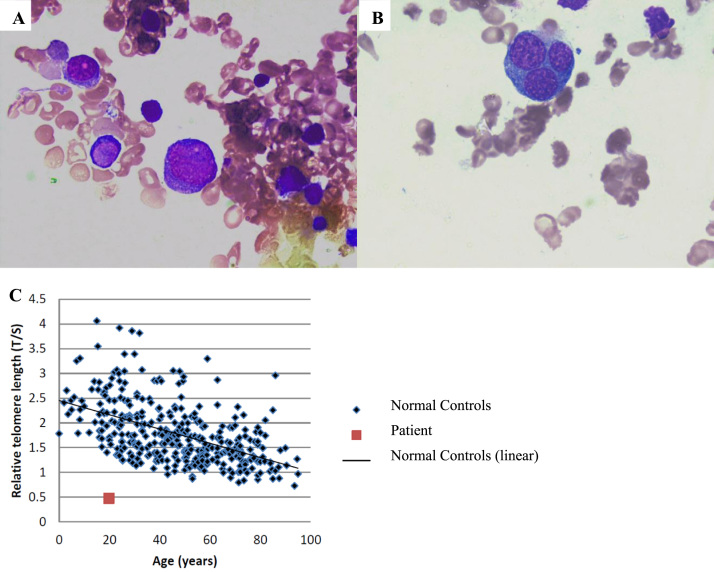
Fig. 1.
Bone marrow aspirate showed numerous blasts (A) and more than 50% dysplastic erythroid precursors (B). Telomere length measured by q-PCR analysis from DNA extracted from peripheral blood mononuclear cells shows that telomere length was less than first percentile (C).
Physical examination revealed a relatively small head (3rd percentile) but no skin pigmentation, nail, teeth or skeletal abnormalities. On further history, there was no failure to thrive and intellectual development was normal. There was no history of steatorrhea or recurrent infections. His only hospitalization was for exacerbation of asthma when he was 7 years old. At that time, full blood count showed anemia and thrombocytopenia but he did not return to a hematology clinic for review. His parents are not consanguineous, and family history was significant for severe aplastic anemia diagnosed in his younger sibling at the age of 16 years old. Bone marrow studies of his brother showed a markedly hypocellular aspirate with trilineage hematopoiesis and normal karyotype on cytogenetics. Chromosomal breakage analysis after incubation of peripheral blood lymphocytes with diepoxybutane (DEB) was negative. Family declined further testing. There was no other family history of hematological conditions.
Patient's subsequent workup for IBMFS revealed that peripheral blood mononuclear cell telomere length measured by quantitative (q-PCR) was less than first percentile compared with normal individuals of the same decade of age. In view of the very short lymphocyte telomeres without clinical phenotype of DC, skin biopsies and blood samples were sent for phenotype-driven targeted exome testing. Results showed biallelic compound heterozygous pathogenic mutations in the SBDS gene, namely c.258 +2T> C (IVS2+2T) and c.183_184delTAinsCT (p. K62X), in both blood and skin samples, confirming a diagnosis of SDS. In addition, two mosaic pathogenic variants in TP53 gene were identified in patient's blood sample but were absent in skin biopsies, indicating that these two TP53 variants were somatic mutations. Our patient was started on Azacitidine while an unrelated donor search was carried out. Unfortunately, he passed away due to neutropenic sepsis. Genetic testing for his sibling confirmed the same compound heterozygous mutations in SBDS gene who is currently under clinical surveillance with serial bone marrow examination.
3. Discussion
Our review of reported cases of SDS in Asia found that the pathogenic variants in SBDS in Asians are similar to that reported in Caucasians. The majority of mutations are caused by gene conversion between SBDS and its unprocessed pseudogene SBDSP1 that contains deletions and nucleotide changes that disrupt coding potential. Our patient is the first molecularly diagnosed case of SDS in Singapore, and harbors the common mutations. Important learning points can be gleaned from this case, as detailed below.
Firstly, SDS likely continues to be underdiagnosed in Asia, because of diverse phenotype, lack of awareness of the condition, and perceived lack of access to gene testing. Although patients with SDS present with cytopenia and classical physical features in early life, adults present subtly because pancreatic function improves with age and neutropenia fluctuates. Similarly, other inherited bone marrow failure syndromes may have a non-specific phenotype and it is critical for hematologists to have a high index of suspicion and understand how to screen for IBMFS given the impact on clinical management. Accurate diagnosis of underlying IBFMS affects therapy for MDS, donor choice and conditioning regimen for transplant, cancer surveillance, and genetic counselling. Clinical findings that should prompt referral and gene testing include early-onset MDS, classical clinical stigmata (e.g. café-au-lait spots), short stature or dysmorphic features, family/personal history of cancer or excess toxicity with chemotherapy, cytopenias with a hypocellular marrow, bone marrow karyotype showing classic cytogenetic or molecular abnormalities, and the presence of short telomeres.
Telomere lengths can be short in bone marrow failure syndromes other than DC, and our case highlights the importance of confirming the genetic diagnosis by identifying the disease causing mutation. Short telomeres, especially in the granulocytes, have been reported in patients with SDS, Diamond-Blackfan anemia, and Fanconi Anemia previously, but telomere lengths are not as short as in DC, where it is consistently below the first percentile compared to age-matched normal controls [19]. The interpretation of the clinical significance of telomere length needs to be considered in the context of concomitant hematological malignancies and also the assay used to measure telomere length. Two common techniques for measuring telomere length are q-PCR and fluorescence in situ hybridization coupled with flow cytometry (flow-FISH). Q-PCR uses total mononuclear cells and provides an average telomere length while FLOW-FISH uses specific lymphocyte subsets to detect individual chromosome lengths. Differing proportions of leucocyte subtypes within each sample could explain the limited sensitivity of qPCR compared to flow-FISH to diagnose DC [20]. The mean peripheral blood leucocyte telomere length by qPCR was shorter among MDS cases, and also shorter in blasts or cases post chemotherapy and should be interpreted with caution [21].
Malignant progression to MDS/AML is associated with very poor outcomes in SDS patients, and further research into better markers for risk stratification are needed to identify patients who may benefit from preemptive transplant. While clonal marrow cytogenetic abnormalities are commonly found in SDS, specific correlation with malignant transformation is lacking. In particular, the commonly found cytogenetic abnormalities in SDS [i(7q) and del(20q)] have not been associated with progression to MDS/AML [22]. On the other hand, recent evidence points towards the acquisition of TP53 mutation as an early initiating event in leukemic transformation [23]. Previous reports have shown that p53 expression is increased in cells with deficient SBDS protein, which likely occurs as a cellular reaction to significant DNA damage, leading to increased apoptosis [24]. Stem cells with TP53 mutations preferentially expand and accumulate more chromosomal aberrations and aneuploidy/polypoidy events in the setting of mitotic spindle dysfunction due to SBDS protein deficiency [25]. This promotes genomic instability and may explain the complex karyotype found in patients with MDS/AML arising from SDS. Further support for the role of somatic TP53 mutations comes from the CIBMTR repository study showing that all of the MDS cases in SDS patients carried TP53 mutations [16]. Other drivers likely synergise to promote the initiation and propagation of malignant clones, since TP53 mutations were found in the majority (80%) of patients who were 10 years or older [19], while the cumulative risk of MDS or AML was estimated to be much lower (19% at 20 years and 36% at 30 years). These drivers, if identified, may allow us to derive better predictors of malignant transformation and inform timing for transplant.
4. Conclusion
SDS remains underdiagnosed in Asia, and specialized clinics for hereditary hematologic malignancies should be initiated to guide genetic testing and support management and surveillance of carriers. Enrollment of affected individuals with SDS into international registry studies is encouraged whenever possible so that prospective data can be generated to optimize management.
Author contributions
SYO drafted the manuscript; STL reviewed cases of SDS in Asia; JN conceived the paper; all authors were involved in the management of the case and contributed critical corrections to the manuscript.
Sources of funding
Not applicable.
References
- 1.Kuijpers T.W., Nannenberg E., Alders M., Bredius R., Hennekam R.C. Congenital aplastic anemia caused by mutations in the SBDS gene: a rare presentation of Shwachman-Diamond syndrome. Pediatrics. 2004;114:e387–e391. doi: 10.1542/peds.2003-0651-F. [DOI] [PubMed] [Google Scholar]
- 2.Carapito R., Konantz M., Paillard C. Mutations in signal recognition particle SRP54 cause syndromic neutropenia with Shwachman-Diamond-like features. J. Clin. Investig. 2017;127:4090–4103. doi: 10.1172/JCI92876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Taneichi H., Kanegane H., Futatani T. Clinical and genetic analyses of presumed Shwachman-Diamond syndrome in Japan. Int. J. Hematol. 2006;84:60–62. doi: 10.1532/IJH97.06043. [DOI] [PubMed] [Google Scholar]
- 4.Jha A.K., Bansal D., Sharda S. Shwachman-Diamond syndrome in India. Pediatr. Blood Cancer. 2012;58:479–480. doi: 10.1002/pbc.23332. [DOI] [PubMed] [Google Scholar]
- 5.Cho W.K., Jung I.A., Kim J. Two cases of Shwachman-Diamond syndrome in adolescents confirmed by genetic analysis. Ann. Lab. Med. 2015;35:269–271. doi: 10.3343/alm.2015.35.2.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kawakami T., Mitsui T., Kanai M. Genetic analysis of Shwachman-Diamond syndrome: phenotypic heterogeneity in patients carrying identical SBDS mutations. Tohoku J. Exp. Med. 2005;206:253–259. doi: 10.1620/tjem.206.253. [DOI] [PubMed] [Google Scholar]
- 7.Lee J.H., Bae S.H., Yu J.J., Lee R., Yun Y.M., Song E.Y. A case of Shwachman-diamond syndrome confirmed with genetic analysis in a Korean child. J. Korean Med. Sci. 2008;23:142–145. doi: 10.3346/jkms.2008.23.1.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shen J., Lin K., An Y. Two cases of Shwachman-diamond syndrome with genetic confirmation and literature review. Zhonghua er ke za zhi = Chin. J. Pediatr. 2013;51:679–683. [PubMed] [Google Scholar]
- 9.Hagiwara S., Watanabe A. A case of shwachman-diamond syndrome distinguished from celiac disease. Pediatr. Rep. 2012;4:e30. doi: 10.4081/pr.2012.e30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kamoda T., Saito T., Kinugasa H. A case of Shwachman-diamond syndrome presenting with diabetes from early infancy. Diabetes Care. 2005;28:1508–1509. doi: 10.2337/diacare.28.6.1508. [DOI] [PubMed] [Google Scholar]
- 11.T. Morishima, K. Watanabe, H. Kanegane, E. Ito, M. Kobayashi, S. Kojima Nationwide study of Shwachman-diamond syndrome in Japan. Presented at New York Academy of Sciences 2011 – 6th International Congress on Shwachman-Diamond Syndrome.
- 12.Kakkar N., Vasishta R.K., Marwaha N., Marwaha R. Shwachman-diamond syndrome: a syndrome of pancreatic insufficiency and bone marrow dysfucntion. Arch. Pediatr. Adolesc. Med. 2001;155(5):611–612. doi: 10.1001/archpedi.155.5.611. [DOI] [PubMed] [Google Scholar]
- 13.Kathuria R., Poddar U., Yachha S.K. Shwachman-diamond syndrome: are we missing many? Indian Pediatr. 2012;49(9):748–749. doi: 10.1007/s13312-012-0138-x. [DOI] [PubMed] [Google Scholar]
- 14.Kwak J.W., Kim S., Lim Y.T. A case of Shwachman-diamond syndrome. K J. Pediatr. 2004;47(8):900–903. [Google Scholar]
- 15.Park S.Y., Chae M.B., Kwack Y.G. Allogeneic bone marrow transplantation in Shwachman-Diamond syndrome with malignant myeloid transformation. A case report. Korean J. Intern. Med. 2002;17:204–206. doi: 10.3904/kjim.2002.17.3.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lindsley R.C., Saber W., Mar B.G. Prognostic mutations in myelodysplastic syndrome after stem-cell transplantation. N. Engl. J. Med. 2017;376:536–547. doi: 10.1056/NEJMoa1611604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Keel S.B., Scott A., Sanchez-Bonilla M. Genetic features of myelodysplastic syndrome and aplastic anemia in pediatric and young adult patients. Haematologica. 2016;101:1343–1350. doi: 10.3324/haematol.2016.149476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guidugli L., Johnson A.K., Alkorta-Aranburu G. Clinical utility of gene panel-based testing for hereditary myelodysplastic syndrome/acute leukemia predisposition syndromes. Leukemia. 2017;31:1226–1229. doi: 10.1038/leu.2017.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alter B.P., Giri N., Savage S.A., Rosenberg P.S. Telomere length in inherited bone marrow failure syndromes. Haematologica. 2015;100:49–54. doi: 10.3324/haematol.2014.114389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gadalla S.M., Khincha P.P., Katki H.A. The limitations of qPCR telomere length measurement in diagnosing dyskeratosis congenita. Mol. Genet. Genom. Med. 2016;4:475–479. doi: 10.1002/mgg3.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rollison D.E., Epling-Burnette P.K., Park J.Y. Telomere length in myelodysplastic syndromes. Leuk. Lymphoma. 2011;52:1528–1536. doi: 10.3109/10428194.2011.568648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maserati E., Pressato B., Valli R. The route to development of myelodysplastic syndrome/acute myeloid leukaemia in Shwachman-diamond syndrome: the role of ageing, karyotype instability, and acquired chromosome anomalies. Br. J. Haematol. 2009;145:190–197. doi: 10.1111/j.1365-2141.2009.07611.x. [DOI] [PubMed] [Google Scholar]
- 23.Xia J., Miller C.A., Baty J. Somatic mutations and clonal hematopoiesis in congenital neutropenia. Blood. 2017 doi: 10.1182/blood-2017-08-801985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dror Y. P53 protein overexpression in Shwachman-Diamond syndrome. Arch. Pathol. Lab. Med. 2002;126:1157–1158. doi: 10.5858/2002-126-1157b-PPOISS. (author reply 1158) [DOI] [PubMed] [Google Scholar]
- 25.Austin K.M., Gupta M.L., Jr., Coats S.A. Mitotic spindle destabilization and genomic instability in Shwachman-Diamond syndrome. J. Clin. Investig. 2008;118:1511–1518. doi: 10.1172/JCI33764. [DOI] [PMC free article] [PubMed] [Google Scholar]