Abstract
Doxorubicin (DOX) is an antitumor drug, associated with cardiomyopathy. Strategies to address DOX-cardiomyopathy are scarce. Here, we identify the effect of forskolin (FSK) on DOX-induced-asymmetric-dimethylarginine (ADMA) accumulation in monocytoid cells. DOX-challenge led to i) augmented cytotoxicity, reactive-oxygen-species (ROS) production and methyltransferase-enzyme-activity identified as ADMA and s-adenosylhomocysteine (SAH) accumulation (SAH-A). However, except cytotoxicity, other DOX effects were decreased by metformin and FSK. FSK, did not alter the DOX-induced cytotoxic effect, but, decreased SAH-A by >50% and a combination of three drugs restored physiological methyltransferase-enzyme-activity. Together, protective effect of FSK against DOX-induced SAH-A is associated with mitigated methyltransferase-activity, a one-of-a-kind report.
Abbreviations: ADMA, asymmetric dimethylarginine; cAMP, cyclic AMP; CVD, cardiovascular disease; CT, chemotherapy; DOX, doxorubicin; DDAH, dimethylarginine diaminohydrolase; eNOS, endothelial nitric oxide synthase; FSK, forskolin; HCY, homocysteine; HTRF, homogenous time-resolved fluorescence; L-arg, L-arginine; L-cit, L-citrulline; MET, metformin; NAD+, nicotinamide adenine dinucleotide; OS, oxidative stress; PRMT1, protein arginine methyltransferase1; ROS, reactive oxygen species; SAH, s-adenosylhomocysteine;; SAH-A, SAH accumulation; SAHH, s-adenosylhomocysteine hydrolase; SAM, s-adenosylmethionine; SIRT1, sirtuin1
Keywords: Metformin, Forskolin, Endothelial dysfunction, Methyltransferase, Cancer, Cardiovascular disease
Graphical abstract
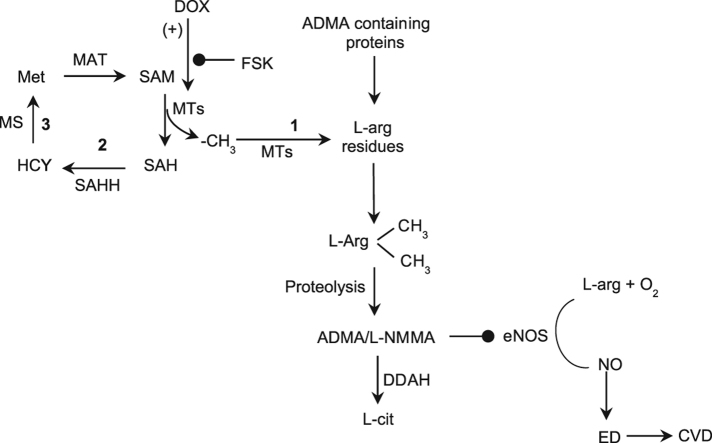
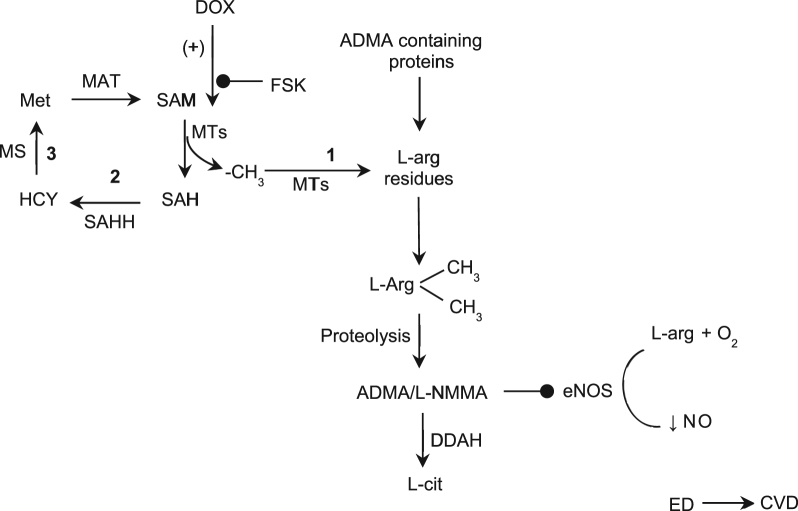
Possible signal pathway by which forskolin regulates arginine methylation. Forskolin prevents doxorubicin-induced methyltransferase activity which mitigates biosynthesis of ADMA and SAH, the intermediate products of protein methylation. ADMA is an endogenous inhibitor of eNOS which catalyses the formation of nitric oxide. Hence, an increase in intracellular or circulating ADMA concentration leads to eNOS uncoupling and endothelial dysfunction. Such an inhibitory effect of FSK has the potential to prevent eNOS uncoupling under DOX insult. (+) – potentiation;  – inhibition; 1 – Methylation pathway; 2 – Transmethylation pathway; 3 – Remethylation pathway; ADMA – asymmetric dimethylarginine; CVD – cardiovascular disease; DDAH – Dimethylarginine dimethylaminohydrolase; DOX – doxorubicin; ED – endothelial dysfunction; eNOS – endothelial nitric oxide; L-cit – L-citrulline; L-NMMA – L-N-monomethylarginine; MAT – methionine adenosyltransferase; MT – methyltransferases; MS – methionine synthase; Met – S-methionine; NO – nitric oxide; SAM – S-adenosylmethionine; SAH – S-adenosylhomocysteine; SAHH – S-adenosylhomocysteine hydrolase; FSK – Forskolin; HCY – homocysteine; L-arg – L-arginine
– inhibition; 1 – Methylation pathway; 2 – Transmethylation pathway; 3 – Remethylation pathway; ADMA – asymmetric dimethylarginine; CVD – cardiovascular disease; DDAH – Dimethylarginine dimethylaminohydrolase; DOX – doxorubicin; ED – endothelial dysfunction; eNOS – endothelial nitric oxide; L-cit – L-citrulline; L-NMMA – L-N-monomethylarginine; MAT – methionine adenosyltransferase; MT – methyltransferases; MS – methionine synthase; Met – S-methionine; NO – nitric oxide; SAM – S-adenosylmethionine; SAH – S-adenosylhomocysteine; SAHH – S-adenosylhomocysteine hydrolase; FSK – Forskolin; HCY – homocysteine; L-arg – L-arginine
Highlights
-
•
Forskolin has a protective role against doxorubicin-induced pathological side-effects.
-
•
Forskolin decreased DXR-induced levels of ADMA and SAH, independent biomarkers in cardiovascular disease.
-
•
Forskolin decreased doxorubicin-induced methyltransferase activity.
-
•
Forskolin does not interfere with the anticancer effect of doxorubicin.
1. Introduction
Chemotherapy (CT) is a prominent anticancer strategy practiced in all stages of cancer treatment. However, CT has significant limitations such as development of drug resistance and lack of sensitivity to anticancer drugs besides the other side effects. Reason being that CT damages normal cells in different organ systems, bone marrow and hair follicles that divide rapidly, thus these cells become sensitive to anti-mitotic drugs [1]. This leads to the prevalent dose-dependent side effects of CT including cardiomyopathy, arrhythmias, nephrotoxicity, dementia and pulmonary toxicity, among the others.
DOX (PubChem CID-31703), a cytotoxic anthracycline antibiotic is a frequent CT medication used to treat both solid and hematologic malignancies. It prevents DNA replication by intercalation as well as elevates free radical production, thus it confers cytotoxicity. However, its therapeutic limitation includes cumulative cardiotoxicity, manifested as acute and chronic events [2]. While molecular mechanism(s) of DOX-induced antineoplastic activity is well established, influence of DOX in chronic cardiotoxicity remains unclear. Previous studies have demonstrated that multiple mechanisms are involved in DOX-induced heart failure, although increased oxidative stress (OS) in cardiomyocytes is a well-known fact. Likewise, DOX-induced cardiotoxicity is associated with different cellular events including increase in iron accumulation, DNA disruption and reduction in eNOS (endothelial nitric oxide synthase) activity via asymmetric dimethylarginine (PubChem CID-123831) build-up [3], [4]. ADMA is a metabolic by-product formed during protein methylation, while s-adenosylmethionine (SAM, PubChem CID-34756)-dependent enzyme, protein arginine methyltransferase-1 (PRMT1) catalyses ADMA biosynthesis in the cytoplasm of all cells [5]. Synthesized free ADMA then proceeds into the blood plasma via the extracellular space. Increased ADMA level reduces nitric oxide production through competitive inhibition of eNOS [6], hence ADMA is associated with different pathologies including cardiovascular and metabolic diseases. In this regard, ADMA lowering strategies have clinical applications. While DOX-induced cardiotoxicity is associated with ADMA accumulation [4] and increased ROS production, strategies to mitigate DOX-induced detrimental effects are less explored. Therefore, a competent approach that will tactfully antagonize DOX-induced cardiotoxicity as well as preserve the anti-cancer effect of DOX needs to be identified. Such an enticing strategy can be exploited to mitigate cardiovascular disease (CVD)-induced mortality in cancer patients.
Forskolin (PubChem CID- 47936) is classified as a potent non-adrenergic activator of adenylyl cyclase and cAMP as well as an antioxidant, besides the fact that FSK activates SIRT1 via cAMP-PKA pathway independent of NAD+ [7]. Impressive characteristics of FSK include being an antihypertensive, a platelet aggregation inhibitor, a smooth muscle relaxant, a vasodilator and a cardiotonic agent [8]. Yet, the effect of FSK in regulating ADMA metabolism remains unexplored. Should FSK modulate intracellular ADMA levels, a novel trait of FSK, over-and-above the existing vasodilatory nature might be revealed. Similar to FSK, experimental and clinical studies have demonstrated that metformin (PubChem CID 4091) mitigates ADMA accumulation by activating the deacetylating enzyme, SIRT1, as well as increasing DDAH (dimethylarginine diaminohydrolase) activity; while DDAH is known to metabolize ADMA to L-citrulline (L-cit) [9]. In support, we have previously reported that SIRT1 potentially deacetylates PRMT1 wherein deacetylation alleviates the enzyme activity of PRMT1 [10].
In the present study, we tested whether FSK, through decreasing methyltransferase activity, could reduce ADMA accumulation without modulating the anti-cancer effect of DOX, in vitro. Reasons for using human monocytoid cells (THP1) for the experiments are that i) these cells are involved in two diseases – cardiovascular disease (ability to produce ADMA, SAH (PubChem CID-439155) and express eNOS) as well as cancer (leukemia), ii) the specific study targets such as methyltransferase and ADMA are also involved in these two pathologies. We found that FSK treatment on monocytoid cells attenuated DOX-induced i) intracellular accumulation of ADMA and SAH and ii) ROS generation while preserving the DOX-induced cytotoxicity.
2. Materials and methods
2.1. Culture and treatment of cells
Human monocytoid cells (THP1) (purchased from National Center for Cell Science, India) were cultured in RPMI-1640 medium containing 10 mM/L HEPES, 0.1 mM/L sodium pyruvate, 2 mM/L glutamine and 50 mM/L 2-mercaptoethanol, supplemented with 10% fetal bovine serum, 100U/ml penicillin and 100 μg/ml streptomycin at 37 °C in a 95% air and 5% CO2 incubator. Cells (1 × 106/ml) in logarithmic growth phase were treated with different concentrations of the drugs (DOX-2 μM, FSK-10 μM, MET-1 mM) or RPMI-1640.
2.2. Cytotoxicity assay
Cell viability was assessed using MTT assay [11], which is based on the reduction of MTT dye [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] to a colored formazon. Levels of reduced MTT were determined by measuring the difference in absorbance at 595 and 650 nm. About 1 × 106 THP1 cells/ml were exposed to increasing concentrations of i) DOX (0.125, 0.25, 0.5, 1, 2μΜ) ii) MET (0.01, 1, 10 mM) and iii) FSK (5, 10 20 μM) for 24 h in CO2 incubator at 37 °C. After incubation, cell viability was determined. For MTT assay, cells were exposed to 0.25 mg/ml MTT in PBS for 30 min at 37 °C then solubilized in 200 μl of 20% (w/v) SDS in 50% (v/v) N, N′-dimethylformamide, pH 4.5. Samples were assayed in triplicate, and the mean value for each experiment was calculated. The obtained results are expressed as a percentage of control, which is considered to be 100%.
2.3. DPPH radical scavenging assay
Free radical scavenging activity of test compounds was determined by the 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical assay. Fresh 1 mM DPPH solution was prepared in methanol and 3 ml of this solution was mixed with 100 μl of different concentrations (3.125, 6.25, 12.5, 50, 100, 200 µg/ml) of the test compounds. Ascorbic acid was used as reference. Samples were incubated for 30 min at 37 °C and absorbance was measured by spectrophotometer at 517 nm.
2.4. SAH conversion assay
Methyltransferase activity was measured from the conversion of SAM to SAH, as reported previously [12], [13]. Cells were treated with DOX, MET or FSK, independently or in combinations, for 24 h at 37 °C. Cells were homogenized in 50 mM Tris-HCl (pH 7.4) and centrifuged at 500×g for 10 min at 4 °C. Supernatant was utilized for the homogenous time-resolved fluorescence (HTRF) assay (Cisbio kit) [14], [15]. Cell lysate, as an enzyme source, was reacted with the methyl donor SAM and incubated for 45 min at room temperature (RT). This facilitates SAH formation, a by-product of transmethylation reaction, through binding of SAM with methyltransferase enzyme in lysate. Then SAH-d2 and SAH-antibody labeled with Lumi4-Tb (anti-SAH-Lumi4-Tb cryptate) were added to the lysate mix and incubated for 1 h at RT. Due to the competition between native SAH and SAH-d2 to bind with SAH antibody, and when SAH-d2 binds with SAH antibody, light emission occurs at 620 nm. However, when SAH produced from the enzyme reaction binds with SAH antibody, due to loss of fluorescence resonance energy transfer (FRET), light emission occurs at 665 nm. Hence, the concentration of SAH formed is inversely proportional to the intensity of fluorescence signals. Three controls were used in the assay as such i) buffer control comprising detection and enzymatic buffers; ii) negative control (no enzyme, no-activity control) had reaction mix without cell lysate; and iii) maximum HTRF ratio control comprising SAH antibody and SAH-d2 with other reagents. Difference in the intensities of HTRF signals in different experimental conditions were measured using a Spectramax i3x (Molecular Devices) multimode plate reader at 665 or 620 nm.
2.5. Measurement of ADMA by HPLC
Monocytoid cells were exposed to DOX, MET or FSK, as previously described. Treated cells were washed thrice, centrifuged at 200×g, 5 min at 4 °C and sonicated in ice-cold 50 Mm Tris-HCl (pH 7.4) followed by ultra-filtration through 3-kDa MWCO filters to remove proteins, as described [16]. The LC ESI-MS analyses were performed with a LC ESI-MS 2020 system equipped with LC10ADVP binary pump (Shimadzu, Japan). Shimadzu HPLC system was coupled on-line with an MS single quadruple ion trap. Identification of ADMA in the lysate was performed using reference ADMA and mass spectrometry, while quantification of cellular ADMA was carried out by HPLC. Samples were separated on a Inertsil NH2 column (250 mm × 4.6 mm, 5 µm Inertsil NH2 column) and detected using a photodiode array detector set at a wavelength of 205 nm. Mobile phase consisted of methanol:water (45:55) run at ambient temperature with a flow rate of 0.5 ml/min. Mass (MS) compartment consisted of single quadruple mass spectrometer with electrospray ionization (ESI) source and nitrogen gas was used to assist nebulization with a flow rate of 1.5 ml/min. Temperature was set for curved desolvation line (CDL) and heat block at 280 °C and 320 °C. Measurements were performed from peak area of the LC chromatogram. Quantification was achieved with the calibration curves obtained from standard solutions prepared at a concentration of (0, 6.25, 12.5, 25, 50, 100, 200 μg/ml). Retention time of ADMA peaks were found to be at 3.7 min [17]. Data were collected and processed using Lab Solution Software (Version 7.1, Shimadzu).
2.6. H2DCFDA assay
The cell-permeant dye, 2′,7′-dichlorofluoresceindiacetate (H2DCFDA) is oxidized by hydrogen peroxide, peroxynitrite, and hydroxyl radicals to yield the fluorescent molecule 2′7′-dichlorofluorescein. Thus, dye oxidation is an indirect measure of the presence of these reactive oxygen intermediates, which is calculated by dividing the mean channel fluorescence of a treated sample by that of the untreated one and multiplying by 100 to obtain the relative change, expressed as a percentage. About 2 × 105 cells/ml were treated with DOX, MET, FSK, either independently or in combinations. To each well, 20 µM of DCFDA solution was added and incubated for 20 min at 37 °C and 5% CO2. Cells were collected and washed once with RPMI containing 10 mM HEPES. Hydrogen peroxide treated cells were used as positive control. A change in fluorescence was assessed with a FACSCalibur flow cytometer (Becton Dickinson, Franklin Lakes, NJ).
3. Results
3.1. FSK does not restrain DOX-induced cytotoxicity
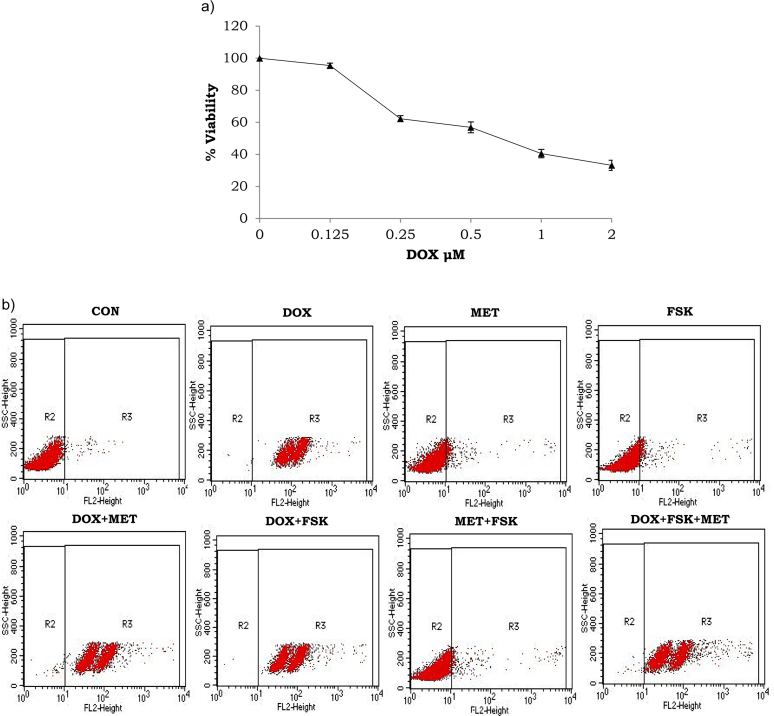
Monocytoid cells were incubated with different concentrations of drugs for 24 h to determine whether DOX, MET or FSK is cytotoxic for monocytoid cells by MTT assay. While THP1 cells were sensitive to DOX (0.25, 0.5, 1 and 2 μM) (Fig. 1A), different concentrations of FSK (5, 10, 20 μM) or MET (0.1, 1, 10 mM) did not induce cell death after 24 h incubation (data not shown). Hence, DOX, FSK and MET at the concentrations of 2 μM, 10 μM and 1 mM, respectively, were chosen for the experiments. Subsequently, apoptosis was measured through flow cytometry of PI-stained cells to identify whether the compound(s) induced apoptosis. Flow cytometry data (Fig. 1B) indicate that DOX, at 2 μM concentration, either independently, or in combination with MET at 1 mM concentration or FSK at 10 μM concentration, strongly induced apoptosis after 24 h treatment. As shown in Fig. 1B MET and FSK, independently or their combination did not induce apoptosis.
Fig. 1.
Forskolin or metformin did not hinder doxorubicin-induced cytotoxicity in monocytoid cells. Cells were incubated with doxorubicin (DOX 2 μM), forskolin (FSK 10 μM) and metformin (MET 1 μM), at 37 °C, 5% CO2 for 24 h and cytotoxicity was analysed using a) MTT assay and b) propidium iodide-flow cytometry.
3.2. FSK possesses radical scavenging activity
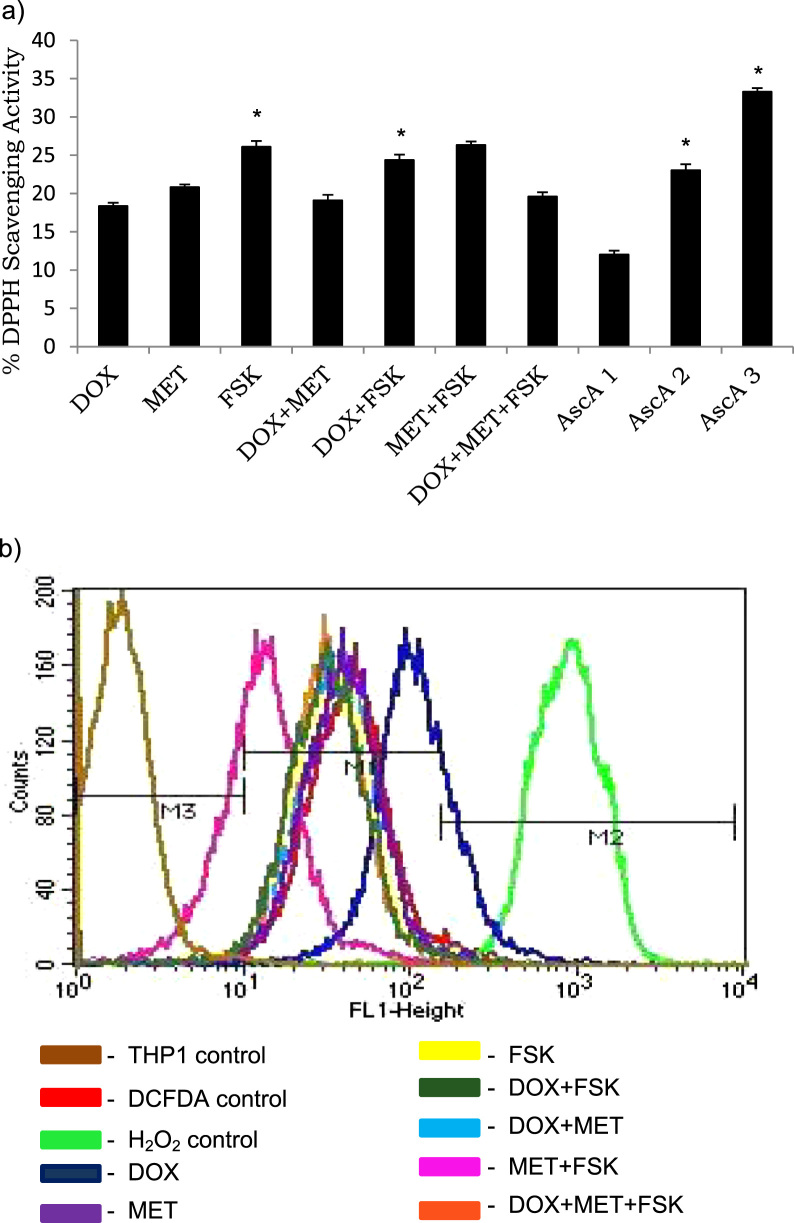
We used non-cellular assays to measure the DPPH radical scavenging capacity of the compounds, either independently or in combination (Fig. 2A). Among the compounds, FSK, per se, exhibited significant radical scavenging activity of 27% at 10 μM concentration (P < 0.05), however, DOX at 20 μM decreased the radical scavenging activity, independently or in combination with either MET or FSK, than FSK alone.
Fig. 2.
Forskolin has radical scavenging activity. a) Free radical scavenging activity of the test compounds (DOX − 2 μM, MET-1 μM, FSK-10 μM) was measured using DPPH assay and spectrophotometry. *P < 0.05 when compared with the respective control. Ascorbic acid (AscA1–3.125 μg/ml, AscA2–6.25 μg/ml, AscA3–12.5 μg/ml) was used as a positive control. b) Forskolin attenuates doxorubicin-induced reactive oxygen species production. THP1 cells were incubated with the test compounds (DOX- 2 μM, MET-1 μM, FSK-10 μM) at 37 °C, 5% CO2 for 24 h. Treated cells were labeled with DCFDA (20 μM) to measure ROS generation by flow cytometry. Three controls comprising THP1 cells alone, DCFDA alone and H2O2 control were included in the assay. M3 represents normal ROS production in THP1 cells, M2 represents H2O2 control and M1 represents compound(s)-induced ROS production.
3.3. FSK mitigates DOX-induced ROS production
Hydrogen peroxide, considered as positive control for ROS production, was found to trigger oxidation of the dye H2DCFDA, indicating the presence of hydrogen peroxide, peroxynitrite and hydroxyl radical (Fig. 2B) in THP1 cells. Since this dye does not distinguish between the free radical species, it was not possible to identify the dominant reactive oxygen species. Among the compounds, DOX was a potent inducer of free radicals than MET and FSK. However, combination of either MET or FSK with DOX mitigated the free radical producing potential of DOX, which is in accordance with previous reports [18].
3.4. FSK weakens DOX-induced SAH formation
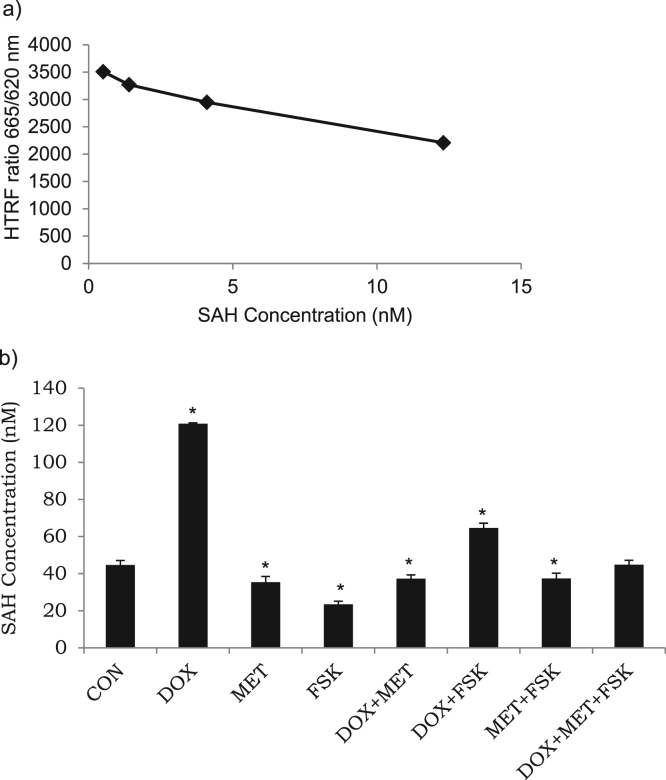
Dose-dependent HTRF signals were measured using different concentrations of reference SAH (0.5, 1.4, 4.1, 12.3 μM) (Fig. 3). Conversion of SAM-to-SAH assay was carried out with pre-treated cell lysates as a source of methyltransferase enzyme, along with SAM and SAH. To directly measure SAH release, anti-SAH antibody labeled with terbium cryptate and SAH-d2 tracer were used. SAH released by the enzymatic reaction decreases HTRF signal due to competitive immuno-binding with d2-SAH.
Fig. 3.
Measurement of SAH concentration through SAM-to-SAH conversion assay. a) Calibration curve for reference SAH. b) Cells were incubated with doxorubicin (DOX, 2 μM), forskolin (FSK, 10 μM) and Metformin (MET, 1 μM), in specific combinations, at 37 °C, 5% CO2 for 24 h. Quantification was performed as a homogenous time resolved fluorescence (HTRF) assay using multimode plate reader at 665 or 620 nm. Data demonstrates the effect of test compounds on the SAM-dependent methyltransferase activity through intracellular SAH concentrations. Results are the mean ± SD of three measurements. *-significantly different at P < 0.05 as compared with the respective control.
To determine whether test compounds regulate SAH formation, we incubated cell lysates with equimolar quantities of SAM and SAH, as described (Epigenous Methyltransferase assay, Cisbio). SAH concentration of both, previously formed native SAH and d2-coupled SAH, were determined by the SAM-to-SAH conversion competitive immunoassay. As shown in Fig. 3, DOX promoted SAH accumulation by about two-fold when compared with the control; while MET and FSK inhibited SAH formation by at least 70% when compared with DOX. MET or FSK, in the presence of DOX, caused about 50% reduction in SAH concentration than DOX. Interestingly, three pharmacological compounds together restored physiological concentration of SAH. These observations, one of its kind, indicate that MET or FSK inhibited the rate and production of SAH from the methyltransferase enzyme in the presence or absence of DOX (Fig. 3). These inhibitory effects were relatively less pronounced when experiments were performed in the presence of DOX. Robust inhibition of methyltransferase activity by FSK in the presence or absence of DOX indicates that under basal conditions or DOX-stimulated methyltransferase activity, FSK exerts an important inhibitory influence on SAH production in monocytoid cells.
3.5. FSK decreases DOX-induced ADMA accumulation
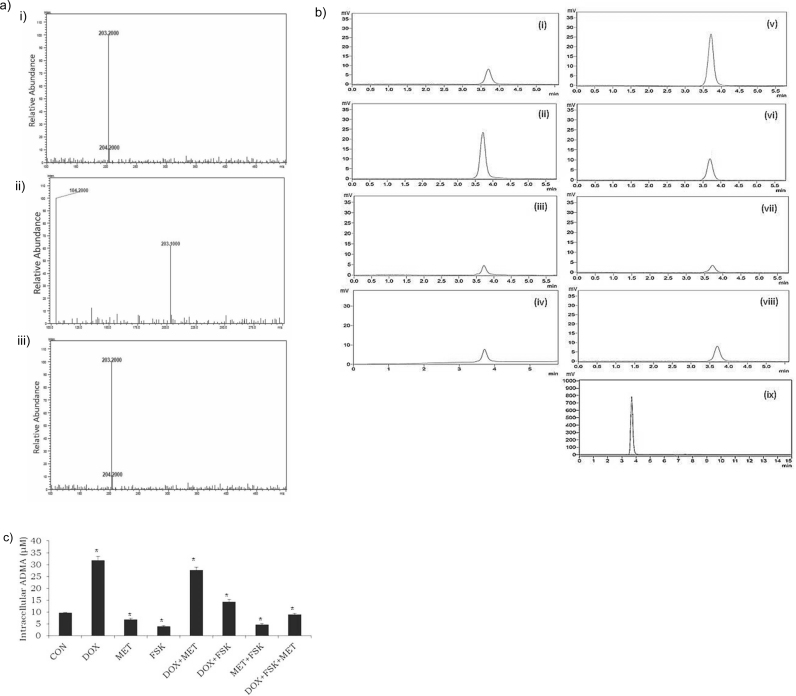
To further verify the observations on methyltransferase enzyme activity and quantitate the SAH inhibitory effects these pharmacological compounds, measurements of intracellular ADMA, a product of SAM-dependent methyltransferase activity were performed. For mass spectroscopic identification of the analyte, since ADMA is an endogenous molecule and that there is no blank sample (THP1 cells without ADMA), we evaluated the authenticity by comparing the reference ADMA and a THP1 cell lysate, which may or may not be spiked with reference ADMA using LC-MS-ESI. Representative MSI spectra of a pure ADMA standard and an extracted cell sample are shown in Fig. 4A. Under the described conditions, the molecular ion peak for reference ADMA was observed at m/z 203.2[M+1]+. A similarity in the pattern of fragmentation mass spectra of cell lysate and lysate spiked with reference ADMA was observed along with a modest increase in the molecular ion peak at m/z 203.2[M+1]+in the spiked sample. These spectra illustrated that the molecular ion peak at m/z 203.2 [M+1]+ in the lysate is indeed ADMA in comparison with the reference ADMA.
Fig. 4.
Measurement of intracellular ADMA in THP1 cells. a) ADMA product ion scan showing the specific fragment at m/z 203.2 which represents the positive ESI tandem mass spectra of i) ADMA reference standard ii) THP1 cell lysate and iii) THP1 cell lysate spiked with reference standard. b) and c) RP-HPLC quantification of ADMA with photodiode detection. Chromatograms of the pre-treated lysates [(i)–Control, (ii)–Doxorubicin (DOX, 2 μM), (iii)–Forskolin (FSK, 10 μM), (iv)–Metformin (MET, 1 μM), (v)–DOX+MET, (vi)–DOX+FSK, (vii)–FSK+MET, (viii)–DOX+MET+FSK] and ADMA reference standard (ix), 200 μg/ml have been reported for comparison of retention time of the standard with that of cell lysates. Data demonstrate the effect of test compounds on intracellular methylarginine concentrations. Results are the mean ± SD of three measurements. *-significantly different at P < 0.05 as compared with the respective control.
For HPLC, calibration curve was generated through different concentrations of reference ADMA, as such, 0, 6.25, 12.5, 25, 50, 100, 200 μg/ml. It was indicated that no significant interference was observed at the retention time of cellular ADMA (3.7 min) while the symmetric peak shape was maintained in the chromatogram (Fig. 4B). Besides, retention time of reference ADMA and cellular ADMA were consistent. Therefore, it was demonstrated that the present LC-MS condition was suitable for the detection of intracellular ADMA in monocytoid cells. We examined whether test compounds modulated the intracellular concentration of ADMA in THP1 cells by HPLC quantification. Significant ADMA concentrations were detected with values of 9.68 ± 0.17 μM in control and 31.8 ± 1.68 μM in DOX-treated cells (Fig. 4B). Chromatogram peaks demonstrated that the levels of ADMA in MET, FSK and MET plus FSK-treated cells were decreased by at least four-fold, when compared with DOX-treated cells. When cells were treated with both the inhibitors, MET and FSK, along with DOX, similar to the observation in SAH formation, methyltransferase activity was restored to physiological state. These results show for the first time that FSK significantly decreased ADMA concentrations independently or in combination with DOX.
Inhibition of SAH formation (Fig. 3) was seen paralleling the HPLC ADMA data (Fig. 4) with more than 50% inhibition seen in the presence of MET, FSK, DOX plus FSK, MET plus FSK and DOX plus FSK plus MET. Taken together, this study demonstrates that with DOX challenge, FSK exerts prominent inhibition of methyltransferase function and arginine methylation reaction as witnessed through reduction in formation of two analytes, intracellular SAH and ADMA concentrations.
4. Discussion
Present study was undertaken to determine the potential effect of FSK in regulating ADMA biosynthesis, under DOX challenge. We found that, DOX led to increased ROS production and methyltransferase activity which resulted in elevated ADMA and SAH formation, while MET and FSK significantly inhibited these effects (Fig. 5), independently and in combination in monocytoid cells.
Fig. 5.
Possible signal pathway by which forskolin regulates arginine methylation. Forskolin prevents doxorubicin-induced methyltransferase activity which mitigates biosynthesis of ADMA and SAH, the intermediate products of protein methylation. ADMA is an endogenous inhibitor of eNOS which catalyses the formation of nitric oxide. Hence, an increase in intracellular or circulating ADMA concentration leads to eNOS uncoupling and endothelial dysfunction. Such an inhibitory effect of FSK has the potential to prevent eNOS uncoupling under DOX insult. (+) – potentiation; – inhibition; 1 – Methylation pathway; 2 – Transmethylation pathway; 3 – Remethylation pathway; ADMA – asymmetric dimethylarginine; CVD – cardiovascular disease; DDAH – Dimethylarginine dimethylaminohydrolase; DOX – doxorubicin; ED – endothelial dysfunction; eNOS – endothelial nitric oxide; L-cit – L-citrulline; L-NMMA – L-N-monomethylarginine; MAT – methionine adenosyltransferase; MT – methyltransferases; MS – methionine synthase; Met – S-methionine; NO – nitric oxide; SAM – S-adenosylmethionine; SAH – S-adenosylhomocysteine; SAHH – S-adenosylhomocysteine hydrolase; FSK – Forskolin; HCY – homocysteine; L-arg – L-arginine.
DOX is an efficient anti-cancer molecule which is well-known to cause cardiotoxicity, principally through elevated OS [19]. Besides, in vitro and animal studies have demonstrated that DOX while inducing DNA damage, it elevates the enzyme activities of PRMT1, histone methyltransferase and DNA methyltransferase [20], [21], [22], [23], which is in support of our observation that DOX elevates methyltransferase activity (Fig. 3). Although extensive research unravels the mechanism and pathophysiology of DOX cardiomyopathy, preventive or restorative strategy for DOX cardiomyopathy remains unclear. It is well-accepted that MET use has better outcomes in DOX-induced cardiotoxicity [19], although the exact mechanism remains elusive. Previous studies have demonstrated that MET contributes to vasoprotection by reducing ADMA generation [24], hence, in this study, MET served as a positive control. Nevertheless, this study reports for the first time that MET regulates methyltransferase activity. Considering the fact that circulating ADMA is an aggregate of the contribution from different types of cells, including monocytes, reducing ADMA formation in any cell type could decrease the individual contribution to circulating ADMA concentration and attenuate the ADMA accumulation-induced disease burden, including CVD. It is possible that MET or FSK regulates ADMA formation via methyltransferase activity in different cell types, which is yet to be understood.
ADMA, a naturally occurring molecule, which is found in all human cells and plasma, can diffuse between cells (easy entry-exit), hence, it can be found in tissues and cells, besides being in circulation [25]. ADMA is an endogenous eNOS inhibitor whose accumulation leads to eNOS uncoupling, subsequent reduction in NO production and endothelial dysfunction [26]. Additionally, ADMA is not only a biomarker, but also a mediator of OS. In clinical studies, compromised NO production due to elevated ADMA level, impaired endothelium-dependent vasodilation and cardiovascular mortality and morbidity has been well established [27]. Other than CVD, elevated plasma ADMA concentration is strongly associated with cancer, Parkinson's disease, preeclampsia, renal disease, rheumatoid arthritis and Duchenne muscular dystrophy [28], [29], [30], [31], [32], [33]. SAH is a functionally important metabolite of homocysteine (HCY) metabolism as well as an accurate indicator and determinant of CVD risk [34]. Parallely, SAH is considered as a key mediator of HCY-associated atherogenesis [35] as it induces endothelial dysfunction by decreasing nitric oxide production and increasing OS [36]. Thus, SAH is associated with CVD [37]. Given that ADMA and SAH are associated with different clinical conditions our intent was to reduce DOX-induced ADMA and SAH accumulation in monocytoid cells as a restorative strategy. So that the outcome of this study can be utilized to develop an approach to reduce the CVD-induced mortality in doxorubicin-receiving cancer patients. Our results demonstrate that FSK mitigated DOX-induced ADMA formation from 33 μM to 4 μM (Fig. 4), an eight-fold decrease in monocytoid cells indicating the ameliorative potential of FSK. Although, FSK has been shown to regulate vasodilation [38], the protective mechanism of FSK in DOX challenge remains unclear. In this study, we have identified the restorative influence of FSK in monocytoid cells under DOX insult by observing the cellular signals including cytotoxicity, ROS production, ADMA accumulation and SAH formation. Our results demonstrated that FSK did not trigger cell death, however, it decreased methyltransferase activity, potentially PRMT1, as determined by the intracellular concentrations of ADMA and SAH. Nonetheless, the precise mechanism of FSK-mediated PRMT1 regulation remains to be determined by PRMT1-specific adenoviral constructs or siRNA. Because, logically two possibilities can exist, FSK can directly down regulate methyltransferase enzyme activity or it can quench free radicals to consequently attenuate the intracellular OS, by modulating the oxidant: antioxidant ratio, thereby altering the activity of the redox-sensitive enzyme, PRMT1. Can FSK directly down regulate methyltransferase activity? We speculate that FSK influences the post translational modification of PRMT1 through SIRT1. Based on the previous reports [10], i) SIRT1, a class III histone deacetylase enzyme, can deacetylate PRMT1 to decrease the methyltransferase activity; and ii) FSK is known to induce the intrinsic SIRT1 activity [7], [39]. On the other hand, the role of SIRT1 in cancer remains unclear as it could act either as tumor promoter or tumor suppressor [40]. Therefore, we hypothesize that FSK increases deacetylation of PRMT1 by up regulating the expression or activity of SIRT1, which deserves to be investigated. Our observations indicated that the cardioprotective effect, i.e., an outcome that causes reduction in ADMA and SAH levels, of FSK in DOX challenge was related to the inhibition of methyltransferase activity, besides activating adenylyl cyclase and scavenging free radicals. Fact is that i) biological models have shown the role of FSK in cancer therapy [41] and obesity [42] while ii) clinical trials have been undertaken to evaluate the efficacy of FSK on the risk factors of metabolic syndrome [20] and as an ocular hypotensive agent [43] in glaucoma patients. Hence, based on the observations from previous studies as well as our in vitro results, FSK can be a promising candidate for future studies on animal models and clinical trials to identify its potential in decreasing the rate of mortality in DOX-cardiomyopathy. Although, we have shown that FSK prevents DOX-induced accumulation of SAH and ADMA, this study does not identify the other critical parameters that regulate the intracellular concentration of SAH and ADMA, in the methylation, re-methylation and trans-methylation pathways, such as, i) status of enzyme activities of dimethylarginine dimethylaminohydrolase (DDAH, converts ADMA to L-cit) and s-adenosylhomocysteine hydrolase (SAHH, converts SAH to HCY); and intracellular concentration of HCY.
While PRMT1 is the primary source of ADMA, one study has demonstrated that along with the increased level of circulating ADMA, enzyme activities of PRMT-1 and −6 were significantly up-regulated in different tumor tissues. Interestingly, suppression of PRMT-1 and −6 by gene silencing siRNAs significantly inhibited the growth of multiple cancer cells [28]. This observation indicates that elevated ADMA concentration may be involved in tumor development and that PRMT-1 and −6 can be a therapeutic target for cancer as well as CVD. Our results are in line with this observation revealing that DOX induced the concentrations of SAH and ADMA by elevating the methyltransferase activity.
Exposure to DOX led to up regulated ROS production as well as cytotoxicity in monocytoid cells, which is in agreement with others [44]. In the present study, DOX-induced ROS production was decreased by FSK, in addition to MET (Fig. 2). The radical scavenging property of FSK can be implicated for the protective effect of FSK against DOX-induced free radical production, which is in agreement with the previous reports [45]. On the other hand, FSK did not interfere with the DOX-induced cytotoxicity because FSK is known to inhibit activation of the apoptotic mechanism while promoting up regulation of the anti-apoptotic pathway [46]. Along with the effect of FSK on ADMA and SAH accumulation, these results proved the hypothesis that FSK could attenuate DOX-induced cardiotoxicity, i.e., reduction of analytes that regulates CVD, without compromising the anticancer property of DOX. However, the endothelium-protective effect of FSK needs to be validated using endothelial cells as well, with DOX challenge.
Physiologically, monocytes are capable of producing i) NO through constitutive gene expression of eNOS [47], ii) ADMA and DDAH [48]. During the pathogenesis of atherosclerosis, studies have suggested that monocytes contribute to ED by two different mechanisms, besides the others: i) When monocytes adhere to EC, monocytes undergo rapid phenotypical modification to resemble EC by expressing eNOS, CD105, CD144 and VEGFR2 [49]. Given that monocytes express eNOS and that DOX causes elevated ADMA formation, we speculate that DOX could influence monocytic-eNOS to undergo uncoupling via ADMA accumulation, leading to endothelial dysfunction. ii) An in vivo study reports that circulating ADMA increases the number of circulating monocytes which in turn affects their adhesion potential, thereby accelerating monocyte-endothelial cell adhesion [50]. This adhesion down regulates the steady state levels of eNOS along with attenuated release of nitric oxide, thus contributing to endothelial dysfunction [51]. These observations demonstrate that a reduction in ADMA biosynthesis in monocytes could significantly alter the intrinsic endothelial dysfunction.
In summary, we report a novel capability of FSK as a protective molecule against DOX-induced cardiotoxicity, as indicated by i) non-interference of FSK in DOX-induced cytotoxicity and ii) reduction in DOX-induced intracellular accumulation of ROS, ADMA and SAH. The protective effect of FSK was related to the down-regulation of methyltransferase activity, resultant decrease in select analytes of methylation and trans-methylation pathways and OS.
4.1. Clinical significance
DOX cardiomyopathy has poor prognosis and is a lethal disease. Principle mechanisms of DOX cardiomyopathy, so far established, are oxidative stress and lipid peroxidation. Hence, the majority of existing treatments focus on attenuating oxidative stress to prevent DOX cardiomyopathy. Our findings, for the first time, revealed a unique molecular mechanism to address DOX cardiomyopathy, wherein FSK, besides being an antioxidant, it mitigates accumulation of ADMA and SAH, which are associated with progression of CVD, under DOX challenge. While DOX causes robust levels of these two molecules, FSK prevents their biosynthesis by reducing the enzyme activity of methyltransferase. Interestingly, when FSK exhibited such protective effect, it did not inhibit the anti-malignancy effect of DOX. Accordingly, we suggest that FSK can be exploited to prevent or treat DOX cardiomyopathy, with appropriate clinical trials.
Funding
This work has been supported by the research grant from i) Department of Biotechnology, India (to MRG, # BT/PR9930/NDB/39/457) and ii) SRM Research Excellence Program (2016) Fund, SRM IST (to KM).
References
- 1.Corrie P.G. Cytotoxic chemotherapy: clinical aspects. Medicine. 2008;36:24–28. [Google Scholar]
- 2.Thandavarayan R.A., Giridharan V.V., Arumugam S. Schisandrin B prevents doxorubicin induced cardiac dysfunction by modulation of DNA damage, oxidative stress and inflammation through inhibition of MAPK/p53 signalling. PLoS One. 2015;10:e0119214. doi: 10.1371/journal.pone.0119214. (PMID: 25742619) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akolkar G., Bagchi A.K., Ayyappan P., Jassal D.S., Singal P.K. Doxorubicin-induced nitrosative stress is mitigated by vitamin C via the modulation of nitric oxide synthases. Am. J. Physiol. Cell Physiol. 2017;312:C418–C427. doi: 10.1152/ajpcell.00356.2016. (PMID: 28100487) [DOI] [PubMed] [Google Scholar]
- 4.Kaida Y., Ueda S., Yamagishi S., Nakayama Y., Ando R., Iwatani R., Fukami K., Okuda S. Proteinuria elevates asymmetric dimethylarginine levels via protein arginine methyltransferase-1 overexpression in a rat model of nephrotic syndrome. Life Sci. 2012;91:301–305. doi: 10.1016/j.lfs.2012.06.015. (PMID: 22749861) [DOI] [PubMed] [Google Scholar]
- 5.Teerlink t. ADMA metabolism and clearance. Vasc. Med. 2005;10:S73–S81. doi: 10.1191/1358863x05vm597oa. (PMID: 16444872) [DOI] [PubMed] [Google Scholar]
- 6.Pope1 Arthur J., Karrupiah1 Kanchana, Kearns1 Patrick N., Xia2 Yong, Cardounel1 Arturo J. ROLE OF dimethylarginine dimethylaminohydrolases IN THE regulation OF endothelial nitric oxide production. J. Biol. Chem. 2009;284(51):35338–35347. doi: 10.1074/jbc.M109.037036. (PMID: 19820234) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gerhart-Hines Z., Dominy J.E., Blattler S.M. The cAMP/PKA pathway rapidly activates SIRT1 to promote fatty acid oxidation independently of changes in NAD+ Mol. Cell. 2011;44:851–863. doi: 10.1016/j.molcel.2011.12.005. (PMID: 22195961) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bhat S.V., Nagasampagi B.A., Sivakumar M. Springer-Narosa; 2005. Chemistry of Natural Products. [Google Scholar]
- 9.Bal F., Bekpinar S., Unlucerci Y. Antidiabetic drug metformin is effective on the metabolism of asymmetric dimethylarginine in experimental liver injury. Diabetes Res. Clin. Pract. 2014;106:295–302. doi: 10.1016/j.diabres.2014.08.028. (PMID:25263501) [DOI] [PubMed] [Google Scholar]
- 10.Soniya C., Vijay R., Jesu A. Caveolin1/protein arginine methyltransferase1/sirtuin1 axis as a potential target against endothelial dysfunction. Pharmacol. Res. 2017;119:1–11. doi: 10.1016/j.phrs.2017.01.022. (PMID:28126510) [DOI] [PubMed] [Google Scholar]
- 11.Cardounel A., Regelson W., Kalimi M. Dehydroepiandrosterone protects hippocampal neurons against neurotoxin-induced cell death: mechanism of action. Proc. Soc. Exp. Biol. Med. 1999;222:145–149. doi: 10.1046/j.1525-1373.1999.d01-124.x. (PMID:10564538) [DOI] [PubMed] [Google Scholar]
- 12.Duchin S., Vershinin Z., Levy D., Aharoni A. A continuous kinetic assay for protein and DNA methyltransferase enzymatic activities. Epigenet. Chromatin. 2015;8:56. doi: 10.1186/s13072-015-0048-y. (PMID:26675044) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hendricks C.L., Ross J.R., Pichersky E., Noel J.P., Zhoud Z.S. An enzyme-coupled colorimetric assay for S-adenosylmethionine-dependent methyltransferases. Anal. Biochem. 2004;326:100–105. doi: 10.1016/j.ab.2003.11.014. (PMID:14769341) [DOI] [PubMed] [Google Scholar]
- 14.Edwards B., Lesnick J., Wang J., Tang N., Peters C. Miniaturization of high throughput epigenetic methyltransferase assays with acoustic liquid handling. J. Lab Autom. 2016;21:208–216. doi: 10.1177/2211068215610861. (PMID:26450876) [DOI] [PubMed] [Google Scholar]
- 15.Kimos M., Burton M., Urbain D. Development of an HTRF assay for the detection and characterization of inhibitors of catechol-o-methyltransferase. J. Biomol. Screen. 2016;21:1–6. doi: 10.1177/1087057115616793. (PMID:26582803) [DOI] [PubMed] [Google Scholar]
- 16.Kinker K.G., Gibson A.M., Bass S.A., Day B.P., Deng J. Overexpression of dimethylarginine dimethylaminohydrolase-1 attenuates airway inflammation in a mouse model of asthma. PLoS One. 2014;9:e85148. doi: 10.1371/journal.pone.0085148. (PMID:24465497) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hecker M., Sessa W., Harris C., Anggard J.H., Vane J.R. The metabolism of L-arginine and its significance for the biosynthesis of endothelium-derived relaxing factor: cultured endothelial cells recycle L-citrulline to L-arginine. Proc. Natl. Acad. Sci. 1990;87:8612–8616. doi: 10.1073/pnas.87.21.8612. (PMID:2236071) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kelleni M.T., Amin E.F., Abdelrahman A.M. Effect of metformin and sitagliptin on doxorubicin-induced cardiotoxicity in rats: impact of oxidative stress, inflammation, and apoptosis. J. Toxicol. 2015;2015:424813. doi: 10.1155/2015/424813. (PMID:26880912) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tseng Y.T. Cardioprotective effect of metformin against doxorubicin cardiotoxicity in rats. Anatol. J. Cardiol. 2016;16:242–243. doi: 10.14744/AnatolJCardiol.2016.18505. (PMID:27111198) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.X. Su, H.N. Shivaprasad, The Effect of Coleus Forskohlii Extract on the Risk Factors of Metabolic Syndrome. ClinicalTrials.gov Identifier:NCT02143349, 〈https://clinicaltrials.gov/ct2/show/〉 NCT02143349, 2014.
- 21.Gros L., Delaporte C., Frey S. Identification of new drug sensitivity genes using genetic suppressor elements: protein arginine N-methyltransferase mediates cell sensitivity to DNA-damaging agents. Cancer Res. 2003;63:164–171. (PMID:12517794) [PubMed] [Google Scholar]
- 22.Zheng Z., Schmidt-Ott K.M., Chua S. A Mendelian locus on chromosome 16 determines susceptibility to doxorubicin nephropathy in the mouse. Proc. Natl. Acad. Sci. 2005;102:2502–2507. doi: 10.1073/pnas.0409786102. (PMID:15699352) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang Y., Gao Y., Zhang G. DNMT3a plays a role in switches between doxorubicin-induced senescence and apoptosis of colorectal cancer cells. Int. J. Cancer. 2011;128:551–561. doi: 10.1002/ijc.25365. (PMID:20473858) [DOI] [PubMed] [Google Scholar]
- 24.Ghosh S., Lakshmanan A.P., Hwang M.J. Metformin improves endothelial function in aortic tissue and microvascular endothelial cells subjected to diabetic hyperglycaemic conditions. Biochem. Pharmacol. 2015;98:412–421. doi: 10.1016/j.bcp.2015.10.008. (PMID:26467186) [DOI] [PubMed] [Google Scholar]
- 25.Raptis V., Kapoulas S., Grekas D. Role of asymmetrical dimethylarginine in the progression of renal disease. Nephrology (Carlton) 2016;18:11–21. doi: 10.1111/j.1440-1797.2012.01659.x. (PMID:23016674) [DOI] [PubMed] [Google Scholar]
- 26.Sitar M.E. Asymmetric dimethylarginine and its relation as a biomarker in nephrologic diseases. Biomark. Insights. 2016;11:131–137. doi: 10.4137/BMI.S38434. (PMID:27980388) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morales Y., Nitzel D.V., Price O.M. Redox control of protein arginine methyltransferase 1 (PRMT1) activity. J. Biol. Chem. 2015;290:14915–14926. doi: 10.1074/jbc.M115.651380. (PMID:25911106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yoshimatsu M., Toyokawa G., Hayami S. Dysregulation of PRMT1 and PRMT6, Type I arginine methyltransferases, is involved in various types of human cancers. Int. J. Cancer. 2011;128:562–573. doi: 10.1002/ijc.25366. (PMID:20473859) [DOI] [PubMed] [Google Scholar]
- 29.Horster I., Weigt-Usinger K., Carmann C. The L-arginine/NO pathway and homoarginine are altered in Duchenne muscular dystrophy and improved by glucocorticoids. Amino Acids. 2015;47:1853–1863. doi: 10.1007/s00726-015-2018-x. (PMID:26066683) [DOI] [PubMed] [Google Scholar]
- 30.Kirbas S., Kirbas A., Tufekci A. Serum levels of homocysteine, asymmetric dimethylarginine and nitric oxide in patients with Parkinson's disease. Acta Clin. Belg. 2016;71:71–75. doi: 10.1080/17843286.2016.1138592. (PMID:27075796) [DOI] [PubMed] [Google Scholar]
- 31.Savvidou M.D., Hingorani A.D., Tsikas D., Frolich J.C., Vallance P., Nicolaides K.H. Endothelial dysfunction and raised plasma concentrations of asymmetric dimethylarginine in pregnant women who subsequently develop pre-eclampsia. Lancet. 2003;361:1511–1517. doi: 10.1016/S0140-6736(03)13177-7. (PMID:12737861) [DOI] [PubMed] [Google Scholar]
- 32.Zheng J.J., Wang H.O., Huang M., Zheng F.Y. Assessment of ADMA, estradiol, and progesterone in severe preeclampsia. Clin. Exp. Hypertens. 2016;38:347–351. doi: 10.3109/10641963.2015.1089880. (PMID:27152507) [DOI] [PubMed] [Google Scholar]
- 33.Dimitroulas T., Sandoo A., Hodson J., Smith J., Douglas K.M., Kitas G.D. Associations between asymmetric dimethylarginine, homocysteine, and the methylenetetrahydrofolate reductase (MTHFR) C677T polymorphism (rs1801133) in rheumatoid arthritis. Scand. J. Rheumatol. 2016;45:267–273. doi: 10.3109/03009742.2015.1086433. (PMID:26599798) [DOI] [PubMed] [Google Scholar]
- 34.Xiao Y., Zhang Y., Wang M. Plasma S-adenosylhomocysteine is associated with the risk of cardiovascular events in patients undergoing coronary angiography: a cohort study. Am. J. Clin. Nutr. 2013;98:1162–1169. doi: 10.3945/ajcn.113.058727. (PMID:24004894) [DOI] [PubMed] [Google Scholar]
- 35.Zawada A.M., Rogacev K.S., Hummel B. S-adenosylhomocysteine is associated with subclinical atherosclerosis and renal function in a cardiovascular low-risk population. Atherosclerosis. 2014;234:17–22. doi: 10.1016/j.atherosclerosis.2014.02.002. (PMID:24589563) [DOI] [PubMed] [Google Scholar]
- 36.Barroso M., Rocha M.S., Esse R. Cellular hypomethylation is associated with impaired nitric oxide production by cultured human endothelial cells. Amino Acids. 2012;42:1903–1911. doi: 10.1007/s00726-011-0916-0. (PMID:21614558) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Castro R., Rivera I., Struys E.A. Increased homocysteine and S-adenosylhomocysteine concentrations and DNA hypomethylation in vascular disease. Clin. Chem. 2003;49:1292–1296. doi: 10.1373/49.8.1292. (PMID:12881445) [DOI] [PubMed] [Google Scholar]
- 38.Schlepper M., Thormann J., Mitrovic M. Cardiovascular effects of forskolin and phosphodiesterase-III inhibitors. Basic Res. Cardiol. 1989;84:197–212. doi: 10.1007/BF02650360. (PMID:2530974) [DOI] [PubMed] [Google Scholar]
- 39.Chao L.C., Tontonoz P. SIRT1 regulation—it ain’t all NAD. Mol. Cell. 2012;45:9–11. doi: 10.1016/j.molcel.2011.12.017. (PMID:22244328) [DOI] [PubMed] [Google Scholar]
- 40.Lin Z., Fang D. The roles of SIRT1 in cancer. Genes Cancer. 2013;4:97–104. doi: 10.1177/1947601912475079. (PMID:24020000) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sapio L., Gallo M., Illiano M. The natural cAMP elevating compound forskolin in cancer therapy: is it time? J. Cell Physiol. 2017;232:922–927. doi: 10.1002/jcp.25650. (PMID:27739063) [DOI] [PubMed] [Google Scholar]
- 42.Shivaprasad H.N., Gopalakrishna S., Mariyanna B., Thekkoot M., Reddy R., Tippeswamy B.S. Effect of coleus forskohlii extract on cafeteria diet-induced obesity in rats. Pharmacogn. Res. 2014;6:42–45. doi: 10.4103/0974-8490.122916. (PMID:24497741) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Majeed M., Nagabhushanam K., Natarajan S., Vaidyanathan P., Karri S.K., Jose J.A. Efficacy and safety of 1% forskolin eye drops in open angle glaucoma–an open label study. Saudi J. Ophthalmol. 2015;29:197–200. doi: 10.1016/j.sjopt.2015.02.003. (PMID:26155078) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ueno M., Kakinuma Y., Yuhki K. Doxorubicin induces apoptosis by activation of caspase-3 in cultured cardiomyocytes in vitro and rat cardiac ventricles in vivo. J. Pharmacol. Sci. 2006;101:151–158. doi: 10.1254/jphs.fp0050980. (PMID:16766856) [DOI] [PubMed] [Google Scholar]
- 45.El-Agroudy N.N., El-Naga R.N., El-Razeq R.A., El-Demerdash E. Forskolin, a hedgehog signalling inhibitor, attenuates carbon tetrachloride-induced liver fibrosis in rats. Br. J. Pharmacol. 2016;173:3248–3260. doi: 10.1111/bph.13611. (PMID: 27590029) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Misra U.K., Pizzo S.V. Coordinate regulation of forskolin-induced cellular proliferation in macrophages by protein kinase A/cAMP-response element-binding protein (CREB) and Epac1-Rap1 signaling: effects of silencing CREB gene expression on Akt activation. J. Biol. Chem. 2005;280:38276–38289. doi: 10.1074/jbc.M507332200. (PMID:16172130) [DOI] [PubMed] [Google Scholar]
- 47.Calo L., Davis P.A., Milani M. Constitutive endothelial nitric oxide synthase (ecNOS) gene expression in human monocytes. Angiology. 1998;49:419–422. doi: 10.1177/000331979804900601. (PMID:9631886) [DOI] [PubMed] [Google Scholar]
- 48.Lambden S., Martin D., Vanezis K. Role of dimethylarginine dimethylaminohydrolase 2 in the regulation of nitric oxide synthesis in animal and observational human models of normobaric hypoxia. Nitric Oxide. 2016;31:59–66. doi: 10.1016/j.niox.2016.06.003. (PMID:27319282) [DOI] [PubMed] [Google Scholar]
- 49.Tso C., Rye K.A., Barter P. Phenotypic and functional changes in blood monocytes following adherence to endothelium. PLoS One. 2012;7:e37091. doi: 10.1371/journal.pone.0037091. (PMID:22615904) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Beutel G., Perthel R., Suntharalingam M. Effect of chronic elevated asymmetric dimethylarginine (ADMA) levels on granulopoiesis. Ann. Hematol. 2013;92:505–508. doi: 10.1007/s00277-012-1636-6. (PMID:23224243) [DOI] [PubMed] [Google Scholar]
- 51.Szabo A., Kovesi T., Gal J. The challenge of breath analysis for clinical diagnosis and therapeutic monitoring. In: Amann A., Smith D., editors. Diagnostic Aspects of Exhaled Nitric Oxide in Cardiothoracic Anesthesia. World Scientific; Singapore: 2005. pp. 171–196. [Google Scholar]