SUMMARY
Dysferlin plays a critical role in the Ca2+-dependent repair of microlesions that occur in the muscle sarcolemma. Of the seven C2 domains in dysferlin, only C2A is reported to bind both Ca2+ and phospholipid, thus acting as a key sensor in membrane repair. Dysferlin C2A exists as two isoforms, the “canonical” C2A and C2A variant 1 (C2Av1). Interestingly, these isoforms have markedly different responses to Ca2+ and phospholipid. Structural and thermodynamic analyses are consistent with the canonical C2A domain as a Ca2+-dependent, phospholipid-binding domain, whereas C2Av1 would likely be Ca2+-independent under physiological conditions. Additionally, both isoforms display remarkably low free energies of stability, indicative of a highly flexible structure. The inverted ligand preference and flexibility for both C2A isoforms suggest the capability for both constitutive and Ca2+-regulated effector interactions, an activity that would be essential in its role as a mediator of membrane repair.
INTRODUCTION
Muscle tissue generates mechanical force. The stress placed on the sarcolemmal membrane as a result of this force introduces microlesions in the muscle cell membrane, which without in vivo repair mechanisms would result in cell death (McNeil, 2002). Repair of membrane lesions depends on the activity of dysferlin, a multiple C2 domain-containing protein proposed to play a role in the vesicle fusion associated with membrane repair (Bansal et al., 2003). Mutations within the 8.5 kb dysferlin (DYSF) gene can result in recessive forms of muscular dystrophy, such as limb-girdle muscular dystrophy (LGMD), Mioshi myopathy (MM), or distal anterior compartment myopathy (DMAT) (Illa et al., 2001). Currently, approximately 500 unique human mutations have been cataloged in the DYSF gene (den Dunnen, 2012). These include point mutations within the predicted folded domains, the interdomain linkers, and premature truncations of the primary sequence (den Dunnen, 2012).
The physiological mechanism of action of dysferlin is not yet understood; however, several hypotheses have been proposed. Dysferlin-deficient mice accumulate subsarcolemmal vacuoles at the surface of muscle cell membranes; consequently, dysferlin has been implicated in vesicle fusion at the site of injury (Bansal et al., 2003; Selcen et al., 2001). Dysferlin could also play a synaptotagmin-like role to interact with SNARE proteins that mediate the fusion of patching vesicles (Evesson et al., 2010; Miyake and McNeil, 1995), or dysferlin may act as a scaffold for other fusion-related proteins, such as MG53 (Cai et al., 2009a, 2009b; Matsuda et al., 2012; McCann et al., 2012). Dysferlin localizes to the t-tubule network (Ampong et al., 2005; Klinge et al., 2010) and may therefore be involved in vesicle trafficking to the sarcolemma through its interactions with tubulin (Azakir et al., 2010). In addition, it may be instrumental in signal transduction pathways related to membrane repair (Covian-Nares et al., 2010). The most prevalent view is that dysferlin mediates the Ca2+-dependent fusion of intracellular vesicles with microtears in the sarcolemma through the concerted interaction of its seven C2 domains with Ca2+ and phospholipid (McNeil et al., 2000).
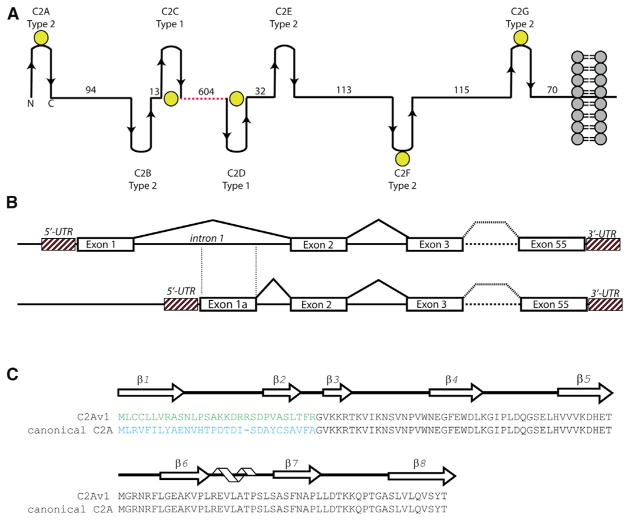
Dysferlin is a 237 kDa protein composed of seven, intermittently spaced C2 domains (C2A–C2G) (Figure 1A). There are 14 isoforms of human dysferlin that are derived from differential splicing events at exons 1, 5a, 17, and 40a (Pramono et al., 2009). Most of these alternative exons encode interdomain linker sequences; however, C2A is the only folded domain in dysferlin with two alternative primary sequences (Pramono et al., 2006). Exon 1 in the dysferlin gene (DYSF) encodes the first 29 amino acids of the “canonical” dysferlin C2A domain, which is currently the most thoroughly characterized (Figure 1B); whereas, exon 1a encodes the first 30 amino acids of the dysferlin C2A variant 1 domain (C2Av1). Approximately 23% of the dysferlin transcripts in skeletal muscle incorporate exon 1a, but no physiological function has been demonstrated for this isoform (Pramono et al., 2006). To determine the differences between the canonical C2A and the C2Av1 isoforms, we merged biophysical studies with in vitro approaches to gain structure-function insight into the role of these two domains in membrane repair.
Figure 1. Overall Schematic of Dysferlin Domain Structure, Exon Structure, and C2A Primary/Secondary Sequence.
(A) Overall modular organization of the full-length dysferlin protein. The spacers between C2 domains have been scaled according to the predicted distances between C2 domains. Yellow spheres correspond to predicted Ca2+-binding C2 domains in dysferlin based on conserved residues in the primary sequence alignments. The dotted red line corresponds to the FerA/B and DysN/DysC domains.
(B) Schematic of the partial exon structure of dysferlin C2A.
(C) Primary sequence alignment of C2A variant 1 versus the canonical C2A domain of dysferlin. The arrows above the sequence correspond to residues that possess β strand secondary structure, whereas the helical cartoon corresponds to residues with α-helical secondary structure.
We solved the X-ray structure of each C2A domain to high resolution, characterized the Ca2+ and phospholipid binding activities of each, and established their subcellular localization in cultured muscle cells. We show that the canonical C2A domain of dysferlin possesses two classes of Ca2+ binding sites, each with distinct biophysical roles. The first class is a single, high-affinity Ca2+ binding site that stabilizes the domain. The second class is a set of low-affinity Ca2+ binding sites that mediate the interactions between the C2A domain and the negatively charged phospholipid membrane, consistent with its role as a Ca2+-dependent phospholipid binding domain. The C2Av1 does not bind Ca2+, but it may become more sensitive to Ca2+ in the presence of phospholipids. Further, both C2A domains exhibit some of the lowest free energies of stability yet measured in a folded protein, suggesting remarkable conformational plasticity (Dunker et al., 2001; Gauer et al., 2012). This flexibility allows the C2A domains of dysferlin to explore different subsets of conformations for maximal ligand and effector binding potential (Gauer et al., 2012). In addition, because both isoforms of dysferlin are coexpressed in the same tissues, and both localize to the plasma membrane, the Ca2+-independent C2Av1 could likely act in concert with the Ca2+-dependent C2A-dysferlin at the plasma membrane to regulate and fine-tune the membrane repair response.
RESULTS
Canonical Dysferlin C2A Is a Type 2 C2 Domain
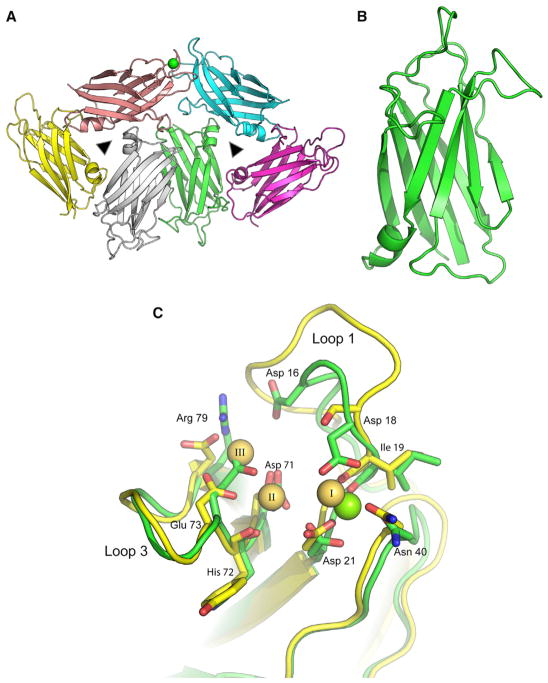
Human dysferlin C2A (residues 1–125) crystallized in 2.8 M sodium formate as two stacked trimers in the asymmetric unit (Table 1). We solved the X-ray structure of these crystals to 2.0 Å resolution (Figure 2A) by molecular replacement, using the nuclear magnetic resonance (NMR) structure of the C2A domain of myoferlin (2DMH) as the search model. The canonical C2A domain is a β sandwich fold of eight β strands connected by a type 2 C2 domain topology (Figure 2B). Three loops at the apex of the domain form the divalent cation binding pocket. Loops 1 and 3 typically possess amino acids that interact with phospholipids. Both the myoferlin (2DMH) (Yokoyama et al., 2000) and the otoferlin C2A domains (3L9B) (Helfmann et al., 2011) share a similar architecture. Primary sequence alignment between dysferlin C2A and myoferlin C2A revealed that the two proteins share 66% sequence similarity between their C2A domains. The root-mean-square deviation (rmsd) between the C2A domains of myoferlin (1DMH) and dysferlin is 0.83 Å over all Cα atoms. Otoferlin C2A is structurally similar to dysferlin C2A; however, its vestigial Ca2+ binding loop 1 is significantly shorter than the homologous loop in either myoferlin or dysferlin (Helfmann et al., 2011).
Table 1.
Crystallographic Parameters
| Canonical Dysferlin C2A | Dysferlin C2Av1 | |
|---|---|---|
| Data Collection | ||
| Space group | I 1 2 1 | P3121 |
| a,b,c (Å) | 102.4, 70.7,118.3 | 71.2, 71.2, 137.5 |
| α,β,γ (°) | 90, 113.4, 90 | 90,90,120 |
| Resolution (Å) | 50–2.04 | 50–1.8 |
| Rsym (%) | 7.3 (41.5) | 7.5 (53.5) |
| I/σI | 11.1 (3.1) | 26 (3.7) |
| Completeness (%) | 99.3 (99.5) | 95.7 (98.9) |
| Redundancy | 3.6 (3.6) | 6.6 (7.0) |
| Refinement | ||
| Resolution (Å) | 33.9–2.04 | 30–1.8 |
| No. reflections | 48,842 | 36,605 |
| Rworking/Rfree (%) | 20.0/24.0 | 19.2/21.5 |
| No. Atoms | ||
| Protein | 5,959 | 5,515 |
| Formate ions (FMT) | 41 | – |
| Ca2+ | 1 | – |
| Water | 261 | 612 |
| Average B Factors (Å2) | ||
| Protein | 49.27 | 26.8 |
| Solvent | 48.0 | 34.6 |
| FMT/Ca2+ | 43.5/65.9 | −/− |
| Rmsds | ||
| Bond lengths (Å) | 0.008 | 0.024 |
| Bond angles (°) | 1.347 | 1.816 |
Figure 2. Crystal Structure of the Canonical Dysferlin C2A Domain.
(A) Ribbon diagram of the stacked trimeric arrangement of the asymmetric unit of human dysferlin C2A. The green sphere is a single Ca2+ coordinated to one of the six domains in the asymmetric unit. Noncrystallographic 3-fold symmetry operators are represented as triangles.
(B) An isolated canonical C2A domain is shown in green.
(C) Ca2+ binding sites in dysferlin C2A. The green sticks correspond to the dysferlin C2A structure. The green sphere corresponds to the high-affinity Ca2+ found in the crystal structure (chain E, 4IHB). The yellow sticks correspond to the three La3+ described in the phospholipase C-δ1 C2 domain (1DJG, residues 628–756) (Essen et al., 1997). The residue numbers refer to the corresponding amino acids in the human dysferlin sequence.
The Variant of C2A Shows Fundamental Structural Differences in the Ca2+-Binding Pocket
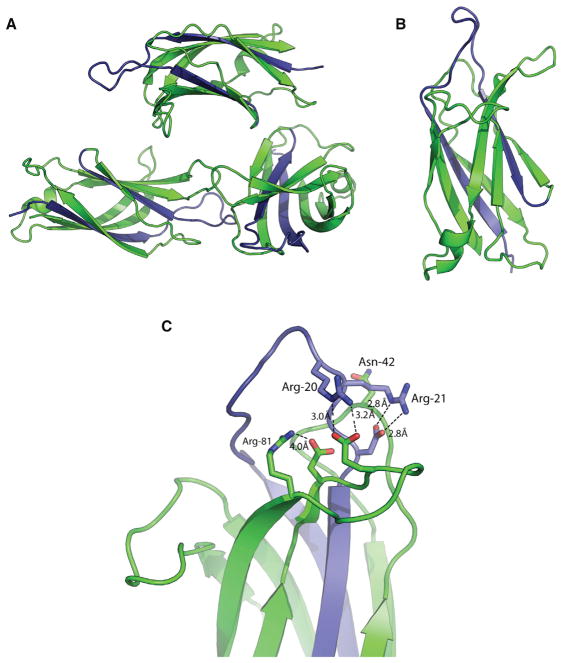
The primary sequence of C2Av1 (residues 1–126) possesses more basic amino acids than does the canonical C2A domain (Figure 1C). At pH 7.4, the calculated isoelectric point (pI) for the canonical C2A domain is 7.66, whereas the calculated pI for C2Av1 is 10.4 (Anandakrishnan et al., 2012). The increase in pI is due to five additional basic residues encoded by the alternate exon. To understand the impact of the alternate residues on the C2 structure, we solved the X-ray crystal structure of C2Av1 to 1.8 Å resolution using a monomer of our dysferlin C2A structure (4IHB) as the search model (Table 1). The C2Av1 domain crystallized as a single trimer in the asymmetric unit under low ionic strength conditions (Figure 3A), whereas the canonical C2A crystal structure crystallized as two stacked trimers under high salt conditions. The overall structure of C2Av1 is similar to that of the canonical C2A domain as the rmsd between both isoforms is 0.493 Å over all Cα atoms (Figure 3B). The resulting primary sequence substitution affects β strand 1, loop 1, and β strand 2. Therefore, despite the substitution of two β strands and one loop, the overall fold of the C2A domain has been conserved. The most significant changes occur in Loop 1, which typically possess the residues for Ca2+ and lipid binding in C2 domains. Upon solving the crystal structure of the C2Av1 isoform, it became clear that the additional arginine residues in loop 1 form salt bridges to each of the remaining Ca2+ binding residues in the C2 domain (Figure 3C). Arg-20 forms a salt bridge interaction with Glu-75; Arg-21 coordinates with Asp-23; and Arg-81 coordinates with Asp-73. Interestingly, Asn-72 contributes to Ca2+ binding in the canonical C2A domain, but it is sterically blocked by the aliphatic chain of Arg-21. Therefore, we predict C2Av1 to have reduced potential for Ca2+ binding relative to that of the canonical C2A domain.
Figure 3. Crystal Structure of the Human Dysferlin C2A Variant 1 Domain.
(A) Asymmetric unit of C2Av1.
(B) Single C2Av1 domain.
(C) Cation binding pocket of dysferlin C2Av1. The variant residues are shown in blue.
Ca2+ and Phospholipid Binding of the Canonical C2A versus C2Av1
In the crystal structure of the canonical C2A domain, one of the six molecules in the asymmetric unit coordinated a single divalent cation (Figures 2A and 2C). The residues that coordinate this cation have an average cation:ligand distance of 2.6 Å; therefore, we modeled and refined it as a calcium ion (Cates et al., 2002). The position of this cation and its coordinating residues superimpose with the X-ray structure of the La3+-bound C2 domain of phospholipase C-δ1 (Essen et al., 1997) (Figure 2C). Based on this structural similarity, the site of an additional cation binding site in dysferlin C2A can be inferred from the “II” position in the phospholipase C-δ1 C2 domain. Asp-18, Ile-19, Asp-21, Asn-40, Asp-71, His-72, and Glu-73 of dysferlin C2A could contribute side-chain or main-chain oxygen atoms to coordinate two divalent cations in dysferlin C2A. The phospholipase C-δ1 C2 domain has the potential for a third ion (Essen et al., 1997); however, two of the homologous residues in dysferlin C2A likely preclude Ca2+ binding at that site. Glu-73 would collide with a third divalent cation at the “III” position (Figure 2C), and Arg-79 would not contribute to productive Ca2+ binding.
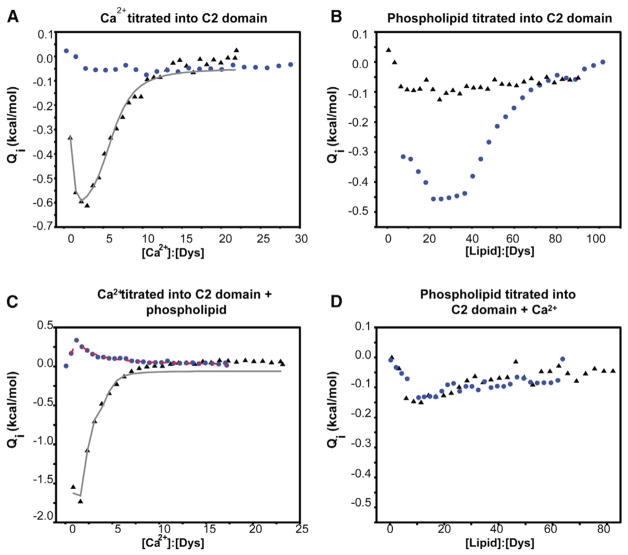
To quantify the ligand binding properties of the two isoforms, we measured the Ca2+ binding affinity of the canonical C2A and the C2Av1 using isothermal titration calorimetry (ITC). The isotherm of the canonical C2A domain exhibited an abrupt “hook” at lower ligand to protein ratios and an exponential curve at the higher end of the isotherm. This overall shape indicates two distinct classes of Ca2+ binding sites (Figure 4A; also see Figure S1 available online). Assuming two classes of independent binding sites, our model predicts n = 1 high-affinity binding site with nanomolar binding affinity (KD) (Table 2) and n = 4.8 low-affinity binding sites with micromolar affinity (Table 2). Accurate fitting of the high-affinity portion of the isotherm is limited by the extreme steepness in that part of the curve (Zubriene et al., 2009) and by the amount of residual Ca2+ that can be removed from the system by Chelex-100, resulting in the large error associated with these values. In addition, the measured value for the high-affinity binding site is at the lower range for standard ITC (KD ~1–10 nM) (Leavitt and Freire, 2001; Zubriene et al., 2009), so accurate measurements of Ca2+ binding affinity would be difficult. A competitive binding assay to better characterize the high-affinity sites was attempted using Mg2+ as a competing ion; however, no significant Mg2+ binding could be measured (Figure S2). Interestingly, under similar experimental conditions, we do not observe Ca2+ binding by C2Av1 (Figure 4A).
Figure 4. Ligand Binding Profiles for the Canonical Dysferlin C2A and the C2Av1 Domains.
The canonical construct is shown above as black triangles, whereas the C2Av1 construct is shown as blue circles. The fits of the canonical C2A data are shown as a gray line, whereas the fits of the C2Av1 data are shown as a dashed red line. All heats were normalized to the concentration of protein in the sample cell at each injection.
(A) Titration of 102 μM dysferlin C2A and the titration of 103 μM C2Av1 with Ca2+.
(B) Titration of 50 μM dysferlin C2A and the titration of 56 μM C2Av1 with LUVs made of a 60:40 mixture of POPC:POPS.
(C) Titration of 80 μM dysferlin C2A and the titration of 108 μM dysferlin C2Av1 with Ca2+ in the presence of 5 mM total lipid composed of LUVs made of a 60:40 mixture of POPC:POPS.
(D) Titration of 47 μM dysferlin C2A and the titration of 90 μM dysferlin C2Av1 with LUVs made of a 60:40 mixture of POPC:POPS in the presence of 2 mM Ca2+.
Table 2.
Summary of ITC Ligand Binding Data and Summary of DSC Ligand Binding Data
| ITC Results | Ca2+ Only | Ca2+ in Lipid Background | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| C2A | C2Av1 | C2A | C2Av1 | |||
| n1 | 0.9 ± 0.1 | – | 1.9 ± 0.2 | 0.5 ± 0.04 | ||
|
| ||||||
| Kd1 (μM) | 0.006 ± 0.008 | – | 0.0010 ± 0.003 | 0.0020 ± 0.0006 | ||
|
| ||||||
| K1 (μM)−1 | 170 ± 240 | – | 1000 ± 300 | 500 ± 100 | ||
|
| ||||||
| ΔH1 (kcal/mol) | −0.43 ± 0.15 | – | −4.1 ± 0.4 | 0.1 ± 0.03 | ||
|
| ||||||
| TΔS1 (kcal/mol) | 10.4 ± 0.7 | – | 7.8 ± 0.2 | 11.5 ± 0.1 | ||
|
| ||||||
| ΔG1 (kcal/mol) | −10.9 ± 0.8 | – | −11.9 ± 0.2 | −11.4 ± 0.2 | ||
|
| ||||||
| n2 | 4.8 ± 0.2 | – | 2.1 + 0.4 | 0.1 ± 0.05 | ||
|
| ||||||
| Kd2 (μM) | 53.2 ± 0.1 | – | 15.5 ± 2.1 | 375 ± 22 | ||
|
| ||||||
| K2 (μM)−1 | 0.019 ± 0.004 | – | 0.064 ± 0.017 | 0.0027 ± 0.002 | ||
|
| ||||||
| ΔH2 (kcal/mol) | −4.6 ± 0.2 | – | −2.2 ± 0.5 | 2.59 ± 0.09 | ||
|
| ||||||
| TΔS2 (kcal/mol) | 1.02 ± 0.06 | – | 4.2 ± 0.4 | 7.11 ± 0.05 | ||
|
| ||||||
| ΔG2 (kcal/mol) | −5.6 ± 0.1 | – | −6.3 ± 0.8 | −4.51 ± 0.03 | ||
|
| ||||||
| DSC Results | ||||||
|
| ||||||
| No ligand (+ EGTA) | ||||||
|
| ||||||
| TM (°C) | ΔH (kcal/mol) | TΔS (kcal/mol) | ΔG (kcal/mol) | % Folded | ||
|
| ||||||
| Dys C2A | 42.2 ± 0.60 | 12.6 ± 0.8 | 12.4 ± 0.9 | 0.17 ± 0.02 | 57 | |
|
| ||||||
| Dys C2Av1 | 55.6 ± 0.01 | 18.3 ± 0.04 | 17.9 ± 0.04 | 0.33 ± 0.03 | 63 | |
|
| ||||||
| 1μM Ca2+ | ||||||
|
| ||||||
| Dys C2A | 44.7 ± 0.8 | 27.8 ± 0.5 | 27.2 ± 0.5 | 0.58 ± 0.04 | 72 | |
|
| ||||||
| Dys C2Av1 | – | – | – | – | – | |
|
| ||||||
| 5mM Ca2+ | ||||||
|
| ||||||
| Dys C2A | 54.9 ± 0.30 | 20.9 ± 0.30 | 20.3 ± 0.30 | 0.66 ± 0.010 | 75 | |
|
| ||||||
| Dys C2Av1 | 55.7 ± 0.80 | 25.4 ± 0.30 | 24. 7 ± 0.30 | 0.73 ± 0.02 | 77 | |
|
| ||||||
| Phospholipid | ||||||
|
| ||||||
| Dys C2A | 44.0 ± 1.00 | 11. 0 ± 1.00 | 11.0 ± 1.00 | 0.18 ± 0.04 | 58 | |
|
| ||||||
| Dys C2Av1 | 51.0 ± 0.40 | 34.4 ± 0.70 | 33.2 ± 0.60 | 1.08 ± 0.008 | 86 | |
|
| ||||||
| Phospholipid + Ca2+ | ||||||
|
| ||||||
| Dys C2A | 55.0 ± 1.00 | 27.4 ± 0.30 | 26.4 ± 0.40 | 1.03 ± 0.03 | 84 | |
|
| ||||||
| Dys C2Av1 | 52.2 ± 0.01 | 19.9 ± 0.20 | 19.5 ± 0.20 | 0.45 ± 0.008 | 68 | |
Thermodynamic parameters for Ca2+ binding of the canonical dysferlin C2A and C2Av1 constructs in both the presence and absence of 60:40 POPC: POPS lipid vesicles. Fit assuming two sets of independent binding sites. Summary of the DSC results. ΔCP is 0.97 ± 0.01 and 1.32 ± 0.02 for the canonical and variant C2A domain, respectively. % folded is the fraction of C2A domain folded under the stated conditions. All errors reported represent the 95% confidence interval of the fits.
In other C2 domains, the presence of acidic phospholipid often enhances Ca2+ binding (Li et al., 2006; Radhakrishnan et al., 2009). For the canonical dysferlin C2A domain, the Ca2+ binding affinity for the lower-affinity class of sites increased from 53 to 15.5 μM in the presence of phospholipid PC:PS (60:40) and fit to n = 2.1 Ca2+ sites (Figure 4C; Table 2). Although C2Av1 has no substantial Ca2+ binding affinity in solution, it does show a robust avidity for negatively charged phospholipids (Figures 4B and S3). In addition, after this domain binds phospholipids, a single high-affinity Ca2+ binding site is uncovered (Figure 4C; Table 2). Once this membrane-induced high-affinity Ca2+ binding site is occupied, the avidity of the C2Av1 for membrane decreases significantly (Figure 4D).
Protein Stability on the Order of Thermal Energy “kT”
Conformational flexibility has been suggested as a mechanism for binding a wide variety of effectors in the C2A domain of synaptotagmin (Gauer et al., 2012). If the dysferlin C2A domains share this property, it should be reflected by an increase in the calculated stability (ΔG° of unfolding) of the domain upon ligand binding, as monitored by changes in the enthalpy (ΔH°) and the transition midpoint (TM) determined by differential scanning calorimetry (DSC). In the case of the canonical dysferlin C2A domain, the TM in the presence of 5 mM EGTA was 42.2°C (Table 2; Figure S4). In the presence of saturating Ca2+, the TM increased to 54.9°C. The net increase in TM of C2A in the presence of saturating Ca2+ is consistent with ligand-dependent stabilization of the canonical C2A domain. With the C2Av1, we measured a TM of 55°C with or without Ca2+, suggesting that Ca2+ does not bind or impart stability to C2Av1 in solution. This is consistent with our ITC results, which show no significant Ca2+ binding by C2Av1 in solution (Figure 4A).
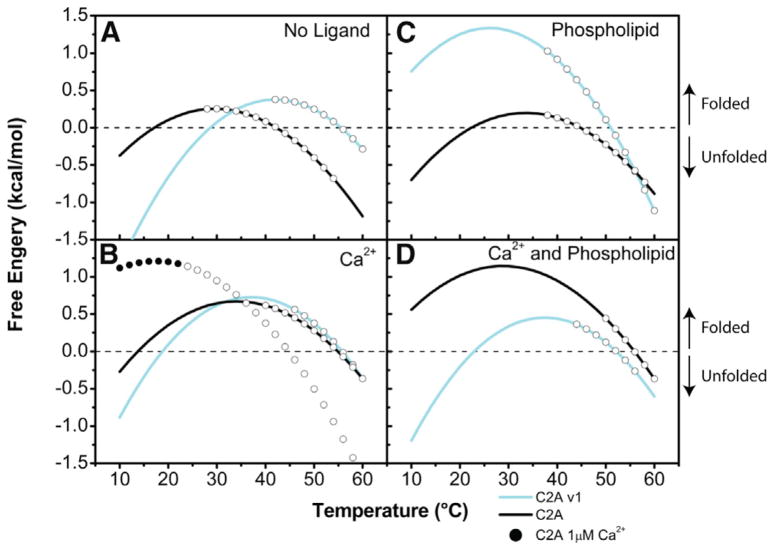
The free energy of stability (ΔG° of unfolding) is a measure of the interactions that maintain the overall fold of a protein. Consequently, the ΔG° of unfolding acts as an energy barrier that must be overcome to unfold the protein. When this barrier is low, the intermolecular interactions stabilizing the protein are small enough to allow the protein to sample multiple subconformations. For most proteins, the ΔG° of unfolding ranges from 5 to 15 kcal/mol (Pace, 1990); however, the stability of the C2A isoforms of dysferlin was found to be markedly lower than these typical values. In the absence of ligands, the ΔG° was measured to be 0.17 kcal/mol and 0.33 kcal/mol for the canonical C2A and C2Av1, respectively (Figure 5A; Table 2; Figure S4). These low free energies are indicative of a significant population of partially or completely unfolded state (Uversky, 2002). Assuming that the two-state model of unfolding is valid in this case (Supplemental Information; Tables S1–S3), at 37°C, 57% of the canonical C2A and 63% of the C2Av1 molecules would exist in a highly ordered/folded state in the absence of ligand. For comparison, in a protein solution with a ΔG° of 5 kcal/mol, 99% of all the protein molecules are in the folded state at this temperature.
Figure 5. Free Energy Diagrams of Dysferlin C2A Domains.
In the absence of Ca2+ (A), Ca2+ (B), phospholipid (C), and phospholipid and Ca2+ (D). At any point along the curve, the native and denatured states of the C2A domains exist at varying ratios. As the temperature changes so does the ratio between the two states. Where the curve crosses 0 kcal/mol on the y axis, the populations of protein in the native and denatured states are equal to one another; below this, the protein is found predominantly in the denatured state, and above this, the protein is predominantly found in the native state. The open circles on each curve represent the temperature over which the protein denatured.
The canonical C2A domain of dysferlin possesses a single high-affinity Ca2+ binding site that would likely be completely occupied at the resting muscle Ca2+ concentrations of ~50 nM (Berchtold et al., 2000). To test the effects of this high-affinity site on the stability of the domain, we measured the stability of the canonical C2A domain in the presence of 1 μM Ca2+. Under these subsaturating conditions, we observed only a slight increase in the TM but a marked increase in the measured enthalpy (Table 2; Figure S4). The increase in the enthalpy in the presence of 1 μM Ca2+ correlates with an increase in the stability (Figure 5B; Table 2; Figure S4) and a concomitant decrease in the plasticity of the domain. Therefore, we propose that the nanomolar Ca2+ binding site in the canonical dysferlin C2A domain predominantly serves a structural role by stabilizing the domain at resting cellular Ca2+ concentrations. In the presence of saturating Ca2+, we measure a further increase in ΔG° for the canonical C2A domain to 0.66 kcal/mol, which was also accompanied by a 12.7°C increase in the TM (Table 2; Figure S4). Interestingly, ΔG° for the C2Av1 domain also increased in the presence of Ca2+ (ΔG° = 0.73 kcal/mol), albeit not to the same extent as that measured in the canonical C2A domain. This small increase in the stability of the C2Av1 domain in the presence of Ca2+ shown in the absence of Ca2+ binding is likely due to the higher ionic strength of the solution restricting the flexibility of the population of partially folded domains, though it could also imply athermal binding.
The canonical C2A domain showed no significant increase in stability in the presence of phospholipids alone (PC:PS 60:40, large unilamellar vesicles) (0.18 kcal/mol) (Figure 5C; Table 2; Figure S4). However, with Ca2+ and phospholipid, the ΔG° for the canonical C2A increased to 1.03 kcal/mol, consistent with Ca2+-dependent phospholipid binding activity. In contrast, we measured a marked increase in ΔG° for C2Av1 in the presence of phospholipid (1.08 kcal/mol) (Figure 5C; Table 2; Figure S4) but a decrease in the presence of Ca2+ and phospholipid (ΔG° = 0.45 kcal/mol) (Figure 5D; Table 2; Figure S4). Regardless, both the canonical C2A and C2Av1, regardless of ligation state, have exceptionally weak stabilizing interactions that sum to no more than twice thermal energy (kT or ~600 cal/mol at ambient temperature), yet will still be in exchange with a significant population of partially structured conformations. The complete thermodynamic analyses of these domains are presented in the Supplemental Information.
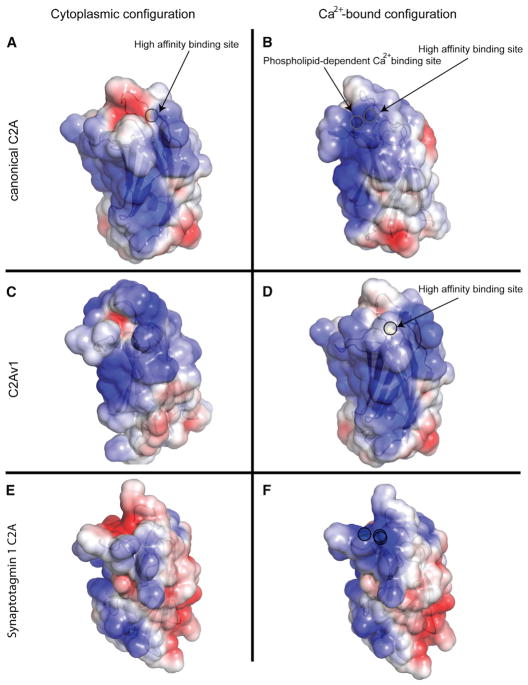
Electrostatic Surface Potential Defines Membrane Interactions of the C2A Domains of Dysferlin
For well-characterized C2 domains, interaction with membrane occurs via an electrostatic switch (Figures 6E and 6F) and hydrophobic anchors located on loops 1 and 3. Because these loops also shape the Ca2+ binding pocket of the C2 domains of synaptotagmin, Ca2+ and lipid binding are effectively linked. Both isoforms of dysferlin C2A possess a single methionine residue (Met-75) on loop 3, but they lack a hydrophobic residue on loop 1. In addition, the surfaces of both the canonical C2A and C2Av1 possess a prominent positive electrostatic feature that could attract the domain to negatively charged phospholipids (Figure 6). This feature is accentuated upon occupancy of the μM affinity Ca2+ binding sites (Figure 6B), consistent with the Ca2+-dependent phospholipid binding activity observed in other C2 domains. A strongly basic electrostatic surface appears to be a constitutive property of C2Av1 (Figure 6C) and is consistent with the ability of the C2Av1 to interact with the membrane in the absence of Ca2+.
Figure 6. Electrostatic Surface Potential of Dysferlin C2 Domains.
(A and B) The canonical C2A domain with (A) a single Ca2+ and canonical C2A with two Ca2+ (B).
(C and D) Dysferlin C2Av1 with no ligand (C) and a single Ca2+ (D). Solvent-accessible surface is colored by the calculated electrostatic potential and displayed at ± 3 kT/e. Calcium ions are highlighted by a circular outline.
(E and F) The electrostatic surface potential representation of synaptotagmin 1 C2A with and without (E) and with (F) saturating Ca2+ (1byn) is shown for comparison (Shao et al., 1998). Calcium ions are highlighted by a circular outline.
C2A and C2Av1 Colocalize in Cultured C2C12 Cells
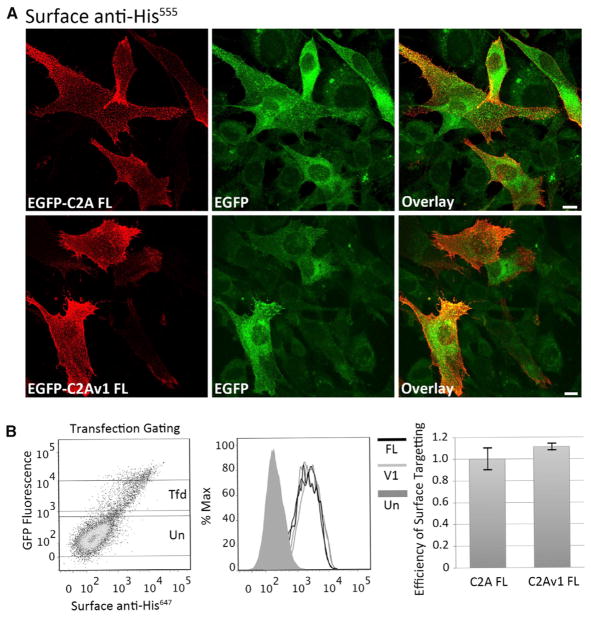
In human muscle tissue, the ratio of canonical C2A to C2Av1 dysferlin transcript is roughly 4:1 (Pramono et al., 2006); however, the relative amount of each isoform varies among other tissues (Figure S5). To determine the differences between the plasma membrane localization of full-length canonical C2A-dysferlin or C2Av1-dysferlin, we monitored the localization of transfected EGFP-dysferlin fusion constructs bearing each isoform in cultured C2C12 myoblasts by confocal microscopy. The efficiency of plasma membrane targeting of each isoform was assessed by flow cytometry. Transfected myoblasts were incubated with antibodies that selectively recognize an extracellular epitope but are unable to cross the plasma membrane. Labeling experiments were performed at <10°C, to prevent vesicular trafficking and endocytosis of the surface-bound antibodies. These surface-binding experiments (immunohistochemistry [IHC] and fluorescence-activated cell sorting [FACs]) showed that both constructs bear a similar intrinsic capacity to target the plasma membrane (Figure 7A). Confocal microscopy shows similar levels of surface-expressed dysferlin and similar subcellular localization of both isoforms as revealed by the autofluorescence of the EGFP fusion protein (Figure 7A). Flow cytometry verified similar levels of plasma membrane expression for both C2A and C2Av1 isoforms by quantifying levels of surface-labeled dysferlin, relative to total levels of EGFP fluorescence (Figure 7B).
Figure 7. Full-Length Dysferlin Proteins Bearing Alternately Spliced Canonical C2A or C2Av1 Show Similar Intrinsic Capacity to Target the Plasma Membrane.
(A) Representative confocal images showing surface-expressed EGFP-FLC2ADysferlinMycHis or EGFP-FLC2Av1DysferlinMycHis. Transfected C2C12 myoblasts were incubated with mouse anti-His to selectively label the extracellular His epitope. Labeling was performed on live cells at <10°C to prevent endosomal internalization of surface-bound antibody. Cells were then washed, fixed, and labeled with goat anti-mouse555. Scale bar 10 μM.
(B) Flow cytometry quantifies similar levels of surface-expressed EGFP-FLC2ADysferlinMycHis or EGFP-FLC2Av1DysferlinMycHis. Transfected C2C12 myoblasts were dissociated from the culture dish and labeled as live cells at <10°C with mouse anti-His followed by an anti-mousealexa647 secondary antibody. Live cells were gated based on impermeability to propidium iodide (data not shown). (Left panel) Shows increasing levels of surface-labeled anti-Hisalexa647 (x axis) is proportional to the levels of EGFP auto-fluorescence (y axis). Gates for transfected (Tfd) and untransfected (Un) cells are shown; very highly transfected cells often show signs of toxicity and were excluded from analysis. (Middle panel) Histogram showing similar normal distributions of surface bound anti-mousealexa647 in populations of transfected cells expressing FLC2A or FLC2Av1 constructs, from duplicate samples labeled on the same day. (Right panel) Pooled data from three experiments performed in duplicate showing similar levels of surface-labeled anti-Hisalexa647 relative to EGFP autofluorescence for both FLC2A or FLC2Av1 constructs. To allow comparison between constructs transfected and labeled on the same day, values derived from canonical C2A were normalized to one. Error bars are reported as the 95% confidence interval.
DISCUSSION
The ferlin proteins have been implicated in various cellular processes ranging from vesicle fusion to membrane repair. Dysferlin is the only member of this family that has been specifically adapted for Ca2+-dependent membrane repair (Lek et al., 2010). Over time, the first C2 domain (C2A) became the sole Ca2+-dependent phospholipid binding domain in the dysferlin protein (Therrien et al., 2009); however, the alternative exon 1a exists within the DYSF gene, which encodes a C2A domain that utilizes Ca2+ differently. Alternate first exons in the DYSF gene are evolutionary preserved within the genomes of fish and mammals (data not shown), indicative of an essential role in the physiological function of dysferlin in eukaryotic cells. Further, the genomic sequences of otoferlin and myoferlin do not possess a homolog to exon 1a, despite their evolutionary relatedness with dysferlin. Although myoferlin is structurally similar to dysferlin and is coexpressed in the same tissues, overexpression of myoferlin does not fully rescue the dysferlin null phenotype (Lostal et al., 2012). These findings highlight the biological significance of the dysferlin protein and the importance of examining the role of C2Av1 in membrane repair. To lay the groundwork for understanding the function of mixed canonical C2A-dysferlin and C2Av1-dysferlin proteins in the cell, we conducted extensive biophysical and thermodynamic analysis of both domains.
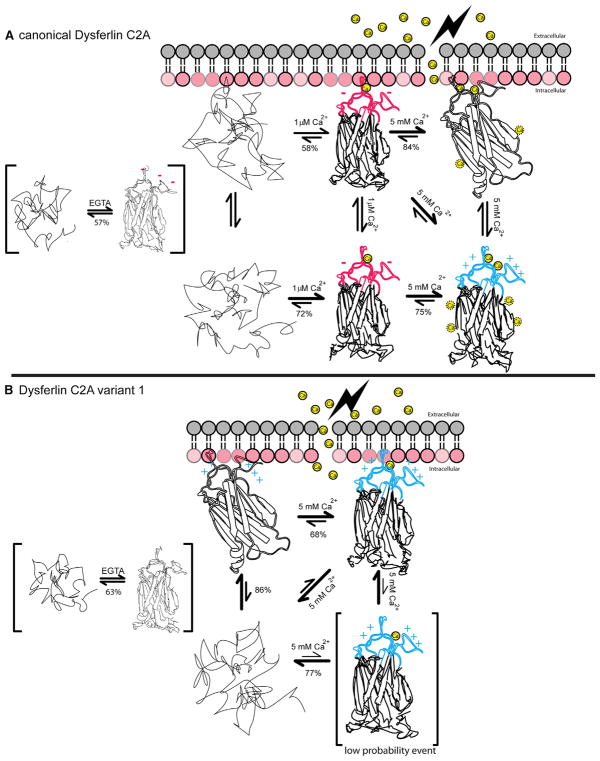
The crystal structure of dysferlin C2A together with structural homology to other cation-bound C2 domains argues for two discrete Ca2+ binding sites within the divalent cation-binding pocket of the canonical dysferlin C2A. However, our ITC binding model fits to two independent classes of binding sites instead of two discrete binding sites. The first class corresponds to a single calcium ion with nanomolar affinity and likely plays a purely structural role in the domain. The other class of sites in the canonical C2A domain of dysferlin binds with micromolar affinity and corresponds to an apparent five calcium ion binding model. In total, our ITC analysis fits to six binding sites. This is clearly inconsistent with total number of cations that can be accommodated in the divalent cation binding pocket of most other C2 domains. To reconcile this perceived discrepancy, we measured the energetic parameters that are required to maintain the fold of these C2A domains by DSC. Our DSC analysis suggests that both dysferlin C2A domains have remarkably low stability. Further, such low stability is indicative of a significant population of highly flexible structures in the unfolded or partially unfolded state, where multiple conformational states are sampled (Gauer et al., 2012; Uversky, 2002). Given this flexibility, the Ca2+ binding loops may be in a receptive conformation to coordinate calcium ions, whereas the remainder of the domain may be able to form binding pockets that appear as structurally ill-defined sites (Figure 8). Indeed, the number of Ca2+ sites decreases upon membrane binding (Table 2), implying that as the number of conformations in the ensemble decreases, some of the accessory low-affinity Ca2+ binding sites are no longer represented.
Figure 8. Summary of Thermodynamic and Structural Results.
(A) Thermodynamic interactions among the canonical C2A domain of dysferlin, Ca2+, and the negatively charged phospholipid (red lollipop representation of a lipid surface). Unfolded protein is shown as a random trace. The various folded states of the C2A domain are shown as a partially unfolded domain with intact Ca2+:phospholipid binding loops at the top of the structure. Calcium ions are shown as yellow spheres. The lightning bolt on the extracellular side of the membrane represents the site of membrane damage. Red color designates net negative charge; blue color designates net positive charge. The relative equilibrium of the various measure states is also shown. Percentages underneath the equilibrium arrows are the % folded protein under the stated equilibrium conditions. The structures within brackets likely do not exist in the muscle cell, but the thermodynamic relationship was described.
(B) Thermodynamic interactions among the C2A variant, Ca2+, and negatively charged phospholipid. The single structure within brackets (C2Av1 + Ca2+) is a low probability event.
Unlike the Ca2+-dependent canonical C2A domain, the alternative loop 1 sequence in C2Av1 effectively mimics the Ca2+-bound canonical C2A dysferlin molecule in the cytosol. Indeed, the guanidino groups of Arg-20 and Arg-21 in the C2Av1 crystal structure may approximate the positions of the two calcium ions in the canonical C2A domain. Although the C2Av1 domain does not bind Ca2+ in solution, it is nonetheless affected by Ca2+. C2Av1 could possess a single high-affinity Ca2+ binding site that is exposed upon binding membrane. Our ITC measurements do not support Ca2+ binding by C2Av1 in the absence of membrane (Figure 4A; Table 2), but the thermodynamic parameters from the DSC experiments do show stabilization of C2Av1 by Ca2+ interaction (Table 2; Figure S4). Because Ca2+ binding by C2Av1 was not demonstrated by ITC, we cannot explicitly state that this stabilization is solely due to binding. Instead, this increase may be due to several processes, such as athermal binding or a restriction of conformational flexibility due to a change in the conditions of the solution. Therefore, the canonical C2A domain utilizes increasing cytoplasmic Ca2+ to initiate membrane binding, while the C2Av1 isoform may use high levels of Ca2+ to alter its conformational ensemble to interact with membrane less efficiently (Figure 5D).
Membrane Binding Model for Dysferlin C2A Isoforms
For other C2 domains, membrane binding involves electrostatic localization followed by membrane anchoring (Murray and Honig, 2002). Comparison of the electrostatic surface potential of the two C2A domains of dysferlin suggests the initial phospholipid binding response is predominantly electrostatic in nature, due to the preponderance of basic charge distributed across their molecular surfaces (Figure 6). This seems to be the case for both the canonical and C2Av1 domains of dysferlin as they show a clear preference for acidic phospholipids but have no avidity for uncharged, PC-only membranes (Figure S3). It is therefore likely that the canonical C2A domain of dysferlin relies on a Ca2+-dependent electrostatic switch mechanism. In contrast, C2Av1 cannot independently bind Ca2+; therefore, the membrane-binding model for C2Av1 must be different. One possibility is that C2Av1 associates with membrane analogous to the nPKC-ε C2 domain. The nPKC-ε domain also possesses membrane-binding properties that are not dependent on the surrounding Ca2+ concentration (Ochoa et al., 2001). Loop 1 of the nPKC-ε C2 domain possesses two arginine residues that are predicted to snorkel into the membrane, whereas loop 3 anchors to the membrane via hydrophobic interactions from Ile-89 and Tyr-91 (Ochoa et al., 2001). In dysferlin C2Av1, Met-77 on loop 3 could penetrate and anchor the domain to the acyl chains of the phospholipid, whereas the three basic residues on loop 1 (Lys-18, Arg-19, and Arg-20) could snorkel and associate with the acidic head group of the phospholipid (Schow et al., 2011).
Sensors of Membrane Damage
One of the most remarkable aspects of our thermodynamic analysis has been the exceptionally low stability of these domains. Although we are not suggesting that the C2A domains of dysferlin are intrinsically disordered, they do share characteristics that classify them as weakly stable, but folded, protein domains (Fisher and Stultz, 2011). Low stability is likely a key feature of these domains that accentuates dysferlins ability to mediate lipid fusion or to interact with other molecules that assist in the membrane repair process. A similar conclusion was drawn concerning synaptotagmin function (Gauer et al., 2012) and other membrane-interacting domains (Halskau et al., 2009). The weak energies that we measure in the C2A domains of dysferlin also predict that pathogenic mutations would be expected to perturb the ensemble distribution, thereby altering the functional, biological response in a nonpredictable manner. This also suggests that experiments to test the properties of dysferlin C2A using mutagenesis should be interpreted carefully.
The inverted ligand preference, ligand binding complexity, and thermodynamic properties that we measure for both isoforms of the dysferlin C2A suggest the potential for diverse effector interactions that range from constitutive membrane interaction to Ca2+-regulated interaction. The canonical C2A domain in dysferlin binds at least two calcium ions, each with a specific function (Figure 8). The high-affinity site measured in the canonical C2A domain would be fully occupied at the resting, intracellular Ca2+ concentration of ~50 nM. Therefore, this site likely stabilizes the domain, whereas the lower affinity Ca2+ binding site (KD = 53 μM) mediates phospholipid binding and environmental sensitivity. At basal Ca2+ concentrations, C2Av1-dysferlin mimics Ca2+-bound canonical C2A and would dominate the initial interactions with the membrane and possibly the interactions with the C2A-dependent repair proteins. However, at high intracellular Ca2+ concentrations following membrane damage, both the canonical and variant isoforms of C2A-dysferlin could be recruited to the membrane, to assemble the repair complex rapidly. The mixed population of canonical and variant C2A isoforms that are present within cells could allow for graded-responses to membrane damage depending on the Ca2+ concentration and the ratio of C2A-dysferlin to C2Av1-dysferlin in any given cell type, enabling the cell to respond more effectively to membrane damage under a wide range of conditions.
EXPERIMENTAL PROCEDURES
Expression Constructs
The gene encoding the human dysferlin canonical C2A domain was obtained from Dr. Elizabeth McNally (Davis et al., 2002). The DNA sequence corresponding to human dysferlin C2A (residues 1–125) was cloned into pGEX-4T. The gene encoding human dysferlin C2Av1 (residues 1–126) was subcloned into a pET28a expression vector with an N-terminal His6-MBP tag and a tobacco etch virus protease cleavage site. Cys2Ala was introduced, as we predicted that it should be exposed to solvent and may interfere with purification and crystallization.
Both the canonical and the C2Av1 constructs were expressed in BL21 (DE3) cells. Ten liters of cells were grown in a BioFlo 3000 fermentor at 30°C in Terrific Broth to an optical density (OD600) of 1.3. The temperature of the cell cultures was then lowered to 18°C, and heterologous protein expression was induced by adding 400 μM isopropyl β-D-1 thiogalactopyranoside. The cells were grown for an additional 12 hr and harvested by centrifugation. The cell pellets were frozen in liquid nitrogen and stored at −80°C until needed.
Crystallization and Data Collection
The canonical C2A domain was crystallized in 2.8 M sodium formate and 0.1 M sodium acetate (pH 5.5) using the hanging droplet method with purified protein at a stock concentration of 10 mg/ml. Crystals were grown at 7°C. Typically, the crystals reached full size overnight. C2Av1 was crystallized in 16% PEG 20000 and 0.1 M sodium citrate (pH 6.2). Crystals were grown using the hanging drop methods with purified protein at 10 mg/ml. Crystals were also grown at 7°C. Typically, the crystals reached full size within 3 days.
The crystals of both C2 domains were captured into nylon loops and frozen in liquid N2. Initial data sets were collected on a Rigaku ScreenMachine. Subsequent data sets were collected at SLAC beamline 7-1. The wavelength of the final data sets was 0.9796 Å, and the data were collected at 90 K. X-ray data were processed with imosflm (Battye et al., 2011), and the data were scaled using SCALA as a part of the CCP4 package (Winn et al., 2011). A summary of the crystal statistics are presented in Table 1.
Isothermal Titration Calorimetry
Isothermal titration calorimetry experiments to determine the binding of Ca2+ and POPC:POPS containing lipid LUVs to dysferlin were performed on a TA Instruments Nano ITC at 15°C. Both the Ca2+ and lipid titrant solutions were prepared in buffer consisting of 20 mM HEPES and 100 mM KCl at pH 7.5 that was passed through Bio-Rad 100 Chelex resin to remove cation impurities and filtered using a 0.2 μm Nalgene PES disposable filter unit. The protein was buffer exchanged into the same 20 mM HEPES and 100 mM KCl buffer using Bio-Rad 10DG disposable chromatography columns. The Ca2+ stock concentrations used in the experiments were verified using a BAPTA fluorescence assay (Invitrogen/Molecular Probes). The titrant lipid concentration was verified by phosphate assay according to standard protocols (Kingsley and Feigenson, 1981). Heats of dilution were conducted with replicate titrations in the absence of protein and subtracted from the corresponding data sets in order to determine the binding parameters.
Differential Scanning Calorimetry Methods
Differential scanning calorimetry (DSC) experiments were performed on a NanoDSC (TA Instruments) at a scan rate of 1°C/min. Representative raw DSC data are presented in Figure S4. DSC methods are described in the Supplemental Experimental Procedures. To see if measured enthalpies varied with concentration or scan rate, both constructs were denatured over a range of concentrations and scan rates (Table S3). All scans were conducted in Chelex-ed 20 mM MOPS and 100 mM KCl (pH 7.5). Scans performed in the absence of Ca2+ contained 5 mM EGTA. All scans in the presence of Ca2+ were done at a concentration of 5 mM to ensure near saturated conditions. The concentration of the Ca2+ stock solution used for all scans was verified using both a calcium ion selective electrode (ThermoScientific) and a BAPTA chelating assay (Invitrogen/Molecular Probes). Scans carried out in the presence of lipid contained LUVs composed of a 60:40 mixture of POPC:POPS and 2 mM Ca2+.
Cellular Localization of C2A versus C2Av1
Antibodies and Cell Culture
C2C12 myoblasts were cultured and transfected as previously described (Evesson et al., 2010). Data presented are from transfected C2C12; identical results were obtained in transfected HEK293 (data not shown). Antibodies to the following proteins were used: mouse anti-Myc (1:200 for IHC, Santa Cruz Biotechnology), rabbit anti-Flag (1:5,000 for IHC, Abcam ab1162), mouse anti-His (1:300 flow cytometry), goat anti-rabbitAlexa647 (1:200 for IHC and flow), and donkey anti-mouseAlexa555 (1:200 for IHC) (Invitrogen).
Surface Labeling of Dysferlin for Confocal Microscopy
Live C2C12 transfected with EGFP-FLC2A DysferlinFLAG or EGFP-FLC2Av1DysferlinMycHis were inverted onto a droplet of primary antibody diluted in blocking reagent (Hank’s balanced salt solution [HBSS] containing 20% FBS) on a parafilm-covered glass plate resting on ice for 90 min. Cells were washed in PBS, fixed in 3% paraformaldehyde in PBS, and incubated in blocking buffer for 15 min. Cells were then incubated with Alexa-conjugated secondary antibodies diluted in block for 60 min, washed in PBS, and mounted onto Fluorsave-mounting reagent (Merck). Confocal microscopy was performed using a Leica SP5 scanning confocal microscope with a 63× HCX Plan Apo oil immersion lens (1.4). Images (1024 × 1024) were captured without subtraction of glow-under. Background was subtracted postcapture using Adobe Photoshop software, through a single adjustment of the levels histogram.
Flow Cytometry
C2C12 transfected with EGFP-FLC2ADysferlinMycHis or EGFP-FLC2Av1 DysferlinMycHis were trypsinized on the morning of the experiment, replated into the same dish, and incubated for 3 hr at 37°C to recover and re-express surface dysferlin. Cells were then dissociated from the plate by incubation for 5 min at 37°C with Versene (0.48 mM EDTA4Na in PBS). Cells were gently triturated from the plate in HBSS + 10% FCS, transferred to a falcon tube, and pelleted by centrifugation at 300 ×g for 2 min. The 3 hr replating step assisted with cell dissociation, yielding >90% live cells as assessed by propidium iodide (PI) exclusion. Cells were resuspended in cold PBS containing 1% BSA with mouse anti-His (1:200), incubated for 90 min at 4°C, washed with PBS, pelleted as before, and then resuspended in buffer containing donkey anti-mouseAlexa647 for 1 hr at 4°C. Cells were then washed twice and resuspended in PBS containing PI (5 μg/ml). Flow cytometry was performed using a Becton Dickenson LSRII cytometer equipped with FACSDiva software (BDBiosciences). Postacquisition analysis was performed using FlowJo software (Tree Star). Gating for live cells was performed based on PI exclusion (data not shown); gating for transfected and untransfected cells is shown in Figure 7B.
Supplementary Material
Acknowledgments
This work was supported by the Jain Foundation (to R.B.S. and S.T.C.); a National Science Foundation CAREER grant (MCB-0845676), the University of Minnesota Grant-in-Aid, and Funding for University of Minnesota Duluth Swenson College of Science and Engineering Faculty Research (to A.H.); and an Australian NHMRC CDF Fellowship (APP1048816; to S.T.C.). Portions of this research were carried out at the Stanford Synchrotron Radiation Light-source (SSRL), a Directorate of SLAC National Accelerator Laboratory and an Office of Science User Facility operated for the U.S. Department of Energy (DOE) Office of Science by Stanford University. The SSRL Structural Molecular Biology Program is supported by the DOE Office of Biological and Environmental Research, the National Institutes of Health (NIH), National Institute of General Medical Sciences (NIGMS; including P41 GM103393), and the National Center for Research Resources (NCRR; P41 RR001209). The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of NIGMS, NCRR, or NIH. The authors thank Dr. Elliott Stollar for helpful discussion.
Footnotes
ACCESSION NUMBERS
Atomic coordinates and structure factors for canonical dysferlin C2A and C2Av1 have been deposited in the Protein Data Bank (http://www.pdb.org) under accession numbers 4IHB and 4IQH, respectively.
Supplemental Information includes Supplemental Experimental Procedures, six figures, three tables, and two 3D molecular structures and can be found with this article online at http://dx.doi.org/10.1016/j.str.2013.10.001.
References
- Ampong BN, Imamura M, Matsumiya T, Yoshida M, Takeda S. Intracellular localization of dysferlin and its association with the dihydropyridine receptor. Acta Myol. 2005;24:134–144. [PubMed] [Google Scholar]
- Anandakrishnan R, Aguilar B, Onufriev AV. H++ 3.0: automating pK prediction and the preparation of biomolecular structures for atomistic molecular modeling and simulations. Nucleic Acids Res. 2012;40:W537–W541. doi: 10.1093/nar/gks375. Web Server issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azakir BA, Di Fulvio S, Therrien C, Sinnreich M. Dysferlin interacts with tubulin and microtubules in mouse skeletal muscle. PLoS ONE. 2010;5:e10122. doi: 10.1371/journal.pone.0010122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bansal D, Miyake K, Vogel SS, Groh S, Chen CC, Williamson R, McNeil PL, Campbell KP. Defective membrane repair in dysferlin-deficient muscular dystrophy. Nature. 2003;423:168–172. doi: 10.1038/nature01573. [DOI] [PubMed] [Google Scholar]
- Battye TG, Kontogiannis L, Johnson O, Powell HR, Leslie AG. iMOSFLM: a new graphical interface for diffraction-image processing with MOSFLM. Acta Crystallogr D Biol Crystallogr. 2011;67:271–281. doi: 10.1107/S0907444910048675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berchtold MW, Brinkmeier H, Müntener M. Calcium ion in skeletal muscle: its crucial role for muscle function, plasticity, and disease. Physiol Rev. 2000;80:1215–1265. doi: 10.1152/physrev.2000.80.3.1215. [DOI] [PubMed] [Google Scholar]
- Cai C, Masumiya H, Weisleder N, Matsuda N, Nishi M, Hwang M, Ko JK, Lin P, Thornton A, Zhao X, et al. MG53 nucleates assembly of cell membrane repair machinery. Nat Cell Biol. 2009a;11:56–64. doi: 10.1038/ncb1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai C, Weisleder N, Ko JK, Komazaki S, Sunada Y, Nishi M, Takeshima H, Ma J. Membrane repair defects in muscular dystrophy are linked to altered interaction between MG53, caveolin-3, and dysferlin. J Biol Chem. 2009b;284:15894–15902. doi: 10.1074/jbc.M109.009589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cates MS, Teodoro ML, Phillips GN., Jr Molecular mechanisms of calcium and magnesium binding to parvalbumin. Biophys J. 2002;82:1133–1146. doi: 10.1016/S0006-3495(02)75472-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covian-Nares JF, Koushik SV, Puhl HL, 3rd, Vogel SS. Membrane wounding triggers ATP release and dysferlin-mediated intercellular calcium signaling. J Cell Sci. 2010;123:1884–1893. doi: 10.1242/jcs.066084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis DB, Doherty KR, Delmonte AJ, McNally EM. Calcium-sensitive phospholipid binding properties of normal and mutant ferlin C2 domains. J Biol Chem. 2002;277:22883–22888. doi: 10.1074/jbc.M201858200. [DOI] [PubMed] [Google Scholar]
- den Dunnen JT. Leiden Muscular Dystrophy pages. 2012 http://www.dmd.nl/
- Dunker AK, Lawson JD, Brown CJ, Williams RM, Romero P, Oh JS, Oldfield CJ, Campen AM, Ratliff CM, Hipps KW, et al. Intrinsically disordered protein. J Mol Graph Model. 2001;19:26–59. doi: 10.1016/s1093-3263(00)00138-8. [DOI] [PubMed] [Google Scholar]
- Essen LO, Perisic O, Lynch DE, Katan M, Williams RL. A ternary metal binding site in the C2 domain of phosphoinositide-specific phospholipase C-delta1. Biochemistry. 1997;36:2753–2762. doi: 10.1021/bi962466t. [DOI] [PubMed] [Google Scholar]
- Evesson FJ, Peat RA, Lek A, Brilot F, Lo HP, Dale RC, Parton RG, North KN, Cooper ST. Reduced plasma membrane expression of dysferlin mutants is attributed to accelerated endocytosis via a syntaxin-4-associated pathway. J Biol Chem. 2010;285:28529–28539. doi: 10.1074/jbc.M110.111120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher CK, Stultz CM. Protein structure along the order-disorder continuum. J Am Chem Soc. 2011;133:10022–10025. doi: 10.1021/ja203075p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauer JW, Sisk R, Murphy JR, Jacobson H, Sutton RB, Gillispie GD, Hinderliter A. Mechanism for calcium ion sensing by the C2A domain of synaptotagmin I. Biophys J. 2012;103:238–246. doi: 10.1016/j.bpj.2012.05.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halskau O, Muga A, Martínez A. Linking new paradigms in protein chemistry to reversible membrane-protein interactions. Curr Protein Pept Sci. 2009;10:339–359. doi: 10.2174/138920309788922199. [DOI] [PubMed] [Google Scholar]
- Helfmann S, Neumann P, Tittmann K, Moser T, Ficner R, Reisinger E. The crystal structure of the C2A domain of otoferlin reveals an unconventional top loop region. J Mol Biol. 2011;406:479–490. doi: 10.1016/j.jmb.2010.12.031. [DOI] [PubMed] [Google Scholar]
- Illa I, Serrano-Munuera C, Gallardo E, Lasa A, Rojas-García R, Palmer J, Gallano P, Baiget M, Matsuda C, Brown RH. Distal anterior compartment myopathy: a dysferlin mutation causing a new muscular dystrophy phenotype. Ann Neurol. 2001;49:130–134. [PubMed] [Google Scholar]
- Kingsley PB, Feigenson GW. 1H-NMR study of the location and motion of ubiquinones in perdeuterated phosphatidylcholine bilayers. Biochim Biophys Acta. 1981;635:602–618. doi: 10.1016/0005-2728(81)90117-1. [DOI] [PubMed] [Google Scholar]
- Klinge L, Harris J, Sewry C, Charlton R, Anderson L, Laval S, Chiu YH, Hornsey M, Straub V, Barresi R, et al. Dysferlin associates with the developing T-tubule system in rodent and human skeletal muscle. Muscle Nerve. 2010;41:166–173. doi: 10.1002/mus.21166. [DOI] [PubMed] [Google Scholar]
- Leavitt S, Freire E. Direct measurement of protein binding energetics by isothermal titration calorimetry. Curr Opin Struct Biol. 2001;11:560–566. doi: 10.1016/s0959-440x(00)00248-7. [DOI] [PubMed] [Google Scholar]
- Lek A, Lek M, North KN, Cooper ST. Phylogenetic analysis of ferlin genes reveals ancient eukaryotic origins. BMC Evol Biol. 2010;10:231. doi: 10.1186/1471-2148-10-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Shin OH, Rhee JS, Araç D, Rah JC, Rizo J, Südhof T, Rosenmund C. Phosphatidylinositol phosphates as co-activators of Ca2+ binding to C2 domains of synaptotagmin 1. J Biol Chem. 2006;281:15845–15852. doi: 10.1074/jbc.M600888200. [DOI] [PubMed] [Google Scholar]
- Lostal W, Bartoli M, Roudaut C, Bourg N, Krahn M, Pryadkina M, Borel P, Suel L, Roche JA, Stockholm D, et al. Lack of correlation between outcomes of membrane repair assay and correction of dystrophic changes in experimental therapeutic strategy in dysferlinopathy. PLoS ONE. 2012;7:e38036. doi: 10.1371/journal.pone.0038036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda C, Miyake K, Kameyama K, Keduka E, Takeshima H, Imamura T, Araki N, Nishino I, Hayashi Y. The C2A domain in dysferlin is important for association with MG53 (TRIM72) PLoS Curr. 2012;4:e5035a. doi: 10.1371/5035add8caff4. dd5038 caff5034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCann JJ, Zheng L, Rohrbeck D, Felekyan S, Kühnemuth R, Sutton RB, Seidel CA, Bowen ME. Supertertiary structure of the synaptic MAGuK scaffold proteins is conserved. Proc Natl Acad Sci USA. 2012;109:15775–15780. doi: 10.1073/pnas.1200254109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeil PL. Repairing a torn cell surface: make way, lysosomes to the rescue. J Cell Sci. 2002;115:873–879. doi: 10.1242/jcs.115.5.873. [DOI] [PubMed] [Google Scholar]
- McNeil PL, Vogel SS, Miyake K, Terasaki M. Patching plasma membrane disruptions with cytoplasmic membrane. J Cell Sci. 2000;113:1891–1902. doi: 10.1242/jcs.113.11.1891. [DOI] [PubMed] [Google Scholar]
- Miyake K, McNeil PL. Vesicle accumulation and exocytosis at sites of plasma membrane disruption. J Cell Biol. 1995;131:1737–1745. doi: 10.1083/jcb.131.6.1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray D, Honig B. Electrostatic control of the membrane targeting of C2 domains. Mol Cell. 2002;9:145–154. doi: 10.1016/s1097-2765(01)00426-9. [DOI] [PubMed] [Google Scholar]
- Ochoa WF, Garcia-Garcia J, Fita I, Corbalan-Garcia S, Verdaguer N, Gomez-Fernandez JC. Structure of the C2 domain from novel protein kinase Cepsilon. A membrane binding model for Ca(2+)-independent C2 domains. J Mol Biol. 2001;311:837–849. doi: 10.1006/jmbi.2001.4910. [DOI] [PubMed] [Google Scholar]
- Pace CN. Conformational stability of globular proteins. Trends Biochem Sci. 1990;15:14–17. doi: 10.1016/0968-0004(90)90124-t. [DOI] [PubMed] [Google Scholar]
- Pramono ZA, Lai PS, Tan CL, Takeda S, Yee WC. Identification and characterization of a novel human dysferlin transcript: dysferlin_v1. Hum Genet. 2006;120:410–419. doi: 10.1007/s00439-006-0230-1. [DOI] [PubMed] [Google Scholar]
- Pramono ZA, Tan CL, Seah IA, See JS, Kam SY, Lai PS, Yee WC. Identification and characterisation of human dysferlin transcript variants: implications for dysferlin mutational screening and isoforms. Hum Genet. 2009;125:413–420. doi: 10.1007/s00439-009-0632-y. [DOI] [PubMed] [Google Scholar]
- Radhakrishnan A, Stein A, Jahn R, Fasshauer D. The Ca2+ affinity of synaptotagmin 1 is markedly increased by a specific interaction of its C2B domain with phosphatidylinositol 4,5-bisphosphate. J Biol Chem. 2009;284:25749–25760. doi: 10.1074/jbc.M109.042499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schow EV, Freites JA, Cheng P, Bernsel A, von Heijne G, White SH, Tobias DJ. Arginine in membranes: the connection between molecular dynamics simulations and translocon-mediated insertion experiments. J Membr Biol. 2011;239:35–48. doi: 10.1007/s00232-010-9330-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selcen D, Stilling G, Engel AG. The earliest pathologic alterations in dysferlinopathy. Neurology. 2001;56:1472–1481. doi: 10.1212/wnl.56.11.1472. [DOI] [PubMed] [Google Scholar]
- Shao X, Fernandez I, Südhof TC, Rizo J. Solution structures of the Ca2+-free and Ca2+-bound C2A domain of synaptotagmin I: does Ca2+ induce a conformational change? Biochemistry. 1998;37:16106–16115. doi: 10.1021/bi981789h. [DOI] [PubMed] [Google Scholar]
- Therrien C, Di Fulvio S, Pickles S, Sinnreich M. Characterization of lipid binding specificities of dysferlin C2 domains reveals novel interactions with phosphoinositides. Biochemistry. 2009;48:2377–2384. doi: 10.1021/bi802242r. [DOI] [PubMed] [Google Scholar]
- Uversky VN. What does it mean to be natively unfolded? Eur J Biochem. 2002;269:2–12. doi: 10.1046/j.0014-2956.2001.02649.x. [DOI] [PubMed] [Google Scholar]
- Winn MD, Ballard CC, Cowtan KD, Dodson EJ, Emsley P, Evans PR, Keegan RM, Krissinel EB, Leslie AG, McCoy A, et al. Overview of the CCP4 suite and current developments. Acta Crystallogr D Biol Crystallogr. 2011;67:235–242. doi: 10.1107/S0907444910045749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoyama S, Hirota H, Kigawa T, Yabuki T, Shirouzu M, Terada T, Ito Y, Matsuo Y, Kuroda Y, Nishimura Y, et al. Structural genomics projects in Japan. Nat Struct Biol Suppl. 2000;7:943–945. doi: 10.1038/80712. [DOI] [PubMed] [Google Scholar]
- Zubriene A, Matuliene J, Baranauskiene L, Jachno J, Torresan J, Michailoviene V, Cimmperman P, Matulis D. Measurement of nanomolar dissociation constants by titration calorimetry and thermal shift assay - radicicol binding to Hsp90 and ethoxzolamide binding to CAII. Int J Mol Sci. 2009;10:2662–2680. doi: 10.3390/ijms10062662. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.