Abstract
Background and objective
Management of combined endodontic–periodontal lesions needs more clinical investigations. The aim of this prospective randomized clinical trial was to evaluate the effect oftime interval between the non-surgical endodontic treatment (ET) and open flap debridement (OFD) on periodontal healing in combined endodontic periodontal lesions with apical communication.
Methods
Forty patients were randomly allocated to two treatment protocols. Group 1(immediate periodontal surgery): OFD was performed at 21 days of initiation of ET and SRP, and Group 2(delayed periodontal surgery): OFD was performed after 3 months of initiation of ET and SRP. The primary parameters included probing pocket depth (PPD), relative attachment level (RAL) and bleeding on probing (BOP) and tooth mobility (TM).
Results
Significantly more reduction in PPD, TM and gain in RAL was observed in Group 1 at 3 months of OFD. (P < 0.05) Whereas at 6 months follow up of OFD (6 and 9 months of ET in Group1 and Group 2, respectively), intergroup analysis showed statistically comparable reduction in BOP (%), PPD, TM and gain in RAL (P > 0.05) in both the groups.
Conclusion
Immediate periodontal surgery may not affect the outcome of the treatment of combined endo-perio lesions with apical communication.
Keywords: Chronic periodontitis, Periodontal attachment loss, Periodontal debridement, Root planing, Root canal therapy, Wound healing
1. Introduction
Combined endodontic-periodontal lesions with communication relate to a tooth that has an infected root canal system with apical periodontitis and having marginal periodontal disease with periodontal pocket formation that extends to the periapical lesion such that the periapical and periodontal diseases communicate with each other.1
Combined lesions have more complex microflora than in teeth with pathosis confined to the periapical region.2 Furthermore, Similar environmental conditions favouring anaerobic growth appear to be present in both deepened periodontal pockets and necrotic pulp and it is difficult to assess which microbiota play a role in the pathogenesis of disease and found in the lesion as the environment favours their selection.3 The periodontal pocket may be a source of bacteria for the root canal system4, 5, 6 or vice versa, and cross-seeding of bacteria can occur in either direction through the anatomical connections between periodontal and pulpal tissues.6
When treating teeth with combined endodontic and periodontal diseases, the effects of the treatment of one tissue on the partner tissue7 and cross seeding of bacteria also need to be considered.
Traditionally, the treatment strategy in management of combined endodontic and periodontal lesion is to first focus on debridement and disinfection of the root canal system followed by an observation period of three months for definitive periodontal therapy.8 Recently, Gupta et al. 9 in a prospective randomized clinical trial in concurrent endodontic-periodontal lesions without communication found that observation period for initiation of periodontal treatment may not be required and there is no negative influence of non-surgical periodontal therapy simultaneously performed with endodontic treatment on periodontal healing.
Management of combined endo-perio lesions with apical communication is more challenging and requires comprehensive treatment with both endodontic and periodontal therapy to reduce possible complications from one disease entity, affecting the outcome of the treatment of the other diseases. Few case reports and review studies1, 10, 11, 12, 13 reported management of such lesions with strategy on timings of endodontic and periodontal treatment. But so far no clinical trial has been conducted. Thus the present study was conducted with the aim to evaluate the effect of immediate and delayed periodontal surgical therapy after non-surgical endodontic treatment,on periodontal healing in concurrent endodontic periodontal lesion with apical communication.
2. Material and methods
2.1. Ethics statement
The study protocol follows the ethical standards outlined in the Helsinki declaration 1975, as revised in 2013. The protocol was approved by the Institutional Review Board and the ethical approval was obtained from the ethical committee. Written and verbal informed consent was obtained from each patient. The clinical trial is registered at ClinicalTrials.gov as NCT02630745.
2.2. Study design and population
This prospective randomized clinical trial was conducted in the department of Periodontics and Oral Implantology in association with department of Conservative Dentistry and Endodontics. Forty patients (32 males and 8 females; aged 22 to 59 years, mean age: 42.10 years) meeting the inclusion criteria were enrolled and the study duration was from June 2015 to October 2016.
Patients with chronic periodontitis14 and having at least one non vital tooth with concurrent endodontic periodontal lesion with apical radiolucency along with communication through periodontal pocket were included in the study. Pulp sensibility testing was performed with a combination of heat test (heated gutta-percha), cold test (Endo-Frost, Coltene Whaledent Switzerland) and electric pulp test (Digitest D626D, Parkell electronics, New York)Teeth not responding to both thermal and electric test were considered nonvital. Diagnosis was made by the investigator (SM) on the basis of clinical and radiographic features of wide base pocket having deep probing pocket depth and apical periodontitis (Fig. 1A) and pulp sensibility tests. Gutta percha number 25 was used for tracing through periodontal pocket to the base of pocket to confirm apical communication with periodontal pocket (Fig. 1B). Radiographs with paralleling cone technique were taken with standardized exposure parameters (70 kvp, 3.5 mAs, and 0.2 s). Stents consisting of a film holding system (a bite block and a ring) and a silicone impression (Affinis, Coltene Whaledent Switzerland) of the occlusal surface of the tooth were prepared to stabilize the film positioning for radiographs at successive followup.
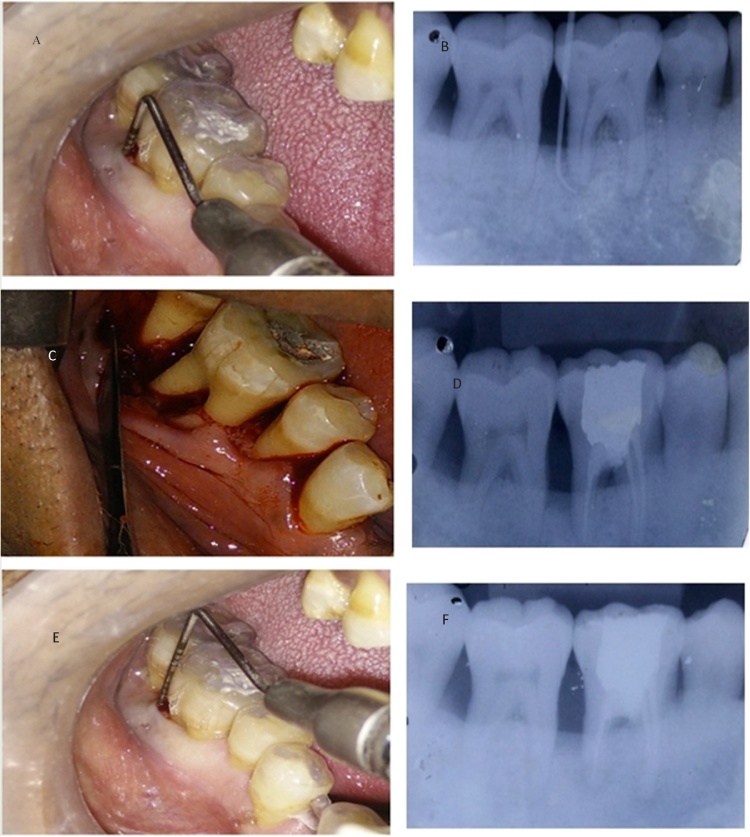
Fig. 1.
Clinical procedure in Mandibular right first molar (#46).
A. Preoperative Periodontal parameter measurements with UNC 15 probe at baseline
B. Preoperative radiograph showing concurrent endo perio lesion with apical communication with gutta percha point in periodontal pocket
C. Open flap debridement
D. Postoperative radiograph at 3 months after OF
E. Postoperative periodontal parameter measurement at 6 months after OFD
F. Postoperative radiograph at 6 months after OFD
Patients were excluded if they presented with systemic illness and on medications such as corticosteroids or calcium channel blockers which are known to affect the periodontium or outcome of periodontal therapy, or on long term NSAID therapy. Pregnant or lactating women, smokers and tobacco chewers (past or current) and patients having history of recent periodontal treatment within 6 months prior to study were also excluded. Grade 3 mobile teeth, unrestorable tooth or those teeth having fractured/perforated roots, developing permanent tooth and previously root canal treated tooth were also not included in the study.
Forty patients/40 teeth with endodontic periodontic involved teeth were randomly allocated to two treatment protocol by using computerized random table by endodontist (ST). All caregivers and outcome assessor were masked regarding patient allocation in both the groups. Only one endo-perio involved tooth per patient was included in the study.
Immediate periodontal surgery group, Group1 (n = 20patients/20teeth) – Non-surgical Endodontic treatment (ET) in endodontic-periodontic involved tooth and supragingival and subgingival scaling and root planning (SRP) were initiated simultaneously. Periodontal surgical treatment in the form of open flap debridement (OFD) was performed at 21 day of initiation of SRP and ET (after 7–10 days of obturation).
Delayed periodontal surgery group, Group 2 (n = 20patients/20 teeth), ET and SRP were performed simultaneously. After 3 months of initiation of ET and SRP, OFD was performed.
2.3. Clinical procedure
All the patients were subjected to SRP and ET simultaneously. SRP was completed with ultrasonic scaler(Suprasson P5 Booster, Satelec, France) and hand instruments (Hu-Friedy, Chicago, IL, USA) until a clinically hard, smooth surface was achieved. ET was performed by another endodontist (SM) using a standardized protocol.Apical patency was achieved with #10 or #15K-files and coronal flare was achieved with # 2 and #3 Gates-Glidden drills (Dentsply Maillefer, Tulsa, OK)Working length of each canal was confirmed with apex locator (Root ZX; J Morita, Irvine, CA) and verified by radiographs. The master apical file size was set at 3 sizes larger than the first binding file at working length. Instrumentation was carried out with K files in a crown-down technique, in conjunction with copious irrigation with 2.5% sodium hypochlorite and intra canal medicamaent calcium hydroxidewas placed for 7–10 days. At the next appointment, calcium hydroxide paste was removed by using circumferential filing with Hedstrom files and copious irrigation was done with 2.5% sodium hypochlorite followed by 5.0 mL 17% ethylenediaminetetraacetic acid. After a final rinse of 5.0 mL of 2.5% sodium hypochlorite the canals were dried with sterile paper points and obturated by using lateral condensation technique with gutta-percha and zinc oxide eugenol (ZOE) sealer.
Open flap debridment was done at 21 days and 3 months of initiation of ET and SRP in Group 1 and Group 2 respectively by the periodontist/investigator (GS). After achieving of local anaesthesia (2% lidocaine with 1:80,000 epinephrine), buccal and lingual/palatal intracrevicular incision were made and full thickness mucoperiosteal flaps were reflected including at least one adjacent tooth on both sides of endo-perio involved tooth (Fig. 1C). Meticulous degranulation and root planing was carried out. Mucoperiosteal flaps was repositioned and secured using 3-0 non absorbable black silk surgical suture. Instructions were given for gentle brushing with soft brush. Sutures were removed after 1 week. Post-surgical follow up were conducted weekly for up to 1 month and again at 3 and 6 months of OFD.
2.4. Assessment of treatment outcome
Periodontal parameters were recorded by a single caliberated investigator/periodontist (ST). Periodontal parameters of endo-perio involved tooth included PI,15 GI, 16 BOP, probing pocket depth (PPD), relative attachment level (RAL) and relative gingival marginal level (RGML) and recorded at baseline, presurgery (at 21 days of ET), 3 and 6 months after surgery in Group1 and at baseline, presurgery (3 months after ET), 3 and 6 months after OFD (6 and 9 months of ET, respectively) in Group 2 with customized stents (Fig. 1E). Measurements were performed with UNC 15 probe by adapting on six grooves (at mesiobuccal, midbuccal, distobuccal, mesiolingual, midlingual, distolingual line angles). Radiographs were taken at each follow-up (Fig. 1D, F). Tooth mobility (TM) was measured using periotest (Medizintechnik gulden, bensheim, Germany).
Determination of the intraexaminer reproducibility was done by performing double clinical periodontal data recording on 10 patients after 48 h. Assessment of the mean difference in the scores indicated good intraexaminer agreement (kappa value 0.77 and 0.84 for PPD and CAL respectively).
2.5. Statistical analyses
A sample size of 15 patients in each group was calculated to be sufficient to detect a clinically important difference in probing pocket depth (PPD) reduction and relative attachment level (RAL) gain (alpha level = 0.05, 80% power, and effect size = 1.0). The effect size was calculated by presuming a 1-mm clinical significant difference in PPD reduction/gain in CAL and a standard deviation of 1 mm. It was decided to enroll 20 patients in each group to compensate for the expected dropouts. The normality of distribution of data was determined using the Shapiro- Wilk test. Data of all the parameters of both the groups were found to be non-normally distributed except PPD and RAL which were in normal distribution. Non parametric analysis was applied for all the variables except PPD and RAL for which parametric analysis was done. Intragroup comparison of variables at different time points were done by applying Friedman analysis followed by Wilcoxin Signed Rank Test and repeated measure ANOVA followed by Paired T test in between two point times. Intergroup comparison was done by Mann-Whitney U test and Independent T test in between two groups. The chi-square test was used to analyze categorical data. Statistical significance was set at the 95% probability level (P < 0.05).
3. Results
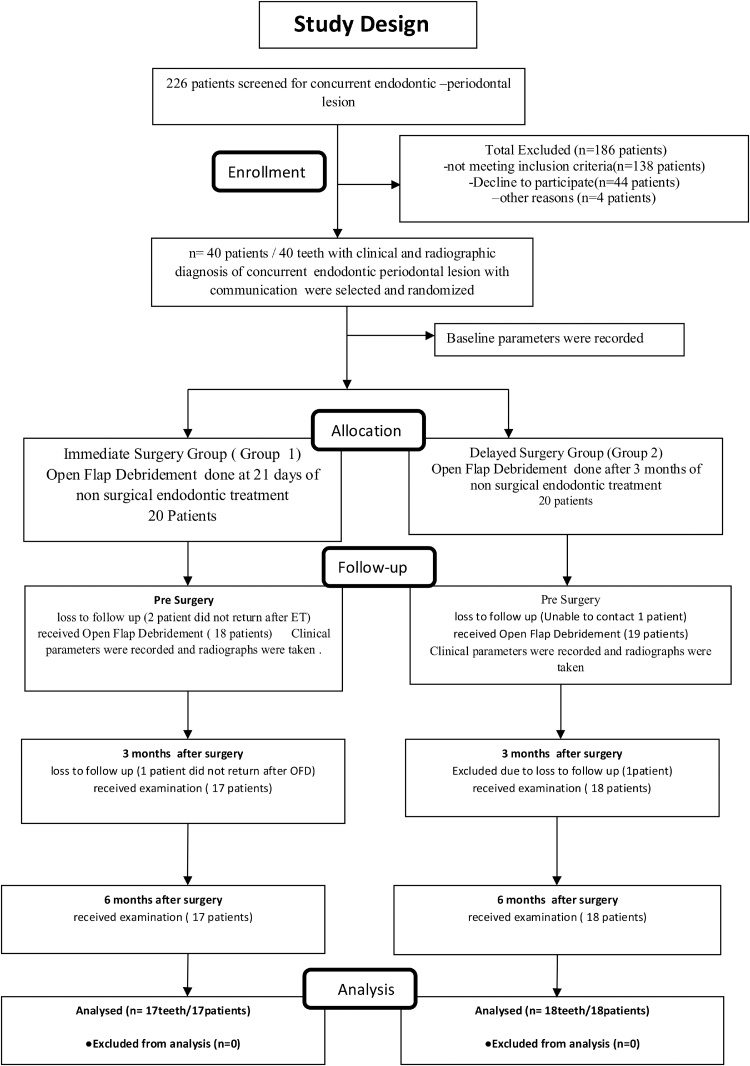
Out of 40 patients initially enrolled in the study, total 35 patients (17 in Group 1 and 18 in Group 2) completed the treatment protocol. Five patients dropped out at different stages as shown in Fig. 2. Unintended effects or any harm was not observed during the treatment and follow ups in each group.
Fig. 2.
Consolidated standards of reporting trials flowchart.
Table 1 depicts demographic and clinical data for each group and no statistical significance difference was detected in all the variables at baseline measurements between the Group 1 and Group 2 (P > 0.05).
Table 1.
Demographic data and Periodontal parameters at baseline.
| Parameters | Group 1 | Group 2 | P Value* |
|---|---|---|---|
| No. of Patients | N = 20 | N = 20 | |
| Age in years (Mean ± Sd (Max.) (Min.) | 42.70 ± 9.88 | 41.50 ± 8.50 | .692 |
| 59 | 58 | ||
| 22 | 27 | ||
| Gender M:F | 14:3 | 13:5 | |
| Tooth involved I: C: PM: M | 1: 0: 2: 17 | 1: 0: 1: 18 | .691 |
| Site Specific Parameters | |||
| PI (mean)mean ± SD | 2.03 ± 0.672 | 2.21 ± 0.61 | 0.39 |
| PI (deepest) mean ± SD | 2.18 ± 0.64 | 2.39 ± 0.61 | 0.32 |
| GI (mean)mean ± SD | 2.03 ± 0.26 | 2.03 ± 0.27 | 0.87 |
| GI (deepest) mean ± SD | 2.06 ± 0.24 | 2.11 ± 0.32 | 0.59 |
| BOP(%)(mean)mean ± SD | 98 ± 5.64 | 95.28 ± 9.77 | 0.39 |
| BOP(%)(deepest) mean ± SD | 100 ± 0.00 | 100 ± 0.00 | 1.00 |
| PPD in mm (mean)mean ± SD | 5.78 ± 0.94 | 5.94 ± 0.84 | 0.75 |
| PPD in mm (deepest) mean ± SD | 11.64 ± 1.22 | 12.17 ± 1.20 | 0.16 |
| RAL in mm (mean)mean ± SD | 7.17 ± 1.03 | 7.04 ± 0.98 | 0.13 |
| RAL in mm (deepest) mean ± SD | 13.06 ± 1.63 | 13.28 ± 1.44 | 0.75 |
| RGML in mm (mean)mean ± SD | 1.40 ± 0.71 | 1.1 ± 0.66 | 0.22 |
| RGML in mm (deepest) mean ± SD | 1.41±.80 | 1.11 ± 0.68 | 0.32 |
| TM | 27.30 ± 5.26 | 26.06 ± 6.15 | 0.46 |
SD, standard deviation.
P > 0.05 nonsignificant.
Table 2 represents intragroup comparison of the improvement in periodontal parameters from baseline to 6 months after periodontal surgery in Group 1 and Group 2. Both the groups demonstrated periodontal healing in terms of significant improvement in periodontal parameters at 6 months after surgery.
Table 2.
Intragroup comparison of periodontal parameters at baseline and 6 months after surgery of Group 1 and Group 2.
| Site specific parameters | GROUP 1 Baseline (mean ± SD) | 6 months after surgery(mean ± SD) | P value | Baseline (mean ± SD) | GROUP 2 6 months after surgery(mean ± SD) | P value |
|---|---|---|---|---|---|---|
| PI (mean) | 2.03 ± 0.67 | 0.60 ± 0.35 | 0.000* | 2.2 ± 6.14 | 0.791 ± 0.43 | 0.000* |
| PI (deepest site) | 2.18 ± 0.64 | 0.47 ± 0.51 | 0.000* | 2.38 ± 0.60 | 0.72 ± 0.46 | 0.000* |
| GI (mean) | 2.03 ± 0.26 | 0.59 ± 0.44 | 0.000* | 2.02 ± 0.61 | 0.63 ± 0.43 | 0.000* |
| GI (deepest site) | 2.06 ± 0.24 | 0.53 ± 0.80 | 0.000* | 2.11 ± 0.32 | 0.66 ± 0.76 | 0.000* |
| BOP(%)(mean) | 98.00 ± 5.65 | 14.47 ± 16.43 | 0.000* | 95.27 ± 9.76 | 15.44 ± 18.30 | 0.000* |
| BOP(%)(deepest site) | 100.00 ± 0.00 | 17.64 ± 39.29 | 0.000* | 100 ± 0.00 | 16.66 ± 38.34 | 0.000* |
| PPD in mm(mean) | 5.78 ± 0.95 | 2.47 ± 0.41 | 0.001* | 5.93 ± 0.84 | 2.52 ± 0.32 | 0.001* |
| PPD in mm (deepest site) | 11.64 ± 1.22 | 4.11 ± 0.78 | 0.000* | 12.16 ± 1.20 | 4.94 ± 1.05 | 0.000* |
| RAL in mm (mean) | 7.17 ± 1.03 | 4.47 ± 0.91 | 0.001* | 7.04 ± 0.98 | 4.35 ± 1.01 | 0.001* |
| RAL in mm(deepest site) | 13.06 ± 1.64 | 6.59 ± 1.27 | 0.000* | 13.27 ± 1.44 | 7.38 ± 1.19 | 0.000* |
| RGML in mm (mean) | 1.40 ± 0.71 | 1.99 ± 0.86 | 0.000* | 1.09 ± 0.66 | 1.84 ± 0.82 | 0.000* |
| RGML in mm (deepest site) | 1.41 ± 0.79 | 2.53 ± 1.12 | 0.001* | 1.11 ± 0.67 | 2.44 ± 0.98 | 0.000* |
| TM | 27.30 ± 5.27 | 9.53 ± 3.18 | 0.000* | 26.05 ± 6.14 | 10.94 ± 3.93 | 0.000* |
P < 0.05 (significant).
Table 3 represents intergroup comparison of improvements in periodontal parameters between Group 1 and 2. Statistically significant more reduction in PPD, TM, and gain in RAL ((P < 0.05) was found in Group 1 between baseline and 3 months after surgery and ET when compared with 6 months of ET in delayed periodontal surgery group(Group 2). Comparison of improvements in periodontal parameters between groups from baseline to 6 months after surgery follow up (6 months in Group 1 & 9months in Group 2) revealed statistically non-significant reduction in BOP (%) (mean and deepest site),PPD (mean and deepest site),TM and gain in RAL(mean and deepest site) (P > 0.05)
Table 3.
Comparison in Improvement (Δ) in Periodontal Parameters between Groups 1 and Group 2.
| Site specific Parameters | Group1 Baseline-3 months after surgery (mean ± SD) | Group 2 Baseline-3 months after surgery (mean ± SD) |
P Value | Group1 Baseline-6 months after surgery (mean ± SD) | Group 2 Baseline-6 months after surgery (mean ± SD) | P value |
|---|---|---|---|---|---|---|
| ΔPI (mean) | 1.30 ± 6.8 | 1.47 ± 0.42 | 0.491 | 1.42 ± 0.642 | 1.42 ± 0.51 | 1 |
| ΔPI (deepest site) | 1.58 ± 0.79 | 1.61 ± 0.84 | 0.873 | 1.71 ± 0.59 | 1.61 ± 0.69 | 0.77 |
| ΔGI (mean) | 1.36 ± 0.50 | 1.31 ± 0.34 | 0.708 | 1.44 ± 0.47 | 1.39 ± 0.44 | 0.63 |
| ΔGI (deepest site) | 1.47 ± 0.79 | 1.27 ± 0.75 | 0.360 | 1.53 ± 0.72 | 1.44 ± 0.70 | 0.65 |
| ΔBOP(%)(mean) | 80.64 ± 16.81 | 78.11 ± 17.07 | 0.678 | 83.52 ± 17.62 | 79.83 ± 20.15 | 0.63 |
| ΔBOP(%)(deepest site) | 82.35 ± 39.29 | 77.77 ± 42.77 | 0.739 | 82.35 ± 39.29 | 83.33 ± 38.3 | 0.94 |
| ΔPPD in mm (mean) | 2.64 ± 0.76 | 2.15 ± 0.70 | 0.07 | 3.31 ± 0.82 | 43.41 ± 0.74 | 0.64 |
| ΔPPD in mm (deepest site) | 6.29 ± 1.10 | 5.33 ± 1.13 | 0.02* | 7.52 ± 1.06 | 7.22 ± 1.17 | 0.54 |
| ΔRAL in mm (mean) | 2.15 ± 0.71 | 1.52 ± 0.72 | 0.02* | 269 ± 0.79 | 2.68 ± 0.84 | 0.84 |
| ΔRAL in mm (deepest site) | 5.29 ± 1.04 | 4.11 ± 1.32 | 0.01* | 6.47 ± 1.007 | 5.89 ± 1.37 | 0.22 |
| ΔRGML in mm (mean) | −0.47 ± 0.49 | −0.62 ± 0.42 | 0.230 | −0.60 ± 0.69 | −0.75 ± 0.61 | 0.21 |
| ΔRGML in mm (deepest site) | −1.00 ± 0.61 | −1.22 ± 0.54 | 0.270 | −1.12 ± 0.78 | −1.33 ± 0.69 | 0.33 |
| ΔTM | 12.41 ± 3.26 | 10.55 ± 3.36 | 0.04* | 17.76 ± 4.51 | 15.11 ± 4.33 | 0.45 |
P < 0.05 (significant).
4. Discussion
Endo-perio lesions with apical communications are a dilemma for the clinician as far as diagnosis and prognosis of the involved teeth are concerned. These lesions require management of both endodontic and periodontal pathogen as infection of periodontal tissue may affect the outcome of endodontic treatment or vice-versa through patent communicating pathways mainly through apical foramina.
The management of combined endodontic periodontal lesions needs more clinical trials. There has been controversy in literature regarding the timing and sequence of endodontic and definite periodontal treatment in case of combined endodontic–periodontal lesions. To the best of our knowledge no prospective randomized clinical study has been conducted till date to evaluate the effect of performing ET and early periodontal surgical therapy in combined endodontic periodontal lesions with communication. Based on the study of Gupta et al. 25 the current trial was undertaken to observe the effect of immediate periodontal surgery at 21 day of ET (7–10 days of obturation of root canals) and SRP in concurrent endodontic periodontal lesions with communication.
In both the groups’ periodontal surgery was performed after an initial phase of SRP which is of 21 days in Group 1 and 3 months in Group 2. Aljateeli et al. 17 in a randomized controlled clinical trial demonstrated that the results of periodontal surgery with initial phase of SRP contributed in greater reduction of inflammation of the gingival tissues. Intracanal dressing of calcium hydroxide was placed for 10 days in both the groups to maintain the environment within the root canal system in a state that is unfavorable for bacterial colonization.18, 19, 20 Furthermore, its temporary obturating action helps in inhibiting periodontal contamination of the instrumented canals via patent channels of communications.
Both the groups depicted significant improvement in periodontal parameters at 3 and 6 month follow up after periodontal surgery. Significantly more reduction in PPD and TM and gain in RAL in immediate periodontal surgery group was observed between baseline and 3 months of follow up after surgery. This indicated that performing periodontal surgery immediately resulted in better periodontal healing at 3 months of follow up of OFD as compared to delayed surgical group patients (6 months of ET).
Intergroup analysis showed similar improvement in periodontal parameters in terms of reduction of BOP (mean and deepest site), PPD (mean and deepest site), TM and gain in RAL (mean and deepest site) when comparing both the groups from baseline to 6 months of OFD follow up (total duration of 6 months in group 1 and 9 months in group 2). There was more than 80% reduction in number of bleeding sites indicating periodontal tissue stability. BOP due to its high degree of specificity is a good predictor of periodontal health and stability.21
The result of the present study indicated that performing both endodontic and early definite periodontal treatment in group 1 resulted in comparable periodontal healing in a shorter time span when compared with group 2 and may not affect the treatment outcome in such lesions.Results of our study reiterate the findings of Gupta et al. 9 that performaing simultaneous endodontic and non-surgical periodontal treatment does not affect the outcome of endo-perio lesions without communications. Further performing periodontal surgery after observation period of 3 months of may also not be required in the management of endodontic periodontal lesions requiring periodontal surgery. Such cases of communication having deep pocket usually more than 7 mm often require periodontal surgery as SRP is not enough to adequately remove the periodontal microflora from deep pockets.
On the contrary an animal histologic and histometric study on beagle dogs,22 observed lesser periodontal healing after modified Widman flap when ET was performed just before periodontal surgical therapy. But in this study unlike clinical conditions, exposed root surfaces were thoroughly denuded of periodontal ligament and cementum using periodontal curettes, files and burs and also grooves and notches were prepared on root surface.
The strength of our study includes its study design being an evidence based study and standardization of calibration of clinical parameters by using stents at each follow up. In both the groups the teeth included are mostly multi-rooted and with furcation involvement to avoid tooth type bias in results. Plaque level in both the groups was comparable at baseline to rule out confounding effect. Smokers were excluded from the study as smoking has been recognized as confounding factors in healing of periodontal diseases.
However, the study has certain limitations such as small sample size, high number of drop outs and no regenerative surgical therapy and use of intracanal medicament. Conventional radiological investigation was taken instead of advanced radiological aids such as digital subtraction radiography and use of cone-beam computed tomographic imaging (CBCT).
5. Conclusion
Within the limits of the study, we conclude that periodontal healing in terms of improvement in periodontal parameters after 6 months of ET in immediate surgical periodontal treatment was comparable to healing after 9 months of ET in delayed surgical treatment in endodontic-periodontal apically communicating lesions which suggested that there may not be need to wait for 3 months after endodontic treatment for definitive periodontal therapy. There may not be any detrimental effect of immediate periodontal surgery on periodontal healing and results of our study are in favour of performing both treatments immediately as it has less treatment duration and better patient compliance. Multicenter study with large sample size and long follow up is warranted for definite conclusions or confirmation of these results.
Conflict of interest
The authors deny any conflicts of interest related to this study.
Acknowledgments
We acknowledge Postgraduate Institute of Dental Sciences, Rohtak for providing all facilities for conducting this trial.
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- 1.Abott P.V., Salgado J.C. Strategies for the endodontic management of concurrent endodontic and periodontal diseases. Aust Dent J. 2009;54:570–585. doi: 10.1111/j.1834-7819.2009.01145.x. [DOI] [PubMed] [Google Scholar]
- 2.Kurihara H., Kobayashi Y., nagai A., Fransisco I.A., Isoshima O., Murayama Y. The microbiological and immunological study of endodontic – periodontic lesion. J Endod. 1995;21:617–621. doi: 10.1016/S0099-2399(06)81115-5. [DOI] [PubMed] [Google Scholar]
- 3.Kipioti A., Nakou M., Legakis N., Mitsis F. Microbiological findings of infected root canals and adjacent periodontal pockets in teeth with advanced periodontitis. Oral Surg Oral Med Oral Pathol. 1984;58:213–220. doi: 10.1016/0030-4220(84)90139-7. [DOI] [PubMed] [Google Scholar]
- 4.Kobayashi T., Hayashi A., Yoshikawa R., Okuda K., Hara K. The microbial flora from root canals and periodontal pockets of non-vital teeth associated with advanced periodontitis. Int Endod J. 1990;23:100–106. doi: 10.1111/j.1365-2591.1990.tb00847.x. [DOI] [PubMed] [Google Scholar]
- 5.Sundqvist G., Johansson E., Sjögren U. Prevalence of black-pigmented bacteroides species in root canal infections. J Endod. 1989;15:13–19. doi: 10.1016/S0099-2399(89)80092-5. [DOI] [PubMed] [Google Scholar]
- 6.Moore W.E.C. Microbiology of periodontal disease. J Periodontal Res. 1987;22:335–341. doi: 10.1111/j.1600-0765.1987.tb01595.x. [DOI] [PubMed] [Google Scholar]
- 7.Kerekes K., Olsen I. Similarities in the microfloras of root canals and deep periodontal pockets. Endod Dent Traumatol. 1990;6:1–5. doi: 10.1111/j.1600-9657.1990.tb00378.x. [DOI] [PubMed] [Google Scholar]
- 8.Zehnder M., Gold S.I., Hasselgren G. Pathologic interactions in pulpal and periodontal tissues. J Clin Periodontol. 2002;29:663–671. doi: 10.1034/j.1600-051x.2002.290801.x. [DOI] [PubMed] [Google Scholar]
- 9.Gupta S., Tewari S., Tewari S., Mittal S. Effect of time lapse between endodontic and periodontal therapies on the healing of concurrent endodontic-periodontal lesions without communication: a prospective randomized clinical trial. J Endod. 2015;41:785–790. doi: 10.1016/j.joen.2015.02.015. [DOI] [PubMed] [Google Scholar]
- 10.Paul B.F., Hutter J.W. The endodontic-periodontal continuum revisited: new insights into etiology, diagnosis and treatment. J Am Dent Assoc. 1997;128:1541–1548. doi: 10.14219/jada.archive.1997.0094. [DOI] [PubMed] [Google Scholar]
- 11.Vakalis S.V., Whitworth J.M., Ellwood R.P., Preshaw P.M. A pilot study of treatment of periodontal-endodontic lesions. Int Dent J. 2005;55:313–318. doi: 10.1111/j.1875-595x.2005.tb00329.x. [DOI] [PubMed] [Google Scholar]
- 12.Oh S.L., Fouad A.F. Treatment strategy for guided tissue regeneration in combined endodontic-periodontal lesions: case report and review. J Endod. 2009;35:1331–1336. doi: 10.1016/j.joen.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 13.Prichard J.F. The diagnosis and management of vertical bone defects. J Periodontol. 1983;54:29–35. doi: 10.1902/jop.1983.54.1.29. [DOI] [PubMed] [Google Scholar]
- 14.Page R.C., Eke P.I. Case definitions for use in population-based surveillance of periodontitis. J Periodontol. 2007;78:1387–1399. doi: 10.1902/jop.2007.060264. [DOI] [PubMed] [Google Scholar]
- 15.Silness J., Loe H. Periodontal disease in pregnancy: II. Correlation between oral hygiene and periodontal condition. Odontol Scand. 1964;22:121–135. doi: 10.3109/00016356408993968. [DOI] [PubMed] [Google Scholar]
- 16.Loe H., Silness J. Periodontal disease in pregnancy: I. Prevalence and severity. Odontol Scand. 1963;21:533–551. doi: 10.3109/00016356309011240. [DOI] [PubMed] [Google Scholar]
- 17.Aljateeli M., Koticha T., Bashutski J. Surgical periodontal therapy with and without initial scaling and root planing in the management of chronic periodontitis: a randomized clinical trial. J Clin Periodontol. 2014;41:693–700. doi: 10.1111/jcpe.12259. [DOI] [PubMed] [Google Scholar]
- 18.Tronstad L., Barnett F., Riso K., Slots L. Extraradicular endodontic infections. Endod Dent Traumatol. 1987;3:86–90. doi: 10.1111/j.1600-9657.1987.tb00549.x. 41. [DOI] [PubMed] [Google Scholar]
- 19.Tronstad L., Barnett F., Cervone F. Periapical bacterial plaque in teeth refractory to endodontic treatment. Endod Dent Traumatol. 1990;6:73–77. doi: 10.1111/j.1600-9657.1990.tb00394.x. 4. [DOI] [PubMed] [Google Scholar]
- 20.Sjögren U., Figdor D., Spångberg L., Sundqvist G. The antimicrobial effect of calcium hydroxide as a short-term intracanal dressing. Int Endod J. 1991;24:119–125. doi: 10.1111/j.1365-2591.1991.tb00117.x. [DOI] [PubMed] [Google Scholar]
- 21.Lang N.P., Adler R., Joss A., Nyman S. Abscence of bleeding on probing- an indicator of periodontal stability. J Clin Periodontol. 1991;17:714–721. doi: 10.1111/j.1600-051x.1990.tb01059.x. [DOI] [PubMed] [Google Scholar]
- 22.Lima L.A., Anderson G.B., Wang M.M. Healing of intrabony defects and its relationship to root canal therapy: a histological and histometric study in dogs. J Periodontol. 1997;68:240–248. doi: 10.1902/jop.1997.68.3.240. [DOI] [PubMed] [Google Scholar]