ABSTRACT
Direct stimulation of the antitumor activity of immune system through checkpoint inhibitors (ICIs) has demonstrated efficacy in the treatment of different cancer types. The activity of these antibodies takes place in the immunological synapse blocking the binding of the negative immunoregulatory proteins, thus leading to the finalization of the immune response. Despite having a favorable toxicity profile, its mechanism of action impedes the negative regulation of the immune activity which can potentially favor autoimmune attacks to normal tissues. Renal toxicity has been described in several ICI but not with atezolizumab, an IgG1 monoclonal antibody targeting PD-L1 (programmed death ligand 1), approved by FDA as a second-line therapy for advanced urothelial carcinoma. Here we present a patient with a single kidney and metastatic renal cell carcinoma treated with atezolizumab and bevacizumab combination, with biopsy-proven acute interstitial nephritis, who had a complete resolution of renal dysfunction after steroid therapy.
KEYWORDS: atezolizumab, immune check-point inhibitors, PD-L1, tubulointerstitial nephritis
Introduction
Despite immune system is able to eradicate cancer, malignant cells manage to escape immune attacks through different strategies. The capacity to exploit the host immune system to treat cancer is known as immunotherapy. Among the different treatments that this concept encompasses, immune checkpoint inhibitors (ICI), such as antibodies anti-cytotoxic T lymphocyte-associated antigen-4 (CTLA-4), anti-programmed cell death protein 1 (PD-1) and anti-programmed cell death ligand 1 (PD-L1), have demonstrated efficacy in the treatment of different cancer types.1 Roughly, the activity of these antibodies takes place in the immunological synapse blocking the binding of the negative immunoregulatory proteins, thus leading to the finalization of the immune response. The physiological function of these proteins is to protect the host against autoimmunity. Unfortunately, one of the strategies that cancer cells use to evade immune system is precisely through the overexpression of these immunoregulatory molecules, such as PD-L1.
Atezolizumab is a fully humanized IgG1 monoclonal antibody targeting PD-L1 that has been approved by FDA as second-line therapy for advanced renal carcinoma.2 Although anti-PD-1/PD-L1 antibodies have a favorable toxicity profile, its mechanism of action impedes the negative regulation of the immune activity which can potentially favors autoimmune attacks to normal tissues, known as immune-related adverse events (irAE). Among them, infrequent renal toxicity has been previously described with the use of other ICI such as nivolumab3 or pembrolizumab,4 but not with atezolizumab.5,6
Here we present, to our knowledge, the first case of immune-mediated acute tubulointerstitial nephritis (ATIN) associated with atezolizumab.
Case report
A 54-year-old Caucasian male, heavy smoker (accumulated dose: 40 packs year), with history of essential hypertension and depressive syndrome, was diagnosed with renal cancer in the context of hematuria. Primary tumor was located in left kidney and was associated with left renal vein invasion; additionally, he had synchronic lung metastases [American Joint Committee of Cancer (AJCC) Stage IV; T3aN0M1]. The patient underwent left radical nephrectomy; histopathological assessment confirmed renal cell carcinoma (RCC) with 20% of sarcomatoid differentiation foci. The patient accepted to participate in the clinical trial NCT02420821, and he was randomized in the treatment arm with atezolizumab-bevacizumab. Subsequently, it was initiated an intravenous infusion combination of atezolizumab (1200 mg) and bevacizumab (15 mg/kg) on days 1 and 22 of each 42-day cycle. Treatment was well tolerated with just few mild adverse events such as grade 1 fatigue, arthralgia, and myalgia, as per CTCAE.7 Disease evaluation performed at the end of the 3rd cycle showed partial response (45% decrease in the sum of target lesions).
In the visit corresponding to cycle 5 day 22, the patient referred to have had general malaise, 39°C fever and grade 1 diarrhea for the previous 2 weeks. Physical examination was unremarkable except for temperature of 37.8°C and mild dehydration. Routine blood tests revealed acute kidney injury stage III (according to Acute Kydney Injury Network classification) with creatinine of 5.6 mg/dL (ULN: 1.3 mg/dL), which represented a sharp increase compared to his baseline value (1.2 mg/dL), and eosinophilia (7.2%, corresponding to 400 eosinophils/mm3). Urinalysis revealed 2+ protein (protein/creatinine ratio 782 mg/g), RBC 8/hpf and WBC 9/hpf. Urine sediments showed 2–3 eosinophils. There had not been recent exposure to nephrotoxic agents such as antibiotics, contrast or analgesics. He was taking, for more than three years, atenolol 50 mg q.d., enalapril 5 mg b.i.d., venlafaxine 75 mg q.d., and lorazepam 1 mg q.d. as needed. Renal ultrasound showed a normal right kidney with no evidence of hydronephrosis. Despite volume repletion and spontaneous remission of diarrhea, the patient persisted with AKI with preserved diuresis; for this reason, a kidney biopsy was performed in order to establish the etiology of the disorder.
In the renal biopsy were observed 18 glomeruli with a preserved architecture. No signs of necrotizing lesions or associated inflammation were observed in the glomerular tufts. An extensive diffuse inflammatory infiltrate consisting of both T-helper cells and cytotoxic T cells some of them with scattered PD1 deposits, plasma cells and eosinophils was observed in the interstitial component, also with tubulitis. Of note, there were some tubular cells that expressed PD-L1. No granulomatous lesions were observed. There were no remarkable findings in the vascular compartment. Immunofluorescence staining did not reveal glomerular immune deposits (Fig. 1).
Figure 1.
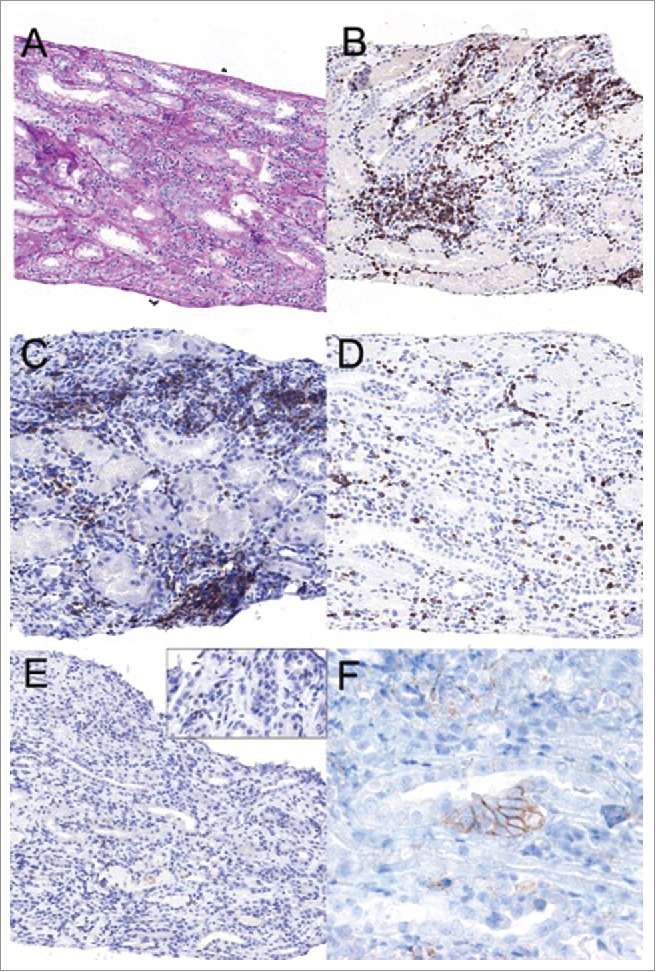
The renal biopsy showed interstitial inflammatory infiltrates with focal tubulitis (A; PAS original magnification × 20). Immunohistochemical stainings revealed a predominant T cell infiltrate (B; CD3, 20x), consisting of both T-helper cells (C; CD4, 20x) and cytotoxic T cells (D; CD8, 20x). There were scattered PD1 positive cells (E; PD1, 20x, the enlarged insert 40x) and some tubular cells expressing PD-L1 (F; PD-L1 80X).
The patient was treated with a steroid pulse [i.v. methylprednisolone 125 mg × 3 days], obtaining clinical and analytical improvement, characterized by resolution of fever and malaise, and a decrease of the creatinine to 1.45 mg/dL after 8–10 weeks of follow-up. Steroids were progressively tapered once periodical creatinine evaluations had shown no impairment of the renal function. Anticancer therapy has not been reintroduced since ulterior radiological evaluations have shown partial response, which is currently maintained by the time our case is reported (PFS: 25 months).
Discussion
PD-L1 is a transmembrane protein widely expressed in antigen-presenting cells, including B cells, dendritic cells, macrophages and tumor cells.2 Its interaction with PD-1 of T cells downregulates the immune response maintaining the peripheral tolerance to self and external antigens, through the inhibition of proliferation and activation of T cells, and inhibiting the cytolytic activity of the immune system.8
This mechanism of avoiding the action of immune system is used by many tumor cells by the expression of PD-L1. In fact, in many tumor types, the more overexpression of PD-L1, the worse cancer prognosis.9 Blocking the pathway of PD-L1/PD-1 activates T cells, and this tool has been used for therapeutic purposes, with the aim of releasing the immune system activity against the tumor tissue.2 But if there is an excessive response, it can also damage healthy tissue, producing irAE.5 This is especially true since PD-L1 is not only restricted to tumor cells, but it is also found in healthy tissue.
The renal tubule epithelial cells (TEC) are one of the few type of epithelial cells that also express major histocompatibility complex class II molecules, which allows TEC to function as antigen presenting cells (APC) for T cells,10 leading to the activation of the immune response. As APC, TEC also has the capacity to modulate T-cell responses negatively. PD-L1 has been described as a negative coestimulatory molecule, and its expression can be induced by activation with IFN-gamma.11,12 PD-L1 was not observed in renal glomerulus.12 Thus, it has been suggested that the TEC PD-L1/T cell PD-1 binding would have a protective role against immune-mediated tubulointerstitial injury.13 Therefore, if anti-PD-L1 antibodies block PD-L1 receptor, T-cells would not be deactivated, which propitiates their activity against antigens presented by TEC. Thus, T cells seems to play a major role in the pathogenesis of ATIN related to ICIs, both CD4+ and CD8+.14 This would be accompanied by an increase of cytokines (IL-18 and IFN-gamma, among others, as observed over the course treatment with atezolizumab),15 and consequently an increase in cytotoxic lymphocytes. These cytokines orchestrate the inflammatory reaction, resulting, as in others drug-induced ATIN, in an interstitial infiltrate of lymphocytes, macrophages, monocytes, eosinophils and/or polymorphonuclear neutrophils, in addition to interstitial edema and disruption of the tubular basement membrane.16
Different hypotheses have been raised about the role of the interaction among lymphocytes and potential antigens in this particular ATIN. It has been reported that 14 of 19 patients17,18 had associated drugs potentially causing tubulointerstitial nephritis. Based on this observation, Izzedine and colleagues19 suggested that ICIs can reactive drug-specific inactive T cells, previously sensitized by nephritogenic antigens. This consequently could produce a response of memory T cells against drugs. Cortazar also suggests that there could be a “reprogramming” of immune system, losing the acquired tolerance against own endogenous antigens. The latter would allow to explain the long latency observed sometimes from the administration of the drug to the development of nephritis.17
Moreover, the combination of atezolizumab and bevacizumab, an inhibitor of the vascular endothelial growth factor (VEGF), seems to be associated with further increase in intra-tumoral CD8+ cells due to enhanced trafficking, and with an increase of intra-tumoral MHC-I, Th1 and T-effector markers.20 In addition, VEGF is known to produce immunosuppressive effects. It has been reported that the VEGF produced in the tumor microenvironment enhances expression of PD-1 and other inhibitory checkpoints involved in CD8+ T cell exhaustion,21 as well as the inhibition of lymphocyte maturation.22 Moreover, VEGF would have the capacity to increase the production of myeloid-derived suppressor cells (MDSC), which are able to decrease T cell function.23 Although the anti-PD-L1 and anti-VEGF association is increasingly supported due to its antitumoral effects, it can favor the development of irAE due to similar mechanisms.24
Anti-VEGF therapies have significant renal implications, but ATIN had been described in very few anecdotic cases.25,26 Bevacizumab and other anti-VEGF drugs are frequently related with proteinuria and hypertension. The most common histopathologic kidney lesion is thrombotic microangiopathy, with other glomerular lesions occurring less frequently. The mechanism for glomerular injury may develop from loss of VEGF effect on maintaining the filtration barrier.27
This case, with no evidence of glomerular injury on renal biopsy, reinforces the hypothesis of the synergistic role of the bevacizumab and atezolizumab combination in inducing an antitumor immune response,20 but also producing an event of ATIN as a side effect. To our knowledge, this is the first case report of biopsy-proven ATIN caused by anti-PD-L1 antibody, confirming the role of this drug as trigger of autoimmunity. The presence of an infiltrate primarily composed of CD4/CD8, PD-1 positive T cells, and some tubular cells expressing PD-L1 and acting as an antigen presenting cell; support the hypothesis of autoimmune disorder associated with this case of ATIN.
This patient has shown susceptibility to atezolizumab, since a partial response was reached in the first radiological evaluation and is currently maintained after 25 months at the expense of a severe irAE. A relationship between developing irAEs and clinical benefit from ICIs has been hypothesized and reported through the last years, firstly with ipilimumab. Beck et al.28 and Downey et al.29 found better response rates in tumor regression in those patients who developed colitis and any irAE, respectively. Contrarily, subsequent ipilimumab data from an expanded access programme30 and a retrospective study31 suggest the opposite. In the case of the anti-PD-1 antibodies, there has been reported a relationship between cutaneous irAE and better outcomes in patients with melanoma.32-36 However, there is still no general agreement between the correlation of clinical benefit from ICIs and outcomes. It is a priority to explore this relationship in the future.
In summary, our case highlights the aggressive T cell-mediated pathophysiology of ATIN in the setting of ICI therapy with atezolizumab. These adverse effects are potentially serious, especially in patients with decreased renal mass, so common in RCC, despite being the incidence of ATIN ICIs-associated in the general population low (∼1%).37 Close follow-up and early treatment with steroids are the key for the management of this complication.
Acknowledgments
CERCA Programme / Generalitat de Catalunya.
References
- 1.Wurz GT, Kao C-J, DeGregorio MW. Novel cancer antigens for personalized immunotherapies: latest evidence and clinical potential. Ther Adv Med Oncol [Internet]. 2016. [cited 2017December7];8:4–31. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26753003 doi: 10.1177/1758834015615514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Inman BA, Longo TA, Ramalingam S, Harrison MR. Atezolizumab: a PD-L1 blocking antibody for bladder cancer. Clin Cancer Res. 2017;23(8):1886–1890. doi: 10.1158/1078-0432.CCR-16-1417. [DOI] [PubMed] [Google Scholar]
- 3.Jung K, Zeng X, Bilusic M. Nivolumab-associated acute glomerulonephritis: a case report and literature review. BMC Nephrol [Internet]. 2016;17:188 Available from: http://www.ncbi.nlm.nih.gov/pubmed/27876011 doi: 10.1186/s12882-016-0408-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Escandon J, Peacock S, Trabolsi A, Thomas DB, Layka A, Lutzky J. Interstitial nephritis in melanoma patients secondary to PD-1 checkpoint inhibitor. J Immunother cancer [Internet]. 2017;5:3 Available from: http://www.ncbi.nlm.nih.gov/pubmed/28105370 doi: 10.1186/s40425-016-0205-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hahn AW, Gill DM, Agarwal N, Maughan BL. PD-1 checkpoint inhibition: Toxicities and management. Urol Oncol. 2017;35(12):701/707. doi: 10.1016/j.urolonc.2017.08.005. [DOI] [PubMed] [Google Scholar]
- 6.Wanchoo R, Karam S, Uppal NN, Barta VS, Deray G, Devoe C, Launay-Vacher V, Jhaveri KD. Adverse Renal Effects of Immune Checkpoint Inhibitors: A Narrative Review. Am J Nephrol. 2017;45:160–9. doi: 10.1159/000455014. PMID:28076863. [DOI] [PubMed] [Google Scholar]
- 7.Institute NC. Common Terminology Criteria for Adverse Events (CTCAE) [Internet]. Available from: http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-%0A06-14_QuickReference_8.5x11.pdf
- 8.Deng R, Bumbaca D, Pastuskovas CV, Boswell CA, West D, Cowan KJ, Chiu H, McBride J, Johnson C, Xin Y, et al.. Preclinical pharmacokinetics, pharmacodynamics, tissue distribution, and tumor penetration of anti-PD-L1 monoclonal antibody, an immune checkpoint inhibitor. MAbs. 2016;8:593–603. doi: 10.1080/19420862.2015.1136043. PMID:26918260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Choueiri TK, Fay AP, Gray KP, Callea M, Ho TH, Albiges L, Bellmunt J, Song J, Carvo I, Lampron M, et al.. PD-L1 expression in nonclear-cell renal cell carcinoma. Ann Oncol Off J Eur Soc Med Oncol [Internet]. 2014;25:2178–84. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25193987 doi: 10.1093/annonc/mdu445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hagerty DT, Allen PM. Processing and presentation of self and foreign antigens by the renal proximal tubule. J Immunol [Internet]. 1992. [cited 2017December7];148:2324–30. Available from: http://www.ncbi.nlm.nih.gov/pubmed/1560195 [PubMed] [Google Scholar]
- 11.Schoop R. Suppressed T-cell activation by IFN- -induced expression of PD-L1 on renal tubular epithelial cells. Nephrol Dial Transplant [Internet]. 2004;19:2713–20. Available from: https://academic.oup.com/ndt/article-lookup/doi/10.1093/ndt/gfh423 doi: 10.1093/ndt/gfh423. [DOI] [PubMed] [Google Scholar]
- 12.Ding H, Wu X, Gao W. PD-L1 is expressed by human renal tubular epithelial cells and suppresses T cell cytokine synthesis. Clin Immunol. 2005;115:184–91. doi: 10.1016/j.clim.2005.01.005. PMID:15885642. [DOI] [PubMed] [Google Scholar]
- 13.Menke J, Lucas JA, Zeller GC, Mary E, Huang XR, Tsuboi N, Mayadas TN, Lan HY, Sharpe AH, Kelley VR. Programmed Death 1 Ligand (PD-L) 1 and PD-L2 Limit Autoimmune Kidney Disease: Distinct Roles. J Immunol. 2007;179:7466–77. doi: 10.4049/jimmunol.179.11.7466. PMID:18025191. [DOI] [PubMed] [Google Scholar]
- 14.Uchida A, Watanabe M, Nawata A, Ikari Y, Sasaki M, Shigemoto K, Hisano S, Nakashima H. Tubulointerstitial nephritis as adverse effect of programmed cell death 1 inhibitor, nivolumab, showed distinct histological findings. CEN Case Rep. 2017;6(2):169–174. doi: 10.1007/s13730-017-0269-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Powles T, Eder JP, Fine GD, Braiteh FS, Loriot Y, Cruz C, Bellmunt J, Burris HA, Petrylak DP, Teng S, et al.. MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature [Internet]. 2014. [cited 2017December7];515:558–62. Available from: http://www.nature.com/doifinder/10.1038/nature13904 doi: 10.1038/nature13904. [DOI] [PubMed] [Google Scholar]
- 16.Spanou Z. Involvement of Drug-Specific T Cells in Acute Drug-Induced Interstitial Nephritis. J Am Soc Nephrol [Internet]. 2006;17:2919–27. Available from: http://www.jasn.org/cgi/doi/10.1681/ASN.2006050418 doi: 10.1681/ASN.2006050418. [DOI] [PubMed] [Google Scholar]
- 17.Cortazar FB, Marrone KA, Troxell ML, Ralto KM, Hoenig MP, Brahmer JR, Le DT Lipson EJ, Glezerman IG, Wolchok J, et al.. Clinicopathological features of acute kidney injury associated with immune checkpoint inhibitors. Kidney Int [Internet]. 2016;90:638–47. Available from: http://www.ncbi.nlm.nih.gov/pubmed/27282937 doi: 10.1016/j.kint.2016.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shirali AC, Perazella MA, Gettinger S. Association of Acute Interstitial Nephritis With Programmed Cell Death 1 Inhibitor Therapy in Lung Cancer Patients. Am J Kidney Dis [Internet]. 2016. [cited 2017December7];68:287–91. Available from: http://www.ncbi.nlm.nih.gov/pubmed/27113507 doi: 10.1053/j.ajkd.2016.02.057. [DOI] [PubMed] [Google Scholar]
- 19.Izzedine H, Mateus C, Boutros C, Robert C, Rouvier P, Amoura Z, Mathian A. Renal effects of immune checkpoint inhibitors. Nephrol Dial Transplant [Internet]. 2016;4:gfw382 Available from: https://academic.oup.com/ndt/article-lookup/doi/10.1093/ndt/gfw382 doi: 10.1093/ndt/gfw382. [DOI] [PubMed] [Google Scholar]
- 20.Wallin JJ, Bendell JC, Funke R, Sznol M, Korski K, Jones S, Hernandez G, Mier J, He X, Hodi FS, et al.. Atezolizumab in combination with bevacizumab enhances antigen-specific T-cell migration in metastatic renal cell carcinoma. Nat Commun. 2016;7:1–8. doi: 10.1038/ncomms12624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Voron T, Colussi O, Marcheteau E, Pernot S, Nizard M, Pointet A-L, Latreche S, Bergaya S, Benhamouda N, Tanchot C, et al.. VEGF-A modulates expression of inhibitory checkpoints on CD8+ T cells in tumors. J Exp Med [Internet]. 2015;212:139–48. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25601652 doi: 10.1084/jem.20140559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li Y-L, Zhao H, Ren X-B, Li Y-L, Zhao H, Ren X-B. Relationship of VEGF/VEGFR with immune and cancer cells: staggering or forward? Cancer Biol Med [Internet]. 2016. [cited 2017December7];13:206–14. Available from: http://www.ncbi.nlm.nih.gov/pubmed/27458528 doi: 10.20892/j.issn.2095-3941.2015.0070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol [Internet]. 2012;12:253–68. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22437938 doi: 10.1038/nri3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Subramanian P, Haas NB. Recent advances in localized RCC: A focus on VEGF and immuno-oncology therapies. Urol Oncol Semin Orig Investig [Internet]. 2017;36:23–30. Available from: https://doi.org/10.1016/j.urolonc.2017.09.008 doi: 10.1016/j.urolonc.2017.09.008. [DOI] [PubMed] [Google Scholar]
- 25.Barakat RK, Singh N, Lal R, Verani RR, Finkel KW, Foringer JR. Interstitial nephritis secondary to bevacizumab treatment in metastatic leiomyosarcoma. Ann Pharmacother [Internet]. 2007. [cited 2017December7];41:707–10. Available from: http://journals.sagepub.com/doi/10.1345/aph.1H635 doi: 10.1345/aph.1H635. [DOI] [PubMed] [Google Scholar]
- 26.Lomax AJ, Hill PA, Ashley DM. Case report of interstitial nephritis induced by bevacizumab therapy for glioblastoma multiforme. J Oncol Pharm Pract [Internet]. 2013. [cited 2017December7];19:365–8. Available from: http://journals.sagepub.com/doi/10.1177/1078155212466421 doi: 10.1177/1078155212466421. [DOI] [PubMed] [Google Scholar]
- 27.Gurevich F, Perazella MA. Renal Effects of Anti-angiogenesis Therapy: Update for the Internist. Am J Med [Internet]. 2009;122:322–8. Available from: https://doi.org/10.1016/j.amjmed.2008.11.025 doi: 10.1016/j.amjmed.2008.11.025. [DOI] [PubMed] [Google Scholar]
- 28.Beck KE, Blansfield JA, Tran KQ, Feldman AL, Hughes MS, Royal RE, Kammula US, Topalian SL, Sherry RM, Kleiner D, et al.. Enterocolitis in patients with cancer after antibody blockade of cytotoxic T-lymphocyte-associated antigen 4. J Clin Oncol [Internet]. 2006;24:2283–9. Available from: http://www.ncbi.nlm.nih.gov/pubmed/16710025 doi: 10.1200/JCO.2005.04.5716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Downey SG, Klapper JA, Smith FO, Yang JC, Sherry RM, Royal RE, Kammula US, Hughes MS, Allen TE, Levy CL, et al.. Prognostic factors related to clinical response in patients with metastatic melanoma treated by CTL-associated antigen-4 blockade. Clin Cancer Res [Internet]. 2007;13:6681–8. Available from: http://www.ncbi.nlm.nih.gov/pubmed/17982122 doi: 10.1158/1078-0432.CCR-07-0187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ascierto PA, Simeone E, Sileni VC, Pigozzo J, Maio M, Altomonte M, Del Vecchio M, Di Guardo L, Marchetti P, Ridolfi R, et al.. Clinical experience with ipilimumab 3 mg/kg: real-world efficacy and safety data from an expanded access programme cohort. J Transl Med [Internet]. 2014;12:116 Available from: http://www.ncbi.nlm.nih.gov/pubmed/24885479 doi: 10.1186/1479-5876-12-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Horvat TZ, Adel NG, Dang T-O, Momtaz P, Postow MA, Callahan MK, Carvajal RD, Dickson MA, D'Angelo SP, Woo KM, et al.. Immune-Related Adverse Events, Need for Systemic Immunosuppression, and Effects on Survival and Time to Treatment Failure in Patients With Melanoma Treated With Ipilimumab at Memorial Sloan Kettering Cancer Center. J Clin Oncol [Internet]. 2015;33:3193–8. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26282644 doi: 10.1200/JCO.2015.60.8448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hua C, Boussemart L, Mateus C, Routier E, Boutros C, Cazenave H, Viollet R, Thomas M, Roy S, Benannoune N, et al.. Association of Vitiligo With Tumor Response in Patients With Metastatic Melanoma Treated With Pembrolizumab. JAMA dermatology [Internet]. 2016;152:45–51. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26501224 doi: 10.1001/jamadermatol.2015.2707. [DOI] [PubMed] [Google Scholar]
- 33.Sanlorenzo M, Vujic I, Daud A, Algazi A, Gubens M, Luna SA, Lin K, Quaglino P, Rappersberger K, Ortiz-Urda S. Pembrolizumab Cutaneous Adverse Events and Their Association With Disease Progression. JAMA dermatology [Internet]. 2015;151:1206–12. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26222619 doi: 10.1001/jamadermatol.2015.1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Teulings H-E, Limpens J, Jansen SN, Zwinderman AH, Reitsma JB, Spuls PI, Luiten RM. Vitiligo-like depigmentation in patients with stage III-IV melanoma receiving immunotherapy and its association with survival: a systematic review and meta-analysis. J Clin Oncol [Internet]. 2015;33:773–81. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25605840 doi: 10.1200/JCO.2014.57.4756. [DOI] [PubMed] [Google Scholar]
- 35.Freeman-Keller M, Kim Y, Cronin H, Richards A, Gibney G, Weber JS. Nivolumab in Resected and Unresectable Metastatic Melanoma: Characteristics of Immune-Related Adverse Events and Association with Outcomes. Clin Cancer Res [Internet]. 2016;22:886–94. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26446948 doi: 10.1158/1078-0432.CCR-15-1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weber JS, Hodi FS, Wolchok JD, Topalian SL, Schadendorf D, Larkin J, Sznol M, Long GV, Li H, Waxman IM, et al.. Safety Profile of Nivolumab Monotherapy: A Pooled Analysis of Patients With Advanced Melanoma. J Clin Oncol [Internet]. 2017;35:785–92. Available from: http://www.ncbi.nlm.nih.gov/pubmed/28068177 doi: 10.1200/JCO.2015.66.1389. [DOI] [PubMed] [Google Scholar]
- 37.Wang PF, Chen Y, Song SY, Wang TJ, Ji WJ, Li SW, Liu N, Yan CX. Immune-related adverse events associated with anti-PD-1/PD-L1 treatment for malignancies: A meta-analysis. Front Pharmacol. 2017;8:1–12. doi: 10.3389/fphar.2017.00730. PMID:28149278. [DOI] [PMC free article] [PubMed] [Google Scholar]


