ABSTRACT
Primary diffuse large B-cell lymphoma of the central nervous system (CNS-DLBCL) is an aggressive disease with a poor prognosis. The status of the tumor immune microenvironment in CNS-DLBCL remains unclear. We investigated the prognostic implications of tumor-associated macrophages (TAMs), regulatory T-cells (Tregs), and indoleamine 2,3-dioxygenase (IDO)+ cells in primary CNS-DLBCL (n = 114) by immunohistochemical analysis. The numbers of tumor-infiltrating immune cells, including CD68+ TAMs, CD163+ or CD204+ M2 macrophages, FOXP3+ Tregs, and IDO+ cells were all significantly lower in CNS-DLBCL versus systemic DLBCL (n = 165; all P < 0.001), but with little difference in the ratio of CD163+/CD68+ or CD204+/CD68+ cells. An increase in CD68+ cell numbers was significantly associated with prolonged progression-free survival (PFS) and overall survival in patients with CNS-DLBCL (P = 0.004 and 0.021, respectively). In contrast, an increase in CD204+ cell numbers or a higher ratio of CD204+/CD68+ cells was related to a shorter PFS (P = 0.020 and 0.063, respectively). An increase in IDO+ cell numbers was associated with a significantly longer PFS (P = 0.019). In combination, the status of low IDO+ cell numbers combined with low CD68+ cell numbers, high CD204+ cell numbers, or a high CD204+/CD68+ cell ratio all predicted poor PFS in multivariate analyses. This study showed that an increase in CD204+ cell numbers, suggestive of M2 macrophages, was associated with poor clinical outcome in CNS-DLBCL, whereas increased CD68+ or IDO+ cell numbers were related to a favorable prognosis. The analysis of tumor-infiltrating immune cells could help in predicting the prognosis of CNS-DLBCL patients and determining therapeutic strategies targeting tumor microenvironment.
KEYWORDS: diffuse large B-cell lymphoma; indoleamine 2,3-dioxygenase; M2 macrophages; primary central nervous system lymphoma; regulatoryT-cells; tumor-associated macrophages; tumor microenvironment
Introduction
Primary central nervous system lymphoma (PCNSL) is one of the most aggressive malignant lymphomas, accounting for 2–3% of non-Hodgkin lymphoma (NHL) cases. More than 90% of PCNSLs are diffuse large B-cell lymphoma (DLBCL), which is a clinicopathologically unique entity, distinct from systemic DLBCL.1 Previous studies have demonstrated that primary CNS-DLBCL has a distinct gene expression signature and mutation status,2–6 including features involved in CNS tropism, B-cell migration, activated B cell-like (ABC) subtype, and activation of B-cell receptor and toll-like receptor signaling. Patients with primary CNS-DLBCL often show refractoriness to chemotherapy and aggressive clinical behavior and poor clinical outcomes,1,7 demanding novel therapeutic approach and risk stratification. Recently, immunotherapy targeting the programmed cell death-1 (PD-1)/PD-1 ligand (PD-L) immune checkpoint pathway has shown clinical benefit in patients with some malignant lymphomas.8 Of note, primary CNS-DLBCL frequently showed alterations in PD-L genes and increased expression of PD-Ls, and PD-1 blockade demonstrated efficacy in patients with primary CNS-DLBCL.6,9 Thus, understanding the tumor immune microenvironment of primary CNS-DLBCL is of importance.
The tumor immune microenvironment is complex and plays an important role in the development and progression of tumors.10 Immunosuppressive mechanisms of tumors include cellular components, such as regulatory T cells (Tregs), myeloid-derived suppressor cells, and M2 macrophages, immunosuppressive cytokines, metabolic enzymes, such as indoleamine 2,3-dioxygenase (IDO), and immune checkpoints, mediated via receptor-ligand interaction.10,11 In many solid tumors, an immunosuppressive microenvironment has been associated with tumor aggressiveness and poor clinical outcome.11 However, the prognostic implications of the immune microenvironment in lymphomas has been conflicting, likely attributed to the fact that lymphoma cells can be influenced by the immunosuppressive mechanisms.11,12 Tumor-associated macrophages (TAMs) produce growth factors, cytokines, and proteases and contribute to tumor initiation and progression.13 Macrophages are classified into two lineages, M1 and M2, which differ in phenotype and function.14 M1 macrophages produce pro-inflammatory cytokines and function primarily as effecter cells that kill invading pathogens. They also exhibit tumor-suppressive functions and stimulate anti-tumor immune responses. In contrast, M2 macrophages expressing the mannose receptor and scavenger receptors (e.g., CD163 and CD204) promote tumor cell survival, invasion, metastasis, and angiogenesis. M2 macrophages also play an immunosuppressive role by downregulating the anti-tumor immune responses of M1 and Th1 cells and recruiting and activating Tregs and Th2 cells.14
In solid tumors, tumor-infiltrating Tregs hamper effective anti-tumor immunity, thereby contributing to tumor progression and poor prognosis in patients.15 However, in several hematolymphoid malignancies, increased quantities of tumor-infiltrating Tregs were associated with favorable clinical outcomes.12,16–20 IDO is a tryptophan-catabolizing enzyme expressed by diverse cells, including myeloid, stromal, and epithelial cells, and functions as a metabolic regulator of immune responses.21,22 IDO exerts its immunosuppressive effects by depleting local tryptophan stores and producing kynurenine, a tryptophan metabolite.23 Tryptophan depletion inhibits the clonal expansion of T cells, leads to T-cell anergy and apoptosis,24 promotes the conversion of naive CD4+ T cells to Tregs, and activates Tregs function.25
Taken together, it seems conceivable that crosstalk among TAMs, Tregs and IDO may affect the constitution of the tumor immune microenvironment and biology of tumor. The CNS microenvironment is unique in that it is in many ways an immunologically privileged site. The blood brain barrier (BBB) limits the entry of immune cells and immune mediators into the CNS.26 However, the immune microenvironment of primary CNS-DLBCL remains unclear. Thus, here, we analyzed the statuses of TAMs, M2 macrophages, Tregs, and IDO in primary CNS-DLBCL to investigate their prognostic implications.
Results
Characteristics of patients with primary CNS-DLBCL
The clinicopathological characteristics of the patients with primary CNS-DLBCL are summarized in Table 1. The male to female ratio was 1.7:1, and the age of the patients ranged from 10 to 82 (mean, 58.7±13.9; median 61) years. Most patients had an Eastern Cooperative Oncology Group (ECOG) performance status of 0, 1 (65.8%), a lack of B symptoms (97.4%), involvement deep brain structures (72.6%), multifocal diseases (61.1%), and ABC immunophenotype (84.8%). The International Extranodal Lymphoma Study Group (IELSG) prognostic index and Nottingham/Barcelona score were 0–2 in 56.1% of patients and 0–2 in 83.3% of patients, respectively. MYD88 mutation (all L265P mutations) was observed in 38.1% and CD79B mutation (all involving Y196) was observed in 23%, of which 52.2% had concomitant MYD88 mutation. Most patients were treated with high-dose methotrexate-containing regimens including combined high-dose methotrexate, vincristine and procarbazine chemotherapy (MVP) (57.9%) or high-dose methotrexate (17.5%).
Table 1.
Clinicopathological features of patients with primary CNS-DLBCL
| Variables |
|
No. (%) |
| Age | ≤60 yr | 53 (46.5) |
| >60 yr | 61 (53.5) | |
| Sex | Male | 71 (62.3) |
| Female | 43 (37.7) | |
| Initial symptom* | H & V | 27 (25.0) |
| Seizure | 7 (6.5) | |
| Neurologic deficit | 74 (68.5) | |
| ECOG performance status | 0, 1 | 75 (65.8) |
| ≥ 2 | 39 (34.2) | |
| B symptom | Absent | 111 (97.4) |
| Present | 3 (2.6) | |
| Serum LDH* | Normal | 67 (62.6) |
| Elevated | 40 (37.4) | |
| CSF cytology* | Negative | 84 (87.5) |
| Positive | 12 (12.5) | |
| CSF protein* | Normal | 45 (48.9) |
| Elevated | 47 (51.1) | |
| Ocular disease* | Absent | 101 (89.4) |
| Present | 12 (10.6) | |
| Involvement of deep structures†,* | Absent | 31 (27.4) |
| Present | 82 (72.6) | |
| Extent of disease* | Unifocal | 44 (38.9) |
| Multifocal | 69 (61.1) | |
| IELSG prognostic index | 0 ∼ 2 | 64 (56.1) |
| 3 ∼ 5 | 50 (43.9) | |
| Nottingham/Barcelona score | 0 ∼ 2 | 95 (83.3) |
| 3 | 19 (16.7) | |
| Radiation | Not done | 40 (35.1) |
| Done | 74 (64.9) | |
| Chemotherapy | MVP | 66 (57.9) |
| HD-MTX | 20 (17.5) | |
| Others†† | 5 (4.4) | |
| No chemotherapy | 23 (20.2) | |
| Rituximab | Not done | 104 (91.2) |
| Done | 10 (8.8) | |
| IT-MTX | Not done | 91 (79.8) |
| Done | 23 (20.2) | |
| Immunophenotype* | GCB | 14 (15.2) |
| ABC | 78 (84.8) | |
| MYD88 mutation* | Absent | 52 (61.9) |
| Present | 32 (38.1) | |
| CD79B mutation* | Absent | 77 (77.0) |
| Present | 23 (23.0) | |
| - concomitant with MYD88 mutation | 12/23 (52.2) |
No., number; H&V, Headache and Vomiting; ECOG, Eastern Cooperative Oncology Group; LDH, lactate dehydrogenase; CSF, cerebrospinal fluid; IELSG, International Extranodal Lymphoma Study Group; MVP, combined chemotherapy regimen of high-dose methotrexate, vincristine and procarbazine; HD-MTX, high-dose methotrexate; IT-MTX, intrathecal methotrexate; GCB, germinal center B cell-like; ABC, activated B cell-like;
Involvement of deep structures of the brain, i.e., basal ganglia and/or corpus callosum and/or brain stem and/or cerebellum.;
Others of chemotherapy includes CHOP, COPADM, etc.;
These variables contain missing values that lacked information about variables.
Quantitative analysis of tumor-infiltrating CD68+, CD163+, and CD204+ TAMs, FOXP3+ Tregs, and IDO+ cells in primary CNS-DLBCL
CD68, CD163, and CD204 immunostaining showed a cytoplasmic and/or membranous pattern in cells presumed to be macrophages (Fig. 1A-F). The mean numbers of tumor-infiltrating CD68+, CD163+, and CD204+ cells in primary CNS-DLBCL were 145.42±70.55 (range, 5.67–385.00; median, 132.00), 149.67±67.76 (range, 21.00–282.67; median, 146.33), and 65.51±61.64 (range, 2.00–278.00; median, 42.00) per unit area, respectively. The mean ratios of CD163+/CD68+ cells and CD204+/CD68+ cells were estimated to be 1.32±1.76 (range, 0.19–17.47; median, 1.06) and 0.46±0.42 (range, 0.02–3.06; median, 0.36), respectively. Overall, the numbers of CD68+ versus CD163+ cells CD68+ versus CD204+ cells, and CD163+ versus CD204+ cells showed significant positive correlations with each other (R = 0.416, 0.552, and 0.656, respectively; all P < 0.001; Fig. 2).
Figure 1.
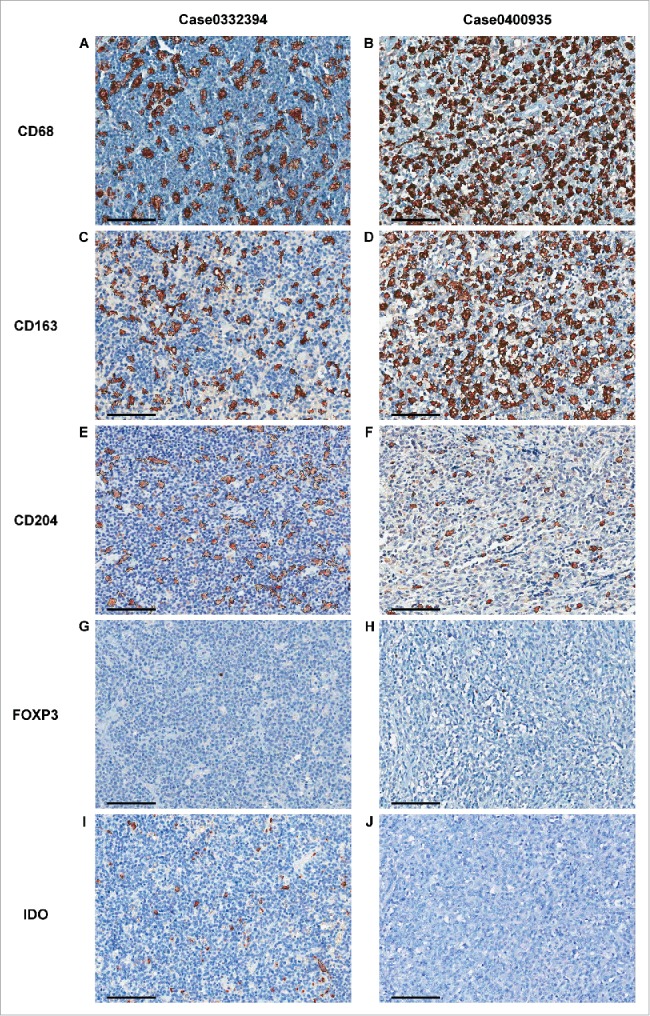
Representative images from the automated enumeration of tumor-infiltrating CD68+, CD163+, CD204+, FOXP3+, and IDO+ cells. Representative images of immune cells from two patients with primary CNS-DLBCL are demonstrated. CD68, CD163, and CD204 were expressed in a granular cytoplasmic pattern by macrophages. FOXP3 showed a nuclear pattern in small lymphoid cells. IDO was expressed in a granular cytoplasmic pattern by suspected macrophages, dendritic cells, small plasmacytoid dendritic cells, and vascular endothelial cells. Images were captured by virtual microscopy and submitted to an image analyzer, which delineated the positive cells by thin black lines, as seen in (A−F), (I) and (J). In the first case, the counts of CD68+ cells (A), CD163+ cells (C), and CD204+ cells (E) were 134, 115, and 115, respectively, per unit area (0.28 mm2). The count of FOXP3+ cells was 1 per unit area (0.28 mm2) (G). The count of IDO+ cells was 75 per unit area (0.28 mm2) (I). In the second case, the counts of CD68+ cells (B), CD163+ cells (D), and CD204+ cells were 294, 257, and 57, respectively, per unit area (0.28 mm2). The count of FOXP3+ cells in this case was 11 per unit area (0.28 mm2) (H). No IDO+ cell was observed in this case (J). (Scale bar, 100 μm, in all images).
Figure 2.
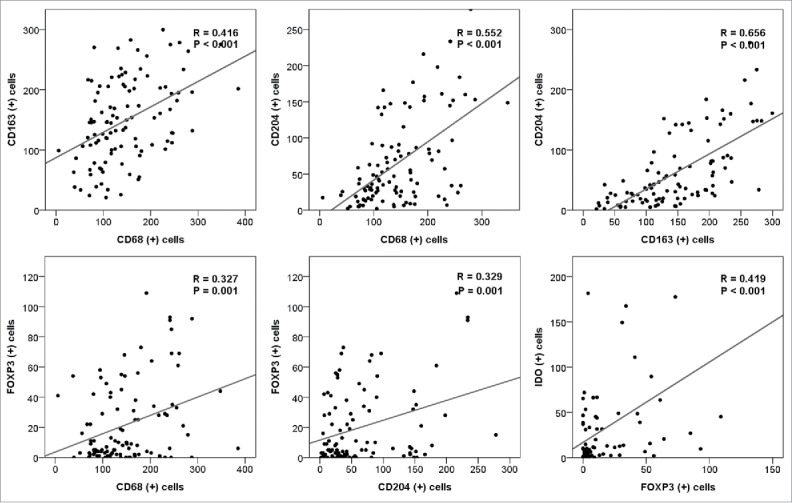
Correlations between the tumor-infiltrating CD68+, CD163+, CD204+, FOXP3+, and IDO+ cells in primary CNS-DLBCL. The counts of CD68+, CD163+, CD204+, FOXP3+, and IDO+ cells for each case were plotted, and correlations between the values were analyzed.
FOXP3 immunostaining was detected in the nuclei of tumor-infiltrating small lymphocytes (Fig. 1G-H). The mean number of FOXP3+ cells per unit area was 21.44±26.24 (range, 0.00–109.0; median, 8.5). The number of FOXP3+ cells showed a positive correlation with the number of CD68+ and CD204+ cells (R = 0.327 and 0.329, respectively; both P = 0.001; Fig. 2), but no correlation with the number of CD163+ cells (Supplementary Fig. S1).
IDO was not expressed in tumor cells. Based on morphology and double immunostaining in representative cases (Supplementary Fig. S2), the IDO+ cells were suspected to be mostly macrophages, dendritic cells, plasmacytoid dendritic cells, or vascular endothelial cells (Fig. 1I-J). Overall, the mean number of IDO+ cells per unit area was 30.88±53.21 (range, 0–340.0; median, 10.0). The number of IDO+ cells was positively correlated with the numbers of FOXP3+ cells and CD68+ cells (R = 0.419; P < 0.001 and R = 0.217; P = 0.058, respectively; Fig. 2 and Supplementary Fig. S1).
Comparative analysis of tumor-infiltrating CD68+, CD163+, and CD204+ TAMs, FOXP3+ Tregs, and IDO+ cells between primary CNS-DLBCL and systemic DLBCL
To compare the numbers of tumor-infiltrating immune cells between primary CNS-DLBCL and systemic DLBCL, systemic DLBCL data from our previous study were used.27, 28 The numbers of tumor-infiltrating CD68+ TAMs, CD163+ or CD204+ M2 TAMs, FOXP3+ Tregs, and IDO+ cells were all significantly lower in primary CNS-DLBCL than in systemic DLBCL (all P < 0.001; Table 2). However, there was no significant difference in the ratio of CD163+/CD68+ cells or CD204+/CD68+ cells between primary CNS-DLBCL and systemic DLBCL.
Table 2.
Comparison of tumor-infiltrating CD68+ cells, CD163+ cells, CD204+ cells, FOXP3+ cells, and IDO+ cells between primary CNS-DLBCL and systemic DLBCL
| Variables |
Primary CNS (n = 114) |
Systemic (n = 165) |
P |
| Age†† (n (%)) | |||
| ≤60 years | 53 (46.5) | 68 (41.2) | n.s. |
| > 60 years | 61 (53.5) | 97 (58.8) | |
| Sex†† (n (%)) | |||
| Male | 71 (62.3) | 91 (55.2) | n.s. |
| Female | 43 (37.7) | 74 (44.8) | |
| ECOG performance status* (n (%)) | |||
| 0, 1 | 75 (65.8) | 125 (76.2) | 0.059 |
| ≥ 2 | 39 (34.2) | 39 (23.8) | |
| B symptoms* (n (%)) | |||
| Absent | 111 (97.4) | 125 (77.6) | <0.001 |
| Present | 3 (2.6) | 36 (22.4) | |
| LDH* (n (%)) | |||
| Normal | 67 (62.6) | 64 (43.5) | 0.003 |
| Elevated | 40 (37.4) | 83 (56.5) | |
| Immunophenotype by the Hans classification* (n (%)) | |||
| GCB | 14 (15.2) | 58 (35.8) | <0.001 |
| ABC | 78 (84.8) | 104 (64.2) | |
| CD68† | |||
| mean ± SD | 145.42 ± 70.55 | 239.18 ± 77.96 | <0.001 |
| CD163†,* | |||
| mean ± SD | 149.67 ± 67.76 | 293.99 ± 222.19 | <0.001 |
| CD163/CD68†,* | |||
| mean ± SD | 1.32 ± 1.76 | 1.21 ± 0.92 | n.s. |
| CD204†,* | |||
| mean ± SD | 65.51 ± 61.64 | 122.44 ± 104.20 | <0.001 |
| CD204/CD68†,* | |||
| mean ± SD | 0.46 ± 0.42 | 0.51 ± 0.43 | n.s. |
| FOXP3†,* | |||
| mean ± SD | 21.44 ± 26.24 | 96.18 ± 109.95 | <0.001 |
| IDO†,* | |||
| mean ± SD | 30.88 ± 53.21 | 112.64 ± 117.27 | <0.001 |
SD, standard deviation; ECOG, Eastern Cooperative Oncology Group; LDH, lactate dehydrogenase; GCB, germinal center B cell-like; ABC, activated B cell-like; n.s., not significant;
These variables were compared using Student's t-test;
These variables were compared using χ2 tests.
These variables contain missing values.
Relationships between clinicopathological features and the numbers of TAMs, M2 macrophages, Tregs, and IDO+ cells in primary CNS-DLBCL
The associations between clinicopathological features and the number of tumor-infiltrating immune cells are summarized in Supplementary Table S1. Briefly, the CD163+ cells was higher in patients with advanced IELSG prognostic index (P = 0.013). The number of FOXP3+ cells was much lower in patients with a higher ECOG performance status (P = 0.039). IDO+ cells were also decreased in number in patients with multifocal diseases (P = 0.002) and in those with involvement of deep structure (P = 0.043). Otherwise, there were no significant associations between the status of tumor-infiltrating immune cells and other clinicopathological and genetic features.
Numbers of tumor-infiltrating TAMs, M2 macrophages, Tregs, and IDO+ cells and the survival of patients with primary CNS-DLBCL
In total, 104 patients with primary CNS-DLBCLs who did not receive rituximab were classified into two groups according to the quantity and status of tumor-infiltrating immune cells, as described in the Materials and Methods, and subjected to a survival analysis. In a Kaplan-Meier analysis, patients with increased CD68+ cell numbers showed better progression-free survival (PFS) (P = 0.004; Fig. 3A) and overall survival (OS) (P = 0.021; Supplementary Fig. S3A). In contrast, an increased number of CD204+ cells and a higher ratio of CD204+/CD68+ cells were associated with worse PFS (P = 0.020 and 0.063, respectively; Fig. 3B, C). Patients with a higher ratio of CD163+/CD68+ cells also showed significantly poor OS (P = 0.029; data not shown). An increased number of FOXP3+ cells tended to be related to prolonged OS (P = 0.057; Supplementary Fig. S4B). An increased number of IDO+ cells was associated with significantly longer PFS (P = 0.019; Fig. 3D) and tended to be related to prolonged OS (Supplementary Fig. S3D).
Figure 3.
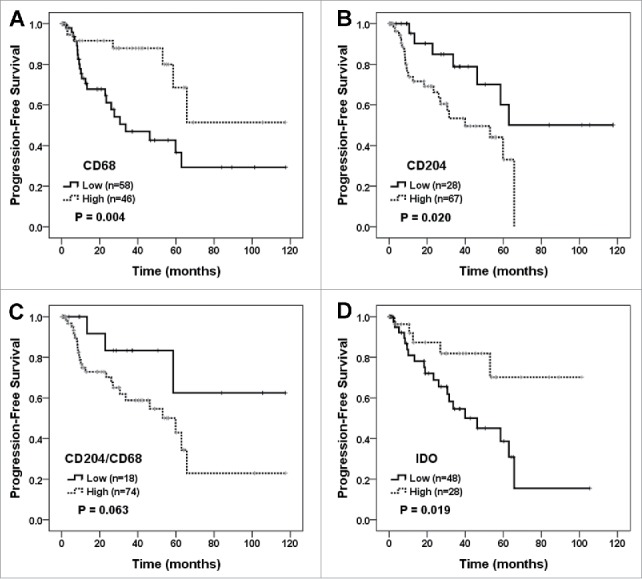
Survival analysis according to the numbers of tumor-infiltrating CD68+, CD204+, and IDO+ cells and the ratio of CD204+/CD68+ cells. PFS of primary CNS-DLBCL patients were evaluated according to the tumor-infiltrating CD68+ cell number (A), CD204+ cell number (B), the ratio of CD204+/CD68+ cells (C), and IDO+ cell number (D). Kaplan-Meier curves are shown with P values generated by log-rank test.
When considering the quantities of IDO+ cells and TAMs (Fig. 4), patients with a low number of IDO+ cells combined with a low number of CD68+ cells, a high number of CD204+ cells, or a high ratio of CD204+/CD68+ cells showed the worst PFS (P < 0.001, = 0.002, and 0.002, respectively; Fig. 4A, C, E). The prognostic significance of the combination of IDO+ with TAM or CD204+ cell numbers was maintained in patients treated with combined MVP and radiotherapy (Fig. 4B, D, F). Patients with high numbers of both IDO+ cells and FOXP3+ cells showed a tendency towards better PFS and OS (Supplementary Fig. S4C, D). The above associations between TAMs, M2 macrophages, Tregs, IDO+ cells and the survival of patients with primary CNS-DLBCL were maintained in ABC group (Supplementary Fig. S6).
Figure 4.
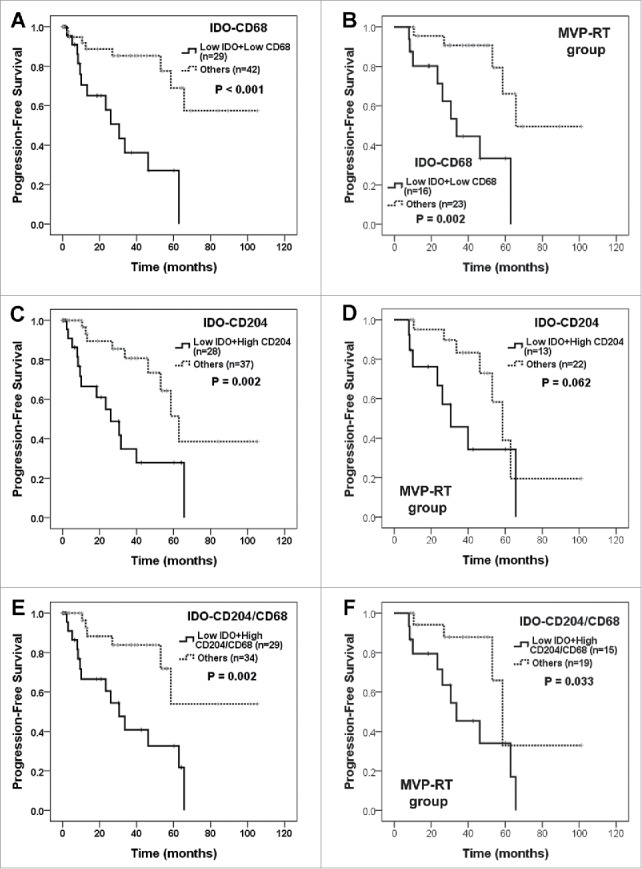
Survival analysis of primary CNS-DLBCL patients according to the combination of IDO+, CD68+, and CD204+ cell numbers and the CD204+/CD68+ cell ratio. Primary CNS-DLBCL patients were classified into two groups: (A and B) low IDO+ and CD68+ cell numbers versus others, (C and D) low IDO+ and high CD204+ cell numbers versus others, (E and F) low IDO+ cell number and high ratio of CD204+/CD68+ cells versus others. Kaplan-Meier curves for PFS in all patients (A, C and E) and in those treated with combined MVP and radiotherapy (MVP-RT) (B, D and F) are shown with P values generated by log-rank test.
In an independently evaluated validation cohort (n = 40) composed of patients with primary CNS-DLBCL and homogeneously treated with rituximab-MVP (Supplementary Table S2), comparable findings were observed in terms of prognostic significance of CD68+, CD204+ and IDO+ cell counts and the ratio of CD204+/CD68+ cells (Fig. 5 and Supplementary Fig. S7). Briefly, increased CD68+ cell numbers were related with better PFS (P = 0.030; Fig. 5A), whereas a higher ratio of CD204+/CD68+ cells was associated with worse PFS (P = 0.017; Fig. 5C). Patients with high CD204+ cells or low IDO+ cells also tended to have poor prognosis, although statistical significance was not reached (Fig. 5B, D).
Figure 5.
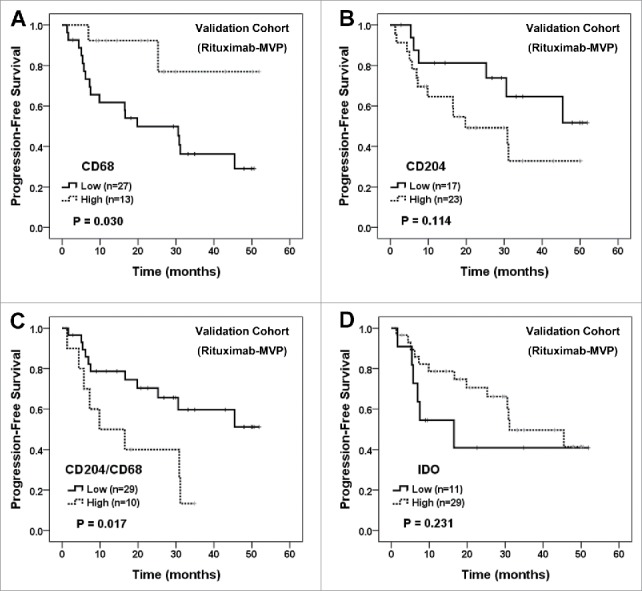
Survival analysis of independent validation cohort of primary CNS-DLBCL patients treated with rituximab-MVP according to CD68+, CD204+, and IDO+ cells and the ratio of CD204+/CD68+ cells. PFS of patients were evaluated according to the tumor-infiltrating CD68+ cell number (A), CD204+ cell number (B), the ratio of CD204+/CD68+ cells (C), and IDO+ cell number (D). Kaplan-Meier curves are shown with P values generated by log-rank test.
Multivariate survival analysis of tumor-infiltrating TAMs, M2 TAMs, Tregs, and IDO+ cells in patients with primary CNS-DLBCL
To further examine the prognostic implications of TAMs, M2 TAMs, and IDO+ cells in primary CNS-DLBCL, we performed univariate and multivariate survival analyses using the Cox proportional hazard model. As summarized in Supplementary Table S3, univariate Cox analysis revealed that the numbers of CD68+ cells and CD204+ cells, the ratio of CD204+/CD68+ cells, and the number of IDO+ cells were predictive of PFS. In multivariate Cox regression analysis, integrating risk factors including the LDH level and multifocal disease, a low CD68+ cell number, a high CD204+ cell number, and a low IDO+ cell number were independent predictors of poor PFS (P = 0.017, 0.032, and 0.035, respectively; Supplementary Table S4). When incorporating the Nottingham/Barcelona score, a low CD68+ cell number and low IDO+ cell number were independent poor prognostic factors for poor PFS (P = 0.011 and 0.030, respectively; Supplementary Table S4).
When incorporating the numbers of IDO+ cells and CD68+ or CD204+ cells, a low IDO+ cell number combined with a low CD68+ cell number, high CD204+ cell number, or a high CD204+/CD68+ cell ratio was found to be an independent predictor of poor PFS (Table 3).
Table 3.
Multivariate analysis of PFS according to clinicopathological parameters and combined IDO+ cell and TAM numbers in patients with primary CNS-DLBCL
| PFS |
|||
| Variables |
HR |
95% CI |
P |
| Low IDO+ and CD68+ cell numbers | |||
| Comparison with risk factors | |||
| Normal LDH | 3.586 | 1.187–10.837 | 0.024 |
| Multifocal disease | 1.091 | 0.370–3.215 | 0.875 |
| Low IDO+ and CD68+ cell numbers | 7.618 | 2.506–23.160 | <0.001 |
| High CD204+ cell numbers | 3.223 | 1.043–9.958 | 0.042 |
| Comparison with Nottingham/Barcelona score | |||
| Nottingham/Barcelona score 3 | 3.785 | 1.170–12.246 | 0.026 |
| Low IDO+ and CD68+ cell numbers | 6.276 | 2.324–16.945 | <0.001 |
| High CD204+ cell numbers | 2.151 | 0.772–5.993 | 0.143 |
| Low IDO+ and high CD204+ cell numbers | |||
| Comparison with risk factors | |||
| Normal LDH | 2.923 | 1.017–8.399 | 0.046 |
| Multifocal disease | 1.542 | 0.474–5.018 | 0.472 |
| Low IDO+ and high CD204+ cell numbers | 3.429 | 1.143–10.287 | 0.028 |
| Low CD68+ cell numbers | 3.156 | 1.075–9.267 | 0.037 |
| Comparison with Nottingham/Barcelona score | |||
| Nottingham/Barcelona score 3 | 4.016 | 1.244–12.971 | 0.020 |
| Low IDO+ and high CD204+ cell numbers | 3.251 | 1.314–8.045 | 0.011 |
| Low CD68+ cell numbers | 3.329 | 1.168–9.484 | 0.024 |
| Low IDO+ cell number and high CD204+/CD68+ cell ratio | |||
| Comparison with risk factors | |||
| Normal LDH | 3.213 | 1.129–9.146 | 0.029 |
| Multifocal disease | 1.369 | 0.486–3.856 | 0.552 |
| Low IDO+ cell number and high CD204+/CD68+ cell ratio | 4.290 | 1.529–12.040 | 0.006 |
| Comparison with Nottingham/Barcelona score | |||
| Nottingham/Barcelona score 3 | 5.187 | 1.609–16.716 | 0.006 |
| Low IDO+ cell number and high CD204+/CD68+ cell ratio | 4.730 | 1.786–12.524 | 0.002 |
PFS, progression-free survival; HR, hazard ratio; CI, confidence interval; LDH, lactate dehydrogenase.
Discussion
In this study, we demonstrated that the numbers of tumor-infiltrating immune cells affect the clinical outcome of patients with primary CNS-DLBCL. Briefly, an increased number of CD68+ TAMs and an increased number of IDO+ cells were associated with a favorable prognosis, whereas an increased number of CD204+ cells and a high ratio of CD204+/CD68+ cells, suggestive of M2 polarization, were associated with a poor prognosis in primary CNS-DLBCL.
In this study, the numbers of tumor-infiltrating immune cells, including CD68+ TAMs, CD163+ or CD204+ M2 TAMs, FOXP3+ Tregs, and IDO+ cells, were significantly lower in primary CNS-DLBCL than in systemic DLBCL. Supporting this, it was recently reported that infiltration of immune cells, including dendritic cells and effector or cytotoxic T cells, was decreased in CNS-DLBCL compared with non-CNS-DLBCL.29 The BBB separates the CNS from the systemic circulation, and thus the recruitment of circulating immune cells is relatively restricted,26 which may account for the decreased number of tumor-associated immune cells in primary CNS-DLBCL. However, the ratio of CD163+/CD68+ cells or CD204+/CD68+ cells, suggestive of M2 polarization of TAMs, was not altered compared with systemic DLBCL, suggesting that the qualitative composition of TAMs may actually be similar between primary CNS and systemic DLBCLs.27,28
In chronic lymphocytic leukemia (CLL), MYD88-mutated CLL cells secreted higher levels of cytokines, involving recruitment of macrophages and T cells, upon toll-like receptor stimulation, compared with MYD88-unmutated CLL cells.30 Although the frequencies of MYD88 and CD79B mutation detected by direct sequencing using formalin-fixed, paraffin-embedded (FFPE) tissue in this study were lower than those previously reported in PCNSL,6 the statuses of tumor-infiltrating immune cells were not significantly different according to the MYD88 and CD79B mutation status in primary CNS-DLBCL.
Increases in TAM numbers have been associated with poor prognoses in patients with solid tumors.13 This prognostic relationship was also observed in several types of malignant lymphomas, including follicular lymphoma and Hodgkin lymphoma.31,32 We previously reported that an increased CD163+ cell number and a high CD163+/CD68+ cell ratio predicted a poor prognosis in patients with systemic DLBCL treated with R-CHOP.27 An increase in the IDO+ cell number was associated with a favorable prognosis in patients with systemic DLBCL.28 Moreover, an integrative analysis of IDO and TAM statuses revealed that a low IDO+ cell count combined with either a high CD163+ cell count or a high CD163+/CD68+ cell ratio was a strong independent predictor of a poor prognosis in systemic DLBCL.28 In this study, an increased CD204+ cell number and a higher ratio of CD204+/CD68+ cells were predictors of a poor prognosis in patients with primary CNS-DLBCL. These findings indicate that M2 TAMs may contribute to lymphoma progression and, consequently, poor prognosis in patients with primary CNS-DLBCL. However, the M2 marker having prognostic significance was different between primary CNS-DLBCL (CD204) and systemic DLBCL (CD163), suggesting a need to use multiple markers when evaluating TAMs. In an intracranial B-cell lymphoma xenograft model, expression of interleukin-4 by malignant B cells potentiated B-cell survival and mediated the polarization of TAMs to M2 TAMs, which was associated with enhanced tumorigenesis.33 In contrast, a favorable prognostic implication of increased CD68+ TAMs might suggest their lymphoma-suppressive function in primary CNS-DLBCL, a finding consistently observed in systemic DLBCL.27 Together, these findings suggest that both the phenotype and number of TAMs may influence the prognosis of patients with primary CNS-DLBCL. Targeting TAM using various approaches are under investigation for cancer therapy, including depletion of TAM, suppression of M2 polarization, and repolarization of TAM from M2 to M1 phenotype.34 Moreover, reprogramming of M2 TAM into M1 TAM enhanced the efficacy of immune checkpoint blockade in preclinical model.35 This study support that targeting M2 TAM may have clinical relevance combined with conventional therapy or immunotherapy in primary CNS-DLBCL.
In a study using NHL tissues, FOXP3 levels were increased markedly alongside elevated IDO levels, suggesting that upregulation of IDO in NHL tissues induces local immune tolerance by favoring development and infiltration of FOXP3+ Tregs.36 Consistently, in this study, the numbers of IDO+ cells and Tregs showed a positive correlation with each other in primary CNS-DLBCL. IDO contributed to tumor progression and metastasis in vivo, and increased IDO expression was associated with a poor prognosis in patients with solid tumors.37 In this study, an increased number of tumor-infiltrating IDO+ cells was associated with a favorable prognosis in patients with primary CNS-DLBCL. Consequently, a decrease in the IDO+ cell number and a concomitant increase in the CD204+ M2 macrophage number in the tumor microenvironment were significant predictors of a poor prognosis in patients with primary CNS-DLBCL. These findings were also consistent with those observed in systemic DLBCL.28 The seemingly paradoxical association of increased IDO+ cell numbers with a favorable prognosis in primary CNS-DLBCL may be explained by previous reports that IDO can suppress B cells in addition to T cells and induce growth arrest and apoptosis of lymphoma B cells.38,39 This hypothesis needs to be addressed by further in vitro and in vivo studies.
Only a few previous studies have reported the status of tumor-infiltrating immune cells in primary CNS-DLBCL. In one, the numbers of CD68+, CD163+, and CD204+ TAMs in primary CNS-DLBCL were not correlated with prognosis.40 However, decreased numbers of tumor-infiltrating S100+ dendritic cells and granzyme B+ cytotoxic T cells were related to poor prognoses in patients with PCNSL,29 suggesting a prognostic role for the tumor microenvironment in PCNSL. Our present study also suggests that TAMs, Tregs, and IDO may influence the biology of primary CNS-DLBCL cooperatively and have prognostic implications. Furthermore, our study has some additional merits in that a relatively large number of patients was included, automatic cell counting was used rather than manual counting, and diverse tumor-infiltrating immune cells were analyzed together. Of note, the combined status of immune cells, rather than their individual statuses, showed stronger association with prognosis in primary CNS-DLBCL. Specifically, combined low IDO+ with low CD68+ cell numbers and a combined low IDO+ cell number with high CD204+/CD68+ cell ratio were related with a poor prognosis.
This study has some limitations. First, it was a retrospective study, and thus the treatment modalities of the patients were not homogeneous. However, subgroup analyses according to treatment modality demonstrated that the prognostic significance of TAMs, M2 macrophages, and IDO+ cells was maintained; particularly, combined low IDO+ with low CD68+ cell numbers and a combined low IDO+ cell numbers with high CD204+/CD68+ cell ratio were significantly associated with poor prognoses in patients homogeneously treated with combined MVP chemotherapy and radiotherapy (Fig. 4 and Supplementary Fig. S5). Moreover, in an independently evaluated validation cohort of patients treated with rituximab-MVP, low CD68+ cell numbers and high CD204+/CD68+ cell ratio were significantly related with poor prognosis (Fig. 5). Second, the prognostic significance of immune cells was observed mainly in terms of PFS, not OS, which may restrict the value of immune cells as a prognostic marker but instead help establish management strategies for patients. Third, some of the patients were administered with steroid before biopsy/surgery, which could affect the viability of tumor cells and immune responses. Fourth, because we did not evaluate the entire area of tumor due to small biopsied samples and capturing representative areas, the heterogeneous distribution of infiltrating immune cells in the tumor could not be fully addressed in this study.
In summary, a decreased number of CD68+ cells, an increased number of CD204+ cells or an increased ratio of CD204+/CD68+ cells, and a decreased number of IDO+ cells were associated with a poor prognoses in patients with primary CNS-DLBCL. An evaluation of the combined statuses of IDO and TAM may be helpful for risk stratification of patients with primary CNS-DLBCL. This study also provides valuable information for developing therapeutic strategies targeting TAMs and IDO in patients with primary CNS-DLBCL.
Materials and methods
Patients
In total, 114 patients who were diagnosed with primary CNS-DLBCL at Seoul National University Hospital (SNUH) between 1996 and 2012 were included in this study. Pathological materials were reviewed by hematopathologists (NSJ, YKJ, and CWK) according to current World Health Organization criteria.41 Clinical data were obtained from the medical records, reviewed by hemato-oncologists (EL, TMK, and DSH). The follow-up periods ranged from 0.2 to 178 months, with a median of 31.35 months.
As an independent validation cohort, a total of 40 patients who were diagnosed at SNUH between 2013 and 2015, and homogeneously treated with rituximab-MVP, were collected. The age of patients ranged from 30 to 81 years and the follow-up duration was from 3 to 55.43 months.
This study followed the World Medical Association Declaration of Helsinki recommendations and the institutional review board at SNUH approved this study (No. 1012-053-344).
Immunohistochemistry
Whole sections of representative FFPE tumor tissue blocks were submitted for immunohistochemistry. The immunohistochemical subgroup of DLBCL was determined to be ABC or GCB type according to Hans' criteria.42 CD68 was used as a marker of TAMs, CD163 or CD204 as a marker of M2 macrophages, and FOXP3 as a marker of Tregs. Immunostaining for CD68 (PG-M1, DakoCytomation, Copenhagen, Denmark), CD163 (10D6, Novocastra, Newcastle Upon Tyne, UK), CD204 (SRA-E5, Transgenic, Kumamoto, Japan) and IDO (1F8.2; Millipore, Billerica, MA, USA) was performed using the Bond-Max autostainer (Leica Microsystems, Melbourne, Australia. Immunostaining for FOXP3 (236 A/E7, Abcam, Cambridge, UK) was performed using the BenchMark XT autostainer (Ventana Medical Systems, Inc., Tucson, AZ, USA).
Double immunostainings for IDO and CD68, IDO and CD204, IDO and CD163, IDO and CD123 (a marker of plasmacytoid dendritic cell) (BR4MS, Novocastra), and IDO and FOXP3 were performed using a BenchMark XT Slide automated system (Ventana Medical Systems, Inc., Tucson, AZ) in representative cases.
Automated quantitation of TAMs, Tregs and IDO+ cells by image analysis
To obtain an unbiased analysis of tumor-infiltrating immune cells with objective and reproducible data, automated enumeration of immune cells was performed using an image analyzer. Briefly, all immunostained slides were subjected to virtual microscope scanning under high-power magnification using the ScanScope CS2 eSlide (Aperio Technologies, Vista, CA, USA). Three different fields of the intratumoral area, excluding necrotic or squeezed area, were captured from virtual microscopic images, and the numbers of CD68+, CD163+, CD204+, and IDO+ cells were counted using ImageJ software (National Institutes of Health, Bethesda, MD, USA), as described previously.27 FOXP3+ cells were enumerated using the nuclear v9 algorithm of the ImageScope software (Aperio Technologies). The average numbers of positive cells per unit area (0.28 mm2) were calculated from values obtained from three areas for each case and used for further statistical analyses.
Direct sequencing for MYD88 and CD79B
Genomic DNA was extracted from 10 μm-thick sections of FFPE tumor tissue using a Maxwell 16 FFPE Plus Tissue LEV DNA Purification kit (Promega, Madison, WI, USA). MYD88 exon 5 and CD79B exon 5 were amplified by PCR using the following primers: for MYD88, forward 5′-CTGGGGTTGAAGACTGGGCT-3′ and reverse 5′-TTGGTGTAGTCGCAGACAGTGA-3′; for CD79B, forward 5′-GGGCTGGGGGACACTAACACTC-3′ and reverse 5′-TGGGTGCTCACCTACAGACCAC-3′. The PCR reactions were performed with EconoTaq PLUS GREEN 2X premix (Lucigen, Middleton, WI, USA) and conditions of 95°C for 5 min followed by 38 cycles of 95°C for 30 seconds, 60°C for 30 seconds and 72°C for 30 seconds. After purifying the PCR products, bi-directionally Sanger sequencing was performed using the ABI3730 DNA analyzer (Applied Biosystems, Carlsbad, CA, USA).
Statistical analysis
All statistical analyses were performed using SPSS (ver. 21; IBM Corp., New York, NY, USA) and R' software (ver. 2.14.0; The R Foundation for Statistical Computing). Non parametric Wilcoxon rank-sum test was used to assess differences in the numbers and ratios of immune cells according to the clinicopathological variables. For survival analysis, we fitted a Kaplan-Meier model after dichotomizing the cases into two groups by cut-off values. The values that maximized the survival difference, including OS and PFS, between the groups according to log-rank test were chosen as the cut-off values. This approach resulted in the following cut-off values: 145.00 for the CD68+ cell number, 120.00 for the CD163+ cell number, 0.81 for the CD163+/CD68+ cell ratio, 27.4 for the CD204+ cell number, 0.169 for the CD204+/CD68+ cell ratio, 24.00 for the FOXP3+ cell number, and 16.00 for the IDO+ cell number. Univariate and multivariate survival analyses were performed using Cox-proportional hazard models. A two-sided P value < 0.05 was considered statistically significant in all analyses.
Supplementary Material
Funding Statement
This work was supported by the Basic Science Research Program (grant No.: NRF-2016R1D1A1B01015964) through the National Research Foundation (NRF) funded by the Ministry of Education, Science and Technology (MEST), and SNU Invitation Program for Distinguished Scholar, Republic of Korea.
Abbreviations
- ABC
activated B cell-like
- BBB
blood brain barrier
- CSF
cerebrospinal fluid
- CNS
central nervous system
- DLBCL
diffuse large B-cell lymphoma
- ECOG
Eastern Cooperative Oncology Group
- GCB
germinal center B cell-like
- IDO
indoleamine 2,3-dioxygenase
- IELSG
International Extranodal Lymphoma Study Group
- IT-MTX
intrathecal methotrexate
- LDH
lactate dehydrogenase
- MVP
combined chemotherapy regimen of high-dose methotrexate, vincristine and procarbazine
- NHL
non-Hodgkin lymphoma
- OS
overall survival
- PCNSL
primary CNS lymphoma
- PFS
progression-free survival
- R-CHOP
combined immunochemotherapy regimen of rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisolone
- TAM
tumor-associated macrophage
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- 1.Rubenstein J, Ferreri AJ, Pittaluga S. Primary lymphoma of the central nervous system: epidemiology, pathology and current approaches to diagnosis, prognosis and treatment. Leuk Lymphoma. 2008;49(Suppl 1):43–51. doi: 10.1080/10428190802311441. PMID:18821432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tun HW, Personett D, Baskerville KA, Menke DM, Jaeckle KA, Kreinest P, Edenfield B, Zubair AC, O'Neill BP, Lai WR, et al.. Pathway analysis of primary central nervous system lymphoma. Blood. 2008;111:3200–10. doi: 10.1182/blood-2007-10-119099. PMID:18184868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rubenstein JL, Fridlyand J, Shen A, Aldape K, Ginzinger D, Batchelor T, Treseler P, Berger M, McDermott M, Prados M, et al.. Gene expression and angiotropism in primary CNS lymphoma. Blood. 2006;107:3716–23. doi: 10.1182/blood-2005-03-0897. PMID:16418334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sung CO, Kim SC, Karnan S, Karube K, Shin HJ, Nam DH, Suh YL, Kim SH, Kim JY, Kim SJ, et al.. Genomic profiling combined with gene expression profiling in primary central nervous system lymphoma. Blood. 2011;117:1291–300. doi: 10.1182/blood-2010-07-297861. PMID:21088137. [DOI] [PubMed] [Google Scholar]
- 5.Soussain C, Hoang-Xuan K. Primary central nervous system lymphoma: an update. Curr Opin Oncol. 2009;21:550–8. doi: 10.1097/CCO.0b013e3283310eb3. PMID:19684518. [DOI] [PubMed] [Google Scholar]
- 6.Chapuy B, Roemer MG, Stewart C, Tan Y, Abo RP, Zhang L, Dunford AJ, Meredith DM, Thorner AR, Jordanova ES, et al.. Targetable genetic features of primary testicular and primary central nervous system lymphomas. Blood. 2016; 127:869–81. doi: 10.1182/blood-2015-10-673236. PMID:26702065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Langner-Lemercier S, Houillier C, Soussain C, Ghesquieres H, Chinot O, Taillandier L, Soubeyran P, Lamy T, Morschhauser F, Benouaich-Amiel A, et al.. Primary CNS lymphoma at first relapse/progression: characteristics, management, and outcome of 256 patients from the French LOC network. Neuro-oncology. 2016;18:1297–303. doi: 10.1093/neuonc/now033. PMID:26951382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goodman A, Patel SP, Kurzrock R. PD-1-PD-L1 immune-checkpoint blockade in B-cell lymphomas. Nat Rev Clin Oncol. 2017;14(4):203–20. doi: 10.1038/nrclinonc.2016.168. PMID:27805626. [DOI] [PubMed] [Google Scholar]
- 9.Nayak L, Iwamoto FM, LaCasce A, Mukundan S, Roemer MGM, Chapuy B, Armand P, Rodig SJ, Shipp MA. PD-1 blockade with nivolumab in relapsed/refractory primary central nervous system and testicular lymphoma. Blood. 2017;129:3071–3. doi: 10.1182/blood-2017-01-764209. PMID:28356247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kerkar SP, Restifo NP. Cellular Constituents of Immune Escape within the Tumor Microenvironment. Cancer Res. 2012;72:3125–30. doi: 10.1158/0008-5472.CAN-11-4094. PMID:22721837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burger JA, Ghia P, Rosenwald A, Caligaris-Cappio F. The microenvironment in mature B-cell malignancies: a target for new treatment strategies. Blood. 2009;114:3367–75. doi: 10.1182/blood-2009-06-225326. PMID:19636060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coutinho R, Clear AJ, Mazzola E, Owen A, Greaves P, Wilson A, Matthews J, Lee A, Alvarez R, da Silva MG, et al.. Revisiting the immune microenvironment of diffuse large B-cell lymphoma using a tissue microarray and immunohistochemistry: robust semi- automated analysis reveals CD3 and FoxP3 as potential predictors of response to R-CHOP. Haematologica. 2015;100:363–9. doi: 10.3324/haematol.2014.110189. PMID:25425693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pollard JW. Tumour-educated macrophages promote tumour progression and metastasis. Nat Rev Cancer. 2004;4:71–8. doi: 10.1038/nrc1256. PMID:14708027. [DOI] [PubMed] [Google Scholar]
- 14.Solinas G, Germano G, Mantovani A, Allavena P. Tumor-associated macrophages (TAM) as major players of the cancer-related inflammation. J Leukoc Biol. 2009;86:1065–73. doi: 10.1189/jlb.0609385. PMID:19741157. [DOI] [PubMed] [Google Scholar]
- 15.Sakaguchi S, Miyara M, Costantino CM, Hafler DA. FOXP3(+) regulatory T cells in the human immune system. Nat Rev Immunol. 2010;10:490–500. doi: 10.1038/nri2785. PMID:20559327. [DOI] [PubMed] [Google Scholar]
- 16.Wahlin BE, Aggarwal M, Montes-Moreno S, Gonzalez LF, Roncador G, Sanchez-Verde L, Christensson B, Sander B, Kimby E. A unifying microenvironment model in follicular lymphoma: outcome is predicted by programmed Death-1-Positive, regulatory, cytotoxic, and helper T cells and macrophages. Clin Cancer Res. 2010;16:637–50. doi: 10.1158/1078-0432.CCR-09-2487. PMID:20068089. [DOI] [PubMed] [Google Scholar]
- 17.Kim WY, Jeon YK, Kim TM, Kim JE, Kim YA, Lee SH, Kim DW, Heo DS, Kim CW. Increased quantity of tumor-infiltrating FOXP3-positive regulatory T cells is an independent predictor for improved clinical outcome in extranodal NK/T-cell lymphoma. Ann Oncol. 2009;20:1688–96. doi: 10.1093/annonc/mdp056. PMID:19542249. [DOI] [PubMed] [Google Scholar]
- 18.Carreras J, Lopez-Guillermo A, Fox BC, Colomo L, Martinez A, Roncador G, Montserrat E, Campo E, Banham AH. High numbers of tumor-infiltrating FOXP3-positive regulatory T cells are associated with improved overall survival in follicular lymphoma. Blood. 2006;108:2957–64. doi: 10.1182/blood-2006-04-018218. PMID:16825494. [DOI] [PubMed] [Google Scholar]
- 19.Ahearne MJ, Bhuller K, Hew R, Ibrahim H, Naresh K, Wagner SD. Expression of PD-1 (CD279) and FoxP3 in diffuse large B-cell lymphoma. Virchows Arch. 2014;465:351–8. doi: 10.1007/s00428-014-1615-5. PMID:25011996. [DOI] [PubMed] [Google Scholar]
- 20.Tzankov A, Meier C, Hirschmann P, Went P, Pileri SA, Dirnhofer S. Correlation of high numbers of intratumoral FOXP3+ regulatory T cells with improved survival in germinal center-like diffuse large B-cell lymphoma, follicular lymphoma and classical Hodgkin's lymphoma. Haematologica. 2008;93:193–200. doi: 10.3324/haematol.11702. PMID:18223287. [DOI] [PubMed] [Google Scholar]
- 21.Munn DH, Mellor AL. Indoleamine 2,3 dioxygenase and metabolic control of immune responses. Trends Immunol. 2013;34:137–43. doi: 10.1016/j.it.2012.10.001. PMID:23103127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Platten M, Wick W, Van den Eynde BJ. Tryptophan Catabolism in Cancer: Beyond IDO and Tryptophan Depletion. Cancer Res. 2012;72:5435–40. doi: 10.1158/0008-5472.CAN-12-0569. PMID:23090118. [DOI] [PubMed] [Google Scholar]
- 23.Wilke CM, Zou WP. T lymphocytes to IDO(+) cells: check. Blood. 2011;117:2082–3. doi: 10.1182/blood-2010-12-322172. PMID:21330479. [DOI] [PubMed] [Google Scholar]
- 24.Frumento G, Rotondo R, Tonetti M, Damonte G, Benatti U, Ferrara GB. Tryptophan-derived catabolites are responsible for inhibition of T and natural killer cell proliferation induced by indoleamine 2,3-dioxygenase. J Exp Med. 2002;196:459–68. doi: 10.1084/jem.20020121. PMID:12186838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sharma MD, Baban B, Chandler P, Hou DY, Singh N, Yagita H, Azurna M, Blazar BR, Mellor AL, Munn DH. Plasmacytoid dendritic cells from mouse tumor-draining lymph nodes directly activate mature Tregs via indoleamine 2,3-dioxygenase. J Clin Invest. 2007;117:2570–82. doi: 10.1172/JCI31911. PMID:17710230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Muldoon LL, Alvarez JI, Begley DJ, Boado RJ, Del Zoppo GJ, Doolittle ND, Engelhardt B, Hallenbeck JM, Lonser RR, Ohlfest JR, et al.. Immunologic privilege in the central nervous system and the blood-brain barrier. J Cereb Blood Flow Metab. 2013;33:13–21. doi: 10.1038/jcbfm.2012.153. PMID:23072749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nam SJ, Go H, Paik JH, Kim TM, Heo DS, Kim CW, Jeon YK. An increase of M2 macrophages predicts poor prognosis in patients with diffuse large B-cell lymphoma treated with rituximab, cyclophosphamide, doxorubicin, vincristine and prednisone. Leuk Lymphoma. 2014;55:2466–76. doi: 10.3109/10428194.2013.879713. PMID:24397616. [DOI] [PubMed] [Google Scholar]
- 28.Nam SJ, Kim S, Paik JH, Kim TM, Heo DS, Kim CW, Jeon YK. An increase in indoleamine 2,3-dioxygenase-positive cells in the tumor microenvironment predicts favorable prognosis in patients with diffuse large B-cell lymphoma treated with rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisolone. Leuk Lymphoma. 2016:1–5. [DOI] [PubMed] [Google Scholar]
- 29.Chang C, Lin CH, Cheng AL, Medeiros LJ, Chang KC. Primary central nervous system diffuse large B-cell lymphoma has poorer immune cell infiltration and prognosis than its peripheral counterpart. Histopathology. 2015;67:625–35. doi: 10.1111/his.12706. PMID:25829022. [DOI] [PubMed] [Google Scholar]
- 30.Puente XS, Pinyol M, Quesada V, Conde L, Ordonez GR, Villamor N, Escaramis G, Jares P, Bea S, Gonzalez-Diaz M, et al.. Whole-genome sequencing identifies recurrent mutations in chronic lymphocytic leukaemia. Nature. 2011;475:101–5. doi: 10.1038/nature10113. PMID:21642962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Canioni D, Salles G, Mounier N, Brousse N, Keuppens M, Morchhauser F, Lamy T, Sonet A, Rousselet MC, Foussard C, et al.. High numbers of tumor-associated macrophages have an adverse prognostic value that can be circumvented by rituximab in patients with follicular lymphoma enrolled onto the GELA-GOELAMS FL-2000 trial. J Clin Oncol. 2008;26:440–6. doi: 10.1200/JCO.2007.12.8298. PMID:18086798. [DOI] [PubMed] [Google Scholar]
- 32.Steidl C, Lee T, Shah SP, Farinha P, Han G, Nayar T, Delaney A, Jones SJ, Iqbal J, Weisenburger DD, et al.. Tumor-associated macrophages and survival in classic Hodgkin's lymphoma. New Engl J Med. 2010;362:875–85. doi: 10.1056/NEJMoa0905680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kadoch C, Dinca EB, Voicu R, Chen LJ, Nguyen D, Parikh S, Karrim J, Shuman MA, Lowell CA, Treseler PA, et al.. Pathologic correlates of primary central nervous system lymphoma defined in an Orthotopic Xenograft Model. Clin Cancer Res. 2009;15:1989–97. doi: 10.1158/1078-0432.CCR-08-2054. PMID:19276270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zheng X, Turkowski K, Mora J, Brune B, Seeger W, Weigert A, Savai R. Redirecting tumor-associated macrophages to become tumoricidal effectors as a novel strategy for cancer therapy. Oncotarget. 2017;18;8(29):48436–52. doi: 10.18632/oncotarget.17061. PMID:28467800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Georgoudaki AM, Prokopec KE, Boura VF, Hellqvist E, Sohn S, Ostling J, Dahan R, Harris RA, Rantalainen M, Klevebring D, et al.. Reprogramming tumor-associated macrophages by antibody targeting inhibits cancer progression and metastasis. Cell Rep. 2016;15:2000–11. doi: 10.1016/j.celrep.2016.04.084. PMID:27210762. [DOI] [PubMed] [Google Scholar]
- 36.Liu XQ, Lu K, Feng LL, Ding M, Gao JM, Ge XL, Wang X. Up-regulated expression of indoleamine 2,3-dioxygenase 1 in non-Hodgkin lymphoma correlates with increased regulatory T-cell infiltration. Leuk Lymphoma. 2014;55:405–14. doi: 10.3109/10428194.2013.804917. PMID:23682557. [DOI] [PubMed] [Google Scholar]
- 37.Godin-Ethier J, Hanafi LA, Piccirillo CA, Lapointe R. Indoleamine 2,3-dioxygenase expression in human cancers: clinical and immunologic perspectives. Clin Cancer Res. 2011;17:6985–91. doi: 10.1158/1078-0432.CCR-11-1331. PMID:22068654. [DOI] [PubMed] [Google Scholar]
- 38.Adikari SB, Lian H, Link H, Huang YM, Xiao BG. Interferon-gamma-modified dendritic cells suppress B cell function and ameliorate the development of experimental autoimmune myasthenia gravis. Clin Exp Immunol. 2004;138:230–6. doi: 10.1111/j.1365-2249.2004.02585.x. PMID:15498031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maby-El Hajjami H, Ame-Thomas P, Pangault C, Tribut O, DeVos J, Jean R, Bescher N, Monvoisin C, Dulong J, Lamy T, et al.. Functional Alteration of the Lymphoma Stromal Cell Niche by the Cytokine Context: Role of Indoleamine-2,3 Dioxygenase. Cancer Res. 2009;69:3228–37. doi: 10.1158/0008-5472.CAN-08-3000. PMID:19276371. [DOI] [PubMed] [Google Scholar]
- 40.Komohara Y, Horlad H, Ohnishi K, Ohta K, Makino K, Hondo H, Yamanaka R, Kajiwara K, Saito T, Kuratsu J, et al.. M2 macrophage/microglial cells induce activation of Stat3 in primary central nervous system lymphoma. J Clin Exp Hematop. 2011;51:93–9. doi: 10.3960/jslrt.51.93. PMID:22104307. [DOI] [PubMed] [Google Scholar]
- 41.Swerdlow S, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J, Vardiman JW. WHO classifi cation of tumours of haematopoietic and lymphoid tissues. Lyon: IARC Press; 2008. [Google Scholar]
- 42.Hans CP, Weisenburger DD, Greiner TC, Gascoyne RD, Delabie J, Ott G, Muller-Hermelink HK, Campo E, Braziel RM, Jaffe ES, et al.. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood. 2004;103:275–82. doi: 10.1182/blood-2003-05-1545. PMID:14504078. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


