ABSTRACT
Soft tissue sarcomas (STS) have minimal expression of PD-L1, a biomarker for PD-1 therapy efficacy. Radiotherapy (RT) has been shown to increase PD-L1 expression pre-clinically. We examined the expression of PD-L1, pre- and post-RT, in 46 Stage II-III STS patients treated with pre-operative RT (50–50.4 Gy in 25–28 fractions) followed by resection. Five additional patients who did not receive RT were utilized as controls. PD-L1 expression on biopsy and resection samples was evaluated by immunochemistry using the anti PD-L1 monoclonal antibody (E1L3 N clone; Cell Signaling). Greater than 1% membranous staining was considered positive PD-L1 expression. Changes in PD-L1 expression were analyzed via the Fisher exact test. Kaplan-Meier statistics were used to correlate PD-L1 expression to distant metastases (DM) rate. The majority of STS were T2b (87.0%), high-grade (80.4%), undifferentiated pleomorphic histology (71.7%), and originated from the extremities (84.6%). Zero patients demonstrated PD-L1 tumor expression pre-RT. Post-RT, 5 patients (10.9%) demonstrated PD-L1 tumor expression (p = 0.056). Tumor associated macrophages (TAM) expression of PD-L1 increased after RT: 15.2% to 45.7% (p = 0.003). Samples from controls demonstrated no baseline (0%) or change in tumor PD-L1 expression. Freedom from DM was lower for patients with PD-L1 TAM expression post-RT (3 years: 49.7% vs. 87.8%, log-rank p = 0.006); TAM PD-L1 positivity remained an independent predictor for DM on multivariate analyses (Hazard ratio – 0.16, 95% confidence interval: 0.034–0.721, p = 0.042). PD-L1 expression on human STS tumor and TAM appears to elevate after pre-operative RT. Expression of PD-L1 on TAM after RT was associated with a higher rate of DM.
KEYWORDS: Soft Tissue Sarcoma, PD-L1, Immunotherapy, Radiation
Introduction
Immune checkpoint inhibitors are a new form of systemic therapy that “release the brakes” on the immune system, allowing enhanced ability of T cells to specifically target and kill cancer cells. Programmed death receptor 1 (PD-1) is a receptor on T cells that, when activated, sends a signal that inhibits T cell anti-neoplastic activity.1 Programmed death receptor ligand 1 (PD-L1) is the cell surface protein that activates PD-1; because it is often expressed on tumor cells, PD-L1 helps promote tumor evasion of the immune system.
Targeting PD-1 with novel antibodies has emerged as a promising anti-neoplastic therapy. Recent studies with anti-PD-1 therapy have demonstrated improved overall survival in various malignancies, including melanoma2 and non-small cell lung cancer.3 Efficacy of anti-PD-1 therapy activity appears to be correlated with PD-L1 tumor expression. Patients with elevated expression have higher response rates4 and improved overall survival5 compared to patients treated with chemotherapy. Conversely, patients with low expression have worse outcomes.3,6
In soft tissue sarcomas (STS), PD-L1 expression has been found to be relatively low compared to other tumors: in a series from Memorial Sloan Kettering, only 12% of patients (6/50) were found to express PD-L1 >1%.7 Of these 6 patients, 4 had gastrointestinal tumors, 1 had a synovial sarcoma, and 1 had a radiation-induced sarcoma. There was no clear correlation demonstrated between PD-L1 expression and tumor type, size, or grade. In corroboration, a recent study by Torabi, et al. demonstrated a lack of PD-L1 expression in liposarcomas, osteosarcomas, chondrosarcomas, and rhabdomyosarcomas.8 By contrast, PD-L1 expression rates as high as 34–52% have been reported for other types of tumors.9,10
Consistent with low PD-L1 expression, anti PD-1 therapy efficacy in STS also appears to be relatively low: in a prospective study of 38 STS, only 19% of patients had a response.11 As a result, relatively few clinical trials investigating immunotherapy agents in STS have been proposed. Methods to increase PD-L1 expression and potentially improve anti PD-1 immunotherapy activity in STS are needed in this under-represented population. In this analysis, we identified pre-operative radiotherapy (RT) as a potential means to increase PD-L1 expression by comparing expression of PD-L1 and other immune biomarkers, before and after RT.
Methods
Patient selection
After obtaining IRB approval, patients treated at Emory University School of Medicine for non-metastatic STS between 2005 and 2015 were analyzed. To be included in the study, patients were required to have tissue material for immunohistochemistry from biopsy and surgical resection samples. Two cohorts of patients were included: Cohort 1 consisted of patients who underwent biopsy, pre-operative RT, followed by surgical resection; Cohort 2 consisted of patients who underwent biopsy followed by surgical resection, without any pre-operative RT. Patients who received pre-operative chemotherapy were excluded from this analysis.
All patient charts were reviewed for the following baseline characteristics: age, gender, size, grade, depth, primary tumor histologic subtype, location and receipt of post-operative chemotherapy. RT treatment parameters were also recorded, including total dose, dose per fraction, time from RT to surgical resection.
Treatment
At Emory University, all STS patients are discussed at a multi-disciplinary tumor board, which includes representatives from radiation oncology, surgical oncology, orthopedic surgical oncology, pathology, diagnostic radiology, and medical oncology. Management decisions for surgery and RT were thus made in a joint manner. A dose of 50–50.4 Gy in 25–28 fractions was prescribed for all patients, with the vast majority receiving 50 Gy in 25 fractions. Surgical resection was performed within 8 weeks (2–8 weeks) after completion of RT.
Immunohistochemistry
Immunohistochemistry was performed using 5 um thick sections from paraffin-embedded tissue. Antibodies used for staining included PD-L1 (E1L3 N clone; Cell Signaling), PD-1 (NAT clone, Abcam), and CD8 (C8/144B clone; Cell Signaling). Positive PD-L1 expression was defined as staining of the plasma membrane in >1% of tumor cells. Macrophages' PD-L1 expression was determined qualitatively only. PD-1 and CD8 expression was defined as staining in >5% of tumor cells and lymphocytes, respectively.
Statistical analysis
Immune biomarker expression levels were directly compared between each patient's biopsy and resection sample. Biomarker expression was recorded as a dichotomous variable (yes vs. no). For categorical variables, the Fisher's exact test was utilized. All statistical analyses were 2-sided, with p-values <0.05 considered statistically significant.
Characteristics were compared between patients with PD-L1 expression after RT and patients without expression by Wilcoxon rank-sum test for continuous variables, and Chi square test or Fisher's exact test for categorical variables, where appropriate. For time to event analysis, outcomes were measured from date of surgery. Distant metastases were defined as development of disease in another organ, outside of the nearest draining lymph node basin and/or primary tumor location. DM was estimated by the Kaplan–Meier product-limit method; the log-rank test was used to assess for differences between treatment groups. Multivariate analysis (MVA) was performed using the Cox proportional hazards model.12 All potentially prognostic covariates were initially entered and a backwards stepwise selection method was employed.13 All statistical analyses were performed using SPSS version 22.
Results
Patient and treatment characteristics
Cohort 1
Forty-six patients with stage II-III STS who received pre-operative RT and had both biopsy and resection samples available for analysis were identified. The median time from completion of RT to surgical resection was 28 days (range: 13–54). Histologic STS subtypes included undifferentiated pleomorphic sarcoma (UPS) (71.7%), liposarcoma (17.4%), leimyosarcoma (6.5%), and 1 each of myxoid, synovial, epitheliod and spindle cell sarcoma. The median patient age was 61.0 years old and 60.9% of patients were male. Most tumors were grade 3 (80.4%), T2b (87.0%), and located in the extremities (84.6%). Table 1 summarizes patient baseline and treatment characteristics.
Table 1.
Baseline patient and treatment characteristics of irradiated patients.
| |
(n = 46) |
|---|---|
| Median Age (range) in years | 61.0 (31.0 – 85.7) |
| Gender | |
| Male | 28 (60.9%) |
| Female | 18 (39.1%) |
| T stage | |
| T1-T1b | 3 (6.5%) |
| T2 a | 3 (6.5%) |
| T2b | 40 (87.0%) |
| Grade | |
| 1–2 | 9 (19.6%) |
| 3 | 37 (80.4%) |
| Histologic Subtypes | |
| Undifferentiated Pleomorphic Sarcoma | 33 (71.7%) |
| Liposarcoma | 8 (17.4%) |
| Leiomyosarcoma | 3 (6.5%) |
| Other | 4 (8.7%) |
| Tumor Location | |
| Upper Extremity | 4 (8.8%) |
| Lower Extremity | 35 (76.1%) |
| Abdomen/Pelvis | 7 (15.2%) |
| Radiation Dose | |
| 50 Gy, 25fractions | 42 (89.7%) |
| 50.4 Gy, 28 fractions | 4 (10.3%) |
| Median (Range), in Days, from Last Day of XRT to Surgery | 28 (13–54) |
| Post-operative Chemotherapy | |
| No | 39 (84.8%) |
| Yes | 7 (15.2%) |
Cohort 2
As described above, five patients with stage II-III STS who underwent post-operative RT were identified to be used as controls. Similar to the pre-operative RT patients, the post-operative RT patients had high grade morphology (100%), high prevalence of UPS (100%), and were located in the extremities (100%).
Immune biomarkers
All patients had biopsy and resection samples. For the 46 patients treated with pre-operative RT, the PD-L1 expression at time of biopsy, prior to RT, was 0% in all cases (Fig. 1c and 1d). On the resected, irradiated sample, PD-L1 expression was elevated in 5 out of 46 patients (10.9%) (Fig. 1g and 1h). This increase in PD-L1 expression was borderline statistically significant (p = 0.056). Table 2 illustrates the characteristics of the 5 patients who had elevated PD-L1 in their irradiated samples. Tumor associated macrophage (TAM) expression of PD-L1 was associated with a statistically significant increase (p = 0.003) from prior to RT (15.2%, 7/46) to after RT (45.6%, 21/46) (Fig. 1). PD-1 expression was similar between biopsied and irradiated samples (30.9%, 30.9%, p = 1.00), as was expression of levels on CD8-positive lymphocytes (33.3% vs. 28.2%, p = 0.806).
Figure 1.
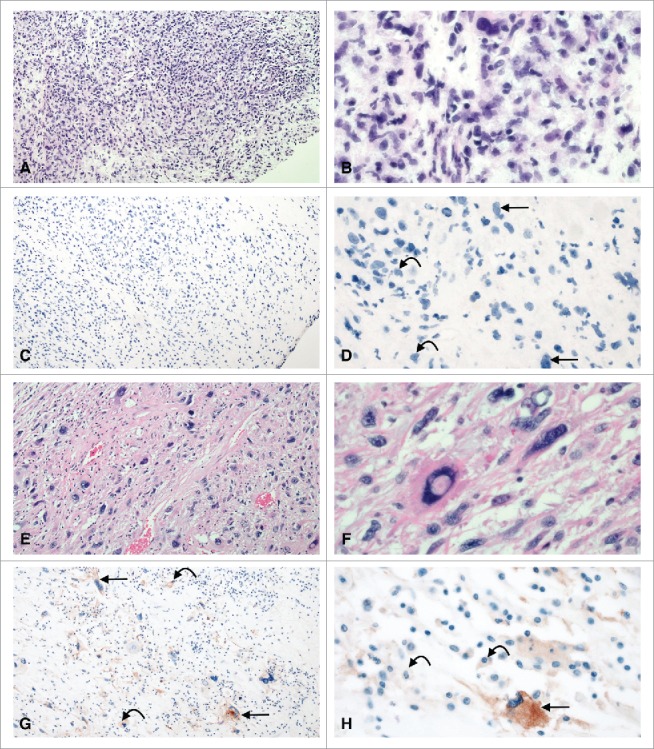
PD-L1 expression in undifferentiated pleomorphic sarcoma pre (A-D) and post (E-H) radiation therapy. Prior to radiation therapy, tumor cells and tumor associated macrophages are negative for PD-L1 (C-D). After radiation therapy, both tumor cells (straight arrows) and macrophages (curved arrows) are positive for PD-L1. A, C, E, G: 10x; B, D, F, H: 40x.
Table 2.
Characteristics of 5 patients with increased PD-L1 expression after pre-operative radiation therapy.
| Gender | Age | Location | T stage | Grade | Histologic Subtype | Time Between Pre-Op RT & Sx (days) | Total Dose | % PDL1 staining at biopsy | % PDL1 staining at surgery/after RT |
|---|---|---|---|---|---|---|---|---|---|
| Male | 36 | Lower Limb | T2b | 3 | Epitheliod | 26 | 50 Gy/ 25 fx | 0 | > 1 |
| Male | 41 | Lower Limb | T2b | 3 | UPS | 27 | 50 Gy/ 25 fx | 0 | > 1 |
| Female | 60 | Lower Limb | T2b | 3 | UPS | 35 | 50 Gy/ 25 fx | 0 | > 1 |
| Male | 56 | Lower Limb | T2b | 3 | UPS | 17 | 50 Gy/ 25 fx | 0 | > 1 |
| Female | 70 | Lower Limb | T2b | 3 | UPS | 42 | 50 Gy/ 25 fx | 0 | > 1 |
Gy/fx = Gray/fraction; RT = radiation therapy; Sx = surgery; UPS = undifferentiated pleomorphic sarcoma.
Of the patients who received post-operative RT, all biopsy and all resection samples did not demonstrate PD-L1 expression on tumor; PD-L1 expression on TAM did not change: 1/5 (20%) expressed PD-L1 on the unirradiated biopsy sample and 1/5 (20%) expressed on the resected, unirradiated sample.
Clinical outcomes
For all patients, median follow up was 69.8 months. Fifteen patients ultimately developed DM. Median time to DM was 11.9 months (0.9-43.7). Twelve patients developed lung metastases; three patients developed liver metastases. Twenty-one patients (45.6%) had elevated PD-L1 TAM expression after RT, while 25 (54.3%) patients did not. Patients with positive PD-L1 expression on TAM were similar in baseline characteristics to patients with PD-L1 negative tumors (Table 3). Fig. 2 illustrates that patients with elevated PD-L1 expression on TAM following RT were associated with lower freedom from DM (at 3 years, 49.7% vs. 87.8%, p = 0.006). On univariate analyses, two other factors were significant for DM: histologic subtype (p = 0.091) and receipt of adjuvant chemotherapy (p = 0.008).
Table 3.
Baseline characteristics of patients with PD-L1 positive and negative expression on tumor-associated macrophages.
| |
PD-L1 positive (n = 21) |
PD-L1 negative (n = 25) |
|
|---|---|---|---|
| Median Age (years) | 58.9 | 61.0 | p = 0.721 |
| Gender | |||
| Male | 11 (52.4%) | 16 (64.0%) | |
| Female | 10 (47.6%) | 9 (36.0%) | p = 0.550 |
| T stage | |||
| T1 a-T1b | 1 (4.8%) | 2 (8.0%) | p = 0.673 |
| T2 a | 1 (4.8%) | 2 (8.0%) | |
| T2b | 19 (90.4%) | 21 (84%) | |
| Grade | |||
| 1–2 | 4 (19.1%) | 7 (28.0%) | p = 0.514 |
| 3 | 17 (80.9%) | 18 (72.0%) | |
| Histologic Subtypes | |||
| Undifferentiated Plemorphic Sarcoma | 14 (66.7%) | 18 (72.0%) | p = 0.755 |
| Liposarcoma | 5 (23.8%) | 3 (12.0%) | |
| Leimyosarcoma | 2 (9.5%) | 1 (4.0%) | |
| Other | 0 (0%) | 3 (12.0%) | |
| Tumor Location | |||
| Upper Extremity | 1 (4.8%) | 2 (8.0%) | p = 0.429 |
| Lower Extremity | 19 (90.4%) | 20 (80%) | |
| Abdomen/Pelvis | 1 (4.8%) | 3 (12.0%) | |
| Post-operative Chemotherapy | |||
| Yes | 4 (19.1%) | 3 (12.0%) | p = 0.880 |
| No | 17 (80.9%) | 22 (88.0%) | |
| Days from Completing XRT to surgery | |||
| Median (range) | 28 (13–42) | 28 (18–54) | p = 0.373 |
Figure 2.
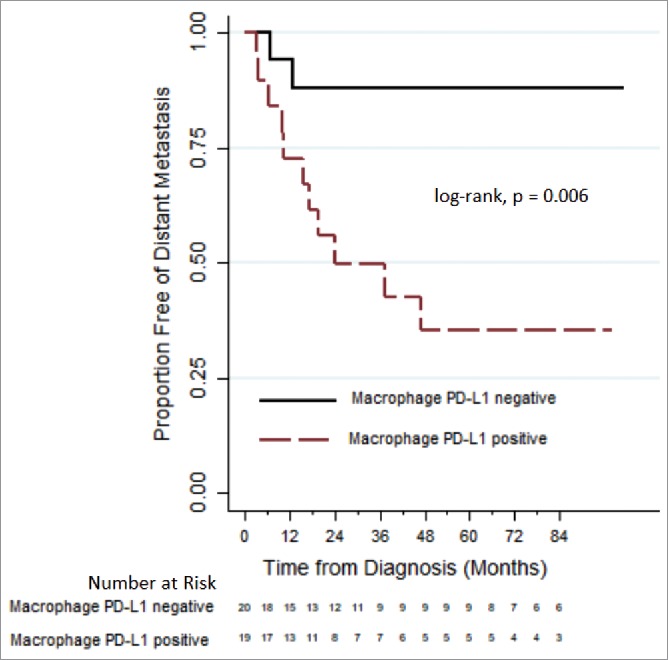
Comparing distant metastases free survival, via Kaplan-Meier plots, between sarcoma patients who express and do not express PD-L1 on macrophages after pre-operative radiation therapy.
On MVA, only TAM PD-L1 expression remained a predictor for DM (Negative staining vs. positive staining; HR 0.16, 95% CI 0.034-0.72, p = 0.017; receipt of adjuvant chemotherapy demonstrated a trend (no chemotherapy vs. receipt of chemotherapy; HR 0.19, p = 0.066). On analysis for overall survival, PD-L1 expression was not a significant predictor on univariate analyses.
Discussion
STS is rare among malignancies in that the usual treatment paradigm grants the capacity to study changes in PD-L1 expression with RT: patients first undergo biopsy, followed by RT, and ultimately surgical resection.14 In this novel study, PD-L1 expression was compared between matched non-radiated (n = 5) and irradiated (n = 46) STS samples. At baseline, no PD-L1 expression was demonstrated on tumor samples. On matched irradiated samples, PD-L1 expression was elevated in some instances: 10.9%, p = 0.056. Furthermore, irradiated samples were associated with higher PD-L1 expression on TAM: with increase from 15.2% to 45.7%, p = 0.003. Control samples, from STS patients treated without pre-operative RT, were not associated with any change in PD-L1 expression. Patients who had elevated PD-L1 expression on TAM after RT had lower freedom from DM (at 3 years, 49.7% vs. 87.8%, p = 0.006); PD-L1 also continued to predict for higher DM on MVA.
PD-L1 is a molecule found on tumor an antigen presenting cells. Its function includes sending an inhibitory signal to suppress the immune anti-neoplastic response. This inhibitory milieu may allow tumor cells to escape and allow for the development of distant metastases. Kim et al investigated PD-L1 expression in STS15; to assess PD-L1, they used an antibody clone H-130. Similar to our findings, they found that STS patients with PD-L1 expression were associated with lower event free survival. Conversely, in a study utilizing the DAKO antibody (clone 22C3), D'Angelo et al found no correlation of PD-L1 expression and clinical outcomes.7 Our study used a third antibody – clone E13LN. It seems likely that differences in the antibody specificity, affinity, and/or epitptopes may contribute to the differences in correlation between expression and clinical outcomes.16 There are two major important differences between our study and those by Kim15 and D'Angelo7 that may contribute to our findings. First, our STS patient population was very specific: we included only stage II and III STS patients who did not receive pre-operative chemotherapy. Second, our analysis found a significant correlation between PD-L1 expression in tumor samples that had been irradiated and the development of DM in tumor samples that had been irradiated. If these findings were to be are validated in prospective samples, using multiple antibodies, it would present PD-L1 as a possible biomarker to stratify patients for possible anti-PD-1/PD-L1 therapy. Finally, it should be noted that we were not able to proceed with analysis correlating tumor PD-L1 expression and clinical outcomes because of the low numbers of patients (n = 5, 10.9%) who were ultimately PD-L1 positive after RT (n = 5, 10.9%).
With growing evidence that PD-L1 is one biomarker for efficacy of anti PD-1 therapy, researchers have investigated methods to enhance PD-L1 expression as a method to increase response rates with PD-1 therapy. In preclinical studies using cell lines and mouse models, RT has been shown to increase tumor PD-L1 expression.17,18 RT appears to induce increased MHC1 mediated antigen presentation and increased tumor lymphocyte infiltration. This radiation response appears partially modulated by increased expression of interferon-Y19; in response to these radiation effects, tumors are hypothesized to increase PD-L1 in order to evade immune recognition. Furthermore, acquired resistance to PD-1 inhibitors can occur via mutations to apelin receptor (APLNR)20 and interferon-receptor–associated Janus kinase 1 (JAK1),21 both of which lead to decreased interferon-Y expression and tumor infiltration by immune cells. Our study builds on these pre-clinical studies by providing the first evidence in human STS samples, that RT modulates PD-L1 expression, which may suggest a potential response to PD-1 therapy. Recently, a secondary analysis of a prospective study found that patients treated with RT and PD-1 inhibitor pembrolizumab had improved outcomes relative to patients treated with pembrolizumab alone.22 Taken together, these data contribute ideological support to clinical trials investigating addition of RT to improve the efficacy of PD-1 inhibitor therapy.
This present study has both weaknesses and strengths. The analysis is limited given it is based on samples that were identified retrospectively, introducing possible selection bias. The matched control arm is also a small sample size due to our institutions' preference to utilize pre-operative RT for stage II-III STS. Pre-clinical evidence suggests that PD-L1 expression peaks 3 days after RT, and then begins to decline.17 Based on this preclinical response, our analysis of PD-L1 expression at median of 28 days after completion of RT may be suboptimal. Nonetheless, PD-L1 expression was still elevated at this later time point. In spite of these limitations, this study examined a relatively homogenous patient population, particularly in terms of radiation dose. In addition, patients who received pre-operative chemotherapy were excluded, thus eliminating potential confounding factors that may modulate PD-L1 expression. Finally, the direct comparison of biomarker expression between pre- and post-RT provides new insight into potential strategies to optimize anti-PD-1 therapies for the treatment of STS.
In conclusion, both tumor cell and TAM expression of PD-L1 in STS is significantly increased after RT compared to pre-RT samples. In light of PD-L1 being a biomarker for PD-1 immunotherapy, these novel findings give further support that adding RT may improve efficacy of agents that target the PD-1/PD-L1 immune cascade. We eagerly await the results from the SARC study that is adding a PD-1 inhibitor with standard course RT (50 Gy in 25 fraction) for non-metastatic STS.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- 1.Patel SP, Kurzrock R. PD-L1 Expression as a Predictive Biomarker in Cancer Immunotherapy. Mol Cancer Ther. 2015;14:847–56. doi: 10.1158/1535-7163.MCT-14-0983. PMID:25695955. [DOI] [PubMed] [Google Scholar]
- 2.Robert C, Ribas A, Wolchok JD, Hodi FS, Hamid O, Kefford R, Weber JS, Joshua AM, Hwu WJ, Gangadhar TC, et al.. Anti-programmed-death-receptor-1 treatment with pembrolizumab in ipilimumab-refractory advanced melanoma: a randomised dose-comparison cohort of a phase 1 trial. Lancet. 2014;384:1109–17. doi: 10.1016/S0140-6736(14)60958-2. PMID:25034862. [DOI] [PubMed] [Google Scholar]
- 3.Garon EB, Rizvi NA, Hui R, Leighl N, Balmanoukian AS, Eder JP, Patnaik A, Aggarwal C, Gubens M, Horn L, et al.. Pembrolizumab for the Treatment of Non-Small-Cell Lung Cancer. N Engl J Med. 2015;372:2018–28. doi: 10.1056/NEJMoa1501824. [DOI] [PubMed] [Google Scholar]
- 4.Garon EB, Rizvi NA, Hui R, Leighl N, Balmanoukian AS, Eder JP, Patnaik A, Aggarwal C, Gubens M, Horn L, et al.. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015;372:2018–28. doi: 10.1056/NEJMoa1501824. PMID:25891174. [DOI] [PubMed] [Google Scholar]
- 5.Reck M, Rodriguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, Gottfried M, Peled N, Tafreshi A, Cuffe S, et al.. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N Engl J Med. 2016;375:1823–33. doi: 10.1056/NEJMoa1606774. PMID:27718847. [DOI] [PubMed] [Google Scholar]
- 6.Robert C, Long GV, Brady B, Dutriaux C, Maio M, Mortier L, Hassel JC, Rutkowski P, McNeil C, Kalinka-Warzocha E, et al.. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372:320–30. doi: 10.1056/NEJMoa1412082. PMID:25399552. [DOI] [PubMed] [Google Scholar]
- 7.D'Angelo SP, Shoushtari AN, Agaram NP, Kuk D, Qin LX, Carvajal RD, Dickson MA, Gounder M, Keohan ML, Schwartz GK, et al.. Prevalence of tumor-infiltrating lymphocytes and PD-L1 expression in the soft tissue sarcoma microenvironment. Hum Pathol. 2015;46:357–65. doi: 10.1016/j.humpath.2014.11.001. PMID:25540867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Torabi A, Amaya CN, Wians FH, Bryan BA. PD-1 and PD-L1 expression in bone and soft tissue sarcomas. Pathology. 2017;49:506–13. doi: 10.1016/j.pathol.2017.05.003. PMID:28688724. [DOI] [PubMed] [Google Scholar]
- 9.Janzic U, Kern I, Janzic A, Cavka L, Cufer T. PD-L1 Expression in Squamous-cell Carcinoma and Adenocarcinoma of the Lung. Radiology and oncology. 2017;51:357–62. doi: 10.1515/raon-2017-0037. PMID:28959173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reddy OL, Shintaku PI, Moatamed NA. Programmed death-ligand 1 (PD-L1) is expressed in a significant number of the uterine cervical carcinomas. Diagnostic pathology. 2017;12:45. doi: 10.1186/s13000-017-0631-6. PMID:28623908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burgess MA, Crowley J, Reinke DK, et al.. SARC 028: A phase II study of the anti-PD1 antibody pembrolizumab (P) in patients (Pts) with 330 advanced sarcomas. Paper presented at: American Society of Clinical Oncology Annual Meeting; 2015. May 31; Chicago, Illinois, USA. doi: 10.1200/jco.2015.33.15_suppl.tps10578. [DOI] [Google Scholar]
- 12.Kalbfleisch JDP R.L. The statistical analysis of failure time data. 2 ed Hoboken (NJ):Wiley-Interscience; 2002. [Google Scholar]
- 13.Cox DR. Regression Models and Life Tables. J Royal Stat Society. 1972;34(2):187–220. [Google Scholar]
- 14.O'Sullivan B, Davis AM, Turcotte R, Bell R, Catton C, Chabot P, Wunder J, Kandel R, Goddard K, Sadura A, et al.. Preoperative versus postoperative radiotherapy in soft-tissue sarcoma of the limbs: a randomised trial. Lancet. 2002;359:2235–41. doi: 10.1016/S0140-6736(02)09292-9. PMID:12103287. [DOI] [PubMed] [Google Scholar]
- 15.Kim JR, Moon YJ, Kwon KS, Bae JS, Wagle S, Kim KM, Park HS, Lee H, Moon WS, Chung MJ, et al.. Tumor infiltrating PD1-positive lymphocytes and the expression of PD-L1 predict poor prognosis of soft tissue sarcomas. PLoS One. 2013;8:e82870. doi: 10.1371/journal.pone.0082870. PMID:24349382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McLaughlin J, Han G, Schalper KA, Carvajal-Hausdorf D, Pelekanou V, Rehman J, Velcheti V, Herbst R, LoRusso P, Rimm DL. Quantitative Assessment of the Heterogeneity of PD-L1 Expression in Non-Small-Cell Lung Cancer. JAMA Oncol. 2016;2:46–54. doi: 10.1001/jamaoncol.2015.3638. PMID:26562159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dovedi SJ, Adlard AL, Lipowska-Bhalla G, McKenna C, Jones S, Cheadle EJ, Stratford IJ, Poon E, Morrow M, Stewart R, et al.. Acquired resistance to fractionated radiotherapy can be overcome by concurrent PD-L1 blockade. Cancer Res. 2014;74:5458–68. doi: 10.1158/0008-5472.CAN-14-1258. PMID:25274032. [DOI] [PubMed] [Google Scholar]
- 18.Sharabi AB, Nirschl CJ, Kochel CM, Nirschl TR, Francica BJ, Velarde E, Deweese TL, Drake CG. Stereotactic Radiation Therapy Augments Antigen-Specific PD-1-Mediated Antitumor Immune Responses via Cross-Presentation of Tumor Antigen. Cancer Immunol Res. 2015;3:345–55. doi: 10.1158/2326-6066.CIR-14-0196. PMID:25527358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dovedi SJ, Illidge TM. The antitumor immune response generated by fractionated radiation therapy may be limited by tumor cell adaptive resistance and can be circumvented by PD-L1 blockade. Oncoimmunology. 2015;4:e1016709. doi: 10.1080/2162402X.2015.1016709. PMID:26140246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Patel SJ, Sanjana NE, Kishton RJ, Eidizadeh A, Vodnala SK, Cam M, Gartner JJ, Jia L, Steinberg SM, Yamamoto TN, et al.. Identification of essential genes for cancer immunotherapy. Nature. 2017;548:537–42. doi: 10.1038/nature23477. PMID:28783722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zaretsky JM, Garcia-Diaz A, Shin DS, Escuin-Ordinas H, Hugo W, Hu-Lieskovan S, Torrejon DY, Abril-Rodriguez G, Sandoval S, Barthly L, et al.. Mutations Associated with Acquired Resistance to PD-1 Blockade in Melanoma. N Engl J Med. 2016;375:819–29. doi: 10.1056/NEJMoa1604958. PMID:27433843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shaverdian N, Lisberg AE, Bornazyan K, Veruttipong D, Goldman JW, Formenti SC, Garon EB, Lee P. Previous radiotherapy and the clinical activity and toxicity of pembrolizumab in the treatment of non-small-cell lung cancer: a secondary analysis of the KEYNOTE-001 phase 1 trial. Lancet Oncol. 2017;18:895–903. doi: 10.1016/S1470-2045(17)30380-7. PMID:28551359. [DOI] [PMC free article] [PubMed] [Google Scholar]


