ABSTRACT
Severe radiation-related lymphopenia is common and associated with decreased survival in patients with several solid tumors. As the mechanisms underlying systemic lymphopenia are poorly understood, we developed an animal model to study the effects of brain radiation on lymphocytes and cytokines. C57 BL/6 and BALB/c mice received focal brain irradiation (4 Gy x 10 fractions or 2 Gy x 30 fractions). Weekly total lymphocyte counts (TLC), lymphocyte subsets and cytokines in blood and lymph nodes were measured. Non-irradiated lymph nodes were collected and examined before, during, and after radiation. We found that systemic TLC decreased rapidly irrespective of mouse strain or radiation schedule. 4 Gy x 10 resulted in a 42% and 75% & 70% and 49% TLC reduction in C57 BL/6 and BALB/c mice respectively. 2 Gy x 30 caused a 70% / 49% decrease in TLC in C57 BL/6 and BALB/c. Similar trends were seen for total T cells, CD4+, regulatory T and CD8+ cells. Changes in lymph node architecture and cellular composition correlated with the development of systemic lymphopenia. Three weeks after radiation, TLC returned to 60–80% of baseline, preceded by increased IL-7 levels in the lymph nodes. Focal brain radiation in mice results in significant systemic lymphodepletion.
KEYWORDS: radiation-related lymphopenia, lymphocytes, IL-7 cytokine, brain radiation, lymphodepletion, immunosuppression
Introduction
Cancer treatment and progression are often associated with immune suppression. Approximately half of patients who receive radiation as part of their anti-neoplastic therapy develop severe and sustained lymphopenia.1 Six studies involving approximately 2,000 patients with newly diagnosed malignant glioma, resected and locally advanced pancreatic adenocarcinoma, non-small cell lung cancer and triple negative breast cancer documented an association between treatment–related lymphopenia (TRL) and shorter survival2,3,4,5,6,7 Multivariate analyses suggested that patients with CD4 counts less than 200 cells/mm3 approximately 2 months after starting radiotherapy (RT) have inferior outcomes related to tumor progression rather than opportunistic infection.
The precise mechanisms by which radiation causes severe and long-lasting lymphopenia in these patients have not yet been defined. There is evidence that this may be related to the inadvertent radiation of blood circulating through the radiation field. Prior animal studies highlighted the central role that extracorporeal radiation of blood plays in the etiology of TRL.8,9,10,11 Yovino et al developed a mathematical model to calculate the radiation dose to circulating lymphocytes.12 This demonstrates that larger target volumes, lower dose rates, and more consecutive days of radiation administered (i.e. number of radiation fractions) increase lymphotoxicity. As a result, a standard treatment plan for patients with high grade gliomas, which is comprised of 30 fractions and affects minimal lymphatic vessels,13 exposes nearly the entire circulating blood pool to lymphotoxic doses. However, this iatrogenic immunosuppression was only recently associated with survival in patients with cancer. The animal model described in this manuscript was developed in an attempt to better understand the underlying mechanism of TRL. These studies showed the effect of focal brain radiation on total circulating lymphocytes, distal lymph node cellularity and cytokine responses and highlight an important difference between mouse and human responses to TRL.
Results
Both short and prolonged radiotherapy regimens are well tolerated
In an effort to create an animal model for radiotherapy treatment related lymphopenia (TRL), we tested two common laboratory mouse strains; C57 BL/6 and BALB/c mice. Each strain was treated with two different doses (Fig. 1A, Fig. 1B) of focal radiation (Fig. 1C) which resulted in the development of transient alopecia on the scalp in the majority of animals (Fig. 1D). Animals maintained standard weight and coordinated movements throughout and after either course of treatment, indicating that the treatments were well-tolerated.
Figure 1.
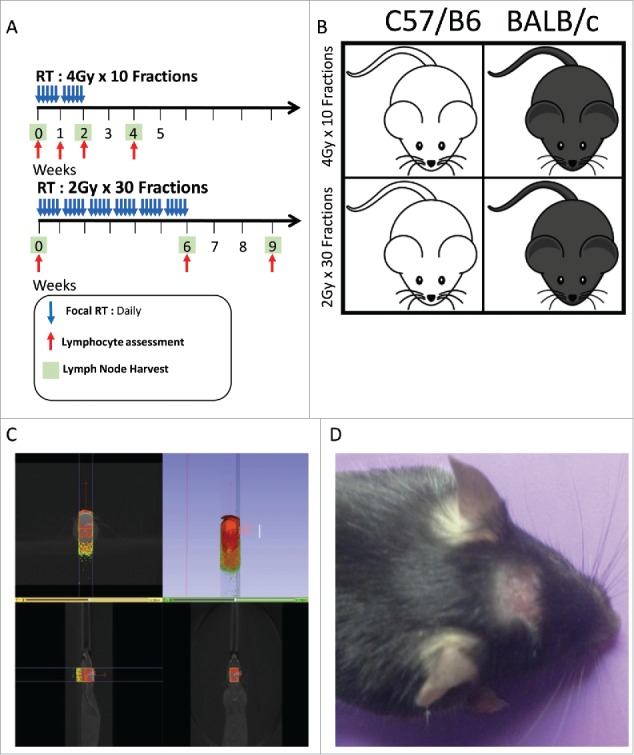
(A) XRT treatment, lymphocyte assessment and lymph node harvest scheme for 4 Gy x 10 fractions(abbreviated) and 2 Gy x 30 fractions(prolonged) groups. (B) Visual representation of treatment scheme and animal strains used in this study. (C) Imaging display from SARRP showing that radiation treatment is extremely precise and that peripheral sites are not exposed to XRT. (D) Focal alopecia is induced in treated animals, highlighting the focused treatment and successful XRT exposure.
Localized radiotherapy results in systemic treatment related lymphopenia (TRL) in animals treated with a short-course (10 fraction) regimen
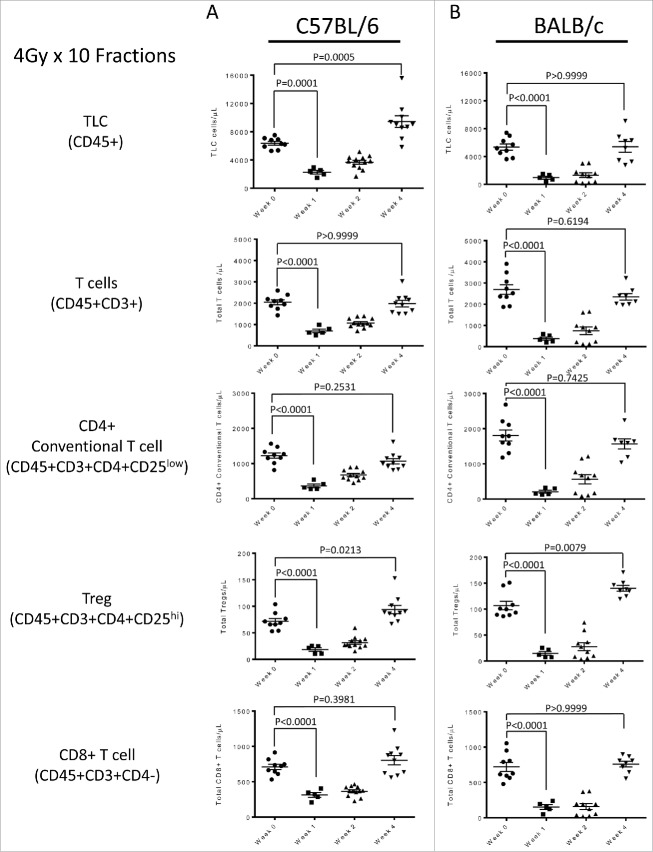
To determine whether focal radiation to the brain would deplete peripheral lymphocytes in mice as previously observed in humans, treatment was first administered using an abbreviated (4 Gy x 10 fractions) regimen. We measured a 42.1% and 75.4% decrease in the total lymphocyte count (TLC; CD45+ cells) in both the C57 BL/6 and BALB/c models respectively when compared to baseline levels (Fig. 2A, Fig. 2B). Similar trends were seen in the total T cell compartment (CD45+CD3+), CD4+ T conventional cells (Tconv; CD45+CD3+CD4+CD25low), CD4+ regulatory T cells (Treg; CD45+CD3+CD4+CD25hi) and CD8+ T cells (CD45+CD3+CD4−) (Fig. 2A, Fig. 2B). This ubiquitous depletion indicates that TRL in these animals is not an effect on specific lymphocyte subsets. Within 3 weeks of the completion of radiation, counts returned to 60–80% of baseline in each model, in opposition to what is observed in one human patients.
Figure 2.
(A) Lymphocyte assessment for the C57 BL/6 strain treated with the 4 Gy x 10 fractions, showing non-specific lymphodepletion in the periphery as response to treatment. (B) Lymphocyte assessment for the Balb/c strain treated with the 4 Gy x 10 fractions, showing non-specific lymphodepletion in the periphery as response to treatment. All experiments were repeated in triplicate. Error bars were calculated using one-way repeated measures analysis of variance. Middle bars represent mean, with upper and lower error bars.
Localized radiotherapy results in systemic treatment related lymphopenia (TRL) in animals treated with a prolonged (30 fraction) regimen
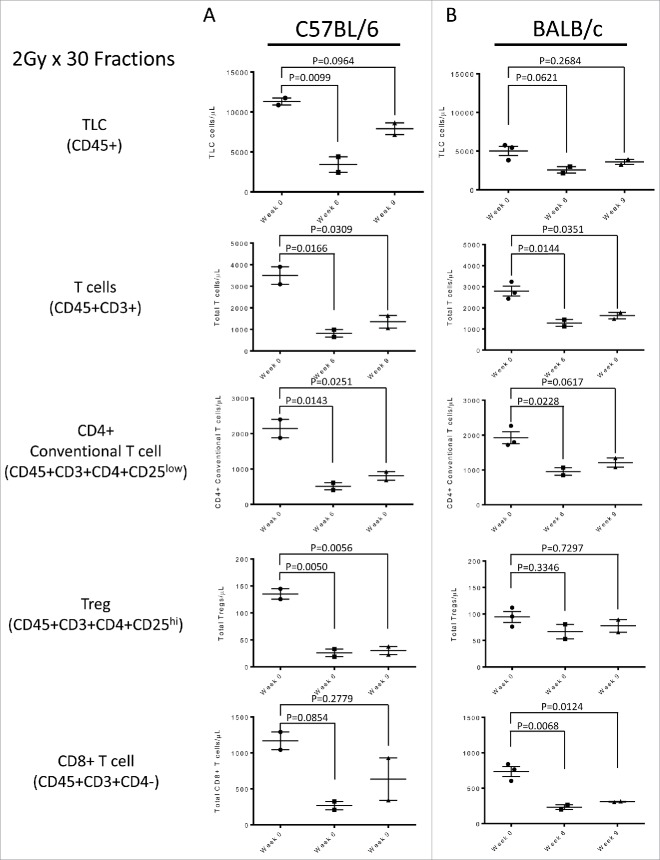
We then set out to test whether a prolonged RT regimen might result in sustained systemic TRL, and repeated the same studies above using a prolonged (30 Fraction) regimen. This prolonged treatment regimen also resulted systemic lymphopenia in the C57 BL/6 and Balb/c models, with total lymphocyte counts (TLC) decreased by 69.7% and 48.8% of baseline respectively (Fig. 3A, Fig. 3B). Total T cells, CD4+ Tconv, Treg and CD8+ T cell subsets were similarly affected (Fig. 3A, Fig. 3B). As was the case for the abbreviated treatment, within 3 weeks of the completion of radiation, counts returned to 60–80% of baseline (Fig. 3A, Fig. 3B). There was no significant trend seen in other hematologic parameters including neutrophils, platelets and red blood cells. Taken together, these data confirm the concept that localized RT can result in systemic lymphopenia in mice. But unlike humans, animals recovered spontaneously without prolonged systemic TRL.
Figure 3.
(A) Lymphocyte assessment for the C57 BL/6 strain treated with the 2 Gy x 30 fractions, showing non-specific lymphodepletion in the periphery as response to treatment. (B) Lymphocyte assessment for the Balb/c strain treated with the 2 Gy x 30 fractions, showing non-specific lymphodepletion in the periphery as response to treatment. All experiments were repeated in triplicate. Error bars were calculated using one-way repeated measures analysis of variance. Middle bars represent mean, with upper and lower error bars.
Distal inguinal lymph nodes are affected by local brain radiation
Since focal radiation has a systemic deleterious effect on lymphocytes, we then investigated its effect on distant secondary lymphoid tissues. Inguinal lymph nodes (LN) of treated mice were harvested at time points throughout their respective RT regimens and examined by a hematopathologist who was blinded to the treatment group. These lymph nodes were far outside the field of radiation, eliminating the chance of direct radiation toxicity. Lymph nodes examined at the end of RT regimens showed architectural distortion by paracortical expansions rich in histiocytes and plasma cells as well as prominent collections of epithelioid histiocytes in hilar regions as compared to baseline (Fig. 4A, Fig. 4B, Fig. 5A, Fig. 5B). Lymph nodes isolated after 3 weeks of recovery showed persistence of the paracortical expansion with mild fibrosis. Tingible body macrophages and ‘sea blue’ histiocytes were seen; these are commonly associated with phagocytosis (Fig. 4C, Fig. 5C). These histopathologic changes in distant lymph nodes, paralleled changes in blood lymphocyte counts and were consistent across animal strains and radiation treatment schemes.
Figure 4.
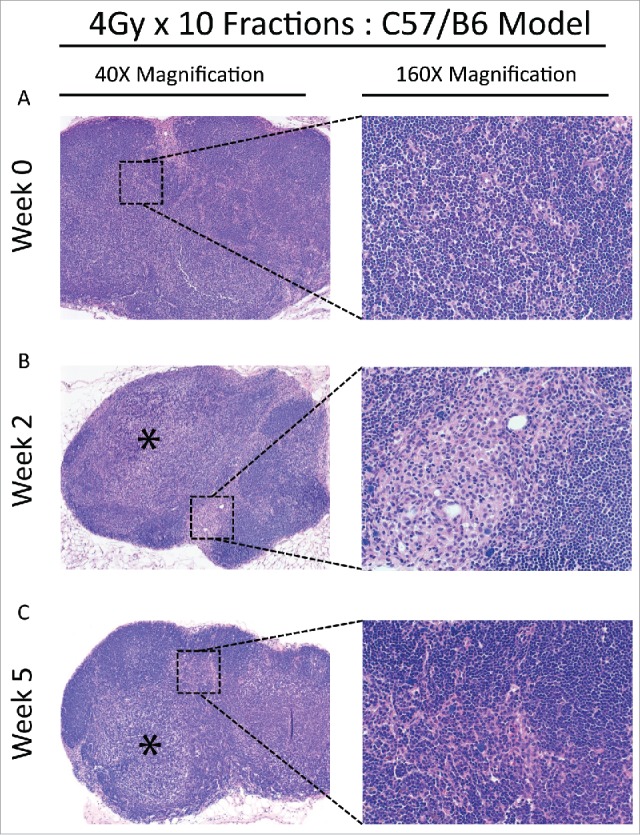
(A) Baseline lymph node assessment for the C57 BL/6 strain treated with the 4 Gy x 10 fractions at 40X and 160X magnification, showing typical LN at baseline. (B) End of treatment lymph node assessment for the C57 BL/6 strain treated with the 4 Gy x 10 fractions. Lymphoid architecture is distorted by a paracortical expansion with a mixed lymphohistiocytic composition (40X, *) and there are prominent aggregates of hilar histiocytes (160X). (C) Recovery lymph node for the C57 BL/6 strain treated with the 4 Gy x 10 fractions. Vaguely nodular, histiocyte-rich paracortical expansions remain prominent (40X, *) with some histiocytes and fibrosis adjacent to organized lymphoid follicles (160X).
Figure 5.
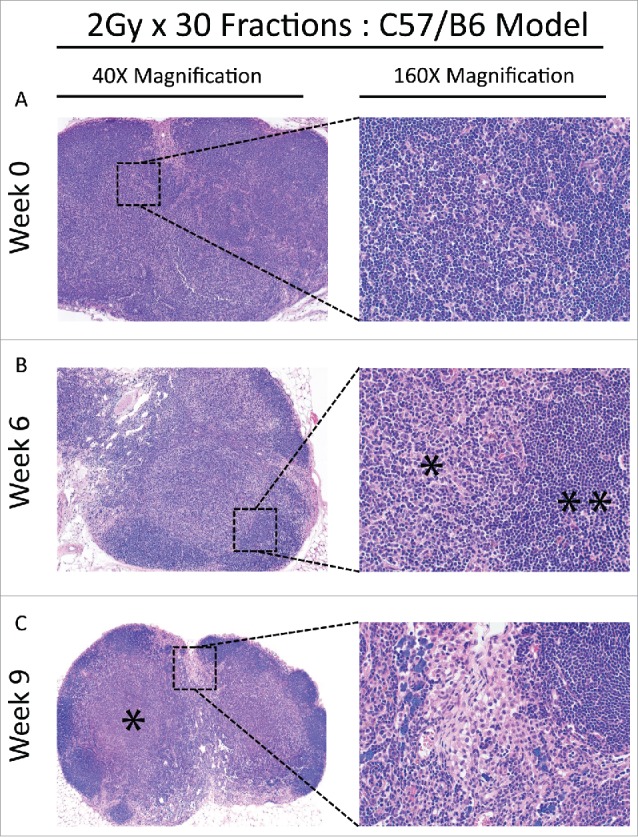
(A) Baseline lymph node assessment for the C57 BL/6 strain treated with the 2 Gy x 30 fractions at 40X and 160X magnification, showing typical architecture at baseline. Cortical areas and paracortical areas are lymphocyte-rich. (B) End of treatment lymph node assessment for the C57 BL/6 strain treated with the 2 Gy x 30 fractions at 40X and 160X magnification. Paracortical, predominantly histiocytic expansions are prominent (*) and lymphocytes are relatively restricted to follicles (**). (C) Recovery lymph node assessment for the C57 BL/6 strain treated with the 2 Gy x 30 fractions at 40X and 160X. Paracortical expansions persist with fibrosis (60X, *) and hilar epitheloid histiocytes and scattered sea-blue histiocytes are prominent. Tingible body macrophages, and pigment-laden macrophages indicating a dermatopathic component to the lymph node changes, were also present (not shown).
Expression of IL-7 correlates with lymphocyte recovery in mice
CD4+ lymphopenia in HIV/AIDS patients is generally accompanied by an increase in Interleukin-7 (IL-7) that promotes homeostatic proliferation,14 and prior studies by our group showed that a compensatory IL-7 increase was absent in patients with TRL.15 To test whether the rapid recovery from lymphopenia seen in our murine models was associated with a compensatory increase in IL-7, we quantified expression of this cytokine from its primary source (lymph nodes) using a sensitive ELISA assay. As a positive control for systemic lymphopenia, we used RAG2−/− mice; these animals lack mature B and T cells and constitutively express high levels of IL-7.16 C57 BL/6 mice indeed displayed a compensatory increase in IL-7 in response to the abbreviated RT regimen, reaching levels seen in the lymphopenic RAG2−/− mice (Fig. 6A). Specifically, IL-7 levels were inversely correlated with TLC (Fig. 6B) throughout treatment, strongly supporting the hypothesis that the recovery of systemic lymphocytes in this animal model was driven by a compensatory increase in IL-7. Due to the identical responses by both animal strains in response to both radiotherapy regimens, IL-7 levels were not tested in the other conditions investigated by this study. We also attempted to measure serum IL-7 levels but these were undetectable using commercially available ELISA reagents.
Figure 6.
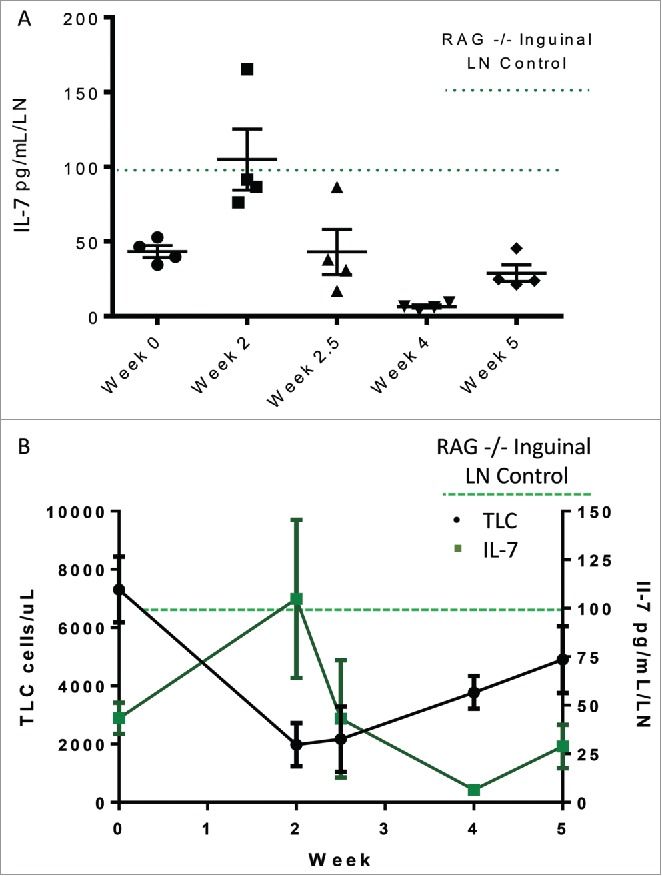
(A) IL-7 levels in distal lymph nodes measured over the course of treatment in C57 Bl/6 animals treated with 4 Gy x 10 fractions. (B) IL-7 levels are inversely correlated with peripheral lymphocyte counts.
Discussion
In these studies, we found that: 1) local radiation to the brain, a region of the body that contains minimal lymphatic vessels, results in marked systemic lymphopenia in two strains of otherwise heathy mice, 2) parallel sampling of lymph nodes from these animals demonstrated that radiation dramatically affects lymphatic tissue architecture in distant non-irradiated regions, and 3) compensatory changes in IL-7 level suggest that this may be an important regulatory factor in the recovery of lymphocytes from treatment-related lymphopenia.
The pre-clinical and clinical observations described above prompted the development of the animal model described in this manuscript to better understand the mechanism of sustained lymphocyte depletion. An advantage of targeting the brain for radiation is that minimal radiation doses are delivered to distal nodal or bone marrow sites. However, even under these conditions we found that virtually all peripheral lymphocyte subsets are substantially affected by focal brain radiation. Specifically, TLC dropped to 40–70% of baseline irrespective of strain or abbreviated versus prolonged treatment plan. This was observed in all lymphocyte subsets studied, including total T cells, CD4+ T conventional cells, CD4+ T regulatory cells, and CD8+ T cells. Similar to patients receiving cranial irradiation, brain radiation in our animals had a disproportionate effect on lymphocytes compared to the effect on neutrophils, platelets and red blood cells.
We next wanted to assess whether radiation-induced lymphopenia was also associated with histopathologic correlates in peripheral lymph nodes. A hematopathologist blinded to the treatment group and time point reviewed hematoxylin and eosin-stained sections of inguinal lymph nodes (LN) from baseline, end of treatment and recovery time periods. Post-treatment lymph nodes displayed paracortical expansions with a relative increase in histiocytes and plasma cells and a relative decrease in lymphocytes. Collections of prominent epithelioid histiocytes in hilar regions were also noted with variably numbers of associated tingible body macrophages and sea blue histiocytes, consistent with phagocytosis. These data underscore that the radiotherapy regimen can have affects outside the radiation field. It is reasonable to conclude that the loss of circulating lymphocytes as well as changes in lymphoid tissues outside the radiation field could leave patients in an immunocompromised state, increasing their susceptibility to infection or additional cancerous growths. This conclusion is consistent with the observations in patients with glioblastoma with post-treatment lymphopenia who are sufficiently immunosuppressed that they are at high risk for opportunistic infections such as pneumocystis pneumonia.17
We found that there is a striking difference between peripheral lymphocyte recovery following radiotherapy in mice and humans. While a significant fraction of patients remain severely lymphopenic for over a year, mice rapidly recovered from lymphopenia and their TLC returned to > 60% of baseline within 3 weeks of the end of radiation. Some changes to lymph node architecture persist, however, at this 3 week time point. This finding highlights that animal models do not always accurately represent disease in humans, and further work investigating TRL needs to be investigated using human subjects.
Since the animal model for TRL is in other ways similar to TRL observed in human patients, we attempted to identify what factor(s) may explain the difference in lymphocyte recovery post-RT. IL-7 is the natural homeostatic response to lymphopenia, leading to increased T cell proliferation.18 IL-7 is required for maintaining homeostasis of T cells and levels are inversely correlated with peripheral CD4 counts in patients with HIV/AIDs. Previous work by our group noted that sustained TRL in patients with high grade gliomas undergoing RT did not have an expected compensatory IL-7 response.15 Therefore, we hypothesized that mice undergoing RT do have a compensatory IL-7 response to lymphodepletion, preceding systemic lymphocyte recovery unlike their human counterparts. Indeed, we could measure IL-7 levels from distal inguinal LN which displayed the expected inverse correlation with total lymphocyte counts. The measured IL-7 levels were on par with RAG2−/− animals, which lack B and T cells and are perpetually in a state of lymphodepletion, making them a strong positive control for our TRL mice. This finding supports the hypothesis that sustained TRL in humans may be due to a lack of IL-7 response after lymphodepletion and raises the question as to whether exogenous IL-7 could help improve CD4 lymphopenia in this population; human studies are underway to determine if this could be helpful in the post-radiation setting (NCT02659800).
Finally, this animal model provides clear evidence that systemic TRL can be directly attributed to local radiation and occurs even without other potentially confounding variables such as an underlying malignancy or the concomitant use of glucocorticoids or chemotherapy. However, given the spontaneous recovery of lymphocyte counts in mice once the radiation is discontinued, this is not an adequate model to test novel interventions to potentiate the recovery of lymphocytes in humans. Further pre-clinical and clinical studies are needed to improve our understanding of this radiation-related lymphopenia and to reduce its clinical impact as nearly 40% of patients receiving radiation will develop severe lymphopenia. The development of new approaches to this inadvertent toxicity of radiation could lead to improved responses to novel therapeutic immunotherapy approaches for cancer.
Materials and methods
Animals
Both BALB/c and C57 BL/6 mice were chosen for their known differences in radiosensitivity and prototypical immune responses. Seven to 12-week-old male BALB/cJ and C57 BL/6 J mice were purchased from The Jackson Laboratory. Rag2−/− mice were used as a positive control for a lympho-depleted model since they lack both B and T cells; these were purchased from Taconic Farms. All animal studies were approved by by the Animal Care and Use Committee of the Johns Hopkins University School of Medicine.
Radiation
Multiple treatment plans were studied including: 1 Gy x 5 fractions, 2 Gy x 5 fractions, 3 Gy x 5 fractions, 2 Gy x 10 fractions, 4 Gy x 10 fractions, and 2 Gy x 30 fractions. A second set of more extended studies focused on a short course of radiation (4 Gy x 10 fractions over 2 weeks) and a longer treatment plan (2 Gy x 30 fractions over 6 weeks).
Small Animal Radiation Research Platform (SARRP)
Radiation was delivered using the Small Animal Radiation Research Platform (SARRP) (Xstrahl, Inc). Animals were anesthetized using isoflurane then immobilized on the rotary bed. The SARRP is equipped with cone beam computed tomography (CBCT) which was used for an image guided and highly conformal radiation delivery to the murine brain. An AP (anterior to posterior) beam of 10 × 10 mm at a depth of 5 mm (345 SSD) was used. The energy of this beam was 220 kVp (voltage) and 13 mA (current). The system's accuracy of the beam position is 0.2 mm. [Fig. 1]
Lymphocyte subtypes
Two techniques were utilized for lymphocyte analysis. Hemavet was used for rapid cell counts during dose finding studies, whereas TruCountTM (Becton Dickinson Biosciences, 340334) was used for detailed subtype analysis in extended studies. BD Trucount tubes were used in accordance with manufacture's specifications; each tube contained a lyophilized pellet of a known number of fluorescent beads. By comparing cellular events to cellular events during FACS analysis, the absolute number of cells can be determined. By measuring cellular events as a function of which cell surface markers are expressed, the absolute number of individual cell subtypes was determined. Hemavet analysis of absolute lymphocyte count was performed via weekly tail vein draws collected in Microtainer Tubes with Dipotassium EDTA (Becton Dickinson Biosciences, 365974) to prevent clotting. A Hemavet 950 (Drew Scientific) was used to analyze multiple hematologic parameters including total lymphocyte count. BD Trucount™ and flow cytometric analysis of lymphocyte subsets was performed using tail vein blood collected in Microtainer Tubes with Dipotassium EDTA (BD Biosciences Cat. 365974). 30 uL of whole blood was transferred to BD Trucount™ (Cat. 340334) along with 30 uL of FITC Hamster Anti-Mouse CD3e (BD 553062, 1:200 dilution), 30 uL of PE Rat Anti-Mouse CD4 (BD 553730, 1:200 dilution), 30 uL of PerCP/Cy5.5 anti-mouse CD25 (Biolegend San, 102029, 1:100 dilution) and 30 uL of APC anti-mouse CD45 (Biolegend 103111, 1:200 dilution). Samples were vortexed and allowed to incubate at room temperature in the dark for 30 minutes. Post incubation, 350 uL of Ammonium-Chloride-Potassium (ACK) buffer was added to each sample. Samples were vortexed and allowed to incubate for 30 minutes at room temperature in the dark. Samples were then analyzed using a BD FacsCalibur instrument (Becton Dickinson [BD) gating on CD45+ (total lymphocytes), CD45+CD3+ (total T cells), CD45+CD3+CD4−(CD8 T cells), CD45+CD3+CD4+CD25low(CD4 T cells), CD45+CD3+CD4+CD25hi (CD4 T regulatory cells). Data were analyzed using FlowJo software (Treestar).
Pathologic analysis of resected inguinal lymph nodes
Inguinal lymph nodes well outside the radiation field were collected: Pre-radiation, end of radiation, and once lymphocyte levels had recovered. Four animals per group were analyzed, using both the left and right nodes. Lymph nodes were fixed in 10% paraformaldehyde for 48 hours at room temperature, then stored in Phosphate Buffered Saline (PBS) until embedding in paraffin. Formalin Fixed Paraffin embedded inguinal lymph nodes were then prepared for hematoxylin and eosin (H&E) staining and analysis was performed in a blinded manner by a trained surgical pathologist (KHB).
Cytokine analysis in blood and lymph nodes
Whole blood was collected in a microcentrifuge tube and allowed to clot at room temperature for 20 minutes before it was centrifuged at 1,500 x g to pellet the clot. Serum was isolated and stored immediately at −80 degrees Celsius. Luminex Assays were performed for IL-1β, IL-2, IL-6, IL-7, IL-17, TNFα (Millipore, MCYTOMAG-70 K). Samples were diluted 1:2 in assay buffer. Standard curves were within the normal ranges expected and quality controls were within the expected ranges. Inguinal lymph node lysate was obtained by harvesting both inguinal lymph nodes and then performing mechanical dissociation in 100 uL of sterile PBS. Whole lysate was then transferred to a 1.7 mL microcentrifuge tube and centrifuged at 500 x g for 8 minutes at room temperature. Supernatant was then removed and transferred to a fresh microcentrifuge tube and stored at −80 °C. Serum and inguinal lymph node lysate IL-7 cytokine levels were measured via ELISA (RayBiotech, Cat. ELM-IL7-1, sensitivity 10 pg/ml – 6000 pg/ml).
Supplementary Material
Funding Statement
This work was supported by the Claudia Fund (Johns Hopkins) NIH/NCI Grant (P30 CA008748).
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed
References
- 1.Grossman SA, Ellsworth S, Campian J, Wild AT, Herman JM, Laheru D, Brock M, Balmanoukian A, Ye X. Survival in Patients With Severe Lymphopenia Following Treatment With Radiation and Chemotherapy for Newly Diagnosed Solid Tumors. J Natl Compr Canc Netw. 2015;13(10):1225–31. Retrieved from http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4778429/ doi: 10.6004/jnccn.2015.0151. PMID:26483062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grossman SA, Ye X, Lesser G, Sloan A, Carraway H, Desideri S, Piantadosi S. Immunosuppression in Patients with High Grade Gliomas Treated with Radiation and Temozolomide. Clin Cancer Res: An Official Journal of the American Association for Cancer Research. 2011;17(16):5473–80. doi: 10.1158/1078-0432.CCR-11-0774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Balmanoukian A, Ye X, Herman J, Laheru D, Grossman SA. The Association Between Treatment-Related Lymphopenia and Survival in Newly Diagnosed Patients with Resected Adenocarcinoma of the Pancreas. Cancer Invest. 2012;30(8):571–6. doi: 10.3109/07357907.2012.700987. PMID:22812722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wild AT, Ye X, Ellsworth SG, Smith JA, Narang AK, Garg T, Campian J, Laheru DA, Zheng L, Wolfgang CL. The Association Between Chemoradiation-related Lymphopenia and Clinical Outcomes in Patients With Locally Advanced Pancreatic Adenocarcinoma. Am J Clin Oncol. 2015;38(3):259–65. doi: 10.1097/COC.0b013e3182940ff9. PMID:23648440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Campian J L, Ye X, Brock M, Grossman SA. Treatment-related Lymphopenia in Patients With Stage III Non-Small-Cell Lung Cancer. Cancer Invest. 2013;31(3):183–8. doi: 10.3109/07357907.2013.767342. PMID:23432821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tang C, Liao Z, Gomez D, Levy L, Zhuang Y, Gebremichael RA, Hong DS, Komaki R, Welsh JW. Lymphopenia Association With Gross Tumor Volume and Lung V5 and Its Effects on Non-Small Cell Lung Cancer Patient Outcomes. Int J Radiat Oncol Biol Phys. 2014;89(5):1084–91. doi: 10.1016/j.ijrobp.2014.04.025. PMID:25035212. [DOI] [PubMed] [Google Scholar]
- 7.Afghahi A, Mathur M, Seto T, Desai M, Kenkare P, Horst KC, Das AK, Thompson CA, Luft HS, Yu PP, et al.. Lymphopenia after adjuvant radiotherapy (RT) to predict poor survival in triple-negative breast cancer (TNBC). J Clin Oncol. 2015;33(15_suppl):1069. [Google Scholar]
- 8.Cronkite EP, Jansen CR, Mather GC, Nielsen NO, Usenik EA, Adamik ER, Sipe CR. Studies on lymphocytes. I. Lymphopenia produced by prolonged extracorporeal irradiation of circulating Blood. 1962;20:203–13. Retrieved from http://www.osti.gov/scitech/servlets/purl/4820729 PMID:13882338. [PubMed] [Google Scholar]
- 9.Oldendorf WH, Burroughs JT, Cassen B, Wetterau LW. Beta Radiation of Circulating Blood by an Implanted Shielded Y90 Source, A Preliminary Report of Technique. J Nucl Med. 1964;5(11):883–6. Retrieved from http://jnm.snmjournals.org/content/5/11/883.short PMID:14247784. [PubMed] [Google Scholar]
- 10.JS W, DM H. Studies of a method of inducing specific lymphopenia in dogs. JAMA. 1965;194(10):1119–21. Retrieved from https://doi.org/ 10.1001/jama.1965.03090230087022 doi: 10.1001/jama.1965.03090230087022. PMID:5897788. [DOI] [PubMed] [Google Scholar]
- 11.Storb R, Ragde H, Thomas ED. Extracorporeal Irradiation of the Blood in Baboons. Radiat Res. 1969;38(1):43–54. doi: 10.2307/3572709. PMID:4976015. [DOI] [PubMed] [Google Scholar]
- 12.Yovino S, Kleinberg L, Grossman SA, Narayanan M, Ford E. The Etiology of Treatment-related Lymphopenia in Patients with Malignant Gliomas: Modeling Radiation Dose to Circulating Lymphocytes Explains Clinical Observations and Suggests Methods of Modifying the Impact of Radiation on Immune Cells. Cancer Invest. 2013;31(2):140–4. doi: 10.3109/07357907.2012.762780. PMID:23362951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Louveau A, Smirnov I, Keyes TJ, Kipnis J. Structural and functional features of central nervous system lymphatic vessels. Nature. 2015;523(7560):337–41. http://www.nature.com/nature/journal/v523/n7560/full/nature14432.html doi: 10.1038/nature14432. PMID:26030524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Napolitano LA, Grant RM, Deeks SG, Schmidt D, De Rosa SC, Herzenberg LA, Herndier BG, Andersson J, McCune JM. Increased production of IL-7 accompanies HIV-1-mediated T-cell depletion: implications for T-cell homeostasis. Nat Med. 2001;7(1):73–79. Retrieved from doi: 10.1038/83381. PMID:11135619. [DOI] [PubMed] [Google Scholar]
- 15.Ellsworth S, Balmanoukian A, Kos F, Nirschl CJ, Nirschl TR, Grossman SA, Luznik L, Drake CG. Sustained CD4(+) T cell-driven lymphopenia without a compensatory IL-7/IL-15 response among high-grade glioma patients treated with radiation and temozolomide. Oncoimmunology. 2014;3(1):e27357. doi: 10.4161/onci.27357. PMID:24790790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Min B, Yamane H, Hu-Li J, Paul WE. Spontaneous and Homeostatic Proliferation of CD4 T Cells Are Regulated by Different Mechanisms. J Immunol. 2005;174(10):6039 LP-6044 Retrieved from http://www.jimmunol.org/content/174/10/6039.abstract doi: 10.4049/jimmunol.174.10.6039. [DOI] [PubMed] [Google Scholar]
- 17.Hughes MA, Parisi M, Grossman S, Kleinberg L. Primary brain tumors treated with steroids and radiotherapy: Low CD4 counts and risk of infection. Int J Radiat Oncol Biol Phys. 2005;62(5):1423–6. doi: 10.1016/j.ijrobp.2004.12.085. PMID:16029802. [DOI] [PubMed] [Google Scholar]
- 18.Mackall CL, Fry TJ, Gress RE. Harnessing the biology of IL-7 for therapeutic application. Nat Rev Immunol. 2011;11(5):330–42. doi: 10.1038/nri2970. PMID:21508983. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.