ABSTRACT
Erdheim-Chester disease (ECD) is a rare histiocytosis, characterized by xanthogranulomatous tissue infiltration by foamy histiocytes. Fibrosis, a histologic hallmark of ECD, is responsible for lesion growth and clinical manifestations. Unraveling molecular fibrotic pathway in ECD would allow the identification of new pharmacologic targets.
In this study, we evaluated serum and tissue samples from a large cohort of ECD patients focusing on two major pro-fibrotic mediators, TGF-β1 and chemokine ligand 18 (CCL18). We found a marked increase in CCL18 but not TGF-β1 levels in serum and lesions of ECD patients (p < 0.001), independently of treatment status and consistently over time. Using a linear mathematical model, we also found that elevated CCL18 serum levels correlate with both number and severity of disease localizations.
These findings suggest the involvement of CCL18-induced fibrosis in ECD pathogenesis, providing a rationale for exploring CCL18 inhibition as a treatment for progressive fibrosis in ECD.
KEYWORDS: Erdheim-Chester disease, fibrosis, CCL-18
Introduction
Erdheim-Chester disease (ECD) is a rare non-Langerhans cells histiocytosis, characterized by xanthogranulomatous infiltration of tissues by CD68-positive, CD1a-/S100-negative foamy histiocytes, surrounded by fibrosis. Since virtually every tissue can be affected by ECD, the clinical picture is protean and the prognosis is often severe.1-3 The oncogenic BRAFV600E mutation and deregulated activation of the mitogen-activated protein kinase (MAPK) pathway are central to ECD pathogenesis.4,5 A pro-inflammatory cytokine-chemokine network, responsible for recruitment and activation of histiocytes, is also a consistent finding in the lesions and serum of ECD patients.6-8
Fibrosis, a histologic hallmark of ECD, is directly responsible for lesion dimensional growth and clinical manifestations, and is not amenable to treatment with currently available strategies. The molecular mechanisms underlying fibrosis in ECD remain undetermined, and previous studies investigating different pro-fibrotic mediators failed to demonstrate a relevance to ECD pathogenesis.6-8 This study investigated the role in the pathogenesis of ECD of two mediators, TGF-β1 and chemokine ligand 18 (CCL18), directly involved in the induction and progression of fibrosis in various physiologic and pathologic conditions.
Results
We first assessed serum levels of TGF-β1 and CCL18 in ECD patients and controls. TGF-β1 levels were not significantly elevated in ECD patients (Fig. 1A; median in ECD patients 33 ng/ml, range 9–74; controls 31 ng/ml; range 9–68), a consistent finding across different time-points during the follow-up (data not shown). Conversely, ECD patients exhibited a significant, 3.4-fold increase in CCL18 serum levels compared to controls (Fig. 1B; median in ECD patients 100 pg/ml, range 35–307; controls 27 pg/ml, range 15–94; p < 0.001). Of note, this elevation was independent of treatment status, as both treated and untreated ECD patients had higher CCL18 levels than controls (p < 0.05 and <0.005, respectively; Fig. 1C), while no significant differences were observed between treated and untreated patients (p = 0.1). Again, this observation was consistent across different time points (data not shown).
Figure 1.
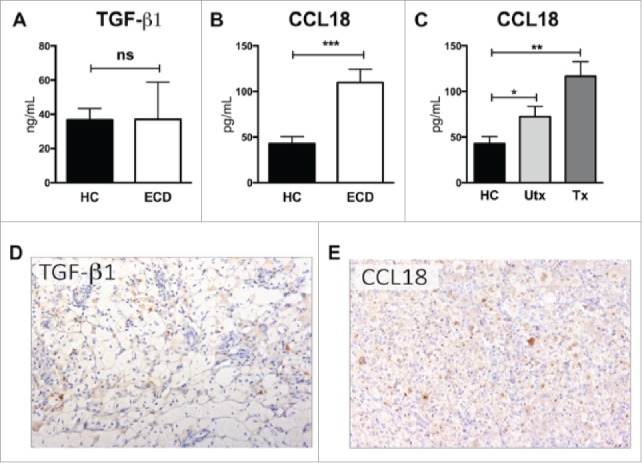
Serum and tissue levels of TGF-β1 and CCL18 in ECD patients. (A) Mean ± SEM plasma levels of TGF-β1 in ECD patients and healthy controls. (B) Mean ± SEM plasma levels of CCL18 in ECD patients and healthy controls. (C) Mean ± SEM plasma levels of CCL18 in treated and untreated ECD patients as compared to healthy controls. Groups were compared using unpaired Student's t-test, data are expressed as mean ± SEM of 20 ECD patients and 20 controls. (D) Expression of TGF-β1 and CCL18 was evaluated in different ECD lesions with immunohistochemistry. Panels D and E show microphotographs of representative lesions (skin biopsies). (D) Immunohistochemical assessments do not show a significant intra-lesional expression of TGF-β1. (E) CCL18 is markedly expressed by macrophages, with cytoplasmic reactivity and a particular reinforcement of the Golgi apparatus. Immunohistochemistry for TGF-β1 and CCL18 was performed with diaminobenzidine and background staining with hematoxylin; original magnification 200 ×. ECD Erdheim-Chester disease, HC healthy control, Utx untreated patients, Tx treated patients; ns non-significant, *p<0.05, **p<0.005, ***p<0.001.
We then evaluated the expression of TGF-β1 and CCL18 in ECD skin lesions with immunohistochemistry. No remarkable TGF- β1 expression was detected, while CCL18 was markedly expressed by intralesional macrophages, with a clear intracellular localization in foamy histiocytes (Fig. 1D–E).
Finally, we developed a linear mathematical model to evaluate correlations between CCL18 serum levels and various epidemiological features, clinical manifestations, and serum markers in ECD patients (Fig. 2, and Supplementary Data). Elevated CCL18 levels positively correlated with central nervous system (CNS) involvement and with the number of disease localizations (p < 0.05). No significant correlations were found with main epidemiologic variables, disease duration, or serum levels of pro-inflammatory mediators or markers.
Figure 2.
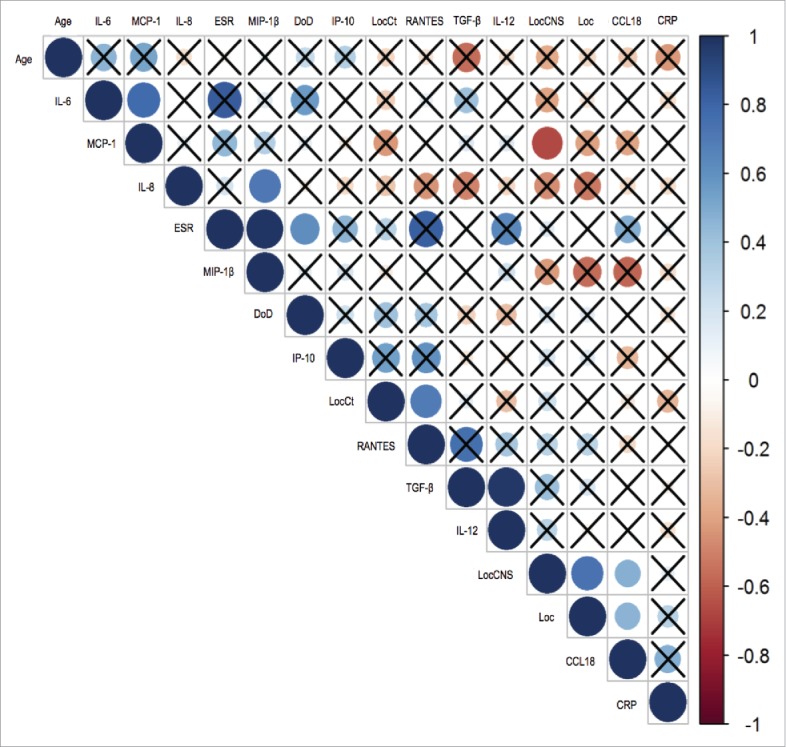
Correlation between CCL18 levels and other variables in ECD patients. Graphic representation of the analysis performed using univariate linear model. The color scale ranges from 1 (correlation coefficient ρli = 1, correlation of the direct type) to -1 (correlation coefficient ρ1, = -1, correlation of the indirect type). A weak correlation is represented with 0<ρ-1<0.3, a moderate correlation with 0.3 <ρ0. <0.7 and a strong correlation with ρ<0> 0.7. The correlation index is 0 if the two variables are independent. The barred boxes represent the pairs of variables whose correlation does not result to be statistically significant with α = 0.05. There is a statistically significant positive correlation between the variables CCL18 and LocCNS and between CCL18 and Locations. IL-6 Interlukin 6, MCP-1 Monocyte chemoattractant protein-1, IL-8 Interlukin 8, ESR Erythrocyte sedimentation rate, MIP-1β Macrophage inflammatory protein-1 beta, DoD Duration of disease, IP-10 Interferon gamma-induced protein 10, LocCt Cutaneous localization, TGF- β transforming growth factor beta, IL-12 Interlukin 12, LocCNS Central nervous system localization, Loc Localisation, CCL18 Chemokine ligand 18, CRP C reactive protein.
Discussion
This study points at the involvement of the pro-fibrotic mediator CCL18 in the pathogenesis of fibrosis that characterizes ECD. CCL18 is produced by macrophages and mainly involved in orchestrating cell-migration and homing.9 Of note, CCL18 expression is induced by Th2-related cytokines,9,10 thus representing a marker for macrophages non-canonically activated by Th2-related stimuli, termed ‘alternatively activated’ macrophages. CCL18 and alternatively activated macrophages are known to stimulate collagen production and fibroblast proliferation, in part through the activation of the MAPK pathway.11,12 In this study, circulating levels of CCL18 were strikingly elevated in ECD patients compared to controls (p < 0.001). Interestingly, this elevation was independent of treatment status, a finding consonant with clinical observations that fibrosis in ECD is poorly responsive to available therapies. In ECD lesions, we observed a marked expression of CCL18 with a prominent localization within infiltrating foamy histiocytes, a finding suggesting that this potent chemo-attractant may also be involved in lymphocyte and macrophage recruitment into ECD lesions. Indeed, elevated levels of CCL18 are encountered in several other diseases characterized by both leucocyte recruitment and fibrosis, including pulmonary fibrosis, systemic sclerosis, and Gaucher disease.13-15
Unexpectedly, we found that serum and tissue levels of the major pro-fibrotic mediator TGF-β1 are not significantly elevated in ECD patients. The effects of TGF-β1 have been extensively described in various diseases characterized by progressive fibrosis, including systemic sclerosis, retroperitoneal fibrosis and idiopathic pulmonary fibrosis.16,17 For example, following tissue damage, TGF-β1 promotes mesenchymal compartment expansion, fibroblasts recruitment, and extracellular matrix deposition.18 Previous studies on ECD patients also reported normal circulating levels of TGF-β1-related cytokines (IL-4, IL-5, IL-13).6-8 We conclude that very low tissue and circulating levels strongly argue against the involvement of TGF-β1 in the pathogenesis of ECD. The role of other regulatory cytokines whose activity overlaps in part with TGF-β1 is to be determined.19-21
Given the role of CCL18 in leukocyte recruitment and fibrosis, we also evaluated potential correlations with disease severity in ECD patients. Elevated CCL18 levels were associated with CNS involvement, an independent predictor of death in ECD patients. Since in cases characterized by CNS involvement ECD tends to have an aggressive course and a multisystem spread,22,23 such elevation may be an expression of greater disease burden, as also suggested by the positive correlation between CCL18 levels and the number of disease localizations. Given this correlation with both number and severity of ECD localizations, further studies should determine whether CCL18 might represent a useful tool in the prospective or prognostic evaluation of ECD, for which no validated biomarker is currently available.24 At present, no treatment available for ECD has clearly demonstrated efficacy against fibrosis.25 Biologic agents blocking the proinflammatory cytokine IL-1 are promising, as this molecule is a central mediator of fibrosis and induces hundreds of chemokines.26,27 Anakinra, the recombinant version of the naturally occurring IL-1 receptor antagonist, blocks the activity of IL-1 in a broad variety of inflammatory disorders28-32 and can be effective in the treatment of ECD as well as other conditions characterized by progressive fibrosis.33,34 Whether anakinra can reduce the fibrotic burden in ECD or dampen levels of CCL18 specifically remains to be determined. Nevertheless, the identification of CCL18 as a target amenable to pharmacologic intervention should encourage exploration of tailored therapeutic strategies, aimed at halting the progression of the fibrotic process. Independent validation studies of CCL18 as a biomarker and therapeutic target in ECD are warranted.
Patients and methods
All studies were approved by Institutional Ethics Committee and conducted in accordance with the Helsinki Declaration. Serum samples were obtained from 20 histologically confirmed ECD cases (15 male and 5 female; median age, 62 years; range, 35–80) evaluated at our Institution between January 2001 and June 2010, and from age- and sex-matched healthy controls.35 Since spontaneous variations in cytokine serum levels may occur over time, we evaluated multiple (at least 5) samples for each patient obtained at different time points during the follow-up, as previously described.27 Levels of CCL18 and TGF-β1 were assessed with specific ELISAs (BioSource Europe Sa, Nivelles, Belgium). Statistical significance was evaluated with the unpaired Student's t-test (Prism version 6.0, GraphPad, San Diego, CA).
Tissue samples were obtained from ECD skin lesions, formalin-fixed, stained with hematoxylin, and incubated with the following monoclonal antibodies: CD68 (Dako, Glostrup, Denmark), p16Ink4a (clone JC8, Santa Cruz Biotechnology, Dallas, TX, USA), CCL18/MIP-4 (clone A7, Santa Cruz Biotechnology), TGF-β1 (clone TB21, AbD Serotec, Raleigh, NC, USA), Ki-67 (MIB-1 clone, Dako), following the manufacturer's instructions.
Correlation of CCL18 serum levels with various epidemiological features, clinical manifestations, and biohumoral markers was assessed with a linear mathematical model. Statistical analyses were performed using R version 3.2.1 for Windows 8 and GraphPad Prism version 5.0b for Macintosh (GraphPad). Data were expressed through statistical descriptors (Spearman's rank correlation coefficient) and, when categorized, by calculating median and range of variability.
Supplementary Material
Funding Statement
This work was supported in part by a grant from the Erdheim-Chester Global Alliance, De Ridder, LA (to MF and LD).
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- 1.Campochiaro C, Tomelleri A, Cavalli G, Berti A, Dagna L. Erdheim-Chester disease. Eur J Intern Med [Internet]. 2015;26:223–9. Available from: http://linkinghub.elsevier.com/retrieve/pii/S0953620515000771. doi: 10.1016/j.ejim.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 2.Cavalli G, Guglielmi B, Berti A, Campochiaro C, Sabbadini MG, Dagna L. The multifaceted clinical presentations and manifestations of Erdheim-Chester disease: comprehensive review of the literature and of 10 new cases. Ann Rheum Dis [Internet]. 2013;72:1691–5. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23396641. doi: 10.1136/annrheumdis-2012-202542. [DOI] [PubMed] [Google Scholar]
- 3.Cavalli G, Berti A, Campochiaro C, Dagna L. Diagnosing Erdheim–Chester disease. Ann Rheum Dis [Internet]. 2013;72:e19 LP-e19 Available from: http://ard.bmj.com/content/72/7/e19.abstract [DOI] [PubMed] [Google Scholar]
- 4.Haroche J, Charlotte F, Arnaud L, Deimling A Von, Hélias-rodzewicz Z, Hervier B, Cohen-aubart F, Launay D, Lesot A, Mokhtari K, et al.. High prevalence of BRAF V600E mutations in Erdheim-Chester disease but not in other non-Langerhans cell histiocytoses Brief report High prevalence of BRAF V600E mutations in Erdheim-Chester disease but not in other non-Langerhans cell histiocytoses. Blood 2012;120:2700–3. [DOI] [PubMed] [Google Scholar]
- 5.Cangi MG, Biavasco R, Cavalli G, Grassini G, Dal-Cin E, Campochiaro C, Guglielmi B, Berti A, Lampasona V, von Deimling A, et al.. BRAFV600E-mutation is invariably present and associated to oncogene-induced senescence in Erdheim-Chester disease. Ann Rheum Dis [Internet]. 2015;74:1–7. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24671772 [DOI] [PubMed] [Google Scholar]
- 6.Stoppacciaro A, Ferrarini M, Salmaggi C, Colarossi C, Praderio L, Tresoldi M, Beretta A A., Sabbadini MG. Immunohistochemical evidence of a cytokine and chemokine network in three patients with Erdheim-Chester disease: Implications for pathogenesis. Arthritis Rheum. 2006;54:4018–22. doi: 10.1002/art.22280. PMID:17133532 [DOI] [PubMed] [Google Scholar]
- 7.Arnaud L, Gorochov G, Charlotte F, Lvovschi V, Parizot C, Larsen M, Ghillani-Dalbin P, Hervier B, Kahn JE, Deback C, et al.. Systemic perturbation of cytokine and chemokine networks in Erdheim-Chester disease: A single-center series of 37 patients. Blood. 2011;117:2783–90. doi: 10.1182/blood-2010-10-313510. PMID:21205927 [DOI] [PubMed] [Google Scholar]
- 8.Cavalli G, Biavasco R, Borgiani B, Dagna L. Oncogene-induced senescence as a new mechanism of disease: The paradigm of Erdheim-Chester disease. Front Immunol. 2014;5:1–6. doi: 10.3389/fimmu.2014.00281. PMID:24474949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schutyser E, Richmond A, Van Damme J. Involvement of CC chemokine ligand 18 (CCL18) in normal and pathological processes. J Leukoc Biol. 2005;78:14–26. doi: 10.1189/jlb.1204712. PMID:15784687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.a Islam S, MF Ling, Leung J, Shreffler WG, Luster AD. Identification of human CCR8 as a CCL18 receptor. J Exp Med [Internet]. 2013;210:1889–98. Available from: http://www.jem.org/cgi/doi/ 10.1084/jem.20130240\npapers3://publication/doi/ doi:. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Atamas SP, Luzina IG, Choi J, Tsymbalyuk N, Carbonetti NH, Singh IS, Trojanowska M, Jimenez S a., White B. Pulmonary and Activation-Regulated Chemokine Stimulates Collagen Production in Lung Fibroblasts. Am J Respir Cell Mol Biol. 2003;29:743–9. doi: 10.1165/rcmb.2003-0078OC. PMID:12805086 [DOI] [PubMed] [Google Scholar]
- 12.Prasse A, Pechkovsky D V., Toews GB, Jungraithmayr W, Kollert F, Goldmann T, Vollmer E, Müller-Quernheim J, Zissel G. A vicious circle of alveolar macrophages and fibroblasts perpetuates pulmonary fibrosis via CCL18. Am J Respir Crit Care Med. 2006;173:781–92. doi: 10.1164/rccm.200509-1518OC. PMID:16415274 [DOI] [PubMed] [Google Scholar]
- 13.Prasse A, Pechkovsky D V., Toews GB, Schäfer M, Eggeling S, Ludwig C, Germann M, Kollert F, Zissel G, Müller-Quernheim J. CCL18 as an indicator of pulmonary fibrotic activity in idiopathic interstitial pneumonias and systemic sclerosis. Arthritis Rheum. 2007;56:1685–93. doi: 10.1002/art.22559. PMID:17469163 [DOI] [PubMed] [Google Scholar]
- 14.Boot RG, Verhoek M, De Fost M, Hollak CEM, Maas M, Bleijlevens B, Van Breemen MJ, Van Meurs M, a Boven L, Laman JD, et al.. Marked elevation of the chemokine CCL18 / PARC in Gaucher disease : a novel surrogate marker for assessing therapeutic intervention. Blood 2004;103:33–9. [DOI] [PubMed] [Google Scholar]
- 15.a. Hägg D, FJ Olson, Kjelldahl J, Jernås M, Thelle DS, Carlsson LMS, Fagerberg B, Svensson PA. Expression of chemokine (C-C motif) ligand 18 in human macrophages and atherosclerotic plaques. Atherosclerosis. 2009;204:15–20. doi: 10.1016/j.atherosclerosis.2008.10.010. [DOI] [PubMed] [Google Scholar]
- 16.Derynck R, Akhurst RJ. Differentiation plasticity regulated by TGF-beta family proteins in development and disease. Nat Cell Biol. 2007;9:1000–4. doi: 10.1038/ncb434. PMID:17762890 [DOI] [PubMed] [Google Scholar]
- 17.Pohlers D, Brenmoehl J, Löffler I, Müller CK, Leipner C, Schultze-Mosgau S, Stallmach A, Kinne RW, Wolf G. TGF-β and fibrosis in different organs – molecular pathway imprints. Biochim Biophys Acta – Mol Basis Dis. 2009;1792:746–56. doi: 10.1016/j.bbadis.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 18.Massagué J. TGFβ signalling in context. Nat Rev Mol Cell Biol. 2012;13:616–30. doi: 10.1038/nrm3434. PMID:22992590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cavalli G, Justice JN, Boyle KE, D'Alessandro A, Eisenmesser EZ, Herrera JJ, Hansen KC, Nemkov T, Stienstra R, Garlanda C, et al.. Interleukin 37 reverses the metabolic cost of inflammation, increases oxidative respiration, and improves exercise tolerance. Proc Natl Acad Sci [Internet]. 2017;114:2313–8. Available from: http://www.pnas.org/lookup/doi/ 10.1073/pnas.1619011114. doi: 10.1073/pnas.1619011114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cavalli G, Dinarello CA. Suppression of inflammation and acquired immunity by IL-37. Immunol Rev [Internet]. 2018;281:179–90. Available from: doi.wiley.com/ 10.1111/imr.12605. doi: 10.1111/imr.12605. [DOI] [PubMed] [Google Scholar]
- 21.Cavalli G, Koenders M, Kalabokis V, Kim J, Tan A, Garlanda C, Mantovani A, Dagna L, Joosten LA, Dinarello CA, et al.. Treating experimental arthritis with the innate immune inhibitor interleukin-37 reduces joint and systemic inflammation. Rheumatology (Oxford). 2016;55:2220–9. doi: 10.1093/rheumatology/kew325. PMID:27567100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cives M, Simone V, Rizzo FM, Dicuonzo F, Cristallo Lacalamita M, Ingravallo G, Silvestris F, Dammacco F. Erdheim–Chester disease: A systematic review. Crit Rev Oncol Hematol [Internet]. 2015; Available from: http://linkinghub.elsevier.com/retrieve/pii/S1040842815000281. doi: 10.1016/j.critrevonc.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 23.Arnaud L, Hervier B, Néel A, Hamidou M a., Kahn JE, Wechsler B, Pérez-Pastor G, Blomberg B, Fuzibet JG, Dubourguet F, et al.. CNS involvement and treatment with interferon-α are independent prognostic factors in Erdheim-Chester disease: A multicenter survival analysis of 53 patients. Blood. 2011;117:2778–82. doi: 10.1182/blood-2010-06-294108. PMID:21239701 [DOI] [PubMed] [Google Scholar]
- 24.Ferrero E, Corti A, Haroche J, Belloni D, Colombo B, Berti A, Cavalli G, Campochiaro C, Villa A, C AF, Amoura Z, et al.. Plasma Chromogranin A as a marker of cardiovascular involvement in Erdheim-Chester disease. Oncoimmunology. 2016;5:e1181244. doi: 10.1080/2162402X.2016.1181244. PMID:27622037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cavalli G, De Luca G DL. Advances in potential targeted therapies for Erdheim-Chester disease. Expert Opin Orphan Drugs. 2017;5:253–60. [Google Scholar]
- 26.Dinarello CA. Interleukin-1 in the pathogenesis and treatment of inflammatory diseases. Blood. 2011;117:3720–32. doi: 10.1182/blood-2010-07-273417. PMID:21304099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cavalli G, Hayashi M, Jin Y, Yorgov D, Santorico SA, Holcomb C, Rastrou M, Erlich H, Tengesdal IW DL, et al.. MHC class II super-enhancer increases surface expression of HLA-DR and HLA-DQ and affects cytokine production in autoimmune vitiligo. Proc Natl Acad Sci U S A. 2016;113:1363–8. doi: 10.1073/pnas.1523482113. PMID:26787888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cavalli G, Franchini S, Aiello P, Guglielmi B, Berti A, Campochiaro C, Sabbadini MG, Baldissera E DL. Efficacy and safety of biological agents in adult-onset Still's disease. Scand J Rheumatol. 2015;44:309–14. doi: 10.3109/03009742.2014.992949. PMID:25656459 [DOI] [PubMed] [Google Scholar]
- 29.Cavalli G, Foppoli M, Cabrini L, Dinarello CA, Tresoldi M DL. Interleukin-1 Receptor Blockade Rescues Myocarditis-Associated End-Stage Heart Failure. Front Immunol. 2017;8:131. doi: 10.3389/fimmu.2017.00131. PMID:28232838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Colafrancesco S, Priori R, Valesini G, Argolini L, Baldissera E, Bartoloni E, Cammelli D, Canestrari G, Cantarini L CE, Cavallaro E, et al.. Response to Interleukin-1 Inhibitors in 140 Italian Patients with Adult-Onset Still's Disease: A Multicentre Retrospective Observational Study. Front Pharmacol. 2017;8:369. doi: 10.3389/fphar.2017.00369. PMID:28659802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cavalli G, Pappalardo F, Mangieri A, Dinarello CA, Dagna L TM. Treating Life-Threatening Myocarditis by Blocking Interleukin-1. Crit Care Med. 2016;44:e751–4. doi: 10.1097/CCM.0000000000001654. PMID:27031379 [DOI] [PubMed] [Google Scholar]
- 32.Cavalli G DC. Treating rheumatological diseases and co-morbidities with interleukin-1 blocking therapies. Rheumatol. 2015;54:2134–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cavalli G, Fallanca F, Dinarello CA DL. Treating pulmonary silicosis by blocking interleukin 1. Am J Respir Crit Care Med. 2015;191:596–8. doi: 10.1164/rccm.201412-2150LE. PMID:25723826 [DOI] [PubMed] [Google Scholar]
- 34.Aouba A, Georgin-Lavialle S, Pagnoux C, Silva NM, Renand A, Galateau-Salle F, Le Toquin S, Bensadoun H, Larousserie F, Silvera S, et al.. Rationale and efficacy of interleukin-1 targeting in Erdheim-Chester disease. Blood [Internet]. 2010;116:4070–6. Available from: https://doi.org/ 10.1182/blood-2010-04-279240. doi: 10.1182/blood-2010-04-279240. [DOI] [PubMed] [Google Scholar]
- 35.Diamond EL, Dagna L, Hyman DM, Cavalli G, Janku F, Estrada-Veras J, Ferrarini M, Abdel-Wahab O, Heaney ML, Scheel PJ, et al.. Consensus guidelines for the diagnosis and clinical management of Erdheim-Chester disease. Blood. 2014;124:483–92. doi: 10.1182/blood-2014-03-561381. PMID:24850756 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


