Abstract
Purpose
Matrix metalloproteinase 14 (MMP14) has been shown to be required for corneal angiogenesis. We hypothesized that the proangiogenic activity of MMP14 may be based on its selective binding to, and cleaving of, vascular endothelial growth factor receptor 1 (VEGFR1), but not VEGFR2 or VEGFR3.
Methods
Recombinant human (rh)VEGFR1, R2, and R3 were incubated with human MMP14, and the reaction mixtures were analyzed by SDS-PAGE and Coomassie blue staining. Surface plasmon resonance was used to determine the equilibrium constants (KD) for binding between MMP14 and VEGFA versus rhVEGFR1, R2, and R3. Extracellular signal-regulated kinase (ERK) phosphorylation was assayed in vascular endothelial cells after incubation with VEGF and various concentrations of MMP14. Ex vivo aortic ring tube formation assays and VEGFA micropocket corneal neovascularization assays were performed using Flk1Cre/Flk1mCherry/MMP14lox and Flk1mCherry/MMP14lox control mice.
Results
Maxtrix metalloproteinase 14 increased VEGFA-induced ERK phosphorylation in a time- and concentration-dependent manner in vascular endothelial cells. Aortic ring assays showed diminished vessel sprouting in vitro in response to VEGFA, but not to basic fibroblast growth factor, in mice with conditional deletion of vascular MMP14 (Flk1creMMP14lox) compared with that in MMP14lox control mice. In addition, diminished VEGFA-induced corneal angiogenesis was seen in flk1creMMP14lox mice compared with MMP14lox mice in vivo.
Conclusions
Our findings indicate that VEGFR1 interaction with MMP14 and the enzymatic activity of MMP14 are necessary for VEGFA-induced angiogenesis. Additionally, selective cleavage of VEGFR1 by MMP14 may play an important role in VEGFA-induced corneal angiogenesis.
Keywords: MT1-MMP, VEGFR1, corneal angiogenesis
Angiogenesis is a critical process involved in many diverse pathological conditions including wound healing, macular degeneration, diabetic retinopathy, tumor growth, and metastasis.1–3 Multiple growth factors, including basic fibroblast growth factor (bFGF), vascular endothelial growth factor A (VEGFA), angiopoietin-2, and platelet-derived growth factor (PDGF) can stimulate angiogenesis by promoting endothelial cell (EC) proliferation, migration, and tube formation.2,4–7 Matrix metalloproteinases (MMPs) also have been shown to be involved in angiogenesis.8–11 These Zn2+-dependent enzymes are produced as proenzymes and are categorized as either secreted MMPs or membrane-type (MT) MMPs.11–14 The first transmembrane-containing MMP to be identified was MMP14 (MT1-MMP),13–15 and MMP14 has been linked with several molecular mechanisms involved in the regulation of angiogenesis.
We previously investigated the role of MMP14 produced by corneal fibroblasts in corneal neovascularization and demonstrated that MMP14 has a proangiogenic function in the cornea.2,3,5,9,16 Specifically, MMP14 potentiates bFGF-induced corneal neovascularization and promotes secretion of VEGFA,5,17–22 a well-known proangiogenic growth factor. Additional research has shown that MMP14 influences extracellular matrix (ECM) remodeling, directly degrading collagens, proteoglycans, and fibronectins, as well as activating other MMPs involved in ECM degradation during angiogenesis.23–26 The importance of the enzymatic functions of MMP14 in corneal angiogenesis was further shown using the corneal pocket assay in MMP14-deficient mice.27 In a recent study, we observed that MMP14 can degrade recombinant mouse VEGF receptor 1 (VEGFR1),28 which acts as a decoy receptor on the surface of vascular ECs.29–31 The binding of MMP14 to VEGFR1 may interfere with binding of VEGFA to VEGFR1.28 Conversely, selective degradation of VEGFR1 by MMP14 may reduce the levels of the decoy receptor, increasing the availability of VEGFA for binding to VEGFR2, thereby promoting angiogenesis. This is consistent with the suggestion that VEGFR1 acts as a negative modulator of VEGFR2 activity, based on the observation that VEGFR1 initiates insignificant levels of tyrosine kinase activity compared with VEGFR2.31 Alteration in the availability of VEGFR1 on the surface of vascular ECs, therefore, has an impact on the VEGFR2-mediated kinase activity in response to VEGFA during angiogenesis.32
In the current study, we evaluated the noncollagenolytic, proteolytic activity of MMP14 for VEGFR1 compared with VEGFR2 and VEGFR3 and observed selective binding of MMP14 to VEGFR1 and selective degradation of VEGFR1 by MMP14. In addition, we used genetically modified Flk1Cre/Flk1mCherry/MMP14lox mice, which lack MMP14 activity on vascular ECs, and observed that MMP14 is necessary for VEGFA-induced tube formation in vitro and corneal neovascularization in vivo. Taken together, our findings demonstrate a novel proangiogenic function for the interaction of MMP14 with VEGFR1 in VEGFA-induced angiogenesis (Fig. 1).
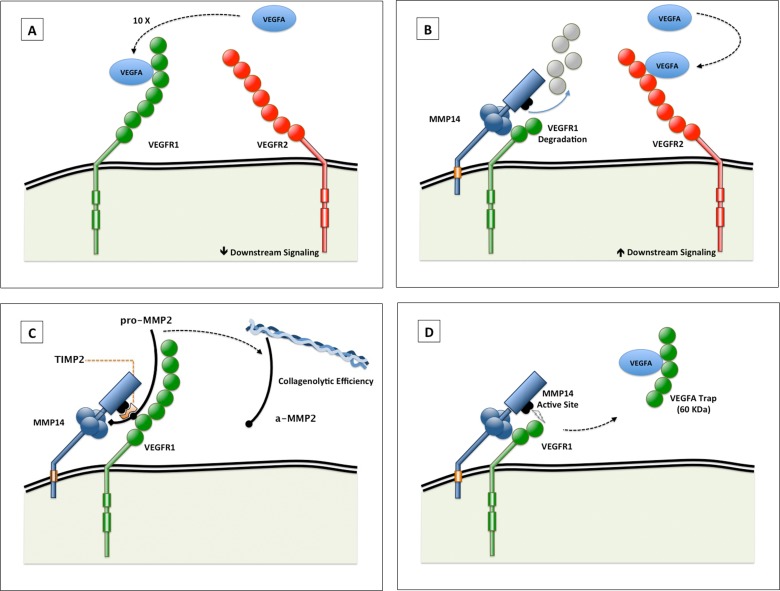
Figure 1.
(A, B) Schematic illustration of the proangiogenic role of VEGFR1 binding to, and selective degradation by, MMP14. Vascular endothelial growth factor A has greater binding affinity for VEGFR1 than VEGFR2 (A). Vascular endothelial growth factor receptor 1 binding to, and degradation by, MMP14 increases the availability of VEGFA to VEGFR2, which increases downstream signaling (B). Vascular endothelial growth factor receptor 1 and VEGFR2 dimers are depicted as monomers in this figure for illustrative purposes. (C, D) Schematic illustration of possible alternative proangiogenic roles of VEGFR1–MMP14 interactions. (C) VEGFR1 interaction with MMP14 altering the activation of pro-MMP2, in the presence of an optimal TIMP2 concentration. The activated MMP2 serves to increase the collagenolytic efficiency of MMP14. (D) Matrix metalloproteinase 14 cleavage of VEGFR1 can free a 60-kDa fragment that may trap VEGFA. The affinity of VEGFA to this fragment is less than its affinity to intact VEGFR1 but more than its affinity to VEGFR2.28
Methods
In Vitro Proteolysis and Staining
Recombinant human (rh)VEGFR1, rhVEGFR2, or rhVEGFR3 (R&D Systems, Minneapolis, MN, USA) was incubated alone or with the catalytic domain of MMP14 (cdMMP14; Calbiochem, Billerica, MA, USA). Pro-MMP2 (Calbiochem) was used as a positive control for these reactions. Reactions of rhVEGFRs with pro-MMP2 were allowed to occur for 4 hours at 37°C. Samples were boiled with loading dye and then separated by 4–20% SDS-PAGE. Coomassie blue was used to stain the gels, which were then destained with acetic acid and methanol.
Coimmunoprecipitation
Human umbilical vein endothelial cells (HUVECs) were maintained in endothelial cell growth medium including supplements (EBM-2; Lonza, Allendale, NJ, USA) and harvested with immunoprecipitation lysis buffer (Pierce/Thermo Fisher Scientific, Waltham, MA, USA). Two milligrams of total protein from cell lysates was cleared with protein A/G beads and incubated with anti-MMP14 antibody (Abcam Inc., Cambridge, MA, USA) overnight in a cold room. The next day, 30 μL protein A/G agarose was added to the mixture and incubated for 4 hours. After the reaction, the agarose was washed with lysis buffer and boiled with loading dye with 100 mM β-mercaptoethanol as a reducing agent before being subjected to SDS-PAGE for Western blotting. For Western blot analyses, separated proteins on SDS-PAGE gels were transferred to nitrocellulose (NC) membranes and blocked using 5% milk or 3% bovine serum albumin. Vascular endothelial growth factor receptor 1, R2, and R3 were detected with specific antibodies (VEGFR1, R&D Systems Inc., Minneapolis, MN, USA; VEGFR2, Cell Signaling Technology, Danvers, MA, USA; VEGFR3, eBiosciences, San Diego, CA, USA). Membranes were incubated with primary antibodies for 1 hour and then washed with 0.05% Tween-20 in Tris-buffered saline. Then the NC membranes were incubated with fluorescence-conjugated secondary antibody (Li-Cor, Lincoln, NE, USA), and protein bands were detected using the Li-Cor Odyssey system (Li-Cor).
Surface Plasmon Resonance Analyses
Surface plasmon resonance (SPR) analyses were performed using a BIAcore T200 instrument (GE Healthcare Bio-Sciences, Pittsburg, PA, USA). Recombinant human VEGFR1, R2, and R3 were immobilized on a CM5 sensor chip (Series S Sensor Chip CM5; GE Healthcare) using standard amine-coupling. Briefly, a 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrocholoride/N-hydroxy succinimide mixture was used to activate flow channels, and each protein was immobilized followed by ethanolamine blocking of the unoccupied surface area at 25°C. Blank immobilization was done with ethanolamine for flow channel 1 as a control. The immobilization levels of flow channels 2 (rhVEGFR1), 3 (rhVEGFR2), and 4 (rhVEGFR3) were 3550, 4939, and 3200 response units (RUs), respectively. Catalytic MMP14 binding was measured for 180 seconds of both association and dissociation at a flow rate of 20 μL/min in running buffer PBS-P (pH 7.4) containing various concentrations (0–250 nM at 2-fold dilution) of analyte proteins, unless otherwise indicated. Vascular endothelial growth factor A was used as a control. Sensorgrams were analyzed using the Biacore T200 evaluation software v3.0, and RU values at each concentration were measured during the equilibration phase for steady-state affinity fittings. Data were referenced with blank channel RU values, and the KD values were determined by fitting the reference subtracted data to a single rectangular hyperbolic curve equation (Equation 1), where y is the RU, ymax is the maximum RU, and x is the MMP14 or VEGFA concentration. Kinetic fittings were done using a 1:1 binding equation embedded in the Biacore T200 evaluation software 2.0 (Biacore/GE Healthcare Bio-Sciences, Pittsburg, PA, USA).
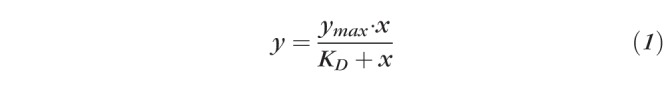 |
Activation of ERK
Human umbilical vein endothelial cells (1 × 106) were seeded and grown on six-well plates overnight. The next day cells were incubated for 24 hours with various concentrations (0, 0.5, 5, and 50 ng/mL) of catalytic MMP14 in M199 medium containing 1% fetal bovine serum (FBS). The cells were then washed with PBS, and 10 ng/mL VEGFA was added for 10 minutes to activate ERK. The cells were again washed with PBS and harvested with cell lysis buffer (1% Triton X-100, 0.2% SDS, 50 mM Tris, and 150 mM NaCl, pH 7.2). Fifty micrograms of lysates with loading dye was boiled and then subjected to SDS-PAGE for Western blotting. Phosphorylated ERK was detected with anti-pERK or ERK (Cell Signaling Technology). For in-cell Western blotting, calf pulmonary endothelial cells (0.7 × 104) were seeded and grown on 96-well clear-bottom plates. The cells were incubated with various concentrations (0, 50, 100, and 200 ng/mL) of MMP14 for 4 hours and then washed with PBS to remove cleaved VEGFR1 fragments. Twenty nanograms of VEGFA was added to the washed cells to activate ERK for 10 minutes. The cells were then fixed and permeabilized with 0.5% Triton X-100 in PBS. Next, a 3% solution of milk in PBS was used as a blocking solution to prevent nonspecific binding of antibodies. Cells were then incubated with anti-phospho ERK or anti-ERK antibody (1:1000 dilutions) for 1 hour, washed three times with 0.05% Tween-20/Tris-buffered saline, and incubated with fluorescent 700CW- or 800CW-conjugated secondary antibody (Li-Cor; 1:2000 in blocking solution). The fluorescence signal of stained cells was detected using the Li-Cor Odyssey system.
Cell Proliferation Assays
Human umbilical vein endothelial cells (5 × 103) were seeded in 96-well clear-bottom plates and incubated overnight. Cells were washed with PBS and then incubated with various concentrations (0, 0.5, 5, and 50 ng/mL) of catalytic MMP14 in 50 μL M199 medium including 1% FBS for 24 hours. The next day, the cells were washed with PBS and then treated with 50 μL 10 ng/mL VEGFA for 24 hours. Cells treated with VEGFA alone were used as a control, which was considered representative of 100% proliferation. Bromodeoxyuridine (Roche, Mannheim, Germany) was added to measure cell proliferation following the manufacturer's instructions.
Generation of Flk1mCherryMMP14lox and Flk1cre/Flk1mCherry/MMP14lox Mice
In the Tg(Flk1mCherry) mice, mCherry expression is driven by a blood endothelial-specific portion of the Flk1 promoter, with the myristoylation (myr) tag directing the fluorescent protein to the cell membrane. Flk1mCherry mice were bred with MMP14lox mice to generate Flk1mCherry/MMP14lox mice. Flk1cre mice were bred with Flk1mCherry/MMP14lox mice to generate Flk1cre/Flk1mCherry/MMP14lox mice.
Animal Care for Surgery and Imaging
Male or female adult (6–8 weeks old, five mice per group) Flk1mCherryMMP14lox and Flk1cre/Flk1mCherry/MMP14lox mice were used in all experiments. Mice were anesthetized with an intraperitoneal injection of ketamine (100 mg/kg) and xylazine (5 mg/kg). Once sedated, one drop of 1% proparacaine was administered topically to the eye for local anesthesia. Eyelashes were clipped above and below the eye in preparation for surgical procedures and in vivo imaging. For all procedures, eyes were imaged in vivo on an Axiozoom V16 microscope (Carl Zeiss Microscopy GmbH, Jena, Germany) on posttreatment days 0, 3, 7, and 10.
Ex Vivo Aorta Ring Assays
Aortic ring assays were performed as described by Kojima et al.33 Briefly, aortas were obtained from MMP14lox and Flk1cre/MMP14lox mice. Fatty tissues around the aorta were carefully removed under a surgical microscope. One-millimeter-long aortic rings were cut and rinsed in five consecutive washes of EBM (Cambrex, Walkersville, MD, USA). Ninety-six-well plates were coated with 100 μL growth factor-reduced Matrigel (BD Biosciences, San Jose, CA, USA), and an aortic ring was placed on its side on top of the gel. Various concentrations of VEGFA (0, 0.1, 1, 10, and 100 ng/mL) or 50 ng/mL bFGF (R&D Systems) in medium containing 2.5% FBS/EBM were added, and the solutions were changed every other day. Sprouting aortas were photographed with a phase contrast microscope equipped with a confocal microscope during the 10-day observation period. Tube formation was analyzed by counting the number of microvessels according to previously reported criteria.34
In Vivo Corneal Micropocket Assays
Vascular endothelial growth factor A pellets used for implantation were prepared according to the methods of Zhu et al.35 with modifications. Pellets were formed from 10% (wt/vol) sulfcralfate (Sigma-Aldrich, St. Louis, MO, USA), 12% (wt/vol) poly-hydroxyethylmethacrylate (HEMA; Sigma-Aldrich) dissolved in absolute ethanol, and rhVEGFA (R&D Systems) by pipetting 0.5 μL poly-HEMA solution onto UV-exposed parafilm, which yielded 20 pellets containing 150 ng VEGFA. Pellets were dried at room temperature before being stored at −80°C or used. Once the mouse was deeply anesthetized and the eye was prepared for surgery, we used a modified von Graefe knife to create a corneal micropocket in the epithelium. The initial incision was made parallel to and approximately 1 mm from the limbus. The knife was inserted into the incision site and advanced laterally, parallel to the limbus, to create a 0.5-mm micropocket within the corneal stroma. The poly-HEMA pellet was carefully inserted into this pocket. Antibiotic erythromycin ointment (Bausch and Lomb, Tampa, FL, USA) was applied to the eye after pellet implantation to prevent infection, and Buprenex (Reckitt Benckiser Healthcare [UK] Ltd., Hull, UK) was administered immediately after the procedure and twice the following day to alleviate postsurgical pain.
Results
Cleavage of rhVEGFR1, but not rhVEGFR2 or R3, by MMP14
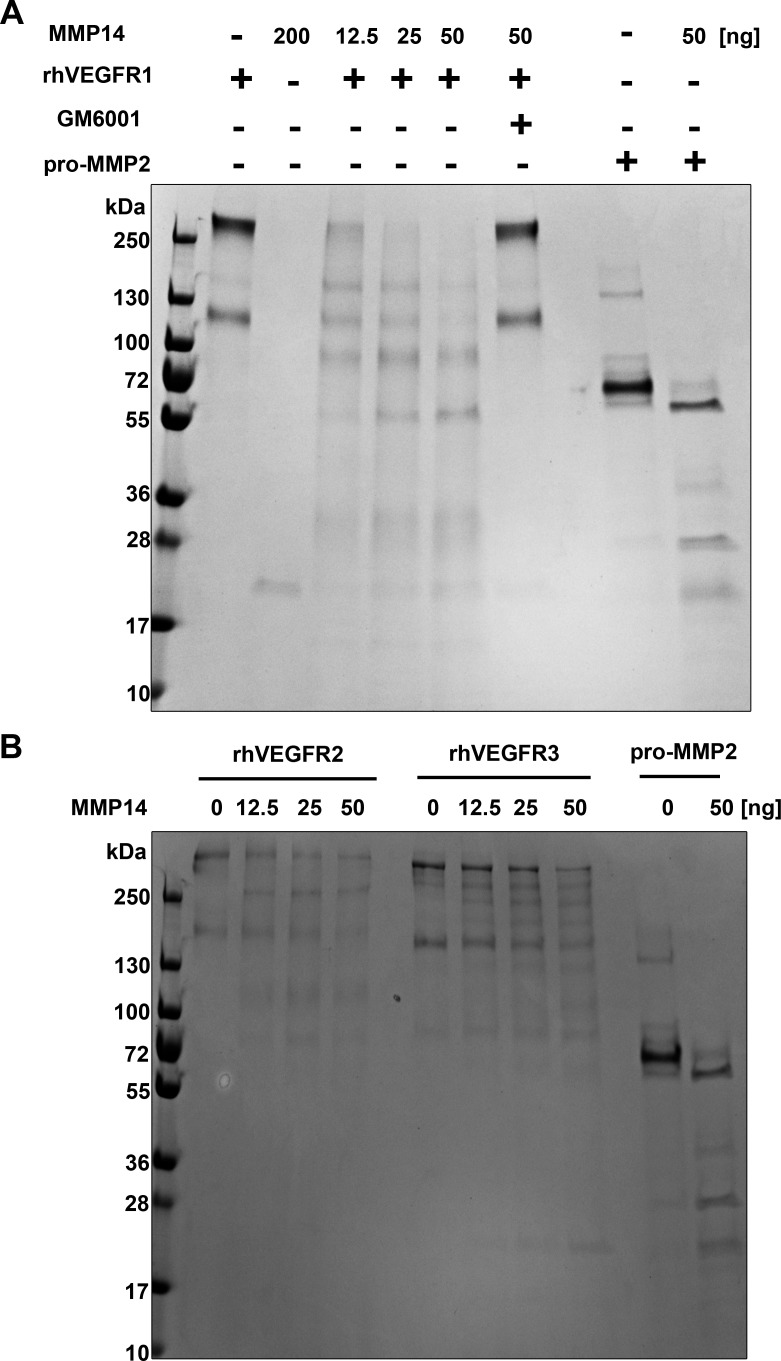
Catalytic MMP14 protein was incubated with rhVEGFR1, R2, and R3, individually, to determine its proteolytic activity. Reactions were observed with various concentrations of MMP14 (2-fold dilution; 0, 12.5, 25, or 50 ng/10 μL per reaction), as well as without MMP14 in combination with 1 μg of each rhVEGFR for 4 hours. Reaction products were subjected to SDS-PAGE and stained with Coomassie blue under nonreducing conditions. The results showed that rhVEGFR1 was cleaved in a concentration-dependent manner under nonreducing conditions (Fig. 2A), and pro-MMP2, a known substrate of MMP14, also was cleaved as an active form. In contrast, rhVEGFR2 and R3 (Fig. 2B) were not cleaved by catalytic MMP14. To verify this proteolytic activity of MMP14, GM6001, a general MMP inhibitor, was added to the reaction and rhVEGFR1 remained intact (Fig. 2A). These findings clearly indicate that MMP14 regulates human VEGFR1 levels and may modulate human VEGFR-mediated angiogenesis.
Figure 2.
Matrix metalloproteinase 14 cleaves recombinant human VEGFR1 in vitro. Cleavage of VEGFR1 produces fragments and requires the catalytic domain of MMP14. Reaction products were subjected to SDS-PAGE and stained with Coomassie blue. (A) Recombinant human VEGFR1 (rhVEGFR1, 1 μg, lane 1) was run alone or incubated with 200 ng MMP14 catalytic domain (lane 2). Undigested rhVEGFR1 was run as several bands, all above 130 kDa (lane 3). Recombinant human VEGFR1 digestion by MMP14 produced three distinct products at approximately 70, 59.8, and 21 kDa (lane 4). Recombinant human VEGFR1 incubation with the MMP14 enzymatic inhibitor GM6001 (lane 6) resulted in no cleavage. Pro-MMP2, a known substrate of MMP14, was used as a control (lanes 8 and 9). (B) Matrix metalloproteinase 14 did not cleave rhVEGFR2 or rhVEGFR3. Reaction products were subjected to SDS-PAGE and stained with Coomassie blue. Recombinant human VEGFR2 or rhVEGFR3 (1 μg) were run as controls. Incubation of recombinant VEGFRs (rhVEGFR2 or rhVEGFR3) with various concentrations of MMP14 (0, 12.5, 25, and 50 ng) resulted in no cleavage. Pro-MMP2, a known substrate of MMP14, was used as a control (lanes 8 and 9).
Binding of MMP14 to VEGFR1 but Not VEGFR2 and R3
Protein–protein interaction is an important step during enzymatic processing to cleave substrate. We explored whether MMP14 directly forms a complex with VEGFRs on cells. Endogenous MMP14 was immunoprecipitated from HUVEC lysates and immunoblotted with anti-VEGFR1, R2, and R3. Vascular endothelial growth factor receptor 1 (Fig. 3A) was present in this coimmunoprecipitation (co-IP) mixture, whereas VEGFR2 (Fig. 3B) and R3 (Fig. 3C) were not. To determine the binding affinity of MMP14 to rhVEGFR1 and verify the co-IP results, we used SPR. Recombinant human VEGFR1, R2, and R3 were immobilized on a CM5 sensor chip. Recombinant human VEGFA was tested first as a control to confirm the immobilized rhVEGFRs were well-folded and functional. The determined KD values for rhVEGFA binding to VEGFR1 and VEGFR2 were 2.31 × 10−11 and 3.98 × 10−10 M, respectively, and VEGFR3 did not bind (Fig. 4D). Next, sensorgrams (Fig. 4) for a series of increasing concentrations of MMP14 flown over immobilized rhVEGFRs on CM5 sensor chips were compared. The determined KD value of MMP14 for VEGFR1 (Fig. 4A) was 2.59 × 10−9 M, and VEGFR2 (Fig. 4B) and VEGFR3 (Fig. 4C) did not show binding. These results support our hypothesis that MMP14 modulates angiogenesis by directly regulating the levels of VEGFRs.
Figure 3.
Matrix metalloproteinase 14 co-IP with VEGFR1, but not VEGFR2 or VEGFR3 in HUVEC lysates. Human umbilical vein endothelial cell (HUVEC) lysates were immunoprecipitated with anti-MMP14 antibodies. The immunocomplexes were immunoblotted with anti-VEGFR1, R2, and R3. (A) Vascular endothelial growth factor receptor 1 was present on anti-MMP14 co-IP, whereas MMP14 did not co-IP with VEGFR2 (B) or VEGFR3 (C). Differences in the band patterns between VEGFR2 and VEGFR3 are due to difference of antibodies species and the IgG heavy chain staining; rabbit anti-VEGFR2 IgG (R&D Systems) and rat anti-VEGFR3 (ebioscience) were used in B and C, respectively.
Figure 4.
Surface plasmon resonance analysis shows MMP14 binding to rhVEGFR1. Sensorgrams of a series of increasing concentrations of MMP14 flown over immobilized rhVEGFRs on a CM5 sensor chip were compared. The determined KD values for MMP14 binding to rhVEGFR1 and rhVEGFR2 were 2.31 × 10−11 (A) and 3.98 × 10−10 M (B), respectively, whereas MMP14 did not show binding affinity for rhVEGFR3 (C). Comparison of MMP14 binding activities to rhVEGFR1, rhVEGFR2, and rhVEGFR3 (D).
Vascular Endothelial Growth Factor Receptor A–Induced ERK Activation After Incubation of HUVECs With MMP14
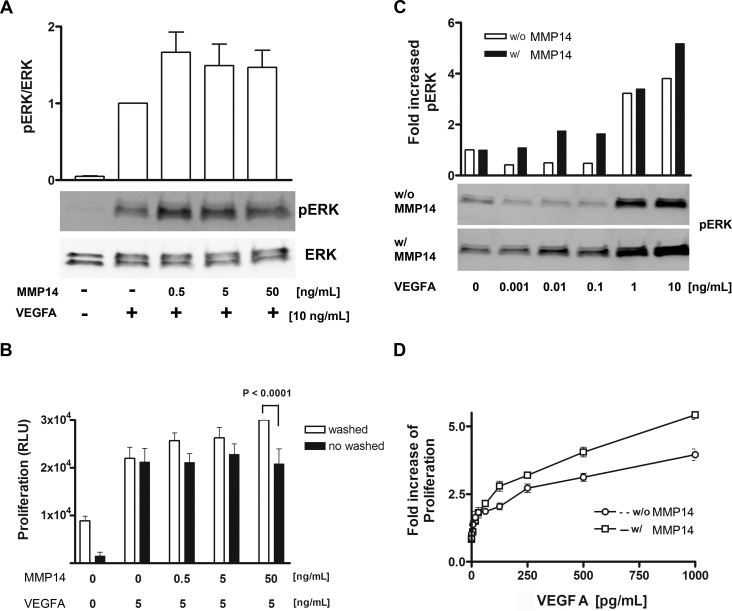
To determine the effect of cleavage of VEGFR1 by MMP14, we compared VEGFA-induced ERK activation in ECs. Human umbilical vein endothelial cells were incubated with various concentrations of MMP14 (0, 0.5, 5, and 50 ng/mL), and the levels of VEGFA-induced phosphorylation of ERK were greater than that without incubation with MMP14 (Fig. 5A). We hypothesized that if the cells were not washed, the fragment of VEGFR1 created on cleavage by MMP14 could trap VEGFA and decrease VEGFA-induced proliferation. Human umbilical vein endothelial cells were washed with PBS after incubation with MMP14 to remove cleaved fragments of VEGFR1, and then VEGFA was added to stimulate proliferation. We observed that the proliferation rates increased for cells that were washed after MMP14 incubation (white bar, Fig. 5B). There was no change in the proliferation rate of unwashed HUVECs (black bar, Fig. 5B). These results indicate that exogenous MMP14 can regulate the level of VEGFR1 on cells and modulate VEGFA-induced angiogenesis.
Figure 5.
Vascular endothelial growth factor A induces ERK activation after cleavage of VEGFR1. (A) Human umbilical vein endothelial cells were incubated with various concentrations of MMP14 (0, 0.5, 5, and 50 ng/mL), and greater VEGFA-induced phosphorylation of ERK was observed with MMP14 than without. (B) Human umbilical vein endothelial cells were washed or not washed serially with PBS after incubation with MMP14, and then VEGFA was added to stimulate proliferation. Vascular endothelial growth factor A at 5 ng/mL could induce proliferation of both washed and unwashed HUVECs. (C) Vascular endothelial growth factor A at 1 ng/mL initiated ERK activation on regular HUVECs without 50 ng/mL MMP-14, but interestingly, a 100× times lower concentration of VEGFA (10 pg/mL) was enough to activate ERK on HUVECs incubated with MMP14 (D).
Vascular endothelial growth factor receptor A has a stronger binding affinity for VEGFR1 than for VEGFR2, and this was confirmed by our SPR analysis. Therefore, we hypothesized that phosphorylation of ERK by VEGFA at low concentrations was significantly different after incubation with exogenous MMP14. Vascular endothelial growth factor receptor A at 1 ng/mL began to induce ERK activation on regular HUVECs without MMP14, but interestingly, a 100× lower concentration of VEGFA (10 pg/mL) was enough to activate ERK on HUVECs incubated with 50 ng/mL MMP14 (Fig. 5C). We determined that VEGFA induced HUVEC proliferation in the same conditions and observed that VEGFA at a low concentration could strongly activate proliferation (Fig. 5D).
Absence of VEGFA-Induced Sprouting From flk1cre-MMP14 Mouse Aortic Rings
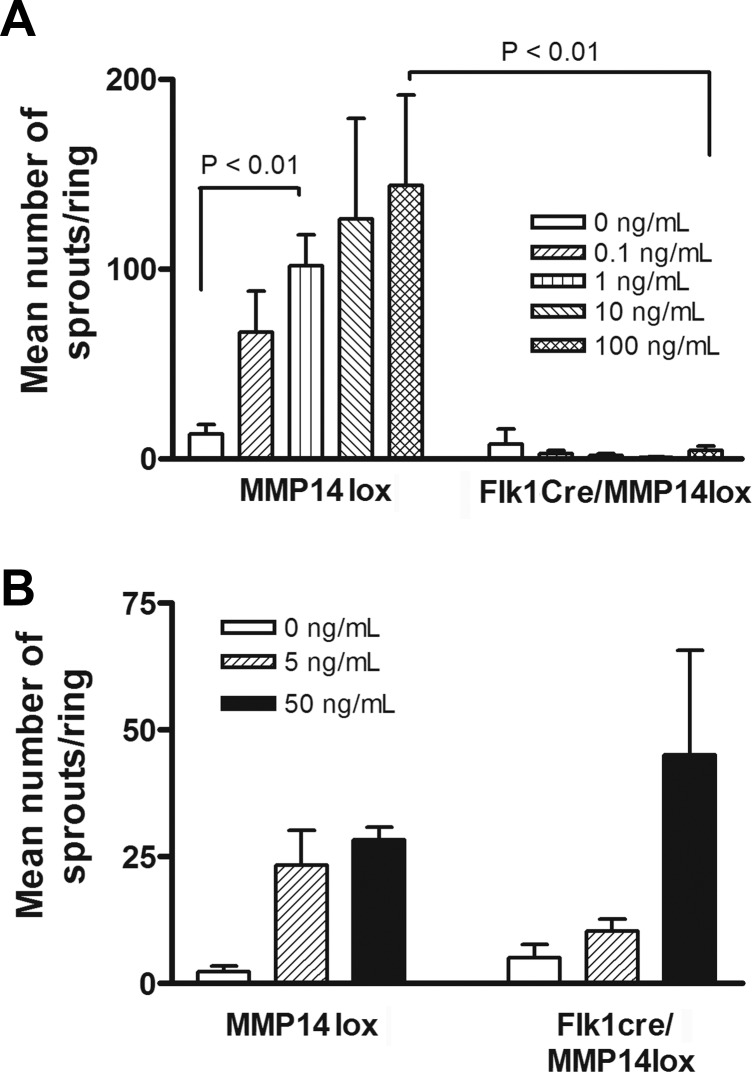
We expected MMP14 conditional knockout ECs to show alterations in VEGFA-induced angiogenesis ex vivo. We analyzed EC growth from aortas of flk1-cre/MMP14lox mice. Endothelial microvessel sprouts were not observed on aortic rings from conditioned MMP14 knockout mice even after stimulation with 100 ng/mL VEGFA. In contrast, microvessels grew from rings of MMP14lox mice as a control in a manner dependent on the VEGFA concentration (Fig. 6A). Moreover, bFGF induced exponential growth of microvessels from aortic rings of flk1-cre/MMP14lox or MMP14lox mice as controls dependent on concentration (Fig. 6B). Next, we added MMP14 to aortic ring cultures with VEGFA to rescue the loss of MMP14 in flk1-cre/MMP14lox mice. Few microvessel sprouts were observed after treatment with VEGFA (Fig. 7B) or MMP14 (Fig. 7C), but interestingly, microvessels were observed after treatment of aortic rings with both (Figs. 7D, 7E, and Supplementary Figures S1–S3).
Figure 6.
No sprouting was observed in flk1cre/MMP14lox mouse aortic ring assays on stimulation with VEGFA. (A) Endothelial microvessel sprouts were not detectable on rings from conditioned MMP14 knockout mice even after stimulation with 100 ng/mL VEGFA. Microvessels from rings of MMP14lox mice (as a control) grew in a manner dependent on VEGFA. (B) Basic fibroblast growth factor at various concentrations (0, 5, and 50 ng/mL) was added to aortic rings from flk1cre/MMP14lox mice and control (MMP14lox) mice.
Figure 7.
Matrix metalloproteinase 14 rescues VEGFA-induced aortic sprouting in Flk1cre/MMP14lox mice. Matrix metalloproteinase 14 was added to the aortic ring in the presence or absence of VEGFA to rescue a loss of MMP14 in flk1cre/MMP14lox mice. (A) No aortic ring sprouting occurred without VEGFA and MMP14. Limited microvessel sprouting occurred after exposure to (B) VEGFA, (C) MMP14, or (D) both VEGFA and MMP14. (E) Quantitated results from the VEGFA-induced aorta ring assay with and without the addition of MMP14.
Reduced Angiogenic Response in Corneas of Flk1cre/Flk1mCherry/MMP14lox Mice Compared With Flk1mCherry/MMP14lox Mice After VEGF Pellet Implantation
We observed corneal angiogenesis after VEGFA pellet implantation in Flk1cre/Flk1mCherry/MMP14lox mice and Flk1mCherry/MMP14lox mice. The percent area of corneal angiogenesis was calculated by observing the normally avascular cornea of Flk1cre/Flk1mCherry/MMP14 mice and Flk1mCherry/MMP14lox mice before and after implantation of a VEGF pellet. A smaller percent area of corneal blood vessels was observed at days 7 and 10 after implantation of a 150 ng VEGF pellet in Flk1cre/Flk1mCherry/MMP14lox mice compared with that in Flk1mCherry/MMP14lox mice (Fig. 8).
Figure 8.
Less blood vessel growth after implantation of VEGFA pellet in corneas of Flk1cre/Flk1mCherry/MMP14lox mice compared with Flk1mCherry/MMP14lox mice. Vascular endothelial growth factor A pellets were implanted in Flk1cre/Flk1mCherry/MMP14lox mice (B, D, F, and H) and Flk1mCherry/MMP14lox control mice (A, C, E, and G). Mice corneas were imaged at days 0, 3, 7, and 10, and the area of corneal blood vessel growth was calculated and analyzed. There were no statistically significant differences between the areas of corneal blood vessels at baseline (day 0) or day 3. (I) The areas of corneal blood vessels at days 7 and 10 were statistically significantly less in the Flk1cre/Flk1mCherry/MMP14lox mice compared with the Flk1mCherry/MMP14lox (P < 0.05).
Here we found that MMP14 knockout on ECs interrupted vessel growth in response to stimulation by VEGFA. Moreover, exogenous MMP14 rescued microvessel sprouting of MMP14-deficient ECs. Together, our results indicate that MMP14 modulates VEGFA-induced angiogenesis by regulating the availability of VEGFR1 to bind with VEGFA.
Discussion
Matrix metalloproteinases, the family of ECM modifying enzymes, belong to a super family of enzymes that can degrade all types of ECM proteins. All MMPs contain conserved domains such as the catalytic domain and hemopexin domain. Many MMPs are secreted by cells and have a wide range of functions. Matrix metalloproteinase 14 can exist as a membrane-anchored MMP, but this form has limited function. When MMP14 binds to its natural inhibitor tissue inhibitor of metalloproteinases 2 (TIMP2), TIMP2 recruits pro-MMP2 to form a tri-molecule complex on the cell surface. Within this complex, MMP14 is not active, but free MMP14 not associated with TIMP2 can cleave a propeptide on MMP2. Cleaved MMP2 is activated and released from the cell surface. Based on this activity, MMP14 was identified as a MMP2 activator36 and has been widely implicated in physiological processes including angiogenesis, inflammation, and cancer invasion and metastasis.19,37,38 Notably, MMP14 expression is high in the wound healing area. Moreover, MMP14 knockout mice die by 21 days after birth due primarily suppressed vascularization and skeletal defects and to a lesser degree due to improper angiogenesis. The importance of MMP14 in angiogenesis is based not only on its role as an activator of MMP2 but also its proteolytic activity toward other ECM and surface proteins, including collagens, decorin, and receptor tyrosine kinase.
We hypothesized that downregulation of VEGFR1 might be achieved through the proteolytic activity of a member of the MMP family. We identified MMP14 as a candidate because it is upregulated in tip cells during angiogenesis but is not expressed in normal, quiescent vascular ECs.39 We found in the current study that MMP14 binds to and cleaves VEGFR1 in vitro and that this proteolytic activity requires only the active MMP14 catalytic domain. Moreover, VEGFR2 and VEGFR3 do not bind with MMP14, nor are they substrates of MMP14 under normal in vitro physiological conditions. The likely purpose of VEGFR1 binding with MMP14 is its cleavage by MMP14, which facilitates increased binding of VEGFA to VEGFR2 on the cell surface to activate VEGFA-induced angiogenesis. Vascular endothelial growth factor A has a greater binding affinity for VEGFR1 than VEGFR2, and thus, VEGFA stimulation of VEGFR2 requires a decrease in available VEGFR1.
Figure 1 illustrates alternative proangiogenic roles of VEGFR1–MMP14 interactions. These include blocking the VEGFR1 binding site (decreasing the decoy function of VEGFR1; Fig. 1A) and promoting MMP14 collagenolytic activity and processing of pro-MMP2 to active MMP2 (Fig. 1C). We have previously shown that MMP14 may generate an antiangiogenic fragment in the mouse (Fig. 1D).
The antiangiogenic decoy effect (Fig. 1D) is counterbalanced by the proangiogenic effect (increased VEGFR2 signaling; Fig. 1B). Our findings suggest that as opposed to keratocytes (in which the decoy effect is not counterbalanced by the proangiogenic R2-mediated effect), the blood vessels are exposed to both the anti- and proangiogenic effects of MMP14 cleavage of VEGFR1. In our model, it is likely the ratio of MMP14 to VEGFR1 plays a role in tilting the balance of MMP14 activity toward angiogenesis. One of the advantages of using the Flk1cre/MMP14lox mouse over using the MMP14 knockout mouse is that the Flk1cre/MMP14lox survive well beyond day 21. In this noninducible mouse model, we inspected the mouse corneas and limbal area for possible vascular developmental abnormalities. However, we were not able to identify alterations in the limbal vasculature arcade, or feeder vessel. Supplementary Figure S4 shows the similarities of the limbal arcades between the Flk1cre/MMP14lox mice and the control mice. Additional studies are needed to determine the exact mechanism of the proangiogenic role of vascular MMP14 in VEGFA-mediated corneal angiogenesis.
In conclusion, our results confirm our hypothesis that MMP14 regulates angiogenesis through cleavage of VEGFR1, and this event represents a new target in the development of treatments to either promote or block angiogenesis.
Supplementary Material
Acknowledgments
The authors thank Wallace Chamon for his contribution to Figure 1.
Supported by grants from the National Institutes of Health EY10101 (DTA), EY023691 (JHC), EY01792, and an unrestricted grant from Research to Prevent Blindness (New York, NY, USA).
Disclosure: K.-Y. Han, None; J.-H. Chang, None; H. Lee, None; D.T. Azar, None
References
- 1. Folkman J. . Angiogenesis. Annu Rev Med. 2006; 57: 1– 18. [DOI] [PubMed] [Google Scholar]
- 2. Azar DT. . Corneal angiogenic privilege: angiogenic and antiangiogenic factors in corneal avascularity vasculogenesis, and wound healing (an American Ophthalmological Society thesis). Trans Am Ophthalmol Soc. 2006; 104: 264– 302. [PMC free article] [PubMed] [Google Scholar]
- 3. Mimura T, Han KY, Onguchi T,et al. . MT1-MMP-mediated cleavage of decorin in corneal angiogenesis. J Vasc Res. 2009; 46: 541– 550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chang JH, Gabison EE, Kato T, Azar DT. . Corneal neovascularization. Curr Opin Ophthalmol. 2001; 12: 242– 249. [DOI] [PubMed] [Google Scholar]
- 5. Onguchi T, Han KY, Chang JH, Azar DT. . Membrane type-1 matrix metalloproteinase potentiates basic fibroblast growth factor-induced corneal neovascularization. Am J Pathol. 2009; 174: 1564– 1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yang JF, Walia A, Huang YH,et al. . Understanding lymphangiogenesis in knockout models, the cornea, and ocular diseases for the development of therapeutic interventions. Surv Ophthalmol. 2016; 61: 272– 296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Walia A, Yang JF, Huang YH, Rosenblatt MI, Chang JH, Azar DT. . Endostatin's emerging roles in angiogenesis lymphangiogenesis, disease, and clinical applications. Biochim Biophys Acta. 2015; 1850: 2422– 2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Raffetto JD, Khalil RA. . Matrix metalloproteinases and their inhibitors in vascular remodeling and vascular disease. Biochem Pharmacol. 2008; 75: 346– 359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chang JH, Huang YH, Cunningham CM,et al. . Matrix metalloproteinase 14 modulates signal transduction and angiogenesis in the cornea. Surv Ophthalmol. 2016; 61: 478– 497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rundhaug JE. . Matrix metalloproteinases and angiogenesis. J Cell Mol Med. 2005; 9: 267– 285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nagase H. . Activation mechanisms of matrix metalloproteinases. Biol Chem. 1997; 378: 151– 160. [PubMed] [Google Scholar]
- 12. Werb Z, Chin JR. . Extracellular matrix remodeling during morphogenesis. Ann N Y Acad Sci. 1998; 857: 110– 118. [DOI] [PubMed] [Google Scholar]
- 13. Sato H, Kinoshita T, Takino T, Nakayama K, Seiki M. . Activation of a recombinant membrane type 1-matrix metalloproteinase (MT1-MMP) by furin and its interaction with tissue inhibitor of metalloproteinases (TIMP)-2. FEBS Lett. 1996; 393: 101– 104. [DOI] [PubMed] [Google Scholar]
- 14. Sato H, Seiki M. . Membrane-type matrix metalloproteinases (MT-MMPs) in tumor metastasis. J Biochem. 1996; 119: 209– 215. [DOI] [PubMed] [Google Scholar]
- 15. Massova I, Kotra LP, Fridman R, Mobashery S. . Matrix metalloproteinases: structures, evolution, and diversification. FASEB J. 1998; 12: 1075– 1095. [PubMed] [Google Scholar]
- 16. Han KY, Fahd DC, Tshionyi M,et al. . MT1-MMP modulates bFGF-induced VEGF-A expression in corneal fibroblasts. Protein Peptide Lett. 2012; 19: 1334– 1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sounni NE, Roghi C, Chabottaux V,et al. . Up-regulation of vascular endothelial growth factor-A by active membrane-type 1 matrix metalloproteinase through activation of Src-tyrosine kinases. J Biol Chem. 2004; 279: 13564– 13574. [DOI] [PubMed] [Google Scholar]
- 18. Hiraoka N, Allen E, Apel IJ, Gyetko MR, Weiss SJ. . Matrix metalloproteinases regulate neovascularization by acting as pericellular fibrinolysins. Cell. 1998; 95: 365– 377. [DOI] [PubMed] [Google Scholar]
- 19. Chun TH, Sabeh F, Ota I,et al. . MT1-MMP-dependent neovessel formation within the confines of the three-dimensional extracellular matrix. J Cell Biol. 2004; 167: 757– 767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Galvez BG, Matias-Roman S, Albar JP, Sanchez-Madrid F, Arroyo AG. . Membrane type 1-matrix metalloproteinase is activated during migration of human endothelial cells and modulates endothelial motility and matrix remodeling. J Biol Chem. 2001; 276: 37491– 37500. [DOI] [PubMed] [Google Scholar]
- 21. Toth M, Hernandez-Barrantes S, Osenkowski P,et al. . Complex pattern of membrane type 1 matrix metalloproteinase shedding. Regulation by autocatalytic cells surface inactivation of active enzyme. J Biol Chem. 2002; 277: 26340– 26350. [DOI] [PubMed] [Google Scholar]
- 22. Langlois S, Gingras D, Beliveau R. . Membrane type 1-matrix metalloproteinase (MT1-MMP) cooperates with sphingosine 1-phosphate to induce endothelial cell migration and morphogenic differentiation. Blood. 2004; 103: 3020– 3028. [DOI] [PubMed] [Google Scholar]
- 23. Chantrain CF, Henriet P, Jodele S,et al. . Mechanisms of pericyte recruitment in tumour angiogenesis: a new role for metalloproteinases. Eur J Cancer. 2006; 42: 310– 318. [DOI] [PubMed] [Google Scholar]
- 24. Davis GE, Senger DR. . Endothelial extracellular matrix: biosynthesis remodeling, and functions during vascular morphogenesis and neovessel stabilization. Circ Res. 2005; 97: 1093– 1107. [DOI] [PubMed] [Google Scholar]
- 25. Heissig B, Hattori K, Friedrich M, Rafii S, Werb Z. . Angiogenesis: vascular remodeling of the extracellular matrix involves metalloproteinases. Curr Opin Hematol. 2003; 10: 136– 141. [DOI] [PubMed] [Google Scholar]
- 26. Han KY, Dugas-Ford J, Seiki M, Chang JH, Azar DT. . Evidence for the involvement of MMP14 in MMP2 processing and recruitment in exosomes of corneal Fibroblasts. Invest Ophthalmol Vis Sci 2015; 56: 5323– 5329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Holmbeck K, Bianco P, Caterina J,et al. . MT1-MMP-deficient mice develop dwarfism, osteopenia, arthritis, and connective tissue disease due to inadequate collagen turnover. Cell. 1999; 99: 81– 92. [DOI] [PubMed] [Google Scholar]
- 28. Han KY, Dugas-Ford J, Lee H, Chang JH, Azar DT. . MMP14 cleavage of VEGFR1 in the cornea leads to a VEGF-trap antiangiogenic effect. Invest Ophthalmol Vis Sci 2015; 56: 5450– 5456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bruns AF, Bao L, Walker JH, Ponnambalam S. . VEGF-A-stimulated signalling in endothelial cells via a dual receptor tyrosine kinase system is dependent on co-ordinated trafficking and proteolysis. Biochem Soc Trans. 2009; 37: 1193– 1197. [DOI] [PubMed] [Google Scholar]
- 30. Hiratsuka S, Minowa O, Kuno J, Noda T, Shibuya M. . Flt-1 lacking the tyrosine kinase domain is sufficient for normal development and angiogenesis in mice. Proc Natl Acad Sci U S A. 1998; 95: 9349– 9354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cudmore MJ, Hewett PW, Ahmad S,et al. . The role of heterodimerization between VEGFR-1 and VEGFR-2 in the regulation of endothelial cell homeostasis. Nat Commun. 2012; 3: 972. [DOI] [PubMed] [Google Scholar]
- 32. Mac Gabhann F, Popel AS. . Systems biology of vascular endothelial growth factors. Microcirculation. 2008; 15: 715– 738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kojima T, Chung TY, Chang JH, Sayegh R, Casanova FH, Azar DT. . Comparison of EphA receptor tyrosine kinases and ephrinA ligand expression to EphB-ephrinB in vascularized corneas. Cornea. 2007; 26: 569– 578. [DOI] [PubMed] [Google Scholar]
- 34. Baker M, Robinson SD, Lechertier T,et al. . Use of the mouse aortic ring assay to study angiogenesis. Nature Protocols. 2012; 7: 89– 104. [DOI] [PubMed] [Google Scholar]
- 35. Zhu J, Dugas-Ford J, Chang M,et al. . Simultaneous in vivo imaging of blood and lymphatic vessel growth in Prox1-GFP/Flk1::myr-mCherry mice. FEBS J. 2015; 282: 1458– 1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Williamson RA, Hutton M, Vogt G,et al. . Tyrosine 36 plays a critical role in the interaction of the AB loop of tissue inhibitor of metalloproteinases-2 with matrix metalloproteinase-14. J Biol Chem. 2001; 276: 32966– 32970. [DOI] [PubMed] [Google Scholar]
- 37. Rooprai HK, Van Meter T, Rucklidge GJ, Hudson L, Everall IP, Pilkington GJ. . Comparative analysis of matrix metalloproteinases by immunocytochemistry immunohistochemistry and zymography in human primary brain tumours. Int J Oncol. 1998; 13: 1153– 1157. [DOI] [PubMed] [Google Scholar]
- 38. Koike T, Vernon RB, Hamner MA, Sadoun E, Reed MJ. . MT1-MMP, but not secreted MMPs, influences the migration of human microvascular endothelial cells in 3-dimensional collagen gels. J Cell Biochem. 2002; 86: 748– 758. [DOI] [PubMed] [Google Scholar]
- 39. Yana I, Sagara H, Takaki S,et al. . Crosstalk between neovessels and mural cells directs the site-specific expression of MT1-MMP to endothelial tip cells. J Cell Sci. 2007; 120: 1607– 1614. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.