Abstract
Background
As a neurodevelopmental disorder, symptoms of ASD likely emerge from a complex interaction between preexisting genetic vulnerabilities and the child’s environment. One way to understand causal paths to ASD is to identify dimensional ASD-related traits that vary in the general population and that predispose individuals with other risk factors toward ASD. Moving beyond behavioral traits to explore underlying neurocognitive processes may further constrain the underlying genetics. Endophenotypes are quantitative, heritable, trait-related differences that are generally assessed with laboratory-based methods, can be identified in the general population, and may be more closely tied to particular causal chains that have a more restricted set of genetic roots. The most fruitful endophenotypes may be those observed in infancy, prior to the emergence of behavioral symptoms that they are hypothesized to cause. Social motivation is an ASD-related trait that is highly heritable. In this study, we investigate whether infant endophenotypes of social attention relate to familial risk for lower social motivation in the general population.
Methods
We examined whether infant social attention (measured using habituation, EEG power, and event-related potential tasks previously used in infants/toddlers with ASD) varies quantitatively with parental social motivation in 117 six-month-old and 106 twelve-month-old typically developing infants assessed cross-sectionally. To assess heritable aspects of social motivation, primary caregiver biological parents completed two self-report measures of social avoidance and discomfort that have shown high heritability in previous work.
Results
Parents with higher social discomfort and avoidance had infants who showed shorter looks to faces but not objects; reduced theta power during naturalistic social attention; and smaller P400 responses to faces versus objects.
Conclusions
Early reductions in social attention are continuously related to lower parental social motivation. Alterations in social attention may be infant endophenotypes of social motivation traits related to ASD.
Keywords: Autism spectrum disorders, infancy, endophenotype, social attention
Introduction
Autism spectrum disorder (ASD) is characterized by impairments in social interaction and communication, and the presence of restrictive and repetitive behaviors and sensory processing difficulties (APA, 2013). As a neurodevelopmental disorder, symptoms of ASD likely emerge from a complex interaction between pre-existing genetic vulnerabilities and the child’s environment. Genetic influences are complex, but include both the aggregate effects of common polygenic risk, and more individually deleterious de novo and rare inherited mutations (Robinson, Neale, & Hyman, 2015). The effects of deleterious mutations are variable, producing symptom clusters in interaction with genetic background (Moreno-De-Luca et al., 2013). For example, children with a 16p11.2 or 22q11.2 deletion show an IQ lower than expected based on parental IQ (Moss et al., 1999; Zufferey et al., 2012). Within families with relatively lower cognitive skills, the child may fall below expected norms and receive a diagnosis of developmental delay; a child from a high-IQ family may function in the typical range. In the context of ASD, children with a penetrant mutation may only receive an ASD diagnosis when their genetic background predisposes them to relatively poorer social communication skills. Similarly, variations in temperament have been proposed as moderating factors of ASD expression (Mundy, Henderson, Inge, & Coman, 2007).
Influences of both aggregated common polygenic risk and interactions between penetrant variants and genetic background on ASD would predict that genetic variance contributing to ASD is the same as that contributing to neurotypical variation in ASD-related traits. This is the case, particularly in the social domain (Constantino, 2011). Furthermore, ASD-related traits show the same etiology in the general population and at quantitative extremes (Lundström et al., 2012; Robinson et al., 2011). Like categorical ASD, quantitative traits are also highly heritable (Colvert et al., 2015; Constantino & Todd, 2005). One way to identify causal paths to ASD is thus to identify dimensional ASD-related traits that vary in the general population. These could provide indicators of the genetic background that predisposes individuals with other risk factors (like deleterious mutations, significant environmental challenges, or accumulated polygenic risk) toward ASD (Cuthbert & Insel, 2013; Jones, Gliga, Bedford, Charman, & Johnson, 2014; London, 2014).
Moving beyond behavioral traits to explore underlying neurocognitive processes may further constrain the underlying genetics. Endophenotypes are quantitative, heritable, trait-related differences that are generally assessed with laboratory-based methods, can be identified in the general population (Bearden & Freimer, 2006), and may be more closely tied to particular causal chains that have a more restricted set of genetic roots (Meyer-Lindenberg & Weinberger, 2006; Viding & Blakemore, 2006). We have selected the term ‘endophenotype’ following Lenzenweger (2013), who argues in favor of the use of this term when discussing traits thought to be both heritable and on the causal path to symptoms of the condition of interest (as contrasted with the more general term ‘biomarker’, a feature associated with the condition but not heritable or necessarily causally linked). A range of endophenotypes for ASD such as atypicalities in face processing, social responsiveness, and executive functioning have been identified in work with older children and adults (e.g. Dawson, Webb, Wijsman, et al., 2005; Lowe, Werling, Constantino, Cantor, & Geschwind, 2014; Rommelse, Geurts, Franke, Buitelaar, & Hartman, 2011). However, the most fruitful endophenotypes may be those observed in infancy, prior to the emergence of behavioral symptoms that they are hypothesized to cause (Gliga, Jones, Bedford, Char-man, & Johnson, 2014).
A common approach to identifying infant endophenotypes of ASD has been to employ prospective longitudinal studies of infants with older siblings with ASD, who have a 20% chance of developing ASD themselves (Ozonoff et al., 2011). An endophenotype should be present in infants with later ASD, in addition to being elevated in unaffected family members relative to the general population. An emerging theme from this research is the early emergence of perturbations in social attention. Infants with later ASD show declining attention to eyes between 2 and 24 months (Jones & Klin, 2013); reduced attention engagement to faces on both cognitive and neural measures at 6 months (Jones et al., 2016); reduced monitoring of social stimuli at 6 months (Chawarska, Macari, & Shic, 2013; Shic, Macari, & Chawarska, 2014); declining attention to people in naturalistic contexts between 6 and 24 months (Ozonoff et al., 2010); and altered neural response to shifts in gaze at 7.5 months (Elsabbagh et al., 2012). These perturbations in social attention may reduce learning about the social world, affecting the later development of social communication skills. Indeed, dimensional relations with later social communication skills within infants at familial risk have been reported in infancy (Jones et al., 2016) and for similar measures in toddlers (Webb et al., 2010, 2011). Thus, perturbations in social attention could be a candidate endophenotype for ASD.
Candidate endophenotypes for ASD should not only be present in infants with a later diagnosis but should also be present at elevated levels in individuals who share genetic risk factors for the condition. As genetic variance contributing to ASD is the same as that contributing to neurotypical variation in autism-related traits (Constantino, 2011), this effect should be apparent in the general population. This is an important distinguishing feature between endophenotypes and other biomarkers, which could include any measurable indicator of a condition and should not be present in individuals without a diagnosis. To test whether infant social attention could be an endophenotype of ASD-related traits, in this study we examine infant social attention and its relation to parental social motivation in a large population of neurotypical infants at low risk for ASD. Social motivation is a core deficit in ASD, and reduced social motivation is part of the ‘broader phenotype’ of the condition shared by some family members (Chevallier, Kohls, Troiani, Brodkin, & Schultz, 2012; Sung et al., 2005). Social motivation encompasses orienting and maintaining attention to the social world, taking pleasure in social interactions and working to foster and maintain social bonds. We focused specifically on parent social motivation rather than a broader measure of ASD-related traits because of evidence that the social communication and restrictive and repetitive behavior domains are separable at the genetic level (Happé & Ronald, 2008; Ronald, Larsson, Anckarsäter, & Lichtenstein, 2011). Critically, social motivation shows high heritability (Sung et al., 2005). Thus, if infant social attention is an endophenotype of ASD-related traits, parent measures of social motivation should relate to infant levels of social attention.
We focused on 6- and 12-month-old infants to examine the same disruptions of social attention observed in infants with later ASD (Jones et al., 2016). To index social attention, we used three metrics that have been previously related to ASD in infants and young children. We hypothesized that infants of parents with low levels of social motivation would show lower levels of social attention in infancy. Specifically, we predicted that infants of parents with lower social motivation would show shorter peak looks to faces during a habituation paradigm (as do 6-month-old infants with later ASD; Jones et al., 2016), smaller and less pro-longed/faster P400 responses to faces versus objects in an event-related potential (ERP) paradigm (as do 6-month-old infants with later ASD; Jones et al., 2016; see also Webb, Dawson, Bernier, & Panagiotides, 2006 Figure 1), and reduced theta power to faces versus objects in an electroencephalography (EEG) study (as do toddlers with ASD; Dawson et al., 2012). Confirmation of these predictions would be consistent with infant social attention being an endophenotype for social motivation difficulties.
Figure 1.
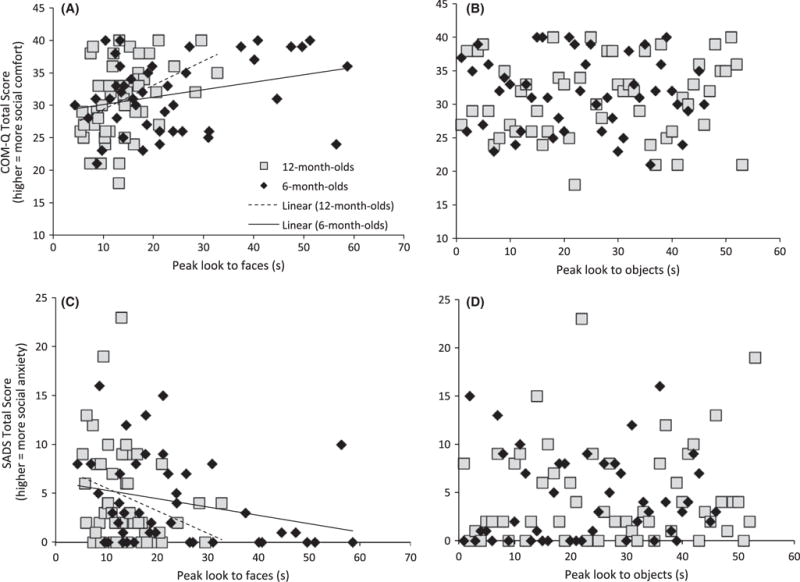
Higher parent social anxiety (SADS) and lower social comfort (COMQ) relate to shorter peak look to faces (A, C) but not objects (B, D) in 6- and 12-month-old infants
Methods
Participants
Six- and 12-month-old typically developing, full-term infants were recruited using a University Infant Participant Pool. Exclusionary criteria included a known family history of ASD in first- or second-degree relatives as we wished to examine traits within the neurotypical population; physical signs of known genetic syndromes; serious medical or neurological conditions; sensory or motor impairments; birth weight <2,000 g; gestational age <37 weeks; history of intraventricular hemorrhage; exposure to neurotoxins; and maternal gestational diabetes. To confirm that infants were typically developing, parents of infants were administered the Vineland Adaptive Behavior Scales (VAB; Sparrow, Cicchetti, & Balla, 2005), a standardized developmental interview that provides norm-referenced scores in the areas of communication, socialization, motor skills, and daily living skills. Table 1 provides final sample size and scores for infants and parents. Of note, only 50% of infants were asked to participate in the habituation task in order to produce comparable participant numbers to the ERP paradigm (given the high attrition rate of this methodology). All procedures performed were in accordance with the ethical standards of the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all parents of infants participating in the study.
Table 1.
Demographic and descriptive data for the subgroups included in particular analyses
| EEG Analysis n = 78 (35 f) |
ERP Analysis n = 42 (17 f) |
Habituation Analysis n = 42 (24 f) |
|
|---|---|---|---|
| 6 months | |||
| Age (days) | 195.3 (1.8) 178-220 |
197.8 (1.6) 178-224 |
195.8 (1.7) 178-224 |
| % Caucasian | 89% | 90% | 89% |
| % (B)PC is female | 90% | 88% | 93% |
| PC COMQ Total score | 31.2 (0.7) 17-40 |
29.5 (1.0) 16-40 |
31.8 (0.9) 21-40 |
| PC SADS Total score | 4.9 (0.6) 0-23 |
6.1 (1.0) 0-23 |
4.0 (0.7) 0-16 |
| Infant VAB Socialization SS | 109.9 (1.0) 91-126 |
110.6 (1.3) 94-126 |
108.4 (1.3) 87-123 |
| Infant VAB Communication SS | 110.1 (1.1) 82-123 |
108.0 (1.7) 82-123 |
112.0 (1.1) 93-123 |
|
| |||
| EEG Analysis n = 66 (29 f) |
ERP Analysis n = 50 (29 f) |
Habituation Analysis n = 47 (24 f) |
|
|
| |||
| 12 months | |||
| Age (days) | 380.1 (2.6) 361-448 |
380.4 (1.5) 361-448 |
379.7 (2.0) 361-448 |
| % Caucasian | 88% | 92% | 88% |
| % (B)PC is female | 90% | 96% | 93% |
| PC COMQ Total score | 30.8 (0.6) 21-40 |
29.9 (0.8) 18-40 |
30.9 (0.84) 18-40 |
| PC SADS Total score | 4.3 (0.5) 0-15 |
5.1 (0.7) 0-23 |
4.5 (0.7) 0-23 |
| Infant VAB Socialization SS | 96.4 (0.7) 84-112 |
96.6 (0.7) 84-112 |
97.4 (0.86) 84-112 |
| Infant VAB Communication SS | 107.9 (1.2) 80-124 |
107.6 (1.5) 80-121 |
110.2 (1.4) 80-124 |
f, female; (B)PC, Biological Primary Caregiver; COMQ, Social Competence Questionnaire; SADS, Social Avoidance and Distress Scale; VAB, Vineland Adaptive Behavior Scale; SS, Standard Score. Values are mean (standard error) and range (minimum-maximum).
Parent measures of social motivation
Primary caregiver, biological parents were asked to complete the Social Competence Questionnaire (COMQ, Sarason, Sarason, Anthony, & Basham, 1985) and the Social Avoidance and Distress Scale (SADS, Watson & Friend, 1969). A previous study of families with a child with ASD had identified strong heritability of the social motivation component of the lengthy in-person BPASS scale. Within this sample, BPASS social motivation scores were very highly correlated with scores on the COMQ and SADS (Sung et al., 2005). Thus, we selected the COMQ and SADS to assess social motivation with minimal parent burden. The COMQ includes 10 statements concerning social comfort that are rated on a scale of 1 to 4 from ‘not at all like me’ to ‘a great deal like me’; higher total scores on this measure reflect greater social comfort (see Appendix S1). The SADS includes 28 true or false questions about experience of social situations; higher total scores indicate greater social avoidance and distress (See Appendix S1). In the present sample, as expected, scores on the questionnaires were significantly related (6 months: r(98) = −.79, p < .001; 12 months r(94) = −.74, p < .001).
Infant social attention tasks
Habituation to faces and objects
As conducted in Jones et al. (2016; Webb et al., 2010), stimuli were colored photographs of female faces and toys. Infants participated in four habituation experiments, in a 2 day, by two stimulus sets repeated measures design. There were no effects of stimulus set, testing day, or order, and so analyses were collapsed to provide a more stable characterization of individual differences (e.g. Colombo, Mitchell, & Horowitz, 1988; Colombo, Mitchell, O’Brien, & Horowitz, 1987). Habituation was met when two consecutive looks (fixation longer than 1 s; e.g. Colombo & Mitchell, 1990) fell below 50% of the average of the child’s longest two looks, requiring a minimum of four looks. Analyses focused on the duration of the longest look (peak look), as shorter peak looks to faces at 6 months were related to ASD at 24 months in a recent longitudinal study (Jones et al., 2016).
EEG during live social interactions
EEG during live social versus nonsocial attention was collected as an index of attention engagement (Jones, Venema, Lowy, Earl, & Webb, 2015). In brief, EEG was recorded from 128-channel Geodesic sensor net, recorded online with reference to the vertex, digitized at 500 Hz, amplified at 1,000×, and band-pass filtered at 0.1 to 100 Hz. During recording, children were seated 60 cm from an experimenter while she sang for two 1-minute periods and held plain infant toys in her hands.
EEG data were segmented into 1-s segments and divided (based on offline coding from video) by whether infant was predominantly looking at social aspects of the display (e.g. the experimenter’s face) or at nonsocial aspects of the display (e.g. the toys in her hand). Looking was coded frame-by-frame from video by a trained experimenter (EJ). Segments were artifact detected for excessive amplitude or artifact (electro-ocular, movement, and muscular); bad channels were interpolated; and data were rereferenced to the average reference. Segments were detrended and subjected to a fast Fourier transform (FFT). Power values were averaged across segments and electrodes over frontal regions (24,28,29,25,20,21,3,4,124,123,119,118); natural logs were calculated to reduce skew. Logged power values were averaged across the theta (3–6 Hz) and alpha (6–9 Hz) frequency ranges to test whether effects were band-specific, and examined for social and nonsocial attention separately.
Event-related potential task
ERPs to faces and objects were collected as a measurement of the speed and depth of processing. The same EEG recording and artifact detection parameters were used as described above. Peaks between 300 and 900 ms were identified for the posterior temporal left (58,59,60,65,66) and right (86,92,97,85,91) regions for the P400 peak amplitude and latency (Dawson et al., 2002; Elsabbagh et al., 2012).
For analysis, we computed a difference score (faces minus objects; hereto referred to as the ‘P400 face/object difference’) for both latency and amplitude. Based on previous work (Elsabbagh et al., 2012; Jones et al., 2016), we predicted that infants with parents with lower social motivation would show a more negative P400 face/object difference for latency (faster response to faces) and a more negative P400 face/object difference for amplitude (smaller response to faces). Attrition rates for the ERP methodology can be found in Appendix S2.
Analysis strategy
We first conducted preliminary repeated measures ANOVAs on each social attention measure by Condition (social, nonsocial), Age and Sex to characterize effects and guide the design of the main analysis. Subsequently, to examine the relation between parent social motivation and infant social attention, data were analyzed with a series of multivariate ANOVAs conducted separately for the primary variable for each social attention measure. In the main multivariate model, parent reports of social motivation (COMQ and SADS total score) were added as the two dependent variables. Infant Age (6, 12) and Infant Sex (male, female) were included as between-subject factors, and the experimental measure was included as a covariate. The models were specified to examine the main effect of Age, Sex, the experimental measure, and the interaction terms (experimental measure by Age, experimental measure by Sex, and the experimental measure by Age and Sex). Thus, variables associated with the parent and variables associated with the child were separated in the models. Where there was a significant interaction between an experimental measure and Age and/or Sex, follow-up analyses examined effects within each subgroup. Of note, there were no significant main effects or interactions with Sex within these multivariate models (see Table S2), and thus Sex is not discussed further. Where there was an effect of an experimental measure on the multivariate term, we report individual significance levels for the COMQ and SADS.
Results
Habituation
The preliminary repeated measures ANOVA showed that peak looks were longer for faces than objects (F(1,87) = 4.9, p = .03), with no significant interactions with Age or Sex (Fs < 0.5, ps > .5). Peak looks were shorter at 12 than 6 months (F(1,87) = 21.8, p < .001).
In the multivariate analysis, infant peak look duration to faces was significantly related to parent social motivation (F(2,82) = 7.13, p = .001, ρ2 = .15). This effect was significant for the COMQ (F(2,83) = 14.42, p < .001, ρ2 = .15; Figure 1A) and the SADS (F(2,83) = 7.65, p = .007, ρ2 = .08; Figure 1C). Specifically, infants of parents with lower levels of social motivation showed shorter peak looks to faces. There was no significant relation between peak look duration to objects and parent social motivation (F(2,84) = 0.06, p = .94, ρ2 = .001; Figure 1B and D), and no significant interaction with age (F(2,82) = 0.50, p = .31, ρ2 = .01).
EEG
The preliminary repeated measures ANOVA confirmed that theta power was greater during social than nonsocial attention (F(1,140) = 26.78, p < .001), and greater at 12 than 6 months (F(1,140) = 27.0, p < .001) (Jones et al., 2015). Alpha power was greater during nonsocial than social attention (F(1,140) = 4.29, p = .04, ρ2 = .03) and there was an interaction between social versus nonsocial attention, Age, and Sex (F(1,140) = 8.57, p = .004, ρ2 = .058). At 12 months, male infants showed a greater difference in alpha power between nonsocial and social stimuli than female infants (F(1,64) = 6.83, p = .011, ρ2 = .096).
In the multivariate ANOVA, infant theta power during live social attention was significantly related to parental social motivation (F(2,136) = 3.51, p = .032, ρ2 = .049); specifically reduced theta during social attention was related to lower parental social motivation. This effect was marginally significant for the COMQ (F(1,137) = 3.66, p = .058, ρ2 = .026, Figure 2A) and significant for the SADS (F(1,137) = 7.04, p = .009, ρ2 = .049, Figure 2C). There was no significant interaction with Age (F(2,136) = 1.91, p = .15, ρ2 = .027).
Figure 2.
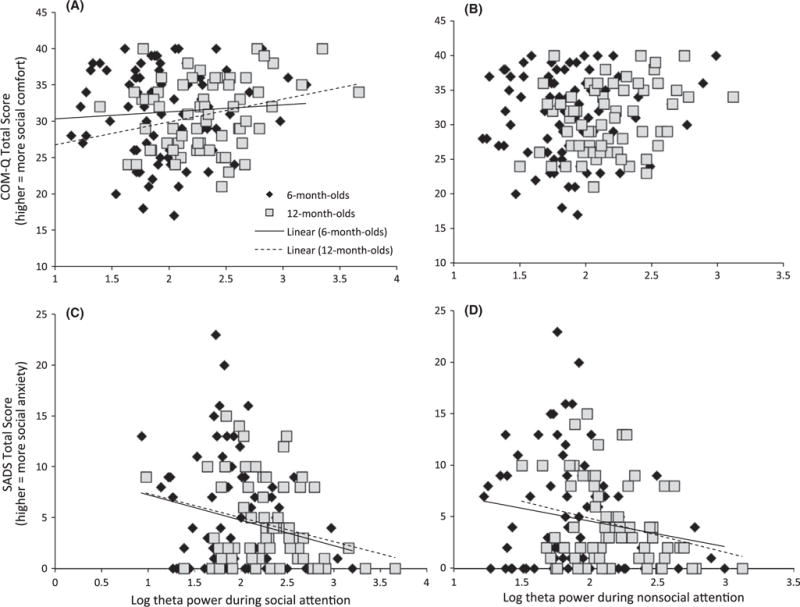
Higher parent social anxiety (SADS) and (with marginal significance) lower social comfort (COMQ) relate to lower theta power during social attention (A, C) in 6- and 12-month-old infants. Higher parent social anxiety (SADS) related to lower theta power during nonsocial attention in a social context (B, D) in 6- and 12-month-old infants
Effects were not significant for alpha power (F(2,136) = 1.11, p = .33, ρ2 = .016), though there was a marginally significant interaction with Age (F(2,136) = −2.48, p = .087). This was not significant for either measure individually, and is not interpreted further.
There was a marginally significant relation between theta power during nonsocial attention and parent social motivation (F(2,136) = 2.79, p = .07, ρ2 = .039), and no significant interaction with Age (F(2, 136)= 1.04, p = .36, ρ2 = .019). The marginal effect was individually significant for the SADS (F(1,137) = 5.56, p = .02, ρ2 = .039, Figure 2D) but not the COMQ (F(1,137) = 2.75, p = .1, ρ2 = .02, Figure 2B); lower theta power during nonsocial attention was related to higher levels of parental social anxiety.
ERP
The preliminary repeated measures ANOVA showed that P400 amplitudes were greater to objects than faces (F(1,90) = 22.1, p < .001) and latencies were faster for faces than objects (F(1,90) = 6.72, p = .011), with no significant interactions with Age or Sex (Fs < 2, ps > .1) (also Dawson et al., 2002; de Haan, Pascalis, & Johnson, 2002; Jones et al., 2016). Latencies were also significantly faster at 12 than 6 months (F(1,90) = 16.6, p < .001).
In the multivariate ANOVA, P400 amplitude ‘face/object difference’ significantly related to parental social motivation as a function of Age (F(2,85) = 3.36, p = .04, ρ2 = .07). This effect was individually significant for the COMQ (F(1,86) = 6.69, p = .011, ρ2 = .07, Figure 3B) and marginally significant for the SADS (F(1,86) = 3.13, p = .08, ρ2 = .035, Figure 3D). Specifically, reduced P400 amplitude to faces relative to objects was related to lower levels of parental social motivation in the 6- but not 12-month-old infants; at 6 months, this effect was significant for the COMQ (F(1,38) = 4.61, p = .038, ρ2 = .108) but not for the SADS (F(1,38) = 2.58, p = .116, ρ2 = .064). Examining responses to faces and objects separately did not reveal any significant effects (Fs < 3, ps > .1).
Figure 3.
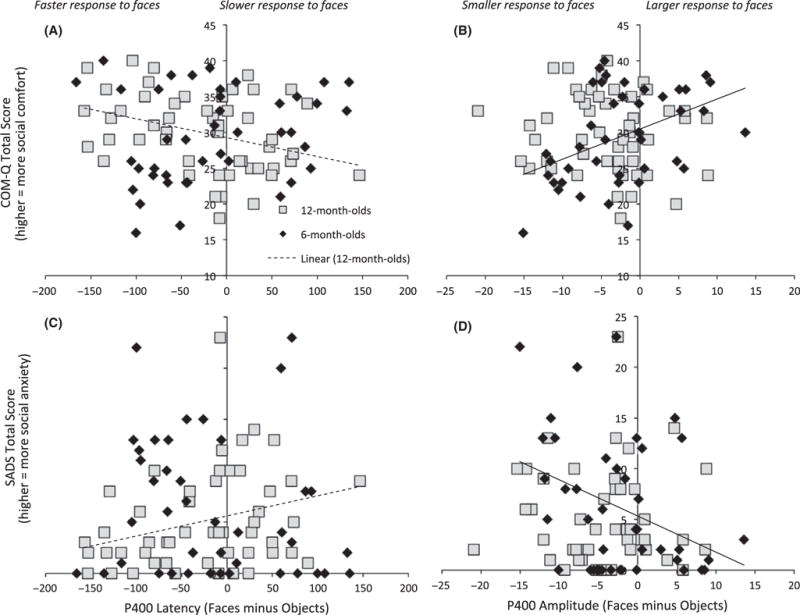
Lower social comfort (COMQ) and (with marginal significance) higher parent social anxiety (SADS) relate to slower responses to faces versus objects at 12 months (A, C). Lower social comfort (COMQ) was associated with smaller responses to faces versus objects at 6 months (B, D)
There was no significant overall relation between P400 latency to faces minus objects and parent phenotype (F(2,82) = 0.85 = 0, p = .88, ρ2 = .003). However, there was a marginally significant interaction between P400 latency and Age in the relation to parent phenotype (F(2,85) = 2.87, p = .062, ρ2 = .063); this was significant for both the COMQ (F(1,86) = 5.53, p = .021, ρ2 = .06, Figure 3A) and SADS (F(1,86) = 4.58, p = .035, ρ2 = .051, Figure 3C) individually. For 12- but not 6-month-old infants, faster P400 latency to faces relative to objects was related to lower levels of parental social motivation at the trend level (6 months: F(2,37) = 0.94, p = .40, ρ2 = .049; 12 months: F(2,45) = 2.67, p = .08, ρ2 = .11); at 12 months, this effect was significant for the COMQ (F(1,46) = 5.33, p = .026, ρ2 = .104) and marginally significant for the SADS (F(1,46) = 3.89, p = .055, ρ2 = .078).
Discussion
We tested whether parental social motivation was related to infant social attention using data from three tasks that reveal social attention difficulties in infants who later develop autism (Jones et al., 2016) and in preschoolers with early ASD (Dawson et al., 2012; Webb et al., 2006, 2010, 2011). Broadly, we confirmed our hypotheses. Infants with parents with lower levels of self-reported social motivation showed shorter peak looks to faces, a reduced P400 amplitude to faces versus objects, and reduced theta power during naturalistic social attention. These findings are consistent with reduced attention engagement to social stimuli in infants of parents with lower levels of social motivation. The quantitative relations observed are consistent with the proposal that variations in social attention are an infant endophenotype of social motivation, an ASD-related trait.
In our habituation task, parents who reported lower levels of social motivation had infants who showed shorter peak look durations to faces. This effect was individually significant for both the COMQ and SADS measures, was specific to faces and not observed for objects, and was not confounded by the number of trials infants took to habituate (See Appendix S3). Previous work with infants diagnosed with ASD at 2 years has shown that a shorter peak look to faces at 6 months is also related to later categorical ASD and dimensional variation in later ASD symptoms (Jones et al., 2016). A shorter peak look to faces likely reflects reduced attention engagement to social stimuli. Thus, in the present study, infants with parents with both lower social comfort and higher social anxiety were less engaged with social stimuli.
Second, we used EEG theta and alpha power to measure brain activity during natural social attention. Specifically, infants watched an experimenter singing and holding toys; EEG was contrasted during social visual attention (e.g. when the infant was looking at the examiner’s face) and nonsocial visual attention (e.g. while the infant was looking at the toys). The use of naturalistic stimuli is rare in infant EEG research, but is critical to establishing the nature of social attention in ecologically valid contexts. Increases in EEG theta power occur during species-relevant behaviors (Orekhova, Stroganova, Posikera & Elam, 2006). Thus, greater attention engagement to social stimuli should be reflected by increased theta power. Indeed, in 6- and 12-month-old typically developing infants, attention to the face of a researcher during a live singing episode produces an increase in frontal theta power relative to attention to objects (Jones et al., 2015). The extent of differential theta activation to faces and objects increases between 6 and 12 months, indicating developing specialization of the social brain. Although this specific paradigm has not yet been reported in high-risk populations, young children with ASD show reduced theta power and elevated alpha power to static faces versus objects; this can be normalized with intensive intervention (Dawson et al., 2012). The present data show that infants of parents with lower levels of social motivation also show smaller frontal theta responses during social attention, consistent with reduced attention engagement. Possibly, lower levels of parental social motivation are associated with slightly slower specialization of social brain regions in their infants. Of note, in this task, there were also effects at a trend level for nonsocial attention; because the experimenter was singing throughout the task, this may reflect the social context in which the nonsocial attention occurred. Supporting this interpretation, analysis of EEG data from the same cohort of infants passively watching naturalistic videos of toys moving shows no relation between theta power and parental social motivation (See Appendix S4).
In the ERP task, we examined P400 responses to faces and objects because responses over this and related components are altered in infants with later ASD (Elsabbagh et al., 2012; Jones et al., 2016), infants with older siblings with ASD (Elsabbagh et al., 2009), and children with ASD (Dawson et al., 2002; Webb et al., 2006). In the present study, 6-month-old infants with parents with lower social motivation showed relatively greater P400 amplitude to objects versus faces, consistent with lesser engagement of social attention. Interestingly, there were marginally significant effects for P400 latency that were strongest at 12 months. We had expected to see shorter P400 latencies to faces at 6 months in infants with parents with lower social motivation levels, as had been previously reported in infants with later ASD (Elsabbagh et al., 2012; Jones et al., 2016); this is the direction observed within 6-month olds in the present report (Figure 3A,C), though this was not significant. In contrast, at 12 months, infants with parents with lower social motivation showed slower responses to faces than objects – an effect in the opposite direction. Older children with ASD also show slower responses to faces than objects over the N170 component (Webb et al., 2006), for which the P400 is thought to be an infant precursor (de Haan, Johnson, & Halit, 2003). Thus, there may be age-related change in optimum endophenotypes of social attention, though further longitudinal work is required to address this hypothesis. Taken together, our study is consistent with the previous work showing that alterations in P400 response to faces are associated with ASD risk, and extends this observation to show a quantitative relationship with liability for lower social motivation within the general population.
Specificity to ASD
One feature of an endophenotype is that it should be more strongly associated with the condition of interest than with other psychiatric conditions. Several lines of evidence suggest that our work is indeed relevant to the study of ASD-associated traits. First, further analysis presented in the online supplementary material is not consistent with more general effects of psychopathology or infant temperament. Measures of social attention were not related to generalized anxiety or depression in parents (See Appendix S5), or more general measures of infant temperament (See Appendix S6); controlling for these variables in the statistical models did not change the pattern of results. Second, in other work, identical and related metrics of social attention relate to later categorical and dimensional features of ASD in infancy and toddlerhood (Dawson et al., 2012; Jones et al., 2016; Webb et al., 2006, 2010, 2011). Third, emerging results show that our selected metrics of social attention can be modified by a parent-mediated intervention designed to ameliorate symptoms of ASD in infants at high familial risk (E.J.H. Jones, G. Dawson, J. Kelly, A. Estes, & S. J. Webb, Personal Communication). Fourth, we used two measures of parent social motivation that relate to a construct that is highly heritable and diminished in individuals with ASD (Sung et al., 2005). Sung’s study used the COMQ and SADS in combination with the in-person Broader Phenotype Autism Symptom Scale (BPASS) interview to assess social motivation in families with ASD (Sung et al., 2005). The BPASS social motivation domain assesses child and adulthood social interest in peers and groups, and was more highly correlated with the COMQ than the SADS (Sung et al., 2005). In the present study, the COMQ was also slightly more closely related to infant social attention than was the SADS. Future work with observational measures of parent social communication would provide more nuanced information about the specific aspects of parent social functioning that might most closely relate to infant social attention. Taken together, our results suggest that infant social attention quantitatively varies with familial social motivation in the general population, and may thus be a candidate endophenotype for ASD-related traits.
Heritability and environment
Within the present study, the relation between parental phenotype and infant measures is likely to be driven by both genetic and environmental factors. Social motivation is known to be heritable (Sung et al., 2005). Altered social attention in infancy may link parent social motivation to the social motivation of their children. Reduced infant social attention may reduce self-directed experience with social stimuli or compromise the processing of those stimuli when experienced, resulting in reduced social motivation over development. In this way, altered infant social attention may be one factor that mediates the heritability of social motivation measured in later development. Alternatively, infant social attention may be an infant expression of a tendency toward reduced social motivation that persists through the life span. Of note, parent report of infant social communication on the Vineland did not reveal any relation between parent social motivation and their perception of their infant’s behaviors (Fs < 2, ps > .2). Changes in overt behavior may emerge downstream of changes in neurocognitive measures of social attention. Indeed, while changes in social attention are observed from 6 months in infants with later ASD (Elsabbagh et al., 2012; Jones et al., 2016), behavioral differences on the Vineland socialization scale are only apparent after 24 months (Estes et al., 2015). The collection of longitudinal observational data on large normative cohorts is required to establish whether there is a developmental link between parent social motivation, infant social attention, and the social motivation in later childhood.
The relation between infant social attention and parent social motivation may also have both direct and indirect contextual environmental components. Further, work should ascertain whether parents with lower social motivation interact differently with their infants, or may take their infants to fewer social events or activities. Given that our parent questionnaires were filled out by a biological parent who was also the infant’s primary caregiver, we cannot disentangle the effect of immediate environment and genetic background. Research with families with more complex family structures (e.g. egg/sperm donors, adoption, nonbiological primary caregivers) will be important in addressing these effects. Furthermore, the majority of our infant population had a female primary caregiver. Studies with a greater proportion of male respondents will be important to understanding whether there are parent-gender differences in the relation between parental social competence and infant social attention.
Further work to probe the nature of the neurocognitive systems underlying the observed individual differences on our social attention tasks will be important. Of note, the three experimental measures did not significantly correlate with each other (Appendix S7). Possibly, each task taps a different face of social attention, all of which are related to parental social functioning. Indeed, there were subtle differences between the measures in their relation to parent phenotype. For example, the ERP measures related differently with parent phenotype at 6 and 12 months; the habituation measure related similarly to both SADS and COMQ at both ages; and the EEG measures related more strongly to SADS than COMQ across both ages. To determine whether these differences represent meaningful divisions between different faces of social attention would require replication. General models of infant attention separate ‘attention’ into different constructs such as alerting, orienting, feature, and spatial attention (Colombo, 2001); how these constructs map onto social attention remains unclear. Jones et al. (2016) propose that while social orienting appears relatively intact in the first few months of life for infants with later ASD, deeper levels of engagement (or feature attention) with social stimuli may be reduced. There may be additional processes relevant to the present dataset (such as affective response), but these have not been well characterized in relation to the present tasks. Alternatively, measurement error associated with each experimental task may make intertask relations difficult to identify, while relations with more stable questionnaire-based measures may be clearer. Very few previous studies of normative infant social attention have used multiple experimental measures, constraining our understanding of the nature of attention development in the social domain. Thus, further work should focus on further specifying the nature of the neurocognitive systems that underlie social attention in infancy.
Of importance, the cohort as a whole scored within the normal range on adaptive social and communicative skills (Table 1; mean = 100, standard deviation 15). Thus, the sample of infants was generally typically developing in terms of their overt behaviors. Consistent with prospectively studied low-risk samples, rates of ASD and other developmental concerns are low (e.g. Ozonoff et al., 2011) and we explicitly excluded families with a history of ASD to ensure that our results reflect normative variation. Thus, we are categorically not proposing that the alterations in social attention in the present study indicate that infants will develop ASD. Rather, we contend that these alterations in social attention may represent background genetic variation that may predispose individuals at the extremes of the population, or who also experience a highly penetrant variant or environmental impact toward ASD. Other children with the same familial tendencies but without the additional risk factors may develop typically, and this is likely the case for the overwhelming majority of low-risk children in this report.
Conclusion
For the first time, we observed significant relations between lower parental social motivation and reduced social attention in their 6- and 12-month- old infants. Taken together, our work suggests that social attention is a quantitative endophenotypes of a core ASD-related domain in the general population. Our work also suggested that some measures of social attention were more sensitive to familial ASD-related traits at 6 or 12 months, again consistent with work with infants who develop ASD. This underlines the importance of considering the developmental nature of infant endophenotypes, and the possibility that different metrics may be needed at different ages. Measures of social attention in infancy have great potential for studies of the relation between genetic and environmental risk factors and the emergence of ASD-related traits over developmental time.
Supplementary Material
Appendix S1. Further information on the COMQ and SADS.
Appendix S2. Attrition rate for the ERP paradigm.
Appendix S3. Effect of number of trials to habituate.
Appendix S4. Relation between parental social motivation and EEG power in response to a dynamic nonsocial video.
Appendix S5. Do parental anxiety or depression confound the present results?
Appendix S6. Could the present results be related to general aspects of infant temperament?
Appendix S7. Correlations between the experimental variables.
Table S1. Relations between social attention measures and parental anxiety and depression symptoms measured with the Brief Symptom Inventory (BSI).
Table S2. Relations between social attention measures and domains of infant temperament.
Table S3. Summary of number of participants providing data for the event-related potential task. In the bottom two rows, figures are mean (standard error).
Key Points.
Efforts to understand autism spectrum disorder (ASD) have emphasized the importance of examining dimensional traits that vary in the general population.
A range of social endophenotypes for ASD have been identified in older children and adults, and prospective longitudinal studies of infants suggest early perturbations in social attention.
To establish whether social attention could be a candidate infant endophenotype for ASD-related traits, we examined the continuous relation between neurocognitive measures of infant social attention and parent self-reported social motivation in the general population.
Lower parental social motivation was related to reduced social attention in their 6- and 12-month-old infants.
Measures of infant social attention have great potential for studies of the relation between genetic and environmental risk factors and emergence of ASD-related traits over developmental time.
Acknowledgments
This project was supported by Autism Speaks and the L’Oreal/UNESCO For Women in Science Award for E. J., the Eunice Kennedy Shriver National Institute of Child Health and Development (P50 HD055782 and R01 HD064820, S.J.W.). The authors thank the families who participated in the University of Washington Early Attention Study and the Seattle Children’s SPARCS study.
Footnotes
Conflict of interest statement: No conflicts declared.
Supporting information
Additional Supporting Information may be found in the online version of this article:
The authors declare that they have no potential or competing conflicts of interests with relation to this report.
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders (DSM-5®) Washington, DC: American Psychiatric Pub; 2013. [Google Scholar]
- Bearden CE, Freimer NB. Endophenotypes for psychiatric disorders: Ready for primetime? Trends in Genetics: TIG. 2006;22:306–313. doi: 10.1016/j.tig.2006.04.004. [DOI] [PubMed] [Google Scholar]
- Chawarska K, Macari S, Shic F. Decreased spontaneous attention to social scenes in 6-month-old infants later diagnosed with autism spectrum disorders. Biological Psychiatry. 2013;74:195–203. doi: 10.1016/j.biopsych.2012.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevallier C, Kohls G, Troiani V, Brodkin ES, Schultz RT. The social motivation theory of autism. Trends in Cognitive Sciences. 2012;16:231–239. doi: 10.1016/j.tics.2012.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombo J. The development of visual attention in infancy. Annual Review of Psychology. 2001;52:337–367. doi: 10.1146/annurev.psych.52.1.337. [DOI] [PubMed] [Google Scholar]
- Colombo J, Mitchell DW. Individual differences in early visual attention: Fixation time and information processing. In: Colombo J, Fagen J, editors. Individual differences in infancy: Reliability, stability, prediction. Mahwah, NJ: Lawrence Erlbaum Associates; 1990. pp. 193–127. [Google Scholar]
- Colombo J, Mitchell DW, Horowitz FD. Infant visual attention in the paired-comparison paradigm: Test-retest and attention-performance relations. Child Development. 1988;59:1198–1210. doi: 10.1111/j.1467-8624.1988.tb01489.x. [DOI] [PubMed] [Google Scholar]
- Colombo J, Mitchell DW, O’Brien M, Horowitz FD. The stability of visual habituation during the first year of life. Child Development. 1987;58:474–487. [PubMed] [Google Scholar]
- Colvert E, Tick B, McEwen F, Stewart C, Curran SR, Woodhouse E, Bolton P. Heritability of autism spectrum disorder in a UK population-based twin sample. JAMA Psychiatry. 2015;72:415–423. doi: 10.1001/jamapsychiatry.2014.3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantino JN. The quantitative nature of autistic social impairment. Pediatric Research. 2011;69(5 Pt 2):55R–62R. doi: 10.1203/PDR.0b013e318212ec6e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantino JN, Todd RD. Intergenerational transmission of subthreshold autistic traits in the general population. Biological Psychiatry. 2005;57:655–660. doi: 10.1016/j.biopsych.2004.12.014. [DOI] [PubMed] [Google Scholar]
- Cuthbert BN, Insel TR. Toward the future of psychiatric diagnosis: The seven pillars of RDoC. BMC Medicine. 2013;11:126. doi: 10.1186/1741-7015-11-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson G, Carver L, Meltzoff AN, Panagiotides H, McPartland J, Webb SJ. Neural correlates of face and object recognition in young children with autism spectrum disorder, developmental delay, and typical development. Child Development. 2002;73:700–717. doi: 10.1111/1467-8624.00433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson G, Jones EJH, Merkle K, Venema K, Lowy R, Faja S, Webb SJ. Early behavioral intervention is associated with normalized brain activity in young children with autism. Journal of the American Academy of Child and Adolescent Psychiatry. 2012;51:1150–1159. doi: 10.1016/j.jaac.2012.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson G, Webb SJ, Wijsman E, Schellenberg G, Estes A, Munson J, Faja S. Neurocognitive and electrophysiological evidence of altered face processing in parents of children with autism: Implications for a model of abnormal development of social brain circuitry in autism. Development and Psychopathology. 2005;17:679–697. doi: 10.1017/S0954579405050327. [DOI] [PubMed] [Google Scholar]
- de Haan M, Johnson MH, Halit H. Development of face-sensitive event-related potentials during infancy: A review. International Journal of Psychophysiology: Official Journal of the International Organization of Psychophysiology. 2003;51:45–58. doi: 10.1016/s0167-8760(03)00152-1. [DOI] [PubMed] [Google Scholar]
- de Haan M, Pascalis O, Johnson MH. Specialization of neural mechanisms underlying face recognition in human infants. Journal of Cognitive Neuroscience. 2002;14:199–209. doi: 10.1162/089892902317236849. [DOI] [PubMed] [Google Scholar]
- Elsabbagh M, Mercure E, Hudry K, Chandler S, Pasco G, Charman T, Johnson MH. Infant neural sensitivity to dynamic eye gaze is associated with later emerging autism. Current Biology. 2012;22:338–342. doi: 10.1016/j.cub.2011.12.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsabbagh M, Volein A, Csibra G, Holmboe K, Garwood H, Tucker L, Johnson MH. Neural correlates of eye gaze processing in the infant broader autism phenotype. Biological Psychiatry. 2009;65:31–38. doi: 10.1016/j.biopsych.2008.09.034. [DOI] [PubMed] [Google Scholar]
- Estes A, Zwaigenbaum L, Gu H, John TS, Paterson S, Elison JT, Network, I Behavioral, cognitive, and adaptive development in infants with autism spectrum disorder in the first 2 years of life. Journal of Neurodevelopmental Disorders. 2015;7:1–10. doi: 10.1186/s11689-015-9117-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gliga T, Jones EJH, Bedford R, Charman T, Johnson MH. From early markers to neuro-developmental mechanisms of autism. Developmental Review. 2014;34:189–207. doi: 10.1016/j.dr.2014.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Happé F, Ronald A. The “fractionable autism triad”: A review of evidence from behavioural, genetic, cognitive and neural research. Neuropsychology Review. 2008;18:287–304. doi: 10.1007/s11065-008-9076-8. [DOI] [PubMed] [Google Scholar]
- Jones EJH, Gliga T, Bedford R, Charman T, Johnson MH. Developmental pathways to autism: A review of prospective studies of infants at risk. Neuroscience and Biobehavioral Reviews. 2014;39:1–33. doi: 10.1016/j.neubiorev.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones W, Klin A. Attention to eyes is present but in decline in 2-6-month-old infants later diagnosed with autism. Nature. 2013;504:427–431. doi: 10.1038/nature12715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones EJH, Venema K, Earl R, Lowy R, Barnes K, Estes A, Webb SJ. Reduced engagement with social stimuli in 6-month-old infants with later autism spectrum disorder: A longitudinal prospective study of infants at high familial risk. Journal of Neurodevelopmental Disorders. 2016;8:7. doi: 10.1186/s11689-016-9139-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones EJH, Venema K, Lowy R, Earl RK, Webb SJ. Developmental changes in infant brain activity during naturalistic social experiences. Developmental Psychobiology. 2015;57:842–853. doi: 10.1002/dev.21336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenzenweger MF. Endophenotype, intermediate phenotype, biomarker: Definitions, concept comparisons, clarifications. Depression and Anxiety. 2013;30:185–189. doi: 10.1002/da.22042. [DOI] [PubMed] [Google Scholar]
- London EB. Categorical diagnosis: A fatal flaw for autism research? Trends in Neurosciences. 2014;37:683–686. doi: 10.1016/j.tins.2014.10.003. [DOI] [PubMed] [Google Scholar]
- Lowe JK, Werling DM, Constantino JN, Cantor RM, Geschwind DH. Social responsiveness, an autism endophenotype: Genomewide significant linkage to two regions on chromosome 8. American Journal of Psychiatry. 2014;172:266–275. doi: 10.1176/appi.ajp.2014.14050576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundström S, Chang Z, Råstam M, Gillberg C, Larsson H, Anckarsäter H, Lichtenstein P. Autism spectrum disorders and autistic like traits: Similar etiology in the extreme end and the normal variation. Archives of General Psychiatry. 2012;69:46–52. doi: 10.1001/archgenpsychiatry.2011.144. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Weinberger DR. Intermediate phenotypes and genetic mechanisms of psychiatric disorders. Nature Reviews Neuroscience. 2006;7:818–827. doi: 10.1038/nrn1993. [DOI] [PubMed] [Google Scholar]
- Moreno-De-Luca A, Myers SM, Challman TD, Moreno-De-Luca D, Evans DW, Ledbetter DH. Developmental brain dysfunction: Revival and expansion of old concepts based on new genetic evidence. Lancet Neurology. 2013;12:406–414. doi: 10.1016/S1474-4422(13)70011-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss EM, Batshaw ML, Solot CB, Gerdes M, McDonald-McGinn DM, Driscoll DA, Wang PP. Psychoeducational profile of the 22q11.2 microdeletion: A complex pattern. The Journal of Pediatrics. 1999;134:193–198. doi: 10.1016/s0022-3476(99)70415-4. [DOI] [PubMed] [Google Scholar]
- Mundy PC, Henderson HA, Inge AP, Coman DC. The modifier model of autism and social development in higher functioning children. Research and Practice for Persons with Severe Disabilities: The Journal of TASH. 2007;32:124. doi: 10.2511/rpsd.32.2.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orekhova EV, Stroganova TA, Posikera IN, Elam M. EEG theta rhythm in infants and preschool children. Clinical Neurophysiology. 2006;117:1047–1062. doi: 10.1016/j.clinph.2005.12.027. [DOI] [PubMed] [Google Scholar]
- Ozonoff S, Iosif AM, Baguio F, Cook IC, Hill MM, Hutman T, Young GS. A prospective study of the emergence of early behavioral signs of autism. Journal of the American Academy of Child and Adolescent Psychiatry. 2010;49:256–266.e2. [PMC free article] [PubMed] [Google Scholar]
- Ozonoff S, Young GS, Carter A, Messinger D, Yirmiya N, Zwaigenbaum L, Stone WL. Recurrence risk for autism spectrum disorders: A baby siblings research consortium study. Pediatrics. 2011;128:e488–e495. doi: 10.1542/peds.2010-2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson EB, Koenen KC, McCormick MC, Munir K, Hallett V, Happe F, Ronald A. Evidence that autistic traits show the same etiology in the general population and at the quantitative extremes (5%, 2.5%, and 1%) Archives of General Psychiatry. 2011;68:1113–1121. doi: 10.1001/archgenpsychiatry.2011.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson EB, Neale BM, Hyman SE. Genetic research in autism spectrum disorders. Current Opinion in Pediatrics. 2015;27:685–691. doi: 10.1097/MOP.0000000000000278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rommelse NNJ, Geurts HM, Franke B, Buitelaar JK, Hartman CA. A review on cognitive and brain endophenotypes that may be common in autism spectrum disorder and attention-deficit/hyperactivity disorder and facilitate the search for pleiotropic genes. Neuroscience and Biobehavioral Reviews. 2011;35:1363–1396. doi: 10.1016/j.neubiorev.2011.02.015. [DOI] [PubMed] [Google Scholar]
- Ronald A, Larsson H, Anckarsaöter H, Lichtenstein P. A twin study of autism symptoms in Sweden. Molecular Psychiatry. 2011;16:1039–1047. doi: 10.1038/mp.2010.82. [DOI] [PubMed] [Google Scholar]
- Sarason BR, Sarason IG, Anthony T, Basham RB. Concomitants of social support: Social skills, physical attractiveness, and gender. Journal of Personality and Social Psychology. 1985;49:469–480. [Google Scholar]
- Shic F, Macari S, Chawarska K. Speech disturbs face scanning in 6-month-old infants who develop autism spectrum disorder. Biological Psychiatry. 2014;75:231–237. doi: 10.1016/j.biopsych.2013.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparrow S, Cicchetti D, Balla D. Vineland adaptive behavior scales: (Vineland II), survey interview form/caregiver rating form. 2005 Pearson Assessments. [Google Scholar]
- Sung YJ, Dawson G, Munson J, Estes A, Schellenberg GD, Wijsman EM. Genetic investigation of quantitative traits related to autism: Use of multivariate polygenic models with ascertainment adjustment. The American Journal of Human Genetics. 2005;76:68–81. doi: 10.1086/426951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viding E, Blakemore SJ. Endophenotype approach to developmental psychopathology: Implications for autism research. Behavior Genetics. 2006;37:51–60. doi: 10.1007/s10519-006-9105-4. [DOI] [PubMed] [Google Scholar]
- Watson D, Friend R. Measurement of social-evaluative anxiety. Journal of Consulting and Clinical Psychology. 1969;33:448–457. doi: 10.1037/h0027806. [DOI] [PubMed] [Google Scholar]
- Webb SJ, Dawson G, Bernier R, Panagiotides H. ERP evidence of atypical face processing in young children with autism. Journal of Autism and Developmental Disorders. 2006;36:881–890. doi: 10.1007/s10803-006-0126-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb SJ, Jones EJH, Merkle K, Namkung J, Toth K, Greenson J, Dawson G. Toddlers with elevated autism symptoms show slowed habituation to faces. Child Neuropsychology: A Journal on Normal and Abnormal Development in Childhood and Adolescence. 2010;16:255–278. doi: 10.1080/09297041003601454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb SJ, Jones EJH, Merkle K, Venema K, Greenson J, Murias M, Dawson G. Developmental change in the ERP responses to familiar faces in toddlers with autism spectrum disorders versus typical development. Child Development. 2011;82:1868–1886. doi: 10.1111/j.1467-8624.2011.01656.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zufferey F, Sherr EH, Beckmann ND, Hanson E, Maillard AM, Hippolyte L, 16p11.2 European Consortium A 600 kb deletion syndrome at 16p11.2 leads to energy imbalance and neuropsychiatric disorders. Journal of Medical Genetics. 2012;49:660–668. doi: 10.1136/jmedgenet-2012-101203. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Further information on the COMQ and SADS.
Appendix S2. Attrition rate for the ERP paradigm.
Appendix S3. Effect of number of trials to habituate.
Appendix S4. Relation between parental social motivation and EEG power in response to a dynamic nonsocial video.
Appendix S5. Do parental anxiety or depression confound the present results?
Appendix S6. Could the present results be related to general aspects of infant temperament?
Appendix S7. Correlations between the experimental variables.
Table S1. Relations between social attention measures and parental anxiety and depression symptoms measured with the Brief Symptom Inventory (BSI).
Table S2. Relations between social attention measures and domains of infant temperament.
Table S3. Summary of number of participants providing data for the event-related potential task. In the bottom two rows, figures are mean (standard error).


