Abstract
Prospective longitudinal studies of infants with older siblings with autism spectrum disorder (ASD) have indicated that differences in the neurocognitive systems underlying social attention may emerge prior to the child meeting ASD diagnostic criteria. Thus, targeting social attention with early intervention might have the potential to alter developmental trajectories for infants at high risk for ASD. Electrophysiological and habituation measures of social attention were collected at 6, 12, and 18 months in a group of high-risk infant siblings of children with ASD (N = 33). Between 9 and 11 months of age, infant siblings received a parent-delivered intervention, promoting first relationships (PFR), (n = 19) or on-going assessment without intervention (n = 14). PFR has been previously shown to increase parental responsivity to infant social communicative cues and infant contingent responding. Compared to infants who only received assessment and monitoring, infants who received the intervention showed improvements in neurocognitive metrics of social attention, as reflected in a greater reduction in habituation times to face versus object stimuli between 6 and 12 months, maintained at 18 months; a greater increase in frontal EEG theta power between 6 and 12 months; and a more comparable P400 response to faces and objects at 12 months. The high-risk infants who received the intervention showed a pattern of responses that appeared closer to the normative responses of two groups of age-matched low-risk control participants. Though replication is necessary, these results suggest that early parent-mediated intervention has the potential to impact the brain systems underpinning social attention in infants at familial risk for ASD.
Keywords: autism, ASD, infant, high-risk, neurocognitive, social attention, promoting first relationships
Introduction
Autism spectrum disorder (ASD) is a neurodevelopmental disorder characterized by impairments in social communication, and the presence of restrictive and repetitive behaviors [American Psychiatric Association, 2013]. With sensitive methods, signatures of ASD should be detectable in early development given the predominantly prenatal peak expression patterns of associated genetic risk factors [Parikshak et al., 2013]. Clear symptoms of ASD likely emerge from a complex interaction between such pre-existing biologically-based vulnerabilities and the child’s environment [Dawson, 2008; Jones, Gliga, Bedford, Charman, & Johnson, 2014]. Studying how ASD unfolds from birth onwards is critical for understanding these developmental mechanisms, identifying children who require early intervention, and defining appropriate intervention targets [Webb, Jones, Kelly, & Dawson, 2014].
Prospective longitudinal studies of infants with older siblings with ASD indicate that approximately 20% will develop ASD themselves [Ozonoff et al., 2011]. Such studies have also suggested that perturbations in the neurocognitive systems underpinning social attention might emerge prior to the detection of any behavioral symptoms of ASD. While very early orienting responses to faces can appear relatively typical [e.g. Elsabbagh et al., 2013; for review see Gliga, Jones, Bedford, Charman, & Johnson, 2014], deeper levels of attention engagement may be altered. For example, infants who meet criteria for ASD between 2 and 3 years of age show declining attention to eyes between 2 and 24 months [Jones & Klin, 2013], reduced sustained attention to faces on both cognitive and neural measures at 6 months [Jones et al., 2016], reduced monitoring of social stimuli at 6 months [Chawarska, Macari, & Shic, 2013; Shic, Macari, & Chawarska, 2014], altered social/ nonsocial attention shifting at 7 months [Elison et al., 2013], declining attention to people in naturalistic contexts between 6 and 24 months [Ozonoff et al., 2010], and altered neural response to shifts in gaze at 6 to 9-months [Elsabbagh et al., 2012]. Such early disruptions to social attention could have cascading consequences for later social development, altering the infant’s experience of their early social environment and constraining learning opportunities [Dawson, 2008; Dawson, Webb, & McPartland, 2005]. Indeed, toddlers with ASD show characteristic disruptions in cognitive and neural measures of social attention (including habituation time and neural responses to faces) that are related to their broader social difficulties [Webb et al., 2010, 2011].
Evidence suggests that early intensive intervention for toddlers with ASD can alter neurophysiological measures related to social attention. Specifically, Dawson et al. [2012] showed that 2 years of intensive therapy using the Early Start Denver Model was associated with normalized neural responses (theta and alpha power) to social stimuli in toddlers with ASD. Further, these responses were related to behavioral outcome measures of social interaction, with higher levels of cortical activation during viewing of social stimuli associated with greater improvements in social behavior. Thus, there are significant clinical implications for improving social attention early in the development of children with ASD.
Interventions that could be applied earlier in development, prior to the emergence of significant behavioral symptoms, have the potential for more pervasive effects [Webb, Jones et al., 2014]. In a small proof-of-principle study, Rogers et al. [2014] showed that 7–15-month-old symptomatic infants who received a low-intensity parent implemented version of the Early Start Denver Model showed a reduction in ASD symptoms at age 3 years. Green et al. [2015] conducted a randomized controlled trial of a parent-mediated intervention focused on parent–child interaction in 54 infants at familial risk for ASD who were not screened for initial level of behavioral symptoms. The intervention had promising effects on behavioral measures of emerging autism symptoms, visual attention shifting, and infant attentiveness to their caregiver between 8 and 14 months, though most effects did not reach standard levels of significance. However, the small number of reports of early intervention in high risk populations stands in stark contrast to the large number of infants enrolled in studies of early ASD, with over 1241 high risk infants included in a recent US Baby Sibs consortium paper alone [Messinger et al., 2015].
One significant challenge to progress has been the lack of sensitive and blinded measures for rapidly assessing the efficacy of early, low-intensity interventions. Parent-report measures are always limited by lack of blinding in an area where parent-mediated intervention is typical. Observational behavioral measures of emerging autism symptoms cover a range of domains, and may be insufficiently sensitive to detect effects of intervention on specific neurocognitive mechanisms that may influence later development. Robust neurocognitive measures of social attention in infancy that are sensitive to the effects of low-intensity intervention could provide early indicators of the success or failure of a particular treatment approach. This could be helpful for developing and/or adapting individualized intervention for this group.
The present study was a randomized clinical trial of a parent-mediated intervention for infants at high familial risk for ASD with treatment between 9 and 11 months. Social attention was an outcome measure at the primary endpoint of 12 months, with a follow-up assessment at 18 months to assess maintenance of effects. The intervention (promoting first relationships [PFR], Kelly, Zuckerman, Sandoval, & Buehlman, 2008) was designed to facilitate parent–infant interaction in populations with a wide range of risk factors (e.g., families living in poverty, or infants with developmental disabilities; see S1.2 for more details). It promotes infant contingent responding, positive affect, self-regulation, and parental responsivity to infant communicative cues. In the present study, infants were provided with intervention based on their familial risk status as there is evidence that a subgroup of these infants will show declining attention to social stimuli in the first year of life. The intervention was designed to proactively stimulate neural systems related to social interaction, including promoting the infant’s ability to attend to and respond to his or her social partner.
In this report, we chose three different tasks that have been widely used in previous work with typically developing infants to provide a comprehensive picture of the effects of intervention on neurocognitive measures of social attention in infants at familial risk for ASD. (1) Habituation to faces and objects was used to measure sustained attention and learning speed. Toddlers with ASD show prolonged habituation times to faces [Webb et al., 2010], indicating slow learning. Thus, we predicted that the PFR intervention would decrease habituation times to faces relative to objects, reflective of increased social learning speed. (2) EEG theta power to social and nonsocial videos was measured as an index of attention engagement. Toddlers with ASD show reduced theta responses to social stimuli and evidence suggests that this pattern of EEG activity can be altered with intensive intervention [Dawson et al., 2012]. Thus, we predicted that the PFR intervention would increase theta responses to social stimuli. (3) Event-related potentials (ERPs) to faces and objects were measured as an index of the speed and depth of processing of social and non-social stimuli. Six-month-old infants who were later diagnosed with ASD show faster and less prolonged neural responses to faces than other infants [Jones et al., 2016]. Thus, we predicted that the PFR intervention would be associated with more prolonged and increased amplitude of responses to faces versus objects (indicating greater depth of processing).
Methods
Participants
Participants were high and low risk infants enrolled at 6 months of age and followed at 12 and 18 months (Table 1, n Fig. 1, see S1.1. for further details). High-risk infants (with an older sibling with ASD) participated in the randomized control trial of the PFR intervention; data from the low-risk groups (with no family history of ASD) were used to confirm normative patterns of performance. Specifically, high-risk infants were randomized after the baseline assessment into two groups: one group received the PFR intervention between 9 and 11 months of age (= 19, PFR), and the other received assessment and monitoring only (n = 14, A + M). Later in the study, an additional randomization group was added who received PFR “late” (between 14 and 17 months of age); due to small sample size (n = 3) this group is not included in the present report. Groups did not differ on sex (χ2 (1) = 0.04, P = 0.95; Hispanic ethnicity (χ2 (1) = 0.11, P = 0.74) or race (χ2 (1) = 0.11, P = 0.74); see Table 1. Inclusion and exclusion criteria and participant numbers per analysis are described in S1.1, available online. At 6 months there were no baseline differences between study groups in terms of adaptive and cognitive skills, or emerging autism symptoms (see Table 1 and S1.3, available online). Randomization occurred after the baseline assessment session at 6 months based on predetermined randomization blocks of 4 (AABB, ABAB); there were no stratification variables for randomization. Other interventions received by infants during the trial are summarized in S1.4 and Table S1, available online.
Table 1.
Clinical and Demographic Characteristics at the Baseline Assessment
| Age in days | Number (% female) |
Ethnicity (% white) |
Mullen verbal standard score | Mullen nonverbal standard score | |
|---|---|---|---|---|---|
| PFR | 192.3 (3.5) | 19 (37%) | 89% | 90.9 (2.1) | 95.2 (2.4) |
| A+M | 194.4 (2.1) | 14 (36%) | 86% | 92.8 (4.0) | 102.1 (3.6) |
| ControlCross | 192.9 (0.9) | 114 (45%) | 76% | 98.9 (1.4) | 104.5 (1.3) |
| ControlLong | 197.8 (3.0) | 36 (47%) | 92% | 92.9 (2.3) | 100.9 (2.8) |
Unless specified, numbers are mean (Standard Error).
Figure 1.
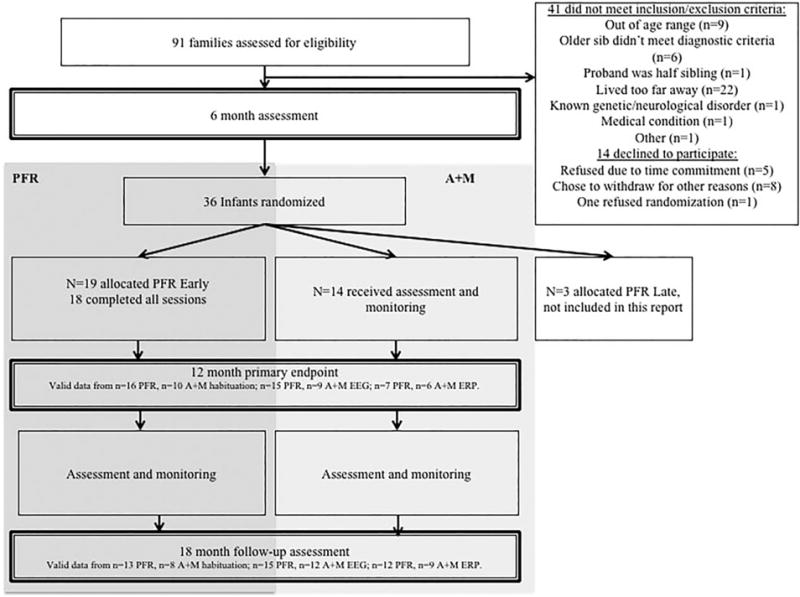
Trial profile.
Two Low-Risk comparison groups (“normative controls”) were enrolled to confirm normative patterns of responding on each task, since neurocognitive measures do not have developmental norms available. The first was infant siblings of children who did not have ASD who were also assessed longitudinally at 6, 12, and 18 months in the same protocol as the High-Risk groups (ControlLong N = 36). The second was a large group of Low-Risk 6 and 12-month-old infants who did not have a family history of ASD recruited from a local participant database (for further details see Jones et al., 2016), and who were studied cross-sectionally (ControlCross). For the ControlCross more infants were enrolled in the EEG and ERP tasks (6 months: N = 114, 51 females; 12 months: N = 104, 50 female) than the behavioral attention tasks (6 months: n = 51, 27 females; 12 months: n = 54, 27 female) given the higher attrition rate for EEG in this age range. We consider this sample size appropriate to define “normative” performance, since it is comparable to that used for individual age bands on the Mullen Scales of Early Learning (a widely used clinical tool; Mullen, 1995).
Promoting First Relationships
Infants who were provided with PFR received intervention for 10 weekly 60 to 85 minute in-home visits by a master’s level mental health provider trained in the promoting first relationships program (http://pfrprogram.org).
The PFR intervention focuses on promoting parenting responsivity to infant social communicative cues and behaviors using strength-based consultation strategies. Caregiver-infant interaction was videotaped and reviewed. The caregiver and the PFR provider discussed parenting strengths and interpretation of the infant’s cues using relationship focused consultation strategies (positive feedback; positive and instructive feedback; reflective comments and questions; validating, responsive statements). Weeks 2–10 focused on reflecting on the prior week’s content and worksheets. The curriculum is fully manualized and fidelity was assessed according to the manual (see S1.2, available online).
Assessments
Infant assessments occurred at 6, 12, and 18 months (PFR, A + M, and ControlLong); see Table 1. All assessment measures were the same at each age unless noted. Assessment staff were naïve with respect to the participant’s intervention group status.
Habituation task
Infants participated in an infant-controlled habituation procedure (see S1.5. available online). Stimuli were colored photographs of neutral forward facing female faces and infant toys. Infants participated in four habituation experiments, in a two stimuli (faces or toys) by two delay (1 second vs. 1 minute) repeated measures design. Looking time was manually coded via button press by two experimenters who viewed the infant in real time through a closed-circuit TV; average intra-class correlation coefficients for the two coders exceeded 0.8 for all tasks and for all groups. Total time to habituate was averaged by stimulus type to provide a more stable characterization of individual differences [Rose, Feldman, & Wallace, 1988]. Data validation procedures and additional methods are detailed in S1.5, available online.
EEG theta power to social and non-social videos
Electroencephalographic (EEG; EGI Inc., Salem, OR) recordings were collected while the infant viewed videos of social stimuli (vignettes of women telling nursery rhymes) and non-social stimuli (dynamic toys such as a ball dropping down a chute; see S1.6, available online). High-density 128 channel EEG was recorded continuously throughout the session, with a concurrent video record of the infant’s behavior time-locked to the EEG record. EEG was segmented into 1-second segments and edited for artifact. Segments were de-trended and processed in a Fast Fourier transform. Power values were then averaged across artifact free segments and electrodes within topographical regions and natural logs were calculated to reduce skew. Infants were only included in the analyses if they provided at least five artifact-free segments per condition. In order to calculate the frontal power indices, natural log (ln) 4–6 Hz theta power data from left and right frontal electrodes were used (Marshall, Bar-Haim, & Fox, 2002; see Fig. S1, available online).
ERP task
Event-related potential data were collected in response to digital photographs of faces and objects (toys; see S1.7, available online). Offline, data were low pass filtered at 20 Hz, segmented, unattended trials and artifact were removed. Data were averaged per stimulus type, re-referenced offline to the average reference, and corrected with respect to a pre-stimulus baseline period.
Posterior temporal left and right regions (see Fig. S1, available online) and components of interest were defined with respect to the previous literature, and inspection of the grand average waveform. We analyzed P400 peak amplitude and latency because these measures have been sensitive to atypicalities in infants with later ASD [Elsabbagh et al., 2012] and children with ASD [Dawson et al., 2002]. The P400 is modulated by dimensions that influence social attention (e.g., social familiarity, de Haan, Johnson, & Halit, 2003); faster and less prolonged neural responses to faces versus objects have been associated with later ASD [Elsabbagh et al., 2012; Jones et al., 2016]. Peaks were identified for each electrode using in-house automatic peak detection software, and verified by visual inspection. Peaks were defined as the most positive point of a deflection between 300 and 900 msec, and the peak had to be present in at least 2/6 electrodes in a group. Peak amplitude and latency values were averaged across regions.
Analysis strategy
For habituation and EEG tasks, initial analyses used repeated-measures ANOVA with Stimulus (face/object) as a within-subject variable, Group (PFR, A + M) as the between-subject variable and difference scores for habituation or EEG (12–6 months or 18–6 months) as the dependent variable. Where there were significant interactions, follow-up analyses on difference scores separated by Stimulus or Group were used to understand the pattern of effects. In addition, we conducted pre-planned cross-sectional analysis of habituation and EEG data at each time-point using repeated measures ANOVA for factors manipulated within-infant (e.g., stimulus, laterality) for comparability to the ERP analysis strategy. For the ERP task, the expected lower rate of inclusion meant that primary analyses were conducted cross-sectionally at 6, 12, and 18 months using repeated-measures ANOVA with Stimulus (face/object) and laterality (left, right) as within-subject variables, group (PFR, A + M) as the between-subject variable, and ERP data at each time-point as the dependent variable. We have included data from the ControlCross and ControlLong groups in the figures to confirm the nature of normative performance on our battery; data from these groups have been previously published [Jones, Venema, Lowy, Earl, & Webb, 2015; Jones et al., 2016]. Supplementary statistical comparisons of performance in the ControlLong and PFR/A + M groups can be found in section S2.1; this was not a pre-planned analysis since the low-risk group were not randomized, and thus is not included in the main manuscript.
Results
Habituation to Faces and Objects
Data were available for 16 infants at both baseline (6 months) and 12 months, and 13 infants at both baseline and 18 months in the PFR condition. Data were available for ten infants at both baseline and 12 months and eight infants at both baseline and 18 months in the A + M condition (see S1.5, available online). Habituation times have been interpreted as a measure of learning rate and information processing speed, and toddlers with ASD show prolonged habituation times to faces [Colombo & Mitchell, 2009; Webb et al., 2010]. We thus predicted that PFR would be associated with reduction in habituation times to faces but not objects.
Primary analyses focused on changes in speed of habituation between the baseline assessment at 6 months and the immediate follow-up assessment (12–6 months) and between baseline and the longer-term follow-up assessment (18–6 months). Habituation difference score (12–6 months; 18–6 months) was the dependent variable, Group (PFR, A + M) as a between-subject variable and Stimulus (Face, Object) as a within-subject variable. Consistent with our prediction, there was a Stimulus by Group interaction on habituation time difference scores between 6 and 12 months (F(1,24) = 5.13, P = 0.033, η2 = 0.17) and between 6 and 18 months (F(1,19) = 4.84, P = 0.04, η2 =0.19; Fig. 2 and Fig. S2). Given the significant interaction, we conducted post-hoc analyses separated by Stimulus that did not produce significant effects. In post-hoc analyses separated by Group, habituation time for the PFR group showed a significantly greater decline between time-points for faces than objects (main effect of Stimulus on difference scores from 6 to 12 months: F(1,15) = 8.4, P = 0.011, η2 = 0.36; 6–18 months: F(1,12) = 14.3, P = 0.003, η2 = 0.54); this was not true for the A + M group (6–12 months: F(1,10) = 0.44, P = 0.52, η2 = 0.04; 6–18 months: F(1,9) = 0.51, P = 0.49, η2 = 0.05).
Figure 2.
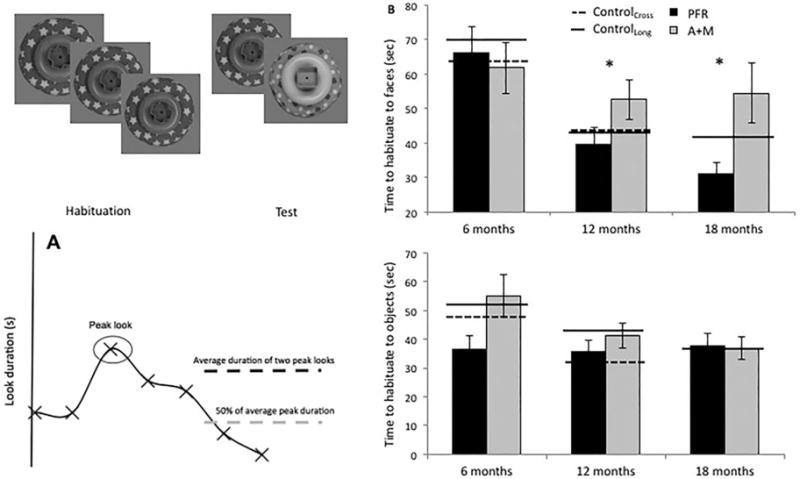
Habituation task and time to habituate to faces and objects. (A) Diagrammatic representation of the habituation task. (B) Total habituation times to faces (top) and objects (bottom). PFR = promoting first relationships; A+M = assessment and monitoring; Cross = cross-sectional; Long. = longitudinal.
Cross sectional comparison using ANOVA on face total habituation times by Group (PFR, A + M) at 6, 12, and 18 months separately (conducted for comparability to the ERP analyses) showed no significant difference between habituation times for the PFR and A + M groups at 6 months (F(1,29) = 0.17, P = 0.67, η2 = 0.06), a trend-level difference at 12 months (F(1,30) = 3.1, P = 0.09, η2 = 0.1), and by 18 months the PFR group had shorter face habituation times than the A + M group (Face: F(1,23) = 7.99, P = 0.01, η2 = 0.29). Parallel analysis for object total habituation times found Group differences in habituation times for the object condition at 6 but not 12 months or 18 months (6 m: F(1,29) = 5.13, P = 0.032, η2 = 0.16; 12 m: F(1,31) = 0.89, P = 0.35, η2 = 0.03; 18 m: F(1,23) = 0.006, P = 0.94, η2 = 0.000).
EEG Theta Power during Viewing of Social and Non-Social Videos
Data were available for 15 infants at both baseline (6 months) and 12 months, and 15 infants at both baseline and 18 months in the PFR condition., Data were available from nine infants at both baseline and 12 months and 12 infants at both baseline and 18 months in the A + M group (see S1.6, available online). Increased or greater theta power has been interpreted as an index of attentional engagement and control [Cava-nagh & Frank, 2014; Orekhova, Stroganova, Posikera, & Elam, 2006; Stroganova, Orekhova, & Posikera, 1998]; typically developing infants show increased frontal theta power to social vs nonsocial stimuli [Jones et al., 2015], and young children with ASD show reduced theta power to social versus nonsocial stimuli [Dawson et al., 2012]. We thus predicted that PFR would be associated with increased theta power to social stimuli.
Primary analyses focused on EEG theta power with log theta difference scores between the baseline and immediate follow-up assessment (12–6 months) and between the baseline and longer-term follow-up (18–6 months) as the dependent variable. Group (PFR, A + M) was the between-subject variable and stimulus (social, non-social) was a within-subject variable.
Across both groups, change in theta power was greater for social than nonsocial videos between 6 and 12 months as would be expected (c.f. Jones et al., 2015; main effect of Stimulus on difference scores: F(1,22) = 12.3, P =0.002, η2 = 0.36; Fig. 3A and Fig. S3). Log theta power increased more between 6 and 12 months in the PFR group than the A + M group across both the social and nonsocial condition (main effect of Group on difference scores; Fig. 2; F(1,22) = 4.67, P = 0.042, η2 = 0.18). Inspection of Fig. 3B indicates that the PFR group responded more like normative controls at 12 months.
Figure 3.
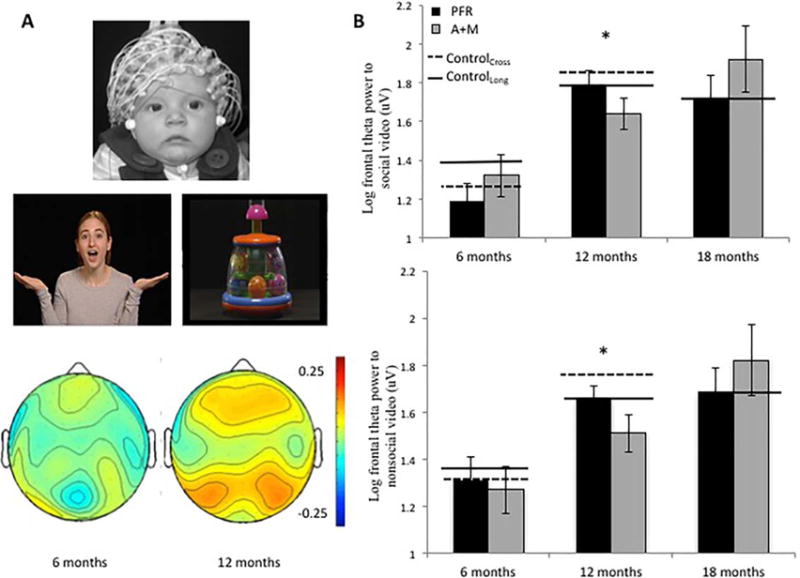
Electroencephalography (EEG) social and non-social video task and theta power. (A) Infant in EEG net (top); screenshot from the stimuli (middle); and topography of EEG theta power to social minus nonsocial videos at 6 and 12 months in the normative cross-sectional control group (bottom, Jones et al., 2015). (B) Frontal theta power to the social (top) and nonsocial (bottom) video. PFR = promoting first relationships; A+M = assessment and monitoring; Cross = cross-sectional; Long. = longitudinal.
Between 6 and 18 months, there was again a significantly greater change in frontal theta power for the social versus nonsocial videos (main effect of stimulus on difference scores, F(1,25) = 4.15, P = 0.05, η2 = 0.14). There were no significant group effects on difference scores between 6 and 18 months (F(1,25) = 0.09, P = 0.77, η2 = 0.004), but there was a trend-level interaction between stimulus and group (F(1,25) = 2.90, P = 0.1, η2 = 0.10). Thus, we conducted post-hoc analyses separated by Group, which showed that the increase in power between 6 and 18 months was greater for social than nonsocial stimuli for the PFR group (main effect of Stimulus on difference scores: F(1,15) = 8.15, P = 0.03, η2 = 0.37, see Fig. S3); this was not the case for the A + M group (F(1,11) = 0.05, P = 0.83, η2 = 0.004). Post-hoc analyses separated by stimulus did not produce significant effects (Ps > 0.4). Cross-sectional analyses conducted for comparability to the ERP analysis indicated that group differences did not reach significance at 6, 12, or 18 months (Ps > 0.3).
ERPs to Faces and Objects
Data were available for eight infants at 6 months, seven infants at 12 months, and 12 infants at 18 months in the PFR group. Data were available for nine infants at 6 months, six infants at 12 months, and nine infants at 18 months from the A + M group. We predicted that PFR would be associated with more prolonged and larger P400 responses to faces, compared to the group who did not receive PFR.
P400 amplitude
There were no effects of Group on P400 amplitude at the 6-month baseline assessment (Fs < 2, Ps > 0.1, η2 < 0.15). However, P400 amplitude differed by group and stimulus at 12 months (F(1,11) = 7.88, P = 0.017, η2 = 0.42). Given this interaction, we conducted post-hoc analyses separated by group and stimulus. Analyses separated by stimulus revealed no significant effects. Analyses separated by Group showed that the A + M group had a significantly larger P400 amplitude to objects than faces (F(1,5) = 9.22, P = 0.029, η2 = 0.65), whilst the PFR group did not (F(1,6) = 0.5, P = 0.49, η2 = 0.08). Inspection of Fig. 4B indicates that the PFR group showed a response to faces that more closely matched controls.
Figure 4.
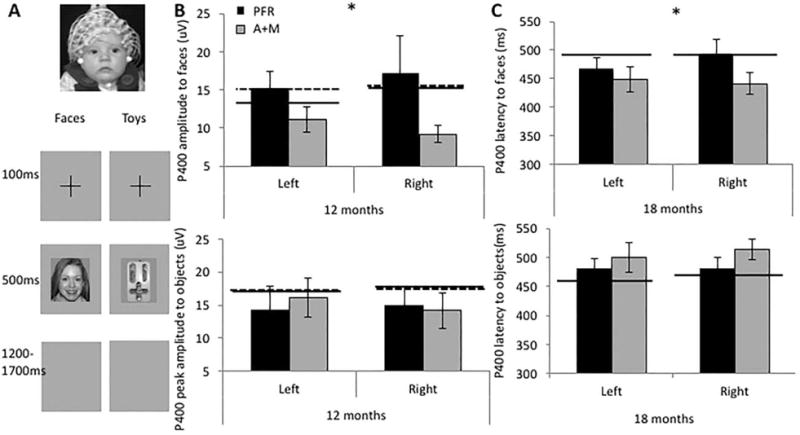
P400 amplitude and latency event-related potentials (ERP) to faces and objects. (A) Illustration of the ERP collection procedure; (B) P400 amplitude to faces (top) and objects (bottom) at 12 months; (C) P400 latency to faces (top) and objects (bottom) at 18 months. PFR = promoting first relationships; A+M = assessment and monitoring; Cross = cross-sectional; Long. = longitudinal.
At 18 months, there were no significant differences for P400 amplitude (Fs < 2, Ps > 0.1).
P400 latency
There were no group differences in P400 latency at 6 months (Fs < 2, Ps > 0.1, η2 < 0.1), or 12 months (Fs < 1, Ps > .5, η2 < 0.2). At 18 months there was a significant interaction between group and stimulus (F(1,19) = 4.76, P = 0.04, η2 = 0.20). Given this interaction, we conducted post-hoc analyses separated by group and stimulus. Analyses separated by stimulus revealed no significant effects. Analyses split by group showed that the A + M group showed less prolonged P400 responses to faces than objects (F(1,8) = 7.59, P = 0.025, η2 = 0.49), whilst the PFR group showed a trend in the opposite direction (F(1,11) = 6.9, P = 0.059, η2 = 0.81; see Fig. 4C).
Discussion
In a randomized clinical trial, we examined the effects of a low-intensity parent-mediated intervention (PFR) on neurophysiological and habituation metrics of social attention in infants at high familial risk for ASD. Broadly consistent with our predictions, compared to infants who only were assessed and monitored, infants who received the intervention from 9 to 11 months of age showed indications of improvement in neurocognitive metrics of social attention at both 12 and 18 months, with a relatively large effect. This was reflected in a greater increase in habituation speed to faces versus objects between 6 and 12 months and 6 and 18 months; a greater increase in frontal EEG-theta power between 6 and 12 months; a comparable (not smaller) P400 amplitude to faces versus objects at 12 months and a marginally prolonged (not less prolonged) P400 latency to faces versus objects at 18 months. The high-risk infants who received the PFR intervention showed responses that appeared broadly more normative, as evident by visual comparison between their responses and those of two groups of low risk control participants. Taken together, these results offer proof-of-concept that a low-intensive parent-mediated intervention delivered prior to the emergence of observable autism symptoms can improve brain- and attention-based measures of social attention in infants at familial risk for ASD. Given that social attention is believed to be a core mechanism by which the infant engages with the social environment and, if altered, can potentially disrupt normal behavioral and brain development, these results are promising that preventive approaches to reducing disability associated with ASD are possible.
In the habituation task, infants who received the intervention not only showed more normative change between 6 and 12 months, but this carried forward to 18 months. Specifically, high-risk infants who received the intervention showed shorter habituation times to face but not object stimuli at 18 months, suggestive of faster learning specific to faces. Using a very similar paradigm, Webb et al. [2010] found that 18–30-month-old toddlers with particularly high levels of ASD symptoms showed prolonged habituation times to faces, consistent with slower learning. Reduced attention to faces in the early development of children with ASD could compromise the ongoing development of the face processing system [Dawson, 2008; Dawson et al., 2002, 2005; Webb, Neuhaus, & Faja, 2016]. By increasing parental responsivity to their infant’s social and communicative cues, PFR may boost early infant social attention and thus support the development of the face processing system. Since individual differences in face processing have been linked to broader social communication delays in toddlers with ASD [Webb et al., 2010, 2011], these improvements in social attention and face processing may have cascading effects on later social communication behavior.
Results from the ERP task also support the possibility that PFR may improve face processing in high-risk infants. Twelve-month-old infants who received PFR showed P400 responses that had similar amplitude to faces and objects whilst other high-risk infants who did not receive PFR continued to show a larger amplitude P400 to objects than faces. These effects on amplitude were not sustained at 18 months. However, there were effects on ERP latency at 18 months. These results indicate that infants who received PFR showed marginally more prolonged P400 responses to faces than objects (similar to the normative controls in the ControlLong and ControlCross groups), whilst other high-risk infants showed less prolonged P400 responses to faces than objects. Notably, others have argued that the amplitude and latency of ERP responses are to an extent interchangeable, since the peak latency is defined by the time at which the ERP component reaches its peak amplitude [Elsabbagh et al., 2012]. Thus, effects on peak amplitude and latency at 12 and 18 months may reflect similar underlying processes, though the lack of longitudinal overlap between infants included at 12 and 18 months makes verification difficult.
The P400 is a positive-going deflection that is sensitive to complex aspects of face processing. Six-month-old infants with later ASD show a P400 that is less prolonged to faces than objects [Elsabbagh et al., 2012; Jones et al., 2016], and P400 amplitude is less modulated by gaze shifts [Elsabbagh et al., 2012]. Both effects are consistent with the proposition that ASD is associated with a reduced depth of processing for social stimuli [Chawarska, Volkmar, & Klin, 2010]. Chawarska et al. [2010] have argued that the depth of processing afforded to social stimuli may be atypical in infants with later ASD, causing cascading consequences for subsequent learning [Webb et al., 2016]. Whilst typically developing toddlers may examine a novel face and spontaneously compute its category (face vs. non-face), familiarity (mother vs. stranger), and affect (happy vs. sad), toddlers with ASD may engage in more limited processing, which in turn may lead to poorer face learning. Depth of processing may explain the observation that poorer processing is associated with faster peak ERP latencies and slower habituation times to faces. Faster peak latencies may be associated with shallower engagement of brain systems as visual processing systems tend to progress from processing simple to more complex features. Further, shallower processing is theoretically associated with slower learning and hence should be reflected in slower habituation. Research with adults indicates that deeper processing facilitates later retention [Bloom & Mudd, 1991]. Indeed, toddlers with ASD show developmental delays in how facial familiarity modulates attention-related neural responses, and this is related to their social ability [Webb et al., 2011]. Taken together, these results are consistent with the notion that PFR intervention facilitated an increase in the depth of processing afforded to faces in high-risk infants, although the limited sample size means these results should be treated with caution.
Results from the EEG task were only partially consistent with our predictions. Theta power increased more in the high-risk infants who received intervention versus those who did not; however, this was not specific to the social video at 12 months (as we had originally predicted). Frontal theta power has been associated with attention engagement to species-relevant stimuli [Orekhova et al., 2006], and young typically developing infants show greater frontal theta power to social than nonsocial videos [Jones et al., 2015]. Possibly, the use of live stimuli would have been more sensitive to condition differences, since frontal theta responses are more differentiated for live than video-based stimuli in infancy [Jones et al., 2015]. Alternatively, since the two tasks that showed social-specific effects all employed simple static stimuli, the differential effects of intervention on social processing may have been easier to detect when examining specific components of social learning, rather than the more complex multiple aspects of social attention elicited by naturalistic stimuli. Of note, infants who received PFR showed a greater increase in frontal theta power for social than nonsocial stimuli between 6 and 18 months, whilst the group that did not receive intervention failed to show this pattern. These data are promising, but since the interaction between group and condition on change scores between 6 and 18 months was only a trend this limits the strength of conclusions that can be drawn. Further work is required to determine whether there are latent effects of the intervention that become more specific to social stimuli with time.
The present study has several limitations that should be addressed in future work. First, sample size is always of concern in clinical trials for ASD, given the heterogeneity in symptoms and outcomes. Our sample, however, did not differ in 6-month behavioral characteristics (with the exception of habituation times to objects), suggesting that changes were not due to a bias in randomization. As well, our use of difference scores for habituation and EEG metrics also allows us to directly address individual change. ERP measures requiring visual attention have a higher attrition rate than other infant tasks, suggesting that alternate protocols for collecting data of this type need to be evaluated.
This study provides further evidence that neurophysiological measures of social attention may be useful as early efficacy biomarkers in clinical trials [Dawson, Bernier, & Ring, 2012]. Developing such biomarkers measures for clinical trials with high-risk infants is essential, because current standardized clinical measures for infants typically rely on either parent report (which is not blinded to treatment status), or cover wide behavioral domains that may be insufficiently sensitive to changes in underlying cognitive mechanisms. Measures of early symptomatology may also be less sensitive to subtle effects associated with less intensive interventions that may have cascading effects on later development. Detecting small effects is also important in indicating the potential efficacy of relatively low intensity (and hence more economically feasible) intervention packages. Switching treatments when individual infants don’t respond is necessary to ensure that infants are enrolled in the most appropriate program, but determining whether or not they are responding cannot wait until full-blown ASD symptoms emerge. Infant outcome measures that can provide rapid intermediate assessments of treatment efficacy will thus be critical to individualized treatment planning. A critical next step is to characterize the test-retest reliability and predictive validity of our measures, in addition to examining their potential for individual-level prediction of treatment success on later behavioral assessments. Longitudinal behavioral data from infants in the current cohort are currently being analyzed and will be the focus of future reports.
In conclusion, we demonstrated improvements in neurophysiological and habituation measures of social attention at 12 months in a group of high-risk infants who received a relatively brief parent-mediated intervention between 6 and 12 months of age. Effect sizes were moderate to relatively large, and some effects were maintained at the 18-month assessment. This suggests that early intervention could be a powerful tool for boosting key attentional mechanisms underlying social communication development in this population. Combined with the success of other available interventions in ameliorating emerging symptomatology [Green et al., 2015; Rogers et al., 2014], our work suggests that the further development and testing of prodromal intervention programs for infants at risk for ASD is promising for improving outcomes for children with ASD.
Supplementary Material
Table S1. Other interventions received by infants in the RCT. Numbers—number of infant in each group who received that type of intervention. In parentheses are total times in hours over each 6-month period received by those infants.
Table S2. Chronological age in days at the 12 and 18-month visits.
Figure S1. Location of electrodes used in the frontal theta and P400 analyses.
Figure S2. Change in habituation time from 6 to 12 and 6 to 18 months for faces and objects. Scores on the y axis represent difference scores; negative scores indicate that habituation times dropped between 6 and 12/18 months. PFR are infants with an older sibling with ASD who received the Promoting First Relationships intervention; A+M are infants with an older sibling with ASD who received Assessment and Monitoring; Controls—Long are longitudinally assessed infants with no family history of ASD.
Figure S3. Change in EEG power from 6 to 12 and 6 to 18 months for social and nonsocial videos. Scores on the y axis represent difference scores; positive scores indicate that EEG power increased between 6 and 12/18 months. PFR are infants with an older sibling with ASD who received the Promoting First Relationships intervention; A+M are infants with an older sibling with ASD who received Assessment and Monitoring; Controls—Long are longitudinally assessed infants with no family history of ASD.
Acknowledgments
We wish to thank the families and young infants who participated in the Early Connections project that was part of the UW NIH Autism Center of Excellence (Dawson (PI)/Transitioned to Webb NICHD P50 HD055782; Webb (PI) R01 HD064820), the Diagnostic and Assessment Core (Estes, PI) clinical assessment staff (Shana Alvarez, PhD, private practice, Jessica Greenson, PhD, Tonya St. John, PhD, Department of Speech and Hearing Sciences, and Karen Barnes, PhD, Seattle Children’s Hospital), Data Analysis and Management Core (Jeffrey Munson, PI, Department of Psychiatry), electrophysiology data collection and analysis staff (Kaitlin Venema, MS, Rachel Kincade Earl, BSc, UW Center on Human Development and Disability, Rachel Lowy, CCC-SLP, Department of Speech and Hearing Sciences, and Michael Murias, PhD, Duke University), and the Promoting First Relations interventionist (Jennifer Rees, MS, of the UW Center on Human Development and Disability). We also thank the Autism Speaks Mentors Program Postdoctoral Fellowship (Jones/Webb) and the L’Oreal/UNESCO for Women in Science Fellowship (Jones) and the UW Center on Human Development and Disability Eunice Shriver Intellectual and Developmental Disability Research Center (U54 HD083091).
Footnotes
Conflict of Interest
Geraldine Dawson receives authorship royalties from Guilford Publications and Oxford University Press and is on the scientific advisory boards of Janssen Research and Development, Roche Pharmaceuticals, Akili, Inc., and Progenity, Inc., for which she receives travel reimbursement and honoraria.
Ethical Approval
All procedures performed were in accordance with the ethical standards of the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. Approval was granted by the University of Washington Institutional Review Board. Informed consent was obtained from all parents of infants participating in the study.
Clinical Trial Registry
Trial title: Early Connections, Early Detection and Intervention in Infants at Risk for Autism; http://clinical-trials.gov; NCT00947700.
Supporting Information
Additional Supporting Information may be found in the online version of this article at the publisher’s web-site:
Contributor Information
Emily J.H. Jones, Centre for Brain & Cognitive Development, Birkbeck, University of London, London, WC1E 7HX, United Kingdom
Geraldine Dawson, Department of Psychiatry and Behavioral Sciences, Duke University, Duke Center for Autism and Brain Development, 2608 Erwin Rd, Suite 300, Durham, North Carolina, 022705.
Jean Kelly, School of Nursing, University of Washington, 1959 NE Pacific St., Seattle, Washington, 98195; Center on Human Development and Disability, University of Washington, Seattle, Washington, 98195.
Annette Estes, Department of Speech and Hearing Sciences, University of Washington, Seattle, Washington, 98195; Center on Human Development and Disability, University of Washington, Seattle, Washington, 98195.
Sara Jane Webb, Department of Psychiatry & Behavioral Sciences, University of Washington, PO Box 5371, M/S CW8-6, Seattle, Washington, 98145; Center on Human Development and Disability, University of Washington, PO Box 5371, M/S CW8-6, Seattle, Washington, 98145; Center on Child Behavior and Development, Seattle Children’s Research Institute, PO Box 5371; M/S CW8-6; Seattle, Washington, 98145.
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th. American Psychiatric Pub; 2013. [Google Scholar]
- Bloom LC, Mudd SA. Depth of processing approach to face recognition: A test of two theories. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1991;17:556–565. https://doi.org/10.1037/0278-7393.17.3.556. [Google Scholar]
- Cavanagh JF, Frank MJ. Frontal theta as a mechanism for cognitive control. Trends in Cognitive Sciences. 2014;18:414–421. doi: 10.1016/j.tics.2014.04.012. https://doi.org/10.1016/j.tics.2014.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chawarska K, Macari S, Shic F. Decreased spontaneous attention to social scenes in 6-month-old infants later diagnosed with autism spectrum disorders. Biological Psychiatry. 2013;74:195–203. doi: 10.1016/j.biopsych.2012.11.022. https://doi.org/10.1016/j.biopsych.2012.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chawarska K, Volkmar F, Klin A. Limited attentional bias for faces in toddlers with autism spectrum disorders. Archives of General Psychiatry. 2010;67:178–185. doi: 10.1001/archgenpsychiatry.2009.194. https://doi.org/10.1001/archgenpsychiatry.2009.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombo J, Mitchell DW. Infant visual habituation. Neurobiology of Learning and Memory. 2009;92:225–234. doi: 10.1016/j.nlm.2008.06.002. https://doi.org/10.1016/j.nlm.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson G. Early behavioral intervention, brain plasticity, and the prevention of autism spectrum disorder. Development and Psychopathology. 2008;20:775–803. doi: 10.1017/S0954579408000370. https://doi.org/10.1017/S0954579408000370. [DOI] [PubMed] [Google Scholar]
- Dawson G, Bernier R, Ring RH. Social attention: A possible early indicator of efficacy in autism clinical trials. Journal of Neurodevelopmental Disorders. 2012;4:11. doi: 10.1186/1866-1955-4-11. https://doi.org/10.1186/1866-1955-4-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson G, Carver L, Meltzoff AN, Panagiotides H, McPartland J, Webb SJ. Neural correlates of face and object recognition in young children with autism spectrum disorder, developmental delay, and typical development. Child Development. 2002;73:700–717. doi: 10.1111/1467-8624.00433. https://doi.org/10.1111/1467-8624.00433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson G, Jones EJH, Merkle K, Venema K, Lowy R, Faja S, Webb SJ. Early behavioral intervention is associated with normalized brain activity in young children with autism. Journal of the American Academy of Child and Adolescent Psychiatry. 2012;51:1150–1159. doi: 10.1016/j.jaac.2012.08.018. https://doi.org/10.1016/j.jaac.2012.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson G, Webb SJ, McPartland J. Understanding the nature of face processing impairment in autism: Insights from behavioral and electrophysiological studies. Developmental Neuropsychology. 2005;27:403–424. doi: 10.1207/s15326942dn2703_6. https://doi.org/10.1207/s15326942dn2703_6. [DOI] [PubMed] [Google Scholar]
- de Haan M, Johnson MH, Halit H. Development of face-sensitive event-related potentials during infancy: A review. International Journal of Psychophysiology: Official Journal of the International Organization of Psychophysiology. 2003;51:45–58. doi: 10.1016/s0167-8760(03)00152-1. [DOI] [PubMed] [Google Scholar]
- Elison JT, Paterson SJ, Wolff JJ, Reznick JS, Sasson NJ, Gu H, IBIS Network White matter microstruc-ture and atypical visual orienting in 7-month-olds at risk for autism. The American Journal of Psychiatry. 2013;170:899–908. doi: 10.1176/appi.ajp.2012.12091150. https://doi.org/10.1176/appi.ajp.2012.12091150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsabbagh M, Gliga T, Pickles A, Hudry K, Charman T, Johnson MH. The development of face orienting mechanisms in infants at-risk for autism. Behavioural Brain Research. 2013;251:147–154. doi: 10.1016/j.bbr.2012.07.030. https://doi.org/10.1016/j.bbr.2012.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsabbagh M, Mercure E, Hudry K, Chandler S, Pasco G, Charman T, Johnson MH. Infant neural sensitivity to dynamic eye gaze is associated with later emerging autism. Current Biology. 2012;22:338–342. doi: 10.1016/j.cub.2011.12.056. https://doi.org/10.1016/j.cub.2011.12.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gliga T, Jones EJH, Bedford R, Charman T, Johnson MH. From early markers to neuro-developmental mechanisms of autism. Developmental Review. 2014;34:189–207. doi: 10.1016/j.dr.2014.05.003. https://doi.org/10.1016/j.dr.2014.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green J, Charman T, Pickles A, Wan MW, Elsabbagh M, Slonims V, Johnson MH. Parent-mediated intervention versus no intervention for infants at high risk of autism: A parallel, single-blind, randomised trial. The Lancet Psychiatry. 2015;2:133–140. doi: 10.1016/S2215-0366(14)00091-1. https://doi.org/10.1016/S2215-0366(14)00091-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones EJH, Gliga T, Bedford R, Charman T, Johnson MH. Developmental pathways to autism: A review of prospective studies of infants at risk. Neuroscience and Biobehavioral Reviews. 2014;39:1–33. doi: 10.1016/j.neubiorev.2013.12.001. https://doi.org/10.1016/j.neubiorev.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones EJH, Venema K, Earl R, Lowy R, Barnes K, Estes A, Webb SJ. Reduced engagement with social stimuli in 6-month-old infants with later autism spectrum disorder: A longitudinal prospective study of infants at high familial risk. Journal of Neurodevelopmental Disorders. 2016;8:7. doi: 10.1186/s11689-016-9139-8. https://doi.org/10.1186/s11689-016-9139-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones EJH, Venema K, Lowy R, Earl RK, Webb SJ. Developmental changes in infant brain activity during naturalistic social experiences. Developmental Psychobiology. 2015;57:842–853. doi: 10.1002/dev.21336. https://doi.org/10.1002/dev.21336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly J, Zuckerman T, Sandoval D, Buehlman K. A curriculum for service providers to help parents and other caregivers meet young children’s social and emotional needs. Seattle, WA: NCAST-AVENUW Publications; 2008. Retrieved from http://pfrprogram.org. [Google Scholar]
- Marshall PJ, Bar-Haim Y, Fox NA. Development of the EEG from 5 months to 4 years of age. Clinical Neurophysiology: Official Journal of the International Federation of Clinical Neurophysiology. 2002;113:1199–1208. doi: 10.1016/s1388-2457(02)00163-3. [DOI] [PubMed] [Google Scholar]
- Messinger DS, Young GS, Webb SJ, Ozonoff S, Bryson SE, Carter A, Zwaigenbaum L. Early sex differences are not autism-specific: A Baby Siblings Research Consortium (BSRC) study. Molecular Autism. 2015;6:32. doi: 10.1186/s13229-015-0027-y. https://doi.org/10.1186/s13229-015-0027-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullen E. Mullen scales of early learning. Circle Pines, MN: AGS; 1995. pp. 58–64. [Google Scholar]
- Orekhova EV, Stroganova TA, Posikera IN, Elam M. EEG theta rhythm in infants and preschool children. Clinical Neurophysiology. 2006;117:1047–1062. doi: 10.1016/j.clinph.2005.12.027. https://doi.org/10.1016/j.clinph.2005.12.027. [DOI] [PubMed] [Google Scholar]
- Ozonoff S, Iosif AM, Baguio F, Cook IC, Hill MM, Hutman T, Young GS. A prospective study of the emergence of early behavioral signs of autism. Journal of the American Academy of Child & Adolescent Psychiatry. 2010;49:256.e2–266.e2. https://doi.org/10.1016/j.jaac.2009.11.009. [PMC free article] [PubMed] [Google Scholar]
- Ozonoff S, Young GS, Carter A, Messinger D, Yirmiya N, Zwaigenbaum L, Stone WL. Recurrence risk for autism spectrum disorders: A baby siblings research consortium study. Pediatrics. 2011;128:e488–e495. doi: 10.1542/peds.2010-2825. https://doi.org/10.1542/peds.2010-2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parikshak NN, Luo R, Zhang A, Won H, Lowe JK, Chandran V, Geschwind DH. Integrative functional genomic analyses implicate specific molecular pathways and circuits in autism. Cell. 2013;155:1008–1021. doi: 10.1016/j.cell.2013.10.031. https://doi.org/10.1016/j.cell.2013.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers SJ, Vismara L, Wagner AL, McCormick C, Young G, Ozonoff S. Autism treatment in the first year of life: A pilot study of infant start, a parent-implemented intervention for symptomatic infants. Journal of Autism and Developmental Disorders. 2014;44:2981–2995. doi: 10.1007/s10803-014-2202-y. https://doi.org/10.1007/s10803-014-2202-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose SA, Feldman JF, Wallace IF. Individual differences in infants’ information processing: reliability, stability, and prediction. Child Development. 1988;59:1177–1197. [PubMed] [Google Scholar]
- Shic F, Macari S, Chawarska K. Speech disturbs face scanning in 6-month-old infants who develop autism spectrum disorder. Biological Psychiatry. 2014;75:231–237. doi: 10.1016/j.biopsych.2013.07.009. https://doi.org/10.1016/j.biopsych.2013.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroganova TA, Orekhova EV, Posikera IN. Externally and internally controlled attention in infants: An EEG study. International Journal of Psychophysiology. 1998;30:339–351. doi: 10.1016/s0167-8760(98)00026-9. https://doi.org/10.1016/S0167-8760(98)00026-9. [DOI] [PubMed] [Google Scholar]
- Webb SJ, Jones EJH, Kelly J, Dawson G. The motivation for very early intervention for infants at high risk for autism spectrum disorders. International Journal of Speech-Language Pathology. 2014;16:36–42. doi: 10.3109/17549507.2013.861018. https://doi.org/10.3109/17549507.2013.861018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb SJ, Jones EJH, Merkle K, Namkung J, Toth K, Greenson J, Dawson G. Toddlers with elevated autism symptoms show slowed habituation to faces. Child Neuropsychology: A Journal on Normal and Abnormal Development in Childhood and Adolescence. 2010;16:255–278. doi: 10.1080/09297041003601454. https://doi.org/10.1080/09297041003601454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb SJ, Jones EJH, Merkle K, Venema K, Greenson J, Murias M, Dawson G. Developmental change in the ERP responses to familiar faces in toddlers with autism spectrum disorders versus typical development. Child Development. 2011;82:1868–1886. doi: 10.1111/j.1467-8624.2011.01656.x. https://doi.org/10.1111/j.1467-8624.2011.01656.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb SJ, Neuhaus E, Faja S. Face perception and learning in autism spectrum disorders. Quarterly Journal of Experimental Psychology. 2016;2006:1–44. doi: 10.1080/17470218.2016.1151059. https://doi.org/10.1080/17470218.2016.1151059. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Other interventions received by infants in the RCT. Numbers—number of infant in each group who received that type of intervention. In parentheses are total times in hours over each 6-month period received by those infants.
Table S2. Chronological age in days at the 12 and 18-month visits.
Figure S1. Location of electrodes used in the frontal theta and P400 analyses.
Figure S2. Change in habituation time from 6 to 12 and 6 to 18 months for faces and objects. Scores on the y axis represent difference scores; negative scores indicate that habituation times dropped between 6 and 12/18 months. PFR are infants with an older sibling with ASD who received the Promoting First Relationships intervention; A+M are infants with an older sibling with ASD who received Assessment and Monitoring; Controls—Long are longitudinally assessed infants with no family history of ASD.
Figure S3. Change in EEG power from 6 to 12 and 6 to 18 months for social and nonsocial videos. Scores on the y axis represent difference scores; positive scores indicate that EEG power increased between 6 and 12/18 months. PFR are infants with an older sibling with ASD who received the Promoting First Relationships intervention; A+M are infants with an older sibling with ASD who received Assessment and Monitoring; Controls—Long are longitudinally assessed infants with no family history of ASD.


