Abstract
Aim
There is conflicting evidence regarding the safety and effectiveness of warfarin for atrial fibrillation (AF) treatment among older end-stage renal disease (ESRD) patients, and differences among subgroups are unclear.
Methods
Older dialysis patients who were newly diagnosed with AF (7/2007–12/2011) were identified in the United States Renal Data System. The adjusted hazard ratios (HR) of the outcomes (any stroke, ischemic stroke, major bleeding, severe gastrointestinal bleeding, and death) by time-varying warfarin use were estimated using Cox regression accounting for the inverse probability of treatment weight.
Results
Among 5,765 older dialysis patients with incident AF, warfarin was associated with significantly increased risk of major bleeding (HR=1.50, 95% CI 1.33–1.68), but was not statistically associated with any stroke (HR=0.92, 95% CI 0.75–1.12), ischemic stroke (HR=0.88, 95%CI 0.70–1.11) or gastrointestinal bleeding (HR=1.03, 95% CI 0.80–1.32). Warfarin use was associated with a reduced risk of mortality (HR=0.72, 95%CI 0.65–0.80). The association between warfarin and major bleeding differed by sex (male: HR=1.29; 95%CI 1.08–1.55; female: HR=1.67; 95%CI 1.44–1.93; P-value for interaction=0.03).
Conclusion
Older ESRD patients with AF who were treated with warfarin had a no difference in stroke risk, lower mortality risk, but increased major bleeding risk. The bleeding risk associated with warfarin was greater among women than men. The risk/benefit ratio of warfarin may be less favorable among older women.
Keywords: atrial fibrillation, anticoagulants, end stage renal disease, warfarin, stroke
INTRODUCTION
Atrial fibrillation (AF) is common among patients with end stage renal disease (ESRD), reaching a prevalence as high as 32% among those aged 65 years or older with ESRD (1). Furthermore, the incidence of AF among older patients on dialysis increased from 11.3% in 1995 to 14.5% in 2007 (2). Patients on dialysis with AF have a 2-fold higher risk for mortality (3) and a 2- to 3-fold higher risk for stroke (4) compared to patients on dialysis without AF.
Warfarin has been used to prevent thrombotic cerebrovascular events in patients with AF without ESRD for decades (5). Despite abundant evidence supporting warfarin use in the general population with AF (6, 7), optimal anticoagulation in patients with ESRD and AF is not well defined because these patients are typically excluded from randomized controlled trials (8). Current evidence from observational studies offers conflicting results. In previous studies, warfarin treatment has been found to be associated with a reduced risk of stroke and mortality (9–12), with no statistically significant association with stroke and mortality, and an increased risk of stroke and mortality (13–17). Pooled estimates from recent meta-analyses of warfarin use among patients of all ages on dialysis with AF suggest that warfarin does not offer stroke and mortality risk reduction, but consistently increases bleeding risk (18–22). Due to recognized limitations of warfarin use such as frequent blood monitoring, food and drug restrictions, uncertain benefit for reducing stroke risk, and increase in bleeding risk, experts have raised concern about warfarin’s safety and effectiveness in patients with AF undergoing dialysis (5, 8, 23–25).
The risks and benefits among older patients undergoing dialysis are less clear. Previous studies of warfarin in cohorts of older patients undergoing dialysis reported that warfarin use was not beneficial in reducing ischemic stroke or mortality risk (14, 15, 26). The bleeding risk among older dialysis patients is unclear. Among older patients undergoing dialysis, warfarin may have differing risks and benefits by sex, age group, and race.
The main goal of this study was to quantify the risk and benefits of warfarin use with respect to stroke, bleeding, and mortality in a national cohort of older patients undergoing dialysis with incident AF. We also compared the impact of warfarin use on these outcomes among subgroups of patients with ESRD by sex, age group, and race. We reasoned that such information might be helpful, given that these basic patient and clinical characteristics could potentially be used to identify particular subpopulations with a distinct risk/benefit balance of warfarin treatment.
MATERIALS AND METHODS
Study population and data
We used the United States Renal Data System (USRDS), a national registry of patients with ESRD, to conduct our analysis. We identified a cohort of 9,784 older adults (aged ≥ 65 years) with ESRD who had an incident AF diagnosis (i.e. exclude pre-existing AF cases) from January 1, 2007 to December 31, 2011 based on 1 inpatient or 2 outpatient diagnosis codes within 30 days of each other indicating AF (International Classification of Diseases, Ninth Revision [ICD-9] code 427.31). We required patients to have uninterrupted Medicare Part A, B, and D coverage from 6 months before through 30 days after AF diagnosis. We excluded patients: 1) with valvular disease associated with AF in 6 months prior to AF diagnosis (n=3,039) (10); 2) who were not on dialysis at AF diagnosis (n=243); 3) warfarin prescription in 6 months prior to AF diagnosis (n=642); 4) with missing Medical Evidence Report (Centers for Medicare & Medicaid Services [CMS] form 2728) (n=84). We further excluded 11 patients who had kidney transplantation or died prior to AF discharge resulting in a final analytic sample of 5,765 patients.
Patient characteristics
We ascertained patients’ demographics, dialysis modality, geographic region, and comorbid conditions from the Medical Evidence Report (Centers for Medicare & Medicaid Services [CMS] form 2728) and all available Medicare inpatient claims data (i.e. from January 1, 1999 to AF diagnosis) using previously published ICD-9 based algorithms (27, 28) (Appendix Table 1). We ascertained history of medication use in the 6 months before AF diagnosis and concomitant use of antiplatelets and NSAIDs within 30 days of AF diagnosis from the Medicare prescription claims data.
Stroke and bleeding risk scores
We calculated the CHA2DS2-VASc (29) for stroke risk stratification and HAS-BLED (30) scores for bleeding risk stratification (Appendix Table 2). We modified the categorization of the CHA2DS2-VASc and HAS-BLED because the original categorization was not designed for older adults on dialysis. The original CHA2DS2-VASc categorizes low risk for score of 0, intermediate risk for score of 1, and high risk for score ≥ 2 (29). Our study population had a minimum CHA2DS2-VASc score of 1 due to age, so we refined the CHA2DS2-VASc categorization: low risk for score of 1, intermediate risk for score 2–3, and high risk for score 4–8. The original HAS-BLED categorizes low risk for score 0, intermediate risk for score of 2–3, and high risk for score ≥ 3 (30). Our study population had a minimum HAS-BLED score of 2 due to age and abnormal kidney function and a maximum HAS-BLED score of 8 due to lack of international normalized ratio (INR). We refined the HAS-BLED categorization: intermediate risk for score 2–3, and high risk for score 4–8.
Warfarin use
We ascertained the date of each warfarin prescription and the days-supply using the Medicare Part D prescription drug events. We considered warfarin treatment as a time-varying exposure such that patients contributed person-time at risk to the untreated group and the treated group according to their warfarin prescription records. Patients were followed from the date of AF discharge (for those identified by inpatient claims) or the second AF diagnosis (for those identified by outpatient claims) until they developed the outcome of interest or the end of follow-up (December 31, 2011). We censored patients at death (for all outcomes other than mortality), kidney transplantation, loss of Medicare coverage, or the end of follow-up. Patients contributed person-time at risk to the untreated group before the date of their first warfarin prescription, and were considered treated after they started using warfarin until they discontinued treatment; if they re-started warfarin treatment, they contributed person-time at risk to the treated group until they discontinued treatment again. In our main analysis, we considered failure to fill a subsequent warfarin prescription within 30 days after their supply ran out as treatment discontinuation (10).
Stroke, bleeding, and mortality
The outcomes of interest were any stroke, ischemic stroke, major bleeding, gastrointestinal bleeding, and all-cause mortality. We defined: 1) any stroke as any inpatient diagnosis of ischemic stroke, cerebral thrombosis, cerebral ischemia and other cerebrovascular disease (31), 2) ischemic stroke as any inpatient diagnosis of ischemic stroke based on previously validated ICD-9 diagnosis (32), 3) major bleeding as any inpatient diagnosis of subarachnoid bleeding, intracerebral bleeding, gastrointestinal bleeding, hematuria, and hemorrhage not otherwise specified (27, 33), and 4) inpatient diagnosis of gastrointestinal bleeding based on the primary ICD-9 diagnosis codes (34) (Appendix Table 1). We ascertained the date of death and the cause of death from the Medical Evidence Report.
Statistical analysis
We evaluated the association of time-varying warfarin use and these outcomes using Cox proportional hazards models with robust standard errors adjusted for potential confounders such as demographics, dialysis characteristics, year of AF diagnosis, history of stroke/bleeding, concomitant use of NSAIDs or antiplatelet agents, comorbid conditions, and CHA2DS2-VASc and HAS-BLED risk categories. We checked the proportional-hazards assumption using the proportionality test based on Schoenfeld residuals.
To visualize the differences in outcomes between warfarin users and non-users, we first graphed the overall cumulative incidence of stroke outcomes, bleeding outcomes, and mortality by warfarin use, and compared the difference in these outcomes by warfarin use through log-rank tests. To account for confounding by indication or channeling bias (35), we generated the stabilized inverse probability of treatment weight (IPTW) for warfarin use (i.e. any use of warfarin during the study follow up) as previously described (36, 37). The probability of being treated with warfarin was estimated using a logistic regression model that included all the potential confounders listed in Table 1. We then compared the measured covariates between warfarin users and nonusers before and after weighting using the standardized differences (38). We also checked the overlap of the IPTW graphically, and we truncated the weights that were outside of the range of complete overlap (i.e. lower than 0.8 or higher than 1.8). Using the weighted sample, we estimated hazard ratios (HR) and the corresponding 95% confidence intervals (95% CI) of these outcomes by warfarin using an adjusted Cox proportional hazards model and the competing risk model with robust standard errors.
Table 1.
Patient characteristics before and after inverse probability treatment weighting (IPTW).
| Patient characteristics | Before IPTW | After IPTW | ||||
|---|---|---|---|---|---|---|
| Warfarin Use (n = 1,651) | No use (n = 4,114) | Standardized difference | Warfarin Use | No use | Standardized difference | |
| Demographics | ||||||
| Age at AF diagnosis, mean (year) | 73.9 | 75.1 | −0.180 | 74.4 | 74.7 | −0.043 |
| Female sex, % | 56.4 | 57.0 | −0.011 | 57.0 | 56.8 | 0.003 |
| Non-white, % | 35.6 | 37.9 | −0.048 | 37.3 | 37.3 | −0.001 |
| Hispanic, % | 19.0 | 18.8 | 0.003 | 18.6 | 18.8 | −0.006 |
| Year of AF diagnosis, % | ||||||
| 2007 | 24.7 | 22.8 | 0.042 | 23.8 | 23.4 | 0.009 |
| 2008 | 28.0 | 26.8 | 0.027 | 26.8 | 27.0 | −0.004 |
| 2009 | 21.6 | 20.8 | 0.020 | 21.4 | 21.0 | 0.011 |
| 2010 | 17.6 | 17.9 | −0.010 | 17.8 | 17.9 | −0.001 |
| 2011 | 8.2 | 11.7 | −0.116 | 10.1 | 10.7 | −0.020 |
| Dialysis vintage, mean (year) | 4.3 | 4.5 | −0.054 | 4.4 | 4.4 | −0.004 |
| Modality, % | ||||||
| Hemodialysis | 96.0 | 96.9 | −0.049 | 96.2 | 96.6 | −0.020 |
| Peritoneal dialysis | 4.0 | 3.1 | 0.049 | 3.8 | 3.4 | 0.020 |
| Geographic region, % | ||||||
| Northeast | 19.2 | 16.9 | 0.059 | 18.1 | 17.6 | 0.013 |
| Midwest | 23.4 | 20.0 | 0.081 | 21.6 | 21.0 | 0.015 |
| South | 41.6 | 45.1 | −0.072 | 43.6 | 44.2 | −0.011 |
| West | 15.9 | 17.9 | −0.054 | 16.7 | 17.3 | −0.015 |
| History of medication use, % | ||||||
| Anticoagulant agent | 0.8 | 0.6 | 0.028 | 0.7 | 0.6 | 0.009 |
| Antiplatelet agent | 25.9 | 25.1 | 0.017 | 25.8 | 25.3 | 0.011 |
| Beta-Blocker | 43.7 | 43.6 | 0.002 | 43.6 | 43.6 | −0.000 |
| Calcium channel blocker | 48.2 | 47.3 | 0.018 | 47.6 | 47.6 | 0.001 |
| Calcium acetate | 31.6 | 31.8 | −0.005 | 31.7 | 31.7 | −0.000 |
| Central acting agonist | 15.7 | 16.6 | −0.024 | 16.2 | 16.4 | −0.003 |
| Diuretic | 20.5 | 20.4 | 0.002 | 20.1 | 20.4 | −0.009 |
| Lipid-lowering agent, nonstatin | 8.0 | 7.9 | 0.004 | 8.1 | 7.9 | 0.007 |
| Nitrate | 23.1 | 23.5 | −0.011 | 23.9 | 23.5 | 0.008 |
| NSAID | 8.7 | 9.3 | −0.021 | 8.7 | 9.0 | −0.012 |
| PPI or H2-blocker | 45.4 | 48.8 | −0.068 | 47.0 | 47.8 | −0.018 |
| Sevelamer | 43.3 | 42.6 | 0.015 | 43.2 | 42.8 | 0.008 |
| Statin | 47.7 | 45.3 | 0.048 | 46.7 | 46.1 | 0.013 |
| History of comorbid conditions, % | ||||||
| Alcohol dependence | 0.8 | 0.8 | −0.004 | 0.8 | 0.8 | 0.001 |
| Tobacco use | 10.2 | 13.0 | −0.086 | 12.1 | 12.2 | −0.006 |
| Concomitant use of antiplatelets or NSAIDs | 15.6 | 16.0 | −0.013 | 15.8 | 15.9 | −0.002 |
| Cancer (excl. non-melanoma skin cancer) | 14.5 | 16.1 | −0.045 | 14.9 | 15.6 | −0.020 |
| Cerebrovascular disease | 18.9 | 22.6 | −0.092 | 21.0 | 21.6 | −0.015 |
| Ischemic heart disease | 63.5 | 67.5 | −0.082 | 65.9 | 66.4 | −0.010 |
| Diabetes mellitus | 68.2 | 73.0 | −0.105 | 70.7 | 71.6 | −0.020 |
| Congestive heart failure | 64.9 | 70.6 | −0.121 | 68.2 | 69.0 | −0.018 |
| Hypertension | 98.1 | 98.8 | −0.058 | 98.4 | 98.6 | −0.017 |
| Liver disease | 5.5 | 8.8 | −0.130 | 7.3 | 7.8 | −0.021 |
| Myocardial infarction | 11.4 | 17.8 | −0.180 | 14.8 | 16.0 | −0.034 |
| Peripheral vascular disease | 39.1 | 43.4 | −0.088 | 41.9 | 42.2 | −0.006 |
| Pulmonary disease | 33.3 | 37.9 | −0.098 | 35.2 | 36.6 | −0.029 |
| Stroke/TIA/TE history | 11.0 | 14.1 | −0.094 | 13.0 | 13.3 | −0.008 |
| Bleeding history | 22.5 | 32.5 | −0.224 | 27.7 | 29.6 | −0.042 |
| Risk Scores, % | ||||||
| CHA2DS2-VASc | ||||||
| Low | 0.2 | 0.2 | 0.003 | 0.2 | 0.2 | −0.003 |
| Intermediate | 18.7 | 14.3 | 0.119 | 16.3 | 15.5 | 0.021 |
| High | 81.2 | 85.6 | −0.118 | 83.5 | 84.3 | −0.021 |
| HAS-BLED | ||||||
| Intermediate | 56.3 | 46.4 | 0.199 | 51.0 | 49.3 | 0.035 |
| High | 43.7 | 53.6 | −0.199 | 49.0 | 50.7 | −0.035 |
IPTW was used to balance the observed baseline characteristics in order to account for potential confounding bias. Standardized difference was a balance assessment that compares the difference in means of the observed covariates.
Given the high mortality rate in older patients with ESRD, we conducted competing-risk regression using the Fine and Gray model based on a semiparametric subdistribution hazards model (39) for the stroke and bleeding outcomes. We considered death from causes other than the outcome of interest as a competing event since it precluded patients from developing stroke or bleeding.
We also tested whether the impact of warfarin on stroke outcomes, bleeding outcomes, and mortality differed by subgroups: sex, age (65 to < 75, 75 to < 85, or ≥ 85), race, and dialysis modality (hemodialysis or peritoneal dialysis) using multiplicative interaction terms in the Cox proportional hazards model with IPTW. However, there were few older peritoneal dialysis (3%) in this study, and these results should be interpreted with caution.
All statistical analyses were performed using Stata 14.0 (StataCorp, College Station, TX). Statistical significance was defined as 2-sided P<0.05.
Sensitivity analysis
For sensitivity analysis, we conducted an intention-to-treat analysis to assess the association of warfarin initiation (i.e. whether warfarin was initiated within 30 days of AF discharge) and stroke outcomes, bleeding outcomes, and mortality. In the multivariate analysis, we adjusted for potential confounders previously mentioned. We also conducted the analysis in the IPTW weighted sample and repeated the competing risk analysis.
RESULTS
Study population
Among the 5,765 older adults undergoing dialysis who were newly diagnosed with AF, 28.6% used warfarin during the study period. The study population had a median age of 74 years (interquartile range [IQR] 69–80 years) at the time of AF diagnosis and 96.7% were undergoing hemodialysis. Almost all patients (99.8%) had a CHA2DS2-VASc score ≥ 2 corresponding to intermediate or high stroke risk in both the ever use (28.6%) and never use (71.4%) groups. Half of the patients (49.5%) were categorized as being at intermediate and half (50.5%) at high risk of bleeding based on HAS-BLED scores. After applying the stabilized weight, all the observed patient characteristics were balanced (Table 1).
Warfarin use and stroke outcomes
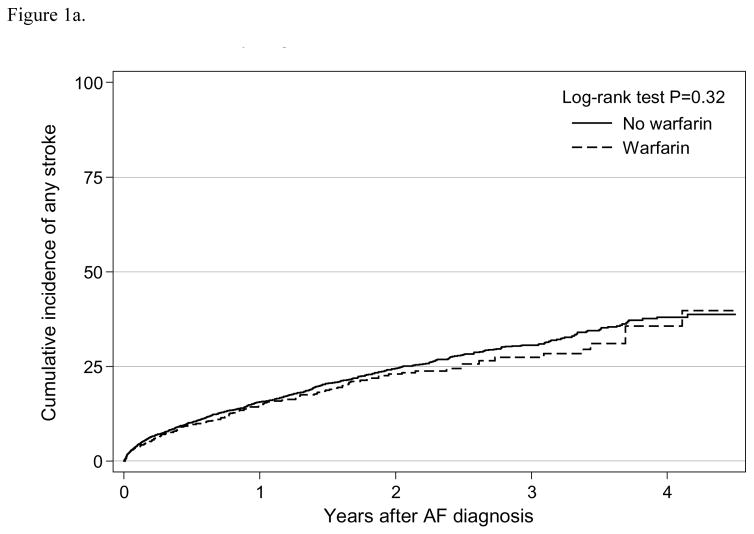
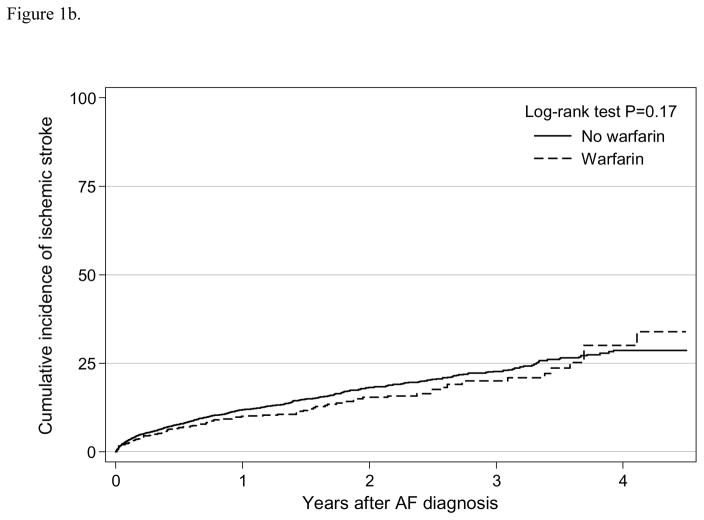
We observed a total of 950 any stroke events for an incidence rate of 14.7 (95% CI: 13.9–15.7) per 100 person-years and 737 ischemic stroke events with a rate of 10.4 (95% CI: 9.6–11.1) per 100 person-years (Table 2). The Kaplan-Meier survival curve suggested that warfarin use was not statistically associated with any stroke event (log-rank test P=0.32; Figure 1a) or ischemic stroke (log-rank test P=0.17; Figure 1b). In the adjusted analysis using weighted sample, warfarin use was not independently associated with any stroke (HR=0.92, 95% CI: 0.75–1.12) or ischemic stroke (HR=0.88, 95% CI: 0.70–1.11) (Table 3). Results were consistent when using a competing risk analysis (any stroke HR=0.96, 95% CI: 0.79–1.17; ischemic stroke HR=0.93, 95% CI: 0.74–1.16). In the intention-to-treat analysis, warfarin initiation was not independently associated with any stroke (HR=0.90, 95% CI: 0.75–1.08) nor ischemic stroke (HR=0.88, 95% CI: 0.71–1.09) (Supplement Table 2).
Table 2.
Time-varying analysis: Incidence rate of stroke outcomes, bleeding outcomes, and all-cause mortality among older dialysis patients (≥65 years) with newly diagnosed atrial fibrillation. Incidence rates were unadjusted.
| Outcome | Comparison group, by treatment status | Number of events | Follow-up time (person-years) | Incidence rate (per 100 person-years, 95% CI) |
|---|---|---|---|---|
| Stroke-related outcomes | ||||
| Any stroke | No warfarin | 826 | 5,780.4 | 14.3 (13.3–15.3) |
| Warfarin | 124 | 1,086.8 | 11.4 (9.6–13.6) | |
| Ischemic stroke | No warfarin | 644 | 5,975.6 | 10.8 (10.0–11.6) |
| Warfarin | 93 | 1,132.9 | 8.2 (6.7–10.0) | |
|
| ||||
| Bleeding-related outcomes | ||||
| Major bleeding | No warfarin | 1,559 | 4,829.6 | 32.3 (30.7–33.9) |
| Warfarin | 407 | 975.7 | 41.7 (37.8–46.0) | |
| Gastrointestinal bleeding | No warfarin | 426 | 6,074.8 | 7.0 (6.4–7.7) |
| Warfarin | 80 | 1,230.3 | 6.5 (5.2–8.1) | |
|
| ||||
| All-cause mortality | No warfarin | 3,349 | 6440.2 | 52.0 (50.3–53.8) |
| Warfarin | 476 | 1287.5 | 37.0 (33.8–40.4) | |
Figure 1.
Figure 1a. Cumulative incidence of any stroke by warfarin use among older patients (≥ 65 years) undergoing dialysis with newly diagnosed atrial fibrillation
Figure 1b. Cumulative incidence of ischemic stroke by warfarin use among older patients (≥ 65 years) undergoing dialysis with newly diagnosed atrial fibrillation
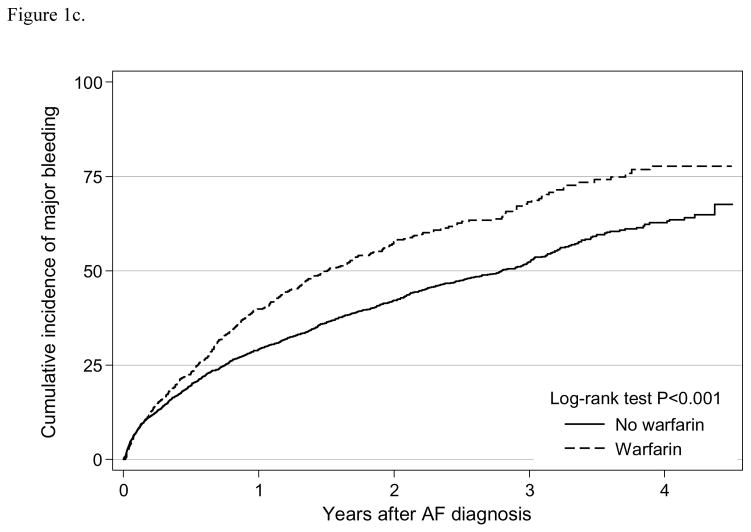
Figure 1c. Cumulative incidence of major bleeding by warfarin use among older patients (≥ 65 years) undergoing dialysis with newly diagnosed atrial fibrillation
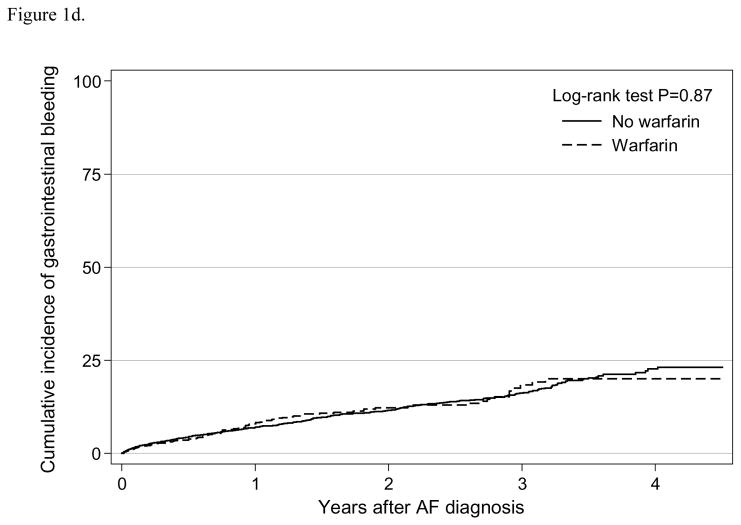
Figure 1d. Cumulative incidence of gastrointestinal bleeding by warfarin use among older patients (≥ 65 years) undergoing dialysis with newly diagnosed atrial fibrillation
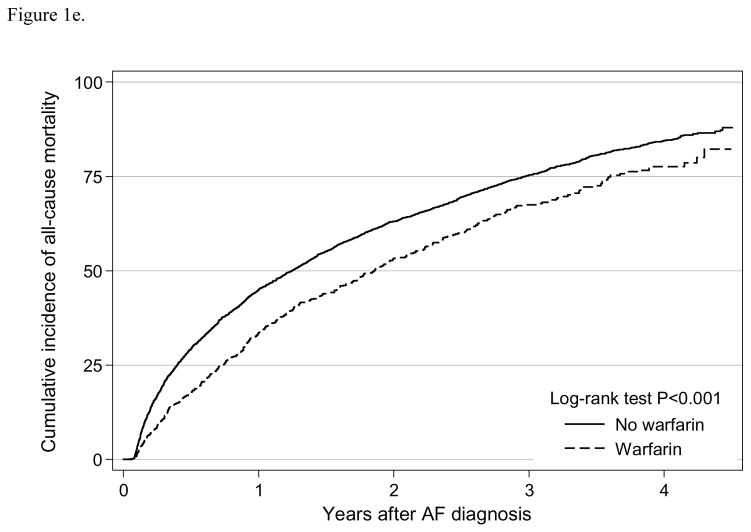
Figure 1e. Cumulative incidence of all-cause mortality by warfarin use among older patients (≥ 65 years) undergoing dialysis with newly diagnosed atrial fibrillation
Table 3.
Time-varying analysis: Association of warfarin use with stroke outcomes, bleeding outcomes, and all-cause mortality among older adults (≥ 65 years) undergoing dialysis with newly diagnosed atrial fibrillation (N = 5,765)
| Outcome | Unadjusted HR (HR, 95% CI) | Adjusted* HR in multivariate analysis (HR, 95% CI) | Adjusted* HR in IPTW weighted sample (HR, 95% CI) | HR after accounting for the competing risk of mortality in IPTW weighted sample (HR, 95% CI) |
|---|---|---|---|---|
| Stroke-related outcomes | ||||
| Any stroke | 0.86 (0.71–1.04) | 0.91 (0.75–1.10) | 0.92 (0.75–1.12) | 0.96 (0.79–1.17) |
| Ischemic stroke | 0.82 (0.66–1.03) | 0.87 (0.70–1.09) | 0.88 (0.70–1.11) | 0.93 (0.74–1.16) |
|
| ||||
| Bleeding-related outcomes | ||||
| Major bleeding | 1.38 (1.23–1.54)* | 1.48 (1.32–1.66)* | 1.50 (1.33–1.68)* | 1.63 (1.45–1.83)* |
| Gastrointestinal bleeding | 0.98 (0.77–1.25) | 1.05 (0.82–1.34) | 1.03 (0.80–1.32) | 1.13 (0.88–1.45) |
|
| ||||
| All-cause mortality | 0.69 (0.62–0.76)* | 0.72 (0.65–0.79)* | 0.72 (0.65–0.80)* | - |
P-value for effect modification statistically significant < 0.05.
Adjusted for age category, sex, race, ethnicity, year of AF diagnosis, region, dialysis modality, dialysis vintage, alcohol dependence, tobacco use, concomitant use of antiplatelets or NSAIDs, cancer (excl. non-melanoma skin cancer), cerebrovascular disease, ischemic heart disease, diabetes mellitus, congestive heart failure, hypertension, liver disease, myocardial infarction, peripheral vascular disease, pulmonary disease, stroke/TIA/TE history, bleeding history, CHA2DS2-VASc score category, HAS-BLED score category
Warfarin use and bleeding outcomes
We observed 1,966 major bleeding events for an incidence rate 33.9 (95% CI:32.4–35.4) per 100 person-years, and 506 gastrointestinal bleeding events with a rate of 6.9 (95% CI:6.3–7.6) per 100 person-years (Table 2). The Kaplan-Meier curve suggested that warfarin use was significantly associated with major bleeding (log-rank test P<0.001; Figure 1c), but not with gastrointestinal bleeding (log-rank test P=0.87; Figure 1d). In the adjusted analysis using weighted sample, warfarin use was independently associated with major bleeding (HR=1.50, 95% CI:1.33–1.68), but not with gastrointestinal bleeding (HR=1.03, 95% CI: 0.80–1.32) (Table 3). In the competing risk analysis, warfarin use was also independently associated with increased risk of major bleeding (HR=1.63, 95% CI: 1.45–1.83), but not with gastrointestinal bleeding (HR=1.13, 95% CI: 0.88–1.45). Similarly in the intention-to-treat analysis, warfarin initiation was associated with increased risk of major bleeding (HR=1.18, 95% CI: 1.05–1.33), but not associated with gastrointestinal bleeding (HR=1.10, 95% CI: 0.87–1.40) (Supplement Table 2).
Warfarin use and mortality
We observed 3,825 deaths for a mortality rate of 49.5 (95% CI: 48.0–51.1) per 100 person-years (Table 2). From the Kaplan-Meier curve, warfarin use was associated with significantly lower mortality risk (log-rank test P<0.001) (Figure 1e). Warfarin use was independently associated with significantly reduced risk of mortality (HR=0.72, 95% CI 0.65–0.80) (Table 3). Similar result was shown in the intention-to-treat analysis (HR=0.80, 95% CI: 0.73–0.88).
Differential effects of warfarin use
The association between warfarin use and any stroke, gastrointestinal bleeding, and mortality outcomes did not differ by age, sex, dialysis type, or race (all P>0.05) (Table 4). The association between warfarin and major bleeding only differed by sex (P-value for interaction=0.03). Warfarin use was associated with a 1.29-fold risk of major bleeding among men (95% CI: 1.08–1.55) and a 1.67-fold risk among women (95% CI: 1.44–1.93). The association between warfarin and ischemic stroke only differed by dialysis modality (P-value for interaction=0.02). Warfarin was not associated with ischemic stroke among older patients on hemodialysis (HR=0.84, 95% CI 0.66–1.07) but associated with increased risk of ischemic stroke among those on peritoneal dialysis (HR=2.59, 95% CI: 1.06–6.32).
Table 4.
Time-varying analysis: Warfarin use and stroke outcomes, bleeding outcomes, and all-cause mortality by sex, age, race and dialysis modality among older adults (≥ 65 years) undergoing dialysis with newly diagnosed atrial fibrillation
| Any stroke | Ischemic stroke | Major bleeding | Gastrointestinal bleeding | All-cause Mortality | |
|---|---|---|---|---|---|
| HR, 95% CI | HR, 95% CI | HR, 95% CI | HR, 95% CI | HR, 95% CI | |
| Sex | |||||
| Male (n=2,491) | 0.80 (0.57–1.11) | 0.74 (0.50–1.08) | 1.29 (1.08–1.55)* | 1.06 (0.74–1.54) | 0.72 (0.62–0.84) |
| Female (n=3,274) | 0.98 (0.77–1.26) | 0.97 (0.73–1.29) | 1.67 (1.44–1.93)* | 1.00 (0.70–1.41) | 0.72 (0.63–0.83) |
|
| |||||
| Age category | |||||
| 65 to < 75 y (n=3,062) | 0.96 (0.74–1.25) | 0.89 (0.66–1.20) | 1.47 (1.27–1.71) | 0.97 (0.70–1.35) | 0.73 (0.64–0.84) |
| 75 to <85 y (n=2,157) | 0.88 (0.64–1.22) | 0.88 (0.61–1.28) | 1.52 (1.25–1.84) | 1.09 (0.71–1.66) | 0.68 (0.57–0.80) |
| ≥ 85 y (n=546) | 0.74 (0.31–1.77) | 0.82 (0.31–2.12) | 1.57 (1.01–2.45) | 1.24 (0.46–3.30) | 0.85 (0.60–1.21) |
|
| |||||
| Race | |||||
| White (n=3,617) | 0.87 (0.67–1.12) | 0.86 (0.64–1.16) | 1.59 (1.38–1.83) | 0.99 (0.72–1.35) | 0.74 (0.65–0.84) |
| Non-white (n=2,148) | 1.00 (0.73–1.35) | 0.92 (0.65–1.30) | 1.36 (1.11–1.65) | 1.10 (0.72–1.67) | 0.69 (0.57–0.83) |
|
| |||||
| Modality | |||||
| Hemodialysis (n=5,572) | 0.89 (0.73–1.09) | 0.84 (0.66–1.07)* | 1.49 (1.33–1.68) | 1.03 (0.80–1.33) | 0.72 (0.65–0.80) |
| Peritoneal dialysis (n=193) | 1.93 (0.82–4.50) | 2.59 (1.06–6.32)* | 1.60 (0.89–2.88) | 1.07 (0.31–3.62) | 0.66 (0.40–1.10) |
P-value for effect modification statistically significant < 0.05.
Analysis was based on the adjusted Cox regression analysis in the IPTW weighted sample.
These differential association was not observed in the intention-to-treat analysis (Supplement Table 3). On the other hand, the association between warfarin initiation and all-cause mortality differed by sex, age group, race, and dialysis modality in the intention-to-treat analysis. Additionally, the younger age group (65 to <75 y) was associated with reduced risk of ischemic stroke (HR=0.76, 95% CI: 0.59–0.99) or any stroke (HR=0.73, 95% CI: 0.54–0.99), and increased risk of major bleeding (HR=1.21, 95% CI: 1.04–1.41); but the older age groups were not associated with these stroke or bleeding outcomes.
DISCUSSION
In this national study of older adults undergoing dialysis with incident AF, 15.5% of patients initiated warfarin therapy within 30 days of AF diagnosis and 46.8% of them discontinued its use after a median treatment length of 8.6 months (40). Warfarin use was associated with 0.72-fold lower risk of mortality, but associated with 1.50-fold higher risk of major bleeding. However, the risk of major bleeding differed for older men and women; warfarin use was associated with an increased risk of major bleeding for both men and women but a greater risk for among women. Warfarin use was not statistically associated with any stroke event or ischemic stroke among older patients on hemodialysis, but was associated with 2.59-fold increased risk of ischemic stroke among older patients on peritoneal dialysis.
To our knowledge, ours is one of only a few recent analyses examining the benefits and risks of warfarin among the older patients with AF and ESRD (14, 15, 26), and is the first study that assessed both the stroke and bleeding outcomes in the presence of the competing risk. In this study, we observed no association between warfarin use and any stroke or ischemic stroke, which was consistent with the results reported by previous cohort studies of patients on dialysis in the U.S. and Canada (10, 14, 15). A report by Shen et al. studied hemodialysis patients of all ages in the USRDS and reported no association between warfarin initiation and any stroke including stroke death or ischemic stroke in the intention-to-treat analysis, and marginal association (HR 0.68, 95% CI 0.47–0.99) in the as-treated analysis (10). Importantly, we extended these previous findings and tested for differential effects of warfarin on these outcomes through effect modification analysis by sex, age group, and race. We found that the impact of warfarin on major bleeding differ by sex. We also observed a difference in ischemic stroke risk by dialysis modality; however interpretation of the PD subgroup data should be cautioned since only a (41, 42) small minority of the study population (3%) had PD as their dialysis modality.
Similar to our analysis of bleeding outcomes, previous cohort studies reported significant association between warfarin use and major bleeding events (15, 43, 44). Our results on the lack of association between warfarin and gastrointestinal bleeding also corroborated previous studies including Shen et al. (10, 14). However, other studies reported lack of association between warfarin use and bleeding events among patients with ESRD (45). These studies reported < 50 bleeding events and < 100 warfarin users, so they might not have enough statistical power to detect significant association. Importantly, we identified differences in the risk of major bleeding by sex which suggests that women may be at an increased risk of major bleeding while treated with warfarin.
We found that warfarin use significantly reduced the risk of mortality, and this result was consistent with previous reports (10, 11, 44, 46, 47). We extended these previous findings to older patients with AF who were undergoing dialysis. Other studies reported lack of association between warfarin use and mortality (3, 13, 14, 45, 47, 48) likely because warfarin use was parameterized as a time-fixed exposure, potentially introducing misclassification. As Genovesi et al. pointed out, it is necessary to consider the actual time of warfarin intake in the analysis for the protective effect of the drug to become evident (47). We accounted for the fact that warfarin users often discontinue or change their treatment at a high rate by defining warfarin use as a time-varying variable. Therefore, our findings may be more generalizable to older adults undergoing dialysis who may have complex oral anticoagulation treatment. While our study did not investigate the cause of death, other reports suggested that the reduction in mortality may be due to a reduction in cardiovascular death rather than stroke death (10, 47).
Our study has several limitations. First, we ascertained warfarin treatment status and duration from the prescription claim status, which might not reflect the true treatment compliance. Because of frequent dosage adjustment according to INR status, the days of supply on claims might not reflect the duration of medication consumption. We mitigated possible misclassification bias of exposure by defining warfarin treatment as a time-varying exposure to appropriately characterize treatment start and discontinuation. When we varied the length of the refill grace period, the results were consistent with our main findings. Because INR data is not available in our study, it is possible that patients discontinued warfarin therapy due to poor control of their INR (10). Previous research suggested that INR monitoring is often inadequate and high INR-variability was associated with increased risk of stroke and bleeding (13, 47, 49). We are not able to understand whether warfarin is associated with stroke and bleeding outcomes because of over- or under-dosing of warfarin or the effect of warfarin per se, so further investigation is required to discern the true effect of warfarin. While we adjusted for most patient characteristics that may confound the association between warfarin use and outcomes, we could not rule out biases due to residual confounding from unknown or unmeasured confounders including the use of over-the-counter aspirin or geriatric syndromes like frailty (50–53) because they were not available in this data. However, to account for differences in the older dialysis patients with AF who did and did not use warfarin we used IPTW to balance the populations on both observed and likely on unobserved differences (54). The stroke and bleeding outcomes were defined using diagnosis code, which may be subject to potential misclassification bias. This limitation is inherent in administrative claims analysis, and we used codes previously validated or published in the literature. The benefits of this study are the large sample size, the new user design of warfarin, and the identification of incident cases of AF among older dialysis patients.
In conclusion, warfarin use was not statistically associated with any stroke, ischemic stroke, or gastrointestinal bleeding, but associated with a significantly increased risk of major bleeding and decreased risk of mortality among older patients on dialysis with incident AF. Warfarin use had a differential effect on major bleeding; while both men and women were at risk of major bleeding while using warfarin, the risk was greater among older women. Our findings provide some evidence in favor of warfarin use among older patients on dialysis with AF due to significant improvement in survival, but the lack of stroke reduction and increased bleeding risk should be taken into consideration. Physicians should balance the risks of bleeding and potential mortality benefit when initiating warfarin among older patients on dialysis who are newly diagnosed with AF.
Supplementary Material
Acknowledgments
The data reported here have been supplied by the United States Renal Data System (USRDS). The interpretation and reporting of these data are the responsibility of the author(s) and in no way should be seen as an official policy or interpretation of the U.S. government.
Funding
This research was supported by the National Heart, Lung, and Blood Institute (T32HL007024) pre-doctoral training grant and the doctoral dissertation fund provided by the Johns Hopkins Bloomberg School of Public Health Department of Epidemiology. Jodi B. Segal was supported by the National Institute on Aging (K24AG049036). Mara McAdams-DeMarco was supported by the American Society of Nephrology Carl W. Gottschalk Research Scholar Grant and Johns Hopkins University Claude D. Pepper Older Americans Independence Center (P30AG021334), and the National Institute on Aging, (R01AG055781 and K01AG043501). Dorry Segev was supported by the National Institute for Diabetes and Digestive and Kidney Disorders (K24DK101828) and the National Institute on Aging (R01AG042504). The funding sources had no role in the design and conduct of the study, analysis, or interpretation of the data; and preparation or final approval of the manuscript prior to publication.
Footnotes
Conflicts of Interest Statement
Jingwen Tan, ScM, PhD: None
Sunjae Bae, KMD, MPH: None
Jodi B. Segal, MD, MPH: None
Junya Zhu, PhD: None
G. Caleb Alexander, MD, MS: G. Caleb Alexander is Chair of the FDA’s Peripheral and Central Nervous System Advisory Committee; serves as a paid consultant to PainNavigator, a mobile startup to improve patients’ pain management; serves as a paid consultant to IMS Health; and serves on an IMS Health scientific advisory board. This arrangement has been reviewed and approved by Johns Hopkins University in accordance with its conflict of interest policies.
Dorry L. Segev, MD, PhD: None
Mara McAdams-DeMarco, PhD: None
Results presented in this paper have not been published previously in whole or part, except in abstract format.
Contributions
Research idea and study design: JT, JBS, JZ, GCA, MMA; data acquisition: JT, SB, DLS; data analysis/interpretation: JT, JBS, JZ, GCA, MMA; statistical analysis: JT; supervision or mentorship: DLS, MMA. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved. JT takes responsibility that this study has been reported honestly, accurately, and transparently; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
References
- 1.Winkelmayer WC, Liu J, Patrick AR, Setoguchi S, Choudhry NK. Prevalence of atrial fibrillation and warfarin use in older patients receiving hemodialysis. J Nephrol. 2012;25(3):341–53. doi: 10.5301/jn.5000010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goldstein BA, Arce CM, Hlatky MA, Turakhia M, Setoguchi S, Winkelmayer WC. Trends in the incidence of atrial fibrillation in older patients initiating dialysis in the United States. Circulation. 2012;126(19):2293–301. doi: 10.1161/CIRCULATIONAHA.112.099606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tanaka A, Inaguma D, Shinjo H, Murata M, Takeda A. Presence of Atrial Fibrillation at the Time of Dialysis Initiation Is Associated with Mortality and Cardiovascular Events. Nephron. 2016 doi: 10.1159/000443314. [DOI] [PubMed] [Google Scholar]
- 4.Zimmerman D, Sood MM, Rigatto C, Holden RM, Hiremath S, Clase CM. Systematic review and meta-analysis of incidence, prevalence and outcomes of atrial fibrillation in patients on dialysis. Nephrol Dial Transplant. 2012;27(10):3816–22. doi: 10.1093/ndt/gfs416. [DOI] [PubMed] [Google Scholar]
- 5.Brancaccio D, Neri L, Bellocchio F, Barbieri C, Amato C, Mari F, et al. Atrial fibrillation in dialysis patients: time to abandon warfarin? Int J Artif Organs. 2016:0. doi: 10.5301/ijao.5000487. [DOI] [PubMed] [Google Scholar]
- 6.Aguilar MI, Hart R. Oral anticoagulants for preventing stroke in patients with non-valvular atrial fibrillation and no previous history of stroke or transient ischemic attacks. Cochrane Database Syst Rev. 2005;(3):Cd001927. doi: 10.1002/14651858.CD001927.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Andersen LV, Vestergaard P, Deichgraeber P, Lindholt JS, Mortensen LS, Frost L. Warfarin for the prevention of systemic embolism in patients with non-valvular atrial fibrillation: a meta-analysis. Heart. 2008;94(12):1607–13. doi: 10.1136/hrt.2007.135657. [DOI] [PubMed] [Google Scholar]
- 8.Sood MM, Komenda P, Sood AR, Rigatto C, Bueti J. The intersection of risk and benefit: is warfarin anticoagulation suitable for atrial fibrillation in patients on hemodialysis? Chest. 2009;136(4):1128–33. doi: 10.1378/chest.09-0730. [DOI] [PubMed] [Google Scholar]
- 9.Olesen JB, Lip GY, Kamper AL, Hommel K, Kober L, Lane DA, et al. Stroke and bleeding in atrial fibrillation with chronic kidney disease. N Engl J Med. 2012;367(7):625–35. doi: 10.1056/NEJMoa1105594. [DOI] [PubMed] [Google Scholar]
- 10.Shen JI, Montez-Rath ME, Lenihan CR, Turakhia MP, Chang TI, Winkelmayer WC. Outcomes After Warfarin Initiation in a Cohort of Hemodialysis Patients With Newly Diagnosed Atrial Fibrillation. Am J Kidney Dis. 2015;66(4):677–88. doi: 10.1053/j.ajkd.2015.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khalid F, Qureshi W, Qureshi S, Alirhayim Z, Garikapati K, Patsias I. Impact of restarting warfarin therapy in renal disease anticoagulated patients with gastrointestinal hemorrhage. Ren Fail. 2013;35(9):1228–35. doi: 10.3109/0886022X.2013.819766. [DOI] [PubMed] [Google Scholar]
- 12.Chan PH, Huang D, Yip PS, Hai J, Tse HF, Chan TM, et al. Ischaemic stroke in patients with atrial fibrillation with chronic kidney disease undergoing peritoneal dialysis. Europace. 2015 doi: 10.1093/europace/euv289. [DOI] [PubMed] [Google Scholar]
- 13.Chan KE, Lazarus JM, Thadhani R, Hakim RM. Warfarin use associates with increased risk for stroke in hemodialysis patients with atrial fibrillation. Journal of the American Society of Nephrology : JASN. 2009;20(10):2223–33. doi: 10.1681/ASN.2009030319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Winkelmayer WC, Liu J, Setoguchi S, Choudhry NK. Effectiveness and safety of warfarin initiation in older hemodialysis patients with incident atrial fibrillation. Clin J Am Soc Nephrol. 2011;6(11):2662–8. doi: 10.2215/CJN.04550511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shah M, Avgil Tsadok M, Jackevicius CA, Essebag V, Eisenberg MJ, Rahme E, et al. Warfarin use and the risk for stroke and bleeding in patients with atrial fibrillation undergoing dialysis. Circulation. 2014;129(11):1196–203. doi: 10.1161/CIRCULATIONAHA.113.004777. [DOI] [PubMed] [Google Scholar]
- 16.Chen CY, Lin TC, Yang YHK. Use of warfarin and risk of stroke and mortality in chronic dialysis patients with atrial fibrillation in taiwan. Pharmacoepidemiology and Drug Safety. 2014;23:457–8. [Google Scholar]
- 17.Wang TKM, Sathananthan J, Hood C, Marshall M, Kerr A. Anticoagulation and outcomes of dialysis patients with atrial fibrillation: 9-year cohort study. European Heart Journal. 2015;36:746. [Google Scholar]
- 18.Liu G, Long M, Hu X, Hu CH, Liao XX, Du ZM, et al. Effectiveness and Safety of Warfarin in Dialysis Patients With Atrial Fibrillation: A Meta-Analysis of Observational Studies. Medicine (Baltimore) 2015;94(50):e2233. doi: 10.1097/MD.0000000000002233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li J, Wang L, Hu J, Xu G. Warfarin use and the risks of stroke and bleeding in hemodialysis patients with atrial fibrillation: A systematic review and a meta-analysis. Nutr Metab Cardiovasc Dis. 2015 doi: 10.1016/j.numecd.2015.03.013. [DOI] [PubMed] [Google Scholar]
- 20.Lee M, Saver JL, Hong KS, Wu YL, Huang WH, Rao NM, et al. Warfarin Use and Risk of Stroke in Patients With Atrial Fibrillation Undergoing Hemodialysis: A Meta-Analysis. Medicine (Baltimore) 2016;95(6):e2741. doi: 10.1097/MD.0000000000002741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dahal K, Kunwar S, Rijal J, Schulman P, Lee J. Stroke, Major Bleeding, and Mortality Outcomes in Warfarin Users With Atrial Fibrillation and Chronic Kidney Disease: A Meta-Analysis of Observational Studies. Chest. 2016;149(4):951–9. doi: 10.1378/chest.15-1719. [DOI] [PubMed] [Google Scholar]
- 22.Tan J, Liu S, Segal JB, Alexander GC, McAdams-DeMarco M. Warfarin use and stroke, bleeding and mortality risk in patients with end stage renal disease and atrial fibrillation: a systematic review and meta-analysis. BMC nephrology. 2016;17(1):157. doi: 10.1186/s12882-016-0368-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bennett WM. Should dialysis patients ever receive warfarin and for what reasons? Clin J Am Soc Nephrol. 2006;1(6):1357–9. doi: 10.2215/CJN.01700506. [DOI] [PubMed] [Google Scholar]
- 24.Shen JI, Turakhia MP, Winkelmayer WC. Anticoagulation for atrial fibrillation in patients on dialysis: are the benefits worth the risks? Curr Opin Nephrol Hypertens. 2012;21(6):600–6. doi: 10.1097/MNH.0b013e32835856fd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang F, Chou D, Schweitzer P, Hanon S. Warfarin in haemodialysis patients with atrial fibrillation: what benefit? Europace. 2010;12(12):1666–72. doi: 10.1093/europace/euq387. [DOI] [PubMed] [Google Scholar]
- 26.Wizemann V, Tong L, Satayathum S, Disney A, Akiba T, Fissell RB, et al. Atrial fibrillation in hemodialysis patients: clinical features and associations with anticoagulant therapy. Kidney Int. 2010;77(12):1098–106. doi: 10.1038/ki.2009.477. [DOI] [PubMed] [Google Scholar]
- 27.Cunningham A, Stein CM, Chung CP, Daugherty JR, Smalley WE, Ray WA. An automated database case definition for serious bleeding related to oral anticoagulant use. Pharmacoepidemiol Drug Saf. 2011;20(6):560–6. doi: 10.1002/pds.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kokotailo RA, Hill MD. Coding of stroke and stroke risk factors using international classification of diseases, revisions 9 and 10. Stroke. 2005;36(8):1776–81. doi: 10.1161/01.STR.0000174293.17959.a1. [DOI] [PubMed] [Google Scholar]
- 29.Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest. 2010;137(2):263–72. doi: 10.1378/chest.09-1584. [DOI] [PubMed] [Google Scholar]
- 30.Pisters R, Lane DA, Nieuwlaat R, De Vos CB, Crijns HJGM, Lip GYH. A novel user-friendly score to assess one-year risk of major bleeding in atrial fibrillation patients: the Euro HeartSurvey. European Heart Journal. 2010;31:923. doi: 10.1378/chest.10-0134. [DOI] [PubMed] [Google Scholar]
- 31.CMS. Chronic Conditions Data Warehouse. Centers for Medicare & Medicaid Services (CMS); Available from: https://www.ccwdata.org/web/guest/condition-categories. [Google Scholar]
- 32.Kumamaru H, Judd SE, Curtis JR, Ramachandran R, Hardy NC, Rhodes JD, et al. Validity of claims-based stroke algorithms in contemporary Medicare data: reasons for geographic and racial differences in stroke (REGARDS) study linked with medicare claims. Circ Cardiovasc Qual Outcomes. 2014;7(4):611–9. doi: 10.1161/CIRCOUTCOMES.113.000743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jasuja GK, Reisman JI, Miller DR, Berlowitz DR, Hylek EM, Ash AS, et al. Identifying major hemorrhage with automated data: results of the Veterans Affairs study to improve anticoagulation (VARIA) Thromb Res. 2013;131(1):31–6. doi: 10.1016/j.thromres.2012.10.010. [DOI] [PubMed] [Google Scholar]
- 34.Yang JY, Lee TC, Montez-Rath ME, Paik J, Chertow GM, Desai M, et al. Trends in acute nonvariceal upper gastrointestinal bleeding in dialysis patients. J Am Soc Nephrol. 2012;23(3):495–506. doi: 10.1681/ASN.2011070658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hernan MA, Hernandez-Diaz S, Robins JM. A structural approach to selection bias. Epidemiology (Cambridge, Mass) 2004;15(5):615–25. doi: 10.1097/01.ede.0000135174.63482.43. [DOI] [PubMed] [Google Scholar]
- 36.Cole SR, Hernan MA. Constructing inverse probability weights for marginal structural models. Am J Epidemiol. 2008;168(6):656–64. doi: 10.1093/aje/kwn164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hernan MA, McAdams M, McGrath N, Lanoy E, Costagliola D. Observation plans in longitudinal studies with time-varying treatments. Stat Methods Med Res. 2009;18(1):27–52. doi: 10.1177/0962280208092345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med. 2009;28(25):3083–107. doi: 10.1002/sim.3697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. Journal of the American statistical association. 1999;94(446):496–509. [Google Scholar]
- 40.Tan J, Bae S, Segal JB, Zhu J, Segev DL, Alexander GC, et al. Treatment of atrial fibrillation with warfarin among older adults with end stage renal disease. Journal of nephrology. 2017 doi: 10.1007/s40620-016-0374-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Olesen JB, Lip Gy, Kamper A-L, Hommel K, Kober L, Lane DA, Lindhardsen J, et al. Stroke and bleeding in atrial fibrillation with chronic kidney disease. [DOI] [PubMed] [Google Scholar]
- 42.Chan PH, Huang D, Yip PS, Hai J, Tse HF, Chan TM, et al. Ischaemic stroke in patients with atrial fibrillation with chronic kidney disease undergoing peritoneal dialysis. Eurpace. 2016;18(5):665–71. doi: 10.1093/europace/euv289. [DOI] [PubMed] [Google Scholar]
- 43.Genovesi S, Rossi E, Gallieni M, Stella A, Badiali F, Conte F, et al. Warfarin use, mortality, bleeding and stroke in haemodialysis patients with atrial fibrillation. Nephrol Dial Transplant. 2015;30(3):491–8. doi: 10.1093/ndt/gfu334. [DOI] [PubMed] [Google Scholar]
- 44.Friberg L, Benson L, Lip GY. Balancing stroke and bleeding risks in patients with atrial fibrillation and renal failure: the Swedish Atrial Fibrillation Cohort study. Eur Heart J. 2015;36(5):297–306. doi: 10.1093/eurheartj/ehu139. [DOI] [PubMed] [Google Scholar]
- 45.Carrero JJ, Evans M, Szummer K, Spaak J, Lindhagen L, Edfors R, et al. Warfarin, kidney dysfunction, and outcomes following acute myocardial infarction in patients with atrial fibrillation. JAMA. 2014;311(9):919–28. doi: 10.1001/jama.2014.1334. [DOI] [PubMed] [Google Scholar]
- 46.Findlay MD, Thomson PC, Fulton RL, Solbu MD, Jardine AG, Patel RK, et al. Risk Factors of Ischemic Stroke and Subsequent Outcome in Patients Receiving Hemodialysis. Stroke. 2015;46(9):2477–81. doi: 10.1161/STROKEAHA.115.009095. [DOI] [PubMed] [Google Scholar]
- 47.Genovesi S, Rebora P, Gallieni M, Stella A, Badiali F, Conte F, et al. Effect of oral anticoagulant therapy on mortality in end-stage renal disease patients with atrial fibrillation: a prospective study. Journal of nephrology. 2017;30(4):573–81. doi: 10.1007/s40620-016-0364-8. [DOI] [PubMed] [Google Scholar]
- 48.Wakasugi M, Kazama JJ, Tokumoto A, Suzuki K, Kageyama S, Ohya K, et al. Association between warfarin use and incidence of ischemic stroke in Japanese hemodialysis patients with chronic sustained atrial fibrillation: a prospective cohort study. Clin Exp Nephrol. 2014;18(4):662–9. doi: 10.1007/s10157-013-0885-6. [DOI] [PubMed] [Google Scholar]
- 49.Kooiman J, van Rein N, Spaans B, van Beers KA, Bank JR, van de Peppel WR, et al. Efficacy and safety of vitamin K-antagonists (VKA) for atrial fibrillation in non-dialysis dependent chronic kidney disease. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McAdams-Demarco MA, Law A, Garonzik-Wang JM, Gimenez L, Jaar BG, Walston JD, et al. Activity of daily living disability and dialysis mortality: better prediction using metrics of aging. Journal of the American Geriatrics Society. 2012;60(10):1981–2. doi: 10.1111/j.1532-5415.2012.04161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McAdams-DeMarco MA, Law A, Salter ML, Boyarsky B, Gimenez L, Jaar BG, et al. Frailty as a novel predictor of mortality and hospitalization in individuals of all ages undergoing hemodialysis. Journal of the American Geriatrics Society. 2013;61(6):896–901. doi: 10.1111/jgs.12266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McAdams-DeMarco MA, Suresh S, Law A, Salter ML, Gimenez LF, Jaar BG, et al. Frailty and falls among adult patients undergoing chronic hemodialysis: a prospective cohort study. BMC nephrology. 2013;14:224. doi: 10.1186/1471-2369-14-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McAdams-DeMarco MA, Tan J, Salter ML, Gross A, Meoni LA, Jaar BG, et al. Frailty and Cognitive Function in Incident Hemodialysis Patients. Clinical journal of the American Society of Nephrology : CJASN. 2015;10(12):2181–9. doi: 10.2215/CJN.01960215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Brookhart MA, Sturmer T, Glynn RJ, Rassen J, Schneeweiss S. Confounding control in healthcare database research: challenges and potential approaches. Medical care. 2010;48(6 Suppl):S114–20. doi: 10.1097/MLR.0b013e3181dbebe3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.