Abstract
DNA methylation is a major mode of epigenetic regulation in the mammalian genome and is essential for embryonic development. The three catalytic DNA methyltransferases (Dnmts), Dnmt1, Dnmt3a, and Dnmt3b, catalyze the methylation of cytosine. Dnmt3b is highly expressed in chondrocytes and global knockout of Dnmt3b led to skeletal deformations and embryonic lethality, suggesting an essential role of Dnmt3b in endochondral bone formation. To further define the role of Dnmt3b in skeletal development, Dnmt3b was deleted in Col2 positive chondrocyte lineage cells. Both axial and appendicular skeletal size were reduced and bone mineralization delayed in Col2Cre+;Dnmt3bf/f (Dnmt3bCol2) mice at E14.5 and E18.5. While Alcian Blue Hematoxylin/Orange G (ABH/OG) staining showed normal chondrocyte columns in control growth plates, the length of hypertrophic chondrocyte zone and type X collagen expression were decreased in E18.5 growth plates from Dnmt3bCol2 mice. TUNEL and PCNA staining demonstrated that the delay in chondrocyte maturation observed in the Dnmt3bCol2 growth plates was not secondary to altered chondrocyte apoptosis or proliferation. Complementary in vitro experiments were performed on primary sternal chondrocytes isolated from control and Dnmt3bCol2 mice. Gene expression studies confirmed delayed terminal maturation as Mmp13 and Col10a1 expression was down-regulated in Dnmt3bCol2 chondrocytes. In addition, alkaline phosphatase (ALP) and Alizarin Red staining confirmed that Dnmt3b deletion in chondrocytes delays in vitro chondrocyte hypertrophic differentiation and matrix mineralization. Mechanistically, Dnmt3b gene deletion resulted in decreased BMP signaling through reduction of Smad1 phosphorylation. These findings show that epigenetic factor, Dnmt3b is necessary for normal chondrocyte hypertrophic maturation and limb development.
Keywords: DNA methyltransferase 3b, chondrocyte, endochondral ossification, embryogenesis
Introduction
Epigentics is the regulation of gene expression in the absence of any changes in DNA sequence (1). Three main mechanisms are involved in epigenetic regulation: 1) post-transcriptional modification of histones which alters chromatin conformation, 2) non-coding RNAs (i.e. microRNAs, long non-coding RNAs) that act post-transcriptionally to regulate of mRNA expression, and 3) DNA methylation (2–6). In DNA methylation CpG dinucleotide sequences are targeted by complementary enzymes that either methylate cytosine in GC rich regions of DNA (DNA methyltransferases; Dnmts) or that demethylate methyl-cytosine nucleotides (Ten-eleven demythylcytosine dioxygenases; TETs). DNA methylation regulates gene expression during embryonic development as well as during postnatal tissue growth and maintenance (7). DNA methylation involves the addition of a methyl group (CH3) from the methyl donor S-adenosyl methionine (SAM) to a cytosine that lies 5′ of guanine (CpG sites) to form methylated cytosine (5mC). CpG islands methylation frequently occurs in promoter, enhancer and gene body regions across the genome, differentially regulating gene expression profile, e.g, repetitive elements silencing, X chromosome inactivation, genomic imprinting, as well as regulation of gene expression via inhibition of transcription initiation (8).
There are three well documented catalytically active DNA methyltransferase (Dnmt) enzymes in mammalian cells, Dnmt1, Dnmt3a and Dnmt3b. Dnmt1 is ubiquitously expressed and is involved in maintaining the methylation signature of cells undergoing proliferation. Dnmt1 is thus important for expansion of various cell populations during development and in adult life (9). In contrast, Dnmt3a and Dnmt3b predominantly act as de novo DNA methyltransferases to establish lineage committed cells with tissue specific gene expression patterns by creating new DNA methylation signatures during cell differentiation (10,11). Deletion of these enzymes in mice results in embryonic (Dnmt1 and Dnmt3b) or post-natal (Dnmt3a) lethality, confirming an essential role during development (12,13).
Given its prominent role in regulating gene expression and maintaining tissue specificity, de novo DNA methylation has been intensively studied in the context of development and disease. In neural stem cells, Dnmt3a is critical for neurogenesis since Dnmt3a-null mice have impaired postnatal neurogenesis and die 4 weeks after birth (13,14). In contrast, Dnmt3b gene deletion in neurons promotes cell differentiation (15). Dnmt3b was shown to be highly expressed in the superficial zone of normal articular cartilage, but with loss of expression occurring in conditions leading to the development of OA (16). Moreover, mice with conditional gene deletion of Dnmt3b in articular cartilage developed spontaneous osteoarthritis, further indicating a role in cartilage tissues (16). In the hematopoietic stem cells (HSCs), deletion of Dnmt3a and double knock out of Dnmt3a and 3b leads to marked decline in differentiation capacity, however, deletion of Dnmt3b alone in HSCs showed minor effect on cell differentiation (17). Global knockout of Dnmt3b in mice results in mid-gestation lethality due to multiple development defects including skeletal malformations. In humans, mutation of Dnmt3b occur in immunodeficiency, centromeric region instability, and facial anomalies (ICF) syndrome (13). This rare autosomal recessive disorder is characterized by variable immunological defects, centromeric heterochromatin instability, and facial anomalies (18), suggesting an essential role of Dnmt3b in endochondral ossification process (13,19,20). Recently, Dnmt3b expression was shown to increase during the endochondral phase of fracture repair, and deletion of Dnmt3b in chondrocytes resulted in impaired fracture healing (21).
Mammalian skeletal and facial development primarily occurs through an endochondral ossification process, with calvaria developing through intramembranous ossification (22–25). The major difference between endochondral and intramembranous ossification is the formation of a cartilage anlagen which is a cartilage template for bone formation. At the outset of a highly ordered and regulated endochondral process, undifferentiated mesenchyme cells condense and differentiate into chondrocytes and form a type II cartilage matrix surrounded by perichondrium. In the cartilage template a zone of proliferation is succeeded by hypertrophic chondrocyte differentiation, type X collagen expression, and calcification of the matrix at the center of the cartilage anlagen. The calcified cartilage stimulates vascular invasion and serves as a subsequently serves as a template for primary bone formation. Eventually the center of the anlagen becomes mature bone and growth plates migrate towards opposite ends of the long bone. Therefore, chondrocyte hypertrophy is the central event bridging between chondrocyte proliferation and bone formation during the long bone elongation process.
In these studies, we determine the importance of Dnmt3b during the cartilage phase of skeletal development in using mice with conditional deletion of Dnmt3b in chondrocytes. Our findings confirm that Dnmt3b is a critical epigenetic factor that regulates chondrocyte hypertrophy, potentially through modulation of the BMP signaling pathway during the endochondral ossification process.
Materials and methods
Animals
Col2a1-Cre mice were obtained from Jackson Laboratory (26,27). Dnmt3bf/f mice, generated by Dr. En Li, were obtained from Mutant Mouse Regional Resource Centers (MMRRC) (28). To generate Col2-Cre;Dnmt3bf/f (Dnmt3bCol2) mice, Dnmt3bf/f mice were crossed with Col2a1-Cre transgenic mice. The Cre-negative littermates were used as controls. All the procedures were approved by Washington University Animal Studies Committee.
Whole skeleton preparation
Whole mount Alizarin red-Alcian blue staining was used to determine the embryonic skeleton composition in embryonic day 14.5 (E14.5) and E18.5 following protocols described by McLeod (29)Briefly, After(29). Briefly, after fixation in 95% ethanol for 24h, s, all the samples were placed in 95% ethanol-Alcian blue and Alizarin red solution (24 and 48h respectively) for cartilage and bone staining. Samples were washed with 95% ethanol (8h) and incubatedand ini d in 1% KOH overnight at 4 °C. Samples were then cleared with 20, 50, and 80% glycerol in 1% KOH for 12h respectively and stored in 100% glycerol. Analyses of the skeletal phenotype between genotypes were performed with Encoded stereo microscopes (Leica M125, Leica Microsystems, US). Quantitative measurements of body size-crown-rump length were performed by Image J 1.46r (Wayne Rasband, National Institutes of Health, US).
Immuno- and histological analyses
Hind limb samples harvested from E18.5 embryos were fixed in 10% formalin overnight. Following decalcification and processing, specimens were sectioned at a thickness of 5μm. Alcian Blue Hematoxylin/Orange G (ABH/OG) staining was performed to analyze skeletal architecture and cartilaginous compartment organization. Stained sections were scanned by Hamamatsu NanoZoomer 2.0-HT System and captured by NDP.view2 Viewing software (U12388-01) for image viewing.
The expression pattern of Dnmt3b, Col10a1, phospho-Smad1 and phospho-Smad3 was examined by immunohistochemistry on E18.5 tibia sections. Briefly, after de-paraffinization and rehydration, antigen retrieval was performed by heat and pressure for Dnmt3b or pepsin digestion for Col10a1. Dnmt3b (LSBio, #LS-B1191, 1:1000), Col10a1 (Quartett, #2031501005, 1:200), phospho-Smad1 (Cell Signaling, #9516, 1:800) and phospho-Smad3 (Abcam, #ab52903, 1:200) antibodies were used respectively for overnight incubation. For Dnmt3b, NovaRED Peroxidase (HRP) Substrate Kit (Vector Laboratories, #SK-4800) was used for colorimetric development and counterstaining was performed using hematoxylin stain (Vector Laboratories, # H-3404). Images were captured with a light microscope (Zeiss Axio Scope A1, Carl Zeiss Co, Ltd, Jena, Germany). Dnmt3b-positive cells were quantified from at least 3 animals of each genotype by Image J 1.46r. For Col10a1, phospho-Smad1 and phospho-Smad3, tyramide Signal Amplification Kit (Invitrogen, #T20912) was used to generate fluorescent signals and the cell nuclei were stained with DAPI (Vector Laboratories, #H-1200). The fluorescence was detected by Microscope Axio Imager.D2 (Carl Zeiss Co, Ltd, Jena, Germany). The length of the Col10a1 positive cartilage compartment was measured by Image J 1.46r.
PCNA and TUNEL staining
PCNA staining was performed to examine cell proliferation from histology sections. After de-paraffinization and rehydration, heat-induced antigen retrieval was performed with citric acid buffer (pH 6.0). Rabbit anti-mouse antibody against PCNA (CST, #13110, 1:500) was incubated overnight at 4℃. Tyramide Signal Amplification Kit (Invitrogen, #T20912) was used to generate fluorescent signals and the cell nuclei were stained with DAPI. Fluorescence was detected using a Microscope Axio Imager.D2. PCNA positive cells were quantified by Image J 1.46r and the percentage of PCNA-positive cell was quantified from at least 3 animals of each genotype.
TUNEL assay was performed on paraffin sections with In Situ Cell Death Detection Kit (Roche, #11684795910) according to the manufacturer’s instructions. Briefly, after de-paraffinization and rehydration, cells were permeabilized with 10μl/ml Proteinase K in 10mM Tris/HCL (PH7.4-8) at room temperature for 20min. TUNEL reaction mixture was then added to each sample and incubated for 60min at 37℃ in a humidified chamber in the dark. The cell nuclei were stained with DAPI (Vector Laboratories, #H-1200) and the fluorescence was detected by Microscope Axio Imager.D2.
Primary murine costal chondrocyte isolation and culture
Costal chondrocytes were isolated from sternae and ribs of P2–P5 WT or Dnmt3bCol2 neonatal pups as previously described(30). Briefly, sternae and ribs were dissected and pre-digested with 2 mg/ml pronase and 3 mg/ml collagenase D at 37 °C for 1h, respectively. This was followed with the second 4-6 h collagenase D digestion. Primary chondrocytes were then harvested and filtered by 40μm cell strainer. The cell suspensions were seeded at a density of 250,000 cells/well in 24-well tissue culture plates in HG-DMEM medium supplemented with 10% FBS. Chondrocytes were subsequently placed in maturation medium, including 50μg/mL L-Ascorbic acid (Sigma, #A4544) and 10mM β-Glycerophosphate disodium salt hydrate (Sigma, #G9422) in HG-DMEM medium (31). The chondrocyte maturation medium was changed every 3 days and the cells were cultured for 5, 14, and 21 days.
Real-time qPCR and Western blot
RNA was isolated from chondrocyte cultures using RNeasy Mini Kit (QIAGEN, #74104). cDNA synthesis and real-time qPCR were performed according to the protocols of the manufacturer. Primer sequences are shown in Table 1. Western blot was performed to examine the protein level of phospho-Smad1, Smad1, phospho-Smad3, Smad3 and β-actin from control and Dnmt3bCol2 chondrocytes. The following primary antibodies were used: phospho-Smad1 (Cell Signaling, #9516, 1:500), Smad1 (Cell Signaling, #6944, 1:1000), phospho-Smad3 (Abcam plc., #ab52903, 1:2000), Smad3 (Abcam plc., #ab40854, 1:2000) and β-actin (Sigma, #2228, 1:2000). Quantifications of the protein expressions (relative intensity) were measured by Image J 1.46r software.
Table 1.
Primer sequences for real-time qPCR
| Genes | Sequences |
|---|---|
| Dnmt1 | 5′- CCT GCC AGG GCT TCA GTG GC-3′ 5′- CAG GCA GCG CAG TGT GAG CT-3′ |
| Dnmt3a | 5′- GCC GAA TTG TGT CTT GGT GGA TGA CA-3′ 5′- CCT GGT GGA ATG CAC TGC AGA AGG A-3′ |
| Dnmt3b | 5′- AAT ACC CAA CTC CTT GAG CAC-3′ 5′- TCT TCA CTA CTG ATC CTG ACC T-3′ |
| Col10a1 | 5′-ATG CCT TGT TCT CCT CTT ACT G-3′ 5′-TGC TGA ACG GTA CCA AAC G-3′ |
| Mmp13 | 5′-AGA CTG GTA ATG GCA TCA AGG-3′ 5′-GCC ATT TCA TGC TTC CTG ATG-3′ |
| Actin | 5′- AGA TGT GGA TCA GCA AGC AG-3′ 5′-GCG CAA GTT AGG TTT TGT CA-3′ |
Alkaline phosphatase (ALP) and Alizarin Red staining
Chondrocyte maturation and mineral deposition were examined by ALP and Alizarin Red staining respectively. Briefly, following fixation with 10% formalin, the cell cultures were stained by NBT/BCIP substrate solution to detect ALP activity or alizarin red solution to detect mineral deposition. After washing each well three times with H2O, images were captured by scanner (EPSON, Perfection 1660).
Statistical Analysis
All quantitative data were presented as mean ± standard deviation with a minimum of three independent experiments. Statistical significance was determined by two-tailed Student’s t test. p<0.05 was considered statistically significant.
Results
Ablation of Dnmt3b in chondrocytes reduces embryonic skeletal growth
In order to investigate the role of Dnmt3b during the endochondral ossification process, Col2Cre; Dnmt3bf/f mice (Dnmt3bCol2) were generated to specifically delete Dnmt3b in chondrogenic lineage cells (32), which compensates for the embryonic lethality of global knockout of Dnmt3b in mice. The deletion efficiency was confirmed by immunohistochemistry (IHC) in the hind limb of E18.5 embryos. IHC showed that Dnmt3b was ubiquitously expressed in E18.5 WT chondrocytes, including both proliferating and hypertrophic chondrocytes, whereas the expression of Dnmt3b was markedly reduced in Dnmt3bCol2 chondrocytes (Fig. 1A and B).
Fig. 1. Ablation of Dnmt3b in chondrocytes inhibited embryonic skeletal growth.
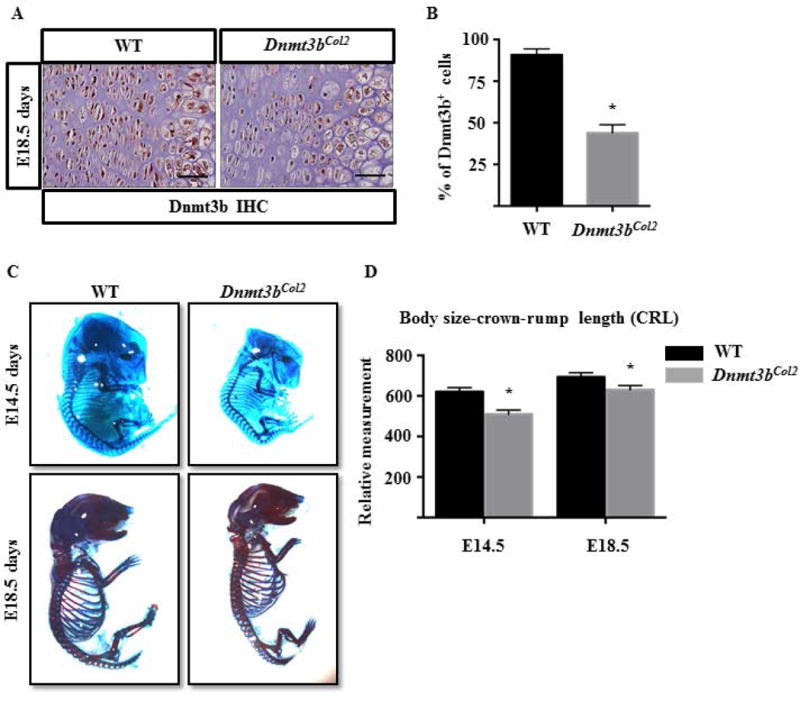
(A) Immunohistochemical analyses of Dnmt3b in hind limbs sections from WT and Dnmt3bCol2 embryos at E18.5. Data are representative of three independent experiments. Scale bar, 50μm. (B) Quantifications of Dnmt3b-positive cells were performed on hind limbs sections from WT and Dnmt3bCol2 embryos at E18.5. Data are expressed as means ± SD of three independent experiments. *, p < 0.05 compared with WT group by two-tailed Student’s t test. (C) The whole mount skeletal staining was performed on WT and Dnmt3bCol2 embryos at E14.5 and E18.5. Data are representative of three independent experiments. (D) Quantifications of body size-crown-rump length (CRL) were performed on WT and Dnmt3bCol2 embryos at E14.5 and E18.5. Data are expressed as means ± SD of three independent experiments. *, p < 0.05 compared with WT group by two-tailed Student’s t test.
The Dnmt3bCol2 mice were born at the expected Mendelian ratio with no gross abnormality. The whole mount skeletal staining of the embryos at E14.5 and E18.5 indicated that the skeletal elements mineralized during development in both WT and Dnmt3bCol2 mice, however, the whole skeletal size was reduced in the mutant embryos compared to their littermate controls (Fig. 1C). Quantitative measurement confirmed that body size-crown-rump length was significantly decreased in Dnmt3bCol2 embryos at E14.5 and E18.5 (Fig. 1D). Further evaluation of the limb skeletal elements demonstrated that the total length (TL) of the humerus, femur, radius, or tibia was shortened by 10% to 25% in Dnmt3bCol2 embryos compared to the littermates (Fig. 2, Fig. S1). Similarly, the width of the diaphysis of the humerus and tibia (BW; measured at the mid-point of the bone shaft) was reduced by 10% in Dnmt3bCol2 embryos (Fig. 2, Fig. S1). Interestingly, while the cartilage length (CL) of each bone was not significantly different between WT and mutant embryos, the bone shaft length (BL) of each long bone was decreased in Dnmt3bCol2 embryos compared to WT embryos (Fig. 2, Fig. S1).
Fig. 2. Ablation of Dnmt3b in chondrocytes results in reduced limb length.
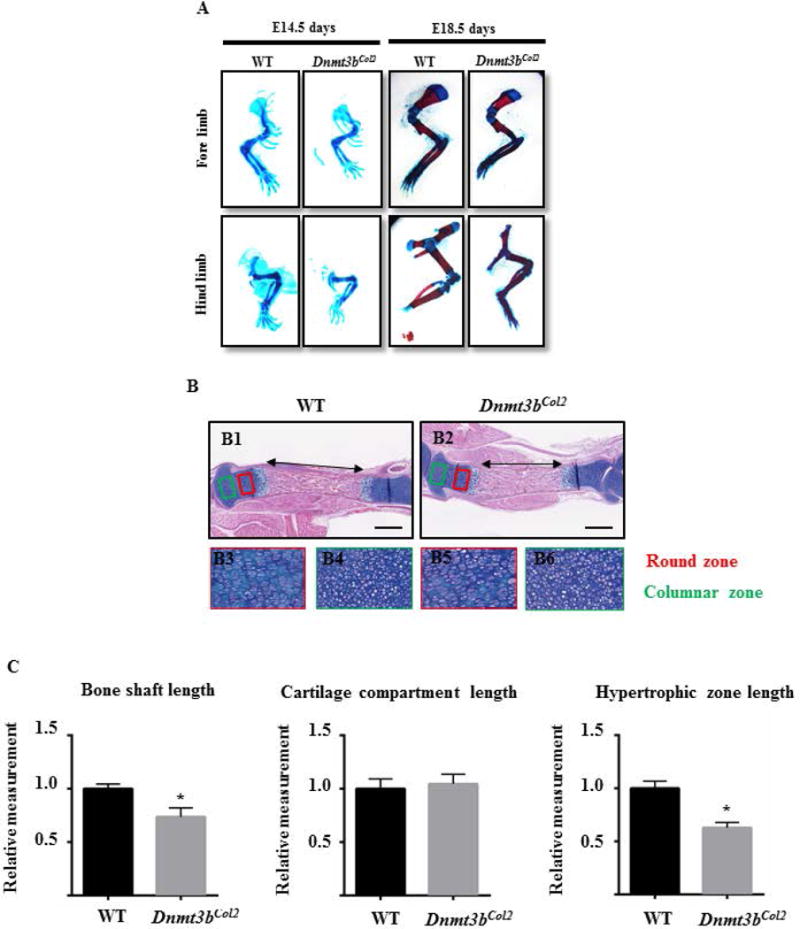
(A) Alizarin red-Alcian blue staining was performed on the fore- and hind limbs of Dnmt3bCol2 and littermate control embryos at E14.5 and E18.5. Data are representative of three independent experiments. (B) Hematoxylin/Orange G (ABH/OG) staining was performed on the hind limbs sections of Dnmt3bCol2 and littermate control embryos at E18.5. Reserve zone chondrocytes (red box) and in proliferating columnar zone of chondrocytes (green box) in panel B1-B2 are shown at high magnification in panels B3-B4 and in panels B5-B6, respectively. Data are representative of five independent experiments. Scale bar, 0.5mm. (C) Measurments of the length of the region of bone formation in the mid-shaft, cartilage compartment and hypertrophic zone were performed on the hind limbs sections of Dnmt3bCol2 and littermate control embryos at E18.5. All results were normalized to respective measurements in the littermate control group at E18.5, which were set at 1.0. Data are expressed as means ± SD of five independent experiments. *, p < 0.05 compared with WT group by two-tailed Student’s t test.
Consistent with the whole skeletal observation, histological analyses of E18.5 tibia by ABH/OG staining confirmed the shortening of bone shaft in Dnmt3bCol2 embryos (Fig. 2B and C). The epiphyseal cartilage contains resting chondrocyte in the round zone and proliferative chondrocytes in the columnar zone (33). Further examination in cartilage tissue revealed no difference in the morphology of the chondrocytes or the organization in either round or columnar zone between the genotypes (Fig. 2B). The cell density in E18.5 Dnmt3bCol2 embryos was similar between in the round zone and in the columnar zone of mutant and WT embryos, suggesting that chondrocyte hypertrophy and maturation is impaired by ablation of Dnmt3b, but not chondrocyte proliferation (Fig. S2). Altogether, Dnmt3b is essential for the embryonic bone growth, but does not affect the morphology and proliferation of cartilage tissue.
Dnmt3b stimulates chondrocyte hypertrophic maturation
Since chondrocyte hypertrophic maturation is required for longitudinal growth of long bones during embryogenesis, we isolated primary murine costal chondrocyte from Dnmt3bCol2 mice and control mice and cultured in chondrocyte maturation medium for up to 21 days to stimulate cell maturation. RT-PCR data confirmed that Dnmt3b expression was reduced by 80% after 5 days culture, whereas, the expression of Dnmt1 and Dnmt3a was not affected in Dnmt3bCol2 chondrocytes (Fig. 3A). The chondrocyte maturation makers, Mmp13 and Col10a1, were suppressed in Dnmt3bCol2 chondrocytes compared to control chondrocytes (Fig. 3A). Consistent with the gene expression profile, ALP staining and Alizarin Red staining also showed a delay in chondrocyte maturation in chondrocytes with deletion of Dnmt3b, with reduced ALP activity and mineral deposition in Dnmt3bCol2 chondrocyte cultures compared to control cultures after 21 days in chondrocyte differentiation medium (Fig. 3B). Furthermore, immunofluorescence for COL10A1 in E18.5 limb revealed that Type X Collagen was highly expressed in the hypertrophic chondrocytes of E18.5 mice, whereas the expression of COL10A1 was significantly reduced in Dnmt3bCol2 embryos (Fig. 3C). Quantification of the length of COL10A1-positive cells demonstrated a 35% reduction in the length of the COL10A1 zone in E18.5 mutant embryos compared to littermate controls (Fig. 3D). Chondrocyte proliferation and apoptosis were examined in Dnmt3bCol2 chondrocytes and no difference was observed in cell proliferation in either chondrocytes in the reserve zone or chondrocytes in the proliferating zone of the proximal tibia in Dnmt3bCol2 and littermate control embryos at E18.5 days (Fig. 4A and B). Similarly, no difference in TUNEL staining was observed (Fig. 4C). Altogether, loss of Dnmt3b suppressed chondrocyte maturation and led to the defect of long bone longitudinal growth.
Fig. 3. Loss of Dnmt3b in chondrocytes suppressed hypertrophic maturation.
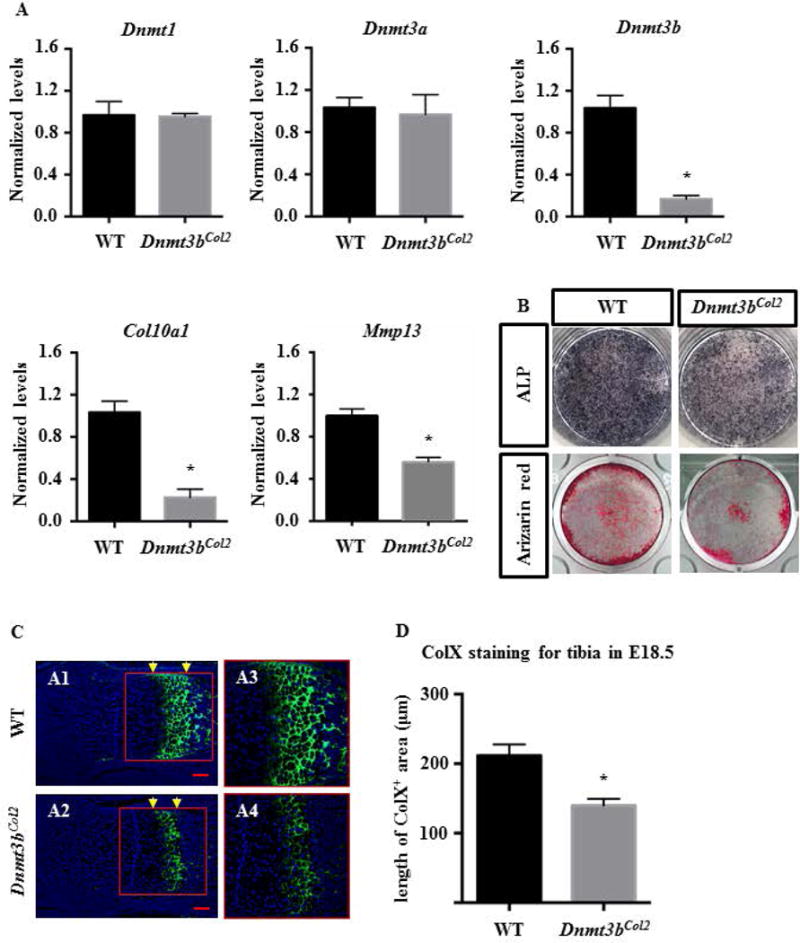
(A) Real-time qPCR analyses were performed to determine the relative expression of Dnmt1, Dnmt3a, Dnmt3b, Col10a1 and Mmp13 in primary chondrocytes isolated primary murine costal chondrocyte from P2–P5 Dnmt3bCol2 mice and littermate control mice, which were cultured in chondrocyte maturation medium up to 21 days. The mRNA levels were normalized to that of the gene coding β-actin and then were normalized to the littermate control group. Data are means ± SD of three independent experiments. *, p < 0.05 compared with WT group by two-tailed Student’s t test. (B) Alkaline phosphatase (ALP) and Alizarin Red staining of Dnmt3bCol2 and littermate control chondrocytes cultured in chondrocyte maturation medium for up to 21 days. Data are representative of three independent experiments. (C) The immunofluorescence for COL10A1 was performed on the hind limbs sections of Dnmt3bCol2 and littermate control embryos at E18.5. Data are representative of five independent experiments. The growth plate (red box) in panels A1-A2 are shown at high magnification in panels A3-A4, respectively. Scale bar, 100μm. (D) Quantifications of the length of COL10A1-positive cells (between yellow arrow) were performed on the hind limbs sections Dnmt3bCol2 and littermate control embryos at E18.5. Data are expressed as means ± SD of five independent experiments. *, p < 0.05 compared with WT group by two-tailed Student’s t test.
Fig. 4. Loss of Dnmt3b does not alter chondrocyte proliferation or apoptosis.

(A) PCNA staining was performed on the hind limbs sections of Dnmt3bCol2 and littermate control embryos at E18.5. Reserve zone chondrocytes (yellow box) and proliferating columnar zone chondrocytes (green box) in panel A1-A2 are shown at high magnification in panels A3-A4 and in panels A5-A6, respectively. Data are representative of five independent experiments. Scale bar, 100μm. (B) Quantifications of the percent of PCNA-positive cells were performed in the reserve zone and columnar proliferating zone of the proximal tibia sections of Dnmt3bCol2 and littermate control embryos at E18.5. Data are expressed as means ± SD of five independent experiments. *, p < 0.05 compared with the littermate control group by two-tailed Student’s t test. (C) TUNEL staining was performed on the hind limbs sections of Dnmt3bCol2 and littermate control embryos at E18.5. Reserve zone chondrocytes (yellow box) and columnar zone chondrocytes (green box) in panel A1-A2 are shown at high magnification in panels A3-A4 and A5-A6, respectively. Data are representative of five independent experiments. Scale bar, 100μm.
Dnmt3b regulates chondrocyte maturation through BMP pathway
TGF-β/BMP pathway is well documented in regulating chondrocyte maturation and homeostasis in vitro and in vivo, therefore, protein was isolated from control and Dnmt3bCol2 chondrocytes to assess the TGF-β/BMP signaling pathway. Western blot was performed to determine the expressions of Smad1, Smad3, phospho-Smad1 and phospho-Smad3. Western blot revealed no difference in the TGF-β pathway but showed down-regulation of the BMP pathway in Dnmt3bCol2 chondrocytes as indicated by decreased expression of phospho-Smad1 (Fig. 5A). Quantifications of the protein expressions confirmed that the ratio of phospho-Smad1/Smad1 was reduced, while the ratio of phospho-Smad3/Smad3 maintained unchanged in Dnmt3bCol2 chondrocytes compared with WT cells (Fig. 5B). Immunofluorescence for phospho-Smad1 and phospho-Smad3 in E18.5 limb confirmed that the expression of phospho-Smad1 was significantly decreased in the hypertrophic chondrocytes of E18.5 mice (Fig. 5C), whereas the expression of phospho-Smad3 was not altered in Dnmt3bCol2 embryos (Fig. S3), indicating Dnmt3b regulates the chondrocyte maturation through BMP pathway during embryonic development.
Fig. 5. Dnmt3b regulates chondrocyte maturation through BMP pathway during embryonic development.
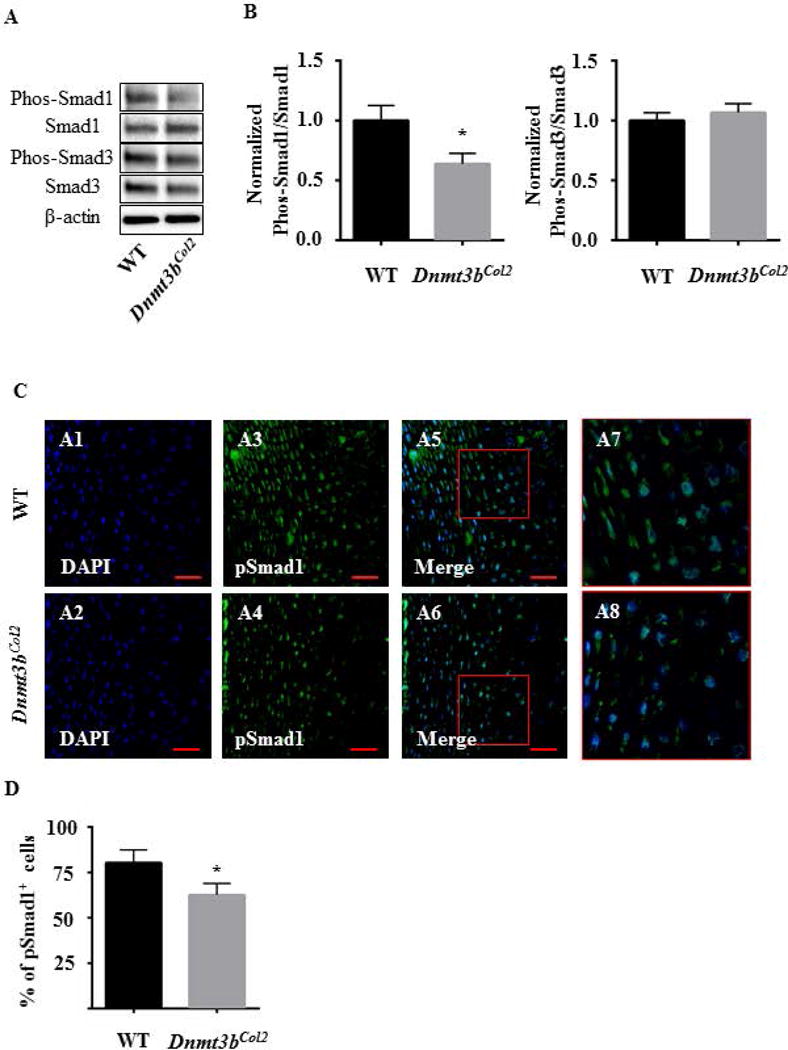
(A) Western blot was performed to examine the protein level of phospho-Smad1, Smad1, phospho-Smad3, Smad3 and β-actin from Dnmt3bCol2 and littermate control chondrocytes cultured in chondrocyte maturation medium for up to 21 days. Data are representative of three independent experiments. (B) Quantifications of the protein expressions were obtained using Image J 1.46r software. Data are expressed as means ± SD of three independent experiments. *, p < 0.05 compared with WT group by two-tailed Student’s t test. (C) The immunofluorescence for phospho-Smad1 was performed on the hind limbs sections of Dnmt3bCol2 and littermate control embryos at E18.5. Data are representative of three independent experiments. The area (red box) in panels A5-A6 are shown at high magnification in panels A7-A8, respectively. Scale bar, 50μm. (D) Quantifications of pSmad1-positive cells were performed on hind limbs sections from WT and Dnmt3bCol2 embryos at E18.5. Data are expressed as means ± SD of three independent experiments. *, p < 0.05 compared with WT group by two-tailed Student’s t test.
Discussion
The DNA methyltransferases are critical in development and normal tissue function, and are involved in aging, cancer, and in disease pathogenesis. Here we defined the biological functions of the de novo Dnmt3b in the regulation of embryonic endochondral bone development. Previous studies from human chondrocytes reported the expression of Dnmts in human chondrocytes (34), however the role of Dnmts in chondrocytes during embryonic skeletal development has not yet been examined. In this study, we examined the function of Dnmt3b in Col2 positive chondrocyte lineage and found that ablation of Dnmt3b in chondrocytes suppressed chondrocyte hypertrophic differentiation and skeletal growth at E14.5 and E18.5, at least partially through down-regulation of BMP signaling pathway during endochondral bone formation process.
In mammals, three conserved catalytically active enzymes, Dnmt1, Dnmt3a and Dnmt3b, are responsible for DNA methylation and maintenance of methylation during cell proliferation and are indispensable for normal development (13,35). During DNA replication, Dnmt1 mediates epigenetic inheritance in dividing cells through the duplication of methylation on the new DNA strand at hemimethylated CpG sites. Thus loss of Dnmt1 leads to whole genome DNA methylation collapse (36). On the other hand, unmethylated CpGs are specifically targeted by Dnmt3a and Dnmt3b during de novo methylation which plays important role to maintain cell proliferation, differentiation as well as tissue homeostasis and specificity (37–39). Recently, high-throughput screenings have characterized the role and distribution of DNA methylation during embryonic development. It has been documented that the methylation primarily occurred in the gene locus which regulates cellular identity and genomic stability during early embryogenesis (40). For developmental transitions, DNA methylation exhibits a repression function on the germline expression program during implantation at the transition from the blastocyst to the post-implantation epiblast and is also targeted to promoters of lineage-specific genes in the blastocyst such as hematopoietic genes with demethylation during terminal differentiation (41). It is worth to note that both of these dynamics on DNA methylation during early embryogenesis is mediated by Dnmt3a and Dnmt3b (41) and in general, Dnmt3b has high activity in vitro (42).
Several studies from mouse embryogenesis indicate an overlapping role Dnmt3a and Dnmt3b in regulating of growth, morphogenesis and gene silencing and yet, Dnmt3a and Dnmt3b also have distinct functions (13,43,44). During early embryonic development, the phonotype that abnormal morphology and lethality of Dnmt3a−/−;Dnmt3b−/− double homozygous embryos was apparently more severe than that of Dnmt3a−/− or Dnmt3b−/− single mutant embryos, demonstrating the presence of overlapping function between Dnmt3a and Dnmt3b (13). The irreplaceable role of Dnmt3a is specifically exhibited in the methylation of imprinted genes in germ cells (45) and the Xist promoter region on the X chromosome (39). Moreover, Dnmt3a expression in neural stem cells is essential for neurogenesis. In neurogenesis, Dnmt3a targets non-proximal promoter methylation sites to maintain an active chromatin state of genes critical for neural development(46). During osteoclastogenesis, osteoclast precursors continuously express mRNA for Dnmt3a, which plays an important role in the regulation of cellular metabolism and differentiation in bone disorders (47). On the other hand, for the minor satellite repeats during the embryonic development, its methylation is primarily in the control of Dnmt3b without contribution from Dnmt3a, indicating Dnmt3b specifically required for methylation of centromeric minor satellite repeats in development (13). Furthermore, in mammalian embryonic stem cells (ESCs), Dnmt3b could also interact with histone modification enzymes to regulate the accessibility of gene transcription machinery to specific DNA loci during embryo development. (48). Despite the redundancy of Dnmt3a and Dnmt3b in cells and tissues, Dnmt3a still needs to be further explored to fully delineate the role of de novo DNA methylation during skeletal development process.
As the most extensively studied epigenetic marks, DNA methylation is considered to be essential to transcriptional gene silencing during mammalian development (49). In mammalian genomes, DNA methylation ordinarily emerges at the special regions of promoters and enhancers of inactive genes, or at repetitive elements and within transcribed gene bodies (50–52). In particular, DNA methylation at the promoter region has been related to gene activity through direct influence on gene expression patterns and cellular phenotype (53). Although DNA methylation are highly studied epigenetic modifications, the exact role of DNA methylation in regulating genome function and how this differs from gene to gene and in genomic context need to be uncovered (49). It still remains to be fully understood how specific DNA methylation patterns are precisely generated, maintained, read, or erased along the genome. In this regard, Genome-wide methylation screen is necessary to identify the methylation patterns of TGF/BMP pathway related molecules, including Smad1, 3, 4 etc. in the promoter, enhancer and gene body region. Moreover, DNA methylation is a dynamic process and Ten-eleven Translocation Enzymes (Tets) are involved in the DNA demethylation, thus, Tets may contribute to the chondrocyte maturation and skeletal development as well.
Supplementary Material
Fig. S1. Ablation of Dnmt3b in chondrocytes results in reduced limb length during embryonic development. (A) Quantifications of the length of the region of bone (bone shaft length; BL), region of cartilage (cartilage length; CL), total skeletal element length (TL), bone width at the midshaft (BW) were measured on the limb skeletal elements from Dnmt3bCol2 and littermate control embryos at E18.5. All results were normalized to respective measurements in the littermate control group at E18.5, which were set at 1.0. Data are expressed as means ± SD of three independent experiments. *, p < 0.05 compared with WT group by two-tailed Student’s t test.
Fig. S2. Ablation of Dnmt3b in chondrocytes did not alter cell density in round and columnar zone chondrocyte populations. (A) Quantifications of cell density were performed on the reserve round zone chondrocytes and the proliferating columnar zone chondrocytes of the proximal tibia sections of WT and Dnmt3bCol2 embryos at E18.5. Data are expressed as means ± SD of five independent experiments. *, p < 0.05 compared with WT group by two-tailed Student’s t test.
Fig. S3. Dnmt3b gene deletion does not modulate the TGFβ signaling during embryonic development (A) The immunofluorescence for phospho-Smad3 was performed on the hind limbs sections of Dnmt3bCol2 and littermate control embryos at E18.5. Data are representative of three independent experiments. The area (red box) in panels A5-A6 are shown at high magnification in panels A7-A8, respectively. Scale bar, 50μm. (B) Quantifications of pSmad3-positive cells were performed on hind limbs sections from WT and Dnmt3bCol2 embryos at E18.5. Data are expressed as means ± SD of three independent experiments. *, p < 0.05 compared with WT group by two-tailed Student’s t test.
Acknowledgments
This work was supported by grants from the National Institutes of Health/National Institute of Arthritis and Musculoskeletal and Skin Diseases (R01 AR069605 to RJO) as well as the China Scholarship Council (CSC NO. 201708330299) and the Cultivation Program for Innovative Talent Graduate Students (Grant NO. 311100G00901).
References
- 1.McCaughan JA, McKnight AJ, Courtney AE, Maxwell AP. Epigenetics: time to translate into transplantation. Transplantation. 2012 Jul 15;94(1):1–7. doi: 10.1097/TP.0b013e31824db9bd. [DOI] [PubMed] [Google Scholar]
- 2.Barter MJ, Bui C, Young DA. Epigenetic mechanisms in cartilage and osteoarthritis: DNA methylation, histone modifications and microRNAs. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2012 May;20(5):339–49. doi: 10.1016/j.joca.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 3.Barter MJ, Young DA. Epigenetic mechanisms and non-coding RNAs in osteoarthritis. Current rheumatology reports. 2013 Sep;15(9):353. doi: 10.1007/s11926-013-0353-z. [DOI] [PubMed] [Google Scholar]
- 4.Jeltsch A, Jurkowska RZ. New concepts in DNA methylation. Trends in biochemical sciences. 2014 Jul;39(7):310–8. doi: 10.1016/j.tibs.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 5.Chatterjee A, Eccles MR. DNA methylation and epigenomics: new technologies and emerging concepts. Genome biology. 2015 May 21;16:103. doi: 10.1186/s13059-015-0674-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ficz G. New insights into mechanisms that regulate DNA methylation patterning. The Journal of experimental biology. 2015 Jan 1;218(Pt 1):14–20. doi: 10.1242/jeb.107961. [DOI] [PubMed] [Google Scholar]
- 7.Kanherkar RR, Bhatia-Dey N, Csoka AB. Epigenetics across the human lifespan. Front Cell Dev Biol. 2014;2:49. doi: 10.3389/fcell.2014.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smith ZD, Meissner A. DNA methylation: roles in mammalian development. Nat Rev Genet. 2013 Mar;14(3):204–20. doi: 10.1038/nrg3354. [DOI] [PubMed] [Google Scholar]
- 9.Robertson KD, Uzvolgyi E, Liang G, Talmadge C, Sumegi J, Gonzales FA, et al. The human DNA methyltransferases (DNMTs) 1, 3a and 3b: coordinate mRNA expression in normal tissues and overexpression in tumors. Nucleic acids research. 1999 Jun 01;27(11):2291–8. doi: 10.1093/nar/27.11.2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bostick M, Kim JK, Esteve PO, Clark A, Pradhan S, Jacobsen SE. UHRF1 plays a role in maintaining DNA methylation in mammalian cells. Science (New York, NY) 2007 Sep 21;317(5845):1760–4. doi: 10.1126/science.1147939. [DOI] [PubMed] [Google Scholar]
- 11.Sharif J, Muto M, Takebayashi S, Suetake I, Iwamatsu A, Endo TA, et al. The SRA protein Np95 mediates epigenetic inheritance by recruiting Dnmt1 to methylated DNA. Nature. 2007 Dec 06;450(7171):908–12. doi: 10.1038/nature06397. [DOI] [PubMed] [Google Scholar]
- 12.Lei H, Oh SP, Okano M, Juttermann R, Goss KA, Jaenisch R, et al. De novo DNA cytosine methyltransferase activities in mouse embryonic stem cells. Development. 1996 Oct;122(10):3195–205. doi: 10.1242/dev.122.10.3195. [DOI] [PubMed] [Google Scholar]
- 13.Okano M, Bell DW, Haber DA, Li E. DNA methyltransferases Dnmt3a and Dnmt3b are essential for de novo methylation and mammalian development. Cell. 1999 Oct 29;99(3):247–57. doi: 10.1016/s0092-8674(00)81656-6. [DOI] [PubMed] [Google Scholar]
- 14.Nguyen S, Meletis K, Fu D, Jhaveri S, Jaenisch R. Ablation of de novo DNA methyltransferase Dnmt3a in the nervous system leads to neuromuscular defects and shortened lifespan. Developmental dynamics : an official publication of the American Association of Anatomists. 2007 Jun;236(6):1663–76. doi: 10.1002/dvdy.21176. [DOI] [PubMed] [Google Scholar]
- 15.Martins-Taylor K, Schroeder DI, LaSalle JM, Lalande M, Xu RH. Role of DNMT3B in the regulation of early neural and neural crest specifiers. Epigenetics. 2012 Jan 01;7(1):71–82. doi: 10.4161/epi.7.1.18750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shen J, Wang C, Li D, Xu T, Myers J, Ashton JM, et al. DNA methyltransferase 3b regulates articular cartilage homeostasis by altering metabolism. JCI insight. 2017 Jun 15;2(12) doi: 10.1172/jci.insight.93612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Challen GA, Sun D, Mayle A, Jeong M, Luo M, Rodriguez B, et al. Dnmt3a and Dnmt3b have overlapping and distinct functions in hematopoietic stem cells. Cell Stem Cell. 2014 Sep 04;15(3):350–64. doi: 10.1016/j.stem.2014.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smeets DF, Moog U, Weemaes CM, Vaes-Peeters G, Merkx GF, Niehof JP, et al. ICF syndrome: a new case and review of the literature. Human genetics. 1994 Sep;94(3):240–6. doi: 10.1007/BF00208277. [DOI] [PubMed] [Google Scholar]
- 19.Li E. Chromatin modification and epigenetic reprogramming in mammalian development. Nature reviews Genetics. 2002 Sep;3(9):662–73. doi: 10.1038/nrg887. [DOI] [PubMed] [Google Scholar]
- 20.Ueda Y, Okano M, Williams C, Chen T, Georgopoulos K, Li E. Roles for Dnmt3b in mammalian development: a mouse model for the ICF syndrome. Development (Cambridge, England) 2006 Mar;133(6):1183–92. doi: 10.1242/dev.02293. [DOI] [PubMed] [Google Scholar]
- 21.Wang C, Abu-Amer Y, O’Keefe RJ, Shen J. Loss of Dnmt3b in Chondrocytes Leads to Delayed Endochondral Ossification and Fracture Repair. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2017 Oct 10; doi: 10.1002/jbmr.3305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Crombrugghe B, Lefebvre V, Behringer RR, Bi W, Murakami S, Huang W. Transcriptional mechanisms of chondrocyte differentiation. Matrix biology : journal of the International Society for Matrix Biology. 2000 Sep;19(5):389–94. doi: 10.1016/s0945-053x(00)00094-9. [DOI] [PubMed] [Google Scholar]
- 23.Goldring MB, Tsuchimochi K, Ijiri K. The control of chondrogenesis. Journal of cellular biochemistry. 2006 Jan 1;97(1):33–44. doi: 10.1002/jcb.20652. Epub 2005/10/11. [DOI] [PubMed] [Google Scholar]
- 24.Lefebvre V, Smits P. Transcriptional control of chondrocyte fate and differentiation. Birth defects research Part C, Embryo today : reviews. 2005 Sep;75(3):200–12. doi: 10.1002/bdrc.20048. [DOI] [PubMed] [Google Scholar]
- 25.Long F, Ornitz DM. Development of the endochondral skeleton. Cold Spring Harbor perspectives in biology. 2013 Jan 01;5(1):a008334. doi: 10.1101/cshperspect.a008334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen M, Lichtler AC, Sheu TJ, Xie C, Zhang X, O’Keefe RJ, et al. Generation of a transgenic mouse model with chondrocyte-specific and tamoxifen-inducible expression of Cre recombinase. Genesis. 2007 Jan;45(1):44–50. doi: 10.1002/dvg.20261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhu M, Chen M, Lichtler AC, O’Keefe RJ, Chen D. Tamoxifen-inducible Cre-recombination in articular chondrocytes of adult Col2a1-CreER(T2) transgenic mice. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2008 Jan;16(1):129–30. doi: 10.1016/j.joca.2007.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dodge JE, Okano M, Dick F, Tsujimoto N, Chen T, Wang S, et al. Inactivation of Dnmt3b in mouse embryonic fibroblasts results in DNA hypomethylation, chromosomal instability, and spontaneous immortalization. J Biol Chem. 2005 May 6;280(18):17986–91. doi: 10.1074/jbc.M413246200. [DOI] [PubMed] [Google Scholar]
- 29.McLeod MJ. Differential staining of cartilage and bone in whole mouse fetuses by alcian blue and alizarin red S. Teratology. 1980 Dec;22(3):299–301. doi: 10.1002/tera.1420220306. [DOI] [PubMed] [Google Scholar]
- 30.Gosset M, Berenbaum F, Thirion S, Jacques C. Primary culture and phenotyping of murine chondrocytes. Nature protocols. 2008;3(8):1253–60. doi: 10.1038/nprot.2008.95. [DOI] [PubMed] [Google Scholar]
- 31.Wang C, Abu-Amer Y, R JOK, Shen J. Loss of Dnmt3b in Chondrocytes Leads to Delayed Endochondral Ossification and Fracture Repair. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2017 Oct 10; doi: 10.1002/jbmr.3305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dao DY, Jonason JH, Zhang Y, Hsu W, Chen D, Hilton MJ, et al. Cartilage-specific beta-catenin signaling regulates chondrocyte maturation, generation of ossification centers, and perichondrial bone formation during skeletal development. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2012 Aug;27(8):1680–94. doi: 10.1002/jbmr.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen J, Holguin N, Shi Y, Silva MJ, Long F. mTORC2 signaling promotes skeletal growth and bone formation in mice. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2015 Feb;30(2):369–78. doi: 10.1002/jbmr.2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sesselmann S, Soder S, Voigt R, Haag J, Grogan SP, Aigner T. DNA methylation is not responsible for p21WAF1/CIP1 down-regulation in osteoarthritic chondrocytes. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2009 Apr;17(4):507–12. doi: 10.1016/j.joca.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 35.Li E, Bestor TH, Jaenisch R. Targeted mutation of the DNA methyltransferase gene results in embryonic lethality. Cell. 1992 Jun 12;69(6):915–26. doi: 10.1016/0092-8674(92)90611-f. [DOI] [PubMed] [Google Scholar]
- 36.Moore LD, Le T, Fan G. DNA methylation and its basic function. Neuropsychopharmacology. 2013 Jan;38(1):23–38. doi: 10.1038/npp.2012.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Avgustinova A, Benitah SA. Epigenetic control of adult stem cell function. Nature reviews Molecular cell biology. 2016 Oct;17(10):643–58. doi: 10.1038/nrm.2016.76. [DOI] [PubMed] [Google Scholar]
- 38.Rinaldi L, Datta D, Serrat J, Morey L, Solanas G, Avgustinova A, et al. Dnmt3a and Dnmt3b Associate with Enhancers to Regulate Human Epidermal Stem Cell Homeostasis. Cell stem cell. 2016 Oct 6;19(4):491–501. doi: 10.1016/j.stem.2016.06.020. [DOI] [PubMed] [Google Scholar]
- 39.Chen T, Ueda Y, Dodge JE, Wang Z, Li E. Establishment and maintenance of genomic methylation patterns in mouse embryonic stem cells by Dnmt3a and Dnmt3b. Molecular and cellular biology. 2003 Aug;23(16):5594–605. doi: 10.1128/MCB.23.16.5594-5605.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hackett JA, Surani MA. DNA methylation dynamics during the mammalian life cycle. Philosophical transactions of the Royal Society of London Series B, Biological sciences. 2013 Jan 05;368(1609):20110328. doi: 10.1098/rstb.2011.0328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Borgel J, Guibert S, Li Y, Chiba H, Schubeler D, Sasaki H, et al. Targets and dynamics of promoter DNA methylation during early mouse development. Nature genetics. 2010 Dec;42(12):1093–100. doi: 10.1038/ng.708. [DOI] [PubMed] [Google Scholar]
- 42.Shen L, Gao G, Zhang Y, Zhang H, Ye Z, Huang S, et al. A single amino acid substitution confers enhanced methylation activity of mammalian Dnmt3b on chromatin DNA. Nucleic acids research. 2010 Oct;38(18):6054–64. doi: 10.1093/nar/gkq456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Oda M, Yamagiwa A, Yamamoto S, Nakayama T, Tsumura A, Sasaki H, et al. DNA methylation regulates long-range gene silencing of an X-linked homeobox gene cluster in a lineage-specific manner. Genes & development. 2006 Dec 15;20(24):3382–94. doi: 10.1101/gad.1470906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li JY, Pu MT, Hirasawa R, Li BZ, Huang YN, Zeng R, et al. Synergistic function of DNA methyltransferases Dnmt3a and Dnmt3b in the methylation of Oct4 and Nanog. Molecular and cellular biology. 2007 Dec;27(24):8748–59. doi: 10.1128/MCB.01380-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kaneda M, Okano M, Hata K, Sado T, Tsujimoto N, Li E, et al. Essential role for de novo DNA methyltransferase Dnmt3a in paternal and maternal imprinting. Nature. 2004 Jun 24;429(6994):900–3. doi: 10.1038/nature02633. [DOI] [PubMed] [Google Scholar]
- 46.Wu H, Coskun V, Tao J, Xie W, Ge W, Yoshikawa K, et al. Dnmt3a-dependent nonpromoter DNA methylation facilitates transcription of neurogenic genes. Science (New York, NY) 2010 Jul 23;329(5990):444–8. doi: 10.1126/science.1190485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nishikawa K, Iwamoto Y, Kobayashi Y, Katsuoka F, Kawaguchi S, Tsujita T, et al. DNA methyltransferase 3a regulates osteoclast differentiation by coupling to an S-adenosylmethionine-producing metabolic pathway. Nature medicine. 2015 Mar;21(3):281–7. doi: 10.1038/nm.3774. [DOI] [PubMed] [Google Scholar]
- 48.Lee JH, Park SJ, Nakai K. Differential landscape of non-CpG methylation in embryonic stem cells and neurons caused by DNMT3s. Scientific reports. 2017 Sep 12;7(1):11295. doi: 10.1038/s41598-017-11800-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ambrosi C, Manzo M, Baubec T. Dynamics and Context-Dependent Roles of DNA Methylation. Journal of molecular biology. 2017 Feb 16; doi: 10.1016/j.jmb.2017.02.008. [DOI] [PubMed] [Google Scholar]
- 50.Jones PA. Functions of DNA methylation: islands, start sites, gene bodies and beyond. Nat Rev Genet. 2012 May 29;13(7):484–92. doi: 10.1038/nrg3230. [DOI] [PubMed] [Google Scholar]
- 51.Jahner D, Stuhlmann H, Stewart CL, Harbers K, Lohler J, Simon I, et al. De novo methylation and expression of retroviral genomes during mouse embryogenesis. Nature. 1982 Aug 12;298(5875):623–8. doi: 10.1038/298623a0. [DOI] [PubMed] [Google Scholar]
- 52.Walsh CP, Chaillet JR, Bestor TH. Transcription of IAP endogenous retroviruses is constrained by cytosine methylation. Nature genetics. 1998 Oct;20(2):116–7. doi: 10.1038/2413. [DOI] [PubMed] [Google Scholar]
- 53.Gibney ER, Nolan CM. Epigenetics and gene expression. Heredity (Edinb) 2010 Jul;105(1):4–13. doi: 10.1038/hdy.2010.54. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Ablation of Dnmt3b in chondrocytes results in reduced limb length during embryonic development. (A) Quantifications of the length of the region of bone (bone shaft length; BL), region of cartilage (cartilage length; CL), total skeletal element length (TL), bone width at the midshaft (BW) were measured on the limb skeletal elements from Dnmt3bCol2 and littermate control embryos at E18.5. All results were normalized to respective measurements in the littermate control group at E18.5, which were set at 1.0. Data are expressed as means ± SD of three independent experiments. *, p < 0.05 compared with WT group by two-tailed Student’s t test.
Fig. S2. Ablation of Dnmt3b in chondrocytes did not alter cell density in round and columnar zone chondrocyte populations. (A) Quantifications of cell density were performed on the reserve round zone chondrocytes and the proliferating columnar zone chondrocytes of the proximal tibia sections of WT and Dnmt3bCol2 embryos at E18.5. Data are expressed as means ± SD of five independent experiments. *, p < 0.05 compared with WT group by two-tailed Student’s t test.
Fig. S3. Dnmt3b gene deletion does not modulate the TGFβ signaling during embryonic development (A) The immunofluorescence for phospho-Smad3 was performed on the hind limbs sections of Dnmt3bCol2 and littermate control embryos at E18.5. Data are representative of three independent experiments. The area (red box) in panels A5-A6 are shown at high magnification in panels A7-A8, respectively. Scale bar, 50μm. (B) Quantifications of pSmad3-positive cells were performed on hind limbs sections from WT and Dnmt3bCol2 embryos at E18.5. Data are expressed as means ± SD of three independent experiments. *, p < 0.05 compared with WT group by two-tailed Student’s t test.


