Abstract
Tendon graft healing in bone tunnels for the fixation of intra-articular ligament reconstructions may limit clinical outcome by delaying healing. This study assesses the effects of hydrogel-mediated delivery of bone anabolic growth factors in a validated model of tendon-to-bone tunnel healing. Forty-five Wistar rats were randomly allocated into three groups (BMP2-treated, GSK126-treated, and placebo). All animals underwent a tendon to bone tunnel reconstruction. Healing was evaluated at 4 weeks by biomechanical assessment, micro-computed tomography (bone mineral density, bone volume, cross sectional area of bone tunnels), and traditional histology. Adverse events associated with the hydrogel-mediated delivery of drugs were not observed. Results of our biomechanical assessment demonstrated favorable trends in animals treated with bone anabolic factors for, energy absorption (p=0.116) and elongation (p=0.054), while results for force to failure (p=0.691) and stiffness (p=0.404) did not show discernible differences. Cross sectional areas for BMP2-treated animals were reduced, but neither BMP2 nor GSK126 administration altered bone mineral density (p=0.492) or bone volume in the bone tunnel. These results suggest a novel and positive effect of bone anabolic factors on tendon to bone tunnel healing. Histological evaluation confirmed absence of collagen fibers crossing the soft tissue-bone interface indicating immature graft integration as expected at this time point. Our study indicates that hydrogel-mediated delivery of BMP2 and GSK126 appears to be safe and has the potential to enhance tendon-to-bone-tunnel healing in ligament reconstructions.
Keywords: GSK126, BMP2, ligament reconstruction, orthopedic, connective tissue, tendon to bone healing
Introduction
Intra-articular ligament injuries are common and challenging to manage. For example, anterior cruciate ligament (ACL) ruptures occur in 35 of 100,000 individuals and cost more than $3 billion to treat in the United States each year[Gianotti et al., 2009]. Within the upper extremity, thescapholunate ligament (SL) in the wrist and rotator cuff in the shoulder are sites of common injury that are difficult to manage[Chim and Moran, 2014; Walsh et al., 2002]. Once injured, ligaments lack the intrinsic ability to heal. If left untreated, joints may become unstable leading to pain, stiffness and post-traumatic arthritis. Although surgical techniques for reconstructing ligaments are advancing, long-term clinical data demonstrate persistent and recurrent joint-related symptoms, pain and degenerative joint disease. Given their limited healing capacity, the current standard of care for ligament reconstruction involves using autograft (self) or allograft (cadaver) tendons. Surgical reconstruction depends on the fixation of two tissues (bone and tendon) with vastly different morphological and biomechanical properties, and depends on new bone formation to anchor the tendon in the bone tunnel during early recovery.
Intra-articular ligament injuries (e.g., anterior cruciate ligament ruptures) are most common among young, active people [Granan et al., 2009]. Severe ligament injury may lead to short-term functional impairment of sport activities and daily living, as well as long-term disability due to early onset osteoarthritis [Lohmander et al., 2004]. The healing potential for intra-articular ligament injuries is low, and surgical reconstruction by tendon graft is often required to restore joint stability and function [Feagin and Curl, 1976; Kiapour and Murray, 2014; Sandberg et al., 1987]. A successful intra-articular ligament reconstruction must withstand multi-directional tensile forces within the joint. In contrast to the native tendon-bone insertion site developed during embryological development, healing of tendon-bone attachment in surgical reconstructions occurs through the formation of fibrovascular scar tissue. The enthesis, a transition zone between native ligaments or tendons and bone, is not regenerated [Apostolakos et al., 2014], and therefore the outcome of reconstruction depends on the secure anchorage of grafted tendon-to-bone tunnel [Ekdahl et al., 2008; Fu et al., 1999; Harryman et al., 1991; Rodeo et al., 1993]. Poor outcomes following tendon-to-bone tunnel healing can be caused by circumferential bone loss, potentially limiting the anchorage of the tendon graft. Collagen formation across the tendon-bone interface can further delay the healing process and limit integration of the graft [Rodeo et al., 1993]. Additional strategies are urgently needed to enhance the fixation of tendon grafts to bone tunnels during the surgical repair of intra-articular ligaments.
Bone anabolic factors, such as bone morphogenic protein (BMP), may enhance tendon-to-bone tunnel healing by increasing new bone formation and reducing circumferential bone tunnel loss. BMPs are part of the transforming growth factor beta superfamily (TGF-β) [Chen et al., 2012; Rahman et al., 2015; Wu et al., 2016], and BMP2 has osteoinductive properties that are well documented in both cell culture and animal models [Tsuji et al., 2006]. Clinically, BMPs are sometimes used to improve bone healing after spine fusions or to repair tibial non-union fractures [Govender et al., 2002]. Exogenous BMPs act via phosphorylation of BMP receptors, which activates the cell signaling pathway via intracellular SMADs to upregulate transcription of target genes and translation of proteins to induce osteoblastogenesis, and therefore increase new bone formation [Wu et al., 2016]. New bone formation in the localized region of a bone tunnel can therefore provide a critical mechanism for the anchoring of tendon grafts used to repair ruptured ligaments and restore joint stability.
Furthermore, RUNX2 is a key transcriptional regulator of the Wnt pathway, involved in osteoblast differentiation and bone formation [Dudakovic et al., 2015]. BMP/SMAD signaling interacts with RUNX2 for co-operative regulation of target genes involved in osteoblastic differentiation of mesenchymal stromal/stem cells (MSCs). The enhancer of zeste homolog 2 (EZH2) is an important epigenetic enzyme that regulates RUNX2 dependent osteoblast differentiation and a principal subunit of the polycomb repressive complex 2 (PRC2) [O’Carroll et al., 2001]. EZH2 catalyzes the trimethylation of histone 3 lysine 27 (H3K27), promoting heterochromatin formation, and silencing the RUNX2 gene. The therapeutic reagent GSK126, a selective inhibitor of EZH2 can unpack chromatin, and increase accessibility of RUNX2 to bone specific genes that stimulate bone-specific transcription, osteoblast differentiation and new bone formation [Dudakovic et al., 2016; Dudakovic et al., 2015; Gordon et al., 2015; Wu et al., 2014]. GSK126 may also suppress osteoclastogenesis, which can further increase new bone formation by silencing cell communication between osteoclasts and osteoblasts [Fang et al., 2016a]. In addition, increased osteoclast activity can contribute to circumferential bone loss in the bone tunnel [Fahey and Indelicato, 1994; Galatz et al., 2005], an undesirable outcome during the tendon graft-based restoration of human joint stability.
Our study aimed to assess the effect of locally administered bone anabolic drugs in a tendon-to-bone tunnel healing model of rat Achilles tendon[Dimmen et al., 2009; Hjorthaug et al., 2014]. This is a novel and clinically applicable approach in the use and evaluation of these bone anabolic factors. Primary measureable outcomes were biomechanical assessment, (i.e., force to failure, stiffness, elongation and energy absorption), quantitative evaluation of cross sectional bone tunnel area, bone mineral density and bone volume, as well as a semi-quantitative histological evaluation of the bone-to-soft tissue interface. We hypothesized that tendon-to-bone tunnel healing would be enhanced by applying bone anabolic growth factors. Specifically, we assessed whether local hydrogel-mediated delivery of BMP2 or GSK126 enhances tendon-to-bone tunnel healing. The main findings of our study were that BMP2 and GSK126 treatments do not have adverse effects, but may result in favorable biomechanical trends during ligament repair. Further, bone tunnel diameters were reduced and bone volumes increased in BMP2 treated animals at 4 weeks after surgery.
Materials and Methods
Animal Handling
All animal work performed during this study was approved by the Mayo Clinic Institutional Animal Care and Use Committee (IACUC #A1182, Mayo Clinic, Rochester, MN). Reduction, replacement and refinement strategies were considered to ensure animal welfare. Animals were maintained according to the guidelines provided by National Institute of Health. Female Wistar rats (n=45), mean weight 239 g (SD 10) were kept three animals per cage, in an accredited animal facility with controlled temperature, humidity and light/dark cycles. Animals were allowed free access to food and water throughout the study period. No restriction to ambulation was applied at any time. The animals were allocated by computerized randomization into three groups (GSK126-treated, BMP2 treated, and saline ‘placebo’), and study members were blinded to animal group assignment until final analyses were completed (Fig. 1). Anesthesia was induced using an intra-peritoneal (IP) administration of ketamine (10 mg/kg), acepromazine (0.3 mg/kg) and xylazine (2 mg/kg) and maintained during the surgical procedure by inhalation of isoflurane (1.0-2.5% and 100% oxygen). Buprenorphine Sustained Release (SR) (0.6 mg/kg) was administered post-operatively to manage pain, and antibiotics were not indicated.
Figure 1.
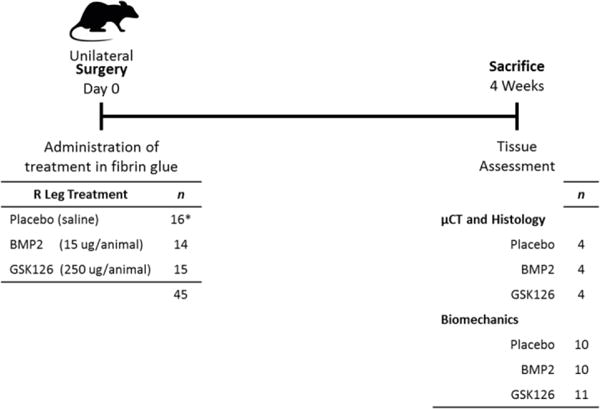
Experimental outline of animal study for tendon to bone tunnel healing. The rat model of ligament repair was designed to have intra-operative delivery of bone anabolic reagents (BMP2 or GSK126) within fibrin glue at Day 0 (45 animals), and animal killing 4 weeks later. Tissues were preserved for analysis by μCT, histology and biomechanical properties (force to failure, stiffness, elongation, and energy absorption). (* 2 animals died during surgery due to anesthesia complications).
2.2 Surgical Procedure
The surgical procedure was done as previously described[Hjorthaug et al., 2014]. Following sterile preparation, a 10 mm longitudinal skin incision was made on the medial side of the right Achilles tendon. The tendon was released proximally at the tendon calf muscle transition zone, while the distal calcaneal insertion was left intact. A holding suture was applied to the proximal end of the Achilles tendon in a Kessler suture fashion using a monofilament non-resorbable suture (Prolene 5-0, Ethicon, Somerville, New Jersey, USA) (Fig. 2a). A drill hole was made in the distal tibia in a dorsoventral direction 3 mm proximal to the ankle joint using a 1.0 mm drill bit followed by drilling with a 1.5 mm drill bit (Fig. 2b). The bone tunnel was irrigated with saline water, before a suture passer was used to guide the sutures through the bone tunnel in a dorsoventral direction and securely sutured to the ventral soft tissue while keeping the ankle joint in a 90° flexed position (Fig 2c).
Figure 2.
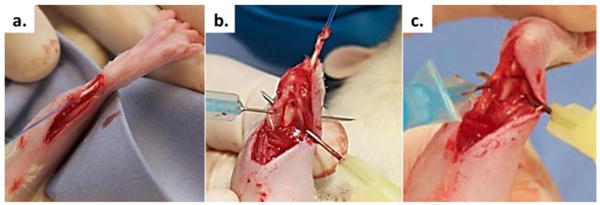
The surgical procedure involved (a) Achilles tendon excision proximally and addition of a holding suture, (b) distal tibia exposure for bone tunnel drilling (dorsal view), and (c) Achilles tendon passage through the bone tunnel.
Selected growth factors were administered locally to the dorsal opening of the bone tunnel, adding 45 μl of fibrin glue containing either drug (BMP2, GSK126) or placebo. Fibrin was prepared as previously described [Samsonraj et al., 2017a]. Clotting of the fibrin glue was confirmed before the skin incision was closed using resorbable sutures (Ethicon, Somerville, New Jersey, USA). Animals were closely observed until awake, and no restrictions to ambulation were applied at any time. All animals were euthanized at 4 weeks by carbon dioxide asphyxiation.
The animal model used for this study allowed assessment of tendon-to-bone tunnel healing at a single insertion site in metaphyseal bone as the distal insertion of the Achilles tendon to the calcaneal bone is preserved. In addition, free load bearing mimics active rehabilitation, which is important to prevent secondary joint stiffness due to immobilization in a clinical setting. GSK126 (BioVision, Inc., Milpitas, CA) and BMP2 (BioVision, Inc., Milpitas, CA) reagents were dissolved in phosphate buffered saline (PBS) to concentrations of 50 μg/μl and 3 μg/μl, respectively. Based on previous studies [Whelan et al., 2014; Yang et al., 2010; Yang et al., 2012], this drug delivery strategy ensures sustained local administration because 5 μl of this solution was mixed and encapsulated into 45 μl of Tisseel fibrin glue (Baxter Healthcare Corp., Deerfield, IL) immediately prior to delivery, which allows in situ clotting. Dosage based on previous experiments in our lab and search of the literature[Samsonraj et al., 2017b; Yasko et al., 1992]
2.3 Biomechanical Assessment
A custom-made electromechanical testing machine was used for assessments of biomechanical properties. Specimens were secured in custom-made clamps, designed to avoid applying pressure to the insertion site on either the tibia or Achilles tendon. Using a 10 lb. ultra-precision mini load cell (Transducer Techniques LLC, CA, Temecula, USA), the displacement rate was set to 0.1 mm/sec and data were collected via linear potentiometer at 100Hz with customized LabView program. This sampling strategy allowed collection of force, time and displacement data for final analyses in MatLab software (MathWorks Inc., Natick, MA, USA).
2.4 μCT Analysis
Skin- and muscle-tissues were removed from all specimens following ex-articulation of legs at the knee joint. The forefoot was resected though the proximal aspect of metatarsal bones. Specimens were preserved in 10% neutral buffered formalin (NBF) until scanning. Bone mineral density (BMD) and cross-sectional area (mm2) of the bone tunnels were measured using microcomputed tomography (μCT). An Inveon CT/PET module scanner (Siemens Medical Solutions, INC., Knoxville, Texas) was used, at pixel size 26.93, energy setting of 60 kV and projection time of 1000 ms. Final analyses of the images were conducted by an experienced biomedical imaging analyst using the software Analyze (AnalyzeDirect Inc., Overland Park, KS, USA). One phantom specimen containing samples with known calcium concentrations was scanned to determine the CT number (density per voxel) for known calcium levels. BMD and bone volume was measured by aligning a cylinder with diameter equal to the drill bit used for the surgery (1.5 mm) along the longitudinal axis of the bone tunnel (Fig 4a). Further, two concentric circles were placed around the cylinder such that each would have approximately the same area, and allowing measurement of Mean CT number for each volume and bone range. To determine the relationship between the measured CT numbers and known CT numbers, we used linear regression to determine ƩCT and βCT. BMD estimates were then calculated using the following relationship: CT number = BMD × ƩCT + βCT.
Figure 4.
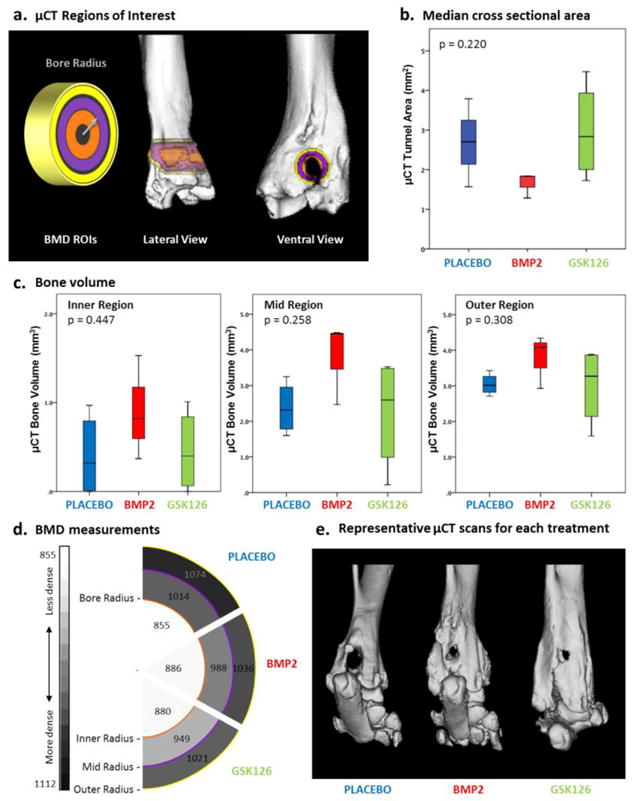
μCT assessment of rat tendon-to-bone tunnel healing. (a) Schematic showing the areas (rings) used to assess bone mineral density (BMD) measurements. The (orange) inner circle has a radius equal to the radius of the bore. Lateral and ventral views of the limb are presented with the measurement areas imposed on top. (b) Box and whisker plot of the median μCT bone tunnel area measurements vs treatment. BMP2 presents the smallest bone tunnel area. (c) Bone volume quantification demonstrates a trend of increased median bone volume values, particularly in BMP2 treated animals. (d) Schematic of the bone mineral density measurements for each treatment group and ring radius, with increasing bone density represented by the darkest intensities. No significant differences in BMD were observed for treatment groups. (e) Representative μ-CT 3D reconstructions, supporting the trend of reduced cross sectional area and increased bone volume in the bone tunnel of BMP2 and GSK126 treated animals.
2.5 Histology
Following μCT scanning, the specimens were used for histological evaluation. Tissues were fixed in 10% NBF, and decalcified using RDO- Rapid Decalcifier Solution (Apex Engineering Products Corporation, Aurora, Illinois) until sufficient decalcification was verified by needle testing. Subsequently, tissues were embedded in paraffin and sectioned along the coronal plane to get a cross-sectional view of the tendon inside the bone tunnel. Slides were stained with H&E (Sigma-Aldrich, St Louis, Missouri) before histological evaluation by a certified veterinary pathologist as well as two additional independent reviewers. Assessments aimed to evaluate bone formation and maturity in the bone tunnel, as well as graft integration on the bone tunnel. More specifically, each sample was assessed for degree of bone tunnel filling (new bone formation) on a scale from 0 to 2, where a score of 2 indicated complete integration of the graft in the bone tunnel, a score of 1 indicated partial integration, and a score of 0 indicated poor attachment of tendon graft in the bone tunnel.
2.6 Statistics
The primary endpoint of biomechanical analysis was pullout strength. Secondary endpoints were stiffness, energy absorption, elongation, bone tunnel cross sectional area, and BMD and bone volume as compared among the groups. Histograms and normal q-q-plots did not demonstrate normal distribution. Hence, results are presented as median and inter quartile range (IQR) distributions. Comparison analysis was done using Kruskal-Wallis tests. The alpha level was set to 0.05 and all statistical analyses were done using IBM SPSS Statistics for Macintosh v. 23.0 (IBM Inc., Chicago, Illinois, USA).
3.0 Results
Successful surgical ligament reconstruction depends on the biomechanical properties of implanted tissue and its osseointegration at origin and insertion sites. Primary reasons for failed ligament reconstruction are graft loosening and incomplete fixation of graft-to-bone. We used rats as a model to assess whether bone-anabolic reagents (BMP2 and GSK126; [Fang et al., 2016b; Tsuji et al., 2006]) would improve the fixation of tendon grafts to bone.
3.1 Animals
Two rats died peri-operatively, most likely due to an anesthesia-related complication. Additionally, two surgical reconstructions failed, which left 41 animals in our final analysis. No post-operative infections or other adverse events were noted. All rats were closely observed throughout the study period to prevent complications and ensure animal welfare, although no additional injections of Buprenorphine were indicated. Overall, animal weights increased significantly (p=0.001) during the study period (final weights = 283.5 ± 17.32 g), and differences in body weight between the study groups were not significant (p=0.931). The absence of treatment related adverse events indicates that the drug regimen does not cause harmful effects.
3.2 Biomechanical Data
Biomechanical assessments were conducted on 31 specimens: BMP2 (n=10), GSK126 (n=11) and placebo (n=10). All tested reconstructions failed at the tendon-bone interface (Fig. 3a). Overall, the median (IQR) force to failure was 10.1N (±8.46). The median (IQR) force to failure was 10.2 (±7.3) N and 13.4 (±5.7) N for BMP2 and GSK126 specimens respectively, compared to 7.4 (±2.2) N for the placebo group (p=0.691) (Fig. 3b, c). Stiffness measurements resulted in median(IQR) values of 6.32 N/mm (±5.53) for placebo, 4.58 N/mm (±3.83) for BMP2, and 6.32 N/mm (± 3.53) for GSK126-treated animals (p=0.404) (Fig. 3b, d). Both BMP2 and GSK126 treated animals had increased median elongation values by 61% and 72% respectively, compared to placebo (p=0.054) (Fig. 3b, e). Energy absorption was higher in BMP2 and GSK126 treated animals, 43% and 67% respectively, however not significant (p=0.116) (Fig. 3).
Figure 3.
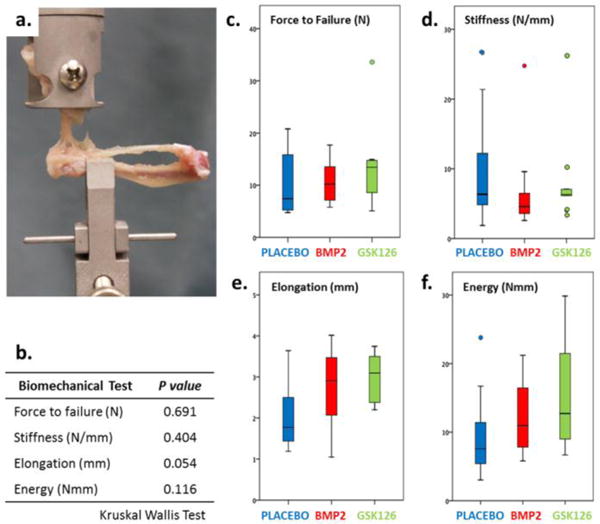
Biomechanical data for tendon-to-bone healing in rats were collected on three groups of animals: placebo (PBS, n=10); GSK126 (n=11); BMP2 (n=10). Measurements were made using (a) custom-made electromechanical testing machine with custom-made clamps, and a 250 g Model 31 load cell, to assess (b) p-values derived using the Kruskal-Wallis test on (c) force to failure, (d) stiffness, (e) elongation, and (f) energy adsorption.
3.3 μCT Imagery
μCT imaging was used to examine bone tunnel area (Fig. 4a,b), bone volume (Fig 4c) and bone mineral density near the bone tunnel (Fig. 4d). Median (IQR) cross sectional area of the bone tunnel was 1.7 (±0.69) mm2 for BMP2, 2.95 (±2.76) mm2 for GSK126 and 2.67 (±1.95) mm2 for the placebo group (Fig. 4b) (p=0.21). Bone volume measurement did not show statistical differences between the groups, however median bone volume was higher in BMP2 and GSK126 in the inner and mid regions compared to placebo (Fig 4c). BMD measurement did not reveal statistically significant differences in comparisons among predefined zones, inner (p=0.957), mid (p=0.492) and outer (p=0.741) (Fig. 4d). Representative μ-CT 3D reformations confirmed trend of reduced cross sectional areal and increased bone volume in the bone tunnel in BMP2 and partly GSK126 treated animals (Fig 4e).
3.4 Histology Outcomes
One score of 0 was noted in the placebo group, and may be related to graft decay/failure to integrate. Additionally, our analysis did not reveal clear differences in graft osseointegration, or new bone formation or maturity, or vascularization among the sample groups (Fig. 5). In general, tissues from the treated animal groups were not histologically dissimilar than the placebo group, supporting the safety of our strategy. Collagen fibers crossing the tendon-bone interface were not observed in any samples or groups. This result indicates indicating an immature fixation of the tendon graft in the bone tunnel that highly depends on new bone formation to anchor the tendon graft in the bone tunnel at this early time point.
Figure 5.
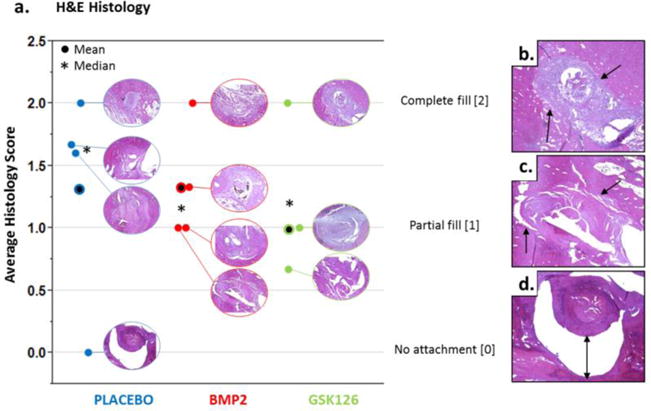
Histological Assessment. (a) The bone tunnels for all histology samples are shown in the graph with the average and median histology scores (n=3 blinded readers) presented. Representative images of (b) complete fill, score of 2; (c) partial fill, score of 1; (d) no attachment, score of 0. Notably, only the placebo group was observed to have a sample with a score of 0 (no attachment).
4.0 Discussion
Because BMP2 and GSK126 are known to increase osteoblast differentiation and new bone formation, we attempted to improve the healing of tendon-to-bone tunnel in a rat model of ligament repair. Although biomechanical analyses yielded no statistical difference between groups for force to failure or stiffness, energy absorption and elongation measurements were marginally significant among the groups. μCT analyses of bone tunnels revealed smaller cross-sectional areas for the BMP2 group (p<0.001), which suggests that BMP2 may improve the short-term integration of tendon-to-bone in a simulated ligament repair model. Tendon-to-bone tunnel healing is a slow process, especially during the early post-operative phase. Thus, early range of motion exercises to prevent joint stiffness limit the initial surgical fixation of the reconstruction. An enhancement of the initial healing phase may therefore improve the early mobilization of the reconstruction, potentially promoting normalization of joint stability and earlier return to daily activities (including sports) for patients.
Using a canine model similar to the rat model we used in this study, Rodeo et al. [Rodeo et al., 1999] demonstrated that BMP can accelerate healing of tendon-to-bone tunnel. In particular, they found higher loads to failure in BMP2 treated animals after only 2 weeks. Histologic and radiographic presentation of more excessive bone formation in these animals confirmed their interpretations. Also using a canine model, Thomopoulos et al. [Thomopoulos et al., 2012] found that BMP did not improve the biomechanical properties of tendon-to-bone tunnel compared to placebo specimens, and concluded that 3 weeks is insufficient for the bone anabolic effect of BMP to overcome post-surgical bone loss. Collagen sponge delivery, anatomic location, and dosing of bone anabolic reagents can have dramatic effects on the outcome of studies designed to recapitulate endogenous tendon osseointegration. For example, gene transfection by adenovirus-infected autografts was shown to enhance tendon graft integration in a bone tunnel. Up-regulation of BMP2 by gene transfection in semitendinosus autografts improved the biomechanical properties and increased bone formation in a rabbit ACL [Martinek et al., 2002]. These studies demonstrate the potential benefit of locally upregulating the BMP2 signaling pathway in tendon-to-bone tunnel, and highlight the need for further research to optimize delivery vehicles that ensure a localized and sustained delivery of growth factor to promote bone ingrowth and minimize adverse events.
Most ACL models involve the complete resection of the tendon including all native vascular elements. The model used for this study maintains critical vascularization to the tendon and leaves the other interface for experimental assessments of the effects of treatments that affect the tendon-bone interface. Our in vivo model improves data reliability because tendon grafts remain alive throughout the healing process by specifically leaving the native tendon intact at the calcaneal bone insertion site. Furthermore, other modifications of our model, including use of custom-made clamps that enable solid fixation of specimens in the testing device, prevent application of excess forces on the tendon –bone interface that is being evaluated for different treatment modalities. The utility of this model is reflected by the previous demonstration that non-steroidal anti-inflammatory drugs have negative effects on tendon-to-bone healing [Dimmen et al., 2009; Hjorthaug et al., 2014].
Biomechanical analysis of the experimentally manipulated tendons indicated a broad range of values for graft rupture in force to failure experiments that prevent our results from reaching statistical significance. Although the median force to failure was 38% higher in BMP2 specimens and 81% higher in GSK126 specimens compared to placebo specimens, these comparisons were not statistically significant. This broad statistical variation of our biomechanical data may be attributable to soft tissue callus formation at the dorsal aspect of the insertion site of the Achilles tendon in the tibia. The latter was not entirely removed, as it is considered a part of the overall tendon-bone reconstruction. Furthermore, the study presented here only evaluated a single dose and administration time for the two bone anabolic factors we investigated. Time and dosage of drug delivery can both have profound effects on healing and integration of the tendon graft in bone tunnel. The used dosage and timing has previously demonstrated positive effect of the evaluated growth factors in in vivo critical bone defect experiments. We analyzed early stage healing, yet this phase can be influenced by inflammation-driven endochondral bone formation. The first healing phase is also characterized by increased osteoclast activity with bone resorption at the perimeter of the bone tunnel. Our μCT data (median cross sectional area of the bone tunnel), indicated that all groups had increased cross sectional areas compared to the size of the largest drill bit (1.5 mm). Taken together, there are a number of factors that cause experimental variation and it is encouraging that our studies reveal interesting numerical trends that warrant follow-up studies on the use of bone anabolic reagents to enhance tendon integration in bone tunnels.
The bioactivity of a growth factor is sometimes limited by short half-life and loss through diffusion. However, as shown by Yang et al. [Yang et al., 2010; Yang et al., 2012], sustained delivery with preserved bioactivity is achievable by encapsulating a growth factor into conjugated fibrin. Drug doses used in our study were identified based on literature and other experiments performed by members of our research group. To prevent any bias of inter-gender difference in drug metabolism, only female animals were used for the experiment. Exogenously delivered BMP and other bone anabolic factors can promote heterotopic ossification, although we did not observe heterotopic ossification in any of our samples, as evaluated by gross anatomic dissection, histology or μCT. No other adverse effects were observed, including seroma, skin healing issues or osteolysis. Thus, we consider fibrin-glue-mediated local administration delivery of bone anabolic factors to be safe and potentially effective.
Mechanically induced signaling is important for bone remodeling and new bone formation, which increases strength by adding collagen fibers that span the soft tissue interface and increase graft integration. The model used for this experiment allows free weight bearing to apply mechanical loading that mimics clinical rehabilitation. Reorganization of microstructural collagen fibers crossing the bone-soft tissue interface were not observed; possibly related to the early timing of our biomechanical evaluation. Additionally, we did not find any differences in BMD for any group comparison, which is consistent with upregulated osteoclast activity and/or increased bone resorption during the initial healing phase measured in this study. As a future strategy, a bone resorption inhibitor could be used to limit the early (healing phase) resorption to accentuate the effects of BMP/GSK126 and promote rapid bone formation. However, co-delivery of multiple reagents can be complicated by dynamic release kinetics and time-dependent interactions among reagents that increase the favorability of single-reagent delivery methods.
EZH2 is a crucial contributor to skeletal development and early bone formation. The inhibitory properties of GSK126 on EZH2 promote commitment of precursor cells into differentiation in the osteoblastic lineage, and thus increase bone formation. This study elucidates the novel approach of using GSK126 to enhance tendon-to-bone tunnel healing. Additional biomaterials may permit sustained release of growth factors and mitogens (e.g., BMP2). Of particular interest are bivalent biopolymer tapes that contact the tendon grafts via a TGFβ1 side and the bone tunnel via a BMP2 side as a strategy for facilitating osseointegration of the graft. Further testing of other bone-anabolic reagents (e.g., PTH) will also have significant translational potential in promoting tendon-to-bone healing because many such compounds are already in clinical use for other indications.
In conclusion, our study presents promising results of fibrin-glue-mediated delivery of bone anabolic drugs, both BMP2 and a novel application of GSK126, in a clinically relevant and validated rodent tendon-to-bone tunnel healing model, with trends of smaller cross sectional areas and increased bone volume in the inner two regions for BMP2 compared to placebo. Further work aimed at characterizing the molecular and cellular differences between tendon and ligament will permit targeted strategies for biologically enhancing tendon tissue in a manner that permits adoption of the mechanical strength characteristic of ligament tissue, and facilitate osseointegration of the tendon graft. Such strategies, including the results presented herein, have potential to improve both short- and long-term clinical outcomes of tendon-based ligament repair by increasing graft fixation and retention.
Acknowledgments
We thank Phillip Edwards for assistance with μCT image analysis and interpretation. Jenny Pattengill and Ronald Marler facilitated histological assessment. In addition, members of the van Wijnen laboratory contributed valuable expertise to our animal experiments.
Funding
This work was supported in part by The Research Council of Norway 239871/F20 (ES) and National Institutes of Health, R01 AR049069 (AVW), F32 AR068154 (EAL), F32 AR066508 (AD), as well as a Career Development Grant from the Robert and Arlene Kogod Center on Aging, Mayo Clinic (RMS). This work was also supported by the Mayo Clinic Materials and Structural Testing Research Core.
References
- Apostolakos J, Durant TJS, Dwyer CR, Russell RP, Weinreb JH, Alaee F, Beitzel K, McCarthy MB, Cote MP, Mazzocca AD. The enthesis: a review of the tendon-to-bone insertion. Muscles Ligaments Tendons J. 2014;4:333–42. [PMC free article] [PubMed] [Google Scholar]
- Chen G, Deng C, Li YP. TGF-beta and BMP signaling in osteoblast differentiation and bone formation. Int J Biol Sci. 2012;8:272–88. doi: 10.7150/ijbs.2929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chim H, Moran SL. Wrist essentials: the diagnosis and management of scapholunate ligament injuries. Plast Reconstr Surg. 2014;134:312e–322e. doi: 10.1097/PRS.0000000000000423. [DOI] [PubMed] [Google Scholar]
- Dimmen S, Nordsletten L, Engebretsen L, Steen H, Madsen JE. The effect of parecoxib and indometacin on tendon-to-bone healing in a bone tunnel: an experimental study in rats. J Bone Joint Surg Br. 2009;91:259–63. doi: 10.1302/0301-620X.91B2.21471. [DOI] [PubMed] [Google Scholar]
- Dudakovic A, Camilleri ET, Riester SM, Paradise CR, Gluscevic M, O’Toole TM, Thaler R, Evans JM, Yan H, Subramaniam M, Hawse JR, Stein GS, Montecino MA, McGee-Lawrence ME, Westendorf JJ, van Wijnen AJ. Enhancer of Zeste Homolog 2 Inhibition Stimulates Bone Formation and Mitigates Bone Loss Caused by Ovariectomy in Skeletally Mature Mice. The Journal of biological chemistry. 2016;291:24594–24606. doi: 10.1074/jbc.M116.740571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudakovic A, Camilleri ET, Xu F, Riester SM, McGee-Lawrence ME, Bradley EW, Paradise CR, Lewallen EA, Thaler R, Deyle DR. Epigenetic control of skeletal development by the histone methyltransferase Ezh2. Journal of Biological Chemistry. 2015;290:27604–27617. doi: 10.1074/jbc.M115.672345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekdahl M, Wang JH, Ronga M, Fu FH. Graft healing in anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2008;16:935–47. doi: 10.1007/s00167-008-0584-0. [DOI] [PubMed] [Google Scholar]
- Fahey M, Indelicato PA. Bone tunnel enlargement after anterior cruciate ligament replacement. Am J Sports Med. 1994;22:410–4. doi: 10.1177/036354659402200318. [DOI] [PubMed] [Google Scholar]
- Fang C, Qiao Y, Mun SH, Lee MJ, Murata K, Bae S, Zhao B, Park-Min KH, Ivashkiv LB. Cutting Edge: EZH2 Promotes Osteoclastogenesis by Epigenetic Silencing of the Negative Regulator IRF8. J Immunol. 2016a;196:4452–6. doi: 10.4049/jimmunol.1501466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang D, Gan H, Lee JH, Han J, Wang Z, Riester SM, Jin L, Chen J, Zhou H, Wang J, Zhang H, Yang N, Bradley EW, Ho TH, Rubin BP, Bridge JA, Thibodeau SN, Ordog T, Chen Y, van Wijnen AJ, Oliveira AM, Xu RM, Westendorf JJ, Zhang Z. The histone H3.3K36M mutation reprograms the epigenome of chondroblastomas. Science. 2016b;352:1344–8. doi: 10.1126/science.aae0065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feagin JA, Jr, Curl WW. Isolated tear of the anterior cruciate ligament: 5-year follow-up study. Am J Sports Med. 1976;4:95–100. doi: 10.1177/036354657600400301. [DOI] [PubMed] [Google Scholar]
- Fu FH, Bennett CH, Lattermann C, Ma CB. Current trends in anterior cruciate ligament reconstruction. Part 1: Biology and biomechanics of reconstruction. Am J Sports Med. 1999;27:821–30. doi: 10.1177/03635465990270062501. [DOI] [PubMed] [Google Scholar]
- Galatz LM, Rothermich SY, Zaegel M, Silva MJ, Havlioglu N, Thomopoulos S. Delayed repair of tendon to bone injuries leads to decreased biomechanical properties and bone loss. J Orthop Res. 2005;23:1441–7. doi: 10.1016/j.orthres.2005.05.005.1100230629. [DOI] [PubMed] [Google Scholar]
- Gianotti SM, Marshall SW, Hume PA, Bunt L. Incidence of anterior cruciate ligament injury and other knee ligament injuries: a national population-based study. J Sci Med Sport. 2009;12:622–7. doi: 10.1016/j.jsams.2008.07.005. [DOI] [PubMed] [Google Scholar]
- Gordon JA, Stein JL, Westendorf JJ, van Wijnen AJ. Chromatin modifiers and histone modifications in bone formation, regeneration, and therapeutic intervention for bone-related disease. Bone. 2015;81:739–45. doi: 10.1016/j.bone.2015.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govender S, Csimma C, Genant HK, Valentin-Opran A, Amit Y, Arbel R, Aro H, Atar D, Bishay M, Borner MG, Chiron P, Choong P, Cinats J, Courtenay B, Feibel R, Geulette B, Gravel C, Haas N, Raschke M, Hammacher E, van der Velde D, Hardy P, Holt M, Josten C, Ketterl RL, Lindeque B, Lob G, Mathevon H, McCoy G, Marsh D, Miller R, Munting E, Oevre S, Nordsletten L, Patel A, Pohl A, Rennie W, Reynders P, Rommens PM, Rondia J, Rossouw WC, Daneel PJ, Ruff S, Ruter A, Santavirta S, Schildhauer TA, Gekle C, Schnettler R, Segal D, Seiler H, Snowdowne RB, Stapert J, Taglang G, Verdonk R, Vogels L, Weckbach A, Wentzensen A, Wisniewski T. Recombinant human bone morphogenetic protein-2 for treatment of open tibial fractures: a prospective, controlled, randomized study of four hundred and fifty patients. The Journal of bone and joint surgery. American. 2002:2123–34. doi: 10.2106/00004623-200212000-00001. [DOI] [PubMed] [Google Scholar]
- Granan LP, Forssblad M, Lind M, Engebretsen L. The Scandinavian ACL registries 2004-2007: baseline epidemiology. Acta Orthop. 2009;80:563–7. doi: 10.3109/17453670903350107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harryman DT, 2nd, Mack LA, Wang KY, Jackins SE, Richardson ML, Matsen FA., 3rd Repairs of the rotator cuff. Correlation of functional results with integrity of the cuff. J Bone Joint Surg Am. 1991;73:982–9. [PubMed] [Google Scholar]
- Hjorthaug GA, Madsen JE, Nordsletten L, Reinholt FP, Steen H, Dimmen S. Tendon to bone tunnel healing-A study on the time-dependent changes in biomechanics, bone remodeling, and histology in a rat model. J Orthop Res. 2014 doi: 10.1002/jor.22756. [DOI] [PubMed] [Google Scholar]
- Kiapour AM, Murray MM. Basic science of anterior cruciate ligament injury and repair. Bone Joint Res. 2014;3:20–31. doi: 10.1302/2046-3758.32.2000241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohmander LS, Ostenberg A, Englund M, Roos H. High prevalence of knee osteoarthritis, pain, and functional limitations in female soccer players twelve years after anterior cruciate ligament injury. Arthritis Rheum. 2004;50:3145–52. doi: 10.1002/art.20589. [DOI] [PubMed] [Google Scholar]
- Martinek V, Latterman C, Usas A, Abramowitch S, Woo SL, Fu FH, Huard J. Enhancement of tendon-bone integration of anterior cruciate ligament grafts with bone morphogenetic protein-2 gene transfer: a histological and biomechanical study. J Bone Joint Surg Am. 2002;84-a:1123–31. doi: 10.2106/00004623-200207000-00005. [DOI] [PubMed] [Google Scholar]
- O’Carroll D, Erhardt S, Pagani M, Barton SC, Surani MA, Jenuwein T. The polycomb-group gene Ezh2 is required for early mouse development. Mol Cell Biol. 2001;21:4330–6. doi: 10.1128/MCB.21.13.4330-4336.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman MS, Akhtar N, Jamil HM, Banik RS, Asaduzzaman SM. TGF-beta/BMP signaling and other molecular events: regulation of osteoblastogenesis and bone formation. Bone Res. 2015;3:15005. doi: 10.1038/boneres.2015.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodeo SA, Arnoczky SP, Torzilli PA, Hidaka C, Warren RF. Tendon-healing in a bone tunnel. A biomechanical and histological study in the dog. J Bone Joint Surg Am. 1993;75:1795–803. doi: 10.2106/00004623-199312000-00009. [DOI] [PubMed] [Google Scholar]
- Rodeo SA, Suzuki K, Deng XH, Wozney J, Warren RF. Use of recombinant human bone morphogenetic protein-2 to enhance tendon healing in a bone tunnel. Am J Sports Med. 1999;27:476–88. doi: 10.1177/03635465990270041201. [DOI] [PubMed] [Google Scholar]
- Samsonraj RM, Dudakovic A, Zan P, Pichurin O, Cool S, van Wijnen AJ. A versatile protocol for studying calvarial bone defect healing in a mouse model. Tissue engineering Part C, Methods. 2017a;24 doi: 10.1089/ten.tec.2017.0205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samsonraj RM, Dudakovic A, Zan P, Pichurin O, Cool SM, van Wijnen AJ. A Versatile Protocol for Studying Calvarial Bone Defect Healing in a Mouse Model. Tissue Eng Part C Methods. 2017b;23:686–693. doi: 10.1089/ten.tec.2017.0205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandberg R, Balkfors B, Nilsson B, Westlin N. Operative versus non-operative treatment of recent injuries to the ligaments of the knee. A prospective randomized study. J Bone Joint Surg Am. 1987;69:1120–6. [PubMed] [Google Scholar]
- Thomopoulos S, Kim HM, Silva MJ, Ntouvali E, Manning CN, Potter R, Seeherman H, Gelberman RH. Effect of bone morphogenetic protein 2 on tendon-to-bone healing in a canine flexor tendon model. J Orthop Res. 2012;30:1702–9. doi: 10.1002/jor.22151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuji K, Bandyopadhyay A, Harfe BD, Cox K, Kakar S, Gerstenfeld L, Einhorn T, Tabin CJ, Rosen V. BMP2 activity, although dispensable for bone formation, is required for the initiation of fracture healing. Nat Genet. 2006;38:1424–9. doi: 10.1038/ng1916. [DOI] [PubMed] [Google Scholar]
- Walsh JJ, Berger RA, Cooney WP. Current status of scapholunate interosseous ligament injuries. J Am Acad Orthop Surg. 2002;10:32–42. doi: 10.5435/00124635-200201000-00006. [DOI] [PubMed] [Google Scholar]
- Whelan D, Caplice NM, Clover AJ. Fibrin as a delivery system in wound healing tissue engineering applications. J Control Release. 2014;196:1–8. doi: 10.1016/j.jconrel.2014.09.023. [DOI] [PubMed] [Google Scholar]
- Wu H, Whitfield TW, Gordon JA, Dobson JR, Tai PW, van Wijnen AJ, Stein JL, Stein GS, Lian JB. Genomic occupancy of Runx2 with global expression profiling identifies a novel dimension to control of osteoblastogenesis. Genome Biol. 2014;15:2014–15. doi: 10.1186/gb-2014-15-3-r52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M, Chen G, Li YP. TGF-beta and BMP signaling in osteoblast, skeletal development, and bone formation, homeostasis and disease. Bone Res. 2016;4:16009. doi: 10.1038/boneres.2016.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang HS, La WG, Bhang SH, Jeon JY, Lee JH, Kim BS. Heparin-conjugated fibrin as an injectable system for sustained delivery of bone morphogenetic protein-2. Tissue Eng Part A. 2010;16:1225–33. doi: 10.1089/ten.TEA.2009.0390. [DOI] [PubMed] [Google Scholar]
- Yang HS, La WG, Cho YM, Shin W, Yeo GD, Kim BS. Comparison between heparin-conjugated fibrin and collagen sponge as bone morphogenetic protein-2 carriers for bone regeneration. Exp Mol Med. 2012;44:350–5. doi: 10.3858/emm.2012.44.5.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasko AW, Lane JM, Fellinger EJ, Rosen V, Wozney JM, Wang EA. The healing of segmental bone defects, induced by recombinant human bone morphogenetic protein (rhBMP-2). A radiographic, histological, and biomechanical study in rats. J Bone Joint Surg Am. 1992;74:659–70. [PubMed] [Google Scholar]


