Abstract
Allogeneic hematopoietic stem cell transplantation (HSCT) reverses the bone marrow failure syndrome due to GATA2 deficiency. The intensity of conditioning required to achieve reliable engraftment and prevent relapse remains unclear. Here, we describe the results of a prospective study of HSCT in 22 patients with GATA2 deficiency using a busulfan-based conditioning regimen. The study includes 2 matched related donor (MRD) recipients, 13 matched unrelated donor (URD) recipients, and 7 haploidentical related donor (HRD) recipients. MRD/URD recipients received 4 days of busulfan and 4 days of fludarabine. HRD recipients received low-dose cyclophosphamide for two days, fludarabine for 5 days, 2–3 days of busulfan depending upon cytogenetics, and 200 cGy total body irradiation (TBI). MRD/URD recipients received tacrolimus/short course methotrexate for graft-versus-host disease (GVHD) prophylaxis. HRD recipients received high-dose post-transplant cyclophosphamide (PT/Cy) followed by tacrolimus and mycophenolate mofetil. At median follow-up of 24 months (range, 9–50), 19/22 patients are alive with reversal of the disease phenotype and correction of the MDS, including eradication of cytogenetic abnormalities. Three patients died: one from refractory acute myelogenous leukemia (AML), one from GVHD, and one from sepsis. There was a 26% incidence of grade III–IV acute GVHD in the MRD/URD groups, and no grade III–IV aGVHD in the HRD cohort. Similarly, there was a 46% incidence of chronic GVHD in the MRD/URD cohort, whereas only 28% of HRD recipients developed cGVHD. Despite excellent overall disease-free survival (86%), GVHD remains a limitation using standard prophylaxis for GVHD. We are currently extending the use of PT/Cy to the MRD/URD cohorts to reduce GVHD.
Keywords: GATA2, HSCT, hematopoietic stem cell transplant, MDS, myelodysplastic syndrome, GVHD, graft-versus host disease
Introduction
In 2011, four groups described a new human disease syndrome, now termed GATA2 deficiency, resulting from heterozygous germline or sporadic mutations in the transcription factor GATA2. Each group approached this syndrome from a distinct clinical perspective, resulting in four different names for processes caused by the same genetic abnormality: autosomal dominant and sporadic monocytopenia and Mycobacterium avium complex (MonoMAC); Dendritic Cell, Monocyte, B and NK Lymphoid (DCML) deficiency; Emberger syndrome (lymphedema and monosomy 7); and familial myelodysplastic syndrome (MDS/acute myelogenous leukemia [AML]).1–6 These phenotypic and hematologic characteristics, now with the addition of mutations in one allele of GATA2 (consistent with haploinsufficiency), constitute a syndrome for which allogeneic hematopoietic stem cell transplantation (HSCT) represents the only curative therapy. However, HSCT remains challenging because of co-morbidities such as infections, pulmonary alveolar proteinosis (PAP), and advanced myelodysplasia including transformation to AML and chronic myelomonocytic leukemia (CMML).7,8
Here, we report the results of a prospective clinical trial of allogeneic HSCT in 22 patients with GATA2 deficiency using a dose-adjusted, busulfan-based conditioning regimen for matched related donors (MRD), unrelated donors (URD), and haploidentical related donor (HRD) recipients. We report an 86% disease-free survival in 22 patients with GATA2 deficiency or MonoMAC treated with this regimen.
Patients and Methods
Data Collection
This study was designed to determine the efficacy and safety of a busulfan-based allogeneic HSCT regimen for patients with GATA2 deficiency or the MonoMAC syndrome, and was approved by the Institutional Review Board of the National Cancer Institute. This study was independently monitored for safety and data accuracy (Clinical Trials. gov number, NCT 009233364).
The following pre-transplant data were collected: age at transplant, duration of illness (years), types of infection, other manifestations of disease, cytogenetics, bone marrow biopsy, GATA2 mutation, and family history suggestive of GATA2 deficiency or MonoMAC syndrome. Pulmonary findings before and after transplant were also analyzed. The transplant data collected consisted of: donor source with either peripheral blood stem cells (PBSC) or bone marrow (BM), HLA match, daily busulfan area under the concentration versus time curve (AUC), targeted at 3600–4800 μMol*min based on a test dose (0.8 mg/kg body weight) of busulfan, total busulfan dose/kg weight of recipient, CD34+ and CD3+ donor cells infused per kg recipient weight, myeloid and CD3+ cell chimerism at day 100, time after transplant in months, GVHD prophylaxis, incidence of acute and chronic GVHD (aGVHD and cGVHD), and current status including the presence or absence of continued immunosuppression.
Patient Characteristics
Eligibility criteria for patients were: age between 8 and 60 years, at least one episode of a life-threatening opportunistic infection and pathologic mutation in the GATA2 gene. In the absence of an identified GATA2 mutation, eligibility consisted of at least one episode of a life-threatening infection and a flow cytometry profile on peripheral blood demonstrating severe monocytopenia and CD19+ B-cell and CD3−/CD56+ NK cell lymphopenia consistent with the MonoMAC profile. If MDS was present, blasts in the bone marrow had to be less than 5% in the absence of granulocyte colony stimulating factor (GCSF).
All recipients needed to have a 10/10 or 9/10 MRD, URD or haploidentical donor. All MRD and URD pairs were matched at 10/10 human leukocyte antigen HLA-A, -B, -C, -DRB1, and -DQB1 loci by high resolution typing Haploidentical donors had HLA compatibility representing a minimum match of 5/10 loci. If more than one haploidentical donor was available, each donor was evaluated for overall health, age, ABO match, and cytomegalovirus (CMV) serostatus to select the best donor. Also, for patients with an EBV-associated tumor, we prefer a donor who is EBV seropositive.
Matched related and haploidentical related donors were excluded if they had a mutation in GATA2, a history of mycobacterial or other opportunistic infections, or abnormal monocyte, NK or B cell counts.
Donor type determined the conditioning regimen. A test dose of busulfan (0.8 mg/kg) approximately one week before conditioning established the conditioning dose, which targeted an AUC between 3600 and 4800 μMol*min. Conditioning similar to that described by de Lima et al. was used for MRD and URD recipients, who received IV fludarabine 40 mg/m2/day given over 30 min (days −6 to −3) followed by busulfan intravenously (IV) once daily administered over 3 hours for 4 days (days −6 to −3).19 HRD recipients received cyclophosphamide 14.5 mg/kg IV for 2 days (days −6 to −5), IV fludarabine 30 mg/m2/day for 5 days (days −6 to −2) given over 30 min, followed by IV busulfan administered over three hours for 2 days (days −4, −3), or 3 days if clonal cytogenetic abnormalities were present (days −4, −3, and −2), and 200 cGy total body irradiation (TBI) on day −1.
Bone marrow was generally preferred as the cell product. For MRD and URD recipients receiving PBSC, donors underwent 5 days of 10 μg/kg/day G-CSF stimulation followed by apheresis to collect at least 5 x 106 CD34+ cells/kg recipient weight. The PBSC from MRDs and URDs were infused fresh on day 0. HRD recipients received fresh bone marrow cells on day 0, targeting 4 x 108 total nucleated cells (TNC)/kg recipient weight.
Graft-versus-host disease (GVHD) prophylaxis in MRD and URD recipients started with tacrolimus from day −3 and methotrexate 5 mg/m2 IV on days +1, 3, 6 and 11. The dose of tacrolimus was titrated to achieve serum levels between 5 and 10 ng/ml. Immunosuppression was stopped at 6 months post-transplant in the absence of GVHD.
Post-transplant immunosuppression for HRD recipients consisted of cyclophosphamide (PT/Cy) 50 mg/kg/d IV for 2 days (+3 and +4), followed by tacrolimus and mycophenolate mofetil, 15 mg/kg IV/PO every 12 hours, starting on day +5–35. Immunosuppression with tacrolimus was stopped at 6 months post-transplant in the absence of GVHD.
Supportive Care
Standard guidelines for supportive care established at the National Institutes of Health Clinical Center for patients undergoing allogeneic HSCT were used. These guidelines agree with international guidelines for preventing infectious complications among hematopoietic cell transplantation recipients.9
The management of non-tuberculous mycobacterial (NTM) infections has been previously described.10 In brief, when possible, the infection was treated to minimize the burden of active infection at transplantation, but in cases in which active infection at transplant was suspected, treatment was continued through and after transplant with regimen modifications to minimize drug interactions.
For viral testing, Epstein-Barr virus (EBV), Cytomegalovirus (CMV), Human Herpes virus 6 (HHV-6), adenovirus and BK virus quantitation was performed on EDTA whole blood and/or urine. The tests were developed and performance characteristics determined by the Department of Laboratory Medicine, NIH and have not been cleared or approved by the US FDA. For EBV and CMV, units of copies/mL were calibrated to International Units (IU)/mL beginning October 2014 and for BK virus in August 2016.
Immune reconstitution of T, B, and NK Cells and monocytes, cytogenetic analysis, and analysis of chimerism were as previously described.10
Statistical Analysis
Descriptive statistics were used for chimerism, monocyte, NK cell and lymphocyte counts. Overall survival and event-free survival were described by Kaplan-Meier estimates.
Results
Demographics
The clinical characteristics of the study population are described in Table 1. There were 9 males and 13 females in the study. The median age was 26 years (range, 17 to 45) with a median duration of clinical illness of 11.2 years (range, 1 to 37). Twenty patients had confirmed GATA2 mutations; two had the MonoMAC phenotype. Seven of the 22 patients had at least one episode of NTM infection. Pre-transplant co-morbidities were frequent, including an EBV-associated smooth muscle tumor in one patient (previously reported).11 Three patients had sensorineural hearing loss, two had Emberger syndrome, and two patients had progressed to AML. Human papillomavirus (HPV) disease, due to both high- and low-risk types, was common: 12 patients had warts of the hands and feet. Cervical, vaginal and anal HPV disease was present at baseline in 7/13 women, including intraepithelial neoplasia stage 2 in 2, stage 3 in 4 and a previously resected squamous cell carcinoma in one. Ten of the 22 patients had a family history of GATA2 deficiency with an affected first-degree relative.
Table 1.
Characteristics of Patients with GATA2 Deficiency Prior to HSCT
| Donor | Pt | Age at HSCT (yrs)/ sex | Duration of Illness (yrs) | Type of Infection | Other | Cytogenetics | Bone Marow | Mutation | Family History | |
|---|---|---|---|---|---|---|---|---|---|---|
| NTM | Viral | |||||||||
| MRD | ||||||||||
| 1 | 24/M | 12 | Disseminated MAC | Skin/ genital HPV | EBV+ spindle cell tumor metastatic to liver, spine | Normal | MDS | R396W | + | |
| 2 | 29/F | 11 | No | HPV Genital, VZV | Pancytopenia | Trisomy 21 Trisomy 1q |
MDS | Intron 5 c. 1017+572 C>T |
+ | |
| URD | ||||||||||
| 1 | 28/F | 17 | No | HPV genital, HSV-2 | Anovulvar CIS | Trisomy 8 | MDS | MonoMAC | + | |
| 2 | 17/M | 4 | No | HPV warts | No | Monosomy 7 | MDS | p.373del5 | + | |
| 3 | 25/F | 7 | No | No | Behcet’s | Trisomy 8 Trisomy 8 + trisomy 20 |
AML Refractory | p.L375S | − | |
| 4 | 22/M | 5 | M. kansasii | HPV warts | No | Normal | MDS | R361H | + | |
| 5 | 18/M | 5 | No | HPV warts | Deafness | Normal | MDS | p.R362X | + | |
| 6 | 21/M | 1 | MAC | HPV warts | Interstitial nephritis | Monosomy 7 | MDS | p.R362X | − | |
| 7 | 25/M | 25 | No | No | Emberger Syndrome | 5q−, 13q+ | AML 1st CR | MonoMAC | − | |
| 8 | 33/M | 3 | No | HPV warts | Abscesses | Normal | MDS | p.R396Q | + | |
| 9 | 24/F | 11 | No | HPV genital | Deafness | Normal | MDS | p.R362X | + | |
| 10 | 28/F | 3 | M. kansasii | HPV genital | C. difficile colitis, Colostomy | Normal | MDS | p.R396W | − | |
| 11 | 38/F | 31 | M. kansasii | HPV genital | Hepatitis C Bell’s Palsy x 2 |
−X, trisomy 1q −1, trisomy 1q −13 |
MDS | p.R398W | + | |
| 12 | 18/F | 5 | No | HPV warts | Pulmonary artery hypertension, Deafness | Normal | MDS | c.1128C>A p.Y376X |
+ | |
| 13 | 30/M | 11 | No | Hand-Foot-Mouth viral | No | Monosomy 7 | MDS | Intron 5 c. 1017+572 C>T |
− | |
| Haplo | ||||||||||
| 1 | 45/F | 37 | None | HPV warts | Emberger syndrome | Normal | MDS | p.R337X | − | |
| 2 | 27/F twin | 18 | Disseminated MAC | HPV genital | Meningococcal bacteremia | Trisomy 8 | MDS | p.R330X | − | |
| 3 | 27/F twin | 18 | None | HPV warts/genital | Streptococcal pharyngitis | Trisomy 8 | MDS | p.R330X | − | |
| 4 | 26/M | 2 | None | HPV warts | West Nile encephalitis | Normal | MDS | p.A341Pfs | − | |
| 5 | 34/F | 5 | M. avium | HPV genital | Cryptococcal meningitis PE | Normal | MDS | c.803delG fs | − | |
| 6 | 16/F | 11 | M. avium | HSV labial | Entamoeba histolytica | Normal | MDS | p.R396W | − | |
| 7 | 17/F | 5 | None | CMV, parvovirus | Pneumonia, HLH | Trisomy 8 | MDS | p.R396Q | − | |
Abbreviations: AML, acute myelogenous leukemia; CIS, carcinoma in situ; CMV, Cytomegalovirus; CR, complete remission; EBV, Epstein-Barr Virus; F, female; fs, frame-shift; GI, gastrointestinal;
Haplo, haploidentical related donor; H, histidine; HLH, hemophagocytic lymphohistiocytosis; HPV, human papilloma virus; MAI, Mycobacterium avium intracellulare; M, male;
MDS, myelodysplastic syndrome; MRD, matched related donor; URD, matched unrelated donor; MDS, myelodysplastic syndrome; NTM, Non-tuberculous Mycobacteria infection;
PE, pulmonary embolism; Q, Glutamine; R, arginine; W, tryptophan; X, stop codon;
Hematologic Status
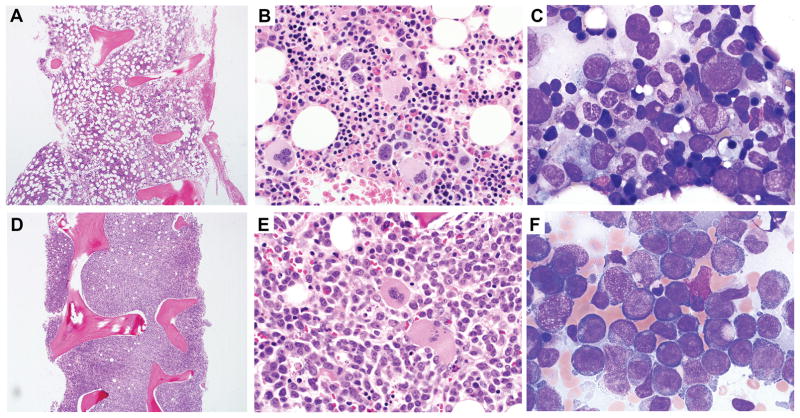
All patients were leukopenic with a median absolute neutrophil count 1.19 K/μl, interquartile range (IQR .84–2.18), absolute lymphocyte count 0.76 K/μl (IQR 0.43–1.16), CD19+ cells 10/μl (IQR 1.75–21.5), monocytes 10 K/μl, (IQR 0–22.5), NK cells 7.5/μl (IQR 0.75–39.8), and were anemic with median hemoglobin 10 g/dl (IQR 8.5–11.6). Bone marrow examination was consistent with MDS in all 22 patients (median cellularity 30%, IQR 18.8–40%). In two patients MDS had transformed to AML. One AML patient was in complete remission at transplant following induction chemotherapy, while the second (URD patient 3) had residual disease at transplant, with a ten-year history of MDS with trisomy 8 prior to the diagnosis of GATA2 deficiency. She transformed from mild MDS to AML M5a with 85% blasts along with new cytogenetic abnormalities over a 4-month interval (Figure 1). Clonal cytogenetic abnormalities were present in 11/22 recipients at the time of transplant (Table 1), with trisomy 8 in 5, monosomy 7 in 3, trisomy 1q, an unfavorable marker, in 2, and one patient (URD 7) with AML and 5q−/13q+. 12
Figure 1.
Rapid evolution from MDS to AML in patient with germline GATA2 mutation. A–C. Baseline bone marrow from a 24-year-old female at first visit to NIH bone marrow failure clinic. Core bone marrow biopsy showing hypocellular bone marrow for age (A). Bone marrow aspirate smear showed atypical large megakaryocytes with separated nuclear lobes (B), mildly atypical myeloid maturation with a subpopulation of hypogranular and hyposegmented forms (C), and no increase in blasts. Cytogenetic analysis revealed trisomy 8. D–E. Bone marrow 4 months later. Core bone marrow biopsy shows a markedly hypercellular marrow (D). Sheets of leukemic blasts are present on the bone marrow aspirate (E and F). By flow cytometric analysis the blasts were of immature monocytic lineage expressing CD33 (bright), CD64, CD36, CD56 (bright), HLA-DR, CD123, CD45, CD11b (partial), CD4 (dim), and CD38; and were negative for myeloperoxidase, CD34, CD13, CD117, CD19, CD3, CD2, CD7 and CD10; and largely negative for CD14. Cytogenetic analysis revealed trisomy 8 and acquisition of trisomy 20.
Pulmonary abnormalities and status
Pulmonary evaluation consisted of chest CT and pulmonary function tests (PFTs). Brochoalveolar lavage (BAL) was performed if indicated prior to transplant. Radiographic abnormalities consisted of ground-glass opacities in 10 cases, tree-in-bud findings in 2, and nodular and/or cystic disease in 9, and pulmonary parenchymal mosaicism and effusions (Supplementary Table 1). The most common PFT abnormality was a decreased diffusion capacity (median DLadj 59% reference, IQR 49–84%), judged to be mild in 6, moderate in 11.
Characteristics of Allogeneic Hematopoietic Stem Cell Transplant
For the 22 recipients, there were 2 matched related donors (MRD), 13 matched unrelated donors (URD) and 7 haploidentical related donors (HRD) (Table 2). Bone marrow was used for all HRD recipients and was increasingly used as the donor source for MRD/URD during the study. In total, 8 MRD/URD patients received PBSC, while 7 received bone marrow. The mean CD34+ cell dose for MRD/URD recipients was 5 x 106 cells/kg recipient body weight (range, 1.3 to 8.6 x 106 CD34+ cells/kg). Similarly, the mean CD3+ dose for MRD/URD recipients receiving PBSC was 2.4 x 108 CD3+cells/kg, whereas the mean CD3+ cell dose for MRD/URD receiving bone marrow was a log lower at 2.9 x 107 CD3+ cell/kg. The cell doses were comparable in the HRD bone marrow recipients with a mean of 5.1 x 106 CD34+ cells/kg and 4.3 x 107 CD3+ cells/kg.
Table 2.
HSCT for Patients with GATA2 Deficiency 14 jan
| Donor | Pt | Donor Source |
HLA Match |
BU AUC |
BU dose/d # of days |
CD34+/kg | CD3+/kg | Myeloid chimerism Day 100 |
CD3+ chimerism Day 100 |
Time after Transplant (months) |
GVHD prophylaxis |
aGVHD | cGVHD | Current Status |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MRD | ||||||||||||||
| 1 RM | PBSC | 10/10 | 4800 | 15.8 mg/kg 4 | 7.67 x 106/kg | 1.3 x 108/kg | 99 | 90 | 45 | Tacro/MTX | Grade 1 skin | None | Alive, off Immunosupp. | |
| 2 AV | BM | 10/10 | 4800 | 16 mg/kg 4 | 6.75 x 106/kg | 4.9 x 107/kg | 99 | 29 | 24 | Tacro/MTX | Grade 3 GI | Vaginal | Alive, off immunosupp. | |
| URD | ||||||||||||||
| 1 SS | PBSC | 10/10 | 4800 | 14.6 mg/kg 4 | 8 x 106/kg | 1.87 x 108/kg | 100 | 83 | 43 | Tacro/MTX | Grade 2 skin and GI | Moderate/severe cGVHD | Alive, on Immunosupp | |
| 2 AT | PBSC | 10/10 | 4800 | 15.8 mg/kg 4 | 5.0 x 106/kg | 4.79 x 107/kg | 100 | 100 | 24 | Tacro/MTX | Grade 4 3 system | Moderate/severe cGVHD | Died, GVHD, 24 mo. | |
| 3 EB | PBSC | 10/10 | 4800 | 13.6 mg/kg 4 | 4.45 x 106/kg | 2.13 x 108/kg | 42 | 98 | 6 | Tacro/MTX | None | None | Died, ref AML | |
| 4 JD | PBSC | 10/10 | 4200 | 11.6 mg/kg 4 | 6.39 x 106/kg | 1.45 x 108/kg | 100 | 89 | 21 | Tacro/MTX | None | Eye, mouth cGVHD | Alive, on Immunosupp | |
| 5 AK | BM | 10/10 | 4137 | 10.3 mg/kg 4 | 1.33 x 106/kg | 2.5 x 107/kg | 99 | 91 | 20 | Tacro/MTX | None | None | Alive, off Immunosupp | |
| 6 CS | BM | 10/10 | 4800 | 13.8 mg/kg 4 | 3.19 x 106/kg | 2.1 x 107/kg | 100 | 93 | 18 | Tacro/MTX | No | Moderate/severe cGVHD | Alive, on Immunosupp | |
| 7 MM | PBSC | 10/10 | 4600 | 12.8 mg/kg 4 | 6.02 x 106/kg | 1.79 x 108/kg | 100 | 100 | 16 | Tacro/MTX | Grade 2 colon | Skin | Alive, on Immunosupp | |
| 8 EG | BM | 10/10 | 4400 | 11.6 mg/kg 4 | 5.24 x 106/kg | 3.2 x 107/kg | 100 | 97 | 15 | Tacro/MTX | Grade 3 skin | Skin | Alive, on Immunosupp | |
| 9 JM | BM | 10/10 | 4000 | 12 mg/kg 4 | 2.67 x 106/kg | 2.48 x 107/kg | 100 | 56 | 14 | Tacro/MTX | Grade 3 skin | Skin | Alive, on Immunosupp | |
| 10 AP | BM | 10/10 | 3900 | 12.8 mg/kg 4 | 1.86 x 106/kg | 3.0 x 107/kg | 100 | 98 | 8 | Tacro/MTX | Grade 3 skin and GI | GI | Died, sepsis 8 mo | |
| 11 SC | PBSC | 10/10 | 4800 | 13.2 mg/kg 4 | 8.69 x 106/kg | 4.51 x 107/kg | 100 | 71 | 12 | Tacro/MTX | Grade 3 3-system | Skin/GI | Alive, on Immunosupp | |
| 12 SD | BM | 10/10 | 4300 | 12 mg/kg 4 | 1.5 x 106/kg | 2.16 x 107/kg | 100 | 100 | 6 | Tacro/MTX | None | None | Alive, on immunosupp | |
| 13 MB | PBSC | 10/10 | 4200 | 10 mg/kg 4 | 7 x 106/kg | 1.57 x 108/kg | 82 | 100 | 3 | Tacro/MTX | None | None | Alive, on immunosupp | |
| Haplo | ||||||||||||||
| 1 JP | BM | 5/10 | 4800 | 7.0 mg/kg 2 | 2.5 x 106/kg | 4.4 x 107/kg | 100 | 99 | 38 | PT/CY | Grade 1 skin | None | Alive, off Immunosupp | |
| 2 KM | BM | 8/10 | 4800 | 6.6 mg/kg 2 | 5.5 x 106/kg | 5.3 x 107/kg | 100 | 100 | 30 | PT/CY | Grade 1 skin Grade 2 GI |
None | Alive, off Immunosupp | |
| 3 LM | BM | 8/10 | 4700 | 6.3 mg/kg 2 | 6.1 x 106/kg | 5.9 x 107/kg | 100 | 100 | 25 | PT/CY | Grade 2 GI | Vaginal | Alive, off Immunosupp | |
| 4 AT | BM | 5/10 | 3800 | 7.4 mg/kg 2 | 6.1 x 106/kg | 3.5 x 107/kg | 100 | 100 | 22 | PT/CY | None | None | Alive, off Immunosupp | |
| 5 WSA | BM | 6/10 | 4400 | 6.8 mg/kg 2 | 2.6 x 106/kg | 3.2 x 107/kg | 100 | 100 | 18 | PT/CY | Grade 3 GI | Moderate/severe cGVHD | Alive, on Immunosupp | |
| 6 GC | BM | 8/10 | 4000 | 9.3 mg/kg 2 | 5.9 x 106/kg | 3.0 x 107/kg | 100 | 100 | 14 | PT/CY | Grade 1 skin | None | Alive, off Immunosupp | |
| 7 NLO | BM | 5/10 | 4400 | 6.6 mg/kg 3 | 7.2 x 106/kg | 5.2 x 107/kg | 100 | 100 | 12 | PT/CY | Grade 2 upper GI | None | Alive, off Immunosupp | |
Abbreviations: MRD, matched related donor; URD, matched unrelated donor; Haplo, haploidentical related donor; PBSC, peripheral blood stem cells; BM, bone marrow; Pt, patient; HLA, human leukocyte antigen; BU, busulfan; AUC, area under curve; aGVHD, acute graft-versus-host disease; cGVHD, chronic graft-versus-host disease; Tacro, tacrolimus, MTX, methotrexate; PT/CY, post-transplant cyclophosphamide;
Busulfan dosing, based upon the area under the concentration versus time curve (AUC) of a 0.8 mg/kg test dose, was targeted at a daily AUC of 3600–4800 μMol*min. MRD/URD recipients received a median daily targeted busulfan AUC of 4600 μMol*min administered over 4 days (cumulative median systemic exposure of busulfan of 18,400 μMol*min) . HRD recipients received a median daily targeted busulfan AUC of 4400 μMol*min administered for 2–3 days (cumulative median systemic exposure of 9400 μMol*min.
Engraftment
Neutrophil and platelet engraftment, defined by recovery of absolute neutrophil count (ANC) to > 500/μL x 3 days and platelets > 20,000/μL without transfusions for MRD/URD recipients occurred at a mean of 11.8 days (range 9–20) for neutrophils and 13.8 days (range 8–26) for platelets. Neutrophil engraftment for HRD recipients occurred at a mean of 15.4 days (range, 14–18) and platelet engraftment at a mean of 16.4 days (range, 11–25).
Adverse events and chimerism
There were no primary graft failures. All recipients had 100% myeloid chimerism by day 100, with the exception of URD recipient 3, who relapsed with AML by day 30, underwent a second transplant, but died from persistent AML. CD3+ cell chimerism was more variable in the MRD/URD cohorts. One MRD patient had 29% donor CD3+ cell chimerism at day 100; this increased to 91% by one year post-transplant, coincident with the tapering of immunosuppression. One URD recipient (URD patient 9) with 56% donor CD3+ cell chimerism at day 100 required additional immunosuppression for skin GVHD and the 6 months chimerism remained low at 59%.
Reversal of the Clinical Phenotype
We anticipated that the infectious complications present in the GATA2 patients might be problematic with HSCT, but that was not the case. Two patients had evidence of post-transplant immune reconstitution affecting sites of previous infection.
Viral infections, especially with HPV, are a hallmark of GATA2 deficiency. Aggressive, refractory and recurrent cervical and vulvovaginal disease with high grade squamous intraepithelial lesions (HGSIL) and intraepithelial neoplasia was present in 7 women pre-transplant. Despite resolution of precancerous lesions, persistence of warts was common. However, 2 patients had persistent or recurrent intraepithelial neoplasia through 1.5 years post-transplant, and one patient developed HGSIL post-transplant. These three patients required additional immunosuppression for aGVHD. A patient with an EBV smooth muscle cell tumor had continued radiographic evidence of control of the tumor in the liver and resolution of other inflammatory lesions through 3 years post-transplant.11
MDS, present in all patients prior to HSCT, and its accompanying cytogenetic changes (9/22 patients, trisomy 8, monosomy 7, and trisomy 1q and multiple abnormalities in two patients), completely reversed by day 100 following HSCT except in the patient with refractory AML.
Pulmonary disease was present in 18/22 patients prior to HSCT and in general responded well to HSCT with URD patient 11 having complete resolution of pulmonary arterial hypertension (Supplementary Table 1).
Emberger syndrome, with lymphedema in 2 patients, was not reversed by HSCT.
Outcomes
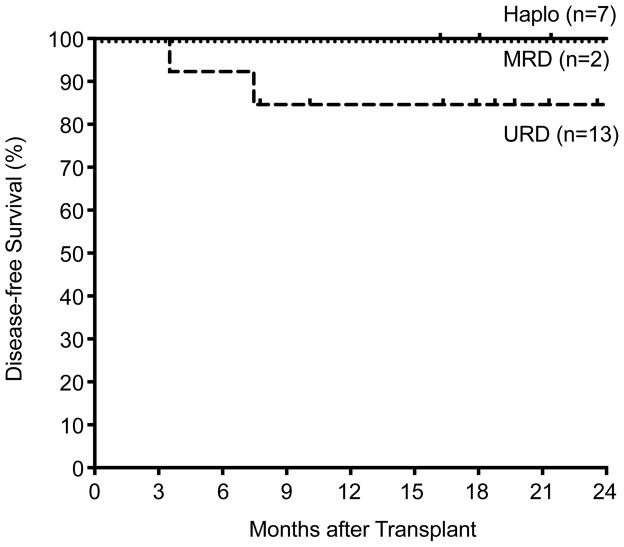
With a mean follow-up of 24 months (range, 9–50 months), 19/22 patients are alive with an 86% overall, disease-free survival (Figure 2). There were three deaths in the URD group, none occurring in the first 100 days post-transplant, indicating the low regimen-related mortality (Table 2). Of the three deaths, one was the result of refractory AML, one from GVHD, and one from sepsis.
Figure 2.
Kaplan-Meier curves showing disease-free survival according to type of donor in GATA2 patients.
GVHD
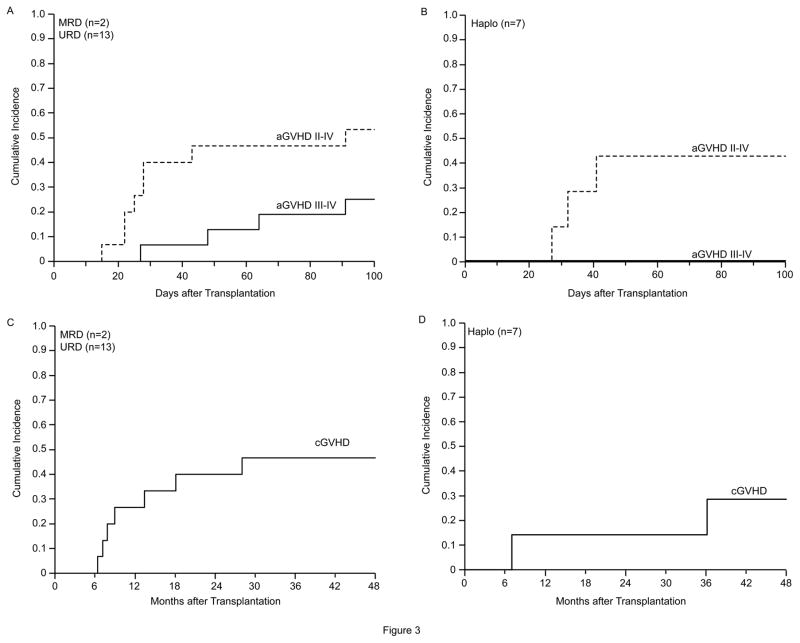
Despite 10/10 HLA matching in all MRD and URD recipients, four of the 15 (26%) developed grade III–IV acute GVHD (aGVHD) following prophylaxis with tacrolimus and methotrexate. All 4 patients required treatment with high-dose corticosteroids, and two received infliximab/basiliximab for steroid-refractory or steroid-requiring disease (Table 2) (Figure 3A and 3B). No HRD recipient developed grade III–IV aGVHD.
Figure 3.
Incidence of acute and chronic GVHD in matched related and unrelated donors and in haploidentical related donor recipients. A. Acute GVHD in matched related and unrelated donor recipients. B. Acute GVHD in haploidentical related donor recipients. C. Chronic GVHD in matched related and unrelated donor recipients. D. Chronic GVHD in haploidentical related donor recipients
Chronic GVHD (cGVHD) also occurred at a considerably higher rate in the MRD/URD recipients than in the HRD recipients, all of whom received post-transplant cyclophosphamide (PT/Cy) (Table 2) (Figure 3C and 3D). Seven of the 15 MRD/URD recipients (46%), and two of the 7 HRD recipients (28%) developed cGVHD.
Reconstitution of Lymphoid and Monocytic Cells Post-Transplant
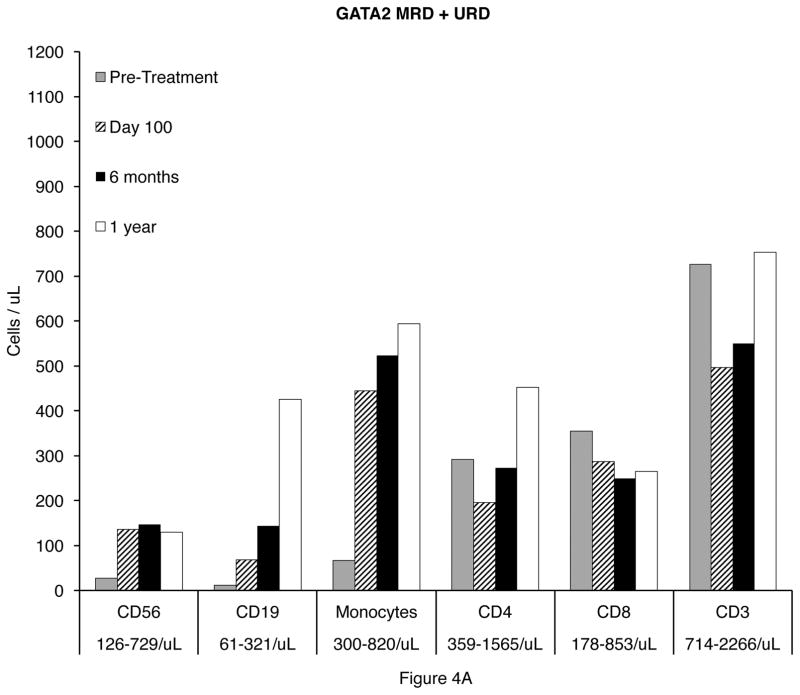
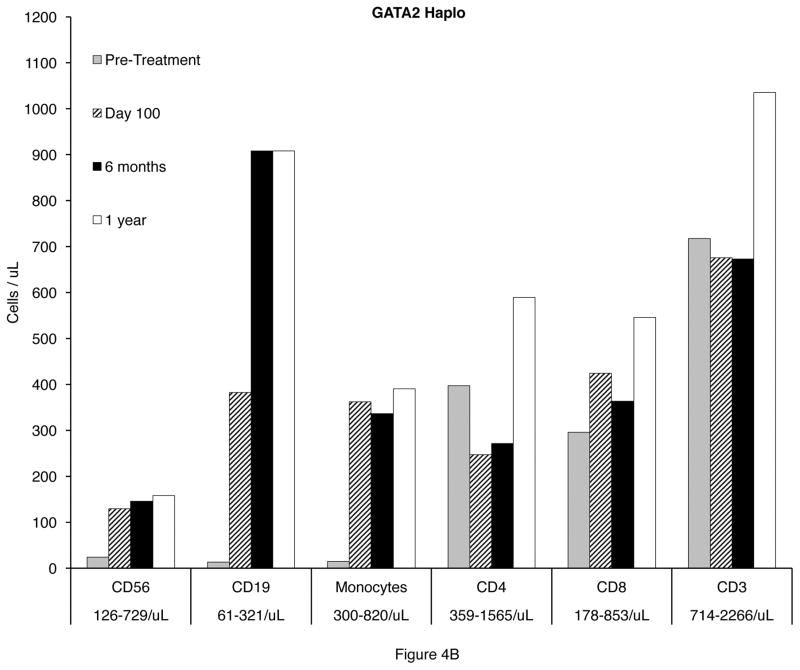
All patients had complete reconstitution of CD56+ NK cells and monocytes by day 30 in all recipients. CD19+ B cell recovery occurred by 6 months in the HRD recipients, but was delayed to one year in MRD and URD recipients. (Figure 4A and 4B).
Figure 4.
Reconstitution of CD19+ B cells, CD4+ T cells, CD8+ T cells, and monocytes 100 days, 6 months, and 1 year after transplantation in GATA2 deficient patients. A. Matched related (MRD) and unrelated donor (URD) recipients. B. Haploidentical (haplo) related donor recipients.
Post-transplant Complications
Two patients, URD recipients 6 and 10, had autoimmune cytopenias following transplant. An autoimmune hemolytic anemia (AIHA) in URD patient 6 developed 6 months post-transplant and required intravenous immunoglobulin (IVIG) after high-dose corticosteroids and erythropoietin were ineffective. Similarly, URD patient 10 developed an AIHA and thrombocytopenia that ultimately resolved following treatment with high-dose corticosteroids, IVIG, eltrombopag, and erythropoietin.
T-Cell Subsets Following HSCT
T-cell populations are generally intact before HSCT in GATA2 deficiency. Most patients had excellent recovery of naive CD4 and CD8 T-cells after HSCT (Supplementary Figs. 1A and 1B), without a major expansion of effector memory (EM) or EM-RA+ cells by one year following HSCT, consistent with the low level of reactivation of viruses such as CMV.
Viral Reactivations
Only one patient had evidence of Epstein-Barr virus (EBV) reactivation requiring intervention, although 9 patients had intermittent viremia pre-transplant. One patient received pre-emptive rituximab for an EBV viremia >4 log10 and concomitant steroid-refractory GVHD, 5 had CMV reactivation (no disease), 5 had reactivations of HHV-6 (generally <3 log10) and there were 11 cases of BK viruria, with 3 episodes of hemorrhagic cystitis (2 HRD recipients). Only one patient reactivated or acquired adenovirus (asymptomatic) as assessed by blood PCR.
Discussion
We present encouraging results with allogeneic HSCT for GATA2 deficiency in 22 recipients of MRD, URD, and HRD grafts. All patients engrafted, with an overall disease-free survival at 24 months of 86%. One URD patient died from refractory AML, one URD patient died from GVHD, and one URD patient died from sepsis. The rates of grade III–IV aGVHD and moderate-to-severe cGVHD in the 10/10 matched donor cohorts were 26% and 46%, respectively. There was no Grade 3–4 aGVHD in the HRD cohort, although 2/7 patients developed moderate-to-severe cGVHD.
The optimum timing and indications for allogeneic HSCT in GATA2 deficiency remain challenging. Moreover, there are limited data in this regard. 10, 13 However, three factors play important roles in the indication and timing of transplant: frequency and severity of infections, secondary organ damage such as pulmonary changes, and myeloid progression of cytopenias and cytogenetic abnormalities.
In approximately one-half of our patients with GATA2 deficiency, infections prompted HSCT. In contrast to our previous report of HSCT in GATA2 deficiency in which 12 of the 14 patients had NTM, many with recurrent episodes, only 7 of the 22 patients in the current study had NTM.10 This is most likely due to earlier syndromic recognition, facilitated by genetic diagnosis.
Progressive organ damage, frequently pulmonary, led to HSCT in several patients. NTM infections, bronchiectasis, and PAP, either alone or in combination, led to abnormal chest CT scans in 19/22 patients. These abnormalities ranged from ground glass opacities, to fibrosis, infiltrates, nodules, and effusions. In our previous study, 3/14 patients received whole lung lavage for PAP prior to transplant, but the risk of pre-transplant whole lung lavage, and the rapid responses in PAP observed in previous GATA2 HSCT recipients. led us to abandon that practice. Pulmonary diffusion capacity post-transplant is complex, as decreases in diffusion are commonly seen post-HSCT.14 This effect will be weighed against improvements related to the reversal of PAP in this patient population.15
Myeloid dysplasia with progressive cytopenias and new cytogenetic changes in the bone marrow prompted HSCT in approximately one-half of the patients in this study. GATA2 plays a central role in the maintenance of hematopoietic stem cells (HSC) in mice and men, and murine HSC which are haploinsufficient for GATA2 expression perform poorly with wild-type GATA2 murine HSC in competitive reconstitution assays.16 Although the progression from a normocellular bone marrow to hypoplastic MDS to AML or CMML is highly variable- perhaps requiring additional somatic mutations for expression of the phenotype – the development of AML or a proliferative CMML occurs frequently enough to warrant close monitoring of GATA2 mutated patients. Illustrative of this point, URD patient 3 progressed from MDS to a refractory AML with 85% monoblasts within four months. MDS was present in all 22 of the patients in this study, and both cases of AML arose from an MDS marrow background.
Cytogenetic changes, present in 50% of this group, (trisomy 8 the most frequent) were frequently progressive. This contrasts with the study by Wlodarski in teenagers with MDS in which monosomy 7 was the most common cytogenetic abnormality. 17 Three patients in our study developed additional cytogenetic changes during the period of observation, including two with trisomy 1q, and prompted HSCT in all three cases.
Ideally, a reduced intensity conditioning regimen would reverse the phenotype of GATA2 deficiency, thus enabling patients with pre-existing, severe, end-organ damage to undergo HSCT. However, utilizing a nonmyeloablative conditioning regimen in 14 patients with GATA2 deficiency, we achieved a 75% disease-free survival in the 8 patients with MRD and URD. 10 There was one relapse requiring a second transplant and one death from graft rejection, suggesting that a more intensive conditioning regimen might result in an improved disease outcome.
The malignant myeloid predisposition of GATA2 deficiency appears to require complete, or near complete, donor chimerism. Thus, GATA2 deficiency differs from many other primary immunodeficiency diseases in which high levels of donor chimerism are not required to correct the phenotype. We adapted the previously described nonmyeloabaltive regimen for haploidentical related donors from Johns Hopkins by adding two to three days of busulfan since the GATA2 deficiency patients had no previous exposure to chemotherapy, and typically were taken directly to transplant. 20
The optimal busulfan exposure in children and young adults prior to hematopoietic stem cell transplantation appears to be coming into focus. In a recent, multicenter, retrospective cohort analysis, Bartelink and colleagues compared the outcome in 790 patients and identified a cumulative AUC of busulfan of 78–101 mg x h/L that resulted in the optimal outcome with 77% event free survival. 18 In our study we administered busulfan at a targeted daily AUC of 3800 to 4800 μMol*min, corresponding to a cumulative AUC of 65–80 mg x h/L.19 Patients with AML or MDS with unfavorable cytogenetics received a targeted daily AUC closer to 4800 μMol*min (80 mg x h/L), whereas patients with a hypocellular MDS and normal, favorable, or intermediate cytogenetics received a daily AUC targeted closer to the lower range, which still led to successful engraftment, despite the use of bone marrow in nearly one-half of the patients. There was no graft failure and one case of refractory AML.
Together with our previous studies using a nonmyeloablative regimen, it appears that if GATA2 deficiency patients are transplanted earlier in the course of disease (with a hypocellular bone marrow with MDS and without cytogenetic changes), a reduced intensity regimen may result in reliable engraftment, since the GATA2 deficient marrow has a proliferative disadvantage.16 However, with the development of clonal progression and unfavorable cytogenetic changes and/or a hypercellular marrow, where a malignant clone may have a proliferative advantage, a higher dose regimen may result in more reliable engraftment and eradication of the malignant clone.10
Haploidentical related donors are our preferred donor in the absence of a fully matched MRD or URD, since our cord blood transplants led to unacceptable outcomes.10 Our results with HRD are notable with engraftment in all 7 recipients and eradication of all cytogenetic abnormalities. The regimen used is based on the non-myeloablative regimen pioneered by Luznik et al., but 2 or 3 days of busulfan have been added to address the need for elimination of cytogenetic/clonal abnormalities, which has been successful .20 The incidence of grade II–IV aGVHD was 57%, with no grade III–IV aGVHD. However, two patients developed moderate-to-severe cGVHD. With post-transplant cyclophosphamide (PT/Cy) there was no grade III–IV aGVHD, a low incidence of cGVHD, and there was an expansion of patients with an acceptable donor.
Allogeneic HSCT remains the only definitive therapy for GATA2 deficiency. However, the decision to undertake allogeneic HSCT should be weighed against the complications inherent to HSCT including regimen related toxicity, GVHD, infection, and death. Similarly, the timing of HSCT is coming into focus. Infections, progressive cytopenias, myeloid progression, and new cytogenetic changes all contribute to the decision to proceed to allogeneic HSCT. HSCT results in a considerably improved outcome if carried out prior to the development of end-organ damage or progression to AML or CMML. Identifying more precise molecular indicators of myeloid transformation would enable earlier HSCT in higher risk individuals.
In summary, we report the results of allogeneic HSCT for GATA2 deficiency in 22 recipients using three different donor types, applying a busulfan-based myeloablative conditioning regimen in the presence of cytogenetic abnormalities, and adding post-transplant cyclophosphamide for HRD recipients. All patients engrafted. Deaths in 3 recipients were related to the persistence of AML in one patient, the development of aGVHD in one patient, and sepsis in a third patient. Longer follow-up will be necessary to define the evolution of pre-transplant morbidities, especially pulmonary and HPV-related disease, but hematologic reconstitution and persistent eradication of clonal cytogenetic abnormalities using this regimen has been observed.
In our current study of HSCT for GATA2 deficiency, we are extending the use of PT/Cy to the MRD and URD cohorts in order to reduce the incidence of aGVHD and cGVHD.
Supplementary Material
Reconstitution of CD4+ and CD8+ T-cell subsets after bone marrow transplantation in patients with GATA2 deficiency, excluding those who received systemic steroids. Solid lines represent patients who underwent HLA-matched related-donor (MRD) and HLA-matched unrelated-donor (URD) transplant. Dashed lines represent patients who underwent haploidentical stem cell transplant. Pre: pre-transplantation. Post: post-transplantation. A: CD4+ central memory (CM) T-cells, B: CD4+ effector memory (EM) T-cells, C: CD4+ naïve T-cells, D: CD4+ terminally differentiated effector memory (EMRA) T-cells, E: CD8+ central memory T-cells, F: CD8+ effector memory T-cells, G: CD8+ naïve T-cells, H: CD8+ terminally differentiated effector memory T-cells.
Highlights.
Multiple phenotypes of disease complicate the choice of conditioning for stem cell transplantation in GATA2 deficiency.
Reduced intensity has been insufficient for engraftment or prevention of relapse of hematologic disorders or cytogenetic abnormalities.
A busulfan-based regimen targeted an AUC betwen 3600 and 4800 μMol*min and added post-transplant high dose cyclophosphamide for graft-versus-host disease prophylaxis in recipients from haploidentical donors.
Engraftment was achieved in all recipients. Disease-free survival was 86%, and the phenotype of GATA2 deficiency was reversed in all recipients except the refractory AML patient.
Post-transplant cyclophosphamide led to successful engraftment with a low incidence of acute GVHD in recipients from haploidentical donors.
Acknowledgments
This project has been funded in whole or in part with federal funds from the National Cancer Institute, National Institutes of Health, under Contract No. HHSN261200800001E. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government. This research was supported [in part] by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research and [in part] by the Division of Intramural Reseach of the National Institute of Allergy and Infectious Diseases.
Footnotes
Authorship
Contribution: M.P., D.H., N.S., and K.B., drafted the manuscript. M.P., N.S., K.B., S.M.H., and D.D.H., and designed the research and supervised the study; T.H., K.C., B.B., H.R., J.C.R., M.K. and M.P. provided critical revision of the manuscript for important intellectual content; K.R.C. reviewed the histopathology.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Conflict of Interest: The authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Vinh DC, Patel SY, Uzel G, et al. Autosomal dominant and sporadic monocytopenia with susceptibility to mycobacteria, fungi, papillomaviruses, and myelodysplasia. Blood. 2010;115(8):1519–1529. doi: 10.1182/blood-2009-03-208629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hsu AP, Sampaio EP, Khan J, et al. Mutations in GATA2 are associated with the autosomal dominant and sporadic monocytopenia and mycobacterial infection (MonoMAC) syndrome. Blood. 2011;118(10):2653–2655. doi: 10.1182/blood-2011-05-356352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dickinson RE, Griffin H, Bigley V, et al. Exome sequencing identifies GATA-2 mutation as the cause of dendritic cell, monocyte, B and NK lymphoid deficiency. Blood. 2011;118(10):2656–2658. doi: 10.1182/blood-2011-06-360313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mansour S, Connell F, Steward C, et al. Emberger syndrome-primary lymphedema with myelodysplasia: report of seven new cases. Am J Med Genet A. 2010;152A(9):2287–2296. doi: 10.1002/ajmg.a.33445. [DOI] [PubMed] [Google Scholar]
- 5.Ostergaard P, Simpson MA, Connell FC, et al. Mutations in GATA2 cause primary lymphedema associated with a predisposition to acute myeloid leukemia (Emberger syndrome) Nat Genet. 2011;43(10):929–931. doi: 10.1038/ng.923. [DOI] [PubMed] [Google Scholar]
- 6.Hahn CN, Chong CE, Carmichael CL, et al. Heritable GATA2 mutations associated with familial myelodysplastic syndrome and acute myeloid leukemia. Nat Genet. 2011;43(10):1012–1017. doi: 10.1038/ng.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Spinner MA, Sanchez LA, Hsu AP, et al. GATA2 deficiency: a protean disorder of hematopoiesis, lymphatics, and immunity. Blood. 2014;123(6):809–821. doi: 10.1182/blood-2013-07-515528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dickinson RE, Milne P, Jardine L, et al. The evolution of cellular deficiency in GATA2 mutation. Blood. 2014;123(6):863–874. doi: 10.1182/blood-2013-07-517151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tomblyn M, Chiller T, Einsele H, et al. Guidelines for preventing infectious complications among hematopoietic cell transplantation recipients: a global perspective. Biol Blood Marrow Transplant. 2009;15(10):1143–1238. doi: 10.1016/j.bbmt.2009.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grossman J, Cuellar-Rodriguez J, Gea-Banacloche J, et al. Nonmyeloablative allogeneic hematopoietic stem cell transplantation for GATA2 deficiency. Biol Blood Marrow Transplant. 2014;20(12):1940–1948. doi: 10.1016/j.bbmt.2014.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parta M, Cuellar-Rodriguez J, Freeman AF, Gea-Banacloche J, Holland SM, Hickstein DD. Resolution of Multifocal Epstein-Barr Virus-Related Smooth Muscle Tumor in a Patient with GATA2 Deficiency Following Hematopoietic Stem Cell Transplantation. J Clin Immunol. 2017;37(1):61–66. doi: 10.1007/s10875-016-0360-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Odish OF, Gotoh A, Liu YC, et al. Recurrent unbalanced whole-arm t(1;10)(q10;p10) in myelodysplastic syndrome: a case report and literature review. Cancer Genet Cytogenet. 2007;172(2):165–167. doi: 10.1016/j.cancergencyto.2006.09.022. [DOI] [PubMed] [Google Scholar]
- 13.Ciullini Mannurita S, Vignoli M, Colarusso G, et al. Timely follow-up of a GATA2 deficiency patient allows successful treatment. J Allergy Clin Immunol. 2016;138(5):1480–1483. e1484. doi: 10.1016/j.jaci.2016.06.004. [DOI] [PubMed] [Google Scholar]
- 14.Barisione G, Bacigalupo A, Brusasco C, et al. Mechanisms for reduced pulmonary diffusing capacity in haematopoietic stem-cell transplantation recipients. Respir Physiol Neurobiol. 2014;194:54–61. doi: 10.1016/j.resp.2014.01.018. [DOI] [PubMed] [Google Scholar]
- 15.Chaulagain CP, Pilichowska M, Brinckerhoff L, Tabba M, Erban JK. Secondary pulmonary alveolar proteinosis in hematologic malignancies. Hematol Oncol Stem Cell Ther. 2014;7(4):127–135. doi: 10.1016/j.hemonc.2014.09.003. [DOI] [PubMed] [Google Scholar]
- 16.Rodrigues NP, Janzen V, Forkert R, et al. Haploinsufficiency of GATA-2 perturbs adult hematopoietic stem-cell homeostasis. Blood. 2005;106(2):477–484. doi: 10.1182/blood-2004-08-2989. [DOI] [PubMed] [Google Scholar]
- 17.Wlodarski MW, Hirabayashi S, Pastor V, et al. Prevalence, clinical characteristics, and prognosis of GATA2-related myelodysplastic syndromes in children and adolescents. Blood. 2016;127(11):1387–1397. doi: 10.1182/blood-2015-09-669937. quiz 1518. [DOI] [PubMed] [Google Scholar]
- 18.Bartelink IH, Lalmohamed A, van Reij EM, et al. Association of busulfan exposure with survival and toxicity after haemopoietic cell transplantation in children and young adults: a multicentre, retrospective cohort analysis. Lancet Haematol. 2016;3(11):e526–e536. doi: 10.1016/S2352-3026(16)30114-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Lima M, Couriel D, Thall PF, et al. Once-daily intravenous busulfan and fludarabine: clinical and pharmacokinetic results of a myeloablative, reduced-toxicity conditioning regimen for allogeneic stem cell transplantation in AML and MDS. Blood. 2004;104(3):857–864. doi: 10.1182/blood-2004-02-0414. [DOI] [PubMed] [Google Scholar]
- 20.Luznik L, O’Donnell PV, Symons HJ, et al. HLA-haploidentical bone marrow transplantation for hematologic malignancies using nonmyeloablative conditioning and high-dose, posttransplantation cyclophosphamide. Biol Blood Marrow Transplant. 2008;14(6):641–650. doi: 10.1016/j.bbmt.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Reconstitution of CD4+ and CD8+ T-cell subsets after bone marrow transplantation in patients with GATA2 deficiency, excluding those who received systemic steroids. Solid lines represent patients who underwent HLA-matched related-donor (MRD) and HLA-matched unrelated-donor (URD) transplant. Dashed lines represent patients who underwent haploidentical stem cell transplant. Pre: pre-transplantation. Post: post-transplantation. A: CD4+ central memory (CM) T-cells, B: CD4+ effector memory (EM) T-cells, C: CD4+ naïve T-cells, D: CD4+ terminally differentiated effector memory (EMRA) T-cells, E: CD8+ central memory T-cells, F: CD8+ effector memory T-cells, G: CD8+ naïve T-cells, H: CD8+ terminally differentiated effector memory T-cells.