Abstract
Patients with follicular lymphoma (FL) experiencing early therapy failure (ETF) within two years of frontline chemoimmunotherapy have poor overall survival (OS). We analyzed data from the Center for International Blood and Marrow Transplant Research (CIBMTR) and the National LymphoCare Study (NLCS) to determine whether autologous hematopoietic cell transplant (autoHCT) can improve outcomes in this high-risk FL subgroup.ETF was defined as failure to achieve at least partial response after frontline chemoimmunotherapy or lymphoma progression within two years of frontline chemoimmunotherapy. We identified two groups: the non-autoHCT cohort (patients from the NLCS with ETF not undergoing autoHCT); and the autoHCT cohort (CIBMTR patients with ETF undergoing autoHCT). All patients received rituximab-based chemotherapy as frontline treatment. 174 non-autoHCT patients and 175 autoHCT patients were identified and analyzed. There was no difference in five year OS between the two groups (60% vs 67% respectively; p=0.16). A planned subgroup analysis showed that patients with ETF receiving autoHCT soon after treatment failure (≤1year of ETF; n=123) had higher five year OS than those without autoHCT (73% vs 60%, p=0.05). On multivariate analysis, early use of autoHCT was associated with significantly reduced mortality (HR=0.63, 95%CI:0.42–0.94, p=0.02). Patients with FL experiencing ETF after frontline chemoimmunotherapy lack optimal therapy. We demonstrate improved OS when receiving autoHCT within one year of treatment failure. Results from this unique collaboration between the NLCS and CIBMTR support consideration of early consolidation with autoHCT in select FL patients experiencing ETF.
Keywords: Follicular lymphoma, early therapy failure, autologous transplantation, early transplant, rituximab, chemoimmunotherapy
INTRODUCTION
Follicular lymphoma (FL) is the most common indolent non-Hodgkin lymphoma (NHL); diagnosed in approximately two-three out of 100,000 people in the Unites States and Europe1. Due to improvements in treatments over the past several decades, patients with FL have increased median overall survival (OS), approaching 20-years2. However, significant disease heterogeneity exists, with a proportion of patients undergoing histologic transformation3, exhibiting refractory disease, or early mortality after frontline chemotherapy.
Among the most relevant features affecting FL prognosis, time to progression has emerged as one of the most robust and reproducible adverse determinants of FL outcomes. Several studies suggest that early treatment failure (ETF) within two years of frontline chemoimmunotherapy occurs in approximately 20% of FL patients, independent of maintenance rituximab4,5. In a pivotal analysis, the National LymphoCare Study (NLCS) demonstrated that patients with disease progression within 24 months of treatment with frontline chemoimmunotherapy including rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP); rituximab, cyclophosphamide, vincristine, and prednisone (R-CVP); or R-Fludarabine had had an inferior five year OS (50%) compared to patients who remained progression-free at 24 months (90%). These findings were validated in an independent cohort of patients (34%, five year OS in the early progressors)6, and subsequently by multiple other independent investigators7,8.
From these data it follows that we lack optimal therapy for patients with FL who experience ETF and there is an unmet need for novel treatments. The National Clinical Trials Network of the National Cancer Institute recently convened a lymphoma clinical trials planning meeting to determine priorities for lymphoma clinical trials research. Consensus from this meeting established that the top priority in FL was to impact these high-risk patients. High dose therapy (HDT) and autologous hematopoietic cell transplantation (autoHCT) is one effective treatment approach in relapsed indolent and aggressive lymphomas9,10. Due to the long natural history of FL, and serious concerns for both short and long term complications of HDT/autoHCT such as second myeloid malignancies11–14, there is lack of consensus as to the optimal patient population to benefit from this approach14–16. However given the durable remissions observed in relapsed/refractory FL with autoHCT, this warrants further investigation and is deserving of consideration in patients with ETF.
As such we hypothesized that autoHCT would be a promising treatment for patients experiencing ETF. Using the Center for International Blood and Marrow Transplant Research [CIBMTR]) registry and the NLCS database as a control, we investigated whether HDT and autoHCT can overcome chemoresistance and improve OS in FL patients with ETF.
MATERIALS AND METHODS
Data sources
Data sources included the observational database of CIBMTR and NCLS (details in supplemental appendix).
Patients
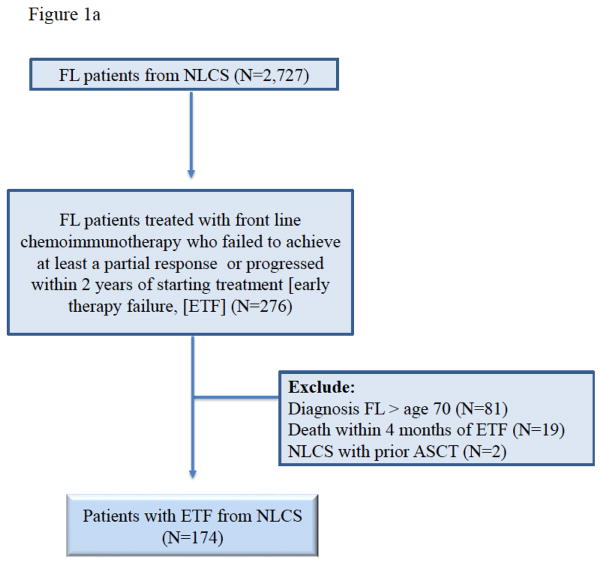
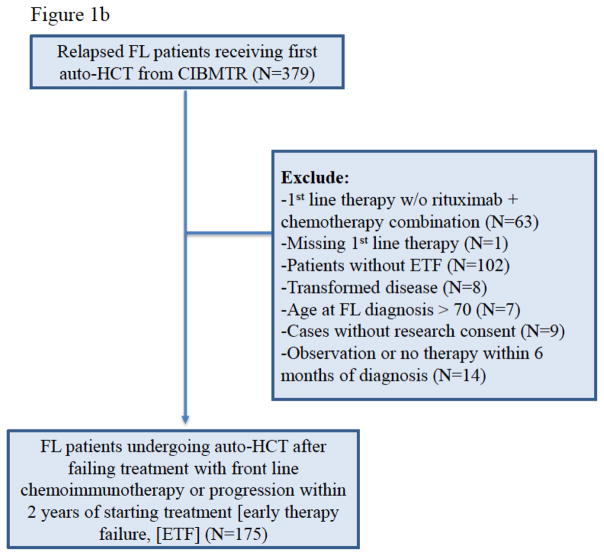
This analysis aimed to compare OS of FL patients who experienced ETF after frontline chemoimmunotherapy and never underwent autoHCT within NLCS, against those FL patients who experienced ETF and subsequently received an autoHCT consolidation within CIBMTR registry. ETF was defined as the failure to achieve at least a partial response (PR) after frontline chemoimmunotherapy, or disease progression within two years of frontline rituximab-based chemoimmunotherapies. Adult FL patients (age ≥18 years) enrolled between 2004–2007 in the NLCS with ETF represent the non-autoHCT cohort of this analysis (CONSORT diagram Figure-1a). NLCS patients meeting these criteria but undergoing autoHCT (n=2) were excluded.
Figure 1a and 1b. CONSORT Diagram for NCLS cohort and CIBMT cohort.
Figure 1a. CONSORT Diagram National Lymphocare Study patients (non-auto-HCT cohort)
Figure 1b. CONSORT Diagram CIBMTR patients (auto-HCT cohort)
FL patients diagnosed during 2002–2009 meeting criteria for ETF and undergoing an autoHCT from the CIBMTR constitute the autoHCT cohort for this study (CONSORT diagram Figure-1b).
Patients older than 70 years of age at the time of FL diagnosis were not eligible for either cohort of this study (NLCS=81 patients, CIBMTR=7 patients) because patients with advanced age are less likely to be referred for autoHCT. As opposed to age restriction at the time of diagnosis, no upper limit for patient age at the time of autoHCT was applied. Since the original NLCS publication demonstrating adverse prognosis of ETF FL6 excluded patients undergoing observation (or watchful waiting) after diagnosis or those with known histologic transformation, the current analysis also excluded such patients from both databases. Patients who died within four months, from the time of ETF were excluded (NLCS=19, CIBMTR=0). The rationale of this criterion was to ensure exclusion of ETF FL patients who did not survive long enough to receive salvage therapy and autoHCT. Patients in the autoHCT cohort with chemorefractory disease before transplantation were not excluded. Patients with grade 3 disease were included, as they were part of the original NLCS analysis.
Definitions and Endpoints
Response to frontline chemoimmunotherapy and disease response before autoHCT was determined using the International Working Group criteria17. The primary endpoint was OS. Death from any cause was considered an event and surviving patients were censored at last follow-up. OS was calculated starting four months after the date of ETF (i.e. the landmark point) to the time of death or last follow-up. Since timing of autoHCT in relapsed/refractory FL is not uniform across transplant centers, the study protocol specified a planned subgroup analysis comparing non-autoHCT cohort (NLCS) patients against CIBMTR patients undergoing early autoHCT consolidation. Early autoHCT was defined as transplantation performed within one year of experiencing ETF. The intent of this preplanned subgroup analysis was to evaluate the impact of early autoHCT in FL patients with ETF.
Statistical analysis
Patient and disease-related variables were compared between the non-autoHCT and autoHCT cohorts using the Chi-square test for categorical variables and the Wilcoxon two sample test for continuous variables. Frequencies of transplantation-related variables were descriptively presented. The autoHCT cohort patients did not enter the study before getting HCT (in other words, they survived long enough to get a transplant). To adjust for this late entry time, the left-truncated Cox model was used for multivariate analysis (MVA), with the clock starting at landmark point (four months after ETF). The late entry time was the time from landmark point to transplantation for patients in the autoHCT cohort. The non-autoHCT cohort had zero late entry time. Because there was no late entry time for the non-autoHCT cohort, the Kaplan-Meier estimator was used to estimate OS probabilities for that group. On the other hand, the Breslow estimator based on the left-truncated Cox model was used to calculate OS probabilities for the autoHCT cohort. Time from diagnosis to ETF was analyzed in the Cox model. The assumption of proportional hazards for each factor in the left-truncated Cox model was tested using time-dependent covariates. A stepwise model selection approach was used to identify all significant risk factors. Each step of model building contained the main effect (i.e. non-autoHCT cohort vs. autoHCT cohort). Factors which were significant at a 5% level were kept in the final model. The potential interactions between main effect and all significant risk factors were tested. The variables considered in MVA are shown in Table S1 of supplemental appendix.
Another MVA was performed using matched data based on propensity scores. The variables significantly associated with the main effect were identified by the logistic regression treating the main effect as a response. The propensity scores were calculated using those variables. The propensity scores were classified into one of the five categories: 0–20%, 20–40%, 40–60%, 60–80%, and 80–100%. Using the five categories, the two arms were matched with one-to-one matching ratio (Table S3). Cluster effect due to matching was adjusted using the marginal left-truncated Cox model. The p-values for survival rates at time points of interest from univariable analysis were obtained from the z-test based on the standard errors of the survival estimates at each time point for each arm, assuming the two arms were independent. False discovery rate (FDR) control was used to further adjust p-values for univariable analysis. The cut-off 10% was used for FDR. All statistical analyses were performed using SAS version 9.3 (SAS Institute, Cary, NC).
RESULTS
Baseline Characteristics
Baseline characteristics of 174 non-autoHCT cohort patients and 175 autoHCT cohort patients are shown in Table 1 and Figure 1a and 1b. There was no significant difference between the two groups in terms of patient gender, proportion of patients with advanced stage disease and bone marrow or extranodal involvement at diagnosis. Compared to the non-autoHCT cohort, the autoHCT cohort more frequently had Caucasian patients (86% vs. 93%; p=0.008), grade 3 histology (23% vs. 35%; p=0.04) and R-CHOP as frontline treatment (57% vs. 79%; P<0.0001). A greater proportion of non-autoHCT cohort patients had normal lactate hydrogenase at diagnosis (46% vs. 19%; p=0.02). Sixty-five (37%) non-autoHCT cohort patients had FLIPI score of 3–5 at diagnosis. FLIPI score was not captured in the CIBMTR registry, during the era of this study. The response to frontline chemoimmunotherapy was complete response (CR) or PR in 67% of non-HCT cohort patients compared to 78% of autoHCT cohort patients (p=0.004).
Table 1.
Baseline characteristics of follicular lymphoma patients with early therapy failure.
| Variable | No AutoHCT- cohort (NLCS) (%) | AutoHCT- Cohort (CIBMTR) (%) | p-value |
|---|---|---|---|
| Number of patients | 174 | 175 | |
| Median age at diagnosis, years (range) | 55 (31–69) | 53 (22–69) | <0.0001 |
| 18–39 | 12 (7) | 23 (13) | |
| 40–49 | 33 (19) | 47 (27) | |
| 50–59 | 66 (38) | 57 (33) | |
| 60–64 | 31 (18) | 31 (18) | |
| 65–70 | 32 (18) | 17 (10) | |
| Median Age at autoHCT, years (range) | N/A | 55 (23–75) | N/A |
| ≥65 | 30 (17) | ||
| Male sex | 100 (57) | 105 (60) | 0.63 |
| KPS at diagnosis | |||
| 90–100% | 58 (33) | Not available | N/A |
| <90% | 52 (30) | ||
| Missing | 64 (37) | ||
| Karnofsky Score at autoHCT | |||
| 90–100% | N/A | 114 (65) | |
| <90% | 46 (26) | ||
| Missing | 15 (9) | ||
| Race | 0.008 | ||
| Caucasian | 149 (86) | 162 (93) | |
| African-American | 12 (7) | 5 (3) | |
| Other1 | 13 (7) | 3 (1) | |
| Unknown | 0 | 5 (3) | |
| Advanced stage (III/IV) at diagnosis | 155 (89) | 140 (80) | 0.06 |
| Missing | 0 | 4 (2) | |
| WHO Grade | 0.04 | ||
| 1/2 | 117 (67) | 104 (59) | |
| 3 | 40 (23) | 62 (35) | |
| Unknown | 17 (10) | 9 (5) | |
| Median time from diagnosis to HCT, months (range) | N/A | 23 (6–86) | |
| Median time from treatment failure to HCT, months (range) | N/A | 6 (2–82) | |
| <1 year | 123 (70) | ||
| ≥1 year | 52 (30) | ||
| Bone marrow positive at diagnosis | 86 (49) | 75 (43) | 0.11 |
| Missing | 15 (8) | 9 (5) | |
| Extranodal sites involved at diagnosis | 120 (69) | 117 (67) | 0.78 |
| Missing | 7 (4) | 9 (5) | |
| Normal LDH at diagnosis | 80 (46) | 34 (19) | 0.02 |
| Unknown | 51 (29) | 104 (59) | |
| FLIPI Score at diagnosis | |||
| Good (0–1) | 24 (14) | Not available | |
| Intermediate (2) | 48 (28) | ||
| Poor (3–5) | 65 (37) | ||
| Unknown | 37 (21) | ||
| Front-line therapy | <0.0001 | ||
| R-CHOP | 100 (57) | 139 (79) | |
| R-CVP | 36 (21) | 20 (11) | |
| R-Fludarabine-containing | 32 (18) | 6 (3) | |
| R-Other2 | 6 (3) | 10 (6) | |
| Response to front-line therapy | 0.004 | ||
| CR/PR | 116 (67) | 136 (78) | |
| Progressive disease | 15 (9) | 5 (3) | |
| Stable disease | 38 (21) | 22 (13) | |
| Unknown | 5 (3) | 12 (7) | |
| Median lines of therapy prior to HCT (range) | N/A | 2 (1–6) | |
| Remission status before HCT | |||
| Complete remission | N/A | 70 (40) | |
| Partial remission | 68 (39) | ||
| Chemorefractory | 30 (17) | ||
| Untreated | 2 (1) | ||
| Missing | 5 (3) | ||
| Conditioning Regimen | |||
| TBI-based | N/A | 23 (13) | |
| BEAM and similar | 119 (68) | ||
| CBV or similar | 27 (15) | ||
| BuMEL/BuCy | 5 (3) | ||
| Carboplatin/Methotrexate/Thiotepa | 1 (<1) | ||
| Year of transplant | |||
| 2002–2003 | N/A | 2 (1) | |
| 2004–2005 | 33 (19) | ||
| 2006–2007 | 65 (37) | ||
| 2008–2009 | 70 (40) | ||
| 2010–2011 | 4 (2) | ||
| 2012 | 1 (<1) | ||
| Median follow-up of survivors (range), months-from 4 months after failure | 76 (2–130) | 71 (6–131) | |
Abbreviations: AutoHCT=autologous hematopoietic cell transplantation; BEAM=carmustine, etoposide, cytarabine, melphalan; BuCy=busulfan, cyclophosphamide; BuMel=busulfan, melpahlan; CBV=cyclophosphamide, carmustine; etoposide; CHOP=cyclophosphamide, doxorubicin, vincristine, prednisone; CIBMTR: Center for International Blood and Marrow Transplant Research; CVP= cyclophosphamide, vincristine, prednisone; FLIPI=follicular lymphoma international prognostic index; KPS=Karnofsky Performance Score; LDH=lactate dehydrogenase; NLCS=National LymphoCare Study; R=rituximab; TBI=total body irradiation.
CIBMTR cohort: Asian (n=1), Native American (n=2); NLCS cohort: Not otherwise specified=13
CIBMTR cohort: Carboplatin/R-CHOP (n=1), R/carboplatin/VP16/ifosphamide (n=1), R-steroids/cyclophosphamide/ara-C/vincristine (n=1), R-ara-c/doxorubicin/cyclophosphamide (n=1), R-cyclophosphamide/pentostatin (n=1), R-cyclophosphamide/vincristine (n=2), R-doxorubicin/vincristine (n=1), R-doxorubicin/bleomycin/dacarbazine/vinblastine (n=1), R-taxol (n=1). NLCS cohort: R/carboplatin/VP16/ifosphamide (n=1), R-cyclophosphamide/ara-c/doxorubin/VP16/steroid/vincristine/methotrexate/bleomycin (n=1), R-cyclophosphamide/vincristine (n=2), R-cisplatin/cyclophosphamide/ara-c/doxorubicin/VP16/methotrexate/steroids/vincristine (n=1), R-bortezomib/leukine (n=1)
Among the autoHCT cohort patients, the median lines of therapy before HCT were two (range: 1–6) and 32 patients (18%) had chemorefractory or untreated relapsed disease at the time of transplantation. BEAM (carmustine, etoposide, cytarabine and melphalan) was the most commonly used conditioning regimen for transplantation (68%). Twenty patients (11%) received planned rituximab therapy after autoHCT. Baseline characteristics of propensity score matched patients are shown in Supplemental Tables S3.
Overall Survival and Predictors of Overall Survival
The median follow-up of survivors in the non-autoHCT and autoHCT cohorts was 76 months (range: 2–130) and 71 months (range: 6–131), respectively. On univariate analysis, the two year OS (calculated from the landmark point) for the non-autoHCT and autoHCT cohorts was 76% and 82%, respectively (p=0.21; Table 2). The respective figures for five year OS are 60% and 67% (p=0.16; Table 2 and Figure-2). On MVA (Table 3), autoHCT in FL with ETF was not associated with a significantly decreased risk of mortality (HR=0.88; 95%CI=0.61–1.26; p=0.49). The only factor independently associated with a higher risk of mortality (i.e. inferior OS) was age ≥60 years at the time of FL diagnosis (HR=2.49; 95%CI=1.18–5.25; p=0.02). Pattern of frontline treatment failure (early relapse/progression vs. primary refractory disease) was not predictive of OS (Supplemental Tables S4 and S5). MVA using the propensity score matched data also showed non-significance (HR=0.84; 95%CI=0.56–1.25; p=0.38).
Table 2.
Univariate comparison of overall survival between non-HCT and auto-HCT cohorts.
| Outcomes | Non-HCT cohort (NLCS Dataset) | Auto-HCT cohort (CIBMTR Dataset) | FDR adjusted p-value | ||
|---|---|---|---|---|---|
| N Eval | Prob (95% CI) | N Eval | Prob (95% CI) | ||
| Overall survival | 174 | 175 | |||
|
| |||||
| 1-year | 84 (78–89)% | 88 (84–93)% | 0.25 | ||
|
| |||||
| 2-year | 76 (69–82)% | 82 (76–88)% | 0.25 | ||
|
| |||||
| 3-year | 70 (63–77)% | 77 (71–83)% | 0.25 | ||
|
| |||||
| 5-year | 60 (52–67)% | 67 (60–75)% | 0.25 | ||
Abbreviations: N Eval=number evaluable, Prob=probability.
Figure 2.
Overall Survival for non-autoHCT cohort vs. autoHCT cohort patients receiving HCT within 1 year of ETF.
Table 3.
Multivariate analysis for overall survival.
| Overall Survival | N Eval | Hazard Ratio | 95% CI Lower Limit | 95% CI Upper Limit | P-value | Overall p-value |
|---|---|---|---|---|---|---|
| Main Effect | ||||||
| Non-HCT | 174 | 1 | 0.49 | |||
| Auto-HCT | 175 | 0.88 | 0.61 | 1.26 | 0.49 | |
| Age at Diagnosis, | ||||||
| years | ||||||
| 18–39 | 35 | 1 | 0.002 | |||
| 40–49 | 80 | 1.10 | 0.49 | 2.47 | 0.82 | |
| 50–59 | 123 | 1.50 | 0.70 | 3.21 | 0.30 | |
| ≥60 | 111 | 2.49 | 1.18 | 5.25 | 0.02 | |
| Contrast | ||||||
| 40–49 vs 50–59 | 0.7339 | 0.4365 | 1.2340 | 0.2433 | ||
| 40–49 vs ≥60 | 0.4427 | 0.2686 | 0.7299 | 0.0014 | ||
| 50–59 vs ≥60 | 0.6033 | 0.4068 | 0.8946 | 0.0119 |
Abbreviations: N Eval=number evaluable, Prob=probability.
Overall Survival (Planned Subgroup Analysis)
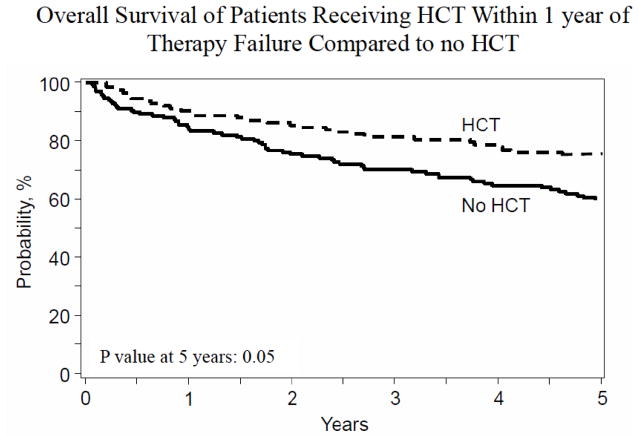
To account for differences in the timing of the application of autoHCT in relapse/refractory FL across various centers, we performed a pre-planned subgroup analysis comparing OS between non-autoHCT cohort patients (n=174) and CIBMTR patients undergoing “early” autoHCT (i.e. HCT within one year of ETF; n=123) (Table S2 for baseline characteristics). On univariate analysis, the two year OS for the non-autoHCT and early autoHCT cohorts was 76% and 84%, respectively (p=0.11; Table 4). The respective figures for five year OS are 60% and 73%, respectively (p=0.05; Table 4 and Figure 2). On MVA, early autoHCT in FL with ETF was the only factor associated with a decreased risk of mortality (HR=0.63; 95%CI=0.42–0.94; p=0.02). Next we performed MVA using the propensity score matched data. The propensity score matched patients (as showing in supplemental Table S3) across two groups had no significant differences in terms of age at diagnosis, race, disease stage, FL grade, LDH, and type or response to frontline chemoimmunotherapies. MVA using the propensity matched data also showed a significantly decreased risk of mortality with early autoHCT in FL with ETF (HR=0.55; 95%CI=0.36–0.84; p=0.005). Characteristics of CIBMTR patients undergoing early vs. late autoHCT are showing in Table S6 in Supplemental Appendix.
Table 4.
Univariate comparison of overall survival between non-HCT and auto-HCT cohort patients receiving transplantation within 1 year of early therapy failure.
| Outcomes | Non-HCT cohort (NLCS Dataset) | Auto-HCT within 1year of ETF cohort (CIBMTR Dataset) | FDR Adjusted p-value* | ||
|---|---|---|---|---|---|
| N Eval | Prob (95% CI) | N Eval | Prob (95% CI) | ||
| Overall survival | 174 | 123 | |||
|
| |||||
| 1-year | 84 (78–89)% | 90 (85–94)% | 0.12 | ||
|
| |||||
| 2-year | 76 (69–82)% | 84 (79–90)% | 0.011 | ||
|
| |||||
| 3-year | 70 (63–77)% | 80 (74–87)% | 0.08 | ||
|
| |||||
| 5-year | 60 (52–67)% | 73 (66–81)% | 0.05 | ||
Abbreviations: ETF=early therapy failure; N Eval=number evaluable, Prob=probability.
DISCUSSION
ETF following chemoimmunotherapy is a new and validated prognostic marker of poor survival in FL6,7,18. Our data, with a median follow-up of over six years, suggest that early use of autoHCT improves OS of FL patients with ETF, if used within one year of ETF in select groups of patients who may be candidates for an aggressive approach. The overall study population showed no significant difference in OS between the non-autoHCT and autoHCT cohorts (60% vs 67% respectively; p=0.16). However a planned subgroup analysis demonstrated that early autoHCT in FL with ETF was the only factor associated with a decreased risk of mortality (HR=0.63; 95%CI=0.42–0.94; p=0.02). These results are reminiscent of studies suggesting autoHCT is most effective when used early in the disease course for relapsed/refractory FL14,19.
Several retrospective series have reported promising outcomes of autoHCT in relapsed/refractory FL14,20,21, however there are few randomized studies evaluating this question15,22. The randomized European CUP study showed an OS advantage for high dose chemotherapy and autoHCT compared to standard chemotherapy in relapsed FL, but accrual was not completed15. In the prospective randomized LYM1 study, favorable disease control was also observed with autoHCT for relapsed FL22. For both these prospective studies, patients either did not receive rituximab, or were required to be rituximab naïve, making results difficult to extrapolate to the modern era. An analysis from the National Comprehensive Cancer Network (NCCN) reported outcomes of autoHCT in relapsed FL treated with modern chemoimmunotherapy induction, and demonstrated plateaus in survival suggestive of cure in a subset of FL patients16.
In the present analysis, the autoHCT and non-autoHCT populations received a variety of both anthracycline and non-anthracycline containing induction regimens. Patients undergoing watchful waiting/observation as part of their induction strategy were not included. Our study did not include patients receiving bendamustine and rituximab (BR) as frontline therapy, as the era of this analysis pre-dates publication of the German STiL trial23. While lack of patients treated with BR, and the more frequent use of R-CHOP in the autoHCT cohort can be perceived as possible limitations of the present analysis, it is critical to highlight that OS was our primary endpoint, and none of the currently used frontline chemoimmunotherapy regimens in FL have shown a survival benefit over each other to date23,24. In line with published data, our MVA found no impact of the type of induction regimen on FL OS.
Grade three FL was more frequent in the autoHCT cohort of this analysis. In the current data set we cannot discriminate between grade 3a or 3b disease, since neither NLCS nor CIBMTR (during the era of this investigation) collected data to differentiate between 3a vs. 3b disease. However as grade 3b disease is very uncommon, we would expect those numbers to be small. Patients with known transformation were excluded from both cohorts. A recent analysis from the PRIMA study suggests that patients with biopsy proven disease progression may be enriched for histologic transformation.25 In that report, autoHCT was of greatest benefit to patients with transformed disease. Publications from the NLCS26 and from other population based studies27 demonstrate that patients with transformation established by clinical versus histological criteria have identical median survival outcomes; and are consistent with prospectively acquired datasets mandating biopsies for transformed FL. This implies reliability on the part of the treating physicians in the CIBMTR and NLCS to recognize and exclude transformed disease, and also of the data quality. It also suggests that unrecognized transformed cases are not over-represented in our study.
In both cohorts, the median age was young (55 in the non-autoHCT group, 53 in the autoHCT group). The younger age may explain in part the observed five year OS in the non-autoHCT cohort of patients (60%), compared to 50% OS at five years in the original NLCS publication, which included many older patients6. However similar to the analysis from the NCCN16, the only factor independently associated with a higher risk of mortality in our series was age ≥60 years at the time of FL diagnosis. To address possible cofounders influencing outcomes between the groups, another MVA was performed using matched data based on propensity scores (see results section). After propensity score matching, we found no significant differences between baseline characteristics of the two groups, and saw consistency with results in our subset analysis without matching.
The fact that only 20 patients (11%) received planned rituximab therapy after autoHCT in the CIBMTR cohort, precludes us from assessing the impact of maintenance rituximab following autoHCT. Since rituximab maintenance post autoHCT was not shown to improve OS in a randomized study22,28, it is unlikely that the infrequent use of rituximab maintenance in CIBMTR cohort would impact survival outcomes of current analysis.
The German Low Grade Lymphoma Study Group (GLSG) has also evaluated autoHCT as an effective second-line treatment option for FL patients with ETF, in a predominantly rituximab-naïve patient cohort29. In their secondary analysis of two randomized trials, autoHCT for patients with ETF was associated with significantly higher five year OS (77% versus 46%). A separate analysis from the NLCS, observed that <2% of patients undergoing any second-line therapy for FL were offered autoHCT as part of their subsequent treatment strategy30. This is also consistent with CIBMTR’s estimate of autoHCT utilization rates in FL of ~1–1.5% (unpublished data), and suggests more widespread underutilization of this potentially effective therapy. In our group of uniformly chemoimmunotherapy treated patients, there was an observed survival benefit with early autoHCT in a select subgroup of patients. The possible reason for the greater benefit of autoHCT earlier in the ETF FL disease course might be that such patient population potentially has biologically less refractory and more chemoresponsive state deriving incrementally greater benefit from HDT, and is in line with observations from pre rituximab era.14,19 On this basis, we feel that auto-HCT should be considered as a possible therapeutic approach for ETF FL patients who are candidates for aggressive treatment strategies.
Currently there are no standard therapeutic options for high-risk early failure FL, and several groups are investigating novel therapies in this space. Despite the plethora of drugs under consideration for relapsed/refractory FL, few have evaluated the ETF patient specifically. In an unplanned subset analysis using idelalisib31, early relapsing FL responded similarly to the rest of the population with median PFS of only 11 months. An abstract evaluating lenalidomide/rituximab in early relapsing FL reported ORR 48%, though many patients received only rituximab as front-line monotherapy. The GADOLIN study used obinutuzumab/bendamustine in relapsed FL, and while demonstrating an OS benefit32, follow-up remains short, and is difficult to apply in this setting to the early progressor population.
Unfortunately the NLCS registry was unable to provide information on second line therapy for ETF patients. However given lack of standardized therapy for FL with ETF, and the possibility of long term OS possible with autoHCT in relapsed FL, we believe that this approach should be considered as a potential therapeutic option in the appropriate setting and for the right patient, ideally in the context of a clinical trial.
Preferably, future treatment approaches for patients with ETF should incorporate a deep understanding of the molecular characteristics of chemoresistance to allow a precision approach, whether or not autoHCT is used as part of the paradigm. The m7-FLIPI, a new clinicopathologic tool, incorporates frequently mutated FL genes paired in a MVA with poor clinical risk factors (poor performance status, high-risk FLIPI) to predict failure-free survival. While the m7-FLIPI groups patients into low or high-risk groups, it captured only about 50% of patients with early relapse7. Further refinement and validation of these biologic predictors can optimize risk-stratified treatments33, with consideration for autoHCT after initial induction therapy. The m7-FLIPI will be prospectively tested in the upcoming SWOG 1608 study, randomizing patients with early relapsing FL to obinutuzumab + CHOP vs. obinutuzumab + lenalidomide vs. obinutuzumab + TGR1202, a PI3kinase inhibitor. AutoHCT is permitted as consolidation on this trial. With refined understanding of high-risk FL biology, and identification of predictive biomarkers at diagnosis of FL, autoHCT could be considered as a component of precision medicine trials with a goal of changing the natural history of high-risk FL.
Supplementary Material
Highlights.
Patients with follicular lymphoma (FL) experiencing early therapy failure (ETF) within two years of frontline chemoimmunotherapy have poor overall survival (OS).
FL patients with ETF receiving autoHCT soon after treatment failure (≤1year of ETF; n=123) had higher five year OS than those without autoHCT (73% vs 60%).
Acknowledgments
CIBMTR Support List
The CIBMTR is supported by Public Health Service Grant/Cooperative Agreement U24-CA076518 from the National Cancer Institute (NCI), the National Heart, Lung and Blood Institute (NHLBI) and the National Institute of Allergy and Infectious Diseases (NIAID); a Grant/Cooperative Agreement 5U10HL069294 from NHLBI and NCI; a contract HHSH250201200016C with Health Resources and Services Administration (HRSA/DHHS); two Grants N00014-13-1-0039 and N00014-14-1-0028 from the Office of Naval Research; and grants from *Actinium Pharmaceuticals; Allos Therapeutics, Inc.; *Amgen, Inc.; Anonymous donation to the Medical College of Wisconsin; Ariad; Be the Match Foundation; *Blue Cross and Blue Shield Association; *Celgene Corporation; Chimerix, Inc.; Fred Hutchinson Cancer Research Center; Fresenius-Biotech North America, Inc.; *Gamida Cell Teva Joint Venture Ltd.; Genentech, Inc.;*Gentium SpA; Genzyme Corporation; GlaxoSmithKline; Health Research, Inc. Roswell Park Cancer Institute; HistoGenetics, Inc.; Incyte Corporation; Jeff Gordon Children’s Foundation; Kiadis Pharma; The Leukemia & Lymphoma Society; Medac GmbH; The Medical College of Wisconsin; Merck & Co, Inc.; Millennium: The Takeda Oncology Co.; *Milliman USA, Inc.; *Miltenyi Biotec, Inc.; National Marrow Donor Program; Onyx Pharmaceuticals; Optum Healthcare Solutions, Inc.; Osiris Therapeutics, Inc.; Otsuka America Pharmaceutical, Inc.; Perkin Elmer, Inc.; *Remedy Informatics; *Sanofi US; Seattle Genetics; Sigma-Tau Pharmaceuticals; Soligenix, Inc.; St. Baldrick’s Foundation; StemCyte, A Global Cord Blood Therapeutics Co.; Stemsoft Software, Inc.; Swedish Orphan Biovitrum; *Tarix Pharmaceuticals; *TerumoBCT; *Teva Neuroscience, Inc.; *THERAKOS, Inc.; University of Minnesota; University of Utah; and *Wellpoint, Inc. The views expressed in this article do not reflect the official policy or position of the National Institute of Health, the Department of the Navy, the Department of Defense, Health Resources and Services Administration (HRSA) or any other agency of the U.S. Government.
*Corporate Members
Morgan Geronime for administrative Support
Funding: CIBMTR
Footnotes
Disclosure of conflict of interest: No disclosures to report.
Author contributions:
Conception and design: Carla Casulo, Jonathan W. Friedberg and Mehdi Hamadani.
Financial support: CIBMTR
Collection and assembly of data: Alyssa DiGilio and Mehdi Hamadani.
Data analysis: Kwang W. Ahn, Alyssa DiGilio and Mehdi Hamadani.
Interpretation: All authors.
Manuscript writing: First draft prepared by Carla Casulo, Jonathan W. Friedberg and Mehdi Hamadani. All authors helped revised the manuscript.
Final approval of manuscript: All authors
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Morton LM, Wang SS, Devesa SS, Hartge P, Weisenburger DD, Linet MS. Lymphoma incidence patterns by WHO subtype in the United States, 1992–2001. Blood. 2006;107(1):265–276. doi: 10.1182/blood-2005-06-2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tan D, Horning SJ, Hoppe RT, et al. Improvements in observed and relative survival in follicular grade 1–2 lymphoma during 4 decades: the Stanford University experience. Blood. 2013;122(6):981–987. doi: 10.1182/blood-2013-03-491514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Casulo C, Burack WR, Friedberg JW. Transformed follicular non-Hodgkin lymphoma. Blood. 2015;125(1):40–47. doi: 10.1182/blood-2014-04-516815. [DOI] [PubMed] [Google Scholar]
- 4.Salles G, Seymour JF, Offner F, et al. Rituximab maintenance for 2 years in patients with high tumour burden follicular lymphoma responding to rituximab plus chemotherapy (PRIMA): a phase 3, randomised controlled trial. Lancet. 2011;377(9759):42–51. doi: 10.1016/S0140-6736(10)62175-7. [DOI] [PubMed] [Google Scholar]
- 5.Press OW, Unger JM, Rimsza LM, et al. Phase III randomized intergroup trial of CHOP plus rituximab compared with CHOP chemotherapy plus (131)iodine-tositumomab for previously untreated follicular non-Hodgkin lymphoma: SWOG S0016. J Clin Oncol. 2013;31(3):314–320. doi: 10.1200/JCO.2012.42.4101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Casulo C, Byrtek M, Dawson KL, et al. Early Relapse of Follicular Lymphoma After Rituximab Plus Cyclophosphamide, Doxorubicin, Vincristine, and Prednisone Defines Patients at High Risk for Death: An Analysis From the National LymphoCare Study. J Clin Oncol. 2015;33(23):2516–2522. doi: 10.1200/JCO.2014.59.7534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jurinovic V, Kridel R, Staiger AM, et al. Clinicogenetic risk models predict early progression of follicular lymphoma after first-line immunochemotherapy. Blood. 2016;128(8):1112–1120. doi: 10.1182/blood-2016-05-717355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maurer MJ, Bachy E, Ghesquieres H, et al. Early event status informs subsequent outcome in newly diagnosed follicular lymphoma. Am J Hematol. 2016;91(11):1096–1101. doi: 10.1002/ajh.24492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Philip T, Guglielmi C, Hagenbeek A, et al. Autologous bone marrow transplantation as compared with salvage chemotherapy in relapses of chemotherapy-sensitive non-Hodgkin's lymphoma. N Engl J Med. 1995;333(23):1540–1545. doi: 10.1056/NEJM199512073332305. [DOI] [PubMed] [Google Scholar]
- 10.Hamadani M, Hari PN, Zhang Y, et al. Early failure of frontline rituximab-containing chemo-immunotherapy in diffuse large B cell lymphoma does not predict futility of autologous hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2014;20(11):1729–1736. doi: 10.1016/j.bbmt.2014.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Friedberg JW, Neuberg D, Gribben JG, et al. Autologous bone marrow transplantation after histologic transformation of indolent B cell malignancies. Biol Blood Marrow Transplant. 1999;5(4):262–268. doi: 10.1053/bbmt.1999.v5.pm10465106. [DOI] [PubMed] [Google Scholar]
- 12.El-Najjar I, Boumendil A, Luan JJ, et al. The impact of total body irradiation on the outcome of patients with follicular lymphoma treated with autologous stem-cell transplantation in the modern era: a retrospective study of the EBMT Lymphoma Working Party. Ann Oncol. 2014;25(11):2224–2229. doi: 10.1093/annonc/mdu440. [DOI] [PubMed] [Google Scholar]
- 13.Waterman J, Rybicki L, Bolwell B, et al. Fludarabine as a risk factor for poor stem cell harvest, treatment-related MDS and AML in follicular lymphoma patients after autologous hematopoietic cell transplantation. Bone Marrow Transplant. 2012;47(4):488–493. doi: 10.1038/bmt.2011.109. [DOI] [PubMed] [Google Scholar]
- 14.Rohatiner AZ, Nadler L, Davies AJ, et al. Myeloablative therapy with autologous bone marrow transplantation for follicular lymphoma at the time of second or subsequent remission: long-term follow-up. J Clin Oncol. 2007;25(18):2554–2559. doi: 10.1200/JCO.2006.09.8327. [DOI] [PubMed] [Google Scholar]
- 15.Schouten HC, Qian W, Kvaloy S, et al. High-dose therapy improves progression-free survival and survival in relapsed follicular non-Hodgkin's lymphoma: results from the randomized European CUP trial. J Clin Oncol. 2003;21(21):3918–3927. doi: 10.1200/JCO.2003.10.023. [DOI] [PubMed] [Google Scholar]
- 16.Evens AM, Vanderplas A, LaCasce AS, et al. Stem cell transplantation for follicular lymphoma relapsed/refractory after prior rituximab: a comprehensive analysis from the NCCN lymphoma outcomes project. Cancer. 2013;119(20):3662–3671. doi: 10.1002/cncr.28243. [DOI] [PubMed] [Google Scholar]
- 17.Cheson BD, Horning SJ, Coiffier B, et al. Report of an international workshop to standardize response criteria for non-Hodgkin's lymphomas. NCI Sponsored International Working Group. J Clin Oncol. 1999;17(4):1244. doi: 10.1200/JCO.1999.17.4.1244. [DOI] [PubMed] [Google Scholar]
- 18.Mauerer MGH, Ansell S, et al. Event-Free Survival at 12 Months (EFS12) from Diagnosis Is a Robust Endpoint for Disease-Related Survival in Patients with Follicular Lymphoma in the Immunochemotherapy Era. ASH abstract # 1664. 2014 [Google Scholar]
- 19.Vose JM, Bierman PJ, Loberiza FR, et al. Long-term outcomes of autologous stem cell transplantation for follicular non-Hodgkin lymphoma: effect of histological grade and Follicular International Prognostic Index. Biol Blood Marrow Transplant. 2008;14(1):36–42. doi: 10.1016/j.bbmt.2007.06.016. [DOI] [PubMed] [Google Scholar]
- 20.Le Gouill S, De Guibert S, Planche L, et al. Impact of the use of autologous stem cell transplantation at first relapse both in naive and previously rituximab exposed follicular lymphoma patients treated in the GELA/GOELAMS FL2000 study. Haematologica. 2011;96(8):1128–1135. doi: 10.3324/haematol.2010.030320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Evens AM, Vanderplas A, LaCascce A, et al. Outcomes with Autologous (Auto) and Allogeneic (Allo) Stem Cell Transplantation (SCT) for Relapsed/Refractory Follicular Lymphoma (FL) in the Post-Rituximab Era: A Comparative Analysis From the National Comprehensive Cancer Network (NCCN) Non-Hodgkin’s Lymphoma (NHL) Outcomes Database Project. Blood (abstract) 2011 [Google Scholar]
- 22.Pettengell R, Schmitz N, Gisselbrecht C, et al. Rituximab purging and/or maintenance in patients undergoing autologous transplantation for relapsed follicular lymphoma: a prospective randomized trial from the lymphoma working party of the European group for blood and marrow transplantation. J Clin Oncol. 2013;31(13):1624–1630. doi: 10.1200/JCO.2012.47.1862. [DOI] [PubMed] [Google Scholar]
- 23.Rummel MJ, Niederle N, Maschmeyer G, et al. Bendamustine plus rituximab versus CHOP plus rituximab as first-line treatment for patients with indolent and mantle-cell lymphomas: an open-label, multicentre, randomised, phase 3 non-inferiority trial. Lancet. 2013;381(9873):1203–1210. doi: 10.1016/S0140-6736(12)61763-2. [DOI] [PubMed] [Google Scholar]
- 24.Flinn IW, van der Jagt R, Kahl BS, et al. Randomized trial of bendamustine-rituximab or R-CHOP/R-CVP in first-line treatment of indolent NHL or MCL: the BRIGHT study. Blood. 2014;123(19):2944–2952. doi: 10.1182/blood-2013-11-531327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sarkozy C, Trneny M, Xerri L, et al. Risk Factors and Outcomes for Patients With Follicular Lymphoma Who Had Histologic Transformation After Response to First-Line Immunochemotherapy in the PRIMA Trial. J Clin Oncol. 2016;34(22):2575–2582. doi: 10.1200/JCO.2015.65.7163. [DOI] [PubMed] [Google Scholar]
- 26.Wagner-Johnston ND, Link BK, Byrtek M, et al. Outcomes of transformed follicular lymphoma in the modern era: a report from the National LymphoCare Study (NLCS) Blood. 2015;126(7):851–857. doi: 10.1182/blood-2015-01-621375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Al-Tourah AJ, Gill KK, Chhanabhai M, et al. Population-based analysis of incidence and outcome of transformed non-Hodgkin's lymphoma. J Clin Oncol. 2008;26(32):5165–5169. doi: 10.1200/JCO.2008.16.0283. [DOI] [PubMed] [Google Scholar]
- 28.Pettengell R. Durable benefit of rituximab maintenance post-autograft in patients with relapsed follicular lymphoma: 12 Year follow-up of the EBMT lymphoma working party LYM1 trial. International Conference on Malignant Lymphoma, abstract 014; 2017. [DOI] [PubMed] [Google Scholar]
- 29.Jurinovic VMB, Pfreundschuh M, et al. Autologous Stem Cell Transplantation for Patients with Early Progression of Follicular Lymphoma- Retrospective Analysis of 2 Randomized Trials of the German Low Grade Lymphoma Study Group (GLSG). American Society of Hematology Annual Meeting; 2016; 2016. Abstract 3464. [DOI] [PubMed] [Google Scholar]
- 30.Link BBM, Cerhan J, et al. Second-line therapy in follicular lymphoma in the United States: Report of NLCS observational study. J Clin Oncol. 2011;29 2011 (suppl; abstr 8049) [Google Scholar]
- 31.Gopal AK, Kahl BS, Flowers CR, et al. Idelalisib is effective in patients with high-risk follicular lymphoma and early relapse after initial chemoimmunotherapy. Blood. 2017;129(22):3037–3039. doi: 10.1182/blood-2016-12-757740. [DOI] [PubMed] [Google Scholar]
- 32.Sehn LH, Chua N, Mayer J, et al. Obinutuzumab plus bendamustine versus bendamustine monotherapy in patients with rituximab-refractory indolent non-Hodgkin lymphoma (GADOLIN): a randomised, controlled, open-label, multicentre, phase 3 trial. Lancet Oncol. 2016;17(8):1081–1093. doi: 10.1016/S1470-2045(16)30097-3. [DOI] [PubMed] [Google Scholar]
- 33.Huet SBT, Jais J, et al. Gene Expression Profiling Predicts Disease Progression in Follicular Lymphoma. International Conference on Malignant Lymphoma, abstract 104; 2017. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.