Abstract
Although cognitive impairment has been well documented in human immunodeficiency virus (HIV) and hepatitis C virus (HCV) mono-infections, research on neurocognitive effects is limited in the context of HIV/HCV co-infection. The aims of this study were to explore the interplay between HIV and HCV infections in the expression of neurocognitive impairment (NCI), and to examine the differences in test performance between HIV/HCV co-infected and HIV or HCV mono-infected patients. A total of 128 participants from southern Brazil underwent a comprehensive neuropsychological (NP) battery comprising 18 tests. Participants were grouped according to their serological status: HCV mono-infected (n=20); HIV mono-infected (n=48); HIV/HCV co-infected (n=12); HIV−/HCV− uninfected controls (n=48). The frequency of HIV subtype B and C between the HIV mono-infected and HIV/HCV co-infected groups were comparable. There was greater prevalence of neuropsychological impairment among all three infection groups compared with the uninfected control group, but no statistically significant differences among mono and co-infected groups were found. HCV infection was associated with cognitive deficits, independently of liver dysfunction. HCV infection did not show an additive effect on neurocognitive function among HIV+. NCI was independent of HCV RNA on peripheral blood, CSF and hepatic injury. While we did not find additive global effect, in the present study there was some evidence of additive HIV/HCV coinfection effects in speed of information processing, executive function and verbal fluency domains when comparing the co-infected group with the other 3 groups. NP impairment was not dependent on HCV subtypes.
Keywords: HCV, HIV associated neurocognitive disorders (HAND), HIV-1
INTRODUCTION
Hepatitis C virus (HCV) belongs to the family Flaviviridae, genus Hepacivirus. The Flaviviridae family includes several viruses with worldwide distribution and known neurotropism, such as the Zika virus (van de Beek and Brouwer 2017), Dengue virus (Puccioni-Sohler et al. 2013), West Nile virus, Saint Louis encephalitis virus, Murray Valley virus, and Japanese encephalitis virus (Solomon 2004). HCV infection is a major public-health problem, worldwide prevalent. A significant portion of patients with chronic HCV experience cognitive difficulties that may interfere with activities of daily living and quality of life (Hilsabeck et al 2005). There is evidence that HCV is neurotropic and may cause NP impairment even in the absence of advanced liver disease (Cherner et al. 2005).
HCV and human immunodeficiency virus (HIV) co-infection is common due to the fact that both viruses are highly prevalent in certain populations, and they share similar routes of transmission. Each virus has been associated with neurocognitive impairment (NCI) among mono-infected individuals. Deficits have been well documented in HIV (Schouten et al. 2011; de Almeida et al. 2013; Heaton et al. 2015) and HCV mono-infections (Forton et al. 2001; Hilsabeck et al. 2002; Hilsabeck et al. 2003; Weissenborn et al. 2004; McAndrews et al. 2005). However, research is more limited in the context of HIV/HCV co-infection and the evidence is mixed about whether HIV/HCV co-infection results in additive deleterious impacts on neurocognition (Hilsabeck et al. 2005; Yarlott et al. 2017).
The aim of this study was to explore and compare individual and possible additive effects of HIV and HCV infections in the expression of global cognitive impairment in a southern Brazilian population, where HIV-B and non B subtypes (chiefly subtype C) co-occur. We hypothesized that the prevalence of impairment would increase with the number of viral risk factors and that HCV seropositivity would contribute to cognitive impairment above and beyond the role of HIV serostatus.
To our knowledge, this is the first study evaluating the relationship between cognitive impairment and HIV/HCV co-infection in a population with mixed HIV subtypes.
DESIGN AND METHODS
This was a cross-sectional study. Standardized clinical and neurocognitive evaluations were performed on prospectively enrolled HCV+, HIV+ and HIV− individuals in Southern Brazil. They were recruited from the Hospital de Clinicas, Universidade Federal do Paraná (HC-UFPR), Curitiba, Paraná, Brazil between 2007 and 2013. The institutional review boards (IRB) at University of California San Diego; Hospital de Clínicas-UFPR, Brazil (IRB) and the Brazilian National Ethics Committee (CONEP) approved this project. All participants provided written informed consent.
1. Subjects
Participants and methods were described previously in detail (de Almeida et al. 2013). All participants underwent a comprehensive neuromedical assessment and none had opportunistic infections in the CNS. Exclusion criteria for all groups included loss of consciousness for greater than 30 minutes, non-HIV-related neurologic injury or disorder (e.g., epilepsy, stroke, developmental delay), psychotic disorder, and significant levels of current substance use defined as more than two alcoholic drinks per day over the past 30 days, or use of any illegal drugs in the past 30 days. For the HCV+ participants, additional exclusion criteria included treatment with interferon alpha and ribavirin in the last 12 months and uncompensated cirrhosis.
A total of 128 participants were studied. The participants belonged to the following groups according to serological status and co-infection. Group characteristics are presented in Table 1.
Table 1.
Demographic and disease characteristics across HIV and/or HCV status groups
| HIV-HCV− | HIV-HCV+ | HIV+HCV− | HIV+HCV+ | p1 | |
|---|---|---|---|---|---|
| N | 48 | 20 | 48 | 12 | |
| Age | 43 (34.5; 48.5) | 46 (39.5; 50) | 42.5 (36; 52) | 44.5 (37; 49) | 0.533 |
| Education, years | 8 (5; 11.5) | 10 (7.5; 11) | 10.5 (4; 12) | 9 (5.5; 12.5) | 0.956 |
| Male, n (%) | 25 (52) | 12 (60) | 24 (50) | 6 (50) | 0.896 |
| History of IDU, n (%) | 0 | 1(5) | 3(6.25) | 3(25) | 0.096 |
| Duration of HIV (months) | – | – | 94 (38; 136) | 78 (18; 135) | 0.573 |
| CD4 Nadir | – | – | 88 (22; 268) | 104 (42; 250) | 0.873 |
| CD4 Current | – | – | 360 (201; 602) | 380 (173; 470) | 0.712 |
| Log plasma HIV viral load | – | – | 1.7 (1.7; 2.6) | 3.14 (1.7; 4.4) | 0.1302 |
| Plasma HIV RNA ≤50 copies/mL, n (%) | – | – | 30 (62.5) | 5 (41.7) | 0.210 |
| Log CSF HIV viral load | – | – | 1.7 (1.7; 2.5) | 2.64 (1.7; 3.2) | 0.1102 |
| CSF HIV RNA ≤50 copies/mL, n (%) | – | – | 28 (58) | 4(33) | 0.20 |
| On ARV, n (%) | – | – | 38 (79) | 9 (75) | 0.710 |
| AIDS diagnosis, n (%) | – | – | 39 (81) | 8 (67) | 0.271 |
| Log plasma HCV viral load | – | 5.9 (5.1; 6.2) | – | 2.9 (1.7; 5.9) | 0.016 |
| CSF HCV viral load | – | – | – | 1.1 (1.1) | |
| HBV co-infection, n (%) | 0 | 0 | 0 | 1(8.3) | |
| Alb serum (g/dL) | 3.9 (3.7; 4.1) | 3.2 (2.9; 3.5) | 2.9 (2.5; 3.2) | 0.0002 | |
| APRI | – | 0.373 (0.223; 2.014) | 0.240 (0.182; 0.343) | 0.404 (0.306; 1.042) | 0.001 |
| FIB-4 | 1.131 (0.774; 3.851) | 0.838 (0.674; 1.139) | 1.385 (1.079; 2.141) | 0.004 | |
| BDI3 | 3 (1; 5.5) | 13 (3; 19.5) | 14 (7; 25) | 13 (10; 28) | <0.00011 |
Data presented in median (IQR) or n (%) as appropriate;
p, comparison between the four groups;
Comparisons of HIV RNA in CSF and plasma between HCV− and HCV+ groups using a log-normal accelerated failure time model; p value adjusted for ARV use;
Comparison between the three seropositives groups, HIV and/or HCV infected (p=0.503);
CD4 counts were quantified by flow cytometry (FACSCalibur-Multitest) at the Clinical Laboratory, Hospital de Clínicas, UFPR, Brazil. Nadir CD4 was extracted from the medical records.
APRI- asparte aminotransferase (AST) to platelet ratio index: APRI score >1.0 had a sensitivity of 76% and specificity of 72% for predicting cirrhosis (Wai et al. 2003; Lin et al. 2011; Chou et al. 2013). FIB-4-Fibrosis-4 score: FIB-4 score <1.45 had a negative predictive value of 90% for advanced fibrosis (Sterling et al. 2006).
IDU- injection drug use.
BDI- Beck depression inventory.
1.1. HCV mono-infected participants (HIV−/HCV+ group), n=20
Hepatitis C virus (HCV) participants were recruited from the hepatology out-clinic of the HC-UFPR, Curitiba, PR, Brazil. The median duration from HCV diagnosis was 63.23(IQR=12.52; 96.60) months. Distribution of hepatitis B (HBV) co-infection is shown in Table 1.
Regarding the HCV genotypes (Abrantes et al. 2013; Wolff et al. 2010), there were 14 cases (70%) with HCV subtype 1 [1a (6 cases); 1b (3 cases); 1 (5 cases)]; and 4 cases with HCV subtype 3 [3a (2 cases); 3 (2 cases)]. The HCV genotype could not be determined for two participants.
In the HIV−/HCV+ group, 10 participants (50%) previously received HCV treatment with interferon alpha (IFN-α) and ribavirin; none of the participants were currently on HCV treatment during NP evaluation. The median interval since IFN-α and ribavirin treatment was 62.20 (IQR=43.87; 94.67) months. The Child-Pugh Score for chronic hepatic disease, used to assess the prognosis of chronic liver disease and cirrhosis, of all HIV−/HCV+ participants was Child-A (Pugh et al. 1973). No one presented with hepatic encephalopathy during the current NP evaluation.
1.2. HIV mono-infected participants (HIV+/HCV− group), n=48
All participants underwent serological testing to confirm their HIV status prior to enrollment, according to guidelines published by the Brazilian Ministry of Health (Brasil, 2016). Cases with opportunistic CNS infections were excluded. HIV genotyping indicated 20 subjects had HIV subtype B, 27 had HIV subtype non B (C (20), BF (4), BC (1), F (2)), and for one subject the subtype was not identified. Table 1 reports HIV disease characteristics and treatment status for both HIV+ groups.
1.3. HIV/HCV co-infected group (HIV+/HCV+), n=12
None of the participants in the HIV+/HCV+ group had ever received HCV treatment and were not on treatment at the time of NP evaluation. HIV genotyping showed six subjects had HIV subtype B while six had HIV subtype non B (C (2), BF (3), CF (1)). The frequencies of HIV subtype B and non-B; and B and C between the HIV+/HCV− vs. HIV+/HCV+ groups are comparable (p= 0.75 and 0.26, respectively). None of HIV disease and treatment variables in Table 1 differ significantly between the HIV monoinfected group and the HIV/HCV co-infected group.
1.4. Uninfected controls (HIV−/HCV− group), n=48
HIV−/HCV− participants were recruited from the HC-UFPR blood bank and tested serologically negative for HIV, HBV, HCV, human T-lymphotrophic virus 1 and 2 (HTLV-1 and 2), and syphilis. HIV+ and HIV− groups were recruited to be comparable with respect to gender, age and years of education.
2. Methods
2.1. Neuropsychological (NP) assessments
All participants underwent a NP evaluation by the same study team from Brazil, supervised by a neuropsychologist. The NP battery assessed seven ability domains with 18 individual tests that have been widely used to study HIV infection in English- and non-English speaking countries (Heaton et al. 2015; Cysique et al. 2007; Ghate et al. 2015; Kanmogne et al. 2010; Royal III et al. 2012). Instruments not already validated for use in Brazil were translated into Brazilian Portuguese, back translated into English, and reviewed by several Brazilian native Portuguese speakers to ensure cultural and linguistic appropriateness (de Almeida et al. 2013).
The domains assessed included: Executive Function (Color Trails Test 2, Wisconsin Card Sorting Test-64 perseverative errors, Stroop Color word); Motor Performance (Grooved Pegboard Test-Dominant Hand (DH) and Non-DH); Verbal Fluency (phonemic: Controlled Oral Word Association Test (COWAT) and category: Animals and Actions); Attention/Working Memory (WMS-III Spatial Span); Learning and Memory (Brief Visuospatial Memory Test-Revised (BVMT-R) learning and delayed recall, Hopkins Verbal Learning Test-Revised (HVLT-R) learning and delayed recall; and Speed of Information Processing (WAIS-III Digit Symbol and Symbol Search, Trail Making Test A, Color Trails Test 1, Stroop Color Naming).
Examiner training
To ensure standardization of NP test administration, examiners (psychologists at UFPR) underwent training administered by the UCSD team from the USA, with at least one bilingual member present to facilitate discussion. Training sessions included demonstrations of each test or interview with a discussion regarding purpose and administration nuances. Several rounds of “mock testing” were conducted as well. Certification sessions subsequently took place using staff or patient volunteers from the hospital as test subjects. There were separate training teams for the neurobehavioral, neuromedical, and psychiatric modules of the battery. All certifications were done in Portuguese.
Demographic corrections for each NP test were developed using data from Brazilian 48 HIV− controls. Norms were statistically derived by methods previously described (de Almeida et al. 2013; Heaton et al. 2004). Briefly, raw scores for the individual tests were converted to normally distributed scaled scores, which have a mean of 10 and a standard deviation of 3. The scaled scores were then converted to T-scores (mean 50, SD 10), with regression-based adjustments for the effects of age, years of education, and sex. The global deficit score (GDS) method was used to classify overall NP impairment status as previously described (Heaton et al. 2004; Carey et al. 2004; Blackstone et al. 2012).
Calculation of deficit score
For each test, demographically corrected, T-scores were converted to deficit scores (DS) as follows: T >39 (normal, DS=0); 35–39 (mild impairment, DS=1); 30–34 (mild to moderate impairment, DS=2); 25–29 (moderate impairment, DS=3); 20–24 (moderate to severe impairment, DS=4); and <20 (severe impairment, DS=5). The Global Deficit Score (GDS) is the average of all test deficit scores. The GDS summarizes the number and severity of impaired test performances across the entire test battery consisting of 18 measures. Based on previous literature, a GDS cut-off ≥0.50 was used to classify overall NP impairment (Heaton et al. 2004; Carey et al. 2004; Carey et al. 2004b); a GDS beyond this cutoff virtually guarantees that the person meets Frascati international criteria for HIV associated neurocognitive disorder (HAND) (Blackstone et al. 2012; Antinori et al. 2007).
2.2. Laboratory Methods
HIV RNA levels in blood and CSF were quantified by branched DNA assay (Siemens) with nominal limit of detection 50 copies/mL. Pol sequences were used for HIV strain genotyping for participants with clinically resistant infection, while env sequences were used for all other participants. HIV genotyping was performed at the Virology Laboratory, Hospital de Clínicas, UFPR, Brazil.
HCV and HBV serostatus was assessed by HCV (anti-HCV) and HBV (antigens HBV, HBe; antibody anti-HBs, anti-HBc total, anti-HBc m, anti-HBe) testing by chemiluminescence (Architect, Abbott, Wiesbaden, Germany), performed at the Clinical Laboratory, Hospital de Clínicas, UFPR, Brazil. CSF HCV RNA was quantified by RealTime HCV (Abbott, Illinois, U.S.A), detection rage 12 to 100000000 UI/mL while plasma was quantified by artus HCV RG RT-PCR (Quiagen, Hilden, Germany), detection range 65 to 1000000 UI/mL. HCV genotyping by Real Time HCV Genotype II Assay (Abbott, Illinois, USA) was performed at the State Central Laboratory (LACEN-PR) Brazil.
2.3. Data analyses
Categorical variables were compared between groups using Fisher’s exact test. Continuous variables were compared with Student’s t-test, Mann–Whitney or Kruskal-Wallis test for non-parametric data, as appropriate. Correlations between variables were calculated using Spearman’s rank-order correlation. Results were considered statistically significant at the 5% alpha level. Cohen’s d or Hedge’s g effect sizes (and 95% confidence intervals), when appropriate, were reported for differences in numeric variables between groups. Results were corrected for multiple comparisons using the Benjamini-Hochberg (BH) method.
RESULTS
Among all HIV+ participants (N=60), 12 (20%) were co-infected with HCV. All four groups were similar with regard to sex, age, and years of education. The HIV+HCV+ and HIV+HCV− groups were comparable on CSF and plasma HIV RNA, duration of HIV infection, nadir and current CD4 (Table 1). Scores for cirrhosis (asparte aminotransferase to platelet ratio index, APRI) (Wai et al. 2003; Lin et al. 2011; Chou et al. 2013) and fibrosis (Fibrosis-4 score, FIB-4) (Sterling et al. 2006), as well as serum albumin are presented on table 1.
Global NP impairment
The frequencies of global NP impairment (GDS>0.5) by group were as follows: HIV-HCV−, n=10 (20.8%); HIV−/HCV+, 10 (50%); HIV+/HCV−, 30 (62.5%); HIV+/HCV+, 8 (66.7%); comparing all four groups, p= 0.0002. The co-infected group HIV+/HCV+ showed the highest percentage of global NP impairment, although the differences between co-infected and mono-infected groups did not reach significance (Figure 1). Logistic regression analysis of global NP impairment indicated that the odds of cognitive impairment for a participant with HIV−/HCV+, HIV+/HCV− and HIV+/HCV+ were 3.8, 6.33, and 7.6 times higher respectively, compared to a person without HIV or HCV infection (p=0.039, p<0.001, and p=0.013 respectively); p values were corrected for multiple comparisons with the BH method.
Figure 1. Frequency of global neuropsychological impairment based on global deficit score (GDS) > 0.5 in the HIV and HCV mono-infected, HIV/HCV co-infected and seronegative groups.
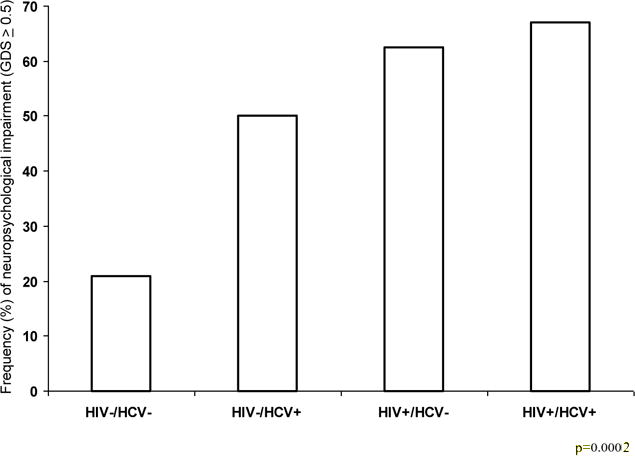
All seropositive groups were significantly worse than the seronegative group p=0.0002, but not different from one another.
All three infected groups, HIV and HCV mono-infected and the co-infected group, had a higher GDS (considered as a continuous variable) compared to the negative control group. For the HIV+/HCV− vs. HIV−/HCV+ comparison, there was a trend-level difference in mean GDS (non-adjusted p=0.058, adjusted for multiple testing p=0.087) (Figure 2).
Figure 2. Global NP impairment in the HIV and HCV mono-infected, HIV/HCV co-infected and serum negative group.
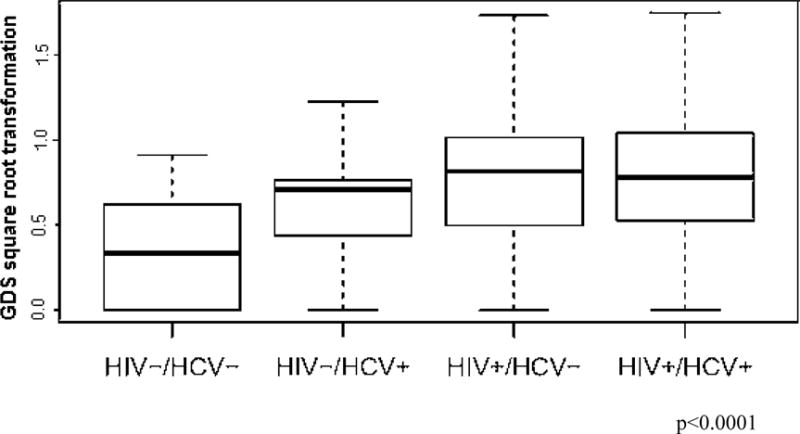
The global deficit scores in the HIV−/HCV+, HIV+/HCV− and HIV+/HCV+ groups were significantly higher than those in the HIV−/HCV− group (p=0.042, p<0.001, and p=0.001 respectively, after Benjamini-Hochberg correction). Differences between groups described above, assessed as Cohen’s d effect size (95% confidence interval), were −0.76(−1.31,−0.20), −1.13(−1.57,−0.69), and −1.27(−1.97,−0.57) respectively. The other comparisons were not significant.
The line in the center of the box represents median; the superior and inferior borders of the box represents IQRs; the whiskers represent the least and greatest values, excluding outliers which are defined as the values falling below the first quartile − 1.5 IQR or above the third quartile + 1.5 IQR.
Concerning the influence of HCV subtype on global NP impairment (GDS > 0.5), the proportions of NP impairment was higher in HCV-3 (3 cases, 75%) than HCV-1 (7 cases, 44%), although do not reach significance (p=0.582) due to the limited number of samples. GDS median (IQR) for HCV-1 was 0.417 (0.1528; 0.5694); for HCV-3 was 0.556 (0.4722 0.7917), p= 0.344; Hedge’s g effect sizes (95% CI) = −0.792 (−0.995, 0.412); GDS was square root transformed. The odds of cognitive impairment are reduced by 72% for a person with HCV-1 compared to a person with HCV-3; although not significant, 95% CI (0.004, 4.346).
The number of samples of different HIV subtypes was insufficient to analyze the influence of HIV subtype on global NP impairment in the HIV/HCV co-infected group.
Group HIV−/HCV+ comparison between groups with history of past IFN-α and Ribavirin treatment and treatment naïve
The HCV+/HIV− group median GDS (IQR) of participant subgroups with past HCV treatment (n=10, 50%) and treatment naïve status were 0.417 (0.028; 0.611) and 0.556 (0.222; 0.694) respectively, Mann-Whitney p= 0.44. Moreover, four treated participants (40%) had impaired GDS whereas six treatment naïve participants (60%) had impaired GDS (p= 0.66).
Cognitive impairment of tests by ability domain
Tables 2 and 3 compares the four groups with respect to impairment (T<40) rates on the individual test measures grouped by ability domain. Six of the 18 measures did not demonstrate significant overall group differences (Color Trails II, WAIS-III Symbol Search, HVLT-R Learning and Delayed Recall, WAIS-III Spatial Span and Grooved Pegboard DH), although the latter two measures evidenced nonsignificant trends for infected groups to have higher impairment rates than controls. The HIV monoinfected group had significantly elevated impairment rates, relative to controls, on 11 tests measures representing six of the seven ability domains, with a trend toward significance in the one test of attention/working memory (Spatial Span).
Table 2.
Proportions of impairment on ability domain and the individual test measures, compared between the four groups.
| Domain/NP Test | A:HIV−/HCV− | B:HIV−/HCV+ | C:HIV+/HCV− | D:HIV+/HCV+ | p Overall | p AxB | p AxC | p AxD | p BxC | p BxD | p CxD |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Executive Function | 11/48(22.9) | 4/20 (20.0) | 22/47(46.8) | 7/12(58.3) | 0.012 | 1.00 | 0.018 | 0.031 | 0.055 | 0.053 | 0.53 |
| Color Trails II | 8/48(16.7) | 3/20(15.0) | 13/47(27.7) | 4/12(33.3) | 0.360 | 1.00 | 0.220 | 0.230 | 0.360 | 0.380 | 0.73 |
| WCST (perseverative errors) | 8/48(16.7) | 0/14(0.00) | 12/43(27.9) | 5/11(45.5) | 0.019 | 0.18 | 0.220 | 0.053 | 0.027 | 0.009 | 0.29 |
| Stroop Color Word | 8/48(16.7) | 8/19(42.1) | 19/47(40.4) | 8/12(66.7) | 0.003 | 0.053 | 0.013 | 0.001 | 1.00 | 0.270 | 0.12 |
| Motor Performance | 6/48 (12.5) | 9/20 (45.0) | 25/48 (52.1) | 4/12(33.3) | <0.001 | 0.008 | <0.001 | 0.10 | 0.79 | 0.71 | 0.34 |
| Grooved Pegboard DH | 10/48(20.8) | 9/20(45.0) | 21/48(43.8) | 3/12(25.0) | 0.060 | 0.073 | 0.028 | 0.71 | 1.00 | 0.45 | 0.33 |
| Grooved Pegboard NDH | 5/48(10.4) | 11/20(55.0) | 27/48(56.2) | 5/12(41.7) | <0.001 | <0.001 | <0.001 | 0.021 | 1.00 | 0.72 | 0.52 |
| Verbal Fluency | 8/48(16.7) | 9/20(45.0) | 18/47(38.3) | 8/12(66.7) | 0.003 | 0.029 | 0.022 | 0.001 | 0.790 | 0.29 | 0.11 |
| Animal Fluency | 7/48(14.6) | 1/18(05.6) | 17/47(36.2) | 4/12(33.3) | 0.014 | 0.430 | 0.019 | 0.210 | 0.014 | 0.13 | 1.00 |
| Action Fluency | 8/47(17.0) | 8/14(57.1) | 18/41(43.9) | 5/10(50.0) | 0.005 | 0.005 | 0.009 | 0.038 | 0.540 | 1.00 | 0.74 |
| Letter Fluency | 6/48(12.5) | 7/20(35.0) | 12/47(25.5) | 6/12(50.0) | 0.023 | 0.045 | 0.120 | 0.009 | 0.550 | 0.47 | 0.16 |
| Attention/Working Memory | |||||||||||
| Spatial Span | 7/48(14.6) | 3/20(15.0) | 14/47(29.8) | 5/12(41.7) | 0.097 | 1.00 | 0.088 | 0.051 | 0.24 | 0.12 | 0.50 |
| Learning and Memory | 7/48(14.6) | 5/20(25.0) | 22/48(45.8) | 4/12(33.3) | 0.008 | 0.320 | 0.002 | 0.210 | 0.170 | 0.70 | 0.53 |
| HVLT-R Learning | 8/48(16.7) | 8/20(40.0) | 16/47(34.0) | 4/12(33.3) | 0.120 | 0.059 | 0.062 | 0.230 | 0.780 | 1.00 | 1.00 |
| BVMT-R Learning | 5/47(10.6) | 5/15(33.3) | 20/48(41.7) | 2/11(18.2) | 0.004 | 0.052 | <0.001 | 0.610 | 0.760 | 0.66 | 0.18 |
| HVLT-R Delayed Recall | 5/48(10.4) | 4/20(20.0) | 14/47(29.8) | 3/12(25.0) | 0.110 | 0.430 | 0.022 | 0.190 | 0.550 | 1.00 | 1.00 |
| BVMT-R Delayed Recall | 6/47(12.8) | 2/14(14.3) | 25/47(53.2) | 4/11(36.4) | <0.001 | 1.00 | <0.001 | 0.083 | 0.014 | 0.35 | 0.50 |
| SIP | 8/48 (16.7) | 5/20(25.0) | 23/48(47.9) | 7/12(58.3) | 0.002 | 0.500 | 0.002 | 0.006 | 0.110 | 0.130 | 0.75 |
| Trail-Making Test A | 7/48(14.6) | 2/18(11.1) | 18/45(40.0) | 4/12(33.3) | 0.015 | 1.00 | 0.009 | 0.210 | 0.036 | 0.180 | 0.75 |
| Color Trails I | 6/48(12.5) | 6/20(30.0) | 21/48(43.8) | 6/12(50.0) | 0.002 | 0.160 | 0.001 | 0.009 | 0.420 | 0.290 | 0.75 |
| WAIS-III Digit Symbol | 9/48(18.8) | 4/19(21.1) | 18/48(37.5) | 7/12(58.3) | 0.025 | 1.00 | 0.068 | 0.010 | 0.260 | 0.056 | 0.21 |
| WAIS-III Symbol Search | 7/48(14.6) | 4/20(20.0) | 13/48(27.1) | 4/12(33.3) | 0.330 | 0.720 | 0.210 | 0.210 | 0.760 | 0.430 | 0.73 |
| Stroop Color | 7/48(14.6) | 7/20(35.0) | 21/47(44.7) | 4/12(33.3) | 0.011 | 0.097 | 0.002 | 0.210 | 0.590 | 1.00 | 0.53 |
Results are N/total participants evaluated (%). Impairment when deficit score >0.5. “P” values were not corrected for multiple comparisons.
Significant differences are in bold typeface.
NP – Neuropsychological; SIP- speed of information processing
Table 3.
“P” values in Table 2 were corrected for multiple comparisons with Benjamini–Hochberg method, separately within individual tests.
| Domain/NP Test | p Overall | p AxB | p AxC | p AxD | p BxC | p BxD | p CxD |
|---|---|---|---|---|---|---|---|
| Executive Function | 0.012 | 1.00 | 0.083 | 0.083 | 0.083 | 0.083 | 0.64 |
| Color Trails II | 0.360 | 1.00 | 0.570 | 0.570 | 0.570 | 0.570 | 0.87 |
| WCST (perseverative errors) | 0.019 | 0.26 | 0.260 | 0.110 | 0.081 | 0.052 | 0.29 |
| Stroop Color Word | 0.003 | 0.11 | 0.038 | 0.008 | 1.00 | 0.330 | 0.18 |
| Motor Performance | <0.001 | 0.024 | <0.001 | 0.200 | 0.79 | 0.79 | 0.51 |
| Grooved Pegboard DH | 0.060 | 0.220 | 0.170 | 0.850 | 1.00 | 0.68 | 0.66 |
| Grooved Pegboard NDH | <0.001 | <0.001 | <0.001 | 0.041 | 1.00 | 0.86 | 0.78 |
| Verbal Fluency | 0.003 | 0.057 | 0.057 | 0.008 | 0.790 | 0.35 | 0.16 |
| Animal Fluency | 0.014 | 0.520 | 0.057 | 0.310 | 0.057 | 0.26 | 1.00 |
| Action Fluency | 0.005 | 0.028 | 0.028 | 0.077 | 0.810 | 1.00 | 0.89 |
| Letter Fluency | 0.023 | 0.130 | 0.240 | 0.055 | 0.550 | 0.55 | 0.24 |
| Attention/Working Memory | |||||||
| Spatial Span | 0.097 | 1.00 | 0.23 | 0.23 | 0.36 | 0.23 | 0.60 |
| Learning and Memory | 0.008 | 0.47 | 0.010 | 0.41 | 0.41 | 0.70 | 0.63 |
| HVLT-R Learning | 0.120 | 0.18 | 0.180 | 0.47 | 1.00 | 1.00 | 1.00 |
| BVMT-R Learning | 0.004 | 0.16 | 0.005 | 0.76 | 0.76 | 0.76 | 0.37 |
| HVLT-R Delayed Recall | 0.110 | 0.83 | 0.130 | 0.57 | 0.83 | 1.00 | 1.00 |
| BVMT-R Delayed Recall | <0.001 | 1.00 | <0.001 | 0.17 | 0.041 | 0.52 | 0.61 |
| SIP | 0.002 | 0.60 | 0.012 | 0.019 | 0.19 | 0.19 | 0.75 |
| Trail-Making Test A | 0.015 | 1.00 | 0.056 | 0.310 | 0.11 | 0.31 | 0.90 |
| Color Trails I | 0.002 | 0.32 | 0.007 | 0.027 | 0.50 | 0.43 | 0.75 |
| WAIS-III Digit Symbol | 0.025 | 1.00 | 0.140 | 0.061 | 0.31 | 0.14 | 0.31 |
| WAIS-III Symbol Search | 0.330 | 0.76 | 0.620 | 0.620 | 0.76 | 0.76 | 0.76 |
| Stroop Color | 0.011 | 0.29 | 0.010 | 0.410 | 0.71 | 1.00 | 0.71 |
A:HIV−/HCV−; B:HIV−/HCV+; C:HIV+/HCV−; D:HIV+/HCV+
NP – Neuropsychological; SIP- speed of information processing
Significant differences are in bold typeface.
Compared to the controls, the HCV monoinfected group showed significantly elevated impairment rates on only three of the 18 individual tests measures representing just two ability domains (Fluency and Motor), although trends in the same direction were seen on five other tests also representing Executive Function, Processing Speed, Learning and Delayed Recall (table 2). Also the HCV monoinfected group evidenced less impairment than one or both HIV infected group on four individual measures involving Processing Speed, Executive Function, Fluency and Delayed Recall.
The dually infected group had significantly elevated impairment rates, relative to controls in only 6 individual test measures in four ability domains (Executive Function, Processing Speed, Fluency and Motor), but with similar trends also in Attention/Working memory and delayed Recall. Notably, impairment rates did not differ significantly between the HIV monoinfection group and the HIV/HCV coinfection group on any test in the battery.
The proportion of impairment (deficit score) by neuropsychological test in the groups studied are showed on supplementary figure 1.
While we did not find additive global effect, in the present study there was some evidence of additive HIV/HCV coinfection effects in speed of information processing, executive function and verbal fluency domains when comparing the co-infected group with the other 3 groups (Tables 2 and 3).
Correlations
In the HIV/HCV coinfected and HCV monoinfected groups, there was no correlation of GDS with blood or CSF HCV RNA(Log), CSF HCV RNA was not quantified in the HCV monoinfected group. There was no correlation of GDS with APRI and FIB-4 scores in the three groups studied (all p>0.05).
In the HIV/HCV coinfected group, there was no correlation of blood or CSF HCV RNA(Log) with CSF or blood HIV RNA(Log); also there was no correlation of blood with CSF HCV RNA (Log).
DISCUSSION
The present study investigated the impact of HCV mono-infection and co-infection with HIV on cognitive functioning in a patient group from Southern Brazil. While each virus represents an independent risk factor for neuropsychological impairment, less is known about whether HCV/HIV co-infection increases the frequency and severity of cognitive impairment.
In the current study, HIV/HCV co-infection was present in 11% of the sample, which was considerably lower than was reported in another study (31%) from Southern Brazil (Wolff et al. 2010). The frequencies of HIV subtype B and C were similar between the HIV mono-infected and HIV/HCV co-infected groups; HCV subtype was predominantly subtype 1 (70%), this rate is similar to that reported in prior studies 79% and 82% (Abrantes et al. 2013; Wolff et al. 2010).
The present study found that HCV infection is associated with cognitive deficits, independently of liver dysfunction. The rates of cognitive impairment in the HCV mono-infected groups were consistent with multiple previous reports (Forton et al. 2001; Hilsabeck et al. 2002; Hilsabeck et al. 2003; Weissenborn et al. 2004; McAndrews et al. 2005), although rates of cognitive impairment can vary widely across studies (0% to 82%), possibly reflecting differences in comorbidities and ascertainment methods (Hilsabeck et al. 2002; Huckans et al. 2009).
In our sample, HCV infection did not show an additive effect on neurocognitive function among HIV+, as the HIV monoinfected and coinfected groups had similar rates of impairment (63% and 67%), and both tended to be greater than HCV alone (50%). GDS was not correlated with HCV RNA on peripheral blood, in the studied sample as observed in another previous study (Clifford et al. 2015). We also found that cognitive impairment was independent of HCV RNA on CSF, and hepatic injury, i.e. cirrhosis or hepatic fibrosis, identified by APRI and FIB-4 score respectively.
In the present study NP impairment was not dependent on HCV subtypes. This was the first study to address the impact of HCV subtypes on cognitive impairment, although the number of cases was small for a definitive conclusion, more studies are necessary.
The literature presents mixed results about whether HCV and HIV have additive consequences for neurocognitive functioning. These discrepant results may be explained by differences in populations studied related to CD4 count, adherence to ARV; and also differences in the severity of HIV/HCV disease or different NP battery applied or test norms. The results of the present study are consistent with studies that do not show additive effects of HIV/HCV coinfection, including the CHARTER study of 1,582 chronically HIV-infected patients and HIV/HCV co-infected in the USA (Clifford et al. 2015). By contrast, several previous investigations have found significantly worse global neuropsychological performance in HIV/HCV co-infected individuals compared to HIV mono-infected patients, suggesting an additive role of the two viruses in the pathogenesis of cognitive disorders (Clifford et al. 2005; Ciccarelli et al. 2013; Hinkin et al. 2008; Letendre et al. 2005; Rempel et al. 2013). In a study with infected women, HIV/HCV co-infected individuals demonstrated greater abnormal NP performance than mono-infected and uninfected, especially in individuals with evidence of CD4 T-lymphocyte immunosuppression (Richardson et al. 2005). Another study of 118 HIV+ adults with advanced HIV disease, 35 of whom were co-infected with HCV, found higher rates of global cognitive impairment in co-infected patients than those infected with HIV alone (63% vs. 43%) (Hinkin et al. 2008).
While we found no differences between HIV monoinfected and HIVHCV coinfected groups, it is important to note that there was a significant HCV effect on global neurocognitive functioning, i.e., the HCV monoinfected group evidenced significantly more global impairment than the uninfected controls (Figure 1), and either significant or trend level impairments (versus controls) were seen on tests of Fluency, Processing Speed, Learning, and Executive and Motor Functions.
Several studies have investigated domain-specific cognitive impairments in HCV+ patients and found deficits in attention, concentration, working memory, executive function and psychomotor speed (Hilsabeck et al. 2002; Hilsabeck et al. 2003; Weissenborn et al. 2005; Perry et al. 2008; Posada et al. 2009).
The literature on the impact of HCV infection on neurocognitive performance in Brazil is scant with only two reported studies (Abrantes et al. 2013; Quarantini et al. 2009). As these studies excluded participants with HIV they could not investigate the contribution of HIV/HCV co-infection to the prevalence of NP impairment. Although Abrantes et al (2013) found no evidence of an association between HCV infection and cognitive impairment, their study differs from the present study because control patients did not undergo blood testing to verify that they were free of viral infections. Additionally, a less extensive NP battery was used, which may have limited the sensitivity to detect impaired performances. The other reported study (Quarantini et al. 2009), compared participants with HCV and HBV but did not include a serum negative control group, they showed that HCV patients had poorer visuospatial memory performance than patients infected with HBV. There were no significant differences in other NP functions between HCV and HBV groups.
Cognitive impairment occurs in a substantial proportion of HIV-infected patients in the cART era from 15 to 62% (Schouten et al. 2011; de Almeida et al. 2013; Heaton et al. 2015). HAND is characterized by broad spanning deficits that include problems with executive function, learning and memory, speed of information processing, attention/working memory, sensorimotor skills, and language/verbal fluency (Grant 2008; Woods et al. 2009; Heaton et al. 2010).
Several hypotheses have been proposed to explain the occurrence of cognitive impairment in HCV infections. Microglia and astrocytes in the CNS express receptors associated with HCV cell entry, and may be particularly vulnerable to infection. HCV has been detected in human brain tissues indicating that blood-derived infected HCV cells trafficked to the brain and incite CNS dysfunction (Murray et al. 2008). The infection of the CNS begins with HCV viral replication in peripheral blood mononuclear cells in the bone marrow, which subsequently serve as precursors of macrophages and microglial cells of the CNS (Senzolo et al. 2011). Another potential contributor to cognitive impairment in chronic HCV is the indirect perpetuation of neurotoxic inflammatory pathways caused by HCV activation of cytokines (Rempel et al. 2013; Cacciarelli et al. 1996; Cohen et al. 2011); also it is thought to be mediated by HCV viral load (Letendre et al. 2005; Sun et al. 2013; Wilkinson et al. 2009). Additionally, there is speculation that brain injury might result from interactions between recreational drug use and hepatitis at multiple levels.
The present study is not free of limitations. Although the overall number of patients and controls was small, the calculated Cohen’s d effect sizes were strong. The cross-sectional design limits our ability to disentangle the neurocognitive effects of viral replication from longstanding individual differences in cognitive functioning. The HCV mono-infected group included four participants with somewhat advanced disease, suggested by a high APRI index and FIB-4 score that could indicate cirrhosis and hepatic fibrosis respectively. Nevertheless, these four participants were neuropsychologically normal. In the present study, 50% of the participants of the HCVmonoinfected group previously received treatment with IFN-α and ribavirin. However, none of the HCV positive participants were currently on HCV treatment during NP evaluation and the median duration since IFN-α and ribavirin treatment was 62 months. Cognitive impairment may improve after 18-months following IFN-α and ribavirin treatment (Cattie et al. 2014).
The positive points of this study include the fact that it compared mono-infected HIV or HCV and co-infected participants with a HIV-HCV− group. To our knowledge, this study was the first to included HIV genotyped participants subtype B and non-B participants chiefly subtype C. Furthermore, other similar Brazilian studies do not present results on global deficit impairment scores (i.e., GDS).
In our sample of 128 Brazilian adults, we observed higher rates of NP impairment among individuals infected with HCV and/or HIV compared to serum negative controls. Both mono-infected groups had elevated global impairment scores, although cognitive deficits appeared to be more associated with HIV than HCV mono-infection. While NP impairment was greatest in HIV/HCV co-infected patients, there was not a significant additive effect of viral co-infection on cognition compared to mono-infected groups.
Supplementary Material
Acknowledgments
FINANCIAL SUPPORT.
This work was supported by the following grants: National Institute of Health, NIH R21 MH76651 (Ellis, Ronald J; Almeida, Sergio M.)
The HIV Neurobehavioral Research Center (HNRC) is supported by Center award P30MH062512 from NIMH. The San Diego HIV Neurobehavioral Research Center [HNRC] group is affiliated with the University of California, San Diego, the Naval Hospital, San Diego, and the Veterans Affairs San Diego Healthcare System, and includes: Director: Robert K. Heaton, Ph.D., Co-Director: Igor Grant, M.D.; Associate Directors: J. Hampton Atkinson, M.D., Ronald J. Ellis, M.D., Ph.D., and Scott Letendre, M.D.; Center Manager: Thomas D. Marcotte, Ph.D.; Jennifer Marquie-Beck, M.P.H.; Melanie Sherman; Neuromedical Component: Ronald J. Ellis, M.D., Ph.D. (P.I.), Scott Letendre, M.D., J. Allen McCutchan, M.D., Brookie Best, Pharm.D., Rachel Schrier, Ph.D., Debra Rosario, M.P.H.; Neurobehavioral Component: Robert K. Heaton, Ph.D. (P.I.), J. Hampton Atkinson, M.D., Steven Paul Woods, Psy.D., Thomas D. Marcotte, Ph.D., Mariana Cherner, Ph.D., David J. Moore, Ph.D., Matthew Dawson; Neuroimaging Component: Christine Fennema-Notestine, Ph.D. (P.I.), Monte S. Buchsbaum, M.D., John Hesselink, M.D., Sarah L. Archibald, M.A., Gregory Brown, Ph.D., Richard Buxton, Ph.D., Anders Dale, Ph.D., Thomas Liu, Ph.D.; Neurobiology Component: Eliezer Masliah, M.D. (P.I.), Cristian Achim, M.D., Ph.D.; Neurovirology Component: David M. Smith, M.D. (P.I.), Douglas Richman, M.D.; International Component: J. Allen McCutchan, M.D., (P.I.), Mariana Cherner, Ph.D.; Developmental Component: Cristian Achim, M.D., Ph.D.; (P.I.), Stuart Lipton, M.D., Ph.D.; Participant Accrual and Retention Unit: J. Hampton Atkinson, M.D. (P.I.), Jennifer Marquie-Beck, M.P.H.; Data Management and Information Systems Unit: Anthony C. Gamst, Ph.D. (P.I.), Clint Cushman; Statistics Unit: Ian Abramson, Ph.D. (P.I.), Florin Vaida, Ph.D. (Co-PI), Bin Tang, Ph.D., Anya Umlauf, M.S.
The views expressed in this article are those of the authors and do not reflect the official policy or position of the Department of the Navy, Department of Defense, nor the United States Government.
Footnotes
CONFLICT OF INTEREST
Sérgio Monteiro de Almeida, the author declare that have no conflict of interest.
Ana Paula de Pereira, the author declare that have no conflict of interest.
Maria Lucia Alves Pedroso, the author declare that have no conflict of interest.
Clea E. Ribeiro, the author declare that have no conflict of interest.
Indianara Rotta, the author declare that have no conflict of interest.
Bin Tang, the author declare that have no conflict of interest.
Anya Umlauf, the author declare that have no conflict of interest.
Donald Franklin, the author declare that have no conflict of interest.
Rowan G Saloner, the author declare that have no conflict of interest.
Maria Geny Ribas Batista, the author declare that have no conflict of interest.
Scott Letendre, the author declare that have no conflict of interest.
Robert K. Heaton, the author declare that have no conflict of interest.
Ronald J. Ellis, the author declare that have no conflict of interest.
Mariana Cherner, the author declare that have no conflict of interest.
References
- Abrantes J, Torres DS, Mello CEB. Patients with hepatitis C infection and normal liver function: an evaluation of cognitive function. Postgrad Med J. 2013;89:433–439. doi: 10.1136/postgradmedj-2012-131185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antinori A, Arendt G, Becker JT, Brew BJ, Byrd DA, Cherner M, et al. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007;69:1789–1799. doi: 10.1212/01.WNL.0000287431.88658.8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackstone K, Moore DJ, Franklin DR, et al. Defining neurocognitive impairment in HIV: deficita scores versus clinical ratings. The clinical Neuropsychologist. 2012:1–15. doi: 10.1080/13854046.2012.694479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brasil, Ministério da Saúde. Programa Nacional de DST/AIDS. 2016 http://www.aids.gov.br/assistencia/manualdst/item12.htm.
- Cacciarelli TV, Martinez OM, Gish RG, Villanueva JC, Krams SM. Immunoregulatory cytokines in chronic hepatitis C virus infection: Pre- and posttreatment with interferon alfa. Hepatology. 1996;24:6–9. doi: 10.1002/hep.510240102. [DOI] [PubMed] [Google Scholar]
- Carey CL, Woods SP, Rippeth JD, Gonzalez R, Moore DJ, Marcotte TD, et al. Initial validation of a screening battery for the detection of HIV-associated cognitive impairment. Clin Neuropsychol. 2004;18:234–48. doi: 10.1080/13854040490501448. [DOI] [PubMed] [Google Scholar]
- Carey CL, Woods SP, Gonzalez R, Conover E, Marcotte TD, Grant I, et al. Predictive validity of global deficit scores in detecting neuropsychological impairment in HIV infection. J Clin Exp Neuropsychol. 2004b;26:307–19. doi: 10.1080/13803390490510031. [DOI] [PubMed] [Google Scholar]
- Cattie JE, Letendre SL, Woods SP, Barakat F, Perry W, Cherner M, et al. Persistent Neurocognitive Decline in a Clinic Sample of Hepatitis C Virus-infected Persons Receiving Interferon and Ribavirin Treatment. J Neurovirol. 2014;20:561–570. doi: 10.1007/s13365-014-0265-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherner M, Letendre S, Heaton RK, Durelle J, Marquie-Beck J, Gragg B. Hepatitis C augments cognitive deficits associated with HIV infection and methamphetamine. Neurology. 2005;64:1343–1347. doi: 10.1212/01.WNL.0000158328.26897.0D. [DOI] [PubMed] [Google Scholar]
- Chou R, Wasson N. Blood tests to diagnose fibrosis or cirrhosis in patients with chronic hepatitis C virus infection: a systematic review. Ann Intern Med. 2013;158:807–20. doi: 10.7326/0003-4819-158-11-201306040-00005. [DOI] [PubMed] [Google Scholar]
- Ciccarelli N, Fabbiani M, Grima P, Falasca K, Tana M, Baldonero E, et al. Comparison of cognitive performance in HIV or HCV mono-infected and HIV–HCV co-infected patients. Infection. 2013;41:1103–1109. doi: 10.1007/s15010-013-0503-2. [DOI] [PubMed] [Google Scholar]
- Clifford DB, Vaida F, Kao YT, Franklin DR, Letendre SL, Collier AC, et al. Absence of neurocognitive effect of hepatitis C infection in HIV-coinfected people. Neurology. 2015;84:241–250. doi: 10.1212/WNL.0000000000001156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suppl 3.Clifford DB, Evans SR, Yang Y, Gulick RM. The neuropsychological and neurological impact of hepatitis C virus co-infection in HIV-infected subjects. AIDS. 2005;19:S64–71. doi: 10.1097/01.aids.0000192072.80572.43. [DOI] [PubMed] [Google Scholar]
- Cohen RA, de la Monte S, Gongvatana A, Ombao H, Gonzalez B, Devlin KN, et al. Plasma cytokine concentrations associated with HIV/hepatitis C coinfection are related to attention, executive and psychomotor functioning. Journal of Neuroimmunology. 2011;233:204–210. doi: 10.1016/j.jneuroim.2010.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cysique LA, Jin H, Franklin DR, Jr, Morgan EE, Shi C, the HNRC Group Neurobehavioral effects of HIV-1 infection in China and the United States: a pilot study. Journal of the International Neuropsychological Society. 2007;13:781–790. doi: 10.1017/S1355617707071007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Almeida SM, Ribeiro CE, de Pereira AP, Badiee J, Cherner M, Smith D, et al. Neurocognitive impairment in HIV-1 clade C- versus B-infected individuals in Southern Brazil. J Neurovirol. 2013;19:550–6. doi: 10.1007/s13365-013-0215-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forton DM, Allsop JM, Main J, Foster GR, Thomas HC, Taylor-Robinson SD. Evidence for a cerebral effect of the hepatitis C virus. Lancet. 2001;358:38–39. doi: 10.1016/S0140-6736(00)05270-3. [DOI] [PubMed] [Google Scholar]
- Ghate M, Mehendale S, Meyer R, Umlauf A, Deutsch R, Kamat R, et al. The effects of antiretroviral treatment initiation on cognition in HIV-infected individuals with advanced disease in Pune, India. Journal of Neurovirology. 2015;21:391–398. doi: 10.1007/s13365-015-0329-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant I. Neurocognitive disturbances in HIV. International Review of Psychiatry. 2008;20:33–47. doi: 10.1080/09540260701877894. [DOI] [PubMed] [Google Scholar]
- Heaton RK, Miller SW, Taylor MJ, Grant I. Revised Comprehensive Norms for an Expanded halstead-Reitan Battery: Demographically Adjusted Neuropsychological Norms for African American and Caucasian Adults. Psychological Assessment Resources, Inc; Lutz: 2004. [Google Scholar]
- Heaton RK, Clifford DB, Franklin DR, Woods SP, Ake C, Vaida F, et al. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology. 2010;75:2087–2096. doi: 10.1212/WNL.0b013e318200d727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Franklin DR, Deutsch R, Letendre S, Ellis RJ, Casaletto K, et al. Neurocognitive change in the era of HIV combination antiretroviral therapy: the longitudinal CHARTER study. Clinical Infectious Diseases. 2015;60:473–480. doi: 10.1093/cid/ciu862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilsabeck RC, Perry W, Hassanein TI. Neuropsychological impairment in patients with chronic hepatitis C. Hepatology. 2002;35:440–6. doi: 10.1053/jhep.2002.31257. [DOI] [PubMed] [Google Scholar]
- Hilsabeck RC, Castellon SA, Hinkin CH. Neuropsychological aspects of coinfection with HIV and hepatitis C virus. Clinical Infectious Diseases. 2005;41(Suppl. 1):S38–S44. doi: 10.1086/429494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilsabeck RC, Hassanein TI, Carlson MD, Ziegler EA, Perry W. Cognitive functioning and psychiatric symptomatology in patients with chronic hepatitis C. J Int Neuropsychol Soc. 2003;9:847–854. doi: 10.1017/S1355617703960048. [DOI] [PubMed] [Google Scholar]
- Hinkin CH, Castellon SA, Levine AJ, Barclay TR, Singer EJ. Neurocognition in individuals coinfected with HIV and hepatitis C. Journal of Addictive Diseases. 2008;27:11–17. doi: 10.1300/j069v27n02_02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huckans M, Seelye A, Parcel T, Mull L, Woodhouse J, Bjornson D, et al. The cognitive effects of hepatitis C in the presence and absence of a history of substance use disorder. J Int Neuropsychol Soc. 2009;15:69–82. doi: 10.1017/S1355617708090085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanmogne GD, Kuate CT, Cysique LA, Fonsah JY, Eta S, Doh R, et al. HIV-associated neurocognitive disorders in sub-Saharan Africa: a pilot study in Cameroon. BMC neurology. 2010;10:60. doi: 10.1186/1471-2377-10-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letendre SL, Cherner M, Ellis RJ, Marquie-Beck J, Gragg B, Marcotte T, et al. The effects of hepatitis C, HIV, and methamphetamine dependence on neuropsychological performance: Biological correlates of disease. AIDS. 2005;19:S72–S78. doi: 10.1097/01.aids.0000192073.18691.ff. [DOI] [PubMed] [Google Scholar]
- Lin ZH, Xin YN, Dong QJ, Wang Q, Jiang XJ, Zhan SH, et al. Performance of the aspartate aminotransferase-to-platelet ratio index for the staging of hepatitis C-related fibrosis: an updated meta-analysis. Hepatology. 2011;53:726–36. doi: 10.1002/hep.24105. [DOI] [PubMed] [Google Scholar]
- McAndrews MP, Farcnik K, Carlen P, Damyanovich A, Mrkonjic M, Jones S. Prevalence and significance of neurocognitive dysfunction in hepatitis C in the absence of correlated risk factors. Hepatology. 2005;41:801–808. doi: 10.1002/hep.20635. [DOI] [PubMed] [Google Scholar]
- Murray J, Fishman SL, Ryan E, Eng FJ, Walewski JL, Branch AD. Clinicopathologic correlates of hepatitisCvirus in brain: A pilot study. J Neurovirol. 2008;14:17–27. doi: 10.1080/13550280701708427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry W, Hilsabeck RC, Hassanein TI. Cognitive dysfunction in chronic hepatitis C: A review. Digestive Diseases and Sciences. 2008;53:307–321. doi: 10.1007/s10620-007-9896-z. [DOI] [PubMed] [Google Scholar]
- Posada C, Morgan EE, Moore DJ, Woods SP, Letendre SL, Grant I. Neurocognitive effects of the hepatitis C virus. Current Hepatitis Reports. 2009;8:18–26. [Google Scholar]
- Puccioni-Sohler M, Rosadas C, Cabral-Castro MJ. Neurological complications in dengue infection: a review for clinical practice. Arq Neuropsiquiatr. 2013;71:667–671. doi: 10.1590/0004-282X20130147. [DOI] [PubMed] [Google Scholar]
- Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. The British journal of surgery. 1973;60:646–9. doi: 10.1002/bjs.1800600817. [DOI] [PubMed] [Google Scholar]
- Quarantini LC, Miranda-Scippa A, Batista-Neves S, Powell VB, Abreu N, Abreu KC, et al. A Neuropsychological Study Comparing Patients Infected With HCV and HBV Without Psychiatric Comorbidities. J Med Virol. 2009;81:1184–1188. doi: 10.1002/jmv.21508. [DOI] [PubMed] [Google Scholar]
- Rempel H, Sun B, Calosing C, Abadjian L, Monto A, Pulliam L. Monocyte activation in HIV/HCV coinfection correlates with cognitive impairment. PloS One. 2013;8:e55776. doi: 10.1371/journal.pone.0055776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson JL, Nowicki M, Danley K, Martin EM, Cohen MH, Gonzalez R, et al. Neuropsychological functioning in a cohort of HIV- and hepatitisC virus-infected women. AIDS. 2005;19:1659–67. doi: 10.1097/01.aids.0000186824.53359.62. [DOI] [PubMed] [Google Scholar]
- Royal W, III, Cherner M, Carr J, Habib AG, Akomolafe A, Abimiku A, et al. Clinical features and preliminary studies of virological correlates of neurocognitive impairment among HIV-infected individuals in Nigeria. Journal of neurovirology. 2012;18:191–199. doi: 10.1007/s13365-012-0097-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senzolo M, Schiff S, D’Aloisio CM, Crivellin C, Cholongitas E, Burra P, et al. Neuropsychological alterations in hepatitis C infection: the role of inflammation. World J Gastroenterol. 2011;17:3369–74. doi: 10.3748/wjg.v17.i29.3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schouten J, Cinque P, Gisslen M, Reiss P, Portegies P. HIV-1 infection and cognitive impairment in the cART-era: A review. AIDS. 2011;25:561–575. doi: 10.1097/QAD.0b013e3283437f9a. [DOI] [PubMed] [Google Scholar]
- Solomon T. Flavivirus encephalitis. N Engl J Med. 2004;351:370–8. doi: 10.1056/NEJMra030476. [DOI] [PubMed] [Google Scholar]
- Sterling RK, Lissen E, Clumeck N, Clumeck N, Sola R, Correa MC, et al. APRICOT Clinical Investigators. Development of a simple noninvasive index to predict significant fibrosis patients with HIV/HCV co-infection. Hepatology. 2006;43:1317–1325. doi: 10.1002/hep.21178. [DOI] [PubMed] [Google Scholar]
- Sun B, Abadjian L, Rempel H, Monto A, Pulliam L. Differential cognitive impairment in HCV coinfected men with controlled HIV compared to HCV monoinfection. Journal of Acquired Immune Deficiency Syndromes. 2013;62:190–196. doi: 10.1097/QAI.0b013e31827b61f1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Beek D, Brouwer MC. CNS Infections in 2016: 2016, the year of Zika virus. Nature Reviews Neurology. 2017;13:69–70. doi: 10.1038/nrneurol.2016.202. [DOI] [PubMed] [Google Scholar]
- Wai CT, Greenson JK, Fontana RJ, Kalbfleisch JD, Marrero JA, Conjeevaram HS, et al. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. 2003;38:518–26. doi: 10.1053/jhep.2003.50346. [DOI] [PubMed] [Google Scholar]
- Weissenborn K, Krause J, Bokemeyer M, Hecker H, Schuler A, Ennen JC, et al. Hepatitis C virus infection affects the brain-evidence from psychometric studies and magnetic resonance spectroscopy. J Hepatol. 2004;41:845–851. doi: 10.1016/j.jhep.2004.07.022. [DOI] [PubMed] [Google Scholar]
- Weissenborn K, Bokemeyer M, Krause J, Ennen J, Ahl B. Neurological and neuropsychiatric syndromes associated with liver disease. AIDS. 2005;19:S93–S98. doi: 10.1097/01.aids.0000192076.03443.6d. [DOI] [PubMed] [Google Scholar]
- Wilkinson J, Radkpwski M, Laskus T. Hepatitis C virus neuroinvasion: identification of infected cells. J Virol. 2009;83:1312–9. doi: 10.1128/JVI.01890-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff FH, Fuchs SC, Barcellos NNT, Alencastro PR, Ikeda MLR, Brandão ABM, et al. Co-infection by hepatitis C virus in HIV-infected patients in Southern Brazil: genotype distribution and clinical correlates. PLoS One. 2010;5:e10494. doi: 10.1371/journal.pone.0010494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods SP, Moore DJ, Weber E, Grant I. Cognitive neuropsychology of HIV-associated neurocognitive disorders. Neuropsychological Review. 2009;19:152–168. doi: 10.1007/s11065-009-9102-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarlott L, Heald E, Forton D. Hepatitis C virus infection, and neurological and psychiatric disorders – A review. Journal of Advanced Research. 2017;8:139–148. doi: 10.1016/j.jare.2016.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


