Abstract
The role of tissue interactions was explored to determine whether epithelial differentiation within the developing human reproductive tract is induced and specified by mesenchyme in tissue recombinants composed of mouse vaginal mesenchyme + human uterine tubal epithelium (mVgM+hTubE). The tissue recombinants were grown in DES-treated ovariectomized athymic mice. After 2 to 4 weeks of in vivo growth, several vaginal specific features were expressed in the human tubal epithelium. The mesenchyme-induced effects included morphological change as well as expression of several immunohistochemical markers. Although the mesenchyme-induced shift in vaginal differentiation in the human tubal epithelium was not complete, the partial induction of vaginal markers in human tubal epithelium verifies the importance of mesenchymal-epithelial interactions in development of the human female reproductive tract.
In a separate experiment, DES-induction of uterine epithelial progesterone receptor (PGR) and estrogen receptor 1 (ESR1) was explored in tissue recombinants composed of wild-type or Esr1KO mouse uterine mesenchyme + human fetal uterine epithelium (wt UtM+hUtE and Esr1KO UtM+hUtE). The rationale of this experiment was to determine whether DES-induction of PGR and ESR1 is mediated directly via epithelial ESR1 or indirectly (paracrine mechanism) via mesenchymal ESR1. DES-induction of uterine epithelial ESR1 and PGR in Esr1KO UtM+hUtE tissue recombinants (devoid of mesenchymal ESR1) formally eliminates the paracrine mechanism and demonstrates that DES induction of human uterine epithelial ESR1 and PGR is directly mediated via epithelial ESR1.
Keywords: Human female fetal reproductive tract, estrogenic response, diethylstilbestrol, mesenchymal-epithelial interactions, estrogen receptor, progesterone receptor
I. Introduction
The tacit, but usually unproven, assumption inherent in animal models is that they are reflective of human biology. This approach is generally useful, even though substantial differences exist between human and animal anatomy, development and pathology. One field for which animal/human pathology is particularly congruent is the effects of exogenous estrogens on the developing female reproductive tract. Administration of the potent synthetic estrogen, diethylstilbestrol (DES), to pregnant women from the 1940s to the 1970s resulted in a broad spectrum of estrogen-induced malformations of the uterine tubes, uterine corpus, cervix and vagina that include T-shaped uterotubal junctions, malformed incompetent cervix, abnormally shaped endometrial cavity, vaginal adenosis as well as clear cell vaginal adenocarcinoma (Jefferies et al., 1984; Rennell, 1979; Stillman, 1982; Titus-Ernstoff et al., 2010; Herbst et al., 1971; Herbst et al., 1975; Robboy et al., 1977; Robboy et al., 1984; Hoover et al., 2011; Robboy et al., 2018). An immense animal literature preceded/confirmed the effects of exogenous estrogens on female reproductive tract development. In addition, animal studies have provided a molecular underpinning for the teratogenic effects of exogenous estrogens on urogenital development (Herbst and Bern, 1981; Bern and Talamantes, 1981; Bern et al., 1984; McLachlan et al., 1975; McLachlan, 1981; Newbold et al., 1983; Newbold and MaLachlan, 1985; Newbold, 1995; Newbold, 2004; 2008; McLachlan et al., 2001; McLachlan and Newbold, 1996; Kurita et al., 2004; Kurita, 2011; Laronda et al., 2012; Laronda et al., 2013). The animal literature on this topic is replete with estrogen-induce vaginal, cervical, uterine and tubal (oviductal) anomalies including cervicovaginal adenosis. While animal models are useful and relevant to many aspects of human biology/pathology, they are frequently the basis of governmental policy designed to protect human health. Accordingly, whenever possible it is important to establish by experimental means the relevance and predictability of animal studies to human biology/pathology.
Even though mouse-human similarities are now appreciated in estrogen-induced anomalies in the female reproductive tract, the molecular mechanisms that lead to human malformations remain enigmatic, despite some clues from animal studies (McLachlan and Newbold, 1996; Kurita et al., 2004; Kurita, 2011; Laronda et al., 2012; Laronda et al., 2013; Terakawa et al., 2016). Direct experimentation on xenografts of human fetal female reproductive tracts treated with DES have provided important insights into the genesis of human malformations and have provided essential bio-endpoints of estrogenic endocrine disruptors. In this regard, we pioneered xenograft methods in 1982 in which human fetal female reproductive tracts were grown in athymic mouse hosts treated with DES and other hormonally active agents (Cunha et al., 1987b; Cunha et al., 1987a; Robboy et al., 1982; Taguchi et al., 1983). Unfortunately molecular and immunohistochemical advances, not yet discovered, prevented exploration of biological mechanisms in our earlier studies. More recently, we have revisited human female reproductive tract development in a series of three papers that included a compendium of differentiation markers and how DES administration affects them in vivo (Cunha et al., 2017a; b; Robboy et al., 2017).
Studies carried out over 40 years ago established that uterine and vaginal mesenchyme induces and specifies epithelial differentiation (Cunha, 1976; Kurita, 2010; 2011; Kurita et al., 2001; Kurita et al., 2005). Accordingly, vaginal mesenchyme instructively induces uterine epithelium to undergo vaginal epithelial differentiation (VgM+UtE⇨vaginal differentiation), and uterine mesenchyme instructively induces vaginal epithelium to undergo uterine epithelial differentiation (UtM+VgE⇨uterine differentiation). These inductive effects involve both morphological as well as molecular effects on the target epithelium. During vaginal development in mice, inductive cues from vaginal mesenchyme elicit epithelial expression of ΔN p63 (an isoform of p63) in Müllerian epithelium, which specifies vaginal squamous epithelial differentiation (Kurita and Cunha, 2001; Kurita et al., 2004; Kurita et al., 2005; Terakawa et al., 2016). p63 is a member of the p53 family of transcription factors. Likewise, immunohistochemical detection of ΔNp63 in fetal and adult human vaginal epithelium suggests a similar role of ΔNp63 in human vaginal differentiation (Kurita et al., 2005; Fritsch et al., 2012; Fritsch et al., 2013; Cunha et al., 2017a). The current paper explores the role of mesenchymal-epithelial interactions in a tissue recombinant model consisting of mouse vaginal mesenchyme + human fetal uterine tube epithelium (mVgM+hTubE). The rationale for this particular experimental model is that expression of several differentiation markers is vastly different in vaginal versus tubal epithelium.
In both human and mouse female reproductive organs, estrogen receptor 1 (ESR1; also known as estrogen receptor α) is the dominant receptor for estrogen (Matsuzaki et al., 1999; Dupont et al., 2000). Earlier, we detailed the ontogeny of ESR1 during human fetal uterine development (Cunha et al., 2017a), demonstrating that ESR1 is first expressed in mesenchymal cells of human uterine corpus. Indeed, ESR1-immunoreactivity in uterine epithelial cells is rarely seen before the 21st gestational week, when the endogenous estrogen levels are elevated (Oakey, 1970), thus suggesting that uterine epithelial ESR1 may be estrogen induced. Analysis of human fetal uterine xenografts treated with DES has verified this prediction (Cunha et al., 2017b). However, given that prior to DES treatment, ESR1 was detected in uterine mesenchyme and not epithelium, there are two potential mechanisms of DES induction of uterine epithelial ESR1: (a) DES may induce epithelial ESR1 directly via epithelial ESR1 whose expression is below the sensitivity of immunohistochemistry. (b) Alternatively, DES may induce epithelial ESR1 indirectly via mesenchymal ESR1 (paracrine mechanism). The same question is relevant to DES induction of epithelial progesterone receptor (PGR) in the developing human female reproductive tract (Cunha et al., 2017b).
The goal of the current paper based on our prior work (Cunha et al., 2017a; Robboy et al., 2017) is to use xenograft models (a) to determine the role of mesenchymal-epithelial interactions in epithelial differentiation during human female reproductive tract development, and (b) to determine whether estrogen regulates human uterine epithelial ESR1 and PGR via direct or paracrine mechanisms using tissue recombinants composed of human fetal uterine epithelium combined with mouse uterine mesenchyme derived from wild-type or Esr1 knockout (Esr1KO) mice.
II. Materials and Methods
A. General comments
The Committee on Human Research at UCSF (IRB# 12-08813) approved the collection of human fetal specimens devoid of patient identifiers after elective termination of pregnancy. Fetal age was estimated using heel-toe length (Drey et al., 2005). Gender was determined by Wolffian and Müllerian duct morphology as previously described (Robboy et al., 2017). Female internal genitalia were identified and isolated from the abortus specimen using a dissecting microscope. For this study 10 human fetal specimens were used at 8, 9, 10, 12, 13, 14, and 18 weeks of gestation.
B. Response of human fetal grafts of uterine corpus to DES in vivo
Intact human fetal reproductive tracts containing the uterine tube, uterine corpus, uterine cervix and vagina were grown for 4 weeks under the renal capsule of untreated and DES-treated (20mg DES subcutaneous pellet) of ovariectomized female athymic mice as described previously (Cunha et al., 2017b). Histology and immunohistochemistry for ESR1 and PGR were performed on tissue sections as described below.
C. Preparation of heterotypic tissue recombinants
Tissue recombinant studies included: (a) mouse vaginal mesenchyme + human uterine tubal epithelium (mVgM+hTubE) and (b) wild-type or Esr1KO mouse uterine mesenchyme + human fetal uterine epithelium (wt UtM+hUtE and Esr1KO UtM+hUtE). For mVgM+hTubE tissue recombinants, mouse vaginal mesenchyme was isolated from 3-day-old neonatal mice and the tube epithelium was derived from 12–13 week specimens. To explore regulation of human uterine epithelial ESR1 and PGR, uterine mesenchyme was isolated from 5-day-old wild-type and Esr1KO neonatal mice and the human uterine epithelium was derived from 10 to 12 week specimens. Heterozygous male and female Esr1KO mice, a gift from Drs. Pierre Chambón and Andrée Krust, were bred to produce the Esr1KO neonatal mice, which were genotyped as described previously (Dupont et al., 2000). Tissue recombinant and xenografting methods have been described previously (Cunha, 1976; Cunha and Baskin, 2016). For mVgM+hTubE, wt UtM+hUtE and Esr1KO UtM+hUtE tissue recombinants, all hosts were ovariectomized at the time of grafting and were treated with a 20mg DES pellet or were untreated (sham). For hosts bearing mVgM+hTubE tissue recombinants, the rationale for treating the hosts with DES was to promote stratified squamous vaginal differentiation. In response to DES grafts of human fetal uterine tube remain simple columnar, while grafts of human fetal vagina differentiate a thick glycogenated stratified epithelium (Cunha et al., 2017b). Thus, should vaginal differentiation be elicited in human tubal epithelium by mouse vaginal mesenchyme, epithelial differentiation should be distinctive. After 2 or 4 weeks of growth under the renal capsules, the tissue recombinants were harvested, fixed in 10% buffered formalin, embedded in paraffin and serially sectioned at 7μm. Every twentieth section was stained with hematoxylin and eosin. Remaining paraffin sections were utilized for immunohistochemical staining with the antibodies indicated (1).
Immunostaining was detected using horseradish peroxidase-based Vectastain kits (Vector Laboratories, Burlingame, CA). Negative controls lacked the primary antibody. Tissue recombinants composed of mouse mesenchyme and human epithelium were stained with Hoechst dye 33258 to verify the species origin of the tissues as described (Cunha and Vanderslice, 1984). This study is based upon the analysis of 18 mVgM+hTubE, 6 wt UtM+hUtE and 5 Esr1KO UtM+hUtE tissue recombinants.
III. Results
A. Epithelial and mesenchymal differentiation in grafts of human fetal female reproductive tracts grown in DES-treated ovariectomized female hosts
1. General comments
Age is an important factor affecting expression of epithelial differentiation markers (Cunha et al., 2017a). All human fetal specimens used for tissue recombinant studies were 12 to 13 weeks of gestation old at which time epithelial differentiation markers are beginning to be expressed, well before terminal epithelial differentiation.
2. Tissue recombinants composed of mouse vaginal mesenchyme plus human uterine tube epithelium (mVgM+hTubE)
Previous studies in mice have shown that differentiation of Müllerian epithelium within the female reproductive tract of the mouse is specified by inductive cues from the mesenchyme (Cunha, 1976; Kurita et al., 2001; Kurita, 2011). To determine whether mesenchymal induction plays a role in differentiation of human Müllerian epithelium, tissue recombinants were prepared with 3-day postnatal mouse vaginal mesenchyme (known to be a vaginal inductor) and human uterine tubal epithelium from 12- to 13-week fetuses. Sections of all six mVgM+hTubE tissue recombinants were screened with Hoechst dye staining to verify that the mesenchyme was mouse and the epithelium was human (Fig. 1), thus eliminating the potential artifact of mouse vaginal mesenchyme contaminated with its own homotypic mouse epithelium.
Figure 1.
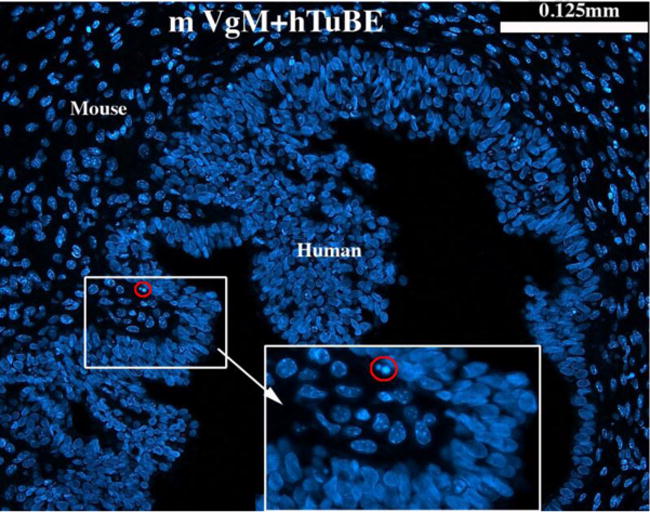
Section of a tissue recombinant composed of neonatal mouse vaginal mesenchyme plus 13 week human fetal uterine tube epithelium (mVgM+hTubE) stained with Hoechst dye 33258 to verify the species origin of the mesenchyme and epithelium. Mouse nuclei contain many bright chromatin bodies, whereas human nuclei lack such intra-nuclear bodies (Cunha and Vanderslice, 1984). The red-circled pycnotic nucleus in the lower magnification images can be seen at higher magnification.
Expression of several epithelial differentiation markers (AR, KRT6, TP63 and RUNX1) is distinctly different in the human tubal versus vaginal epithelium (Table 2). Figure 2 depicts mVgM+hTubE recombinants grown in DES-treated female athymic mouse hosts and indicates that mouse vaginal mesenchyme elicited a partial shift in epithelial histo-differentiation and differentiation marker expression from uterine tubal epithelial differentiation to vaginal epithelial differentiation (Table 2). Expression of some tubal epithelial markers (KRT14 and KRT19) were unchanged in mVgM+hTubE recombinants, suggesting that mouse vaginal mesenchyme was able to elicit only a subset of vaginal differentiation markers.
Table 2.
Epithelial differentiation of human uterine tube, human vagina and mVgM+hTubE tissue recombinants.
| Feature | Uterine tube | Vagina | mVgM+hTubE |
|---|---|---|---|
| Simple columnar epithelium | Yes | No | No |
| Stratified squamous epithelium | No | Yes | Yes |
| KRT6 | No | Yes | Yes |
| TP63 | No | Yes | Yes |
| Androgen receptor | Yes | No | No |
| RUNX1 | No | Yes | Yes |
| KRT14 | No | Yes | No |
| KRT19 | Yes | No | Yes |
Uterine tube epithelial features are shaded red, vaginal epithelial feature are shaded green.
Figure 2.
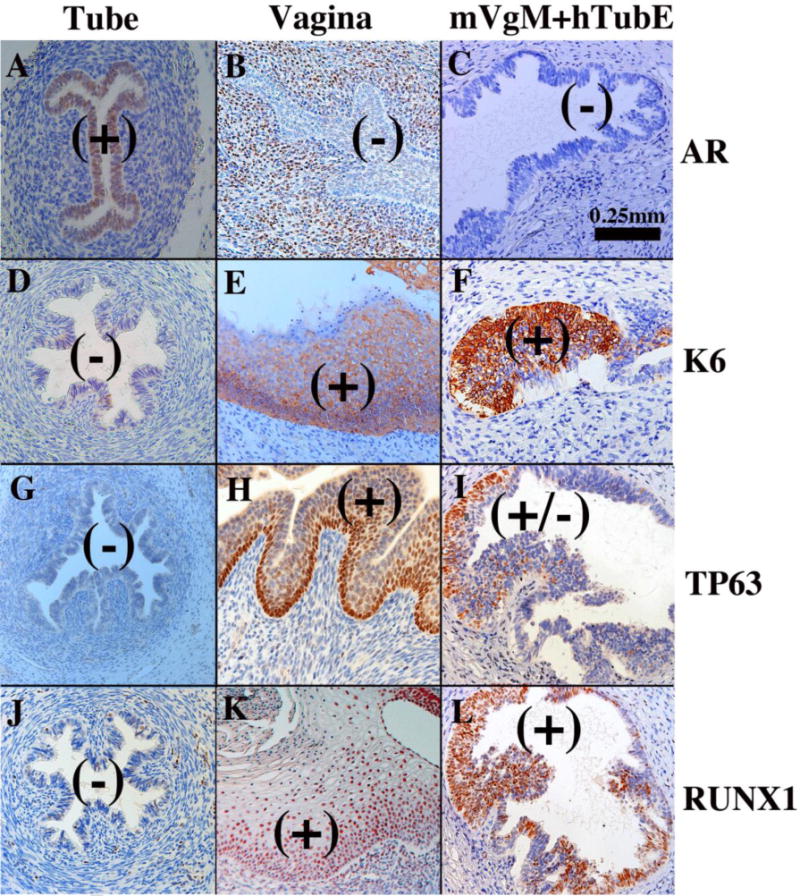
Tissue recombinants composed of neonatal mouse vaginal mesenchyme plus 13 week human fetal uterine tube epithelium (mVgM+hTubE) grown for 4 weeks in DES-treated hosts and immunostained for various vaginal epithelial markers as indicated. Human uterine tube (A, D, G, J) and vagina (B, E, H, K) at 16 to 18 weeks of gestation serve as controls. Note induction of KRT6, TP63 and RUNX1 and down regulation of AR in epithelium of the mVgM+hTubE recombinants, indicative of an effect of mouse vaginal mesenchyme on expression of differentiation markers in human tubal epithelium. (+) and (−) indicate epithelial marker expression.
2. Tissue recombinants composed of epithelium of the human uterine corpus plus wild-type or Esr1KO uterine mesenchyme (wt UtM+hUtE and Esr1KO UtM+hUtE)
The background for this experiment is based upon the ontogeny of ESR1 in the human fetal uterine corpus. From 8 to 13 weeks ESR1 is undetectable in both the epithelium and mesenchyme of the human fetal uterine corpus (Fig. 3A–C). Subsequently, ~14 weeks to 18 weeks the mesenchyme of the human fetal uterine corpus become ESR1-positive (Fig. 3D], while the epithelium remains mostly ESR1-negative with the exception of rare ESR1-positive epithelial cells interspersed within the ESR1-negative uterine epithelial cells (Cunha et al., 2017a]. When 10- and 13-week human fetal female reproductive tracts are grown for 4 weeks in DES-treated ovariectomized female hosts, ESR1 was induced in epithelium of the uterine corpus (Fig. 3E & F) (Cunha et al., 2017b). To determine whether DES induction of human fetal uterine epithelial ESR1 is mediated indirectly via mesenchymal ESR1 (paracrine effect) or directly via epithelial ESR1, we prepared tissue recombinants composed of epithelium of the human corpus (hUtE) and uterine mesenchyme from either Esr1-positive wild-type mice or Esr1-negative Esr1KO mice (wt UtM+hUtE and Esr1KO UtM+hUtE). For this experiment we used hUtE from 10- and 12-week fetuses, an age before epithelial ESR1 was detectable (see Fig. 3A–C).
Figure 3.
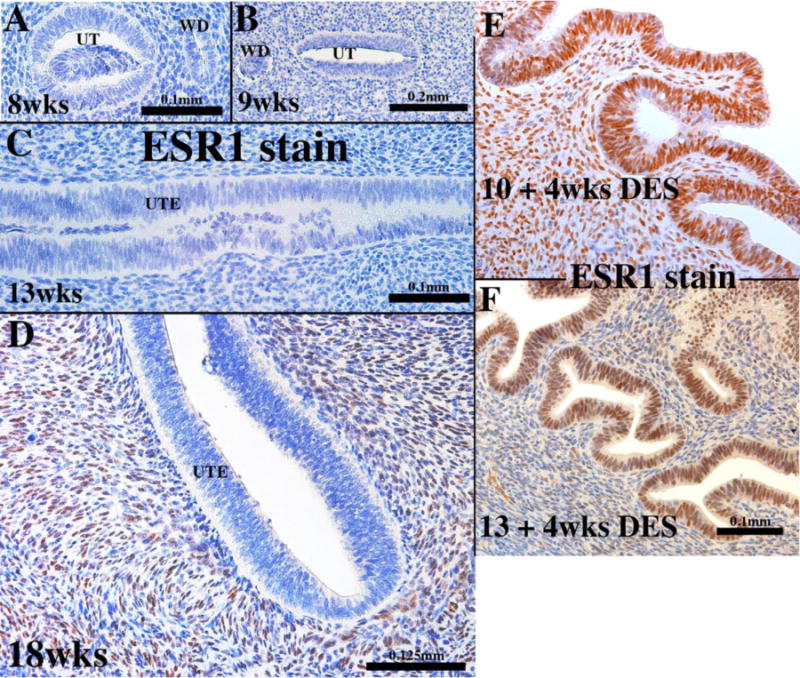
Sections of developing human fetal uterine corpus immunostained for ESR1 at the ages indicated (A-D). At all stages (8 to 18 weeks) ESR1 is undetectable in the uterine epithelium. At 18 weeks, the stroma is ESR1-positive. (E & F) are sections of grafts of 10 week (E) and 13 week (F) human fetal uterine corpus grown for 4 weeks in DES-treated ovariectomized hosts and immunostained for ESR1. Note induction of epithelial ESR1.
wt UtM+hUtE and Esr1KO UtM+hUtE tissue recombinants were grown under the renal capsule of female athymic mice that were ovariectomized at the time of grafting and implanted subcutaneously with a 20mg DES pellet. After 1 month of growth, sections of all tissue recombinants were stained with Hoechst dye 332598 to verify that the stroma of the harvested grafts was mouse and the epithelium was human (Fig. 4E) as described previously (Cunha and Vanderslice, 1984). ESR1 immunstaining of all tissue recombinants verified the mesenchymal genotype (Fig. 4A & C): wt UtM+hUtE tissue recombinants contained ESR1-positive mesenchymal cells (Fig. 4A), whereas Esr1KO UtM+hUtE tissue recombinants were devoid of ESR1-positive mesenchymal cells (Figs. 4C). wt UtM+hUtE tissue recombinants consistently (6/6) contained an ESR1-positive human uterine epithelium (Fig. 4A). Surprisingly, Esr1KO UtM+hUtE tissue recombinants also (5/5) contained an ESR1-positive human uterine epithelium even though the surrounding stromal cells were ESR1-negative (Fig. 4C).
Figure 4.
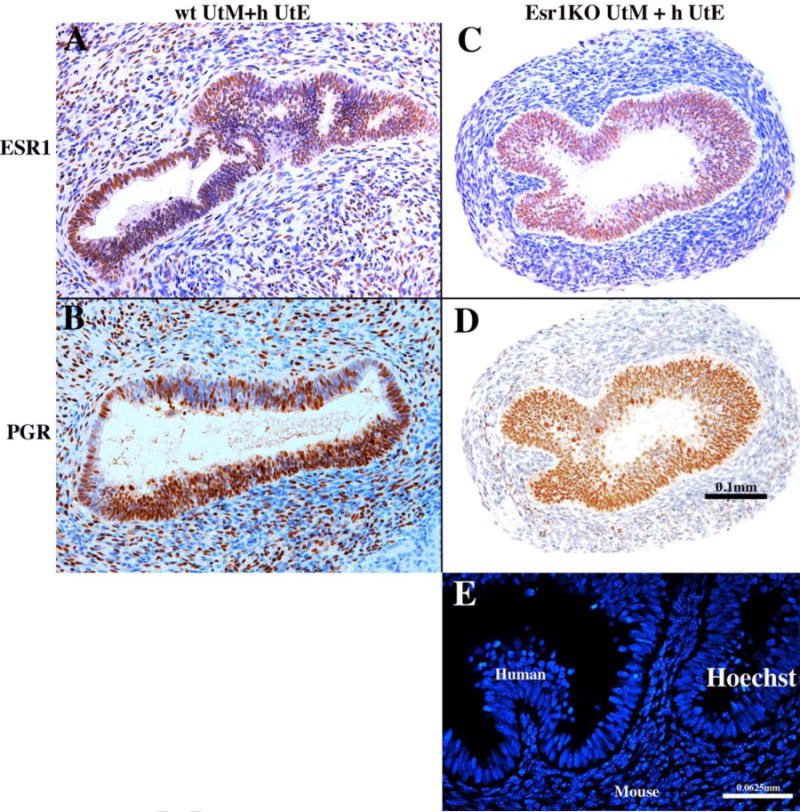
Tissue recombinants composed of neonatal mouse wild-type uterine mesenchyme plus human fetal uterine tube epithelium (wt UtM+hUtE) (A &B) and aERKO uterine mesenchyme plus human uterine tube epithelium Esr1KO UtM+ h UtE) (C & D) grown in DES-treated hosts and immunostained for ESR1 (A & C) and PGR (B & D). DES induced ESR1 and PGR even when the mesenchyme was genetically devoid of ESR1. Sections (C) and (D) are adjacent sections stained for ESR1 (C) and PGR (D). (E) is a section of a graft of Esr1KO UtM+hUtE tissue recombinants stained with Hoechst dye 33258 to verify the tissue origin.
To further explore the cellular mechanism of uterine epithelial steroid receptor expression (direct versus paracrine), we examined regulation of the PGR in wt UtM+hUtE and Esr1KO UtM+hUtE tissue recombinants. During the time frame of 8 to 14 weeks of gestation, PGR is undetectable (Fig. 5A-B) in both epithelium and mesenchyme of all organs of the developing human female reproductive tract (Cunha et al., 2017a). However, when human fetal female reproductive tracts were grown in DES-treated ovariectomized female hosts, PGR was induced globally within epithelial and stromal cells throughout the female reproductive tract, especially in the uterine corpus (Fig. 5C) (Cunha et al., 2017b). This observation is in keeping with the established idea that PGR is an estrogen inducible protein (Janne et al., 1975; Horwitz and McGuire, 1979). Epithelial PGR was detected in both wt UtM+ human UtE (Fig. 4B, 6/6) and Esr1KO UtM+ human UtE (Fig. 4D, 5/5) tissue recombinants following growth for 1 month in DES-treated hosts, demonstrating the PGR is induced by DES directly via epithelial ESR1.
Figure 5.
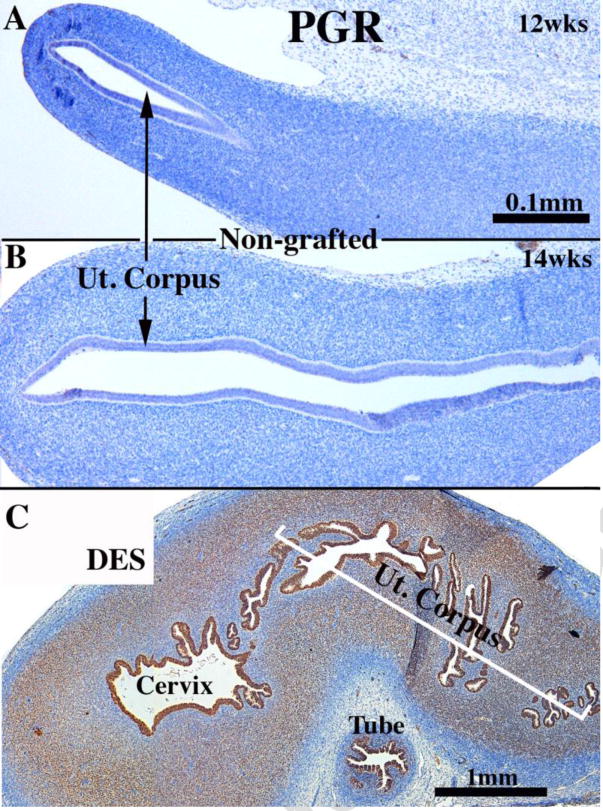
Sections of non-grafted human female fetal reproductive tracts (A and B) at the ages specified immunostained for PGR. (C) A 13 week human fetal uterine corpus grown for 4 weeks in DES-treated ovariectomized hosts and immunostained for PGR. Note DES induction of epithelial and stromal PGR.
IV. Discussion
Since epithelial differentiation within the female mouse reproductive tract is induced by cues from the mesenchyme (Cunha, 1976), we examined whether this concept also applies to the developing human female fetal reproductive tract through analysis of tissue recombinants composed of mouse vaginal mesenchyme and human tubal epithelium (mVgM+hTubE). The rationale of this experiment was to focus on two organs whose epithelial differentiation is markedly different (uterine tube=simple columnar epithelium versus vagina=stratified squamous epithelium) and whose profile of differentiation markers is also substantially different (see Table 2).
Our current studies extend prior works indicating that mesenchyme of various female reproductive organs induces and specifies the developmental fate of epithelium in laboratory animals. In our current studies neonatal mouse vaginal mesenchyme was grown in association with human fetal epithelium of uterine tubal origin, and the resulting epithelium expressed vaginal epithelial differentiation both morphologically and with vaginal immunohistochemical markers. Thus, mVgM+hTubE tissue recombinants formed a stratified epithelium that expressed KRT6, TP63, RUNX1, while concurrently abolishing expression of AR, an epithelial differentiation marker normally present in human fetal uterine tube epithelium, but not in human vaginal epithelium (Cunha et al., 2017a). However, the epithelium that formed was not perfectly vaginal as a few tubal pattern markers (absence of KRT14 and presence of KRT19) were retained in the partially induced epithelium. There are several reasons for this partial mesenchyme-induced shift in epithelial differentiation: (a) The mVgM+hTubE tissue recombinants were grown for either 2 or 4 weeks in host mice, and this may be insufficient to achieve full vaginal differentiation. Studies of longer duration, e.g., 1–2 months, may be required to achieve full vaginal epithelial differentiation. (b) Use of uterine tubal epithelium younger in age might result in a more complete vaginal epithelial differentiation as age can affect epithelial responsiveness (Cunha, 1976). Nonetheless, simultaneous induction of partial vaginal differentiation coupled with vaginal mesenchyme-induced loss of at least one tubal differentiation marker formally validates the concept that mesenchymal-epithelial interactions play an important role in differentiation within the human female fetal reproductive tract, perhaps via molecular mechanisms similar to those revealed in mouse studies. In any case, the use of mouse/human heterospecific tissue interaction studies, particularly those employing mutant mouse tissues, could be used in the future to dissect molecular mechanisms of human female (and male) reproductive tract development.
The second experiment in this paper deals with the mechanism of estrogenic induction of ESR1 and PGR in human fetal uterine epithelium, that is, whether DES elicits uterine epithelial ESR1 and PGR (a) directly via epithelial ESR1 or (b) indirectly (paracrine mechanism) via ESR1 in adjacent uterine mesenchyme. This question becomes even more interesting in so far as ESR1 was undetectable by immunohistochemistry in the human fetal uterine corpus at the age of the human uterine epithelium used to prepare the tissue recombinant (Fig. 3).
The background and rationale for this experiment is that ESR1 and PGR, whose expression is normally undetectable in second trimester human fetal uterine epithelium (Cunha et al., 2017a), were induced by DES treatment (Cunha et al., 2017b). Estrogen is known to up-regulate ESR1 mRNA and protein (Read et al., 1989; Saceda et al., 1988) and PGR (Janne et al., 1975; Horwitz and McGuire, 1979) via binding of liganded ESR1 to estrogen-response elements associated in the genes encoding ESR1 and PGR (Liu et al., 2003; Mehta et al., 2016).
We found that uterine epithelial ESR1 and PGR were induced by DES in Esr1KO UtM+hUtE tissue recombinants (devoid of mesenchymal ESR1), which formally eliminates the paracrine mechanism and demonstrates that DES induction of human uterine epithelial ESR1 and PGR is directly mediated via epithelial ESR1. With respect to estrogen-induced epithelial PGR in human uterine epithelium, we have previously addressed this question with heterospecific tissue recombinants consisting of adult human uterine epithelium and Esr1KO UtM. However, our earlier study was inconclusive as the Esr1KO mouse strain utilized for the previous study expressed a truncated form of Esr1 in UtM (Kurita et al. 2005b). Since the Esr1KO mice utilized in the current study are truly Esr1 null (Dupont et al., 2000), regulation of PGR in human UtE unquestionably does not require mesenchymal Esr1. Thus, epithelial PGR was prominently expressed Esr1KO UtM+ human UtE tissue recombinants (5/5) having Esr1-negative mesenchyme even though Esr1 was undetectable in the human fetal uterine epithelium used to construct the Esr1KO UtM+ human UtE tissue recombinants. DES-induction of ESR1 in human fetal uterine epithelium was also observed in Esr1KO UtM+ human UtE tissue recombinants suggesting that estrogenic induction of ESR1 is a direct effect mediated by epithelial Esr1. It is perhaps worth noting that the design of this experiment does not exclude a possible role of mesenchymal ESR2.
It is perhaps worth noting that the mechanism of regulating uterine epithelial PGR in mice is vastly different than that in human. In mice uterine epithelial PGR is strongly expressed in untreated ovariectomized mice and is profoundly down regulated upon administration of estrogen, and effect that is mediated indirectly via stromal ESR1 (paracrine mechanism) (Kurita et al., 2000). This finding in mice contrasts strikingly with the direct estrogenic induction of PGR in human cells and tissues.
Table 1.
Antibodies used in this study
| Antibody # | Source | Catalogue # | Dilution |
|---|---|---|---|
| AR (Androgen receptor) | GeneTax | EPR1535(2) | 1/100 |
| ESR1 (Estrogen receptor 1) | Dako | Ab16660 | 1/100 |
| KRT14 (Keratin 14) | BioGenex | LL002 | 1/100 |
| KRT19 (Keratin 19) | EB Lane | LP2K | 1/100 |
| PGR (Progesterone receptor) | Abcam | Ab16661 | 1/100 |
| RUNX1 (runt-related transcription factor 1) | Abcam | Ab92336 | 1/100 |
| TP63 (Tumor protein 63) | Santa Cruz Biotechnology | sc-8343 | 1/100 |
Acknowledgments
This study was supported by NIH grant DK058105 to Dr. Baskin, R01 CA154358 to Dr. Kurita and HD088006 to Paul S Cooke. The authors thank Drs. Pierre Chambon and Andrée Krust (Institut de Génétique et de Biologie Moléculaire et Cellulaire, Illkirch, France) for Esr1 knockout mice.
Abbreviations
- ESR1
Estrogen receptor alpha
- DES
diethylstilbestrol
- PGR
progesterone receptor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bern HA, Mills KT, Ostrander PI, Schoenrock B, Graveline B, Plapinger L. Cervicovaginal abnormalities in BALB/c mice treated neonatally with sex hormones. Teratology. 1984;30:267–274. doi: 10.1002/tera.1420300214. [DOI] [PubMed] [Google Scholar]
- Bern HA, Talamantes FJ. Neonatal mouse models and their relation to disease in the humal female. In: Herbst A, Bern HA, editors. Developmental Effects of Diethylstilbestrol (DES) in Pregnancy. Thieme Stratton Inc.; New York: 1981. pp. 129–147. [Google Scholar]
- Cunha GR. Stromal induction and specification of morphogenesis and cytodifferentiation of the epithelia of the Mullerian ducts and urogenital sinus during development of the uterus and vagina in mice. J Exp Zool. 1976;196:361–370. doi: 10.1002/jez.1401960310. [DOI] [PubMed] [Google Scholar]
- Cunha GR, Baskin L. Use of sub-renal capsule transplantation in developmental biology. Differentiation; research in biological diversity. 2016;91:49–9. doi: 10.1016/j.diff.2015.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha GR, Kurita T, Cao M, Shen J, Robboy S, Baskin L. Molecular mechanisms of development of the human fetal female reproductive tract. Differentiaiton. 2017a;97:54–72. doi: 10.1016/j.diff.2017.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha GR, Kurita T, Cao M, Shen J, Robboy S, Baskin L. Response of xenografts of developing human female reproductive tracts to the synthetic estrogen, diethylstilbestrol. Differentiation; research in biological diversity. 2017b doi: 10.1016/j.diff.2017.10.001. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha GR, Taguchi O, Namikawa R, Nishizuka Y, Robboy SJ. Teratogenic effects of Clomid, tamoxifen, and diethylstilbestrol on the developing human female genital tract. Human Pathol. 1987a;18:1132–1143. doi: 10.1016/s0046-8177(87)80381-7. [DOI] [PubMed] [Google Scholar]
- Cunha GR, Taguchi O, Sugimura Y, Lawrence DW, Mahmood F, Robboy SJ. Absence of teratogenic effects of progesterone on the developing gential tract of the human female fetus. Human Pathol. 1987b;19:777–783. doi: 10.1016/s0046-8177(88)80260-0. [DOI] [PubMed] [Google Scholar]
- Cunha GR, Vanderslice KD. Identification in histological sections of species origin of cells from mouse, rat and human. Stain Technol. 1984;59:7–12. doi: 10.3109/10520298409113823. [DOI] [PubMed] [Google Scholar]
- Drey EA, Kang MS, McFarland W, Darney PD. Improving the accuracy of fetal foot length to confirm gestational duration. Obstetrics and gynecology. 2005;105:773–778. doi: 10.1097/01.AOG.0000154159.75022.11. [DOI] [PubMed] [Google Scholar]
- Dupont S, Krust A, Gansmuller A, Dierich A, Chambon P, Mark M. Effect of single and compound knockouts of estrogen receptors alpha (ERalpha) and beta (ERbeta) on mouse reproductive phenotypes. Development. 2000;127:4277–4291. doi: 10.1242/dev.127.19.4277. [DOI] [PubMed] [Google Scholar]
- Fritsch H, Hoermann R, Bitsche M, Pechriggl E, Reich O. Development of epithelial and mesenchymal regionalization of the human fetal utero-vaginal anlagen. Journal of anatomy. 2013;222:462–472. doi: 10.1111/joa.12029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritsch H, Richter E, Adam N. Molecular characteristics and alterations during early development of the human vagina. Journal of anatomy. 2012;220:363–371. doi: 10.1111/j.1469-7580.2011.01472.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbst A, Bern H. Developmental effects of DES in pregnancy. Thieme Stratton; New York: 1981. [Google Scholar]
- Herbst AL, Scully RE, Robboy SJ. Vaginal adenosis and other diethylstilbestrol-related abnormalities. Clin Obstet Gynecol. 1975;18:185–194. doi: 10.1097/00003081-197509000-00021. [DOI] [PubMed] [Google Scholar]
- Herbst AL, Ulfelder H, Poskanzer DC. Adenocarcinoma of the vagina: Association of maternal stilbestrol therapy with tumor appearance in young women. New Eng J Med. 1971;284:878–881. doi: 10.1056/NEJM197104222841604. [DOI] [PubMed] [Google Scholar]
- Hoover RN, Hyer M, Pfeiffer RM, Adam E, Bond B, Cheville AL, Colton T, Hartge P, Hatch EE, Herbst AL, Karlan BY, Kaufman R, Noller KL, Palmer JR, Robboy SJ, Saal RC, Strohsnitter W, Titus-Ernstoff L, Troisi R. Adverse health outcomes in women exposed in utero to diethylstilbestrol. The New England journal of medicine. 2011;365:1304–1314. doi: 10.1056/NEJMoa1013961. [DOI] [PubMed] [Google Scholar]
- Horwitz KB, McGuire WL. Estrogen control of progesterone receptor induction in human breast cancer: role of nuclear estrogen receptor. Adv Exp Med Biol. 1979;117:95–110. doi: 10.1007/978-1-4757-6589-2_5. [DOI] [PubMed] [Google Scholar]
- Janne O, Kontula K, Luukkainen T, Vihko R. Oestrogen-induced progesterone receptor in human uterus. Journal of steroid biochemistry. 1975;6:501–509. doi: 10.1016/0022-4731(75)90179-x. [DOI] [PubMed] [Google Scholar]
- Jefferies JA, Robboy SJ, O’Brien PC, Bergstralh EJ, Labarthe DR, Barnes AB, Noller KL, Hatab PA, Kaufman RH, Townsend DE. Structural anomalies of the cervix and vagina in women enrolled in the Diethylstilbestrol Adenosis (DESAD) Project. American journal of obstetrics and gynecology. 1984;148:59–66. doi: 10.1016/s0002-9378(84)80033-2. [DOI] [PubMed] [Google Scholar]
- Kurita T. Developmental origin of vaginal epithelium. Differentiation; research in biological diversity. 2010;80:99–105. doi: 10.1016/j.diff.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurita T. Normal and abnormal epithelial differentiation in the female reproductive tract. Differentiation; research in biological diversity. 2011;82:117–126. doi: 10.1016/j.diff.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurita T, Cooke PS, Cunha GR. Epithelial-stromal tissue interaction in paramesonephric (Mullerian) epithelial differentiation. Developmental biology. 2001;240:194–211. doi: 10.1006/dbio.2001.0458. [DOI] [PubMed] [Google Scholar]
- Kurita T, Cunha GR. Roles of p63 in differentiation of Mullerian duct epithelial cells. Ann N Y Acad Sci. 2001;948:9–12. doi: 10.1111/j.1749-6632.2001.tb03982.x. [DOI] [PubMed] [Google Scholar]
- Kurita T, Cunha GR, Robboy SJ, Mills AA, Medina RT. Differential expression of p63 isoforms in female reproductive organs. Mechanisms of development. 2005;122:1043–1055. doi: 10.1016/j.mod.2005.04.008. [DOI] [PubMed] [Google Scholar]
- Kurita T, Lee KJ, Cooke PS, Taylor JA, Lubahn DB, Cunha GR. Paracrine regulation of epithelial progesterone receptor by estradiol in the mouse female reproductive tract. Biology of reproduction. 2000;62:821–830. doi: 10.1093/biolreprod/62.4.821. [DOI] [PubMed] [Google Scholar]
- Kurita T, Mills AA, Cunha GR. Roles of p63 in the diethylstilbestrol-induced cervicovaginal adenosis. Development. 2004;131:1639–1649. doi: 10.1242/dev.01038. [DOI] [PubMed] [Google Scholar]
- Laronda MM, Unno K, Butler LM, Kurita T. The development of cervical and vaginal adenosis as a result of diethylstilbestrol exposure in utero. Differentiation; research in biological diversity. 2012;84:252–260. doi: 10.1016/j.diff.2012.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laronda MM, Unno K, Ishi K, Serna VA, Butler LM, Mills AA, Orvis GD, Behringer RR, Deng C, Sinha S, Kurita T. Diethylstilbestrol induces vaginal adenosis by disrupting SMAD/RUNX1-mediated cell fate decision in the Mullerian duct epithelium. Developmental biology. 2013;381:5–16. doi: 10.1016/j.ydbio.2013.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D, Zhang Z, Gladwell W, Teng CT. Estrogen stimulates estrogenrelated receptor alpha gene expression through conserved hormone response elements. Endocrinology. 2003;144:4894–4904. doi: 10.1210/en.2003-0432. [DOI] [PubMed] [Google Scholar]
- Matsuzaki S, Fukaya T, Suzuki T, Murakami T, Sasano H, Yajima A. Oestrogen receptor alpha and beta mRNA expression in human endometrium throughout the menstrual cycle. Mol Hum Reprod. 1999;5:559–564. doi: 10.1093/molehr/5.6.559. [DOI] [PubMed] [Google Scholar]
- McLachlan J. Rodent models for perinatal exposure to diethylstilbestrol and their relation to human disease in the male. In: Herbst A, Bern HA, editors. Developmental Effects of Diethylstilbestrol (DES) in Pregnancy. Thieme Stratton Inc.; New York: 1981. pp. 148–157. [Google Scholar]
- McLachlan JA, Newbold RR. Cellular and molecular mechanisms of cancers of the uterus in animals. Prog Clin Biol Res. 1996;394:175–182. [PubMed] [Google Scholar]
- McLachlan JA, Newbold RR, Bullock BC. Reproductive tract lesions in male mice exposed prenatally to diethylstilbestrol. Science. 1975;190:991–992. doi: 10.1126/science.242076. [DOI] [PubMed] [Google Scholar]
- McLachlan JA, Newbold RR, Burow ME, Li SF. From malformations to molecular mechanisms in the male: three decades of research on endocrine disrupters. APMIS. 2001;109:263–272. doi: 10.1034/j.1600-0463.2001.d01-119.x. [DOI] [PubMed] [Google Scholar]
- Mehta FF, Son J, Hewitt SC, Jang E, Lydon JP, Korach KS, Chung SH. Distinct functions and regulation of epithelial progesterone receptor in the mouse cervix, vagina, and uterus. Oncotarget. 2016;7:17455–17467. doi: 10.18632/oncotarget.8159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newbold R. Cellular and molecular effects of developmental exposure to diethylstilbestrol: Implications for other environmental estrogens. Environ Health Perspect. 1995;103:83–87. doi: 10.1289/ehp.95103s783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newbold RR. Lessons learned from perinatal exposure to diethylstilbestrol. Toxicology and applied pharmacology. 2004;199:142–150. doi: 10.1016/j.taap.2003.11.033. [DOI] [PubMed] [Google Scholar]
- Newbold RR. Prenatal exposure to diethylstilbestrol (DES) Fertility and sterility. 2008;89:e55–56. doi: 10.1016/j.fertnstert.2008.01.062. [DOI] [PubMed] [Google Scholar]
- Newbold RR, MaLachlan JA. Diethylstilbestrol-associated defects in murine genital tract development. In: McLachlan JA, editor. Estrogens in the Environment II. Elsevier; New York: 1985. pp. 288–318. [Google Scholar]
- Newbold RR, Tyrey S, Haney AF, McLachlan JA. Developmentally arrested oviduct: a structural and functional defect in mice following prenatal exposure to diethystilbestrol. Teratology. 1983;27:417–426. doi: 10.1002/tera.1420270316. [DOI] [PubMed] [Google Scholar]
- Oakey RE. The progressive increase in estrogen production in human pregnancy: an appraisal of the factors responsible. Vitam Horm. 1970;28:1–36. doi: 10.1016/s0083-6729(08)60887-0. [DOI] [PubMed] [Google Scholar]
- Read LD, Greene GL, Katzenellenbogen BS. Regulation of estrogen receptor messenger ribonucleic acid and protein levels in human breast cancer cell lines by sex steroid hormones, their antagonists, and growth factors. Molecular endocrinology. 1989;3:295–304. doi: 10.1210/mend-3-2-295. [DOI] [PubMed] [Google Scholar]
- Rennell CL. T-shaped uterus in diethylstilbestrol (DES) exposure. AJR American journal of roentgenology. 1979;132:979–980. doi: 10.2214/ajr.132.6.979. [DOI] [PubMed] [Google Scholar]
- Robboy SJ, Cunha GR, Kurita T, Strickland KC. Vagina. In: Mills S, editor. Histology for Pathologists. Kluver; Philadelphia: 2018. In Press. [Google Scholar]
- Robboy SJ, Kurita T, Baskin L, Cunha GR. New insights insights into human female reproductive tract development. Differentiation; research in biological diversity. 2017;97:9–22. doi: 10.1016/j.diff.2017.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robboy SJ, Noller KLRHK, Barnes AB, Townsend D, Gunderson JH, Kurland L, Nash S. An atlas of findings in the human female after intrauterine exposure to diethylstilbestrol. DHEW publication 84-2344 1984 [Google Scholar]
- Robboy SJ, Scully RE, Welch WR, Herbst AL. Intrauterine diethylstilbestrol exposure and its consequences: pathologic characteristics of vaginal adenosis, clear cell adenocarcinoma, and related lesions. Arch Pathol Lab Med. 1977;101:1–5. [PubMed] [Google Scholar]
- Robboy SJ, Taguchi O, Cunha GR. Normal development of the human female reproductive tract and alterations resulting from experimental exposure to diethylstilbestrol. Human Pathol. 1982;13:190–198. doi: 10.1016/s0046-8177(82)80177-9. [DOI] [PubMed] [Google Scholar]
- Saceda M, Lippman ME, Chambon P, Lindsey RL, Ponglikitmongkol M, Puente M, Martin MB. Regulation of the estrogen receptor in MCF-7 cells by estradiol. Molecular endocrinology. 1988;2:1157–1162. doi: 10.1210/mend-2-12-1157. [DOI] [PubMed] [Google Scholar]
- Stillman RJ. In utero exposure to diethylstilbestrol: adverse effects on the reproductive tract and reproductive performance and male and female offspring. American journal of obstetrics and gynecology. 1982;142:905–921. doi: 10.1016/s0002-9378(16)32540-6. [DOI] [PubMed] [Google Scholar]
- Taguchi O, Cunha GR, Robboy SJ. Experimental study of the effect of diethylstilbestrol on the development of the human female reproductive tract. International J Biological Res in Pregnancy. 1983;4:56–70. [PubMed] [Google Scholar]
- Terakawa J, Rocchi A, Serna VA, Bottinger EP, Graff JM, Kurita T. FGFR2IIIb-MAPK Activity Is Required for Epithelial Cell Fate Decision in the Lower Mullerian Duct. Molecular endocrinology. 2016;30:783–795. doi: 10.1210/me.2016-1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titus-Ernstoff L, Troisi R, Hatch EE, Palmer JR, Hyer M, Kaufman R, Adam E, Noller K, Hoover RN. Birth defects in the sons and daughters of women who were exposed in utero to diethylstilbestrol (DES) International journal of andrology. 2010;33:377–384. doi: 10.1111/j.1365-2605.2009.01010.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


