Abstract
AIM
To assess associations between white matter properties and prereading skills (phonological awareness and receptive and expressive language) in children born preterm and at term at the onset of reading acquisition.
METHOD
Six-year-old children born preterm (n=36; gestational age 22–32wk) and at term (n=43) underwent diffusion magnetic resonance imaging and behavioural assessments. Tracts were selected a priori based on findings from a study of 6-year-old children born at term: the left hemisphere arcuate fasciculus and superior longitudinal fasciculus, and right hemisphere uncinate fasciculus. Using linear regression, we assessed associations between fractional anisotropy of tracts and phonological awareness and receptive and expressive language scores. We investigated whether associations were moderated by prematurity.
RESULTS
Fractional anisotropy of the left hemisphere arcuate fasciculus contributed unique variance to phonological awareness across birth groups. The association between fractional anisotropy of the right hemisphere uncinate fasciculus and receptive and expressive language was significantly moderated by prematurity.
INTERPRETATION
A left-hemisphere tract was associated with phonological awareness in both birth groups. A right-hemisphere tract was associated with language only in the term group, suggesting that expressive and receptive language is mediated by different white matter pathways in 6-year-old children born preterm. These findings provide novel insights into similarities and differences of the neurobiology of prereading skills between preterm and term born children at reading onset.
Preterm birth affects nearly 10% of infants born in the USA.1 Children born preterm are at risk for adverse neurodevelopmental outcomes, including deficits in attention, working memory, executive function, language, and reading.2,3 A recent meta-analysis confirmed that children born preterm, on average, scored 0.48 standard deviations below children born at term in reading.3 A second meta-analysis found a significant gap between preterm and term groups in reading that increased between the age of 6 to 12 years, especially for reading comprehension.4 Precursor skills for reading include phonological awareness and language abilities. Measures of each of these precursors in isolation significantly predict later reading abilities in children born at term.5 Compared with children born at term, 6-year-old children born preterm have demonstrated deficits in early prereading skills, including phonological awareness and language abilities.6 These early behavioural differences likely account for why children born preterm are unlikely to achieve reading levels similar to their typically developing peers.7 Understanding the neurobiological basis of early prereading abilities in both children born preterm and at term may provide insight into the process of learning to read. Studies to date have primarily examined how early prereading skills relate to neurobiological properties, such as metrics of white matter pathways, in children born at term but not in children born preterm at the early stages of learning to read.8,9
Preterm birth is associated with injury to the white matter of the brain.10 Microstructural properties of white matter tracts can be quantified with diffusion magnetic resonance imaging (MRI) using metrics such as fractional anisotropy and mean diffusivity.11 Using diffusion MRI, studies of neonates have found increased mean diffusivity among children born preterm.12 Among children and adolescents, studies have observed altered fractional anisotropy values in preterm versus term participants.13 Recently, in the current sample of 6-year-old children we replicated a similar pattern of both increased and decreased fractional anisotropy in children born preterm versus at term that had been previously observed in a cohort of older children and adolescents.13,14
Reading and its related subskills are known to activate a distributed network of cortical grey matter areas that are connected via complex networks of white matter pathways (tracts).15–17 Fractional anisotropy of specific white matter tracts, such as the arcuate, superior longitudinal, and uncinate fasciculi, have been associated with reading, phonological awareness, and language skills in older, typically developing children and adults.18–21 Studies involving children born preterm have also observed associations between white matter properties and reading.19,22 However, a recent study found that the direction of these associations varied between the two birth groups, suggesting a differing neurobiology for reading in children and adolsecents born preterm and at term.19 We recently demonstrated in the current sample of 6-year-old children born at term that fractional anisotropy of the left arcuate fasciculus (AF-L) and left superior longitudinal fasciculus (SLF-L) were associated with phonological awareness skills.8 In addition, we found that fractional anisotropy of the right uncinate fasciculus (UF-R) and SLF-L were associated with receptive and expressive language skills.8 To our knowledge, no study to date has examined whether children born preterm versus their term-born peers demonstrate similar or different patterns of associations between fractional anisotropy of white matter tracts and prereading skills at the age at which children are learning to read.
The present study explored the degree of association between individual variation in fractional anisotropy of white matter tracts and phonological awareness and language abilities in children born preterm and at term. Based on the findings in the term group,8 we specifically examined the associations between fractional anisotropy of the AF-L and SLF-L and phonological awareness. In addition, we examined the associations between fractional anisotropy of the UF-R and SLF-L and language. Given previous evidence that older children born preterm and at term exhibit different patterns of associations with reading,19 we hypothesized that children born preterm would have significantly different patterns of associations than children born at term. Thus, we predicted that birth group would moderate the association between fractional anisotropy of the selected tract and the prereading measure. We replicated the analyses using mean diffusivity to establish whether there were novel findings using a different metric of white matter. These analyses were performed to determine whether children born preterm and at term have distinctive neurobiological profiles associated with variation in their level of skill in these critical prereading abilities. Findings from this study will help to elucidate the neural basis of prereading skills in children born preterm in the early stages of reading development.
METHOD
Participants
Participants were children born preterm (n=54) and at term (n=50), recruited from 2012 to 2015 as part of a longitudinal study investigating the neural basis of reading in children born preterm and at term. To be included in the present study, participants were required to have useable MRI data and complete behavioural testing. Of the participants born preterm, 18 were not included in the present analyses because the children underwent a different MRI protocol (n=10), were unable to perform MRI scans (n=6), or moved too much during scanning (n=2). Of the participants born at term, seven were not included in the present analyses because they underwent a different MRI protocol (n=4), were unable to perform MRI scans (n=2), or moved too much during scanning (n=1). Thus, the final sample consisted of 36 participants born preterm (23 males, mean age 6y 1.8mo) and 43 participants born at term (16 males, mean age 6y 2.3mo). Data from 41 participants born at term were previously reported by Travis et al.8
Preterm birth was defined as gestational age at 32 weeks or earlier (22–32wk; 470–2180g). This criteria for defining prematurity was used to capture children at greatest risk for white matter injury.23 Term birth was defined as gestational age at 37 weeks or later or birthweight greater than or equal to 2500g (36–42wk; 2720–4370g). One child in the term group was born at 36 weeks by parent report but was above our weight threshold (2780g). See Dodson et al. for recruitment methods.14 Exclusion criteria for all participants included neurological factors unrelated to preterm birth that would account for white matter differences or potential reading disorders among participants, including congenital anomalies, active seizure disorder, hydrocephalus, or sensorineural hearing loss. Diagnosis of cerebral palsy (CP) was not an exclusion criterion, as CP is a known risk factor of preterm birth. One participant born preterm had mild CP. The experimental protocol was approved by the Stanford University Institutional Review Board (#IRB-22233). A parent or legal guardian provided informed written consent and participants were compensated for participation.
Demographic characteristics assessed in this sample included socio-economic status (SES), as measured using a modified Four-Factor Hollingshead Index (Appendix S1, online supporting information), and ethnicity. At the time of participation, children were either enrolled in kindergarten or first grade. Participants were considered to have a family history of reading delay if a parent reported diagnosed or suspected reading problems in one or more immediate biological family member, including mother, father, or siblings.24 Children were classified as bilingual if parents reported that children could conduct conversation fluently in a language other than English. Because of the high proportion of bilingual children in the Bay Area of California, we did not exclude children based on bilingual status but included such children only if they had been enrolled in an English-speaking daycare, preschool, or elementary school for a minimum of 2 years and were not classified at school as an English language learner. Handedness was measured using the Edinburgh Handedness Inventory.25
Behavioural measures
All participants completed a battery of norm-referenced behavioural tests to characterize their phonological awareness and receptive and expressive language skills. Studies have found that intelligence scores are, on average, lower in preterm than in matched term samples.2 Non-verbal IQ has also been associated with reading ability.26 For this reason, we assessed all participants’ general intelligence.
Phonological awareness was assessed using three subtests of the Comprehensive Test of Phonological Processing: Elision, Sound Matching, and Blending Words.27 Receptive and expressive language skills were assessed with the Clinical Evaluation of Language Fundamentals, 4th Edition.28 These skills were summarized using the Core Language composite score, which consists of four subtests of the Clinical Evaluation of Language Fundamentals, 4th Edition: Concepts and Following Directions, Word Structure, Recalling Sentences, and Formulating Sentences. Non-verbal intellectual abilities were assessed using the Wechsler Abbreviated Scale of Intelligence, a nationally standardized test of general intellectual abilities, with the Matrix Reasoning subtest and the Block Design subtest.29
As part of the longitudinal study, we assessed real-word and pseudo-word reading abilities with the Word Attack and Word Identification subtests of Woodcock Reading Mastery Test respectively.30 Exploratory analyses revealed that 44% of children born preterm in the current sample could not read more than one pseudo-word, demonstrating that many were not yet able to read. Based on this finding, we chose not to examine associations between white matter properties and reading scores, owing to limited variation in reading from floor effects and a non-normal distribution of reading scores among children born preterm.
Diffusion MRI data acquisition and analyses
All participants were scanned for research purposes without the use of sedation. For each participant, we collected a high-resolution T1-weighted anatomical image using a 5-minute inversion recovery-prep three-dimensional fast-spoiled gradient sequence and a 30-direction diffusion MRI scan with a b-value of 1000 seconds/mm2. Methods for imaging parameters, data preprocessing steps, analysis of motion, and individual native-space tractography are described in several previous publications and are included in Appendix S1 (online supporting information).8,13,14,19 White matter pathways (AF-L, SLF-L, UF-R) for analysis were selected a priori based on evidence for associations with prereading skills in this sample of children born at term at 6 years of age.8
Statistical analyses
Characteristics of the preterm and term groups
We computed two-tailed t-tests for independent samples for each of the four continuous demographic measures of age, gestational age, birthweight, and SES. We computed separate χ2 analyses for each of the five categorical demographic variables: sex, ethnicity, grade, family history of reading delays, and handedness. We computed two-tailed t-tests for independent samples for each of the three behavioural measures: phonological awareness, core language, and non-verbal IQ. All results comparing demographic and behavioural characteristics were considered significant at p<0.05.
Hierarchical linear regressions
We computed four separate hierarchical linear regression models to assess associations between tract fractional anisotropy and prereading measures. We separately entered mean fractional anisotropy of the AF-L and SLF-L as predictor variables to phonological awareness, and mean fractional anisotropy of the UF-R and SLF-L as predictor variables to core language. To examine whether birth group significantly moderated the association between mean fractional anisotropy of each brain tract and prereading measure, we entered an interaction term as the last step in each model.
We included SES, sex, and non-verbal IQ as covariates in each model because they have previously been associated with reading and prereading abilities.26,31,32 We employed a Bonferroni correction to control for the two regression models computed per prereading measure. Significance level adjusted for multiple comparisons was thus set to p<0.025.
RESULTS
Characteristics of the preterm and term groups
Detailed medical information was available for 32 of the 36 participants born preterm. Medical complications at birth in the preterm group were respiratory distress syndrome (n=29); bronchopulmonary dysplasia or chronic lung disease (n=7); hyperbilirubinaemia (n=20); patent ductus arteriosus (n=15); retinopathy of prematurity or immature retinae (n=14); necrotizing enterocolitis (n=6); small for gestational age (≤third centile birthweight for gestational age; n=2). In terms of early neuroimaging findings, 15 had one or more mildly abnormal findings on head ultrasounds or MRIs (grade 1 intraventricular haemorrhage [n=8]; grade 2 intraventricular haemorrhage [n=2]; small periventricular lesions [n=1]; mild white matter injury [n=4]; transient vascular malformation [n=1]; mildly enlarged ventricles [n=3]), and one had abnormal findings on head ultrasound or MRI (bilateral grade 3 intraventricular haemorrhage). To follow-up on the early abnormalities seen in participants born preterm on head ultrasound or MRI, a neuroradiologist assessed T1-weighted MRI scans collected at 6 years of age for five features associated with white matter injury: white matter signal abnormality, periventricular white matter volume loss, cystic abnormalities, ventricular dilation, and thinning of the corpus callosum.13,33 Excessive punctate lesions were considered mild periventricular white matter volume loss. Individuals born preterm were categorized as normal if they had less than or equal to one abnormal feature and abnormal if they had two or more abnormal features. Of the 36 participants born preterm, five had abnormal T1-weighted scans at 6 years of age, including the participant with mild CP.
Results of group analyses for demographic and behavioural measures are presented in Table I. By design, children born preterm had significantly decreased gestational age and birthweight than children born at term. SES was significantly lower in the preterm than in the term group. However, on average, both participants born preterm and at term were from high socio-economic backgrounds. There were significantly more males in the preterm than in the term group. There was a significantly lower proportion of participants with a family history of reading delays in the preterm compared to the term group. Children in the preterm group did not differ significantly from the term group with regard to age, ethnicity, grade, bilingual status, and handedness.
Table I.
Demographic information, prereading performance, and intelligence scores for the preterm and term groups
| Preterm (n=36) GA 22–32wk |
Term (n=43) GA 36–42wk |
t or χ2 | p | |
|---|---|---|---|---|
| Demographic measures | ||||
|
| ||||
| Mean (y:m) ± SD age | 6:2 ± 1.8mo | 6:2 ± 2.3mo | 0.27 | 0.786 |
| Mean ± SD GA (wk) | 29.4 ± 2.4 | 39.5 ± 1.5 | 22.18d | <0.001 |
| Mean ± SD birthweight (g) | 1325 ± 461 | 3320 ± 412 | 20.31d | <0.001 |
| Mean ± SD SES (HI) | 50.8 ± 15.1 | 58.5 ± 9.5 | 2.65d | 0.011 |
| Males | 23 (64) | 16 (37) | 5.58d | 0.018 |
| Non-white | 12 (33) | 12 (28) | 0.27 | 0.601 |
| Kindergarten | 26 (72) | 31 (72) | <0.01 | 0.990 |
| Family history of reading delays | 1 (3) | 9 (21) | 5.84d | 0.016 |
| Bilingual | 12 (33) | 22 (51) | 2.54 | 0.111 |
| Right handed | 34 (94) | 41 (95) | 0.03 | 0.855 |
|
| ||||
| Prereading measures (mean ± SD) | ||||
|
| ||||
| Phonological awarenessa | 109.5 ± 13.2 | 112.6 ± 13.5 | 1.01 | 0.315 |
| Core languageb | 102.4 ± 10.8 | 111.6 ± 15.5 | 3.00d | 0.004 |
|
| ||||
| Intelligence measures (mean ± SD) | ||||
|
| ||||
| Non-verbal IQc | 100.4 ± 13.7 | 112.0 ± 15.8 | 3.43d | 0.001 |
Data are n (%) unless otherwise indicated.
Comprehensive Test of Phonological Processing.
Clinical Evaluation of Language Fundamentals, 4th Edition.
Wechsler Abbreviated Scale of Intelligence, 2nd Edition.
p<0.05.
GA, gestational age; SES, socioeconomic status; HI, Hollingshead Index.
Children in the preterm group had lower phonological awareness scores than term peers, but the difference was not significant. Children in the preterm group had significantly lower core language scores than the term group. The preterm group had general intelligence mean scores within the normal range but had significantly lower non-verbal IQ scores than the term group.
Hierarchical linear regressions
In preparation for regression analyses, exploratory analyses confirmed that the present data did not violate assumptions of collinearity, continuity of variables, and normality. Results of hierarchical linear regressions for associations between mean fractional anisotropy of the AF-L and SLF-L and phonological awareness are presented in Table II as Models 1 to 5. Results of the hierarchical linear regressions for associations between mean fractional anisotropy of the UF-R and SLF-L and core language are presented in Table III as Models 6 to 10. Scatter plots for significant associations are presented in Figure 1.
Table II.
Associations between mean fractional anisotropy of the left arcuate fasciculus (AF-L) and left superior longitudinal fasciculus (SLF-L) and phonological awareness in 6-year-old children born preterm and at term, controlling for socio-economic status (SES), sex and non-verbal IQ.
| Model 1 | Model 2 | Model 3 | Model 4 | Model 5 | |
|---|---|---|---|---|---|
| SES | 0.07 (0.13) | −0.02 (0.12) | −0.01 (0.12) | 0.06 (0.13) | 0.07 (0.12) |
| Sex | 2.61 (2.91) | 2.63 (2.68) | 2.57 (2.67) | 1.66 (2.90) | 1.37 (2.89) |
| Non-verbal IQ | 0.34 (0.11)a | 0.38 (0.10)a | 0.38 (0.10)a | 0.32 (0.11)a | 0.32 (0.11)a |
| Group | 2.07 (3.06) | 4.66 (2.89) | 54.88 (41.66) | 1.17 (3.03) | 39.45 (29.24) |
| AF-L | – | 180.74 (43.58)a | 238.72 (64.73)a | – | – |
| AF-L × group | – | – | −104.24 (86.26) | – | – |
| SLF-L | – | – | – | 68.54 (34.96)b | 109.78 (46.81)a |
| SLF-L × group | – | – | – | – | −89.71 (68.13) |
|
| |||||
| ∆ R2 (%) | – | 15.4a | 1.3 | 4.0b | 1.8 |
| Total R2 (%) | 20.2a | 35.5a | 36.8a | 24.2a | 26.0a |
Data are unstandardized coefficients (SE) unless otherwise indicated.
p<0.025, corrected.
p<0.1.
Table III.
Associations between mean fractional anisotropy of the right uncinate fasciculus (UF-R) and left superior longitudinal fasciculus (SLF-L) and core language in 6-year-old children born preterm and at term, controlling for socio-economic status (SES), sex, and non-verbal IQ (Unstandardized Coefficients; SE)
| Model 6 | Model 7 | Model 8 | Model 9 | Model 10 | |
|---|---|---|---|---|---|
| SES | 0.30 (0.12)a | 0.28 (0.12)a | 0.28 (0.11)a | 0.29 (0.12)a | 0.30 (0.12)a |
| Sex | 3.74 (2.70) | 4.60 (2.75)b | 3.81 (2.69) | 2.94 (2.70) | 2.70 (2.70) |
| Non-verbal IQ | 0.32 (0.10)a | 0.32 (0.10)a | 0.31 (0.10)a | 0.30 (0.10)a | 0.31 (0.10)a |
| Group | −2.22 (2.83) | −1.38 (2.88) | 99.50 (43.06)a | −2.98 (2.82) | 28.59 (27.25) |
| UF-R | – | 65.13 (47.56) | 217.77 (79.73)a | – | – |
| UR-R × group | – | – | −228.65 (97.38)a | – | – |
| SLF-L | – | – | – | 57.51 (32.50)b | 91.51 (43.63)b |
| SLF-L × group | – | – | – | – | −73.97 (63.51) |
|
| |||||
| ∆ R2 (%) | – | 1.5 | 4.2a | 2.5b | 1.1 |
| Total R2 (%) | 39.7a | 41.2a | 45.4a | 42.2a | 43.3a |
Data are unstandardized coefficients (SE) unless otherwise indicated.
p<0.025, corrected.
p<0.1.
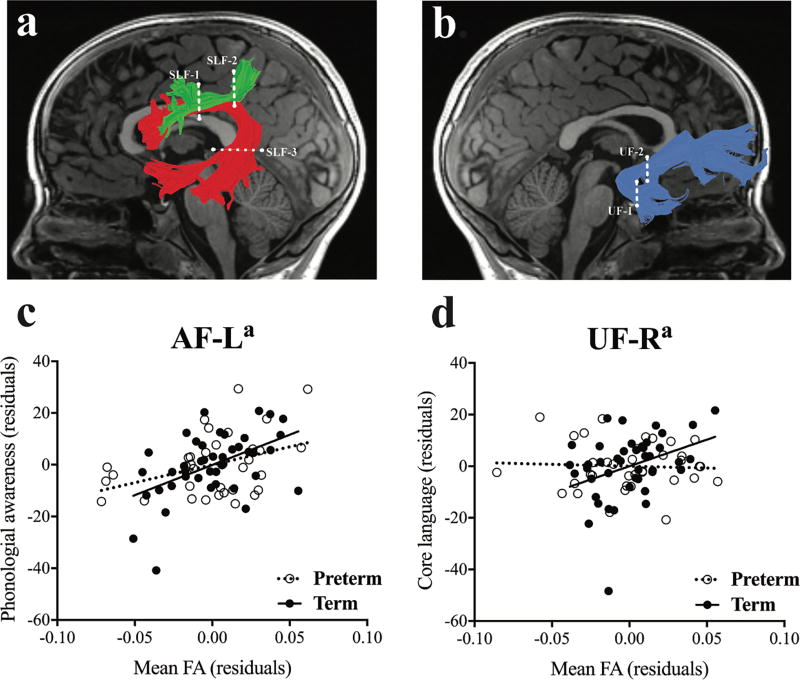
Figure 1.
Associations between white matter properties and prereading abilities in children born preterm and at term. Tract renderings (a, b, top row) and corresponding associations between tract mean fractional anisotropy (FA) and prereading measures after controlling for socio-economic status (SES), sex, and non-verbal IQ (c, d, bottom row). (a, b) Tract renderings are displayed on a mid-sagittal T1 image from a representative child in the preterm group. Dashed lines represent the location of the two regions of interest (ROIs) used to segment each cerebral tract from the whole-brain fibre group. (a) Left hemisphere arcuate fasciculus (AF-L; red [ROIs: superior longitudinal fasciculus {SLF-1} and SLF-3]) and left hemisphere SLF (SLF-L; green [ROIs: SLF-1 and SLF-2]). (b) Right hemisphere uncinate fasciculus (UF-R; blue). Partial correlations controlling for SES, sex, and non-verbal IQ are visualized as a scatter plot between residual mean FA values and residual values for each prereading measure. Children born preterm are represented with open circles and dashed lines, and children born at term are represented with closed circles and solid lines. (c) Associations between phonological awareness and mean FA of AF-L were similar in preterm and term groups after controlling for SES, sex, and non-verbal IQ. (d) Associations between core language and mean FA of the UF-R differed between preterm and term groups after controlling for SES, sex, and non-verbal IQ. ap<0.025.
Model 1 demonstrated that non-verbal IQ was the only covariate that significantly contributed to phonological awareness (p=0.002). Model 2 demonstrated that mean fractional anisotropy of the AF-L significantly contributed 15.4% unique variance to phonological awareness in the whole sample (p=0.001), and Model 3 demonstrated that the effect was not moderated by birth group (Table II; Fig. 1a, c). The scatter plot presented in Figure 1(c) illustrates that children born preterm and at term demonstrated similar patterns of associations between mean fractional anisotropy of the AF-L and phonological awareness. Model 4 demonstrated that the SLF-L contributed 4.0% unique variance to phonological awareness in the whole sample, trending toward significance (p=0.054). Model 5 demonstrated that the relation between mean fractional anisotropy of the SLF-L and phonological awareness was not moderated by birth group (Table II; Fig. 1a).
Model 6 demonstrated that the covariates of SES and non-verbal IQ significantly contributed to core language (p=0.014 and p=0.002 respectively). Model 7 demonstrated that mean fractional anisotropy of the UF-R did not significantly contribute to core language in the whole sample, but Model 8 demonstrated that the addition of the interaction term significantly predicted 4.2% unique variance (p=0.022). Thus, the relation between mean fractional anisotropy of the UF-R and core language was significantly moderated by birth group (Table III; Fig. 1b, d). The scatter plot presented in Figure 1(d) illustrates that the associations between mean fractional anisotropy of the UF-R and core language differed significantly between preterm and term groups. Model 9 demonstrated that mean fractional anisotropy of the SLF-L contributed 2.5% unique variance to core language in the whole sample, trending towards significance (p=0.081). Model 10 demonstrated that the relationship between mean fractional anisotropy of the SLF-L and core language was not moderated by birth group (Table III). Results of regression analyses repeated removing the five participants born preterm with abnormal white matter at 6 years of age, or the two participants born preterm and two participants born at term who were left-handed, demonstrated the same pattern of significant findings for both phonological awareness and core language (Tables SI–SII and Tables SIII–SIV respectively [online supporting information]). Results of regression analyses repeated using mean diffusivity demonstrated the same pattern of significant findings for phonological awareness (Table SV, online supporting information) but not for core language, which did not show a significant moderation of birth group in the UF-R (Table SVI, online supporting information).
Secondary analyses: hierarchical linear regressions
In order to understand the significant UF-R mean fractional anisotropy by birth group interaction, we repeated regression analyses separately in the preterm and term groups (Table SVII, online supporting information). Regression analyses performed in the preterm group demonstrated that non-verbal IQ was the only covariate to significantly contribute to core language (p=0.015; Table SVII, Model 1 [online supporting information]) and that mean fractional anisotropy of the UF-R was not a unique predictor for core language (p=0.617 Table SVII, Model 2 [online supporting information]). By contrast, regression analyses performed in the term group separately demonstrated that SES was the only covariate that significantly contributed to core language (p=0.005; Table SVII, Model 3 [online supporting information]) and that mean fractional anisotropy of the UF-R significantly contributed 7.3% unique variance to core language scores (p=0.028; Table SVII, Models 3 and 4 [online supporting information]).
DISCUSSION
This study investigated the neural basis of prereading skills in a group of children born preterm and at term at the onset of learning to read. Mean fractional anisotropy of the AF-L significantly contributed unique variance to phonological awareness scores across both groups, and the effect was not moderated by birth group. In contrast, mean fractional anisotropy of the UF-R did not contribute unique variance to core language scores across groups, but the relationship was significantly moderated by birth group. The pattern of results remained unchanged after removing the five participants born preterm with abnormal white matter at 6 years of age or the two participants born preterm and two born at term who were left-handed. The pattern of results was also similar when mean diffusivity was substituted for fractional anisotropy as the metric of interest except for the association between UF-R and core language, which was no longer significantly moderated by birth group.
Contrary to our hypothesis, preterm and term groups showed similar patterns of association between fractional anisotropy of the AF-L and SLF-L and phonological awareness scores and similar patterns of association between fractional anisotropy of the SLF-L and core language scores. These tracts are collectively assumed to be part of the dorsal stream in cognitive models of language and reading,34 and are known to provide connections between inferior frontal, inferior parietal, and posterior temporal cortices activated during phonological tasks in children born preterm and at term.35,36 These findings were consistent with many studies of term-born developmental samples that find positive associations between left hemisphere dorsal tracts and reading and related skills.18 Dorsal tracts were also implicated in learning to read in studies of dyslexia, as fractional anisotropy was reduced in these tracts in populations with developmental dyslexia versus healthy controls.16,37 A few studies have assessed behavioural associations with properties of dorsal white matter tracts in older preterm versus term samples. One such study found that fractional anisotropy of the SLF-L was associated with reading-related skills across birth groups, but that decreased volume of the tract was associated with poorer reading performance in children born preterm.38 The association in the current sample between phonological awareness and properties of dorsal tracts suggested that the neural basis of phonological awareness was similar across groups at this early stage of learning to read.
Consistent with our hypothesis, the association between mean fractional anisotropy of the UF-R and core language scores was moderated by birth group. The exact function of the ucinate fasciculus is debated; however, the present findings are consistent with prior studies that have found associations between properties of the ucinate fasciculus and language and reading abilities.20,39 The ucinate fasciculus connects with anterior projections of the inferior fronto-occipital fasciculus, a tract considered in cognitive models of language and reading to be part of the ventral stream,34 and relevant for mapping sound to meaning. A previous study demonstrated altered white matter properties in the ucinate fasciculus in adolescents born preterm compared with term-born peers, and found associations between fractional anisotropy of the UF-R and scores on a semantic language task.40 This evidence suggested that the ucinate fasciculus may be involved in language and reading abilities in older children born preterm and at term. Compared with posterior tracts, the ucinate fasciculus myelinates late and is slow to develop.41 It may be that ucinate fasciculus associations with reading in older preterm samples were easier to detect because of greater variability in white matter maturation in this tract. In the current sample, we observed preterm/term differences in core language performance, which suggested that the differences in the underlying neurobiology may have been related to differences in behaviour. We did not think that the lack of association in the preterm sample was because of a lack of power; we were likely powered to see the association given that we observed associations with other measures, and the range in scores was similar across measures and groups. To explain the lack of association in the preterm group, future studies should explore whether expressive and receptive language abilities may rely on a different balance of white matter pathways and white matter properties in children born preterm versus children born at term.
A recent study found that reading skills were positively associated with fractional anisotropy of dorsal pathways in a sample of 9 to 16-year-old children and adolescents born preterm, but negatively associated with fractional anisotropy in children born at term.19 We recognize that findings in this study differ from the patterns of associations between fractional anisotropy and reading-related tasks seen in the sample of older children and adolescents.19 This difference may be because of differences between the present sample and the older cohort, including less severe white matter injury in the children born preterm included in the current study. Also, the present sample was of younger age and thus at a different stage of reading development. The tasks assessed in this study were different from those assessed in the older sample, so the skill set that children rely on may differ across ages and birth groups. Further, fractional anisotropy has multiple contributors, including myelin, axonal diameter, and crossing fibres, each of which may be important to reading during different stages of reading development and may vary between preterm and term groups.
This study is novel because we assessed behavioural associations with white matter properties in children born preterm at the onset of learning to read. It is important to investigate which prereading skills would be associated with brain measures in children born preterm to understand neurobiological and behavioural differences in later reading outcomes. At the early stages of reading, we saw similarities and differences in the associations between brain measures and behavioural measures in children born preterm and at term. The neurobiological properties assessed with diffusion MRI are dynamic; there may be differences in the properties that fractional anisotropy captures across birth group, age group, or white matter tract. For example, there may be age- or birth-group-dependent effects of myelin or fibre pruning that change in relation to clinical factors or experience.42,43
Limitations of this study include a modest sample size. Also, these children were evaluated on a single occasion at the age of 6 years. Future studies should employ a longitudinal design to assess whether fractional anisotropy associations with behavioiral outcomes observed at 6 years of age persist or change in association with specific clinical or environmental factors. Future studies will also apply additional quantitative MRI methods to better understand how different properties of white matter relate to reading abilities.
In summary, the present findings provided evidence that metrics of a left-hemisphere dorsal tract, the AF-L, were associated with phonological awareness across birth groups. The study also provided evidence that metrics of a right-hemisphere ventral pathway, the UF-R, were associated with core language only in the term group. Taken together, these findings suggest that there are both similarities and differences in the neural basis of prereading abilities in children born preterm and at term. Overall, these findings have important implications for understanding how both brain and behaviour may shape developmental outcomes in children born preterm and at term.
Supplementary Material
Appendix S1: Supplementary materials
Table SI: Associations between fractional anisotropy of the left arcuate fasciculus and left superior longitudinal fasciculus and phonological awareness
Table SII: Associations between fractional anisotropy of the right uncinate fasciculus and left superior longitudinal fasciculus and core language
Table SIII: Associations between fractional anisotropy of the left arcuate fasciculus and left superior longitudinal fasciculus and phonological awareness
Table SIV: Associations between fractional anisotropy of the right uncinate fasciculus and left superior longitudinal fasciculus and core language
Table SV: Associations between mean diffusivity of the left arcuate fasciculus and left superior longitudinal fasciculus and phonological awareness
Table SVI Associations between mean diffusivity of the right uncinate fasciculus and left superior longitudinal fasciculus and core language
Table SVII: Associations between fractional anisotropy of the right uncinate fasciculus and core language
What this paper adds.
White matter properties and prereading abilities were associated in children born preterm at the onset of reading.
The neurobiology of phonological awareness was similar in children born preterm versus children born at term at 6 years.
The neurobiology of language was different in children born preterm versus children born at term at 6 years.
Acknowledgments
The authors received funding support from the National Institute of Child Health and Human Development (Grant # R01HD069162 [HMF], 5K99HD084749 [KET]). Funders were not involved in study design, data collection, data analysis, manuscript preparation, or publication decisions. The authors have stated that they had no interests that might be perceived as posing a conflict or a bias.
ABBREVIATIONS
- AF-L
Left arcuate fasciculus
- SES
Socio-economic status
- SLF-L
Left superior longitudinal fasciculus
- UF-R
Right uncinate fasciculus
References
- 1.Hamilton BE. Births: Provisional data for 2016 [Internet] U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics; [accessed 19 March 2018]. Available from: https://www.cdc.gov/nchs/data/vsrr/report002.pdf. [Google Scholar]
- 2.Aylward GP. Neurodevelopmental outcomes of infants born prematurely. J Dev Behav Pediatr. 2014;35:394–407. doi: 10.1097/01.DBP.0000452240.39511.d4. [DOI] [PubMed] [Google Scholar]
- 3.Aarnoudse-Moens CS, Weisglas-Kuperus N, van Goudoever JB, Oosterlaan J. Meta-Analysis of neurobehavioral outcomes in very preterm and/or very low birth weight children. Pediatrics. 2009;124:717–28. doi: 10.1542/peds.2008-2816. [DOI] [PubMed] [Google Scholar]
- 4.Kovachy VN, Adams JN, Tamaresis JS, Feldman HM. Reading abilities in school-aged preterm children: a review and meta-analysis. Dev Med Child Neurol. 2015;57:410–9. doi: 10.1111/dmcn.12652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Olofsson Å, Niedersøe J. Early language development and kindergarten phonological awareness as predictors of reading problems:from 3 to 11 years of age. J Learn Disabil. 1999;32:464–72. doi: 10.1177/002221949903200512. [DOI] [PubMed] [Google Scholar]
- 6.Wolke D, Meyer R. Cognitive status, language attainment, and prereading skills of 6-year-old very preterm children and their peers: the Bavarian Longitudinal Study. Dev Med Child Neurol. 1999;41:94–109. doi: 10.1017/s0012162299000201. [DOI] [PubMed] [Google Scholar]
- 7.O'Connor RE, Harty KR, Fulmer D. Tiers of intervention in kindergarten through third grade. J Learn Disabil. 2005;38:532–8. doi: 10.1177/00222194050380060901. [DOI] [PubMed] [Google Scholar]
- 8.Travis KE, Adams JN, Kovachy VN, Ben-Shachar M, Feldman HM. White matter properties differ in 6-year old Readers and Pre-readers. Brain Struct Funct. 2017;222:1685–703. doi: 10.1007/s00429-016-1302-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saygin ZM, Norton ES, Osher DE, et al. Tracking the roots of reading ability: white matter volume and integrity correlate with phonological awareness in prereading and early-reading kindergarten children. J Neurosci. 2013;33:13251–8. doi: 10.1523/JNEUROSCI.4383-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Volpe JJ. Brain injury in premature infants: a complex amalgam of destructive and developmental disturbances. Lancet Neurol. 2009;8:110–24. doi: 10.1016/S1474-4422(08)70294-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Basser PJ, Pierpaoli C. Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. 1996. J Magn Reson Imaging. 2011;213:560–70. doi: 10.1016/j.jmr.2011.09.022. [DOI] [PubMed] [Google Scholar]
- 12.Pannek K, Scheck SM, Colditz PB, Boyd RN, Rose SE. Magnetic resonance diffusion tractography of the preterm infant brain: a systematic review. Dev Med Child Neurol. 2014;56:113–24. doi: 10.1111/dmcn.12250. [DOI] [PubMed] [Google Scholar]
- 13.Travis KE, Adams JN, Ben-Shachar M, Feldman HM. Decreased and increased anisotropy along major cerebral white matter tracts in preterm children and adolescents. PLoS ONE. 2015;10:e0142860. doi: 10.1371/journal.pone.0142860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dodson CK, Travis KE, Ben-Shachar M, Feldman HM. White matter microstructure of 6-year old children born preterm and full term. Neuroimage Clin. 2017;16:268–75. doi: 10.1016/j.nicl.2017.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wandell BA, Yeatman JD. Biological development of reading circuits. Curr Opin Neurobiol. 2013;23:261–8. doi: 10.1016/j.conb.2012.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vandermosten M, Boets B, Wouters J, Ghesquière P. A qualitative and quantitative review of diffusion tensor imaging studies in reading and dyslexia. Neurosci Biobehav Rev. 2012;36:1532–52. doi: 10.1016/j.neubiorev.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 17.Dehaene S. Reading in the brain: the science and evolution of human invention. New York: Viking Penguin; 2009. [Google Scholar]
- 18.Yeatman JD, Dougherty RF, Rykhlevskaia E, et al. Anatomical properties of the arcuate fasciculus predict phonological and reading skills in children. J Cogn Neurosci. 2011;23:3304–17. doi: 10.1162/jocn_a_00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Travis KE, Ben-Shachar M, Myall NJ, Feldman HM. Variations in the neurobiology of reading in children and adolescents born full term and preterm. Neuroimage Clin. 2016;11:555–65. doi: 10.1016/j.nicl.2016.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nikki Arrington C, Kulesz PA, Juranek J, Cirino PT, Fletcher JM. White matter microstructure integrity in relation to reading proficiency. Brain Lang. 2017;174:103–11. doi: 10.1016/j.bandl.2017.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saur D, Kreher BW, Schnell S, et al. Ventral and dorsal pathways for language. Proc Natl Acad Sci U S A. 2008;105:18035–40. doi: 10.1073/pnas.0805234105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Feldman HM, Lee ES, Yeatman JD, Yeom KW. Language and reading skills in school-aged children and adolescents born preterm are associated with white matter properties on diffusion tensor imaging. Neuropsychologia. 2012;50:3348–62. doi: 10.1016/j.neuropsychologia.2012.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Back SA. White matter injury in the preterm infant: pathology and mechanisms. Acta Neuropathol. 2017;134:331–49. doi: 10.1007/s00401-017-1718-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Snowling M, Dawes P, Nash H, Hulme C. Validity of a protocol for adult self-report of dyslexia and related difficulties. Dyslexia. 2012;18:1–15. doi: 10.1002/dys.1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oldfield RC. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 26.Wang Y, Mauer MV, Raney T, et al. Development of tract-specific white matter pathways during early reading development in at-risk children and typical controls. Cereb Cortex. 2017;27:2469–85. doi: 10.1093/cercor/bhw095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wagner RK, Torgesen JK, Rashotte CA. Comprehensive Test of Phonological Processing: CTOPP. Austin, TX: Pro-Ed; 1999. [Google Scholar]
- 28.Semel E, Wiig EH, Secord WA. Clinical Evaluation of Lanugage Fundamentals (CELF-4) San Antonio, TX: Harcourt Psychology Corporation Assessments; 2003. [Google Scholar]
- 29.Wechsler D, Hsiao-pin C. WASI-II: Wechsler Abbreviated Scale of Intelligence. Second. San Antonio, TX: Pearson; 2011. [Google Scholar]
- 30.Woodcock RW. Woodcock Reading Mastery Test. 3. San Antonio, TX: Pearson; 2011. [Google Scholar]
- 31.Rutter M, Caspi A, Fergusson D, et al. Sex differences in developmental reading disability: New findings from 4 epidemiological studies. JAMA. 2004;291:2007–12. doi: 10.1001/jama.291.16.2007. [DOI] [PubMed] [Google Scholar]
- 32.Bowey JA. Socioeconomic status differences in preschool phonological sensitivity and first-grade reading achievement. J Educ Psychol. 1995;87:476–87. [Google Scholar]
- 33.Inder TE, Wells SJ, Mogridge NB, Spencer C, Volpe JJ. Defining the nature of the cerebral abnormalities in the premature infant: a qualitative magnetic resonance imaging study. J Pediatr. 2003;143:171–9. doi: 10.1067/S0022-3476(03)00357-3. [DOI] [PubMed] [Google Scholar]
- 34.Hickok G, Poeppel D. Dorsal and ventral streams: a framework for understanding aspects of the functional anatomy of language. Cognition. 2004;92:67–99. doi: 10.1016/j.cognition.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 35.Houdé O, Rossi S, Lubin A, Joliot M. Mapping numerical processing, reading, and executive functions in the developing brain: an fMRI meta-analysis of 52 studies including 842 children. Dev Sci. 2010;13:876–85. doi: 10.1111/j.1467-7687.2009.00938.x. [DOI] [PubMed] [Google Scholar]
- 36.Ment LR, Peterson BS, Vohr B, et al. Cortical recruitment patterns in children born prematurely compared with control subjects during a passive listening functional magnetic resonance imaging task. J Pediatr. 2006;149:490–8. doi: 10.1016/j.jpeds.2006.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vandermosten M, Boets B, Poelmans H, Sunaert S, Wouters J, Ghesquière P. A tractography study in dyslexia: neuroanatomic correlates of orthographic, phonological and speech processing. Brain. 2012;135:935–48. doi: 10.1093/brain/awr363. [DOI] [PubMed] [Google Scholar]
- 38.Frye RE, Hasan K, Malmberg B, et al. Superior longitudinal fasciculus and cognitive dysfunction in adolescents born preterm and at term. Dev Med Child Neurol. 2010;52:760–6. doi: 10.1111/j.1469-8749.2010.03633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McDonald CR, Ahmadi ME, Hagler DJ, et al. Diffusion tensor imaging correlates of memory and language impairments in temporal lobe epilepsy. Neurology. 2008;71:1869–76. doi: 10.1212/01.wnl.0000327824.05348.3b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mullen KM, Vohr BR, Katz KH, et al. Preterm birth results in alterations in neural connectivity at age 16 years. Neuroimage. 2011;54:2563–70. doi: 10.1016/j.neuroimage.2010.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Olson IR, Von Der Heide RJ, Alm KH, Vyas G. Development of the uncinate fasciculus: Implications for theory and developmental disorders. Dev Cogn Neurosci. 2015;14:50–61. doi: 10.1016/j.dcn.2015.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yeatman JD, Wandell BA, Mezer AA. Lifespan maturation and degeneration of human brain white matter. Nat Commun. 2014;5:4932. doi: 10.1038/ncomms5932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yeatman JD, Dougherty RF, Ben-Shachar M, Wandell BA. Development of white matter and reading skills. Proc Natl Acad Sci U S A. 2012;109:E3045–53. doi: 10.1073/pnas.1206792109. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1: Supplementary materials
Table SI: Associations between fractional anisotropy of the left arcuate fasciculus and left superior longitudinal fasciculus and phonological awareness
Table SII: Associations between fractional anisotropy of the right uncinate fasciculus and left superior longitudinal fasciculus and core language
Table SIII: Associations between fractional anisotropy of the left arcuate fasciculus and left superior longitudinal fasciculus and phonological awareness
Table SIV: Associations between fractional anisotropy of the right uncinate fasciculus and left superior longitudinal fasciculus and core language
Table SV: Associations between mean diffusivity of the left arcuate fasciculus and left superior longitudinal fasciculus and phonological awareness
Table SVI Associations between mean diffusivity of the right uncinate fasciculus and left superior longitudinal fasciculus and core language
Table SVII: Associations between fractional anisotropy of the right uncinate fasciculus and core language