Abstract
Objective
On/off motor fluctuations in Parkinson’s disease (PD) can be associated with extreme mood fluctuations and severe dysphoria. The impact of these affective symptoms may be overlooked in the treatment of motor fluctuations. Our goal was to examine the relationship between motor fluctuations, their treatment status, and suicidality in PD participants.
Methods
We analyzed data from the Methods of Optimal Depression Detection in Parkinson’s Disease (MOOD-PD) study of 223 individuals with PD. Suicidality was measured using items from four depression scales: Hamilton Depression Rating Scale (HAM-D-17); Montgomery-Åsberg Depression Rating Scale (MADRS); Inventory for Depressive Symptomatology (IDS-C); and the self-rated Beck Depression Inventory (BDI). Multivariable Poisson regression analyses tested whether self-reported motor fluctuations and their treatment status were associated with suicidality while controlling for recognized risk factors.
Results
Thirty-seven participants (16.6%) self-reported suicidality and 89 (39.5%) self-reported motor fluctuations, of whom 21 (23.6%) perceived their fluctuations as untreated. Participants reporting untreated motor fluctuations more frequently had a current depressive disorder (p < 0.001) and endorsed suicidality (p = 0.006) than participants with treated or no fluctuations. They also had significantly higher total scores on the HAM-D-17, MADRS, IDS-C, and BDI depression scales (p < 0.001 for each). Regression analyses showed significant associations between untreated motor fluctuations and higher scores on suicide questions extracted from the HAM-D-17, MADRS, and IDS-C (p < 0.01 for each).
Conclusions
PD patients with untreated motor fluctuations are at increased risk for suicidal thoughts and should be monitored for mood changes as treatment is adapted.
Keywords: Parkinson’s disease, suicide, depression, fluctuations
Introduction
Parkinson’s disease (PD) is characterized by motor signs of resting tremor, rigidity, and bradykinesia, but non-motor symptoms contribute greatly to disability.1 Neurodegeneration of limbic structures that depend on normal dopaminergic or noradrenergic neurotransmission is implicated in comorbid depression in PD patients.2 Major depression in PD has an estimated point-prevalence of 17%3 and increases the risk of suicidal ideation,4 which has a point-prevalence of at least 10% in PD patients.4–6 Earlier reports7,8 of post-operative suicidality in patients receiving deep brain stimulation (DBS) — despite clear motor improvement — helped to direct clinical focus to the non-motor aspects of PD management. However, knowledge of clinically useful correlates of suicidality in PD is incomplete.
Over 40% of PD patients experience motor fluctuations within 6 years of dopamine replacement therapy (DRT),9 although time of onset varies widely.10 Fluctuations in mood, anxiety, pain, autonomic, and other symptoms besides motor impairment are collectively called “non-motor” fluctuations.11 Typically, patients experience exacerbation of non-motor symptoms during “off” periods;12 for example, depressed mood and anxiety are temporarily worsened.12,13 Patients with mood fluctuations are at increased risk for depression after adjusting for age and disease duration.14,15 Therefore, it is plausible that motor fluctuations and the mood changes that accompany them may increase the risk of suicidal ideation in PD patients. Using data from a sample of 223 participants with idiopathic PD who were assessed by psychiatrists and neurologists, this analysis examined relationships between the character of fluctuations (including mood fluctuations), their treatment, and the presence of suicidal thoughts.
Methods
Population and Study Design
We used the dataset from the Methods of Optimal Depression Detection in PD (MOOD-PD) study, which investigated the psychometric performance of self-rated depression scales in participants with idiopathic PD16 and is described in previous reports.17 Supplemental table 1 (S1) contains an overview of depression and PD symptomatology scales from the MOOD-PD study pertinent to this manuscript.
Participants were recruited from the movement disorder practices of three community-based neurologists in the Baltimore, Maryland area. The initial visit included completion of the Mini-Mental State Exam (MMSE), Unified Parkinson’s Disease Rating Scale (UPDRS) Activities of Daily Living (ADL, part-II), and Complications of Therapy (part-IV) subsections by the study coordinator, UPDRS Motor ratings (part-III) and Hoehn and Yahr stage by a neurologist, self-report depression rating scales,17 and the 8-item Parkinson’s Disease Quality of Life Questionnaire-8 (PDQ-8).18 Presence or absence of motor fluctuations and their treatment status were queried on a self-report questionnaire about PD history completed prior to neurologist evaluation; participants were asked to report if they were currently experiencing motor fluctuations and, if yes, whether they were aware of receiving treatment specifically addressing these motor fluctuations. This questionnaire was included in the MOOD-PD study to investigate differences between clinician assessment of the presence of motor fluctuations and patient perception and reporting—the UPDRS-IV includes examiner-rating of fluctuations but does not address management. Based on questionnaire responses, participants were grouped for analysis: Non-fluctuators (NF), treated fluctuators (TF), and untreated fluctuators (UF).
A psychiatric diagnostic interview using the Schedule for Clinical Interview and Diagnosis (SCID) was conducted by a geriatric psychiatrist who also completed the following clinician-rated depression rating scales: Hamilton Depression Rating Scale (HAM-D-17),19 the Montgomery-Åsberg Depression Rating Scale (MADRS),20 and the Inventory for Depressive Symptomatology (IDS-C).21 The Beck Depression Inventory (BDI) was also completed by participants. The time-frame for symptom ratings was the prior two weeks. Final consensus current and lifetime best-estimate psychiatric diagnoses,22 based on the Diagnostic and Statistical Manual of Mental Disorders, version IV, text revision (DSM-IV TR) criteria, were established by a panel of six psychiatrists with expertise in geriatrics and/or movement disorders that met monthly to review each patient’s history and all collected data. Supplemental questions were used to obtain additional psychiatric history; included among these was a single question regarding the presence and intensity of mood fluctuations (0=not present, 1=mild, 2=moderate, 3=severe, see also Table S1). Participants scoring ≥ 1 were counted as having mood fluctuations.
Measures
For primary analyses, independent variables included self-reported presence or absence of motor fluctuations—and their perceived treatment status—and the presence or absence of mood fluctuations. Dependent variables of interest were based on the scores from the suicidality questions (one per scale) extracted from the HAM-D-17, MADRS, IDS-C, and BDI depression scales (Table S1). When reporting the prevalence of suicidal thoughts in our sample, participants were regarded as having endorsed suicidality if at least one of the four suicide questions was scored ≥ 1.
Secondary analyses were based on the UPDRS-IV (Complications of Therapy) responses regarding presence of motor fluctuations, as indicated by “yes” responses to at least one of questions 36, 37, or 38 of the UPDRS- IV, or a score of ≥ 1 on UPDRS Item 39 (Table S1). Fluctuation magnitude was quantified using the difference between the UPDRS-II (ADL) total “on” and “off” scores. The total UPDRS-III (Motor impairment “on”) score measured motoric impairment.
Statistical Analysis
Demographic makeup and clinical characteristics were described using means and standard 95% confidence intervals. As appropriate, Kruskal-Wallis, Mann-Whitney U, or Fisher’s exact tests were used to assess group differences. Correlation between UPDRS-IV and self-reported clinical fluctuation responses were quantified using the Kendall’s rank correlation coefficient (Kendall’s τ). Non-parametric analysis of covariance (ANCOVA)23 was used to produce significance estimates for UF vs. TF group differences in depression or PDQ-8 scores after accounting for a covariate (UPDRS-II or –III scores). Smoothing parameters and p-values for ANCOVA tests are in supplemental table 2 (Table S2). P-value for relative risk was based on Z-statistic derived from log-scale ratio estimate and confidence interval.24
Poisson regression analyses modeled associations between suicidal thought intensity (outcome variables based on the scores from the four suicidality questions) and the following independent variables: (1) on/off fluctuations and their treatment status (NF, TF, and UF groups); (2) presence or absence of mood fluctuations, (3) total UPDRS-III scores, and (4) motor fluctuation magnitude. Poisson regression was chosen to accurately model the distribution of our ordinal data outcome variables (suicidality question scores). Each model included the following five covariates or potential confounders: levodopa equivalent daily dosage (LEDD) (mg/day), sex, disease duration, current depressive disorder diagnosis, and history of alcohol dependence. Alcoholism is an important risk factor for suicidal ideation in the general population25 and noted as a potential risk factor for suicidal ideation in prior PD studies.4,6 A binomial regression using the presence of suicidality was also performed to estimate adjusted odds of suicidality related to the perception of untreated fluctuations. Odds ratios were calculated as exponentiated regression coefficients and confidence interval endpoints. Statistical significance was accepted at p < 0.05.
Standard Protocol Approvals, Registrations, and Patient Consents
The Johns Hopkins Institutional Review Board approved the study; participants—and when available, informants—provided written informed consent.
Results
Clinical characteristics of participants
Of the 250 participants with idiopathic PD and a Mini-Mental State Exam (MMSE) score ≥ 24 who completed diagnostic interviews, 27 were excluded because of missing data items on the UPDRS-II (n=8), UPDRS-III (n=1), and UPDRS IV (n=18), leaving 223 participants with complete datasets for this analysis. Of those, 89 (39.5%) self-reported on/off fluctuations and 21 of those 89 participants (23.6%) perceived that their fluctuations were untreated. Table 1 provides details of demographic and clinical characteristics data for the three groups based on fluctuation and treatment status. NF and TF participants had the shortest and longest disease durations, respectively. The NF group had the lowest LEDD and a later age of PD onset than the TF and UF groups.
Table 1.
Subject Characteristics, Grouped by Presence of Self-Reported On/Off Symptoms (n=223)
| Variable | No fluctuations (NF), (n=134) | Treated fluctuations (TF) (n=68) | Untreated fluctuations (UF) (n=21) | p |
|---|---|---|---|---|
| Demographic Information | ||||
| Age, years† | 69.0 (67.3–70.7) | 62.0 (59.8–64.1) | 66.4 (63.0–69.9) | *** |
| Age at PD onset, years† | 64.5 (62.7–66.3) | 50.9 (48.4–53.5) | 59.4 (55.0–63.8) | *** |
| Sex, male‡ | 93 (69.4%) | 46 (67.6%) | 12 (57.1%) | 0.515 |
| Mini-Mental State Exam† | 28.4 (28.1–28.6) | 28.1 (27.7–28.6) | 28.4 (27.7 (29.1) | 0.895 |
| Family history of PD‡ | 33 (24.6%) | 17 (25.0%) | 7 (35.0%) | 0.656 |
| Parkinson’s disease characteristics | ||||
| Disease duration† | 4.5 (3.9–5.1) | 11.0 (9.4–12.6) | 7.0 (4.8–9.1) | *** |
| Hoehn & Yahr stage† | 2.1 (2.0–2.2) | 2.4 (2.3–2.6) | 2.3 (2.0–2.6) | 0.007** |
| UPDRS-III Motor Examination score† | 16.8 (15.2–18.4) | 19.6 (16.4–22.8) | 22.0 (17.7–26.4) | 0.069 |
| Quality of Life (PDQ-8)† | 4.9 (4.1–5.6) | 8.0 (6.9–9.1) | 9.2 (6.9–11.4) | *** |
| Levodopa Usage (yes/no) | 118 (88.1%) | 68 (100%) | 20 (95.2%) | 0.003** |
| LEDD (mg/day)† | 510 (457–564) | 897 (775–1018) | 721 (596–846) | *** |
| Dopamine agonist usage | 41 (30.6%) | 28 (43.8%) | 4 (19.0%) | 0.0691 |
| MAO inhibitor usage | 17 (12.7%) | 16 (24.2%) | 0 | 0.009**1 |
| COMT inhibitor usage | 36 (26.9%) | 49 (74.2%) | 4 (19.0%) | ***1 |
| # With Bilateral DBS (n, %) | 7 (5.2%) | 19 (27.9%) | 0 | — |
| Fluctuations | ||||
| UPDRS-II ADL “on” vs. “off” score difference† | 1.24 (0.94–1.54) | 4.50 (3.47–5.53) | 3.38 (1.19–5.57) | *** |
| Mood Fluctuations (non-UPDRS)‡ | 2 (1.5%) | 10 (14.7%) | 4 (19.0%) | *** |
| Psychiatric Measures | ||||
| HAM-D-17 total score† | 6.0 (5.3–6.8) | 7.7 (6.5–8.8) | 13.0 (10.1–15.9) | *** |
| MADRS total score† | 6.5 (5.4–7.7) | 8.3 (6.6–10.0) | 16.0 (11.5–20.6) | *** |
| IDS-C total score | 10.9 (9.4–12.3) | 12.8 (10.7–14.8) | 22.8 (17.0–28.6) | *** |
| BDI total score | 9.0 (7.7–10.4) | 10.4 (8.8–12.0) | 16.9 (13.0–20.8) | *** |
| Any current depressive disorder (DSM- IV TR diagnosis)‡ | 50 (37.3%) | 37 (54.4%) | 15 (71.4%) | 0.003** |
| Suicidality Present‡ (yes/no) | 18 (13.4%) | 9 (13.2%) | 10 (47.6%) | 0.001** |
| SSRI use | 33 (24.6%) | 27 (39.7%) | 9 (42.9%) | 0.040* |
| Past or current alcohol abuse | 7 (5.2%) | 3 (4.4%) | 0 | 0.889 |
Patients with a score of 1 or greater on any of the four depression scale suicide items were counted as having suicidality present. SSRI = Selective Serotonin Reuptake Inhibitor; MAO = monoamine oxidase; COMT = catechol-O-methyltransferase. For the no fluctuations group, n=133 for PDQ-8 assessment and n=132 for BDI total score. For the treated fluctuations group, n=66 for the BDI scale, MAO inhibitor use, COMT inhibitor use, and dopamine agonist use. For the untreated fluctuations group, n=20 for family history of PD.
p-values derived from Kruskal-Wallis tests (df = 2) calculated in reference to χ2.
p-value derived from Fisher’s exact tests.
p-values represent Fisher’s exact tests between treated fluctuators and untreated fluctuators only.
p<0.05,
p<0.01,
p<0.001.
Thirty-seven participants (16.6%) endorsed suicidality on at least one depression scale; UF participants most frequently indicated suicidality and more frequently had current depressive disorder diagnoses than NF or TF participants. The UF group also had higher total HAM-D-17, MADRS, IDS-C, and BDI scores. TF and UF groups had comparable frequencies of selective serotonin reuptake inhibitor (SSRI) use. Mood fluctuations were least common and PDQ-8 quality of life scores were lowest (high quality of life) in the NF group.
Consistent with their perceived untreated fluctuations status, no UF participants had undergone deep brain stimulation surgery, compared with 7 (5.2%) of the NF and 19 (27.9%) of the TF participants. Additionally, no UF participants were using a monoamine-oxidase (MAO) inhibitor, compared to 16 (24.2%) of TF participants, and significantly fewer UF participants (19.0%) were using catechol-O-methyltransferase (COMT) inhibitors than TF participants (74.2%).
In terms of fluctuation magnitude, Mann-Whitney U-tests comparing the UPDRS-II total “on” and “off” score differences showed no significant differences between the TF and UF groups (U = 873.5; p = 0.120). Fluctuation magnitude was significantly greater in both the UF group (U = 617.5; p < 0.001) and TF group (U = 1208.5; p < 0.001) relative to the NF group.
Fluctuations perceived as untreated were significantly associated with suicidality
Table 2 presents Poisson regression analysis results from four separate types of models—each with different independent variables. The first model used group (NF, TF, or UF) as the independent variable of interest. Independent variables for the other three model types included: (1) UPDRS-II “on” and “off” total score differences (fluctuation magnitude); (2) UPDRS-III motor examination scores; or (3) the presence of mood fluctuations.
Table 2.
Poisson Regression: Suicidality and UPDRS, Self-Report, or Mood Fluctuation Data (n=223)
| Suicide Scale Question (Outcome) | Independent | Odds ratio | 95% CI | Z-value | p |
|---|---|---|---|---|---|
| HAM-D-17 | UPDRS-II: On vs. off total score difference | 1.04 | 0.95–1.12 | 0.99 | 0.322 |
| UPDRS-III: Motor examination total score | 1.03 | 1.01–1.05 | 2.59 | 0.01** | |
| Endorsed Mood Fluctuations (non-UPDRS) | 3.66 | 1.64–7.68 | 3.32 | *** | |
| Self-reported no on/off symptoms (reference) | |||||
| Self-reported treated on/off symptoms | 1.16 | 0.47–2.77 | 0.34 | 0.736 | |
| Self-reported untreated on/off symptoms | 3.73 | 1.62–8.45 | 3.15 | 0.002** | |
| MADRS | UPDRS-II: On vs. off total score difference | 1.03 | 0.95–1.09 | 0.70 | 0.482 |
| UPDRS-III: Motor examination total score | 1.03 | 1.01–1.05 | 2.87 | 0.004** | |
| Endorsed Mood Fluctuations (non-UPDRS) | 3.14 | 1.59–5.92 | 3.42 | *** | |
| Self-reported no on/off symptoms (reference) | |||||
| Self-reported treated on/off symptoms | 1.08 | 0.50–2.28 | 0.22 | 0.826 | |
| Self-reported untreated on/off symptoms | 3.93 | 1.98–7.76 | 3.94 | *** | |
| IDS-C | UPDRS-II: On vs. off total score difference | 1.07 | 0.98–1.15 | 1.69 | 0.092 |
| UPDRS-III: Motor examination total score | 1.03 | 1.01–1.06 | 2.60 | 0.009** | |
| Endorsed Mood Fluctuations (non-UPDRS) | 3.84 | 1.66–8.31 | 3.29 | *** | |
| Self-reported no on/off symptoms (reference) | |||||
| Self-reported treated on/off symptoms | 1.23 | 0.47–3.07 | 0.44 | 0.657 | |
| Self-reported untreated on/off symptoms | 3.80 | 1.58–8.97 | 3.04 | 0.002** | |
| BDI | UPDRS-II: On vs. off total score difference | 1.04 | 0.93–1.14 | 0.73 | 0.463 |
| UPDRS-III: Motor examination total score | 1.03 | 1.00–1.06 | 2.19 | 0.028* | |
| Endorsed Mood Fluctuations (non-UPDRS) | 2.50 | 0.86–6.32 | 1.82 | 0.068 | |
| Self-reported no on/off symptoms (reference) | |||||
| Self-reported treated on/off symptoms | 0.49 | 0.14–1.48 | −1.20 | 0.232 | |
| Self-reported untreated on/off symptoms | 2.46 | 0.92–6.20 | 1.87 | 0.061 |
Each model includes the following additional covariates: current depressive disorder (DSM-IV TR diagnosis), history of alcohol dependence, LEDD, disease duration, and sex. Odds ratios and confidence intervals represent the exponentiated coefficients and interval endpoints. Z-value was computed as the regression coefficient divided by its standard error.
p<0.05,
p<0.01,
p<0.001.
There was consistency among results using different clinician-rated depression scale items as the outcome of interest (HAM-D, MADRS, and IDS-C). For each corresponding suicidality outcome, significant adjusted associations were noted for participants with untreated motor fluctuations, mood fluctuations, greater motor impairment (higher UPDRS-III score), but not with fluctuation magnitude (Table 2). Regression analyses using UPDRS-IV fluctuation data were also produced, but failed to show significant associations with suicidality. When the self-rated BDI suicidality item was used as the outcome of interest in regression, the only model type showing significant association with the independent variable was that testing UPDRS-III score.
To measure the clinical relevance of these findings, we first produced relative risk estimates using the presence (yes or no) of suicidality and the treatment group among self-reported fluctuators (TV vs. UF). The relative risk of endorsing suicidality in the UF group was 3.60 (95% CI: 1.69–7.66; Z = 3.32; p < 0.001) compared to the TF group.
UF participants have more severe depression, but not worse quality of life
We next addressed the possibility of reverse causation; e.g., perhaps worse depression or more pessimistic self-assessment of their condition prompted participants to identify as having untreated fluctuations. We found that UF participants did not report worse overall quality of life than the TF patients (Figure 1) despite scoring significantly higher on all scales of depression. Scores on the UPDRS-II, another self-report measure, were also not significantly different. To further test whether UF participants were more inclined to report adverse health status than TF participants, we modeled depression scores and quality of life scores predicted based on either UPDRS-III or UPDRS-II scores (Figure 2). The results show that whereas UF participants had higher depression scores with respect to their UPDRS-III and UPDRS-II scores than did the TF participants, this was not true of their quality of life self-assessment. This was consistent across scales of depression (supplemental table 2). LEDD was not significantly different between TF and UF participants (U = 810; p = 0.356)
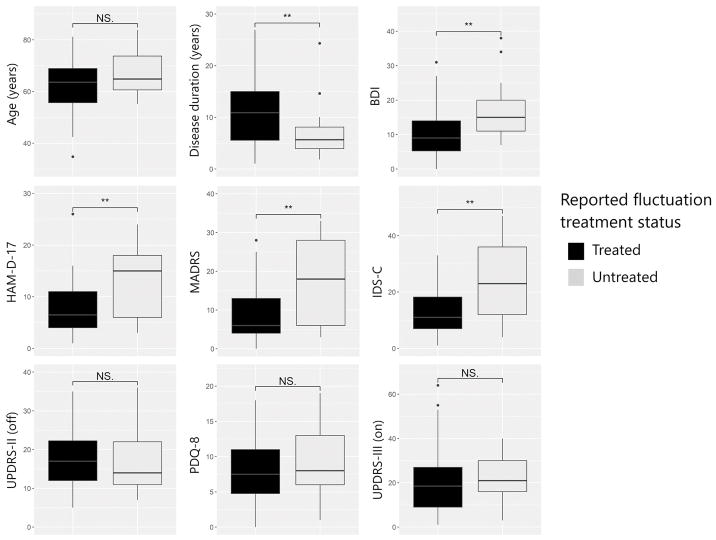
Figure 1. Comparison of participants with self-reported fluctuations based on perception of treatment.
Participants perceiving untreated fluctuations (UF group) score higher on all three clinician-rated scales of depression (HAM-D-17, MADRS, IDS-C) and a self-rated scale of depression (BDI). However, they do not report worse quality of life (PDQ-8) or more severe impairment in ADL (UPDRS-II, measured “off” medication). They also do not exhibit worse neurologist-rated motor impairment (UPDRS-III, measured “on” medication). P-values derived from Mann-Whitney U tests. NS. = not significant. ** = p < 0.01.
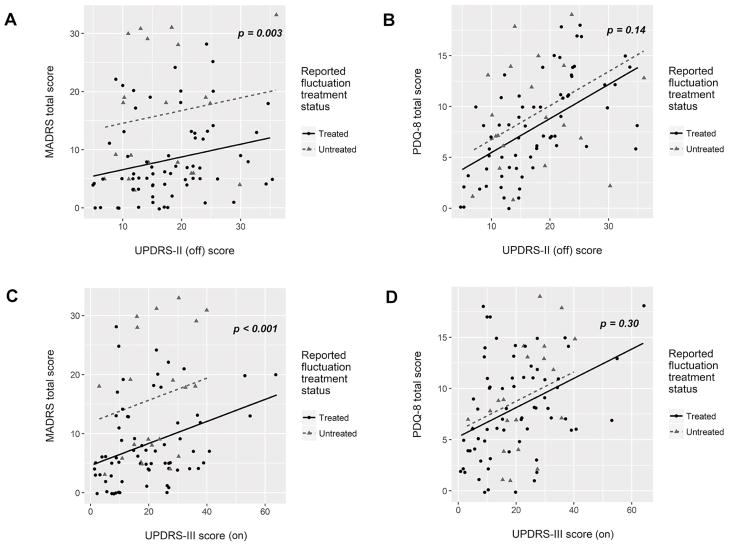
Figure 2. Perceived untreated fluctuations are associated with worse depression but not worse quality of life.
Depression score (A, C) or quality of life score (B, D) was linearly modeled based on either impairment in ADL (UPDRS-II, measured “off” medication) or neurologist-rated motor impairment (UPDRS-III, measured “on” medication). P-values derived from non-parametric analysis of covariance. All other scales of depression (HAM-D-17, IDS-C, and BDI) were also significantly higher in UF group (see supplemental table 2 for smoothing parameters and p-values for all depression scales).
To ascertain adjusted estimates (as odds ratios, OR) of the relative impacts of perceived treatment status and the presence of a current depressive disorder on suicidality, we produced a binomial regression with these factors as independent variables and the presence of suicidality as the outcome, using only TF and UF participants (n=89). Perceived UF status remained significantly associated with suicidality (OR = 5.5; 95% CI: 1.7–18.9; Z = 2.82; p = 0.005), as did current depressive disorder diagnosis (OR = 7.9; CI: 1.9–54.3; Z = 2.56; p = 0.011).
Self-reported fluctuation responses versus UPDRS-IV ratings
Self-reported on/off symptom data (Table 3) were highly correlated with responses indicated during the neurologist-conducted UPDRS-IV evaluation (Kendall’s tau = 0.78; p < 0.001).
Table 3.
Comparison of self-reported on/off symptom data and UPDRS-IV interview responses (n=223)
| UPDRS-IV Group | Self-reported on/off symptoms | No self-reported on/off symptoms | Total | τ | p |
|---|---|---|---|---|---|
| Any “off” periods | 83 | 19 | 102 | 0.78 | < 0.001 |
| No “off” periods | 6 | 115 | 121 | ||
| Total | 89 | 134 | 223 |
Participants scoring >1 on any of questions 36–39 of the UPDRS-IV (fluctuation items) were considered as having any “off” periods. τ = Kendall’s rank correlation (Kendall’s tau coefficient) between UPDRS-IV measures and self-report responses.
Table 4 presents data used to compare the validity of the self-reported fluctuation responses to results from the UPDRS-IV assessment by measuring differences between total “on” and total “off” scores of the UPDRS-II. Among the 102 participants with neurologist-rated fluctuations, 83 self-reported fluctuations and 19 denied them. Fluctuation magnitude was significantly greater in those with concurrent UPDRS-IV and self-reported fluctuations relative to the 19 UPDRS-IV fluctuators who denied on/off symptoms on self-report. Additionally, none of those 19 participants endorsed presence of mood fluctuations, as compared to 14 of the 83 (16.9%) with self-reported fluctuations.
Table 4.
UPDRS and Mood Fluctuation Data by Concordance of UPDRS-IV and Self-Report (n=102)
| UPDRS Section | “Yes” to any UPDRS-IV fluctuation item and “Yes” to self-reported on/off (n=83) | “Yes” to any UPDRS-IV fluctuation item; “No” to self-reported on/off (n=19) | p |
|---|---|---|---|
| UPDRS-II ADL “on” vs. “off” score difference | 4.54 (3.56–5.52) | 1.21 (0.41–2.01) | < 0.001 |
| Mood fluctuations‡ | 14 (16.9%) | 0 (0%) | 0.067 |
p-values derived from Kruskal-Wallis test (df = 1) calculated in reference to χ2.
p-value derived from Fisher’s exact tests.
Discussion
Reducing suicidality is an important means of improving PD patient quality of life and reducing caregiver stress.5 In this analysis of community-based PD participants, mood fluctuations—as well as motor fluctuations that were perceived by the patient as untreated—were associated with an increased likelihood of suicidal ideation and higher ratings on suicidality items from three clinician-rated depression scales. The association was similar in direction but not significant when analyzing the suicide item from the single self-reported depression scale (BDI), suggesting that clinician involvement in assessing the degree of suicidality influenced our findings. Untreated fluctuations conferred a relative risk of 3.6 for suicidality, measured binomially using aggregated responses to these depression scale items, and increased the odds of suicidality by 5.5 after adjusting for depressive disorder diagnosis. These data suggest that mood fluctuations and motor fluctuations that are perceived as being untreated are independent risk factors for suicidality in PD. Our findings also suggest that worse overall motoric impairment, as measured by higher UPDRS III scores, are associated with suicidality.
At least 40% of PD patients experience motor fluctuations within 6 years of levodopa therapy, but the exact onset is unpredictable.9 We show that suicidality is increased in patients for whom fluctuations are perceived as untreated. We recognize that some of these patients may have been unaware of objective interventions to address motor fluctuations. While it was not possible for us to reconstruct the intended effects of prescribed medication regimens, we did note that certain classes of medications that might be used to address fluctuations—COMT inhibitors, MAO inhibitors, and dopamine agonists—were not used or used less frequently in participants who described their fluctuations as untreated, suggesting that participants did use objective data to ascertain their treatment status. Similarly, no UF participants had undergone deep brain stimulation (DBS) surgery—which is consistent with their perceived undertreated status—compared to 28% of TF participants. Given the lack of clearly superior medications for fluctuation treatment, our data suggest another latent factor may contribute to perceived treatment status. Regardless, these data serve as a reminder that patient perceptions and expectations, whether consistent with clinical assessment or not, are important indicators of disease impact and should be accounted for. Furthermore, our results suggest that there is a window of time, starting at fluctuation onset, during which vulnerability for suicidal thinking is increased. PD duration, on average, was 7.0 years in the UF group versus 4.5 and 11.0 years in the NF and TF groups, respectively. This indicates that screening for fluctuating mood states and suicidal thought patterns is important when the effectiveness of DRT begins to wane and motor fluctuations are endorsed or become apparent. Nonetheless, it is important to avoid conflating treatment for fluctuations with treatment for depression specifically—aggressive depression management remains a critical aspect of PD therapy.
The present study incorporated subjective treatment assessment as an independent variable. Even when motor fluctuations are addressed clinically, patients may be unaware of the treatment purpose or regard their symptoms as untreated. Previous reports advocate for questionnaires regarding the patient experience of wearing-off and fluctuation phenomena, critiquing the lack of relevant questions in the original UPDRS interview.26,27 Similarly, a Patient-Reported Outcome (PRO) scale has explored the role of patient expectations when receiving complex care for advanced PD.28 The Patient-Centered Outcomes Research Institute (PCORI) actively promotes the use of such tools to produce knowledge of how the expected effects of treatment impact disease-related symptoms. In our study, participants who perceived their fluctuations as untreated were more likely to experience suicidal thoughts. Since UPDRS-II “on” vs. “off” score differences were comparable in the UF and TF groups, the magnitude or severity of fluctuations does not appear to be a key differentiator.
Our approach using a self-report questionnaire to assess perceived treatment status of fluctuations appears to have face validity, as the UF group had comparable LEDD to the TF group but was much less likely to be prescribed medications commonly used to treat fluctuations. Additionally, the UF participants scored worse on all measures of depression, but not worse on a validated quality-of-life measure (PDQ-8). This difference remained when adjusting for either another self-report (UPDRS-II) or neurologist-rated (UPDRS-III) measure of disease severity. These findings argue against reverse causation: i.e., that a participant’s decision to report motor fluctuations as being untreated might be ascribed to the general pessimism or dissatisfaction that occurs with depression. We acknowledge that antidepressant medication use was comparably frequent among these groups, which could be interpreted as evidence for undertreated depression in the UF participants. However, it is difficult to assess the adequacy or efficacy of pharmacologic intervention in this population based on the available data, which do not include information on dosages or therapy duration.
Data from the neurologist-rated UPDRS parts II, III, and IV were also used to evaluate the validity of the self-reported fluctuation data. The latter were recorded prior to neurologist evaluation to preclude influence of clinician judgment on participant self-evaluation. High correlation between these measures suggest general concordance between clinical and patient assessments of symptoms. Discrepant neurologist and patient assessments were similarly observed in a study comparing neurologist-rated changes in mood and motor symptoms to hourly diary entries by patients.29 Symptom severity may also contribute to differences in patient- versus clinician-derived data. For example, in our study, patients who did not self-report motor fluctuations—regardless of their UPDRS-IV ratings—had significantly less fluctuation-related ADL dysfunction per their UPDRS-II ratings. It is also observed that PD patients can fail to recognize dyskinesias and motor fluctuations, even when obvious to a clinician, causing discrepancy between patient-reported and clinician-rated assessments of these phenomena.30 Decreased self-awareness of motor fluctuations may further contribute to observed differences between self-reported and clinician-rated phenomena.
Prevalence data on suicide and suicidal ideation in PD relative to the general population are inconsistent; some indicate lower31,32 and others report greater prevalence.33,34 Factors such as diverse patient samples and metrics that do not evaluate suicidal ideation directly may account for these discrepancies.6 Apathy, decreased motivation to act upon suicidal thinking, and impaired executive function to plan a potential suicide are also proposed as explanations when lower prevalence of suicide is observed.31 In the present report, we extracted individual items from four scales of depression to test the strength of the association across different instruments. Across the three clinician-rated scales (HAM-D, MADRS, and IDS-C), the consistent association between suicidality measures and untreated motor fluctuations, mood fluctuations, and UPDRS-III motor scores suggests that the associations are robust and detectable using a variety of clinician-rated suicidality metrics. By contrast, a patient-rated depression scale, such as the BDI in this study, may not be sensitive to this clinically important association.
The cross-sectional design and the limited extent of the suicidality data restrict interpretations of this analysis. Furthermore, clinical data on history of suicide attempts (or longitudinal data following attempts subsequent to study participation) were not collected, precluding any analyses of their prevalence among our participants. The present analysis focused on depressive disorders and alcoholism as psychiatric comorbidities that may predispose patients to suicidality in a variety of medical illness contexts.35 It is possible that other comorbid neuropsychiatric disorders, cognitive impairment, or pain could also impact suicidality in PD and represent areas for future investigation. While the MOOD-PD depression scales are validated for detecting depression in PD, the MOOD-PD study did not include a dedicated metric to assess suicidal thoughts or tendencies. This is because the primary objective of the MOOD-PD study was to evaluate and compare psychometric performance of depression scales in PD and provide guidance on their use in clinical and research settings to improve depression detection.17 The use of self-report measures does perhaps also complicate interpretation of our results, although this is inherent to any investigation into the clinical significance of patient perception relative to objectively assessed phenomena. Finally, it is possible that the use of DBS—often indicated to improve fluctuations—by TF participants may have introduced non-motor changes that we have not accounted for.
Our findings should encourage assessment of both motor and non-motor fluctuations as potential targets for treatment and as metrics of treatment efficacy. In our data, self-reported motor fluctuations were associated with worse quality of life and more disabling fluctuations. The updated MDS-UPDRS interview36 evaluates the functional impact of motor fluctuations and is a significant advance in measuring their effects on patient quality of life. As the new MDS-UPDRS does not include items that address non-motor fluctuations, other approaches to assess their presence are warranted given our findings associating mood fluctuations with suicidality. Others have also shown that non-motor fluctuations disrupt patient quality of life more so than motor fluctuations.12,37,38 Active research on therapies to minimize on/off fluctuations provides reason for optimism.39
Supplementary Material
Highlights.
We examined the relationships among suicidality, mood fluctuations, motor fluctuations, and the perception of treatment for motor fluctuations in patients with parkinson’s disease (PD).
Regression analyses showed associations between fluctuations perceived to be untreated and higher scores on suicide questions extracted from three clinician-rated depression scales.
Worse overall motoric impairment and the presence of mood fluctuations were also significantly associated with suicidality.
We conclude that the perception of treatment for motor fluctuations in PD is critical for patient well-being and depression severity; monitoring for mood changes and suicidality is critical as treatment is adapted to fluctuations.
Acknowledgments
Study Funding: Supported by grants from the NIH [R01-MH069666 (MOOD-PD Study), P50-NS-58377 (the Morris K. Udall Parkinson’s Disease Research Center of Excellence at Johns Hopkins), NIH/NIA K23AG044441, and the Donna Jeanne Gault Baumann Fund.
Footnotes
Statistical analysis conducted by Jared Hinkle, B.S., Johns Hopkins Medical Scientist Training Program
Author Contributions:
Jared Hinkle: Design & execution of statistical analysis; writing of the first draft.
Kate Perepezko: Review and critique of the analysis and all drafts.
Laura Marsh: Review and critique of the analysis and all drafts; acquisition of funding.
Zoltan Mari: Review and critique of the analysis and all drafts.
Gregory Pontone: Conception, organization, and execution of the manuscript. Review and critique of the analysis and all drafts.
Author Disclosures:
Jared Hinkle: No relevant disclosures. Supported by the Medical Scientist Training Program at the Johns Hopkins School of Medicine, NIH/NINDS 5 T32 GM007309
Kate Perepezko: No relevant disclosures.
Zoltan Mari: No relevant disclosures.
Laura Marsh: No relevant disclosures
Gregory Pontone: No relevant disclosures.
Currently supported by NIH/NIA K23AG044441 for research in Parkinson’s disease.
Conflict of Interest Statement: The authors report that the above funding sources were not involved in the collection or analysis of data reported in this manuscript. There are no financial conflicts of interest to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chaudhuri KR, Healy DG, Schapira AHV. Non-motor symptoms of Parkinson’s disease: diagnosis and management. Lancet Neurol. 2006;5:235–245. doi: 10.1016/S1474-4422(06)70373-8. [DOI] [PubMed] [Google Scholar]
- 2.Remy P, Doder M, Lees A, Turjanski N, Brooks D. Depression in Parkinson’s disease: loss of dopamine and noradrenaline innervation in the limbic system. Brain. 2005;128:1314–1322. doi: 10.1093/brain/awh445. [DOI] [PubMed] [Google Scholar]
- 3.Reijnders J, Ehrt U, Weber W, Aarsland D, Leentjens A. A systematic review of prevalence studies of depression in Parkinson’s disease. Mov Disord. 2008;23:183–9. doi: 10.1002/mds.21803. quiz 313. [DOI] [PubMed] [Google Scholar]
- 4.Kostić VS, Pekmezović T, Tomić A, et al. Suicide and suicidal ideation in Parkinson’s disease. J Neurol Sci. 2009;289:40–43. doi: 10.1016/j.jns.2009.08.016. [DOI] [PubMed] [Google Scholar]
- 5.Leentjens AFG, Marinus J, Van Hilten JJ, Lousberg R, Verhey FRJ. The contribution of somatic symptoms to the diagnosis of depressive disorder in Parkinson’s disease: a discriminant analytic approach. J Neuropsychiatry Clin Neurosci. 2003;15:74–77. doi: 10.1176/jnp.15.1.74. [DOI] [PubMed] [Google Scholar]
- 6.Nazem S, Siderowf AD, Duda JE, et al. Suicidal and death ideation in Parkinson’s disease. Mov Disord. 2008;23:1573–1579. doi: 10.1002/mds.22130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berney A, Vingerhoets F, Perrin A, et al. Effect on mood of subthalamic DBS for Parkinson’s disease A consecutive series of 24 patients. Neurology. 2002;59:1427–1429. doi: 10.1212/01.wnl.0000032756.14298.18. [DOI] [PubMed] [Google Scholar]
- 8.Ch Wolters E. Deep brain stimulation and continuous dopaminergic stimulation in advanced Parkinson’s disease. Park Relat Disord. 2007;13:S18–S23. doi: 10.1016/j.parkreldis.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 9.Ahlskog JE, Muenter MD. Frequency of levodopa-related dyskinesias and motor fluctuations as estimated from the cumulative literature. Mov Disord. 2001;16:448–458. doi: 10.1002/mds.1090. [DOI] [PubMed] [Google Scholar]
- 10.Stocchi F, Jenner P, Obeso JA. When do levodopa motor fluctuations first appear in Parkinson’s disease? Eur Neurol. 2010;63:257–266. doi: 10.1159/000300647. [DOI] [PubMed] [Google Scholar]
- 11.Martínez-Fernández R, Schmitt E, Martinez-Martin P, Krack P. The hidden sister of motor fluctuations in Parkinson’s disease: A review on nonmotor fluctuations. Mov Disord. doi: 10.1002/mds.26731. Epub 2016. [DOI] [PubMed] [Google Scholar]
- 12.Witjas T, Kaphan E, Azulay JP, et al. Nonmotor fluctuations in Parkinson’s disease: frequent and disabling. Neurology. 2002;59:408–413. doi: 10.1212/wnl.59.3.408. [DOI] [PubMed] [Google Scholar]
- 13.Maricle RA, Nutt JG, Carter JH. Mood and anxiety fluctuation in Parkinson’s disease associated with levodopa infusion: preliminary findings. Mov Disord. 1995;10:329. doi: 10.1002/mds.870100316. [DOI] [PubMed] [Google Scholar]
- 14.Racette BA, Hartlein JM, Hershey T, Mink JW, Perlmutter JS, Black KJ. Clinical features and comorbidity of mood fluctuations in Parkinson’s disease. J Neuropsychiatry Clin Neurosci. 2002;14:438–442. doi: 10.1176/jnp.14.4.438. [DOI] [PubMed] [Google Scholar]
- 15.Marsh L. Depression and Parkinson’s Disease: Current Knowledge. Curr Neurol Neurosci Rep. 2013;13:409. doi: 10.1007/s11910-013-0409-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease : a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992;55:181–184. doi: 10.1136/jnnp.55.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Williams JR, Hirsch ES, Anderson K, et al. A comparison of nine scales to detect depression in Parkinson disease: Which scale to use? Neurology. 2012;78:998–1006. doi: 10.1212/WNL.0b013e31824d587f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jenkinson C, Fitzpatrick R, Peto V, Greenhall R, Hyman N. The PDQ-8: development and validation of a short-form Parkinson’s disease questionnaire. Psychol Heal. 1997;12:805–814. [Google Scholar]
- 19.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–389. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- 21.Rush AJ, Gullion CM, Basco MR, Jarrett RB, Trivedi MH. The Inventory of Depressive Symptomatology (IDS): psychometric properties. Psychol Med. 1996;26:477–486. doi: 10.1017/s0033291700035558. [DOI] [PubMed] [Google Scholar]
- 22.Leckman JF, Sholomskas D, Thompson WD, Belanger A, Weissman MM. Best estimate of lifetime psychiatric diagnosis: a methodological study. Arch Gen Psychiatry. 1982;39:879–883. doi: 10.1001/archpsyc.1982.04290080001001. [DOI] [PubMed] [Google Scholar]
- 23.Young SG, Bowman AW. Non-parametric Analysis of Covariance. Biometrics. 1995;51:920–931. [Google Scholar]
- 24.Altman DG, Bland JM. How to obtain the P value from a confidence interval. BMJ. 2011;343:d2304. doi: 10.1136/bmj.d2304. [DOI] [PubMed] [Google Scholar]
- 25.Barraclough B, Bunch J, Nelson B, Sainsbury P. A hundred cases of suicide: clinical aspects. Br J Psychiatry. 1974;125:355–373. doi: 10.1192/bjp.125.4.355. [DOI] [PubMed] [Google Scholar]
- 26.Chaudhuri KR, Yates L, Martinez-Martin P. The non-motor symptom complex of Parkinson’s disease: a comprehensive assessment is essential. Curr Neurol Neurosci Rep. 2005;5:275–283. doi: 10.1007/s11910-005-0072-6. [DOI] [PubMed] [Google Scholar]
- 27.Stacy M, Hauser R. Development of a patient questionnaire to facilitate recognition of motor and non-motor wearing-off in Parkinson’s disease. J Neural Transm. 2007;114:211–217. doi: 10.1007/s00702-006-0554-y. [DOI] [PubMed] [Google Scholar]
- 28.Reddy P, Martinez-Martin P, Brown RG, et al. Perceptions of symptoms and expectations of advanced therapy for Parkinson’s disease: preliminary report of a Patient-Reported Outcome tool for Advanced Parkinson’s disease (PRO-APD) Health Qual Life Outcomes. 2014;12:11. doi: 10.1186/1477-7525-12-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Richard IH, Frank S, McDermott MP, et al. The ups and downs of Parkinson disease: a prospective study of mood and anxiety fluctuations. Cogn Behav Neurol. 2004;17:201–207. [PubMed] [Google Scholar]
- 30.Pietracupa S, Latorre A, Berardelli A, Fabbrini G. Parkinsonian patients and poor awareness of dyskinesias. Front Neurol. 2014;5:1–3. doi: 10.3389/fneur.2014.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Myslobodsky M, Lalonde FM, Hicks L. Are patients with Parkinson’s disease suicidal? J Geriatr Psychiatry Neurol. 2001;14:120–124. doi: 10.1177/089198870101400304. [DOI] [PubMed] [Google Scholar]
- 32.Stenager EN, Wermuth L, Stenager E, Boldsen J. Suicide in patients with Parkinson’s disease. An epidemiological study. Acta Psychiatr Scand. 1994;90:70–72. doi: 10.1111/j.1600-0447.1994.tb01558.x. [DOI] [PubMed] [Google Scholar]
- 33.Stensman R, Sundqvist-Stensman UB. Physical disease and disability among 416 suicide cases in Sweden. Scand J Soc Med. 1988;16:149–153. doi: 10.1177/140349488801600305. [DOI] [PubMed] [Google Scholar]
- 34.Lee T, Lee HB, Ahn MH, et al. Parkinsonism Relat Disord. Vol. 32. Elsevier Ltd; 2016. Increased suicide risk and clinical correlates of suicide among patients with Parkinson’s disease; pp. 102–107. [DOI] [PubMed] [Google Scholar]
- 35.Druss B, Pincus H. Suicidal ideation and suicide attempts in general medical illnesses. Arch Intern Med. 2000;160:1522–1526. doi: 10.1001/archinte.160.10.1522. [DOI] [PubMed] [Google Scholar]
- 36.Goetz CG, Tilley BC, Shaftman SR, et al. Movement Disorder Society-Sponsored Revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): Scale presentation and clinimetric testing results. Mov Disord. 2008;23:2129–2170. doi: 10.1002/mds.22340. [DOI] [PubMed] [Google Scholar]
- 37.Storch A, Schneider CB, Wolz M, et al. Nonmotor fluctuations in Parkinson disease: Severity and correlation with motor complications. Neurology. 2013;80:800–809. doi: 10.1212/WNL.0b013e318285c0ed. [DOI] [PubMed] [Google Scholar]
- 38.Müller B, Assmus J, Herlofson K, Larsen JP, Tysnes OB. Importance of motor vs. non-motor symptoms for health-related quality of life in early Parkinson’s disease. Park Relat Disord. 2013;19:1027–1032. doi: 10.1016/j.parkreldis.2013.07.010. [DOI] [PubMed] [Google Scholar]
- 39.Oertel W, Schulz JB. Current and experimental treatments of Parkinson disease: A guide for neuroscientists. J Neurochem. 2016;139:1–13. doi: 10.1111/jnc.13750. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.