Abstract
Genetic research generates results with implications for relatives. Recommendations addressing relatives’ access to a participant’s genetic research findings include eliciting participant preferences about access, and choosing a representative to make decisions about access upon participant incapacity/death. Representatives are likely to be blood relatives or spouse/partners (who may share genetically-related children). This raises the question of whether relatives hold similar attitudes about access or divergent attitudes that may yield conflict. We surveyed pancreatic cancer biobank participants (probands) and relatives in a family registry (blood relatives and spouse/partners of probands); 1,903 (>55%) surveys were returned. Results revealed few attitudinal differences between the groups. A slightly higher proportion of blood relatives agreed with statements reflecting proband privacy. In conclusion, probands’ decisions on access are likely to be accepted by relatives; in choosing a representative, probands may not face major differences in attitudes about privacy/sharing between a blood relative and a spouse/partner.
Keywords: cancer biobank, genetic research, return of results, biological family, attitudes, pancreatic cancer
Introduction
Disclosure of valid, clinically significant genetic research results to individual research participants, including both individual research results and incidental or secondary findings, is now widely justified (Bredenoord, Kroes, Cuppen, Parker, & van Delden, 2011; Knoppers, Deschenes, Zawati, & Tasse, 2013; Quaid, Jessup, & Meslin, 2004; S. M. Wolf et al., 2012; S.M. Wolf et al., 2008). However, the issue of disclosure of genetic research results to family members of research participants, both before and after death of the participant, has received less analysis (Black & McClellan, 2011; Chan et al., 2012; Milner, Liu, & Garrison, 2013; Tasse, 2011; S. M. Wolf et al., 2015). The importance of this emerging issue is now increasingly recognized, as genetic and genomic studies generate a growing volume of results that have potential clinical significance for individual participants as well as participants’ relatives. In addition, the common research practice of archiving data and biospecimens for reanalysis in the future means that findings discovered both before and after participant death may raise questions of sharing with relatives. In the context of cancer, genetic and genomic studies hold promise for risk prediction and the development of targeted therapies. However, research participants may die prior to genetic results being known, especially participants with cancers that remain highly lethal and rapidly fatal, such as pancreatic cancer. Researchers thus face complex questions of whether and how to share participant results with relatives. Because little is known about attitudes toward disclosure of genetic research results to family members of deceased research participants in the cancer context (Hallowell et al., 2013; Radecki Breitkopf et al., 2015) devising approaches to this question that are in accord with research participants’ wishes and likely to be accepted by families has been difficult. This study addresses the need for additional information by performing group-based comparisons of attitudes about family disclosure of genetic research results among cancer probands, blood relatives of probands who may share genetic risk, and spouse/partners of probands who may share children who are biologically related to the proband, to determine whether attitudes and preferences about family sharing tend to be concordant or divergent.
This study emanates from a project supported by the National Institutes of Health (NIH) using mixed methods to: (1) collect empirical data on sharing research results with relatives, and (2) develop normative guidance on this issue, informed by the empirical work. The project has previously published an analysis of the survey data (Radecki Breitkopf et al., 2015) and consensus guidance based on ethical and legal analysis of whether and how researchers might offer a participant’s genetic research results to relatives, including after participant death (S. M. Wolf et al., 2015).
In the consensus guidance paper, Wolf et al. (S. M. Wolf et al., 2015) recommended that researchers address the issue of sharing results with relatives by inviting participants (probands) to state their preferences and to designate a personal representative to make decisions about access to the proband’s results if the proband becomes decisionally incapacitated or deceased. This raises several issues of importance. The first is the question of family sharing, which potentially pits proband privacy against family members’ interest in or need for information, and has the potential to elicit discordant attitudes and conflict within the family. The second issue is how researchers should counsel participants faced with choosing a personal representative and potential concern about choosing someone who might make decisions at variance with their preferences. A third issue that emerges is how researchers should counsel personal representatives faced with decisions about family access after proband incapacity or death.
Personal representatives who are asked to make decisions about a family member’s access to genetic results on the participant’s behalf in the research context would be counseled to make a decision that follows the expressed wishes of the proband on sharing results with family, made known when the proband was alive and competent (S. M. Wolf et al., 2015). However, if the proband expressed no such preferences, we recommended that the representative balance the proband’s privacy and personal interests with the family member’s interests, including after the proband’s death. We thus rejected the 3-tier decisional standard that surrogate decision makers are customarily asked to use in making treatment decisions for decisionally incapacitated patients (following expressed wish, applying substituted judgment, or analyzing best interests), in favor of a simpler 2-tier standard for deciding about sharing genetic research results with family. However, even under this simpler standard, the ability and willingness of the representative to either follow the proband’s expressed wishes or consider the proband’s interests and balance those against a relative’s interests is important. Empirical research on the ability of next-of-kin surrogate decision makers to ascertain and follow patients’ wishes in the clinical context (e.g., in the domain of end-of-life decision making) has been shown to be modest (Shalowitz, Garrett-Mayer, & Wendler, 2006). In the context of deciding whether to share a research participant’s genetic results with relatives, although the representative would be instructed to follow the proband’s expressed preferences (if any) or balance the research participant’s interests with the interests of relatives wishing to learn genetic results, representatives’ decisions are vulnerable to cognitive biases and heuristics, particularly when they are made under conditions of uncertainty (Baron, 2007). The representatives could project their own attitudes and preferences onto the participant to guide decisions about family disclosure. Therefore, it seems important to consider attitude similarity between participants/probands and potential representatives with regard to views on privacy vs. sharing genetic information and weighing individual wishes vs. family benefit.
In a related empirical study of participants affected by pancreatic cancer, Radecki Breitkopf and colleagues (Radecki Breitkopf et al., 2015) surveyed participants’ general preferences regarding who should serve as a research participant’s representative. The survey asked, “What if a research participant dies without saying whether his/her genetic information can be offered to family members; who should make decisions about the genetic information obtained from the blood sample?” and gave the following six potential response options: “spouse/partner,” “blood relative,” “personal representative/executor of estate,” “research participant’s primary care provider,” “the researcher,” and “other.” Three-fourths of survey respondents indicated a family member, with 39% choosing “spouse/partner” and 36% choosing “blood relative” (Radecki Breitkopf et al., 2015). These findings suggest that in most cases, the individual serving as a research participant’s representative is likely to be a member of the participant’s family: either a blood relative who shares genetic material with the participant or a spouse/partner who does not share genetic material with the participant, but may share biologically-related children. Clearly, both blood relatives and spouse/partners with shared children have a “vested interest” in learning or permitting the disclosure of the participant’s genetic research results. However, no prior research has examined whether research participants’ attitudes regarding sharing genetic research results are similar to those of blood relatives or spouse/partners.
The purpose of this paper is to examine attitude similarity regarding offering genetic research results to relatives among three groups of individuals: probands in a pancreatic cancer biobank, and two potential groups from which the proband’s representative could arise: blood relatives and spouse/partners of probands. Attitude similarity was evaluated by analyzing survey responses obtained in our empirical study (Radecki Breitkopf et al., 2015). Although the representative’s own attitudes are to be put aside when deciding about family sharing of the proband’s genetic results on behalf of a deceased or incapacitated proband, the potential for bias in judgment exists; if the representative’s own attitudes may be projected onto the proband, it seems important to understand concordance with probands. Moreover, lack of concordance between probands, blood relatives, and spouse/partners on key issues regarding privacy vs. sharing could lead to family conflict.
Method
Participants and Procedure
The study population consisted of 6,103 individuals from three groups: 1) participants in a pancreatic cancer biobank based at Mayo Clinic in Rochester, MN, or “probands” (n=840), 2) family members of probands who were invited to enroll in a companion pancreatic cancer family registry also based at Mayo Clinic (n=2,471), and 3) healthy individuals who served as matched “controls” for the probands in genetic epidemiologic studies (n=2,792). For the present study, “control” participants were excluded from analysis; only probands and their family members (dichotomized as blood relatives or spouse/partners) were included.
Survey development and methodology are described in detail in Radecki Breitkopf et al. (Radecki Breitkopf et al., 2015). Briefly, survey content was guided by qualitative interviews conducted with 51 pancreatic cancer probands and their family members, with subsequent pilot testing and review of survey design and content by pancreatic cancer patient advocates. Survey booklets accompanied by an invitation letter were mailed to the sample population in several waves during the Fall of 2013, with two repeat mailings to non-responders at approximately 1 and 2 months post-initial mailing. Survey responses received by the end of February 2014 were included (allowing for a 4-6 month response window), after which time the database was considered final and locked for analysis.
Measures
Survey booklets contained 50 numbered questions, many with sub-parts or branching logic, for a total of 136 items. As described previously (Radecki Breitkopf et al., 2015), survey questions were organized into sections to orient respondents to various topics: Research Participation and Opinions, About Your Family, Views on Genes and Health, Genetic Testing Experience, An Example from Genetic Research, Practical Considerations in Genetic Research, How to Return Genetic Research Results, Genetic Research Results and Privacy, About You. The “Views on Genes and Health” section contained five items reflecting genetic knowledge and understanding and followed by the response options “True,” “False,” or “Not Sure.” Genetic knowledge scores were computed by allocating 1 point for each correct response, and 0 points for incorrect or not sure responses, and summing across the 5 items. The items were as follows: “If a person has a genetic mutation for a disease, the person will always get the disease” (False), “Only mothers can pass on genetic diseases” (False), “People can be healthy even if they have a genetic mutation for a disease” (True), “Genetic testing can be used in adults to find out if they have a greater than average chance of developing certain kinds of cancer” (True), and “Genetic testing can be used during pregnancy to find out whether the baby will develop sickle cell disease or cystic fibrosis” (True). The first three items were selected from Furr (Furr & Kelly, 1999) and the last two items were selected from Singer (Singer, Antonucci, & Van Hoewyk, 2004).
The section “An Example from Genetic Research” mirrored a real-life scenario through a hypothetical gender-neutral research participant named Pat, who was diagnosed with pancreatic cancer and gave a blood sample to be used for research. “Pat’s Story” unfolded in three parts, describing how Pat’s blood sample was found to contain a new gene associated with pancreatic cancer risk (unspecified), a mutation in the Breast cancer 2 gene (BRCA2), and a gene indicating carrier-status for the Cystic fibrosis transmembrane conductance regulator gene (CFTR). A series of questions addressed offering the research results to family members for each gene type (discovery gene, BRCA2, CFTR) under conditions when Pat’s wishes about sharing were known vs. unknown and under conditions when the discovery was made while Pat was alive vs. deceased. Response options for the items included a 5-point “strongly agree” to “strongly disagree” Likert-type rating scale.
Attitudes toward privacy were also assessed with three additional items outside of the Pat scenario: “I would NOT want my blood relatives to know about my genetic results [assuming they would be medically useful],” “I would want my genetic results [assuming they were medically useful] to be kept PRIVATE, even after my death” and “How concerned would you be if your biological family members learned your genetic research results?” The first two items were assessed using a 5-point “strongly agree” to “strongly disagree” response scale, while the third item included the response options “not at all concerned,” “slightly concerned,” “quite concerned,” and “extremely concerned.” Attitudes toward sharing were assessed with two items: “I would be OK with sharing my genetic research results with blood relatives who wanted to know them” and “I would feel OBLIGATED to share my genetic research results with my blood relatives” using the same 5-point “strongly agree” to “strongly disagree” rating scale.
Respondents were also asked to choose between two broad considerations as “the most important factor to consider” with regard to sharing genetic information with relatives: “the wishes of an individual research participant” or “whether blood relatives will benefit.” The use of a forced-choice item encouraged respondents to reflect on their attitudes toward each consideration, psychologically weigh them against each other, and choose only one to prevail. Additional survey items addressed attitudes toward return of an individual research participant’s genetic results to the individual and to family members, including: “Genetic information belongs to all blood relatives, not just the person who gave the blood sample,” “No matter how much money it costs, researchers SHOULD offer results to research participants,” and “When entering a research registry, participants should be given the choice to say whether or not their research results may be offered to biological family members.” These items used the 5-point agree-disagree scale described above.
Statistical Analysis
Descriptive data are presented as frequencies (n) and percentages (%), excluding missing data. Means are presented ±standard deviations. Overall, missing data were minimal (1%–3%) throughout the survey. For some analyses, response scales were collapsed for ease of reporting. Specifically, 5-point agreement scales were dichotomized by grouping “agree” and “strongly agree” responses to reflect agreement with the statement and grouping “neither agree nor disagree,” “disagree,” and “strongly disagree” responses to reflect lack of agreement. Overall respondent group differences in categorical variables were evaluated using chi-square tests (X2). For proportions, 95% confidence limits were calculated using the Wilson method for small sample sizes (Brown, Cai, & DasGupta, 2001) and denoted as 95% CL; these are presented when overall respondent group differences were statistically significant. Overall respondent group differences in continuous variables were evaluated using analysis of variance (ANOVA (F)), with Bonferroni-corrected pairwise comparisons. P values ≤0.05 were considered statistically significant. Statistical analyses were performed using SPSS version 22 (IBM Corp.).
Results
The survey response rates were 55.2% among probands (464 of 840), 57.8% among spouse/partners (399 of 690), and 58.4% among blood relatives (1,040 of 1,781) (P=0.31), yielding a total sample size of 1,903 for analysis. Approximately 40% of the blood relative group were siblings of probands (n=401), 35% were adult children (n=362), and 15% were nieces or nephews (n=165). Additionally, blood relatives included cousins (n=32), parents (n=20), grandchildren (n=20), aunts/uncles (n=4), and small numbers of other, more distant and half-relatives. Self-reported socio-demographic characteristics of survey respondents, stratified by respondent group are shown in Table 1. A majority of the sample was female (62.8%), of non-Hispanic ethnicity (99.0%) and white race (98.1%), married/partnered (68.5%), and had post-secondary education (81.3%). Ninety-eight percent had health insurance, and 13.1% had prior experience with genetic counseling (received genetic counseling or had met with a genetic counselor). Genetic knowledge was generally high, with an overall mean score of 3.62 ±1.2 (range 0 to 5). Mean genetic knowledge scores differed overall by respondent group (F (2, 1897)=3.33, P=0.04). Pairwise comparisons revealed genetic knowledge scores were lower among probands (3.50 ±1.3; 95% CL 3.38, 3.62) relative to blood relatives (3.68 ±1.2; 95% CL 3.60, 3.75); however, the 95% CLs slightly overlap. In the spouse/partner group, the mean genetic knowledge score was 3.60 ±1.2 (95% CL 3.49, 3.72). Genetic knowledge was associated with genetic counseling experience (F (2, 1865)=23.76, P<0.0001). Specifically, higher knowledge scores were observed among those reporting experience with genetic counseling (4.08 ±0.96; 95% CL 3.96, 4.20) as compared to those who reported no experience (3.57 ±1.2; 95% CL 3.51, 3.63) and those who answered “not sure” (3.08 ±1.4; 95% CL 2.69, 3.47).
Table 1.
Participant characteristics ascertained from survey responses (N=1903)
| Characteristic | All Respondents N=1903 n (%) |
Proband n=464 n (%) |
Blood Relative n=1040 n (%) |
Spouse/Partner n=399 n (%) |
|---|---|---|---|---|
|
| ||||
| Sex | ||||
| Male | 707 (37.2) | 231 (49.8) | 339 (32.6) | 137 (34.3) |
| Female | 1196 (62.8) | 233 (50.2) | 701 (67.4) | 262 (65.7) |
|
| ||||
| Age, years | ||||
| Mean (Standard Deviation) | 63.6 (12.8) | 66.4 (11.3) | 60.3 (13.4) | 69.1 (10.4) |
| Range | 23–99 | 29–94 | 23–99 | 38–94 |
| Median | 64 | 66 | 61 | 70 |
|
| ||||
| Race | ||||
| White | 1841 (98.1) | 444 (97.4) | 1012 (98.4) | 385 (98.0) |
| Black/African American | 8 (0.4) | 4 (0.9) | 4 (0.4) | 0 (0) |
| Asian | 10 (0.5) | 3 (0.7) | 4 (0.4) | 3 (0.8) |
| Hawaiian/Pacific Islander | 2 (0.1) | 0 (0) | 0 (0) | 2 (0.5) |
| American Indian/Alaska Native | 10 (0.5) | 3 (0.7) | 5 (0.5) | 2 (0.5) |
| Other | 6 (0.3) | 2 (0.4) | 3 (0.3) | 1 (0.3) |
|
| ||||
| Hispanic Ethnicity | ||||
| Yes | 18 (1.0) | 6 (1.3) | 8 (0.8) | 4 (1.0) |
| No | 1854 (99.0) | 446 (98.7) | 1020 (99.2) | 388 (99.0) |
|
| ||||
| Marital status | ||||
| Married/Life partner | 1279 (68.5) | 377 (83.2) | 797 (78.0) | 105 (26.9) |
| Separated/Divorced | 115 (6.2) | 37 (8.2) | 78 (7.6) | 0 (0) |
| Widowed | 395 (21.2) | 27 (6.0) | 82 (8.0) | 286 (73.1) |
| Single/never married | 77 (4.1) | 12 (2.6) | 65 (6.4) | 0 (0) |
|
| ||||
| Education | ||||
| High school or less | 350 (18.6) | 111 (24.4) | 157 (15.2) | 82 (20.9) |
| 2 year college/technical school | 627 (33.4) | 140 (30.8) | 343 (33.3) | 144 (36.6) |
| 4 year college or greater | 900 (47.9) | 203 (44.7) | 530 (51.5) | 167 (42.5) |
|
| ||||
| Employment† | ||||
| Not employed | 142 (7.6) | 62 (13.7) | 67 (6.5) | 13 (3.3) |
| Employed | 833 (44.6) | 141 (31.2) | 577 (56.2) | 115 (29.4) |
| Retired | 894 (47.8) | 249 (55.1) | 382 (37.2) | 263 (67.3) |
|
| ||||
| Health insurance coverage | ||||
| No | 31 (1.7) | 3 (0.7) | 22 (2.1) | 6 (1.5) |
| Yes (private, employer, public) | 1844 (98.3) | 451 (99.3) | 1006 (97.9) | 387 (98.5) |
|
| ||||
| Prior experience with genetic counseling | ||||
| Yes | 246 (13.1) | 56 (12.3) | 158 (15.4) | 32 (8.2) |
| No/Unsure | 1625 (86.9) | 398 (87.7) | 871 (84.6) | 356 (91.8) |
|
| ||||
| Genetic knowledge§ | ||||
| Mean (Standard Deviation) | 3.62 (1.2) | 3.50 (1.3) | 3.68 (1.2) | 3.60 (1.2) |
Not employed = full time or part time student, unemployed, homemaker, unable to work due to disability; Employed = full time or part time employment, employed but on medical leave; Retired = retired, or retired but working part time.
5-item scale, range is 0-5 items correct; higher scores reflect greater knowledge.
Proband Privacy and Sharing with Family
Proband Privacy
Fewer than 10% of respondents agreed with the statement: “I would NOT want my blood relatives to know about my genetic results [assuming they would be medically useful],” with similar proportions of agreement between probands, blood relatives, and spouse/partners (6.5%, 8.4%, and 6.5%, respectively), X2 (2)=2.23, P=0.327. After death, privacy within the family appeared to be even less of a concern – overall agreement with the item: “I would want my genetic results [assuming they were medically useful] to be kept PRIVATE, even after my death” was <5% with no significant differences observed between probands, blood relatives, and spouse/partners (4.1%, 4.5%, and 4.3%, respectively), X2 (2)=0.096, P=0.953. Finally, in a more passively-worded third item: “How concerned would you be if your biological family members learned your genetic research results?” fewer than 10% of respondents indicated concern (“quite” or “extremely” concerned responses were combined), with no significant differences in proportions indicating concern between probands, blood relatives, and spouse/partners (8.1%, 7.0%, and 7.7%, respectively), X2 (2)=0.662, P=0.718.
Family sharing
Overall, greater than 94% of respondents agreed with the statement: “I would be OK with sharing my genetic research results with blood relatives who wanted to know them,” with no significant differences in percent agreement observed between probands, blood relatives, and spouse/partners (95.4%, 94.1%, and 94.2%, respectively), X2 (2)=1.19, P=0.552. When “OK with sharing” was changed to “feel OBLIGATED to share,” agreement was lower in each of the respondent groups and did not significantly differ between probands, blood relatives, and spouse/partners (90.2%, 86.5%, and 88.7%, respectively), X2 (2)=4.41, P=0.110.
The context of the hypothetical “Pat” scenario offered insight regarding attitudes toward privacy and family sharing while the participant is alive and after death, and with and without explicit participant permission to share. The percentage agreement with each statement, by respondent group, is shown in Table 2 for the three different gene contexts: a discovery gene related to pancreatic cancer risk, a known cancer risk gene mutation (BRCA2), and a gene mutation revealing carrier status (CFTR) with reproductive implications (cystic fibrosis). Overall, these analyses reveal few differences between probands, blood relatives, and spouse/partners, with the exception of the item addressing Pat’s ability to keep genetic information private from others in the family, presumably while Pat was still alive. For this item, a consistent pattern of responses (agreement) emerged across each of the three gene contexts (see Table 2, first row). Specifically, a greater proportion of blood relatives expressed agreement that Pat should be able to keep the genetic information private from others in the family, compared to probands and spouse/partners, who did not significantly differ. It is important to note, however, that the proportion agreeing with privacy, even among blood relatives, was modest and ranged from 32.4% to 37.2% across the three gene contexts. For this item (not shown in table), the proportion of disagreement (combining “strongly disagree” and “disagree” responses) among blood relatives was below 50% (42.3%–49.2% across the three gene contexts), with the remainder of blood relatives (18%–20%) choosing “neither agree nor disagree.” In contrast, among probands and spouse/partners, over 50% indicated disagreement, and less than 20% indicated “neither agree nor disagree” for this item. Following a similar pattern (though not reaching statistical significance), attitudes regarding honoring Pat’s expressed wishes to keep genetic information private (see Table 2, last row) revealed the highest proportions of agreement among blood relatives (compared to probands and spouse/partners) across each of the gene contexts, but the range again reflected modest overall agreement, ranging from 29.6% to 35.5%.
Table 2.
Percent agreementa (95% confidence limits (CL)) for each statement by respondent group and genetic finding
| Statement (emphases in original survey) | Proband % (95% CL) |
Blood Relative % (95% CL) |
Spouse/Partner % (95% CL) |
Pb |
|---|---|---|---|---|
|
| ||||
| Pat should be able to keep information about the [genetic finding] private from others in the family | ||||
| Pancreatic cancer discovery gene | 28.7 (24.7, 32.9) | 37.2 (34.3, 40.2) | 25.8 (21.7, 30.3) | 0.000 |
| BRCA2 | 27.0 (23.1, 31.3) | 33.1 (30.3, 36.0) | 24.6 (20.1, 29.1) | 0.002 |
| CFTR | 27.5 (23.7, 31.8) | 32.4 (29.6, 35.3) | 25.3 (21.3, 29.8) | 0.016 |
|
| ||||
| Researchers should ONLY offer Pat’s information about the [genetic finding] to blood relatives if Pat has given EXPLICIT PERMISSION to share genetic results | ||||
| Pancreatic cancer discovery gene | 69.7 (65.4, 73.7) | 69.4 (66.5, 72.2) | 67.2 (62.4, 71.6) | 0.665 |
| BRCA2 | 59.0 (54.4, 63.4) | 57.0 (54.0, 60.0) | 56.9 (52.0, 61.7) | 0.757 |
| CFTR | 58.4 (53.8, 62.8) | 55.4 (52.3, 58.4) | 56.5 (51.5, 61.3) | 0.560 |
|
| ||||
| If the new discovery is made AFTER PAT’S DEATH, the information about the [genetic finding] should be offered to Pat’s spouse | ||||
| Pancreatic cancer discovery gene | 90.5 (87.5, 92.8) | 89.7 (87.7, 91.4) | 92.2 (89.1, 94.4) | 0.375 |
| BRCA2 | 92.8 (90.0, 94.8) | 92.5 (90.7, 94.0) | 95.9 (93.5, 97.5) | 0.060 |
| CFTR | 93.3 (90.6, 95.2) | 92.6 (90.8, 94.0) | 97.5 (95.4, 98.6) | 0.003 |
|
| ||||
| If Pat’s spouse REFUSES the offer of information about the [genetic finding], researchers should offer the results directly to Pat’s children | ||||
| Pancreatic cancer discovery gene | 73.4 (69.2, 77.2) | 74.8 (72.1, 77.4) | 71.8 (67.2, 76.0) | 0.496 |
| BRCA2 | 79.9 (76.0, 83.3) | 81.3 (78.8, 83.6) | 80.8 (76.6, 84.3) | 0.810 |
| CFTR | 79.6 (75.7, 83.0) | 80.0 (77.5, 82.4) | 79.9 (75.7, 83.6) | 0.982 |
|
| ||||
| If the new discovery is made AFTER PAT’S DEATH, and Pat’s wishes about sharing genetic information are UNKNOWN, the information about the [genetic finding] should be offered to Pat’s blood relatives | ||||
| Pancreatic cancer discovery gene | 86.0 (82.5, 88.8) | 86.5 (84.2, 88.4) | 87.4 (83.7, 90.3) | 0.835 |
| BRCA2 | 87.8 (84.5, 90.5) | 89.1 (87.1, 90.9) | 89.9 (86.5, 92.5) | 0.601 |
| CFTR | 88.5 (85.3, 91.1) | 89.7 (87.7, 91.4) | 90.1 (86.7, 92.7) | 0.719 |
|
| ||||
| If the new discovery is made AFTER PAT’S DEATH, and Pat previously said NOT TO SHARE genetic information, the information about the [genetic finding] should NOT be offered to Pat’s blood relatives | ||||
| Pancreatic cancer discovery gene | 32.1 (28.0, 36.5) | 35.5 (32.6, 38.5) | 29.8 (25.5, 34.5) | 0.096 |
| BRCA2 | 27.2 (23.4, 31.5) | 31.4 (28.6, 34.3) | 25.3 (21.2, 30.0) | 0.046 |
| CFTR | 28.8 (24.8, 33.1) | 29.6 (26.9, 32.5) | 25.4 (21.4, 30.0) | 0.291 |
Combines the responses “strongly agree” and “agree” on a 5-point Likert scale that included the responses “strongly agree,” “agree,” “neither agree nor disagree,” “disagree,” and “strongly disagree.”
P value associated with 3 (respondent group) × 2 (strongly agree/agree vs. strongly disagree/disagree/neutral) chi-square test.
Individual Participant Wishes vs. Family Benefit
Overall, a majority (61.6%) of respondents chose “whether blood relatives will benefit” in response to the item presenting a forced choice between individual wishes vs. family benefit with regard to offering genetic research results. A lower proportion of blood relatives selected “whether blood relatives will benefit” (58.2%, 95% CL 55.1, 61.2) as compared to probands (65.4%, 95% CL 60.9, 69.6) and spouse/partners (65.8%, 95% CL 61.0, 70.3) (X2 (2)=10.82, P=0.004) (Figure 1). Slightly overlapping confidence intervals imply a cautionary note in interpreting the overall P value as indicating group differences.
Figure 1.
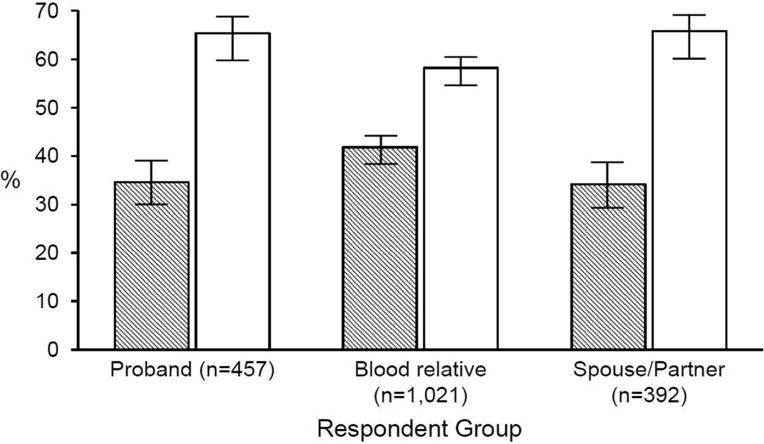
Most important factor to consider in returning genetic research results, forced-choice item, by respondent group.
Participant responses to the forced-choice item: “Please tell us which ONE of the following two statements better reflects your opinion about offering results: The most important factor to consider in returning genetic research results is the wishes of the person who provided the blood sample OR The most important factor to consider in returning genetic research results is whether blood relatives will benefit.” The mean percentage is represented along with 95% confidence limits. The open bars represent the percent choosing “whether blood relatives will benefit” and the shaded bars represent the percent choosing “the wishes of the person who provided the blood sample.”
The same respondent group pattern was observed for the statement: “Genetic information belongs to all blood relatives, not just the person who gave the blood sample.” Specifically, 59.7% (95% CL 56.6, 62.6) of blood relatives expressed agreement with the statement, as compared to 66.2% (95% CL 61.7, 70.4) of probands, and 66.0% (95% CL 61.2, 70.5) of spouse/partners (X2 (2)=8.12, P=0.017). Once again, the overall test statistic is indicative of significant group differences in proportions; however, the confidence intervals demonstrate slight overlap.
Over three-fourths of probands (76.3%, 95% CL 72.2, 80.0) indicated agreement with the statement: “No matter how much money it costs, researchers SHOULD offer results to research participants,” compared to 68% (95% CL 65.1, 70.8) of blood relatives and 75.5% (95% CL 71.0, 79.5) of spouse/partners (X2 (2)=14.42, P=0.001). About two-thirds of respondents indicated agreement with the statement: “When entering a research registry, participants should be given the choice to say whether or not their research results may be offered to biological family members.” No significant differences in agreement were observed between probands, blood relatives, and spouse/partners (65.2%, 67.0%, and 64.9%, respectively), X2 (2)=0.78, P=0.678.
Discussion
This investigation examined whether attitudes and preferences regarding sharing genetic research results with family are concordant between cancer probands and two family groups from which our prior work demonstrates a representative may be drawn to speak on behalf of the deceased or incapacitated proband, namely, blood relatives and spouse/partners (Radecki Breitkopf et al., 2015). In general, the results revealed few attitudinal differences on issues regarding sharing a proband’s research-derived genetic information within families. The absence of large respondent group differences in this study is somewhat reassuring, as it suggests that despite the potential for different “vested interests” in the disclosure of the proband’s genetic results between blood relatives and spouse/partners, attitudes about privacy and sharing were quite similar between the groups.
Where overall group differences emerged, the findings suggested that probands, as a group, were more attitudinally similar to the group of spouse/partners than to the group of blood relatives. However, these observed differences were generally small in magnitude (<10 percentage points). This pattern of findings may be understood in the context of partner selection and attraction based on attitude similarity, (Byrne et al., 1971) although it is unlikely that prior to partnering, individuals would realistically initiate discussion on such topics so as to reveal whether attitudes are shared. It is more likely that as individuals share their lives in close proximity with a spouse/partner, shared experiences, decisions, and environments shape attitudes and bring them to greater similarity, although longitudinal data would be needed to empirically examine a process of attitude convergence (Feng & Baker, 1994).
Through the use of a hypothetical scenario that presented three different gene contexts (a discovery gene related to pancreatic cancer risk, BRCA2, and CFTR), a consistent pattern was identified whereby blood relatives (compared to probands and spouse/partners) showed greater agreement with individual privacy and lesser agreement that the proband’s genetic information should be shared with family against the prior wishes of the proband. One possible explanation for this finding involves a heightened perception among blood relatives in this study of their own risk of pancreatic cancer, a devastating disease for which early detection is rare. Feelings of vulnerability among blood relatives of probands could have sensitized this group and manifested in attitudes that are more aligned with cancer risk information staying private. Blood relatives may also be more fearful of learning potential self-threatening information from the proband’s results, even though learning genetic risk information could empower blood relatives to pursue their own clinical genetic testing and proactively address their risk. Post-hoc analysis of responses to a survey item regarding perceived risk of pancreatic cancer confirmed higher values among blood relatives than spouse/partners (data not shown); thus, the role of perceived personal vulnerability in attitudes toward privacy vs. sharing genetic information may warrant further exploration in research on the attitudes of blood relatives, probands, and spouse/partners. Importantly, though, we observed the same pattern of results (i.e., blood relatives indicating greater agreement with privacy) for the BRCA2 and CFTR genetic findings in the hypothetical scenario. These genetic findings were not directly related to risk of pancreatic cancer at the time this survey was conducted (though subsequent research has shown BRCA2 status to bear on pancreatic cancer risk (Martinez-Useros & Garcia-Foncillas, 2016; Petersen, 2016) therefore, other explanations are needed for our findings beyond perceived personal vulnerability. In our study, blood relatives had higher genetic knowledge scores, on average, than probands and spouse/partners; it is possible that greater understanding of inheritance among blood relatives influenced attitudes in this group. There is a need to further explore potential explanatory mechanisms of the pattern suggesting greater agreement with privacy among blood relatives of probands, and to test the replicability of this pattern in disease contexts beyond pancreatic cancer.
Best Practices
The decision to offer research results to participants or family members of deceased or incapacitated participants in genetic research is complex. Careful and full consideration of ethical, economic, and practical issues is warranted. Several papers by members of our team offer guidance as to this process (Wolf et al., 2008, 2012, 2015). This NIH-funded project has published consensus guidelines recommending that a participant’s personal representative make decisions about family access once the proband has lost decisional capacity or has died (Wolf et al., 2015). Empirical research on the ability of potential representatives to fulfill that function is therefore important. In addition, research can shed light on the likelihood of family agreement or disagreement on individual proband privacy and family sharing.
In this study of pancreatic cancer probands and family members, the absence of large respondent group differences suggests that broaching issues of sharing proband results with relatives is unlikely to produce family disagreement, that proband decisions on sharing with relatives are generally likely to be accepted by relatives, and that probands choosing between a spouse/partner and a blood relative in selecting a personal representative will generally not face major differences in attitudes about privacy and sharing. Though blood relatives may learn genetic information germane to their own health, a spouse or partner may show the attitude similarity documented in couples and may learn genetic information germane to the health of offspring shared with the proband.
There are important limitations to the present study. First, survey respondents were enrolled in a pancreatic cancer biobank or family registry at Mayo Clinic and reflected a relatively homogeneous sample with regard to sociodemographic characteristics. Specifically, participants were predominantly non-Hispanic White, nearly all reported having health insurance, >80% had post-secondary education, and on average, genetic knowledge was high. While this limits generalizability, our sample reflects the lack of diversity reported internationally in biobank populations and those enrolled in genome-wide association studies (Haga, 2010; Knerr, Wayman, & Bonham, 2011; Need & Goldstein, 2009) therefore, the attitudes and preferences we report may in fact reflect those in other currently existing family cancer registries and biobanks. We recognize the urgent need to examine the perspectives of racial and ethnic minorities on return of genetic research results to relatives, including after death of the proband, and have already begun some of these efforts using qualitative inquiry. Similarly, there is a need to examine the attitudes and preferences regarding disclosure of genetic information to family in a variety of contexts (e.g., families constituted through remarriage that may include half-siblings or step-siblings, families built through adoption, and families created through reproductive technologies using gamete donation) as well as to consider who may constitute “family” to a participant who belongs to a blended family or whose genetically-related kin reside in another country.
Second, our sampling frame was comprised of individuals in a pancreatic cancer biobank and its associated family registry, thus our results reflect attitudes and preferences of individuals within a single cancer context. The views of individuals affected by pancreatic cancer may not be representative of individuals affected by other cancers for which strategies for prevention or early detection exist and for which the prognosis is less bleak. Further research in cancers of varying survival is warranted. Third, we examined concordance in attitudes and preferences by conducting comparisons between respondent groups. A complementary approach to examining concordance would be to survey or interview families as the unit of analysis in order to compare attitudes of multiple family members, exploring similarities and differences. More work is needed to delineate the range of relevant concerns within families in order to more fully assess attitudes and preferences.
Research Agenda
Further work is needed to develop and evaluate sensitive, effective, and pragmatic processes for eliciting research participant’s preferences for family sharing and designation of a representative. Additionally, implementation and evaluation research is needed to examine participant and family experiences with decision making concerning sharing the proband’s genetic results with family, including after loss of decisional capacity or death.
Educational Implications
The growing importance of biobanks, registries, and data archives retaining genomic data and interpreted results for research use into the future, including after proband death, makes the development of acceptable procedures for governing access to proband information a crucial next step.
Acknowledgments
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The funding agencies (NCI, NHGRI, NCATS) were not involved in the design of the study, the collection, analysis, or interpretation of the data, nor the writing of the manuscript. We acknowledge, with gratitude, the significant contributions of the pancreatic cancer patient advocate group known as RAPPORT in reviewing and providing critical and valuable feedback on the survey content, design, and format. We thank Maggie Breslin for providing expertise in graphic design and layout of the survey content.
Sources of support: Research reported in this publication was supported by the National Cancer Institute (NCI) and the National Human Genome Research Institute (NHGRI) of the National Institutes of Health under award numbers: R01CA154517, P50CA102701, R01CA97075 (NCI), P20HG007243 (NHGRI), and by UL1TR000135 from the National Center for Advancing Translational Sciences (NCATS).
References
- Baron J. Thinking and deciding. 4th. New York City: Cambridge University Press; 2007. [Google Scholar]
- Black L, McClellan KA. Familial communication of research results: A need to know? Journal of Law, Medicine & Ethics. 2011;39(4):605–613. doi: 10.1111/j.1748-720X.2011.00627.x. [DOI] [PubMed] [Google Scholar]
- Bredenoord AL, Kroes HY, Cuppen E, Parker M, van Delden JJ. Disclosure of individual genetic data to research participants: the debate reconsidered. Trends in genetics: TIG. 2011;27(2):41–47. doi: 10.1016/j.tig.2010.11.004. [DOI] [PubMed] [Google Scholar]
- Brown LD, Cai TT, DasGupta A. Interval Estimation for a Binomial Proportion. Statist Sci. 2001;16(2):101–133. doi: 10.1214/ss/1009213286. [DOI] [Google Scholar]
- Byrne D, Gouaux C, Griffitt W, Lamberth J, Murakawa N, B PM. The ubiquitous relationship: attitude similarity and attraction: a cross-cultural study. Human Relations. 1971;24:201–207. [Google Scholar]
- Chan B, Facio FM, Eidem H, Hull SC, Biesecker LG, Berkman BE. Genomic inheritances: disclosing individual research results from whole-exome sequencing to deceased participants’ relatives. The American journal of bioethics: AJOB. 2012;12(10):1–8. doi: 10.1080/15265161.2012.699138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng D, Baker L. Spouse similarity in attitudes, personality, and psychological well-being. Behavior Genetics. 1994;24(4):357–364. doi: 10.1007/BF01067537. [DOI] [PubMed] [Google Scholar]
- Furr LA, Kelly SE. The Genetic Knowledge Index: developing a standard measure of genetic knowledge. Genet Test. 1999;3(2):193–199. doi: 10.1089/gte.1999.3.193. [DOI] [PubMed] [Google Scholar]
- Haga SB. Impact of limited population diversity of genome-wide association studies. Genetics in medicine: official journal of the American College of Medical Genetics. 2010;12(2):81–84. doi: 10.1097/GIM.0b013e3181ca2bbf. [DOI] [PubMed] [Google Scholar]
- Hallowell N, Alsop K, Gleeson M, Crook A, Plunkett L, Bowtell D, Young MA. The responses of research participants and their next of kin to receiving feedback of genetic test results following participation in the Australian Ovarian Cancer Study. Genetics in medicine: official journal of the American College of Medical Genetics. 2013;15(6):458–465. doi: 10.1038/gim.2012.154. [DOI] [PubMed] [Google Scholar]
- Knerr S, Wayman D, Bonham VL. Inclusion of racial and ethnic minorities in genetic research: advance the spirit by changing the rules? J Law Med Ethics. 2011;39(3):502–512. doi: 10.1111/j.1748-720X.2011.00617.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoppers BM, Deschenes M, Zawati MH, Tasse AM. Population studies: return of research results and incidental findings Policy Statement. European journal of human genetics: EJHG. 2013;21(3):245–247. doi: 10.1038/ejhg.2012.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Useros J, Garcia-Foncillas J. The Role of BRCA2 Mutation Status as Diagnostic, Predictive, and Prognosis Biomarker for Pancreatic Cancer. Biomed Res Int. 2016;2016:1869304. doi: 10.1155/2016/1869304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner LC, Liu EY, Garrison NA. Relationships matter: ethical considerations for returning results to family members of deceased subjects. The American journal of bioethics: AJOB. 2013;13(10):66–67. doi: 10.1080/15265161.2013.828533. [DOI] [PubMed] [Google Scholar]
- Need AC, Goldstein DB. Next generation disparities in human genomics: concerns and remedies. Trends in genetics: TIG. 2009;25(11):489–494. doi: 10.1016/j.tig.2009.09.012. [DOI] [PubMed] [Google Scholar]
- Petersen GM. Familial pancreatic cancer. Semin Oncol. 2016;43(5):548–553. doi: 10.1053/j.seminoncol.2016.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quaid KA, Jessup NM, Meslin EM. Disclosure of genetic information obtained through research. Genetic Testing. 2004;8(3):347–355. doi: 10.1089/gte.2004.8.347. [DOI] [PubMed] [Google Scholar]
- Radecki Breitkopf C, Petersen GM, Wolf SM, Chaffee KG, Robinson ME, Gordon DR, Koenig BA. Preferences regarding return of genomic results to relatives of research participants, including after participant death: Empirical results from a cancer biobank. Journal of Law, Medicine & Ethics, Fall. 2015:464–475. doi: 10.1111/jlme.12289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalowitz DI, Garrett-Mayer E, Wendler D. The accuracy of surrogate decision makers: a systematic review. Arch Intern Med. 2006;166(5):493–497. doi: 10.1001/archinte.166.5.493. [DOI] [PubMed] [Google Scholar]
- Singer E, Antonucci T, Van Hoewyk J. Racial and ethnic variations in knowledge and attitudes about genetic testing. Genet Test. 2004;8(1):31–43. doi: 10.1089/109065704323016012. [DOI] [PubMed] [Google Scholar]
- Tasse AM. The return of results of deceased research participants. Journal of Law, Medicine & Ethics. 2011;39(4):621–630. doi: 10.1111/j.1748-720X.2011.00629.x. [DOI] [PubMed] [Google Scholar]
- Wolf SM, Branum R, Koenig BA, Petersen GM, Berry SA, Beskow LM, Wilfond BS. Returning a research participant’s genomic results to relatives: Analysis and recommendations. Journal of Law, Medicine & Ethics, Fall. 2015:440–463. doi: 10.1111/jlme.12288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf SM, Crock BN, Van Ness B, Lawrenz F, Kahn JP, Beskow LM, Wolf WA. Managing incidental findings and research results in genomic research involving biobanks and archived data sets. Genetics in medicine: official journal of the American College of Medical Genetics. 2012;14(4):361–384. doi: 10.1038/gim.2012.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf SM, Lawrenz FP, Nelson CA, Kahn JP, Cho MK, Clayton EW, Wilfond BS. Managing incidental findings in human subjects research: Analysis and recommendations. Journal of Law, Medicine & Ethics. 2008:219–248. doi: 10.1111/j.1748-720X.2008.00266.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


