Abstract
Adolescents and females experience worse outcomes of drug use compared to adults and males. This could result from age- and sex-specific consequences of drug exposure on brain function and cognitive behavior. In the current study, we examined whether a history of intravenous methamphetamine (METH) self-administration impacted cognitive flexibility and 5-HT2CR localization in the orbitofrontal cortex (OFC) in an age- and sex-dependent manner. Strategy shifting was assessed in male and female Sprague-Dawley rats that had self-administered METH (0.08 mg/kg/inf) or received non-contingent infusions of saline during periadolescence or young adulthood. After all rats reached adulthood, they were tested in an operant strategy shifting task and their brains were subsequently analyzed using immunofluorescence to quantify co-localization of 5-HT2C receptors with parvalbumin interneurons in the OFC. We found that adolescent-onset females were the only group impaired during discrimination and reversal learning, but they did not exhibit changes in localization of 5-HT2C receptors. In contrast, adult-onset males exhibited a significant increase in co-localization of 5-HT2C receptors within parvalbumin interneurons in the left hemisphere of the OFC. These studies reveal that age and sex differences in drug-induced deficits in reversal learning and 5-HT2CR co-localization with parvalbumin interneurons are dissociable and can manifest independently. In addition, these data highlight the potential for certain treatment approaches to be more suitable in some populations compared to others, such as alleviating drug-induced cognitive deficits as a focus for treatment in adolescent females.
Keywords: Adolescence, Sex differences, Cognitive flexibility, Orbitofrontal cortex, Serotonin-2C receptors, Parvalbumin
1. Introduction
Females and adolescents disproportionately account for users that experience poor outcomes of drug use (Hillhouse et al., 2007; Rawson et al., 2007; Dluzen and Liu, 2008; Chen et al., 2009) and in these populations methamphetamine (METH) use is especially problematic (Gonzales et al., 2008). Females initiate METH use at younger ages and transition to disordered use more rapidly than males (Sommers et al., 2006; Dluzen and Liu, 2008), which is of particular concern given that METH use during adolescence is associated with increases in risky sexual behaviors, teen pregnancy, and the use of other drugs (Embry et al., 2009). Moreover, the use of METH among adolescents has remained constant in recent years even though the rates of alcohol and tobacco use have declined (SAMHSA, 2014). To better understand and address the potentially unique complications of METH and other substance misuse in adolescents and females, a more complete understanding of drug-induced cognitive deficits and neuroadaptations in these populations is needed. In fact, one approach that has been used to understand differential vulnerability to substance use disorders (SUDs) has been investigation of the mechanisms underlying drug-induced cognitive dysfunction (Selby and Azrin, 1998; Rogers and Robbins, 2001; Cadet and Bisagno, 2016; Hankosky and Gulley, 2016), particularly since deficits in working memory, attention, and inhibitory control are associated with poorer treatment outcomes (Aharonovich et al., 2003, 2006; Streeter et al., 2008; Verdejo-García et al., 2012).
In laboratory rodents, psychostimulant exposure during adolescence induces similar (Hankosky et al., 2013; Hammerslag et al., 2014) or more severe (Vorhees et al., 2005; Harvey et al., 2009; Counotte et al., 2011; Sherrill et al., 2013) cognitive impairments than exposure during adulthood (for review see Hankosky and Gulley, 2016). Disrupted inhibitory control of prefrontal cortex (PFC) neural circuits has been hypothesized to underlie drug-induced cognitive deficits (Enomoto et al., 2011; Gonzalez-Burgos et al., 2011) and recent work from our lab is consistent with this. Using in vitro whole-cell recording we found that repeated amphetamine exposure during adolescence attenuated D1 receptor-mediated inhibition in medial PFC pyramidal neurons in adulthood (Kang et al., 2016a; Paul et al., 2016), potentially through reductions in D1 receptor expression (Kang et al., 2016b). The important role of adaptations in medial PFC D1 receptors in drug-induced cognitive dysfunctions was previously described by Fletcher et al. (2007) who demonstrated that amphetamine-induced deficits in attention were alleviated by intra-PFC infusion of a D1 receptor agonist.
In addition to dopamine, serotonergic signaling is critical for normal PFC function (Puig and Gulledge, 2011; Celada et al., 2013) and is thus another target through which vulnerability to develop SUDs may be influenced (Kirby et al., 2011). Modulation of inhibition in the PFC by serotonin is accomplished via multiple receptor subtypes that regulate excitability in a variety of neuronal populations (Puig et al., 2005; Celada et al., 2013; Leiser et al., 2015). This inhibitory modulation is mediated in part by 5-HT2C receptors (5-HT2CRs; Feng et al., 2001), likely due to their preferential expression on parvalbumin-immunoreactive (PV-ir) interneurons (Liu et al., 2007). These interneurons are the most abundant inhibitory neuron subtype in the PFC and they provide tight regulatory control of pyramidal cell output to subcortical structures (Kubota and Kawaguchi, 1994; Markram et al., 2004). As such, the preferential expression of 5-HT2CRs on PV neurons suggests that the behavioral effects associated with influencing their function may be the result of modulating inhibitory tone in the PFC. Reversal learning is one behavior that is sensitive to both 5-HT2CR manipulations (Boulougouris et al., 2008; Boulougouris and Robbins, 2010) and disrupted inhibitory transmission (Bissonette et al., 2015) in the orbitofrontal cortex (OFC), and is known to be impaired following repeated exposure to amphetamine or METH (Izquierdo et al., 2010; Parsegian et al., 2011; Hankosky et al., 2013; Cox et al., 2016).
There is indirect behavioral evidence to suggest that repeated METH exposure changes 5-HT2CRs (Napier and Istre, 2008; Graves and Napier, 2012), but the impact of this exposure on 5-HT2CR expression in the OFC is unknown. Based on the cellular distribution of 5-HT2CRs in the PFC (Liu et al., 2007) and evidence that antagonism of these receptors in the OFC improves reversal learning (Boulougouris and Robbins, 2010), we hypothesized that drug-induced increases in the co-localization of 5-HT2CRs on PV-ir interneurons could be a mechanism through which drug-induced deficits in cognitive flexibility manifest. To test this hypothesis, we examined strategy shifting and 5-HT2CR co-localization with PV-ir interneurons in the OFC in rats with a history of intravenous METH self-administration that was initiated in adolescence or adulthood.
2. Methods
2.1 Subjects
Subjects were a total of 82 Sprague-Dawley rats (32 male, 50 female) that had previously been trained to self-administer d-methamphetamine HCl (0.08 mg/kg) or received non-contingent infusions of saline beginning during either adolescence or adulthood. The full methods outlining treatment of the METH rats used in this study and analysis of age- and sex-differences in METH self-administration was reported previously (Hankosky et al., 2018). Of the 46 rats that initiated METH self-administration, 15 males and 25 females met acquisition criteria to be included in this study. The remaining male (n=17) and female (n=25) rats were littermates of the METH groups treated identically with the exception that they received non-contingent infusions of saline approximated to match the number of infusions earned by their METH counterparts. Briefly, subjects were offspring of rats bred in our colony, weaned on postnatal day (P) 22, and housed 2–3 per cage. Rats were housed on a 12 h light/dark cycle (lights off at 0900) and behavior was assessed during the dark cycle (0900–1700). Rats were implanted with indwelling jugular vein catheters on P32 or 82 (± 2 days) and had daily 2-h access to METH under increasing fixed ratio (FR) schedules of reinforcement (FR1 to FR5) or received non-contingent infusions of saline between P41–55 or P91–105, for adolescent- and adult-onset groups, respectively (Fig. 1). The last 7 days of self-administration (P56–62 and P106–112) consisted of four daily progressive ratio sessions (5-h) each separated by one 2-h maintenance session with METH available on an FR5 schedule. Rats were allowed ad libitum access to food and water except during the limited periods (≤4 h) noted below. Experimental procedures were approved by the Institutional Animal Care and Use Committee at the University of Illinois, Urbana–Champaign, and were consistent with the Guide for the Care and Use of Laboratory Animals (National Research Council, 2011).
Figure 1.
Experimental timeline (A) and table indicating sample sizes for rats tested in the strategy shifting task (B). The timeline shows the approximate age (in days) of adolescent- and adult-onset rats for the previously reported (Hankosky et al., 2018) self-administration phase, the strategy shifting task phase, and the age when tissue was collected for immunofluorescence. Surgery and behavioral testing were done ± 2 days and tissue collection varied by ± 4 days. The table shows the subset of rats from the original sample (Hankosky et al., 2018) that met acquisition criterion for METH self-administration and were subsequently trained and tested for strategy shifting.
2.3 Apparatus
Strategy shifting took place in standard operant chambers (Coulbourn Instruments, Whitehall, PA) that were enclosed in sound-attenuating cubicles. The cubicles were equipped with fans that provided ventilation and masked extraneous noise. One wall of each operant chamber was equipped with a centrally located food trough outfitted on either side with retractable levers that were equidistant (87 mm) from the trough. White cue lights were located above each lever and a 2.9 kHz Sonalert speaker was located directly above the food trough. A white houselight was located near the chamber ceiling on the wall opposite the nosepoke ports. Graphic State software (v3.1; Coulbourn Instruments) was used for automated chamber control and data collection.
2.4 Strategy Shifting
Five days after completion of self-administration sessions (on P67 or P117), male (n = 6–10/group) and female (n = 10–15/group) rats that received non-contingent infusions of saline or met acquisition criterion for METH self-administration (unit dose: 0.08 mg/kg/inf) began training for strategy shifting (Fig. 1). Food was removed 2 h prior to each daily session and returned 30 min to 2 h following its completion; rats were not otherwise food restricted and this procedure has no overall impact on body weight compared to rats fed ad libitum (Westbrook et al., in press). As we have described previously (Hankosky et al., 2013), rats were first trained across 4 sessions to lever press for a 45-mg food pellet (BioServ, F0021, Frenchtown, NJ). During the next 5 sessions, rats had to press the lever within 10-sec of its extension into the chamber to earn reinforcement. Subsequently, each rat’s side bias was then established in a single session as described by Floresco et al. (2008).
Daily strategy shifting sessions consisted of 120 trials that were separated by a 10-sec intertrial interval. Trials began when both levers were inserted into the chamber concurrent with illumination of one of the two cue lights located above the levers. Trials ended after either the rat pressed a lever or 10 sec elapsed. Individual cue lights were presented pseudorandomly across trials, such that no cue light could be presented on more than two consecutive trials. Because all rats had prior exposure to cue lights during self-administration, the cue light illuminated 2-sec prior to trial onset during visual strategy sessions in order to increase its saliency (Floresco et al., 2008).
Rats were first trained to use a visual strategy by reinforcing them (with delivery of a food pellet) for pressing the lever that had a cue light illuminated above it, regardless of the lever’s spatial location (i.e., left or right side of pellet delivery trough). Trials continued until rats achieved eight consecutive correct choices and had completed at least 30 trials. On the day following acquisition of this performance criterion, rats were required to shift to an egocentric response strategy. This required that rats press a lever relative to its position, regardless of cue light location (e.g. always press the left lever). The location of the reinforced lever for the response strategy was determined individually for each rat such that it was the lever opposite of the rat’s side bias that was observed during initial training (see above). Trials continued until rats achieved a criterion of eight consecutive correct choices.
Following acquisition of the response strategy, rats were overtrained on this response during a single session consisting of 150 trials. One day following the overtraining session, rats completed a within-session reversal of the learned response. During this session, rats were first required to demonstrate retention of the overtrained response by pressing the appropriate lever eight consecutive times. Once retention of the overtrained response was confirmed, the stimulus-reward association was reversed such that pressing on the previously non-rewarded lever now resulted in reinforcement (e.g. always press the right lever). As before, trials continued until rats achieved a criterion of eight consecutive correct choices. For all stages of the task, trials where rats failed to respond on a lever within 10-sec of trial onset were terminated, scored as an omission, and led to the intertrial interval.
2.5 5-HT2CR co-expression with PV-ir interneurons
A subset of rats that completed strategy shifting (n = 6/group) were matched for cumulative METH intake and used for immunohistochemical analysis of 5-HT2CR co-expression with PV-ir cells. Rats were chosen for this analysis based on cumulative METH intake, such that groups did not differ significantly (Fig. 2). Seven to ten days following completion of operant strategy shifting (at P96 ± 4 or P146 ± 4), which was ~5 weeks after the last METH self-administration session, rats were deeply anesthetized (100 mg/kg pentobarbital) and transcardially perfused with phosphate buffered saline (PBS; 10 mmol/L; pH 7.2) and 3% paraformaldehyde in PBS. Their brains were removed and post-fixed for at least 2 h at room temperature in 3% paraformaldehyde in PBS, followed by immersion in PBS containing 30% sucrose at 4 °C for 3 days until the brains sunk. Subsequently, a freezing microtome was used to collect 40-μm thick sections including the OFC and these were stored in cryoprotectant (30% sucrose, 0.1% polyvinylpyrrolidone-40, and 30% ethylene glycol in 10mmol PBS) at −20 °C until processed.
Figure 2.
CumulativeMETH intake across all sessions for individual rats used in tests of strategy shifting. All rats acquired self-administration at a unit dose of 0.08 mg/kg/inf and mean intake for each group is indicated by the horizontal line with error bars representing SEM. Individual rats indicated with a square symbol (n = 6/group) were those used for immunohistochemical analysis of 5-HT2CR localization.
For each animal, 2 free-floating, anatomically matched coronal sections (40 μm) containing the OFC (between +3.72 and +4.68 from bregma; Fig. 5-A) were stained with 5-HT2CR antibody and the calcium-binding protein antibody, PV, as described previously (Liu et al. 2007). The anti-5HT2C polyclonal antibody raised in goat (1:100 dilution; sc-15081, Santa Cruz Biotechnology) and the anti-PV monoclonal antibody raised in mouse (1:2500 dilution; PV 235, SWANT) were used with fluorescent secondary antibodies Alexa Fluor® 488 (anti-goat, 1:500 dilution; A-11055 Invitrogen Corporation) and Alexa Fluor® 555 (anti-mouse, 1:2000 dilution; A-31570, Invitrogen Corporation) to visualize 5-HT2CR expression and co-localization to PV-ir interneurons (Bubar et al., 2005; Liu et al., 2007). Sections were rinsed out of cryoprotectant with tris-buffered saline (TBS: pH 7.6) 5 times for 10 min each. Sections were then blocked in TBS containing 20% normal donkey serum, 1% bovine serum albumin and 1% hydrogen peroxide for 60 min at room temperature. Sections were then incubated in a primary antibody solution, diluted with TBS containing 2% normal donkey serum and 0.3% triton-x-100, for 44 hours at 4°C. Then, sections were rinsed 6 times in TBS (6 min each) and incubated in a secondary antibody solution (diluted in TBS) for 60 min at room temperature. Following secondary antibody incubation, sections were rinsed 3 times in TBS (10 min each) and mounted on gelatin-coated slides with Vectashield reagent with a DAPI counterstain (H-1200, Vector Laboratories). To rule out secondary antibody cross-reactivity, control sections were stained with the secondary antibodies in the presence of only one primary antibody. Exclusion of an individual primary antibody resulted in no observable immunoreactivity for that protein. Images were collected using a 20× objective with a fluorescent confocal microscope (Zeiss LSM 880) and processed with the Zen software package. Sixty PV-ir cells from layer V of the lateral OFC of each rat were counted from 1–2 sections (bregma +4.2 mm). We chose to count from the lateral OFC due its role in reversal learning (Izquierdo, 2017). The number of PV-ir cells that also co-expressed the 5-HT2CR was then counted and the percentage of co-localized cells was calculated.
Figure 5.
5-HT2CR co-localization with parvalbumin (PV) interneurons. (A) Schematic drawing of a representative section, adapted from Paxinos and Watson (2007), from which PV-ir cells from layer V of the lateral OFC were sampled (gray boxes). Brains from a subset of rats (see Fig. 2) were stained for (B) 5-HT2CRs (green) and (C) PV (red). Subsequently, co-localization of the proteins was assessed (D). Arrows indicate cells co-expressing 5-HT2CRs and the PV calcium-binding protein (inset: enlarged image of double-labeled cell). Mean percentage of 5-HT2CR co-localization with PV-ir interneurons in both hemispheres of the OFC is shown for (E)males (n = 3–6/hemisphere) and (F) females (n = 5–6/hemisphere). * p < 0.05.
2.6 Data Analysis
Cumulative METH intake was calculated by multiplying the unit dose of METH (0.08 mg/kg) by the number of infusions earned and summing across all days of self-administration. It was analyzed using two-way ANOVA (age × sex). Dependent measures used to assess strategy shifting included the number of trials and errors to criterion. Errors following the shift to the response strategy were classified as perseverative or never-reinforced. Perseverative errors were defined as those that would have been correct when the visual strategy was in effect (e.g., responding in the left port when the left cue light was illuminated, but the right port response was the reinforced response). All other errors were scored as never-reinforced, as they would not have been correct in a visual strategy and they included responses in the non-reinforced port during the response strategy. Because drug-induced deficits are sometimes evident early rather than later in training (Kondrad and Burk, 2004; Hankosky and Gulley, 2013; Hankosky et al., 2013), errors during the first 30 trials of the shift to response and response reversal were analyzed. Trials and errors for each strategy were analyzed in three-way ANOVAs (treatment × sex × age-of-onset). Error types were analyzed separately in males and females using three-way repeated measures ANOVA (treatment × age × type). Pre-planned comparisons were used to investigate a priori hypotheses that METH would impair PFC-sensitive cognition (Hankosky and Gulley, 2013; Hankosky et al., 2013).
For 5-HT2CR localization, the dependent measure was percent of PV-ir interneurons that co-expressed 5-HT2CRs (number of PV-ir cells co-expressing 5-HT2CRs divided by the number of PV-ir neurons counted * 100). Sixty PV-ir interneurons were counted for each rat. Percent co-localization was analyzed with a four-way repeated measures ANOVA (treatment × sex × age-of-onset × hemisphere). Significant main effects and interactions were followed up with Tukey’s post-hoc tests. The percent of 5-HT2CRs co-localized to PV-ir interneurons was correlated using simple linear regression with METH intake and errors committed early (first 30 trials) in reversal learning. For 6 rats, all PV-ir interneurons were counted from only one hemisphere.
3. Results
3.1 Strategy Shifting
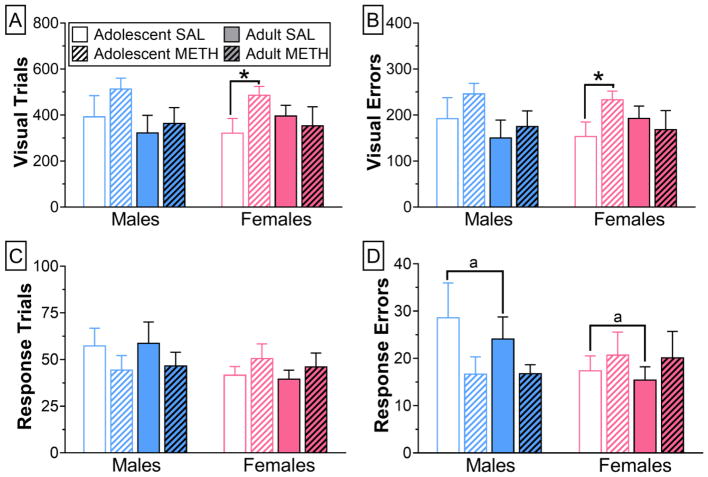
Training in an operant strategy shifting task began 5 days after the last session in which rats received non-contingent infusions of saline or self-administered METH (Fig. 1). For the latter groups of rats, cumulative METH intake was not significantly different (Fig. 2). Three-way ANOVA of trials and errors to criterion during visual strategy acquisition revealed no significant main effects or interactions. However, pre-planned comparisons revealed that adolescent-onset females with a history of METH self-administration required significantly more trials (t46 = 2.11; p = 0.0381) and committed significantly more errors (t46 = 2.02; p = 0.0467) than their sex- and age-matched controls (Fig. 3-A,B).
Figure 3.
Acquisition of visual strategy and shift to response strategy in males (n=6–10/group) and females (n=10–15/group) with a history of METH self-administration (n=6–15/group) or exposure to saline (n=7–13/group). Shown are trials (A) and errors (B)to reach acquisition criterion of the visual strategy and trials (C)and errors ( D)to criterion during the shift from visual to response strategy. * p < 0.05 for indicated comparison; matching letters indicate p < 0.05 for the indicated sex difference collapsed across age.
Cognitive flexibility was then assessed by requiring rats to shift from a visual to a response strategy. Three-way ANOVA of trials to criterion (Fig. 3-C,D) revealed no significant main effects or interactions. Three-way ANOVA of errors to criterion, however, revealed a significant treatment by sex interaction (F1,74= 4.21; p < 0.0464), such that control males made significantly more errors during the shift from visual to response than control females (Fig. 3-D). Errors that occurred during the shift were characterized as either perseverative or never reinforced. For males and females, three-way repeated measures ANOVAs revealed no significant main effects or interactions (data not shown). There were also no significant main effects or interactions when performance during the first 30 trials (early training) was assessed (data not shown).
Reversal learning, which is known to be sensitive to OFC function (Birrell and Brown, 2000), was next tested by overtraining and then reversing the response strategy. Three-way ANOVA of trials (Fig. 4-A) and errors (Fig. 4-B) to criterion revealed no significant main effects or interactions. However, when performance during the first 30 trials (early training) was examined, three-way ANOVA revealed a significant treatment by sex by age-of-onset interaction (F1,74= 6.36; p = 0.0138). Specifically, early in training adolescent-onset females committed significantly more errors than their age-matched controls and adult-onset females (Fig. 4-C). In contrast, adult-onset females committed significantly fewer errors than their controls.
Figure 4.
Reversal of the overtrained response in males (n=6–10/group) and females (n=10–15/group) with a history of METH self-administration (n=6–15/group) or exposure to saline (n=7–13/group). Shown are trials (A) and errors (B) to criterion for reversal of the response strategy, as well as errors committed during the first 30 trials of the reversal (“early errors”; C). * p < 0.05 vs. age-matched control; ** p < 0.01 adolescent- vs. adult-onset.
3.2 5-HT2CR co-expression with PV-ir interneurons
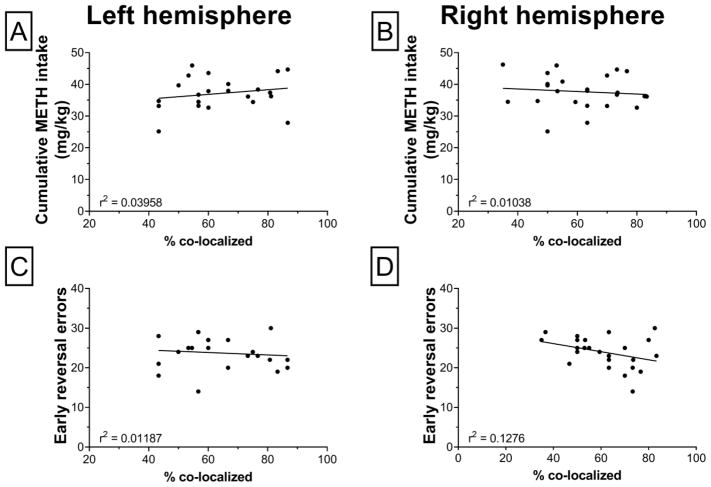
A subset of brains from rats that completed the strategy shifting experiment were stained for 5-HT2CRs and the calcium binding protein PV (Fig. 5). Four-way repeated measures ANOVA revealed a significant treatment by sex by age-of-onset by hemisphere interaction (F1,34 = 4.24; p = 0.0472). In the left hemisphere, adult-onset males had significantly higher co-localization of 5-HT2CRs with PV-ir interneurons than their controls and adolescent-onset males (Fig. 5-E). In females, there were no significant group differences in 5-HT2CR co-localization with PV interneurons (Fig. 5-F). In rats with a history of METH self-administration, percent co-localization was not significantly correlated with METH intake or reversal errors committed early in training overall or when stratified by hemisphere (Fig. 6).
Figure 6.
Scatterplots showing the relationship of (A,B) cumulative METH intake and (C,D) early reversal errors with percent co-localization (5-HT2CRs in PV interneurons) in the left (A,C) and right (B,D) hemisphere of the OFC in rats with a history of METH self-administration. None of these relationships were statistically significant.
4. Discussion
This study used rats with a history of METH self-administration or non-contingent saline exposure to investigate the impact of age and sex on METH-induced changes in cognitive flexibility and 5-HT2CR localization in the OFC. As we describe elsewhere (Hankosky et al., 2018), females in our sample acquired self-administration of this METH dose (0.08 mg/kg/inf) more readily than males and this effect was driven by low levels of acquisition in adolescent-onset males. In contrast, adults of both sexes reached higher breakpoints during progressive ratio responding than adolescents. Importantly, differences in acquisition did not translate to significant differences in intake.
In the current study, we found that the effects of METH self-administration on reversal learning and 5-HT2CR localization in the OFC were dissociable and depended on age and sex. Adolescent-onset females were significantly impaired during acquisition of the visual strategy and during early stages of the reversal, compared to their saline-exposed controls. However, this difference was not associated with a change in 5-HT2CR localization. We instead found that adult-onset males had significantly increased co-expression of 5-HT2CRs with PV-ir interneurons in the left hemisphere of the OFC. In addition, we found no significant correlations between cumulative METH intake, early reversal errors, and co-localization measures. Our other results suggest that these relationships may be dissociable within each group, however data in the present study were collapsed across age and sex factors to achieve a large enough sample size for meaningful correlational analyses. The dissociation between cognitive impairments and changes in 5-HT2CR co-expression with PV-ir interneurons is not consistent with the hypothesis that these structural adaptations contribute to drug-induced deficits in reversal learning via modulation of OFC inhibition. Alternative possibilities for the involvement of 5-HT2CRs and PV-ir interneurons in the modulation of OFC inhibition include functional changes to the receptor (Werry et al., 2008), such as alterations in ligand affinity or constitutive activity. Although the precise neurobiological mechanisms remain unknown, our results suggest that increased susceptibility to drug-induced cognitive deficits is a potential mechanism by which adolescent females may experience worse outcomes of drug use.
4.1 Strategy Shifting
Assessment of cognitive flexibility in rats with a history of METH self-administration revealed age- and sex-dependent deficits in initial discrimination learning and reversal learning early in training. Discrimination learning depends on intact corticostriatal circuits (Winocur and Eskes, 1998; Fidalgo et al., 2014) and reversal learning relies on the OFC (McAlonan and Brown, 2003; Boulougouris et al., 2007), suggesting that METH self-administration in adolescent-onset females disrupted corticostriatal and OFC-sensitive functions. The putative deficits in adolescent-onset females is consistent with age- and sex-dependent differences in the development of corticostriatal circuits, reflecting research demonstrating that development of these circuits is ongoing during adolescence (Huttenlocher and Dabholkar, 1997; Giedd et al., 1999; Casey et al., 2000; Markham et al., 2007; Rubinow and Juraska, 2009; Willing and Juraska, 2015) and this process is different in males and females (Giedd et al., 1999; Markham et al., 2007; Willing and Juraska, 2015). That the reversal deficit was only observed early in training is consistent with other studies showing transient amphetamine-induced deficits in PFC-sensitive tasks (Kondrad and Burk, 2004; Hankosky and Gulley, 2013; Hankosky et al., 2013). Moreover, antagonism of 5-HT2CRs in the OFC reduces the number of early reversal errors committed (Alsiö et al., 2015). Coupled with our analysis of 5-HT2CR co-localization, this suggests that METH exposure may result in functional, but not structural changes to 5-HT2CRs in the OFC of adolescent females. In addition, the finding that no other METH exposed groups exhibited deficits in reversal learning is consistent with a recent report (Izquierdo et al., 2016), but inconsistent with many previous studies (Izquierdo et al., 2010; Parsegian et al., 2011; Hankosky et al., 2013; Ye et al., 2014; Cox et al., 2016). The inconsistent findings might be related to these studies differences in task difficulty, total drug intake, drug-free interval prior to testing, or the method of drug exposure (i.e., volitional vs. experimenter-administered). The age-dependency of the deficits we observed is consistent with evidence demonstrating that exposure to psychostimulants during adolescence, compared to adulthood, results in greater cognitive impairments (Harvey et al., 2009; Counotte et al., 2011; Sherrill et al., 2013; Hammerslag et al., 2014). However, other studies (Hankosky et al., 2013; Hammerslag et al., 2014) have failed to observe age-dependent deficits of psychostimulant exposure in male rats. This discrepancy may be due to methodological differences such as route of drug administration (Robinson et al., 2002; Harvey et al., 2009) and withdrawal duration prior to testing (Santucci et al., 2004; Paul et al., 2016).
Adult-onset females with a history of METH self-administration committed fewer early errors than their counterparts. Interestingly, this is consistent with two studies that reported facilitation of operant strategy reversal in males exposed to amphetamine during adolescence (Hankosky et al., 2013) and in males that self-administered cocaine during adulthood (Kantak et al., 2014). Although the cause of this facilitation is not known, it is possible that adult-onset females did not learn the original strategy as well and subsequently shifted faster due to reduced proactive interference (Sherrill et al., 2013).
4.2 5-HT2CR co-expression with PV-ir interneurons
In the OFC, 5-HT2CR mRNA is localized to both glutamatergic and GABAergic cell types located primarily in deep layers with some expression in intermediate and superficial layers as well (Santana & Artigas, 2017). Assessment of 5-HT2CR co-localization with PV-ir interneurons revealed that in the left hemisphere, adult-onset males with a history of METH self-administration had significantly higher co-localization than their age-matched controls and adolescent-onset males. The significant impact of hemisphere was not anticipated, but is consistent with a large body of literature demonstrating laterality of monoamine levels (Staiti et al., 2011), receptor distribution (Schneider et al., 1982; Drew et al., 1986; Glick et al., 1988), innervation (Neddens et al., 2004), and function (Andersen and Teicher, 1999; Neddens et al., 2004; Sullivan et al., 2009). Increased co-expression of 5-HT2CRs with PV-ir interneurons in adult-onset males with a history of METH self-administration is consistent with previous studies demonstrating psychostimulant-induced changes in 5-HT2CRs (Filip et al., 2004; Napier and Istre, 2008; Graves and Napier, 2012) and PFC GABAergic interneurons (Mohila and Onn, 2005; Morshedi and Meredith, 2007; Cass et al., 2013; Kang et al., 2016a). Although these changes were observed in the absence of cognitive deficits, it is possible that with a more challenging task METH-induced deficits would have been detectable (Hankosky et al., 2013; Kantak et al., 2014). However, due to the lack of cognitive impairment, the functional implication of the redistribution of 5-HT2CRs is unknown.
Age- and sex-dependent differences in the structure and/or function of serotonergic (Biver et al., 1996; Zhang et al., 1999; Moll et al., 2000; Klink et al., 2002) or GABAergic systems (Cruz et al., 2003; Tseng and O’Donnell, 2007; Cholanian et al., 2014; Gonzalez-Burgos et al., 2015) at baseline or following METH self-administration may account for why 5-HT2CR upregulation on PV-ir interneurons was only observed in adult-onset males. Alternatively, increased function (e.g. constitutive activity) of 5-HT2CRs localized to PV-ir interneurons could have the same outcome on OFC inhibition as structural upregulation of receptors. Indeed, there is evidence to suggest that chronic exposure to elevated levels of 5-HT, as would occur with repeated psychostimulant exposure, increases constitutive activity of 5-HT2CRs (Devlin et al., 2004). As such, distinct mechanisms of 5-HT2CR-mediated OFC dysregulation, alone or in combination with changes to other systems, could constitute alternatives to receptor upregulation that disrupt reversal learning.
4.3 Conclusion
The current examination of differences in METH-induced cognitive deficits and changes in 5-HT2CR localization on PV-ir interneurons revealed dissociable interactions dependent on age and sex. Adolescent-onset females were most affected by the impact of METH self-administration on strategy shifting. This suggests that increased susceptibility to the deleterious effects of METH exposure on cognition may contribute to worse outcomes in adolescent females. In fact, a recent study in adult rats demonstrated that reduced cognitive flexibility predicted subsequent intake and escalation of METH self-administration (Istin et al., 2016). Future studies will be needed to determine the impact of drug-induced cognitive dysfunction on subsequent drug-seeking. We hypothesized a priori that METH-induced cognitive impairments would be associated with an upregulation of 5-HT2CRs on PV-ir interneurons, but the only group that exhibited these drug-induced neuroadaptations was adult-onset males. In the absence of cognitive deficits, the impact of these changes is unknown and future work should investigate alternative 5-HT2CR adaptations that may alter inhibitory control in the OFC. One implication of our findings is that certain treatment approaches may be more beneficial in some populations of drug users than in others and that alleviating cognitive deficits may be especially efficacious in adolescent females.
Highlights.
Drug-induced neuroadaptations and cognitive impairment depend on age and sex
Methamphetamine self-administration impairs reversal learning in adolescent females
Methamphetamine self-administration changes 5-HT2CR localization in adult males
Reversal learning deficits and 5-HT2CR expression changes manifest independently
Acknowledgments
Funding
This work was supported by the National Institutes of Health [F31 DA036330 to E.R.H and R01 DA029815 to J.M.G.].
The authors thank Adam Gold, Courtney Hong, Laura Cortes, Kristen Hughes, Ashley Wehrheim, Sarah Rahman, Shawn Kurian, and Emily Kroeger for excellent technical assistance. We also thank Dr. Bob Switzer III at NeuroScience Associates (Knoxville, TN) for generously providing the SWANT parvalbumin antibody.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aharonovich E, Hasin DS, Brooks AC, Liu X, Bisaga A, Nunes EV. Cognitive deficits predict low treatment retention in cocaine dependent patients. Drug Alcohol Depend. 2006;81:313–322. doi: 10.1016/j.drugalcdep.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Aharonovich E, Nunes E, Hasin D. Cognitive impairment, retention and abstinence among cocaine abusers in cognitive-behavioral treatment. Drug Alcohol Depend. 2003;71:207–211. doi: 10.1016/s0376-8716(03)00092-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen SL, Teicher MH. Serotonin laterality in amygdala predicts performance in the elevated plus maze in rats. Neuroreport. 1999;10:3497–3500. doi: 10.1097/00001756-199911260-00006. [DOI] [PubMed] [Google Scholar]
- Birrell JM, Brown VJ. Medial frontal cortex mediates perceptual attentional set shifting in the rat. J Neurosci. 2000;20:4320–4324. doi: 10.1523/JNEUROSCI.20-11-04320.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissonette GB, Schoenbaum G, Roesch MR, Powell EM. Interneurons are necessary for coordinated activity during reversal learning in orbitofrontal cortex. Biol Psychiatry. 2015;77:454–464. doi: 10.1016/j.biopsych.2014.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biver F, Lotstra F, Monclus M, Wikler D, Damhaut P, Mendlewicz J, Goldman S. Sex difference in 5HT2 receptor in the living human brain. Neurosci Lett. 1996;204:25–28. doi: 10.1016/0304-3940(96)12307-7. [DOI] [PubMed] [Google Scholar]
- Boulougouris V, Dalley JW, Robbins TW. Effects of orbitofrontal, infralimbic and prelimbic cortical lesions on serial spatial reversal learning in the rat. Behav Brain Res. 2007;179:219–228. doi: 10.1016/j.bbr.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Boulougouris V, Glennon JC, Robbins TW. Dissociable effects of selective 5-HT2A and 5-HT2C receptor antagonists on serial spatial reversal learning in rats. Neuropsychopharmacology. 2008;33:2007–2019. doi: 10.1038/sj.npp.1301584. [DOI] [PubMed] [Google Scholar]
- Boulougouris V, Robbins TW. Enhancement of spatial reversal learning by 5-HT2C receptor antagonism is neuroanatomically specific. J Neurosci. 2010;30:930–938. doi: 10.1523/JNEUROSCI.4312-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bubar MJ, Seitz PK, Thomas ML, Cunningham KA. Validation of a selective serotonin 5-HT(2C) receptor antibody for utilization in fluorescence immunohistochemistry studies. Brain Res. 2005;1063:105–113. doi: 10.1016/j.brainres.2005.09.050. [DOI] [PubMed] [Google Scholar]
- Cadet JL, Bisagno V. Neuropsychological consequences of chronic drug use: Relevance to treatment approaches. Front Psychiatry. 2016;6:1–10. doi: 10.3389/fpsyt.2015.00189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, Giedd JN, Thomas KM. Structural and functional brain development and its relation to cognitive development. Biol Psychol. 2000;54:241–257. doi: 10.1016/s0301-0511(00)00058-2. [DOI] [PubMed] [Google Scholar]
- Cass DK, Thomases DR, Caballero A, Tseng KY. Developmental disruption of gamma-aminobutyric acid function in the medial prefrontal cortex by noncontingent cocaine exposure during early adolescence. Biol Psychiatry. 2013;74:490–501. doi: 10.1016/j.biopsych.2013.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celada P, Puig MV, Artigas F. Serotonin modulation of cortical neurons and networks. Front Integr Neurosci. 2013;7:25. doi: 10.3389/fnint.2013.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CY, Storr CL, Anthony JC. Early-onset drug use and risk for drug dependence problems. Addict Behav. 2009;34:319–322. doi: 10.1016/j.addbeh.2008.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cholanian M, Lobzova A, Das B, Yelleswarapu C, Donaldson ST. Digital holographic microscopy discriminates sex differences in medial prefrontal cortex GABA neurons following amphetamine sensitization. Pharmacol Biochem Behav. 2014;124:326–332. doi: 10.1016/j.pbb.2014.06.026. [DOI] [PubMed] [Google Scholar]
- Counotte DS, Goriounova NA, Li KW, Loos M, van der Schors RC, Schetters D, Schoffelmeer ANM, Smit AB, Mansvelder HD, Pattij T, Spijker S. Lasting synaptic changes underlie attention deficits caused by nicotine exposure during adolescence. Nat Neurosci. 2011;14:417–419. doi: 10.1038/nn.2770. [DOI] [PubMed] [Google Scholar]
- Cox BM, Cope ZA, Parsegian A, Floresco SB, Aston-Jones G, See RE. Chronic methamphetamine self-administration alters cognitive flexibility in male rats. Psychopharmacology. 2016;233:2319–2327. doi: 10.1007/s00213-016-4283-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz DA, Eggan SM, Lewis DA. Postnatal development of pre- and postsynaptic GABA markers at chandelier cell connections with pyramidal neurons in monkey prefrontal cortex. J Comp Neurol. 2003;465:385–400. doi: 10.1002/cne.10833. [DOI] [PubMed] [Google Scholar]
- Devlin MG, Smith NJ, Ryan OM, Guida E, Sexton PM, Christopoulos A. Regulation of serotonin 5-HT2C receptors by chronic ligand exposure. Eur J Pharmacol. 2004;498:59–69. doi: 10.1016/j.ejphar.2004.07.102. [DOI] [PubMed] [Google Scholar]
- Dluzen DE, Liu B. Gender Differences in Methamphetamine Use and Responses : A Review. Gend Med. 2008;5:24–35. doi: 10.1016/s1550-8579(08)80005-8. [DOI] [PubMed] [Google Scholar]
- Drew KL, Lyon RA, Titeler M, Glick SD. Asymmetry in D-2 binding in female rat striata. Brain Res. 1986;363:192–195. doi: 10.1016/0006-8993(86)90678-5. [DOI] [PubMed] [Google Scholar]
- Embry D, Hankins M, Biglan A, Boles S. Behavioral and social correlates of methamphetamine use in a population-based sample of early and later adolescents. Addict Behav. 2009;34:343–351. doi: 10.1016/j.addbeh.2008.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enomoto T, Tse MT, Floresco SB. Reducing prefrontal gamma-aminobutyric acid activity induces cognitive, behavioral, and dopaminergic abnormalities that resemble schizophrenia. Biol Psychiatry. 2011;69:432–441. doi: 10.1016/j.biopsych.2010.09.038. [DOI] [PubMed] [Google Scholar]
- Feng J, Cai X, Zhao J, Yan Z. Serotonin Receptors Modulate GABAA Receptor Channels through Activation of Anchored Protein Kinase C in Prefrontal Cortical Neurons. J Neurosci. 2001;21:6502–6511. doi: 10.1523/JNEUROSCI.21-17-06502.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fidalgo C, Conejo NM, González-Pardo H, Arias JL. Dynamic functional brain networks involved in simple visual discrimination learning. Neurobiol Learn Mem. 2014;114:165–170. doi: 10.1016/j.nlm.2014.06.001. [DOI] [PubMed] [Google Scholar]
- Filip M, Bubar MJ, Cunningham KA. Contribution of Serotonin (5-Hydroxytryptamine; 5-HT) 5-HT2 Receptor Subtypes to the Hyperlocomotor Effects of Cocaine : Acute and Chronic Pharmacological Analyses. J Pharmacol Exp Ther. 2004;310:1246–1254. doi: 10.1124/jpet.104.068841. [DOI] [PubMed] [Google Scholar]
- Fletcher PJ, Tenn CC, Sinyard J, Rizos Z, Kapur S. A sensitizing regimen of amphetamine impairs visual attention in the 5-choice serial reaction time test: reversal by a D1 receptor agonist injected into the medial prefrontal cortex. Neuropsychopharmacology. 2007;32:1122–1132. doi: 10.1038/sj.npp.1301221. [DOI] [PubMed] [Google Scholar]
- Floresco SB, Block AE, Tse MT. Inactivation of the medial prefrontal cortex of the rat impairs strategy set-shifting, but not reversal learning, using a novel, automated procedure. Behav Brain Res. 2008;190:85–96. doi: 10.1016/j.bbr.2008.02.008. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, Paus T, Evans AC, Rapoport JL. Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci. 1999;2:861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Glick SD, Lyon RA, Hinds PA, Sowek C, Titeler M. Correlated asymmetries in striatal D1 and D2 binding: relationship to apomorphine-induced rotation. Brain Res. 1988;455:43–48. doi: 10.1016/0006-8993(88)90112-6. [DOI] [PubMed] [Google Scholar]
- Gonzales R, Ang A, McCann MJ, Rawson RA. An Emerging Problem: Methamphetamine Abuse Among Treatment Seeking Youth. Subst Abus. 2008;29:71–80. doi: 10.1080/08897070802093312. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Burgos G, Fish KN, Lewis DA. GABA neuron alterations, cortical circuit dysfunction and cognitive deficits in schizophrenia. Neural Plast. 2011;2011:723184. doi: 10.1155/2011/723184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Burgos G, Miyamae T, Pafundo DE, Yoshino H, Rotaru DC, Hoftman G, Datta D, Zhang Y, Hammond M, Sampson AR, Fish KN, Ermentrout GB, Lewis DA. Functional Maturation of GABA Synapses During Postnatal Development of the Monkey Dorsolateral Prefrontal Cortex. Cereb cortex. 2015;25:4076–4093. doi: 10.1093/cercor/bhu122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves SM, Napier TC. SB 206553, a putative 5-HT2C inverse agonist, attenuates methamphetamine-seeking in rats. BMC Neurosci. 2012;13:65. doi: 10.1186/1471-2202-13-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammerslag LR, Waldman AJ, Gulley JM. Effects of amphetamine exposure in adolescence or young adulthood on inhibitory control in adult male and female rats. Behav Brain Res. 2014;263:22–33. doi: 10.1016/j.bbr.2014.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hankosky ER, Westbrook SR, Haake RM, Marinelli M, Gulley JM. Reduced sensitivity to reinforcement in adolescent compared to adult Sprague-Dawley rats of both sexes. Psychopharmacology. 2018 doi: 10.1007/s00213-017-4804-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hankosky ER, Gulley JM. Performance on an impulse control task is altered in adult rats exposed to amphetamine during adolescence. Dev Psychobiol. 2013;55:733–744. doi: 10.1002/dev.21067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hankosky ER, Gulley JM. Adolescent Exposure to Amphetamines and Vulnerability to Addiction. In: Preedy VR, editor. Neuropathology of Drug Addictions and Substance Misuse. Vol. 2. London: Elsevier Inc; 2016. pp. 292–299. [Google Scholar]
- Hankosky ER, Kofsky NM, Gulley JM. Age of exposure-dependent effects of amphetamine on behavioral flexibility. Behav Brain Res. 2013;252:117–125. doi: 10.1016/j.bbr.2013.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey RC, Dembro KA, Rajagopalan K, Mutebi MM, Kantak KM. Effects of self-administered cocaine in adolescent and adult male rats on orbitofrontal cortex-related neurocognitive functioning. Psychopharmacology. 2009;206:61–71. doi: 10.1007/s00213-009-1579-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillhouse MP, Marinelli-Casey P, Gonzales R, Ang A, Rawson RA. Predicting in-treatment performance and post-treatment outcomes in methamphetamine users. Addiction. 2007;102:84–95. doi: 10.1111/j.1360-0443.2007.01768.x. [DOI] [PubMed] [Google Scholar]
- Huttenlocher PR, Dabholkar AS. Regional differences in synaptogenesis in human cerebral cortex. J Comp Neurol. 1997;387:167–178. doi: 10.1002/(sici)1096-9861(19971020)387:2<167::aid-cne1>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Istin M, Thiriet N, Solinas M. Behavioral flexibility predicts increased ability to resist excessive methamphetamine self-administration. Addict Biol. 2016;22:958–966. doi: 10.1111/adb.12384. [DOI] [PubMed] [Google Scholar]
- Izquierdo A, Belcher AM, Scott L, Cazares VA, Chen J, O’Dell SJ, Malvaez M, Wu T, Marshall JF. Reversal-specific learning impairments after a binge regimen of methamphetamine in rats: possible involvement of striatal dopamine. Neuropsychopharmacology. 2010;35:505–514. doi: 10.1038/npp.2009.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izquierdo A, Pozos H, de Torre AL, DeShields S, Cevallos J, Rodriguez J, Stolyarova A. Sex differences, learning flexibility, and striatal dopamine D1 and D2 following adolescent drug exposure in rats. Behav Brain Res. 2016;308:104–114. doi: 10.1016/j.bbr.2016.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izquierdo A. Functional heterogeneity within rat orbitofrontal cortex in reward learning and decision making. J Neurosci. 2017;37:10529–10540. doi: 10.1523/JNEUROSCI.1678-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang S, Paul K, Hankosky ER, Cox CL, Gulley JM. D1 receptor-mediated inhibition of medial prefrontal cortex neurons is disrupted in adult rats exposed to amphetamine in adolescence. Neuroscience. 2016a;324:40–49. doi: 10.1016/j.neuroscience.2016.02.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang S, Paul K, Hankosky ER, Cox CL, Gulley JM. Timing of amphetamine exposure in relation to puberty onset determines its effects on anhedonia, exploratory behavior, and dopamine D1 receptor expression in young adulthood. Neuroscience. 2016b;339:72–84. doi: 10.1016/j.neuroscience.2016.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantak KM, Barlow N, Tassin DH, Brisotti MF, Jordan CJ. Performance on a strategy set shifting task in rats following adult or adolescent cocaine exposure. Psychopharmacology. 2014;231:4489–4501. doi: 10.1007/s00213-014-3598-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby LG, Zeeb FD, Winstanley CA. Contributions of serotonin in addiction vulnerability. Neuropharmacology. 2011;61:421–432. doi: 10.1016/j.neuropharm.2011.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klink R, Robichaud M, Debonnel G. Gender and gonadal status modulation of dorsal raphe nucleus serotonergic neurons. Part I: Effects of gender and pregnancy. Neuropharmacology. 2002;43:1119–1128. doi: 10.1016/s0028-3908(02)00219-8. [DOI] [PubMed] [Google Scholar]
- Kondrad RL, Burk JA. Transient disruption of attentional performance following escalating amphetamine administration in rats. Psychopharmacology. 2004;175:436–442. doi: 10.1007/s00213-004-1857-z. [DOI] [PubMed] [Google Scholar]
- Kubota Y, Kawaguchi Y. Three Classes of GABAergic Interneurons in Neocortex and Neostriatum. Jpn J Physiol. 1994;44:145–148. [PubMed] [Google Scholar]
- Leiser SC, Li Y, Pehrson AL, Dale E, Smagin G, Sanchez C. Serotonergic Regulation of Prefrontal Cortical Circuitries Involved in Cognitive Processing: A Review of Individual 5-HT Receptor Mechanisms and Concerted Effects of 5-HT Receptors Exemplified by the Multimodal Antidepressant Vortioxetine. ACS Chem Neurosci. 2015;6:970–986. doi: 10.1021/cn500340j. [DOI] [PubMed] [Google Scholar]
- Liu S, Bubar MJ, Lanfranco MF, Hillman GR, Cunningham KA. Serotonin2C receptor localization in GABA neurons of the rat medial prefrontal cortex: implications for understanding the neurobiology of addiction. Neuroscience. 2007;146:1677–1688. doi: 10.1016/j.neuroscience.2007.02.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markham JA, Morris JR, Juraska JM. Neuron number decreases in the rat ventral, but not dorsal, medial prefrontal cortex between adolescence and adulthood. Neuroscience. 2007;144:961–968. doi: 10.1016/j.neuroscience.2006.10.015. [DOI] [PubMed] [Google Scholar]
- Markram H, Toledo-Rodriguez M, Wang Y, Gupta A, Silberberg G, Wu C. Interneurons of the neocortical inhibitory system. Nat Rev Neurosci. 2004;5:793–807. doi: 10.1038/nrn1519. [DOI] [PubMed] [Google Scholar]
- McAlonan K, Brown VJ. Orbital prefrontal cortex mediates reversal learning and not attentional set shifting in the rat. Behav Brain Res. 2003;146:97–103. doi: 10.1016/j.bbr.2003.09.019. [DOI] [PubMed] [Google Scholar]
- Mohila CA, Onn SP. Increases in the density of parvalbumin-immunoreactive neurons in anterior cingulate cortex of amphetamine-withdrawn rats: evidence for corticotropin-releasing factor in sustained elevation. Cereb cortex. 2005;15:262–274. doi: 10.1093/cercor/bhh128. [DOI] [PubMed] [Google Scholar]
- Moll GH, Mehnert C, Wicker M, Bock N, Rothenberger A, Ruther E, Huether G. Age-associated changes in the densities of presynaptic monoamine transporters in different regions of the rat brain from early juvenile life to late adulthood. Dev Brain Res. 2000;119:251–257. doi: 10.1016/s0165-3806(99)00182-0. [DOI] [PubMed] [Google Scholar]
- Morshedi MM, Meredith GE. Differential laminar effects of amphetamine on prefrontal parvalbumin interneurons. Neuroscience. 2007;149:617–624. doi: 10.1016/j.neuroscience.2007.07.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napier TC, Istre ED. Methamphetamine-Induced Sensitization Includes a Functional Upregulation of Ventral Pallidal 5-HT2A/2C Receptors. Synapse. 2008;62:14–21. doi: 10.1002/syn.20460. [DOI] [PubMed] [Google Scholar]
- National Research Council (US) Committee. Guide for the Care and Use of Laboratory Animals. 8. Washington, DC: National Academies Press (US); 2011. [Google Scholar]
- Neddens J, Dawirs RR, Bagorda F, Busche A, Horstmann S, Teuchert-Noodt G. Postnatal maturation of cortical serotonin lateral asymmetry in gerbils is vulnerable to both environmental and pharmacological epigenetic challenges. Brain Res. 2004;1021:200–208. doi: 10.1016/j.brainres.2004.06.050. [DOI] [PubMed] [Google Scholar]
- Parsegian A, Glen WB, Lavin A, See RE. Methamphetamine self-administration produces attentional set-shifting deficits and alters prefrontal cortical neurophysiology in rats. Biol Psychiatry. 2011;69:253–259. doi: 10.1016/j.biopsych.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul K, Kang S, Cox CL, Gulley JM. Repeated exposure to amphetamine during adolescence alters inhibitory tone in the medial prefrontal cortex following drug re-exposure in adulthood. Behav Brain Res. 2016;309:9–13. doi: 10.1016/j.bbr.2016.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain Atlas. 6. London, UK: Elsevier Inc; 2007. [Google Scholar]
- Puig MV, Artigas F, Celada P. Modulation of the activity of pyramidal neurons in rat prefrontal cortex by raphe stimulation in vivo: Involvement of serotonin and GABA. Cereb Cortex. 2005;15:1–14. doi: 10.1093/cercor/bhh104. [DOI] [PubMed] [Google Scholar]
- Puig MV, Gulledge AT. Serotonin and prefrontal cortex function: neurons, networks, and circuits. Mol Neurobiol. 2011;44:449–464. doi: 10.1007/s12035-011-8214-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawson RA, Gonzales R, McCann M, Ling W. Use of methamphetamine by young people: is there reason for concern? Addiction. 2007;102:1021–1022. doi: 10.1111/j.1360-0443.2007.01899.x. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Gorny G, Savage VR, Kolb B. Widespread but regionally specific effects of experimenter- versus self-administered morphine on dendritic spines in the nucleus accumbens, hippocampus, and neocortex of adult rats. Synapse. 2002;46:271–279. doi: 10.1002/syn.10146. [DOI] [PubMed] [Google Scholar]
- Rogers RD, Robbins TW. Investigating the neurocognitive deficits associated with chronic drug misuse. Curr Opin Neurobiol. 2001;11:250–257. doi: 10.1016/s0959-4388(00)00204-x. [DOI] [PubMed] [Google Scholar]
- Rubinow MJ, Juraska JM. Neuron and glia numbers in the basolateral nucleus of the amygdala from preweaning through old age in male and female rats: A stereological study. J Comp Neurol. 2009;512:717–725. doi: 10.1002/cne.21924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration (SAMHSA) Results from the 2012 National Survey on Drug Use and Health. Rockville MD: Substance Abuse and Mental Health Services Administration; 2013. NSDUH Series H-47, HHS Publication No. (SMA) 13–4805. [Google Scholar]
- Santana N, Artigas F. Expression of serotonin2C receptors in pyramidal and GABAergic neurons of rat prefrontal cortex: A comparison with striatum. Cereb Cortex. 2017;27:3125–3139. doi: 10.1093/cercor/bhw148. [DOI] [PubMed] [Google Scholar]
- Santucci AC, Capodilupo S, Bernstein J, Gomez-Ramirez M, Milefsky R, Mitchell H. Cocaine in adolescent rats produces residual memory impairments that are reversible with time. Neurotoxicol Teratol. 2004;26:651–661. doi: 10.1016/j.ntt.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Schneider LH, Murphy RB, Coons EE. Lateralization of striatal, dopamine (D2) receptors in normal rats. Neurosci Lett. 1982;33:281–284. doi: 10.1016/0304-3940(82)90385-8. [DOI] [PubMed] [Google Scholar]
- Selby MJ, Azrin RL. Neuropsychological functioning in drug abusers. Drug Alcohol Depend. 1998;50:39–45. doi: 10.1016/s0376-8716(98)00002-7. [DOI] [PubMed] [Google Scholar]
- Sherrill LK, Stanis JJ, Gulley JM. Age-dependent effects of repeated amphetamine exposure on working memory in rats. Behav Brain Res. 2013;242:84–94. doi: 10.1016/j.bbr.2012.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommers I, Baskin D, Baskin-Sommers A. Methamphetamine use among young adults: Health and social consequences. Addict Behav. 2006;31:1469–1476. doi: 10.1016/j.addbeh.2005.10.004. [DOI] [PubMed] [Google Scholar]
- Staiti AM, Morgane PJ, Galler JR, Grivetti JY, Bass DC, Mokler DJ. A microdialysis study of the medial prefrontal cortex of adolescent and adult rats. Neuropharmacology. 2011;61:544–549. doi: 10.1016/j.neuropharm.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streeter CC, Terhune DB, Whitfield TH, Gruber S, Sarid-Segal O, Silveri MM, Tzilos G, Afshar M, Rouse ED, Tian H, Renshaw PF, Ciraulo DA, Yurgelun-Todd DA. Performance on the Stroop predicts treatment compliance in cocaine-dependent individuals. Neuropsychopharmacology. 2008;33:827–836. doi: 10.1038/sj.npp.1301465. [DOI] [PubMed] [Google Scholar]
- Sullivan RM, Duchesne A, Hussain D, Waldron J, Laplante F. Effects of unilateral amygdala dopamine depletion on behaviour in the elevated plus maze: Role of sex, hemisphere and retesting. Behav Brain Res. 2009;205:115–122. doi: 10.1016/j.bbr.2009.07.023. [DOI] [PubMed] [Google Scholar]
- Tseng KY, O’Donnell P. Dopamine modulation of prefrontal cortical interneurons changes during adolescence. Cereb Cortex. 2007;17:1235–1240. doi: 10.1093/cercor/bhl034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdejo-García A, Betanzos-Espinosa P, Lozano OM, Vergara-Moragues E, González-Saiz F, Fernández-Calderón F, Bilbao-Acedos I, Pérez-García M. Self-regulation and treatment retention in cocaine dependent individuals: A longitudinal study. Drug Alcohol Depend. 2012;122:142–148. doi: 10.1016/j.drugalcdep.2011.09.025. [DOI] [PubMed] [Google Scholar]
- Vorhees CV, Reed TM, Morford LL, Fukumura M, Wood SL, Brown CA, Skelton MR, McCrea AE, Rock SL, Williams MT. Periadolescent rats (P41–50) exhibit increased susceptibility to D-methamphetamine-induced long-term spatial and sequential learning deficits compared to juvenile (P21–30 or P31–40) or adult rats (P51–60) Neurotoxicol Teratol. 2005;27:117–134. doi: 10.1016/j.ntt.2004.09.005. [DOI] [PubMed] [Google Scholar]
- Werry TD, Loiacono R, Sexton PM, Christopoulos A. RNA editing of the serotonin 5HT2C receptor and its effects on cell signalling, pharmacology and brain function. Pharmacol Ther. 2008;119:7–23. doi: 10.1016/j.pharmthera.2008.03.012. [DOI] [PubMed] [Google Scholar]
- Westbrook SR, Hankosky ER, Dwyer MR, Gulley JM. Age and sex differences in behavioral flexibility, sensitivity to reward value, and risky decision-making. Behav Neurosci. 2018 doi: 10.1037/bne0000235. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willing J, Juraska JM. The timing of neuronal loss across adolescence in the medial prefrontal cortex of male and female rats. Neuroscience. 2015;301:268–275. doi: 10.1016/j.neuroscience.2015.05.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winocur G, Eskes G. Prefrontal cortex and caudate nucleus in conditional associative learning: dissociated effects of selective brain lesions in rats. Behav Neurosci. 1998;112:89–101. doi: 10.1037//0735-7044.112.1.89. [DOI] [PubMed] [Google Scholar]
- Ye T, Pozos H, Phillips TJ, Izquierdo A. Long-term effects of exposure to methamphetamine in adolescent rats. Drug Alcohol Depend. 2014;138:17–23. doi: 10.1016/j.drugalcdep.2014.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Ma W, Barker JL, Rubinow DR. Sex differences in expression of serotonin receptors (subtypes 1A and 2A) in rat brain: a possible role of testosterone. Neuroscience. 1999;94:251–259. doi: 10.1016/s0306-4522(99)00234-1. [DOI] [PubMed] [Google Scholar]