Summary
Bacterial pathogens have developed a wide range of strategies to survive within human cells. A number of pathogens multiply in a vacuolar compartment, while others can rupture the vacuole and replicate in the host cytosol. A common theme among many bacterial pathogens is the use of specialized secretion systems to deliver effector proteins into the host cell. These effectors can manipulate the host’s membrane trafficking pathways to remodel the vacuole into a replication-permissive niche and prevent degradation. As master regulators of eukaryotic membrane traffic, Rab GTPases are principal targets of bacterial effectors. This review highlights the manipulation of Rab GTPases that regulate host recycling endocytosis by several bacterial pathogens including Chlamydia pneumoniae, Chlamydia trachomatis, Shigella flexneri, Salmonella enterica serovar Typhimurium, Uropathogenic Escherichia coli, and Legionella pneumophila. Recycling endocytosis plays key roles in a variety of cellular aspects such as nutrient uptake, immunity, cell division, migration and adhesion. Though much remains to be understood about the molecular basis and the biological relevance of bacterial pathogens exploiting Rab GTPases, current knowledge supports the notion that endocytic recycling Rab GTPases are differentially targeted to avoid degradation and support bacterial replication. Thus, future studies of the interactions between bacterial pathogens and host endocytic recycling pathways are poised to deepen our understanding of bacterial survival strategies.
A brief overview of endocytic pathways and recycling endocytosis
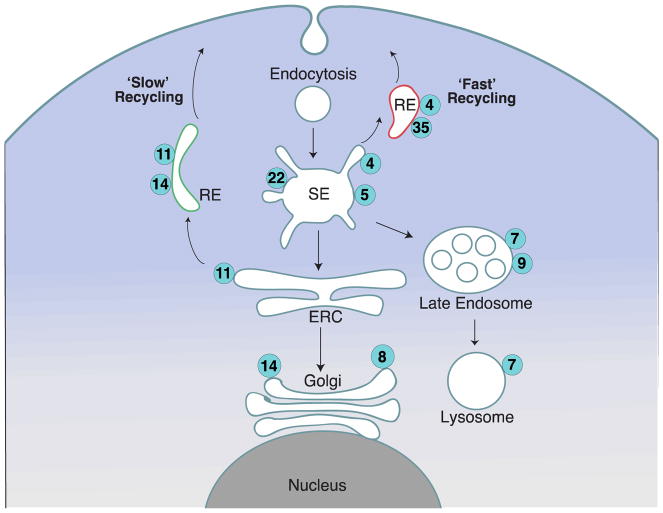
Membrane trafficking is fundamentally important for the normal function of mammalian cells, mediating essential processes such as nutrient uptake, immunity, cell migration, adhesion, and division. Proteins integrated at the plasma membrane allow cells to sense and respond to changes in their extracellular environment. These proteins frequently travel into the cell through endocytosis, ushering in nutrients and relaying extracellular information through intracellular signaling cascades. Upon internalization through a range of endocytic mechanisms, much of the protein cargo converges at early endosomes, which then undergo homotypic fusion (Grant et al., 2009). At this stage, cargo is segregated into microdomains and chartered to specific membrane trafficking routes. Cargoes can be returned back to the plasma membrane via recycling endocytosis, routed towards the trans-Golgi network (TGN) via retrograde traffic, or packaged in intraluminal vesicles of late endosomes/multivesicular bodies that ultimately fuse with lysosomes leading to cargo degradation (Fig. 1).
Figure 1. Schematic of endosomal traffic and Rab GTPases present on different membrane compartments (not a comprehensive view).
Cargo is internalized into vesicles that undergo homotypic fusion giving rise to early/sorting endosomes (SE), where distinct cargoes are then segregated into microdomains. Subsequently, tubular recycling endosomes (RE) carry the sorted cargo back to the plasma membrane. Rab4 and Rab35 regulate fast recycling from these sorting hubs through recycling endosomes back to the plasma membrane. Alternatively cargo can be routed to the endocytic recycling compartment (ERC) from where it can be recycled back to the plasma membrane through Rab11-positive tubular recycling endosomes. Other endosomes move segregated cargoes from sorting endosomes toward the trans-Golgi network through retrograde traffic, or towards late endosome to ultimately be degraded by fusion with lysosomes.
The directionality of the membrane traffic and cargo sorting relies on the precise spatiotemporal regulation of endosomal identity. Endosomal compartments have unique molecular identities that can rapidly shift to ensure the flow of membrane traffic. The molecular identity of endosomes is largely regulated by the differential recruitment and activation of Rab GTPases (hereafter Rabs), a class of (>60) proteins that belongs to the Ras superfamily of small GTPases (Pereira-Leal et al., 2001, Seabra et al., 2002, Pfeffer, 2013). Rabs act as molecular switches alternating between two conformations: active when GTP-bound and inactive when GDP-bound. The nucleotide binding state of Rabs is regulated by guanine exchange factors (GEFs), which facilitate the swapping of GDP with GTP and by GTPase activating proteins (GAPs), which stimulate the GTP hydrolysis activity of Rabs (Stenmark, 2009). Dedicated sets of GEFs and GAPs tightly regulate the activation status of each Rab. Active Rabs interact with downstream effectors involved in cargo selection, vesicle movement, and membrane fusion (Zhen et al., 2015). For example, Rab5 localization to early/sorting endosomes is critical for progression through the endocytic/phagocytic pathway, regulating homotypic fusion as well as fusion of early endosomes with the early phagosomes, while Rab7 regulates fusion of late endosomes with the phagosomes driving their maturation into a phagolysosomal compartment (Scott et al., 2003). An emerging concept in endocytic maturation is the role of Rab cascades to progressively change the identity of endosomes. In this paradigm an upstream Rab (e.g. Rab5) recruits the GEF that activates the subsequent Rab (e.g. Rab7), which then co-opts a GAP that inactivates the upstream Rab (Pfeffer, 2013).
The ‘fast’ and ‘slow’ pathways of recycling endocytosis in mammalian cells
Endocytic recycling of cargoes involves membrane compartments known as recycling endosomes which mediate the return of cargo back to the plasma membrane. Recycling endosomes differ in terms of the cargoes they carry, their molecular identity, and their itinerary to the plasma membrane. Two major pathways recycle cargo back to the plasma membrane: the so-called ‘slow’ and ‘fast’ pathways. As implied by the terminology, the two pathways differ kinetically and this is largely due to the cargo’s itinerary through the endocytic recycling system (Hopkins et al., 1983). The ‘fast’ recycling pathway returns cargo back to the plasma membrane directly from early endosomes, while in the ‘slow’ recycling route, membrane tubules carry the cargo to a perinuclear location known as the endocytic recycling compartment (ERC) (Ren et al., 1998, Grant et al., 2009). Often localized near the microtubule-organizing center (MTOC), the ERC is composed of tubular and vesicular membrane compartments, and gives rise to recycling endosomes destined for the plasma membrane. The two endocytic recycling pathways are regulated by distinct sets of Rabs. We will briefly highlight several recycling Rabs that are emerging as common targets of bacterial pathogens: Rab4, Rab11, Rab14, and Rab35.
Rab4 and Rab35 have been identified as major regulators of the fast recycling pathway (van der Sluijs et al., 1992, Choudhury et al., 2004, Chaineau et al., 2013). Both Rabs influence iron levels by regulating recycling of the transferrin receptor, which binds transferrin to mediate cellular uptake of iron (Grant et al., 2009). In addition, important immune molecules such as the T-cell receptor (TCR) and Major Histocompatibility Complex I and II (MHC-I and MHC-II) are recycled back to the cell surface through Rab35-dependent pathways (Patino-Lopez et al., 2008, Walseng et al., 2008, Allaire et al., 2010). Furthermore, Rab35 is a key regulator of phagosome maturation, a process by which microbial cargo is degraded through progressive fusions of the phagosome with early endosomes, late endosomes, and ultimately with lysosomes (Egami et al., 2011, Verma et al., 2017). Rab14 is involved in controlling Golgi-to-endosome transport and endosome-phagosome fusion (Junutula et al., 2004, Kyei et al., 2006). More recent evidence shows that Rab14 defines an intermediate recycling compartment that functions after Rab4/Rab5 but before Rab11 and regulates recycling of the transmembrane protease ADAM10 back to the cell surface (Linford et al., 2012).
The ‘slow’ recycling pathway is thought to be mediated primarily through Rab11a, which localizes to tubular endosomes, the ERC, and the trans-Golgi network (TGN) compartment (Ullrich et al., 1996, Grant et al., 2009). Rab11a mediates transport of molecules from sorting endosomes to the ERC (Horgan et al., 2010), and regulates the TGN-to-ERC or TGN-to-plasma membrane traffic (Chen et al., 1998). Rab11a controls recycling of a wide range of cell surface proteins including the transferrin receptor, ion channels, junctional proteins, integrins, and immune receptors (Campa et al., 2017). Through interactions with adaptor proteins, Rab11a couples with various motor proteins that provide the mechanical force to move vesicles along microtubules or actin filaments (Welz et al., 2014). Ultimately, Rab11a influences a variety of cellular processes including cytokinesis, phagocytosis, cell migration, immunological synapse and primary cilia formation.
It is important to note that the membrane trafficking circuits regulated by these Rabs are not entirely isolated from each other, often crossing paths at intermediate hybrid vesicles (Sonnichsen et al., 2000, Linford et al., 2012). Thus, cargo returning to the plasma membrane may not all follow a simple linear transport pathway. As such, the precise number of distinct pathways and the manner in which these pathways intersect is not completely understood.
Bacterial pathogens target Rabs that regulate endocytic recycling
Targeting of Rabs is a well-established strategy that bacterial pathogens use to exploit specific membrane trafficking routes (Pizarro-Cerda et al., 2014). Bacterial effector proteins translocated by specialized secretion systems into the host cell can selectively target and manipulate the activity of Rabs (Brumell et al., 2007, Cossart et al., 2010, Stein et al., 2012, Sherwood et al., 2013). Fluorescence microscopy of infected host cells transiently overproducing fluorescently tagged Rabs has been the approach most broadly used to determine whether Rabs associate with bacteria-containing vacuoles. Higher resolution imaging is typically needed to discern whether membrane compartments marked by a particular Rab fuse with the vacuolar membrane or merely accumulate in the vicinity of the vacuole. As detailed in the next section, overproduction of wild-type and mutant forms of Rabs, locked in either the active (GTP-bound) or inactive (GDP-bound) state, has been widely applied to determine the extent to which the activity status of a particular Rab impacts bacterial survival or vacuolar properties such as expansion or association with host membrane compartments. RNAi screens have been useful in identifying Rabs important for bacterial growth, however these data must be interpreted with caution since Rabs are involved in a myriad of cellular functions and they often regulate membrane traffic through Rab cascades (Pfeffer, 2013). Targeting of recycling Rabs by bacterial pathogens leads to a salient question: is endocytic recycling affected during infection? Although this has not yet been addressed for all the pathogens reviewed here, current knowledge suggests that endocytic recycling is generally affected during infection. The transferrin receptor is widely used as a marker for recycling endosomes and its binding partner, transferrin, is commonly used to track endocytic recycling (Mayle et al., 2012). Using methods based on recycling of transferrin, it was determined that recycling endocytosis is inhibited in human macrophage-like cells infected with L. pneumophila (Allgood et al., 2017) and human epithelial cells infected with S. flexneri (Mounier et al., 2012), whereas Coxiella burnetti (not reviewed here) did not affect transferrin recycling despite transferrin-positive vesicles accumulating close to its vacuole (Larson et al., 2017). Since the transferrin receptor is recycled through both the ‘slow’ and ‘fast’ recycling pathways, the extent to which each pathway is impacted during infection can instead be determined by quantifying the recycling rate of plasma membrane proteins known to be recycled predominantly through one of the two pathways. In this review we will focus on how various bacterial pathogens including Chlamydia pneumoniae, Chlamydia trachomatis, Shigella flexneri, Salmonella enterica serovar Typhimurium, Uropathogenic E. coli, and Legionella pneumophila target Rabs that are known to regulate endocytic recycling.
CHLAMYDIA
Chlamydia spp. are obligate intracellular pathogens that cause a wide range of diseases. Here we focus on two important human pathogens leading to distinct pathologies, Chlamydia pneumoniae and Chlamydia trachomatis. C. pneumoniae causes acute respiratory tract infections and it has been linked to chronic diseases such as arthritis, atherosclerosis, and asthma (Elwell et al., 2016). C. trachomatis is a major cause of preventable blindness in underdeveloped parts of the world (Hu et al., 2013) and the most prevalent cause for sexually transmitted infection (Elwell et al., 2016), leading to pelvic inflammatory disease, ectopic pregnancies, or sterility in women.
The developmental cycle of both C. pneumoniae and C. trachomatis includes two morphologically and functionally distinct forms known as the elementary body (EB) and reticulate body (RB) (Fig. 2A). The infectious EB form, resistant to the harsh conditions of the extracellular environment, adheres to epithelial cells and promotes invasion by injecting the host with preformed effector proteins that induce cytoskeletal rearrangements. Hours following uptake into the plasma membrane-derived compartment known as the inclusion, EBs differentiate into the non-infectious, replicative RB form. Over the next 2–3 days, RBs continue to replicate and then differentiate back into EBs before exiting the host through lysis or extrusion. During infection, C. trachomatis and C. pneumoniae remodel the inclusion membrane by secreting effector proteins into the host via a type III secretion system (T3SS).
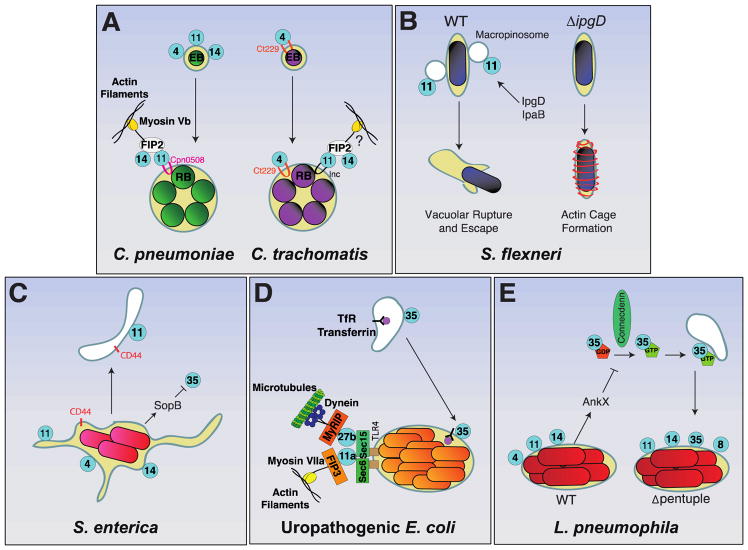
Figure 2. Bacterial pathogens selectively target endocytic recycling Rabs.
A) C. pneumoniae and C. trachomatis – Upon uptake into the host cell, the infectious elementary body (EB) of C. pneumoniae forms the inclusion body. The early inclusion body associates with Rab4, Rab11, and Rab14 through unknown mechanisms. Subsequently, EBs differentiate into the non-infectious replicative bodies (RB). At this later stage the inclusion is associated with Rab11 through direct interaction with the C. pneumoniae inclusion protein Cpn0508. As a result, the Rab11 adaptor proteins Rab11FIP2 (FIP2) is recruited leading to recruitment of Rab14 to the inclusion body and interaction with the actin motor protein Myosin Vb. The developmental cycle of infection of C. trachomatis is similar to C. pneumoniae. Rab4 is recruited to the early inclusion by the C. trachomatis inclusion protein CT229. The Rab11-FIP2-Rab14 complex is also recruited to the inclusion body, but it is unclear if it facilitates interactions with the cytoskeleton.
B) S. flexneri – The nascent vacuole containing S. flexneri associates with Rab11-positive macropinosomes. Recruitment of macropinosomes is critical for vacuolar rupture and escape of S. flexneri into the cytosol. The effector protein IpgD, a PI(4,5)P2 phosphatase, is responsible for recruitment of Rab11-positive macropinosomes to the invasion site. In the absence of IpgD, Rab11-positive macropinosomes are not recruited and instead the vacuole becomes enclosed in an actin cage. S. flexneri effector IpaB is thought to directly bind cholesterol to induce formation of Rab11 positive compartments while the bacterium enters a replicative state.
C) S. enterica serovar Typhimurium – replicates within the Salmonella-containing vacuole (SCV). The SCV matures to resemble a late endosome, but does not fuse with lysosomes. The SCV interacts with endocytic recycling pathways through the early recruitment of Rab4 and Rab11. The cell adhesion molecule CD44 is recycled from the SCV back to the plasma membrane through a Rab11-dependent pathway. The effector SopB, a PI(4,5)P2 phosphatase, prevents Rab35 recruitment to the SCV by modifying the SCV’s surface charge.
D) Uropathogenic E. coli – can reside within a vacuole that co-localizes with Rab35 and the transferrin receptors, a major transporter of iron within the host cell. UPEC is also capable of being expelled from the cell without damage to the bacterium or the host. TLR4 present on the vacuole interacts with Sec6 and Sec15 which recruit Rab11a and Rab27b to the UPEC-containing vacuole. Rab11a can then bind its adaptor protein Rab11FIP3 (FIP3) to recruit the actin motor protein Myosin VIIa while Rab27b recruits the microtubule motor protein dynein through its adaptor protein MyRIP.
E) L. pneumophila – The Legionella-containing vacuole (LCV) associates with Rab4a, Rab11a and Rab14 through an unknown mechanism. The effector protein AnkX phosphocholinates Rab35 preventing activation by the cognate GEF, Connecdenn, and thereby inhibiting Rab35 activation. A mutant of L. pneumophila missing five of its genomic islands encoding effector proteins (Δpentuple) still recruited Rab4a, Rab11a and Rab14 and did not prevent Rab35 recruitment.
Recent insight into how the membrane of the inclusion is modified to avoid endolysosomal maturation suggests Chlamydiae actively alter the cytosolic façade of the inclusion membrane to resemble that of recycling endosomes (Molleken et al., 2017). This study analyzed the localization of Rabs at the inclusion membrane in C. pneumoniae-infected Hep-2 cells transiently overproducing GFP-tagged versions of Rabs. Focusing on early infection, by 15 minutes, C. pneumoniae-containing inclusions had acquired Rab4, Rab5, Rab7, Rab11, and Rab14 (Fig. 2A). However, Rab4 and Rab7 were removed by 60 minutes, while Rab11 and Rab14 were retained on the inclusion. Thus, based on its Rab composition, it would seem that the inclusion shifts from initially resembling early/sorting endosomes to later resembling recycling endosomes. Although Rab4 was absent from the inclusion at 30 minutes post-infection, it was recruited later during infection suggesting that it plays roles at different stages of infection (Cortes et al., 2007). However, the mechanism responsible for recruiting Rab4 to the inclusion and the functional significance of Rab4 during C. pneumoniae requires further study. An important event occurring early during infection is the relocation of the inclusion from the cell periphery to a perinuclear region. This is believed to be mediated by association of the inclusion with Fip2, a Rab11/Rab14 adaptor protein, typically involved in intracellular transport of cargo within the endocytic recycling system (Cullis et al., 2002, Baetz et al., 2013). Rab11 and Rab14 recruit Fip2 to the nascent inclusion, which in turn binds to the actin motor protein Myosin Vb; Fip2 or MyoVb depletion caused deficient internalization and infectivity (Fig. 2A) (Molleken et al., 2017). At later stages of infection Rab11 recruitment to the inclusion is mediated by Cpn0585 (Cortes et al., 2007), but it is unclear whether this inclusion protein is responsible for early recruitment of Rab11. In host cells overproducing Rab11 locked in the inactive GDP-bound state, internalization and infectivity was dramatically reduced compared to cells expressing Rab11 locked in the active GTP-bound state (Molleken et al., 2017). It remains to be determined if targeting Rab11-positive endosomes allows C. pneumoniae to gain access to host cellular resources carried by these compartments.
The inclusion formed by the closely related C. trachomatis also associates with recycling Rabs (Fig. 2A). Pioneering work showed that C. trachomatis-containing inclusions in HeLa 229 cells are positive for Rab4, Rab11, and Rab14 at 18 hours post infection (Rzomp et al., 2006). Rab4 and Rab11 associated with the inclusion within the first 2 hours post-infection (Rejman Lipinski et al., 2009), suggesting that they might play a role in remodeling the inclusion at the start of infection to prevent trafficking through endocytic maturation pathways. A combined approach including a yeast two-hybrid screen, pull-down, and co-localization experiments, demonstrated that the inclusion protein CT229 is responsible for recruiting Rab4 (Rzomp et al., 2006). CT229 (CpoS) also associates with several other Rabs including Rab35 (Mirrashidi et al., 2015, Sixt et al., 2017), although the biological relevance of the interaction between CT229 and Rab35 is unknown, and it remains to be determined whether Rab35 is indeed recruited to the inclusion. Notably, disruption of CT229 compromises the integrity of the inclusion membrane leading to the early release of bacteria into the host cytosol and resulting in host cell death suggesting that CT229 plays an important role in the stability of the inclusion and prevention of host cell death (Sixt et al., 2017, Weber et al., 2017).
Though it is unclear how Rab11 is recruited to the C. trachomatis-containing inclusion, it was found to interact with the adaptor protein Fip2, which also localizes on the inclusion (Leiva et al., 2013). This interaction appeared to stimulate recruitment of Rab14, similar to C. pneumonia infection. The purpose of this interaction has not yet been elucidated, although it could be that, analogous to C. pneumoniae, the Fip2 interaction with Rab11 and Rab14 facilitates movement along the host cytoskeleton. Rab11 and Rab14 may be involved in chlamydial inclusion development since individually depleting them led to formation of a smaller inclusion or to delayed enlargement of the inclusion. This is believed to be caused by deficient delivery of sphingolipids to the inclusion membrane (Rejman et al., 2009a; Capmany and Damiani, 2010. This idea is supported by data showing that Rab14-positive vesicles carry the sphingolipid precursor ceramide. Fluorescence microscopy revealed that BODIPY-TR ceramide vesicles co-localizing with Rab14 were found within the inclusion in the proximity of bacteria (Capmany and Damiani, 2010). Rab4, Rab11, and Rab14 regulate recycling of the transferrin receptor (TfR), but it is unknown whether Chlamydiae manipulate these Rabs to obtain iron. By electron microscopy, TfR positive tubular recycling endosomes were observed in intimate contact with the inclusion, but they did not seem to fuse with the inclusion membrane (Al-Younes et al., 1999, Scidmore et al., 2003, Ouellette et al., 2010). These observations support the notion that association of the inclusion with recycling endosomes does not necessarily lead to fusion of the inclusion membrane with these compartments. Interestingly, a screen for low-molecular weight inhibitors of Chlamydia growth revealed that FR179254 diminishes the formation of Rab4/11 hybrid vesicles that carry transferrin, thereby causing a delay in the slow transferrin recycling pathway (Ouellette et al., 2010). Unexpectedly, growth inhibition caused by this compound was relieved upon removal of the transferrin-containing fraction of the serum. Overproducing dominant negative mutants of Rab4 and Rab11 inhibited transferrin recycling and led to accumulation of transferrin-positive vesicles around the inclusion and ultimately resulted in impaired Chlamydial growth (Ouellette et al., 2010). These data are consistent with the idea that overaccumulation of transferrin-containing endosomes at the inclusion may be deleterious for the development of C. trachomatis and that interactions with this trafficking pathway need to be closely regulated to ensure proper development.
Overall, these studies demonstrate that the Chlamydia inclusion associates with recycling endosomes, but much remains to be learned about the biological relevance of this association and the molecular mechanisms that mediate their recruitment to the inclusion. Current evidence suggests that Rab11-positive recycling endosomes do not fuse with the inclusion membrane (Ouellette et al., 2010), but they remain in close proximity to the inclusion and seem to be important for moving the inclusion along microtubules. This observation is intriguing, yet how this is physically accomplished if Rab11-vesicles do not fuse with the inclusion has not been determined. A recent study demonstrated that Rab11a-containing endosomes are tethered to the ERC by contiguous “membrane bridges” (Xie et al., 2016). We could envision that perhaps Rab11-positive endosomes connect with the inclusion membrane in a similar way, enveloping the inclusion in a “mantle” of recycling endosomes that allows the inclusion to interface with the host cytoskeleton. Future super-resolution microscopy studies could readily address this hypothesis. Recycling endosomes shuttle a range of molecules between the plasma membrane and intracellular organelles including receptors for nutrient acquisition and innate immune signaling. Beyond sphingolipid acquisition, it remains to be established whether Chlamydiae target recycling Rabs to obtain nutrients or manipulate innate immune signaling pathways.
SHIGELLA
The facultative intracellular bacterium Shigella flexneri, the causative agent of dysentery, invades epithelial cells of the human colon. Upon contact with the host cell, S. flexneri swiftly injects a number of effector proteins through a T3SS. These effectors promote major rearrangements of actin and the plasma membrane driving the formation of a vacuole that initially houses the pathogen. The vacuole ruptures within minutes of infection releasing bacteria into the nutrient-rich cytosol where they replicate and then spread cell-to-cell (Sansonetti, 2001, Ray et al., 2010). Since vacuolar escape occurs so quickly after entry, the events that lead up to it have been challenging to study in detail. A high-content siRNA screen for host proteins involved in Shigella uptake and vacuolar rupture identified Rab11 as a key player in the unfolding of early events (Mellouk et al., 2014). Immunofluorescence staining revealed that Rab11-positive compartments abundantly accumulate at the invasion site and Rab11 recruitment depends on the Shigella effector IpgD, a phosphatidylinositol(4,5)bisphosphate (PI(4,5)P2) phosphatase that generates PI(5)P (Mellouk et al., 2014). In the absence of IpgD, actin structures assemble around vacuoles leading to delayed vacuolar rupture (Fig. 2B). A similar phenotype occurred in cells infected with a mutant strain encoding a catalytically inactive IpgD or Rab11-depleted cells. Large volume correlative light electron microscopy and dynamic microscopy revealed that the Rab11-positive compartments accumulating at the invasion site are large endocytic structures known as macropinosomes, and that shortly before vacuolar rupture, macropinosomes are found in direct contact with the bacteria-containing vacuole (Fig. 2B) (Weiner et al., 2016). Formation of Rab11-positive compartments was also stimulated by IpaB, which localizes at the tip of the T3SS and directly binds cholesterol (Mounier et al., 2012). Fluorescence microscopy of S. flexneri-infected epithelial cells transiently producing GFP-tagged Rabs showed that IpaB induced formation of Rab11-positive tubules when S. flexneri reached the replicative stage (Mounier et al., 2012). This resulted in drastic impairment of traffic of E-cadherin, a core intercellular junction component crucial for epithelial barrier integrity. This striking phenotype suggests that IpaB could also disrupt Rab11-dependent traffic of other cell surface receptors or secreted proteins. Since both IpaB and IpaD lead to accumulation of Rab11-positive compartments one question that needs to be addressed is whether IpaB and IpaD work together to manipulate Rab11-dependent pathways.
In conclusion, although most intravacuolar pathogens exploit Rab11-dependent events to support vacuole biogenesis, Shigella does just the opposite, manipulating Rab11 to destabilize the vacuole. Precisely how Rab11-positive vesicles stimulate vacuolar rupture is unclear, but it may involve Rab11 interaction with the exocyst, an octameric protein complex involved in the tethering of secretory vesicles to the plasma membrane (Lopez-Montero et al., 2016).
SALMONELLA
A major cause of gastroenteritis in humans, Salmonella enterica serovar Typhimurium is a facultative intracellular pathogen that infects a variety of cell types including epithelial cells and macrophages using two distinct T3SS. Upon entry into the host cell, the bacteria settle into a Salmonella-containing vacuole (SCV) that matures into a late endosome-like compartment, but does not fuse with lysosomes (LaRock et al., 2015). The SCV interacts with several components of the recycling endocytosis pathway. In Salmonella-infected HeLa cells transiently producing GFP-tagged Rab constructs, Rab4 localized transiently to the SCV within the first hour of infection, whereas the number of Rab11-positive vacuoles gradually increased until about 60 minutes post infection (Fig. 2C) (Smith et al., 2005). During infection, Rab11 regulated recycling of the cell adhesion receptor CD44, but not the immune molecule MHC-I, from the SCV back to the cell surface (Smith et al., 2005), despite previous studies reporting that MHC-I recycling is partly controlled by Rab11 (Weigert et al., 2004, Smith et al., 2005). Moreover, ectopic expression of constitutively inactive Rab11 inhibited maturation of the SCV. This effect was only partial, however, and did not disrupt replication, suggesting that redundant pathways regulate the endocytic recycling events associated with phagosome maturation. A screen for fluorescently-tagged Rabs associated with SCVs during infection of HeLa cells demonstrated that Salmonella effectors selectively and dynamically alter the Rab composition of the vacuolar membrane (Smith et al., 2007). Interestingly, the SCV abundantly accumulated Rabs associated with early, late, and recycling endosomes (Rab5a, Rab5b, Rab5c, Rab7a, Rab11a, Rab11b) (Smith et al., 2007). Meanwhile, a translocation-deficient mutant, unable to inject effectors into the host cell and consequently targeted for degradation through phagosome maturation, accumulated a distinct set of Rabs including Rab8b, Rab13, Rab23, Rab32, and Rab35 (Fig. 2C). Strikingly, with the exception of Rab32, all of these Rabs have been implicated in regulating recycling endocytosis. The Salmonella effector SopB contributes to regulating association of Rab35 with the SCV (Bakowski et al., 2010). SopB reduces the levels of PI(4,5)P2 and phosphatidylserine on the SCV membrane through its phosphoinositide phosphatase activity, altering the electrostatic surface charge of nascent SCVs (Fig. 2C). This change is thought to prevent binding of Rab35, which had been previously shown to directly bind negatively charged phosphoinositides (Heo et al., 2006). Furthermore, Kobayashi et al proposed that Rab35 functions as a master Rab that promotes recruitment of other Rabs (including Rab8 and Rab13) to recycling endosomes through recruitment of MICAL-L1, a protein that interacts with multiple Rabs (Kobayashi et al., 2014). Localization of these Rabs on SCVs occupied by translocation-deficient mutants is consistent with the hypothesis that these particular Rabs are not only regulators of recycling endocytosis, but are also involved in phagosome maturation. Thus, although recycling Rabs have been shown to be selectively recruited to the SCV membrane, the biological relevance of these interactions is not understood and merits further study. Drawing on knowledge about the importance of these pathways for other bacterial pathogens, association with Rab11-positive endosomes, for instance, could be of nutritional benefit potentially providing the SCV with access to host resources such as iron or sphingolipids.
E. COLI
The uropathogenic E. coli (UPEC) is frequently the cause of recurrent urinary tract infections. Though mostly extracellular, UPEC can also persist intracellularly. The pathogen penetrates bladder epithelial cells by forming intracellular bacterial communities and quiescent intracellular reservoirs, both being sources of recurrent and persistent infections that can often evade the host immune response (Hunstad et al., 2010). Following internalization, UPEC are retained in a vacuole that at late time points after mouse infections (>2 weeks) display late endosome characteristics including the presence of LAMP-1, but absence of the lysosomal protein Cathepsin D (Mysorekar et al., 2006) and this phenotype was recapitulated in an in vitro cell culture infection system (Dikshit et al., 2015). Depletion of Rab35 in cultured bladder epithelial cells (BEC) led to accumulation of lysosomal markers on the UPEC vacuole and resulted in decreased survival of UPEC. Fluorescence microscopy of BEC cells overproducing GFP-tagged Rab GTPases and infected with UPEC revealed that throughout infection a gradually increasing percentage of UCVs accumulated Rab35, consistent with the idea that Rab35 contributes to the intracellular persistence of UPEC. Rab35 is a key regulator of transferrin receptor (TfR) recycling and TfR is located on the UPEC vacuole (Fig. 2D). UPEC seems to utilize the Rab35-dependent traffic of TfR to gain access to the host’s supply of iron in addition to its own iron acquisition system (Dikshit et al., 2015). This notion is supported by observations that UPEC survival is dramatically reduced in Rab35 or TfR depleted cells and bacterial growth could not be rescued by iron supplementation. In addition to associating with Rab35, the UPEC vacuole also associates with Rab11a, which has been linked to the intriguing ability of BEC cells to expel UPEC without disrupting cell viability (Bishop et al., 2007). A recent study demonstrated that UPEC extrusion through the plasma membrane, known to be triggered by the pattern recognition receptor, Toll-like receptor 4 (TLR4), is accomplished through a mechanism that involves Rab11a and Rab27b (Miao et al., 2017). Remarkably, this mechanism of bacterial clearance seems to simultaneously involve the motor proteins dynein and myosin. On one hand, Rab11a recruits Rab11FIP3 and the microtubule motor protein dynein, and on the other hand Rab27b interacts with MyRIP and actin-associated motor myosin VIIa (Fig. 2D). Rab11a and Rab27b are recruited and activated by the exocyst complex components SEC6/SEC15, which in turn interacts with TLR4 present on the UPEC vacuole. It is unclear whether this mechanism is simply a host defense strategy or whether this is a UPEC-driven strategy to infect neighboring cells.
LEGIONELLA
The facultative intracellular bacterium Legionella pneumophila is an opportunistic human pathogen that causes a severe type of pneumonia known as Legionnaires’ disease. In the lung, L. pneumophila infects cells of the innate immune system including macrophages and neutrophils. Bacteria internalized by these professional phagocytes remodel the nascent phagosome into a replicative niche (So et al., 2015). Recruitment of vesicles derived from the endoplasmic reticulum (ER) is a hallmark of vacuolar remodeling and it begins occurring within minutes of infection onset (Swanson et al., 1995). The Legionella-containing vacuole (LCV) avoids phagosomal maturation and fusion with lysosomes, however, the mechanism behind this process is not well understood. The prevailing view is that L. pneumophila manipulates host membrane traffic to support vacuole biogenesis and maintenance (Ham et al., 2011, Hilbi et al., 2011, Spano et al., 2017). Though more is known about how L. pneumophila effectors exploit ER-to-Golgi traffic, how other membrane trafficking pathways might be manipulated is less clear. In a recent study using a pulse-chase approach, transferrin recycling was found to be drastically diminished in U937 macrophages infected with wild type L. pneumophila, but not in those infected with a translocation-deficient mutant (Allgood et al., 2017). This is consistent with the notion that L. pneumophila disrupts one ore more host endocytic recycling pathway since TfR is recycled through both the ‘fast’ and ‘slow’ recycling pathways. Although it is not yet known precisely which pathway is disrupted, this phenotype was largely attributed to the effector protein AnkX since the inhibitory effect on recycling was significantly relieved when macrophages were infected with the ΔankX mutant. AnkX had been previously shown to directly modify Rab35 by covalent addition of a phosphocholine moiety (Mukherjee et al., 2011). Phosphocholinated GDP-bound Rab35 no longer interacts with its cognate GEF, Connecdenn, persisting in an inactive state (Fig. 2E). Thus, it would be expected that during infection, the Rab35 pool accessed by AnkX remains locked in an inactive GDP-bound conformation, which presumably contributes to inhibition of transferrin recycling. In support of this notion, transferrin recycling was inhibited in macrophages infected with the ΔankX mutant complemented with wild type AnkX, but not when complemented with the catalytically inactive AnkXH229A (Allgood et al., 2017). This directly links post-translational modification of host proteins to an inhibitory effect on recycling endocytosis, but it is unclear if this effect was mediated by targeting other recycling Rabs in addition to Rab35.
Proteomic studies of intact LCVs isolated from RAW264.7 murine macrophages revealed the presence of a number of Rabs including several associated with recycling endocytosis (Bruckert et al., 2015, Schmolders et al., 2017). A recent study compared the proteome of LCVs from macrophages infected with either wild type or a pentuple deletion mutant, a strain lacking 5 gene clusters covering 31% of the L. pneumophila effector proteins (O’Connor et al., 2011), which replicates within macrophages, but not within amoebae, their natural host. In this study, Rab11a was identified in the proteome of LCVs regardless of the presence of a full complement of effectors. This was also the case for LCVs purified from the amoeba, Dictyostelium discoideum. According to a previous study, Rab11a was still part of the LCV proteome at 4 h post-infection in RAW264.7 macrophages (Bruckert et al., 2015). Notably, Rab11a was ubiquitinated in a manner that depended on the activity of the effector AnkB (Bruckert et al., 2015), although it is unclear whether Rab11a is a direct substrate of AnkB. Rab14 was present on LCVs of wild type, the Δpentuple mutant, and the ΔankB mutant. The fact that Rab11a and Rab14 have been consistently found on LCVs during infection of macrophages or amoebae points to a conserved function of these Rabs in vacuole biogenesis. Depletion of Rab11a led to defective intracellular growth, whereas depletion of Rab14 did not cause a significant growth phenotype (Hoffmann et al., 2014). However, the precise function of Rab11 and Rab14 and the mechanisms responsible for their recruitment to the LCV remain to be determined. Rab4a was also found to associate with the LCV when ectopically expressed in host cells, but Rab4a knockdown did not affect intracellular growth (Hoffmann et al., 2014).
Interestingly, both the Δpentuple mutant and the ΔankB mutant, but not the wild type LCVs, were positive for Rab35 at 1h and 4 h, respectively. This suggests that host protein uqibuitination is important for controlling association of Rab35 with the LCV. It is also possible that one of the effectors missing in the Δpentuple mutant is responsible for inhibiting binding of Rab35 to the LCV. We could then speculate that Rab35 is involved in the early stages of phagosome maturation, as reported in other systems (Verma et al., 2017).
Though proteomic studies are a rich and valuable resource for understanding how Legionella alters the protein composition of the vacuole, this approach provides only a snapshot of the LCV’s protein content. Deciphering the roles of Rab11a, Rab14, and Rab35 in vacuole biogenesis and maintenance necessitates a better temporal resolution of the dynamics of these Rabs on the LCV. Further study is needed to clarify how these Rabs are recruited to the LCV, how L. pneumophila distinguishes different pathways of recycling endocytosis, and why L. pneumophila inhibits recycling endocytosis.
Concluding remarks
Mammalian cells rely on an intricate and highly dynamic network of membrane trafficking pathways to perform essential functions. Within this tapestry of trafficking routes, the function and movement of vesicles is defined in large part by the Rabs present on their membrane. Intracellular bacterial pathogens access specific trafficking routes by selectively targeting Rabs, but how precisely they impact intracellular traffic is only beginning to be understood. Regulated by multiple Rabs, recycling endocytosis mediates the transport of membrane proteins back to the plasma membrane. Secreted bacterial effectors target several recycling related Rabs and, in this review, we have highlighted how particular intracellular bacterial pathogens manipulate one or more of these Rabs. Currently there is strong evidence in support of selective recruitment of endocytic recycling Rabs to the vacuole of bacterial pathogens described here. However, much remains to be understood about how Rabs are recruited to vacuole and what is the functional role of these interactions. Nevertheless, based on present knowledge of interactions between intracellular bacterial pathogens and endocytic recycling regulators, several clear parallels emerge among pathogens. First, Rab11 is a prominent target, common to almost all pathogens reviewed here, highlighting its key role in normal function of mammalian cells. The multifunctional nature of Rab11 seems to translate into a number of benefits for bacterial pathogens that manipulate it, including entry and exit from the host cell, vacuole biogenesis or rupture, acquisition of nutrients, and evasion of immune detection, depending on the particular needs of the pathogen. A second common trend observed is the transient recruitment of Rab4 early during infection and an ensuing, more persistent wave of Rab11. This sequential recruitment suggests the presence of a common mechanism potentially involving endogenous or bacterial regulatory proteins that activate or deactivate Rabs in a specific order. Third, vacuoles of degradation-destined Salmonella and Legionella mutants both acquire Rab35 as well as Rab8 and Rab13. Previous studies showed that Rab35 promotes recruitment of Rab8 and Rab13 to recycling endosomes through MICAL-L1, an endogenous protein that serves as a scaffold. This further strengthens the possibility that Rab cascades regulating endogenous membrane traffic are recapitulated on the membranes of bacteria-baring vacuoles. An alternative explanation for the recruitment of these Rabs could be that the electrostatic charge of the vacuolar membrane is altered in a way that allows recruitment of Rabs with polybasic clusters (Heo et al., 2006). Finally, because Rab35 is a key regulator of recycling endocytosis and it has recently been demonstrated to play a major role in phagosome maturation, pathogens could exploit it to both gain access to host resources and to prevent their degradation. How bacterial pathogens distinguish between recycling endocytosis pathways that are functionally distinct is a central question that is far from being well understood and addressing this question in more depth will bring important insights into mechanisms of intracellular survival of bacterial pathogens.
Acknowledgments
We thank Rebecca Noll for critical reading of this manuscript. Because of word limitations many papers were not cited. We apologize to the authors whose publications were not included. Research in our laboratory is supported by the University of Delaware Research Foundation, the NIH-NIGMS under awards P20GM103446 and P20GM104316. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors have no conflict to declare.
References
- Al-Younes HM, Rudel T, Meyer TF. Characterization and intracellular trafficking pattern of vacuoles containing Chlamydia pneumoniae in human epithelial cells. Cell Microbiol. 1999;1:237–247. doi: 10.1046/j.1462-5822.1999.00024.x. [DOI] [PubMed] [Google Scholar]
- Allaire PD, Marat AL, Dall’Armi C, Di Paolo G, McPherson PS, Ritter B. The Connecdenn DENN domain: a GEF for Rab35 mediating cargo-specific exit from early endosomes. Mol Cell. 2010;37:370–382. doi: 10.1016/j.molcel.2009.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allgood SC, Romero Dueñas BP, Noll RR, Pike C, Lein S, Neunuebel MR. Legionella Effector AnkX Disrupts Host Cell Endocytic Recycling in a Phosphocholination-Dependent Manner. Frontiers in Cellular and Infection Microbiology. 2017:7. doi: 10.3389/fcimb.2017.00397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baetz NW, Goldenring JR. Rab11-family interacting proteins define spatially and temporally distinct regions within the dynamic Rab11a-dependent recycling system. Mol Biol Cell. 2013;24:643–658. doi: 10.1091/mbc.E12-09-0659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakowski MA, Braun V, Lam GY, Yeung T, Heo WD, Meyer T, et al. The phosphoinositide phosphatase SopB manipulates membrane surface charge and trafficking of the Salmonella-containing vacuole. Cell Host Microbe. 2010;7:453–462. doi: 10.1016/j.chom.2010.05.011. [DOI] [PubMed] [Google Scholar]
- Bishop BL, Duncan MJ, Song J, Li G, Zaas D, Abraham SN. Cyclic AMP-regulated exocytosis of Escherichia coli from infected bladder epithelial cells. Nat Med. 2007;13:625–630. doi: 10.1038/nm1572. [DOI] [PubMed] [Google Scholar]
- Bruckert WM, Abu Kwaik Y. Complete and ubiquitinated proteome of the Legionella-containing vacuole within human macrophages. J Proteome Res. 2015;14:236–248. doi: 10.1021/pr500765x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brumell JH, Scidmore MA. Manipulation of rab GTPase function by intracellular bacterial pathogens. Microbiol Mol Biol Rev. 2007;71:636–652. doi: 10.1128/MMBR.00023-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campa CC, Hirsch E. Rab11 and phosphoinositides: A synergy of signal transducers in the control of vesicular trafficking. Adv Biol Regul. 2017;63:132–139. doi: 10.1016/j.jbior.2016.09.002. [DOI] [PubMed] [Google Scholar]
- Chaineau M, Ioannou MS, McPherson PS. Rab35: GEFs, GAPs and effectors. Traffic. 2013;14:1109–1117. doi: 10.1111/tra.12096. [DOI] [PubMed] [Google Scholar]
- Chen W, Feng Y, Chen D, Wandinger-Ness A. Rab11 is required for trans-golgi network-to-plasma membrane transport and a preferential target for GDP dissociation inhibitor. Mol Biol Cell. 1998;9:3241–3257. doi: 10.1091/mbc.9.11.3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhury A, Sharma DK, Marks DL, Pagano RE. Elevated endosomal cholesterol levels in Niemann-Pick cells inhibit rab4 and perturb membrane recycling. Mol Biol Cell. 2004;15:4500–4511. doi: 10.1091/mbc.E04-05-0432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortes C, Rzomp KA, Tvinnereim A, Scidmore MA, Wizel B. Chlamydia pneumoniae inclusion membrane protein Cpn0585 interacts with multiple Rab GTPases. Infect Immun. 2007;75:5586–5596. doi: 10.1128/IAI.01020-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cossart P, Roy CR. Manipulation of host membrane machinery by bacterial pathogens. Curr Opin Cell Biol. 2010;22:547–554. doi: 10.1016/j.ceb.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullis DN, Philip B, Baleja JD, Feig LA. Rab11-FIP2, an adaptor protein connecting cellular components involved in internalization and recycling of epidermal growth factor receptors. J Biol Chem. 2002;277:49158–49166. doi: 10.1074/jbc.M206316200. [DOI] [PubMed] [Google Scholar]
- Dikshit N, Bist P, Fenlon SN, Pulloor NK, Chua CE, Scidmore MA, et al. Intracellular Uropathogenic E. coli Exploits Host Rab35 for Iron Acquisition and Survival within Urinary Bladder Cells. PLoS Pathog. 2015;11:e1005083. doi: 10.1371/journal.ppat.1005083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egami Y, Fukuda M, Araki N. Rab35 regulates phagosome formation through recruitment of ACAP2 in macrophages during FcgammaR-mediated phagocytosis. J Cell Sci. 2011;124:3557–3567. doi: 10.1242/jcs.083881. [DOI] [PubMed] [Google Scholar]
- Elwell C, Mirrashidi K, Engel J. Chlamydia cell biology and pathogenesis. Nat Rev Microbiol. 2016;14:385–400. doi: 10.1038/nrmicro.2016.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant BD, Donaldson JG. Pathways and mechanisms of endocytic recycling. Nat Rev Mol Cell Biol. 2009;10:597–608. doi: 10.1038/nrm2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ham H, Sreelatha A, Orth K. Manipulation of host membranes by bacterial effectors. Nat Rev Microbiol. 2011;9:635–646. doi: 10.1038/nrmicro2602. [DOI] [PubMed] [Google Scholar]
- Heo WD, Inoue T, Park WS, Kim ML, Park BO, Wandless TJ, Meyer T. PI(3,4,5)P3 and PI(4,5)P2 lipids target proteins with polybasic clusters to the plasma membrane. Science. 2006;314:1458–1461. doi: 10.1126/science.1134389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilbi H, Weber S, Finsel I. Anchors for effectors: subversion of phosphoinositide lipids by legionella. Front Microbiol. 2011;2:91. doi: 10.3389/fmicb.2011.00091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann C, Finsel I, Otto A, Pfaffinger G, Rothmeier E, Hecker M, et al. Functional analysis of novel Rab GTPases identified in the proteome of purified Legionella-containing vacuoles from macrophages. Cell Microbiol. 2014;16:1034–1052. doi: 10.1111/cmi.12256. [DOI] [PubMed] [Google Scholar]
- Hopkins CR, Trowbridge IS. Internalization and processing of transferrin and the transferrin receptor in human carcinoma A431 cells. J Cell Biol. 1983;97:508–521. doi: 10.1083/jcb.97.2.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horgan CP, Hanscom SR, Jolly RS, Futter CE, McCaffrey MW. Rab11-FIP3 links the Rab11 GTPase and cytoplasmic dynein to mediate transport to the endosomal-recycling compartment. J Cell Sci. 2010;123:181–191. doi: 10.1242/jcs.052670. [DOI] [PubMed] [Google Scholar]
- Hu VH, Holland MJ, Burton MJ. Trachoma: protective and pathogenic ocular immune responses to Chlamydia trachomatis. PLoS Negl Trop Dis. 2013;7:e2020. doi: 10.1371/journal.pntd.0002020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunstad DA, Justice SS. Intracellular lifestyles and immune evasion strategies of uropathogenic Escherichia coli. Annu Rev Microbiol. 2010;64:203–221. doi: 10.1146/annurev.micro.112408.134258. [DOI] [PubMed] [Google Scholar]
- Junutula JR, De Maziere AM, Peden AA, Ervin KE, Advani RJ, van Dijk SM, et al. Rab14 is involved in membrane trafficking between the Golgi complex and endosomes. Mol Biol Cell. 2004;15:2218–2229. doi: 10.1091/mbc.E03-10-0777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi H, Etoh K, Ohbayashi N, Fukuda M. Rab35 promotes the recruitment of Rab8, Rab13 and Rab36 to recycling endosomes through MICAL-L1 during neurite outgrowth. Biol Open. 2014;3:803–814. doi: 10.1242/bio.20148771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyei GB, Vergne I, Chua J, Roberts E, Harris J, Junutula JR, Deretic V. Rab14 is critical for maintenance of Mycobacterium tuberculosis phagosome maturation arrest. EMBO J. 2006;25:5250–5259. doi: 10.1038/sj.emboj.7601407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaRock DL, Chaudhary A, Miller SI. Salmonellae interactions with host processes. Nat Rev Microbiol. 2015;13:191–205. doi: 10.1038/nrmicro3420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson CL, Heinzen RA. High-Content Imaging Reveals Expansion of the Endosomal Compartment during Coxiella burnetii Parasitophorous Vacuole Maturation. Front Cell Infect Microbiol. 2017;7:48. doi: 10.3389/fcimb.2017.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leiva N, Capmany A, Damiani MT. Rab11-family of interacting protein 2 associates with chlamydial inclusions through its Rab-binding domain and promotes bacterial multiplication. Cell Microbiol. 2013;15:114–129. doi: 10.1111/cmi.12035. [DOI] [PubMed] [Google Scholar]
- Linford A, Yoshimura S, Nunes Bastos R, Langemeyer L, Gerondopoulos A, Rigden DJ, Barr FA. Rab14 and its exchange factor FAM116 link endocytic recycling and adherens junction stability in migrating cells. Dev Cell. 2012;22:952–966. doi: 10.1016/j.devcel.2012.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Montero N, Enninga J. Diverted recycling-Shigella subversion of Rabs. Small GTPases. 2016:1–10. doi: 10.1080/21541248.2016.1240494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayle KM, Le AM, Kamei DT. The intracellular trafficking pathway of transferrin. Biochim Biophys Acta. 2012;1820:264–281. doi: 10.1016/j.bbagen.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellouk N, Weiner A, Aulner N, Schmitt C, Elbaum M, Shorte SL, et al. Shigella subverts the host recycling compartment to rupture its vacuole. Cell Host Microbe. 2014;16:517–530. doi: 10.1016/j.chom.2014.09.005. [DOI] [PubMed] [Google Scholar]
- Miao Y, Bist P, Wu J, Zhao Q, Li QJ, Wan Y, Abraham SN. Collaboration between Distinct Rab Small GTPase Trafficking Circuits Mediates Bacterial Clearance from the Bladder Epithelium. Cell Host Microbe. 2017;22:330–342. e334. doi: 10.1016/j.chom.2017.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirrashidi KM, Elwell CA, Verschueren E, Johnson JR, Frando A, Von Dollen J, et al. Global Mapping of the Inc-Human Interactome Reveals that Retromer Restricts Chlamydia Infection. Cell Host Microbe. 2015;18:109–121. doi: 10.1016/j.chom.2015.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molleken K, Hegemann JH. Acquisition of Rab11 and Rab11-Fip2-A novel strategy for Chlamydia pneumoniae early survival. PLoS Pathog. 2017;13:e1006556. doi: 10.1371/journal.ppat.1006556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mounier J, Boncompain G, Senerovic L, Lagache T, Chretien F, Perez F, et al. Shigella effector IpaB-induced cholesterol relocation disrupts the Golgi complex and recycling network to inhibit host cell secretion. Cell Host Microbe. 2012;12:381–389. doi: 10.1016/j.chom.2012.07.010. [DOI] [PubMed] [Google Scholar]
- Mukherjee S, Liu X, Arasaki K, McDonough J, Galan JE, Roy CR. Modulation of Rab GTPase function by a protein phosphocholine transferase. Nature. 2011 doi: 10.1038/nature10335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mysorekar IU, Hultgren SJ. Mechanisms of uropathogenic Escherichia coli persistence and eradication from the urinary tract. Proc Natl Acad Sci U S A. 2006;103:14170–14175. doi: 10.1073/pnas.0602136103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor TJ, Adepoju Y, Boyd D, Isberg RR. Minimization of the Legionella pneumophila genome reveals chromosomal regions involved in host range expansion. Proc Natl Acad Sci U S A. 2011;108:14733–14740. doi: 10.1073/pnas.1111678108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouellette SP, Carabeo RA. A Functional Slow Recycling Pathway of Transferrin is Required for Growth of Chlamydia. Front Microbiol. 2010;1:112. doi: 10.3389/fmicb.2010.00112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patino-Lopez G, Dong X, Ben-Aissa K, Bernot KM, Itoh T, Fukuda M, et al. Rab35 and its GAP EPI64C in T cells regulate receptor recycling and immunological synapse formation. J Biol Chem. 2008;283:18323–18330. doi: 10.1074/jbc.M800056200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira-Leal JB, Seabra MC. Evolution of the Rab family of small GTP-binding proteins. J Mol Biol. 2001;313:889–901. doi: 10.1006/jmbi.2001.5072. [DOI] [PubMed] [Google Scholar]
- Pfeffer SR. Rab GTPase regulation of membrane identity. Curr Opin Cell Biol. 2013;25:414–419. doi: 10.1016/j.ceb.2013.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizarro-Cerda J, Kuhbacher A, Cossart P. Phosphoinositides and host-pathogen interactions. Biochim Biophys Acta. 2014 doi: 10.1016/j.bbalip.2014.09.011. [DOI] [PubMed] [Google Scholar]
- Ray K, Bobard A, Danckaert A, Paz-Haftel I, Clair C, Ehsani S, et al. Tracking the dynamic interplay between bacterial and host factors during pathogen-induced vacuole rupture in real time. Cell Microbiol. 2010;12:545–556. doi: 10.1111/j.1462-5822.2010.01428.x. [DOI] [PubMed] [Google Scholar]
- Rejman Lipinski A, Heymann J, Meissner C, Karlas A, Brinkmann V, Meyer TF, Heuer D. Rab6 and Rab11 regulate Chlamydia trachomatis development and golgin-84-dependent Golgi fragmentation. PLoS Pathog. 2009;5:e1000615. doi: 10.1371/journal.ppat.1000615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren M, Xu G, Zeng J, De Lemos-Chiarandini C, Adesnik M, Sabatini DD. Hydrolysis of GTP on rab11 is required for the direct delivery of transferrin from the pericentriolar recycling compartment to the cell surface but not from sorting endosomes. Proc Natl Acad Sci U S A. 1998;95:6187–6192. doi: 10.1073/pnas.95.11.6187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rzomp KA, Moorhead AR, Scidmore MA. The GTPase Rab4 interacts with Chlamydia trachomatis inclusion membrane protein CT229. Infect Immun. 2006;74:5362–5373. doi: 10.1128/IAI.00539-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sansonetti PJ. Rupture, invasion and inflammatory destruction of the intestinal barrier by Shigella, making sense of prokaryote-eukaryote cross-talks. FEMS Microbiol Rev. 2001;25:3–14. doi: 10.1111/j.1574-6976.2001.tb00569.x. [DOI] [PubMed] [Google Scholar]
- Schmolders J, Manske C, Otto A, Hoffmann C, Steiner B, Welin A, et al. Comparative Proteomics of Purified Pathogen Vacuoles Correlates Intracellular Replication of Legionella pneumophila with the Small GTPase Ras-related protein 1 (Rap1) Mol Cell Proteomics. 2017;16:622–641. doi: 10.1074/mcp.M116.063453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scidmore MA, Fischer ER, Hackstadt T. Restricted fusion of Chlamydia trachomatis vesicles with endocytic compartments during the initial stages of infection. Infect Immun. 2003;71:973–984. doi: 10.1128/IAI.71.2.973-984.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott CC, Botelho RJ, Grinstein S. Phagosome maturation: a few bugs in the system. J Membr Biol. 2003;193:137–152. doi: 10.1007/s00232-002-2008-2. [DOI] [PubMed] [Google Scholar]
- Seabra MC, Mules EH, Hume AN. Rab GTPases, intracellular traffic and disease. Trends Mol Med. 2002;8:23–30. doi: 10.1016/s1471-4914(01)02227-4. [DOI] [PubMed] [Google Scholar]
- Sherwood RK, Roy CR. A Rab-centric perspective of bacterial pathogen-occupied vacuoles. Cell Host Microbe. 2013;14:256–268. doi: 10.1016/j.chom.2013.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sixt BS, Bastidas RJ, Finethy R, Baxter RM, Carpenter VK, Kroemer G, et al. The Chlamydia trachomatis Inclusion Membrane Protein CpoS Counteracts STING-Mediated Cellular Surveillance and Suicide Programs. Cell Host Microbe. 2017;21:113–121. doi: 10.1016/j.chom.2016.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AC, Cirulis JT, Casanova JE, Scidmore MA, Brumell JH. Interaction of the Salmonella-containing vacuole with the endocytic recycling system. J Biol Chem. 2005;280:24634–24641. doi: 10.1074/jbc.M500358200. [DOI] [PubMed] [Google Scholar]
- Smith AC, Heo WD, Braun V, Jiang X, Macrae C, Casanova JE, et al. A network of Rab GTPases controls phagosome maturation and is modulated by Salmonella enterica serovar Typhimurium. J Cell Biol. 2007;176:263–268. doi: 10.1083/jcb.200611056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- So EC, Mattheis C, Tate EW, Frankel G, Schroeder GN. Creating a customized intracellular niche: subversion of host cell signaling by Legionella type IV secretion system effectors. Can J Microbiol. 2015;61:617–635. doi: 10.1139/cjm-2015-0166. [DOI] [PubMed] [Google Scholar]
- Sonnichsen B, De Renzis S, Nielsen E, Rietdorf J, Zerial M. Distinct membrane domains on endosomes in the recycling pathway visualized by multicolor imaging of Rab4, Rab5, and Rab11. J Cell Biol. 2000;149:901–914. doi: 10.1083/jcb.149.4.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spano S, Galan JE. Taking control: Hijacking of Rab GTPases by intracellular bacterial pathogens. Small GTPases. 2017:1–10. doi: 10.1080/21541248.2017.1336192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein MP, Muller MP, Wandinger-Ness A. Bacterial pathogens commandeer Rab GTPases to establish intracellular niches. Traffic. 2012;13:1565–1588. doi: 10.1111/tra.12000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenmark H. Rab GTPases as coordinators of vesicle traffic. Nat Rev Mol Cell Biol. 2009;10:513–525. doi: 10.1038/nrm2728. [DOI] [PubMed] [Google Scholar]
- Swanson MS, Isberg RR. Association of Legionella pneumophila with the macrophage endoplasmic reticulum. Infect Immun. 1995;63:3609–3620. doi: 10.1128/iai.63.9.3609-3620.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullrich O, Reinsch S, Urbe S, Zerial M, Parton RG. Rab11 regulates recycling through the pericentriolar recycling endosome. J Cell Biol. 1996;135:913–924. doi: 10.1083/jcb.135.4.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Sluijs P, Hull M, Webster P, Male P, Goud B, Mellman I. The small GTP-binding protein rab4 controls an early sorting event on the endocytic pathway. Cell. 1992;70:729–740. doi: 10.1016/0092-8674(92)90307-x. [DOI] [PubMed] [Google Scholar]
- Verma K, Datta S. The monomeric GTPase Rab35 regulates phagocytic cup formation and phagosomal maturation in Entamoeba histolytica. J Biol Chem. 2017 doi: 10.1074/jbc.M117.775007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walseng E, Bakke O, Roche PA. Major histocompatibility complex class II-peptide complexes internalize using a clathrin- and dynamin-independent endocytosis pathway. J Biol Chem. 2008;283:14717–14727. doi: 10.1074/jbc.M801070200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber MM, Lam JL, Dooley CA, Noriea NF, Hansen BT, Hoyt FH, et al. Absence of Specific Chlamydia trachomatis Inclusion Membrane Proteins Triggers Premature Inclusion Membrane Lysis and Host Cell Death. Cell Rep. 2017;19:1406–1417. doi: 10.1016/j.celrep.2017.04.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigert R, Yeung AC, Li J, Donaldson JG. Rab22a regulates the recycling of membrane proteins internalized independently of clathrin. Mol Biol Cell. 2004;15:3758–3770. doi: 10.1091/mbc.E04-04-0342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner A, Mellouk N, Lopez-Montero N, Chang YY, Souque C, Schmitt C, Enninga J. Macropinosomes are Key Players in Early Shigella Invasion and Vacuolar Escape in Epithelial Cells. PLoS Pathog. 2016;12:e1005602. doi: 10.1371/journal.ppat.1005602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welz T, Wellbourne-Wood J, Kerkhoff E. Orchestration of cell surface proteins by Rab11. Trends Cell Biol. 2014;24:407–415. doi: 10.1016/j.tcb.2014.02.004. [DOI] [PubMed] [Google Scholar]
- Xie S, Bahl K, Reinecke JB, Hammond GR, Naslavsky N, Caplan S. The endocytic recycling compartment maintains cargo segregation acquired upon exit from the sorting endosome. Mol Biol Cell. 2016;27:108–126. doi: 10.1091/mbc.E15-07-0514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhen Y, Stenmark H. Cellular functions of Rab GTPases at a glance. J Cell Sci. 2015;128:3171–3176. doi: 10.1242/jcs.166074. [DOI] [PubMed] [Google Scholar]