Abstract
Internal drug states/cues can impact drug taking, as pretreatment with a moderate to high alcohol dose (i.e., loading dose) can decrease subsequent alcohol self-administration, alcohol-seeking, and relapse-like drinking. The insular cortex (IC) is implicated in processing information about internal states and findings show that silencing the IC and its projections to the nucleus accumbens core (AcbC) enhance sensitivity to the interoceptive effects of alcohol. Therefore, the goal of the present work was to determine the functional role of IC-AcbC projections in modulating the effects of alcohol pretreatment on operant alcohol self-administration. Long-Evans rats were trained to self-administer a sweetened alcohol solution (15% alcohol (v/v) + 2% sucrose (w/v)) and on test sessions received a pretreatment with an alcohol loading dose. A chemogenetic strategy (i.e., hM4D Designer Receptors Exclusively Activated by Designer Drugs [DREADDs]) was implemented to silence the IC-AcbC projections and test the functional role of the insular-striatal circuitry in regulating self-administration following the alcohol loading doses. Alcohol self-administration decreased following pre-session treatment with alcohol, confirming titration of alcohol drinking following a loading dose of alcohol. Chemogenetic silencing of IC-AcbC projections decreased alcohol self-administration under baseline conditions (i.e., water loading dose) and the reduction in self-administration of an alcohol loading dose, implicating a role for this circuit in the maintenance of alcohol self-administration and suggesting increased sensitivity to the alcohol loading dose. These findings provide evidence for the critical nature of insular-striatal circuitry in ongoing alcohol self-administration, and specifically in relation to interoceptive/internal cues that can impact alcohol drinking.
Keywords: insular cortex, priming, drinking, nucleus accumbens, reinforcement
1. INTRODUCTION
An important consideration when investigating the neurobiological mechanisms underlying alcohol use are the unique interoceptive/subjective (i.e., discriminative stimulus) effects produced by the pharmacological effects of alcohol, as such cues can serve as internal drug states/cues to impact alcohol-taking, seeking, and relapse [1–4]. Numerous preclinical and clinical studies have demonstrated that pretreatment with a low alcohol dose can prime alcohol-related behaviors, by inducing craving, promoting relapse, and leading to additional or increased alcohol consumption [5–11]. Conversely, pretreatment with a moderate to high alcohol dose (i.e., loading dose) can decrease alcohol self-administration, alcohol-seeking, and relapse-like drinking, likely related to processes such as satiation [12–14]. Together, these studies demonstrate the titration of alcohol drinking and seeking behaviors relative to the interoceptive effects produced by alcohol pretreatment. As such, the present work investigates the underlying neurobiological circuitry modulating the effects of alcohol pretreatment (i.e., alcohol loading dose) on alcohol self-administration.
The focus of the present study are the projections from the insular cortex (IC) to the nucleus accumbens core (AcbC; IC-AcbC), two regions previously implicated in modulating the interoceptive effects of alcohol [15] and alcohol self-administration [16]. The IC is proposed to integrate internal and external stimuli into interoceptive states to drive motivated behavior [17, 18], and is implicated in modulating drug self-administration, reinstatement, and seeking behavior [19]. The AcbC is proposed to play a central role in modulating the interoceptive effects of alcohol [20–24] and alcohol self-administration [25, 26]. As such the insular-striatal circuit is implicated in modulating goal-directed behavior in conditions under interoceptive control [15], such as drug self-administration [16].
The goal of the present work was to test the functional role of IC-AcbC projections in modulating the effects of an alcohol loading dose on maintenance of ongoing operant alcohol self-administration. As such, male Long Evans rats were trained to self-administer a sweetened alcohol solution and a chemogenetic strategy (i.e., hM4D Designer Receptors Exclusively Activated by Designer Drugs [DREADDs]) was implemented to silence the IC-AcbC projections and test the functional role of the insular-striatal circuitry in regulating self-administration following alcohol loading doses. Given that chemogenetic silencing of these regions and projections increases sensitivity to alcohol [22] and decreases alcohol self-administration [16], we hypothesized that chemogenetic silencing of these projections to the AcbC, would potentiate sensitivity to an alcohol loading dose in effect resulting in decreased alcohol self-administration. Given the role of interoceptive effects as potent modulators of drug-related behaviors, understanding the circuitry of these internal cues and their functional role in modulating self-administration is important to better understand the neural mechanisms driving drug taking.
2. MATERIALS AND METHODS
2.1. Animals
Male Long Evans rats (n=23; Harlan Sprague–Dawley, Indianapolis, IN, USA) were double housed, in ventilated cages. Following cannulae implantation surgery rats were individually housed. Water and food were available ad libitum in the home cage. The colony room was maintained on a 12-h light/dark cycle, with lights on at 07:00. All experiments were conducted during the light cycle. Animals were under continuous care and monitoring by veterinary staff from the UNC Division of Comparative Medicine. All procedures were carried out in accordance with the NIH Guide to Care and Use of Laboratory Animals and institutional guidelines, and approved by the UNC Institutional Animal Care and Use Committee.
2.2. Viral Vectors
hM4D-DREADDs (AAV8-hSyn-DIO-hM4Di-mCherry; UNC Vector Core, Chapel Hill, NC, USA [lot #4980H]) or mCherry-Control (AAV8-hSyn-DIO-mCherry; UNC Vector Core, Chapel Hill, NC, USA [lot#4981CD]) previously described by [27, 28] were expressed through combination with Cre recombinase (AAV8-CMV-Cre-GFP; Vector Biolabs, Malvern, PA, USA or Addgene, Cambridge, MA, USA) in a ratio of 7:3 (v/v). The combination of viral vectors (DREADD+Cre or mCherry+Cre) were bilaterally infused into the IC (2 μl/side; AP +3.2 mm, ML ±4.0 mm, DV −6.0 mm from skull; [29], as we previously report [15, 16]. We have previously confirmed projections from the IC to AcbC at these coordinates [15]; however, it is important to consider that there is likely anatomical overlap with the orbitofrontal cortex [30]. This injection volume was selected based on a previous rat study that infused hM4D DREADDs into the IC (albeit at a volume of 3 μl/side; [31]), and on previous studies in our lab [15, 16]. This volume was necessary to ensure effective DREADD expression and is likely related to our approach in which two AAV viruses (i.e., DREADD + Cre) are co-administered [32].
2.3. Surgical Procedures
Site-specific microinjections were delivered by a microinfusion pump (Harvard Apparatus, Holliston, MA, USA) through 1.0 μl Hamilton syringes (Hamilton Robotic, Reno, NV, USA) connected to injectors (Plastics One, Roanoke, VA, USA) as described in [15, 16, 22]. For viral vector delivery, rats were anesthetized with isoflurane/oxygen combination and received bilateral microinjection of viral constructs into the IC through 33-gauge injectors at a 0.2 μl/min flow rate across 10-min. The injector was left in place for 10 min following the end of the 10 min infusion, as these are important strategies to further limit the spread of the viral injection [32]. In a separate surgery rats were also implanted with injector 26-gauge guide cannulae (Plastics One, Roanoke, VA, USA) aimed to terminate 2 mm above the AcbC (AP +1.7 mm, ML +1.5 mm, DV −6.8 mm from skull; [29]. Naproxen (10 mg/kg, IP) was administered after the surgeries and 24-hours post-surgery to minimize discomfort. Rats had at least 7 days to recover between surgeries and training was withheld during recovery.
2.4 Behavioral Training Procedures
2.4.1. Self-Administration Training
Rats were trained using the same two lever (i.e., active lever and inactive lever) chambers previously described in [12, 16, 33]. Self-administration sessions (30 min) took place 5 days/week (M−F) with active lever responses on a fixed ratio 2 (FR2) schedule of reinforcement such that every second response on the lever resulted in delivery of alcohol (0.1 ml) into a liquid receptacle. Responses on the inactive lever were recorded, but produced no programmed consequences. Locomotor activity was measured during the self-administration sessions by infrared photobeams that divided the behavioral chamber into 4 parallel zones. A sucrose fading procedure was used in which alcohol was gradually added to a 10% (w/v) sucrose solution. The exact order of exposure was as follows: 10% sucrose (w/v)/2% (v/v) alcohol (10S/2A), 10S/5A, 10S/10A, 5S/10A, 5S/15A, 2S/15A. There were one or two sessions at each concentration. Following sucrose fading, sweetened alcohol (2S/15A) was the reinforcer for the remainder of the study, as in our experience we find that the sweetened alcohol solution results in stable self-administration over time. Based on our previous findings using similar self-administration procedures, we typically observe moderate daily alcohol intake ranging from 0.5 to 1.0 g/kg [12, 16, 33].
2.5. Experimental Procedures
2.5.1. Experiment 1: Examination of the functional role of IC-AcbC projections on alcohol-loading doses prior to alcohol self-administration
Prior to or at the start of self-administration training, rats were infused with hM4D-DREADDs (n=11). After approximately 1.5 months of self-administration training, animals received cannulae implantation surgery (followed by a week of recovery). Testing was only conducted following stable self-administration behavior, (i.e., defined as no change greater than 15% in the total number of responses during the session prior to testing). On the test days, rats received intra-AcbC infusion of CNO (0 or 3 μM/side) 5 min prior to an alcohol loading dose (0, 0.5, 1.0 g/kg, IG). 10-min later rats underwent a self-administration session. CNO microinjections were delivered through 33-gauge injectors that extended 2 mm below the bilateral AcbC cannulae at a volume of 0.5 μl/side across 1 min. The injectors remained in place for an additional 2-min after the infusion to allow for diffusion. For each experiment, a repeated measures design was used such that each rat received each dose in a randomized order, with at least two training sessions between testing days.
2.5.2. Experiment 2: Examination of the functional role of IC-AcbC projections on alcohol-loading doses prior to alcohol self-administration – mCherry Controls
To follow-up the findings from Experiment 1 implicating IC-AcbC projections in changes in alcohol self-administration following pretreatment with the alcohol loading doses, rats in this experiment served as mCherry-Controls. This experiment was important in order to examine potential nonspecific effects of the viral vector and CNO. Rats (n=12) underwent the same self-administration training and surgical procedures as the rats in Experiment 1, but were infused with the mCherry-Control virus in the IC. The same alcohol and CNO testing protocol as in Experiment 1 was used.
2.6. Tissue Preparation for Viral Vector and Cannulae Confirmation
Tissue collection, immunofluorescent and Nissl staining were similar as previously described in [15, 16, 22]. To obtain brain tissue, rats were deeply anesthetized with pentobarbital and perfused with 0.1 M PBS, followed by 4% paraformaldehyde, 4°C; pH=7.4. The brains were removed from the skull and placed in the same fixative solution for approximately 24 h. Next, they were transferred to 30% (w/v) sucrose in a 0.1 m PBS solution, and subsequently sliced on a freezing microtome into 40 μm coronal sections. Tissue was then stored in cryoprotectant (−20 °C) until immunohistochemistry (IHC) processing. The brain regions examined were the IC (AP: +2.8 to +1.9 mm), and the AcbC (AP: +2.3 to +1.3 mm) according to [29]. Free-floating coronal sections (40 μm) were incubated in rabbit anti-DSRed (1:2,500; Clontech, CA) for 24 h at 4 °C. Sections were then incubated at RT in fluorescent conjugated secondary antibody (1:200; goat anti-rabbit 594; Life Technologies, MA). hM4D-mCherry or mCherry-Control expression was confirmed by immunofluorescence (individual expression represented as 20% opacity [Figure 1B and 2B]) with a Nikon 80i Upright microscope (Nikon Instruments, NY). Cannulae placements were confirmed by Nissl staining (injector placements represented by circles in Figure 1C and 2C). Only rats with accurate viral injections and cannulae placements were included in the analyses and data presentation.
Figure 1. Chemogenetic silencing of IC-AcbC projections decreases alcohol self-administration and decreases the effects of a moderate alcohol loading dose.
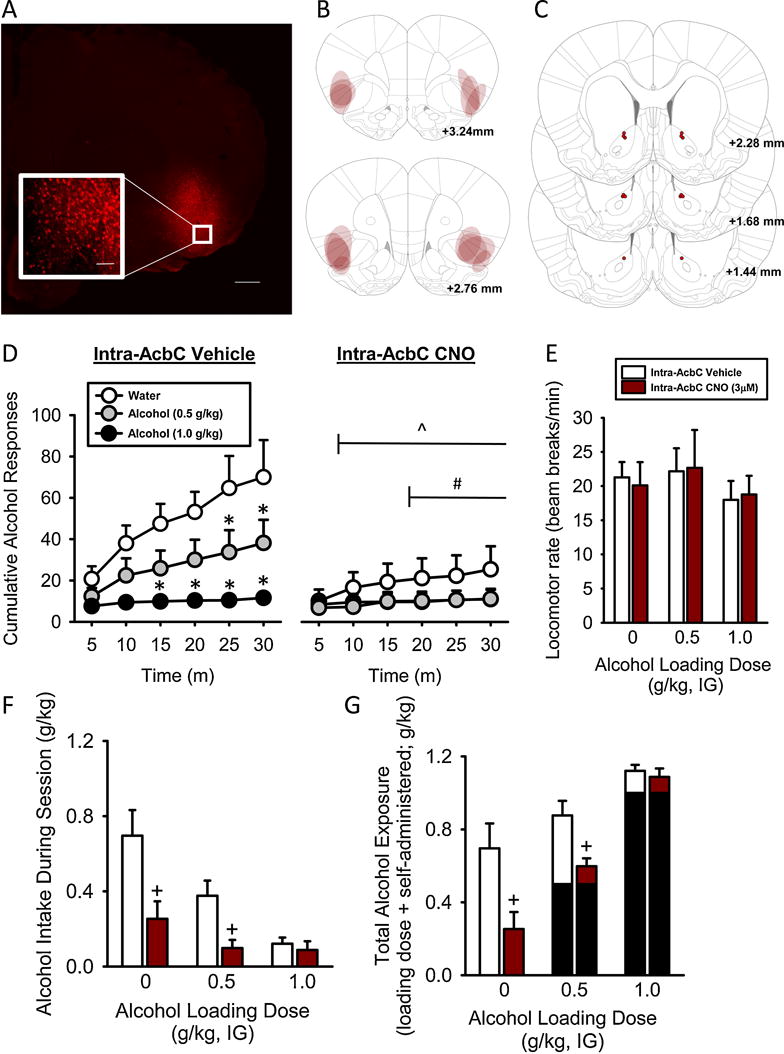
(A) Representative hM4D-mCherry expression in the IC at 2X magnification (500 μm scale bar). Inset shows 20X magnification (1000 μm scale bar). (B) Schematic demonstrating individual intra-IC hM4D-mCherry bilateral expression and (C) AcbC injector tip placements (depicted as circles) from individual rats in the hM4D-DREADD group trained to self-administer alcohol. (D) The pattern of alcohol-reinforced responses across the self-administration session demonstrate decreased responses following an alcohol loading dose. After silencing IC-AcbC projections, by intra-AcbC CNO, there were decreased responses and a potentiated reduction of the 0.5 g/kg loading dose. (E) General locomotor activity was unaffected. (F) Alcohol intake during session (g/kg) show decreased intake after silencing the IC-AcbC and potentiated reduction of the 0.5 loading dose. (G) Total alcohol exposure (g/kg; alcohol intake that was self-administered + experimenter-administered loading dose in black), increased by the alcohol loading dose and intra-AcbC CNO. *Significant difference from water in the intra-AcbC vehicle condition, ˆSignificant difference between intra-AcbC vehicle and CNO condition following water loading dose. # Significant difference between intra-AcbC vehicle and CNO condition following 0.5 g/kg loading dose. +Significant difference from vehicle. Values on graphs represent mean ± S.E.M. (n=7; p≤0.05).
Figure 2. Decreased alcohol self-administration following alcohol loading doses and no effect of CNO in mCherry-Control group.

(A) Representative mCherry expression in the IC 2X magnification (500 μm scale bar). Inset shows 20× magnification (1000 μm scale bar). (B) Schematic demonstrating individual intra-IC mCherry bilateral expression and (C) bilateral AcbC injector tip placements (depicted as circles) from individual rats in the mCherry-Control group trained to self-administer alcohol. (C) The pattern of alcohol-reinforced responses across the self-administration session demonstrate decreased responses following an alcohol loading dose with no effects following intra-AcbC CNO. (D) General locomotor activity was unaffected. (E) Alcohol intake during session (g/kg) decreased and (F) Total alcohol exposure (g/kg; alcohol intake that was self-administered + experimenter-administered loading dose in black), increased following an alcohol loading dose with no effects following intra-AcbC CNO. Values on graphs represent mean ± S.E.M. (n=8; p≤0.05).
2.7. Drugs
Alcohol [95 percent (w/v); Pharmco-AAPER, Shelbyville, KY, USA] was diluted in distilled water to 15 % (v/v) for self-administration sessions. For loading dose injections, alcohol was diluted to 20% (v/v) with distilled water and administered via intragastric gavage (IG) with volume varied by rat weight to achieve the desired dose. For intracranial administration (NIDA Drug Supply Program), CNO was dissolved in aCSF. The CNO dose was chosen based on our previous work [15, 16, 27].
2.8. Data Analysis
Alcohol intake during session (g/kg) was approximated based on body weight and number of reinforcements delivered. Total alcohol (g/kg) was approximated based on self-administered alcohol intake + experimenter-administered loading dose. Three-way repeated measures analysis of variance (ANOVA) with CNO treatment, cumulative time, and loading dose as within-subject factors, was used to analyze cumulative alcohol responses. Two-way RM ANOVA was used to explore significant three-way interactions as detailed in the Results. Two-way repeated measures ANOVA with CNO dose and loading dose as within-subject factor, was used to analyze total session inactive lever responses, alcohol intake, and locomotor rate. Tukey post hoc analyses were used to explore significant main effects and interactions. Data are represented as means ± S.E.M. and significance was declared at p≤0.05.
3. RESULTS
3.1. Experiment 1: Examination of the functional role of IC➔AcbC projections on alcohol loading doses prior to alcohol self-administration
hM4D-mCherry expression and bilateral AcbC injector placements (red circles) in the DREADD group are represented in Figure 1A–C (n=7). Three rats died prior to completion of testing and one rat had inefficient hM4D-DREADD infusions (i.e., no hM4D-mCherry expression), and are not included in analyses or in Figure 1.
The pattern of alcohol lever responses across the self-administration session is shown in Figure 1D. A three-way RM ANOVA was used to analyze the data, however, for ease of presentation, cumulative alcohol lever responding for the intra-AcbC vehicle and intra-AcbC CNO treatment is shown separately. There was a significant main effect of CNO dose [F(1,6)=19.76, p=0.004], alcohol loading dose [F(2,12)=7.60, p=0.007], and time [F(5,30)=13.31, p<0.001]. The CNO dose by alcohol loading dose interaction [F(2,12)=6.12, p=0.01], the CNO dose by time interaction [F(5,30)=12.24, p<0.001], the alcohol loading dose by time interaction [F(10,60)=8.39, p<0.001], and the three-way interaction (CNO dose by alcohol loading dose by time) were significant [F(10,60)=5.77, p<0.001]. To explore this three-way interaction, two-way RM ANOVAs on cumulative alcohol responses for the vehicle and the CNO condition were conducted separately. For the vehicle condition, there was significant main effect of loading dose [F(2,60)=10.79, p<0.02], time [F(5,60)=14.11, p<0.001], and significant interaction [F(10,60)=9.15, p<0.001]. There were reduced alcohol lever responses following the highest alcohol loading dose (1 g/kg) from 10–30 minutes, and following the 0.5 g/kg alcohol loading dose at 25–30 min relative to the water loading dose (p<0.05). These results show that self-administration behavior is sensitive to the alcohol loading doses, consistent with previous work [12, 13]. For the CNO condition, there was a significant main effect of time [F(5,60)=6.52, p<0.001], and a significant interaction [F(10,60)=2.35, p=0.02]; however, post hoc analyses did not show significant differences relative to the water loading dose at any of the time points.
Based on our hypothesis that silencing the IC-AcbC projections would potentiate sensitivity to the alcohol loading dose, the three-way interaction was also explored by comparing the effects of intra-AcbC vehicle vs. CNO for each alcohol loading dose (separate two-way RM ANOVAs). Following the water loading dose, there was a significant CNO-induced reduction in cumulative alcohol lever responses 10–30 min (p<0.05; main effect of CNO dose [F(1,30)=19.89, p=0.004], time [F(5,30)=11.15, p<0.001, interaction [F(5,30)=10.46, p<0.001]), indicating that silencing IC-AcbC projections significantly reduces alcohol self-administration, consistent with our previous findings [16]. Following the 0.5 g/kg alcohol loading dose, there was a significant CNO-induced reduction in cumulative alcohol lever responses at 20–30 min (p<0.05; main effect of CNO dose [F(1,30)=6.83, p=0.04], time [F(5,30)=11.62, p<0.001], interaction [F(5,30)=6.20, p<0.001]). This result indicates that silencing IC-AcbC projections potentiates the reduction in self-administration following the 0.5 g/kg alcohol loading dose. Additionally, because the cumulative responses at the 30 min time point represent the total session alcohol responses, these data show that silencing IC-AcbC projections resulted in a significant decrease in total session alcohol lever responses (consistent with our previous work) and potentiated the reduction in alcohol self-administration following the 0.5 g/kg alcohol loading dose. Following the 1 g/kg alcohol loading dose there was a significant main effect of time [F(5,30)=4.36, p=0.004], but no main effect of CNO or interaction, indicating that silencing the IC-AcbC projections did not further potentiate the reduction in alcohol self-administration, likely due to a floor effect given the low rate of behavior.
Total locomotor activity during the self-administration sessions is illustrated in Figure 1E. There was no effect of the alcohol loading doses or CNO on general locomotor activity or inactive lever responding (Table 1). Total alcohol intake (g/kg) during the self-administration sessions is shown in Figure 1F. The two-way RM ANOVA showed a significant main effect of alcohol loading dose [F(2,12)=10.67, p<0.002], and a significant main effect of CNO [F(1,12)=8.22, p≤0.03], indicating that alcohol intake decreased with loading dose and CNO treatment. There was no significant interaction. An important consideration is the approximate amount of total alcohol exposure which is illustrated in Figure 1G (loading dose [shown in black] + self-administered (amount self-administered during the session). Therefore, although alcohol intake is reduced following pretreatment with the alcohol loading doses, the approximate amount of total alcohol exposure is increased (two-way RM ANOVA: significant main effect of alcohol loading dose [F(2,12)=27.46, p<0.001], significant main effect of CNO [F(1,12)=7.75, p≤0.03]). There was no significant two-way interaction. However, based on our a priori hypothesis that silencing IC-AcbC projections would potentiate sensitivity to the alcohol loading dose, we compared intake and total alcohol exposure at each alcohol loading dose. There was a significant reduction in alcohol intake at the water and the 0.5 g/kg alcohol loading doses (p<0.05), and a significant increase in total alcohol exposure following water and the 0.5 g/kg alcohol loading dose (p<0.05). These data indicate that silencing IC-AcbC projections reduced alcohol intake and potentiated the effects of the 0.5 g/kg alcohol loading dose (p<0.05), corresponding to the reductions in alcohol lever responding (Figure 1D). Together, these data show that 1) alcohol self-administration behavior is sensitive to alcohol pretreatment, 2) activity of IC-AcbC projections regulate, in part, alcohol self-administration, and 3) silencing IC-AcbC projections potentiates the effects of a 0.5 g/kg alcohol loading dose.
Table 1.
Total test session inactive lever responses following CNO-induced IC-AcbC silencing
| Intra-AcbC Treatment: | Alcohol Loading Dose (g/kg, IG) | |||||
|---|---|---|---|---|---|---|
| 0 | 0.5 | 1.0 | ||||
| Vehicle | CNO | Vehicle | CNO | Vehicle | CNO | |
|
|
||||||
| hM4D-DREADDs | 0.4±0.2 | 0.3±0.2 | 0.4±0.3 | 0.1±0.1 | 0.1±0.1 | 0.3±0.3 |
| Control-mCherry | 1.4±0.9 | 0.6±0.6 | 1.1±0.5 | 0.1±0.1 | 0.4±0.3 | 0.4±0.3 |
Data represent mean ± standard error of the mean (p≤0.05)
3.2. Experiment 2: Examination of the functional role of IC-AcbC projections on alcohol-loading doses prior to alcohol self-administration – mCherry Controls
mCherry expression and bilateral AcbC injector placements (blue circles) in the mCherry-Control group are represented in Figure 2A–C (n=8). One rat died prior to completion of testing and 3 rats had inaccurate placements or inefficient mCherry-Control infusions (i.e., no mCherry expression), and are not included in any analyses and not shown in Figure 2.
The pattern of alcohol lever responses across the self-administration session is shown in Figures 2D. The three-way RM ANOVA showed a significant main effect of alcohol loading dose [F(2,14)=5.24, p=0.02] and time [F(5,35)=42.22, p<0.001]. There was no main effect of CNO dose. There was a significant loading dose by time interaction [F(10,70)=2.86, p=0.005], with lower cumulative alcohol lever responses at the 0.5 g/kg loading dose vs. water at 15, 20, and 25 min, and at the 1.0 g/kg loading dose vs. water at 20 – 30 min (p<0.05). Additionally, because the cumulative responses at the 30 min time point represent the total session alcohol responses, these data show that the 1 g/kg alcohol loading dose significantly reduced total session alcohol lever responses relative to the water loading dose. There were no other significant interactions, indicating that self-administration behavior was under the control of the alcohol loading dose and unaffected by CNO, demonstrating no off-target effect by CNO. Additionally, the two-way RM ANOVA showed no effects on locomotor activity (Figure 2E) or inactive lever responding (Table 1). Total alcohol intake (g/kg) during the session is shown in Figure 2F. There was a main effect of alcohol loading dose [F(2,14)=11.86, p≤0.001], indicating decreased alcohol intake following pretreatment with the alcohol loading doses. There was no main effect of CNO treatment and no significant interaction. Similarly, there was a significant main effect of alcohol loading dose on total alcohol exposure (loading dose [shown in black] + self-administered; g/kg; Figure 2G; [F(2,14)=64.66, p≤0.001]), indicating that as total alcohol exposure increased following pretreatment with the alcohol loading doses. There was no main effect of CNO or interaction. These data confirm decreased alcohol self-administration following pretreatment with alcohol loading doses, and importantly show that CNO had no behavioral effect in the mCherry-Control group.
4. DISCUSSION
The present findings demonstrate that alcohol self-administration decreases following pre-session treatment with alcohol, confirming previous findings and demonstrating titration of alcohol drinking following a loading dose of alcohol [12, 13]. Through specific chemogenetic silencing of IC-AcbC projections, we demonstrate decreased alcohol self-administration, implicating a role for insular-striatal circuitry in modulating operant alcohol self-administration, consistent with previous work from our lab [16]. Additionally, we show that alcohol self-administration was reduced following the 0.5 g/kg loading dose and further reduced following silencing of IC-AcbC projections, suggesting possible enhanced sensitivity to the alcohol loading dose.
An important aspect of this study was to assess sensitivity to alcohol pretreatment (i.e., loading dose) on ongoing alcohol self-administration. Previous work from our lab has shown a decrease in alcohol self-administration following an experimenter administered (1 g/kg) alcohol loading dose, but not a 0.3 g/kg alcohol loading dose [12]. Here we confirm and expand our findings by demonstrating a decrease in alcohol self-administration following a 0.5 and 1.0 (g/kg) loading dose of alcohol. Other studies utilizing similar moderate alcohol loading doses also demonstrate decreased alcohol intake under both experimenter-administered and self-administered loading conditions [13, 14, 34], implicating the postingestive interoceptive effects of an alcohol loading dose, regardless of route of administration. Although a study has attributed a loading dose-induced decrease in self-administration to gastric distention [13]; albeit lower alcohol dose and higher volume), others have attributed the effects to reinforcer-specific pharmacological processes, as the effects are specific to alcohol loading doses and not sucrose or water [14, 34]. Additionally, utilizing sham ingestion (open gastric fistula) to minimize gastric absorption of alcohol, [35] demonstrates acquisition of increased alcohol intake in rats, interpreted as an attempt to titrate consumption in the absence of the postingestive pharmacological effects of alcohol. Additionally, devaluation of alcohol reinforcement through the use of alcohol paired with lithium chloride to induce malaise, results in decreased alcohol consumption [36]. Together, these studies demonstrate that postingestive interoceptive effects and internal/interoceptive cues associated with alcohol directly contribute to ongoing alcohol intake.
To this end, given the importance of internal cues in regulating alcohol self-administration behavior, we hypothesized that silencing IC-AcbC projections would increase sensitivity to the interoceptive effects of a loading dose of alcohol. This hypothesis was based on our previous findings showing that silencing IC-AcbC projections fully substitutes for the interoceptive effects of 1 g/kg alcohol and potentiates the effects of alcohol [15]. First, under “control” conditions (i.e., water loading dose), we found that silencing of IC-AcbC projections resulted in approximately a 50% decrease in self-administration, implicating a role of this circuit in driving ongoing alcohol self-administration, consistent with our previous work [16]. Therefore, a possible explanation for the decrease in alcohol self-administration is that silencing these IC-AcbC projections produced interoceptive effects similar to an alcohol loading dose, subsequently resulting in decreased self-administration. Second, following the 0.5 g/kg loading dose, alcohol self-administration was reduced and further reduced following silencing of IC-AcbC projections. However, analysis of cumulative alcohol lever responses across the self-administration session in the CNO group did not show a main effect of the alcohol loading dose (i.e., no difference between 0.5 g/kg and 0 g/kg). Together, this suggests that silencing IC-AcbC projections may have increased sensitivity to the 0.5 g/kg dose, but this effect is not necessarily a potentiation of the loading dose as silencing IC-AcbC projections produced an overall robust suppression of alcohol self-administration. Lastly, silencing the IC-AcbC projections did not change the effects of 1 g/kg loading dose (likely because self-administration was already at a floor level following pretreatment).
Importantly, while decreased self-administration following the alcohol loading doses was also observed in the mCherry-Control group, there was no effect of CNO in this group. This is important as CNO can convert to the anti-psychotic drug clozapine and lead to off target effects [37–40], that can influence alcohol intake [41–43] and produce its own interoceptive effects [44, 45]. Thus, the inclusion of the mCherry-Control group confirms that the changes in behavior in the hM4D-DREADD group were due to chemogenetic silencing of the IC-AcbC circuit. An important consideration is that in the mCherry-Control group alcohol self-administration was slightly lower than the DREADD group. Consequently, it is possible that under conditions with a lower rate of behavior it is more difficult to observe a CNO-induced reduction in self-administration behavior, than under conditions with a higher rate of behavior. However, the fact that there were reductions in alcohol self-administration following the alcohol loading doses lessen this concern.
Together with the previous findings [15, 16], this work implicates IC-AcbC projections as a site of action of alcohol, suggesting that alcohol may silence IC-AcbC activity to induce and potentiate the interoceptive effects of alcohol which in turn regulates alcohol self-administration/drinking. Interestingly, optogenetic silencing of IC to AcbC inputs has been shown to decrease foot-shock resistant alcohol self-administration, but to have no effect on baseline self-administration (pre-shock) [46]. This latter outcome is in contrast to the present findings in which we observe a reduction in alcohol self-administration following chemogenetically silencing IC-AcbC projections. The discrepancy between the findings may be related to differences in neuronal manipulation (optogenetic vs. chemogenetic), and to the different alcohol drinking methods as that study uses intermittent alcohol access prior to operant self-administration training vs. only operant self-administration in the present study [46]. This finding along with [47] demonstrating a role for the IC-AcbC in outcome devaluation and our present findings, implicate a role of the insular-striatal projections in modulating alcohol intake in conditions under strong interoceptive control.
While the present study was focused on IC projections to the AcbC, future studies will be needed to investigate and characterize the AcbC cells recruited by the IC input. Given that dopamine antagonists in the AcbC decrease self-administration [48, 49] and D1 and D2 knockout mice show alterations in alcohol self-administration [50] it is possible that decreased IC input onto dopamine expressing AcbC cells may be mediating the present findings. Additionally, it will be important to further investigate other IC outgoing projections (e.g. IC projections to amygdala and mPFC), to confirm specificity of the findings to the IC-AcbC, as an anatomical control was not included in this study. Additionally, although strategies were taken to localize chemogenetic manipulations to the IC, the potential for the functional contribution of neighboring regions needs to be considered. Lastly, previous work has shown that silencing IC-AcbC projections does not affect ongoing sucrose self-administration [16], however, given that a sweetened alcohol reinforcer was used in this work, it will be interesting for future work to examine the consequences of alcohol pretreatment on self-administration of a non-alcohol reinforcer (e.g., sucrose).
4.1. CONCLUSION
Together these data demonstrate titration of alcohol self-administration relative to alcohol pretreatment, and a functional role for the IC-AcbC circuit in modulating self-administration and sensitivity to the alcohol pretreatment. Given that silencing IC-AcbC projections enhances sensitivity to alcohol [15], the present data suggest that the changes in self-administration may be related to enhanced sensitivity to the interoceptive effects of alcohol. These findings provide evidence for the critical nature of insular-striatal circuitry in modulating alcohol self-administration, and specifically in relation to interoceptive/internal cues that impact alcohol drinking.
Highlights.
Decreased alcohol self-administration following alcohol loading doses.
Silencing IC to AcbC projections decreased alcohol self-administration.
Silencing IC to AcbC projections enhanced sensitivity to alcohol loading dose reductions.
Acknowledgments
The authors would like to thank the NIDA Drug Supply Program for providing the CNO.
FUNDING SOURCES
This work was supported by the National Institute of Health [R01AA019682, F31AA024973, F32AA024674]; the National Science Foundation [DGE-1144081], and by the Bowles Center for Alcohol Studies.
Abbreviations
- AcbC
nucleus accumbens core
- ANOVA
repeated measures analysis of variance
- CNO
clozapine-N-oxide
- DREADDs
Designer Receptors Activated by Designer Drugs
- IC
insular cortex
- IG
intragastric
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declarations of interest: none.
References
- 1.Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35(1):217–38. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.DiChiara TJ, Reinhart PH. Distinct effects of Ca2+ and voltage on the activation and deactivation of cloned Ca(2+)-activated K+ channels. J Physiol. 1995;489(Pt 2):403–18. doi: 10.1113/jphysiol.1995.sp021061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jackson A, Duka T, Stephens DN. Effects of alcohol and lorazepam during extinction of alcohol self-administration in rats. J Psychopharmacol. 2003;17(3):293–9. doi: 10.1177/02698811030173011. [DOI] [PubMed] [Google Scholar]
- 4.Stolerman I. Drugs of abuse: behavioural principles, methods and terms. Trends Pharmacol Sci. 1992;13(5):170–6. doi: 10.1016/0165-6147(92)90059-f. [DOI] [PubMed] [Google Scholar]
- 5.Bigelow GE, Griffiths RR, Liebson IA. Pharmacological influences upon human ethanol self-administration. Adv Exp Med Biol. 1977;85B:523–38. doi: 10.1007/978-1-4615-9038-5_33. [DOI] [PubMed] [Google Scholar]
- 6.de Wit H, Chutuape MA. Increased ethanol choice in social drinkers following ethanol preload. Behav Pharmacol. 1993;4(1):29–36. [PubMed] [Google Scholar]
- 7.Gass JT, Olive MF. Reinstatement of ethanol-seeking behavior following intravenous self-administration in Wistar rats. Alcohol Clin Exp Res. 2007;31(9):1441–5. doi: 10.1111/j.1530-0277.2007.00480.x. [DOI] [PubMed] [Google Scholar]
- 8.Hodgson R, Rankin H, Stockwell T. Alcohol dependence and the priming effect. Behav Res Ther. 1979;17(4):379–87. doi: 10.1016/0005-7967(79)90009-3. [DOI] [PubMed] [Google Scholar]
- 9.Kirk JM, de Wit H. Individual differences in the priming effect of ethanol in social drinkers. J Stud Alcohol. 2000;61(1):64–71. doi: 10.15288/jsa.2000.61.64. [DOI] [PubMed] [Google Scholar]
- 10.Le AD, Quan B, Juzytch W, Fletcher PJ, Joharchi N, Shaham Y. Reinstatement of alcohol-seeking by priming injections of alcohol and exposure to stress in rats. Psychopharmacology (Berl) 1998;135(2):169–74. doi: 10.1007/s002130050498. [DOI] [PubMed] [Google Scholar]
- 11.Vosler PS, Bombace JC, Kosten TA. A discriminative two-lever test of dizocilpine’s ability to reinstate ethanol-seeking behavior. Life Sci. 2001;69(5):591–8. doi: 10.1016/s0024-3205(01)01150-x. [DOI] [PubMed] [Google Scholar]
- 12.Randall PA, Jaramillo AA, Frisbee S, Besheer J. The role of varenicline on alcohol-primed self-administration and seeking behavior in rats. Psychopharmacology (Berl) 2015 doi: 10.1007/s00213-015-3878-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Czachowski CL, Prutzman S, DeLory MJ. Volume and dose effects of experimenter-administered ethanol preloads on ethanol seeking and self-administration. Alcohol. 2006;40(1):35–40. doi: 10.1016/j.alcohol.2006.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Samson HH, Denning C, Czachowski CL. Effects of self-administered ethanol or water preloads on appetitive and consummatory behavior in the alcohol-preferring (P) rat. J Stud Alcohol. 2003;64(1):105–10. doi: 10.15288/jsa.2003.64.105. [DOI] [PubMed] [Google Scholar]
- 15.Jaramillo AA, Agan VE, Makhijani VH, Pedroza S, McElligott ZA, Besheer J. Functional role for suppression of the insular-striatal circuit in modulating interoceptive effects of alcohol. Addict Biol. 2017 doi: 10.1111/adb.12551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jaramillo AA, Randall PA, Stewart S, Fortino B, VanVoorhies K, Besheer J. Functional role for cortical-striatal circuitry in modulating alcohol self-administration. Neuropharmacology. 2017 doi: 10.1016/j.neuropharm.2017.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Paulus MP, Stewart JL. Interoception and drug addiction. Neuropharmacology. 2014;76(Pt B):342–50. doi: 10.1016/j.neuropharm.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Craig AD. How do you feel–now? The anterior insula and human awareness. Nat Rev Neurosci. 2009;10(1):59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- 19.Droutman V, Read SJ, Bechara A. Revisiting the role of the insula in addiction. Trends Cogn Sci. 2015;19(7):414–20. doi: 10.1016/j.tics.2015.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Besheer J, Grondin JJ, Salling MC, Spanos M, Stevenson RA, Hodge CW. Interoceptive effects of alcohol require mGlu5 receptor activity in the nucleus accumbens. J Neurosci. 2009;29(30):9582–91. doi: 10.1523/JNEUROSCI.2366-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hodge CW, Cox AA. The discriminative stimulus effects of ethanol are mediated by NMDA and GABA(A) receptors in specific limbic brain regions. Psychopharmacology (Berl) 1998;139(1–2):95–107. doi: 10.1007/s002130050694. [DOI] [PubMed] [Google Scholar]
- 22.Jaramillo AA, Randall PA, Frisbee S, Besheer J. Modulation of sensitivity to alcohol by cortical and thalamic brain regions. Eur J Neurosci. 2016;44(8):2569–2580. doi: 10.1111/ejn.13374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Besheer J, Cox AA, Hodge CW. Coregulation of ethanol discrimination by the nucleus accumbens and amygdala. Alcohol Clin Exp Res. 2003;27(3):450–6. doi: 10.1097/01.ALC.0000057036.64169.C1. [DOI] [PubMed] [Google Scholar]
- 24.Hodge CW, Nannini MA, Olive MF, Kelley SP, Mehmert KK. Allopregnanolone and pentobarbital infused into the nucleus accumbens substitute for the discriminative stimulus effects of ethanol. Alcohol Clin Exp Res. 2001;25(10):1441–7. doi: 10.1097/00000374-200110000-00006. [DOI] [PubMed] [Google Scholar]
- 25.Chaudhri N, Sahuque LL, Cone JJ, Janak PH. Reinstated ethanol-seeking in rats is modulated by environmental context and requires the nucleus accumbens core. Eur J Neurosci. 2008;28(11):2288–98. doi: 10.1111/j.1460-9568.2008.06517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chaudhri N, Sahuque LL, Schairer WW, Janak PH. Separable roles of the nucleus accumbens core and shell in context- and cue-induced alcohol-seeking. Neuropsychopharmacology. 2010;35(3):783–91. doi: 10.1038/npp.2009.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roth BL. DREADDs for Neuroscientists. Neuron. 2016;89(4):683–94. doi: 10.1016/j.neuron.2016.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krashes MJ, Koda S, Ye C, Rogan SC, Adams AC, Cusher DS, Maratos-Flier E, Roth BL, Lowell BB. Rapid, reversible activation of AgRP neurons drives feeding behavior in mice. J Clin Invest. 2011;121(4):1424–8. doi: 10.1172/JCI46229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 6th. Academic Press/Elsevier; Amsterdam; Boston: 2007. [Google Scholar]
- 30.Schoenbaum G, Roesch MR, Stalnaker TA. Orbitofrontal cortex, decision-making and drug addiction. Trends Neurosci. 2006;29(2):116–24. doi: 10.1016/j.tins.2005.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mizoguchi H, Katahira K, Inutsuka A, Fukumoto K, Nakamura A, Wang T, Nagai T, Sato J, Sawada M, Ohira H, Yamanaka A, Yamada K. Insular neural system controls decision-making in healthy and methamphetamine-treated rats. Proc Natl Acad Sci U S A. 2015;112(29):E3930–9. doi: 10.1073/pnas.1418014112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smith KS, Bucci DJ, Luikart BW, Mahler SV. DREADDS: Use and application in behavioral neuroscience. Behav Neurosci. 2016;130(2):137–55. doi: 10.1037/bne0000135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Randall PA, Stewart RT, Besheer J. Sex differences in alcohol self-administration and relapse-like behavior in Long-Evans rats. Pharmacol Biochem Behav. 2017;156:1–9. doi: 10.1016/j.pbb.2017.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Samson HH, Chappell A, Legg B. Effects of self-administered alcohol or sucrose preloads on subsequent consumption in the rat. J Stud Alcohol. 2002;63(1):107–13. [PubMed] [Google Scholar]
- 35.Rowland NE, Barnett M. Sham ingestion of alcohol in rats. Alcohol. 1992;9(1):75–7. doi: 10.1016/0741-8329(92)90013-z. [DOI] [PubMed] [Google Scholar]
- 36.Samson HH, Cunningham CL, Czachowski CL, Chappell A, Legg B, Shannon E. Devaluation of ethanol reinforcement. Alcohol. 2004;32(3):203–12. doi: 10.1016/j.alcohol.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 37.Chang WH, Lin SK, Lane HY, Wei FC, Hu WH, Lam YW, Jann MW. Reversible metabolism of clozapine and clozapine N-oxide in schizophrenic patients. Prog Neuropsychopharmacol Biol Psychiatry. 1998;22(5):723–39. doi: 10.1016/s0278-5846(98)00035-9. [DOI] [PubMed] [Google Scholar]
- 38.Jann MW, Lam YW, Chang WH. Rapid formation of clozapine in guinea-pigs and man following clozapine-N-oxide administration. Arch Int Pharmacodyn Ther. 1994;328(2):243–50. [PubMed] [Google Scholar]
- 39.MacLaren DA, Browne RW, Shaw JK, Krishnan Radhakrishnan S, Khare P, Espana RA, Clark SD. Clozapine N-Oxide Administration Produces Behavioral Effects in Long-Evans Rats: Implications for Designing DREADD Experiments. eNeuro. 2016;3(5) doi: 10.1523/ENEURO.0219-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gomez JL, Bonaventura J, Lesniak W, Mathews WB, Sysa-Shah P, Rodriguez LA, Ellis RJ, Richie CT, Harvey BK, Dannals RF, Pomper MG, Bonci A, Michaelides M. Chemogenetics revealed: DREADD occupancy and activation via converted clozapine. Science. 2017;357(6350):503–507. doi: 10.1126/science.aan2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chau DT, Gulick D, Xie H, Dawson R, Green AI. Clozapine chronically suppresses alcohol drinking in Syrian golden hamsters. Neuropharmacology. 2010;58(2):351–6. doi: 10.1016/j.neuropharm.2009.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chau DT, Khokhar JY, Dawson R, Ahmed J, Xie H, Green AI. The comparative effects of clozapine versus haloperidol on initiation and maintenance of alcohol drinking in male alcohol-preferring P rat. Alcohol. 2013;47(8):611–8. doi: 10.1016/j.alcohol.2013.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Green AI, Chau DT, Keung WM, Dawson R, Mesholam RI, Schildkraut JJ. Clozapine reduces alcohol drinking in Syrian golden hamsters. Psychiatry Res. 2004;128(1):9–20. doi: 10.1016/j.psychres.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 44.Goudie AJ, Smith JA, Taylor A, Taylor MA, Tricklebank MD. Discriminative stimulus properties of the atypical neuroleptic clozapine in rats: tests with subtype selective receptor ligands. Behav Pharmacol. 1998;9(8):699–710. doi: 10.1097/00008877-199812000-00006. [DOI] [PubMed] [Google Scholar]
- 45.Prus AJ, Baker LE, Meltzer HY. Discriminative stimulus properties of 1.25 and 5.0 mg/kg doses of clozapine in rats: examination of the role of dopamine, serotonin, and muscarinic receptor mechanisms. Pharmacol Biochem Behav. 2004;77(2):199–208. doi: 10.1016/j.pbb.2003.10.010. [DOI] [PubMed] [Google Scholar]
- 46.Seif T, Chang SJ, Simms JA, Gibb SL, Dadgar J, Chen BT, Harvey BK, Ron D, Messing RO, Bonci A, Hopf FW. Cortical activation of accumbens hyperpolarization-active NMDARs mediates aversion-resistant alcohol intake. Nat Neurosci. 2013;16(8):1094–100. doi: 10.1038/nn.3445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Parkes SL, Bradfield LA, Balleine BW. Interaction of insular cortex and ventral striatum mediates the effect of incentive memory on choice between goal-directed actions. J Neurosci. 2015;35(16):6464–71. doi: 10.1523/JNEUROSCI.4153-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rassnick S, Pulvirenti L, Koob GF. Oral ethanol self-administration in rats is reduced by the administration of dopamine and glutamate receptor antagonists into the nucleus accumbens. Psychopharmacology (Berl) 1992;109(1–2):92–8. doi: 10.1007/BF02245485. [DOI] [PubMed] [Google Scholar]
- 49.Hodge CW, Samson HH, Chappelle AM. Alcohol self-administration: further examination of the role of dopamine receptors in the nucleus accumbens. Alcohol Clin Exp Res. 1997;21(6):1083–91. doi: 10.1111/j.1530-0277.1997.tb04257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Crabbe JC, Phillips TJ, Harris RA, Arends MA, Koob GF. Alcohol-related genes: contributions from studies with genetically engineered mice. Addict Biol. 2006;11(3–4):195–269. doi: 10.1111/j.1369-1600.2006.00038.x. [DOI] [PubMed] [Google Scholar]


