Abstract
Superoxide anion radical is generated as a natural byproduct of aerobic metabolism, but is also produced as part of the oxidative burst of the innate immune response design to kill pathogens. In living systems, superoxide is largely managed through superoxide dismutases (SODs), families of metalloenzymes that use Fe, Mn, Ni or Cu cofactors to catalyze the disproportionation of superoxide to oxygen and hydrogen peroxide. Given the bursts of superoxide faced by microbial pathogens, it comes as no surprise that SOD enzymes play important roles in microbial survival and virulence. Interestingly, microbial SOD enzymes not only detoxify host superoxide, but may also participate in signaling pathways that involve reactive oxygen species derived from the microbe itself, particularly in the case of eukaryotic pathogens. In this review, we will discuss the chemistry of superoxide radicals and the role of diverse SOD metalloenzymes in bacterial, fungal, and protozoan pathogens. We will highlight the unique features of microbial SOD enzymes that have evolved to accommodate the harsh lifestyle at the host-pathogen interface. Lastly, we will discuss key non-SOD superoxide scavengers that specific pathogens employ for defense against host superoxide.
Keywords: superoxide dismutase, host-pathogen interface, redox biology, hydrogen peroxide, NADPH oxidase
The origins of superoxide in biology and in infectious disease
Two to three billion years ago, cyanobacteria evolved a remarkable capacity to split H2O, and the O2 gas emitted from this reaction dramatically altered the chemical composition of the planet and the course of evolution 1. With O2 in the atmosphere, organisms evolved methods to harness energy through O2 reduction, providing the fuel to drive evolution of multicellularity 2–3. Yet in biological systems, O2 can also be detrimental through its conversion to reactive oxygen species (ROS), including superoxide (O2·−), hydrogen peroxide (H2O2), and hydroxyl radical (·OH). O2·− is generated from the single electron reduction of O2 and while O2·− cannot generally cross biological membranes, it becomes membrane permeable if protonated to hydroperoxyl (HO2·) and can dismutate spontaneously to generate H2O2 and O2. H2O2 can also cross biological membranes and may be further reduced through metal-catalyzed Fenton chemistry to generate the highly reactive ·OH. These ROS have the capacity to oxidize virtually all organic as well as many inorganic components of the cell, and the effects can range from mild reversible modifications to protein thiols, to irreversible and lethal damage to DNA, enzymes, and cellular membranes. There are many excellent reviews on the topic of ROS in biology 4–10. Here we shall focus on the first product of oxygen reduction, O2·−, its sources and reactivity in biology, and the special circumstances surrounding O2·− at the host-pathogen interface.
All aerobic organisms generate O2·− as a natural byproduct of metabolism, and leakage of electrons from the respiratory chain is a just one origin of intracellular O2·− 11. Aside from these endogenous or metabolic sources, microbial pathogens are faced with exogenous insults of O2·− from the host. As part of the innate immune process, host phagocytic cells produce O2·− deliberately as a means of defense against invading microorganisms and the pathogen must efficiently remove this O2·− before formation of species that are even more reactive. The so-called oxidative burst generated by macrophages and neutrophils involves activation of transmembrane NADPH oxidase (NOX) enzymes that use electrons from NADPH to reduce oxygen to O2·− 12. The O2·− is produced vectorially away from the host cell cytosol in the direction of the invading pathogen either into the extracellular space or the phagolyosomal compartment, an intracellular vesicle resulting from the engulfment of microbes by phagocytes 12–13. There are four NOX isoforms in humans. Each is a multi-subunit complex that is membrane bound and utilizes NADPH, FADH2, and two molecules of heme to shuttle electrons to O2. Catalysis is accomplished by flavocytochrome b558, which is comprised of a 91 kDa glycoprotein (gp91) and a non-glycoslyated 22 kDa subunit (p22). gp91phox−/− mice missing the gp91 subunit of phagocytic NOX (phox) can no longer produce bursts of O2·− and have a greater susceptibility towards infection by bacteria and fungi 14–15.
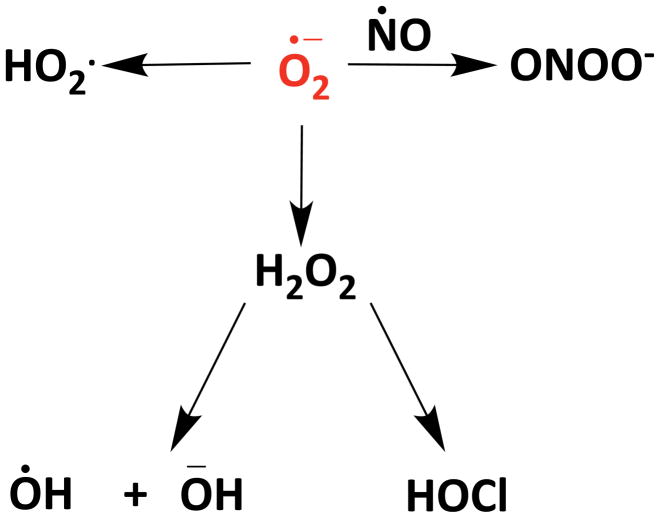
O2·− on its own can be toxic to pathogens. For example, O2·− can disrupt microbial Fe-S clusters, elevate reactive iron levels, cause mismetallation of mononuclear Fe enzymes, or it can be converted to other reactive species to attack the invading microbe 16–20. Phagocytic cells can also produce nitric oxide (NO·) from nitric oxide synthase (NOS) 21, a freely diffusible and labile radical that can readily react with O2·− generated by neighboring NOX enzymes to produce highly reactive peroxynitrite (ONOO-) 22 (Scheme 1). ONOO- in turn can oxidize and damage polypeptide side chains, DNA, and transition metal centers and, if protonated, can cross biological membranes 23–24. Another possible fate of O2·− in the immune response is its reduction to the membrane permeable H2O2, which can either react on its own with microbial macromolecules or be converted to the highly reactive ·OH through Fenton chemistry 25–26. Additionally, in neutrophils, H2O2 can be converted to the membrane permeable hypochlorous acid (HOCl) via myeloperoxidase 27. HOCL can trigger oxidative unfolding of proteins 28 and is genotoxic to microbes29. Overall, through the production of these various reactive oxygen and nitrogen species, O2·− lies at the heart of immune cell chemical warfare (Scheme 1).
Scheme 1.
Superoxide is central to the production of highly reactive oxygen and nitrogen species
ROS management through superoxide dismutase enzymes
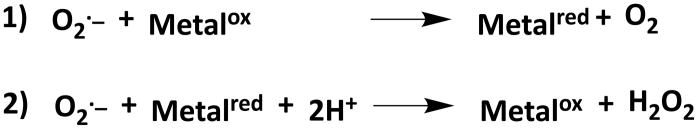
In virtually all living organisms, O2·− is managed through the action of a single enzyme class, the superoxide dismutase (SOD) metalloenzymes that catalyze the two-step disproportionation of O2·− anion to H2O2 and O2 (Scheme 2).
Scheme 2.
Two half reactions for the disproportionation of O2·− by SOD enzymes
Since the initial discovery of a Cu-containing SOD by Fridovich and colleagues in 1969, there has been an explosion in the literature regarding the multiple families of SOD enzymes with varying metal cofactors and their multifaceted roles in ROS biology 30–32. Due to their widespread importance, SOD enzymes have evolved on several occasions to utilize different redox-active metal cofactors. Importantly, when these proteins are substituted with other metals they typically become inactivated.33 This occurs because the protein must be able to specifically tune the redox potential of the active site metal to accommodate its reduction midpoint potential for catalysis34.
The most ancient of these enzymes are thought to have utilized Fe as the metal cofactor based on the abundant bioavailability of ferrous Fe in primordial oceans 35–36. The large Fe-containing family has since expanded to also include Mn-SODs and in some instances “cambialistic” SODs that can function with either Fe or Mn (see details below) 36–40. However, cambialistic SODs tend to have lower enzyme activity for the reasons described above31. Unrelated families of Cu-containing and Ni-containing SODs have also evolved over time 31, 41–42, and this expansion of cofactor options in SOD enzymes is likely linked to geological changes in metal bioavailability that accompanied oxygenation of the biosphere 43–44. Given the virtually ubiquitous existence of O2·− in aerobic organisms, it is of no surprise that SOD enzymes are found in every domain of life and have even been discovered in viral genomes 45. Importantly, since O2·− is not freely diffusible across membranes, each compartment requires its own SOD enzyme. In the case of microbial pathogens, the extracellular SODs are particularly important as they must directly engage the O2·− produced by host NOX. Below we summarize the various ways microbial pathogens have evolved to exploit SOD enzymes for survival and pathogenesis.
Bacterial Pathogens
Bacterial pathogens have been reported with Mn-, Fe-, and Cu-containing SODs, typically referred to as SodA, SodB, and SodC respectively. With few exceptions (listed below) SodA and SodB are restricted to intracellular/cytoplasmic compartments while Cu-containing SodC is exclusively in the extracellular/periplasmic space. In bacteria, Cu is generally excluded from the cytosol and bacterial cuproenzymes, including Cu-containing SodC, are restricted to extracytoplasmic compartments 46–49.
Extracellular Bacterial SODs
The extracellular/periplasmic SODs of bacteria are particularly important in that they constitute the first line of defense against the O2·− attack of host cells. With gram-positive bacteria, the extracellular SODs can be in direct contact with host O2·−. The periplasmic SODs of gram-negative bacteria can also remove host O2·− that crosses the bacterial outer membrane through either porins or a membrane that has been permeabilized by antimicrobial peptides50–51. Additionally in the low pH of the macrophage phagolysosome, protonation of host O2·− will facilitate its crossing of the bacterial outer membrane52. The vast majority of what is known regarding extracellular SODs and bacterial pathogenesis has stemmed from studies on Cu-containing SodC. Bacterial SodC enzymes are closely related to the Cu/Zn-SODs of eukaryotes in terms of overall protein fold, an intramolecular disulfide bond, and an active site that contains both the catalytic Cu and a Zn ion that assists in Cu-catalysis and helps stabilize the protein53. SodC can exist in a soluble form in the periplasmic space of gram-negative bacteria 46, 54 or can be tethered to the cell surface through lipid moieties 55. In studies conducted in gram-negative bacteria, SodC is exported from the bacterial cytosol through the “Sec” pathway for unfolded proteins and arrives in the periplasm in an apo form that is then activated in this compartment through Cu and Zn insertion and disulfide oxidation 56–57 (Figure 1). There are numerous examples where loss of SodC results in decreased pathogenesis of gram-negative bacteria 54, 58–63, firmly establishing SodC as virulence factor.
Figure 1. Bacterial secreted SODs differ in their modes of export and metal acquisition.
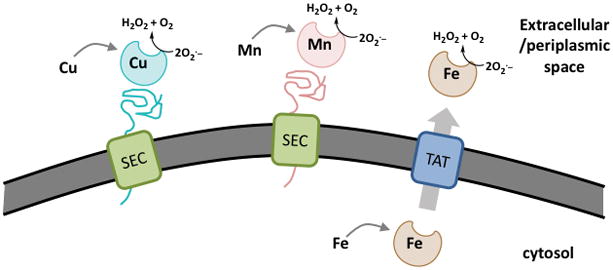
Model showing differential secretion and metal insertion of three classes of bacterial extracytoplasmic SODs. Cu-SODs (blue) and Mn-SODs (pink) are secreted in the unfolded state by Sec translocation and become folded and activated with their respective metal cofactors in the periplasm or extracellular space. Fe-SODs (brown) appear to acquire their metal cofactor in the intracellular/cytosolic space and are translocated in the native and enzymatically active from by the TAT system.
Interestingly, some bacterial pathogens express multiple forms of SodC. For example, the highly virulent E. coli strain O517:H7, the causative agent of hemorrhagic colitis, has three SodC encoding genes 64. One (denoted SodC) is highly similar to the SodC of non-pathogenic E. coli, while the other two (SodC-F1, and SodC-F2) were acquired from lambdoid phage and have biophysical properties that render them more resistant to the harsh environment of the host. SodC-F1 and SodC-F2 proteins are dimeric compared to monomeric SodC, are more resistant to proteases and low pH, and more stably bind their Cu cofactor compared to SodC 64. Additionally, SodC-F1 and SodC-F2 isomers contain a lipid modification that helps tether the proteins to the bacterial membrane unlike the soluble SodC64. These unique SodC-F1 and SodC-F2 isoforms are expressed in anaerobic log phase cultures, while SodC is expressed exclusively in aerobic cultures that are in stationary phase64. SodC-F1 and SodC-F2 are also favorably induced in intracellular environments of an animal host cell 64. A similar set of SodC isoforms has been well studied in Salmonella enterica. This food-born pathogen expresses two Cu/Zn SODs in the periplasm (SodCI and SodCII), only one of which (SodC1) contributes to virulence in vivo. SodCI appears to be the equivalent to the aforementioned SodC-F isoforms and becomes tethered to the membrane by binding to peptidoglycan and is more stable against protease degradation 65–69. SodCI is particularly important for allowing Salmonella enterica survival in host macrophages 70.
A particularly unusual form of SodC is expressed in Mycobacterium tuberculosis, the causative agent of tuberculosis. Interestingly, SodC of M. tuberculosis is Cu-only, as there is no Zn binding site in the enzyme and no requirement for Zn in catalysis 71. To date, Mycobacterium species are the only bacteria known to express Cu-only SODs; all other SodC enzymes are documented as Cu/Zn-SODs 72. In M. tuberculosis, the Cu-only isoform may offer an advantage to the pathogen under conditions of fluctuating Zn pools in the host (see below, section 4.1)
SodC is not the only form of extracellular SOD in bacteria. Certain species express Mn- or Fe-containing SODs that are secreted to help defend against the O2·− burst of the host. In some cases, the Mn or Fe-SOD can work together with SodC to maximize oxidative stress defense as has been shown in M. tuberculosis 73–76 and related Mycobacterium 77–78, as well as in spores of Bacillus anthracis, the causative agent of anthrax 79. In other cases, the bacterium lacks an obvious SodC and the Mn- or Fe-SOD is the sole defense against extracellular O2·−. Examples of this strategy include the secreted Mn-SodA of the Streptococcus pyrogenes, a causative agent of skin and mucosal infections 80–81, and the Fe-SodB of the plant pathogen Agrobacterium tumefaciens 82. Although Mn- and Fe-containing SODs are highly similar enzymes at the primary sequence and tertiary structure level, they appear to use distinct modes for protein secretion and acquisition of their metal cofactors (Figure 1). With Mn-SodA, the polypeptide is exported in an unfolded apo state through the Sec pathway and the Mn cofactor is then acquired in the extracellular/periplasmic location 75, precisely as is done with Cu containing SodC 56–57 (Figure 1). By contrast, the closely related Fe-SodB can acquire its Fe cofactor before exiting the cytosol, and the enzyme is exported through the TAT system in a mature folded state, arriving outside the cell in an enzymatically active form 82–83 (Figure 1). The rationale for distinct modes of secreting SodA and SodC versus SodB may reflect bioavailability of their corresponding metal cofactor. In bacteria, Cu is known to be largely bioavailable in extracellular locations 84, and the same may be true for Mn in those species that secrete SodA, while Fe availability for SodB is prevalent in the intracellular/cytosolic location.
Intracellular SODs and bacterial pathogenesis
Intracellular SODs in pathogens are thought to act mostly on O2·− generated as byproducts of aerobic metabolism. Since O2·− is not generally thought to cross biological membranes, the intracellular SODs are not expected to defend against the oxidative burst of the host immune system. Nevertheless, many cytosolic Fe- and Mn-containing SODs have been shown to be virulence factors for bacterial pathogens 59, 79, 85–95. Why are intracellular SODs important for bacterial pathogenesis when they are not in direct contact with host O2·− ? Even more paradoxical, the intracellular SODs are typically not required for growth under laboratory conditions, but only in the context of the host 85, 94. It is possible that at low pH, as may be encountered in the host macrophage phagolysosome, host derived O2·− can be protonated to HO·2 which can cross biological membranes, thereby providing a substrate for intracellular SODs 52. This is feasible given the pKa of HO2· is 4.88 and it is known that the macrophage phagolysosome can be acidified below that pH. However, studies with Salmonella have argued that phagocytic superoxide specifically reacts with extracytosolic and not intracellular targets67. As a more potent oxidant than superoxide anion, protonated superoxide is likely to oxidize and damage molecules in extracytoplasmic compartments, without crossing the membrane. Alternatively, the intracellular SODs may not react with host derived O2−·. Instead, metabolic constraints experienced in a host setting may alter the endogenous intracellular O2−· generated by the bacteria, placing a greater demand on intracellular SODs, although this has not yet been demonstrated. Finally, it is possible that certain SODs presumed to be intracellular may actually shuttle to the cell surface or are secreted in specific instances.
Obligate anaerobic bacteria that thrive in low oxygen represent an interesting case in that the endogenous metabolic production of O2−· appears to be lower than that of aerobic bacteria96. Nevertheless these microbes can be transiently subjected to oxygenated environments and assaulted by ROS of the innate immune response and have therefore evolved with SOD enzymes as part of an oxidative stress defense, similar to aerobic bacteria97–98. The oxidative stress challenges of anaerobic bacterial pathogens have been reviewed elsewhere99–100.
Clever adaptations to handling O2·− in the harsh metal environment of the host
A bacterial pathogen is completely dependent on its host for acquiring the metal cofactors for its SOD metalloenzymes. This acquisition of metals can be quite challenging. During infection, the host attempts to restrict metal nutrients such as Mn, Cu and Fe from pathogens or attacks the microbes with high doses of metal toxicity. Many excellent reviews have been written on the topic of metal extremes at the host-pathogen interface 101–106. Here we provide some examples of how bacteria and fungi (see ahead) have adapted to these extremes to maintain oxidative stress protection.
As mentioned above, bacterial SodA and SodB are closely related enzymes and most Mn/Fe-SODs can bind either metal. However, mis-incorporation of the wrong metal (ie., Fe in the active site of SodA or vice versa) usually results in an inactive enzyme due to differences in redox tuning33–34. Nevertheless, there exists a subset of bacterial Mn/Fe-dependent SOD enzymes that are capable of maintaining SOD activity with either metal, so called cambialistic SODs 38, 40, 107. Cambialistic SODs have been shown to be important for the virulence of some pathogenic bacteria38. One example involves the cytosolic SodA and SodM of the skin and soft tissue pathogen Staphylococcus aureus. During host invasion, S. aureus is subjected to severe Mn limitation caused by the host’s metal-binding protein calprotectin108. Although Mn-SodA is vulnerable to Mn deprivation, S. aureus maintains intracellular SOD activity through SodM, a cambialistic SOD that switches to utilizing Fe as its cofactor under Mn-limiting conditions 38. Another example includes the secreted Mn-SodA of M. tuberculosis that appears to maintain activity with Fe as its cofactor and is thus also classified as cambialistic 73. The use of cambialistic SODs may be widespread among bacteria faced with host-imposed metal nutrient restriction.
SOD metalloenzymes are not the only means of O2·− defense for pathogenic bacteria as certain species have evolved non-SOD methods for dealing with host O2·−. Neisseria gonorrhoeae, the causative agent of gonorrhea, derives most of its resistance to O2·− stress by accumulating high levels of non-proteinaceous complexes of Mn that can serve as anti-oxidants 109. Many articles have been written on the topic of Mn antioxidants as alternatives to SODs in oxidative stress protection 110–112. Other bacteria have found a way to take advantage of host-imposed Cu toxicity by forming non-SOD antioxidants. As mentioned above, the host can either withhold metals from microbes or attack pathogens with metal toxicity, a classic example being the Cu burst of macrophages 84, 113–115. Yersiniabactin, a siderophore so named for its discovery in Yersinia sp, is a virulence factor in Yersinia pestis, the causative agent of the black plague116–117. It is also secreted from uropathogenic E. coli where it has been shown to bind host copper, and in doing so, generate a SOD-mimic that can protect the bacteria against host-derived O2·− 118–119. Yersiniabactin may be used similarly in other species including Y. pestis. In spite of the extremes in metal bioavailability at the host-pathogen interface, successful pathogens have evolved methods of adapting and even exploiting fluctuations in metals to optimize oxidative stress defenses.
Fungal Pathogens
Fungal pathogens are eukaryotic and thus require SOD enzymes within numerous intracellular organelles to accommodate the many origins of O2·−. Unlike bacteria, where Cu-containing SODs are exclusively extracellular, the major Cu-containing SOD of fungi and other eukaryotes is intracellular. This Cu/Zn-SOD, typically known as SOD1, is largely cytosolic and also present in the intermembrane space of the mitochondria, mimicking the periplasmic location of bacterial Cu-containing SODs46, 120–121. Within the matrix of the mitochondria is a separate Mn-containing SOD2, and the combined action of SOD1 and SOD2 effectively remove O2·− produced during mitochondrial respiration. Although eukaryotic Mn-SODs are typically mitochondrial, there are rare cases of cytosolic Mn-SODs in fungal pathogens as described below. In addition to the aforementioned intracellular SODs, many fungal pathogens also express extracellular SODs. Analogous to the extracellular SODs of bacteria pathogens, these fungal SODs represent the first line of defense against host-derived O2·−.
Extracellular Fungal SODs
Fungi produce an unusual form of extracellular SOD that is Cu-only and does not require Zn, similar to that described for Mycobacteria above 72. These SODs can be either attached to the cell wall through GPI-anchors or secreted122–123. Interestingly, Cu-only SODs are the only type of extracellular SOD in the fungal kingdom and there have been no reports of Cu/Zn-, Mn-, or Fe-containing SODs secreted from any fungal species72. Cu-only extracellular SODs are widespread among fungal pathogens and in fungal-like oomycetes species 72, 124–125. Like Mycobacterium Cu-only SODs, the fungal SODs are missing two histidine residues necessary to coordinate zinc in the active site, and additionally lack protein sequences spanning a structure known as the electrostatic loop (ESL), which in Cu/Zn SODs helps channel the O2·− substrate and stabilize metal binding to the enzyme126,124–125. Because they lack an ESL covering the active site, the Cu site of fungal Cu-only SODs is uniquely solvent exposed. Despite these deviations from Cu/Zn-SODs, fungal Cu-only SODs are highly active enzymes with rates of O2·− disproportionation that reach diffusion limits (1.8 × 109M−1s−1) 124–125.
Another distinguishing feature of fungal Cu-only SODs is their mode of enzyme activation through Cu-insertion. In animals, the extracellular SOD is a Cu/Zn-SOD that acquires its metal cofactors in the secretory pathway and arrives at the cell surface in a fully active state 127–128. By comparison, the fungal Cu-only SODs can be loaded with Cu outside of the cell 125, similar to what is observed in bacteria for the periplasmic Cu/Zn SOD 56 and (Figure 1). During infection, the host can attack pathogens with high concentrations of Cu and the microbe may, in turn, use this host Cu to charge its extracellular SODs to defend against O2·− produced by the host 113–115, 129. Since Cu-only SODs have no requirement for Zn, their activity is not impacted by Zn bioavailability, which can vary greatly within the host during infection 102, 130–132.
Extracellular Cu-only SODs have been shown to be virulence factors for pathogenic fungi. The best studied are those in the opportunistic fungal pathogen, Candida albicans. C. albicans encodes three extracellular Cu-only SOD enzymes (SOD4, SOD5, and SOD6). During host invasion, SOD4 and SOD5 are both abundantly expressed, suggesting they play important roles in eliminating O2·− produced at sites of infection 133–136. SOD5 has been shown to be a virulence factor for C. albicans in a systemic model of infection and is important for fungal survival against the oxidative attack of macrophages and neutrophils 137–139. Why C. albicans encodes three different extracellular Cu-SOD enzymes is currently not understood, but each SOD may function in distinct host niches. In addition to C. albicans, the pulmonary fungal pathogen Histoplasma capsulatum expresses a single Cu-only SOD3 that has been shown to be important for fungal survival against the O2·− attack of macrophages and for virulence in a mouse model of lung infection122, 140. Cu-only SODs are also important for virulence of the pulmonary fungal pathogen Paracocidiodes brasiliensis141 and of the arthropod fungal pathogen Beauveria bassiana142 that can cause keratitis in humans 143–144. It is important to note that not all fungal pathogens have extracellular SOD enzymes, an example being Cryptococcus neoformans, a pulmonary and central nervous system pathogen. Interestingly, the largely cytosolic Cu/Zn-SOD1 of C. neoformans can be enriched in lipid rafts and may shuttle to the cell surface to deal with host-derived ROS 145.
O2·− produced by host cells may not be the only substrate for fungal extracellular SODs. Recent studies have shown that C. albicans produces a burst of extracellular O2·− by a fungal NOX enzyme (FRE8) during morphogenesis and that this O2·− serves as a substrate for extracellular Cu-only SOD5. The diffusible H2O2 product generated by SOD5 then acts as a signal to modulate morphogenesis through a mechanism that is currently not known 146. In fact, SOD5 is specifically induced during the fungal oxidative burst, indicating that SOD5 may have evolved to deal with fungal-derived ROS 138. Overall, the role of Cu-only SODs at the host-pathogen interface is complex: these SODs can both protect the fungus from host-derived O2·− as well as function in fungal signaling pathways involving ROS (Figure 2). In any case, these SODs are important for fungal virulence and based on the biophysical properties that distinguish them from the Cu/Zn SOD of humans, this unique class of SODs represent a possible therapeutic target for treating fungal infections.
Figure 2. Fungal Cu-only SODs can react with O2·− from both the host and the fungus.
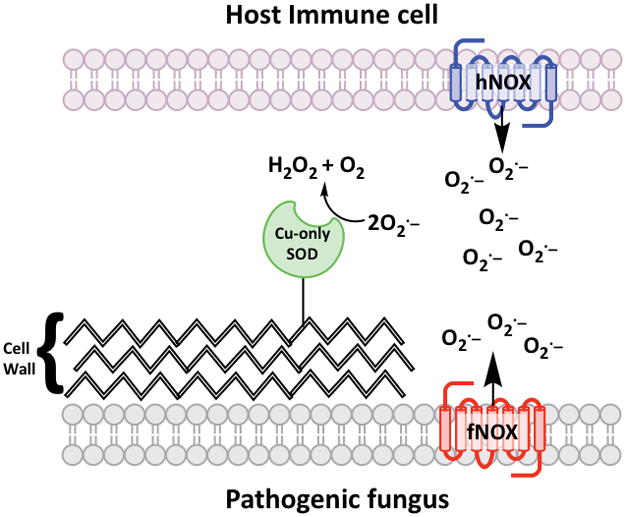
The extracellular Cu-only SODs of fungi (green) are linked to the fungal cell wall through GPI anchors or are secreted. These SODs are known to remove O2·− from the host NOX enzyme (hNOX, blue) of immune cells including macrophages and neutrophils. In addition, studies with C. albicans have shown that Cu-only SODs can remove O2·− from fungal NOX enzymes (fNOX, red) and the concomitant production of H2O2 can promote morphogenesis of the fungus.
Intracellular Fungal SODs
Intracellular SODs of fungi have also been shown to be virulence factors. Deletion of the mitochondrial matrix SOD2 reduces virulence in mouse and insect models of C. neoformans and B. bassiana infection, respectively, likely due to the importance of detoxifying ROS byproducts of respiration 147–149. Cu/Zn-SOD1 has been shown to be a virulence factor in mouse models for both C. albicans and C. neoformans infection and is also important for fungal survival in macrophages 150–152. As described above for bacterial pathogens, the requirement for intracellular SODs during infection may reflect the protonation and subsequent membrane permeability of host-derived O2·−, or changes in fungal production of metabolic O2·− to accommodate life within the host. It is also possible that the intracellular SODs shuttle to the cell surface to remove host derived O2·−, as proposed above for C. neoformans SOD1145. Lastly, as described in more detail below with protozoans, intracellular SODs may participate in cell signaling pathways that involve ROS and drive pathogenesis.
Since SOD enzymes are metalloproteins, microbes must adapt to changes in host metals to maintain SOD activity. As described above, the S. aureus bacterium adapts to low Mn availability by substituting Fe as a cofactor for Mn-SOD enzymes38 and a similar process of metal cofactor swapping has been described for the cytosolic SODs of certain fungal pathogens. Specifically, C. albicans and closely related fungi express a rare form of Mn-SOD (SOD3) that resides in the cytosol, the same location as Cu/Zn-SOD1. C. albicans will switch from expressing Cu/Zn-SOD1 to cytosolic Mn-SOD3 when Cu is low, a condition that occurs in the kidney during fungal infection 153–154. This adaptation allows C. albicans to maintain cytosolic SOD activity even in times of Cu depletion153–154. The arthropod fungal pathogen B. bassiana also contains dual cytosolic Mn and Cu/Zn-containing SODs and, moreover, expresses a pair of mitochondrial matrix SODs that use either Mn or Fe as a cofactor. Mitochondrial Fe-SODs are extremely rare among fungi and B. bassiana may alternate between Fe and Mn SODs to accommodate changes in availability of these metals. All five of the B. bassiana SODs are important for virulence, including the extracellular Cu-only and the four intracellular Cu/Zn-, Mn- and Fe-SODs142, 149, 155. This large repertoire of SOD enzymes with varying metal cofactors may aide in the pathogens ability to infect many different niches in diverse arthropods as well as humans.
Protist Pathogens
Protozoans are unicellular eukaryotic microbes and, as is the case with pathogenic bacteria and fungi, parasitic species have evolved intriguing strategies involving SOD enzymes to promote virulence within the host. Curiously, all the SOD enzymes characterized to date in protist pathogens utilize Fe as their cofactor; there has been no documentation of Mn- or Cu-containing SODs in infectious protozoans 36. There is some evidence for secretion of the Fe-SODs, as was shown with Trypanosoma cruzi, the pathogen responsible for Chagas disease 156. Yet much of what is known on protozoan SODs focuses on the intracellular enzymes, including Fe-SODs of the mitochondria and extra-mitochondrial compartments, eg., cytosol and glycosomes/peroxisomes. These intracellular SODs have been shown to be virulence factors in multiple species157–158.
Studies on the mitochondrial Fe-containing SOD of Leishmania amazonensis, a causative agent of leishmaniasis, have provided insight into how a mitochondrial SOD can be important for virulence. L. amazonensis switches between non-virulent (promastigotes) and virulent (amastigotes) states and morphogenesis into the virulent state is triggered by a signaling pathway involving mitochondrial ROS and mitochondrial Fe-SODA, although the mechanism is not known 157. Removal of just one allele of SODA was sufficient to disrupt this signaling pathway, inhibit protist replication in macrophages and decrease virulence in a mouse model of cutaneous infection 157. These findings underscore the notion that microbial SODs are not just for guarding against the oxidative attack of the host; the SODs also promote pathogenesis through signaling pathways involving ROS.
Trypanosoma cruzi has four predicted isoforms of Fe-SOD, two of which, SODA and SODB, reside in the mitochondria and cytosol respectively. Remarkably, SODB has been shown to be resistant to inactivation by ONOO-. As mentioned above (Scheme 1), macrophage attack of microbes involves formation of diffusible NO that can react with either host- or pathogen-derived O2·− to generate ONOO-. Fe and Mn SODs from bacteria to humans are notoriously susceptible to inactivation by peroxynitrite-mediated nitration of an active site tyrosine residue 159–160 (Figure 3). The unusual resistance of T. cruzi SODB to ONOO- reflects repair of nitrated Tyr35 at the active site via an intramolecular electron transfer event involving nearby Cys83 161 (Figure 3). The closely related Trypanosoma brucei, the causative agent of African sleeping sickness, has also been shown to encode four highly similar Fe-SOD enzymes that may play analogous roles as the isoforms of T. cruzi 162.
Figure 3. Resistance of T. cruzi Fe-SODB to inactivation by peroxynitrite.
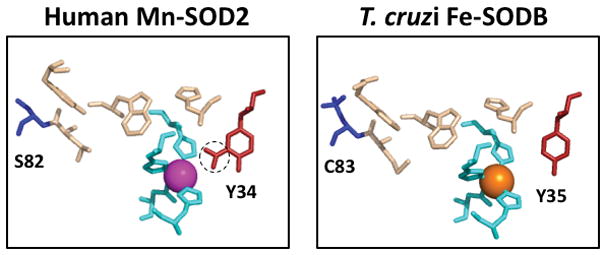
A comparison of the active site of human mitochondrial Mn-SOD2 (left) versus T. cruzi cytosolic Fe-SOD (right) generated using PDB ID: 2ADP and 2GPC, respectively. The Mn and Fe atoms are shown as magenta and orange balls respectively, and the metal coordinating residues are in aqua. A tyrosine near the active site (red) is nitrated in the case of Mn-SOD2 (circle), but not with T. cruzi Fe-SOD where the nitrated tyrosine is repaired through an intramolecular electron transfer involving cysteine 83 (blue). This cysteine is replaced by serine 82 (blue) in human Mn-SOD2.
Given the importance of the Fe-containing SODs of protozoans during infection, they have become targets of interest for drug design. Although the Fe-SODs are closely related to the Mn-SOD2 of human mitochondria, there are key differences that may be targeted. For example, in the substrate channel leading to the active site, a lysine residue caps the active site of protozoan Fe-SODs, but not that of mammalian SOD2 163–164. Strategies exploiting such differences between protist and mammalian SODs are being used in the design of inhibitors against Plasmodium, Leishmania, and Trypanosoma sp.163–165, and one suspected inhibitor of T. cruzi cystosolic Fe-SODB was able to deplete parasite levels in a mouse infection model 166–167. Curiously, some parasitic protozoans lack SODs altogether. Such is the case for the intestinal pathogen and anaerobe Giardia intestinalis, which instead contains a cytosolic superoxide reductase (SOR) 168. SORs catalyze the conversion of O2·− into H2O2 in the presence of a reductant. They have been found in many archaea and bacteria, but G. intestinalis has the first SOR characterized in a eukaryote 31. Given that SORs only cause the reduction of O2·− to H2O2 without production of O2 like SODs, this might be a protective adaptation for an oxygen-sensitive organism such as G. intestinalis 169.
Concluding Remarks
It is clear that SODs are important for pathogens to protect against the oxidative burst generated by the host. These metalloenzymes have evolved multiple times to utilize different metal cofactors and pathogens have selected for SOD enzymes that maintain activity in the face of many different environments that vary widely in metal availability. In certain bacterial and fungal microbes, success of the pathogen relies on its ability to switch cofactor usage in SOD enzymes when the host attempts to starve the microbe of metal nutrients such as Mn, Fe and Cu. In most pathogens, extracellular SODs are the first line of defense against host-derived O2·−, yet intracellular SODs are also virulence factors for bacterial, fungal and protozoan pathogens. These SODs may guard against protonated host O2·− that can cross the cell membrane, or may promote microbial survival by managing endogenous or metabolic ROS. Although microbial SODs clearly thwart the oxidative attack of the host, they can also promote pathogenesis in ways that do not involve oxidative stress protection. In eukaryotic pathogens, including fungi and protists, SODs can participate in signaling pathways that enhance virulence. With the many ways that microbial SODs support virulence, they have become attractive targets for drug development. Strategies currently being explored to inhibit the Fe-SODs of protozoa may be expanded in the future to include other specialized SODs of microbial pathogens, including the Cu-only SODs of fungi that are clearly distinct from their mammalian Cu/Zn-counterpart. Lastly, it is important to remember that SOD enzymes are not the only method for handling O2·−, and non-SOD forms of O2·− removal can be seen in microbial pathogens such as SORs in certain protists and non- proteinaceous SOD-mimics in bacterial pathogens. It is possible that additional mechanisms for dealing with O2·− during infection are yet to be discovered in successful pathogens that lack an obvious extracellular SOD enzyme.
Acknowledgments
We are grateful to Dr. Angelique Besold for helpful discussions. This work was supported by grants from the National Institutes of Health, RO1 GM50016 and RO1 AI119949 (to VCC).
Abbreviations
- ESL
electrostatic loop
- GPI
glycosylphosphatidylinositol
- NOX
NADPH oxidase
- ROS
reactive oxygen species
- Sec
secretory translocon
- SOD
superoxide dismutase
- SOR
superoxide reductase
- TAT
twin-arginine translocation
Footnotes
Conflict of Interest
The authors declare that they have no conflicts of interest with the contents of this article.
References
- 1.Lyons TW, Reinhard CT, Planavsky NJ. The rise of oxygen in Earth’s early ocean and atmosphere. Nature. 2014;506(7488):307–15. doi: 10.1038/nature13068. [DOI] [PubMed] [Google Scholar]
- 2.Holland HD. The oxygenation of the atmosphere and oceans. Philos Trans R Soc Lond B Biol Sci. 2006;361(1470):903–15. doi: 10.1098/rstb.2006.1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schirrmeister BE, de Vos JM, Antonelli A, Bagheri HC. Evolution of multicellularity coincided with increased diversification of cyanobacteria and the Great Oxidation Event. Proc Natl Acad Sci U S A. 2013;110(5):1791–6. doi: 10.1073/pnas.1209927110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39(1):44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 5.Cadet J, Davies KJA. Oxidative DNA damage & repair: An introduction. Free Radic Biol Med. 2017;107:2–12. doi: 10.1016/j.freeradbiomed.2017.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thannickal VJ, Fanburg BL. Reactive oxygen species in cell signaling. Am J Physiol Lung Cell Mol Physiol. 2000;279(6):L1005–28. doi: 10.1152/ajplung.2000.279.6.L1005. [DOI] [PubMed] [Google Scholar]
- 7.Imlay JA. The molecular mechanisms and physiological consequences of oxidative stress: lessons from a model bacterium. Nat Rev Microbiol. 2013;11(7):443–54. doi: 10.1038/nrmicro3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Imlay JA. The mismetallation of enzymes during oxidative stress. J Biol Chem. 2014;289(41):28121–8. doi: 10.1074/jbc.R114.588814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fridovich I. Oxygen toxicity: a radical explanation. J Exp Biol. 1998;201(Pt 8):1203–9. doi: 10.1242/jeb.201.8.1203. [DOI] [PubMed] [Google Scholar]
- 10.Fridovich I. Superoxide radical: an endogenous toxicant. Annu Rev Pharmacol Toxicol. 1983;23:239–57. doi: 10.1146/annurev.pa.23.040183.001323. [DOI] [PubMed] [Google Scholar]
- 11.Imlay JA, Fridovich I. Superoxide production by respiring membranes of Escherichia coli. Free Radic Res Commun. 1991;12–13(Pt 1):59–66. doi: 10.3109/10715769109145768. [DOI] [PubMed] [Google Scholar]
- 12.Panday A, Sahoo MK, Osorio D, Batra S. NADPH oxidases: an overview from structure to innate immunity-associated pathologies. Cell Mol Immunol. 2015;12(1):5–23. doi: 10.1038/cmi.2014.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thomas DC. The phagocyte respiratory burst: Historical perspectives and recent advances. Immunol Lett. 2017;192:88–96. doi: 10.1016/j.imlet.2017.08.016. [DOI] [PubMed] [Google Scholar]
- 14.Shiloh MU, MacMicking JD, Nicholson S, Brause JE, Potter S, Marino M, Fang F, Dinauer M, Nathan C. Phenotype of mice and macrophages deficient in both phagocyte oxidase and inducible nitric oxide synthase. Immunity. 1999;10(1):29–38. doi: 10.1016/S1074-7613(00)80004-7. [DOI] [PubMed] [Google Scholar]
- 15.France MP, Muir D. An outbreak of pulmonary mycosis in respiratory burst-deficient (gp91(phox−/−)) mice with concurrent acidophilic macrophage pneumonia. J Comp Pathol. 2000;123(2–3):190–194. doi: 10.1053/jcpa.2000.0393. [DOI] [PubMed] [Google Scholar]
- 16.Gu M, Imlay JA. Superoxide poisons mononuclear iron enzymes by causing mismetallation. Mol Microbiol. 2013;89(1):123–34. doi: 10.1111/mmi.12263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Keyer K, Imlay JA. Inactivation of dehydratase [4Fe-4S] clusters and disruption of iron homeostasis upon cell exposure to peroxynitrite. J Biol Chem. 1997;272(44):27652–9. doi: 10.1074/jbc.272.44.27652. [DOI] [PubMed] [Google Scholar]
- 18.Srinivasan C, Liba A, Imlay JA, Valentine JS, Gralla EB. Yeast lacking superoxide dismutase(s) show elevated levels of “free iron” as measured by whole cell electron paramagnetic resonance. J Biol Chem. 2000;275(38):29187–92. doi: 10.1074/jbc.M004239200. [DOI] [PubMed] [Google Scholar]
- 19.Keyer K, Imlay JA. Superoxide accelerates DNA damage by elevating free-iron levels. Proc Natl Acad Sci U S A. 1996;93(24):13635–40. doi: 10.1073/pnas.93.24.13635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sobota JM, Gu M, Imlay JA. Intracellular hydrogen peroxide and superoxide poison 3-deoxy-D-arabinoheptulosonate 7-phosphate synthase, the first committed enzyme in the aromatic biosynthetic pathway of Escherichia coli. J Bacteriol. 2014;196(11):1980–91. doi: 10.1128/JB.01573-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kwon NS, Nathan CF, Gilker C, Griffith OW, Matthews DE, Stuehr DJ. L-citrulline production from L-arginine by macrophage nitric oxide synthase. The ureido oxygen derives from dioxygen. J Biol Chem. 1990;265(23):13442–5. [PubMed] [Google Scholar]
- 22.Huie RE, Padmaja S. The reaction of no with superoxide. Free Radic Res Commun. 1993;18(4):195–9. doi: 10.3109/10715769309145868. [DOI] [PubMed] [Google Scholar]
- 23.Ferrer-Sueta G, Radi R. Chemical biology of peroxynitrite: kinetics, diffusion, and radicals. ACS Chem Biol. 2009;4(3):161–77. doi: 10.1021/cb800279q. [DOI] [PubMed] [Google Scholar]
- 24.Bartesaghi S, Radi R. Fundamentals on the biochemistry of peroxynitrite and protein tyrosine nitration. Redox Biol. 2018;14:618–625. doi: 10.1016/j.redox.2017.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Imlay JA, Chin SM, Linn S. Toxic DNA damage by hydrogen peroxide through the Fenton reaction in vivo and in vitro. Science. 1988;240(4852):640–2. doi: 10.1126/science.2834821. [DOI] [PubMed] [Google Scholar]
- 26.Repine JE, Fox RB, Berger EM. Hydrogen peroxide kills Staphylococcus aureus by reacting with staphylococcal iron to form hydroxyl radical. J Biol Chem. 1981;256(14):7094–6. [PubMed] [Google Scholar]
- 27.Harrison JE, Schultz J. Studies on the chlorinating activity of myeloperoxidase. J Biol Chem. 1976;251(5):1371–4. [PubMed] [Google Scholar]
- 28.Winter J, Ilbert M, Graf PC, Ozcelik D, Jakob U. Bleach activates a redox-regulated chaperone by oxidative protein unfolding. Cell. 2008;135(4):691–701. doi: 10.1016/j.cell.2008.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kellner S, DeMott MS, Cheng CP, Russell BS, Cao B, You D, Dedon PC. Oxidation of phosphorothioate DNA modifications leads to lethal genomic instability. Nat Chem Biol. 2017;13(8):888–894. doi: 10.1038/nchembio.2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McCord JM, Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein) J Biol Chem. 1969;244(22):6049–55. [PubMed] [Google Scholar]
- 31.Sheng Y, Abreu IA, Cabelli DE, Maroney MJ, Miller AF, Teixeira M, Valentine JS. Superoxide dismutases and superoxide reductases. Chem Rev. 2014;114(7):3854–918. doi: 10.1021/cr4005296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Case AJ. On the Origin of Superoxide Dismutase: An Evolutionary Perspective of Superoxide-Mediated Redox Signaling. Antioxidants (Basel) 2017;6(4) doi: 10.3390/antiox6040082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miller AF. Superoxide dismutases: active sites that save, but a protein that kills. Curr Opin Chem Biol. 2004;8(2):162–8. doi: 10.1016/j.cbpa.2004.02.011. [DOI] [PubMed] [Google Scholar]
- 34.Vance CK, Miller AF. Novel insights into the basis for Escherichia coli superoxide dismutase’s metal ion specificity from Mn-substituted FeSOD and its very high E(m) Biochemistry. 2001;40(43):13079–87. doi: 10.1021/bi0113317. [DOI] [PubMed] [Google Scholar]
- 35.Isley Ann E, Abbott DH. Plume-related mafic volcanism and the deposition of banded iron formation. J Geophys Res. 1999;104(B7):15461–15477. [Google Scholar]
- 36.Miller AF. Superoxide dismutases: ancient enzymes and new insights. FEBS Lett. 2012;586(5):585–95. doi: 10.1016/j.febslet.2011.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Keele BB, Jr, McCord JM, Fridovich I. Superoxide dismutase from escherichia coli B. A new manganese-containing enzyme. J Biol Chem. 1970;245(22):6176–81. [PubMed] [Google Scholar]
- 38.Garcia YM, Barwinska-Sendra A, Tarrant E, Skaar EP, Waldron KJ, Kehl-Fie TE. A Superoxide Dismutase Capable of Functioning with Iron or Manganese Promotes the Resistance of Staphylococcus aureus to Calprotectin and Nutritional Immunity. PLoS Pathog. 2017;13(1):e1006125. doi: 10.1371/journal.ppat.1006125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yost FJ, Jr, Fridovich I. An iron-containing superoxide dismutase from Escherichia coli. J Biol Chem. 1973;248(14):4905–8. [PubMed] [Google Scholar]
- 40.Martin ME, Byers BR, Olson MO, Salin ML, Arceneaux JE, Tolbert C. A Streptococcus mutans superoxide dismutase that is active with either manganese or iron as a cofactor. J Biol Chem. 1986;261(20):9361–7. [PubMed] [Google Scholar]
- 41.Barondeau DP, Kassmann CJ, Bruns CK, Tainer JA, Getzoff ED. Nickel superoxide dismutase structure and mechanism. Biochemistry. 2004;43(25):8038–47. doi: 10.1021/bi0496081. [DOI] [PubMed] [Google Scholar]
- 42.Youn HD, Kim EJ, Roe JH, Hah YC, Kang SO. A novel nickel-containing superoxide dismutase from Streptomyces spp. Biochem J. 1996;318(Pt 3):889–96. doi: 10.1042/bj3180889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mak A, Saito DMS, François M, Morel M. The bioinorganic chemistry of the ancient ocean: the co-evolution of cyanobacterial metal requirements and biogeochemical cycles at the Archean–Proterozoic boundary? Inorganica Chimica Acta. 2003;356:308–318. [Google Scholar]
- 44.Anbar AD, Knoll AH. Proterozoic ocean chemistry and evolution: a bioinorganic bridge? Science. 2002;297(5584):1137–42. doi: 10.1126/science.1069651. [DOI] [PubMed] [Google Scholar]
- 45.Lartigue A, Burlat B, Coutard B, Chaspoul F, Claverie JM, Abergel C. The megavirus chilensis Cu, Zn-superoxide dismutase: the first viral structure of a typical cellular copper chaperone-independent hyperstable dimeric enzyme. J Virol. 2015;89(1):824–32. doi: 10.1128/JVI.02588-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Benov L, Chang LY, Day B, Fridovich I. Copper, zinc superoxide dismutase in Escherichia coli: periplasmic localization. Arch Biochem Biophys. 1995;319(2):508–11. doi: 10.1006/abbi.1995.1324. [DOI] [PubMed] [Google Scholar]
- 47.Benov LT, Fridovich I. Escherichia coli expresses a copper- and zinc-containing superoxide dismutase. J Biol Chem. 1994;269(41):25310–4. [PubMed] [Google Scholar]
- 48.Arguello JM, Raimunda D, Padilla-Benavides T. Mechanisms of copper homeostasis in bacteria. Front Cell Infect Microbiol. 2013;3:73. doi: 10.3389/fcimb.2013.00073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ladomersky E, Petris MJ. Copper tolerance and virulence in bacteria. Metallomics. 2015;7(6):957–64. doi: 10.1039/c4mt00327f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Slauch JM. How does the oxidative burst of macrophages kill bacteria? Still an open question. Mol Microbiol. 2011;80(3):580–3. doi: 10.1111/j.1365-2958.2011.07612.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Korshunov S, Imlay JA. Detection and quantification of superoxide formed within the periplasm of Escherichia coli. J Bacteriol. 2006;188(17):6326–34. doi: 10.1128/JB.00554-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Korshunov SS, Imlay JA. A potential role for periplasmic superoxide dismutase in blocking the penetration of external superoxide into the cytosol of Gram-negative bacteria. Mol Microbiol. 2002;43(1):95–106. doi: 10.1046/j.1365-2958.2002.02719.x. [DOI] [PubMed] [Google Scholar]
- 53.Pesce A, Capasso C, Battistoni A, Folcarelli S, Rotilio G, Desideri A, Bolognesi M. Unique structural features of the monomeric Cu, Zn superoxide dismutase from Escherichia coli, revealed by X-ray crystallography. J Mol Biol. 1997;274(3):408–20. doi: 10.1006/jmbi.1997.1400. [DOI] [PubMed] [Google Scholar]
- 54.Wilks KE, Dunn KL, Farrant JL, Reddin KM, Gorringe AR, Langford PR, Kroll JS. Periplasmic superoxide dismutase in meningococcal pathogenicity. Infect Immun. 1998;66(1):213–7. doi: 10.1128/iai.66.1.213-217.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.D’Orazio M, Folcarelli S, Mariani F, Colizzi V, Rotilio G, Battistoni A. Lipid modification of the Cu, Zn superoxide dismutase from Mycobacterium tuberculosis. Biochem J. 2001;359(Pt 1):17–22. doi: 10.1042/0264-6021:3590017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Osman D, Patterson CJ, Bailey K, Fisher K, Robinson NJ, Rigby SE, Cavet JS. The copper supply pathway to a Salmonella Cu, Zn-superoxide dismutase (SodCII) involves P(1B)-type ATPase copper efflux and periplasmic CueP. Mol Microbiol. 2013;87(3):466–77. doi: 10.1111/mmi.12107. [DOI] [PubMed] [Google Scholar]
- 57.Sakurai Y, Anzai I, Furukawa Y. A primary role for disulfide formation in the productive folding of prokaryotic Cu, Zn-superoxide dismutase. J Biol Chem. 2014;289(29):20139–49. doi: 10.1074/jbc.M114.567677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gee JM, Valderas MW, Kovach ME, Grippe VK, Robertson GT, Ng WL, Richardson JM, Winkler ME, Roop RM. 2nd, The Brucella abortus Cu, Zn superoxide dismutase is required for optimal resistance to oxidative killing by murine macrophages and wild-type virulence in experimentally infected mice. Infect Immun. 2005;73(5):2873–80. doi: 10.1128/IAI.73.5.2873-2880.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kang IH, Kim JS, Lee JK. The virulence of Vibrio vulnificus is affected by the cellular level of superoxide dismutase activity. J Microbiol Biotechnol. 2007;17(8):1399–402. [PubMed] [Google Scholar]
- 60.Melillo AA, Mahawar M, Sellati TJ, Malik M, Metzger DW, Melendez JA, Bakshi CS. Identification of Francisella tularensis live vaccine strain CuZn superoxide dismutase as critical for resistance to extracellularly generated reactive oxygen species. J Bacteriol. 2009;191(20):6447–56. doi: 10.1128/JB.00534-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pratt AJ, DiDonato M, Shin DS, Cabelli DE, Bruns CK, Belzer CA, Gorringe AR, Langford PR, Tabatabai LB, Kroll JS, Tainer JA, Getzoff ED. Structural, Functional, and Immunogenic Insights on Cu, Zn Superoxide Dismutase Pathogenic Virulence Factors from Neisseria meningitidis and Brucella abortus. J Bacteriol. 2015;197(24):3834–47. doi: 10.1128/JB.00343-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Figueroa-Bossi N, Bossi L. Inducible prophages contribute to Salmonella virulence in mice. Mol Microbiol. 1999;33(1):167–76. doi: 10.1046/j.1365-2958.1999.01461.x. [DOI] [PubMed] [Google Scholar]
- 63.San Mateo LR, Toffer KL, Orndorff PE, Kawula TH. Neutropenia restores virulence to an attenuated Cu, Zn superoxide dismutase-deficient Haemophilus ducreyi strain in the swine model of chancroid. Infect Immun. 1999;67(10):5345–51. doi: 10.1128/iai.67.10.5345-5351.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.D’Orazio M, Scotti R, Nicolini L, Cervoni L, Rotilio G, Battistoni A, Gabbianelli R. Regulatory and structural properties differentiating the chromosomal and the bacteriophage-associated Escherichia coli O157:H7 Cu, Zn superoxide dismutases. BMC Microbiol. 2008;8:166. doi: 10.1186/1471-2180-8-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tidhar A, Rushing MD, Kim B, Slauch JM. Periplasmic superoxide dismutase SodCI of Salmonella binds peptidoglycan to remain tethered within the periplasm. Mol Microbiol. 2015;97(5):832–843. doi: 10.1111/mmi.13067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Krishnakumar R, Kim B, Mollo EA, Imlay JA, Slauch JM. Structural properties of periplasmic SodCI that correlate with virulence in Salmonella enterica serovar Typhimurium. J Bacteriol. 2007;189(12):4343–52. doi: 10.1128/JB.00010-07. JB.00010-07 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Craig M, Slauch JM. Phagocytic superoxide specifically damages an extracytoplasmic target to inhibit or kill Salmonella. PLoS One. 2009;4(3):e4975. doi: 10.1371/journal.pone.0004975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Krishnakumar R, Craig M, Imlay JA, Slauch JM. Differences in enzymatic properties allow SodCI but not SodCII to contribute to virulence in Salmonella enterica serovar Typhimurium strain 14028. J Bacteriol. 2004;186(16):5230–8. doi: 10.1128/JB.186.16.5230-5238.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rushing MD, Slauch JM. Either periplasmic tethering or protease resistance is sufficient to allow a SodC to protect Salmonella enterica serovar Typhimurium from phagocytic superoxide. Mol Microbiol. 2011;82(4):952–63. doi: 10.1111/j.1365-2958.2011.07884.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fenlon LA, Slauch JM. Phagocyte roulette in Salmonella killing. Cell Host Microbe. 2014;15(1):7–8. doi: 10.1016/j.chom.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Spagnolo L, Toro I, D’Orazio M, O’Neill P, Pedersen JZ, Carugo O, Rotilio G, Battistoni A, Djinovic-Carugo K. Unique features of the sodC-encoded superoxide dismutase from Mycobacterium tuberculosis, a fully functional copper-containing enzyme lacking zinc in the active site. J Biol Chem. 2004;279(32):33447–55. doi: 10.1074/jbc.M404699200. [DOI] [PubMed] [Google Scholar]
- 72.Robinett NG, Peterson RL, Culotta VC. Eukaryotic Cu-only superoxide dismutases (SODs): A new class of SOD enzymes and SOD-like protein domains. J Biol Chem. 2017 doi: 10.1074/jbc.TM117.000182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Padilla-Benavides T, Long JE, Raimunda D, Sassetti CM, Arguello JM. A novel P(1B)-type Mn2+-transporting ATPase is required for secreted protein metallation in mycobacteria. J Biol Chem. 2013;288(16):11334–47. doi: 10.1074/jbc.M112.448175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Edwards KM, Cynamon MH, Voladri RK, Hager CC, DeStefano MS, Tham KT, Lakey DL, Bochan MR, Kernodle DS. Iron-cofactored superoxide dismutase inhibits host responses to Mycobacterium tuberculosis. Am J Respir Crit Care Med. 2001;164(12):2213–9. doi: 10.1164/ajrccm.164.12.2106093. [DOI] [PubMed] [Google Scholar]
- 75.Braunstein M, Espinosa BJ, Chan J, Belisle JT, Jacobs WR., Jr SecA2 functions in the secretion of superoxide dismutase A and in the virulence of Mycobacterium tuberculosis. Mol Microbiol. 2003;48(2):453–64. doi: 10.1046/j.1365-2958.2003.03438.x. [DOI] [PubMed] [Google Scholar]
- 76.Liao D, Fan Q, Bao L. The role of superoxide dismutase in the survival of Mycobacterium tuberculosis in macrophages. Jpn J Infect Dis. 2013;66(6):480–8. doi: 10.7883/yoken.66.480. [DOI] [PubMed] [Google Scholar]
- 77.Liu X, Feng Z, Harris NB, Cirillo JD, Bercovier H, Barletta RG. Identification of a secreted superoxide dismutase in Mycobacterium avium ssp. paratuberculosis. FEMS Microbiol Lett. 2001;202(2):233–8. doi: 10.1111/j.1574-6968.2001.tb10809.x. [DOI] [PubMed] [Google Scholar]
- 78.Kang SK, Jung YJ, Kim CH, Song CY. Extracellular and cytosolic iron superoxide dismutase from Mycobacterium bovis BCG. Clin Diagn Lab Immunol. 1998;5(6):784–9. doi: 10.1128/cdli.5.6.784-789.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cybulski RJ, Jr, Sanz P, Alem F, Stibitz S, Bull RL, O’Brien AD. Four superoxide dismutases contribute to Bacillus anthracis virulence and provide spores with redundant protection from oxidative stress. Infect Immun. 2009;77(1):274–85. doi: 10.1128/IAI.00515-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.McMillan DJ, Davies MR, Good MF, Sriprakash KS. Immune response to superoxide dismutase in group A streptococcal infection. FEMS Immunol Med Microbiol. 2004;40(3):249–56. doi: 10.1016/S0928-8244(04)00003-3. [DOI] [PubMed] [Google Scholar]
- 81.Gerlach D, Reichardt W, Vettermann S. Extracellular superoxide dismutase from Streptococcus pyogenes type 12 strain is manganese-dependent. FEMS Microbiol Lett. 1998;160(2):217–24. doi: 10.1111/j.1574-6968.1998.tb12914.x. [DOI] [PubMed] [Google Scholar]
- 82.Saenkham P, Eiamphungporn W, Farrand SK, Vattanaviboon P, Mongkolsuk S. Multiple superoxide dismutases in Agrobacterium tumefaciens: functional analysis, gene regulation, and influence on tumorigenesis. J Bacteriol. 2007;189(24):8807–17. doi: 10.1128/JB.00960-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fournier M, Zhang Y, Wildschut JD, Dolla A, Voordouw JK, Schriemer DC, Voordouw G. Function of oxygen resistance proteins in the anaerobic, sulfate-reducing bacterium Desulfovibrio vulgaris hildenborough. J Bacteriol. 2003;185(1):71–9. doi: 10.1128/JB.185.1.71-79.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fu Y, Chang FM, Giedroc DP. Copper transport and trafficking at the host-bacterial pathogen interface. Acc Chem Res. 2014;47(12):3605–13. doi: 10.1021/ar500300n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Poyart C, Pellegrini E, Gaillot O, Boumaila C, Baptista M, Trieu-Cuot P. Contribution of Mn-cofactored superoxide dismutase (SodA) to the virulence of Streptococcus agalactiae. Infect Immun. 2001;69(8):5098–106. doi: 10.1128/IAI.69.8.5098-5106.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yesilkaya H, Kadioglu A, Gingles N, Alexander JE, Mitchell TJ, Andrew PW. Role of manganese-containing superoxide dismutase in oxidative stress and virulence of Streptococcus pneumoniae. Infect Immun. 2000;68(5):2819–26. doi: 10.1128/iai.68.5.2819-2826.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mahdi LK, Van der Hoek MB, Ebrahimie E, Paton JC, Ogunniyi AD. Characterization of Pneumococcal Genes Involved in Bloodstream Invasion in a Mouse Model. PLoS One. 2015;10(11):e0141816. doi: 10.1371/journal.pone.0141816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tang Y, Zhang X, Wu W, Lu Z, Fang W. Inactivation of the sodA gene of Streptococcus suis type 2 encoding superoxide dismutase leads to reduced virulence to mice. Vet Microbiol. 2012;158(3–4):360–6. doi: 10.1016/j.vetmic.2012.02.028. [DOI] [PubMed] [Google Scholar]
- 89.Martin DW, Baumgartner JE, Gee JM, Anderson ES, Roop RM. 2nd, SodA is a major metabolic antioxidant in Brucella abortus 2308 that plays a significant, but limited, role in the virulence of this strain in the mouse model. Microbiology. 2012;158(Pt 7):1767–74. doi: 10.1099/mic.0.059584-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Roggenkamp A, Bittner T, Leitritz L, Sing A, Heesemann J. Contribution of the Mn-cofactored superoxide dismutase (SodA) to the virulence of Yersinia enterocolitica serotype O8. Infect Immun. 1997;65(11):4705–10. doi: 10.1128/iai.65.11.4705-4710.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bakshi CS, Malik M, Regan K, Melendez JA, Metzger DW, Pavlov VM, Sellati TJ. Superoxide dismutase B gene (sodB)-deficient mutants of Francisella tularensis demonstrate hypersensitivity to oxidative stress and attenuated virulence. J Bacteriol. 2006;188(17):6443–8. doi: 10.1128/JB.00266-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Purdy D, Cawthraw S, Dickinson JH, Newell DG, Park SF. Generation of a superoxide dismutase (SOD)-deficient mutant of Campylobacter coli: evidence for the significance of SOD in Campylobacter survival and colonization. Appl Environ Microbiol. 1999;65(6):2540–6. doi: 10.1128/aem.65.6.2540-2546.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Seyler RW, Jr, Olson JW, Maier RJ. Superoxide dismutase-deficient mutants of Helicobacter pylori are hypersensitive to oxidative stress and defective in host colonization. Infect Immun. 2001;69(6):4034–40. doi: 10.1128/IAI.69.6.4034-4040.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Esteve-Gassent MD, Elliott NL, Seshu J. sodA is essential for virulence of Borrelia burgdorferi in the murine model of Lyme disease. Mol Microbiol. 2009;71(3):594–612. doi: 10.1111/j.1365-2958.2008.06549.x. [DOI] [PubMed] [Google Scholar]
- 95.Karavolos MH, Horsburgh MJ, Ingham E, Foster SJ. Role and regulation of the superoxide dismutases of Staphylococcus aureus. Microbiology. 2003;149(Pt 10):2749–58. doi: 10.1099/mic.0.26353-0. [DOI] [PubMed] [Google Scholar]
- 96.Lu Z, Imlay JA. The Fumarate Reductase of Bacteroides thetaiotaomicron, unlike That of Escherichia coli, Is Configured so that It Does Not Generate Reactive Oxygen Species. MBio. 2017;8(1) doi: 10.1128/mBio.01873-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Carlsson J, Wrethen J, Beckman G. Superoxide dismutase in Bacteroides fragilis and related Bacteroides species. J Clin Microbiol. 1977;6(3):280–4. doi: 10.1128/jcm.6.3.280-284.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ohara N, Kikuchi Y, Shoji M, Naito M, Nakayama K. Superoxide dismutase-encoding gene of the obligate anaerobe Porphyromonas gingivalis is regulated by the redox-sensing transcription activator OxyR. Microbiology. 2006;152(Pt 4):955–66. doi: 10.1099/mic.0.28537-0. [DOI] [PubMed] [Google Scholar]
- 99.Henry LG, Boutrin MC, Aruni W, Robles A, Ximinies A, Fletcher HM. Life in a Diverse Oral Community - Strategies for Oxidative Stress Survival. J Oral Biosci. 2014;56(2):63–71. doi: 10.1016/j.job.2014.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Brioukhanov AL, Netrusov AI. Catalase and superoxide dismutase: distribution, properties, and physiological role in cells of strict anaerobes. Biochemistry (Mosc) 2004;69(9):949–62. doi: 10.1023/b:biry.0000043537.04115.d9. [DOI] [PubMed] [Google Scholar]
- 101.Hood MI, Skaar EP. Nutritional immunity: transition metals at the pathogen-host interface. Nat Rev Microbiol. 2012;10(8):525–37. doi: 10.1038/nrmicro2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kehl-Fie TE, Skaar EP. Nutritional immunity beyond iron: a role for manganese and zinc. Curr Opin Chem Biol. 2010;14(2):218–24. doi: 10.1016/j.cbpa.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Neumann W, Hadley RC, Nolan EM. Transition metals at the host-pathogen interface: how Neisseria exploit human metalloproteins for acquiring iron and zinc. Essays Biochem. 2017;61(2):211–223. doi: 10.1042/EBC20160084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Besold AN, Culbertson EM, Culotta VC. The Yin and Yang of copper during infection. J Biol Inorg Chem. 2016;21(2):137–44. doi: 10.1007/s00775-016-1335-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Palmer LD, Skaar EP. Transition Metals and Virulence in Bacteria. Annu Rev Genet. 2016;50:67–91. doi: 10.1146/annurev-genet-120215-035146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Malavia D, Crawford A, Wilson D. Nutritional Immunity and Fungal Pathogenesis: The Struggle for Micronutrients at the Host-Pathogen Interface. Adv Microb Physiol. 2017;70:85–103. doi: 10.1016/bs.ampbs.2017.01.006. [DOI] [PubMed] [Google Scholar]
- 107.Tu WY, Pohl S, Gray J, Robinson NJ, Harwood CR, Waldron KJ. Cellular iron distribution in Bacillus anthracis. J Bacteriol. 2012;194(5):932–40. doi: 10.1128/JB.06195-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Corbin BD, Seeley EH, Raab A, Feldmann J, Miller MR, Torres VJ, Anderson KL, Dattilo BM, Dunman PM, Gerads R, Caprioli RM, Nacken W, Chazin WJ, Skaar EP. Metal chelation and inhibition of bacterial growth in tissue abscesses. Science. 2008;319(5865):962–5. doi: 10.1126/science.1152449. [DOI] [PubMed] [Google Scholar]
- 109.Tseng HJ, Srikhanta Y, McEwan AG, Jennings MP. Accumulation of manganese in Neisseria gonorrhoeae correlates with resistance to oxidative killing by superoxide anion and is independent of superoxide dismutase activity. Mol Microbiol. 2001;40(5):1175–86. doi: 10.1046/j.1365-2958.2001.02460.x. [DOI] [PubMed] [Google Scholar]
- 110.Archibald FS, Fridovich I. Manganese and defenses against oxygen toxicity in Lactobacillus plantarum. J Bacteriol. 1981;145(1):442–51. doi: 10.1128/jb.145.1.442-451.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Barnese K, Gralla EB, Valentine JS, Cabelli DE. Biologically relevant mechanism for catalytic superoxide removal by simple manganese compounds. Proc Natl Acad Sci U S A. 2012;109(18):6892–7. doi: 10.1073/pnas.1203051109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Culotta VC, Daly MJ. Manganese complexes: diverse metabolic routes to oxidative stress resistance in prokaryotes and yeast. Antioxid Redox Signal. 2013;19(9):933–44. doi: 10.1089/ars.2012.5093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hodgkinson V, Petris MJ. Copper homeostasis at the host-pathogen interface. J Biol Chem. 2012;287(17):13549–55. doi: 10.1074/jbc.R111.316406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.White C, Lee J, Kambe T, Fritsche K, Petris MJ. A role for the ATP7A copper-transporting ATPase in macrophage bactericidal activity. J Biol Chem. 2009;284(49):33949–56. doi: 10.1074/jbc.M109.070201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Achard ME, Stafford SL, Bokil NJ, Chartres J, Bernhardt PV, Schembri MA, Sweet MJ, McEwan AG. Copper redistribution in murine macrophages in response to Salmonella infection. Biochem J. 2012;444(1):51–7. doi: 10.1042/BJ20112180. [DOI] [PubMed] [Google Scholar]
- 116.Bearden SW, Fetherston JD, Perry RD. Genetic organization of the yersiniabactin biosynthetic region and construction of avirulent mutants in Yersinia pestis. Infect Immun. 1997;65(5):1659–68. doi: 10.1128/iai.65.5.1659-1668.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Haag H, Hantke K, Drechsel H, Stojiljkovic I, Jung G, Zahner H. Purification of yersiniabactin: a siderophore and possible virulence factor of Yersinia enterocolitica. J Gen Microbiol. 1993;139(9):2159–65. doi: 10.1099/00221287-139-9-2159. [DOI] [PubMed] [Google Scholar]
- 118.Chaturvedi KS, Hung CS, Giblin DE, Urushidani S, Austin AM, Dinauer MC, Henderson JP. Cupric yersiniabactin is a virulence-associated superoxide dismutase mimic. ACS Chem Biol. 2014;9(2):551–61. doi: 10.1021/cb400658k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Chaturvedi KS, Hung CS, Crowley JR, Stapleton AE, Henderson JP. The siderophore yersiniabactin binds copper to protect pathogens during infection. Nat Chem Biol. 2012;8(8):731–6. doi: 10.1038/nchembio.1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Sturtz LA, Diekert K, Jensen LT, Lill R, Culotta VC. A fraction of yeast Cu, Zn-superoxide dismutase and its metallochaperone, CCS, localize to the intermembrane space of mitochondria. A physiological role for SOD1 in guarding against mitochondrial oxidative damage. J Biol Chem. 2001;276(41):38084–9. doi: 10.1074/jbc.M105296200. [DOI] [PubMed] [Google Scholar]
- 121.Okado-Matsumoto A, Fridovich I. Subcellular distribution of superoxide dismutases (SOD) in rat liver: Cu, Zn-SOD in mitochondria. J Biol Chem. 2001;276(42):38388–93. doi: 10.1074/jbc.M105395200. [DOI] [PubMed] [Google Scholar]
- 122.Youseff BH, Holbrook ED, Smolnycki KA, Rappleye CA. Extracellular superoxide dismutase protects Histoplasma yeast cells from host-derived oxidative stress. PLoS Pathog. 2012;8(5):e1002713. doi: 10.1371/journal.ppat.1002713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Richard ML, Plaine A. Comprehensive analysis of glycosylphosphatidylinositol-anchored proteins in Candida albicans. Eukaryot Cell. 2007;6(2):119–33. doi: 10.1128/EC.00297-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Peterson RL, Galaleldeen A, Villarreal J, Taylor AB, Cabelli DE, Hart PJ, Culotta VC. The Phylogeny and Active Site Design of Eukaryotic Copper-only Superoxide Dismutases. J Biol Chem. 2016;291(40):20911–20923. doi: 10.1074/jbc.M116.748251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Gleason JE, Galaleldeen A, Peterson RL, Taylor AB, Holloway SP, Waninger-Saroni J, Cormack BP, Cabelli DE, Hart PJ, Culotta VC. Candida albicans SOD5 represents the prototype of an unprecedented class of Cu-only superoxide dismutases required for pathogen defense. Proc Natl Acad Sci U S A. 2014;111(16):5866–71. doi: 10.1073/pnas.1400137111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Getzoff ED, Tainer JA, Weiner PK, Kollman PA, Richardson JS, Richardson DC. Electrostatic recognition between superoxide and copper, zinc superoxide dismutase. Nature. 1983;306(5940):287–90. doi: 10.1038/306287a0. [DOI] [PubMed] [Google Scholar]
- 127.Petersen SV, Kristensen T, Petersen JS, Ramsgaard L, Oury TD, Crapo JD, Nielsen NC, Enghild JJ. The folding of human active and inactive extracellular superoxide dismutases is an intracellular event. J Biol Chem. 2008;283(22):15031–6. doi: 10.1074/jbc.M801548200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Qin Z, Itoh S, Jeney V, Ushio-Fukai M, Fukai T. Essential role for the Menkes ATPase in activation of extracellular superoxide dismutase: implication for vascular oxidative stress. FASEB J. 2006;20(2):334–6. doi: 10.1096/fj.05-4564fje. [DOI] [PubMed] [Google Scholar]
- 129.Ding C, Festa RA, Chen YL, Espart A, Palacios O, Espin J, Capdevila M, Atrian S, Heitman J, Thiele DJ. Cryptococcus neoformans copper detoxification machinery is critical for fungal virulence. Cell Host Microbe. 2013;13(3):265–76. doi: 10.1016/j.chom.2013.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Besold AN, Gilston BA, Radin JN, Ramsoomair C, Culbertson EM, Li CX, Cormack BP, Chazin WJ, Kehl-Fie TE, Culotta VC. The role of calprotectin in withholding zinc and copper from Candida albicans. Infect Immun. 2017 doi: 10.1128/IAI.00779-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Subramanian Vignesh K, Deepe GS., Jr Immunological orchestration of zinc homeostasis: The battle between host mechanisms and pathogen defenses. Arch Biochem Biophys. 2016;611:66–78. doi: 10.1016/j.abb.2016.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Capdevila DA, Wang J, Giedroc DP. Bacterial Strategies to Maintain Zinc Metallostasis at the Host-Pathogen Interface. J Biol Chem. 2016;291(40):20858–20868. doi: 10.1074/jbc.R116.742023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Amorim-Vaz S, du Tran VT, Pradervand S, Pagni M, Coste AT, Sanglard D. RNA Enrichment Method for Quantitative Transcriptional Analysis of Pathogens In Vivo Applied to the Fungus Candida albicans. MBio. 2015;6(5):e00942–15. doi: 10.1128/mBio.00942-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Zakikhany K, Naglik JR, Schmidt-Westhausen A, Holland G, Schaller M, Hube B. In vivo transcript profiling of Candida albicans identifies a gene essential for interepithelial dissemination. Cell Microbiol. 2007;9(12):2938–54. doi: 10.1111/j.1462-5822.2007.01009.x. [DOI] [PubMed] [Google Scholar]
- 135.Cheng S, Clancy CJ, Xu W, Schneider F, Hao B, Mitchell AP, Nguyen MH. Profiling of Candida albicans gene expression during intra-abdominal candidiasis identifies biologic processes involved in pathogenesis. J Infect Dis. 2013;208(9):1529–37. doi: 10.1093/infdis/jit335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Fanning S, Xu W, Solis N, Woolford CA, Filler SG, Mitchell AP. Divergent targets of Candida albicans biofilm regulator Bcr1 in vitro and in vivo. Eukaryot Cell. 2012;11(7):896–904. doi: 10.1128/EC.00103-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Frohner IE, Bourgeois C, Yatsyk K, Majer O, Kuchler K. Candida albicans cell surface superoxide dismutases degrade host-derived reactive oxygen species to escape innate immune surveillance. Mol Microbiol. 2009;71(1):240–52. doi: 10.1111/j.1365-2958.2008.06528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Martchenko M, Alarco AM, Harcus D, Whiteway M. Superoxide dismutases in Candida albicans: transcriptional regulation and functional characterization of the hyphal-induced SOD5 gene. Mol Biol Cell. 2004;15(2):456–67. doi: 10.1091/mbc.E03-03-0179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Fradin C, De Groot P, MacCallum D, Schaller M, Klis F, Odds FC, Hube B. Granulocytes govern the transcriptional response, morphology and proliferation of Candida albicans in human blood. Mol Microbiol. 2005;56(2):397–415. doi: 10.1111/j.1365-2958.2005.04557.x. [DOI] [PubMed] [Google Scholar]
- 140.Garfoot AL, Rappleye CA. Histoplasma capsulatum surmounts obstacles to intracellular pathogenesis. FEBS J. 2016;283(4):619–33. doi: 10.1111/febs.13389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Tamayo D, Munoz JF, Lopez A, Uran M, Herrera J, Borges CL, Restrepo A, Soares CM, Taborda CP, Almeida AJ, McEwen JG, Hernandez O. Identification and Analysis of the Role of Superoxide Dismutases Isoforms in the Pathogenesis of Paracoccidioides spp. PLoS Negl Trop Dis. 2016;10(3):e0004481. doi: 10.1371/journal.pntd.0004481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Li F, Shi HQ, Ying SH, Feng MG. Distinct contributions of one Fe- and two Cu/Zn-cofactored superoxide dismutases to antioxidation, UV tolerance and virulence of Beauveria bassiana. Fungal Genet Biol. 2015;81:160–71. doi: 10.1016/j.fgb.2014.09.006. [DOI] [PubMed] [Google Scholar]
- 143.Lara Oya A, Medialdea Hurtado ME, Rojo Martin MD, Aguilera Perez A, Alastruey-Izquierdo A, Miranda Casas C, Rubio Prats M, Medialdea Marcos S, Navarro Mari JM. Fungal Keratitis Due to Beauveria bassiana in a Contact Lenses Wearer and Review of Published Reports. Mycopathologia. 2016;181(9–10):745–52. doi: 10.1007/s11046-016-0027-2. [DOI] [PubMed] [Google Scholar]
- 144.Oh JY, Lee MJ, Wee WR, Heo JW. A case of necrotizing sclerokeratitis and endophthalmitis caused by Beauveria bassiana. Jpn J Ophthalmol. 2009;53(5):551–3. doi: 10.1007/s10384-009-0715-2. [DOI] [PubMed] [Google Scholar]
- 145.Siafakas AR, Wright LC, Sorrell TC, Djordjevic JT. Lipid rafts in Cryptococcus neoformans concentrate the virulence determinants phospholipase B1 and Cu/Zn superoxide dismutase. Eukaryot Cell. 2006;5(3):488–98. doi: 10.1128/EC.5.3.488-498.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Rossi DCP, Gleason JE, Sanchez H, Schatzman SS, Culbertson EM, Johnson CJ, McNees CA, Coelho C, Nett JE, Andes DR, Cormack BP, Culotta VC. Candida albicans FRE8 encodes a member of the NADPH oxidase family that produces a burst of ROS during fungal morphogenesis. PLoS Pathog. 2017;13(12):e1006763. doi: 10.1371/journal.ppat.1006763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Narasipura SD, Chaturvedi V, Chaturvedi S. Characterization of Cryptococcus neoformans variety gattii SOD2 reveals distinct roles of the two superoxide dismutases in fungal biology and virulence. Mol Microbiol. 2005;55(6):1782–800. doi: 10.1111/j.1365-2958.2005.04503.x. [DOI] [PubMed] [Google Scholar]
- 148.Giles SS, Batinic-Haberle I, Perfect JR, Cox GM. Cryptococcus neoformans mitochondrial superoxide dismutase: an essential link between antioxidant function and high-temperature growth. Eukaryot Cell. 2005;4(1):46–54. doi: 10.1128/EC.4.1.46-54.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Xie XQ, Li F, Ying SH, Feng MG. Additive contributions of two manganese-cored superoxide dismutases (MnSODs) to antioxidation, UV tolerance and virulence of Beauveria bassiana. PLoS One. 2012;7(1):e30298. doi: 10.1371/journal.pone.0030298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Cox GM, Harrison TS, McDade HC, Taborda CP, Heinrich G, Casadevall A, Perfect JR. Superoxide dismutase influences the virulence of Cryptococcus neoformans by affecting growth within macrophages. Infect Immun. 2003;71(1):173–80. doi: 10.1128/IAI.71.1.173-180.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Hwang CS, Rhie GE, Oh JH, Huh WK, Yim HS, Kang SO. Copper- and zinc-containing superoxide dismutase (Cu/ZnSOD) is required for the protection of Candida albicans against oxidative stresses and the expression of its full virulence. Microbiology. 2002;148(Pt 11):3705–13. doi: 10.1099/00221287-148-11-3705. [DOI] [PubMed] [Google Scholar]
- 152.Narasipura SD, Ault JG, Behr MJ, Chaturvedi V, Chaturvedi S. Characterization of Cu, Zn superoxide dismutase (SOD1) gene knock-out mutant of Cryptococcus neoformans var. gattii: role in biology and virulence. Mol Microbiol. 2003;47(6):1681–94. doi: 10.1046/j.1365-2958.2003.03393.x. [DOI] [PubMed] [Google Scholar]
- 153.Li CX, Gleason JE, Zhang SX, Bruno VM, Cormack BP, Culotta VC. Candida albicans adapts to host copper during infection by swapping metal cofactors for superoxide dismutase. Proc Natl Acad Sci U S A. 2015;112(38):E5336–42. doi: 10.1073/pnas.1513447112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Broxton CN, Culotta VC. An Adaptation to Low Copper in Candida albicans Involving SOD Enzymes and the Alternative Oxidase. PLoS One. 2016;11(12):e0168400. doi: 10.1371/journal.pone.0168400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Xie XQ, Wang J, Huang BF, Ying SH, Feng MG. A new manganese superoxide dismutase identified from Beauveria bassiana enhances virulence and stress tolerance when overexpressed in the fungal pathogen. Appl Microbiol Biotechnol. 2010;86(5):1543–53. doi: 10.1007/s00253-010-2437-2. [DOI] [PubMed] [Google Scholar]
- 156.Villagran ME, Marin C, Rodriguez-Gonzalez I, De Diego JA, Sanchez-Moreno M. Use of an iron superoxide dismutase excreted by Trypanosoma cruzi in the diagnosis of Chagas disease: seroprevalence in rural zones of the state of Queretaro, Mexico. Am J Trop Med Hyg. 2005;73(3):510–6. [PubMed] [Google Scholar]
- 157.Mittra B, Laranjeira-Silva MF, Miguel DC, Perrone Bezerra de Menezes J, Andrews NW. The iron-dependent mitochondrial superoxide dismutase SODA promotes Leishmania virulence. J Biol Chem. 2017;292(29):12324–12338. doi: 10.1074/jbc.M116.772624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Ghosh S, Goswami S, Adhya S. Role of superoxide dismutase in survival of Leishmania within the macrophage. Biochemical Journal. 2003;369:447–452. doi: 10.1042/BJ20021684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Demicheli V, Moreno DM, Jara GE, Lima A, Carballal S, Rios N, Batthyany C, Ferrer-Sueta G, Quijano C, Estrin DA, Marti MA, Radi R. Mechanism of the Reaction of Human Manganese Superoxide Dismutase with Peroxynitrite: Nitration of Critical Tyrosine 34. Biochemistry. 2016;55(24):3403–17. doi: 10.1021/acs.biochem.6b00045. [DOI] [PubMed] [Google Scholar]
- 160.Surmeli NB, Litterman NK, Miller AF, Groves JT. Peroxynitrite mediates active site tyrosine nitration in manganese superoxide dismutase. Evidence of a role for the carbonate radical anion. J Am Chem Soc. 2010;132(48):17174–85. doi: 10.1021/ja105684w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Martinez A, Peluffo G, Petruk AA, Hugo M, Pineyro D, Demicheli V, Moreno DM, Lima A, Batthyany C, Duran R, Robello C, Marti MA, Larrieux N, Buschiazzo A, Trujillo M, Radi R, Piacenza L. Structural and molecular basis of the peroxynitrite-mediated nitration and inactivation of Trypanosoma cruzi iron-superoxide dismutases (Fe-SODs) A and B: disparate susceptibilities due to the repair of Tyr35 radical by Cys83 in Fe-SODB through intramolecular electron transfer. J Biol Chem. 2014;289(18):12760–78. doi: 10.1074/jbc.M113.545590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Dufernez F, Yernaux C, Gerbod D, Noel C, Chauvenet M, Wintjens R, Edgcomb VP, Capron M, Opperdoes FR, Viscogliosi E. The presence of four iron-containing superoxide dismutase isozymes in trypanosomatidae: characterization, subcellular localization, and phylogenetic origin in Trypanosoma brucei. Free Radic Biol Med. 2006;40(2):210–25. doi: 10.1016/j.freeradbiomed.2005.06.021. [DOI] [PubMed] [Google Scholar]
- 163.Phan IQ, Davies DR, Moretti NS, Shanmugam D, Cestari I, Anupama A, Fairman JW, Edwards TE, Stuart K, Schenkman S, Myler PJ. Iron superoxide dismutases in eukaryotic pathogens: new insights from Apicomplexa and Trypanosoma structures. Acta Crystallogr F Struct Biol Commun. 2015;71(Pt 5):615–21. doi: 10.1107/S2053230X15004185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Bachega JF, Navarro MV, Bleicher L, Bortoleto-Bugs RK, Dive D, Hoffmann P, Viscogliosi E, Garratt RC. Systematic structural studies of iron superoxide dismutases from human parasites and a statistical coupling analysis of metal binding specificity. Proteins. 2009;77(1):26–37. doi: 10.1002/prot.22412. [DOI] [PubMed] [Google Scholar]
- 165.Boucher IW, Brzozowski AM, Brannigan JA, Schnick C, Smith DJ, Kyes SA, Wilkinson AJ. The crystal structure of superoxide dismutase from Plasmodium falciparum. BMC Struct Biol. 2006;6:20. doi: 10.1186/1472-6807-6-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Olmo F, Urbanova K, Rosales MJ, Martin-Escolano R, Sanchez-Moreno M, Marin C. An in vitro iron superoxide dismutase inhibitor decreases the parasitemia levels of Trypanosoma cruzi in BALB/c mouse model during acute phase. Int J Parasitol Drugs Drug Resist. 2015;5(3):110–6. doi: 10.1016/j.ijpddr.2015.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Sanchez-Moreno M, Gomez-Contreras F, Navarro P, Marin C, Olmo F, Yunta MJ, Sanz AM, Rosales MJ, Cano C, Campayo L. Phthalazine derivatives containing imidazole rings behave as Fe-SOD inhibitors and show remarkable anti-T. cruzi activity in immunodeficient-mouse mode of infection. J Med Chem. 2012;55(22):9900–13. doi: 10.1021/jm3011004. [DOI] [PubMed] [Google Scholar]
- 168.Mastronicola D, Falabella M, Forte E, Testa F, Sarti P, Giuffre A. Antioxidant defence systems in the protozoan pathogen Giardia intestinalis. Mol Biochem Parasitol. 2016;206(1–2):56–66. doi: 10.1016/j.molbiopara.2015.12.002. [DOI] [PubMed] [Google Scholar]
- 169.Testa F, Mastronicola D, Cabelli DE, Bordi E, Pucillo LP, Sarti P, Saraiva LM, Giuffre A, Teixeira M. The superoxide reductase from the early diverging eukaryote Giardia intestinalis. Free Radic Biol Med. 2011;51(8):1567–74. doi: 10.1016/j.freeradbiomed.2011.07.017. [DOI] [PubMed] [Google Scholar]