Abstract
Despite its importance in studies of migrant health, selectivity of migrants—also known as migration health selection—has seldom been examined in sub-Saharan Africa (SSA). This neglect is problematic because several features of the context in which migration occurs in SSA—very high levels of HIV, in particular—differ from contextual features in regions that have been studied more thoroughly. To address this important gap, we use longitudinal panel data from Malawi to examine whether migrants differ from nonmigrants in pre-migration health, assessed via SF-12 measures of mental and physical health. In addition to overall health selection, we focus on three more-specific factors that may affect the relationship between migration and health (1) whether migration health selection differs by destination (rural-rural, rural-town, and rural-urban), (2) whether HIV infection moderates the relationship between migration and health, and (3) whether circular migrants differ in pre-migration health status. We find evidence of the healthy migrant phenomenon in Malawi, where physically healthier individuals are more likely to move. This relationship varies by migration destination, with healthier rural migrants moving to urban and other rural areas. We also find interactions between HIV-infected status and health: HIV-infected women moving to cities are physically healthier than their nonmigrant counterparts.
Keywords: Migration, Physical health, Mental health, Selection, HIV infection
Introduction
A strong association between migration and health has been found in many settings (Chen 2011; Jasso et al. 2004; Landale et al. 2000; Lu 2008, Lu and Qin 2014; Nauman et al. 2015; Palloni and Morenoff 2001). Although studies in sub-Saharan Africa (SSA) have examined the relationship between migration and outcomes such as fertility, child mortality, and HIV infection (e.g., Boerma et al. 2002; Brockerhoff 1990, 1995a; Lagarde et al. 2003; Lurie et al. 2003; Rokicki et al. 2014), very little research has focused on the relationship between migration and general health in this setting. This neglect is of consequence because the SSA context has several distinctive characteristics—in particular, very high levels of HIV infection—that are likely to influence the relationship between migration and health.
In this article, we focus on health selection and internal migration in Malawi. We use longitudinal panel data with pre-migration information to examine whether healthier (or less healthy) residents of rural Malawi are more likely to migrate and whether this relationship varies by migration destination (rural, town, urban). Given previous research finding that HIV-infected individuals are more likely to migrate, we also investigate whether HIV infection moderates the relationship between migration and health in Malawi. Finally, we examine whether return migrants differ in health status from nonmigrants and those migrants who remain at destinations.
Background
Migration and Health, and the Role of Selection
With increasing population mobility across the globe and greater attention paid to the consequences of this mobility transition, migration has become widely recognized as a major determinant of health and well-being (Gushulak and MacPherson 2011; Zimmerman et al. 2011). Migration has also been increasingly recognized as a highly selective process, particularly with respect to health and health-related behaviors. Systematic differences between migrants and their nonmigrant counterparts—even before migration occurs—have been reported by studies that have the longitudinal data required to measure them (e.g., Anglewicz 2012; Lu 2008; Lu and Qin 2014; Nauman et al. 2015).
Migration research in settings outside SSA has often found strong support for the healthy migrant hypothesis, reporting a tendency for migrants to be healthier than peers at both origin and destination (Jasso et al. 2004; Landale et al. 2000; Lu 2008; Nauman et al. 2016; Palloni and Morenoff 2001). The health advantage of migrants before moving is thought to be due in part to considerations of what the move will entail: frail individuals are less likely than healthy individuals to decide to undertake the journey and accept the inevitable migration-related uncertainties and costs (Palloni and Morenoff 2001). Furthermore, because health is rewarded in the labor market (and in other markets, such as the marriage market), the returns from migration might be higher for healthy individuals. This is particularly the case in contexts where migration is undertaken to find employment or work opportunities (Lu and Qin 2014).
In addition to health selection during this pre-migration stage, another selection mechanism occurs later, among those who decided to migrate. Differences between individuals who choose to remain at destination versus those who return home (circular migrants) are typically not random. Those who return often do so because they were poorly suited for the move in the first place; the long-standing moniker for this type of selection is “salmon bias.”1 Both of these types of health selection can confound the effects of the actual move on the health of those who undertake it (Palloni and Arias 2004; Turra and Elo 2008).
Migration and the health selection that accompanies it have important implications for public health. Rapid urban population growth in developing regions—including in SSA—exerts pressure on local governments to take care of increasing number of people, straining already limited health resources, which can be exacerbated if migrants arrive with serious health issues (Gushulak and MacPherson 2011; White and Lindstrom 2005). Mobile populations are also tied to the spread of disease: migrants are often at greater risk of infectious diseases and facilitate the spread of diseases between urban centers and rural areas (Githeko et al. 2000; Johnson and Appleton 2000; Lagarde et al. 2003; Pison et al. 1993; Wilson 1995).
The Sub-Saharan African Context
Sub-Saharan Africa is undergoing rapid demographic change, and migration is a driving force underlying this change. African cities are expected to triple in population by 2050 (United Nations 2014), and rural-to-urban migration is a key component of this growth (Barrios et al. 2006). Short- and long-term internal migration is thus common throughout the region (Boerma et al. 2002; Coffee et al. 2005; Kahn et al. 2007) and appears to be increasing in some settings (Kahn et al. 2007; Schuyler et al. 2015). Although rural-to-urban migration is widespread, SSA will remain mostly rural for many years to come, and rural-rural migration for work, schooling, marriage, or other reasons has been—and will continue to be—the dominant internal migration stream within much of the region (Anglewicz 2012; Oucho and Gould 1993; Schuyler et al. 2015).
In contrast with other regions where selection forces favor healthy individuals to migrate, some recent studies have suggested that the healthy migrant phenomenon may not apply—or may apply only in modified form—in SSA. Internal migrants in SSA tend to be younger (Chalasani et al. 2013; Collinson et al. 2007; Lu 2008; Reed et al. 2010; Schuyler et al. 2015), unmarried and with fewer children (Anglewicz 2012; Arnoldo 2004; Boerma et al. 2002; Chalasani et al. 2013; Reed et al. 2010), and better-educated compared with nonmigrants (Anglewicz 2012; Brockerhoff and Eu 1993; Chalasani et al. 2013; Guilmoto 1998). However, in contrast to regions without a generalized HIV epidemic, an elevated HIV prevalence among migrants in SSA is likely to weaken the health and well-being both of infected migrants and their survivors when they migrate after the infected individual dies (Floyd et al. 2008; Gregson et al. 2007; Ford and Hosegood 2005; Urassa et al. 2001). Individuals who become sick (from HIV/AIDS or other illness) often return home to receive palliative care (Chimwaza and Watkins 2004; Clark et al. 2007; Urassa et al. 2001). Marital dissolution disproportionately affects households affected by HIV (Floyd et al. 2008; Gregory et al. 2007; Lopman et al. 2009; Porter et al. 2004), and members of these households—whether infected with HIV or not—often move after divorce, separation, and widowhood (Anglewicz 2012; Boerma et al. 2002). HIV infection notwithstanding, those experiencing divorce and widowhood have relatively worse mental and physical health (Myroniuk 2017). All these factors suggest that the relationship between migration and health in SSA is likely to be distinct from that reported in other regions and that the healthy migrant phenomenon may be absent or strictly different in SSA than in other contexts.
Limitations of Studies on Migration and Health in SSA
The dearth of information on the healthy migrant hypothesis in SSA is primarily due to limitations of data and study designs. Much of the research on migration and health is conducted with cross-sectional data, comparing health outcomes between nonmigrants and migrants after moving (e.g., Brockerhoff and Biddlecom 1999; Chirwa 1997; Coffee et al. 2005; Li et al. 2007; Roux and van Tonder 2006; Yang et al. 2007) or using retrospective migration histories (e.g., Mberu and White 2011; Reed et al. 2010). Cross-sectional analysis cannot tell us whether migration affects health, or healthier individuals are more likely to migrate, or both. Retrospective migration histories do not include health status prior to migration. Testing the healthy migrant hypothesis is possible with longitudinal data, which permit examining the health of migrants before moving as well as afterward. Longitudinal studies that include health status for individuals before migration are rare in any setting, although they have become more common in recent years (e.g., Chen 2011; Ginsburg et al. 2016; Lu 2008; Lu and Qin 2014; Nauman et al. 2015).
In addition to limitations of study designs, health measures used in much of the existing research have been quite narrowly focused. Although some recent studies in SSA have examined migration and mortality (Collinson et al. 2014; Ginsburg et al. 2016), health outcomes have historically and predominantly focused on HIV infection or HIV risk behavior (e.g., Boerma et al. 2002; Lagarde et al. 2003; Lurie et al. 2003; Pison et al. 1993), infant/child mortality (Brockerhoff 1990, 1995a; Ssengonzi et al. 2002), or reproductive health (Agadjanian et al. 2011; Brockerhoff 1995b; Chattopadhyay et al. 2006; Lee 1992; Rokicki et al. 2014). The broader non-SSA migration and health literature, however, has identified other dimensions of health that are importantly related to the migration process but have been examined much less frequently, particularly for those moving to rapidly growing urban centers in the region. One such measure is mental health (Kohler et al. 2017), which has been connected to migration in several settings outside SSA (e.g., Lu 2010; Nauman et al. 2015). Research in SSA has shown that the HIV-infected are more likely to experience mental distress than uninfected individuals (Brandt 2009; Freeman et al. 2008) and that the HIV-infected are more likely to move (Anglewicz 2012), but research in SSA has not examined whether migrants have relatively worse mental health. Similarly, although research in other settings has found a strong relationship between migration and overall physical health before moving (Lu 2008; Lu and Qin 2014; Nauman et al. 2015), this has not been empirically studied in SSA.
A final limitation of existing research on migration in SSA is the scarcity of population-based data on health and migration. Instead, migration research has often sampled specific migrant groups. Much of the migration research in SSA has focused on labor migration (Chirwa 1997; Kahn et al. 2003; Weine and Kashuba 2012; Yabiku et al. 2011) despite the fact that many migrate for marriage-, climate-, and household-related reasons (Arnoldo 2004; Boerma et al. 2002; Coffee et al. 2005; Reniers 2003; Watts 1983). Due in part to the interest in labor migration, the spatial movement of interest has primarily been rural-urban migration (Coast 2006; Luke 2012[), and the gender focus has often been on male migrants (Agadjanian et al. 2011; Luke 2012; Lurie et al. 2003). South Africa has also contributed disproportionately to research on internal migration in SSA (e.g., Gelderblom and Kok 1994; Reed 2013) despite the fact that the apartheid system, with its severe restrictions on mobility for the black population, created internal migratory patterns that likely differ from other countries in SSA (Posel 2003; Reed 2013).
The Country Setting: Migration, Urbanization, and HIV Infection in Malawi
Malawi exemplifies the rapid transition from rural to urban throughout SSA and much of the developing world. Currently one of the least urbanized countries in the world, Malawi has one of the highest rates of urban population growth (United Nations 2014). Yet, Malawi is similar to most other developing countries in that the vast majority of rural migrants move (at least initially) to other rural areas instead of urban centers (Anglewicz 2012; Chalasani et al. 2013).
Internal migration in Malawi occurs primarily for work and marriage-related reasons (Anglewicz 2012; Chalasani et al. 2013; Englund 2002). Differences by sex have been observed, with men more likely to move for work, and women moving for marriage-related reasons (Anglewicz 2012). Marriage-related migration typically occurs at the beginning or end of a marital union, but patterns of marital migration vary by gender and ethnicity. The Tumbuka, who are the majority ethnic group of the northern region, practice a patrilocal tradition, in which the wife moves to the home of her husband upon marriage; in contrast, the Yao primarily reside in the southern region and typically have a matrilocal marriage pattern (Mtika and Doctor 2002; Reniers 2003). Divorce, widowhood, and remarriage are common throughout the country, leading to frequent post-marriage–related migration (Anglewicz and Reniers 2014; Reniers 2003).
HIV infection and migration are closely connected in Malawi. Malawi has a generalized HIV epidemic, with a recent prevalence estimate of 9.1 % (UNAIDS 2016) and significant differences in prevalence between rural and urban areas. The 2010 Malawi Demographic and Health Survey estimated an HIV prevalence of 17.4 % in urban centers and 8.9 % in rural areas (National Statistical Office and ICF Macro 2011). As elsewhere, migrants in Malawi are significantly more likely to be HIV-infected than nonmigrants: HIV-infected individuals from rural Malawi had more than two times greater odds of migration than those who were HIV-uninfected (Anglewicz et al. 2016). This pattern is primarily due to the greater likelihood of migration for HIV-infected individuals rather than to migration resulting in higher HIV incidence (Anglewicz 2012; Anglewicz et al. 2016).
In this article, we examine several related questions on migration health selection in Malawi. We begin with the simple question of whether migration within Malawi is selective of individuals with better or worse health. Next, given that rural-to-rural migrants may be different from those moving to cities, we investigate whether migration health selection differs by migration destination (rural, town, urban). Third, because previous research has shown that HIV-infected individuals are more likely to migrate than those who are uninfected, we examine whether HIV moderates the relationship between internal migration and health in Malawi, and we do this by each type of destination. Finally, we turn to salmon bias and investigate whether circular migrants differ in health status before migration compared with permanent migrants and nonmigrants.
Methods
Data
Examining migration health selection requires a data set with several features: (1) longitudinal panel data to measure pre-migration health status; (2) physical and mental health measures; (3) variation in and information about migration destinations to allow for a distinction between among rural-rural, rural-town, and rural-urban movement; and (4) information on pre-migration HIV status to examine whether HIV status moderates the relationship between migration and health.
We use data from two related sources: the Malawi Longitudinal Study of Families and Health (MLSFH)2 and the Migration and Health in Malawi (MHM) Project. The MLSFH was designed in 1998 as a longitudinal couples’ survey, targeting a population-based representative sample of approximately 1,500 ever-married women and 1,000 of their husbands in three rural sites of Malawi. Following a household enumeration in the three designated survey sites in 1998, a random sample of approximately 500 ever-married women aged 15–49 were selected to be interviewed at each site, along with all their spouses. The first follow-up in 2001 included all respondents from the first wave, along with any new spouses. The MLSFH returned to interview all respondents and new spouses in 2004, 2006, 2008, and 2010; and also added two new samples: (1) approximately 1,500 young adults aged 15–27 in 2004 (both ever- and never-married) and (2) approximately 800 parents of existing MSLFH respondents in 2008. MLSFH initiated HIV testing for all respondents in 2004, with follow-up testing in 2006 and 2008. MLSFH HIV testing procedures followed guidelines given by the Malawi Ministry of Health and the World Health Organization (WHO), in which consenting respondents provided finger-prick rapid tests using parallel Determine HIV/1–2 (Abbott Laboratories, USA) and UniGold HIV (Trinity Biotech, Ireland) test kits. All HIV tests were preceded and followed by a counseling session (Kohler et al. 2015). Descriptions of the MLSFH data, sampling, and HIV testing are presented in Watkins et al. (2003) and Kohler et al. (2015). Bignami-Van Assche et al. (2003), Anglewicz et al. (2009), and Kohler et al. (2015) discussed sample characteristics and presented analyses of data quality.
In all waves of the MLSFH, the most common reason for nonresponse was migration out of the study sites. Migrants were identified through attempts to interview all respondents in the MLSFH target sample. While visiting the house of a respondent, the MLSFH team was informed of migration by friends and family members who remained in the MLSFH pre-migration village of the respondent. To qualify as a “migrant,” friends and family members reported that the individual had moved from the MLSFH village to another location outside at least 20km of the MLSFH study site, with the expectation that the move is permanent. The MLSFH survey also provides information on return migration: in all waves, the MLSFH asked respondents whether they had lived someplace outside the current district for one month or more in the past year.
Starting in 2012, the MHM Project was designed to trace and interview MLSFH respondents who previously resided in an MLSFH sample village but moved to another part of rural or urban Malawi. Using data from the 2010 MLSFH, the MHM identified 1,096 individuals who were interviewed by MSLFH at least once since the third wave (in 2004) but were identified by friends or family members as migrants when attempting to interview them in the sixth wave, in 2010.
The MHM attempted to trace the location of these migrants by using a migration tracking technique. The MHM returned to the previous MLSFH village of residence for these migrants and requested information on their current location from friends and family members who remained in the pre-migration village. The MHM was able to collect detailed information on the current location for the vast majority of migrants; the location was unknown for only 77 of 1,096 migrants (5.1 %). An additional 83 (5.5 %) were reported to have moved internationally,3 and 39 (2.6 %) reportedly died. See Anglewicz et al. (2017) for information on MHM sampling, study design, and characteristics of migrants.
The migration tracking data collected by the MHM permit us to identify migration destination. Rural-rural migrants are those who moved to another rural part of Malawi. Individuals moving to one of Malawi’s three regional capitals (Mzuzu in the north, Lilongwe in the central region, and Blantyre in the south), and the third largest city (and former capital, Zomba), are considered rural-urban migrants. Rural-town migrants are those who moved to the capital of one of Malawi’s 22 districts.
Overall, this study uses information from 3,039 men and women from the MLSFH: 1,833 women and 1,206 men. We use MLSFH data from Waves 4–6 (2006, 2008, and 2010), which include all measures of interest, SF-12 scores , and HIV serostatus.4 A total of 2,381 and 2,351 respondents had complete information for all measures in 2006 and 2008, respectively (including 1,693 who were interviewed in both waves).
We use data from subsequent waves of the MLSFH (2008 and 2010) to identify men and women who migrated. Overall, 219 men and 261 women moved within Malawi by the next wave of MLSFH and had complete MLSFH/MHM survey information. We then use information from the MHM study to identify the destination of the migrant (rural, town, or city). We also identify return migrants, comprising 448 women and 323 men who stayed outside a MLSFH sample village before returning to the village.5 This measure is based on self-reports of having stayed outside the current district of residence for one month or more in the past year.
Measures
Measures of primary interest are physical and mental health, produced by the SF-12 instrument. The SF-12 has been shown to accurately capture physical and mental health status in a wide range of settings (Jenkinson et al. 2001; Ware et al. 1996, 1998) and comprises more robust measures of health than the single five-point scale of health that is commonly used in migration research (Jasso et al. 2004). SF-12 summary measures range from 0 to 100 and are normed for a particular population, with the mean score for both mental and physical health set to 50 and higher scores indicating better health. Two summary measures—a mental health component summary (MCS) score and a physical health component summary (PCS) score—are calculated by aggregating data from the eight subscales. Research has shown that SF-12 scores are strongly associated with health outcomes, such as depression and anxiety disorders (Gill et al. 2007; Kohler et al. 2017), arthritis (Gandhi et al. 2001), physical pain (Luo et al. 2003), and overall self-assessed health (Jenkinson et al. 2001). In addition, the SF-12 scores are responsive to differences in or changes to a range of health outcomes (Ware et al. 1996). Although SF-12 scores are not used for formal clinical diagnosis, it is important to note that a score 2 standard deviations below the SF-12 mental health scale mean is strongly linked to clinical depression (Ware et al. 1998), and a score of 45 or less—approximately 1 standard deviation below the mean score for women and men in our sample—has been used as a general cutoff for depression screening (Gill et al. 2007).
Because of the consistent connection between migration and HIV infection in SSA, another health measure of interest is HIV status. More than 90 % of MLSFH respondents at each wave consented to be tested (Obare et al. 2009). Nearly all those tested received their HIV test results: approximately 68 % of respondents received their HIV test result in 2004, compared with 98 % in 2006 and 93 % in 2008. For more information about MLSFH testing procedures and outcomes, see Obare et al. (2009) and Kohler et al. (2015).
Analytic Methods
We conduct our analysis in three steps. First, we examine whether healthier (or less healthy) individuals are more likely to migrate. To do so, we use the longitudinal MLSFH data from 2006 (the year that MLSFH started collecting SF-12 measures) through 2010. To establish the time-order between these measures in this analysis, we measure migration (the dependent variable) from a future wave and health status (SF-12 summary scores of mental and physical health) from a prior wave (i.e., before migration). To facilitate interpretation of SF-12 scores in our analysis—and in particular, the main effects for models with interaction terms—we convert the scores to differences from the mean.
Using this approach, we run random-effects logistic regressions, which are used to account for correlation in the residual due to multiple observations of the same individual over time. We then run five separate regression models. Model 1 includes only three independent variables: SF-12 measures of mental health and physical health, and a binary indicator for MLSFH survey year (with 2006 as the reference category). Then, in two additional sequential regression models, we add measures of age and HIV status to examine whether health selection is still evident after we control for potential differences in age and HIV status between migrants and nonmigrants.6 We are also interested in whether HIV status moderates the relationship between migration and health. To test this, we add two regressions that include interactions for HIV status and health status (both mental and physical health, separately). By including these measures, we examine whether HIV-infected individuals with differing health status are more likely to move. Results are shown in odds ratios (ORs) with standard errors and 95 % confidence intervals (CIs). To facilitate interpretation, we include marginal effects (MEs) for selected results, as adjusted predictions at the means. We run all models separately for men and women.7
The second step is to examine differences in migration health selection by migrant destination. We use an approach similar to that mentioned earlier, in which health and HIV status are measured prior to migration. Instead of using the binary measure of migrant or nonmigrant, however, we separate migrants by destination: rural-rural, rural-town, and rural-urban migration (with nonmigrants as the reference category). With this four-category dependent variable, we run random-effects multinomial logistic regressions, using the same pooled MLSFH data from 2006 to 2010. As before, in the first sets of models, we include physical and mental health measures and control for differences by MLSFH survey wave (2008 compared with 2006). We then add age and HIV infection in subsequent models. Finally, as noted earlier, we also include interactions for HIV infection and both health status measures in regression Models 4 and 5. Results for these regressions are shown in relative risk ratios (RRRs) with standard errors and 95 % CIs.
Third, we examine whether the relationship between migration and health differs for return migrants compared with permanent migrants and nonmigrants. Using the same lagged-variable regression approach, we again conduct random-effects multinomial logistic regressions with a three-category dependent variable of return migrants (reference group) compared with permanent migrants and nonmigrants. Independent variables are the same as the second step, including age, the health measures, HIV-infected status, and the interactions between HIV and health. As in the previous step, results are shown in RRRs with standard errors and 95 % CIs.
Results
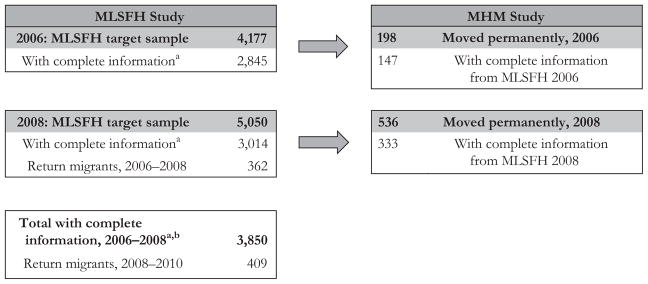
Figure 1 describes the sampling for the MLSFH and MHM study populations. Our sample begins with 2,845 individuals who were interviewed and tested for HIV in the MLSFH in 2006. Similarly, 3,014 respondents were interviewed and tested in MLSFH 2008. A subset of 480 moved permanently (147 moving between 2006 and 2008, and 333 moving between 2008 and 2010), and the MHM study identified their destination. The total sample size for our analysis is 3,850: 2,009 were interviewed in both 2006 and 2008; 836 were interviewed only in 2006; and 1,005 were interviewed only in 2008. The MLSFH also identified 771 return migrants, with 362 moving between 2006 and 2008 and the remainder moving between 2008 and 2010.8
Fig. 1.
MLSFH and MHM Study sample flow chart. aComplete information: includes data for measures used in the analysis and an HIV test result. Those without complete information were (1) not interviewed or tested for HIV by MLSFH, (2) interviewed but not tested for HIV, or (3) tested for HIV but not interviewed. Detailed flow charts and information on each of these categories and analyses of attrition and nonresponse are available in Kohler et al. (2015) (for MLSFH) and Anglewicz et al. (2017) (MHM). bOf the 3,850 with complete information, 2,009 were interviewed in both 2006 and 2008; 836 were interviewed only in 2006; and 1,005 were interviewed only in 2008
Table 1 shows characteristics for MLSFH migrants who moved permanently from the MLSFH study areas and the comparison group consisting of MLSFH nonmigrants. The MLSFH nonmigrants include respondents who did not move from the study areas as well as return migrants who temporarily moved between 2006 and 2008 and between 2008 and 2010. Among nonmigrants, 55 % were female, compared with 48 % of migrants (2006) and 57 % (2008) of individuals migrating by the next wave of MLSFH. Approximately 7 % (2006) and 9 % (2008) of migrants were infected with HIV, and 5 % (2006 and 2008) of nonmigrants were HIV-positive. Regarding migration destinations, rural-rural migration was the most common stream, followed by rural-town and rural-urban.
Table 1.
Pre-migration background characteristics for migrants and nonmigrants: MLSFH women and men, 2006 and 2008
| 2006 | 2008 | |||
|---|---|---|---|---|
|
|
||||
| Nonmigrant | Migrant | Nonmigrant | Migrant | |
| Female (%) | 56.5 | 47.6 | 60.7 | 57.4 |
| Mean Physical Health | 52.4 | 53.7 | 51.4 | 52.6 |
| (7.5) | (5.9) | (7.7) | (6.2) | |
| Mean Mental Health | 55.6 | 56.2 | 54.1 | 54.4 |
| (7.9) | (7.3) | (8.7) | (8.5) | |
| Mean Age | 35.5 | 29.9 | 41.4 | 36.7 |
| (13.4) | (12.4) | (16.7) | (15.3) | |
| HIV-Infected (%) | 5.3 | 6.8 | 5.2 | 8.7 |
| Migration Destination (%) | ||||
| Rural-rural | 67.3 | 80.2 | ||
| Rural-town | 21.2 | 14.7 | ||
| Rural-urban | 11.5 | 5.1 | ||
| N | 2,698 | 147 | 2,681 | 333 |
Note: Standard deviations are shown in parentheses.
Health Selection
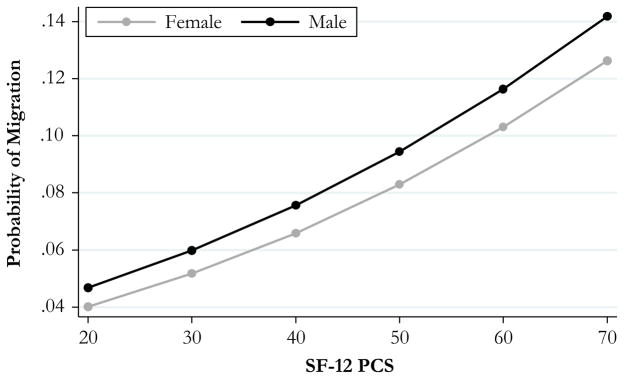
Our first set of analysis shows evidence for the selection of healthier men and women into migration from rural Malawi. Table 2 shows results from random-effects logistic regressions predicting migration in a future wave of MLSFH. As shown in the first set of regressions, which include only mental and physical health and a dummy variable measure for the 2008 MLSFH wave, physically healthier men (OR = 1.06, 95 % CI = 1.01–1.11, ME = 0.003) and women (OR = 1.03, 95 % CI = 1.00–1.07, ME = 0.001) were significantly more likely to move. In Fig. 2 in the appendix, we plot the predictive marginal probability of migrating for values of SF-12 physical health scores ranging from 20 to 70, separately for men and women.
Table 2.
Random-effects logistic regression results (odds ratios) for migration health selection: MLSFH women and men, 2006–2010
| Women | Men | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||
| Model 1 | Model 2 | Model 3 | Model 4 | Model 5 | Model 1 | Model 2 | Model 3 | Model 4 | Model 5 | |
| Physical Health | 1.03* | 1.01 | 1.02 | 1.01 | 1.01 | 1.06* | 1.04* | 1.04† | 1.04 | 1.04† |
| Standard error | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.03 | 0.02 |
| 95 % confidence interval | 1.00–1.07 | 0.98–1.05 | 0.98–1.05 | 0.98–1.05 | 0.98–1.05 | 1.01–1.11 | 1.00–1.09 | 0.99–1.09 | 0.99–1.09 | 0.99–1.09 |
| Mental Health | 1.00 | 0.99 | 0.99 | 0.99 | 0.99 | 1.00 | 1.00 | 1.00 | 1.00 | 1.01 |
| Standard error | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.02 | 0.02 | 0.02 | 0.02 |
| 95 % confidence interval | 0.98–1.03 | 0.97–1.02 | 0.97–1.02 | 0.97–1.02 | 0.97–1.02 | 0.97–1.03 | 0.96–1.03 | 0.97–1.03 | 0.97–1.03 | 0.97–1.04 |
| Age | 0.86** | 0.84** | 0.85** | 0.84** | 0.96** | 0.96** | 0.96** | 0.96** | ||
| Standard error | 0.03 | 0.03 | 0.03 | 0.03 | 0.01 | 0.01 | 0.01 | 0.01 | ||
| 95 % confidence interval | 0.79–0.92 | 0.78–0.91 | 0.79–0.91 | 0.78–0.91 | 0.94–0.98 | 0.93–0.99 | 0.94–0.99 | 0.94–0.98 | ||
| Age, Squared | 1.00** | 1.00** | 1.00** | 1.00** | ||||||
| Standard error | 0.00 | 0.00 | 0.00 | 0.00 | ||||||
| 95 % confidence interval | 1.00–1.01 | 1.00–1.01 | 1.00–1.01 | 1.00–1.01 | ||||||
| HIV-Infected | 2.11† | 2.18† | 2.14† | 4.33** | 4.10* | 3.89* | ||||
| Standard error | 0.98 | 1.02 | 1.04 | 2.64 | 2.62 | 2.29 | ||||
| 95 % confidence interval | 0.89–5.34 | 0.87–5.48 | 0.88–5.48 | 1.30–14.36 | 1.17–14.33 | 1.23–12.03 | ||||
| MLSFH Survey 2008 | 7.61** | 8.48** | 10.00** | 9.29** | 9.78** | 3.59** | 5.53** | 5.16** | 5.16** | 5.11** |
| Standard error | 2.20 | 3.46 | 3.09 | 2.94 | 3.02 | 1.61 | 2.15 | 2.62 | 2.92 | 2.15 |
| 95 % confidence interval | 4.31–13.42 | 3.80–18.86 | 5.45–18.35 | 5.00–17.27 | 5.34–17.90 | 1.49–8.64 | 2.58–11.83 | 1.91–13.98 | 1.70–15.63 | 2.23–11.65 |
| HIV-Infected × Physical Health | 1.03 | 1.04 | ||||||||
| Standard error | 0.05 | 0.08 | ||||||||
| 95 % confidence interval | 0.93–1.13 | 0.90–1.20 | ||||||||
| HIV-Infected × Mental Health | 1.01 | 0.94 | ||||||||
| Standard error | 0.05 | 0.05 | ||||||||
| 95 % confidence interval | 0.92–1.10 | 2.23–11.65 | ||||||||
Notes: Women: n = 2,240. Men: n = 1,610. Quadratic measure of age for men was not statistically significant, so it is not included in the models in this table.
p < .10;
p < .05;
p < .01
For women, the selection of physically healthier individuals appears to be attenuated by age. After age is included, the association between physical health and migration has smaller odds and CIs that include the value 1.0 for women (OR = 1.02, 95 % CI = 0.99–1.06), and it is not statistically significant at the p < .10 level. We find a nonlinear relationship with age, with younger women less likely to move, and women at later ages were more likely to move. There is not a nonlinear relationship between age and migration for men; older men are less likely to move. For men, however, the relationship between physical health and future migration remains statistically significant even after age is controlled for (OR = 1.04, 95 % CI = 1.00–1.09).
In Model 3, we find that HIV-infected men and women had greater odds of migrating than the uninfected (men OR = 4.33, 95 % CI = 1.30–14.36, ME = 0.075; women OR = 2.11, 95 % CI = 0.89–5.34, ME = 0.029). Finally, in Models 4 and 5, we do not find a relationship between the interactions of HIV infection and health status and migration, which suggests that HIV infection did not moderate the relationship between migration and health for migrants (without separating migrants by destination).
Health Selection by Destination
When separated by migration destination, health selection depends partly on where the migrant moves. Both women (Table 3) and men (Table 4) who moved from rural to urban (women RRR = 1.11, 95 % CI = 1.03–1.20; men RRR = 1.17, 95 % CI = 1.06–1.29) and other rural areas (women RRR = 1.07, 95 % CI = 1.01–1.14; men RRR = 1.09, 95 % CI = 1.02–1.16) were physically healthier (before migration) than those who remained in the rural MLSFH villages. For men, those moving to towns were also physically healthier (RRR = 1.08, 95 % CI = 1.00–1.17).
Table 3.
Random-effects multinomial logistic regression results (relative risk ratios) for migration health selection, by migration destination: MLSFH women, 2006–2010
| Model 1 | Model 2 | Model 3 | Model 4 | Model 5 | |
|---|---|---|---|---|---|
| Rural-Rural Migrant vs. Nonmigrant | |||||
| Physical health | 1.07* | 1.04 | 1.04 | 1.03 | 1.04 |
| Standard error | 0.03 | 0.03 | 0.03 | 0.03 | 0.03 |
| 95 % confidence interval | 1.01–1.14 | 0.99–1.09 | 0.99–1.09 | 0.97–1.08 | 0.99–1.09 |
| Mental health | 0.99 | 0.99 | 0.99 | 0.99 | 0.99 |
| Standard error | 0.03 | 0.02 | 0.02 | 0.02 | 0.02 |
| 95 % confidence interval | 0.94–1.04 | 0.95–1.03 | 0.95–1.03 | 0.95–1.03 | 0.95–1.03 |
| Age | 0.81** | 0.80** | 0.80** | 0.80** | |
| Standard error | 0.05 | 0.05 | 0.05 | 0.05 | |
| 95 % confidence interval | 0.71–0.91 | 0.71–0.91 | 0.70–0.91 | 0.71–0.91 | |
| Age, squared | 1.00** | 1.00** | 1.00** | 1.00** | |
| Standard error | 0.00 | 0.00 | 0.00 | 0.00 | |
| 95 % confidence interval | 1.00–1.01 | 1.00–1.01 | 1.00–1.01 | 1.00–1.01 | |
| HIV-infected | 2.28* | 2.20† | 2.22† | ||
| Standard error | 1.05 | 1.15 | 1.19 | ||
| 95 % confidence interval | 1.01–9.02 | 0.92–8.01 | 0.89–9.17 | ||
| MLSFH survey 2008 | 63.01* | 28.85** | 27.79** | 30.47** | 27.65** |
| Standard error | 105.88 | 17.73 | 17.14 | 18.85 | 17.04 |
| 95 % confidence interval | 2.34–1697.23 | 8.65–96.23 | 8.27–93.10 | 9.09–102.14 | 8.26–92.50 |
| HIV-infected × Physical health | 1.08 | ||||
| Standard error | 0.08 | ||||
| 95 % confidence interval | 0.95–1.24 | ||||
| HIV-infected × Mental health | 1.01 | ||||
| Standard error | 0.06 | ||||
| 95 % confidence interval | 0.89–1.14 | ||||
| Rural-Town Migrant vs. Nonmigrant | |||||
| Physical health | 1.04 | 1.01 | 1.01 | 1.01 | 1.01 |
| Standard error | 0.04 | 0.03 | 0.03 | 0.03 | 0.03 |
| 95 % confidence interval | 0.98–1.12 | 0.95–1.07 | 0.95–1.07 | 0.94–1.07 | 0.95–1.07 |
| Mental health | 0.96* | 0.95* | 0.96* | 0.95* | 0.94* |
| Standard error | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 |
| 95 % confidence interval | 0.91–0.99 | 0.91–0.99 | 0.91–0.99 | 0.91–0.99 | 0.89–0.99 |
| Age | 0.76** | 0.76** | 0.75** | 0.76** | |
| Standard error | 0.06 | 0.06 | 0.06 | 0.06 | |
| 95 % confidence interval | 0.66–0.89 | 0.65–0.88 | 0.65–0.87 | 0.65–0.88 | |
| Age, squared | 1.00** | 1.00** | 1.00** | 1.00** | |
| Standard error | 0.00 | 0.00 | 0.00 | 0.00 | |
| 95 % confidence interval | 1.00–1.01 | 1.00–1.01 | 1.00–1.01 | 1.00–1.01 | |
| HIV-infected | 2.50 | 2.10 | 2.70 | ||
| Standard error | 2.08 | 1.80 | 2.37 | ||
| 95 % confidence interval | 0.49–12.74 | 0.39–11.31 | 0.48–15.13 | ||
| MLSFH survey 2008 | 29.09* | 16.11** | 15.54** | 16.78** | 15.36** |
| Standard error | 49.73 | 10.93 | 10.58 | 11.30 | 10.48 |
| 95 % confidence interval | 1.02–823.53 | 4.27–60.87 | 4.09–59.00 | 4.48–62.84 | 4.03–58.53 |
| HIV-infected × Physical health | 1.02 | ||||
| Standard error | 0.08 | ||||
| 95 % confidence interval | 0.88–1.18 | ||||
| HIV-infected × Mental health | 1.14 | ||||
| Standard error | 0.10 | ||||
| 95 % confidence interval | 0.96–1.36 | ||||
| Rural-Urban Migrant vs. Nonmigrant | |||||
| Physical health | 1.11** | 1.08* | 1.09* | 1.06† | 1.09* |
| Standard error | 0.04 | 0.04 | 0.04 | 0.04 | 0.04 |
| 95 % confidence interval | 1.03–1.20 | 1.01–1.16 | 1.01–1.16 | 0.99–1.15 | 1.01–1.17 |
| Mental health | 0.99 | 0.98 | 0.98 | 0.99 | 0.99 |
| Standard error | 0.03 | 0.03 | 0.03 | 0.03 | 0.03 |
| 95 % confidence interval | 0.93–1.05 | 0.93–1.03 | 0.94–1.03 | 0.94–1.04 | 0.94–1.05 |
| Age | 0.68** | 0.68** | 0.68** | 0.68** | |
| Standard error | 0.05 | 0.05 | 0.05 | 0.05 | |
| 95 % confidence interval | 0.59–0.78 | 0.59–0.78 | 0.59–0.78 | 0.59–0.78 | |
| Age, squared | 1.00** | 1.00** | 1.00** | 1.00** | |
| Standard error | 0.00 | 0.00 | 0.00 | 0.00 | |
| 95 % confidence interval | 1.00–1.01 | 1.00–1.01 | 1.00–1.01 | 1.00–1.01 | |
| HIV-infected | 1.49 | 0.06 | 0.12 | ||
| Standard error | 1.48 | 0.15 | 0.32 | ||
| 95 % confidence interval | 1.92–27.87 | 0.00–11.31 | 0.00–26.22 | ||
| MLSFH survey 2008 | 14.39 | 7.64** | 7.32** | 8.13** | 7.60** |
| Standard error | 24.51 | 5.20 | 4.99 | 5.54 | 5.19 |
| 95 % confidence interval | 0.51–405.09 | 2.01–28.98 | 1.92–27.76 | 2.14–30.95 | 1.99–28.98 |
| HIV-infected × Physical health | 2.07* | ||||
| Standard error | 0.75 | ||||
| 95 % confidence interval | 1.02–4.23 | ||||
| HIV-infected × Mental health | 0.80 | ||||
| Standard error | 0.13 | ||||
| 95 % confidence interval | 0.58–1.09 | ||||
Note: n = 2,240.
p < .10;
p < .05;
p < .01
Table 4.
Random-effects multinomial logistic regression results (relative risk ratios) for migration health selection, by migration destination: MLSFH men, 2006–2010
| Model 1 | Model 2 | Model 3 | Model 4 | Model 5 | |
|---|---|---|---|---|---|
| Rural-Rural Migrant vs. Nonmigrant | |||||
| Physical health | 1.09** | 1.06* | 1.06† | 1.05† | 1.06† |
| Standard error | 0.03 | 0.03 | 0.03 | 0.03 | 0.03 |
| 95 % confidence interval | 1.02–1.16 | 1.00–1.12 | 1.00–1.12 | 0.99–1.12 | 1.00–1.12 |
| Mental health | 1.01 | 1.00 | 1.00 | 1.00 | 1.00 |
| Standard error | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 |
| 95 % confidence interval | 0.97–1.05 | 0.96–1.04 | 0.96–1.05 | 0.96–1.05 | 0.96–1.04 |
| Age | 0.94** | 0.94** | 0.94** | 0.94** | |
| Standard error | 0.02 | 0.02 | 0.02 | 0.02 | |
| 95 % confidence interval | 0.96–1.04 | 0.91–0.97 | 0.91–0.97 | 0.96–1.04 | |
| HIV-infected | 5.26* | 4.85† | 5.34* | ||
| Standard error | 4.10 | 3.94 | 4.35 | ||
| 95 % confidence interval | 1.14–24.22 | 0.99–23.87 | 1.08–26.35 | ||
| MLSFH survey 2008 | 16.97** | 22.08** | 20.25** | 20.36** | 21.03** |
| Standard error | 10.88 | 13.56 | 12.40 | 12.45 | 13.16 |
| 95 % confidence interval | 4.83–59.60 | 6.62–73.59 | 6.10–67.26 | 6.14–67.50 | 6.17–71.67 |
| HIV-infected × Physical health | 1.08 | ||||
| Standard error | 0.12 | ||||
| 95 % confidence interval | 0.87–1.33 | ||||
| HIV-infected × Mental health | 1.02 | ||||
| Standard error | 0.10 | ||||
| 95 % confidence interval | 0.84–1.24 | ||||
| Rural-Town Migrant vs. Nonmigrant | |||||
| Physical health | 1.08* | 1.06 | 1.06 | 1.07 | 1.06 |
| Standard error | 0.04 | 0.04 | 0.04 | 0.05 | 0.04 |
| 95 % confidence interval | 1.00–1.17 | 0.99–1.15 | 0.98–1.14 | 0.98–1.16 | 0.98–1.15 |
| Mental health | 0.99 | 0.99 | 0.99 | 0.99 | 1.00 |
| Standard error | 0.03 | 0.03 | 0.03 | 0.03 | 0.03 |
| 95 % confidence interval | 0.93–1.05 | 0.93–1.04 | 0.94–1.04 | 0.93–1.04 | 0.94–1.06 |
| Age | 0.95* | 0.95* | 0.95* | 0.95* | |
| Standard error | 0.02 | 0.02 | 0.02 | 0.02 | |
| 95 % confidence interval | 0.92–0.99 | 0.91–0.99 | 0.91–0.99 | 0.91–0.99 | |
| HIV-infected | 5.33† | 5.59† | 4.17 | ||
| Standard error | 5.04 | 5.39 | 4.45 | ||
| 95 % confidence interval | 0.83–33.99 | 0.84–37.03 | 0.51–33.79 | ||
| MLSFH survey 2008 | 4.18* | 5.11* | 4.71* | 4.66* | 4.72* |
| Standard error | 2.89 | 3.40 | 3.13 | 3.07 | 3.18 |
| 95 % confidence interval | 1.08–16.19 | 1.39–18.84 | 1.28–17.32 | 1.28–16.95 | 1.26–17.70 |
| HIV-infected × Physical health | 0.98 | ||||
| Standard error | 0.11 | ||||
| 95 % confidence interval | 0.80–1.22 | ||||
| HIV-infected × Mental health | 0.92 | ||||
| Standard error | 0.10 | ||||
| 95 % confidence interval | 0.75–1.15 | ||||
| Rural-Urban Migrant vs. Nonmigrant | |||||
| Physical health | 1.17** | 1.14** | 1.14* | 1.13* | 1.13* |
| Standard error | 0.06 | 0.06 | 0.06 | 0.06 | 0.06 |
| 95 % confidence interval | 1.06–1.29 | 1.03–1.27 | 1.03–1.26 | 1.02–1.25 | 1.02–1.25 |
| Mental health | 1.01 | 1.00 | 1.00 | 1.00 | 1.02 |
| Standard error | 0.03 | 0.03 | 0.03 | 0.03 | 0.03 |
| 95 % confidence interval | 0.95–1.07 | 0.94–1.06 | 0.94–1.06 | 0.95–1.06 | 0.96–1.09 |
| Age | 0.88** | 0.88** | 0.88** | 0.88** | |
| Standard error | 0.02 | 0.02 | 0.02 | 0.02 | |
| 95 % confidence interval | 0.84–0.93 | 0.84–0.92 | 0.84–0.92 | 0.84–0.92 | |
| HIV-infected | 5.72* | 2.85 | 1.52 | ||
| Standard error | 4.23 | 4.53 | 2.88 | ||
| 95 % confidence interval | 1.00–48.42 | 0.13–64.14 | 0.04–62.07 | ||
| MLSFH survey 2008 | 2.70 | 4.43* | 4.08* | 4.14* | 4.08* |
| Standard error | 1.84 | 2.95 | 2.71 | 2.76 | 2.78 |
| 95 % confidence interval | 0.70–10.33 | 1.20–16.31 | 1.11–15.02 | 1.12–15.26 | 1.07–15.55 |
| HIV-infected × Physical health | 1.19 | ||||
| Standard error | 0.24 | ||||
| 95 % confidence interval | 0.80–1.78 | ||||
| HIV-infected × Mental health | 0.81* | ||||
| Standard error | 0.09 | ||||
| 95 % confidence interval | 0.65–0.99 | ||||
Notes: n = 1610. Quadratic measure of age for men was not statistically significant, so it is not included in the models in this table.
p < .10;
p < .05;
p < .01
After age is controlled for, positive physical health selection is still evident for men and women, particularly those moving to urban areas. Both men and women moving to cities in Malawi were physically healthier before migration than those who remained in MLSFH villages of origin, even after age is included in the models. The same is true for men moving to other rural areas of Malawi. The relationship with age (Model 2 in Tables 3 and 4) is consistent across migration destinations for men and women: as before, we find a nonlinear association with migration for women and a negative association between migration and age for men.
Although we find that migration health selection is more common for physical health, we also find statistically significant patterns for mental health. Women who moved to towns compared with female nonmigrants had significantly worse mental health, a result that is consistent across all models. We do not, however, find any statistically significant relationships between mental health and migration among men.
HIV status has been strongly linked to migration in previous studies (see Introduction). Our study additionally shows that the association of HIV status with migration varies by destination (Model 3). HIV-infected women were significantly more likely to move to another rural area (instead of staying in a MLSFH village) than HIV-uninfected women (RRR = 2.28, 95 % CI = 1.01–9.02). Among men, those who were HIV-infected were more likely than the uninfected to move to all three destinations: urban, town, and another rural area.
In the final two models, our analyses document that HIV status moderates the relationship between migration and health in Malawi for one specific migration pattern: rural to urban. As shown in Model 4, among HIV-infected women, better physical health is associated with higher likelihood of moving to urban areas than nonmigration (RRR = 2.07, 95 % CI = 1.02–4.23, ME = 0.003). We also find that the main effect for physical health is statistically significant in Model 4 for both men and women, which indicates that migration to urban areas also selected individuals with better physical health (regardless of HIV status). For men, we find no relationship for the interaction with physical health. However, results for mental health (Table 4, Model 5) show that for HIV-infected men, better mental health before migration is associated with lower likelihood of urban migration than not moving, compared with those not infected with HIV (RRR = 0.81, 95 % CI = 0.65–0.99, ME = −0.002).
Return Migration and Health
Finally, we examine the relationship between return migration and health (Tables 5 and 6). Among women (Table 5), we do not find differences in health status between return migrants and permanent migrants or nonmigrants for physical health, mental health, HIV status, or the interactions. For men (Table 6), we find that healthier men (pre-migration) were more likely to be permanent migrants compared with return migrants (Model 1) (RRR = 1.04, 95 % CI = 1.00–1.10). HIV-infected men were more likely to be permanent migrants compared with return migrants than HIV-uninfected men. Another factor associated with return migration is the MLSFH survey wave: the relative risk of not moving (compared with return migration) is significantly less between 2008 and 2010 than between 2006 and 2008. Age is significantly associated with return migration. At higher ages, men were less likely to be permanent migrants instead of return migrants; the relationship for women is nonlinear, with the likelihood of being a permanent migrant (instead of return migrant) first decreasing and then increasing at higher ages.
Table 5.
Random-effects multinomial logistic regression results (relative risk ratios) for migration health selection, by migration destination: MLSFH women, 2006–2010
| Model 1 | Model 2 | Model 3 | Model 4 | Model 5 | |
|---|---|---|---|---|---|
| Nonmigrant vs. Return Migrant | |||||
| Physical health | 0.98 | 0.98 | 0.98 | 0.98 | 0.98 |
| Standard error | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 |
| 95 % confidence interval | 0.95–1.01 | 0.96–1.01 | 0.95–1.01 | 0.95–1.01 | 0.95–1.01 |
| Mental health | 0.99 | 0.99 | 0.99 | 0.99 | 0.99 |
| Standard error | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 |
| 95 % confidence interval | 0.96–1.01 | 0.96–1.01 | 0.96–1.01 | 0.96–1.01 | 0.96–1.01 |
| Age | 0.99 | 0.99 | 0.99 | 0.99 | |
| Standard error | 0.04 | 0.04 | 0.04 | 0.04 | |
| 95 % confidence interval | 0.93–1.07 | 0.93–1.07 | 0.93–1.07 | 0.93–1.07 | |
| Age, squared | 1.00 | 1.00 | 1.00 | 1.00 | |
| Standard error | 0.00 | 0.00 | 0.00 | 0.00 | |
| 95 % confidence interval | 0.99–1.00 | 0.99–1.00 | 0.99–1.00 | 0.99–1.00 | |
| HIV-infected | 0.81 | 0.79 | 0.77 | ||
| Standard error | 0.20 | 0.40 | 0.40 | ||
| 95 % confidence interval | 0.31–2.11 | 0.31–2.04 | 0.30–2.01 | ||
| MLSFH survey 2008 | 0.32** | 0.32** | 0.32** | 0.32** | 0.32** |
| Standard error | 0.06 | 0.06 | 0.06 | 0.06 | 0.06 |
| 95 % confidence interval | 0.22–0.47 | 0.21–0.47 | 0.21–0.47 | 0.21–0.47 | 0.21–0.47 |
| HIV-infected × Physical health | 0.95 | ||||
| Standard error | 0.05 | ||||
| 95 % confidence interval | 0.85–1.06 | ||||
| HIV-infected × Mental health | 0.97 | ||||
| Standard error | 0.04 | ||||
| 95 % confidence interval | 0.89–1.06 | ||||
| Permanent Migrant vs. Return Migrant | |||||
| Physical health | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Standard error | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 |
| 95 % confidence interval | 0.97–1.04 | 0.96–1.00 | 0.96–1.04 | 0.96–1.04 | 0.96–1.04 |
| Mental health | 0.99 | 0.98 | 0.98 | 0.98 | 0.98 |
| Standard error | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 |
| 95 % confidence interval | 0.96–1.02 | 0.95–1.01 | 0.95–1.01 | 0.95–1.01 | 0.95–1.02 |
| Age | 0.91* | 0.90* | 0.90* | 0.90* | |
| Standard error | 0.04 | 0.04 | 0.04 | 0.04 | |
| 95 % confidence interval | 0.83–0.98 | 0.83–0.98 | 0.83–0.98 | 0.83–0.98 | |
| Age, squared | 1.00† | 1.00† | 1.00† | 1.00† | |
| Standard error | 0.00 | 0.00 | 0.00 | 0.00 | |
| 95 % confidence interval | 1.00–1.01 | 1.00–1.01 | 1.00–1.01 | 1.00–1.01 | |
| HIV-infected | 1.19 | 1.18 | 1.06 | ||
| Standard error | 0.66 | 0.66 | 0.61 | ||
| 95 % confidence interval | 0.40–3.56 | 0.40–3.53 | 0.34–3.27 | ||
| MLSFH survey 2008 | 0.89 | 1.04 | 1.04 | 1.03 | 1.04 |
| Standard error | 0.23 | 0.28 | 0.28 | 0.27 | 0.28 |
| 95 % confidence interval | 0.54–1.49 | 0.62–1.75 | 0.62–1.75 | 0.61–1.74 | 0.61–1.77 |
| HIV-infected × Physical health | 0.98 | ||||
| Standard error | 0.07 | ||||
| 95 % confidence interval | 0.86–1.12 | ||||
| HIV-infected × Mental health | 0.96 | ||||
| Standard error | 0.05 | ||||
| 95 % confidence interval | 0.86–1.06 | ||||
Note: n = 1,692.
p < .10;
p < .05;
p < .01
Table 6.
Random-effects multinomial logistic regression results (relative risk ratios) for migration health selection, by migration destination: MLSFH men, 2006–2010
| Model 1 | Model 2 | Model 3 | Model 4 | Model 5 | |
|---|---|---|---|---|---|
| Nonmigrant vs. Return Migrant | |||||
| Physical health | 1.00 | 1.00 | 1.00 | 1.01 | 1.00 |
| Standard error | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 |
| 95 % confidence interval | 0.96–1.04 | 0.97–1.04 | 0.97–1.04 | 0.97–1.05 | 0.97–1.04 |
| Mental health | 1.00 | 1.00 | 1.01 | 1.00 | 1.01 |
| Standard error | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 |
| 95 % confidence interval | 0.97–1.04 | 0.97–1.04 | 0.97–1.04 | 0.97–1.04 | 0.98–1.05 |
| Age | 1.01 | 1.01 | 1.01 | 1.01 | |
| Standard error | 0.01 | 0.01 | 0.01 | 0.01 | |
| 95 % confidence interval | 0.99–1.02 | 0.99–1.02 | 0.99–1.02 | 0.99–1.03 | |
| HIV-infected | 1.52 | 1.62 | 1.55 | ||
| Standard error | 1.13 | 1.23 | 1.16 | ||
| 95 % confidence interval | 0.35–6.54 | 0.36–7.16 | 0.36–6.70 | ||
| MLSFH survey 2008 | 0.23** | 0.22** | 0.23** | 0.23** | 0.22** |
| Standard error | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 |
| 95 % confidence interval | 0.15–0.36 | 0.13–0.36 | 0.14–0.36 | 0.14–0.36 | 0.14–0.35 |
| HIV-infected × Physical health | 0.94 | ||||
| Standard error | 0.09 | ||||
| 95 % confidence interval | 0.78–1.13 | ||||
| HIV-infected × Mental health | 0.89 | ||||
| Standard error | 0.08 | ||||
| 95 % confidence interval | 0.75–1.05 | ||||
| Permanent Migrant vs. Return Migrant | |||||
| Physical health | 1.04* | 1.03 | 1.03 | 1.04 | 1.03 |
| Standard error | 0.03 | 0.03 | 0.03 | 0.03 | 0.03 |
| 95 % confidence interval | 1.00–1.10 | 0.98–1.08 | 0.98–1.08 | 0.98–1.09 | 0.98–1.08 |
| Mental health | 0.99 | 0.99 | 0.99 | 0.99 | 1.00 |
| Standard error | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 |
| 95 % confidence interval | 0.96–1.03 | 0.95–1.00 | 0.96–1.03 | 0.95–1.03 | 0.96–1.05 |
| Age | 0.98* | 0.97* | 0.97* | 0.97* | |
| Standard error | 0.01 | 0.01 | 0.01 | 0.01 | |
| 95 % confidence interval | 0.95–0.99 | 0.95–1.00 | 0.95–1.00 | 0.95–1.00 | |
| HIV-infected | 4.16† | 4.56† | 3.85† | ||
| Standard error | 3.36 | 3.74 | 3.17 | ||
| 95 % confidence interval | 0.85–20.24 | 0.91–22.80 | 0.77–19.36 | ||
| MLSFH survey 2008 | 0.55* | 0.61† | 0.61† | 0.62 | 0.60 |
| Standard error | 0.16 | 0.18 | 0.18 | 0.18 | 0.18 |
| 95 % confidence interval | 0.31–0.98 | 0.34–1.09 | 0.34–1.10 | 0.35–1.11 | 0.33–1.07 |
| HIV-infected × Physical health | 0.92 | ||||
| Standard error | 0.09 | ||||
| 95 % confidence interval | 0.75–1.12 | ||||
| HIV-infected × Mental health | 0.86 | ||||
| Standard error | 0.08 | ||||
| 95 % confidence interval | 0.72–1.04 | ||||
Notes: n = 1,148. Quadratic measure of age for men was not statistically significant, so it is not included in the models in this table.
p < .10;
p < .05;
p < .01
Discussion
Drawing on exceptional data for SSA that include pre-migration information on health combined with information on migrant destinations, our analyses find that the healthy migrant hypothesis generally applies in Malawi. Healthier men and women from rural areas are more likely to migrate. Among women, this phenomenon appears to be due to the selection of women from young and healthy age groups into migration. For men, this relationship is maintained even after age and other covariates are controlled for. This positive health selection, however, applies only to physical health; our analyses do not find that men and women with better mental health are more likely to move.
The healthy migrant phenomenon differs by migration destination. Our results are strongest for migration to cities: men and women moving to urban areas are significantly healthier than nonmigrants. We also find that rural-to-rural male and female migrants are significantly healthier before moving. The results are weakest for movement to towns. Similarly, the moderating role of HIV infection in the relationship between migration and health applies only to rural-to-urban migrants.
HIV infection plays a major role in migration health selection in Malawi. As elsewhere, we find that HIV-infected individuals are generally more likely to migrate than those who are HIV-uninfected (Anglewicz 2012; Anglewicz et al. 2016), but this relationship varies by the sex of the migrant and destination. Our results show that HIV-infected women are more likely to move to other rural areas compared with HIV-uninfected women; and HIV-infected men are more likely to move to other rural areas, towns, and cities in Malawi than men who are uninfected. We also find important interactions between HIV status and health among those moving to cities. For HIV-infected women, compared with those who are HIV-uninfected, better physical health is associated with higher likelihood of urban migration than nonmigration; and, as shown in the main effects for physical health, healthier HIV-uninfected women and men are also more likely to move to cities. Interestingly, HIV-infected men with better mental health are less likely to move to cities, suggesting that the HIV-infected men who move to cities have worse mental health.
We do not find health differences between return migrants and nonmigrants, but we do find that male permanent migrants have better pre-migration physical health than return migrants. This may suggest that better physical health may be required in order for migration to be successful; the lack of pre-migration health advantage for return migrants may be the reason why they move back to the MLSFH village of origin.
We suspect that the greater likelihood of HIV-infected individuals to migrate has more than one explanation. As previous research suggests, HIV-infected individuals are more likely to experience marital dissolution, after which they return to rural homes (Anglewicz 2012). However, HIV-infected individuals who move to towns or cities may do so to gain better access to antiretroviral therapy (ART). This may explain why physically healthier HIV-infected women move to cities (as suggested elsewhere; Vearey 2008) because better physical health may be required for the HIV-infected to successfully transition to urban residence.
Our research demonstrates the utility of more-detailed information on migrant destination and HIV status in examining migration health selection, but there are some important limitations as well. Our sample size is limited, resulting in large standard errors for several of our relationships of interest. Our MLSFH/MHM data include only migrants originating from rural areas. Those who move from cities and towns may differ in the relationship between migration, health, and HIV status. We also do not compare the health status of these migrants with those in areas of destination. We do not explore here the effects of migration on health net of these selection effects, but we plan to in future work. Our research cannot identify the causal effects of health on migration, or vice versa. Although we can generally establish the time order in this relationship, which is unusual in SSA migration research, other characteristics might still be associated with both migration and health that we do not control for in this research.
Another challenge in migration and health research is the duration between interview and subsequent migration, when health status could change. HIV status is unlikely to have changed for many during this period. The HIV incidence rate for the MLSFH is very small, at 0.7 per 100 person-years between 2004 and 2006 (95 % CI = 0.4 to 1.0) (Obare et al. 2009). However, the health status of migrants may change during this period, which potentially has implications for our results. If, for example, health improved for migrants between the time of interview and migration, we would potentially underestimate the healthy migrant phenomenon in this analysis.
Our research contributes to the findings on the importance of migration-health interrelations for demographic and social change in SSA, and also has important implications for research and programs. Policies and programs have often targeted migrants in HIV prevention efforts, but this may be too late if migrants are already infected at the time of moving. However, the sexual behavior of HIV-infected migrants after moving may have implications for the further spread of the epidemic. Urban population growth has important implications for health, planning, and development; and the flow of HIV-infected individuals into cities adds to this issue (White and Lindstrom 2005). If HIV-infected individuals are indeed moving to cities to better access ART, programs may seek to quickly connect migrants to HIV treatment facilities shortly after migration, while also shifting resources to accommodate greater demand for these services in urban areas. These programs may also benefit from a focus on mental health, which appears to be worse among HIV-infected urban migrants (although physical health is better than rural nonmigrants). Similarly, the permanence of these migrants in cities and return migration are important issues, given that governments are often concerned with the extent of job availability in cities (White and Lindstrom 2005). Finally, our results show that research and programs should be aware of the diversity among migrants in health status: the exact relationship between migration and health differs by destination and gender, which may help to appropriately target interventions.
Acknowledgments
The authors thank Invest in Knowledge Initiative, which collected data for the MHM and MLSFH. We are also very grateful to the reviewers for their very insightful and helpful comments. This research was funded by National Institute of Child Health and Development (NICHD) (MHM Grant No. R21HD071471-01; MLSFH Grant Nos. R03 HD05 8976, R21 HD050652, R01 HD044228, R01 HD053781, and R24 HD-044964).
Appendix
Fig. 2.
Predictive margins of the probability of migration at SF-12 PCS of 20–70, by gender
Table 7.
Logistic regression results for previous migration, measured as lived outside the district for one or more months in past year
| Women | Men | |||||||
|---|---|---|---|---|---|---|---|---|
|
|
||||||||
| 2006 | 2008 | 2006 | 2008 | |||||
|
|
||||||||
| Odds Ratio | SE | OR | SE | Odds Ratio | SE | Odds Ratio | SE | |
| Physical Health/Previous Migration | ||||||||
| Physical health | 1.17 | 0.10 | 1.03 | 0.03 | 1.01 | 0.05 | 1.10 | 0.06 |
| Mental health | 1.01 | 0.03 | 1.01 | 0.01 | 1.00 | 0.02 | 0.99 | 0.02 |
| Previous migration | 1.43 | 0.64 | 0.66 | 0.21 | 2.09* | 0.74 | 0.76 | 0.32 |
| Inter: previous migration × physical health | 0.93 | 0.08 | 0.95 | 0.04 | 1.01 | 0.06 | 0.92 | 0.07 |
| Mental Health/Previous Migration | ||||||||
| Physical health | 1.16 | 0.09 | 1.01 | 0.01 | 1.02 | 0.03 | 1.04 | 0.03 |
| Mental health | 1.02 | 0.04 | 1.02 | 0.02 | 1.00 | 0.04 | 1.01 | 0.02 |
| Previous migration | 1.10 | 0.34 | 0.65 | 0.15 | 2.16* | 0.76 | 0.77 | 0.23 |
| Inter: previous migration × mental health | 0.98 | 0.07 | 0.97 | 0.02 | 0.99 | 0.05 | 0.94 | 0.04 |
p < .05;
p < .01
Table 8.
Logistic regression results for previous migration, measured as lived outside the current residence for six or more months since age 15
| Women | Men | |||||||
|---|---|---|---|---|---|---|---|---|
|
|
||||||||
| 2006 | 2008 | 2006 | 2008 | |||||
|
|
||||||||
| Odds Ratio | SE | Odds Ratio | SE | Odds Ratio | SE | Odds Ratio | SE | |
| Physical Health/Previous Migration | ||||||||
| Physical health | 1.05* | 0.03 | 1.02 | 0.02 | 1.04 | 0.04 | 1.06 | 0.03 |
| Mental health | 0.99 | 0.02 | 1.01 | 0.01 | 0.99 | 0.03 | 0.99 | 0.02 |
| Previous migration | 0.68 | 0.24 | 0.65 | 0.20 | 1.05 | 0.70 | 1.99 | 1.00 |
| Previous migration × Physical health | 0.93 | 0.04 | 0.96 | 0.04 | 1.02 | 0.12 | 0.99 | 0.08 |
| Mental Health/Previous Migration | ||||||||
| Physical health | 1.06 | 0.04 | 1.01 | 0.01 | 1.04 | 0.03 | 1.04 | 0.02 |
| Mental health | 0.99 | 0.02 | 1.00 | 0.01 | 0.99 | 0.02 | 0.99 | 0.02 |
| Previous migration | 2.07 | 1.19 | 0.67 | 0.21 | 0.52 | 0.59 | 0.63 | 0.23 |
| Previous migration × Mental health | 1.00 | 0.07 | 1.01 | 0.04 | 1.21 | 0.25 | 0.98 | 0.04 |
p < .05;
p < .01
Table 9.
Pre-migration background characteristics for individuals in the return migration sample compared with the full sample: MLSFH men and women, 2006 and 2008
| 2006 | 2008 | |||
|---|---|---|---|---|
|
|
||||
| Return Migration Sample Only | Full Sample | Return Migration Sample Only | Full Sample | |
| Female (%) | 50.6 | 57.6** | 56.3 | 62.9** |
| Mean Physical Health | 52.3 | 52.5 | 51.6 | 51.5 |
| Mean Mental Health | 55.5 | 55.6 | 54.3 | 54.1 |
| Mean Age | 36.2 | 34.9* | 40.6 | 41.1 |
| HIV-Infected | 5.2% | 5.4% | 4.9% | 6.0% |
| N | 633 | 2212 | 1176 | 1838 |
Note: Asterisks indicate that t tests and chi-squared tests of the difference between return migration sample and full sample are statistically significant.
p < .05;
p < .01
Footnotes
Nauman et al. (2015) referred to this phenomenon as the “midnight train effect” instead of “salmon bias” because it is often the persons least fit for the trip in the first place who return to origin, whereas for salmon, those who make it back to origin are the most fit.
Between 1998 and 2004, the MLSFH was known as the Malawi Diffusion and Ideational Change Project (MDICP).
Most migrants moving internationally were MLSFH respondents from the central region, Mchinji, which boarders on Zambia, and moved a relatively short distance across the border.
Although the MHM study traced migrants who were interviewed in MLSFH 2004 and migrated afterward, the MLSFH did not include SF-12 scores until 2006. Therefore, these migrants are not included in the analysis here.
We also examined an alternative measure of return migration, living outside the current residence for six months or more since age 15. Analysis of this measure of return migration yielded results that were not substantively different.
We also include a quadratic measure of age to test for a nonlinear relationship with future migration.
We also examined whether individuals who migrated previously have different health and are more likely to move again. To do so, we ran regressions similar to those in Step 1, but ran them separately for each year (2006 and 2008) instead of using pooled random-effects regressions, and included measures of previous migration (lived outside the district for one month or more in the past year, and lived outside the current residence for six months or more since age 15). We also included interactions between these previous migration measures and both mental and physical health, which test whether those who previously migrated and have different health are more likely to move again. The results (Tables 7 and 8 in the appendix) show that previous migrants do not have greater odds of moving again (for either measure of migration), and the interactions between previous migration and health are not statistically significant in any of the models.
The MLSFH survey that included information on return migration (staying outside the district for one month or more in the past year) was administered separately from the HIV test and measure of SF-12 score. As a result, fewer respondents answered the question on previous migration, and our overall sample size for this analysis is reduced (n = 2,840). We compare the characteristics of individuals in the full sample compared with the sample of return migration (Table 9 in the appendix) and find no statistically significant differences in SF-12 health status or HIV infection, although there are differences in gender and age.
References
- Agadjanian V, Yabiku ST, Cau B. Men’s migration and women’s fertility in rural Mozambique. Demography. 2011;48:1029–1048. doi: 10.1007/s13524-011-0039-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anglewicz P. Migration, marital change and HIV infection in Malawi. Demography. 2012;49:239–265. doi: 10.1007/s13524-011-0072-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anglewicz P, Adams J, Obare F, Kohler HP, Watkins S. The Malawi Diffusion and Ideational Change Project 2004–06: Data collection, data quality, and analysis of attrition. Demographic Research. 2009;20(article 21):503–540. doi: 10.4054/demres.2009.20.21. https://doi.org/10.4054/DemRes.2009.20.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anglewicz P, Reniers G. HIV status, gender, and marriage dynamics among adults in rural Malawi. Studies in Family Planning. 2014;45:415–428. doi: 10.1111/j.1728-4465.2014.00005.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anglewicz P, VanLandingham M, Manda-Taylor L, Kohler HP. Migration and HIV infection in Malawi. AIDS. 2016;30:2099–2105. doi: 10.1097/QAD.0000000000001150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anglewicz P, VanLandingham M, Manda-Taylor L, Kohler HP. Cohort profile: Internal migration in sub-Saharan Africa—The Migration and Health in Malawi (MHM) Study. BMJ Open. 2017;7(5):e014799. doi: 10.1136/bmjopen-2016-014799. http://doi.org/10.1136/bmjopen-2016-014799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnoldo C. Ethnicity and marriage patterns in Mozambique. African Population Studies. 2004;19(1):143–164. [Google Scholar]
- Barrios S, Bertinelli L, Strobl E. Climatic change and rural-urban migration: The case of sub-Saharan Africa. Journal of Urban Economics. 2006;60:357–371. [Google Scholar]
- Bignami-Van Assche S, Reniers G, Weinreb AA. An assessment of the KDICP and MDICP data quality: Interviewer effects, question reliability and sample attrition. Demographic Research, Special Collection. 2003;1(article 2):31–76. https://doi.org/10.4054/DemRes.2003.S1.2. [Google Scholar]
- Boerma JT, Urassa M, Nnko S, Ng’weshemi J, Isingo R, Zaba B, Mwaluko G. Sociodemographic context of the AIDS epidemic in a rural area in Tanzania with a focus on people’s mobility and marriage. Sexually Transmitted Infections. 2002;78(Suppl 1):i97–i105. doi: 10.1136/sti.78.suppl_1.i97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt R. The mental health of people living with HIV/AIDS in Africa: A systematic review. African Journal of AIDS Research. 2009;8:123–133. doi: 10.2989/AJAR.2009.8.2.1.853. [DOI] [PubMed] [Google Scholar]
- Brockerhoff M. Rural-to-urban migration and child survival in Senegal. Demography. 1990;27:601–616. [PubMed] [Google Scholar]
- Brockerhoff M. Fertility and family planning in African cities: The impact of female migration. Journal of Biosocial Science. 1995a;27:347–358. doi: 10.1017/s0021932000022872. [DOI] [PubMed] [Google Scholar]
- Brockerhoff M. Child survival in big cities: The disadvantages of migrants. Social Science & Medicine. 1995b;40:1371–1383. doi: 10.1016/0277-9536(94)00268-x. [DOI] [PubMed] [Google Scholar]
- Brockerhoff M, Biddlecom AE. Migration, sexual behavior and the risk of HIV in Kenya. International Migration Review. 1999;33:833–856. [Google Scholar]
- Brockerhoff M, Eu H. Demographic and socioeconomic determinants of female rural to urban migration in sub-Saharan Africa. International Migration Review. 1993;27:557–577. [PubMed] [Google Scholar]
- Chalasani S, Mensch BS, Hewett PC. Migration among adolescents from rural Malawi. Paper presented at the annual meeting of the Population Association of America; New Orleans, LA. 2013. Apr, [Google Scholar]
- Chattopadhyay A, White MJ, Debpuur C. Migrant fertility in Ghana: Selection versus adaptation and disruption as causal mechanisms. Population Studies. 2006;60:189–203. doi: 10.1080/00324720600646287. [DOI] [PubMed] [Google Scholar]
- Chen J. Internal migration and health: Re-examining the healthy migrant phenomenon in China. Social Science & Medicine. 2011;72:1294–1301. doi: 10.1016/j.socscimed.2011.02.016. [DOI] [PubMed] [Google Scholar]
- Chimwaza AF, Watkins SC. Giving care to people with symptoms of AIDS in rural sub-Saharan Africa. AIDS Care. 2004;16:795–807. doi: 10.1080/09540120412331290211. [DOI] [PubMed] [Google Scholar]
- Chirwa WC. Migrant labor, sexual networking and multi-partnered sex in Malawi. Health Transition Review. 1997;7(S3):5–15. [Google Scholar]
- Clark SJ, Collinson MA, Kahn K, Drullinger K, Tollman SM. Returning home to die: Circular labour migration and mortality in South Africa. Scandinavian Journal of Public Health. 2007;35(Suppl 69):35–44. doi: 10.1080/14034950701355619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coast E. Local understandings of, and responses to, HIV: Rural-urban migrants in Tanzania. Social Science & Medicine. 2006;63:1000–1010. doi: 10.1016/j.socscimed.2006.03.009. [DOI] [PubMed] [Google Scholar]
- Coffee M, Garnett G, Mlilo M, Voeten H, Chandiwana S, Gregson S. Patterns of movement and risk of HIV infection in rural Zimbabwe. Journal of Infectious Diseases. 2005;191(Suppl 1):159–167. doi: 10.1086/425270. [DOI] [PubMed] [Google Scholar]
- Collinson MA, Tollman SM, Kahn K. Migration, settlement change and health in post-apartheid South Africa: Triangulating health and demographic surveillance with national census data. Scandinavian Journal of Public Health. 2007;35(Suppl 69):77–84. doi: 10.1080/14034950701356401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collinson MA, White MJ, Bocquier P, McGarvey ST, Afolabi SA, Clark SJ, … Tollman SM. Migration and the epidemiological transition: Insights from the Agincourt sub-district of northeast South Africa. Global Health Action. 2014;7(1) doi: 10.3402/gha.v7.23514. https://doi.org/10.3402/gha.v7.23514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Englund H. The village in the city, the city in the village: Migrants in Lilongwe. Journal of Southern African Studies. 2002;28:137–154. [Google Scholar]
- Floyd S, Crampin A, Glynn J, Mwenebabu M, Mnkhondia S, Ngwira B, … Fine PE. The long-term social and economic impact of HIV on the spouses of infected individuals in northern Malawi. Tropical Medicine and International Health. 2008;13(4):1–12. doi: 10.1111/j.1365-3156.2008.02030.x. [DOI] [PubMed] [Google Scholar]
- Ford K, Hosegood V. AIDS mortality and the mobility of children in KwaZulu Natal, South Africa. Demography. 2005;42:757–768. doi: 10.1353/dem.2005.0029. [DOI] [PubMed] [Google Scholar]
- Freeman M, Nkomo N, Kafaar Z, Kelly K. Mental disorder in people living with HIV/AIDS in South Africa. South African Journal of Psychology. 2008;38:489–500. doi: 10.1080/09540120701426482. [DOI] [PubMed] [Google Scholar]
- Gandhi SK, Salmon JW, Zhao SZ, Lambert BL, Gore PR, Conrad K. Psychometric evaluation of the 12-item Short-Form Health Survey (SF-12) in osteoarthritis and rheumatoid arthritis clinical trials. Clinical Therapeutics. 2001;23:1080–1098. doi: 10.1016/s0149-2918(01)80093-x. [DOI] [PubMed] [Google Scholar]
- Gelderblom D, Kok PC. Urbanization: South Africa’s challenge: Volume 1, Dynamics. Pretoria, South Africa: HSRC Publishers; 1994. [Google Scholar]
- Gill S, Butterworth P, Rodgers B, Mackinnon A. Validity of the mental health component of the 12-item Short-Form Health Survey (MCS-12) as a measure of common mental disorders in the general population. Psychiatry Research. 2007;152:63–71. doi: 10.1016/j.psychres.2006.11.005. [DOI] [PubMed] [Google Scholar]
- Ginsburg C, Bocquier P, Béguy D, Afolabi S, Augusto O, Derra K, … Collinson MA. Healthy or unhealthy migrants? Identifying internal migration effects on mortality in Africa using health and demographic surveillance systems of the INDEPTH network. Social Science & Medicine. 2016;164:59–73. doi: 10.1016/j.socscimed.2016.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Githeko AK, Lindsay SW, Confalonieri UE, Patz JA. Climate change and vector-borne diseases: A regional analysis. Bulletin of the World Health Organization. 2000;78:1136–1147. [PMC free article] [PubMed] [Google Scholar]
- Gregory R, Isingo R, Marston M, Urassa M, Changalucha J, Ndege M, … Zaba B. HIV and marital outcomes: Dissolution and remarriage in Kisesa, Tanzania. Presented at the annual meeting of the Population Association of America; New York, NY. 2007. Mar, [Google Scholar]
- Gregson S, Mushati P, Nyamukapa C. Adult mortality and erosion of household viability in AIDS-afflicted towns, estates, and villages in eastern Zimbabwe. Journal of Acquired Immune Deficiency Syndromes. 2007;44:188–195. doi: 10.1097/01.qai.0000247230.68246.13. [DOI] [PubMed] [Google Scholar]
- Guilmoto CZ. Institutions and migrations: Short-term versus long-term moves in rural west Africa. Population Studies. 1998;52:85–103. [Google Scholar]
- Gushulak BD, MacPherson DW. Health aspects of the pre-departure phase of migration. PLoS Medicine. 2011;8(5):e1001035. doi: 10.1371/journal.pmed.1001035. https://doi.org/10.1371/journal.pmed.1001035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasso G, Massey DS, Rosenzweig MR, Smith JP. Immigrant health: Selectivity and acculturation. In: Anderson NB, Bulatao RA, Cohen B, editors. Critical perspectives on racial and ethnic differences in health in late life. Washington, DC: National Academies Press; 2004. pp. 227–266. [PubMed] [Google Scholar]
- Jenkinson C, Chandola T, Coulter A, Bruster S. An assessment of the construct validity of the SF-12 summary scores across ethnic groups. Journal of Public Health. 2001;23:187–194. doi: 10.1093/pubmed/23.3.187. [DOI] [PubMed] [Google Scholar]
- Johnson CL, Appleton CC. Schistosomiasis and rural-urban migration. Abstract presented at the annual conference of the Epidemiological Society of Southern Africa; East London, UK. 2000. Feb, [Google Scholar]
- Kahn K, Collinson M, Tollman S, Wolff B, Garenne M, Clark S. Health consequences of migration: Evidence from South Africa’s rural northeast (Agincourt). Paper prepared for Conference on African Migration in Comparative Perspective; Johannesburg, South Africa. 2003. Jun, [Google Scholar]
- Kahn K, Tollman SM, Collinson MA, Clark SJ, Twine R, Clark BD, … Garenne ML. Research into health, population and social transitions in rural South Africa: Data and methods of the Agincourt Health and Demographic Surveillance System. Scandinavian Journal of Public Health. 2007;35(Suppl 69):8–20. doi: 10.1080/14034950701505031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler HP, Watkins SC, Behrman JR, Anglewicz P, Kohler IV, Thornton RL, … Kalilani-Phiri L. Cohort profile: The Malawi Longitudinal Study of Families and Health (MLSFH) International Journal of Epidemiology. 2015;44:394–404. doi: 10.1093/ije/dyu049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler IV, Payne CF, Bandawe C, Kohler HP. The demography of mental health among mature adults in a low-income high-HIV-prevalence context. Demography. 2017;54:1529–1558. doi: 10.1007/s13524-017-0596-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagarde E, Schim van der Loeff MS, Enel C, Holmgren B, Dray-Spira R, Pison G … MECORA Group. Mobility and the spread of human immunodeficiency virus into rural areas of west Africa. International Journal of Epidemiology. 2003;32:744–752. doi: 10.1093/ije/dyg111. [DOI] [PubMed] [Google Scholar]
- Landale NS, Oropesa RS, Gorman BK. Migration and infant death: Assimilation or selective migration among Puerto Ricans? American Sociological Review. 2000;65:888–909. [Google Scholar]
- Lee BS. The influence of rural-urban migration on migrant’s fertility behavior in Cameroon. International Migration Review. 1992;26:1416–1447. [PubMed] [Google Scholar]
- Li X, Zhang L, Stanton B, Fang X, Xiong Q, Lin D. HIV/AIDS-related sexual risk behaviors among rural residents in China: Potential role of rural-to-urban migration. AIDS Education and Prevention. 2007;19:396–408. doi: 10.1521/aeap.2007.19.5.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopman BA, Nyamukapa C, Hallett TB, Mushati P, Spark-du Preez N, Kurwa F, … Gregson S. Role of widows in the heterosexual transmission of HIV in Manicaland, Zimbabwe, 1998–2003. Sexually Transmitted Infections. 2009;85:i41–i48. doi: 10.1136/sti.2008.033043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y. Test of the “healthy migrant hypothesis”: A longitudinal analysis of health selectivity of internal migration in Indonesia. Social Science & Medicine. 2008;67:1331–1339. doi: 10.1016/j.socscimed.2008.06.017. [DOI] [PubMed] [Google Scholar]
- Lu Y. Rural-urban migration and health: evidence from longitudinal data in Indonesia. Social Science & Medicine. 2010;70:412–419. doi: 10.1016/j.socscimed.2009.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Qin L. Healthy migrant and salmon bias hypotheses: A study of health and internal migration in China. Social Science & Medicine. 2014;102:41–48. doi: 10.1016/j.socscimed.2013.11.040. [DOI] [PubMed] [Google Scholar]
- Luke N, Xu H, Mberu BU, Goldberg RE. Migration experience and premarital sexual initiation in urban Kenya: An event history analysis. Studies in Family Planning. 2012;43:115–126. doi: 10.1111/j.1728-4465.2012.00309.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo X, George ML, Kakouras I, Edwards CL, Pietrobon R, Richardson W, Hey L. Reliability, validity, and responsiveness of the short form 12-item survey (SF-12) in patients with back pain. Spine. 2003;28:1739–1745. doi: 10.1097/01.BRS.0000083169.58671.96. [DOI] [PubMed] [Google Scholar]
- Lurie MN, Williams BG, Zuma K, Mkaya-Mwamburi D, Garnett G, Sturm AW, … Abdool Karim SS. The impact of migration on HIV-1 transmission in South Africa: A study of migrant and nonmigrant men and their partners. Sexually Transmitted Diseases. 2003;30:149–156. doi: 10.1097/00007435-200302000-00011. [DOI] [PubMed] [Google Scholar]
- Mberu B, White M. Internal migration and health: Premarital sexual initiation in Nigeria. Social Science & Medicine. 2011;72:1284–1293. doi: 10.1016/j.socscimed.2011.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mtika M, Doctor H. Matriliny, patriliny, and wealth flow variations in rural Malawi. African Sociological Review. 2002;6(2):71–97. [Google Scholar]
- Myroniuk TW. Marital dissolutions and the health of older individuals in a rural African context. Journals of Gerontology, Series B: Psychological Sciences and Social Sciences. 2017;72:656–664. doi: 10.1093/geronb/gbw077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Statistical Office (NSO), & ICF Macro. Malawi Demographic and Health Survey 2010. Zomba, Malawi: NSO/Malawi and ICF Macro; 2011. [Google Scholar]
- Nauman E, VanLandingham M, Anglewicz P. Migration, urbanization and health. In: White M, editor. The handbook of migration and population distribution. Dordrecht, the Netherlands: Springer; 2016. pp. 451–463. [Google Scholar]
- Nauman E, VanLandingham M, Anglewicz P, Patthavanit U, Punpuing S. Rural-to-urban migration and changes in health among young adults in Thailand. Demography. 2015;52:233–257. doi: 10.1007/s13524-014-0365-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obare F, Fleming P, Anglewicz P, Thornton R, Martinson F, Kapatuka A, … Kohler HP. Acceptance of repeat population-based voluntary counselling and testing for HIV in rural Malawi. Sexually Transmitted Infections. 2009;85:139–144. doi: 10.1136/sti.2008.030320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oucho JO, Gould WTS. Internal migration, urbanization, and population distribution. In: Foote KA, Hill KH, Martin LG, editors. Demographic change in sub-Saharan Africa. Washington DC: National Research Council; 1993. pp. 256–296. [Google Scholar]
- Palloni A, Arias E. Paradox lost: Explaining the Hispanic adult mortality advantage. Demography. 2004;41:385–415. doi: 10.1353/dem.2004.0024. [DOI] [PubMed] [Google Scholar]
- Palloni A, Morenoff JD. Interpreting the paradoxical in the Hispanic paradox: Demographic and epidemiologic approaches. Annals of the New York Academy of Sciences. 2001;954:140–174. doi: 10.1111/j.1749-6632.2001.tb02751.x. [DOI] [PubMed] [Google Scholar]
- Pison G, Le Guenno B, Legarde E, Enel C, Seck C. Seasonal migration: A risk factor for HIV in rural Senegal. Journal of Acquired Immune Deficiency Syndrome. 1993;6:196–200. [PubMed] [Google Scholar]
- Porter L, Hao LX, Bishai D, Serwadda D, Wawer MJ, Lutalo T, Gray R. HIV status and union dissolution in sub-Saharan Africa: The case of Rakai, Uganda. Demography. 2004;41:465–482. doi: 10.1353/dem.2004.0025. [DOI] [PubMed] [Google Scholar]
- Posel G. Have migration patterns in post-apartheid South Africa changed?. Paper presented at the Conference on African Migration in Comparative Perspective; Johannesburg, South Africa. 2003. Jun, [Google Scholar]
- Reed HE. Moving across boundaries: Migration in South Africa, 1950–2000. Demography. 2013;50:71–95. doi: 10.1007/s13524-012-0140-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed HE, Andrzejewski CS, White MJ. Men’s and women’s migration in coastal Ghana: An event history analysis. Demographic Research. 2010;22(article 25):771–812. doi: 10.4054/DemRes.2010.22.25. https://doi.org/10.4054/DemRes.2010.22.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reniers G. Divorce and remarriage in rural Malawi. Demographic Research. 2003;S1:175–206. (article 6) https://doi.org/10.4054/DemRes.2003.S1.6. [Google Scholar]
- Rokicki S, Montana L, Fink G. Impact of migration on fertility and abortion: Evidence from the Household and Welfare Study of Accra. Demography. 2014;51:2229–2254. doi: 10.1007/s13524-014-0339-0. [DOI] [PubMed] [Google Scholar]
- Roux N, van Tonder L. Migration and health in South Africa. In: Kok P, Gelderblom D, van Zyl J, editors. Migration in South and southern Africa: Dynamics and determinants. Cape Town, South Africa: HSRC Press; 2006. pp. 120–146. [Google Scholar]
- Schuyler AC, Edelstein ZR, Mathur S, Sekasanvu J, Nalugoda F, Gray R, … Santelli JS. Mobility among youth in Rakai, Uganda: Trends, characteristics, and associations with behavioural risk factors for HIV. Global Public Health. 2015;12:1033–1050. doi: 10.1080/17441692.2015.1074715. https://doi.org/10.1080/17441692.2015.1074715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ssengonzi R, De Jong G, Stokes S. The effect of female migration on infant and child survival in Uganda. Population Research and Policy Review. 2002;21:403–431. [Google Scholar]
- Turra CM, Elo IT. The impact of salmon bias on the Hispanic mortality advantage: New evidence from Social Security data. Population Research and Policy Review. 2008;27:515–530. doi: 10.1007/s11113-008-9087-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- United Nations. World urbanization prospects: The 2014 revision. New York, NY: United Nations Department of Economics and Social Affairs; 2014. [Google Scholar]
- UNAIDS. HIV and AIDS estimates (2015) Malawi: 2016. Retrieved from http://www.unaids.org/en/regionscountries/countries/malawi. [Google Scholar]
- Urassa M, Boerma JT, Isingo R, Ngalula J, Ng’weshemi J, Mwaluko G, Zaba B. The impact of HIV/AIDS on mortality and household mobility in rural Tanzania. AIDS. 2001;15:2017–2023. doi: 10.1097/00002030-200110190-00015. [DOI] [PubMed] [Google Scholar]
- Vearey J. Migration, access to ART, and survivalist livelihood strategies in Johannesburg. African Journal of AIDS Research. 2008;7:361–374. doi: 10.2989/AJAR.2008.7.3.13.660. [DOI] [PubMed] [Google Scholar]
- Ware JE, Jr, Gandek B, Kosinski M, Aaronson NK, Apolone G, Brazier J, … Thunedborg K. The equivalence of SF-36 summary health scores estimated using standard and country-specific algorithms in 10 countries: Results from the IQOLA Project. Journal of Clinical Epidemiology. 1998;51:1167–1170. doi: 10.1016/s0895-4356(98)00108-5. [DOI] [PubMed] [Google Scholar]
- Ware JE, Kosinski M, Keller SD. A 12-item Short-Form Health Survey: Construction of scales and preliminary tests of reliability and validity. Medical Care. 1996;34:220–233. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- Watkins S, Behrman JR, Kohler H-P, Zulu EM. Introduction to “Research on demographic aspects of HIV/AIDS in rural Africa”. Demographic Research. 2003;S1:1–30. (article 1) https://doi.org/10.4054/DemRes.2003.S1.1. [Google Scholar]
- Watts SJ. Marriage migration, a neglected form of long-term mobility: A case study from Ilorin, Nigeria. International Migration Review. 1984;17:682–698. [PubMed] [Google Scholar]
- Weine SM, Kashuba AB. Labor migration and HIV risk: A systematic review of the literature. AIDS and Behavior. 2012;16:1605–1621. doi: 10.1007/s10461-012-0183-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White MJ, Lindstrom DP. Internal migration. In: Poston DL Jr, Micklin M, editors. Handbook of population. New York, NY: Springer; 2005. pp. 311–346. [Google Scholar]
- Wilson ME. Infectious diseases: An ecological perspective. BMJ. 1995;311:1681–1684. doi: 10.1136/bmj.311.7021.1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yabiku ST, Agadjanian V, Sevoyan A. Husbands’ labour migration and wives’ autonomy, Mozambique 2000–2006. Population Studies. 2010;64:293–306. doi: 10.1080/00324728.2010.510200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Derlega VJ, Luo H. Migration, behaviour change and HIV/STD risks in China. AIDS Care. 2007;19:282–288. doi: 10.1080/09540120600909414. [DOI] [PubMed] [Google Scholar]
- Zimmerman C, Kiss L, Hossain M. Migration and health: A framework for 21st century policy-making. PLoS Med. 2011;8(5):e1001034. doi: 10.1371/journal.pmed.1001034. https://doi.org/10.1371/journal.pmed.1001034. [DOI] [PMC free article] [PubMed] [Google Scholar]