Abstract
Rationale
The clinical course of cerebral cavernous malformations (CCMs) is highly unpredictable, with few cross-sectional studies correlating pro-inflammatory genotypes and plasma biomarkers with prior disease severity.
Objective
We hypothesize that a panel of 24 candidate plasma biomarkers, with a reported role in the physiopathology of CCMs, may predict subsequent clinically relevant disease activity.
Methods and Results
Plasma biomarkers were assessed in non-fasting peripheral venous blood collected from consecutive CCM subjects followed for one year after initial sample collection. A first cohort (N=49) was used to define the best model of biomarker level combinations to predict a subsequent symptomatic lesional hemorrhagic expansion within a year following the blood sample. We generated the receiver operating characteristic curves and area under curves (AUC) for each biomarker individually and each weighted linear combination of relevant biomarkers. The best model to predict lesional activity was selected as that minimizing the Akaike Information Criterion (AIC). In this cohort, 11 subjects experienced symptomatic lesional hemorrhagic expansion (5 bleeds and 10 lesional growths) within a year after the blood draw. Subjects had lower soluble cluster of differentiation 14 (sCD14; p=0.05), interleukin-6 (IL-6; p=0.04), vascular endothelial growth factor (VEGF; p=0.0003) levels along with higher plasma levels of interleukin-1 beta (IL-1β; p=0.008) and roundabout guidance receptor 4 (sROBO4; p=0.03). Among the 31 weighted linear combinations of these 5 biomarkers, the best model (with the lowest AIC value=25.3) was the weighted linear combination including sCD14, IL-1β, VEGF and sROBO4, predicting a symptomatic hemorrhagic expansion with a sensitivity of 86% and specificity of 88% (AUC=0.90, p<0.0001). We then validated our best model in the second sequential independent cohort (N=28).
Conclusions
This is the first study reporting a predictive association between plasma biomarkers and subsequent CCM disease clinical activity. This may be applied in clinical prognostication, and stratification of cases in clinical trials.
Keywords: Cerebral cavernous malformation, plasma biomarker, neuroinflammation, prognostic biomarker, cerebrovascular disease/stroke
Subject Terms: Biomarkers, Cerebrovascular Malformations, Intracranial Hemorrhage
INTRODUCTION
Cerebral cavernous malformations (CCMs) are a common cerebrovascular pathology predisposing 0.5% of the population, predisposing to a lifetime risk of intracerebral hemorrhage, seizures and progressive focal neurological deficits.1, 2 A sporadic form of the disease, with solitary lesions, accounts for almost two thirds of cases, while a familial form manifests multifocal lesions developing throughout the patient’s lifetime in different regions of the brain. The familial phenotype is associated with an autosomal dominant Mendelian inheritance with germ line mutations at one of the three CCM gene loci (CCM1/KRIT1, CCM2/MGC4607 and CCM3/PDCD10).3
The lesions harbored in the two forms are histologically indistinguishable and consist of clusters of thin-walled blood filled vascular caverns lined by endothelium, and lacking mature vessel wall angio-architecture.4, 5 Endothelium of sporadic and familial lesions harbors somatic mutations in one of the three documented CCM genes, indicating common pathogenesis mechanisms.5 CCM genes encode proteins involved in maintaining endothelial barrier integrity by inhibiting RhoA associated kinase (ROCK) activation.6 A loss of CCM gene function results in increased ROCK activity leading to endothelial cell-cell junction dysregulation and defective vascular permeability.3 Experimental evidence also suggests a complex interplay between vascular permeability, inflammatory, and angiogenic processes in the neuroglial milieu as central features of CCM.7
Acute lesional bleeding or proliferative hemorrhagic expansion result in clinically significant sequelae. However, the molecular mechanisms underlying such relevant lesional activity remain unclear, and the behavior of CCMs remains highly variable and unpredictable. Pro-inflammatory gene variants have been associated with higher lesion counts in cases with common familial CCM1 (Q455X) mutation.8, 9 Our group has also reported associations of brain vascular permeability,1, 10 and clustered pro-inflammatory biomarkers in peripheral blood plasma7, 11 with cumulative CCM disease severity during a patient’s life. Yet specific biomarker signatures predicting subsequent clinically relevant disease behavior would be most relevant clinically and have remained elusive. Herein, we studied the prognostic association between the plasma levels of a panel of inflammatory and angiogenic proteins previously related to CCM disease pathobiology,7 and the occurrence of a CCM-related clinically significant event in the subsequent year.
METHODS
Because of the sensitive nature of the data collected for this study, requests to access the dataset from qualified researchers trained in human subject confidentiality protocols may be sent to the University of Chicago Medicine at Dr. Issam Awad (e-mail iawad@uchicago.edu).
Patients recruitment
From July 2014 to February 2018, 172 consecutive CCM subjects were evaluated clinically at a single referral center and enrolled in biomarker studies (www.uchospitals.edu/ccm). Of these, 77 CCM subjects (42 sporadic and 35 familial) were followed for 1 year (± 30 days) after biomarker collection, and considered as 2 independent cohorts based on their period of enrollment. The first cohort (Group 1-Algorithm definition) included 49 patients enrolled and biomarker collected between July 2014 to May 2016. The second cohort entitled Group 2-Algorithm testing included an additional 28 patients enrolled between June 2016 and February 2018 (Online Figure I and Online Table I).
All patients gave written informed consent to participate in this research in accordance to the Declaration of Helsinki, and approved by The University of Chicago Institutional Review Board (IRB). The ethical principles guiding the IRB are consistent with The Belmont Report, and comply with the rules and regulations of The Federal Policy for the Protection of Human Subjects (56 FR 28003).
As per currently accepted disease categorization, cases were classified as sporadic if they harbored a solitary lesion on the most sensitive susceptibility weighted magnetic resonance imaging (MRI) sequences, or a cluster of lesions associated with a developmental venous anomaly. They were classified as familial if they harbored multifocal CCMs, a family history of CCM in a first-degree blood relative or a mutation genotyped at a CCM gene locus. Patients with partial or complete CCM lesion resection or any prior brain irradiation were excluded.1, 7, 10
The best weighted combination of biomarkers to predict subsequent disease activity within 1 year (± 30 days) was defined in Group 1-Algorithm definition and then tested in Group 2-Algorithm testing.
For each patient enrolled in the study, the aforementioned features were assessed during the clinical follow-up visit. These were reviewed and adjudicated by the senior author with experience in the care of CCM (IAA), blinded to any knowledge about the biomarker levels, and electronically stored in a secure database for subsequent analysis. For more methodological details, refer to Supplemental Material.
RESULTS
Demographic and CCM lesions characteristics
In the first cohort (Group 1-Algorithm definition), 11 of the 49 subjects manifested a hemorrhagic expansion. One subject experienced a new symptomatic hemorrhage from a known CCM per adjudicated criteria,12 5 developed lesional growth by >3 mm diameter on T2-weighted MRI sequences, and 5 had both symptomatic hemorrhage and lesional growth during the subsequent year after biomarker collection.2, 10 Thirty-eight subjects (78%) did not experience any change in CCM lesions during the same period and were defined as stable.
In the second cohort (Group 2-Algorithm testing), 7 of the 28 subjects experienced hemorrhagic expansion as previously described (2 subjects experienced a new symptomatic hemorrhage, 3 a lesional growth, and 2 both) within one year after biomarker collection.
Among the familial cohort, 8 subjects developed new lesions on the most sensitive MRI susceptibility weighted imaging. Cases with hemorrhagic expansion had higher T2-weighted (p=0.001) and total (p= 0.002) lesion counts, and non-significant trends toward higher prevalence of brainstem lesion location and recent symptomatic hemorrhage in the prior year. There were no significant differences in the age, gender or mean follow-up time between the stable subjects and the ones who experienced a hemorrhagic expansion within the year after the blood sample. However, the cohort of familial cases that developed new lesions within a year were older than familial patients that did not (p=0.02).
Soluble cluster of differentiation 14 (sCD14), vascular endothelial growth factor (VEGF) and interleukin-6 (IL-6) plasma levels were lower while interleukin-1 beta (IL-1β) and soluble roundabout guidance receptor 4 (sROBO4) were higher in subjects who experienced clinical lesional activity within the year following the initial blood sample.
After correction for batch effect when appropriate, subjects experiencing a hemorrhagic expansion showed lower plasma levels of sCD14 (p=0.05), IL-6 (p=0.04) and VEGF (p=0.0003), along with higher IL-1β (p=0.008) and sROBO4 (p=0.03) plasma levels (Online Figure II). There were no potential confounders affecting the plasma levels of the 24 biomarkers such as age of enrollment, phenotype (solitary/sporadic or multifocal/familial), genotype (sporadic, CCM1, 2 or 3) or sex (male or female). In addition, plasma levels of these biomarkers were not associated with brainstem lesion location or symptomatic bleed within the preceding year, known risk factors of lesional activity.4 We did not identify significant association between the formation of new CCMs and the plasma levels of any of the selected biomarkers in the familial cohort.
The combination of sCD14, IL-1β, VEGF and sROBO4 is the best predictor of a future lesional activity
The receiving operating characteristic (ROC) curves for sCD14 [area under the curve (AUC)=0.68, p=0.04), IL-6 (AUC=0.66, p=0.007] showed ‘poor’ accuracy. The accuracies to predict lesional activity were considered as “fair” for sROBO4 (AUC=0.76, p=0.004) and VEGF (AUC=0.77, p=0.01) while it was “good” for IL-1β (AUC=0.82, p=0.0003) (Online Figure II). Further analysis showed that among the 31-possible weighted linear combinations of these 5 biomarkers, the best model (Equation 1) defined by the lowest Akaike information criterion (AIC) value (AIC=25.3) (Figure 1A) was achieved by combining sCD14, IL-1β, VEGF and sROBO4 (AUC=0.90, p<0.0001).
Figure 1. The linear combination including sCD14, IL-1β, VEGF and sROBO4 was the best predictor of future lesional activity within 1 year.
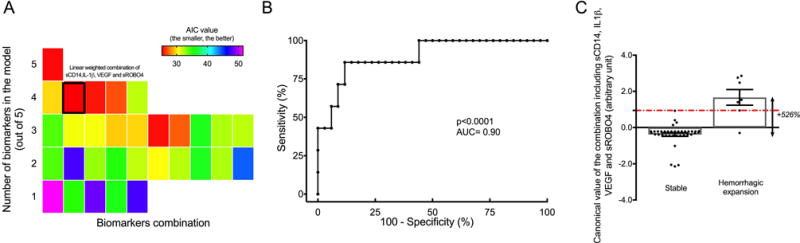
(A) Each colored square represents an optimal weighted combination of biomarkers with its associated Akaike Information Criterion (AIC) value. The lowest AIC value (25.3) was achieved with −0.135x[sCD14] + 7.73x[IL-1β] - 0.775x[VEGF] + 0.658x[sROBO4]. (B) The receiving operating characteristic curve generated for the best linear combination was able to predict a clinical lesional activity within a year (± 30 days) [area under the curve (AUC)=0.90, p=0.0001] with a sensitivity of 86% and specificity of 88%. (C) The mean estimated combination value was 5-fold higher in subjects who experienced a hemorrhagic expansion than stable cases (p<0.0001).
| Equation 1 |
The ROC analysis differentiated subjects who experienced a lesional hemorrhagic expansion from stable subjects with a sensitivity of 86% and specificity of 88% (Figure 1B). Further analysis showed that the mean estimated combination value (Equation 1) was 5-fold higher (p<0.0001) in subjects who experienced hemorrhagic expansion (mean estimated value=1.67 ± 1.13) within the following year compared to subjects that remained stable (mean estimated value=−0.39 ± 0.59) (Figure 1C). The mean estimated combination value calculated with the best model was also 3-fold higher (p<0.01) in patients of the independent cohort (i.e., Group 2 - Algorithm testing) who experienced a subsequent hemorrhagic expansion. In addition, the logistic regression analysis showed that the best linear combination was able to correctly predict the subsequent respective stable or hemorrhagic expansion status in 83.3% and 75.0% of the cases in the independent cohort (Online Table III). This result was also supported by ROC analysis which showed 90% sensitivity and 71% specificity (Online Figure III) of the weighted biomarker equation predicting a hemorrhagic expansion within 1-year (± 30 days) in the independent cohort.
For more results, refer to Supplemental Material.
DISCUSSION
We evaluated the prognostic association between the plasma levels of 24 biomarkers and the occurrence of an impending clinically relevant lesional activity during the subsequent year. The biomarkers were selected based on a systematic literature review of demonstrated associations with relevant disease mechanisms in CCM or brain hemorrhage (Online Table IV).7 Results showed that the plasma levels of sCD14, IL-1β, sROBO4, VEGF and IL-6 were differently expressed in subjects who experienced a CCM-related event in the year following the initial blood sample. We then calculated the AIC, a robust and traditional likelihood-based model-selection, to determine the best linear combination to predict future lesional activity. The best model was achieved by the linear weighted combination including sCD14, IL-1β, sROBO4 and VEGF.
The influence of pro-inflammatory genotypes and neuro-inflammation in the physiopathology and clinical course of CCM disease has been described in recent years.7–9 Single-nucleotide polymorphisms of CD14, IL1β and IL6 genes have been associated with aggressive phenotypes of hemorrhagic cerebrovascular disease including CCM, brain arteriovenous malformation and aneurysm.8, 9, 13 Recently, Tang et al. in collaboration with our group showed that functional gene variants causing increased expression of CD14-anchored membrane glycoprotein is associated with greater lesion counts in familial CCM1 sharing the same mutation (Q455X).9 Here we report lower levels of sCD14 as a predictor of subsequent lesional activity. The association between the levels of circulating CD14 and the anchored membrane form remains unclear and may not be correlated.14 Higher levels of sCD14 may have anti-inflammatory effects by inhibiting lipopolysaccharide-mediated functional responses.14–16
IL-1β is a pro-inflammatory cytokine with a role in mediating inflammation-induced angiogenic responses that indirectly regulates the synthesis of pro-angiogenic factors, and facilitates endothelial cell (EC) migration, proliferation and organization into blood vessel-like structures.17, 18 IL-1β has also been shown to increase the endothelial permeability promoting leukocyte transmigration via multiple direct and indirect pathways.19, 20 We recently reported that an increased permeability assessed by MRI at follow-up correlated with symptomatic lesional hemorrhage or growth.10 Soluble IL-6 is a multifunctional cytokine mediating pro- and anti-inflammatory processes, as well as regenerative, neural and metabolic pathways.21 As with CD14, SNP in IL6R gene have been correlated with a greater number of CCM lesions.8 The roles of sIL-6 versus IL-6 receptor in CCM lesion development and hemorrhage will require further investigation. Our results showing higher IL-1β and lower IL-6 predicting subsequent CCM clinical activity support the hypothesis that an increased endothelial permeability may occur via inflammatory mechanisms, resulting in imminent symptomatic lesional bleeding or expansion.
ROBO4 is an endogenous inhibitor of VEGF signaling expressed by vascular endothelial cells.22, 23 This protein has been shown to dynamically maintain vascular network stability, during pathological angiogenesis and pro-inflammatory processes24–26 by modulating the expression of tight junction proteins such as zona occludens-1, occludin and claudin-5.27 In CCM disease, lesions harbor structurally defective tight junctions,28 and decreased mRNA expression of occludin, claudin-5, and ZO-1.29 An increase in sROBO4 may reflect pro-inflammatory processes enhancing endothelial permeability, consistent with its prognostic association with CCM bleeding and growth.
VEGF has direct mitogenic effects on ECs, and is a key regulator of angiogenesis.17 Dysregulation of VEGF expression has been widely studied in CCM disease and has been shown to worsen the blood-brain barrier permeability and promote progression of CCM lesions.6, 30 A modulatory effect of VEGF in the hemorrhagic brain has also been described.7, 31, 32 However, no difference in lesional VEGF expression was observed in a study comparing unstable versus CCM lesions resected surgically.33 Here we observed that decreased plasma level of VEGF is a predictor of subsequent lesional hemorrhage or growth in CCM disease. This is somewhat counter-intuitive, and motivates a hypothesis about how lower VEGF plasma levels might reflect an imbalance in vascular integrity, associated with subsequent aggressive lesion behavior.
The combined model including weighted contribution of four biomarkers sCD14, IL-1β, VEGF and sROBO4 was the best predictor of future lesional activity. The absence of co-correlation among these four protein levels suggest that their relative contribution to CCM lesional activity is independent and additive (Online Table V).
Previous studies had shown that brainstem lesion location and recent symptomatic hemorrhage are predictors of future bleeding risk.4 Also, familial cases with aggressive genotype are associated with greater lesion burden and hemorrhagic risk.3 In our study, patients with these risk factors were also more likely to manifest prospective symptomatic hemorrhage or lesional growth. However, we noted no association of the biomarkers with these or other baseline clinical features of disease, suggesting that the biomarker prognostication was independent of these clinical features. To best explore these interactions, future studies will need to be powered to detect prognostic associations of the weakest biomarker in the smallest relevant disease subgroup.
None of the biomarkers showed association with de novo lesion genesis in familial cases. We had previously shown an association of focal brain vascular hyperpermeability with subsequent lesion genesis in that same brain region.10 Our study included a small number of familial cases with lesion growth. If confirmed in a larger cohort, it is possible that such focal brain vascular hyperpermeability may not be reflected in our assayed biomarkers.
Our single site study does not exclude referral bias. And cases with biomarker sampling may have undergone lesion resection or otherwise not been available for prospective follow-up at our center. These cases without follow-up did not have significantly different biomarker levels nor clinical features, reassuring us that follow-up bias did not likely impact our results. Beyond larger sample sizes, with sufficient number of cases in relevant subgroups, future multi-site studies will be designed to better control for these potential biases.
Notwithstanding these limitations, this study is the largest to date in this rare disease. And this is the first report of a strong prognostic association of a peripheral blood biomarker with clinical activity in a defined cerebrovascular pathology. We will pursue validation of these results in conjunction with multi-site clinical trial readiness project already underway, funded by the U.S. National Institutes of Health, and in other prospective question-driven studies. These will consider specific clinical scenarios and risk versus benefit of biomarker clinical application.34 In addition, the assay methodologies and batch effect correction have generated arbitrary units that could not be extended directly into clinical practice. Future studies will assess the differences in plasma levels between healthy controls and cohorts of CCM patients with stable and unstable clinical activity, providing reference ranges of biomarker values applicable to positive and negative clinical prognostic risk.
The correlations herein do not imply a specific causality related to CCM disease. But they generate cogent hypotheses about mechanism of disease risk, to be pursued in future laboratory and clinical studies. Finally, the chosen biomarkers were selected based on current knowledge about CCM disease mechanims.17 More recent investigations have highlighted additional key molecules involved in pathobiology.9, 35 Respective biomarkers related to these novel mechanisms may further refine the predictive power of clinical behavior in this disease.
Supplementary Material
SUMMARY.
Our results support an extensive literature on the influence of inflammatory and angiogenic processes in the pathobiology of CCM disease. They define a mechanistic-based conceptual model to predict short-term future clinical activity based on a panel of inflammatory and angiogenic cytokines. If validated in multi-site studies, this model may play a direct role in selecting patients for aggressive therapies, and in the stratification of cohorts in clinical trials. This same approach may be useful in other cerebrovascular pathologies including hemorrhagic microangiopathy, amyloid angiopathy and aging where similar mechanisms have been postulated.36
NOVELTY AND SIGNIFICANCE.
What Is Known?
Cerebral cavernous malformations (CCMs) is a common neurovascular pathology affecting 0.5% of the worldwide population.
The clinical course of CCMs is highly unpredictable.
The central and systemic inflammatory processes influence the etiology and progression of CCMs.
What New Information Does This Article Contribute?
It is the first study reporting a mathematical model predicting short-term subsequent clinical CCM lesional activity.
This model may play a direct role in selecting CCM patients for aggressive therapies, and in the stratification of cohorts in clinical trials.
These results may be critical to define biological targets for therapies.
Herein, we report a predictive association between plasma biomarkers and subsequent lesional clinical activity. Our results support an extensive literature on the influence of inflammatory and angiogenic processes in the pathobiology of CCM disease. They also define a likelihood-based computational model to predict short-term future clinical activity based on a panel of preselected inflammatory and angiogenic cytokines. If validated in multi-site studies, this model may play a direct role in selecting patients for aggressive therapies, and in the stratification of cohorts in clinical trials. The same approach may be useful in other cerebrovascular pathologies including hemorrhagic microangiopathy, amyloid angiopathy and aging, where similar mechanisms have been postulated.
Acknowledgments
We would like to thank Mark H. Ginsberg from The University of California San Diego for helpful discussions.
SOURCES OF FUNDING
This work was partially supported by a grant from the NIH (R21NS087328) to IAA, by the University of Chicago Medicine Comprehensive Cancer Center Support Grant (P30 CA14599), by the William and Judith Davis Fund in Neurovascular Surgery Research, and by the Safadi Translational Fellowship to RG. Funding sources played no role in the formulation of research questions nor the interpretation of results.
Nonstandard Abbreviations and Acronyms
- AIC
akaike information criterion
- AUC
area under the curve
- CCM
cerebral cavernous malformations
- EC
endothelial cell
- IL-1β
interleukin-1 beta
- IL-6
interleukin-6
- ROC
receiving operating characteristic
- ROCK
rhoA associated kinase
- sCD14
soluble cluster of differentiation 14
- sROBO4
soluble roundabout guidance receptor 4
- VEGF
vascular endothelial growth factor
Footnotes
DISCLOSURES
The authors declare no conflict of interest.
References
- 1.Mikati AG, Khanna O, Zhang L, Girard R, Shenkar R, Guo X, Shah A, Larsson HB, Tan H, Li L, Wishnoff MS, Shi C, Christoforidis GA, Awad IA. Vascular permeability in cerebral cavernous malformations. J Cereb Blood Flow Metab. 2015;35:1632–1639. doi: 10.1038/jcbfm.2015.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akers A, Al-Shahi Salman R, Awad IA, et al. Synopsis of guidelines for the clinical management of cerebral cavernous malformations: Consensus recommendations based on systematic literature review by the angioma alliance scientific advisory board clinical experts panel. Neurosurgery. 2017;80:665–680. doi: 10.1093/neuros/nyx091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shenkar R, Shi C, Rebeiz T, et al. Exceptional aggressiveness of cerebral cavernous malformation disease associated with pdcd10 mutations. Genet Med. 2015;17:188–196. doi: 10.1038/gim.2014.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Al-Shahi Salman R, Hall JM, Horne MA, Moultrie F, Josephson CB, Bhattacharya JJ, Counsell CE, Murray GD, Papanastassiou V, Ritchie V, Roberts RC, Sellar RJ, Warlow CP, Scottish Audit of Intracranial Vascular Malformations (SAIVMs) collaborators Untreated clinical course of cerebral cavernous malformations: A prospective, population-based cohort study. Lancet Neurol. 2012;11:217–224. doi: 10.1016/S1474-4422(12)70004-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McDonald DA, Shi C, Shenkar R, Gallione CJ, Akers AL, Li S, De Castro N, Berg MJ, Corcoran DL, Awad IA, Marchuk DA. Lesions from patients with sporadic cerebral cavernous malformations harbor somatic mutations in the ccm genes: Evidence for a common biochemical pathway for ccm pathogenesis. Hum Mol Genet. 2014;23:4357–4370. doi: 10.1093/hmg/ddu153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Whitehead KJ, Chan AC, Navankasattusas S, Koh W, London NR, Ling J, Mayo AH, Drakos SG, Jones CA, Zhu W, Marchuk DA, Davis GE, Li DY. The cerebral cavernous malformation signaling pathway promotes vascular integrity via rho gtpases. Nat Med. 2009;15:177–184. doi: 10.1038/nm.1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Girard R, Zeineddine HA, Fam MD, et al. Plasma biomarkers of inflammation reflect seizures and hemorrhagic activity of cerebral cavernous malformations. Transl Stroke Res. 2018;9:34–43. doi: 10.1007/s12975-017-0561-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Choquet H, Pawlikowska L, Nelson J, McCulloch CE, Akers A, Baca B, Khan Y, Hart B, Morrison L, Kim H, Brain Vascular Malformation Consortium S Polymorphisms in inflammatory and immune response genes associated with cerebral cavernous malformation type 1 severity. Cerebrovasc Dis. 2014;38:433–440. doi: 10.1159/000369200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tang AT, Choi JP, Kotzin JJ, et al. Endothelial tlr4 and the microbiome drive cerebral cavernous malformations. Nature. 2017;545:305–310. doi: 10.1038/nature22075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Girard R, Fam MD, Zeineddine HA, Tan H, Mikati AG, Shi C, Jesselson M, Shenkar R, Wu M, Cao Y, Hobson N, Larsson HB, Christoforidis GA, Awad IA. Vascular permeability and iron deposition biomarkers in longitudinal follow-up of cerebral cavernous malformations. J Neurosurg. 2016;127:1–9. doi: 10.3171/2016.5.JNS16687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Girard R, Khanna O, Shenkar R, et al. Peripheral plasma vitamin d and non-hdl cholesterol reflect the severity of cerebral cavernous malformation disease. Biomark Med. 2016;10:255–264. doi: 10.2217/bmm.15.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Al-Shahi Salman R, Berg MJ, Morrison L, Awad IA, Angioma Alliance Scientific Advisory B Hemorrhage from cavernous malformations of the brain: Definition and reporting standards. Angioma alliance scientific advisory board. Stroke. 2008;39:3222–3230. doi: 10.1161/STROKEAHA.108.515544. [DOI] [PubMed] [Google Scholar]
- 13.Kim H, Hysi PG, Pawlikowska L, Poon A, Burchard EG, Zaroff JG, Sidney S, Ko NU, Achrol AS, Lawton MT, McCulloch CE, Kwok PY, Young WL. Common variants in interleukin-1-beta gene are associated with intracranial hemorrhage and susceptibility to brain arteriovenous malformation. Cerebrovasc Dis. 2009;27:176–182. doi: 10.1159/000185609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lloyd-Jones KL, Kelly MM, Kubes P. Varying importance of soluble and membrane cd14 in endothelial detection of lipopolysaccharide. J Immunol. 2008;181:1446–1453. doi: 10.4049/jimmunol.181.2.1446. [DOI] [PubMed] [Google Scholar]
- 15.Leveque M, Simonin-Le Jeune K, Jouneau S, Moulis S, Desrues B, Belleguic C, Brinchault G, Le Trionnaire S, Gangneux JP, Dimanche-Boitrel MT, Martin-Chouly C. Soluble cd14 acts as a damp in human macrophages: Origin and involvement in inflammatory cytokine/chemokine production. FASEB J. 2017;31:1891–1902. doi: 10.1096/fj.201600772R. [DOI] [PubMed] [Google Scholar]
- 16.Haziot A, Rong GW, Bazil V, Silver J, Goyert SM. Recombinant soluble cd14 inhibits lps-induced tumor necrosis factor-alpha production by cells in whole blood. J Immunol. 1994;152:5868–5876. [PubMed] [Google Scholar]
- 17.Voronov E, Carmi Y, Apte RN. The role il-1 in tumor-mediated angiogenesis. Front Physiol. 2014;5:114. doi: 10.3389/fphys.2014.00114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carmi Y, Voronov E, Dotan S, Lahat N, Rahat MA, Fogel M, Huszar M, White MR, Dinarello CA, Apte RN. The role of macrophage-derived il-1 in induction and maintenance of angiogenesis. J Immunol. 2009;183:4705–4714. doi: 10.4049/jimmunol.0901511. [DOI] [PubMed] [Google Scholar]
- 19.Abbott NJ, Patabendige AA, Dolman DE, Yusof SR, Begley DJ. Structure and function of the blood-brain barrier. Neurobiol Dis. 2010;37:13–25. doi: 10.1016/j.nbd.2009.07.030. [DOI] [PubMed] [Google Scholar]
- 20.Wang Y, Jin S, Sonobe Y, Cheng Y, Horiuchi H, Parajuli B, Kawanokuchi J, Mizuno T, Takeuchi H, Suzumura A. Interleukin-1beta induces blood-brain barrier disruption by downregulating sonic hedgehog in astrocytes. PLoS One. 2014;9:e110024. doi: 10.1371/journal.pone.0110024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scheller J, Chalaris A, Schmidt-Arras D, Rose-John S. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim Biophys Acta. 2011;1813:878–888. doi: 10.1016/j.bbamcr.2011.01.034. [DOI] [PubMed] [Google Scholar]
- 22.Acevedo LM, Weis SM, Cheresh DA. Robo4 counteracts vegf signaling. Nat Med. 2008;14:372–373. doi: 10.1038/nm0408-372. [DOI] [PubMed] [Google Scholar]
- 23.Koch AW, Mathivet T, Larrivee B, et al. Robo4 maintains vessel integrity and inhibits angiogenesis by interacting with unc5b. Dev Cell. 2011;20:33–46. doi: 10.1016/j.devcel.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 24.London NR, Li DY. Robo4-dependent slit signaling stabilizes the vasculature during pathologic angiogenesis and cytokine storm. Curr Opin Hematol. 2011;18:186–190. doi: 10.1097/MOH.0b013e328345a4b9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yadav SS, Narayan G. Role of robo4 signalling in developmental and pathological angiogenesis. Biomed Res Int. 2014;2014:683025. doi: 10.1155/2014/683025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shirakura K, Ishiba R, Kashio T, Sakai M, Fukushima Y, Yamamoto N, Manabe S, Shigesada N, Tanaka T, Hino N, Aird WC, Doi T, Okada Y. Endothelial robo4 regulates il-6 production by endothelial cells and monocytes via a crosstalk mechanism in inflammation. Biochem Biophys Res Commun. 2018;495:801–806. doi: 10.1016/j.bbrc.2017.11.067. [DOI] [PubMed] [Google Scholar]
- 27.Cai H, Liu W, Xue Y, Shang X, Liu J, Li Z, Wang P, Liu L, Hu Y, Liu Y. Roundabout 4 regulates blood-tumor barrier permeability through the modulation of zo-1, occludin, and claudin-5 expression. J Neuropathol Exp Neurol. 2015;74:25–37. doi: 10.1097/NEN.0000000000000146. [DOI] [PubMed] [Google Scholar]
- 28.Dejana E, Tournier-Lasserve E, Weinstein BM. The control of vascular integrity by endothelial cell junctions: Molecular basis and pathological implications. Dev Cell. 2009;16:209–221. doi: 10.1016/j.devcel.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 29.Schneider H, Errede M, Ulrich NH, Virgintino D, Frei K, Bertalanffy H. Impairment of tight junctions and glucose transport in endothelial cells of human cerebral cavernous malformations. J Neuropathol Exp Neurol. 2011;70:417–429. doi: 10.1097/NEN.0b013e31821bc40e. [DOI] [PubMed] [Google Scholar]
- 30.Zhu Y, Wu Q, Fass M, Xu JF, You C, Muller O, Sandalcioglu IE, Zhang JM, Sure U. In vitro characterization of the angiogenic phenotype and genotype of the endothelia derived from sporadic cerebral cavernous malformations. Neurosurgery. 2011;69:722–731. doi: 10.1227/NEU.0b013e318219569f. [DOI] [PubMed] [Google Scholar]
- 31.Matsuo R, Ago T, Kamouchi M, et al. Clinical significance of plasma vegf value in ischemic stroke - research for biomarkers in ischemic stroke (rebios) study. BMC Neurol. 2013;13:32–39. doi: 10.1186/1471-2377-13-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jung KH, Chu K, Jeong SW, Park HK, Bae HJ, Yoon BW. Cerebral cavernous malformations with dynamic and progressive course: Correlation study with vascular endothelial growth factor. Arch Neurol. 2003;60:1613–1618. doi: 10.1001/archneur.60.11.1613. [DOI] [PubMed] [Google Scholar]
- 33.Maiuri F, Cappabianca P, Gangemi M, De Caro Mdel B, Esposito F, Pettinato G, de Divitiis O, Mignogna C, Strazzullo V, de Divitiis E. Clinical progression and familial occurrence of cerebral cavernous angiomas: The role of angiogenic and growth factors. Neurosurg Focus. 2006;21:1–9. doi: 10.3171/foc.2006.21.1.4. [DOI] [PubMed] [Google Scholar]
- 34.Leptak C, Menetski JP, Wagner JA, et al. What evidence do we need for biomarker qualification? Sci Transl Med. 2017;9 doi: 10.1126/scitranslmed.aal4599. [DOI] [PubMed] [Google Scholar]
- 35.Zhou Z, Tang AT, Wong WY, et al. Cerebral cavernous malformations arise from endothelial gain of mekk3-klf2/4 signalling. Nature. 2016;532:122–126. doi: 10.1038/nature17178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Poggesi A, Pasi M, Pescini F, Pantoni L, Inzitari D. Circulating biologic markers of endothelial dysfunction in cerebral small vessel disease: A review. J Cereb Blood Flow Metab. 2016;36:72–94. doi: 10.1038/jcbfm.2015.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


