Abstract
Adolescent stress exposure is a risk factor for drug abuse, and sex differences contribute to psychostimulant responses. Although many studies have utilized the Wistar rat strain in adolescent stress paradigms, the impact of adolescent stress exposure on addiction-like outcomes has not been rigorously tested in female Wistar rats. In this study, locomotor sensitization was assessed in adolescent and adult female Wistar rats following either chronic stress during adolescence (CAS) or no stress (NS). Adolescent, but not adult, female Wistar rats developed locomotor sensitization to 15 mg/kg cocaine over 5 days of treatment, regardless of stress history. CAS reduced the initial locomotor response to novelty in both adolescent and adult rats compared to NS controls but had no effect on locomotor sensitization to cocaine in adolescents or adult female rats. These studies expand our understanding of age and adolescent stress on cocaine-induced behavioral plasticity in female Wistar rats.
Keywords: Stress, cocaine, sensitization, adolescent, female, Wistar
1. Introduction
Chronic stress is an important risk factor in the development of addiction [1]. Due to the extensive neuronal maturation that occurs during the adolescent period, exposure to stress during adolescence may result in more severe drug abuse outcomes [1-3]. Rodents have been useful in studying the impact of adolescent stress exposure on the behavioral response to drugs of abuse, but the majority of these studies have focused on males [3, 4]. Given the sex differences observed in the behavioral and molecular response to chronic adolescent stress (CAS) exposure, it is essential to include females in studies examining the interaction between adolescent stress and addictive drugs [5, 6].
Locomotor sensitization is considered a behavioral representation of drug-induced plasticity, and it is well established that cross-sensitization between stress and drug responses occurs [1]. While previous studies have examined locomotor sensitization to cocaine in adult females, there are far fewer studies that have assessed adolescent female rats, and the majority of these employed the Sprague Dawley or Long Evans strain [7-14]. By contrast, the Wistar strain has been essential in experiments evaluating the impact of CAS exposure in adolescents [5], but cocaine sensitization in female Wistar rats has only been evaluated in adults [15-17]. Because a number of studies report variable sensitization responses in adolescents and adults, assessing the behavioral response to cocaine in adolescent female Wistar rats is important for integrating the adolescent stress, sensitization, and sex differences literature [7, 8].
2. Material and Methods
Timed-pregnant Wistar rats were purchased from Charles River (Raleigh, NC) and housed on a 12:12 light:dark cycle with food and water available ad libitum. All experiments were performed in an AAALAC approved facility, and experiments were approved by the Institutional Animal Care and Use Committee of Emory University and were conducted according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Litters from timed-pregnant females were culled to 5 female and 3 male (or 6 female and 2 male) pups and weaned on postnatal day (PND) 21 into same-sex pairs. Only females were used for the reported experiments. On PND 35, consistent with the established paradigm [5, 6], rats in the CAS group were individually housed, while non-stress controls (NS) remained pair-housed. All rats were weighed weekly throughout the study. On PND 38-49, rats in the CAS group were exposed to 12 days of a mixed-modality CAS paradigm as previously described [5, 6]. Briefly, CAS consisted of a pseudorandom alternating schedule of 6 days of social defeat and 6 days of restraint. For each social defeat session, adolescent female Wistar rats were individually placed in the home cage of an ovariectomized female Long Evans rat (Charles River) for 2 min, separated by a clear plastic barrier that allowed both visual and olfactory cues. The barrier was then removed, and the rats were allowed to physically interact for 5 min. The barrier was then replaced for an additional 25 min before the Wistar rat was returned to its home cage. Each female Long Evans rat was housed with a male retired breeder Long Evans rat that was removed prior to each session. This housing arrangement is consistent with previous experiments [5, 6]. For the restraint paradigm, each rat was placed in a clear plastic rodent restraint for 60 min.
Separate groups of rats were used for sensitization testing in adolescence and adulthood. On the day following the final day of the CAS paradigm or during adulthood (PND 92) (Figure 1), female rats were habituated to the locomotor chamber (8 × 17 inches) for 2 h. Consecutive beam breaks (ambulations) in automated locomotor chambers (8 y-axis beams and 4 x-axis beams separated by 1 and 15/16”, San Diego Instruments, La Jolla, CA) were recorded in 5-min bins to assess the locomotor response to novelty. Rats were habituated to locomotor chambers on the second day for 2 h. On the third day and recurring every day for 5 days, rats were habituated to the locomotor chamber for 30-60 min prior to administration of saline or cocaine (15 mg/kg, i.p.), and ambulations were assessed for the following 2 h. In adult rats, an additional cocaine challenge was assessed after 7 days of abstinence (PND 105). One adolescent rat included in novelty testing did not complete the sensitization paradigm and was excluded from sensitization analysis. Estrous cycle was tracked in adult females from PND 85-105. Adolescents were not tracked because they would not exhibit regular estrous cycles at the age of testing [18]. Total ambulations from x and y beams from 5-min bins were summed over the 2-h testing session following cocaine or saline administration. Two-way ANOVA was used to assess statistical significance (α=0.05). Adjusted p-values from Dunnett’s post-hoc multiple comparison tests were used to assess post-hoc differences between days for NS and CAS groups, and a Sidak’s test was used to assess differences between CAS and NS groups within bins. GraphPad Prism Version 7.02 was used for all statistical analyses.
Figure 1.
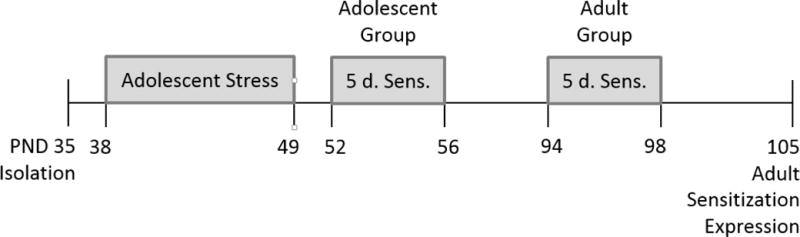
Timeline for CAS and testing. On PND 35, female CAS rats were isolation-housed, exposed to social defeat and restraint from PND 38-49, and tested for cocaine sensitization from PND 52 to 56 (adolescents) or PND 94 to 98 (adults). Expression of sensitization following abstinence was assessed on PND 105 in adults.
3. Results
We first assessed the impact of CAS on the locomotor response to a novel environment in adolescent females (Figure 2A) and adult females (Figure 2B). A history of CAS and bin number significantly interacted to impact locomotor activity in both adolescents (F(23,276) = 2.37, p <0.001) and adults (F(23,138) = 1.87, p=0.014), although there was only a trend towards a main effect of CAS history alone (adolescents: F(1,12) = 4.68, p =0.052; adults: F(1,6) = 2.30, p = 0.18). Post-hoc Sidak’s multiple comparisons test revealed that CAS reduced initial locomotor activity (i.e. the first 5-min bin) after being placed in the locomotor chamber in adolescents (p<0.001) and adults (p=0.008) compared NS controls. As expected, locomotor activity habituated over time in both groups (adolescents: F(23,276) = 61.2, p<0.001; adults: F(23,138) = 21.91, p <0.001).
Figure 2.
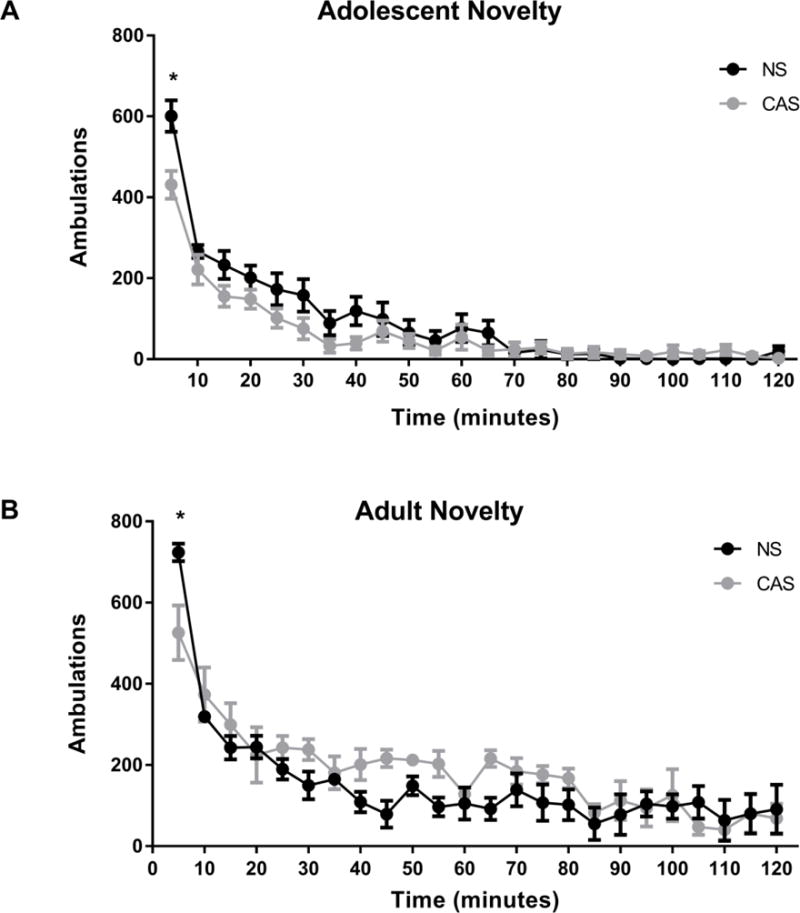
CAS attenuates novelty-induced locomotion in adolescent and adult female Wistar rats. Locomotor activity following exposure to a novel environment was assessed in adolescent, n=6-8 (A) and adult, n=4 (B) female Wistar rats with a history of CAS or NS for 2 h. Locomotor activity was significantly impacted by an interaction between CAS history and bin in both adolescents (A) and adults (B). Data are presented as mean ± SEM. α = 0.05, *denotes significant effect in Sidak’s post-hoc test (CAS vs NS).
We next assessed whether age of testing impacted locomotor sensitization to cocaine. Adolescents sensitized to cocaine across the 5 day regimen (main effect of treatment day, F(4,20) = 5.74, p=0.003) (Figure 3A), and Dunnett’s post-hoc tests revealed that locomotor activity was significantly higher on day 5 compared to day 1 in both NS and CAS exposed rats (NS: p=0.0094, CAS: p=0.0162). To confirm that the observed sensitization was due to the pharmacological properties of cocaine rather than sensitization to repeated injections, we repeated the experiment but administered saline instead of cocaine. As expected, there was no effect of testing day on locomotor activity in saline-treated adolescent female rats (F(4,16) = 0.74, p > 0.05). In contrast to adolescents, adults did not sensitize to cocaine; a significant main effect of test day was observed (F(5,70) = 2.50, p = 0.038), but Dunnett’s post-hoc testing did not reveal a significant effect on locomotor activity when comparing any two days, including day 4 or day 5 vs. day 1.
Figure 3.
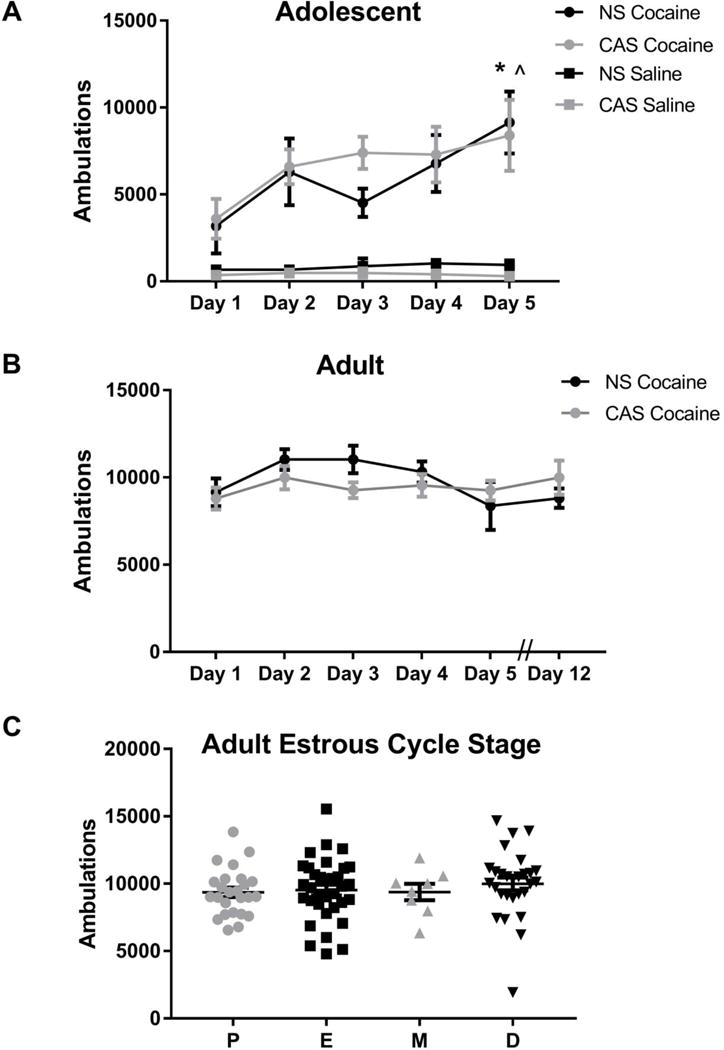
Adolescent, but not adult female Wistar rats sensitize to cocaine, with no effect of CAS. Locomotor activity (summed ambulations) was assessed for 2 h following cocaine administration (15 mg/kg/d, i.p., for 5 days) in adolescent, n=3-4 (A) and adult, n=8 (B) female Wistar rats or saline (once/day for 5 days) in adolescent females, n=3 (A). Adolescent rats exhibited enhanced locomotor activity on Day 5 compared to Day 1. Adult rats did not exhibit sensitized locomotor activity (Days 4, 5, or 12 compared to Day 1). Data in (A) and (B) are presented as mean ± SEM. α = 0.05, a significant effect in Dunnett’s multiple comparisons post-hoc test compared to Day 1 for the NS group is denoted with ^ and for the CAS CAS group with *. Estrous cycle was tracked in adult female Wistar rats (C), and locomotor activity (summed ambulations) was graphed by cycle stage (Proestrus (P), Estrus (E), Metestrus (M), Diestrus (D)) pooled across stress group and test day. Estrous cycle stage did not impact locomotor activity (p>0.05).
Because CAS exposure has been shown to impact behavior in female Wistar rats, we assessed whether a history of CAS exposure altered the locomotor response to cocaine in both adolescent and adult females. We found no effect of CAS exposure on sensitization in adolescents (F(1,5) = 0.19, p > 0.05) (Figure 3A) or adults (F(1,14) = 0.18, p > 0.05) (Figure 3B). We also confirmed that there was no effect of CAS history (F(1,4) = 2.55, p > 0.05) on locomotor activity in saline-treated adolescent female rats (Figure 3A). Finally, we found no effect of estrous cycle stage on locomotor activity (F3,91=0.42, p>0.05, Figure 3C) in adults pooled across stress group and test day.
4. Discussion
Here, we report that adolescent female Wistar rats sensitized to 15 mg/kg cocaine across 5 days of testing, but that adult female Wistars did not. While most sensitization studies have employed males, we focused on females given the sex differences observed in the behavioral and molecular response to CAS exposure [5, 6]. In addition to sex, there are 3 important variables that must be considered in sensitization paradigms: age, strain, and dosing regimen (Table 1).
Table 1.
Summary of studies assessing locomotor sensitization to repeated cocaine administration in adolescent and adult female rats.
| Age on First Day of Treatment Paradigm | Strain | Dose | Treatment Paradigm | Reference | Result |
|---|---|---|---|---|---|
| Adult ~PND 71 | Wistar | 15 mg/kg | 8 days cocaine, day 19 challenge | [15] | Sensitization |
| Adult 175-200g | Wistar | 20, 40 mg/kg | 5 days cocaine | [16] | Sensitization |
| Adult ~PND 111 | Wistar | 10 mg/kg | 10 days cocaine | [17] | Sensitization |
| PND 41 | Sprague Dawley | 3, 7.5, 15 mg/kg | 1 day saline, 6 days cocaine, day 11 challenge | [7] | Sensitization on day 7 (7.5mg/kg), day 11 (7.5, 15 mg/kg) |
| PND 33 | Sprague Dawley | 10, 20 mg/kg | 4 days cocaine, day 6 challenge with 10 mg/kg cocaine | [8] | Sensitization on day 6 |
| PND 20 | Sprague Dawley | 30 mg/kg day 1, 20 mg/kg day 2 | 2 days cocaine | [12] | Sensitization on day 2 compared to 20 mg/kg first cocaine exposure group |
| PND 25 | Sprague Dawley | 1-17.8 mg/kg | 4 cocaine doses every 15 min on 6 test days once weekly | [9] | Sensitization trend; stats not available |
| 70-80g (~PND25- 27) | Sprague Dawley | 1-17.8 mg/kg | 4 cocaine doses every 15 min on 6 test days once weekly | [13] | Sensitization |
| PND 34 | Sprague Dawley | 30 mg/kg day 1, 20 mg/kg day 2 | 2 days cocaine | [11] | No sensitization; Significant in first 10-min bin |
| PND 32 | Sprague Dawley | 5 and 15 mg/kg | 5 days cocaine, day 20 challenge | [10] | Sensitization trend, no significance on day 20 |
| PND 27 | Long Evans | 7 and 15 mg/kg | two rounds of 5 days cocaine separated by 2 days | [14] | Sensitization on day 4, 5, and 9 to 15 mg/kg |
Studies concerning whether locomotor activity following psychostimulant exposure is higher in adults or adolescents have yielded inconsistent results. Following acute cocaine exposure, some report higher locomotor activity in adults [7, 19] while others find similar activity levels across ages [14]. We observed higher locomotor activity in the adult group, consistent with the work by McDougall et al [19]. In chronic treatment paradigms, adolescent female rats may exhibit locomotor sensitization to cocaine more readily than adult females [7, 8], although in other studies, adult females have been found to exhibit similar sensitization to adolescents [14]. It is unlikely that a ceiling effect prevented adult sensitization in the current studies because a moderate dose of cocaine was used (15 mg/kg) that is still on the ascending limb of the dose-response curve [20-22]. It is important to note that the adult rats used in the study by King et al. [7] had previous adolescent exposure to cocaine which is not the case in our current study. Although the mechanisms underlying these age differences are not clear, one possibility is that adolescence is a period of intense synaptic remodeling in the brain, and thus provides a particularly hospitable environment for cocaine-induced plasticity [23]. Although the sample sizes we used in the adolescent sensitization experiment were on the low side compared with some other studies, the results were very consistent and robust, and statistical analysis confirmed that the study was adequately powered to detect significant sensitization in both the CAS and NS groups.
Rat strain also plays a critical role in determining the magnitude of sensitization. Few studies have examined cocaine sensitization in adult Wistar female rats, though sensitization in adult females across other rat strains has been more widely studied. Long Evans rats may sensitize more readily than Fischer rats [14, 24]. For example, in a five-day (15 mg/kg/d) treatment paradigm, Wiley et al. reported sensitization on days 4 and 5 in adult female Long Evans rats [14], while Zhou et al. failed to observe sensitization after five days with a similar regimen in female Fischer rats [24]. Strain-specific changes in dopamine receptor binding [25] and dendritic spine morphology [26] may also contribute to differences in behavioral response to cocaine.
Cocaine dose and length of administration schedules vary considerably between sensitization studies (Table 1), and in general, higher cocaine doses and more administration days produce greater sensitization. Three previous papers reported sensitization in Wistar females, but all of them used either a higher cocaine dose (e.g. 20-40 mg/kg) [16] or a longer sensitization period (e.g. 8-10 days) [15, 17] than in the current study.
CAS did not impact the locomotor response to acute cocaine (Day 1, NS vs. CAS), or cocaine sensitization in adolescent or adult female Wistar rats. Previous studies have shown that adolescent stress exposure increases cocaine self-administration in male Long- Evans rats [27] and cocaine-induced locomotor activity in male Wistar rats [3], but a pilot study did not reveal an effect of CAS on cocaine self-administration in female Wistar rats (our unpublished data). These results suggest that the ability of adolescent stress to alter behavioral responses to drugs of abuse may be more potent in males than females, but additional studies are needed to confirm.
While previous work has indicated a facilitating effect of estradiol and an impact of estrous cycle stage on on cocaine-induced locomotor activity and sensitization [28, 29], we did not observe an effect of estrous cycle stage on locomotor activity in our data pooled across day and stress group. Other studies report locomotor sensitization in adult female rats that are normally cycling [30], and some report that only normal cycling females, rather than females with ovariectomy and estradiol replacement, exhibit locomotor sensitization [15]. Thus, the influence of estrous cycle is unlikely to explain the failure of our adult cohort to exhibit cocaine sensitization.
We also assessed the locomotor response to novelty in adolescent and adult female Wistar rats (Figure 2) with or without a history of CAS and found that initial exploratory activity (in the first 5-min bin) following exposure to a novel environment was attenuated in both adolescent and adult CAS rats. One study found that males exposed to prenatal stress exhibited an enhanced locomotor response to a novel environment compared to NS controls [31], while another reported that adolescent female isolation-housed rats traveled an increased distance following exposure to a novel environment compared to pair-housed females [32]. Our finding of reduced exploratory activity suggests a unique effect of the mixed-modality stress paradigm during adolescence, and that isolation housing alone is unlikely to drive the altered initial response to novelty. The reduced locomotor response to novelty in CAS rats was surprisingly long-lasting; we observed a reduction in locomotor activity in adulthood, weeks removed from exposure to the stressors.
Combined with the existing literature, these data demonstrate that careful consideration should be given to age and strain when designing and interpreting the results of cocaine sensitization experiments. In addition, the findings reported here contribute evidence of age-dependent sensitization to the collective understanding of cocaine-induced behavioral plasticity in adolescent and adult female Wistar rats.
Highlights.
Adolescent but not adult female Wistar rats sensitize to 15 mg/kg cocaine.
Chronic adolescent stress attenuates the initial locomotor response to novelty.
Chronic adolescent stress does not impact locomotor sensitization to cocaine.
Acknowledgments
SAR was supported by training grant T32-GM008602. This work was supported by the National Institutes of Health National Institute of Nursing Research (NR014886 to GNN) and Drug Abuse (DA038453 and DA040788 to DW). The authors thank Daniel Manvich, Jacki Rorabaugh, Jason Schroeder, and Kirsten Porter-Stransky for their assistance in developing these experiments.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest
The authors have no conflicts of interest to disclose.
References
- 1.Burke AR, Miczek KA. Stress in adolescence and drugs of abuse in rodent models: role of dopamine, CRF, and HPA axis. Psychopharmacology. 2014;231(8):1557–80. doi: 10.1007/s00213-013-3369-1. Epub 2013/12/29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yajie D, Lin K, Baoming L, Lan M. Enhanced cocaine self-administration in adult rats with adolescent isolation experience. Pharmacol Biochem Behav. 2005;82(4):673–7. doi: 10.1016/j.pbb.2005.11.007. Epub 2006/01/03. [DOI] [PubMed] [Google Scholar]
- 3.Lepsch LB, Gonzalo LA, Magro FJ, Delucia R, Scavone C, Planeta CS. Exposure to chronic stress increases the locomotor response to cocaine and the basal levels of corticosterone in adolescent rats. Addiction biology. 2005;10(3):251–6. doi: 10.1080/13556210500269366. Epub 2005/08/20. [DOI] [PubMed] [Google Scholar]
- 4.Frantz KJ, O’Dell LE, Parsons LH. Behavioral and neurochemical responses to cocaine in periadolescent and adult rats. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2007;32(3):625–37. doi: 10.1038/sj.npp.1301130. Epub 2006/06/24. [DOI] [PubMed] [Google Scholar]
- 5.Bourke CH, Neigh GN. Behavioral effects of chronic adolescent stress are sustained and sexually dimorphic. Hormones and behavior. 2011;60(1):112–20. doi: 10.1016/j.yhbeh.2011.03.011. Epub 2011/04/07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bourke CH, Raees MQ, Malviya S, Bradburn CA, Binder EB, Neigh GN. Glucocorticoid sensitizers Bag1 and Ppid are regulated by adolescent stress in a sex-dependent manner. Psychoneuroendocrinology. 2013;38(1):84–93. doi: 10.1016/j.psyneuen.2012.05.001. Epub 2012/06/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.King LN, Dafny N, Yang PB, Swann AC. Does a rat’s exposure to cocaine during adolescence affect its response to cocaine in adulthood? The International journal of neuroscience. 2009;119(6):879–907. doi: 10.1080/00207450701591016. Epub 2009/03/28. [DOI] [PubMed] [Google Scholar]
- 8.Laviola G, Wood RD, Kuhn C, Francis R, Spear LP. Cocaine sensitization in periadolescent and adult rats. The Journal of pharmacology and experimental therapeutics. 1995;275(1):345–57. Epub 1995/10/01. [PubMed] [Google Scholar]
- 9.Serafine KM, Bentley TA, Koek W, France CP. Eating high fat chow, but not drinking sucrose or saccharin, enhances the development of sensitization to the locomotor effects of cocaine in adolescent female rats. Behavioural pharmacology. 2015;26(3):321–5. doi: 10.1097/fbp.0000000000000114. Epub 2014/12/09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Franke RM, Belluzzi JD, Leslie FM. Gestational exposure to nicotine and monoamine oxidase inhibitors influences cocaine-induced locomotion in adolescent rats. Psychopharmacology. 2007;195(1):117–24. doi: 10.1007/s00213-007-0876-y. Epub 2007/07/27. [DOI] [PubMed] [Google Scholar]
- 11.Kozanian OO, Gutierrez A, Mohd-Yusof A, McDougall SA. Ontogeny of methamphetamine-induced and cocaine-induced one-trial behavioral sensitization in preweanling and adolescent rats. Behavioural pharmacology. 2012;23(4):367–79. doi: 10.1097/FBP.0b013e32835651c9. Epub 2012/06/27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mohd-Yusof A, Gonzalez AE, Veliz A, McDougall SA. Role of the D1 receptor for the dopamine agonist-induced one-trial behavioral sensitization of preweanling rats. Psychopharmacology. 2014;231(21):4167–77. doi: 10.1007/s00213-014-3561-y. Epub 2014/04/18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Serafine KM, Labay C, France CP. Dietary supplementation with fish oil prevents high fat diet-induced enhancement of sensitivity to the locomotor stimulating effects of cocaine in adolescent female rats. Drug and alcohol dependence. 2016;165:45–52. doi: 10.1016/j.drugalcdep.2016.05.013. Epub 2016/06/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wiley JL, Evans RL, Grainger DB, Nicholson KL. Locomotor activity changes in female adolescent and adult rats during repeated treatment with a cannabinoid or club drug. Pharmacological reports : PR. 2011;63(5):1085–92. doi: 10.1016/s1734-1140(11)70627-2. Epub 2011/12/20. [DOI] [PubMed] [Google Scholar]
- 15.Souza MF, Couto-Pereira NS, Freese L, Costa PA, Caletti G, Bisognin KM, Nin MS, Gomez R, Barros HM. Behavioral effects of endogenous or exogenous estradiol and progesterone on cocaine sensitization in female rats. Brazilian journal of medical and biological research = Revista brasileira de pesquisas medicas e biologicas. 2014;47(6):505–14. doi: 10.1590/1414-431X20143627. Epub 2014/06/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dow-Edwards D, Fico TA, Osman M, Gamagaris Z, Hutchings DE. Comparison of oral and subcutaneous routes of cocaine administration on behavior, plasma drug concentration and toxicity in female rats. Pharmacol Biochem Behav. 1989;33(1):167–73. doi: 10.1016/0091-3057(89)90446-2. Epub 1989/05/01. [DOI] [PubMed] [Google Scholar]
- 17.van Haaren F, Meyer ME. Sex differences in locomotor activity after acute and chronic cocaine administration. Pharmacology, biochemistry, and behavior. 1991;39(4):923–7. doi: 10.1016/0091-3057(91)90054-6. Epub 1991/08/01. [DOI] [PubMed] [Google Scholar]
- 18.Gabriel SM, Roncancio JR, Ruiz NS. Growth hormone pulsatility and the endocrine milieu during sexual maturation in male and female rats. Neuroendocrinology. 1992;56(5):619–25. doi: 10.1159/000126284. Epub 1992/11/01. [DOI] [PubMed] [Google Scholar]
- 19.McDougall SA, Eaton SE, Mohd-Yusof A, Crawford CA. Age-dependent changes in cocaine sensitivity across early ontogeny in male and female rats: possible role of dorsal striatal D2(High) receptors. Psychopharmacology. 2015;232(13):2287–301. doi: 10.1007/s00213-014-3860-3. Epub 2015/01/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kalivas PW, Duffy P, DuMars LA, Skinner C. Behavioral and neurochemical effects of acute and daily cocaine administration in rats. The Journal of pharmacology and experimental therapeutics. 1988;245(2):485–92. Epub 1988/05/01. [PubMed] [Google Scholar]
- 21.Witkin JM, Goldberg SR. Effects of cocaine on locomotor activity and schedule-controlled behaviors of inbred rat strains. Pharmacol Biochem Behav. 1990;37(2):339–42. doi: 10.1016/0091-3057(90)90345-i. Epub 1990/10/01. [DOI] [PubMed] [Google Scholar]
- 22.Lau CE, Imam A, Ma F, Falk JL. Acute effects of cocaine on spontaneous and discriminative motor functions: relation to route of administration and pharmacokinetics. The Journal of pharmacology and experimental therapeutics. 1991;257(1):444–56. Epub 1991/04/01. [PubMed] [Google Scholar]
- 23.Crews F, He J, Hodge C. Adolescent cortical development: a critical period of vulnerability for addiction. Pharmacology, biochemistry, and behavior. 2007;86(2):189–99. doi: 10.1016/j.pbb.2006.12.001. Epub 2007/01/16. [DOI] [PubMed] [Google Scholar]
- 24.Zhou L, Sun WL, Weierstall K, Minerly AC, Weiner J, Jenab S, Quinones-Jenab V. Sex differences in behavioral and PKA cascade responses to repeated cocaine administration. Psychopharmacology. 2016;233(19-20):3527–36. doi: 10.1007/s00213-016-4387-6. Epub 2016/08/25. [DOI] [PubMed] [Google Scholar]
- 25.Zamudio S, Fregoso T, Miranda A, De La Cruz F, Flores G. Strain differences of dopamine receptor levels and dopamine related behaviors in rats. Brain research bulletin. 2005;65(4):339–47. doi: 10.1016/j.brainresbull.2005.01.009. Epub 2005/04/07. [DOI] [PubMed] [Google Scholar]
- 26.Selvas A, Coria SM, Kastanauskaite A, Fernaud-Espinosa I, DeFelipe J, Ambrosio E, Miguens M. Rat-strain dependent changes of dendritic and spine morphology in the hippocampus after cocaine self-administration. Addiction biology. 2017;22(1):78–92. doi: 10.1111/adb.12294. Epub 2015/09/04. [DOI] [PubMed] [Google Scholar]
- 27.Burke AR, DeBold JF, Miczek KA. CRF type 1 receptor antagonism in ventral tegmental area of adolescent rats during social defeat: prevention of escalated cocaine self-administration in adulthood and behavioral adaptations during adolescence. Psychopharmacology. 2016;233(14):2727–36. doi: 10.1007/s00213-016-4336-4. Epub 2016/06/03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sell SL, Scalzitti JM, Thomas ML, Cunningham KA. Influence of ovarian hormones and estrous cycle on the behavioral response to cocaine in female rats. The Journal of pharmacology and experimental therapeutics. 2000;293(3):879–86. Epub 2000/06/28. [PubMed] [Google Scholar]
- 29.Sell SL, Thomas ML, Cunningham KA. Influence of estrous cycle and estradiol on behavioral sensitization to cocaine in female rats. Drug and alcohol dependence. 2002;67(3):281–90. doi: 10.1016/s0376-8716(02)00085-6. Epub 2002/07/20. [DOI] [PubMed] [Google Scholar]
- 30.Thomas MB, Hu M, Lee TM, Bhatnagar S, Becker JB. Sex-specific susceptibility to cocaine in rats with a history of prenatal stress. Physiology & behavior. 2009;97(2):270–7. doi: 10.1016/j.physbeh.2009.02.025. Epub 2009/03/10. [DOI] [PubMed] [Google Scholar]
- 31.Deminiere JM, Piazza PV, Guegan G, Abrous N, Maccari S, Le Moal M, Simon H. Increased locomotor response to novelty and propensity to intravenous amphetamine self-administration in adult offspring of stressed mothers. Brain research. 1992;586(1):135–9. doi: 10.1016/0006-8993(92)91383-p. Epub 1992/07/17. [DOI] [PubMed] [Google Scholar]
- 32.Zakharova E, Starosciak A, Wade D, Izenwasser S. Sex differences in the effects of social and physical environment on novelty-induced exploratory behavior and cocaine-stimulated locomotor activity in adolescent rats. Behav Brain Res. 2012;230(1):92–9. doi: 10.1016/j.bbr.2012.01.052. Epub 2012/02/14. [DOI] [PMC free article] [PubMed] [Google Scholar]


