Abstract
Level of education is often regarded as a proxy for cognitive reserve in older adults. This implies that brain degeneration has a smaller effect on cognitive decline in those with more education but this has not been directly tested in previous research. We examined how education, quantitative MRI based measurement of brain degeneration, and their interaction affect cognitive decline in diverse older adults spanning the spectrum from normal cognition to dementia. Gray matter atrophy was strongly related to cognitive decline. While education was not related to cognitive decline, brain atrophy had a stronger effect on cognitive decline in those with more education. Importantly, high education was associated with slower decline in individuals with lesser atrophy but with faster decline in those with greater atrophy. This moderation effect was observed in Hispanics (who had high heterogeneity of education) but not in African Americans or Caucasians. These results suggest that education is an indicator of cognitive reserve in individuals with low levels of brain degeneration but the protective effect of higher education is rapidly depleted as brain degeneration progresses.
Keywords: Aging, cognitive change, education, cognitive reserve, MRI, gray matter change
1. Introduction
Education has been shown to have robust effects on a broad range of health outcomes, and specifically, has been identified in many studies in different countries and populations as having a protective effect with respect to development of dementia. That is, dementia incidence rates are lower in more educated individuals (EClipSE Collaborative Members et al., 2010; Prince et al., 2012; Xu et al., 2016) and average age of dementia onset is delayed (Amieva et al., 2014; Xu et al., 2016). Education also has been widely regarded as a factor that promotes cognitive reserve (Foubert-Samier et al., 2012; Stern, 2006) and often is considered a direct proxy for cognitive reserve (Valenzuela & Sachdev, 2006a, 2006b). Cognitive reserve is a construct that refers to intra-individual characteristics that buffer against or confer resilience to effects of brain injury associated with diseases of aging. Much of the literature on education and cognitive reserve does not directly address whether education modifies effects of brain injury on cognitive trajectories. Rather, this modification is inferred as an explanation for complex interrelations of education, clinical diagnosis, and cognitive trajectories. In this study, we directly evaluated the hypothesis that education modifies the effect of brain degeneration on decline of cognitive function in older adults.
A major, widely repeated finding in the literature on education and cognitive reserve is that higher education is associated with more rapid decline after a clinical diagnosis of Alzheimer's disease or dementia. This was described in seminal work by Stern et al. (1999) and subsequently has been replicated in a larger sample from the same population (Scarmeas, Albert, Manly, & Stern, 2006) and in different populations using different methods (Amieva et al., 2014; Ye et al., 2013). The explanation for this finding is that education promotes resilience to brain changes associated with the developing dementia and consequently delays the onset of clinical symptoms. In effect, the reserve effect of education protects against early decline, but reserve is more depleted in highly educated individuals once clinical symptoms are manifest.
There also is a body of literature that doesn't support the hypothesis that education promotes cognitive reserve. Specifically, there are publications from different populations using different methods that do not show an association of education with late life cognitive decline (Early et al., 2013; Gross et al., 2015; Masel & Peek, 2009; Mungas, Early, Glymour, Zeki Al Hazzouri, & Haan, 2017). These studies show robust effects of education on baseline levels of cognitive function, but not on decline over time. This is problematic for reserve models that posit a protective effect against age associated brain pathology.
Recent studies have examined rates of cognitive decline both before and after clinical symptom onset. A multi-site clinical study in Korea showed that higher education was associated with faster cognitive decline in late stage amnestic mild cognitive impairment (MCI), but with slower decline in early stage MCI (Ye et al., 2013). Amieva et al. (2014), in a large, population based study from France, reported that higher education was associated with slower decline preceding an incident diagnosis of AD and with a substantial delay of the diagnosis of AD, but rate of decline was faster in those with higher education after an AD diagnosis. Yu et al. (2012) applied random change point modeling to combined results from two large US longitudinal cohort studies and reported that higher education delayed the onset of cognitive decline in incident MCI and AD cases but also was associated with faster decline after the onset of decline. This study, like Ye et al. (2013), would suggest that depletion of the reserve effect occurs prior to the diagnosis of dementia. These studies collectively provide an evidence base to show differential effects of education before and after onset of cognitive decline and clinical symptoms. This raises a question of whether the lack of education effects on cognitive decline in studies of the full spectrum of cognitive function is a result of averaging of two different trajectories, one positively and one negatively associated with education.
The literature showing differential education effects before and after diagnosis is largely based on incident or prevalent dementia cases while much of the literature showing no association of education with cognitive decline is based on samples spanning the spectrum of cognitive function from normal to demented. The studies showing differential rates of cognitive decline before and after symptom onset do not directly address whether education modifies the effects of brain degeneration on cognitive decline. The current study examined the joint and interactive effects of education and progressive brain atrophy on cognitive decline in a demographically and cognitively diverse cohort that spanned a spectrum of late life cognitive function from normal cognition to dementia. Previous work with this cohort has shown that education is not associated with rate of cognitive decline (Early et al., 2013; Gross et al., 2015), but also has shown that progressive brain atrophy measured by longitudinal magnetic resonance imaging (MRI) is strongly associated with cognitive decline (Fletcher et al., 2018) . We tested the hypothesis that education would modify the association of brain atrophy with cognitive decline such that higher education would be associated with slower decline resulting from milder levels of atrophy but faster decline related to more rapid atrophy.
2. Materials and Methods
2.1. Participants
Participants were from the UC Davis Diversity Cohort, a longitudinal study that includes substantial numbers of Hispanic, African American, and non-Hispanic White older adults. This cohort is heterogenous in race/ethnicity and educational attainment and spans a spectrum of cognitive function from normal to mildly impaired to demented. Cohort composition and recruitment methods are described in Hinton et al. (2010). Participants were 460 persons who had received at least two cognitive evaluations and at least one MRI brain scan; 295 had two or more scans. There were 212 Caucasians, 111 Hispanics, and 121 African Americans and 16 other races/ethnicities; 64 Hispanics were tested in Spanish, and all others were tested in English. A community screening program designed to identify and recruit individuals with cognitive functioning representative of the community dwelling population in a six-county catchment area in the central Sacramento/San Joaquin valley and east San Francisco Bay area of Northern California identified 313 individuals (97 Caucasians, 98 Hispanics, 107 African Americans, 11 Other). The remaining 147 (115 Caucasians, 13 Hispanics, 14 African Americans, 5 Other) were initially seen for clinical evaluation at a university memory/dementia clinic and referred for research.
Participants in this study were evaluated and followed within the research program of the University of California at Davis Alzheimer’s Disease Center. Enrollment began in 2001 and a rolling enrollment design was used to build the cohort with substantial enrollment continuing through 2010. All participants in this study had at least two evaluations but due to rolling enrollment there was variability in the number of evaluations completed by each participant. Inclusion criteria for the longitudinal cohort included age 60 or older at their first examination and ability to speak English or Spanish. Exclusion criteria included unstable major medical illness, major primary psychiatric disorder, and substance abuse or dependence in the last five years. All participants signed informed consent, and all human subject involvement was overseen by institutional review boards at University of California at Davis, the Veterans Administration Northern California Health Care System and San Joaquin General Hospital in Stockton, California.
Sample characteristics are presented in Table 1.
Table 1.
Sample characteristics.
| Dementia | MCI | Normal | Total | |
|---|---|---|---|---|
| Gender - Female | 33 (57.9%) | 82 (49.4%) | 153 (66.2%) | 268 (59.0%) |
| Gender - Male | 24 (42.1%) | 84 (50.6%) | 78 (33.8%) | 186 (41.0%) |
| Age_IA - Mean (SD) | 77.5 (±7.0) | 75.0 (±7.0) | 73.4 (±6.7) | 74.5 (±7.0) |
| Education - Mean (SD) | 11.5 (±4.7) | 13.8 (±4.4) | 12.8 (±4.4) | 13.0 (±4.5) |
| Recruitment Source - Clinic | 28 (49.1%) | 85 (51.2%) | 32 (13.9%) | 145 (31.9%) |
| Recruitment Source - Community | 29 (50.9%) | 81 (48.8%) | 199 (86.1%) | 309 (68.1%) |
| Race/Ethnicity - African American (N=121) | 15 (26.3%) | 37 (22.3%) | 68 (29.4%) | 120 (26.4%) |
| Race/Ethnicity - Hispanic (N=111) | 19 (33.3%) | 17 (10.2%) | 72 (31.2%) | 108 (23.8%) |
| Race/Ethnicity - Other (N=16) | 0 (0.0%) | 7 (4.2%) | 9 (3.9%) | 16 (3.5%) |
| Race/Ethnicity - White (N=212) | 23 (40.4%) | 105 (63.3%) | 82 (35.5%) | 210 (46.3%) |
| APOE ε4 - 0 | 22 (38.6%) | 88 (53.0%) | 161 (69.7%) | 271 (59.7%) |
| APOE ε4 - 1 | 35 (61.4%) | 78 (47.0%) | 70 (30.3%) | 183 (40.3%) |
| Global Gray Change (raw)** - Mean (SD) | −1.3 (±0.7) | −1.0 (±0.7) | −0.6 (±0.4) | −0.8 (±0.6) |
| Global Gray Change (standardized) - Mean (SD) | −0.6 (±0.8) | −0.3 (±0.9) | 0.1 (±0.6) | −0.1 (±0.8) |
| Global Gray Baseline (standardized) - Mean (SD) | −0.6 (±0.9) | −0.1 (±1.0) | 0.3 (±1.0) | 0.0 (±1.0) |
| Episodic Memory BL - Mean (SD) | −1.0 (±0.5) | −0.4 (±0.7) | 0.5 (±0.8) | −0.0 (±0.9) |
| Semantic Memory BL - Mean (SD) | −0.8 (±1.0) | 0.0 (±0.8) | 0.2 (±0.9) | 0.0 (±0.9) |
| Executive Function BL - Mean (SD) | −0.7 (±0.9) | −0.1 (±0.7) | 0.4 (±0.9) | 0.1 (±0.9) |
| Spatial BL - Mean (SD) | −0.6 (±1.0) | −0.1 (±0.9) | 0.2 (±0.9) | 0.0 (±1.0) |
log jacobian X 100
2.2. Cognitive Assessment
The cognitive outcomes in this study were composite measures of episodic memory, semantic memory, executive function, and spatial ability derived from the Spanish and English Neuropsychological Assessment Scales (SENAS). The SENAS has undergone extensive development as a battery of cognitive tests relevant to cognitive aging that allow for valid comparisons across race/ethnic groups (Mungas, Reed, Haan, & Gonzalez, 2005; Mungas, Reed, Crane, Haan, & González, 2004; Mungas, Reed, Marshall, & González, 2000; Mungas, Reed, Tomaszewski Farias, & DeCarli, 2005; Mungas, Widaman, Reed, & Tomaszewski Farias, 2011). Item response theory and confirmatory factor analysis methods were used to evaluate reliability across a broad range of ability relevant to older adults and incorporate items that effectively measure over this ability continuum. This development process yielded composite measures that are psychometrically matched across domains in terms of level of reliability across the ability continuum. Importantly, these composite scores do not have floor and ceiling effect and are normally distributed. The episodic memory composite score is derived from a multi-trial word-list-learning test (Mungas et al., 2004). The semantic memory composite is derived from highly correlated verbal (object-naming) and nonverbal (picture association) tasks. The executive function composite is constructed from component tasks of category fluency, phonemic (letter) fluency, and working memory (digit-span backward, visual-span backward, list sorting). Spatial ability was measured using the SENAS Spatial Localization scale which assesses ability to perceive and reproduce two-dimensional spatial relationships that are increasingly complex. These measures were administered at all evaluations. Language of test administration was determined by an algorithm that combined information regarding each participant’s language preference in several specific contexts (e.g., conversing at home, listening to radio or television, conversing outside the home, preferred language for reading). Administration procedures, measure development and psychometric characteristics of the SENAS battery are described in more detail elsewhere (Mungas et al., 2004).
2.3. MRI Measures
2.3.1. MRI Sequence Acquisition
All brain imaging was performed at the University of California Davis (UCD) Imaging Research Center on a 1.5T GE Signa Horizon LX system, obtaining 3D T1-weighted spoiled gradient recalled echo acquisition (T1 SPGR: TR 9.1 ms, flip angle 15°, field of view 24 and slice thickness 1.5mm). MRI baseline measurements were derived as part of our in-house processing pipeline described previously (Fletcher, Carmichael, Pasternak, Maier-Hein, & DeCarli, 2014; Lee et al., 2012). Briefly, structural MRI images were processed to remove the skull using an atlas-based method and consensus-voting algorithm (Aljabar, Heckemann, Hammers, Hajnal, & Rueckert, 2009; Aljabar, Heckemann, Hammers, Hajnal, & Rueckert, 2007). Human analysts provided quality control and minimal cleanup as needed. The stripped brain images were nonlinearly deformed to a minimal deformation template (MDT) synthetic image (Kochunov et al., 2001) using cubic B-spline registrations (Rueckert, Aljabar, Heckemann, Hajnal, & Hammers, 2006). Parameters from this transformation were later used to automatically delineate regions of interest (ROIs) in each subject native space by reverse transforming the regions from the MDT image. Lobar ROIs in MDT space were drawn by an experienced neurologist, as described previously (Lee et al., 2010).
2.3.2. Gray Matter Volume Change
For participants having at least two longitudinal structural MRI scan acquisitions, we computed longitudinal structural change between the most widely separated time points. We used a tensor-based morphometry (TBM) method designed to enhance sensitivity and specificity for biological change by incorporating estimates of likely tissue boundaries (Fletcher, 2014; Fletcher et al., 2013). TBM generates deformation fields by registering brain scans at differing time points and using these to estimate local volume changes between the scans (Ashburner & Friston, 2000). This processing was done via an in-house processing pipeline that has been previously described (Fletcher et al., 2016). Briefly, we linearly aligned images at time 1 and time 2 to a “halfway space” to avoid interpolation biases when only one image is transformed. Each brain scan was then corrected for field intensity inhomogeneities using an atlas-based technique and finally tissue-segmented using an algorithm sensitive to edge presence. The log-transformed determinant of the 3x3 Jacobian matrix of the TBM deformation at each voxel (i.e. log-Jacobian) quantifies local brain change.
To perform voxel-wise longitudinal change analysis across subjects in a common space, we transformed subject native-space log-Jacobian images onto MDT template space as described above for baseline volumes. Statistical analysis of longitudinal change in native space was performed using ROIs transformed to native space also as described above, then calculating the mean log-Jacobian for each subject in segmented GM on each native ROI.
Gray matter (GM) volume change was computed over a cortical GM ROI, delineated in native space as described above, that included prefrontal regions, the parietal lobe posterior to the post-central gyrus, the temporal lobe, and the occipital lobe. Log-Jacobians from these ROIs from both hemispheres were averaged to constitute a global cortical gray matter change measure. Longitudinal change over these regions was computed as the mean log-Jacobian over the ROI intersected with the segmented GM. Cortical gray matter change defined in this manner had the strongest effect on cognitive decline in a previous study based on this cohort (Fletcher et al., 2018). We calculated cortical gray matter volume for the baseline scan by summing segmented GM volumes in the same ROIs used for measuring cortical gray matter change. This sum was then regressed on total intracranial volume and the residual was used as a measure of gray matter volume adjusted for intracranial volume.
2.4. APOE Genotyping
Apolipoprotein E (APOE) genotyping was carried out using the LightCycler ApoE mutation detection kit (Roche Diagnostics, Indianapolis, IN).
2.5. Data Analysis
2.5.1. Measures and Data Processing
SENAS measures of episodic memory, semantic memory, executive function, and spatial ability were the primary dependent variables. MRI gray matter volume change (average of PreFrontal, Temporal, Parietal minus post-central gyrus, and Occipital ROIs) and education were the primary independent variables. Cognitive variables and gray matter change were reasonably normally distributed. We applied the Blom inverse normal rank order transformation to these variables to normalize the variables and establish a common scale (mean=0, sd=1). Education was centered at 12 years. Age (centered at 70 years) was a continuous covariate. Gender, ethnicity, language of test administration and APOE ε4 status were categorical covariates coded using indicator variables. Ethnicity was coded using three indicator variables: African American (1 = yes, 0 = no), Hispanic (1 = yes, 0 = no), and Other minority (1=yes, 0=no); non-Hispanic white was represented by 0’s for all three indicator variables. Gender (male=1, female=0), language of test administration (Spanish=1, English=0) and APOE (≥1 ε4 alleles = 1, 0 ε4 = 0) were represented by single indicator variables. We also created indicator variables to identify individuals who were lost to follow-up (continuously enrolled = 0, lost = 1) and individuals who died (continuously enrolled = 0, deceased = 1) and included these variables as covariates. This coding establishes a continuously enrolled White female, 70 years of age, with 12 years of education, tested in English, and APOE ε4 negative as a reference.
2.5.2. Longitudinal Modeling of Cognitive Trajectories
Mixed effects, parallel process longitudinal analyses were performed using MPlus version 7.4 multilevel modeling (Muthén & Muthén, 1998–2012). In the Within part of the model, each of the four cognitive outcomes was regressed on time in study. This generated person-specific intercept and linear slope random effects for each outcome. These random effects then served as dependent variables in the Between part of the model. All parameters in the model, including Within and Between components, were estimated simultaneously.
Model building proceeded in steps. Step 1 developed a base model to estimate intercept and slope random effects for all four outcomes. It included a within-subjects term to account for practice effects and a term to model a practice effect by Spanish test administration interaction that has has been identified in previous studies with this sample (Brewster et al., 2014; Early et al., 2013; Melrose et al., 2015). The initial model included correlated intercept and slope random effects for each of the four outcomes, but we then evaluated whether second order latent variables (one with intercepts as indicators, one with slopes) explained the correlations among the random effects. Previous studies with this cohort have shown that cognitive trajectories are best described by individual intercepts but a global slope variable that represents a weighted combination of slopes of the four cognitive outcomes (Fletcher et al., 2018; Gavett et al., In Press).
In Step 2, we added covariates including APOE genotype, age, gender, race/ethnicity, language, and indicators for loss to follow-up and deceased status as fixed effect independent variables to explain cognition baseline and change. Effects of being lost to follow-up or deceased were included to adjust for potential bias in the rate of cognitive change associated with these forms of drop out. We examined interaction effects involving ethnicity and other covariates and retained significant interaction effects in subsequent models. In Step 3 we added global gray matter change as an independent variable to explain cognitive decline, continuous education (centered at 12 years), and an effect to represent the interaction of brain change with continuous education. Clinical diagnosis was not used as a grouping variable or covariate in these primary analyses.
Complete data was not available on all variables. The largest component of missing data was for longitudinal MRI scans, where 165 of the 460 participants did not have longitudinal scans. The full sample of 460 was used for the primary data analyses and the missing data analysis option of Mplus was used. This approach effectively used all available data to estimate cognitive trajectories and effects of baseline brain variables and covariates on those trajectories. It maximized precision of estimation of cognitive intercept and slope parameters and statistical power for detecting education and covariate effects. Power for detecting brain change effects is lower because of the reduced sample size for the brain change variables. Mplus uses full information maximum likelihood estimation, which provides unbiased parameter estimates in the context of missing at random (Newman, 2003). Missing at random is satisfied when missingness can be explained by observed variables in the model, and is a reasonable assumption in this study since we included comprehensive measures of cognitive status and change, demographic variables, and genetic risk that could be associated with present versus absent longitudinal scans. As a sensitivity analysis, we performed a secondary analysis to evaluate whether results were different when we excluded those participants who did not have longitudinal scans.
We performed a secondary analysis to evaluate the effects of education, gray matter change, and their interaction within the three main racial/ethnic groups (African Americans, Hispanics, Caucasians). We used a multiple group analysis to simultaneously estimate Model 3 in the three groups, and effects involving education and gray matter change were freely estimated.
An additional secondary analysis examined non-linear effects of education by replacing the continuous measure of years of education with discrete, ordered categories. For example, this might identify a threshold effect where education below a certain level has an impact on cognitive change but differences in education above that threshold have no effect. Years of education was recoded into three indicator variables: less than 12 years, 12 years, and 13–15 years; 16+ years was the reference and was coded as 0 on all three indicator variables. The education main effect in Model 3 was replaced with these 3 indicator variables, and the education by gray matter change interaction was replaced by interaction terms for each indicator variable by gray matter change. A multiple group analysis using categorical education was not performed due to small N's in some of the cells of the group-by-education group cross tabulation.
Finally, we examined joint and interactive effects of education and clinical diagnosis on rate of gray matter change and on baseline gray matter volume. Clinical diagnosis was a categorical variable with the Normal group as the reference group. Gray matter change was the dependent variable in a regression analysis that included years of education, clinical diagnosis (MCI versus Normal and Dementia versus Normal), and the education-by-clinical diagnosis interaction as independent variables.
3. Results
3.1. Sample Characteristics
Sample characteristics are presented in Table 1. About 59% were females. Gender differed across diagnosis groups (χ2[2]=11.356, p=0.003); Normals and Demented cases were more likely to be female but MCI cases were evenly divided among males and females. About 26% were African Americans, 24% were Hispanics, 46% were Caucasians, and 3% were Other ethnicities. Ethnicity differed by diagnosis (χ2[6]=41.050, p=0.001) with Whites more likely to have a diagnosis of MCI. Approximately two thirds of the sample was recruited from the community (68%). Recruitment source differed by diagnosis (χ2[2]=70.848, p=0.001), with MCI cases more likely to be clinic referrals. Average age was about 75 years and this differed across groups (F[2,451]=9.110, p=0.001) with Dementia older than MCI who were older than Normals. Average education was 12.9 and differed across diagnosis groups (F[2,451]=6.256, p=0.002), with highest education in MCI, lowest in Dementia, and Normals in between. APOE ε4 differed by diagnosis (χ2[2]=23.231, p=0.001) with highest ε4 prevalence in Demented cases (61%) and lowest in Normals (30%). Gray matter volume change, baseline gray matter volume, and baseline cognitive test scores all differed across diagnostic groups (p's < 0.001), with a consistent pattern of Normal > MCI > Dementia.
There were 65 single domain amnestic MCI cases, 54 multiple domain amnestic MCI, 27 single domain non-amnestic MCI, and 20 multiple domain non-amnestic MCI. Etiologic diagnosis for dementia was Alzheimer's disease for 45, vascular disease for 2, Lewy body disease for 3, frontotemporal degeneration for 1, and Alzheimer's disease mixed with another etiology for 5.
3.2. Model for Cognitive Intercepts and Slopes
A model that included individual intercepts for the four cognitive outcomes and a second order factor to summarize cognitive slopes provided optimal model fit according to three commonly used indices that have different balances between absolute model fit and model parsimony (the Akaike Information Criterion (Akaike, 1987), the Bayesian Information Criterion (Schwarz, 1978), and the Sample Size Adjusted Bayesian Information Criterion (Sclove, 1987)). Correlations in the unconditional Step 1 model with separate intercepts and slopes ranged from 0.53 to 0.80 for intercepts. In contrast, correlations among slope random effects ranged from 0.87 to 0.96. These results show that intercepts, while correlated, are dissociable, but that slopes are very highly correlated and reflect a unidimensional decline process. Subsequent results examine determinants of cognitive decline measured by this global slope factor. While effects of independent variables and covariates on cognitive intercepts were estimated, we report results pertaining to global cognitive change.
3.3. Education, Brain Change, and Covariate Effects on Cognitive Decline
None of the covariate by ethnicity interaction effects on global slope were significant (p's > 0.15), and so, these interactions were not included in subsequent models. The reference person in the sample (female, white, 12 years of education, 70 years of age, English speaking, no APOE ε4, continuously followed) declined about 6% of a standard deviation annually. frican Americans declined at a slower rate on average (about 0.01 SD per year). Older age, APOE ε4, clinical recruitment, and deceased status all were associated with faster decline.
Table 2 shows effects on cognitive decline of global gray matter change, education, and their interaction. The education main effect on cognitive change was quite small and was not significant. Global Gray Matter Change had a large effect; a 1.0 SD increase in rate of atrophy was associated with a 0.056 SD annual decline. Over 5 years, an individual with gray matter atrophy 1.0 SD faster than average would decline 0.28 SD more than an individual with average gray matter change. The education by gray matter change interaction was significant. Brain change had a stronger effect on cognitive change in individuals with higher education. Results were essentially the same when individuals who did not have longitudinal MRI scans were excluded from the analysis.
Table 2.
Main and Interaction Effects of Education and Global Gray Matter Change on Global Cognitive Change.
| variable | estimate | s.e. | p |
|---|---|---|---|
| Global Gray Change | 0.056 | 0.009 | 0.001 |
| Education by Global Gray Change | 0.005 | 0.001 | 0.001 |
| Education (centered at 12 years) | −0.001 | 0.001 | 0.495 |
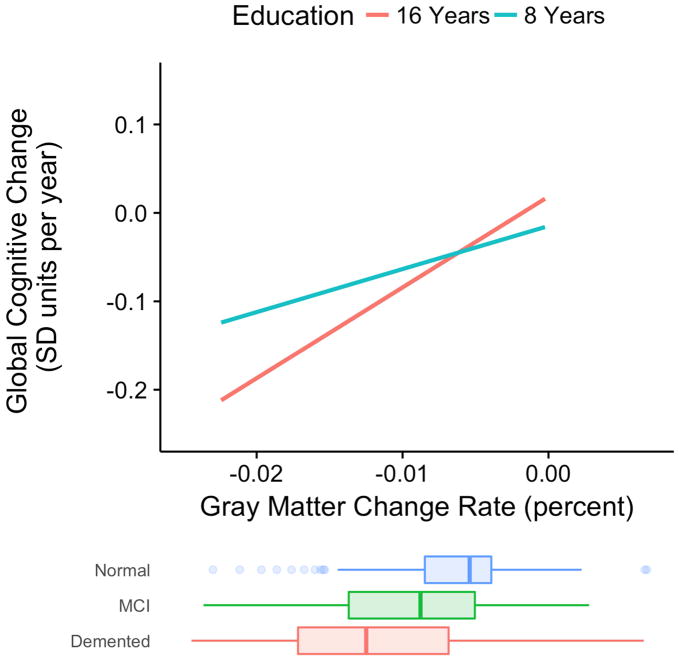
Figure 1 shows the brain atrophy by education interaction. It presents the expected annual rate of cognitive decline as a function of brain atrophy rate for two different levels of education, 8 and 16 years. Less than high school education and college education are clear landmarks for level of educational attainment, and these values were chosen to demonstrate how education level affects cognitive trajectories. There are two notable aspects of Figure 1. First, cognitive decline is more strongly related to brain atrophy rate (steeper slope) at the higher education level. But importantly, rate of cognitive decline is slower for the high education individual when rate of brain atrophy is low, but cognitive decline is faster when brain atrophy rate is high.
Figure 1.
Expected rate of cognitive decline by cortical gray matter atrophy rate for two specific levels of education (8 and 16 years). Cognitive decline is annual decline in standard deviation units of baseline cognitive scores. Distributions of gray matter change rates in diagnostic groups are superimposed at the bottom. The education by gray matter change interaction effect on rate of cognitive decline was stististically significant (p<0.001).
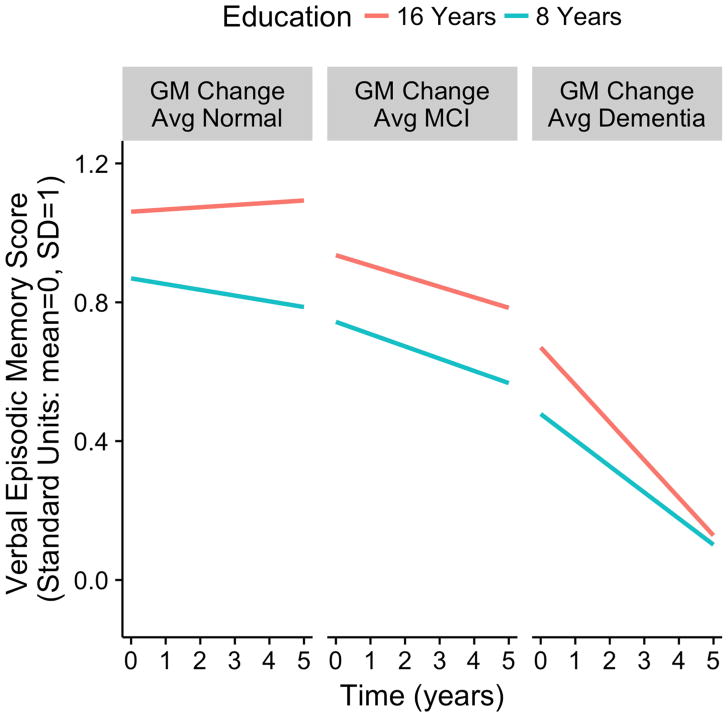
Figure 2 shows how the complex interaction of education and brain atrophy rate influences cognitive trajectories. This graph presents predicted trajectories for individuals with 16 and 8 years of education and with atrophy rates that were average for Normals in this sample, average for MCI, and average for demented cases in this sample. These results are shown for one cognitive outcome, verbal episodic memory, but would be similar for the other outcomes due to the high correlations of change in the four cognitive measures. A high education person with average brain change for Normals would exhibit a slight improvement over 5 years of follow-up; a high education individual with a brain atrophy rate typical for MCI would decline about 0.15 SD in 5 years, and a high education case with brain atrophy typical for dementia in this sample would decline more than 0.5 SD in 5 years. A low education Normal person would be expected to decline slowly, but more than a high Education Normal. Rate of expected decline for low and high education MCI would be about the same, but rate of decline for a low education dementia case would be less than for high education. The typical high education demented person would score about 0.2 SD higher at baseline, but would be at about the same level of performance as the typical low education demented case after 5 years.
Figure 2.
Expected 5-year longitudinal trajectories for verbal episodic memory scores for specific education levels (8 and 16 years) and specific brain atrophy values (average atrophy rates in this sample for Normal, MCI, and Dementia groups). Estimated model parameters were used to demonstrate the effects of education, brain atrophy, and their interaction on longitudinal change in this one specific cognitive outcome. The education by gray matter change interaction effect on rate of cognitive decline was stististically significant (p<0.001).
3.4. Education and Brain Change Effects on Cognitive Decline Within Racial/Ethnic Groups
A multiple group analysis evaluated the effects of education, gray matter change, and their interaction within racial/ethnic groups. Gray matter change was associated with cognitive change in all three groups (Table 3), and education had no main effect in any group. The education by gray matter change effect was significant only in Hispanics, and this result for Hispanics mirrored what was found in the full sample.
Table 3.
Main and Interaction Effects (SEs) of Education and Global Gray Matter Change on Global Cognitive Change by Ethnicity.
| variable | African American | Hispanic | Caucasian |
|---|---|---|---|
| Education | 0.000 (0.001) | 0.000 (0.001) | 0.000 (0.001) |
| Gray Matter Change | 0.054 (0.014)+++ | 0.031 (0.012)++ | 0.091 (0.018)+++ |
| Gray Matter Change by Education | 0.000 (0.003) | 0.003 (0.002)+ | −0.002 (0.004) |
p<0.05,
p<0.01,
p<0.001
3.5. Secondary Analyses
3.5.1. Exclusion of cases with missing longitudinal scans
Results were unchanged when we restricted the analysis to those individuals who had longitudinal MRI scans.
3.5.2. Non-linear Effects of Education
None of the main effects for capturing categorical education effects on cognitive change were significant, indicating that the three lower levels of education (<12 years, 12 years, 13–15 years) did not differ from 16+ years (p's = 0.12, 0.68, 0.65). The interaction of gray matter change with less than 12 years of education was significantly related to global cognitive decline (estimate (se) = −0.048 (0.017), p = 0.006) but the other two interaction effects were not signficant (0.68, 0.9). As expected the gray matter change main effect was significant (estimate (se) = 0.07 (0.012), p = 0). These results show that the effect of gray matter change on cognitive change was smaller in the lowest education group compared to the highest, but this effect did not differ across the three higher education groups.
3.5.3. Gray Matter Change by Education and Clinical Diagnosis
Rate of gray matter change differed by clinical diagnosis (average standardized gray matter change in Normals = 0.145, MCI = −0.28, Dementia = −0.626, Dementia and MCI were significantly different from Normal (p's < 0.001)). Education was not related to gray matter change (estimate=−0.004, s.e.=0.012, p=0.724), nor was there a significant interaction of education with clinical diagnosis (p’s > 0.75). The simple correlation of education with gray matter change was −0.096. For baseline gray matter volume, there were similar group differences (mean standardized Normal = 0.347, MCI = −0.112, Dementia = −0.642, Dementia and MCI were different from Normal (p’s< 0.001). There was a trend for higher education to be associated with lower gray matter volume (estimate=−0.024, s.e.=0.014, p=0.088) independent of clinical diagnosis, and this effect did not differ across diagnosis groups (p’s > 0.53). The simple correlation of education with baseline gray matter was −0.059.
4. Discussion
4.1 Summary of Results
This study examined whether education modifies the impact of brain degeneration on cognitive decline in a demographically diverse sample that spanned the spectrum of cognitive function from normal cognition to dementia. We found that education modified the brain atrophy effect, and actually increased the impact of rate of gray matter atrophy by 9% per year of education. That is, gray matter atrophy had a stronger impact on cognitive decline in participants with higher education. This effect is complex because education was associated with slower cognitive decline in those with low rates of atrophy, but this advantage was reversed by the education-related heightened sensitivity to atrophy as the atrophy rate became higher, eventually resulting in faster cognitive decline in high education individuals with moderate to high rates of atrophy. These results mirror previously reported findings that high education individuals decline slower prior to a diagnosis of MCI or dementia, but decline faster after clinical disease is diagnosed (Amieva et al., 2014; Scarmeas et al., 2006; Stern et al., 1999), and raise two important questions. First, what is the magnitude and real world significance of the education impact on late life cognitive trajectories? Second, since education is a complex, multifactorial variable that affects health and cognitive function in many different ways, what are the mechanisms by which education influences cognitive trajectories?
4.2. Impact of Education Effects
Figure 2 can help to address the impact of education on late life cognitive trajectories. It shows predicted cognitive trajectories for individuals with average rates of brain atrophy in Normal, MCI, and Dementia groups. The expected trajectory for a typical high education Normal starts above average and does not decline, while the expected trajectory for a typical low education Normal starts lower and declines slightly. Even when absolute differences in rates of decline in the high education and low education trajectories are relatively small, a lower start point combined with faster decline, in the context of disease processes that progress over decades, could result in substantially earlier clinical expression of dementia in low education individuals. This is consistent with the conclusions of Amieva et al. (2014). They argued that cognitive decline in both low and high education groups starts at the time of onset of AD disease pathology, but progression to dementia occurred in about 7 years in low education individuals in their study in contrast to about 16 years on average in those with high education. Dementia free survival for 9 additional years represents a major impact at personal, familial, and societal levels. Even though high education individuals might decline more rapidly as brain degeneration progresses and eventually become more impaired on average, the disability free life span is substantially extended. Consequently, increasing population levels of education could have major public health benefits (de la Fuente-Fernandez, 2006).
4.3. Mechanisms of Education Effects
Results of this study raise important questions about mechanistic pathways by which education might influence cognition. Education could impact late life cognitive health through promoting brain maintenance, a relatively new concept that refers to structural and functional brain integrity associated with successful cognitive aging (Nyberg, Lovden, Riklund, Lindenberger, & Backman, 2012). Several studies have shown a link between education and brain health. Rolstad et al. (2010) reported that CSF total tau level in stable MCI cases differed by education level and suggested that education might modify the level of AD pathology. Foubert-Samier et al. (2012) showed an association of education with temporoparietal and orbitofrontal volumes and with white matter tracts connected to these gray matter regions. Liu et al. (2012) reported regional differences in cortical thickness associated with education. Negative results also have been reported. EClipSE Collaborative Members et al. (2010) did not find an association between education and specific neuropathologies despite showing that education protected against incident dementia.
In our study, education was not associated with brain atrophy rate, and in a secondary analysis, did not modify atrophy rate differences among clinical diagnosis groups. This suggests that accelerated cognitive decline in highly educated individuals with dementia is not simply because brain atrophy rate is greater when the landmark of a clinical diagnosis of dementia is reached, and points to mechanisms outside of brain structural integrity as principal mediators of the education effect on the rate of cognitive decline. Specifically, cognitive and brain compensatory processes that are influenced by education merit consideration as important parts of the pathways that link education, brain degeneration, and cognitive decline.
Education might develop skills and knowledge that confer lifelong advantages for problem solving and the ability to adapt to environmental demands and minimize effects of brain degeneration. Brain function might also be an important mediator by providing the neural substrate of these cognitively protective processes. Specifically, cognitive reserve has been proposed to have two brain mechanisms – neural reserve and neural compensation (Steffener, Reuben, Rakitin, & Stern, 2011) – each of which may be affected by education. First, education has been related to greater gray matter volume in cortical regions, including the anterior cingulate gyrus and insula, along with greater gray matter metabolism and functional connectivity of the anterior cingulate with other brain regions (Arenaza-Urquijo et al., 2013). Thus education may contribute to neural reserve via increased efficiency and capacity of existing neural resources (Steffener et al., 2011). Second, education has been related to better brain network recruitment and efficiency (Steffener et al., 2011), suggesting that it may contribute to neural compensation via increased recruitment of resources from non-target brain regions.
In sum, education may contribute to cognitive reserve through increased capacity for processing cognitive tasks and more efficient recruitment of neural resources (Springer, McIntosh, Winocur, & Grady, 2005). This efficiency and redundancy in brain function may explain how individuals with high cognitive reserve can maintain intact cognitive abilities in the context of neuropathology that would otherwise cause cognitive impairment. However, once pathology begins to interfere with the brain’s enhanced efficiency and functional connectivity, decline may be more rapid than it is in those without elevated levels of these two functional attributes. In effect, highly developed, complex functional networks that promote resilience are also possibly more sensitive to effects of developing brain injury. A study correlating cognitive reserve composite scores (in which one component was education) with fMRI activations (Bosch et al., 2010) found patterns consistent with the effects of education reported here. In normal controls, high cognitive reserve was associated with more efficient use (i.e. less activation) for cognitive networks along with more modest deactivation of the default mode network (DMN). Conversely patients with mild cognitive impairment showed an inverse relation of cognitive reserve with network activation. Cognitive networks were more activated in high cognitive reserve (indicating less efficiency), while the DMN activations had a more negative correlation with cognitive reserve than among normals.
Future studies are needed to more comprehensively delineate paths by which education might modify late life cognitive trajectories. Longitudinal studies that carefully measure cognitive change and include measures of important mediators including biomarkers of brain diseases and imaging measures of brain structure and function are especially needed. However, broad diversity of education levels is critical in these studies, and a limitation of the literature to date is that studies with biomarkers and imaging are often of relatively homogenous, high education populations while studies with greater diversity of education do not have comprehensive biomarker and imaging data. The cohort described in this study is very heterogenous in educational background, has well characterized longitudinal cognitive trajectories, and has comprehensive MRI based imaging, and so, future studies with this cohort might help to address some of these questions.
The importance of having a broad range of education in studies of education effects on cognitive trajectories is highlighted by results of the analyses that compared education effects across racial/ethnic groups. Education modified the effects of brain atrophy on cognitive decline in the combined sample in this study and in Hispanics, but not in African Americans or Caucasians. This is not just a sample size issue because this was observed in the Hispanic sub group but not the larger Caucasian group. Education levels were relatively high in African Americans (mean = 13.3, SD = 3.3) and Caucasians (mean = 14.8, SD = 3.3). Mean education was substantially lower and variance was substantially higher in Hispanics (mean = 8.6, SD = 5.3). Variance also was relatively high in the combined sample ( SD =4.6). The secondary analysis that replaced continuous education with categorical education showed that the education by gray matter change interaction was present for the lowest versus the highest education groups but not for the intermediate groups. This provides evidence that range of education differences is an important factor underlying this interaction and that this is not unique to Hispanics. This finding also highlights the importance of having a broad range of education in studies of education effects on cognitive trajectories.
4.4. Education, Cognitive Reserve, Brain Reserve, and Brain Maintenance
This study has theoretical implications for understanding how education relates to the constructs of brain maintenance, brain reserve, and cognitive reserve. Cognitive reserve is best conceptualized as a multifactorial combination of compensatory processes encompassing brain function and cognitive processes that protect against cognitive decline associated with brain diseases of aging (Barulli & Stern, 2013). An expectation that cognitive reserve should dynamically change as brain pathology and compensatory processes change is inherent in this conceptualization. Our results showed that brain maintenance (which can be operationalized as a relative lack of decrease of gray matter volume) was strongly associated with less cognitive decline. Education was associated with greater resilience of cognition to early and mild brain degeneration but less cognitive resilience to higher rates of brain degeneration. Cognitive reserve refers to resilience to cognitive decline resulting from brain injury, so our results suggest that education was associated with greater cognitive reserve at higher levels of brain maintenance but with less reserve as brain integrity declined. This suggests that education is not a simple proxy for cognitive reserve because its effect on cognitive decline changes in degree and direction as structural brain integrity declines.
Measurement of cognitive reserve coupled with measurement of brain integrity can provide important information for understanding prognosis for future cognitive decline but this presupposes concurrent measurement of both cognitive reserve and brain integrity. Education is a static variable that cannot reflect dynamic changes, but there are other approaches to measuring dynamic reserve. First, dynamic proxies for cognitive reserve might have value. For example, recent studies have operationalized cognitive reserve as a composite of measures of vocabulary and reading ability, which can change over time, and education, and have shown that this measure moderates the effects of biomarkers of Alzheimer’s disease (AD) on incident dementia in pre-symptomatic individuals at high risk for AD (Pettigrew et al., 2013; Soldan, Pettigrew, & Albert, 2018). It is noteworthy that education had relatively weak effects on risk for dementia in comparison with the dynamic components, vocabulary and reading (Pettigrew et al., 2013). A second approach is to indirectly measure cognitive reserve as the difference between observed cognitive function and that expected on the basis of brain structure and demographic variables (Barulli & Stern, 2013; Reed et al., 2011). A third approach is to directly measure cognitive and brain function processes underlying reserve. As these mechanisms become better understood, measurement of these processes will yield dynamic measures of reserve. This approach is dependent on research advances to understand reserve, but has a clear advantage of direct measurement of processes that are shown to buffer against declining brain integrity.
This study also raises questions about how education is related to brain reserve. Brain reserve refers to “differences in brain size and other quantitative aspects of the brain that explain differential susceptibility to functional impairment in the presence of pathology or other neurological insult” (Barulli & Stern, 2013). We examined baseline gray matter volume as a simple proxy for brain reserve. Our results did not show a clear association of education to gray matter volume, and indeed there was a trend for higher education to be associated with lower volume. This is a specific but limited measure of brain reserve and there clearly is a need for future studies to examine the association between education and brain structure in a more comprehensive manner. Nevertheless, our results do not support the hypothesis that education contributes to greater brain reserve through greater amounts of grey matter volume.
Cognitive decline and dementia are major public health problems. Studies to clarify mechanisms that protect against late life cognitive decline are important for identifying interventions to promote late life cognitive health. This study shows that educational experience can modify the effects of brain degeneration on cognitive decline, and points to future studies that might identify more granular mechanisms that could enhance cognitive reserve and late life cognitive health.
Supplementary Material
Brain atrophy had a stronger effect on cognitive decline in older adults with higher education
Higher education was associated with slower cognitive decline in individuals with low rates of brain atrophy
Cognitive decline was more sensitive to brain atrophy in those with higher education, and was faster in high education individuals with moderate to high atrophy rates
Education is a useful proxy for cognitive reserve in those with low brain atrophy rates but does not accurately indicate cognitive reserve as brain atrophy increases
Acknowledgments
We would like to acknowledge the devotion of the participants in this study who volunteered their time for comprehensive annual evaluations and repeated MRI scans. Many staff of the UC Davis Alzheimer's Disease Center made this study a reality. Esther Lara supervised all aspects of study implementation from participant recruitment through retention over time leading to successful longitudinal follow-up.
Funding
This work was supported by multiple grants from the National Institute on Aging (NIA) (P30 AG10129, R01 AG021028, and R01 AG047827, C DeCarli, PI; R01 AG10220, D Mungas, PI; R01 AG031563, B Reed/D Mungas, PI; R01 AG031252, S Tomaszewski Farias, PI; R01 AG051170, R Jones, PI). Analysis and manuscript development were supported by a NIA Resource Centers for Minority Aging Research grant (P30 AG043097, L Hinton, PI).
Footnotes
Disclosure
None of the authors have financial or personal conflicts of interest related to this work.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Dan Mungas, Department of Neurology, University of California, Davis.
Brandon Gavett, Department of Psychology, University of Colorado, Colorado Springs.
Evan Fletcher, Department of Neurology, University of California, Davis.
Sarah Tomaszewski Farias, Department of Neurology, University of California, Davis.
Charles DeCarli, Department of Neurology, University of California, Davis.
Bruce Reed, Center for Scientific Review, National Institutes of Health.
References
- Akaike H. Factor analysis and aic. Psychometrika. 1987;52:317–332. [Google Scholar]
- Aljabar P, Heckemann Ra, Hammers a, Hajnal JV, Rueckert D. Multi-atlas based segmentation of brain images: Atlas selection and its effect on accuracy. NeuroImage. 2009;46:726–38. doi: 10.1016/j.neuroimage.2009.02.018. https://doi.org/10.1016/j.neuroimage.2009.02.018. [DOI] [PubMed] [Google Scholar]
- Aljabar P, Heckemann R, Hammers A, Hajnal JV, Rueckert D. Classifier selection strategies for label fusion using large atlas databases. Med Image Comput Comput Assist Interv. 2007;10(Pt 1):523–31. doi: 10.1007/978-3-540-75757-3_64. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/18051099. [DOI] [PubMed] [Google Scholar]
- Amieva H, Mokri H, Le Goff M, Meillon C, Jacqmin-Gadda H, Foubert-Samier A, … Dartigues JF. Compensatory mechanisms in higher-educated subjects with alzheimer’s disease: A study of 20 years of cognitive decline. Brain. 2014;137(Pt 4):1167–75. doi: 10.1093/brain/awu035. https://doi.org/10.1093/brain/awu035. [DOI] [PubMed] [Google Scholar]
- Arenaza-Urquijo EM, Landeau B, La Joie R, Mevel K, Mezenge F, Perrotin A, … Chetelat G. Relationships between years of education and gray matter volume, metabolism and functional connectivity in healthy elders. Neuroimage. 2013;83:450–7. doi: 10.1016/j.neuroimage.2013.06.053. https://doi.org/10.1016/j.neuroimage.2013.06.053. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Voxel-based morphometry–the methods. Neuroimage. 2000;11(6 Pt 1):805–21. doi: 10.1006/nimg.2000.0582. https://doi.org/10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- Barulli D, Stern Y. Efficiency, capacity, compensation, maintenance, plasticity: Emerging concepts in cognitive reserve. Trends Cogn Sci. 2013;17(10):502–9. doi: 10.1016/j.tics.2013.08.012. https://doi.org/10.1016/j.tics.2013.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch B, Bartres-Faz D, Rami L, Arenaza-Urquijo EM, Fernandez-Espejo D, Junque C, … Molinuevo JL. Cognitive reserve modulates task-induced activations and deactivations in healthy elders, amnestic mild cognitive impairment and mild alzheimer’s disease. Cortex. 2010;46(4):451–61. doi: 10.1016/j.cortex.2009.05.006. https://doi.org/10.1016/j.cortex.2009.05.006. [DOI] [PubMed] [Google Scholar]
- Brewster PW, Melrose RJ, Marquine MJ, Johnson JK, Napoles A, MacKay-Brandt A, … Mungas D. Life experience and demographic influences on cognitive function in older adults. Neuropsychology. 2014;28(6):846–58. doi: 10.1037/neu0000098. https://doi.org/2014-24826-001 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Fuente-Fernandez R. Impact of neuroprotection on incidence of alzheimer’s disease. PLoS One. 2006;1:e52. doi: 10.1371/journal.pone.0000052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Early DR, Widaman KF, Harvey D, Beckett L, Park LQ, Farias ST, … Mungas D. Demographic predictors of cognitive change in ethnically diverse older persons. Psychol Aging. 2013;28(3):633–45. doi: 10.1037/a0031645. https://doi.org/10.1037/a0031645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EClipSE Collaborative Members. Brayne C, Ince PG, Keage HA, McKeith IG, Matthews FE, … Sulkava R. Education, the brain and dementia: Neuroprotection or compensation? Brain. 2010;133(Pt 8):2210–6. doi: 10.1093/brain/awq185. https://doi.org/10.1093/brain/awq185. [DOI] [PubMed] [Google Scholar]
- Fletcher E. Using prior information to enhance sensitivity of longitudinal brain change computation. In: Chen CH, editor. Frontiers of medical imaging. Book Section: World Scientific; 2014. pp. 63–81. https://doi.org/10.1142/9789814611107_0004. [Google Scholar]
- Fletcher E, Carmichael O, Pasternak O, Maier-Hein KH, DeCarli C. Early brain loss in circuits affected by alzheimer’s disease is predicted by fornix microstructure but may be independent of gray matter. Frontiers in Aging Neuroscience. 2014;6:1–9. doi: 10.3389/fnagi.2014.00106. https://doi.org/10.3389/fnagi.2014.00106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher E, Gavett B, Harvey D, Farias ST, Olichney J, Beckett L, … Mungas D. Brain volume change and cognitive trajectories in aging. Neuropsychology. 2018 doi: 10.1037/neu0000447. https://doi.org/10.1037/neu0000447. [DOI] [PMC free article] [PubMed]
- Fletcher E, Knaack A, Singh B, Lloyd E, Wu E, Carmichael O, DeCarli C. Combining boundary-based methods with tensor-based morphometry in the measurement of longitudinal brain change. Medical Imaging, IEEE Transactions on. 2013;32(2):223–236. doi: 10.1109/TMI.2012.2220153. https://doi.org/10.1109/tmi.2012.2220153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher E, Villeneuve S, Maillard P, Harvey D, Reed B, Jagust W, Decarli C. Beta-amyloid, hippocampal atrophy and their relation to longitudinal brain change in cognitively normal individuals. Neurobiology of Aging. 2016;40:173–180. doi: 10.1016/j.neurobiolaging.2016.01.133. https://doi.org/10.1016/j.neurobiolaging.2016.01.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foubert-Samier A, Catheline G, Amieva H, Dilharreguy B, Helmer C, Allard M, Dartigues JF. Education, occupation, leisure activities, and brain reserve: A population-based study. Neurobiol Aging. 2012;33(2):423e15–25. doi: 10.1016/j.neurobiolaging.2010.09.023. https://doi.org/10.1016/j.neurobiolaging.2010.09.023. [DOI] [PubMed] [Google Scholar]
- Gavett B, Fletcher E, Harvey D, Tomaszewski Farias S, Olichney J, Beckett L, … Mungas D. Ethnoracial differences in brain structure change and cognitive change. Neuropsychology. doi: 10.1037/neu0000452. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross AL, Mungas DM, Crane PK, Gibbons LE, MacKay-Brandt A, Manly JJ, … Jones RN. Effects of education and race on cognitive decline: An integrative study of generalizability versus study-specific results. Psychol Aging. 2015;30(4):863–80. doi: 10.1037/pag0000032. https://doi.org/10.1037/pag0000032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinton L, Carter K, Reed BR, Beckett L, Lara E, DeCarli C, Mungas D. Recruitment of a community-based cohort for research on diversity and risk of dementia. Alzheimer Dis Assoc Disord. 2010;24(3):234–241. doi: 10.1097/WAD.0b013e3181c1ee01. https://doi.org/10.1097/WAD.0b013e3181c1ee01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochunov P, Lancaster JL, Thompson P, Woods R, Mazziotta J, Hardies J, Fox P. Regional spatial normalization: Toward and optimal target. Journal of Computer Assisted Tomography. 2001;25(5):805–816. doi: 10.1097/00004728-200109000-00023. [DOI] [PubMed] [Google Scholar]
- Lee DY, Fletcher E, Carmichael OT, Singh B, Mungas D, Reed B, … Decarli C. Sub-regional hippocampal injury is associated with fornix degeneration in alzheimer’s disease. Front Aging Neurosci. 2012;4:1. doi: 10.3389/fnagi.2012.00001. https://doi.org/10.3389/fnagi.2012.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DY, Fletcher E, Martinez O, Zozulya N, Kim J, Tran J, … DeCarli C. Vascular and degenerative processes differentially affect regional interhemispheric connections in normal aging, mild cognitive impairment, and alzheimer disease. Stroke. 2010;41(8):1791–7. doi: 10.1161/STROKEAHA.110.582163. https://doi.org/10.1161/STROKEAHA.110.582163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Julkunen V, Paajanen T, Westman E, Wahlund LO, Aitken A … AddNeuroMed, C. Education increases reserve against alzheimer’s disease–evidence from structural mri analysis. Neuroradiology. 2012;54(9):929–38. doi: 10.1007/s00234-012-1005-0. https://doi.org/10.1007/s00234-012-1005-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masel MC, Peek MK. Ethnic differences in cognitive function over time. Ann Epidemiol. 2009;19(11):778–83. doi: 10.1016/j.annepidem.2009.06.008. https://doi.org/10.1016/j.annepidem.2009.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melrose RJ, Brewster P, Marquine MJ, MacKay-Brandt A, Reed B, Farias ST, Mungas D. Early life development in a multiethnic sample and the relation to late life cognition. J Gerontol B Psychol Sci Soc Sci. 2015;70(4):519–31. doi: 10.1093/geronb/gbt126. https://doi.org/10.1093/geronb/gbt126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mungas D, Early DR, Glymour MM, Zeki Al Hazzouri A, Haan MN. Education, bilingualism, and cognitive trajectories: Sacramento area latino aging study (salsa) Neuropsychology. 2017 doi: 10.1037/neu0000356. https://doi.org/10.1037/neu0000356. [DOI] [PMC free article] [PubMed]
- Mungas D, Reed BR, Haan MN, Gonzalez H. Spanish and english neuropsychological assessment scales: Relationship to demographics, language, cognition, and independent function. Neuropsychology. 2005;19(4):466–75. doi: 10.1037/0894-4105.19.4.466. Retrieved from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=16060821. [DOI] [PubMed] [Google Scholar]
- Mungas D, Reed B, Crane P, Haan M, González H. Spanish and english neuropsychological assessment scales (senas): Further development and psychometric characteristics. Psychological Assessment. 2004;16(4):347–359. doi: 10.1037/1040-3590.16.4.347. [DOI] [PubMed] [Google Scholar]
- Mungas D, Reed B, Marshall S, González H. Development of psychometrically matched english and spanish neuropsychological tests for older persons. Neuropsychology. 2000;14:209–223. doi: 10.1037//0894-4105.14.2.209. [DOI] [PubMed] [Google Scholar]
- Mungas D, Reed B, Tomaszewski Farias S, DeCarli C. Criterion-referenced validity of a neuropsychological test battery: Equivalent performance in elderly hispanics and non-hispanic whites. Journal of the International Neuropsychological Society. 2005;11:620–630. doi: 10.1017/S1355617705050745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mungas D, Widaman KF, Reed BR, Tomaszewski Farias S. Measurement invariance of neuropsychological tests in diverse older persons. Neuropsychology. 2011;25(2):260–9. doi: 10.1037/a0021090. https://doi.org/10.1037/a0021090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthén L, Muthén B. Mplus user’s guide. 7. Book, Los Angeles, CA: Muthén & Muthén; 1998–2012. [Google Scholar]
- Nyberg L, Lovden M, Riklund K, Lindenberger U, Backman L. Memory aging and brain maintenance. Trends Cogn Sci. 2012;16(5):292–305. doi: 10.1016/j.tics.2012.04.005. https://doi.org/10.1016/j.tics.2012.04.005. [DOI] [PubMed] [Google Scholar]
- Pettigrew C, Soldan A, Li S, Lu Y, Wang MC, Selnes OA … The Biocard Research, T. Relationship of cognitive reserve and apoe status to the emergence of clinical symptoms in preclinical alzheimer’s disease. Cogn Neurosci. 2013;4(3–4):136–42. doi: 10.1080/17588928.2013.831820. https://doi.org/10.1080/17588928.2013.831820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince M, Acosta D, Ferri CP, Guerra M, Huang Y, Llibre Rodriguez JJ, … Liu Z. Dementia incidence and mortality in middle-income countries, and associations with indicators of cognitive reserve: A 10/66 dementia research group population-based cohort study. Lancet. 2012;380(9836):50–8. doi: 10.1016/S0140-6736(12)60399-7. https://doi.org/10.1016/S0140-6736(12)60399-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed BR, Dowling M, Tomaszewski Farias S, Sonnen J, Strauss M, Schneider JA, … Mungas D. Cognitive activities during adulthood are more important than education in building reserve. J Int Neuropsychol Soc. 2011;17(4):615–24. doi: 10.1017/S1355617711000014. https://doi.org/S1355617711000014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolstad S, Nordlund A, Eckerstrom C, Gustavsson MH, Blennow K, Olesen PJ, … Wallin A. High education may offer protection against tauopathy in patients with mild cognitive impairment. J Alzheimers Dis. 2010;21(1):221–8. doi: 10.3233/JAD-2010-091012. https://doi.org/10.3233/JAD-2010-091012. [DOI] [PubMed] [Google Scholar]
- Rueckert D, Aljabar P, Heckemann RA, Hajnal JV, Hammers A. Diffeomorphic registration using b-splines. MICCAI 2006; Conference Proceedings; Springer-Verlag; 2006. pp. 702–709. [DOI] [PubMed] [Google Scholar]
- Scarmeas N, Albert SM, Manly JJ, Stern Y. Education and rates of cognitive decline in incident alzheimer’s disease. J Neurol Neurosurg Psychiatry. 2006;77(3):308–16. doi: 10.1136/jnnp.2005.072306. Retrieved from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=16484637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz G. Estimating the dimension of a model. Annals of Statistics. 1978;6:461–464. [Google Scholar]
- Sclove SL. Application of model-selection criteria to some problems in multivariate analysis. Psychometrika. 1987;52:333–343. [Google Scholar]
- Soldan A, Pettigrew C, Albert M. Evaluating cognitive reserve through the prism of preclinical alzheimer disease. Psychiatr Clin North Am. 2018;41(1):65–77. doi: 10.1016/j.psc.2017.10.006. https://doi.org/10.1016/j.psc.2017.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer MV, McIntosh AR, Winocur G, Grady CL. The relation between brain activity during memory tasks and years of education in young and older adults. Neuropsychology. 2005;19(2):181–92. doi: 10.1037/0894-4105.19.2.181. https://doi.org/10.1037/0894-4105.19.2.181. [DOI] [PubMed] [Google Scholar]
- Steffener J, Reuben A, Rakitin BC, Stern Y. Supporting performance in the face of age-related neural changes: Testing mechanistic roles of cognitive reserve. Brain Imaging Behav. 2011;5(3):212–21. doi: 10.1007/s11682-011-9125-4. https://doi.org/10.1007/s11682-011-9125-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern Y. Cognitive reserve and alzheimer disease. Alzheimer Dis Assoc Disord. 2006;20(2):112–7. doi: 10.1097/01.wad.0000213815.20177.19. Retrieved from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=16772747. [DOI] [PubMed] [Google Scholar]
- Stern Y, Albert S, Tang MX, Tsai WY. Rate of memory decline in ad is related to education and occupation: Cognitive reserve? Neurology. 1999;53(9):1942–7. doi: 10.1212/wnl.53.9.1942. Retrieved from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=10599762. [DOI] [PubMed] [Google Scholar]
- Valenzuela MJ, Sachdev P. Brain reserve and cognitive decline: A non-parametric systematic review. Psychol Med. 2006a;36(8):1065–73. doi: 10.1017/S0033291706007744. https://doi.org/10.1017/S0033291706007744. [DOI] [PubMed] [Google Scholar]
- Valenzuela MJ, Sachdev P. Brain reserve and dementia: A systematic review. Psychol Med. 2006b;36(4):441–54. doi: 10.1017/S0033291705006264. https://doi.org/10.1017/S0033291705006264. [DOI] [PubMed] [Google Scholar]
- Xu W, Tan L, Wang HF, Tan MS, Tan L, Li JQ, … Yu JT. Education and risk of dementia: Dose-response meta-analysis of prospective cohort studies. Mol Neurobiol. 2016;53(5):3113–3123. doi: 10.1007/s12035-015-9211-5. https://doi.org/10.1007/s12035-015-9211-5. [DOI] [PubMed] [Google Scholar]
- Ye BS, Seo SW, Cho H, Kim SY, Lee JS, Kim EJ, … Na DL. Effects of education on the progression of early- versus late-stage mild cognitive impairment. Int Psychogeriatr. 2013;25(4):597–606. doi: 10.1017/S1041610212002001. https://doi.org/10.1017/S1041610212002001. [DOI] [PubMed] [Google Scholar]
- Yu L, Boyle P, Wilson RS, Segawa E, Leurgans S, De Jager PL, Bennett DA. A random change point model for cognitive decline in alzheimer’s disease and mild cognitive impairment. Neuroepidemiology. 2012;39(2):73–83. doi: 10.1159/000339365. https://doi.org/10.1159/000339365. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.